Apolipoprotein D Expression Dynamics During Cuprizone-Induced Demyelination and Remyelination in a Mouse Model of Multiple Sclerosis
Abstract
1. Introduction
2. Results
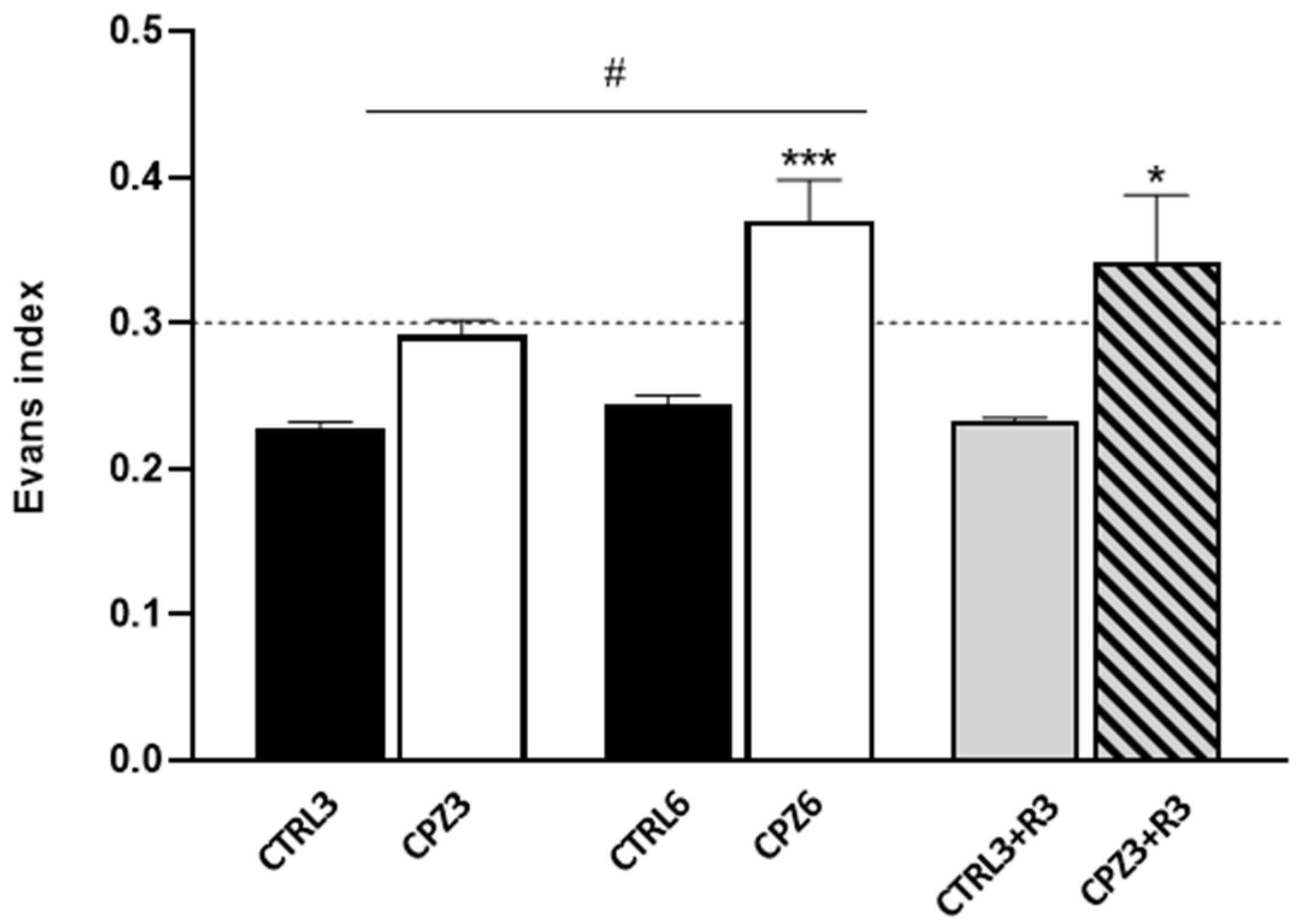
2.1. Cuprizone Administration Induces Demyelination and Other Brain Alterations, Which Are Reversed upon Treatment Discontinuation
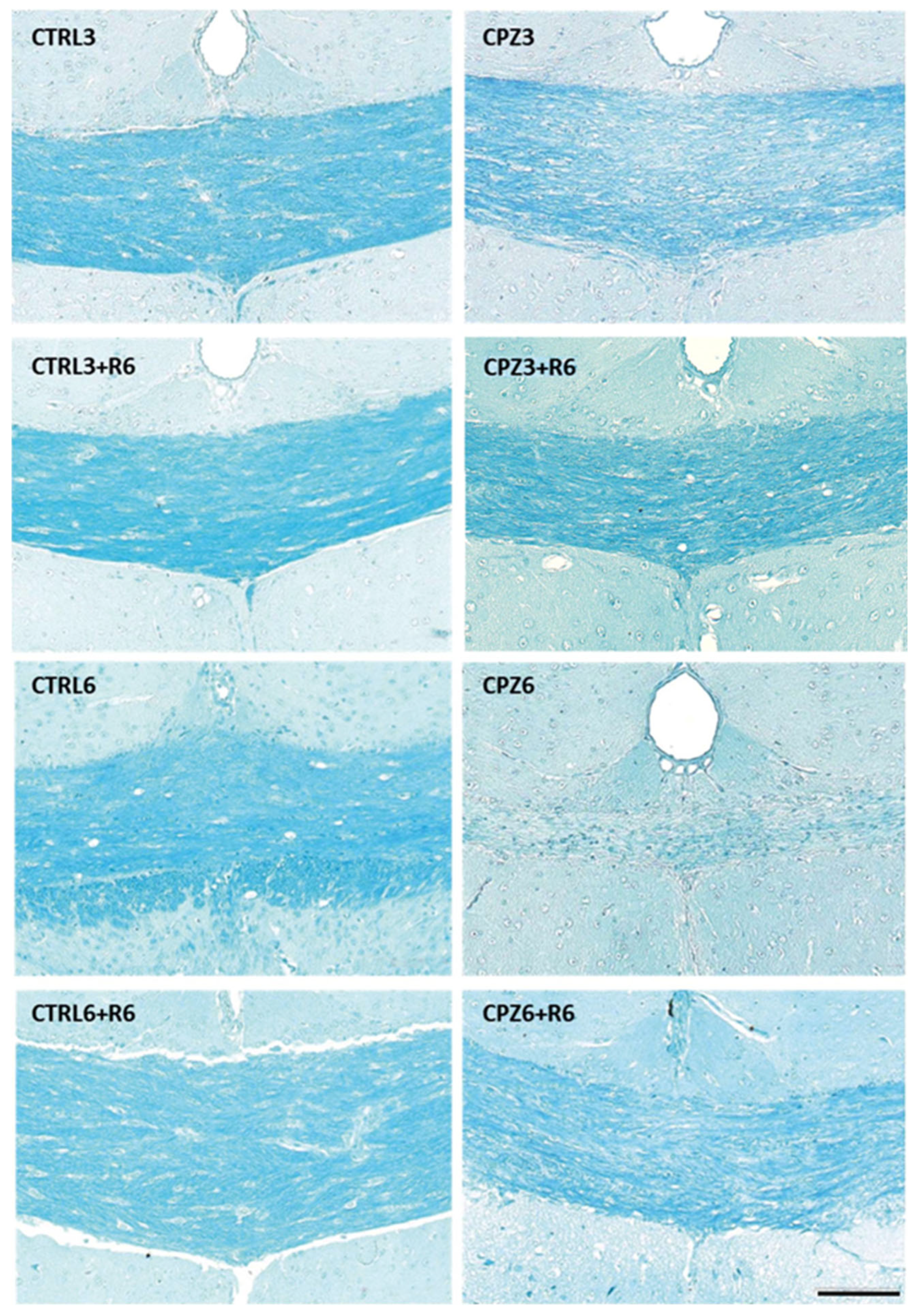
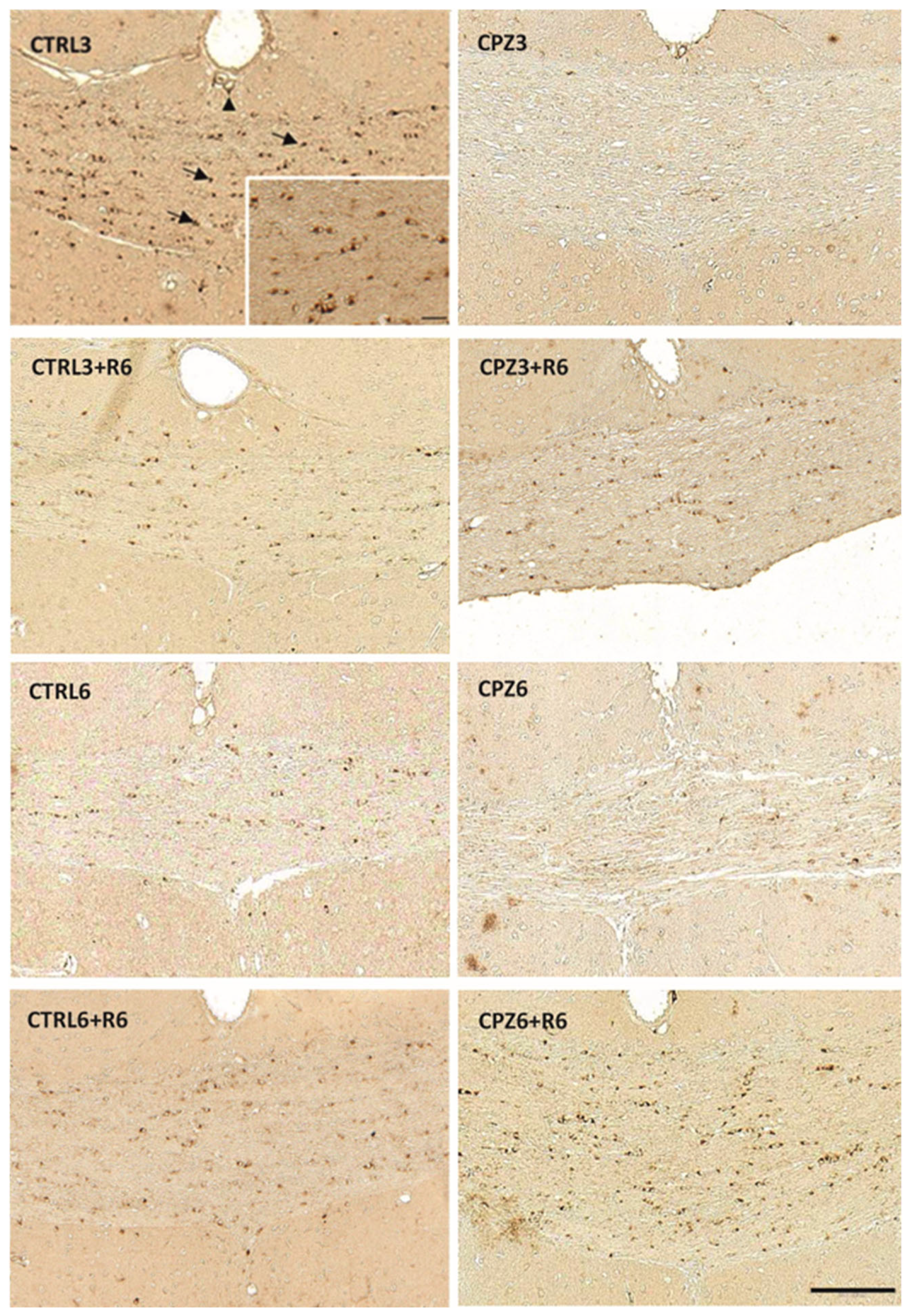
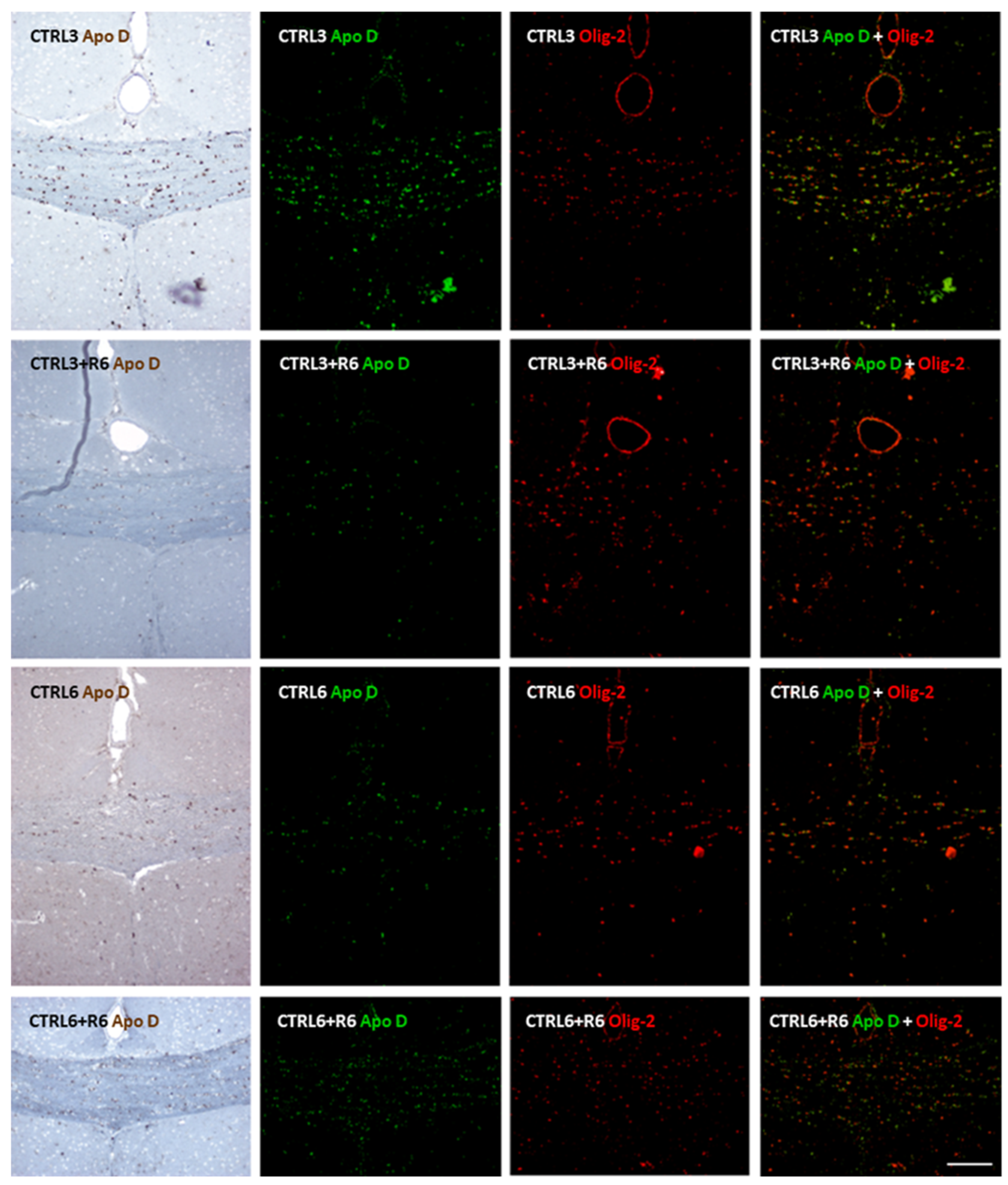
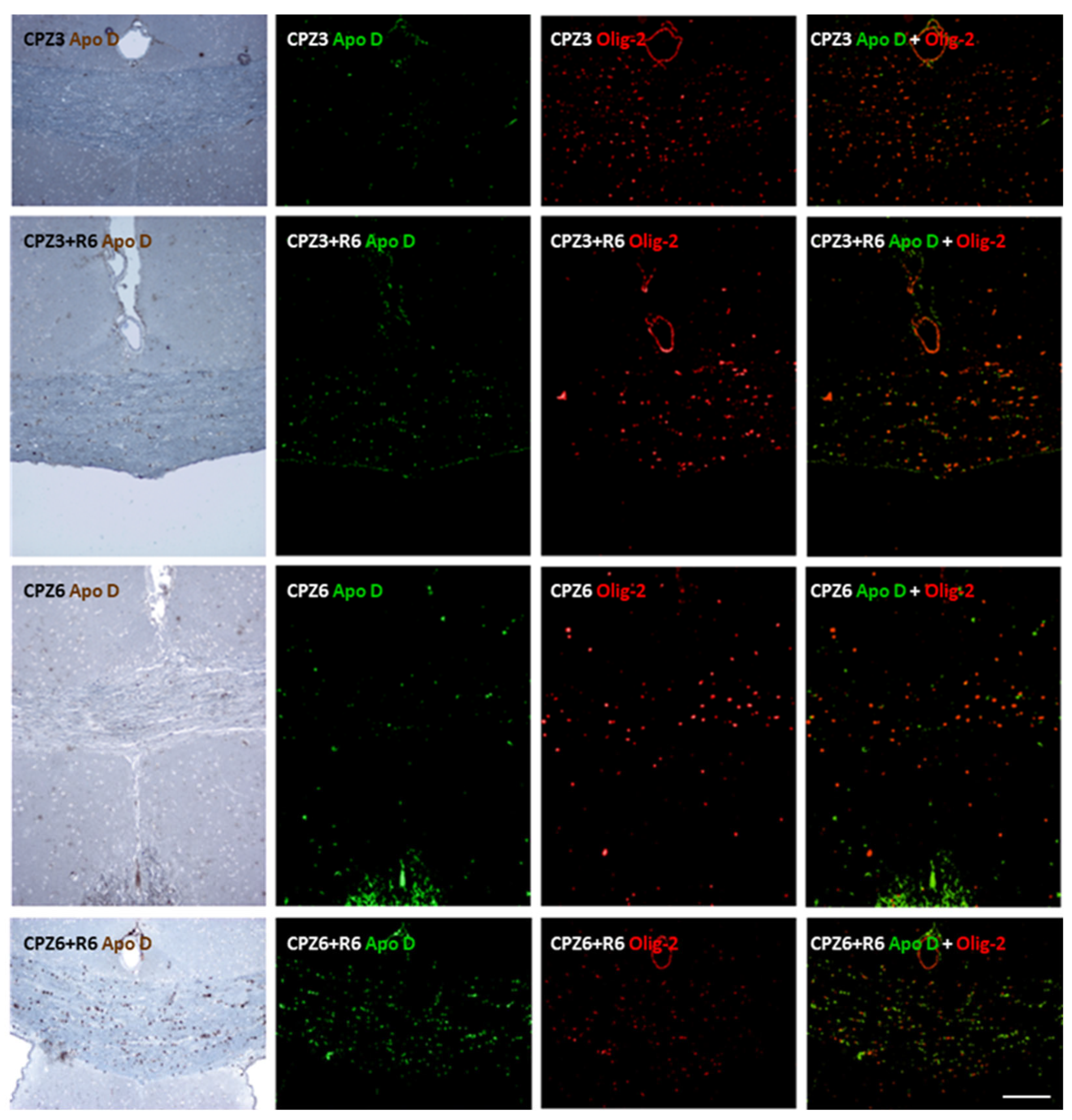
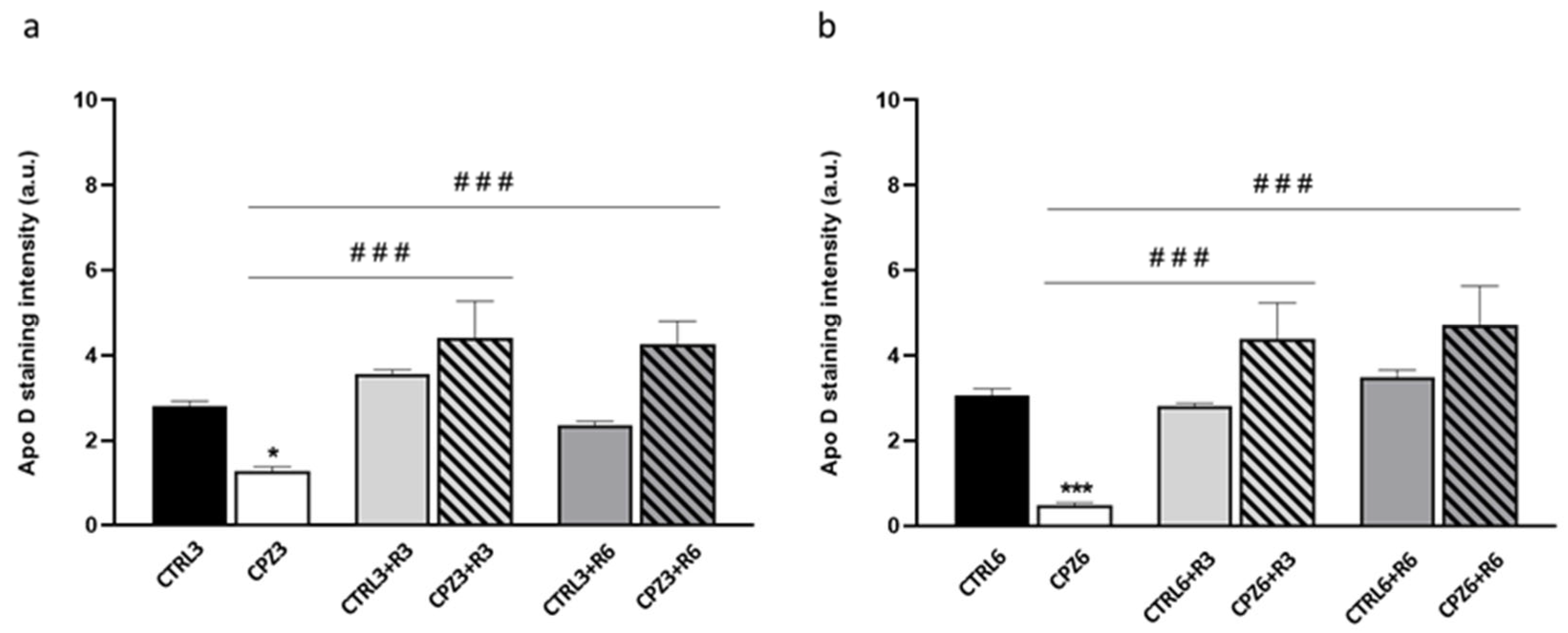
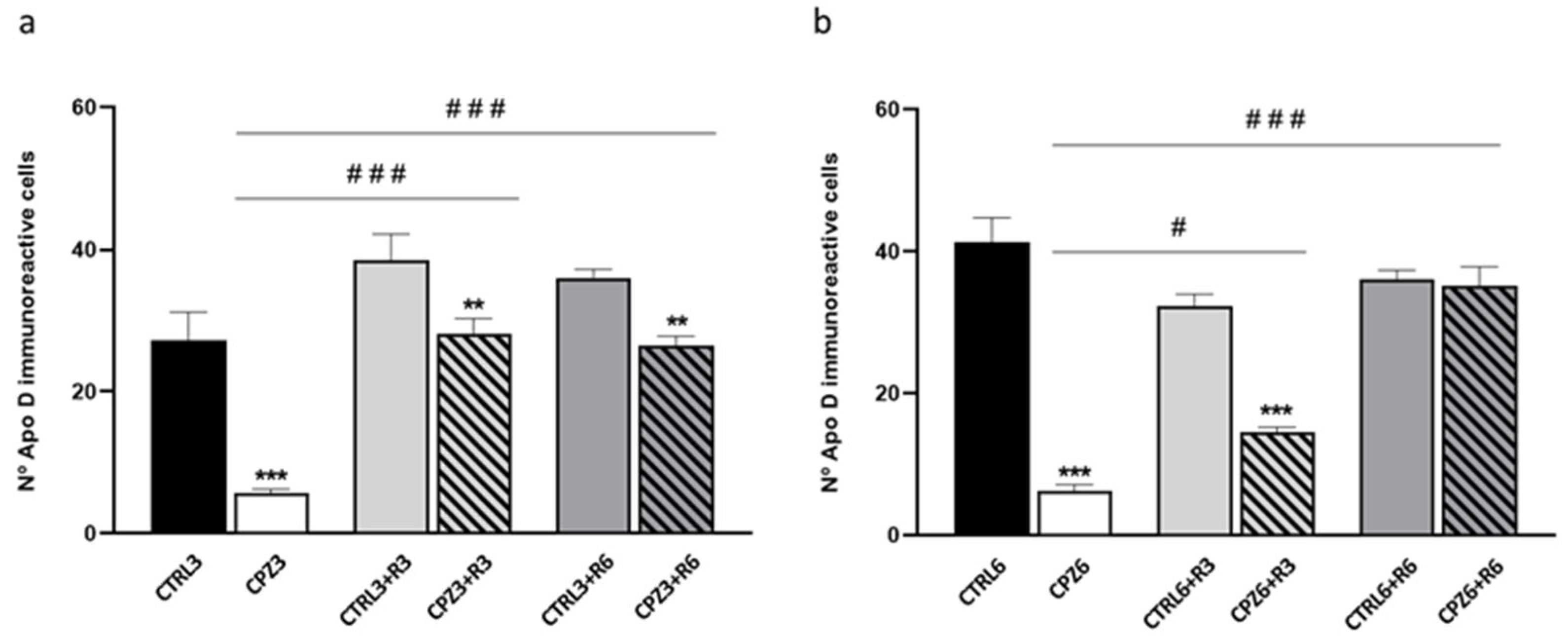
2.2. Cuprizone Administration Reduces Apolipoprotein D Expression in the Corpus Callosum, Which Recovers After Treatment Discontinuation
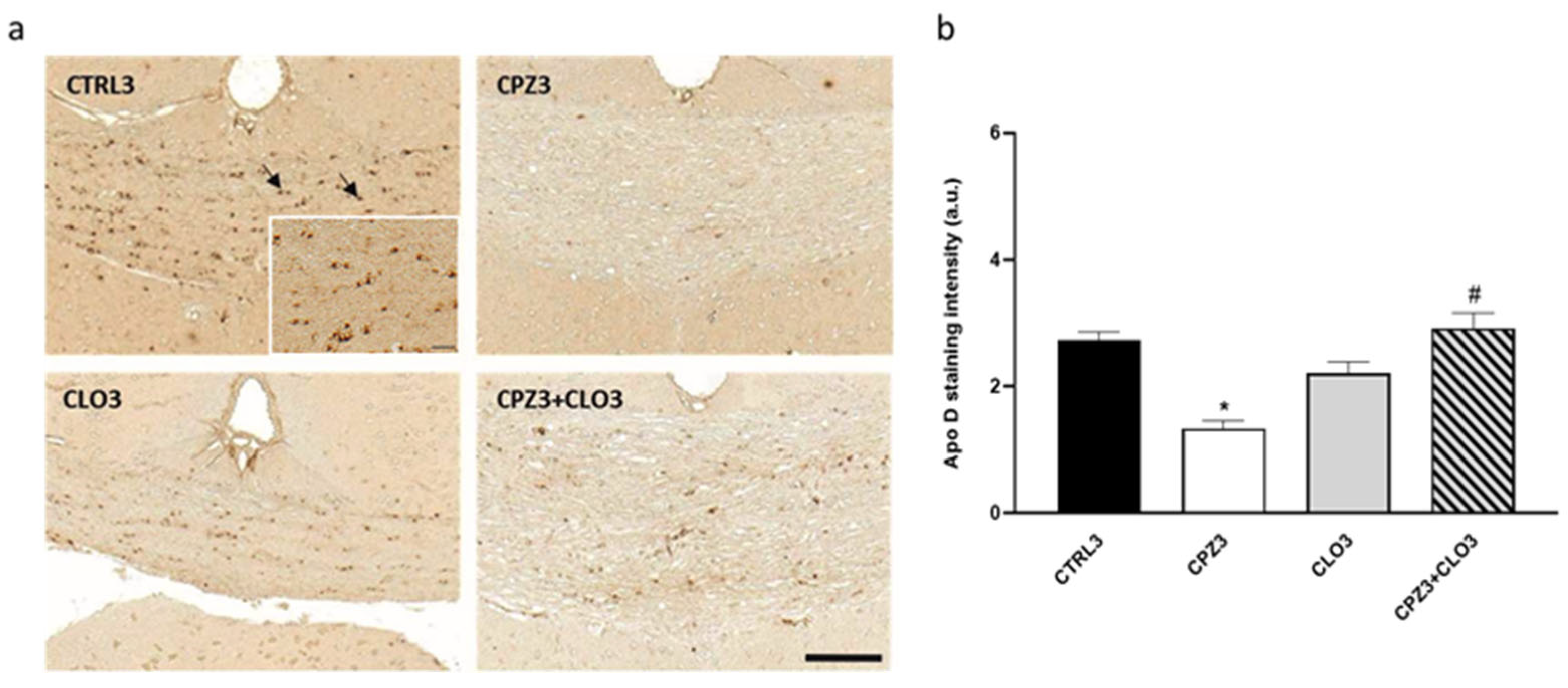
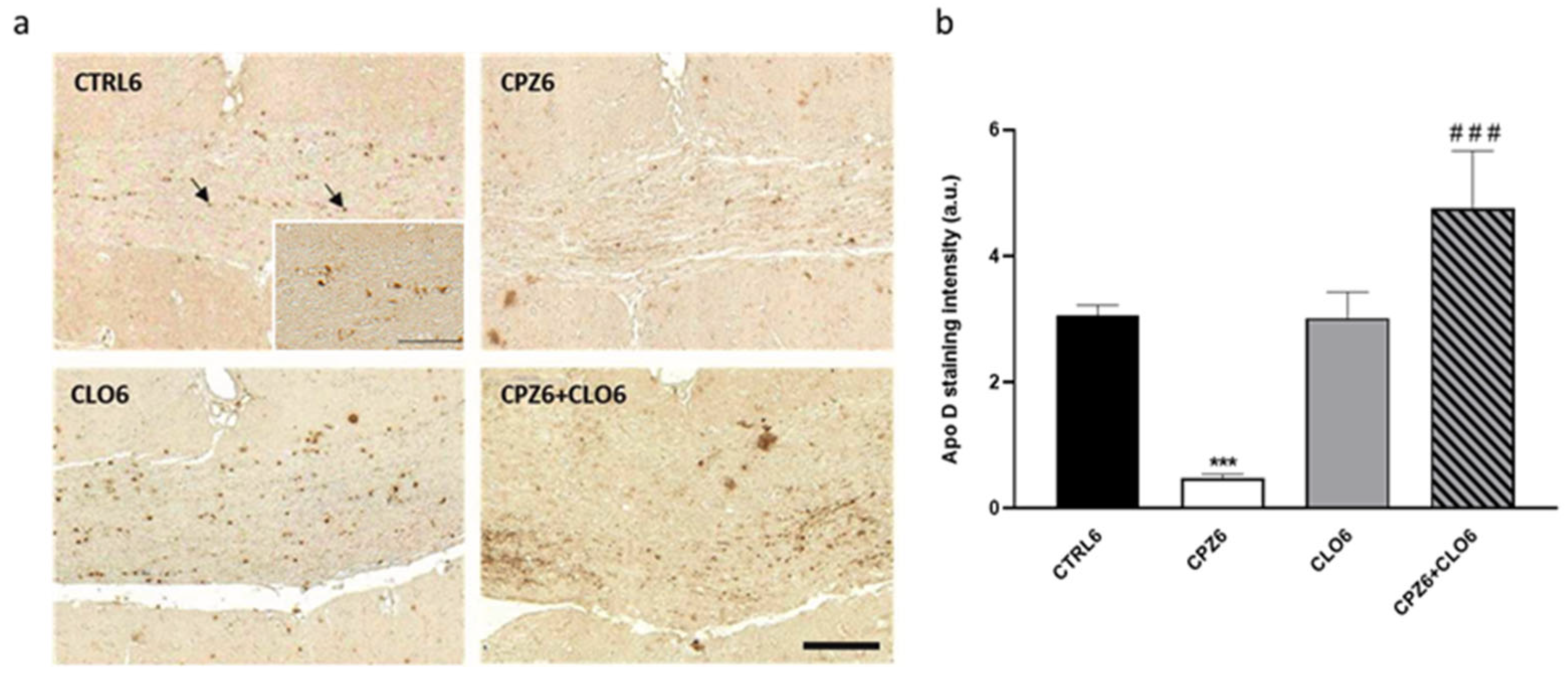
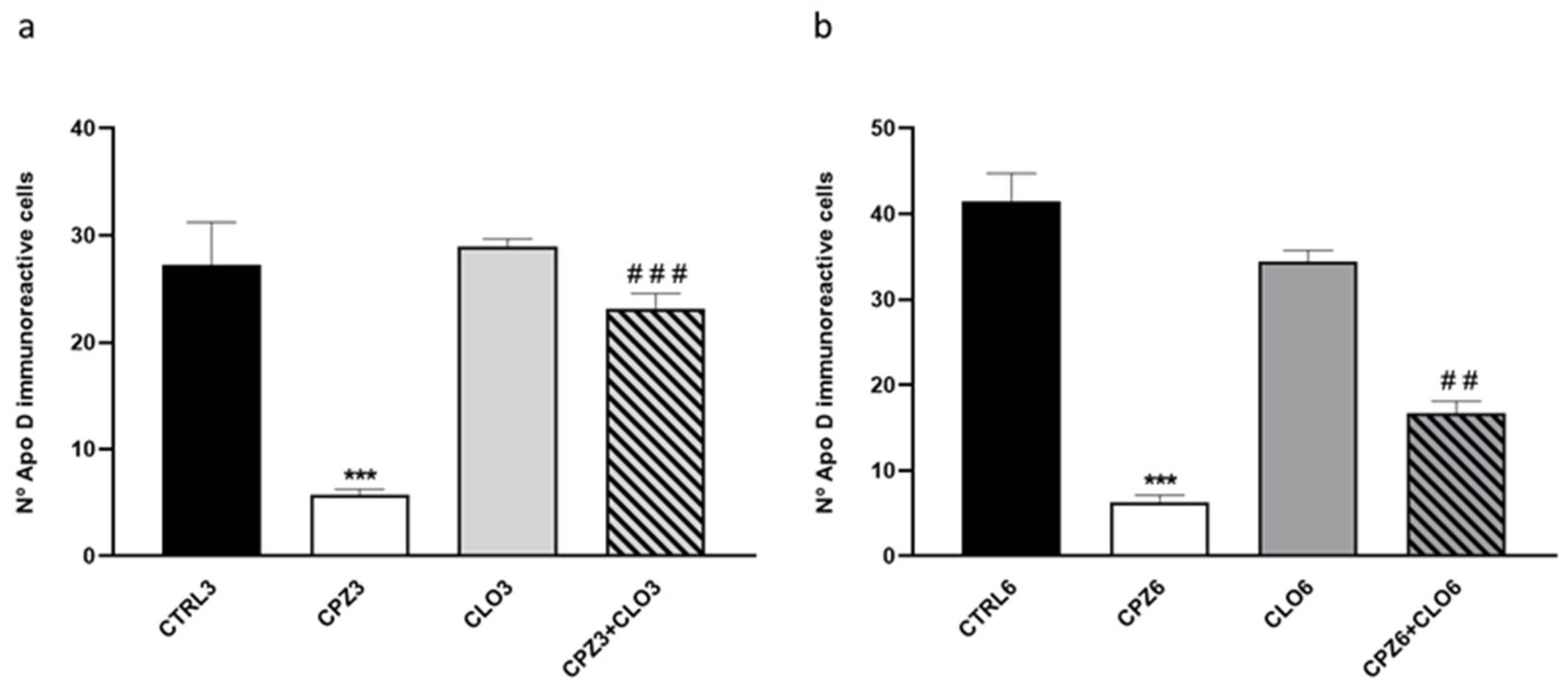
2.3. Clozapine Counteracts the Decrease in Apolipoprotein D Expression Caused by Cuprizone Treatment in the Corpus Callosum
3. Discussion
4. Materials and Methods
4.1. Animal Model and Experimental Design
4.2. Magnetic Resonance Imaging
4.3. Tissue Processing and Histochemical Staining
4.4. Immunohistochemistry for Apolipoprotein D and GFAP
4.5. Immunohistochemical Staining Quantification
4.6. Data Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s disease |
| Apo D | apolipoprotein D |
| BSA | bovine serum albumin |
| CLO | clozapine |
| CNS | central nervous system |
| CPZ | cuprizone |
| CSF | cerebrospinal fluid |
| DAB | diaminobenzidine |
| LFB | luxol fast blue |
| MRI | magnetic resonance imaging |
| MS | multiple sclerosis |
| NS | nervous system |
| NSCs | neural stem cells |
| OLG | oligodendrocyte |
| OPCs | oligodendroglial progenitor cells |
| PBS | phosphate-buffer saline |
| PFA | paraformaldehyde |
| PNS | peripheral nervous system |
| RRMS | relapsing-remitting MS |
| SCTs | scientific and technical services |
References
- Reich, D.S.; Lucchinetti, C.F.; Calabresi, P.A. Multiple sclerosis. N. Engl. J. Med. 2018, 378, 169–180. [Google Scholar] [CrossRef]
- Calabrese, M.; Magliozzi, R.; Ciccarelli, O.; Geurts, J.J.G.; Reynolds, R.; Martin, R. Exploring the origins of grey matter damage in multiple sclerosis. Nat. Rev. Neurosci. 2015, 16, 147–158. [Google Scholar] [CrossRef]
- Cao, Y.; Diao, W.; Tian, F.; Zhang, F.; He, L.; Long, X.; Zhou, F.; Jia, Z. Gray Matter Atrophy in the Cortico-Striatal-Thalamic Network and Sensorimotor Network in Relapsing–Remitting and Primary Progressive Multiple Sclerosis. Neuropsychol. Rev. 2021, 31, 703–720, Erratum in Neuropsychol. Rev. 2021, 31, 721. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.; Vidal-Jordana, A.; Montalban, X. Multiple sclerosis: Clinical aspects. Curr. Opin. Neurol. 2018, 31, 752–759. [Google Scholar] [CrossRef]
- Andica, C.; Hagiwara, A.; Kamagata, K.; Yokoyama, K.; Shimoji, K.; Saito, A.; Takenaka, Y.; Nakazawa, M.; Hori, M.; Cohen-Adad, J.; et al. Gray Matter Alterations in Early and Late Relapsing-Remitting Multiple Sclerosis Evaluated with Synthetic Quantitative Magnetic Resonance Imaging. Sci. Rep. 2019, 9, 8147. [Google Scholar] [CrossRef]
- Lublin, F.D.; Reingold, S.C.; Cohen, J.A.; Cutter, G.R.; Sørensen, P.S.; Thompson, A.J.; Wolinsky, J.S.; Balcer, L.J.; Banwell, B.; Barkhof, F.; et al. Defining the clinical course of multiple sclerosis: The 2013 revisions. Neurology 2014, 83, 278–286. [Google Scholar] [CrossRef]
- Correale, J.; Gaitán, M.I.; Ysrraelit, M.C.; Fiol, M.P. Progressive multiple sclerosis: From pathogenic mechanisms to treatment. Brain 2017, 140, 527–546. [Google Scholar] [CrossRef]
- Ford, H. Clinical presentation and diagnosis of multiple sclerosis. Clin. Med. J. R. Coll. Physicians Lond. 2020, 20, 380–383. [Google Scholar] [CrossRef]
- Mathieu, P.A.; Almeira Gubiani, M.F.; Rodríguez, D.; Gómez Pinto, L.I.; Calcagno, M.D.L.; Adamo, A.M. Demyelination-remyelination in the Central Nervous System: Ligand-dependent Participation of the Notch Signaling Pathway. Toxicol. Sci. 2019, 171, 172–192. [Google Scholar] [CrossRef]
- Fancy, S.P.J.; Chan, J.R.; Baranzini, S.E.; Franklin, R.J.M.; Rowitch, D.H. Myelin regeneration: A recapitulation of development? Annu. Rev. Neurosci. 2011, 34, 21–43. [Google Scholar] [CrossRef]
- Gautier, H.O.B.; Evans, K.A.; Volbracht, K.; James, R.; Sitnikov, S.; Lundgaard, I.; James, F.; Lao-Peregrin, C.; Reynolds, R.; Franklin, R.J.M.; et al. Neuronal activity regulates remyelination via glutamate signalling to oligodendrocyte progenitors. Nat. Commun. 2015, 6, 8518. [Google Scholar] [CrossRef]
- Fernandez-Castaneda, A.; Gaultier, A. Adult oligodendrocyte progenitor cells—Multifaceted regulators of the CNS in health and disease. Brain. Behav. Immun. 2016, 57, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Chari, D.M. Remyelination In Multiple Sclerosis. Int. Rev. Neurobiol. 2007, 79, 589–620. [Google Scholar]
- Dulamea, A.O. Role of oligodendrocyte dysfunction in demyelination, remyelination and neurodegeneration in multiple sclerosis. In Multiple Sclerosis: Bench to Bedside; Advances in Experimental Medicine and Biology; Springer: Cham, Switzerland, 2017; Volume 958, pp. 91–127. [Google Scholar]
- Gruchot, J.; Weyers, V.; Göttle, P.; Förster, M.; Hartung, H.P.; Küry, P.; Kremer, D. The Molecular Basis for Remyelination Failure in Multiple Sclerosis. Cells 2019, 8, 825. [Google Scholar] [CrossRef] [PubMed]
- Navarro, A.; Rioseras, B.; del Valle, E.; Martínez-Pinilla, E.; Astudillo, A.; Tolivia, J. Expression pattern of Myelin-Related Apolipoprotein D in human multiple sclerosis lesions. Front. Aging Neurosci. 2018, 10, 254. [Google Scholar] [CrossRef] [PubMed]
- Matsushima, G.K.; Morell, P. The neurotoxicant, cuprizone, as a model to study demyelination and remyelination in the central nervous system. Brain Pathol. 2001, 11, 107–116. [Google Scholar] [CrossRef]
- Sachs, H.H.; Bercury, K.K.; Popescu, D.C.; Narayanan, S.P.; Macklin, W.B. A new model of Cuprizone-Mediated demyelination/remyelination. ASN Neuro 2014, 6, 1759091414551955. [Google Scholar] [CrossRef]
- van der Star, B.; Vogel, D.; Kipp, M.; Puentes, F.; Baker, D.; Amor, S. In Vitro and In Vivo Models of Multiple Sclerosis. CNS Neurol. Disord. Drug Targets 2012, 11, 570–588. [Google Scholar] [CrossRef]
- Osorio-Querejeta, I.; Sáenz-Cuesta, M.; Muñoz-Culla, M.; Otaegui, D. Models for Studying Myelination, Demyelination and Remyelination. NeuroMolecular Med. 2017, 19, 181–192. [Google Scholar] [CrossRef]
- Vega-Riquer, J.M.; Mendez-Victoriano, G.; Morales-Luckie, R.A.; Gonzalez-Perez, O. Five Decades of Cuprizone, an Updated Model to Replicate Demyelinating Diseases. Curr. Neuropharmacol. 2019, 17, 129–141. [Google Scholar] [CrossRef]
- Nyamoya, S.; Schweiger, F.; Kipp, M.; Hochstrasser, T. Cuprizone as a model of myelin and axonal damage. Drug Discov. Today Dis. Model. 2017, 25–26, 63–68. [Google Scholar] [CrossRef]
- Kipp, M.; Clarner, T.; Dang, J.; Copray, S.; Beyer, C. The cuprizone animal model: New insights into an old story. Acta Neuropathol. 2009, 118, 723–736. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Yoshida, S. Mechanisms of remyelination: Recent insight from experimental models. Biomol. Concepts 2014, 5, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Franklin, R.J.M.; Gallo, V. The translational biology of remyelination: Past, present, and future. Glia 2014, 62, 1905–1915. [Google Scholar] [CrossRef]
- Suess, T.; Remschmidt, C.; Schink, S.; Luchtenberg, M.; Haas, W.; Krause, G.; Buchholz, U. Facemasks and intensified hand hygiene in a German household trial during the 2009/2010 influenza A(H1N1) pandemic: Adherence and tolerability in children and adults. Epidemiol. Infect. 2011, 139, 1895–1901. [Google Scholar] [CrossRef]
- Berghoff, S.A.; Spieth, L.; Sun, T.; Hosang, L.; Depp, C.; Sasmita, A.O.; Vasileva, M.H.; Scholz, P.; Zhao, Y.; Krueger-Burg, D.; et al. Neuronal cholesterol synthesis is essential for repair of chronically demyelinated lesions in mice. Cell Rep. 2021, 37, 109889. [Google Scholar] [CrossRef]
- Gonzalez, G.A.; Hofer, M.P.; Syed, Y.A.; Amaral, A.I.; Rundle, J.; Rahman, S.; Zhao, C.; Kotter, M.R.N. Tamoxifen accelerates the repair of demyelinated lesions in the central nervous system. Sci. Rep. 2016, 6, 31599. [Google Scholar] [CrossRef]
- Rassart, E.; Desmarais, F.; Najyb, O.; Bergeron, K.F.; Mounier, C. Apolipoprotein D. Gene 2020, 756, 144874. [Google Scholar] [CrossRef]
- Rassart, E.; Bedirian, A.; Do Carmo, S.; Guinard, O.; Sirois, J.; Terrisse, L.; Milne, R. Apolipoprotein D. Biochim. Biophys. Acta Protein Struct. Mol. Enzymol. 2000, 1482, 185–198. [Google Scholar] [CrossRef]
- Fernández-Ruiz, J.; Gómez-Ruiz, M.; García, C.; Hernández, M.; Ramos, J.A. Modeling Neurodegenerative Disorders for Developing Cannabinoid-Based Neuroprotective Therapies. Methods Enzymol. 2017, 593, 175–198. [Google Scholar]
- Nasreen, A. Solubility engineering and crystallization of human apolipoprotein D. Protein Sci. 2006, 15, 190–199. [Google Scholar] [CrossRef]
- Eichinger, A.; Nasreen, A.; Kim, H.J.; Skerra, A. Structural insight into the dual ligand specificity and mode of high density lipoprotein association of apolipoprotein D. J. Biol. Chem. 2007, 282, 31068–31075. [Google Scholar] [CrossRef] [PubMed]
- Kielkopf, C.S.; Low, J.K.K.; Mok, Y.-F.; Bhatia, S.; Palasovski, T.; Oakley, A.J.; Whitten, A.E.; Garner, B.; Brown, S.H.J. Identification of a novel tetrameric structure for human apolipoprotein-D. J. Struct. Biol. 2018, 203, 205–218. [Google Scholar] [CrossRef] [PubMed]
- Kielkopf, C.S.; Whitten, A.E.; Garner, B.; Brown, S.H.J. Small angle X-ray scattering analysis of ligand-bound forms of tetrameric apolipoprotein-D. Biosci. Rep. 2021, 41, BSR20201423. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, D.; Ganfornina, M.D. The Lipocalin Apolipoprotein D Functional Portrait: A Systematic Review. Front. Physiol. 2021, 12, 738991. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cong, Y.; Wang, S.; Zhang, S. Antioxidant activities of recombinant amphioxus (Branchiostoma belcheri) apolipoprotein D. Mol. Biol. Rep. 2011, 38, 1847–1851. [Google Scholar] [CrossRef]
- Oakley, A.J.; Bhatia, S.; Ecroyd, H.; Garner, B. Molecular dynamics analysis of apolipoprotein-D—lipid hydroperoxide interactions: Mechanism for selective oxidation of Met-93. PLoS ONE 2012, 7, 34057. [Google Scholar] [CrossRef]
- Thomas, E.A.; George, R.C.; Gregor Sutcliffe, J. Apolipoprotein D modulates arachidonic acid signaling in cultured cells: Implications for psychiatric disorders. Prostaglandins Leukot. Essent. Fat. Acids 2003, 69, 421–427. [Google Scholar] [CrossRef]
- Bhatia, S.; Knoch, B.; Wong, J.; Kim, W.S.; Else, P.L.; Oakley, A.J.; Garner, B. Selective reduction of hydroperoxyeicosatetraenoic acids to their hydroxy derivatives by apolipoprotein D: Implications for lipid antioxidant activity and Alzheimer’s disease. Biochem. J. 2012, 442, 713–721. [Google Scholar] [CrossRef]
- Waldner, A.; Dassati, S.; Redl, B.; Smania, N.; Gandolfi, M. Apolipoprotein D Concentration in Human Plasma during Aging and in Parkinson’s Disease: A Cross-Sectional Study. Parkinsons. Dis. 2018, 2018, 3751516. [Google Scholar] [CrossRef]
- Corraliza-Gomez, M.; Sanchez, D.; Ganfornina, M.D. Lipid-Binding Proteins in Brain Health and Disease. Front. Neurol. 2019, 10, 1152. [Google Scholar] [CrossRef] [PubMed]
- Pascua-Maestro, R.; Diez-Hermano, S.; Lillo, C.; Ganfornina, M.D.; Sanchez, D. Protecting cells by protecting their vulnerable lysosomes: Identification of a new mechanism for preserving lysosomal functional integrity upon oxidative stress. PLoS Genet. 2017, 13, e1006603. [Google Scholar] [CrossRef] [PubMed]
- Ganfornina, M.D.; Do Carmo, S.; Lora, J.M.; Torres-Schumann, S.; Vogel, M.; Allhorn, M.; González, C.; Bastiani, M.J.; Rassart, E.; Sanchez, D. Apolipoprotein D is involved in the mechanisms regulating protection from oxidative stress. Aging Cell 2008, 7, 506–515. [Google Scholar] [CrossRef] [PubMed]
- Dassati, S.; Waldner, A.; Schweigreiter, R. Apolipoprotein D takes center stage in the stress response of the aging and degenerative brain. Neurobiol. Aging 2014, 35, 1632–1642. [Google Scholar] [CrossRef]
- Salami, M.; Bandegi, A.R.; Sameni, H.R.; Vafaei, A.A.; Pakdel, A. Hippocampal Up-Regulation of Apolipoprotein D in a Rat Model of Maternal Hypo- and Hyperthyroidism: Implication of Oxidative Stress. Neurochem. Res. 2019, 44, 2190–2201. [Google Scholar] [CrossRef]
- Pascua-Maestro, R.; González, E.; Lillo, C.; Ganfornina, M.D.; Falcón-Pérez, J.M.; Sanchez, D. Extracellular vesicles secreted by astroglial cells transport apolipoprotein D to neurons and mediate neuronal survival upon oxidative stress. Front. Cell. Neurosci. 2019, 12, 526. [Google Scholar] [CrossRef]
- Rickhag, M.; Deierborg, T.; Patel, S.; Ruscher, K.; Wieloch, T. Apolipoprotein D is elevated in oligodendrocytes in the peri-infarct region after experimental stroke: Influence of enriched environment. J. Cereb. Blood Flow Metab. 2008, 28, 551–562. [Google Scholar] [CrossRef]
- Ganfornina, M.D.; Do Carmo, S.; Martínez, E.; Tolivia, J.; Navarro, A.; Rassart, E.; Sanchez, D. ApoD, a glia-derived apolipoprotein, is required for peripheral nerve functional integrity and a timely response to injury. Glia 2010, 58, 1320–1334. [Google Scholar] [CrossRef]
- Fyfe-Desmarais, G.; Desmarais, F.; Rassart, É.; Mounier, C. Apolipoprotein D in Oxidative Stress and Inflammation. Antioxidants 2023, 12, 1027. [Google Scholar] [CrossRef]
- Muffat, J.; Walker, D.W. Apolipoprotein D: An overview of its role in aging and age-related diseases. Cell Cycle 2010, 9, 269–273. [Google Scholar] [CrossRef]
- Li, H.; Ruberu, K.; Karl, T.; Garner, B. Cerebral apolipoprotein-D Is hypoglycosylated compared to peripheral tissues and is variably expressed in mouse and human brain regions. PLoS ONE 2016, 11, e0148238. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.Y.; Ong, W.Y.; Sundaram, R.K.; Chan, C.; Patel, S.C. Immunocytochemical localization of apolipoprotein D in oligodendrocyte precursor-like cells, perivascular cells, and pericytes in the human cerebral cortex. J. Neurocytol. 2001, 30, 209–218. [Google Scholar] [CrossRef] [PubMed]
- García-Mateo, N.; Pascua-Maestro, R.; Pérez-Castellanos, A.; Lillo, C.; Sanchez, D.; Ganfornina, M.D. Myelin extracellular leaflet compaction requires apolipoprotein D membrane management to optimize lysosomal-dependent recycling and glycocalyx removal. Glia 2018, 66, 670–687. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.; Kim, W.S.; Shepherd, C.E.; Halliday, G.M. Apolipoprotein D Upregulation in Alzheimer’s Disease but Not Frontotemporal Dementia. J. Mol. Neurosci. 2018, 67, 125. [Google Scholar] [CrossRef]
- de Magalhães, J.P.; Curado, J.; Church, G.M. Meta-analysis of age-related gene expression profiles identifies common signatures of aging. Bioinformatics 2009, 25, 875–881. [Google Scholar] [CrossRef]
- Kalman, J.; McConathy, W.; Araoz, C.; Kasa, P.; Lacko, A.G. Apolipoprotein D in the aging brain and in Alzheimer’s dementia. Neurol. Res. 2000, 22, 330–336. [Google Scholar] [CrossRef]
- Navarro, A.; Del Valle, E.; Juárez, A.; Martinez, E.; Ordóñez, C.; Astudillo, A.; Tolivia, J. Apolipoprotein D synthesis progressively increases in frontal cortex during human lifespan. Age 2010, 32, 85–96. [Google Scholar] [CrossRef]
- Bhatia, S.; Jenner, A.M.; Li, H.; Ruberu, K.; Spiro, A.S.; Shepherd, C.E.; Kril, J.J.; Kain, N.; Don, A.; Garner, B. Increased apolipoprotein D dimer formation in alzheimer’s disease hippocampus is associated with lipid conjugated diene levels. J. Alzheimer’s Dis. 2013, 35, 475–486. [Google Scholar] [CrossRef]
- Reindl, M.; Knipping, G.; Wicher, I.; Dilitz, E.; Egg, R.; Deisenhammer, F.; Berger, T. Increased intrathecal production of apolipoprotein D in multiple sclerosis. J. Neuroimmunol. 2001, 119, 327–332. [Google Scholar] [CrossRef]
- Pascua-Maestro, R.; Corraliza-Gomez, M.; Fadrique-Rojo, C.; Ledesma, M.D.; Schuchman, E.H.; Sanchez, D.; Ganfornina, M.D. Apolipoprotein D-mediated preservation of lysosomal function promotes cell survival and delays motor impairment in Niemann-Pick type A disease. Neurobiol. Dis. 2020, 144, 105046. [Google Scholar] [CrossRef]
- Thomas, E.A.; Dean, B.; Pavey, G.; Sutcliffe, J.G. Increased CNS levels of apolipoprotein D in schizophrenic and bipolar subjects: Implications for the pathophysiology of psychiatric disorders. Proc. Natl. Acad. Sci. USA 2001, 98, 4066–4071. [Google Scholar] [CrossRef]
- Lee, H.; Rhee, S.J.; Kim, J.; Lee, Y.; Kim, H.; Lee, J.; Lee, K.; Shin, H.; Kim, H.; Lee, T.Y.; et al. Predictive protein markers for depression severity in mood disorders: A preliminary trans-diagnostic approach study. J. Psychiatr. Res. 2021, 142, 63–72. [Google Scholar] [CrossRef] [PubMed]
- del Valle, E.; Rubio-Sardón, N.; Menéndez-Pérez, C.; Martínez-Pinilla, E.; Navarro, A. Apolipoprotein D as a Potential Biomarker in Neuropsychiatric Disorders. Int. J. Mol. Sci. 2023, 24, 15631. [Google Scholar] [CrossRef] [PubMed]
- Thomas, E.A.; Danielson, P.E.; Austin Nelson, P.; Pribyl, T.M.; Hilbush, B.S.; Hasel, K.W.; Gregor Sutcliffe, J. Clozapine increases apolipoprotein D expression in rodent brain: Towards a mechanism for neuroleptic pharmacotherapy. J. Neurochem. 2001, 76, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Ciapparelli, A.; Dell’Osso, L.; Pini, S.; Chiavacci, M.C.; Fenzi, M.; Cassano, G.B. Clozapine for treatment-refractory schizophrenia, schizoaffective disorder, and psychotic bipolar disorder: A 24-month naturalistic study. J. Clin. Psychiatry 2000, 61, 329–334. [Google Scholar] [CrossRef]
- García-Mateo, N.; Ganfornina, M.D.; Montero, O.; Gijón, M.A.; Murphy, R.C.; Sanchez, D. Schwann cell-derived Apolipoprotein D controls the dynamics of post-injury myelin recognition and degradation. Front. Cell. Neurosci. 2014, 8, 374. [Google Scholar] [CrossRef]
- Boyles, J.K.; Notterpek, L.M.; Anderson, L.J. Accumulation of apolipoproteins in the regenerating and remyelinating mammalian peripheral nerve: Identification of apolipoprotein D, apolipoprotein A-IV, apolipoprotein E, and apolipoprotein A-I. J. Biol. Chem. 1990, 265, 17805–17815. [Google Scholar] [CrossRef]
- Spreyer, P.; Schaal, H.; Kuhn, G.; Rothe, T.; Unterbeck, A.; Olek, K.; Muller, H.W. Regeneration-associated high level expression of apolipoprotein D mRNA in endoneurial fibroblasts of peripheral nerve. EMBO J. 1990, 9, 2479–2484. [Google Scholar] [CrossRef]
- Stoop, M.P.; Singh, V.; Dekker, L.J.; Titulaer, M.K.; Stingl, C.; Burgers, P.C.; Sillevis Smitt, P.A.E.; Hintzen, R.Q.; Luider, T.M. Proteomics comparison of cerebrospinal fluid of relapsing remitting and primary progressive multiple sclerosis. PLoS ONE 2010, 5, e12442. [Google Scholar] [CrossRef]
- Xu, H.; Yang, H.J.; Zhang, Y.; Clough, R.; Browning, R.; Li, X.M. Behavioral and Neurobiological Changes in C57BL/6 Mice Exposed to Cuprizone. Behav. Neurosci. 2009, 123, 418–429. [Google Scholar] [CrossRef]
- Sanabria-Castro, A.; Flores-Díaz, M.; Alape-Girón, A. Biological models in multiple sclerosis. J. Neurosci. Res. 2020, 98, 491–508. [Google Scholar] [CrossRef] [PubMed]
- Skripuletz, T.; Gudi, V.; Hackstette, D.; Stangel, M. De- and remyelination in the CNS white and grey matter induced by cuprizone: The old, the new, and the unexpected. Histol. Histopathol. 2011, 26, 1585–1597. [Google Scholar] [PubMed]
- Sen, M.K.; Almuslehi, M.S.M.; Coorssen, J.R.; Mahns, D.A.; Shortland, P.J. Behavioural and histological changes in cuprizone-fed mice. Brain Behav. Immun. 2020, 87, 508–523. [Google Scholar] [CrossRef] [PubMed]
- Franco-Pons, N.; Torrente, M.; Colomina, M.T.; Vilella, E. Behavioral deficits in the cuprizone-induced murine model of demyelination/remyelination. Toxicol. Lett. 2007, 169, 205–213. [Google Scholar] [CrossRef]
- Remington, L.T.; Babcock, A.A.; Zehntner, S.P.; Owens, T. Microglial recruitment, activation, and proliferation in response to primary demyelination. Am. J. Pathol. 2007, 170, 1713–1724. [Google Scholar] [CrossRef]
- Selvaraju, R.; Bernasconi, L.; Losberger, C.; Graber, P.; Kadi, L.; Avellana-Adalid, V.; Picard-Riera, N.; Van Evercooren, A.B.; Cirillo, R.; Kosco-Vilbois, M.; et al. Osteopontin is upregulated during in vivo demyelination and remyelination and enhances myelin formation in vitro. Mol. Cell. Neurosci. 2004, 25, 707–721. [Google Scholar] [CrossRef]
- Xiao, L.; Xu, H.; Zhang, Y.; Wei, Z.; He, J.; Jiang, W.; Li, X.; Dyck, L.E.; Devon, R.M.; Deng, Y.; et al. Quetiapine facilitates oligodendrocyte development and prevents mice from myelin breakdown and behavioral changes. Mol. Psychiatry 2008, 13, 697–708. [Google Scholar] [CrossRef]
- Torkildsen; Brunborg, L.A.; Myhr, K.M.; Bø, L. The cuprizone model for demyelination. Acta Neurol. Scand. 2008, 117, 72–76. [Google Scholar] [CrossRef]
- Praet, J.; Guglielmetti, C.; Berneman, Z.; Van der Linden, A.; Ponsaerts, P. Cellular and molecular neuropathology of the cuprizone mouse model: Clinical relevance for multiple sclerosis. Neurosci. Biobehav. Rev. 2014, 47, 485–505. [Google Scholar] [CrossRef]
- O’Brien, T.; Paine, M.; Matotek, K.; Byrne, E. Apparent hydrocephalus and chronic multiple sclerosis: A report of two cases. Clin. Exp. Neurol. 1993, 30, 137–143. [Google Scholar]
- Bateman, G.A.; Lechner-Scott, J.; Lea, R.A. A comparison between the pathophysiology of multiple sclerosis and normal pressure hydrocephalus: Is pulse wave encephalopathy a component of MS? Fluids Barriers CNS 2016, 13, 18. [Google Scholar] [CrossRef] [PubMed]
- Kesterson, J.W.; Carlton, W.W. Cuprizone toxicosis in mice-Attempts to antidote the toxicity. Toxicol. Appl. Pharmacol. 1972, 22, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Evans, W.A. An encephalographic ratio for estimating ventricular enlargement and cerebral atrophy. Arch. Neurol. Psychiatry 1942, 47, 931–937. [Google Scholar] [CrossRef]
- Kesterson, J.W.; Carlton, W.W. Aqueductal stenosis as the cause of hydrocephalus in mice fed the substituted hydrazine, cuprizone. Exp. Mol. Pathol. 1970, 13, 281–294. [Google Scholar] [CrossRef]
- Hemm, R.D.; Carlton, W.W.; Welser, J.R. Ultrastructural changes of cuprizone encephalopathy in mice. Toxicol. Appl. Pharmacol. 1971, 18, 869–882. [Google Scholar] [CrossRef]
- Pong, A.C.; Jugé, L.; Bilston, L.E.; Cheng, S. Development of acute hydrocephalus does not change brain tissue mechanical properties in adult rats, but in juvenile rats. PLoS ONE 2017, 12, e0182808. [Google Scholar] [CrossRef]
- Stys, P.K.; Zamponi, G.W.; Van Minnen, J.; Geurts, J.J.G. Will the real multiple sclerosis please stand up? Nat. Rev. Neurosci. 2012, 13, 507–514. [Google Scholar] [CrossRef]
- Zirngibl, M.; Assinck, P.; Sizov, A.; Caprariello, A.V.; Plemel, J.R. Oligodendrocyte death and myelin loss in the cuprizone model: An updated overview of the intrinsic and extrinsic causes of cuprizone demyelination. Mol. Neurodegener. 2022, 17, 34. [Google Scholar] [CrossRef]
- Carassiti, D.; Altmann, D.R.; Petrova, N.; Pakkenberg, B.; Scaravilli, F.; Schmierer, K. Neuronal loss, demyelination and volume change in the multiple sclerosis neocortex. Neuropathol. Appl. Neurobiol. 2018, 44, 377–390. [Google Scholar] [CrossRef]
- Cerina, M.; Muthuraman, M.; Gallus, M.; Koirala, N.; Dik, A.; Wachsmuth, L.; Hundehege, P.; Schiffler, P.; Tenberge, J.G.; Fleischer, V.; et al. Myelination- And immune-mediated MR-based brain network correlates. J. Neuroinflamm. 2020, 17, 186. [Google Scholar] [CrossRef]
- Radetz, A.; Mladenova, K.; Ciolac, D.; Gonzalez-Escamilla, G.; Fleischer, V.; Ellwardt, E.; Krämer, J.; Bittner, S.; Meuth, S.G.; Muthuraman, M.; et al. Linking Microstructural Integrity and Motor Cortex Excitability in Multiple Sclerosis. Front. Immunol. 2021, 12, 748357. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cai, L.; Fan, K.; Fan, B.; Li, N.; Gao, W.; Yang, X.; Ma, J. The Spatial and Temporal Characters of Demyelination and Remyelination in the Cuprizone Animal Model. Anat. Rec. 2019, 302, 2020–2029, Erratum in Anat. Rec. 2023, 306, 697. https://doi.org/10.1002/ar.25154. [Google Scholar] [CrossRef] [PubMed]
- Brousse, B.; Magalon, K.; Durbec, P.; Cayre, M. Region and dynamic specificities of adult neural stem cells and oligodendrocyte precursors in myelin regeneration in the mouse brain. Biol. Open 2016, 5, 980–992. [Google Scholar] [CrossRef] [PubMed]
- Dedoni, S.; Scherma, M.; Camoglio, C.; Siddi, C.; Dazzi, L.; Puliga, R.; Frau, J.; Cocco, E.; Fadda, P. An overall view of the most common experimental models for multiple sclerosis. Neurobiol. Dis. 2023, 184, 106230. [Google Scholar] [CrossRef]
- Steelman, A.J.; Thompson, J.P.; Li, J. Demyelination and remyelination in anatomically distinct regions of the corpus callosum following cuprizone intoxication. Neurosci. Res. 2012, 72, 32–42. [Google Scholar] [CrossRef]
- Skripuletz, T.; Lindner, M.; Kotsiari, A.; Garde, N.; Fokuhl, J.; Linsmeier, F.; Trebst, C.; Stangel, M. Cortical demyelination is prominent in the murine cuprizone model and is strain-dependent. Am. J. Pathol. 2008, 172, 1053–1061. [Google Scholar] [CrossRef]
- Zhan, J.; Mann, T.; Joost, S.; Behrangi, N.; Frank, M.; Kipp, M. The Cuprizone Model: Dos and Do Nots. Cells 2020, 9, 843. [Google Scholar] [CrossRef]
- Fallier-Becker, P.; Bonzheim, I.; Pfeiffer, F. Cuprizone feeding induces swollen astrocyte endfeet. Pflugers Arch. Eur. J. Physiol. 2022, 474, 1275–1283. [Google Scholar] [CrossRef]
- Zindler, E.; Zipp, F. Neuronal injury in chronic CNS inflammation. Best Pract. Res. Clin. Anaesthesiol. 2010, 24, 551–562. [Google Scholar] [CrossRef]
- Popescu, B.F.G.; Pirko, I.; Lucchinetti, C.F. Pathology of multiple sclerosis: Where do we stand? Contin. Lifelong Learn. Neurol. 2013, 19, 901–921. [Google Scholar] [CrossRef]
- Taraboletti, A.; Walker, T.; Avila, R.; Huang, H.; Caporoso, J.; Manandhar, E.; Leeper, T.C.; Modarelli, D.A.; Medicetty, S.; Shriver, L.P. Cuprizone Intoxication Induces Cell Intrinsic Alterations in Oligodendrocyte Metabolism Independent of Copper Chelation. Biochemistry 2017, 56, 1518–1528. [Google Scholar] [CrossRef]
- Franco, R.; Aguinaga, D.; Jiménez, J.; Lillo, J.; Martínez-Pinilla, E.; Navarro, G. Biased receptor functionality versus biased agonism in G-protein-coupled receptors. Biomol. Concepts 2018, 9, 143–154. [Google Scholar] [CrossRef]
- Gudi, V.; Gingele, S.; Skripuletz, T.; Stangel, M. Glial response during cuprizone-induced de- and remyelination in the CNS: Lessons learned. Front. Cell. Neurosci. 2014, 8, 73. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Ruberu, K.; Muñoz, S.S.; Jenner, A.M.; Spiro, A.; Zhao, H.; Rassart, E.; Sanchez, D.; Ganfornina, M.D.; Karl, T.; et al. Apolipoprotein D modulates amyloid pathology in APP/PS1 Alzheimer’s disease mice. Neurobiol. Aging 2015, 36, 1820–1833. [Google Scholar] [CrossRef] [PubMed]
- Thomas, E.A.; Yao, J.K. Clozapine specifically alters the arachidonic acid pathway in mice lacking apolipoprotein D. Schizophr. Res. 2007, 89, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Do Carmo, S.; Jacomy, H.; Talbot, P.J.; Rassart, E. Neuroprotective effect of apolipoprotein D against human coronavirus OC43-induced encephalitis in mice. J. Neurosci. 2008, 28, 10330–10338. [Google Scholar] [CrossRef]
- Martínez-Pinilla, E.; Navarro, A.; Ordóñez, C.; del Valle, E.; Tolivia, J. Apolipoprotein D subcellular distribution pattern in neuronal cells during oxidative stress. Acta Histochem. 2015, 117, 536–544. [Google Scholar] [CrossRef]
- Najyb, O.; Do Carmo, S.; Alikashani, A.; Rassart, E. Apolipoprotein D Overexpression Protects Against Kainate-Induced Neurotoxicity in Mice. Mol. Neurobiol. 2017, 54, 3948–3963. [Google Scholar] [CrossRef]
- Navarro, A.; Del Valle, E.; Tolivia, J. Differential expression of apolipoprotein D in human astroglial and oligodendroglial cells. J. Histochem. Cytochem. 2004, 52, 1031–1036. [Google Scholar] [CrossRef]
- Sanchez, D.; Bajo-Grañeras, R.; Del Caño-Espinel, M.; Garcia-Centeno, R.; Garcia-Mateo, N.; Pascua-Maestro, R.; Ganfornina, M.D. Aging without apolipoprotein d: Molecular and cellular modifications in the hippocampus and cortex. Exp. Gerontol. 2015, 67, 19–47. [Google Scholar] [CrossRef]
- Patel, J.; Balabanov, R. Molecular Mechanisms of Oligodendrocyte Injury in Multiple Sclerosis and Experimental Autoimmune Encephalomyelitis. Int. J. Mol. Sci. 2012, 13, 10647–10659. [Google Scholar] [CrossRef]
- Clausen, B.H.; Lambertsen, K.L.; Meldgaard, M.; Finsen, B. A quantitative in situ hybridization and polymerase chain reaction study of microglial-macrophage expression of interleukin-1β mRNA following permanent middle cerebral artery occlusion in mice. Neuroscience 2005, 132, 879–892. [Google Scholar] [CrossRef]
- Elo, T.; Sipilä, P.; Valve, E.; Kujala, P.; Toppari, J.; Poutanen, M.; Härkönen, P. Fibroblast growth factor 8b causes progressive stromal and epithelial changes in the epididymis and degeneration of the seminiferous epithelium in the testis of transgenic mice. Biol. Reprod. 2012, 86, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Pajaniappan, M.; Glober, N.K.; Kennard, S.; Liu, H.; Zhao, N.; Lilly, B. Endothelial cells downregulate apolipoprotein D expression in mural cells through paracrine secretion and Notch signaling. Am. J. Physiol. Hear. Circ. Physiol. 2011, 301, 784–793. [Google Scholar] [CrossRef] [PubMed]
- López-Boado, Y.S.; Tolivia, J.; López-Otín, C. Apolipoprotein D gene induction by retinoic acid is concomitant with growth arrest and cell differentiation in human breast cancer cells. J. Biol. Chem. 1994, 269, 26871–26878. [Google Scholar] [CrossRef] [PubMed]
- Kosacka, J.; Gericke, M.; Nowicki, M.; Kacza, J.; Borlak, J.; Spanel-Borowski, K. Apolipoproteins D and E3 exert neurotrophic and synaptogenic effects in dorsal root ganglion cell cultures. Neuroscience 2009, 162, 282–291. [Google Scholar] [CrossRef]
- Ceylan, U.; Haupeltshofer, S.; Kämper, L.; Dann, J.; Ambrosius, B.; Gold, R.; Faissner, S. Clozapine Regulates Microglia and Is Effective in Chronic Experimental Autoimmune Encephalomyelitis. Front Immunol. 2021, 12, 656941. [Google Scholar] [CrossRef]
- Templeton, N.; Kivell, B.; McCaughey-Chapman, A.; Connor, B.; La Flamme, A.C. Clozapine administration enhanced functional recovery after cuprizone demyelination. PLoS ONE 2019, 14, e0216113. [Google Scholar] [CrossRef]
- Hu, X.; Zhou, H.; Zhang, D.; Yang, S.; Qian, L.; Wu, H.M.; Chen, P.S.; Wilson, B.; Gao, H.M.; Lu, R.B.; et al. Clozapine protects dopaminergic neurons from inflammation-induced damage by inhibiting microglial overactivation. J. Neuroimmune Pharmacol. 2012, 7, 187–201. [Google Scholar] [CrossRef]
- Chang, H.X.; Wei, Y.Z.; Chen, Y.J.; Du, L.; Cong, H.R.; Zhang, X.H.; Geng, X.C.; Yin, L.L. The antipsychotic-like effects of clozapine in C57BL/6 mice exposed to cuprizone: Decreased glial activation. Behav. Brain Res. 2019, 364, 157–161. [Google Scholar] [CrossRef]
- Xu, H.; Yang, H.J.; McConomy, B.; Browning, R.; Li, X.M. Behavioral and neurobiological changes in C57BL/6 mouse exposed to cuprizone: Effects of antipsychotics. Front. Behav. Neurosci. 2010, 4, 1332. [Google Scholar] [CrossRef]
- Martínez-Pinilla, E.; Rubio-Sardón, N.; Peláez, R.; García-Álvarez, E.; del Valle, E.; Tolivia, J.; Larráyoz, I.M.; Navarro, A. Neuroprotective Effect of Apolipoprotein D in Cuprizone-Induced Cell Line Models: A Potential Therapeutic Approach for Multiple Sclerosis and Demyelinating Diseases. Int. J. Mol. Sci. 2021, 22, 1260. [Google Scholar] [CrossRef] [PubMed]
- Steiner, J.; Martins-de-Souza, D.; Schiltz, K.; Sarnyai, Z.; Westphal, S.; Isermann, B.; Dobrowolny, H.; Turck, C.W.; Bogerts, B.; Bernstein, H.G.; et al. Clozapine promotes glycolysis and myelin lipid synthesis in cultured oligodendrocytes. Front. Cell. Neurosci. 2014, 8, 384. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Yang, H.J.; Li, X.M. Differential effects of antipsychotics on the development of rat oligodendrocyte precursor cells exposed to cuprizone. Eur. Arch. Psychiatry Clin. Neurosci. 2014, 264, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.K.; Thomas, E.A.; Reddy, R.D.; Keshavan, M.S. Association of plasma apolipoproteins D with RBC membrane arachidonic acid levels in schizophrenia. Schizophr. Res. 2005, 72, 259–266. [Google Scholar] [CrossRef]
- Bajo-Grañeras, R.; Sanchez, D.; Gutierrez, G.; González, C.; Do Carmo, S.; Rassart, E.; Ganfornina, M.D. Apolipoprotein D alters the early transcriptional response to oxidative stress in the adult cerebellum. J. Neurochem. 2011, 117, 949–960. [Google Scholar] [CrossRef]
- He, X.; Jittiwat, J.; Kim, J.H.; Jenner, A.M.; Farooqui, A.A.; Patel, S.C.; Ong, W.Y. Apolipoprotein D modulates F2-isoprostane and 7-ketocholesterol formation and has a neuroprotective effect on organotypic hippocampal cultures after kainate-induced excitotoxic injury. Neurosci. Lett. 2009, 455, 183–186. [Google Scholar] [CrossRef]
- Muffat, J.; Walker, D.W.; Benzer, S. Human Apo D, an apolipoprotein up-regulated in neurodegenerative diseases, extends lifespan and increases stress resistance in Drosophila. Proc. Natl. Acad. Sci. USA 2008, 105, 7088–7093. [Google Scholar] [CrossRef]
- Jeon, S.; Kim, S.H.; Shin, S.Y.; Lee, Y.H. Clozapine reduces Toll-like receptor 4/NF-κB-mediated inflammatory responses through inhibition of calcium/calmodulin-dependent Akt activation in microglia. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2018, 81, 477–487. [Google Scholar] [CrossRef]
- Zeng, Z.; Wang, X.; Bhardwaj, S.K.; Zhou, X.; Little, P.J.; Quirion, R.; Srivastava, L.K.; Zheng, W. The Atypical Antipsychotic Agent, Clozapine, Protects Against Corticosterone-Induced Death of PC12 Cells by Regulating the Akt/FoxO3a Signaling Pathway. Mol. Neurobiol. 2017, 54, 3395–3406. [Google Scholar] [CrossRef]
- Topic, A.; Vasic, M.; Markovic, B.; Milinkovic, N.; Dincic, E. The effects of disease-modifying therapies on oxidative stress in patients with relapsing-remitting multiple sclerosis. Clin. Neuropharmacol. 2022, 45, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Taraboletti, A.; Shriver, L.P. Dimethyl fumarate modulates antioxidant and lipid metabolism in oligodendrocytes. Redox Biol. 2015, 5, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Kihara, Y.; Chun, J. Molecular and neuroimmune pharmacology of S1P receptor modulators and other disease-modifying therapies for multiple sclerosis. Pharmacol. Ther. 2023, 246, 108432. [Google Scholar] [CrossRef] [PubMed]
- Manai, F.; Amadio, M. Dimethyl fumarate triggers the antioxidant defense system in human retinal endothelial cells through Nrf2 activation. Antioxidants 2022, 11, 1924. [Google Scholar] [CrossRef]
- Groves, A.; Kihara, Y.; Chun, J. Fingolimod: Direct CNS effects of sphingosine 1-phosphate (S1P) receptor modulation and implications in multiple sclerosis therapy. J. Neurol. Sci. 2013, 328, 9–18. [Google Scholar] [CrossRef]
- Brunkhorst, R.; Vutukuri, R.; Pfeilschifter, W. Fingolimod for the treatment of neurological diseases—State of play and future perspectives. Front. Cell. Neurosci. 2014, 8, 283. [Google Scholar] [CrossRef]
- Navarro, A.; Ordóñez, C.; Martínez, E.; Pérez, C.; Astudillo, A.; Tolivia, J. Apolipoprotein d expression absence in degenerating neurons of human central nervous system. Histol. Histopathol. 2008, 23, 995–1001. [Google Scholar]
- Tolivia, J.; Navarro, A.; Del Valle, E.; Perez, C.; Ordoñez, C.; Martínez, E. Application of photoshop and scion image analysis to quantification of signals in histochemistry, immunocytochemistry and hybridocytochemistry. Anal. Quant. Cytol. Histol. 2006, 28, 43–53. [Google Scholar]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Pinilla, E.; Rubio-Sardón, N.; Fernández-García, G.; Villar-Conde, S.; Menéndez-Pérez, C.; Tolivia, J.; del Valle, E.; Navarro, A. Apolipoprotein D Expression Dynamics During Cuprizone-Induced Demyelination and Remyelination in a Mouse Model of Multiple Sclerosis. Int. J. Mol. Sci. 2025, 26, 8692. https://doi.org/10.3390/ijms26178692
Martínez-Pinilla E, Rubio-Sardón N, Fernández-García G, Villar-Conde S, Menéndez-Pérez C, Tolivia J, del Valle E, Navarro A. Apolipoprotein D Expression Dynamics During Cuprizone-Induced Demyelination and Remyelination in a Mouse Model of Multiple Sclerosis. International Journal of Molecular Sciences. 2025; 26(17):8692. https://doi.org/10.3390/ijms26178692
Chicago/Turabian StyleMartínez-Pinilla, Eva, Nuria Rubio-Sardón, Gemma Fernández-García, Sandra Villar-Conde, Carlota Menéndez-Pérez, Jorge Tolivia, Eva del Valle, and Ana Navarro. 2025. "Apolipoprotein D Expression Dynamics During Cuprizone-Induced Demyelination and Remyelination in a Mouse Model of Multiple Sclerosis" International Journal of Molecular Sciences 26, no. 17: 8692. https://doi.org/10.3390/ijms26178692
APA StyleMartínez-Pinilla, E., Rubio-Sardón, N., Fernández-García, G., Villar-Conde, S., Menéndez-Pérez, C., Tolivia, J., del Valle, E., & Navarro, A. (2025). Apolipoprotein D Expression Dynamics During Cuprizone-Induced Demyelination and Remyelination in a Mouse Model of Multiple Sclerosis. International Journal of Molecular Sciences, 26(17), 8692. https://doi.org/10.3390/ijms26178692





