Neuroaxonal Degeneration as a Converging Mechanism in Motor Neuron Diseases (MNDs): Molecular Insights into RNA Dysregulation and Emerging Therapeutic Targets
Abstract
1. Introduction
2. Molecular Mechanisms of Neuroaxonal Degeneration in MNDs
2.1. RNA Dysregulation
2.1.1. RNA Metabolism
2.1.2. RBP Pathology
2.1.3. Axonal Transport
2.2. Prion-like Propagation of Misfolded Proteins in MNDs
2.3. Kinase Signaling Abnormalities
2.4. DNA Damage and Repair Deficits
2.5. Mitochondrial Dynamics and Dysfunctions
2.6. Immune Activation, T Cell Involvement, and Glial Contribution
3. Pharmacological Treatments and Clinical Trials
3.1. RNA-Targeted Therapies and Antisense Oligonucleotides (ASOs)
3.2. Proteostasis Restoration Strategies
3.3. Mitochondrial Therapeutics
3.4. Kinase Signaling Modulators
3.5. Immune Modulation Strategies
3.6. Integrating Biomarkers and Precision Trial Design
3.7. Emerging Clinical Trials Across the MND Spectrum
4. Discussion
5. Future Directions
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chiò, A.; Logroscino, G.; Hardiman, O.; Swingler, R.; Mitchell, D.; Beghi, E.; Traynor, B.G. Prognostic factors in ALS: A critical review. Amyotroph. Lateral Scler. 2009, 10, 310–323. [Google Scholar] [CrossRef]
- Mélé, N.; Berzero, G.; Maisonobe, T.; Salachas, F.; Nicolas, G.; Weiss, N.; Beaudonnet, G.; Ducray, F.; Psimaras, D.; Lenglet, T. Motor neuron disease of paraneoplastic origin: A rare but treatable condition. J. Neurol. 2018, 265, 1590–1599. [Google Scholar] [CrossRef]
- Saxena, S.; Caroni, P. Selective neuronal vulnerability in neurodegenerative diseases: From stressor thresholds to degeneration. Neuron 2011, 71, 35–48. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.P.; Brown, R.H., Jr.; Cleveland, D.W. Decoding ALS: From genes to mechanism. Nature 2016, 539, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.B.; Taylor, J.P. RNA metabolism in neurological disease. Brain Res. 2014, 1584, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.O.; Mandrioli, J.; Benatar, M.; Abramzon, Y.; Van Deerlin, V.M.; Trojanowski, J.Q.; Gibbs, J.R.; Brunetti, M.; Gronka, S.; Wuu, J.; et al. Exome sequencing reveals VCP mutations as a cause of familial ALS. Neuron 2010, 68, 857–864. [Google Scholar] [CrossRef]
- Katsuno, M.; Adachi, H.; Minamiyama, M.; Waza, M.; Doi, H.; Kondo, N.; Mizoguchi, H.; Nitta, A.; Yamada, K.; Banno, H.; et al. Disrupted transforming growth factor-beta signaling in spinal and bulbar muscular atrophy. J. Neurosci. 2010, 30, 5702–5712. [Google Scholar] [CrossRef]
- Ikenaka, K.; Katsuno, M.; Kawai, K.; Ishigaki, S.; Tanaka, F.; Sobue, G. Disruption of axonal transport in motor neuron diseases. Int. J. Mol. Sci. 2012, 13, 1225–1238. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, X.; Huo, Z.; Chen, Y.; Liu, J.; Zhao, Z.; Meng, F.; Su, Q.; Bao, W.; Zhang, L.; et al. The Impact of Mitochondrial Dysfunction in Amyotrophic Lateral Sclerosis. Cells 2022, 11, 2049. [Google Scholar] [CrossRef]
- Beers, D.R.; Appel, S.H. Immune dysregulation in amyotrophic lateral sclerosis: Mechanisms and emerging therapies. Lancet Neurol. 2019, 18, 211–220. [Google Scholar] [CrossRef]
- Xu, Z.; Henderson, R.D.; David, M.; McCombe, P.A. Neurofilaments as Biomarkers for Amyotrophic Lateral Sclerosis: A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0164625. [Google Scholar] [CrossRef]
- Prasad, A.; Bharathi, V.; Sivalingam, V.; Girdhar, A.; Patel, B.K. Molecular Mechanisms of TDP-43 Misfolding and Pathology in Amyotrophic Lateral Sclerosis. Front. Mol. Neurosci. 2019, 12, 25. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.C.; Ng, C.S.; Xiang, P.; Liu, H.; Zhang, K.; Mohamud, Y.; Luo, H. Dysregulation of RNA-Binding Proteins in Amyotrophic Lateral Sclerosis. Front. Mol. Neurosci. 2020, 13, 78. [Google Scholar] [CrossRef]
- Park, J.; Desai, H.; Liboy-Lugo, J.M.; Gu, S.; Jowhar, Z.; Xu, A.; Floor, S.N. IGHMBP2 deletion suppresses translation and activates the integrated stress response. Life Sci. Alliance 2024, 7, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Jaiswal, M.K.; Chien, J.F.; Kozlenkov, A.; Jung, J.; Zhou, P.; Gardashli, M.; Pregent, L.J.; Engelberg-Cook, E.; Dickson, D.W.; et al. Divergent single cell transcriptome and epigenome alterations in ALS and FTD patients with C9orf72 mutation. Nat. Commun. 2023, 14, 5714. [Google Scholar] [CrossRef]
- Cooper-Knock, J.; Walsh, M.J.; Higginbottom, A.; Robin Highley, J.; Dickman, M.J.; Edbauer, D.; Ince, P.G.; Wharton, S.B.; Wilson, S.A.; Kirby, J.; et al. Sequestration of multiple RNA recognition motif-containing proteins by C9orf72 repeat expansions. Brain 2014, 137, 2040–2051. [Google Scholar] [CrossRef]
- Mehta, A.R.; Gregory, J.M.; Dando, O.; Carter, R.N.; Burr, K.; Nanda, J.; Story, D.; McDade, K.; Smith, C.; Morton, N.M.; et al. Mitochondrial bioenergetic deficits in C9orf72 amyotrophic lateral sclerosis motor neurons cause dysfunctional axonal homeostasis. Acta Neuropathol. 2021, 141, 257–279. [Google Scholar] [CrossRef]
- Butti, Z.; Patten, S.A. RNA Dysregulation in Amyotrophic Lateral Sclerosis. Front. Genet. 2018, 9, 712. [Google Scholar] [CrossRef]
- Raman, R.; Allen, S.P.; Goodall, E.F.; Kramer, S.; Ponger, L.L.; Heath, P.R.; Milo, M.; Hollinger, H.C.; Walsh, T.; Highley, J.R.; et al. Gene expression signatures in motor neurone disease fibroblasts reveal dysregulation of metabolism, hypoxia-response and RNA processing functions. Neuropathol. Appl. Neurobiol. 2015, 41, 201–226. [Google Scholar] [CrossRef]
- de Boer, E.M.J.; de Vries, B.S.; Pennings, M.; Kamsteeg, E.J.; Veldink, J.H.; van den Berg, L.H.; van Es, M.A. Genetic characterization of primary lateral sclerosis. J. Neurol. 2023, 270, 3970–3980. [Google Scholar] [CrossRef]
- Li, Y.; Sun, S. RNA dysregulation in neurodegenerative diseases. EMBO J. 2025, 44, 613–638. [Google Scholar] [CrossRef]
- Kara, E.; Tucci, A.; Manzoni, C.; Lynch, D.S.; Elpidorou, M.; Bettencourt, C.; Chelban, V.; Manole, A.; Hamed, S.A.; Haridy, N.A.; et al. Genetic and phenotypic characterization of complex hereditary spastic paraplegia. Brain 2016, 139, 1904–1918. [Google Scholar] [CrossRef]
- Toupenet Marchesi, L.; Stockholm, D.; Esteves, T.; Leblanc, M.; Auger, N.; Branchu, J.; El Hachimi, K.H.; Stevanin, G. Transcriptomic analysis reinforces the implication of spatacsin in neuroinflammation and neurodevelopment. Sci. Rep. 2025, 15, 2370. [Google Scholar] [CrossRef]
- Krumm, L.; Pozner, T.; Zagha, N.; Coras, R.; Arnold, P.; Tsaktanis, T.; Scherpelz, K.; Davis, M.Y.; Kaindl, J.; Stolzer, I.; et al. Neuroinflammatory disease signatures in SPG11-related hereditary spastic paraplegia patients. Acta Neuropathol. 2024, 147, 28. [Google Scholar] [CrossRef] [PubMed]
- Wali, G.; Li, Y.; Liyanage, E.; Kumar, K.R.; Day, M.L.; Sue, C.M. Pharmacological rescue of mitochondrial and neuronal defects in SPG7 hereditary spastic paraplegia patient neurons using high throughput assays. Front. Neurosci. 2023, 17, 1231584. [Google Scholar] [CrossRef] [PubMed]
- Grohmann, K.; Schuelke, M.; Diers, A.; Hoffmann, K.; Lucke, B.; Adams, C.; Bertini, E.; Leonhardt-Horti, H.; Muntoni, F.; Ouvrier, R.; et al. Mutations in the gene encoding immunoglobulin μ-binding protein 2 cause spinal muscular atrophy with respiratory distress type 1. Nat. Genet. 2001, 29, 75–77. [Google Scholar] [CrossRef]
- Tian, Y.; Xing, J.; Shi, Y.; Yuan, E. Exploring the relationship between IGHMBP2 gene mutations and spinal muscular atrophy with respiratory distress type 1 and Charcot-Marie-Tooth disease type 2S: A systematic review. Front. Neurosci. 2023, 17, 1252075. [Google Scholar] [CrossRef]
- Jablonka, S.; Yildirim, E. Disease Mechanisms and Therapeutic Approaches in SMARD1-Insights from Animal Models and Cell Models. Biomedicines 2024, 12, 845. [Google Scholar] [CrossRef]
- Guenther, U.P.; Handoko, L.; Laggerbauer, B.; Jablonka, S.; Chari, A.; Alzheimer, M.; Ohmer, J.; Plöttner, O.; Gehring, N.; Sickmann, A.; et al. IGHMBP2 is a ribosome-associated helicase inactive in the neuromuscular disorder distal SMA type 1 (DSMA1). Hum. Mol. Genet. 2009, 18, 1288–1300. [Google Scholar] [CrossRef]
- Beijer, D.; Kim, H.J.; Guo, L.; O’Donovan, K.; Mademan, I.; Deconinck, T.; Van Schil, K.; Fare, C.M.; Drake, L.E.; Ford, A.F.; et al. Characterization of HNRNPA1 mutations defines diversity in pathogenic mechanisms and clinical presentation. JCI Insight 2021, 6, e148363. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, N.C.; Wang, Y.D.; Scarborough, E.A.; Moore, J.; Diaz, Z.; MacLea, K.S.; Freibaum, B.; Li, S.; Molliex, A.; et al. Mutations in prion-like domains in hnRNPA2B1 and hnRNPA1 cause multisystem proteinopathy and ALS. Nature 2013, 495, 467–473. [Google Scholar] [CrossRef]
- Pfeffer, G.; Lee, G.; Pontifex, C.S.; Fanganiello, R.D.; Peck, A.; Weihl, C.C.; Kimonis, V. Multisystem Proteinopathy Due to VCP Mutations: A Review of Clinical Heterogeneity and Genetic Diagnosis. Genes 2022, 13, 963. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.A.; Klickstein, J.A.; Khanna, R.; Gou, Y.; Raman, M. The Cure VCP Scientific Conference 2021: Molecular and clinical insights into neurodegeneration and myopathy linked to multisystem proteinopathy-1 (MSP-1). Neurobiol. Dis. 2022, 169, 105722. [Google Scholar] [CrossRef] [PubMed]
- La Spada, A.R.; Wilson, E.M.; Lubahn, D.B.; Harding, A.E.; Fischbeck, K.H. Androgen receptor gene mutations in X-linked spinal and bulbar muscular atrophy. Nature 1991, 352, 77–79. [Google Scholar] [CrossRef]
- Li, M.; Miwa, S.; Kobayashi, Y.; Merry, D.E.; Yamamoto, M.; Tanaka, F.; Doyu, M.; Hashizume, Y.; Fischbeck, K.H.; Sobue, G. Nuclear inclusions of the androgen receptor protein in spinal and bulbar muscular atrophy. Ann. Neurol. 1998, 44, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Pennuto, M.; Rinaldi, C. From gene to therapy in spinal and bulbar muscular atrophy: Are we there yet? Mol. Cell. Endocrinol. 2018, 465, 113–121. [Google Scholar] [CrossRef]
- Hirunagi, T.; Sahashi, K.; Meilleur, K.G.; Katsuno, M. Nucleic Acid-Based Therapeutic Approach for Spinal and Bulbar Muscular Atrophy and Related Neurological Disorders. Genes 2022, 13, 109. [Google Scholar] [CrossRef]
- Ramaswami, M.; Taylor, J.P.; Parker, R. Altered ribostasis: RNA-protein granules in degenerative disorders. Cell 2013, 154, 727–736. [Google Scholar] [CrossRef]
- Schweingruber, C.; Nijssen, J.; Mechtersheimer, J.; Reber, S.; Lebœuf, M.; O’Brien, N.L.; Mei, I.; Hedges, E.; Keuper, M.; Benitez, J.A.; et al. Single-cell RNA-sequencing reveals early mitochondrial dysfunction unique to motor neurons shared across FUS- and TARDBP-ALS. Nat. Commun. 2025, 16, 4633. [Google Scholar] [CrossRef]
- Nalbandian, A.; Ghimbovschi, S.; Radom-Aizik, S.; Dec, E.; Vesa, J.; Martin, B.; Knoblach, S.; Smith, C.; Hoffman, E.; Kimonis, V.E. Global gene profiling of VCP-associated inclusion body myopathy. Clin. Transl. Sci. 2012, 5, 226–234. [Google Scholar] [CrossRef]
- Chu, S.; Xie, X.; Payan, C.; Stochaj, U. Valosin containing protein (VCP): Initiator, modifier, and potential drug target for neurodegenerative diseases. Mol. Neurodegener. 2023, 18, 52. [Google Scholar] [CrossRef]
- Neveling, K.; Martinez-Carrera, L.A.; Hölker, I.; Heister, A.; Verrips, A.; Hosseini-Barkooie, S.M.; Gilissen, C.; Vermeer, S.; Pennings, M.; Meijer, R.; et al. Mutations in BICD2, which encodes a golgin and important motor adaptor, cause congenital autosomal-dominant spinal muscular atrophy. Am. J. Hum. Genet. 2013, 92, 946–954. [Google Scholar] [CrossRef] [PubMed]
- Maciel, R.; Bis, D.M.; Rebelo, A.P.; Saghira, C.; Züchner, S.; Saporta, M.A. The human motor neuron axonal transcriptome is enriched for transcripts related to mitochondrial function and microtubule-based axonal transport. Exp. Neurol. 2018, 307, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Wolozin, B.; Ivanov, P. Stress granules and neurodegeneration. Nat. Rev. Neurosci. 2019, 20, 649–666. [Google Scholar] [CrossRef] [PubMed]
- Rummens, J.; Da Cruz, S. RNA-binding proteins in ALS and FTD: From pathogenic mechanisms to therapeutic insights. Mol. Neurodegener. 2025, 20, 64. [Google Scholar] [CrossRef]
- Kim, N.C.; Tresse, E.; Kolaitis, R.M.; Molliex, A.; Thomas, R.E.; Alami, N.H.; Wang, B.; Joshi, A.; Smith, R.B.; Ritson, G.P.; et al. VCP is essential for mitochondrial quality control by PINK1/Parkin and this function is impaired by VCP mutations. Neuron 2013, 78, 65–80. [Google Scholar] [CrossRef]
- Kannan, A.; Gangadharan Leela, S.; Branzei, D.; Gangwani, L. Role of senataxin in R-loop-mediated neurodegeneration. Brain Commun. 2024, 6, fcae239. [Google Scholar] [CrossRef]
- Marrone, L.; Drexler, H.C.A.; Wang, J.; Tripathi, P.; Distler, T.; Heisterkamp, P.; Anderson, E.N.; Kour, S.; Moraiti, A.; Maharana, S.; et al. FUS pathology in ALS is linked to alterations in multiple ALS-associated proteins and rescued by drugs stimulating autophagy. Acta Neuropathol. 2019, 138, 67–84. [Google Scholar] [CrossRef]
- Sleigh, J.N.; Rossor, A.M.; Fellows, A.D.; Tosolini, A.P.; Schiavo, G. Axonal transport and neurological disease. Nat. Rev. Neurol. 2019, 15, 691–703. [Google Scholar] [CrossRef]
- Gicking, A.M.; Ma, T.C.; Feng, Q.; Jiang, R.; Badieyan, S.; Cianfrocco, M.A.; Hancock, W.O. Kinesin-1, -2, and -3 motors use family-specific mechanochemical strategies to effectively compete with dynein during bidirectional transport. Elife 2022, 11, e82228. [Google Scholar] [CrossRef]
- Cason, S.E.; Holzbaur, E.L.F. Selective motor activation in organelle transport along axons. Nat. Rev. Mol. Cell Biol. 2022, 23, 699–714. [Google Scholar] [CrossRef]
- Gibbs, K.L.; Greensmith, L.; Schiavo, G. Regulation of Axonal Transport by Protein Kinases. Trends Biochem. Sci. 2015, 40, 597–610. [Google Scholar] [CrossRef]
- McAlary, L.; Plotkin, S.S.; Yerbury, J.J.; Cashman, N.R. Prion-Like Propagation of Protein Misfolding and Aggregation in Amyotrophic Lateral Sclerosis. Front. Mol. Neurosci. 2019, 12, 262. [Google Scholar] [CrossRef] [PubMed]
- Harrison, A.F.; Shorter, J. RNA-binding proteins with prion-like domains in health and disease. Biochem. J. 2017, 474, 1417–1438. [Google Scholar] [CrossRef] [PubMed]
- Münch, C.; O’Brien, J.; Bertolotti, A. Prion-like propagation of mutant superoxide dismutase-1 misfolding in neuronal cells. Proc. Natl. Acad. Sci. USA 2011, 108, 3548–3553. [Google Scholar] [CrossRef] [PubMed]
- Neupane, K.; Narayan, A.; Sen Mojumdar, S.; Adhikari, G.; Garen, C.R.; Woodside, M.T. Direct observation of prion-like propagation of protein misfolding templated by pathogenic mutants. Nat. Chem. Biol. 2024, 20, 1220–1226. [Google Scholar] [CrossRef]
- Pillai, M.; Jha, S.K. Conformational Enigma of TDP-43 Misfolding in Neurodegenerative Disorders. ACS Omega 2024, 9, 40286–40297. [Google Scholar] [CrossRef]
- Arai, T.; Hasegawa, M.; Akiyama, H.; Ikeda, K.; Nonaka, T.; Mori, H.; Mann, D.; Tsuchiya, K.; Yoshida, M.; Hashizume, Y.; et al. TDP-43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem. Biophys. Res. Commun. 2006, 351, 602–611. [Google Scholar] [CrossRef]
- Afjadi, M.N.; Dabirmanesh, B.; Uversky, V.N. Chapter Eleven—Therapeutic approaches in proteinopathies. In Progress in Molecular Biology and Translational Science; Dabirmanesh, B., Uversky, V.N., Eds.; Academic Press: Los Angeles, CA, USA, 2024; Volume 206, pp. 341–388. [Google Scholar]
- Mann, J.R.; Gleixner, A.M.; Mauna, J.C.; Gomes, E.; DeChellis-Marks, M.R.; Needham, P.G.; Copley, K.E.; Hurtle, B.; Portz, B.; Pyles, N.J.; et al. RNA Binding Antagonizes Neurotoxic Phase Transitions of TDP-43. Neuron 2019, 102, 321–338.e8. [Google Scholar] [CrossRef]
- Gendron, T.F.; Petrucelli, L. Rodent models of TDP-43 proteinopathy: Investigating the mechanisms of TDP-43-mediated neurodegeneration. J. Mol. Neurosci. 2011, 45, 486–499. [Google Scholar] [CrossRef]
- Zhang, K.; Donnelly, C.J.; Haeusler, A.R.; Grima, J.C.; Machamer, J.B.; Steinwald, P.; Daley, E.L.; Miller, S.J.; Cunningham, K.M.; Vidensky, S.; et al. The C9orf72 repeat expansion disrupts nucleocytoplasmic transport. Nature 2015, 525, 56–61. [Google Scholar] [CrossRef]
- Cantara, S.; Simoncelli, G.; Ricci, C. Antisense Oligonucleotides (ASOs) in Motor Neuron Diseases: A Road to Cure in Light and Shade. Int. J. Mol. Sci. 2024, 25, 4809. [Google Scholar] [CrossRef]
- Mackenzie, I.R.A.; Briemberg, H. TDP-43 pathology in primary lateral sclerosis. Amyotroph. Lateral Scler. Front. Degener. 2020, 21 (Suppl. S1), 52–58. [Google Scholar] [CrossRef] [PubMed]
- Namkoong, S.; Ho, A.; Woo, Y.M.; Kwak, H.; Lee, J.H. Systematic Characterization of Stress-Induced RNA Granulation. Mol. Cell 2018, 70, 175–187.e8. [Google Scholar] [CrossRef] [PubMed]
- Mann, J.R.; Donnelly, C.J. RNA modulates physiological and neuropathological protein phase transitions. Neuron 2021, 109, 2663–2681. [Google Scholar] [CrossRef] [PubMed]
- Marcelo, A.; Koppenol, R.; de Almeida, L.P.; Matos, C.A.; Nóbrega, C. Stress granules, RNA-binding proteins and polyglutamine diseases: Too much aggregation? Cell Death Dis. 2021, 12, 592. [Google Scholar] [CrossRef]
- Wali, G.; Kumar, K.R.; Liyanage, E.; Davis, R.L.; Mackay-Sim, A.; Sue, C.M. Mitochondrial Function in Hereditary Spastic Paraplegia: Deficits in SPG7 but Not SPAST Patient-Derived Stem Cells. Front. Neurosci. 2020, 14, 820. [Google Scholar] [CrossRef]
- Awuah, W.A.; Tan, J.K.; Shkodina, A.D.; Ferreira, T.; Adebusoye, F.T.; Mazzoleni, A.; Wellington, J.; David, L.; Chilcott, E.; Huang, H.; et al. Hereditary spastic paraplegia: Novel insights into the pathogenesis and management. SAGE Open Med. 2024, 12, 20503121231221941. [Google Scholar] [CrossRef]
- Ricardez Hernandez, S.M.; Ahmed, B.; Al Rawi, Y.; Torres, F.J.L.; Garro Kacher, M.O.; Smith, C.L.; Al Rawi, Z.; Garcia, J.; Nichols, N.L.; Lorson, C.L.; et al. Ighmbp2 mutations and disease pathology: Defining differences that differentiate SMARD1 and CMT2S. Exp. Neurol. 2025, 383, 115025. [Google Scholar] [CrossRef]
- Sierra-Delgado, J.A.; Sinha-Ray, S.; Kaleem, A.; Ganjibakhsh, M.; Parvate, M.; Powers, S.; Zhang, X.; Likhite, S.; Meyer, K. In Vitro Modeling as a Tool for Testing Therapeutics for Spinal Muscular Atrophy and IGHMBP2-Related Disorders. Biology 2023, 12, 867. [Google Scholar] [CrossRef]
- Picchiarelli, G.; Dupuis, L. Role of RNA Binding Proteins with prion-like domains in muscle and neuromuscular diseases. Cell Stress 2020, 4, 76–91. [Google Scholar] [CrossRef]
- Harley, J.; Patani, R. Stress-Specific Spatiotemporal Responses of RNA-Binding Proteins in Human Stem-Cell-Derived Motor Neurons. Int. J. Mol. Sci. 2020, 21, 8346. [Google Scholar] [CrossRef] [PubMed]
- Ju, J.S.; Miller, S.E.; Hanson, P.I.; Weihl, C.C. Impaired protein aggregate handling and clearance underlie the pathogenesis of p97/VCP-associated disease. J. Biol. Chem. 2008, 283, 30289–30299. [Google Scholar] [CrossRef] [PubMed]
- Chivet, M.; Marchioretti, C.; Pirazzini, M.; Piol, D.; Scaramuzzino, C.; Polanco, M.J.; Romanello, V.; Zuccaro, E.; Parodi, S.; D’Antonio, M.; et al. Polyglutamine-Expanded Androgen Receptor Alteration of Skeletal Muscle Homeostasis and Myonuclear Aggregation Are Affected by Sex, Age and Muscle Metabolism. Cells 2020, 9, 325. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, T.; Nagai, Y. Protein Misfolding and Aggregation as a Therapeutic Target for Polyglutamine Diseases. Brain Sci. 2017, 7, 128. [Google Scholar] [CrossRef]
- Arnold, F.J.; Merry, D.E. Molecular Mechanisms and Therapeutics for SBMA/Kennedy’s Disease. Neurotherapeutics 2019, 16, 928–947. [Google Scholar] [CrossRef]
- Hu, C.; Yan, Y.; Jin, Y.; Yang, J.; Xi, Y.; Zhong, Z. Decoding the Cellular Trafficking of Prion-like Proteins in Neurodegenerative Diseases. Neurosci. Bull. 2024, 40, 241–254. [Google Scholar] [CrossRef]
- Iannibelli, E.; Gibertini, S.; Cheli, M.; Blasevich, F.; Cavaliere, A.; Riolo, G.; Ruggieri, A.; Maggi, L. VCP-related myopathy: A case series and a review of literature. Acta Myol. 2023, 42, 2–13. [Google Scholar]
- Matsubara, S.; Shimizu, T.; Komori, T.; Mori-Yoshimura, M.; Minami, N.; Hayashi, Y.K. Nuclear inclusions mimicking poly(A)-binding protein nuclear 1 inclusions in a case of inclusion body myopathy associated with Paget disease of bone and frontotemporal dementia with a novel mutation in the valosin-containing protein gene. Neuromuscul. Disord. 2016, 26, 436–440. [Google Scholar] [CrossRef]
- Davis, A.A.; Leyns, C.E.G.; Holtzman, D.M. Intercellular Spread of Protein Aggregates in Neurodegenerative Disease. Annu. Rev. Cell Dev. Biol. 2018, 34, 545–568. [Google Scholar] [CrossRef]
- Falcão de Campos, C.; de Carvalho, M. Distal myopathy and rapidly progressive dementia associated with a novel mutation in the VCP gene: Expanding inclusion body myopathy with early-onset Paget disease and frontotemporal dementia spectrum. J. Clin. Neurosci. 2019, 64, 8–10. [Google Scholar] [CrossRef] [PubMed]
- Yi, J.; Zhao, X.; Noell, C.R.; Helmer, P.; Solmaz, S.R.; Vallee, R.B. Role of Nesprin-2 and RanBP2 in BICD2-associated brain developmental disorders. PLoS Genet. 2023, 19, e1010642. [Google Scholar] [CrossRef] [PubMed]
- Becker, L.-L.; Dafsari, H.S.; Schallner, J.; Abdin, D.; Seifert, M.; Petit, F.; Smol, T.; Bok, L.; Rodan, L.; Krapels, I.; et al. The clinical-phenotype continuum in DYNC1H1-related disorders—Genomic profiling and proposal for a novel classification. J. Hum. Genet. 2020, 65, 1003–1017. [Google Scholar] [CrossRef] [PubMed]
- Möller, B.; Becker, L.L.; Saffari, A.; Afenjar, A.; Coci, E.G.; Williamson, R.; Ward-Melver, C.; Gibaud, M.; Sedláčková, L.; Laššuthová, P.; et al. The expanding clinical and genetic spectrum of DYNC1H1-related disorders. Brain 2025, 148, 597–612. [Google Scholar] [CrossRef]
- Rhine, K.; Al-Azzam, N.; Yu, T.; Yeo, G.W. Aging RNA granule dynamics in neurodegeneration. Front. Mol. Biosci. 2022, 9, 991641. [Google Scholar] [CrossRef]
- Kellett, E.A.; Bademosi, A.T.; Walker, A.K. Molecular mechanisms and consequences of TDP-43 phosphorylation in neurodegeneration. Mol. Neurodegener. 2025, 20, 53. [Google Scholar] [CrossRef]
- Park, J.I. MAPK-ERK Pathway. Int. J. Mol. Sci. 2023, 24, 9666. [Google Scholar] [CrossRef]
- Tenreiro, S.; Eckermann, K.; Outeiro, T.F. Protein phosphorylation in neurodegeneration: Friend or foe? Front. Mol. Neurosci. 2014, 7, 42. [Google Scholar] [CrossRef]
- Burk, K.; Pasterkamp, R.J. Disrupted neuronal trafficking in amyotrophic lateral sclerosis. Acta Neuropathol. 2019, 137, 859–877. [Google Scholar] [CrossRef]
- Rzepnikowska, W.; Kaminska, J.; Kochański, A. The molecular mechanisms that underlie IGHMBP2-related diseases. Neuropathol. Appl. Neurobiol. 2024, 50, e13005. [Google Scholar] [CrossRef]
- Sahana, T.G.; Zhang, K. Mitogen-Activated Protein Kinase Pathway in Amyotrophic Lateral Sclerosis. Biomedicines 2021, 9, 969. [Google Scholar] [CrossRef]
- Lee, S.; Huang, E.J. Modeling ALS and FTD with iPSC-derived neurons. Brain Res. 2017, 1656, 88–97. [Google Scholar] [CrossRef]
- Wu, X.; Yang, Z.; Zou, J.; Gao, H.; Shao, Z.; Li, C.; Lei, P. Protein kinases in neurodegenerative diseases: Current understandings and implications for drug discovery. Signal Transduct. Target. Ther. 2025, 10, 146. [Google Scholar] [CrossRef]
- Choi, H.J.; Cha, S.J.; Lee, J.W.; Kim, H.J.; Kim, K. Recent Advances on the Role of GSK3β in the Pathogenesis of Amyotrophic Lateral Sclerosis. Brain Sci. 2020, 10, 675. [Google Scholar] [CrossRef]
- Lee, A.; Henderson, R.; Arachchige, B.J.; Robertson, T.; McCombe, P.A. Proteomic investigation of ALS motor cortex identifies known and novel pathogenetic mechanisms. J. Neurol. Sci. 2023, 452, 120753. [Google Scholar] [CrossRef]
- Mackenzie, I.R.A. Neuropathology of primary lateral sclerosis. Amyotroph. Lateral Scler. Front. Degener. 2020, 21 (Suppl. S1), 47–51. [Google Scholar] [CrossRef]
- Mishra, H.K.; Prots, I.; Havlicek, S.; Kohl, Z.; Perez-Branguli, F.; Boerstler, T.; Anneser, L.; Minakaki, G.; Wend, H.; Hampl, M.; et al. GSK3ß-dependent dysregulation of neurodevelopment in SPG11-patient induced pluripotent stem cell model. Ann. Neurol. 2016, 79, 826–840. [Google Scholar] [CrossRef]
- Güner, F.; Pozner, T.; Krach, F.; Prots, I.; Loskarn, S.; Schlötzer-Schrehardt, U.; Winkler, J.; Winner, B.; Regensburger, M. Axon-Specific Mitochondrial Pathology in SPG11 Alpha Motor Neurons. Front. Neurosci. 2021, 15, 680572. [Google Scholar] [CrossRef]
- Kim, E.K.; Choi, E.-J. Pathological roles of MAPK signaling pathways in human diseases. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2010, 1802, 396–405. [Google Scholar] [CrossRef]
- Ackerley, S.; Grierson, A.J.; Banner, S.; Perkinton, M.S.; Brownlees, J.; Byers, H.L.; Ward, M.; Thornhill, P.; Hussain, K.; Waby, J.S.; et al. p38α stress-activated protein kinase phosphorylates neurofilaments and is associated with neurofilament pathology in amyotrophic lateral sclerosis. Mol. Cell. Neurosci. 2004, 26, 354–364. [Google Scholar] [CrossRef]
- Le Ber, I.; Van Bortel, I.; Nicolas, G.; Bouya-Ahmed, K.; Camuzat, A.; Wallon, D.; De Septenville, A.; Latouche, M.; Lattante, S.; Kabashi, E.; et al. hnRNPA2B1 and hnRNPA1 mutations are rare in patients with “multisystem proteinopathy” and frontotemporal lobar degeneration phenotypes. Neurobiol. Aging 2014, 35, 934.e5–934.e6. [Google Scholar] [CrossRef]
- Ludtmann, M.H.R.; Arber, C.; Bartolome, F.; de Vicente, M.; Preza, E.; Carro, E.; Houlden, H.; Gandhi, S.; Wray, S.; Abramov, A.Y. Mutations in valosin-containing protein (VCP) decrease ADP/ATP translocation across the mitochondrial membrane and impair energy metabolism in human neurons. J. Biol. Chem. 2017, 292, 8907–8917. [Google Scholar] [CrossRef]
- Malik, B.; Devine, H.; Patani, R.; La Spada, A.R.; Hanna, M.G.; Greensmith, L. Gene expression analysis reveals early dysregulation of disease pathways and links Chmp7 to pathogenesis of spinal and bulbar muscular atrophy. Sci. Rep. 2019, 9, 3539. [Google Scholar] [CrossRef]
- Todd, T.W.; Kokubu, H.; Miranda, H.C.; Cortes, C.J.; La Spada, A.R.; Lim, J. Nemo-like kinase is a novel regulator of spinal and bulbar muscular atrophy. Elife 2015, 4, e08493. [Google Scholar] [CrossRef][Green Version]
- Rinaldi, C.; Malik, B.; Greensmith, L. Targeted Molecular Therapies for SBMA. J. Mol. Neurosci. 2016, 58, 335–342. [Google Scholar] [CrossRef]
- Albert-Gascó, H.; Ros-Bernal, F.; Castillo-Gómez, E.; Olucha-Bordonau, F.E. MAP/ERK Signaling in Developing Cognitive and Emotional Function and Its Effect on Pathological and Neurodegenerative Processes. Int. J. Mol. Sci. 2020, 21, 4471. [Google Scholar] [CrossRef]
- Saklatvala, J. The p38 MAP kinase pathway as a therapeutic target in inflammatory disease. Curr. Opin. Pharmacol. 2004, 4, 372–377. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, W.Z.; Liu, T.; Feng, X.; Yang, N.; Zhou, H.F. Signaling pathway of MAPK/ERK in cell proliferation, differentiation, migration, senescence and apoptosis. J. Recept. Signal Transduct. Res. 2015, 35, 600–604. [Google Scholar] [CrossRef]
- Punetha, J.; Monges, S.; Franchi, M.E.; Hoffman, E.P.; Cirak, S.; Tesi-Rocha, C. Exome Sequencing Identifies DYNC1H1 Variant Associated With Vertebral Abnormality and Spinal Muscular Atrophy With Lower Extremity Predominance. Pediatr. Neurol. 2015, 52, 239–244. [Google Scholar] [CrossRef]
- Frasquet, M.; Camacho, A.; Vílchez, R.; Argente-Escrig, H.; Millet, E.; Vázquez-Costa, J.F.; Silla, R.; Sánchez-Monteagudo, A.; Vílchez, J.J.; Espinós, C.; et al. Clinical spectrum of BICD2 mutations. Eur. J. Neurol. 2020, 27, 1327–1335. [Google Scholar] [CrossRef]
- Unger, A.; Roos, A.; Gangfuß, A.; Hentschel, A.; Gläser, D.; Krause, K.; Doering, K.; Schara-Schmidt, U.; Hoffjan, S.; Vorgerd, M.; et al. Microscopic and Biochemical Hallmarks of BICD2-Associated Muscle Pathology toward the Evaluation of Novel Variants. Int. J. Mol. Sci. 2023, 24, 6808. [Google Scholar] [CrossRef]
- León, M.; Prieto, J.; Molina-Navarro, M.M.; García-García, F.; Barneo-Muñoz, M.; Ponsoda, X.; Sáez, R.; Palau, F.; Dopazo, J.; Izpisua Belmonte, J.C.; et al. Rapid degeneration of iPSC-derived motor neurons lacking Gdap1 engages a mitochondrial-sustained innate immune response. Cell Death Discov. 2023, 9, 217. [Google Scholar] [CrossRef] [PubMed]
- Ropert, B.; Gallrein, C.; Schumacher, B. DNA repair deficiencies and neurodegeneration. DNA Repair 2024, 138, 103679. [Google Scholar] [CrossRef] [PubMed]
- Konopka, A.; Atkin, J.D. DNA Damage, Defective DNA Repair, and Neurodegeneration in Amyotrophic Lateral Sclerosis. Front. Aging Neurosci. 2022, 14, 786420. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Kim, D.Y.; Lee, K.H. RNA-Binding Proteins and the Complex Pathophysiology of ALS. Int. J. Mol. Sci. 2021, 22, 2598. [Google Scholar] [CrossRef]
- Kawaguchi, T.; Rollins, M.G.; Moinpour, M.; Morera, A.A.; Ebmeier, C.C.; Old, W.M.; Schwartz, J.C. Changes to the TDP-43 and FUS Interactomes Induced by DNA Damage. J. Proteome Res. 2020, 19, 360–370. [Google Scholar] [CrossRef]
- Stavgiannoudaki, I.; Goulielmaki, E.; Garinis, G.A. Broken strands, broken minds: Exploring the nexus of DNA damage and neurodegeneration. DNA Repair 2024, 140, 103699. [Google Scholar] [CrossRef]
- Kim, B.W.; Jeong, Y.E.; Wong, M.; Martin, L.J. DNA damage accumulates and responses are engaged in human ALS brain and spinal motor neurons and DNA repair is activatable in iPSC-derived motor neurons with SOD1 mutations. Acta Neuropathol. Commun. 2020, 8, 7. [Google Scholar] [CrossRef]
- He, L.; Liang, J.; Chen, C.; Chen, J.; Shen, Y.; Sun, S.; Li, L. C9orf72 functions in the nucleus to regulate DNA damage repair. Cell Death Differ. 2023, 30, 716–730. [Google Scholar] [CrossRef]
- Yu, H.; Ren, K.; Jin, Y.; Zhang, L.; Liu, H.; Huang, Z.; Zhang, Z.; Chen, X.; Yang, Y.; Wei, Z. Mitochondrial DAMPs: Key mediators in neuroinflammation and neurodegenerative disease pathogenesis. Neuropharmacology 2025, 264, 110217. [Google Scholar] [CrossRef]
- Silani, V.; Corcia, P.; Harms, M.B.; Rouleau, G.; Siddique, T.; Ticozzi, N. Genetics of primary lateral sclerosis. Amyotroph. Lateral Scler. Front. Degener. 2020, 21 (Suppl. S1), 28–34. [Google Scholar] [CrossRef]
- Cunha-Oliveira, T.; Montezinho, L.; Mendes, C.; Firuzi, O.; Saso, L.; Oliveira, P.J.; Silva, F.S.G. Oxidative Stress in Amyotrophic Lateral Sclerosis: Pathophysiology and Opportunities for Pharmacological Intervention. Oxid. Med. Cell. Longev. 2020, 2020, 5021694. [Google Scholar] [CrossRef]
- Renvoisé, B.; Chang, J.; Singh, R.; Yonekawa, S.; FitzGibbon, E.J.; Mankodi, A.; Vanderver, A.; Schindler, A.; Toro, C.; Gahl, W.A.; et al. Lysosomal abnormalities in hereditary spastic paraplegia types SPG15 and SPG11. Ann. Clin. Transl. Neurol. 2014, 1, 379–389. [Google Scholar] [CrossRef]
- Brochier, C.; Langley, B. Chromatin modifications associated with DNA double-strand breaks repair as potential targets for neurological diseases. Neurotherapeutics 2013, 10, 817–830. [Google Scholar] [CrossRef]
- Behrouzi, A.; Kelley, M.R.; Fehrenbacher, J.C. Oxidative DNA Damage: A Role in Altering Neuronal Function. J. Cell Signaling 2022, 3, 160–166. [Google Scholar]
- Guenther, U.P.; Handoko, L.; Varon, R.; Stephani, U.; Tsao, C.Y.; Mendell, J.R.; Lützkendorf, S.; Hübner, C.; von Au, K.; Jablonka, S.; et al. Clinical variability in distal spinal muscular atrophy type 1 (DSMA1): Determination of steady-state IGHMBP2 protein levels in five patients with infantile and juvenile disease. J. Mol. Med. 2009, 87, 31–41. [Google Scholar] [CrossRef]
- Nizzardo, M.; Simone, C.; Rizzo, F.; Salani, S.; Dametti, S.; Rinchetti, P.; Del Bo, R.; Foust, K.; Kaspar, B.K.; Bresolin, N.; et al. Gene therapy rescues disease phenotype in a spinal muscular atrophy with respiratory distress type 1 (SMARD1) mouse model. Sci. Adv. 2015, 1, e1500078. [Google Scholar] [CrossRef]
- Perego, M.G.L.; Galli, N.; Nizzardo, M.; Govoni, A.; Taiana, M.; Bresolin, N.; Comi, G.P.; Corti, S. Current understanding of and emerging treatment options for spinal muscular atrophy with respiratory distress type 1 (SMARD1). Cell. Mol. Life Sci. 2020, 77, 3351–3367. [Google Scholar] [CrossRef]
- Meyer, H.; Bug, M.; Bremer, S. Emerging functions of the VCP/p97 AAA-ATPase in the ubiquitin system. Nat. Cell Biol. 2012, 14, 117–123. [Google Scholar] [CrossRef]
- Ju, J.S.; Fuentealba, R.A.; Miller, S.E.; Jackson, E.; Piwnica-Worms, D.; Baloh, R.H.; Weihl, C.C. Valosin-containing protein (VCP) is required for autophagy and is disrupted in VCP disease. J. Cell Biol. 2009, 187, 875–888. [Google Scholar] [CrossRef]
- Salton, M.; Lerenthal, Y.; Wang, S.Y.; Chen, D.J.; Shiloh, Y. Involvement of Matrin 3 and SFPQ/NONO in the DNA damage response. Cell Cycle 2010, 9, 1568–1576. [Google Scholar] [CrossRef] [PubMed]
- Aguilera, A.; García-Muse, T. R loops: From transcription byproducts to threats to genome stability. Mol. Cell 2012, 46, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Kiskinis, E.; Sandoe, J.; Williams, L.A.; Boulting, G.L.; Moccia, R.; Wainger, B.J.; Han, S.; Peng, T.; Thams, S.; Mikkilineni, S.; et al. Pathways disrupted in human ALS motor neurons identified through genetic correction of mutant SOD1. Cell Stem Cell 2014, 14, 781–795. [Google Scholar] [CrossRef]
- Belikov, S.; Bott, L.C.; Fischbeck, K.H.; Wrange, Ö. The polyglutamine-expanded androgen receptor has increased DNA binding and reduced transcriptional activity. Biochem. Biophys. Rep. 2015, 3, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Ranganathan, S.; Fischbeck, K.H. Therapeutic approaches to spinal and bulbar muscular atrophy. Trends Pharmacol. Sci. 2010, 31, 523–527. [Google Scholar] [CrossRef]
- Meerang, M.; Ritz, D.; Paliwal, S.; Garajova, Z.; Bosshard, M.; Mailand, N.; Janscak, P.; Hübscher, U.; Meyer, H.; Ramadan, K. The ubiquitin-selective segregase VCP/p97 orchestrates the response to DNA double-strand breaks. Nat. Cell Biol. 2011, 13, 1376–1382. [Google Scholar] [CrossRef]
- Tresse, E.; Salomons, F.A.; Vesa, J.; Bott, L.C.; Kimonis, V.; Yao, T.P.; Dantuma, N.P.; Taylor, J.P. VCP/p97 is essential for maturation of ubiquitin-containing autophagosomes and this function is impaired by mutations that cause IBMPFD. Autophagy 2010, 6, 217–227. [Google Scholar] [CrossRef]
- Kimonis, V. Inclusion Body Myopathy with Paget Disease of Bone and/or Frontotemporal Dementia. In GeneReviews(®); Adam, M.P., Feldman, J., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 1993. [Google Scholar]
- Huynh, W.; Vale, R.D. Disease-associated mutations in human BICD2 hyperactivate motility of dynein-dynactin. J. Cell Biol. 2017, 216, 3051–3060. [Google Scholar] [CrossRef]
- Malamos, P.; Papanikolaou, C.; Gavriatopoulou, M.; Dimopoulos, M.A.; Terpos, E.; Souliotis, V.L. The Interplay between the DNA Damage Response (DDR) Network and the Mitogen-Activated Protein Kinase (MAPK) Signaling Pathway in Multiple Myeloma. Int. J. Mol. Sci. 2024, 25, 6991. [Google Scholar] [CrossRef]
- Lanz, M.C.; Dibitetto, D.; Smolka, M.B. DNA damage kinase signaling: Checkpoint and repair at 30 years. EMBO J. 2019, 38, e101801. [Google Scholar] [CrossRef]
- Schon, E.A.; Przedborski, S. Mitochondria: The next (neurode)generation. Neuron 2011, 70, 1033–1053. [Google Scholar] [CrossRef]
- Smith, E.F.; Shaw, P.J.; De Vos, K.J. The role of mitochondria in amyotrophic lateral sclerosis. Neurosci. Lett. 2019, 710, 132933. [Google Scholar] [CrossRef]
- Wang, W.; Li, L.; Lin, W.L.; Dickson, D.W.; Petrucelli, L.; Zhang, T.; Wang, X. The ALS disease-associated mutant TDP-43 impairs mitochondrial dynamics and function in motor neurons. Hum. Mol. Genet. 2013, 22, 4706–4719. [Google Scholar] [CrossRef]
- Wang, W.Y.; Pan, L.; Su, S.C.; Quinn, E.J.; Sasaki, M.; Jimenez, J.C.; Mackenzie, I.R.; Huang, E.J.; Tsai, L.H. Interaction of FUS and HDAC1 regulates DNA damage response and repair in neurons. Nat. Neurosci. 2013, 16, 1383–1391. [Google Scholar] [CrossRef]
- Bustamante-Barrientos, F.A.; Luque-Campos, N.; Araya, M.J.; Lara-Barba, E.; de Solminihac, J.; Pradenas, C.; Molina, L.; Herrera-Luna, Y.; Utreras-Mendoza, Y.; Elizondo-Vega, R.; et al. Mitochondrial dysfunction in neurodegenerative disorders: Potential therapeutic application of mitochondrial transfer to central nervous system-residing cells. J. Transl. Med. 2023, 21, 613. [Google Scholar] [CrossRef]
- Shanmughapriya, S.; Langford, D.; Natarajaseenivasan, K. Inter and Intracellular mitochondrial trafficking in health and disease. Ageing Res. Rev. 2020, 62, 101128. [Google Scholar] [CrossRef]
- Vanderhaeghe, S.; Prerad, J.; Tharkeshwar, A.K.; Goethals, E.; Vints, K.; Beckers, J.; Scheveneels, W.; Debroux, E.; Princen, K.; Van Damme, P.; et al. A pathogenic mutation in the ALS/FTD gene VCP induces mitochondrial hypermetabolism by modulating the permeability transition pore. Acta Neuropathol. Commun. 2024, 12, 161. [Google Scholar] [CrossRef] [PubMed]
- Ozdinler, P.H.; Gautam, M.; Gozutok, O.; Konrad, C.; Manfredi, G.; Area Gomez, E.; Mitsumoto, H.; Erb, M.L.; Tian, Z.; Haase, G. Better understanding the neurobiology of primary lateral sclerosis. Amyotroph. Lateral Scler. Front. Degener. 2020, 21 (Suppl. S1), 35–46. [Google Scholar] [CrossRef]
- Obrador, E.; Salvador, R.; López-Blanch, R.; Jihad-Jebbar, A.; Vallés, S.L.; Estrela, J.M. Oxidative Stress, Neuroinflammation and Mitochondria in the Pathophysiology of Amyotrophic Lateral Sclerosis. Antioxidants 2020, 9, 901. [Google Scholar] [CrossRef] [PubMed]
- Votsi, C.; Ververis, A.; Nicolaou, P.; Christou, Y.P.; Christodoulou, K.; Zamba-Papanicolaou, E. A Novel SPG7 Gene Pathogenic Variant in a Cypriot Family With Autosomal Recessive Spastic Ataxia. Front. Genet. 2021, 12, 812640. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Brangulí, F.; Mishra, H.K.; Prots, I.; Havlicek, S.; Kohl, Z.; Saul, D.; Rummel, C.; Dorca-Arevalo, J.; Regensburger, M.; Graef, D.; et al. Dysfunction of spatacsin leads to axonal pathology in SPG11-linked hereditary spastic paraplegia. Hum. Mol. Genet. 2014, 23, 4859–4874. [Google Scholar] [CrossRef] [PubMed]
- Saladini, M.; Nizzardo, M.; Govoni, A.; Taiana, M.; Bresolin, N.; Comi, G.P.; Corti, S. Spinal muscular atrophy with respiratory distress type 1: Clinical phenotypes, molecular pathogenesis and therapeutic insights. J. Cell. Mol. Med. 2020, 24, 1169–1178. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Dhakal, K.; Yi, J. Mitochondrial Ca(2+) uptake in skeletal muscle health and disease. Sci. China Life Sci. 2016, 59, 770–776. [Google Scholar] [CrossRef]
- Bartolome, F.; Wu, H.C.; Burchell, V.S.; Preza, E.; Wray, S.; Mahoney, C.J.; Fox, N.C.; Calvo, A.; Canosa, A.; Moglia, C.; et al. Pathogenic VCP mutations induce mitochondrial uncoupling and reduced ATP levels. Neuron 2013, 78, 57–64. [Google Scholar] [CrossRef]
- Paillusson, S.; Gomez-Suaga, P.; Stoica, R.; Little, D.; Gissen, P.; Devine, M.J.; Noble, W.; Hanger, D.P.; Miller, C.C.J. α-Synuclein binds to the ER-mitochondria tethering protein VAPB to disrupt Ca(2+) homeostasis and mitochondrial ATP production. Acta Neuropathol. 2017, 134, 129–149. [Google Scholar] [CrossRef]
- Zilio, E.; Piano, V.; Wirth, B. Mitochondrial Dysfunction in Spinal Muscular Atrophy. Int. J. Mol. Sci. 2022, 23, 10878. [Google Scholar] [CrossRef]
- Beitel, L.K.; Alvarado, C.; Mokhtar, S.; Paliouras, M.; Trifiro, M. Mechanisms mediating spinal and bulbar muscular atrophy: Investigations into polyglutamine-expanded androgen receptor function and dysfunction. Front. Neurol. 2013, 4, 53. [Google Scholar] [CrossRef]
- Katsuno, M.; Adachi, H.; Kume, A.; Li, M.; Nakagomi, Y.; Niwa, H.; Sang, C.; Kobayashi, Y.; Doyu, M.; Sobue, G. Testosterone reduction prevents phenotypic expression in a transgenic mouse model of spinal and bulbar muscular atrophy. Neuron 2002, 35, 843–854. [Google Scholar] [CrossRef]
- Du, Y.; Wang, J.; Xiong, J.; Fang, N.; Ji, W.K. VPS13D interacts with VCP/p97 and negatively regulates endoplasmic reticulum-mitochondria interactions. Mol. Biol. Cell 2021, 32, 1474–1486. [Google Scholar] [CrossRef]
- Moughamian, A.J.; Holzbaur, E.L. Dynactin is required for transport initiation from the distal axon. Neuron 2012, 74, 331–343. [Google Scholar] [CrossRef]
- Strickland, A.V.; Schabhüttl, M.; Offenbacher, H.; Synofzik, M.; Hauser, N.S.; Brunner-Krainz, M.; Gruber-Sedlmayr, U.; Moore, S.A.; Windhager, R.; Bender, B.; et al. Mutation screen reveals novel variants and expands the phenotypes associated with DYNC1H1. J. Neurol. 2015, 262, 2124–2134. [Google Scholar] [CrossRef]
- Hoogenraad, C.C.; Wulf, P.; Schiefermeier, N.; Stepanova, T.; Galjart, N.; Small, J.V.; Grosveld, F.; de Zeeuw, C.I.; Akhmanova, A. Bicaudal D induces selective dynein-mediated microtubule minus end-directed transport. EMBO J. 2003, 22, 6004–6015. [Google Scholar] [CrossRef]
- Kodavati, M.; Wang, H.; Hegde, M.L. Altered Mitochondrial Dynamics in Motor Neuron Disease: An Emerging Perspective. Cells 2020, 9, 1065. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.R.; Wu, T.; Chen, T.H.; Wang, Y. Neuroimmune interactions and their roles in neurodegenerative diseases. Fundam. Res. 2024, 4, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Ferraiuolo, L.; Kirby, J.; Grierson, A.J.; Sendtner, M.; Shaw, P.J. Molecular pathways of motor neuron injury in amyotrophic lateral sclerosis. Nat. Rev. Neurol. 2011, 7, 616–630. [Google Scholar] [CrossRef] [PubMed]
- Henkel, J.S.; Beers, D.R.; Wen, S.; Rivera, A.L.; Toennis, K.M.; Appel, J.E.; Zhao, W.; Moore, D.H.; Powell, S.Z.; Appel, S.H. Regulatory T-lymphocytes mediate amyotrophic lateral sclerosis progression and survival. EMBO Mol. Med. 2013, 5, 64–79. [Google Scholar] [CrossRef]
- McCauley, M.E.; Baloh, R.H. Inflammation in ALS/FTD pathogenesis. Acta Neuropathol. 2019, 137, 715–730. [Google Scholar] [CrossRef]
- Philips, T.; Robberecht, W. Neuroinflammation in amyotrophic lateral sclerosis: Role of glial activation in motor neuron disease. Lancet Neurol. 2011, 10, 253–263. [Google Scholar] [CrossRef]
- Hiew, J.Y.; Lim, Y.S.; Liu, H.; Ng, C.S. Integrated transcriptomic profiling reveals a STING-mediated Type II Interferon signature in SOD1-mutant amyotrophic lateral sclerosis models. Commun. Biol. 2025, 8, 347. [Google Scholar] [CrossRef]
- Rossi, D.; Volterra, A. Astrocytic dysfunction: Insights on the role in neurodegeneration. Brain Res. Bull. 2009, 80, 224–232. [Google Scholar] [CrossRef]
- Paganoni, S.; Alshikho, M.J.; Zürcher, N.R.; Cernasov, P.; Babu, S.; Loggia, M.L.; Chan, J.; Chonde, D.B.; Garcia, D.I.; Catana, C.; et al. Imaging of glia activation in people with primary lateral sclerosis. Neuroimage Clin. 2018, 17, 347–353. [Google Scholar] [CrossRef]
- Frakes, A.E.; Ferraiuolo, L.; Haidet-Phillips, A.M.; Schmelzer, L.; Braun, L.; Miranda, C.J.; Ladner, K.J.; Bevan, A.K.; Foust, K.D.; Godbout, J.P.; et al. Microglia induce motor neuron death via the classical NF-κB pathway in amyotrophic lateral sclerosis. Neuron 2014, 81, 1009–1023. [Google Scholar] [CrossRef]
- Mahmoud, S.; Gharagozloo, M.; Simard, C.; Gris, D. Astrocytes Maintain Glutamate Homeostasis in the CNS by Controlling the Balance between Glutamate Uptake and Release. Cells 2019, 8, 184. [Google Scholar] [CrossRef]
- Toupenet Marchesi, L.; Leblanc, M.; Stevanin, G. Current Knowledge of Endolysosomal and Autophagy Defects in Hereditary Spastic Paraplegia. Cells 2021, 10, 1678. [Google Scholar] [CrossRef] [PubMed]
- Goutman, S.A.; Hardiman, O.; Al-Chalabi, A.; Chió, A.; Savelieff, M.G.; Kiernan, M.C.; Feldman, E.L. Emerging insights into the complex genetics and pathophysiology of amyotrophic lateral sclerosis. Lancet Neurol. 2022, 21, 465–479. [Google Scholar] [CrossRef] [PubMed]
- Sansa, A.; Miralles, M.P.; Beltran, M.; Celma-Nos, F.; Calderó, J.; Garcera, A.; Soler, R.M. ERK MAPK signaling pathway inhibition as a potential target to prevent autophagy alterations in Spinal Muscular Atrophy motoneurons. Cell Death Discov. 2023, 9, 113. [Google Scholar] [CrossRef] [PubMed]
- Korn, T.; Kallies, A. T cell responses in the central nervous system. Nat. Rev. Immunol. 2017, 17, 179–194. [Google Scholar] [CrossRef]
- Cortes, C.J.; Ling, S.C.; Guo, L.T.; Hung, G.; Tsunemi, T.; Ly, L.; Tokunaga, S.; Lopez, E.; Sopher, B.L.; Bennett, C.F.; et al. Muscle expression of mutant androgen receptor accounts for systemic and motor neuron disease phenotypes in spinal and bulbar muscular atrophy. Neuron 2014, 82, 295–307. [Google Scholar] [CrossRef]
- Palazzolo, I.; Burnett, B.G.; Young, J.E.; Brenne, P.L.; La Spada, A.R.; Fischbeck, K.H.; Howell, B.W.; Pennuto, M. Akt blocks ligand binding and protects against expanded polyglutamine androgen receptor toxicity. Hum. Mol. Genet. 2007, 16, 1593–1603. [Google Scholar] [CrossRef]
- Sharma, B.; Dabur, R. Role of Pro-inflammatory Cytokines in Regulation of Skeletal Muscle Metabolism: A Systematic Review. Curr. Med. Chem. 2020, 27, 2161–2188. [Google Scholar] [CrossRef]
- Lai, J.J.; Lai, K.P.; Zeng, W.; Chuang, K.H.; Altuwaijri, S.; Chang, C. Androgen receptor influences on body defense system via modulation of innate and adaptive immune systems: Lessons from conditional AR knockout mice. Am. J. Pathol. 2012, 181, 1504–1512. [Google Scholar] [CrossRef]
- Korb, M.K.; Kimonis, V.E.; Mozaffar, T. Multisystem proteinopathy: Where myopathy and motor neuron disease converge. Muscle Nerve 2021, 63, 442–454. [Google Scholar] [CrossRef] [PubMed]
- Rossor, A.M.; Sleigh, J.N.; Groves, M.; Muntoni, F.; Reilly, M.M.; Hoogenraad, C.C.; Schiavo, G. Loss of BICD2 in muscle drives motor neuron loss in a developmental form of spinal muscular atrophy. Acta Neuropathol. Commun. 2020, 8, 34. [Google Scholar] [CrossRef] [PubMed]
- Hafezparast, M.; Klocke, R.; Ruhrberg, C.; Marquardt, A.; Ahmad-Annuar, A.; Bowen, S.; Lalli, G.; Witherden, A.S.; Hummerich, H.; Nicholson, S.; et al. Mutations in dynein link motor neuron degeneration to defects in retrograde transport. Science 2003, 300, 808–812. [Google Scholar] [CrossRef] [PubMed]
- LaMonte, B.H.; Wallace, K.E.; Holloway, B.A.; Shelly, S.S.; Ascaño, J.; Tokito, M.; Van Winkle, T.; Howland, D.S.; Holzbaur, E.L.F. Disruption of Dynein/Dynactin Inhibits Axonal Transport in Motor Neurons Causing Late-Onset Progressive Degeneration. Neuron 2002, 34, 715–727. [Google Scholar] [CrossRef]
- Castro-Gomez, S.; Heneka, M.T. Innate immune activation in neurodegenerative diseases. Immunity 2024, 57, 790–814. [Google Scholar] [CrossRef]
- Carpentier, P.A.; Palmer, T.D. Immune Influence on Adult Neural Stem Cell Regulation and Function. Neuron 2009, 64, 79–92. [Google Scholar] [CrossRef]
- Mercuri, E.; Darras, B.T.; Chiriboga, C.A.; Day, J.W.; Campbell, C.; Connolly, A.M.; Iannaccone, S.T.; Kirschner, J.; Kuntz, N.L.; Saito, K.; et al. Nusinersen versus Sham Control in Later-Onset Spinal Muscular Atrophy. N. Engl. J. Med. 2018, 378, 625–635. [Google Scholar] [CrossRef]
- Everett, W.H.; Bucelli, R.C. Tofersen for SOD1 ALS. Neurodegener. Dis. Manag. 2024, 14, 149–160. [Google Scholar] [CrossRef]
- Ayala, Y.M.; Nguyen, A.D. RNA-Based Therapies for Neurodegenerative Diseases. Mo. Med. 2021, 118, 340–345. [Google Scholar]
- Huang, S.; Hao, X.Y.; Li, Y.J.; Wu, J.Y.; Xiang, D.X.; Luo, S. Nonviral delivery systems for antisense oligonucleotide therapeutics. Biomater. Res. 2022, 26, 49. [Google Scholar] [CrossRef] [PubMed]
- Menzies, F.M.; Fleming, A.; Caricasole, A.; Bento, C.F.; Andrews, S.P.; Ashkenazi, A.; Füllgrabe, J.; Jackson, A.; Jimenez Sanchez, M.; Karabiyik, C.; et al. Autophagy and Neurodegeneration: Pathogenic Mechanisms and Therapeutic Opportunities. Neuron 2017, 93, 1015–1034. [Google Scholar] [CrossRef] [PubMed]
- Chernega, T.; Choi, J.; Salmena, L.; Andreazza, A.C. Mitochondrion-targeted RNA therapies as a potential treatment strategy for mitochondrial diseases. Mol. Ther. Nucleic Acids 2022, 30, 359–377. [Google Scholar] [CrossRef] [PubMed]
- Steele, H.; Gomez-Duran, A.; Pyle, A.; Hopton, S.; Newman, J.; Stefanetti, R.J.; Charman, S.J.; Parikh, J.D.; He, L.; Viscomi, C.; et al. Metabolic effects of bezafibrate in mitochondrial disease. EMBO Mol. Med. 2020, 12, e11589. [Google Scholar] [CrossRef]
- Harding, E.C.; Chen, H.-J.C.; Shepilov, D.; Zhang, S.O.; Rowley, C.; Mali, I.; Chen, J.; Stewart, N.; Swinden, D.; Washer, S.J.; et al. Unbiased preclinical phenotyping reveals neuroprotective properties of pioglitazone. bioRxiv 2024. [Google Scholar] [CrossRef]
- Duda, P.; Wiśniewski, J.; Wójtowicz, T.; Wójcicka, O.; Jaśkiewicz, M.; Drulis-Fajdasz, D.; Rakus, D.; McCubrey, J.A.; Gizak, A. Targeting GSK3 signaling as a potential therapy of neurodegenerative diseases and aging. Expert Opin. Ther. Targets 2018, 22, 833–848. [Google Scholar] [CrossRef]
- Sousa, C.; Golebiewska, A.; Poovathingal, S.K.; Kaoma, T.; Pires-Afonso, Y.; Martina, S.; Coowar, D.; Azuaje, F.; Skupin, A.; Balling, R.; et al. Single-cell transcriptomics reveals distinct inflammation-induced microglia signatures. EMBO Rep. 2018, 19, e46171. [Google Scholar] [CrossRef]
- Guo, N.; Huang, W.; Huang, J.; Liu, Y.; Zhu, K.; Gao, W. Global research trends in biomarkers, therapeutic targets, and drugs for amyotrophic lateral sclerosis: A bibliometric and visualization analysis. Front. Pharmacol. 2025, 16, 1588968. [Google Scholar] [CrossRef]
- uniQure. uniQure Announces Favorable Recommendation from Independent Data Monitoring Committee for Its Phase I/II EPISOD1 Clinical Trial of AMT-162 for the Treatment of SOD1-ALS. Available online: https://uniqure.gcs-web.com/news-releases/news-release-details/uniqure-announces-favorable-recommendation-independent-data (accessed on 30 January 2025).
- Spinogenix. Spinogenix Announces FDA Clearance of IND Application for SPG302, a Novel Therapy for the Treatment of ALS. Available online: https://www.spinogenix.com/spinogenix-announces-fda-authorized-expanded-access-programfor-spg302-the-first-synaptic-regenerative-therapy-to-treat-als/ (accessed on 5 May 2025).
- Gerometta, M.; Henderson, R.D.; Friend, R.; Cooper, L.T.; Zhao, J.; Boyd, A.W.; Bartlett, P.F. Evaluation of NUN-004, a Novel Engineered Ephrin Antagonist, in Healthy Volunteers and Patients with Amyotrophic Lateral Sclerosis: A Phase I/Ib, Open-Label, Escalating Dose and Extended Access Study. Clin. Drug Investig. 2025, 45, 17–28. [Google Scholar] [CrossRef]
- Wu, Y.F.; Chen, J.A.; Jong, Y.J. Treating neuromuscular diseases: Unveiling gene therapy breakthroughs and pioneering future applications. J. Biomed. Sci. 2025, 32, 30. [Google Scholar] [CrossRef]
- Byrne, B.J.; Flanigan, K.M.; Matesanz, S.E.; Finkel, R.S.; Waldrop, M.A.; D’Ambrosio, E.S.; Johnson, N.E.; Smith, B.K.; Bönnemann, C.; Carrig, S.; et al. Current clinical applications of AAV-mediated gene therapy. Mol. Ther. 2025, 33, 2479–2516. [Google Scholar] [CrossRef]
- Cristofani, R.; Tedesco, B.; Ferrari, V.; Chierichetti, M.; Cozzi, M.; Pramaggiore, P.; Cornaggia, L.; Mohamed, A.; Casarotto, E.; Brodnanova, M.; et al. Targeting androgen receptor stability and degradation: Approaches for developing a therapy for spinal and bulbar muscular atrophy. Cell Commun. Signal. 2025, 23, 344. [Google Scholar] [CrossRef] [PubMed]
- NeurologyLive. AJ201 Demonstrates Promising Phase 1/2 Results in Spinal and Bulbar Muscular Atrophy. Available online: https://www.neurologylive.com/view/aj201-demonstrates-promising-phase-1-2-results-spinal-bulbar-muscular-atrophy (accessed on 4 June 2025).
- Reash, N.; Iammarino, M.; Pietruszewski, L.; Lowes, L.; Mendell, J.; Connolly, A.; Adderley, K.; Peck, N.; Peck, A.; Alfano, L. O06 Clinical trial readiness and validation of onsite and remote evaluation in valosin-containing protein-associated multisystem proteinopathy: A 24-month longitudinal study. Neuromuscul. Disord. 2023, 33, S68. [Google Scholar] [CrossRef]
- Vantaggiato, C.; Guarato, G.; Brivio, F.; Panzeri, E.; Speltoni, B.; Gumeni, S.; Orso, G.; Santorelli, F.M.; Bassi, M.T. Naringenin and SMER28 target lysosomal reformation and rescue SPG11 and SPG15 hereditary spastic paraplegia phenotypes. Pharmacol. Res. 2025, 218, 107836. [Google Scholar] [CrossRef]
- Lemmens, R.; Moore, M.J.; Al-Chalabi, A.; Brown, R.H., Jr.; Robberecht, W. RNA metabolism and the pathogenesis of motor neuron diseases. Trends Neurosci. 2010, 33, 249–258. [Google Scholar] [CrossRef]
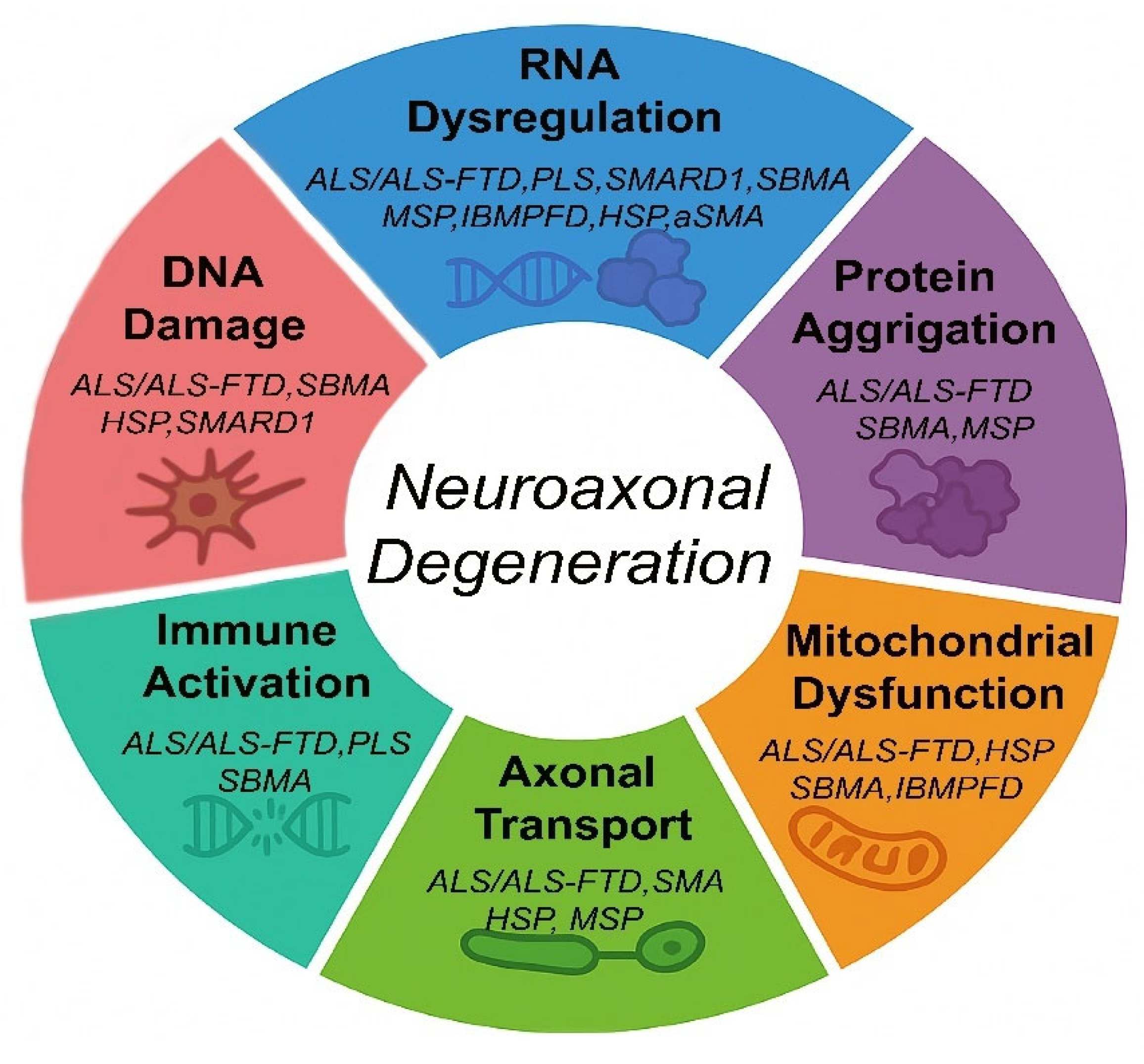
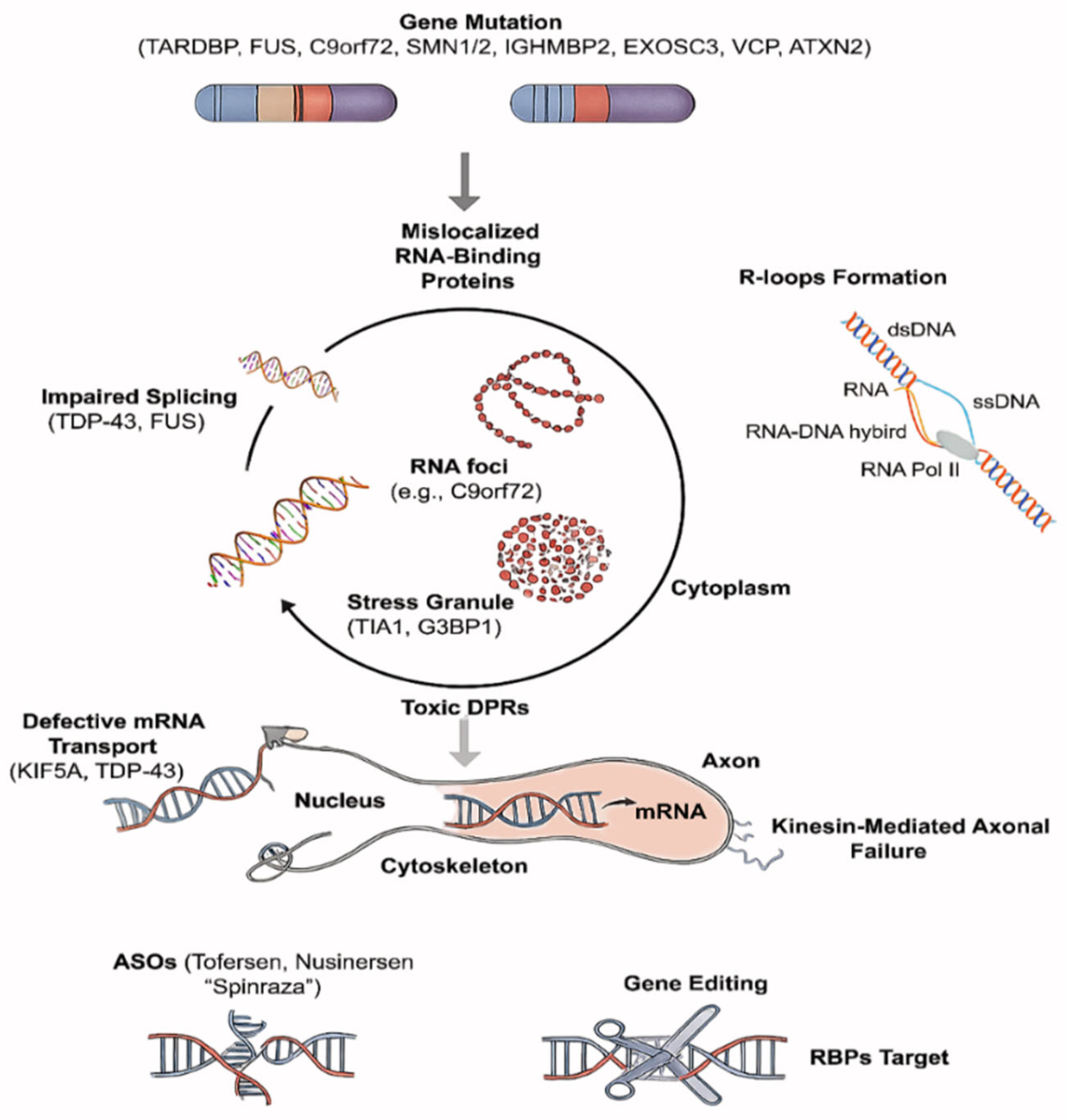
| Mechanism | Diseases | Molecular Features |
|---|---|---|
| RNA Dysregulation | ALS-FTD, SMARD1 | TDP-43, IGHMBP2, EXOSC3 |
| Protein Misfolding | SBMA, MSP, ALS | AR-polyQ, VCP, TDP-43 |
| Mitochondrial Dysfunction | HSP, SMA, ALS | SPG7, DYNC1H1, SOD1 |
| Kinase Signaling Defects | ALS, SBMA | p38, AKT, ERK dysregulation |
| Axonal Transport Failure | SMA, ALS-FTD | DYNC1H1, BICD2, IGHMBP2 |
| Disease | RNA Dysregulation | Protein Misfolding/Aggregation | Axonal Transport Defects | Mitochondrial Dysfunction | Glial/T Cell Immune Involvement |
|---|---|---|---|---|---|
| ALS-FTD spectrum | TDP-43 mislocalization, FUS mutations, C9orf72 RNA foci | TDP-43, SOD1, FUS aggregates | KIF5A, TUBA4A, C9orf72 affect transport | SOD1, C9orf72, and CHCHD10 impair ETC and calcium buffering | Astrocyte dysfunction, T cell infiltration, microgliosis |
| PLS | Shared RBP changes with ALS (e.g., TDP-43 pathology) | Less prominent, but misfolded neurofilaments may occur | Degeneration of long CST axons, slow axoplasmic flow | Emerging data suggest mild mitochondrial stress | Cortical microglia activation: immune transcripts upregulated |
| HSP (SPG11, SPG7) | SPG11 affects RNA metabolism, with potential spliceosome involvement | SPG11 may secondarily cause proteostasis stress | SPG11 affects autophagosomes and cargo delivery | SPG7 encodes mitochondrial protease; SPG11 impacts lysosome–mitochondria axis | Spastic paraplegia with variable neuroinflammation |
| SMARD1 | IGHMBP2 mutation disrupts RNA helicase activity, and mRNA decay | IGHMBP2 loss leads to stalled ribosome-associated protein aggregates | Disrupted ribosome transport and NMJ targeting | Energy failure from ribosomal/mitochondrial collapse | Early microglial priming in the spinal cord: interferon signatures |
| MSP (VCP, HNRNPA1/2B1) | Mutant HNRNPA1/2B1 disrupts RNP granules, RNA export | VCP and RBPs form cytoplasmic inclusions | VCP mutations impair dynein-dependent cargo transport | Impaired mitophagy and ATP supply in VCP mutants | CNS inflammation, T cell-mediated degeneration |
| SBMA (Kennedy’s Disease) | AR polyQ expansion interferes with splicing and RBP dynamics | Misfolded AR protein aggregates in nuclei and cytoplasm | Impaired AR nuclear shuttling affects retrograde signaling | AR aggregates impair mitochondrial membrane integrity | Androgen-linked immunomodulation; possible glial stress |
| IBMPFD (VCP) | VCP impacts RNA surveillance and stress granule resolution | VCP-associated aggregates disrupt proteostasis | Autophagy and organelle trafficking are disrupted | Mitochondrial clustering and UPR activation in muscle and neurons | Neuroinflammation and glial reactivity in cortex and muscle |
| Diseases | Prion-Like Proteins/Aggregates | Mechanistic Implications |
|---|---|---|
| ALS | TDP-43, SOD1, FUS, C9orf72-associated DPRs | Template-directed misfolding, propagation via axons, and extracellular vesicles |
| PLS | TDP-43 (shared pathology with ALS), potential RBP granules | May amplify UMN pathology via chronic stress granule persistence |
| HSP (SPG11, SPG7) | SPG11-linked spatacsin may impair granule clearance, promoting indirect RBP aggregation | Likely contributes to proteostasis collapse in advanced HSP forms |
| SMARD1 | Ribosome-associated stalling may promote noncanonical RNP aggregation | Stalled RNA–protein complexes may trigger aggregation |
| MSP (VCP, HNRNPA1/2B1) | Cytoplasmic stress granules with prion-like domains (hnRNPA1, hnRNPA2B1, VCP) | Self-propagating RBPs induce degeneration across motor neuron pools |
| SBMA (Kennedy’s Disease) | PolyQ-expanded AR forms nuclear/cytoplasmic inclusions with seeding capacity | Nuclear AR inclusions recruit splicing factors and disrupt proteostasis |
| IBMPFD (VCP) | VCP-linked aggregates exhibit prion-like spreading behavior in muscle and brain | Disruption of UPS and autophagy enables aggregate accumulation and spread |
| Adult-onset SMA (BICD2, DYNC1H1) | Not classical prion-like; possible cytoskeletal or transport-linked misfolding stress | Limited evidence suggests that aggregation may occur secondary to axonal transport stress |
| Disease | Therapeutic Strategies | Clinical Trial Status | NfL as Biomarker | Disease Detection Method |
|---|---|---|---|---|
| ALS | Riluzole, Edaravone, Tofersen, AMX0035 | Multiple Phase III trials, NfL endpoint | Validated in serum/CSF; prognostic marker | Clinical exam, EMG, MRI, genetic testing (SOD1, C9orf72) |
| PLS | Spasticity relief, Riluzole, neuroimaging biomarkers | Small observational studies, Riluzole trials | Elevated but lower than ALS; progression tracking | UMN signs on clinical exam, TMS, diffusion tensor imaging (DTI) |
| HSP (SPG11, SPG7) | Spasticity meds, mTOR/HDAC inhibitors, autophagy enhancers | Limited trials; EU registries (SPATAX) | Mildly elevated in complex cases | Spastic gait, family history, genetic panels, brain/spinal MRI |
| SMARD1 | Gene therapy, ASOs, and ventilation support | Gene therapy in preclinical and early human use | Correlates with axonal loss; used in models | Neonatal hypotonia, phrenic nerve EMG, IGHMBP2 gene testing |
| MSP (VCP, HNRNPA1/2B1) | Proteostasis modulation, autophagy inducers | Emerging early-phase and biomarker trials. | Elevated in ALS-like forms; correlates with decline | Muscle biopsy, genetic testing (VCP, hnRNPA genes), family history |
| SBMA (Kennedy’s Disease) | Anti-androgens, ASOs, mitochondrial protectants | Phase III Leuprorelin completed; ASOs under study | Low/modest; progression marker in subtypes | Genetic confirmation of AR gene CAG repeat; EMG and hormonal profile |
| IBMPFD (VCP) | Autophagy and proteostasis targets, immunomodulators | Trial preparation underway | Elevated in cognitive/motor cases | Clinical triad (myopathy, Paget disease, FTD), VCP mutation testing |
| Adult-onset SMA (BICD2, DYNC1H1) | Transport modulators, ER-mitochondrial therapies | No clinical trials; iPSC modelling. | Potential marker, preclinical interest | Axonal neuropathy on EMG, genetic analysis (BICD2, DYNC1H1) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharbafshaaer, M.; Pepe, R.; Notariale, R.; Canale, F.; Tessitore, A.; Tedeschi, G.; Trojsi, F. Neuroaxonal Degeneration as a Converging Mechanism in Motor Neuron Diseases (MNDs): Molecular Insights into RNA Dysregulation and Emerging Therapeutic Targets. Int. J. Mol. Sci. 2025, 26, 7644. https://doi.org/10.3390/ijms26157644
Sharbafshaaer M, Pepe R, Notariale R, Canale F, Tessitore A, Tedeschi G, Trojsi F. Neuroaxonal Degeneration as a Converging Mechanism in Motor Neuron Diseases (MNDs): Molecular Insights into RNA Dysregulation and Emerging Therapeutic Targets. International Journal of Molecular Sciences. 2025; 26(15):7644. https://doi.org/10.3390/ijms26157644
Chicago/Turabian StyleSharbafshaaer, Minoo, Roberta Pepe, Rosaria Notariale, Fabrizio Canale, Alessandro Tessitore, Gioacchino Tedeschi, and Francesca Trojsi. 2025. "Neuroaxonal Degeneration as a Converging Mechanism in Motor Neuron Diseases (MNDs): Molecular Insights into RNA Dysregulation and Emerging Therapeutic Targets" International Journal of Molecular Sciences 26, no. 15: 7644. https://doi.org/10.3390/ijms26157644
APA StyleSharbafshaaer, M., Pepe, R., Notariale, R., Canale, F., Tessitore, A., Tedeschi, G., & Trojsi, F. (2025). Neuroaxonal Degeneration as a Converging Mechanism in Motor Neuron Diseases (MNDs): Molecular Insights into RNA Dysregulation and Emerging Therapeutic Targets. International Journal of Molecular Sciences, 26(15), 7644. https://doi.org/10.3390/ijms26157644










