The Inositol-5-Phosphatase SHIP1: Expression, Regulation and Role in Acute Lymphoblastic Leukemia
Abstract
1. Introduction
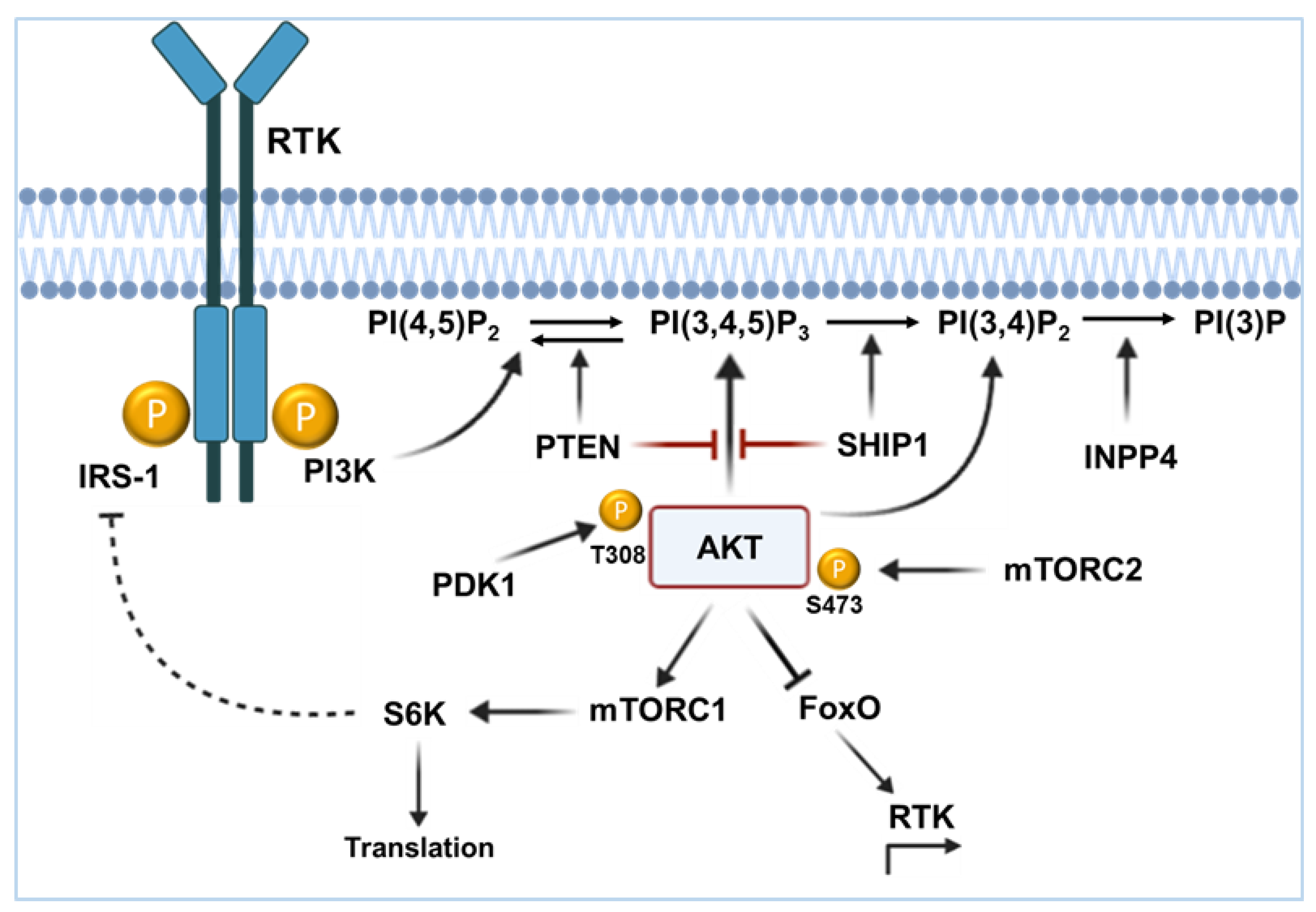
2. The PI3K/AKT Signaling Pathway
2.1. Enzymatic Activity of SHIP1
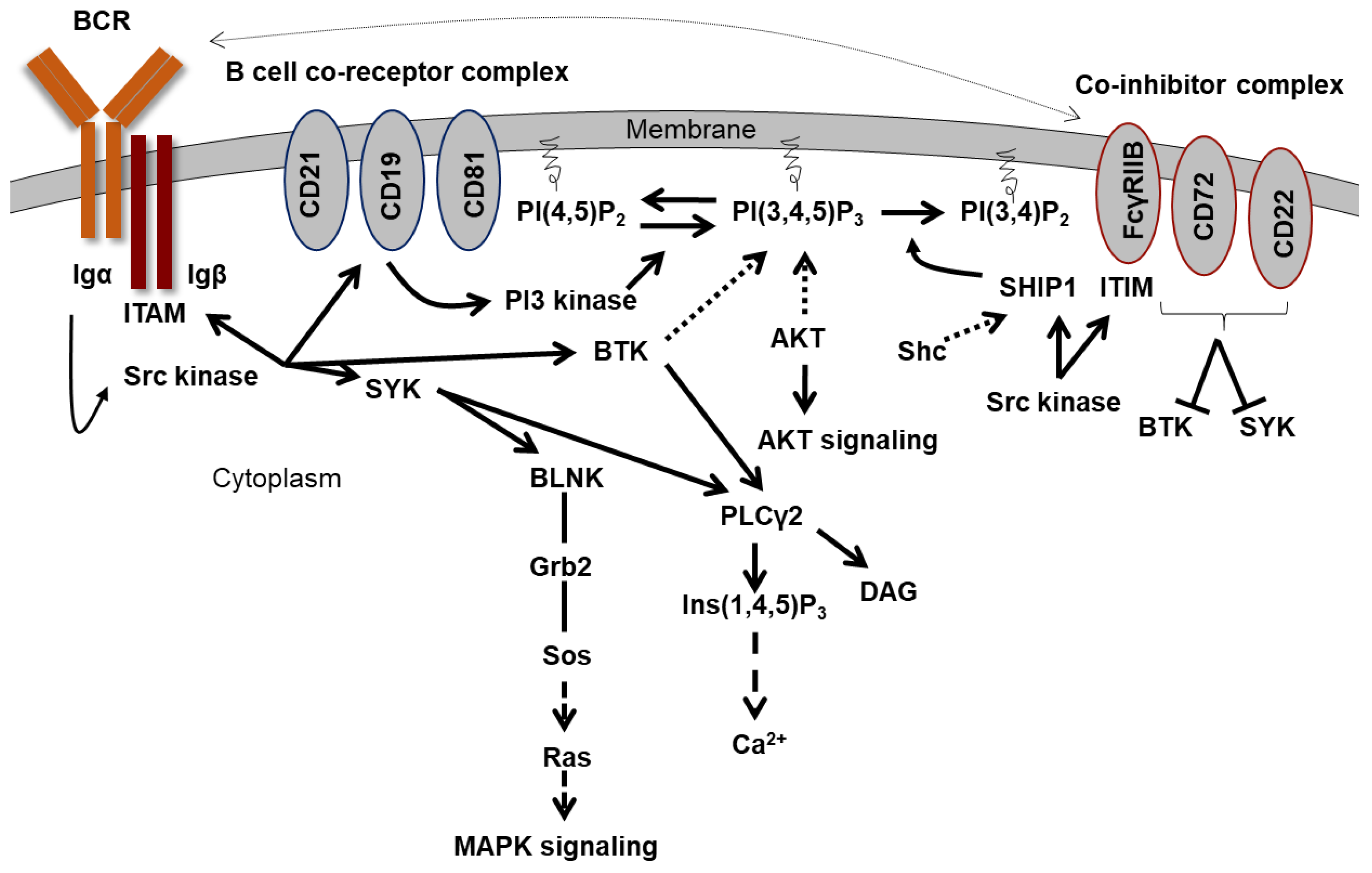
2.2. The B Cell Activation
2.3. Recruitment of SHIP1 to the Membrane
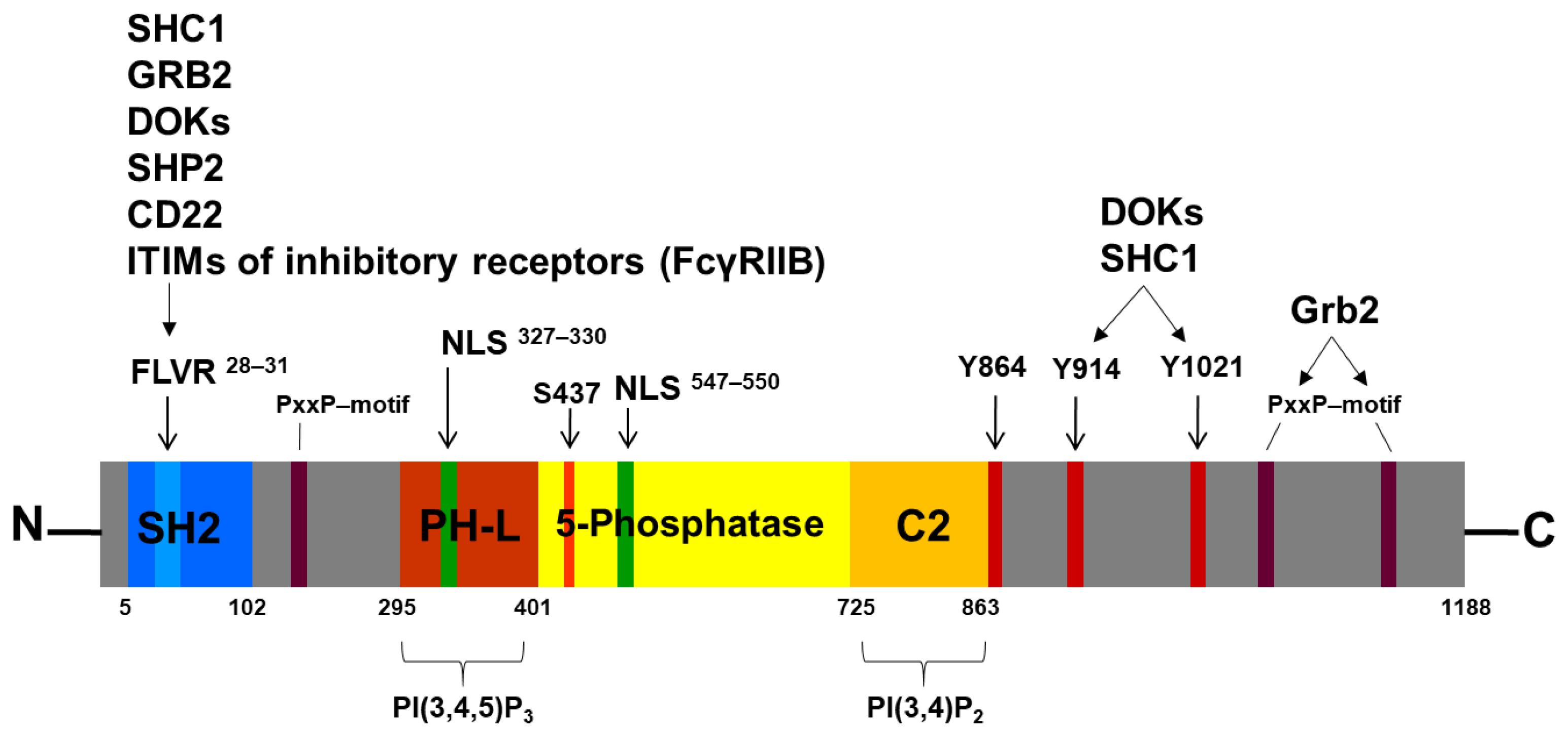
3. The Human Inositol-5-Phosphatase (SHIP1) and Its Structure
3.1. The SH2 Domain and the FLVR Motif
3.2. The 5-Phosphatase Domain
3.3. The NPXY and PxxP Motifs
3.4. The Nuclear Localization Signals (NLS) and Nuclear Export Signals
4. The SHIP1 Mutation Status
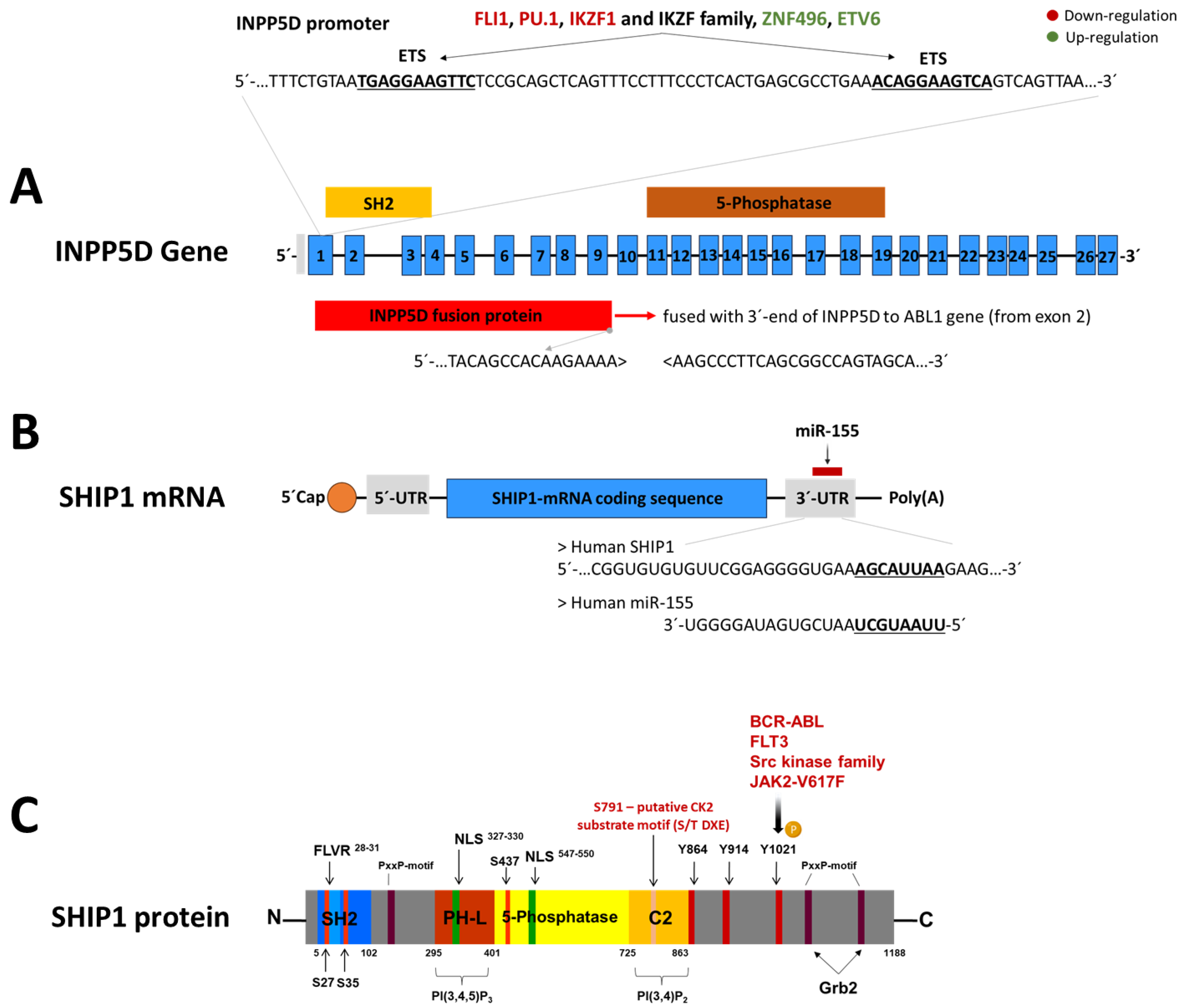
5. The Regulation of SHIP1
5.1. Regulation of SHIP1 Protein by Tyrosine and Serine Kinases
5.2. Regulation of SHIP1 mRNA by miR-155
5.3. Transcriptional Regulation of INPP5D
6. Expression Status of SHIP1 in the Different Subtypes of ALL
6.1. ETV6-RUNX1 Subroup
6.2. TCF3-PBX1 Subgroup
6.3. KMT2A-r Subgroup
6.4. Ph-Positive and Ph-like Subgroup
6.5. T-ALL
7. Beyond Leukemia: SHIP1 Expression in Carcinoma
8. Ikaros Regulates SHIP1 Expression and Influences Cell Metabolism by Mediating the AKT Pathway
9. Discussion
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Roberts, K.G.; Li, Y.; Payne-Turner, D.; Harvey, R.C.; Yang, Y.L.; Pei, D.; McCastlain, K.; Ding, L.; Lu, C.; Song, G.; et al. Targetable kinase-activating lesions in Ph-like acute lymphoblastic leukemia. N. Engl. J. Med. 2014, 371, 1005–1015. [Google Scholar] [CrossRef] [PubMed]
- Irving, J.; Matheson, E.; Minto, L.; Blair, H.; Case, M.; Halsey, C.; Swidenbank, I.; Ponthan, F.; Kirschner-Schwabe, R.; Groeneveld-Krentz, S.; et al. Ras pathway mutations are prevalent in relapsed childhood acute lymphoblastic leukemia and confer sensitivity to MEK inhibition. Blood 2014, 124, 3420–3430. [Google Scholar] [CrossRef] [PubMed]
- Ehm, P.; Grottke, A.; Bettin, B.; Jücker, M. Investigation of the function of the PI3-Kinase/AKT signaling pathway for leukemogenesis and therapy of acute childhood lymphoblastic leukemia (ALL). Cell. Signal. 2022, 93, 110301. [Google Scholar] [CrossRef] [PubMed]
- Grüninger, P.K.; Uhl, F.; Herzog, H.; Gentile, G.; Andrade-Martinez, M.; Schmidt, T.; Han, K.; Morgens, D.W.; Bassik, M.C.; Cleary, M.L.; et al. Functional characterization of the PI3K/AKT/MTOR signaling pathway for targeted therapy in B-precursor acute lymphoblastic leukemia. Cancer Gene Ther. 2022, 29, 1751–1760. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Imanaga, H.; Semba, Y.; Sasaki, K.; Setoguchi, K.; Maniriho, H.; Yamauchi, T.; Terasaki, T.; Hirabayashi, S.; Nakao, F.; Nogami, J.; et al. Central role of the mTORC1 pathway in glucocorticoid activity against B-ALL cells. Blood Neoplasia 2024, 1, 100015. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vanhaesebroeck, B.; Perry, M.W.D.; Brown, J.R.; André, F.; Okkenhaug, K. PI3K inhibitors are finally coming of age. Nat. Rev. Drug Discov. 2021, 20, 741–769, Erratum in Nat. Rev. Drug Discov. 2021, 20, 798. https://doi.org/10.1038/s41573-021-00300-7. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, Y.; Rozen, V.; Zhao, Y.; Wang, Z. Oncogenic activation of PIK3CA in cancers: Emerging targeted therapies in precision oncology. Genes Dis. 2024, 12, 101430. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kandoth, C.; McLellan, M.D.; Vandin, F.; Ye, K.; Niu, B.; Lu, C.; Xie, M.; Zhang, Q.; McMichael, J.F.; Wyczalkowski, M.A.; et al. Mutational landscape and significance across 12 major cancer types. Nature 2013, 502, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.; Yunes, J.A.; Cardoso, B.A.; Martins, L.R.; Jotta, P.Y.; Abecasis, M.; Nowill, A.E.; Leslie, N.R.; Cardoso, A.A.; Barata, J.T. PTEN posttranslational inactivation and hyperactivation of the PI3K/Akt pathway sustain primary T cell leukemia viability. J. Clin. Investig. 2008, 118, 3762–3774. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.M.; Soares, M.V.; Ribeiro, P.; Caldas, J.; Póvoa, V.; Martins, L.R.; Melão, A.; Serra-Caetano, A.; de Sousa, A.B.; Lacerda, J.F.; et al. Adult B-cell acute lymphoblastic leukemia cells display decreased PTEN activity and constitutive hyperactivation of PI3K/Akt pathway despite high PTEN protein levels. Haematologica 2014, 99, 1062–1068. [Google Scholar] [CrossRef] [PubMed]
- Ehm, P.A.H.; Horn, S.; Hoffer, K.; Kriegs, M.; Horn, M.; Giehler, S.; Nalaskowski, M.; Rehbach, C.; Horstmann, M.A.; Jücker, M. Ikaros sets the threshold for negative B-cell selection by regulation of the signaling strength of the AKT pathway. Cell Commun. Signal. 2024, 22, 360. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Franks, S.E.; Cambier, J.C. Putting on the Brakes: Regulatory Kinases and Phosphatases Maintaining B Cell Anergy. Front. Immunol. 2018, 9, 665. [Google Scholar] [CrossRef] [PubMed]
- Thorpe, L.M.; Spangle, J.M.; Ohlson, C.E.; Cheng, H.; Roberts, T.M.; Cantley, L.C.; Zhao, J.J. PI3K-p110α mediates the oncogenic activity induced by loss of the novel tumor suppressor PI3K-p85α. Proc. Natl. Acad. Sci. USA 2017, 114, 7095–7100. [Google Scholar] [CrossRef] [PubMed]
- Pungsrinont, T.; Kallenbach, J.; Baniahmad, A. Role of PI3K-AKT-mTOR Pathway as a Pro-Survival Signaling and Resistance-Mediating Mechanism to Therapy of Prostate Cancer. Int. J. Mol. Sci. 2021, 22, 11088. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, T.T.; Li, H.; Cheung, S.M.; Costantini, J.L.; Hou, S.; Al-Alwan, M.; Marshall, A.J. Phosphoinositide 3-kinase-regulated adapters in lymphocyte activation. Immunol. Rev. 2009, 232, 255–272. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.; Cheung, S.M.; Marshall, A.J.; Duronio, V. PI(3,4,5)P3 and PI(3,4)P2 levels correlate with PKB/akt phosphorylation at Thr308 and Ser473, respectively; PI(3,4)P2 levels determine PKB activity. Cell. Signal. 2008, 20, 684–694. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.L.; Wang, Z.G.; Hu, Y.; Xin, Y.; Singaram, I.; Gorai, S.; Zhou, X.; Shim, Y.; Min, J.H.; Gong, L.W.; et al. Quantitative Lipid Imaging Reveals a New Signaling Function of Phosphatidylinositol-3,4-Bisphophate: Isoform- and Site-Specific Activation of Akt. Mol. Cell 2018, 71, 1092–1104.e5. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Srivastava, N.; Sudan, R.; Kerr, W.G. Role of inositol poly-phosphatases and their targets in T cell biology. Front. Immunol. 2013, 4, 288. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shorning, B.Y.; Dass, M.S.; Smalley, M.J.; Pearson, H.B. The PI3K-AKT-mTOR Pathway and Prostate Cancer: At the Crossroads of AR, MAPK, and WNT Signaling. Int. J. Mol. Sci. 2020, 21, 4507. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Devanathan, N.; Jones, S.; Kaur, G.; Kimble-Hill, A.C. Using Phosphatidylinositol Phosphorylation as Markers for Hyperglycemic Related Breast Cancer. Int. J. Mol. Sci. 2020, 21, 2320. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, M.; Ceyhan, Y.; Mei, S.; Hirz, T.; Sykes, D.B.; Agoulnik, I.U. Regulation of EZH2 Expression by INPP4B in Normal Prostate and Primary Prostate Cancer. Cancers 2023, 15, 5418. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Morishita, N.; Tsukahara, H.; Chayama, K.; Ishida, T.; Washio, K.; Miyamura, T.; Yamashita, N.; Oda, M.; Morishima, T. Activation of Akt is associated with poor prognosis and chemotherapeutic resistance in pediatric B-precursor acute lymphoblastic leukemia. Pediatr. Blood Cancer 2012, 59, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Yang, A.; Ma, J.; Wu, M.; Xu, H.; Wu, K.; Jin, Y.; Xie, Y. Akt2 mediates glucocorticoid resistance in lymphoid malignancies through FoxO3a/Bim axis and serves as a direct target for resistance reversal. Cell Death Dis. 2019, 9, 1013. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, R.; Chen, Y.; Liu, G.; Li, C.; Song, Y.; Cao, Z.; Li, W.; Hu, J.; Lu, C.; Liu, Y. PI3K/AKT pathway as a key link modulates the multidrug resistance of cancers. Cell Death Dis. 2020, 11, 797. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, L.; Zhou, S.; Zhou, T.; Li, X.; Tang, J. Targeting the lncRNA DUXAP8/miR-29a/PIK3CA Network Restores Doxorubicin Chemosensitivity via PI3K-AKT-mTOR Signaling and Synergizes With Inotuzumab Ozogamicin in Chemotherapy-Resistant B-Cell Acute Lymphoblastic Leukemia. Front. Oncol. 2022, 12, 773601. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sarno, J.; Domizi, P.; Liu, Y.; Merchant, M.; Pedersen, C.B.; Jedoui, D.; Jager, A.; Nolan, G.P.; Gaipa, G.; Bendall, S.C.; et al. Dasatinib overcomes glucocorticoid resistance in B-cell acute lymphoblastic leukemia. Nat. Commun. 2023, 14, 2935. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Damen, J.E.; Liu, L.; Rosten, P.; Humphries, R.K.; Jefferson, A.B.; Majerus, P.W.; Krystal, G. The 145-kDa protein induced to associate with Shc by multiple cytokines is an inositol tetraphosphate and phosphatidylinositol 3,4,5-triphosphate 5-phosphatase. Proc. Natl. Acad. Sci. USA 1996, 93, 1689–1693. [Google Scholar] [CrossRef] [PubMed]
- Diop, A.; Santorelli, D.; Malagrinò, F.; Nardella, C.; Pennacchietti, V.; Pagano, L.; Marcocci, L.; Pietrangeli, P.; Gianni, S.; Toto, A. SH2 Domains: Folding, Binding and Therapeutical Approaches. Int. J. Mol. Sci. 2022, 23, 15944. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bates, L.; Wiseman, E.; Whetzel, A.; Carroll, D.J. A Novel Method to Profile Transcripts Encoding SH2 Domains in the Patiria miniata Mature Egg Transcriptome. Cells 2024, 13, 1898. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mehra, S.; Nicholls, M.; Taylor, J. The Evolving Role of Bruton’s Tyrosine Kinase Inhibitors in B Cell Lymphomas. Int. J. Mol. Sci. 2024, 25, 7516. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- L’Estrange-Stranieri, E.; Gottschalk, T.A.; Wright, M.D.; Hibbs, M.L. The dualistic role of Lyn tyrosine kinase in immune cell signaling: Implications for systemic lupus erythematosus. Front. Immunol. 2024, 15, 1395427. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sadras, T.; Martin, M.; Kume, K.; Robinson, M.E.; Saravanakumar, S.; Lenz, G.; Chen, Z.; Song, J.Y.; Siddiqi, T.; Oksa, L.; et al. Developmental partitioning of SYK and ZAP70 prevents autoimmunity and cancer. Mol. Cell 2021, 81, 2094–2111.e9. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Leveille, E.; Chan, L.N.; Mirza, A.S.; Kume, K.; Müschen, M. SYK and ZAP70 kinases in autoimmunity and lymphoid malignancies. Cell. Signal. 2022, 94, 110331. [Google Scholar] [CrossRef] [PubMed]
- Seiler, C.; Stainthorp, A.K.; Ketchen, S.; Jones, C.M.; Marks, K.; Quirke, P.; Ladbury, J.E. The Grb2 splice variant, Grb3-3, is a negative regulator of RAS activation. Commun. Biol. 2022, 5, 1029. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Harwood, S.J.; Smith, C.R.; Lawson, J.D.; Ketcham, J.M. Selected Approaches to Disrupting Protein–Protein Interactions within the MAPK/RAS Pathway. Int. J. Mol. Sci. 2023, 24, 7373. [Google Scholar] [CrossRef] [PubMed]
- Tkachenko, A.; Kupcova, K.; Havranek, O. B-Cell Receptor Signaling and Beyond: The Role of Igα (CD79a)/Igβ (CD79b) in Normal and Malignant B Cells. Int. J. Mol. Sci. 2024, 25, 10. [Google Scholar] [CrossRef] [PubMed]
- Hamdan, T.A.; Lang, P.A.; Lang, K.S. The Diverse Functions of the Ubiquitous Fcγ Receptors and Their Unique Constituent, FcRγ Subunit. Pathogens 2020, 9, 140. [Google Scholar] [CrossRef] [PubMed]
- Fei, C.; Zwozdesky, M.A.; Stafford, J.L. A Fish Leukocyte Immune-Type Receptor Uses a Novel Intracytoplasmic Tail Networking Mechanism to Cross-Inhibit the Phagocytic Response. Int. J. Mol. Sci. 2020, 21, 5146. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Keller, C.W.; Chuquisana, O.; Derdelinckx, J.; Gross, C.C.; Berger, K.; Robinson, J.; Nimmerjahn, F.; Wiendl, H.; Willcox, N.; Lünemann, J.D. Impaired B Cell Expression of the Inhibitory Fcγ Receptor IIB in Myasthenia Gravis. Ann. Neurol. 2022, 92, 1046–1051. [Google Scholar] [CrossRef] [PubMed]
- Glück, M.; Dally, L.; Jücker, M.; Ehm, P. JAK2-V617F is a negative regulation factor of SHIP1 protein and thus influences the AKT signaling pathway in patients with Myeloproliferative neoplasm (MPN). Int. J. Biochem. Cell Biol. 2022, 149, 106229. [Google Scholar] [CrossRef] [PubMed]
- Ehm, P.; Bettin, B.; Jücker, M. Activated Src kinases downstream of BCR-ABL and Flt3 induces proteasomal degradation of SHIP1 by phosphorylation of tyrosine 1021. Biochim. Biophys. Acta Mol. Cell Res. 2023, 1870, 119467. [Google Scholar] [CrossRef] [PubMed]
- Padarti, A.; Abou-Fadel, J.; Zhang, J. Resurgence of phosphotyrosine binding domains: Structural and functional properties essential for understanding disease pathogenesis. Biochim. Biophys. Acta Gen. Subj. 2021, 1865, 129977. [Google Scholar] [CrossRef] [PubMed]
- Rauh, M.J.; Sly, L.M.; Kalesnikoff, J.; Hughes, M.R.; Cao, L.P.; Lam, V.; Krystal, G. The role of SHIP1 in macrophage programming and activation. Biochem. Soc. Trans. 2004, 32 Pt 5, 785–788. [Google Scholar] [CrossRef] [PubMed]
- Weerawarna, P.M.; Richardson, T.I. Lyn Kinase Structure, Regulation, and Involvement in Neurodegenerative Diseases: A Mini Review. Kinases Phosphatases 2023, 1, 23–38. [Google Scholar] [CrossRef]
- Tridandapani, S.; Kelley, T.; Pradhan, M.; Cooney, D.; Justement, L.B.; Coggeshall, K.M. Recruitment and phosphorylation of SH2-containing inositol phosphatase and Shc to the B-cell Fc gamma immunoreceptor tyrosine-based inhibition motif peptide motif. Mol. Cell. Biol. 1997, 17, 4305–4311. [Google Scholar] [CrossRef] [PubMed]
- DeLuca, J.M.; Murphy, M.K.; Wang, X.; Wilson, T.J. FCRL1 Regulates B Cell Receptor-Induced ERK Activation through GRB2. J. Immunol. 2021, 207, 2688–2698. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Olufunmilayo, E.O.; Holsinger, R.M.D. INPP5D/SHIP1: Expression, Regulation and Roles in Alzheimer’s Disease Pathophysiology. Genes 2023, 14, 1845. [Google Scholar] [CrossRef] [PubMed]
- Drayer, A.L.; Pesesse, X.; De Smedt, F.; Woscholski, R.; Parker, P.; Erneux, C. Cloning and expression of a human placenta inositol 1,3,4,5-tetrakisphosphate and phosphatidylinositol 3,4,5-trisphosphate 5-phosphatase. Biochem. Biophys. Res. Commun. 1996, 225, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Geier, S.J.; Algate, P.A.; Carlberg, K.; Flowers, D.; Friedman, C.; Trask, B.; Rohrschneider, L.R. The human SHIP gene is differentially expressed in cell lineages of the bone marrow and blood. Blood 1997, 89, 1876–1885. [Google Scholar] [CrossRef] [PubMed]
- Horn, S.; Meyer, J.; Heukeshoven, J.; Fehse, B.; Schulze, C.; Li, S.; Frey, J.; Poll, S.; Stocking, C.; Jucker, M. The inositol 5-phosphatase SHIP is expressed as 145 and 135 kDa proteins in blood and bone marrow cells in vivo, whereas carboxyl-truncated forms of SHIP are generated by proteolytic cleavage in vitro. Leukemia 2001, 15, 112–120. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Filippakopoulos, P.; Kofler, M.; Hantschel, O.; Gish, G.D.; Grebien, F.; Salah, E.; Neudecker, P.; Kay, L.E.; Turk, B.E.; Superti-Furga, G.; et al. Structural coupling of SH2-kinase domains links Fes and Abl substrate recognition and kinase activation. Cell 2008, 134, 793–803. [Google Scholar] [CrossRef] [PubMed]
- Filippakopoulos, P.; Müller, S.; Knapp, S. SH2 domains: Modulators of nonreceptor tyrosine kinase activity. Curr. Opin. Struct. Biol. 2009, 19, 643–649. [Google Scholar] [CrossRef] [PubMed]
- Sondka, Z.; Dhir, N.B.; Carvalho-Silva, D.; Jupe, S.; Madhumita McLaren, K.; Starkey, M.; Ward, S.; Wilding, J.; Ahmed, M.; Argasinska, J.; et al. COSMIC: A curated database of somatic variants and clinical data for cancer. Nucleic Acids Res. 2024, 52, D1210–D1217. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Brauer, H.; Strauss, J.; Wegner, W.; Müller-Tidow, C.; Horstmann, M.; Jücker, M. Leukemia-associated mutations in SHIP1 inhibit its enzymatic activity, interaction with the GM-CSF receptor and Grb2, and its ability to inactivate PI3K/AKT signaling. Cell. Signal. 2012, 24, 2095–2101. [Google Scholar] [CrossRef] [PubMed]
- Jaber Chehayeb, R.; Wang, J.; Stiegler, A.L.; Boggon, T.J. The GTPase-activating protein p120RasGAP has an evolutionarily conserved “FLVR-unique” SH2 domain. J. Biol. Chem. 2020, 295, 10511–10521. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ehm, P.A.H.; Lange, F.; Hentschel, C.; Jepsen, A.; Glück, M.; Nelson, N.; Bettin, B.; de Bruyn Kops, C.; Kirchmair, J.; Nalaskowski, M.; et al. Analysis of the FLVR motif of SHIP1 and its importance for the protein stability of SH2 containing signaling proteins. Cell. Signal. 2019, 63, 109380. [Google Scholar] [CrossRef] [PubMed]
- Candotti, F.; Oakes, S.A.; Johnston, J.A.; Giliani, S.; Schumacher, R.F.; Mella, P.; Fiorini, M.; Ugazio, A.G.; Badolato, R.; Notarangelo, L.D.; et al. Structural and functional basis for JAK3-deficient severe combined immunodeficiency. Blood 1997, 90, 3996–4003. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, S.; Suzuki-Fujimoto, T.; Minowa, A.; Ueno, H.; Katamura, K.; Koyasu, S. Temperature-sensitive ZAP70 mutants degrading through a proteasome-independent pathway. Restoration of a kinase domain mutant by Cdc37. J. Biol. Chem. 1999, 274, 34515–34518. [Google Scholar] [CrossRef] [PubMed]
- Lappalainen, I.; Thusberg, J.; Shen, B.; Vihinen, M. Genome wide analysis of pathogenic SH2 domain mutations. Proteins 2008, 72, 779–792. [Google Scholar] [CrossRef] [PubMed]
- Gencer Akçok, E.B.; Güner, H.; Akçok, İ. Determination of promising inhibitors for N-SH2 domain of SHP2 tyrosine phosphatase: An in silico study. Mol. Divers. 2024, 28, 3393–3407. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hornbeck, P.V.; Zhang, B.; Murray, B.; Kornhauser, J.M.; Latham, V.; Skrzypek, E. PhosphoSitePlus, 2014: Mutations, PTMs and recalibrations. Nucleic Acids Res. 2015, 43, D512–D520. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Holt, L.J. Regulatory modules: Coupling protein stability to phopshoregulation during cell division. FEBS Lett. 2012, 586, 2773–2777. [Google Scholar] [CrossRef] [PubMed]
- Bradshaw, W.J.; Kennedy, E.C.; Moreira, T.; Smith, L.A.; Chalk, R.; Katis, V.L.; Benesch, J.L.P.; Brennan, P.E.; Murphy, E.J.; Gileadi, O. Regulation of inositol 5-phosphatase activity by the C2 domain of SHIP1 and SHIP2. Structure 2024, 32, 453–466.e6. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Müller, S.M.; Nelson, N.; Jücker, M. Functional Characterization of the SHIP1-Domains Regarding Their Contribution to Inositol 5-Phosphatase Activity. Biomolecules 2025, 15, 105. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ming-Lum, A.; Shojania, S.; So, E.; McCarrell, E.; Shaw, E.; Vu, D.; Wang, I.; McIntosh, L.P.; Mui, A.L. A pleckstrin homology-related domain in SHIP1 mediates membrane localization during Fcγ receptor-induced phagocytosis. FASEB J. 2012, 26, 3163–3177. [Google Scholar] [CrossRef] [PubMed]
- Ehm, P.; Nelson, N.; Giehler, S.; Schaks, M.; Bettin, B.; Kirchmair, J.; Jücker, M. Reduced expression and activity of patient-derived SHIP1 phosphatase domain mutants. Cell. Signal. 2023, 101, 110485. [Google Scholar] [CrossRef] [PubMed]
- Pauls, S.D.; Marshall, A.J. Regulation of immune cell signaling by SHIP1: A phosphatase, scaffold protein, and potential therapeutic target. Eur. J. Immunol. 2017, 47, 932–945. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ravichandran, K.S.; Garrison, J.C. A key role for the phosphorylation of Ser440 by the cyclic AMP-dependent protein kinase in regulating the activity of the Src homology 2 domain-containing Inositol 5′-phosphatase (SHIP1). J. Biol. Chem. 2010, 285, 34839–34849. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Hu, L.; Mi, B.; Panayi, A.C.; Xue, H.; Hu, Y.; Liu, G.; Chen, L.; Yan, C.; Zha, K.; et al. SHIP1 Activator AQX-1125 Regulates Osteogenesis and Osteoclastogenesis Through PI3K/Akt and NF-κb Signaling. Front. Cell Dev. Biol. 2022, 10, 826023. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pedicone, C.; Fernandes, S.; Matera, A.; Meyer, S.T.; Loh, S.; Ha, J.H.; Bernard, D.; Chisholm, J.D.; Paolicelli, R.C.; Kerr, W.G. Discovery of a novel SHIP1 agonist that promotes degradation of lipid-laden phagocytic cargo by microglia. iScience 2022, 25, 104170. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lemm, E.A.; Valle-Argos, B.; Smith, L.D.; Richter, J.; Gebreselassie, Y.; Carter, M.J.; Karolova, J.; Svaton, M.; Helman, K.; Weston-Bell, N.J.; et al. Preclinical Evaluation of a Novel SHIP1 Phosphatase Activator for Inhibition of PI3K Signaling in Malignant B Cells. Clin. Cancer Res. 2020, 26, 1700–1711. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Negro, R.; Gobessi, S.; Longo, P.G.; He, Y.; Zhang, Z.Y.; Laurenti, L.; Efremov, D.G. Overexpression of the autoimmunity-associated phosphatase PTPN22 promotes survival of antigen-stimulated CLL cells by selectively activating AKT. Blood 2012, 119, 6278–6287. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, O.; Weingarten, L.; Padberg, I.; Pracht, C.; Sinha, R.; Hochdörfer, T.; Kuppig, S.; Backofen, R.; Reth, M.; Huber, M. The SH2-domain of SHIP1 interacts with the SHIP1 C-terminus: Impact on SHIP1/Ig-α interaction. Biochim. Biophys. Acta 2012, 1823, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Ruschmann, J.; Ho, V.; Antignano, F.; Kuroda, E.; Lam, V.; Ibaraki, M.; Snyder, K.; Kim, C.; Flavell, R.A.; Kawakami, T.; et al. Tyrosine phosphorylation of SHIP promotes its proteasomal degradation. Exp. Hematol. 2010, 38, 392–402. [Google Scholar] [CrossRef] [PubMed]
- Waddell, G.L.; Drew, E.E.; Rupp, H.P.; Hansen, S.D. Mechanisms controlling membrane recruitment and activation of the autoinhibited SHIP1 inositol 5-phosphatase. J. Biol. Chem. 2023, 299, 105022. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Müller, S.M.; Jücker, M. The Functional Roles of the Src Homology 2 Domain-Containing Inositol 5-Phosphatases SHIP1 and SHIP2 in the Pathogenesis of Human Diseases. Int. J. Mol. Sci. 2024, 25, 5254. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nalaskowski, M.M.; Metzner, A.; Brehm, M.A.; Labiadh, S.; Brauer, H.; Grabinski, N.; Mayr, G.W.; Jücker, M. The inositol 5-phosphatase SHIP1 is a nucleo-cytoplasmic shuttling protein and enzymatically active in cell nuclei. Cell. Signal. 2012, 24, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Nalaskowski, M.M.; Ehm, P.; Rehbach, C.; Nelson, N.; Täger, M.; Modest, K.; Jücker, M. Nuclear accumulation of SHIP1 mutants derived from AML patients leads to increased proliferation of leukemic cells. Cell. Signal. 2018, 49, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Ehm, P.; Nalaskowski, M.M.; Wundenberg, T.; Jücker, M. The tumor suppressor SHIP1 colocalizes in nucleolar cavities with p53 and components of PML nuclear bodies. Nucleus 2015, 6, 154–164. [Google Scholar] [CrossRef] [PubMed]
- Nalaskowski, M.M.; Ehm, P.; Giehler, S.; Mayr, G.W. A toolkit for graded expression of green fluorescent protein fusion proteins in mammalian cells. Anal. Biochem. 2012, 428, 24–27. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Cheng, H.; Roberts, T.M.; Zhao, J.J. Targeting the phosphoinositide 3-kinase (PI3K) pathway in cancer. Nat. Rev. Drug Discov. 2009, 8, 627–644. [Google Scholar] [CrossRef] [PubMed]
- Papa, A.; Pandolfi, P.P. The PTEN–PI3K Axis in Cancer. Biomolecules 2019, 9, 153. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Graff, S.L.; Wang, Y. New Emerging Therapies Targeting PI3K/AKT/mTOR/PTEN Pathway in Hormonal Receptor-Positive and HER2-Negative Breast Cancer—Current State and Molecular Pathology Perspective. Cancers 2025, 17, 16. [Google Scholar] [CrossRef] [PubMed]
- Glaviano, A.; Foo, A.S.C.; Lam, H.Y.; Yap, K.C.H.; Jacot, W.; Jones, R.H.; Eng, H.; Nair, M.G.; Makvandi, P.; Geoerger, B.; et al. PI3K/AKT/mTOR signaling transduction pathway and targeted therapies in cancer. Mol. Cancer 2023, 22, 138. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Badoiu, S.C.; Greabu, M.; Miricescu, D.; Stanescu-Spinu, I.-I.; Ilinca, R.; Balan, D.G.; Balcangiu-Stroescu, A.-E.; Mihai, D.-A.; Vacaroiu, I.A.; Stefani, C.; et al. PI3K/AKT/mTOR Dysregulation and Reprogramming Metabolic Pathways in Renal Cancer: Crosstalk with the VHL/HIF Axis. Int. J. Mol. Sci. 2023, 24, 8391. [Google Scholar] [CrossRef] [PubMed]
- Leiphrakpam, P.D.; Are, C. PI3K/Akt/mTOR Signaling Pathway as a Target for Colorectal Cancer Treatment. Int. J. Mol. Sci. 2024, 25, 3178. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhong, H.; Zhou, Z.; Wang, H.; Wang, R.; Shen, K.; Huang, R.; Wang, Z. The Biological Roles and Clinical Applications of the PI3K/AKT Pathway in Targeted Therapy Resistance in HER2-Positive Breast Cancer: A Comprehensive Review. Int. J. Mol. Sci. 2024, 25, 13376. [Google Scholar] [CrossRef] [PubMed]
- Versari, I.; Salucci, S.; Bavelloni, A.; Battistelli, M.; Traversari, M.; Wang, A.; Sampaolesi, M.; Faenza, I. The Emerging Role and Clinical Significance of PI3K-Akt-mTOR in Rhabdomyosarcoma. Biomolecules 2025, 15, 334. [Google Scholar] [CrossRef] [PubMed]
- Garg, P.; Ramisetty, S.; Nair, M.; Kulkarni, P.; Horne, D.; Salgia, R.; Singhal, S.S. Strategic advancements in targeting the PI3K/AKT/mTOR pathway for Breast cancer therapy. Biochem. Pharmacol. 2025, 236, 116850. [Google Scholar] [CrossRef] [PubMed]
- Dilawari, A.; Buturla, J.; Osgood, C.; Gao, X.; Chen, W.; Ricks, T.K.; Schaefer, T.; Avasarala, S.; Reyes Turcu, F.; Pathak, A.; et al. US Food and Drug Administration Approval Summary: Capivasertib With Fulvestrant for Hormone Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative Locally Advanced or Metastatic Breast Cancer With PIK3CA/AKT1/PTEN Alterations. J. Clin. Oncol. 2024, 42, 4103–4113. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kakadia, P.M.; Tizazu, B.; Mellert, G.; Harbott, J.; Röttgers, S.; Quentmeier, H.; Spiekermann, K.; Bohlander, S.K. A novel ABL1 fusion to the SH2 containing inositol phosphatase-1 (SHIP1) in acute lymphoblastic leukemia (ALL). Leukemia 2011, 25, 1645–1649. [Google Scholar] [CrossRef] [PubMed]
- Poukka, M.; Lund-Aho, T.; Raittinen, P.; Nikkilä, A.; Kivinen, K.; Lundán, T.; Porkka, K.; Lohi, O. Acute Lymphoblastic Leukemia With INPP5D-ABL1 Fusion Responds to Imatinib Treatment. J. Pediatr. Hematol. Oncol. 2019, 41, e481–e483. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, S.; Srivastava, N.; Sudan, R.; Middleton, F.A.; Shergill, A.K.; Ryan, J.C.; Kerr, W.G. SHIP1 Deficiency in Inflammatory Bowel Disease Is Associated With Severe Crohn’s Disease and Peripheral T Cell Reduction. Front. Immunol. 2018, 9, 1100. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chouvarine, P.; Antić, Ž.; Lentes, J.; Schröder, C.; Alten, J.; Brüggemann, M.; Carrillo-de Santa Pau, E.; Illig, T.; Laguna, T.; Schewe, D.; et al. Transcriptional and Mutational Profiling of B-Other Acute Lymphoblastic Leukemia for Improved Diagnostics. Cancers 2021, 13, 5653. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shojaee, S.; Chan, L.N.; Buchner, M.; Cazzaniga, V.; Cosgun, K.N.; Geng, H.; Qiu, Y.H.; von Minden, M.D.; Ernst, T.; Hochhaus, A.; et al. PTEN opposes negative selection and enables oncogenic transformation of pre-B cells. Nat. Med. 2016, 22, 379–387. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, R.M.; Chaudhuri, A.A.; Rao, D.S.; Baltimore, D. Inositol phosphatase SHIP1 is a primary target of miR-155. Proc. Natl. Acad. Sci. USA 2009, 106, 7113–7118. [Google Scholar] [CrossRef] [PubMed]
- Alinikula, J.; Kohonen, P.; Nera, K.P.; Lassila, O. Concerted action of Helios and Ikaros controls the expression of the inositol 5-phosphatase SHIP. Eur. J. Immunol. 2010, 40, 2599–2607. [Google Scholar] [CrossRef] [PubMed]
- Lakhanpal, G.K.; Vecchiarelli-Federico, L.M.; Li, Y.J.; Cui, J.W.; Bailey, M.L.; Spaner, D.E.; Dumont, D.J.; Barber, D.L.; Ben-David, Y. The inositol phosphatase SHIP-1 is negatively regulated by Fli-1 and its loss accelerates leukemogenesis. Blood 2010, 116, 428–436. [Google Scholar] [CrossRef] [PubMed]
- Sattler, M.; Verma, S.; Byrne, C.H.; Shrikhande, G.; Winkler, T.; Algate, P.A.; Rohrschneider, L.R.; Griffin, J.D. BCR/ABL directly inhibits expression of SHIP, an SH2-containing polyinositol-5-phosphatase involved in the regulation of hematopoiesis. Mol. Cell. Biol. 1999, 19, 7473–7480. [Google Scholar] [CrossRef] [PubMed]
- Amarante-Mendes, G.P.; Rana, A.; Datoguia, T.S.; Hamerschlak, N.; Brumatti, G. BCR-ABL1 Tyrosine Kinase Complex Signaling Transduction: Challenges to Overcome Resistance in Chronic Myeloid Leukemia. Pharmaceutics 2022, 14, 215. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hu, Y.; Liu, Y.; Pelletier, S.; Buchdunger, E.; Warmuth, M.; Fabbro, D.; Hallek, M.; Van Etten, R.A.; Li, S. Requirement of Src kinases Lyn, Hck and Fgr for BCR-ABL1-induced B-lymphoblastic leukemia but not chronic myeloid leukemia. Nat. Genet. 2004, 36, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Giuriato, S.; Bodin, S.; Erneux, C.; Woscholski, R.; Plantavid, M.; Chap, H.; Payrastre, B. pp60c-src associates with the SH2-containing inositol-5-phosphatase SHIP1 and is involved in its tyrosine phosphorylation downstream of alphaIIbbeta3 integrin in human platelets. Biochem. J. 2000, 348 Pt 1, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Robinson, L.J.; Xue, J.; Corey, S.J. Src family tyrosine kinases are activated by Flt3 and are involved in the proliferative effects of leukemia-associated Flt3 mutations. Exp. Hematol. 2005, 33, 469–479. [Google Scholar] [CrossRef] [PubMed]
- Kazi, J.U.; Rönnstrand, L. The role of SRC family kinases in FLT3 signaling. Int. J. Biochem. Cell Biol. 2019, 107, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Voisset, E.; Brenet, F.; Lopez, S.; de Sepulveda, P. SRC-Family Kinases in Acute Myeloid Leukaemia and Mastocytosis. Cancers 2020, 12, 1996. [Google Scholar] [CrossRef] [PubMed]
- Roberts, K.G. Genetics and prognosis of ALL in children vs adults. Hematol. Am. Soc. Hematol. Educ. Program 2018, 2018, 137–145. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ho, J.M.; Nguyen, M.H.; Dierov, J.K.; Badger, K.M.; Beattie, B.K.; Tartaro, P.; Haq, R.; Zanke, B.W.; Carroll, M.P.; Barber, D.L. TEL-JAK2 constitutively activates the extracellular signal-regulated kinase (ERK), stress-activated protein/Jun kinase (SAPK/JNK), and p38 signaling pathways. Blood 2002, 100, 1438–1448. [Google Scholar] [PubMed]
- Buontempo, F.; McCubrey, J.A.; Orsini, E.; Ruzzene, M.; Cappellini, A.; Lonetti, A.; Evangelisti, C.; Chiarini, F.; Evangelisti, C.; Barata, J.T.; et al. Therapeutic targeting of CK2 in acute and chronic leukemias. Leukemia 2018, 32, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Grygier, P.; Pustelny, K.; Nowak, J.; Golik, P.; Popowicz, G.M.; Plettenburg, O.; Dubin, G.; Menezes, F.; Czarna, A. Silmitasertib (CX-4945), a Clinically Used CK2-Kinase Inhibitor with Additional Effects on GSK3β and DYRK1A Kinases: A Structural Perspective. J. Med. Chem. 2023, 66, 4009–4024. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cesaro, L.; Zuliani, A.M.; Bosello Travain, V.; Salvi, M. Exploring Protein Kinase CK2 Substrate Recognition and the Dynamic Response of Substrate Phosphorylation to Kinase Modulation. Kinases Phosphatases 2023, 1, 251–264. [Google Scholar] [CrossRef]
- Bradley, D.; Garand, C.; Belda, H.; Gagnon-Arsenault, I.; Treeck, M.; Elowe, S.; Landry, C.R. The substrate quality of CK2 target sites has a determinant role on their function and evolution. Cell Syst. 2024, 15, 544–562.e8. [Google Scholar] [CrossRef] [PubMed]
- Meyer, R.; Nalaskowski, M.M.; Ehm, P.; Schröder, C.; Naj, X.; Brehm, M.A.; Mayr, G.W. Nucleocytoplasmic shuttling of human inositol phosphate multikinase is influenced by CK2 phosphorylation. Biol. Chem. 2012, 393, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, T.; Kamata, Y.; Ohtsuki, K. Casein kinase 2 (CK2)-mediated reduction of the activities of Src family tyrosine kinases in vitro. Biol. Pharm. Bull. 2004, 27, 1895–1899. [Google Scholar] [CrossRef] [PubMed]
- Stam, R.W.; den Boer, M.L.; Schneider, P.; Nollau, P.; Horstmann, M.; Beverloo, H.B.; van der Voort, E.; Valsecchi, M.G.; de Lorenzo, P.; Sallan, S.E.; et al. Targeting FLT3 in primary MLL-gene-rearranged infant acute lymphoblastic leukemia. Blood 2005, 106, 2484–2490. [Google Scholar] [CrossRef] [PubMed]
- Suematsu, M.; Yagyu, S.; Yoshida, H.; Osone, S.; Nakazawa, Y.; Sugita, K.; Imamura, T.; Iehara, T. Targeting FLT3-specific chimeric antigen receptor T cells for acute lymphoblastic leukemia with KMT2A rearrangement. Cancer Immunol. Immunother. 2023, 72, 957–968. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kotecha, R.S.; Pieters, R.; Stutterheim, J. KMT2A-rearranged acute lymphoblastic leukaemia. EJC Paediatr. Oncol. 2024, 4, 100204. [Google Scholar] [CrossRef]
- Fedders, H.; Alsadeq, A.; Schmäh, J.; Vogiatzi, F.; Zimmermann, M.; Möricke, A.; Lenk, L.; Stadt, U.Z.; Horstmann, M.A.; Pieters, R.; et al. The role of constitutive activation of FMS-related tyrosine kinase-3 and NRas/KRas mutational status in infants with KMT2A-rearranged acute lymphoblastic leukemia. Haematologica 2017, 102, e438–e442. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Mao, J.; Ye, X.; Zhang, F.; Kerr, W.G.; Zheng, T.; Zhu, Z. SHIP-1, a target of miR-155, regulates endothelial cell responses in lung fibrosis. FASEB J. 2020, 34, 2011–2023. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Monnot, G.C.; Martinez-Usatorre, A.; Lanitis, E.; Lopes, S.F.; Cheng, W.C.; Ho, P.C.; Irving, M.; Coukos, G.; Donda, A.; Romero, P. miR-155 Overexpression in OT-1 CD8+ T Cells Improves Anti-Tumor Activity against Low-Affinity Tumor Antigen. Mol. Ther. Oncolytics 2019, 16, 111–123. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pedersen, I.M.; Otero, D.; Kao, E.; Miletic, A.V.; Hother, C.; Ralfkiaer, E.; Rickert, R.C.; Gronbaek, K.; David, M. Onco-miR-155 targets SHIP1 to promote TNFα-dependent growth of B cell lymphomas. EMBO Mol. Med. 2009, 1, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Costinean, S.; Zanesi, N.; Pekarsky, Y.; Tili, E.; Volinia, S.; Heerema, N.; Croce, C.M. Pre-B cell proliferation and lymphoblastic leukemia/high-grade lymphoma in E(mu)-miR155 transgenic mice. Proc. Natl. Acad. Sci. USA 2006, 103, 7024–7029. [Google Scholar] [CrossRef] [PubMed]
- Costinean, S.; Sandhu, S.K.; Pedersen, I.M.; Tili, E.; Trotta, R.; Perrotti, D.; Ciarlariello, D.; Neviani, P.; Harb, J.; Kauffman, L.R.; et al. Src homology 2 domain-containing inositol-5-phosphatase and CCAAT enhancer-binding protein beta are targeted by miR-155 in B cells of Emicro-MiR-155 transgenic mice. Blood 2009, 114, 1374–1382. [Google Scholar] [CrossRef] [PubMed]
- Golovina, E.; Kokavec, J.; Kazantsev, D.; Yurikova, O.; Bajecny, M.; Savvulidi, F.G.; Simersky, R.; Lenobel, R.; Tost, J.; Herynek, V.; et al. Deficiency of miR-155 in Leukemic B-Cells Results in Cell Cycle Arrest and Deregulation of MIR155HG/TP53INP1/CDKN1A/CCND1 network. Arch. Med. Res. 2025, 56, 103124. [Google Scholar] [CrossRef] [PubMed]
- Gerloff, D.; Grundler, R.; Wurm, A.A.; Bräuer-Hartmann, D.; Katzerke, C.; Hartmann, J.U.; Madan, V.; Müller-Tidow, C.; Duyster, J.; Tenen, D.G.; et al. NF-κB/STAT5/miR-155 network targets PU.1 in FLT3-ITD-driven acute myeloid leukemia. Leukemia 2015, 29, 535–547. [Google Scholar] [CrossRef] [PubMed]
- Trino, S.; Lamorte, D.; Caivano, A.; Laurenzana, I.; Tagliaferri, D.; Falco, G.; Del Vecchio, L.; Musto, P.; De Luca, L. MicroRNAs as New Biomarkers for Diagnosis and Prognosis, and as Potential Therapeutic Targets in Acute Myeloid Leukemia. Int. J. Mol. Sci. 2018, 19, 460. [Google Scholar] [CrossRef] [PubMed]
- Ostrycharz, E.; Hukowska-Szematowicz, B. Micro-Players of Great Significance—Host microRNA Signature in Viral Infections in Humans and Animals. Int. J. Mol. Sci. 2022, 23, 10536. [Google Scholar] [CrossRef] [PubMed]
- Mullighan, C.G.; Miller, C.B.; Radtke, I.; Phillips, L.A.; Dalton, J.; Ma, J.; White, D.; Hughes, T.P.; Le Beau, M.M.; Pui, C.H.; et al. BCR-ABL1 lymphoblastic leukaemia is characterized by the deletion of Ikaros. Nature 2008, 453, 110–114. [Google Scholar] [CrossRef] [PubMed]
- Conserva, M.R.; Redavid, I.; Anelli, L.; Zagaria, A.; Tarantini, F.; Cumbo, C.; Tota, G.; Parciante, E.; Coccaro, N.; Minervini, C.F.; et al. IKAROS in Acute Leukemia: A Positive Influencer or a Mean Hater? Int. J. Mol. Sci. 2023, 24, 3282. [Google Scholar] [CrossRef] [PubMed]
- Paolino, J.; Tsai, H.K.; Harris, M.H.; Pikman, Y. IKZF1 Alterations and Therapeutic Targeting in B-Cell Acute Lymphoblastic Leukemia. Biomedicines 2024, 12, 89. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Gowda, C.; Pan, X.; Ding, Y.; Tong, Y.; Tan, B.H.; Wang, H.; Muthusami, S.; Ge, Z.; Sachdev, M.; et al. Targeting casein kinase II restores Ikaros tumor suppressor activity and demonstrates therapeutic efficacy in high-risk leukemia. Blood 2015, 126, 1813–1822. [Google Scholar] [CrossRef] [PubMed]
- Oksuz, O.; Henninger, J.E.; Warneford-Thomson, R.; Zheng, M.M.; Erb, H.; Vancura, A.; Overholt, K.J.; Hawken, S.W.; Banani, S.F.; Lauman, R.; et al. Transcription factors interact with RNA to regulate genes. Mol. Cell 2023, 83, 2449–2463.e13. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.Y.; Ellegast, J.M.; Ross, K.N.; Malone, C.F.; Lin, S.; Mabe, N.W.; Dharia, N.V.; Meyer, A.; Conway, A.; Su, A.H.; et al. The ETS transcription factor ETV6 constrains the transcriptional activity of EWS-FLI to promote Ewing sarcoma. Nat. Cell Biol. 2023, 25, 285–297. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yang, Y.; Han, X.; Sun, L.; Shao, F.; Yin, Y.; Zhang, W. ETS Transcription Factors in Immune Cells and Immune-Related Diseases. Int. J. Mol. Sci. 2024, 25, 10004. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Xu, B.; Yu, J.; Huang, L.; Zeng, X.; Shen, X.; Ren, C.; Ben-David, Y.; Luo, H. Fli-1 Activation through Targeted Promoter Activity Regulation Using a Novel 3′, 5′-diprenylated Chalcone Inhibits Growth and Metastasis of Prostate Cancer Cells. Int. J. Mol. Sci. 2020, 21, 2216. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Oda, Y.; Kasakura, K.; Fujigaki, I.; Kageyama, A.; Okumura, K.; Ogawa, H.; Yashiro, T.; Nishiyama, C. The effect of PU.1 knockdown on gene expression and function of mast cells. Sci. Rep. 2018, 8, 2005. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.R.; Yang, W.; Gocho, Y.; John, A.; Rowland, L.; Smart, B.; Williams, H.; Maxwell, D.; Hunt, J.; Yang, W.; et al. Pharmacotypes across the genomic landscape of pediatric acute lymphoblastic leukemia and impact on treatment response. Nat. Med. 2023, 29, 170–179. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Brady, S.W.; Roberts, K.G.; Gu, Z.; Shi, L.; Pounds, S.; Pei, D.; Cheng, C.; Dai, Y.; Devidas, M.; Qu, C.; et al. The genomic landscape of pediatric acute lymphoblastic leukemia. Nat. Genet. 2022, 54, 1376–1389. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ehm, P.; Rietow, R.; Wegner, W.; Bußmann, L.; Kriegs, M.; Dierck, K.; Horn, S.; Streichert, T.; Horstmann, M.; Jücker, M. SHIP1 Is Present but Strongly Downregulated in T-ALL, and after Restoration Suppresses Leukemia Growth in a T-ALL Xenotransplantation Mouse Model. Cells 2023, 12, 1798. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Inaba, H.; Mullighan, C.G. Pediatric acute lymphoblastic leukemia. Haematologica 2020, 105, 2524–2539. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schäfer, D.; Olsen, M.; Lähnemann, D.; Stanulla, M.; Slany, R.; Schmiegelow, K.; Borkhardt, A.; Fischer, U. Five percent of healthy newborns have an ETV6-RUNX1 fusion as revealed by DNA-based GIPFEL screening. Blood 2018, 131, 821–826. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kuster, L.; Grausenburger, R.; Fuka, G.; Kaindl, U.; Krapf, G.; Inthal, A.; Mann, G.; Kauer, M.; Rainer, J.; Kofler, R.; et al. ETV6/RUNX1-positive relapses evolve from an ancestral clone and frequently acquire deletions of genes implicated in glucocorticoid signaling. Blood 2011, 117, 2658–2667. [Google Scholar] [CrossRef] [PubMed]
- Montaño, A.; Ordoñez, J.L.; Alonso-Pérez, V.; Hernández-Sánchez, J.; Santos, S.; González, T.; Benito, R.; García-Tuñón, I.; Hernández-Rivas, J.M. ETV6/RUNX1 Fusion Gene Abrogation Decreases the Oncogenicity of Tumour Cells in a Preclinical Model of Acute Lymphoblastic Leukaemia. Cells 2020, 9, 215. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Montero, O.; Ayllon, V.; Lamolda, M.; López-Onieva, L.; Montes, R.; Bueno, C.; Ng, E.; Guerrero-Carreno, X.; Romero, T.; Romero-Moya, D.; et al. RUNX1c Regulates Hematopoietic Differentiation of Human Pluripotent Stem Cells Possibly in Cooperation with Proinflammatory Signaling. Stem Cells 2017, 35, 2253–2266. [Google Scholar] [CrossRef] [PubMed]
- Speck, N.A.; Gilliland, D.G. Core-binding factors in haematopoiesis and leukaemia. Nat. Rev. Cancer 2002, 2, 502–513. [Google Scholar] [CrossRef] [PubMed]
- Bresciani, E.; Carrington, B.; Yu, K.; Kim, E.M.; Zhen, T.; Guzman, V.S.; Broadbridge, E.; Bishop, K.; Kirby, M.; Harper, U.; et al. Redundant mechanisms driven independently by RUNX1 and GATA2 for hematopoietic development. Blood Adv. 2021, 5, 4949–4962. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gonzalez Galofre, Z.N.; Kilpatrick, A.M.; Marques, M.; Sá da Bandeira, D.; Ventura, T.; Gomez Salazar, M.; Bouilleau, L.; Marc, Y.; Barbosa, A.B.; Rossi, F.; et al. Runx1+ vascular smooth muscle cells are essential for hematopoietic stem and progenitor cell development in vivo. Nat. Commun. 2024, 15, 1653. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mori, H.; Colman, S.M.; Xiao, Z.; Ford, A.M.; Healy, L.E.; Donaldson, C.; Hows, J.M.; Navarrete, C.; Greaves, M. Chromosome translocations and covert leukemic clones are generated during normal fetal development. Proc. Natl. Acad. Sci. USA 2002, 99, 8242–8247. [Google Scholar] [CrossRef] [PubMed]
- Greaves, M.F.; Wiemels, J. Origins of chromosome translocations in childhood leukaemia. Nat. Rev. Cancer 2003, 3, 639–649. [Google Scholar] [CrossRef] [PubMed]
- Ford, A.M.; Palmi, C.; Bueno, C.; Hong, D.; Cardus, P.; Knight, D.; Cazzaniga, G.; Enver, T.; Greaves, M. The TEL-AML1 leukemia fusion gene dysregulates the TGF-beta pathway in early B lineage progenitor cells. J. Clin. Investig. 2009, 119, 826–836. [Google Scholar] [PubMed]
- Kohlmann, A.; Kipps, T.J.; Rassenti, L.Z.; Downing, J.R.; Shurtleff, S.A.; Mills, K.I.; Gilkes, A.F.; Hofmann, W.K.; Basso, G.; Dell’orto, M.C.; et al. An international standardization programme towards the application of gene expression profiling in routine leukaemia diagnostics: The Microarray Innovations in LEukemia study prephase. Br. J. Haematol. 2008, 142, 802–807. [Google Scholar] [CrossRef] [PubMed]
- Haferlach, T.; Kohlmann, A.; Wieczorek, L.; Basso, G.; Kronnie, G.T.; Béné, M.C.; De Vos, J.; Hernández, J.M.; Hofmann, W.K.; Mills, K.I.; et al. Clinical utility of microarray-based gene expression profiling in the diagnosis and subclassification of leukemia: Report from the International Microarray Innovations in Leukemia Study Group. J. Clin. Oncol. 2010, 28, 2529–2537. [Google Scholar] [CrossRef] [PubMed]
- Burmeister, T.; Gröger, D.; Gökbuget, N.; Spriewald, B.; Starck, M.; Elmaagacli, A.; Hoelzer, D.; Keller, U.; Schwartz, S. Molecular characterization of TCF3::PBX1 chromosomal breakpoints in acute lymphoblastic leukemia and their use for measurable residual disease assessment. Sci. Rep. 2023, 13, 15167. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kusterer, M.; Lahnalampi, M.; Voutilainen, M.; Brand, A.; Pennisi, S.; Norona, J.; Gentile, G.; Herzog, H.; Greve, G.; Lübbert, M.; et al. Dynamic evolution of TCF3-PBX1 leukemias at the single-cell level under chemotherapy pressure. Hemasphere 2025, 9, e70071. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shochat, C.; Tal, N.; Bandapalli, O.R.; Palmi, C.; Ganmore, I.; te Kronnie, G.; Cario, G.; Cazzaniga, G.; Kulozik, A.E.; Stanulla, M.; et al. Gain-of-function mutations in interleukin-7 receptor-α (IL7R) in childhood acute lymphoblastic leukemias. J. Exp. Med. 2011, 208, 901–908. [Google Scholar] [CrossRef] [PubMed]
- Fischer, U.; Forster, M.; Rinaldi, A.; Risch, T.; Sungalee, S.; Warnatz, H.J.; Bornhauser, B.; Gombert, M.; Kratsch, C.; Stütz, A.M.; et al. Genomics and drug profiling of fatal TCF3-HLF-positive acute lymphoblastic leukemia identifies recurrent mutation patterns and therapeutic options. Nat. Genet. 2015, 47, 1020–1029. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Horváth, M.; Kertész, G.; Kassa, C.; Goda, V.; Csordás, K.; Hau, L.; Kövér, A.; Stréhn, A.; Horváth, O.; Kállay, K.; et al. Peripheral Bone Relapse of Paediatric TCF3-HLF Positive Acute Lymphoblastic Leukaemia during Haematopoietic Stem Cell Transplantation: A Case Report. Children 2022, 9, 1919. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Waardenberg, A.J.; Demuth, M.; Osteil, P.; Sun, J.Q.J.; Loebel, D.A.F.; Graham, M.; Tam, P.P.L.; Fossat, N. TWIST1 Homodimers and Heterodimers Orchestrate Lineage-Specific Differentiation. Mol. Cell. Biol. 2020, 40, e00663-19. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- de Martin, X.; Sodaei, R.; Santpere, G. Mechanisms of Binding Specificity among bHLH Transcription Factors. Int. J. Mol. Sci. 2021, 22, 9150. [Google Scholar] [CrossRef] [PubMed]
- Piskacek, M.; Vasku, A.; Hajek, R.; Knight, A. Shared structural features of the 9aaTAD family in complex with CBP. Mol. Biosyst. 2015, 11, 844–851. [Google Scholar] [CrossRef] [PubMed]
- Crisafulli, L.; Brindisi, M.; Liturri, M.G.; Sobacchi, C.; Ficara, F. PBX1: A TALE of two seasons-key roles during development and in cancer. Front. Cell Dev. Biol. 2024, 12, 1372873. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hernández-Sánchez, A.; González, T.; Sobas, M.; Sträng, E.; Castellani, G.; Abáigar, M.; Valk, P.J.M.; Villaverde Ramiro, Á.; Benner, A.; Metzeler, K.H.; et al. Rearrangements involving 11q23.3/KMT2A in adult AML: Mutational landscape and prognostic implications-a HARMONY study. Leukemia 2024, 38, 1929–1937. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, C.W.; Koche, R.P.; Sinha, A.U.; Deshpande, A.J.; Zhu, N.; Eng, R.; Doench, J.G.; Xu, H.; Chu, S.H.; Qi, J.; et al. DOT1L inhibits SIRT1-mediated epigenetic silencing to maintain leukemic gene expression in MLL-rearranged leukemia. Nat. Med. 2015, 21, 335–343. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Godfrey, L.; Kerry, J.; Thorne, R.; Repapi, E.; Davies, J.O.; Tapia, M.; Ballabio, E.; Hughes, J.R.; Geng, H.; Konopleva, M.; et al. MLL-AF4 binds directly to a BCL-2 specific enhancer and modulates H3K27 acetylation. Exp. Hematol. 2017, 47, 64–75. [Google Scholar] [CrossRef] [PubMed] [PubMed Central][Green Version]
- Zoghbi, A.; Zur Stadt, U.; Winkler, B.; Müller, I.; Escherich, G. Lineage switch under blinatumomab treatment of relapsed common acute lymphoblastic leukemia without MLL rearrangement. Pediatr. Blood Cancer 2017, 64, e26594. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Trahan, G.D.; Howell, E.D.; Speck, N.A.; Jones, K.L.; Gillen, A.E.; Riemondy, K.; Hesselberth, J.; Bryder, D.; Ernst, P. Enhancing Hematopoiesis from Murine Embryonic Stem Cells through MLL1-Induced Activation of a Rac/Rho/Integrin Signaling Axis. Stem Cell Rep. 2020, 14, 285–299. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Coad, J.E.; Arthur, D.C.; Gajl-Peczalska, K.J.; Litz, C.E. Late-developing Philadelphia chromosomes in a case of T-cell acute lymphoblastic leukemia. Leukemia 1994, 8, 889–894. [Google Scholar] [PubMed]
- Tchirkov, A.; Bons, J.M.; Chassagne, J.; Schoepfer, C.; Kanold, J.; Briançon, G.; Giollant, M.; Malet, P.; Deméocq, F. Molecular detection of a late-appearing BCR-ABL gene in a child with T-cell acute lymphoblastic leukemia. Ann. Hematol. 1998, 77, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Verrma, S.P.; Dutta, T.K.; Vinod, K.V.; Dubashi, B.; Ariga, K.K. Philadelphia chromosome positive pre-T cell acute lymphoblastic leukemia: A rare case report and short review. Indian J. Hematol. Blood Transfus. 2014, 30 (Suppl. 1), 177–179. [Google Scholar] [CrossRef] [PubMed]
- Amin, H.; Ahmed, S. Characteristics of BCR-ABL gene variants in patients of chronic myeloid leukemia. Open Med. 2021, 16, 904–912. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- McLaughlin, J.; Chianese, E.; Witte, O.N. Alternative forms of the BCR-ABL oncogene have quantitatively different potencies for stimulation of immature lymphoid cells. Mol. Cell. Biol. 1989, 9, 1866–1874. [Google Scholar] [PubMed]
- Kantarjian, H.M.; Talpaz, M.; Dhingra, K.; Estey, E.; Keating, M.J.; Ku, S.; Trujillo, J.; Huh, Y.; Stass, S.; Kurzrock, R. Significance of the P210 versus P190 molecular abnormalities in adults with Philadelphia chromosome-positive acute leukemia. Blood 1991, 78, 2411–2418. [Google Scholar] [CrossRef] [PubMed]
- Adnan-Awad, S.; Kim, D.; Hohtari, H.; Javarappa, K.K.; Brandstoetter, T.; Mayer, I.; Potdar, S.; Heckman, C.A.; Kytölä, S.; Porkka, K.; et al. Characterization of p190-Bcr-Abl chronic myeloid leukemia reveals specific signaling pathways and therapeutic targets. Leukemia 2021, 35, 1964–1975. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Padmakumar, A.; Thankamony, P.; Vasudevan, J.A.; Gopinath, P.; Chandraprabha, V.R.; Devi, A.R.T.V.; Anitha, G.R.J.; Sreelatha, M.M.; Padmakumar, D.; Sreedharan, H. Double Philadelphia chromosome: A rare and sole abnormality in pediatric B-acute lymphoblastic leukemia. 3 Biotech 2024, 14, 75. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Baccarani, M.; Iacobucci, I.; Chiaretti, S.; Foà’, R.; Balasubramanian, P.; Paietta, E.; Foroni, L.; Jeromin, S.; Izzo, B.; Spinelli, O.; et al. In Ph+BCR-ABL1P210+ acute lymphoblastic leukemia the e13a2 (B2A2) transcript is prevalent. Leukemia 2020, 34, 929–931. [Google Scholar] [CrossRef] [PubMed]
- Irgit, A.; Kamıs, R.; Sever, B.; Tuyun, A.F.; Otsuka, M.; Fujita, M.; Demirci, H.; Ciftci, H. Structure and Dynamics of the ABL1 Tyrosine Kinase and Its Important Role in Chronic Myeloid Leukemia. Arch. Pharm. 2025, 358, e70005. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Druker, B.J.; Guilhot, F.; O’Brien, S.G.; Gathmann, I.; Kantarjian, H.; Gattermann, N.; Deininger, M.W.; Silver, R.T.; Goldman, J.M.; Stone, R.M.; et al. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N. Engl. J. Med. 2006, 355, 2408–2417. [Google Scholar] [CrossRef] [PubMed]
- Braun, T.P.; Eide, C.A.; Druker, B.J. Response and Resistance to BCR-ABL1-Targeted Therapies. Cancer Cell 2020, 37, 530–542. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jones, D.; Thomas, D.; Yin, C.C.; O’Brien, S.; Cortes, J.E.; Jabbour, E.; Breeden, M.; Giles, F.J.; Zhao, W.; Kantarjian, H.M. Kinase domain point mutations in Philadelphia chromosome-positive acute lymphoblastic leukemia emerge after therapy with BCR-ABL kinase inhibitors. Cancer 2008, 113, 985–994. [Google Scholar] [CrossRef] [PubMed]
- Hochhaus, A.; Larson, R.A.; Guilhot, F.; Radich, J.P.; Branford, S.; Hughes, T.P.; Baccarani, M.; Deininger, M.W.; Cervantes, F.; Fujihara, S.; et al. Long-Term Outcomes of Imatinib Treatment for Chronic Myeloid Leukemia. N. Engl. J. Med. 2017, 376, 917–927. [Google Scholar] [CrossRef] [PubMed]
- Saleh, K.; Fernandez, A.; Pasquier, F. Treatment of Philadelphia Chromosome-Positive Acute Lymphoblastic Leukemia in Adults. Cancers 2022, 14, 1805. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Navas-Acosta, J.; Hernández-Sánchez, A.; González, T.; Villaverde Ramiro, Á.; Santos, S.; Miguel, C.; Ribera, J.; Granada, I.; Morgades, M.; Sánchez, R.; et al. Preferential Genetic Pathways Lead to Relapses in Adult B-Cell Acute Lymphoblastic Leukemia. Cancers 2024, 16, 4200. [Google Scholar] [CrossRef] [PubMed]
- Zabriskie, M.S.; Eide, C.A.; Tantravahi, S.K.; Vellore, N.A.; Estrada, J.; Nicolini, F.E.; Khoury, H.J.; Larson, R.A.; Konopleva, M.; Cortes, J.E.; et al. BCR-ABL1 compound mutations combining key kinase domain positions confer clinical resistance to ponatinib in Ph chromosome-positive leukemia. Cancer Cell 2014, 26, 428–442. [Google Scholar] [CrossRef] [PubMed]
- Østergaard, A.; Boer, J.M.; van Leeuwen, F.N.; Pieters, R.; Den Boer, M.L. IKZF1 in acute lymphoblastic leukemia: The rise before the fall? Leuk. Lymphoma 2024, 65, 2077–2087. [Google Scholar] [CrossRef] [PubMed]
- Mullighan, C.; Wangondu, R.; Ashcraft, E.; Chang, T.C.; Roberts, K.; Brady, S.; Fan, Y.; Evans, W.; Relling, M.; Crews, K.; et al. Heterogeneity of IKZF1 genomic alterations and risk of relapse in childhood B-cell precursor acute lymphoblastic leukemia. Leukemia 2025, 39, 1595–1606. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mullighan, C.G.; Phillips, L.A.; Su, X.; Ma, J.; Miller, C.B.; Shurtleff, S.A.; Downing, J.R. Genomic analysis of the clonal origins of relapsed acute lymphoblastic leukemia. Science 2008, 322, 1377–1380. [Google Scholar] [CrossRef] [PubMed]
- Mullighan, C.G.; Su, X.; Zhang, J.; Radtke, I.; Phillips, L.A.; Miller, C.B.; Ma, J.; Liu, W.; Cheng, C.; Schulman, B.A.; et al. Deletion of IKZF1 and prognosis in acute lymphoblastic leukemia. N. Engl. J. Med. 2009, 360, 470–480. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Shojaee, S.; Buchner, M.; Geng, H.; Lee, J.W.; Klemm, L.; Titz, B.; Graeber, T.G.; Park, E.; Tan, Y.X.; et al. Signalling thresholds and negative B-cell selection in acute lymphoblastic leukaemia. Nature 2015, 521, 357–361. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.H.; Loh, M.L. Ph-like acute lymphoblastic leukemia. Hematol. Am. Soc. Hematol. Educ. Program 2016, 2016, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Jain, N.; Roberts, K.G.; Jabbour, E.; Patel, K.; Eterovic, A.K.; Chen, K.; Zweidler-McKay, P.; Lu, X.; Fawcett, G.; Wang, S.A.; et al. Ph-like acute lymphoblastic leukemia: A high-risk subtype in adults. Blood 2017, 129, 572–581. [Google Scholar] [CrossRef] [PubMed]
- Mullighan, C.G.; Zhang, J.; Harvey, R.C.; Collins-Underwood, J.R.; Schulman, B.A.; Phillips, L.A.; Tasian, S.K.; Loh, M.L.; Su, X.; Liu, W.; et al. JAK mutations in high-risk childhood acute lymphoblastic leukemia. Proc. Natl. Acad. Sci. USA 2009, 106, 9414–9418. [Google Scholar] [CrossRef] [PubMed]
- Ziętara, K.J.; Wróblewska, K.; Zajączkowska, M.; Taczała, J.; Lejman, M. The Role of the JAK–STAT Pathway in Childhood B-Cell Acute Lymphoblastic Leukemia. Int. J. Mol. Sci. 2024, 25, 6844. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.T.; Zhang, F.; Fang, H.; Li, J.F.; Lu, G.; Jiang, L.; Chen, B.; Mao, D.D.; Liu, Y.F.; Wang, J.; et al. Transcriptome-wide subtyping of pediatric and adult T cell acute lymphoblastic leukemia in an international study of 707 cases. Proc. Natl. Acad. Sci. USA 2022, 119, e2120787119. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lo, T.C.; Barnhill, L.M.; Kim, Y.; Nakae, E.A.; Yu, A.L.; Diccianni, M.B. Inactivation of SHIP1 in T-cell acute lymphoblastic leukemia due to mutation and extensive alternative splicing. Leuk. Res. 2009, 33, 1562–1566. [Google Scholar] [CrossRef] [PubMed]
- Girardi, T.; Vicente, C.; Cools, J.; De Keersmaecker, K. The genetics and molecular biology of T-ALL. Blood 2017, 129, 1113–1123. [Google Scholar] [CrossRef] [PubMed]
- Bermúdez Brito, M.; Goulielmaki, E.; Papakonstanti, E.A. Focus on PTEN Regulation. Front. Oncol. 2015, 5, 166. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- González-García, A.; Garrido, A.; Carrera, A.C. Targeting PTEN Regulation by Post Translational Modifications. Cancers 2022, 14, 5613. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, J.; Pan, Y.; Liu, Y.; Wei, W.; Hu, X.; Xin, W.; Chen, N. The regulation of PTEN: Novel insights into functions as cancer biomarkers and therapeutic targets. J. Cell. Physiol. 2023, 238, 1693–1715. [Google Scholar] [CrossRef] [PubMed]
- Bose, S.; Deininger, M.; Gora-Tybor, J.; Goldman, J.M.; Melo, J.V. The presence of typical and atypical BCR-ABL fusion genes in leukocytes of normal individuals: Biologic significance and implications for the assessment of minimal residual disease. Blood 1998, 92, 3362–3367. [Google Scholar] [CrossRef] [PubMed]
- Smeets, M.F.; Chan, A.C.; Dagger, S.; Bradley, C.K.; Wei, A.; Izon, D.J. Fli-1 overexpression in hematopoietic progenitors deregulates T cell development and induces pre-T cell lymphoblastic leukaemia/lymphoma. PLoS ONE 2013, 8, e62346. [Google Scholar] [CrossRef] [PubMed]
- Mavrakis, K.J.; Van Der Meulen, J.; Wolfe, A.L.; Liu, X.; Mets, E.; Taghon, T.; Khan, A.A.; Setty, M.; Rondou, P.; Vandenberghe, P.; et al. A cooperative microRNA-tumor suppressor gene network in acute T-cell lymphoblastic leukemia (T-ALL). Nat. Genet. 2011, 43, 673–678. [Google Scholar] [CrossRef] [PubMed]
- Mansour, M.R.; Sanda, T.; Lawton, L.N.; Li, X.; Kreslavsky, T.; Novina, C.D.; Brand, M.; Gutierrez, A.; Kelliher, M.A.; Jamieson, C.H.; et al. The TAL1 complex targets the FBXW7 tumor suppressor by activating miR-223 in human T cell acute lymphoblastic leukemia. J. Exp. Med. 2013, 210, 1545–1557. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Nie, L.; Zhang, L.; Li, Y. The notch pathway promotes NF-κB activation through Asb2 in T cell acute lymphoblastic leukemia cells. Cell. Mol. Biol. Lett. 2018, 23, 37. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Toribio, M.L.; González-García, S. Notch Partners in the Long Journey of T-ALL Pathogenesis. Int. J. Mol. Sci. 2023, 24, 1383. [Google Scholar] [CrossRef] [PubMed]
- Sade, H.; Krishna, S.; Sarin, A. The anti-apoptotic effect of Notch-1 requires p56lck-dependent, Akt/PKB-mediated signaling in T cells. J. Biol. Chem. 2004, 279, 2937–2944. [Google Scholar] [CrossRef] [PubMed]
- Schaks, M.; Allgoewer, K.; Nelson, N.; Ehm, P.; Heumann, A.; Ewald, F.; Schumacher, U.; Simon, R.; Sauter, G.; Jücker, M. Ectopic Expression of Hematopoietic SHIP1 in Human Colorectal Cancer. Biomedicines 2020, 8, 215. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ehm, P.A.H.; Linnebacher, M.; Block, A.; Rehbach, C.; Jücker, M. Targeted hyperactivation of AKT through inhibition of ectopic expressed SHIP1 induces cell death in colon carcinoma cells and derived metastases. Cell. Signal. 2023, 108, 110720. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Huang, Y.; Ge, C.; Li, Z.; Tian, H.; Li, Q.; Li, H.; Li, R.; Tao, X.; Xue, Y.; et al. SHIP1 inhibits cell growth, migration, and invasion in non-small cell lung cancer through the PI3K/AKT pathway. Oncol. Rep. 2019, 41, 2337–2350. [Google Scholar] [CrossRef] [PubMed]
- Jorissen, R.N.; Gibbs, P.; Christie, M.; Prakash, S.; Lipton, L.; Desai, J.; Kerr, D.; Aaltonen, L.A.; Arango, D.; Kruhøffer, M.; et al. Metastasis-Associated Gene Expression Changes Predict Poor Outcomes in Patients with Dukes Stage B and C Colorectal Cancer. Clin. Cancer Res. 2009, 15, 7642–7651. [Google Scholar] [CrossRef] [PubMed]
- Bailet, O.; Fenouille, N.; Abbe, P.; Robert, G.; Rocchi, S.; Gonthier, N.; Denoyelle, C.; Ticchioni, M.; Ortonne, J.P.; Ballotti, R.; et al. Spleen tyrosine kinase functions as a tumor suppressor in melanoma cells by inducing senescence-like growth arrest. Cancer Res. 2009, 69, 2748–2756. [Google Scholar] [CrossRef] [PubMed]
- Gewinner, C.; Wang, Z.C.; Richardson, A.; Teruya-Feldstein, J.; Etemadmoghadam, D.; Bowtell, D.; Barretina, J.; Lin, W.M.; Rameh, L.; Salmena, L.; et al. Evidence that inositol polyphosphate 4-phosphatase type II is a tumor suppressor that inhibits PI3K signaling. Cancer Cell 2009, 16, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Alimonti, A.; Nardella, C.; Chen, Z.; Clohessy, J.G.; Carracedo, A.; Trotman, L.C.; Cheng, K.; Varmeh, S.; Kozma, S.C.; Thomas, G.; et al. A novel type of cellular senescence that can be enhanced in mouse models and human tumor xenografts to suppress prostate tumorigenesis. J. Clin. Investig. 2010, 120, 681–693. [Google Scholar] [CrossRef] [PubMed]
- Di Micco, R.; Fumagalli, M.; Cicalese, A.; Piccinin, S.; Gasparini, P.; Luise, C.; Schurra, C.; Garre’, M.; Nuciforo, P.G.; Bensimon, A.; et al. Oncogene-induced senescence is a DNA damage response triggered by DNA hyper-replication. Nature 2006, 444, 638–642. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.S.; Baek, J.; Han, S.-Y. The Role of Kinase Modulators in Cellular Senescence for Use in Cancer Treatment. Molecules 2017, 22, 1411. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.L.; Ding, J.; Meng, L.H. Oncogene-induced senescence: A double edged sword in cancer. Acta Pharmacol. Sin. 2018, 39, 1553–1558. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chan, K.T.; Blake, S.; Zhu, H.; Kang, J.; Trigos, A.S.; Madhamshettiwar, P.B.; Diesch, J.; Paavolainen, L.; Horvath, P.; Hannan, R.D.; et al. A functional genetic screen defines the AKT-induced senescence signaling network. Cell Death Differ. 2020, 27, 725–741. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chan, A.S.L.; Zhu, H.; Narita, M.; Cassidy, L.D.; Young, A.R.J.; Bermejo-Rodriguez, C.; Janowska, A.T.; Chen, H.C.; Gough, S.; Oshimori, N.; et al. Titration of RAS alters senescent state and influences tumour initiation. Nature 2024, 633, 678–685. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Srinivasan, L.; Sasaki, Y.; Calado, D.P.; Zhang, B.; Paik, J.H.; DePinho, R.A.; Kutok, J.L.; Kearney, J.F.; Otipoby, K.L.; Rajewsky, K. PI3 kinase signals BCR-dependent mature B cell survival. Cell 2009, 139, 573–586. [Google Scholar] [CrossRef] [PubMed]
- Le, Y.; Zhu, S.; Peng, H.; Wang, Z. Unveiling the omics tapestry of B-acute lymphoblastic leukemia: Bridging genomics, metabolomics, and immunomics. Sci. Rep. 2025, 15, 3188. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Burnet, F.M. The Clonal Selection Theory of Acquired Immunity; Cambridge University Press: Cambridge, UK, 1959. [Google Scholar]
- Schuler, F.; Weiss, J.G.; Lindner, S.E.; Lohmüller, M.; Herzog, S.; Spiegl, S.F.; Menke, P.; Geley, S.; Labi, V.; Villunger, A. Checkpoint kinase 1 is essential for normal B cell development and lymphomagenesis. Nat. Commun. 2017, 8, 1697. [Google Scholar] [CrossRef] [PubMed]
- Wardemann, H.; Yurasov, S.; Schaefer, A.; Young, J.W.; Meffre, E.; Nussenzweig, M.C. Predominant autoantibody production by early human B cell precursors. Science 2003, 301, 1374–1377. [Google Scholar] [CrossRef] [PubMed]
- Shojaee, S.; Caeser, R.; Buchner, M.; Park, E.; Swaminathan, S.; Hurtz, C.; Geng, H.; Chan, L.N.; Klemm, L.; Hofmann, W.K.; et al. Erk Negative Feedback Control Enables Pre-B Cell Transformation and Represents a Therapeutic Target in Acute Lymphoblastic Leukemia. Cancer Cell 2015, 28, 114–128. [Google Scholar] [CrossRef] [PubMed]
- Ecker, V.; Stumpf, M.; Brandmeier, L.; Neumayer, T.; Pfeuffer, L.; Engleitner, T.; Ringshausen, I.; Nelson, N.; Jücker, M.; Wanninger, S.; et al. Targeted PI3K/AKT-hyperactivation induces cell death in chronic lymphocytic leukemia. Nat. Commun. 2021, 12, 3526. [Google Scholar] [CrossRef] [PubMed]
- Kerr, W.G.; Pedicone, C.; Dormann, S.; Pacherille, A.; Chisholm, J.D. Small molecule targeting of SHIP1 and SHIP2. Biochem. Soc. Trans. 2020, 48, 291–300. [Google Scholar] [CrossRef] [PubMed]
- So, E.Y.; Sun, C.; Wu, K.Q.; Dubielecka, P.M.; Reginato, A.M.; Liang, O.D. Inhibition of lipid phosphatase SHIP1 expands myeloid-derived suppressor cells and attenuates rheumatoid arthritis in mice. Am. J. Physiol. Cell Physiol. 2021, 321, C569–C584. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chowdhury, B.P.; Das, S.; Bodhale, N.; Prakash Pandey, S.; Sudan, R.; Srivastava, N.; Chisholm, J.D.; Kerr, W.G.; Majumdar, S.; Saha, B. SHIP1 inhibition via 3-alpha-amino-cholestane enhances protection against Leishmania infection. Cytokine 2023, 171, 156373. [Google Scholar] [CrossRef] [PubMed]
- Miao, J.; Lin, J.; Dong, J.; Amarasinghe, O.; Mason, E.R.; Chu, S.; Qu, Z.; Cullers, C.C.; Putt, K.S.; Zhang, Z.Y. Discovery and evaluation of novel SHIP-1 inhibitors. Bioorg. Med. Chem. 2024, 114, 117965. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jesudason, C.D.; Lin, P.B.C.; Soni, D.; Perkins, B.M.; Lee-Gosselin, A.; Ingraham, C.M.; Hamilton, W.; Mason, E.R.; Jordi, O.E.; Souza, S.; et al. Optimization of SHIP1 Inhibitors for the treatment of Alzheimer’s disease. Alzheimers Dement. 2025, 20 (Suppl. 6), e087553. [Google Scholar] [CrossRef] [PubMed Central]
- Chan, L.N.; Müschen, M. B-cell identity as a metabolic barrier against malignant transformation. Exp. Hematol. 2017, 53, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Chan, L.N.; Chen, Z.; Braas, D.; Lee, J.W.; Xiao, G.; Geng, H.; Cosgun, K.N.; Hurtz, C.; Shojaee, S.; Cazzaniga, V.; et al. Metabolic gatekeeper function of B-lymphoid transcription factors. Nature 2017, 542, 479–483. [Google Scholar] [CrossRef] [PubMed]
- Gale, K.B.; Ford, A.M.; Repp, R.; Borkhardt, A.; Keller, C.; Eden, O.B.; Greaves, M.F. Backtracking leukemia to birth: Identification of clonotypic gene fusion sequences in neonatal blood spots. Proc. Natl. Acad. Sci. USA 1997, 94, 13950–13954. [Google Scholar] [CrossRef] [PubMed]
- Wiemels, J.L.; Cazzaniga, G.; Daniotti, M.; Eden, O.B.; Addison, G.M.; Masera, G.; Saha, V.; Biondi, A.; Greaves, M.F. Prenatal origin of acute lymphoblastic leukaemia in children. Lancet 1999, 354, 1499–1503. [Google Scholar] [CrossRef] [PubMed]
- Cazzaniga, G.; van Delft, F.W.; Lo Nigro, L.; Ford, A.M.; Score, J.; Iacobucci, I.; Mirabile, E.; Taj, M.; Colman, S.M.; Biondi, A.; et al. Developmental origins and impact of BCR-ABL1 fusion and IKZF1 deletions in monozygotic twins with Ph+ acute lymphoblastic leukemia. Blood 2011, 118, 5559–5564. [Google Scholar] [CrossRef] [PubMed]
- Martin-Lorenzo, A.; Auer, F.; Chan, L.N.; García-Ramírez, I.; González-Herrero, I.; Rodríguez-Hernández, G.; Bartenhagen, C.; Dugas, M.; Gombert, M.; Ginzel, S.; et al. Loss of Pax5 Exploits Sca1-BCR-ABLp190 Susceptibility to Confer the Metabolic Shift Essential for pB-ALL. Cancer Res. 2018, 78, 2669–2679. [Google Scholar] [CrossRef] [PubMed]
- Biernaux, C.; Loos, M.; Sels, A.; Huez, G.; Stryckmans, P. Detection of major bcr-abl gene expression at a very low level in blood cells of some healthy individuals. Blood 1995, 86, 3118–3122. [Google Scholar] [CrossRef] [PubMed]
- Takagi, M.; Shinoda, K.; Piao, J.; Mitsuiki, N.; Takagi, M.; Matsuda, K.; Muramatsu, H.; Doisaki, S.; Nagasawa, M.; Morio, T.; et al. Autoimmune lymphoproliferative syndrome-like disease with somatic KRAS mutation. Blood 2011, 117, 2887–2890. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Kishton, R.J.; Macintyre, A.N.; Gerriets, V.A.; Xiang, H.; Liu, X.; Abel, E.D.; Rizzieri, D.; Locasale, J.W.; Rathmell, J.C. Glucose transporter 1-mediated glucose uptake is limiting for B-cell acute lymphoblastic leukemia anabolic metabolism and resistance to apoptosis. Cell Death Dis. 2014, 5, e1470. [Google Scholar] [CrossRef] [PubMed]
- Xiao, G.; Chan, L.N.; Klemm, L.; Braas, D.; Chen, Z.; Geng, H.; Zhang, Q.C.; Aghajanirefah, A.; Cosgun, K.N.; Sadras, T.; et al. B-Cell-Specific Diversion of Glucose Carbon Utilization Reveals a Unique Vulnerability in B Cell Malignancies. Cell 2018, 173, 470–484.e18. [Google Scholar] [CrossRef] [PubMed]
- Marke, R.; Havinga, J.; Cloos, J.; Demkes, M.; Poelmans, G.; Yuniati, L.; van Ingen Schenau, D.; Sonneveld, E.; Waanders, E.; Pieters, R.; et al. Tumor suppressor IKZF1 mediates glucocorticoid resistance in B-cell precursor acute lymphoblastic leukemia. Leukemia 2016, 30, 1599–1603. [Google Scholar] [CrossRef] [PubMed]
- Karim, S.; Burzangi, A.S.; Ahmad, A.; Siddiqui, N.A.; Ibrahim, I.M.; Sharma, P.; Abualsunun, W.A.; Gabr, G.A. PI3K-AKT Pathway Modulation by Thymoquinone Limits Tumor Growth and Glycolytic Metabolism in Colorectal Cancer. Int. J. Mol. Sci. 2022, 23, 2305. [Google Scholar] [CrossRef] [PubMed]
- Piovan, E.; Yu, J.; Tosello, V.; Herranz, D.; Ambesi-Impiombato, A.; Da Silva, A.C.; Sanchez-Martin, M.; Perez-Garcia, A.; Rigo, I.; Castillo, M.; et al. Direct reversal of glucocorticoid resistance by AKT inhibition in acute lymphoblastic leukemia. Cancer Cell 2013, 24, 766–776. [Google Scholar] [CrossRef] [PubMed]
- Paradoski, B.T.; Hou, S.; Mejia, E.M.; Olayinka-Adefemi, F.; Fowke, D.; Hatch, G.M.; Saleem, A.; Banerji, V.; Hay, N.; Zeng, H.; et al. PI3K-dependent reprogramming of hexokinase isoforms controls glucose metabolism and functional responses of B lymphocytes. iScience 2024, 27, 110939. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lu, Z.; Xie, J.; Wu, G.; Shen, J.; Collins, R.; Chen, W.; Kang, X.; Luo, M.; Zou, Y.; Huang, L.J.; et al. Fasting selectively blocks development of acute lymphoblastic leukemia via leptin-receptor upregulation. Nat. Med. 2017, 23, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Orgel, E.; Tucci, J.; Alhushki, W.; Malvar, J.; Sposto, R.; Fu, C.H.; Freyer, D.R.; Abdel-Azim, H.; Mittelman, S.D. Obesity is associated with residual leukemia following induction therapy for childhood B-precursor acute lymphoblastic leukemia. Blood 2014, 124, 3932–3938. [Google Scholar] [CrossRef] [PubMed]
- Tsilingiris, D.; Vallianou, N.G.; Spyrou, N.; Kounatidis, D.; Christodoulatos, G.S.; Karampela, I.; Dalamaga, M. Obesity and Leukemia: Biological Mechanisms, Perspectives, and Challenges. Curr. Obes. Rep. 2024, 13, 1–34. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Han, J.; Jin, R.; Zhang, M.; Guo, Q.; Zhou, F. Ikaros 6 protects acute lymphoblastic leukemia cells against daunorubicin-induced apoptosis by activating the Akt-FoxO1 pathway. J. Leukoc. Biol. 2017, 101, 675–681. [Google Scholar] [CrossRef] [PubMed]
- Bateman, C.M.; Colman, S.M.; Chaplin, T.; Young, B.D.; Eden, T.O.; Bhakta, M.; Gratias, E.J.; van Wering, E.R.; Cazzaniga, G.; Harrison, C.J.; et al. Acquisition of genome-wide copy number alterations in monozygotic twins with acute lymphoblastic leukemia. Blood 2010, 115, 3553–3558. [Google Scholar] [CrossRef] [PubMed]
- de Smith, A.J.; Wiemels, J.L.; Mead, A.J.; Roberts, I.; Roy, A.; Spector, L.G. Backtracking to the future: Unraveling the origins of childhood leukemia. Leukemia 2024, 38, 416–419. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- de Smith, A.J.; Spector, L.G. In Utero Origins of Acute Leukemia in Children. Biomedicines 2024, 12, 236. [Google Scholar] [CrossRef] [PubMed]
- Ford, A.M.; Colman, S.; Greaves, M. Covert pre-leukaemic clones in healthy co-twins of patients with childhood acute lymphoblastic leukaemia. Leukemia 2023, 37, 47–52. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Arenzana, T.L.; Schjerven, H.; Smale, S.T. Regulation of gene expression dynamics during developmental transitions by the Ikaros transcription factor. Genes Dev. 2015, 29, 1801–1816. [Google Scholar] [CrossRef] [PubMed]


| Gene | Melanoma SNP [%] (Cases) | Colorectal SNP [%] (Cases) | Breast SNP [%] (Cases) | AML SNP [%] (Cases) | CLL SNP [%] (Cases) | T-ALL SNP [%] (Cases) | B-ALL SNP [%] (Cases) |
|---|---|---|---|---|---|---|---|
| PIK3CA | 8.16 (7063) | 14.16 (24,318) | 28.93 (23,448) | 0.32 (1567) | 0.51 (2344) | 1.17 (1367) | 0.21 (1456) |
| PIK3CB | 4.9 (3426) | 2.69 (4878) | 2.88 (6212) | 0.79 (1399) | 0.35 (1738) | 0.45 (662) | 0.22 (1395) |
| PIK3CD | 4.96 (2640) | 3.83 (4595) | 2.08 (5720) | 0.79 (1399) | 0.35 (1737) | 2.05 (876) | 0.5 (1395) |
| PIK3CG | 10.82 (3004) | 5.62 (4606) | 1.74 (5919) | 0.78 (1410) | 0.17 (1737) | 0 (662) | 0.07 (1395) |
| PIK3R1 | 4.25 (4497) | 4.59 (5683) | 2.6 (9735) | 0.79 (1510) | 0.41 (1947) | 3.44 (1104) | 0.53 (1502) |
| AKT1 | 3.82 (5760) | 1.85 (9227) | 3.92 (13077) | 0.35 (2021) | 0.05 (2086) | 1.17 (1277) | 0.2 (1520) |
| AKT2 | 4.53 (3624) | 1.87 (5020) | 1.21 (6190) | 0.26 (1512) | 0.12 (1737) | 0.41 (737) | 0.07 (1395) |
| AKT3 | 4.01 (3537) | 3.8 (4947) | 4.88 (6251) | 0.53 (1512) | 1.84 (1737) | 0 (654) | 0.14 (1395) |
| mTOR | 10.93 (3771) | 7.37 (5562) | 3.36 (8102) | 0.99 (1412) | 0.69 (1737) | 1.82 (991) | 0.69 (1453) |
| PTEN | 8.1 (5696) | 5.23 (10,101) | 5.63 (11,038) | 0.33 (4191) | 0.42 (2122) | 12.6 (2336) | 0.2 (1527) |
| INPP5D | 9.47 (2143) | 5.35 (3122) | 4.69 (2918) | 1.28 (1409) | 0.92 (1737) | 0.15 (653) | 0.29 (1395) |
| INPP4B | 4.73 (2750 | 2.96 (4466) | 6.4 (5378) | 1.7 (1410) | 6.1 (1737) | 0.46 (654) | 0.65 (1395) |
| NRAS | 15.56 (2760) | 3.82 (17,606) | 0.39 (8160) | 13.73 (10,262) | 0.66 (2591) | 7.81 (1856) | 13.11 (3256) |
| KRAS | 3.11 (8522) | 33.24 (82,451) | 1.3 (11,759) | 5.12 (7791) | 2.06 (2625) | 2.71 (1810) | 12.6 (3325) |
| DUSP6 | 1.05 (2185) | 1.38 (3122) | 0.45 (3092) | 0 (1398) | 0 (1737) | 0 (653) | 0.07 (1395) |
| PTPN11 | 3.79 (4279) | 1.83 (6209) | 1.25 (7125) | 6.1 (7733) | 1.68 (1842) | 1.2 (1335) | 5.34 (2791) |
| FLT3 | 8.42 (4726) | 2.9 (6787) | 1.53 (7298) | 23.57 (71,050) | 0.33 (1811) | 3.53 (2123) | 5.52 (3318) |
| EBF1 | 7.37 (2143) | 5.09 (3122) | 10.56 (2784) | 0.85 (1409) | 2.99 (1737) | 0 (653) | 0.5 (1396) |
| PAX5 | 7.95 (3672) | 2.98 (4837) | 2.68 (5856) | 0.54 (2019) | 1.67 (1795) | 0.21 (963) | 9.17 (2781) |
| IKZF1 | 8.36 (3122 | 3.55 (4765) | 2.59 (5518) | 0.95 (3787) | 0.62 (1785) | 1.3 (1311) | 14.46 (3444) |
| TP53 | 25.99 (6945) | 44.48 (21,508) | 26.61 (20,079) | 7.16 (6135) | 13.28 (4338) | 5.04 (1132) | 4.15 (2384) |
| Subtype | Be Found: Infant | Be Found: Pediatric | Be Found: Adult | SHIP1 RNA | SHIP1 Protein | Trans-Location | Mutation and Deletion | Outcome |
|---|---|---|---|---|---|---|---|---|
| ETV6-RUNX1 | +++ | +++ | +++ | ETV6-RUNX1 | KRAS, NRAS, WHSC1, PAX5 del, TBL1XR1 del | favorable | ||
| TCF3-PBX1 | ++ | + | + | + | TCF3-PBX1 | TP53 | intermediate | |
| KMT2A-r | +++ | + | ++ | + | + | KMT2A fused to AFF1, MLLT1, MLLT3 and other | NRAS, KRAS, FLT3 | poor |
| Ph-positive | + | +++ | ++ | +++ | BCR-ABL1 | PAX5 del, IKZF1 mut + del, CDKN2A/2B del | poor | |
| Ph-like | ++ | +++ | ++ | N/A | CRLF2, ABL, JAK2 EPOR PDGFRB | NRAS, KRAS, JAK2, PTPN11, IKZF1 mut + del, CDKN2A/2B del | poor |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ehm, P.; Jücker, M. The Inositol-5-Phosphatase SHIP1: Expression, Regulation and Role in Acute Lymphoblastic Leukemia. Int. J. Mol. Sci. 2025, 26, 6935. https://doi.org/10.3390/ijms26146935
Ehm P, Jücker M. The Inositol-5-Phosphatase SHIP1: Expression, Regulation and Role in Acute Lymphoblastic Leukemia. International Journal of Molecular Sciences. 2025; 26(14):6935. https://doi.org/10.3390/ijms26146935
Chicago/Turabian StyleEhm, Patrick, and Manfred Jücker. 2025. "The Inositol-5-Phosphatase SHIP1: Expression, Regulation and Role in Acute Lymphoblastic Leukemia" International Journal of Molecular Sciences 26, no. 14: 6935. https://doi.org/10.3390/ijms26146935
APA StyleEhm, P., & Jücker, M. (2025). The Inositol-5-Phosphatase SHIP1: Expression, Regulation and Role in Acute Lymphoblastic Leukemia. International Journal of Molecular Sciences, 26(14), 6935. https://doi.org/10.3390/ijms26146935





