Knocking on Cells’ Door: Strategic Approaches for miRNA and siRNA in Anticancer Therapy
Abstract
1. Introduction
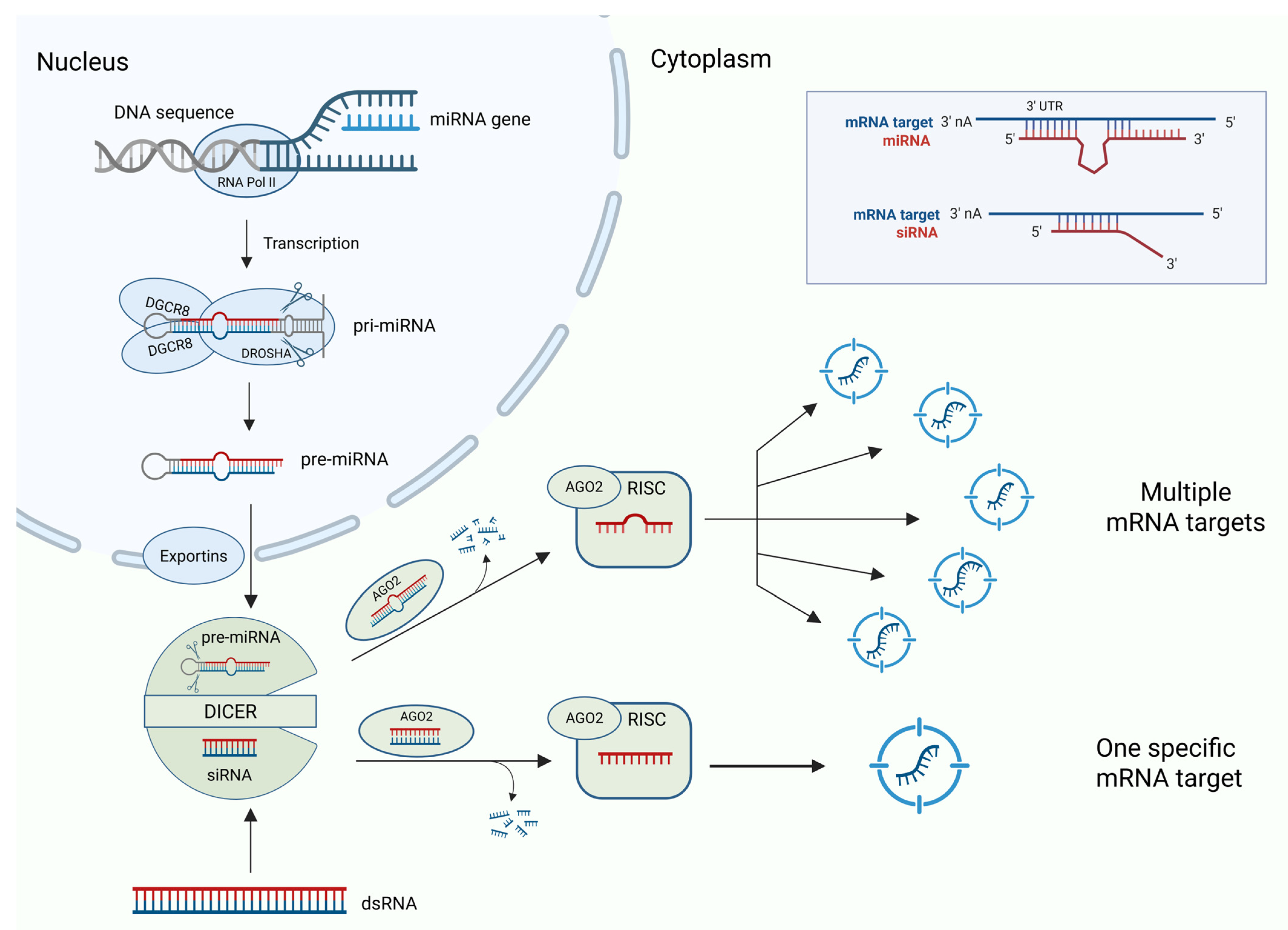
2. Micro RNAs (miRNA)
3. Short Interfering RNAs (siRNAs)
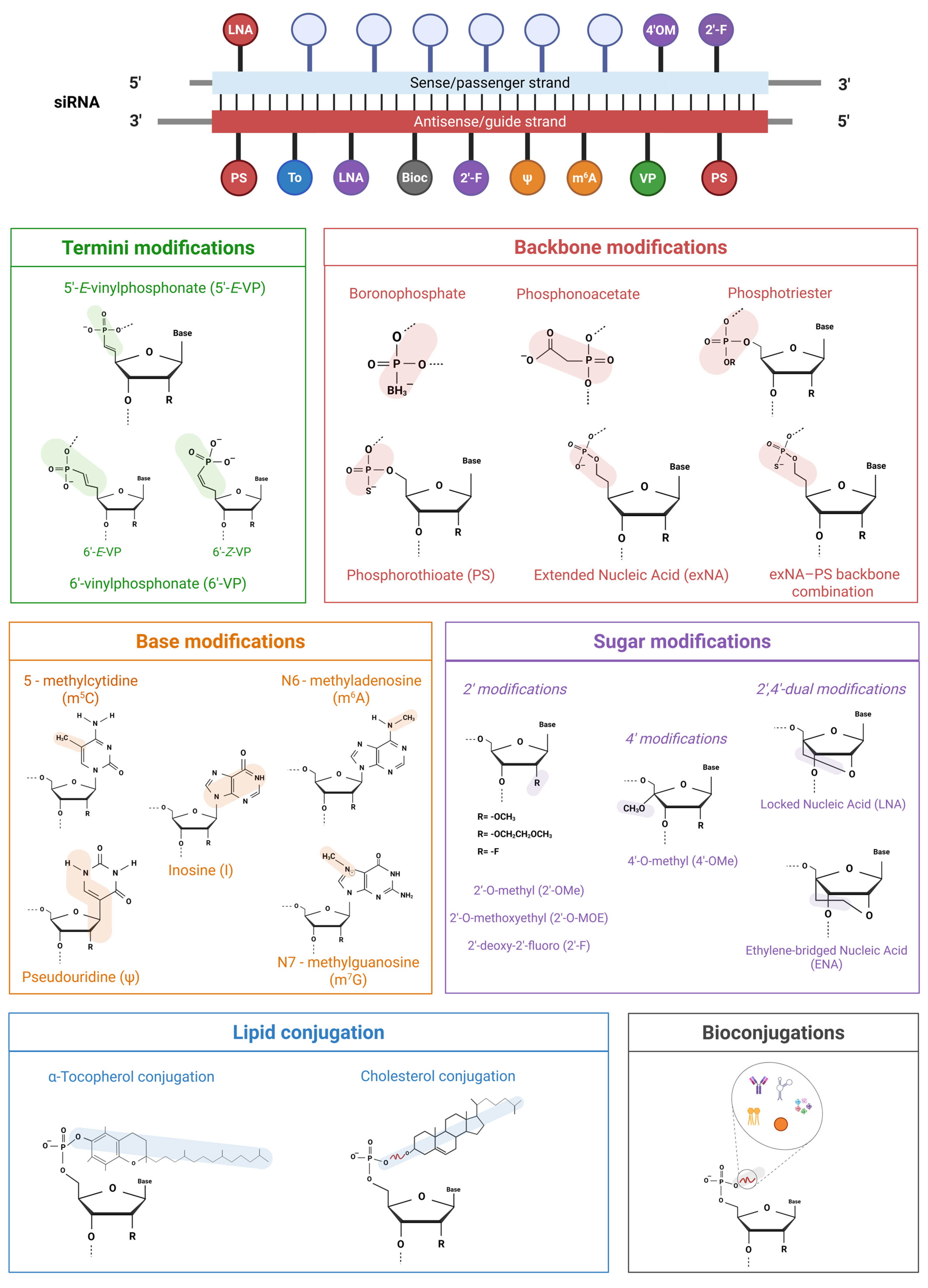
3.1. Beyond Native siRNA: A Chemical Optimization Guide
3.1.1. Termini Modifications
3.1.2. Backbone Modifications
3.1.3. Sugar Modifications
3.1.4. Base Modifications
3.1.5. Lipid Conjugation
3.1.6. Bioconjugations
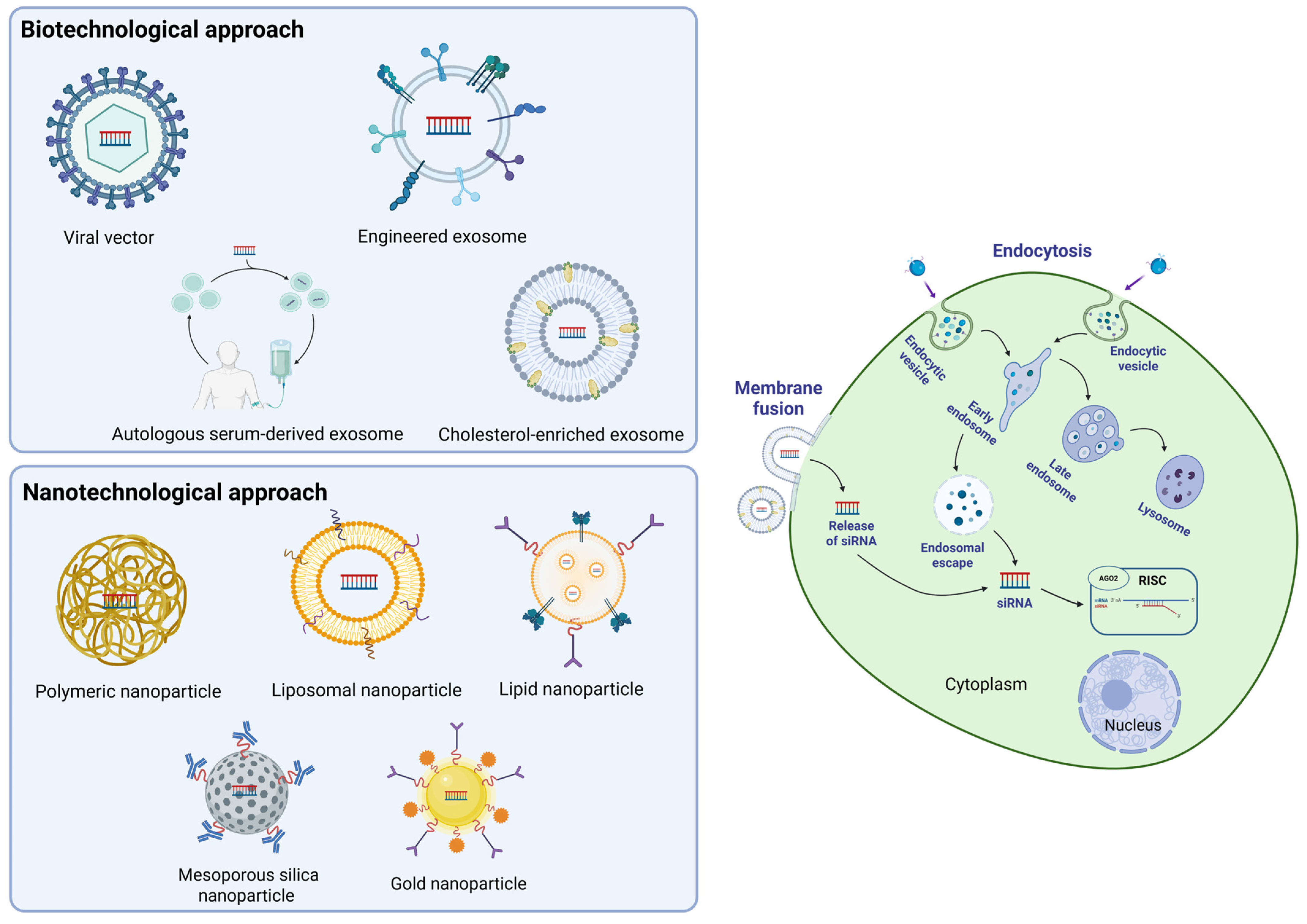
3.2. siRNA Delivery
3.2.1. Biotechnological Approach
3.2.2. Nanotechnological Approach
| Clinical Trials NCT Number | Status and Study Start | Drug or Biological | Biological Function | Diseases | Results | References |
|---|---|---|---|---|---|---|
| NCT03608631 | Active, not recruiting, 27 January 2021 | Drug: Mesenchymal stromal cells (MSC)-derived exosomes (iEXs) loaded with KRASG12D siRNA | siRNA silences KRASG12D oncogene mutation to inhibit cancer cell proliferation and survival. | Metastatic pancreatic ductal adenocarcinoma | Results are expected in 2027. | [73,77] |
| NCT00672542 | Completed, January 2008 | Biological: vaccine of autologous dendritic cells (DCs) transfected with iP-targeting siRNAs and TAA-encoding RNAs | siRNA targeting iP beta subunits (LMP2, LMP7, and MECL1) is used to modify the expression of iP-mediated antigen processed by dendritic cells, in combination with RNAs encoding melanoma TAAs (MART-1, tyrosinase, gp100, and MAGE-3), to enhance antigen-specific T cell responses. | Metastatic melanoma | The open-access abstract available in PubMed summarizes the results, mentioning two treated subjects as partially and completely responsive. No results are published on ClinicalTrials.gov | [77,78] |
| NCT02166255 | Completed, December 2014 | Biological: autologous peripheral blood mononuclear cells (PBMCs) transfected with CBLB siRNA (APN401) | siRNA silences CBLB mRNA to enhance T cells function by reducing CBLB brake. CBLB is an E3 ubiquitin ligase that functions as an intracellular checkpoint restraining lymphocyte activation. | Advanced solid tumors, e.g., metastatic melanoma, metastatic kidney cancer, and metastatic pancreatic cancer | The meeting abstract available in BMJ Journal reported that APN401 infusion seems feasible and well-tolerated. No results are published on ClinicalTrials.gov | [77,79] |
| NCT03087591 | Completed, 28 April 2017 | Biological: autologous peripheral blood mononuclear cells (PBMCs) transfected with CBLB siRNA (APN401) | siRNA silences CBLB mRNA to enhance T cells function by reducing CBLB brake. CBLB is an E3 ubiquitin ligase that functions as an intracellular checkpoint restraining lymphocyte activation. | Advanced solid tumors, e.g., metastatic pancreatic and metastatic colorectal cancer | The treatment was found to induce significant immunological activation without increasing the risk of autoimmunity or systemic toxicity. No deaths were reported; 82% of participants completed the trial, one subject was removed, and another withdrew. | [77] |
| NCT00363714 | Completed, November 2004 | Drug: siRNA-027 (AGN211745) | siRNA-027 targets and silences VEGFR1 mRNA, thereby inhibiting macular neovascularization development. | Age-related macular degeneration (AMD) | The article in AJO reported that siRNA-027 was well-tolerated with no dose-limiting effects, while visual acuity and foveal thickness were stabilized or improved. No results are published on ClinicalTrials.gov | [77,80] |
| NCT01437007 | Completed, 26 August 2011 | Drug: Lipid nanoparticle (LNP) formulated with PLK1 siRNA (TKM-080301) | siRNA targeting the polo-like kinase-1 (PLK1) gene impairs cancer cell proliferation, leading to mitotic arrest and apoptosis. | Primary or secondary liver cancer, e.g., colorectal liver metastasis, pancreas liver metastasis | The trial was limited to one participant, and, unfortunately, no results were published. | [69,77] |
| NCT02110563 | Terminated, April 2014 | Drug: Lipid nanoparticle (LNP) formulated with MYC siRNA (DCR-MYC) | siRNA silences the mRNA expression of the oncogene c-MYC, thereby inhibiting cancer cell proliferation, differentiation, and apoptosis. | Solid tumors, multiple myeloma, and lymphoma | Failed to meet therapeutic criteria | [77,81,82] |
4. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kalla, R.; Ventham, N.T.; Kennedy, N.A.; Quintana, J.F.; Nimmo, E.R.; Buck, A.H.; Satsangi, J. MicroRNAs: New players in IBD. Gut 2015, 64, 504–513. [Google Scholar] [CrossRef]
- Weng, Y.T.; Chang, Y.M.; Chern, Y. The Impact of Dysregulated microRNA Biogenesis Machinery and microRNA Sorting on Neurodegenerative Diseases. Int. J. Mol. Sci. 2023, 24, 3443. [Google Scholar] [CrossRef]
- Kim, T.; Croce, C.M. MicroRNA: Trends in clinical trials of cancer diagnosis and therapy strategies. Exp. Mol. Med. 2023, 55, 1314–1321. [Google Scholar] [CrossRef]
- Saliminejad, K.; Khorram Khorshid, H.R.; Soleymani Fard, S.; Ghaffari, S.H. An overview of microRNAs: Biology, functions, therapeutics, and analysis methods. J. Cell Physiol. 2019, 234, 5451–5465. [Google Scholar] [CrossRef] [PubMed]
- Medley, J.C.; Panzade, G.; Zinovyeva, A.Y. microRNA strand selection: Unwinding the rules. Wiley Interdiscipl. Rev. RNA 2021, 12, e1627. [Google Scholar] [CrossRef]
- Chakrabortty, A.; Patton, D.J.; Smith, B.F.; Agarwal, P. miRNAs: Potential as Biomarkers and Therapeutic Targets for Cancer. Genes 2023, 14, 1375. [Google Scholar] [CrossRef]
- Menon, A.; Abd-Aziz, N.; Khalid, K.; Poh, C.L.; Naidu, R. miRNA: A Promising Therapeutic Target in Cancer. Int. J. Mol. Sci. 2022, 23, 11502. [Google Scholar] [CrossRef]
- Gambari, R.; Brognara, E.; Spandidos, D.A.; Fabbri, E. Targeting oncomiRNAs and mimicking tumor suppressor miRNAs: New trends in the development of miRNA therapeutic strategies in oncology (Review). Int. J. Oncol. 2016, 49, 5–32. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.; Elzallat, M.; Aboushousha, T.; Elhusseny, Y.; El-Ahwany, E. MicroRNA-122 mimic/microRNA-221 inhibitor combination as a novel therapeutic tool against hepatocellular carcinoma. Noncoding RNA Res. 2023, 8, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Ranasinghe, P.; Addison, M.L.; Dear, J.W.; Webb, D.J. Small interfering RNA: Discovery, pharmacology and clinical development—An introductory review. Br. J. Pharmacol. 2023, 180, 2697–2720. [Google Scholar] [CrossRef]
- Friedrich, M.; Aigner, A. Therapeutic siRNA: State-of-the-Art and Future Perspectives. BioDrugs 2022, 36, 549–571. [Google Scholar] [CrossRef]
- Hu, B.; Zhong, L.; Weng, Y.; Peng, L.; Huang, Y.; Zhao, Y.; Liang, X.J. Therapeutic siRNA: State of the art. Signal Transduct. Target. Ther. 2020, 5, 101. [Google Scholar] [CrossRef]
- Selvam, C.; Mutisya, D.; Prakash, S.; Ranganna, K.; Thilagavathi, R. Therapeutic potential of chemically modified siRNA: Recent trends. Chem. Biol. Drug Des. 2017, 90, 665–678. [Google Scholar] [CrossRef] [PubMed]
- Deleavey, G.F.; Watts, J.K.; Damha, M.J. Chemical Modification of siRNA. Curr. Protoc. Nucleic Acid. Chem. 2009, 39, 16.3.1–16.3.22. [Google Scholar] [CrossRef]
- Tatiparti, K.; Sau, S.; Kashaw, S.; Iyer, A. siRNA Delivery Strategies: A Comprehensive Review of Recent Developments. Nanomaterials 2017, 7, 77. [Google Scholar] [CrossRef]
- Kliuchnikov, E.; Maksudov, F.; Zuber, J.; Hyde, S.; Castoreno, A.; Waldron, S.; Schlegel, M.K.; Marx, K.A.; Maier, M.A.; Barsegov, V. Improving the potency prediction for chemically modified siRNAs through insights from molecular modeling of individual sequence positions. Mol. Ther. Nucleic Acids 2025, 36, 102415. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Zhang, M.; Huang, Y. Three ‘E’ challenges for siRNA drug development. Trends Mol. Med. 2024, 30, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Varley, A.J.; Desaulniers, J.P. Chemical strategies for strand selection in short-interfering RNAs. RSC Adv. 2021, 11, 2415–2426. [Google Scholar] [CrossRef]
- Haraszti, R.A.; Roux, L.; Coles, A.H.; Turanov, A.A.; Alterman, J.F.; Echeverria, D.; Godinho, B.M.D.C.; Aronin, N.; Khvorova, A. 5′-Vinylphosphonate improves tissue accumulation and efficacy of conjugated siRNAs in vivo. Nucleic Acids Res. 2017, 45, 7581–7592. [Google Scholar] [CrossRef]
- Parmar, R.; Willoughby, J.L.S.; Liu, J.; Foster, D.J.; Brigham, B.; Theile, C.S.; Charisse, K.; Akinc, A.; Guidry, E.; Pei, Y.; et al. 5′-(E)-Vinylphosphonate: A Stable Phosphate Mimic Can Improve the RNAi Activity of siRNA–GalNAc Conjugates. ChemBioChem 2016, 17, 985–989. [Google Scholar] [CrossRef]
- Datta, D.; Kundu, J.; Miller, P.; Khan, M.S.; Salinas, J.; Qin, J.; LeBlanc, S.; Nguyen, T.; Peng, H.; Theile, C.S.; et al. Expanding the binding space of argonaute-2: Incorporation of either E or Z. isomers of 6′-vinylphosphonate at the 5′ end of the antisense strand improves RNAi activity. Chem. Commun. 2025, 61, 6659–6662. [Google Scholar] [CrossRef]
- Berk, C.; Civenni, G.; Wang, Y.; Steuer, C.; Catapano, C.V.; Hall, J. Pharmacodynamic and Pharmacokinetic Properties of Full Phosphorothioate Small Interfering RNAs for Gene Silencing In Vivo. Nucleic Acid. Ther. 2021, 31, 237–244. [Google Scholar] [CrossRef]
- Springer, A.D.; Dowdy, S.F. GalNAc-siRNA Conjugates: Leading the Way for Delivery of RNAi Therapeutics. Nucleic Acid. Ther. 2018, 28, 109–118. [Google Scholar] [CrossRef]
- Lorenzer, C.; Dirin, M.; Winkler, A.M.; Baumann, V.; Winkler, J. Going beyond the liver: Progress and challenges of targeted delivery of siRNA therapeutics. J. Control. Release 2015, 203, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Hariharan, V.N.; Caiazzi, J.; Miller, R.; Ferguson, C.M.; Sapp, E.; Fakih, H.H.; Tang, Q.; Yamada, N.; Furgal, R.C.; et al. Enhancing siRNA efficacy in vivo with extended nucleic acid backbones. Nat. Biotechnol. 2025, 43, 904–913. [Google Scholar] [CrossRef]
- Meade, B.R.; Gogoi, K.; Hamil, A.S.; Palm-Apergi, C.; van den Berg, A.; Hagopian, J.C.; Springer, A.D.; Eguchi, A.; Kacsinta, A.D.; Dowdy, C.F.; et al. Efficient delivery of RNAi prodrugs containing reversible charge-neutralizing phosphotriester backbone modifications. Nat. Biotechnol. 2014, 32, 1256–1261. [Google Scholar] [CrossRef]
- Goroshchuk, O.; Vidarsdottir, L.; Björklund, A.C.; Hamil, A.S.; Kolosenko, I.; Dowdy, S.F.; Palm-Apergi, C. Targeting Plk1 with siRNNs in primary cells from pediatric B-cell acute lymphoblastic leukemia patients. Sci. Rep. 2020, 10, 2688. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Dong, M.; Chen, P. Advances in structural-guided modifications of siRNA. Bioorg. Med. Chem. 2024, 110, 117825. [Google Scholar] [CrossRef]
- Valenzuela, R.A.P.; Suter, S.R.; Ball-Jones, A.A.; Ibarra-Soza, J.M.; Zheng, Y.; Beal, P.A. Base Modification Strategies to Modulate Immune Stimulation by an siRNA. ChemBioChem 2015, 16, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Robbins, M.; Judge, A.; Liang, L.; McClintock, K.; Yaworski, E.; MacLachlan, I. 2′-O-methyl-modified RNAs Act as TLR7 Antagonists. Mol. Ther. 2007, 15, 1663–1669. [Google Scholar] [CrossRef]
- Malek-Adamian, E.; Fakhoury, J.; Arnold, A.E.; Martínez-Montero, S.; Shoichet, M.S.; Damha, M.J. Effect of Sugar 2′,4′-Modifications on Gene Silencing Activity of siRNA Duplexes. Nucleic Acid. Ther. 2019, 29, 187–194. [Google Scholar] [CrossRef]
- Takahashi, M.; Nagai, C.; Hatakeyama, H.; Minakawa, N.; Harashima, H.; Matsuda, A. Intracellular stability of 2′-OMe-4′-thioribonucleoside modified siRNA leads to long-term RNAi effect. Nucleic Acids Res. 2012, 40, 5787–5793. [Google Scholar] [CrossRef] [PubMed]
- Elmén, J.; Thonberg, H.; Ljungberg, K.; Frieden, M.; Westergaard, M.; Xu, Y.; Wahren, B.; Liang, Z.; Ørum, H.; Koch, T.; et al. Locked nucleic acid (LNA) mediated improvements in siRNA stability and functionality. Nucleic Acids Res. 2005, 33, 439–447. [Google Scholar] [CrossRef]
- Mook, O.R.; Baas, F.; de Wissel, M.B.; Fluiter, K. Evaluation of locked nucleic acid–modified small interfering RNA in vitro and in vivo. Mol. Cancer Ther. 2007, 6, 833–843. [Google Scholar] [CrossRef] [PubMed]
- Robbins, M.; Judge, A.; MacLachlan, I. siRNA and Innate Immunity. Oligonucleotides 2009, 19, 89–102. [Google Scholar] [CrossRef] [PubMed]
- Rydzik, A.M.; Riether, D.; Gottschling, D. Synthesis of 2′-modified N6-methyladenosine phosphoramidites and their incorporation into siRNA. Bioorg. Med. Chem. Lett. 2023, 81, 129126. [Google Scholar] [CrossRef]
- Kaushal, A. Innate immune regulations and various siRNA modalities. Drug Deliv. Transl. Res. 2023, 13, 2704–2718. [Google Scholar] [CrossRef]
- Lee, H.S.; Seok, H.; Lee, D.H.; Ham, J.; Lee, W.; Youm, E.M.; Yoo, J.S.; Lee, Y.S.; Jang, E.S.; Sung, W.C. Abasic pivot substitution harnesses target specificity of RNA interference. Nat. Commun. 2015, 6, 10154. [Google Scholar] [CrossRef]
- Sarett, S.M.; Werfel, T.A.; Lee, L.; Jackson, M.A.; Kilchrist, K.V.; Brantley-Sieders, D.; Duvall, C.L. Lipophilic siRNA targets albumin in situ and promotes bioavailability, tumor penetration, and carrier-free gene silencing. Proc. Natl. Acad. Sci. USA 2017, 114, E6490–E6497. [Google Scholar] [CrossRef]
- Chernikov, I.V.; Gladkikh, D.V.; Meschaninova, M.I.; Ven’yaminova, A.G.; Zenkova, M.A.; Vlassov, V.V.; Chernolovskaya, E.L. Cholesterol-Containing Nuclease-Resistant siRNA Accumulates in Tumors in a Carrier-free Mode and Silences MDR1 Gene. Mol. Ther. Nucleic Acids 2017, 6, 209–220. [Google Scholar] [CrossRef]
- Nishina, K.; Unno, T.; Uno, Y.; Kubodera, T.; Kanouchi, T.; Mizusawa, H.; Yokota, T. Efficient In Vivo Delivery of siRNA to the Liver by Conjugation of α-Tocopherol. Mol. Ther. 2008, 16, 734–740. [Google Scholar] [CrossRef]
- Chernikov, I.V.; Vlassov, V.V.; Chernolovskaya, E.L. Current Development of siRNA Bioconjugates: From Research to the Clinic. Front. Pharmacol. 2019, 10, 444. [Google Scholar] [CrossRef]
- Lee, J.W.; Choi, J.; Kim, E.H.; Choi, J.; Kim, S.H.; Yang, Y. Design of siRNA Bioconjugates for Efficient Control of Cancer-Associated Membrane Receptors. ACS Omega 2023, 8, 36435–36448. [Google Scholar] [CrossRef]
- Nguyen, G.N.; Everett, J.K.; Kafle, S.; Roche, A.M.; Raymond, H.E.; Leiby, J.; Wood, C.; Assenmacher, C.A.; Merricks, E.P.; Long, C.T.; et al. A long-term study of AAV gene therapy in dogs with hemophilia A identifies clonal expansions of transduced liver cells. Nat. Biotechnol. 2021, 39, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Ubanako, P.; Mirza, S.; Ruff, P.; Penny, C. Exosome-mediated delivery of siRNA molecules in cancer therapy: Triumphs and challenges. Front. Mol. Biosci. 2024, 11, 1447953. [Google Scholar] [CrossRef]
- Wang, J.; Li, W.; Lu, Z.; Zhang, L.; Hu, Y.; Li, Q.; Du, W.; Feng, X.; Jia, H.; Liu, B.F. The use of RGD-engineered exosomes for enhanced targeting ability and synergistic therapy toward angiogenesis. Nanoscale 2017, 9, 15598–15605. [Google Scholar] [CrossRef] [PubMed]
- Bergqvist, M.; Park, K.S.; Karimi, N.; Yu, L.; Lässer, C.; Lötvall, J. Extracellular vesicle surface engineering with integrins (ITGAL & ITGB2) to specifically target ICAM-1-expressing endothelial cells. J. Nanobiotechnol. 2025, 23, 64. [Google Scholar] [CrossRef]
- Kundu, S.; Guo, J.; Islam, M.S.; Rohokale, R.; Jaiswal, M.; Guo, Z. A New Strategy to Functionalize Exosomes via Enzymatic Engineering of Surface Glycans and its Application to Profile Exosomal Glycans and Endocytosis. Adv. Sci. 2025, 12, 2415942. [Google Scholar] [CrossRef]
- Zhuo, Y.; Luo, Z.; Zhu, Z.; Wang, J.; Li, X.; Zhang, Z.; Guo, C.; Wang, B.; Nie, D.; Gan, Y.; et al. Direct cytosolic delivery of siRNA via cell membrane fusion using cholesterol-enriched exosomes. Nat. Nanotechnol. 2024, 19, 1858–1868. [Google Scholar] [CrossRef]
- Hazekawa, M.; Nishinakagawa, T.; Hosokawa, M.; Ishibashi, D. Development of an Organ-Directed Exosome-Based siRNA-Carrier Derived from Autologous Serum for Lung Metastases and Testing in the B16/BL6 Spontaneous Lung Metastasis Model. Pharmaceutics 2022, 14, 815. [Google Scholar] [CrossRef]
- Zhao, L.; Gu, C.; Gan, Y.; Shao, L.; Chen, H.; Zhu, H. Exosome-mediated siRNA delivery to suppress postoperative breast cancer metastasis. J. Control. Release 2020, 318, 1–15. [Google Scholar] [CrossRef]
- Zhang, C.; Wu, Q.; Gong, Y.; Qin, Q.; Han, Q.; Cheng, Z.; Yan, Z. Biomimetic exosomal vesicles loaded with siRNA improves antitumor immune responses by inhibiting the secretion of tumor-derived exosome PD-L1. Int. Immunopharmacol. 2024, 129, 111659. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, G.; Yu, T.; He, J.; Liu, J.; Chai, X.; Zhao, G.; Yin, D.; Zhang, C. Exosomes deliver lncRNA DARS-AS1 siRNA to inhibit chronic unpredictable mild stress-induced TNBC metastasis. Cancer Lett. 2022, 543, 215781. [Google Scholar] [CrossRef]
- Dong, Y.; Siegwart, D.J.; Anderson, D.G. Strategies, design, and chemistry in siRNA delivery systems. Adv. Drug Deliv. Rev. 2019, 144, 133–147. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Xu, X.; Feng, J.; Liu, M.; Hu, K. Chitosan and chitosan coating nanoparticles for the treatment of brain disease. Int. J. Pharm. 2019, 560, 282–293. [Google Scholar] [CrossRef]
- Lin, S.; Jing, H.; Du, X.; Yang, X.; Wang, J. Optimization of lipid assisted polymeric nanoparticles for siRNA delivery and cancer immunotherapy. Biomater. Sci. 2024, 12, 2057–2066. [Google Scholar] [CrossRef] [PubMed]
- Mrksich, K.; Padilla, M.S.; Mitchell, M.J. Breaking the final barrier: Evolution of cationic and ionizable lipid structure in lipid nanoparticles to escape the endosome. Adv. Drug Deliv. Rev. 2024, 214, 115446. [Google Scholar] [CrossRef]
- Kulkarni, J.A.; Witzigmann, D.; Chen, S.; Cullis, P.R.; van der Meel, R. Lipid Nanoparticle Technology for Clinical Translation of siRNA Therapeutics. Acc. Chem. Res. 2019, 52, 2435–2444. [Google Scholar] [CrossRef] [PubMed]
- Maurer, M.S.; Kale, P.; Fontana, M.; Berk, J.L.; Grogan, M.; Gustafsson, F.; Hung, R.R.; Gottlieb, R.L.; Damy, T.; González-Duarte, A.; et al. Patisiran Treatment in Patients with Transthyretin Cardiac Amyloidosis. N. Engl. J. Med. 2023, 389, 1553–1565. [Google Scholar] [CrossRef]
- Rungta, R.L.; Choi, H.B.; Lin, P.J.; Ko, R.W.Y.; Ashby, A.; Nair, J.; Manoharan, M.; Cullis, P.R.; MacVicar, B.A. Lipid Nanoparticle Delivery of siRNA to Silence Neuronal Gene Expression in the Brain. Mol. Ther.—Nucleic Acids 2013, 2, e136. [Google Scholar] [CrossRef]
- Nabhan, J.F.; Wood, K.M.; Rao, V.P.; Morin, J.; Bhamidipaty, S.; LaBranche, T.P.; Gooch, R.L.; Bozal, F.; Bulawa, C.E.; Guild, B.C. Intrathecal delivery of frataxin mRNA encapsulated in lipid nanoparticles to dorsal root ganglia as a potential therapeutic for Friedreich’s ataxia. Sci. Rep. 2016, 6, 20019. [Google Scholar] [CrossRef]
- Bari, E.; Serra, M.; Paolillo, M.; Bernardi, E.; Tengattini, S.; Piccinini, F.; Lanni, C.; Sorlini, M.; Bisbano, G.; Calleri, E.; et al. Silk Fibroin Nanoparticle Functionalization with Arg-Gly-Asp Cyclopentapeptide Promotes Active Targeting for Tumor Site-Specific Delivery. Cancers 2021, 13, 1185. [Google Scholar] [CrossRef]
- Li, S.D.; Chono, S.; Huang, L. Efficient Oncogene Silencing and Metastasis Inhibition via Systemic Delivery of siRNA. Mol. Ther. 2008, 16, 942–946. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; You, J.O.; Yang, J.; Jia, D.; Moses, M.A.; Auguste, D.T. Inhibiting Metastatic Breast Cancer Cell Migration via the Synergy of Targeted, pH-triggered siRNA Delivery and Chemokine Axis Blockade. Mol. Pharm. 2014, 11, 755–765. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, Y.; Hada, T.; Kato, A.; Hagino, Y.; Mizumura, W.; Harashima, H. Effective Therapy Using a Liposomal siRNA that Targets the Tumor Vasculature in a Model Murine Breast Cancer with Lung Metastasis. Mol. Ther. Oncolytics 2018, 11, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Li, S.D.; Chono, S.; Huang, L. Efficient gene silencing in metastatic tumor by siRNA formulated in surface-modified nanoparticles. J. Control. Release 2008, 126, 77–84. [Google Scholar] [CrossRef]
- Morry, J.; Ngamcherdtrakul, W.; Gu, S.; Reda, M.; Castro, D.J.; Sangvanich, T.; Gray, J.W.; Yantasee, W. Targeted Treatment of Metastatic Breast Cancer by PLK1 siRNA Delivered by an Antioxidant Nanoparticle Platform. Mol. Cancer Ther. 2017, 16, 763–772. [Google Scholar] [CrossRef]
- Zeng, L.; Li, J.; Wang, Y.; Qian, C.; Chen, Y.; Zhang, Q.; Wu, W.; Lin, Z.; Liang, J.; Shuai, X.; et al. Combination of siRNA-directed Kras oncogene silencing and arsenic-induced apoptosis using a nanomedicine strategy for the effective treatment of pancreatic cancer. Nanomedicine 2014, 10, 463–472. [Google Scholar] [CrossRef]
- Xie, J.; Wang, S. Small Interfering RNA in Colorectal Cancer Liver Metastasis Therapy. Technol. Cancer Res. Treat. 2022, 21, 15330338221103318. [Google Scholar] [CrossRef]
- Kang, S.H.; Revuri, V.; Lee, S.J.; Cho, S.; Park, I.K.; Cho, K.J.; Bae, W.K.; Lee, Y.K. Oral siRNA Delivery to Treat Colorectal Liver Metastases. ACS Nano 2017, 11, 10417–10429. [Google Scholar] [CrossRef]
- Wang, F.; Pang, J.; Huang, L.; Wang, R.; Jiang, Q.; Zhang, L.; Sun, K. Inhibition of osteosarcoma growth and metastasis using a polysaccharide derivative of Amy-g-PLLD for the delivery of AEG-1 siRNA. Nano Res. 2018, 11, 3886–3898. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhu, J.S.; Gao, C.P.; Li, L.P.; Zhou, C.; Wang, H.; Liu, X.G. siRNA targeting YAP gene inhibits gastric carcinoma growth and tumor metastasis in SCID mice. Oncol. Lett. 2016, 11, 2806–2814. [Google Scholar] [CrossRef]
- Kamerkar, S.; LeBleu, V.S.; Sugimoto, H.; Yang, S.; Ruivo, C.F.; Melo, S.A.; Lee, J.J.; Kalluri, R. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature 2017, 546, 498–503. [Google Scholar] [CrossRef]
- Rossi, J.J.; Rossi, D.J. siRNA Drugs: Here to Stay. Mol. Ther. 2021, 29, 431–432. [Google Scholar] [CrossRef]
- Larson, M.H.; Gilbert, L.A.; Wang, X.; Lim, W.A.; Weissman, J.S.; Qi, L.S. CRISPR interference (CRISPRi) for sequence-specific control of gene expression. Nat. Protoc. 2013, 8, 2180–2196. [Google Scholar] [CrossRef] [PubMed]
- Ebenezer, O.; Oyebamiji, A.K.; Olanlokun, J.O.; Tuszynski, J.A.; Wong, G.K.S. Recent Update on siRNA Therapeutics. Int. J. Mol. Sci. 2025, 26, 3456. [Google Scholar] [CrossRef]
- National Library of Medicine (US). ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ (accessed on 28 May 2025).
- Dannull, J.; Haley, N.R.; Archer, G.; Nair, S.; Boczkowski, D.; Harper, M.; De Rosa, N.; Pickett, N.; Mosca, P.J.; Burchette, J.; et al. Melanoma immunotherapy using mature DCs expressing the constitutive proteasome. J. Clin. Investig. 2013, 123, 3135–3145. [Google Scholar] [CrossRef] [PubMed]
- Triozzi, P.; Kooshki, M.; Alistar, A.; Bitting, R.; Neal, A.; Lametschwandtner, G.; Loibner, H. Phase I clinical trial of adoptive cellular immunotherapy with APN401 in patients with solid tumors. J. Immunother. Cancer 2015, 3 (Suppl. S2), P175. [Google Scholar] [CrossRef]
- Kaiser, P.K.; Symons, R.C.A.; Shah, S.M.; Quinlan, E.J.; Tabandeh, H.; Do, D.V.; Reisen, G.; Lockridge, J.A.; Short, B.; Guerciolini, B.; et al. RNAi-Based Treatment for Neovascular Age-Related Macular Degeneration by Sirna-027. Am. J. Ophthalmol. 2010, 150, 33–39.e2. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Yu, T.; Li, X.; Zhang, N.; Foster, L.J.; Peng, C.; Huang, W.; He, G. Recent advances in targeting the “undruggable” proteins: From drug discovery to clinical trials. Signal Transduct. Target. Ther. 2023, 8, 335. [Google Scholar] [CrossRef]
- National Cancer Institute (U.S.). MYC-Targeting siRNA DCR-MYC. Available online: https://www.cancer.gov/publications/dictionaries/cancer-drug/def/myc-targeting-sirna-dcr-myc (accessed on 28 May 2025).
- Serra, M.; Rubes, D.; Schinelli, S.; Paolillo, M. Small Molecules against Metastatic Tumors: Concrete Perspectives and Shattered Dreams. Cancers 2023, 15, 4173. [Google Scholar] [CrossRef] [PubMed]
- Ombrato, L.; Nolan, E.; Kurelac, I.; Mavousian, A.; Bridgeman, V.L.; Heinze, I.; Chakravarty, P.; Horswell, S.; Gonzalez-Gualda, E.; Matacchione, G.; et al. Metastatic-niche labelling reveals parenchymal cells with stem features. Nature 2019, 572, 603–608, Erratum in Nature 2019, 575, E8. [Google Scholar] [CrossRef] [PubMed]
- Massara, M.; Dolfi, B.; Wischnewski, V.; Nolan, E.; Held, W.; Malanchi, I.; Joyce, J.A. Investigation of a fluorescent reporter microenvironment niche labeling strategy in experimental brain metastasis. iScience 2024, 27, 110284. [Google Scholar] [CrossRef] [PubMed]
- Passaro, D.; Garcia-Albornoz, M.; Diana, G.; Chakravarty, P.; Ariza-McNaughton, L.; Batsivari, A.; Borràs-Eroles, C.; Abarrategi, A.; Waclawiczek, A.; Ombrato, L.; et al. Integrated OMICs unveil the bone-marrow microenvironment in human leukemia. Cell Rep. 2021, 35, 109119. [Google Scholar] [CrossRef]
- Han, Y.; Wang, D.; Peng, L.; Huang, T.; He, X.; Wang, J.; Ou, C. Single-cell sequencing: A promising approach for uncovering the mechanisms of tumor metastasis. J. Hematol. Oncol. 2022, 15, 59. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serra, M.; Buccellini, A.; Paolillo, M. Knocking on Cells’ Door: Strategic Approaches for miRNA and siRNA in Anticancer Therapy. Int. J. Mol. Sci. 2025, 26, 8703. https://doi.org/10.3390/ijms26178703
Serra M, Buccellini A, Paolillo M. Knocking on Cells’ Door: Strategic Approaches for miRNA and siRNA in Anticancer Therapy. International Journal of Molecular Sciences. 2025; 26(17):8703. https://doi.org/10.3390/ijms26178703
Chicago/Turabian StyleSerra, Massimo, Alessia Buccellini, and Mayra Paolillo. 2025. "Knocking on Cells’ Door: Strategic Approaches for miRNA and siRNA in Anticancer Therapy" International Journal of Molecular Sciences 26, no. 17: 8703. https://doi.org/10.3390/ijms26178703
APA StyleSerra, M., Buccellini, A., & Paolillo, M. (2025). Knocking on Cells’ Door: Strategic Approaches for miRNA and siRNA in Anticancer Therapy. International Journal of Molecular Sciences, 26(17), 8703. https://doi.org/10.3390/ijms26178703









