Disruption of Human Papillomavirus 16 E6/E7 Genes Using All-in-One Adenovirus Vectors Expressing Eight Double-Nicking Guide RNAs
Abstract
1. Introduction
2. Results
2.1. In Vitro Cleavage of HPV16 DNA by Cas9, tracrRNA, and crRNAs
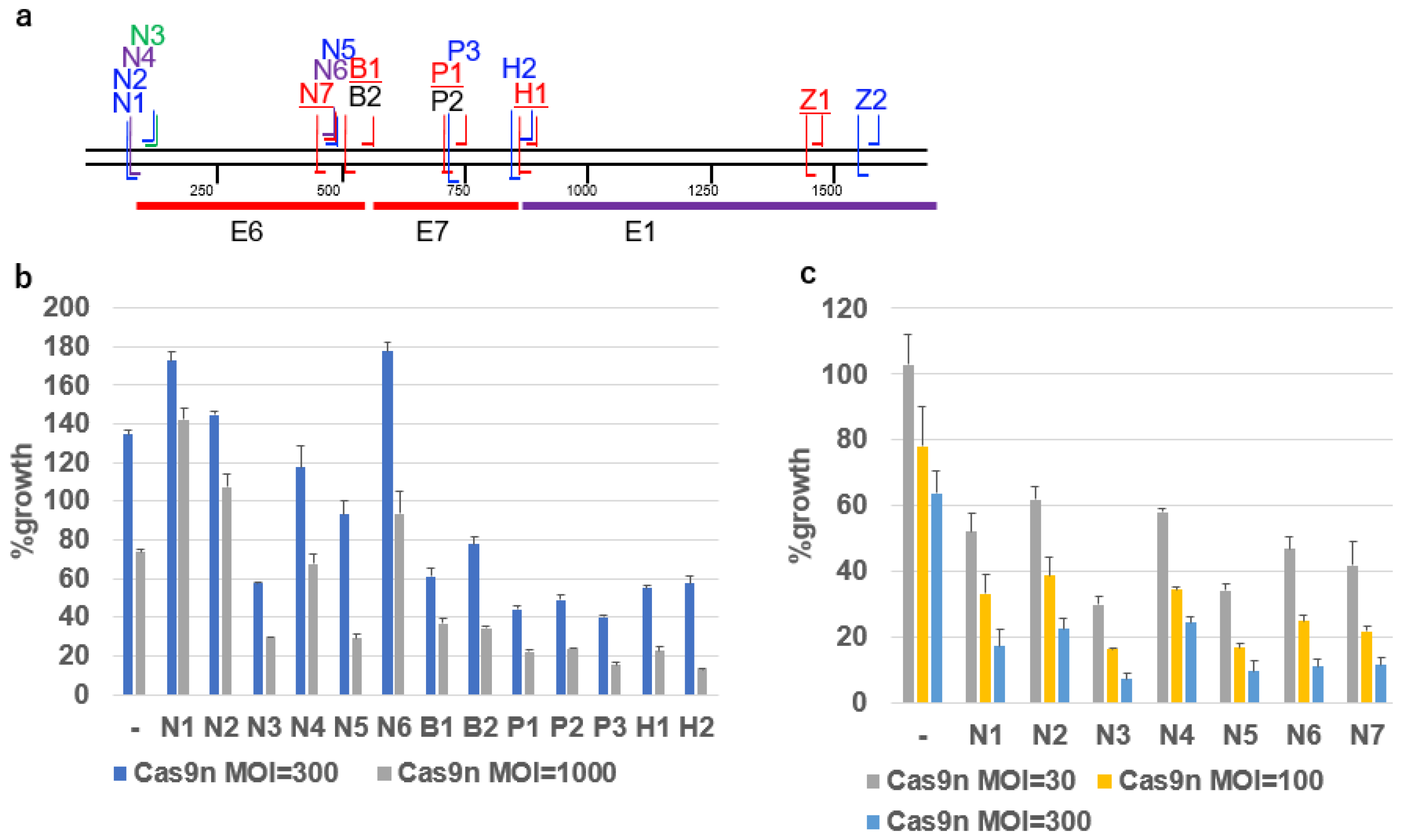
2.2. Growth Inhibition by gRNAs and Cas9n
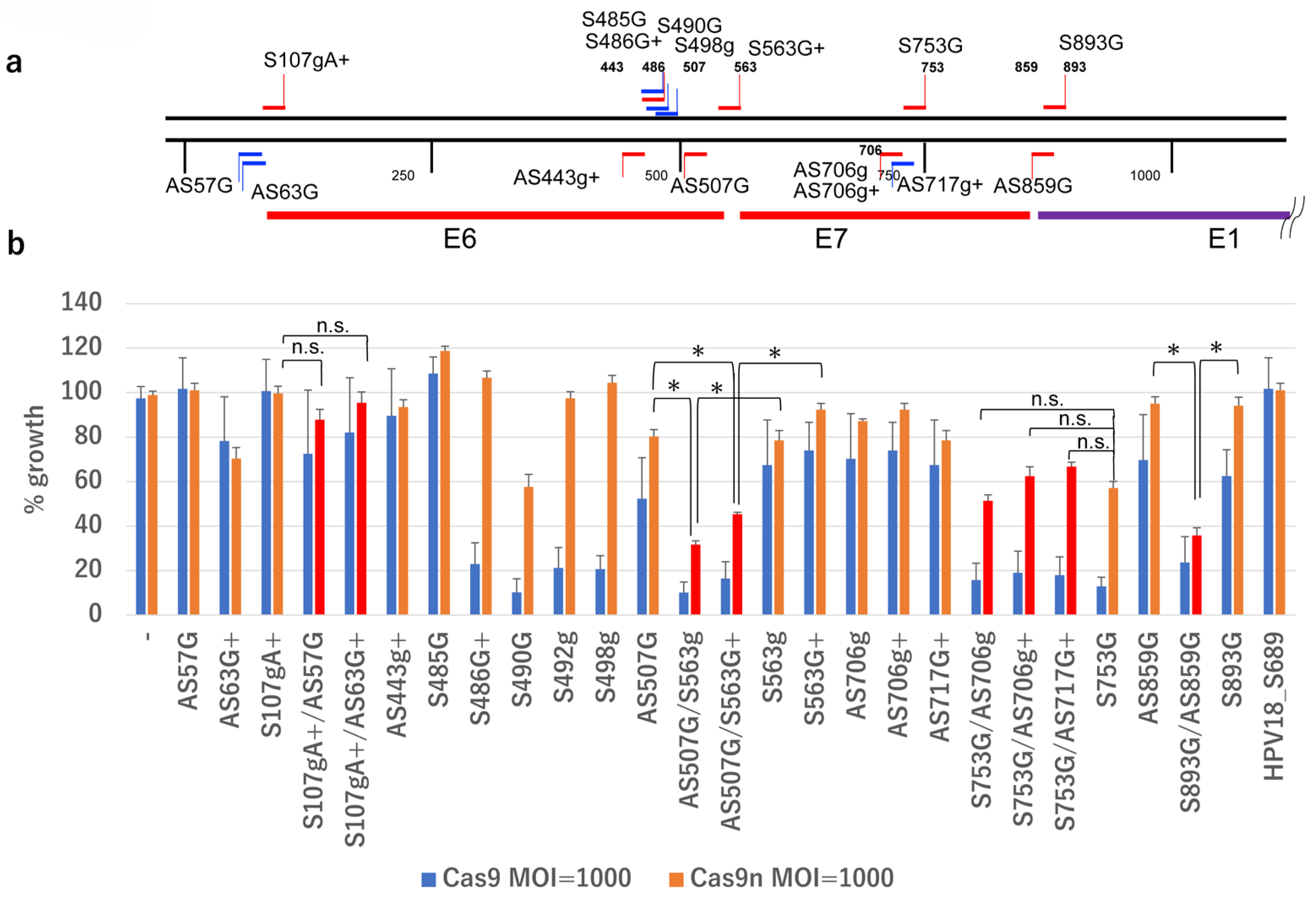
2.3. Design of DN-gRNA Pairs Targeting the E6/E7 Region of HPV16
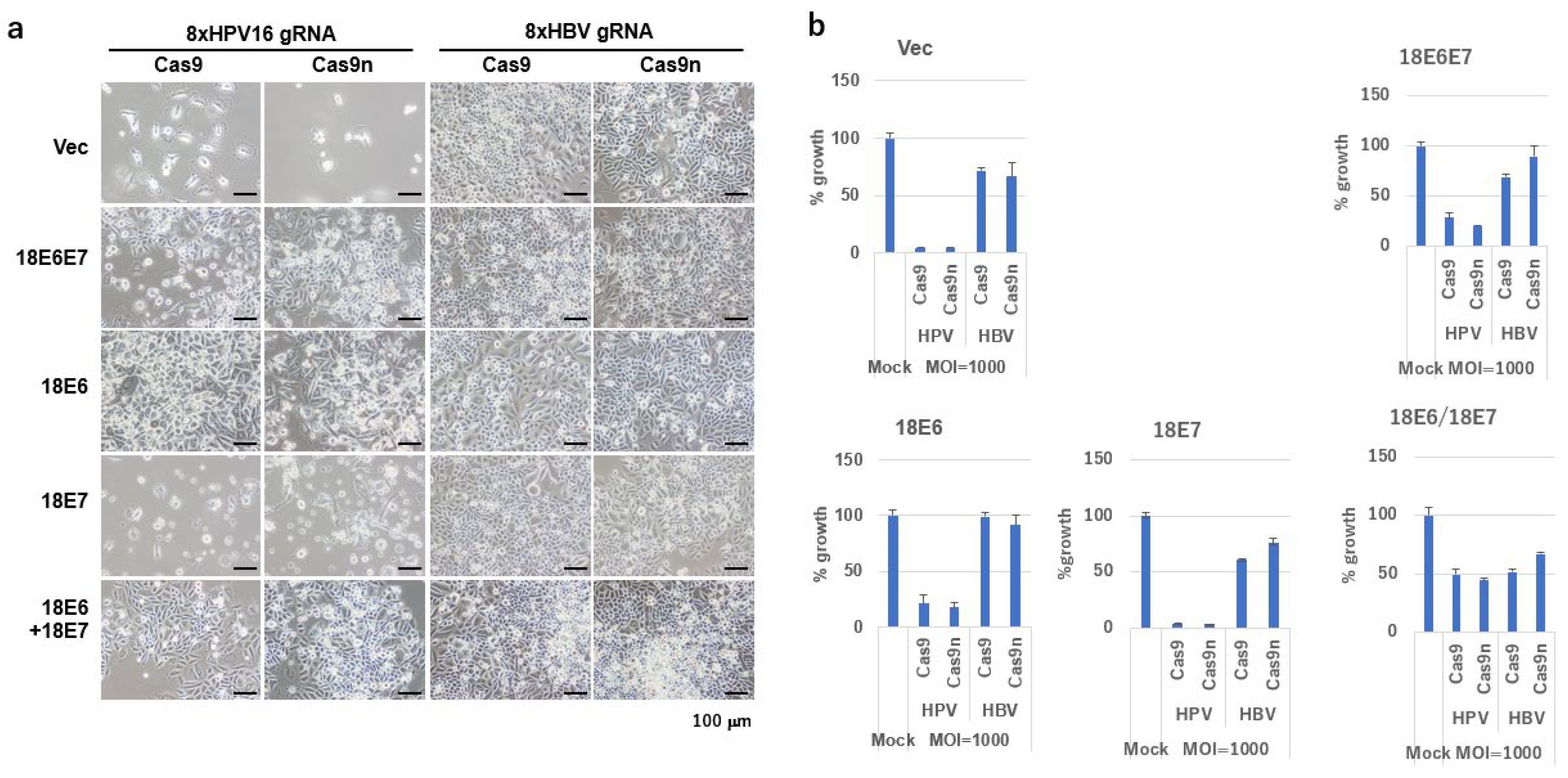
2.4. Growth Inhibition of SiHa Cells by DN-gRNA Pairs and Cas9n
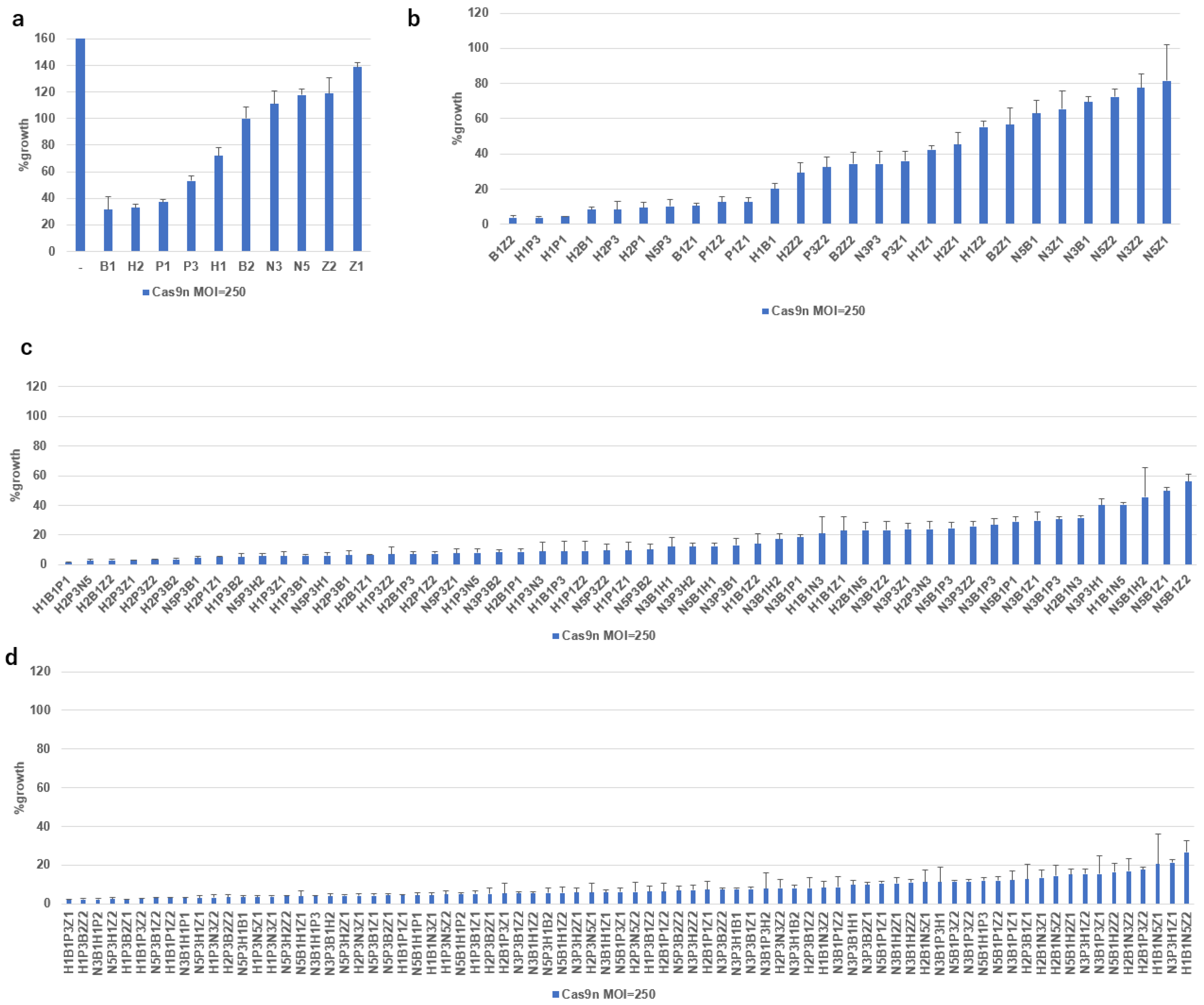
2.5. Growth Inhibition of SiHa Cells by Multiplex DN-gRNA Pair Combinations
2.6. Growth Inhibition by Co-Infection of All-in-One AdVs Simultaneously Expressing Four DN-gRNA Pairs and AdV Expressing Cas9n
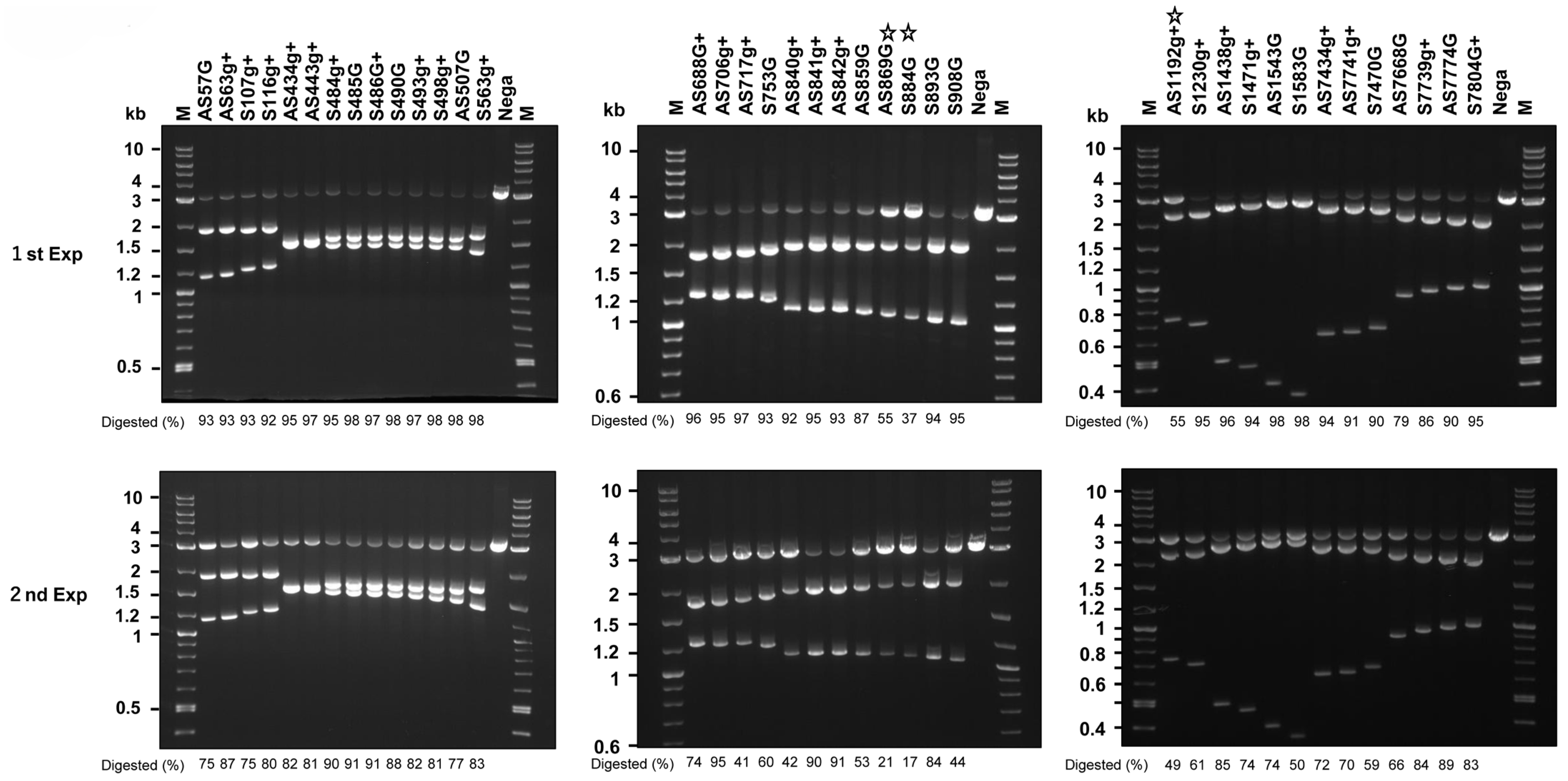
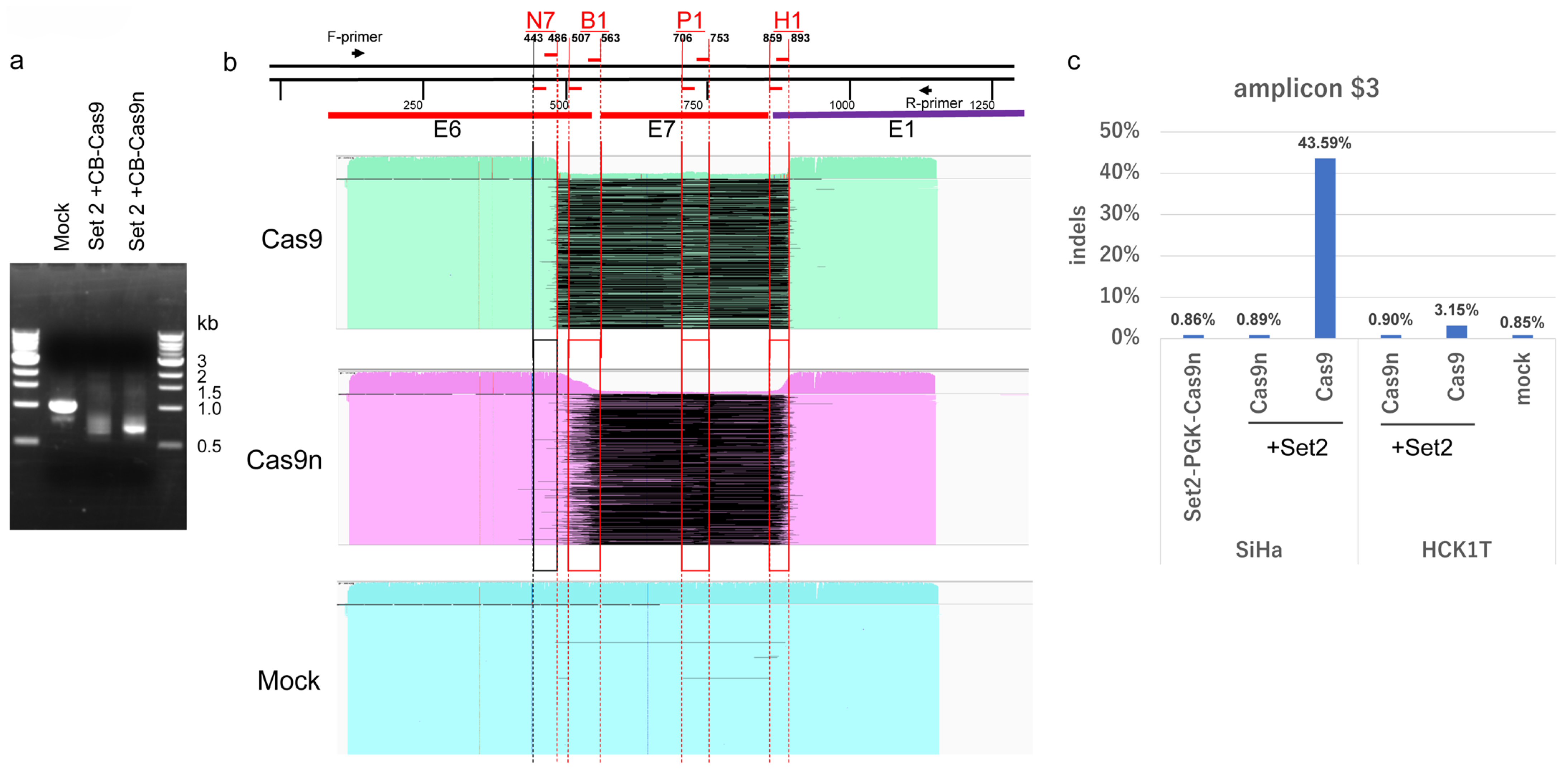
2.7. Disruption of the HPV16 Genome by DN-gRNAs and Cas9n Expressed by All-in-One AdVs
2.8. Large Deletion of HPV16 Genome Induced by Four DN-gRNAs and Cas9n
2.9. Comparison of Off-Target Effects Induced by Cas9n and Wild-Type Cas9
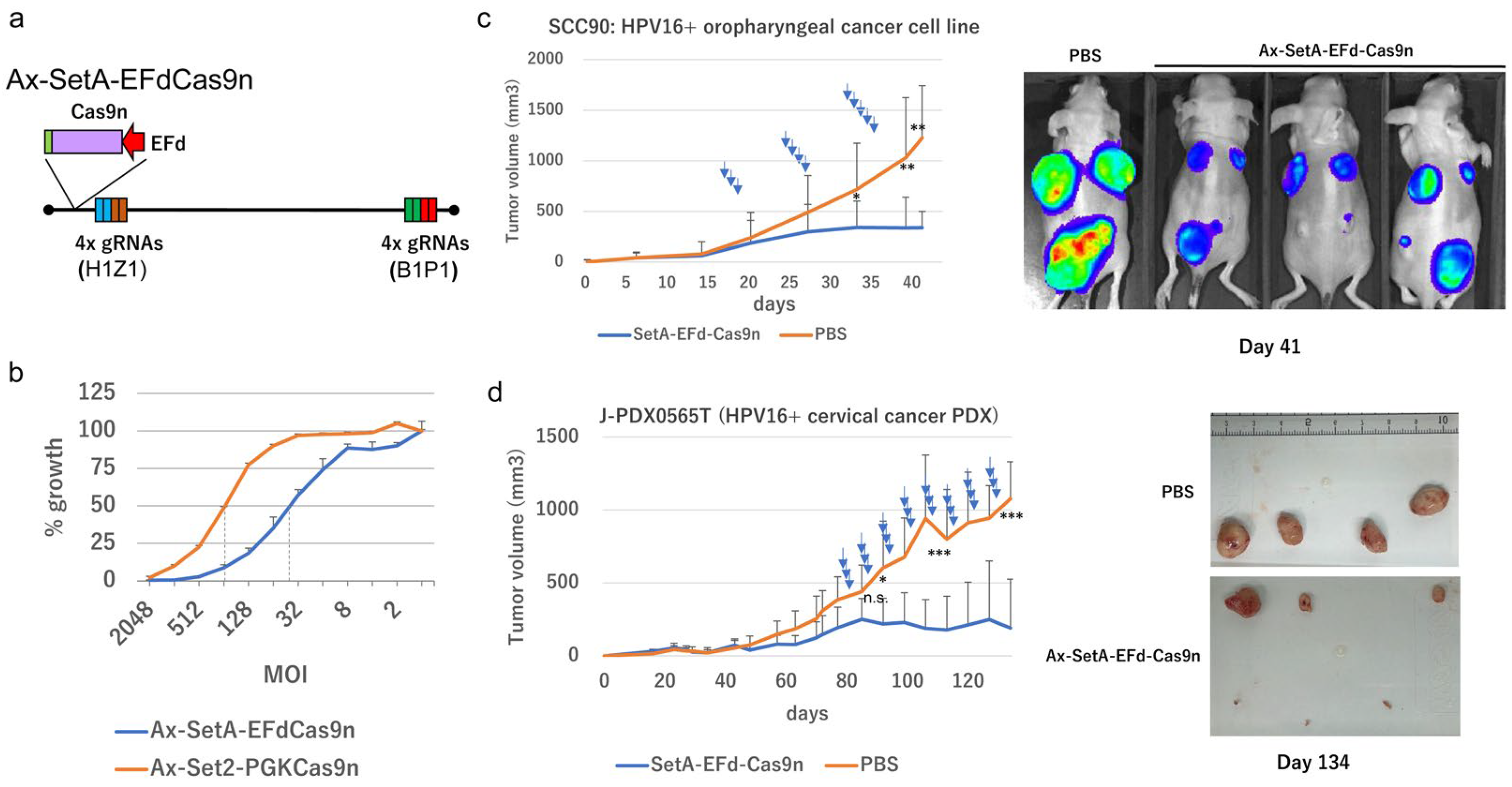
2.10. Tumor Suppression In Vivo
3. Discussion
4. Materials and Methods
4.1. Plasmids
4.2. Retrovirus and Lentivirus Infection
4.3. Cell Culture
4.4. Colorimetric Cytotoxicity Assay
4.5. Construction of Cosmids Containing AdV Genome Bearing Multiplex gRNA Units
4.6. Production of AdVs
4.7. Purification of AdVs
4.8. Titration of AdVs
4.9. In Vitro Cleavage of HPV16 DNA with Cas9, tracrRNA and Selected crRNAs
4.10. Patient Specimens and PDX Establishment
4.11. In Vivo Experiments
4.12. Selection of Putative Off-Target Sites
4.13. Detection of Indels at On-Target Sites
5. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AdV | Adenovirus vector |
| gRNA | Guide RNA |
| crRNA | CRISPR RNA |
| QOL | Quality of Life |
| PDX | Patient-derived xenograft |
| HPV | Human Papillomavirus |
References
- Singh, D.; Vignat, J.; Lorenzoni, V.; Eslahi, M.; Ginsburg, O.; Lauby-Secretan, B.; Arbyn, M.; Basu, P.; Bray, F.; Vaccarella, S. Global estimates of incidence and mortality of cervical cancer in 2020: A baseline analysis of the WHO Global Cervical Cancer Elimination Initiative. Lancet Glob. Health 2023, 11, e197–e206. [Google Scholar] [CrossRef]
- Narisawa-Saito, M.; Kiyono, T. Basic mechanisms of high-risk human papillomavirus-induced carcinogenesis: Roles of E6 and E7 proteins. Cancer Sci. 2007, 98, 1505–1511. [Google Scholar] [CrossRef]
- zur Hausen, H.; de Villiers, E.M. Human papillomaviruses. Annu. Rev. Microbiol. 1994, 48, 427–447. [Google Scholar] [CrossRef] [PubMed]
- Hoxbroe Michaelsen, S.; Gronhoj, C.; Hoxbroe Michaelsen, J.; Friborg, J.; von Buchwald, C. Quality of life in survivors of oropharyngeal cancer: A systematic review and meta-analysis of 1366 patients. Eur. J. Cancer 2017, 78, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Poorvu, P.D.; Frazier, A.L.; Feraco, A.M.; Manley, P.E.; Ginsburg, E.S.; Laufer, M.R.; LaCasce, A.S.; Diller, L.R.; Partridge, A.H. Cancer Treatment-Related Infertility: A Critical Review of the Evidence. JNCI Cancer Spectr. 2019, 3, pkz008. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wang, J.; Yang, W.; Hou, F.; Feng, X. Precision therapeutic targets for HPV-positive cancers: An overview and new insights. Infect. Agents Cancer 2025, 20, 17. [Google Scholar] [CrossRef]
- Ran, F.A.; Hsu, P.D.; Lin, C.Y.; Gootenberg, J.S.; Konermann, S.; Trevino, A.E.; Scott, D.A.; Inoue, A.; Matoba, S.; Zhang, Y.; et al. Double nicking by RNA-guided CRISPR Cas9 for enhanced genome editing specificity. Cell 2013, 154, 1380–1389. [Google Scholar] [CrossRef]
- Hryhorowicz, M.; Lipianki, D.; Zeyland, J.; Slomski, R. CRISPR/Cas9 Immune System as a Tool for Genome Engineering. Arch. Immunol. Ther. Exp. 2017, 65, 233–240. [Google Scholar] [CrossRef]
- Nakahara, T.; Tabata, H.; Kato, Y.; Fuse, R.; Nakamura, M.; Yamaji, M.; Hattori, N.; Kiyono, T.; Saito, I.; Nakanishi, T. Construction and Stability of All-in-One Adenovirus Vectors Simultaneously Expressing Four and Eight Multiplex Guide RNAs and Cas9 Nickase. Int. J. Mol. Sci. 2024, 25, 8783. [Google Scholar] [CrossRef]
- Zhen, S.; Hua, L.; Takahashi, Y.; Narita, S.; Liu, Y.H.; Li, Y. In vitro and in vivo growth suppression of human papillomavirus 16-positive cervical cancer cells by CRISPR/Cas9. Biochem. Biophys. Res. Commun. 2014, 450, 1422–1426. [Google Scholar] [CrossRef]
- Gao, C.; Wu, P.; Yu, L.; Liu, L.; Liu, H.; Tan, X.; Wang, L.; Huang, X.; Wang, H. The application of CRISPR/Cas9 system in cervical carcinogenesis. Cancer Gene Ther. 2022, 29, 466–474, Correction in Cancer Gene Ther. 2022, 29, 625–626. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Noroozi, Z.; Shamsara, M.; Valipour, E.; Esfandyari, S.; Ehghaghi, A.; Monfaredan, A.; Azizi, Z.; Motevaseli, E.; Modarressi, M.H. Antiproliferative effects of AAV-delivered CRISPR/Cas9-based degradation of the HPV18-E6 gene in HeLa cells. Sci. Rep. 2022, 12, 2224. [Google Scholar] [CrossRef]
- Yoshiba, T.; Saga, Y.; Urabe, M.; Uchibori, R.; Matsubara, S.; Fujiwara, H.; Mizukami, H. CRISPR/Cas9-mediated cervical cancer treatment targeting human papillomavirus E6. Oncol. Lett. 2019, 17, 2197–2206. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, T.; Maekawa, A.; Suzuki, M.; Tabata, H.; Sato, K.; Mori, M.; Saito, I. Construction of adenovirus vectors simultaneously expressing four multiplex, double-nicking guide RNAs of CRISPR/Cas9 and in vivo genome editing. Sci. Rep. 2021, 11, 3961. [Google Scholar] [CrossRef] [PubMed]
- Hirose, Y.; Onuki, M.; Tenjimbayashi, Y.; Yamaguchi-Naka, M.; Mori, S.; Tasaka, N.; Satoh, T.; Morisada, T.; Iwata, T.; Kiyono, T.; et al. Whole-Genome Analysis of Human Papillomavirus Type 16 Prevalent in Japanese Women with or without Cervical Lesions. Viruses 2019, 11, 350. [Google Scholar] [CrossRef]
- Burk, R.D.; Harari, A.; Chen, Z. Human papillomavirus genome variants. Virology 2013, 445, 232–243. [Google Scholar] [CrossRef]
- Kato, Y.; Tabata, H.; Sato, K.; Nakamura, M.; Saito, I.; Nakanishi, T. Adenovirus Vectors Expressing Eight Multiplex Guide RNAs of CRISPR/Cas9 Efficiently Disrupted Diverse Hepatitis B Virus Gene Derived from Heterogeneous Patient. Int. J. Mol. Sci. 2021, 22, 10570. [Google Scholar] [CrossRef] [PubMed]
- Ferris, R.L.; Martinez, I.; Sirianni, N.; Wang, J.; Lopez-Albaitero, A.; Gollin, S.M.; Johnson, J.T.; Khan, S. Human papillomavirus-16 associated squamous cell carcinoma of the head and neck (SCCHN): A natural disease model provides insights into viral carcinogenesis. Eur. J. Cancer 2005, 41, 807–815. [Google Scholar] [CrossRef]
- Walline, H.M.; Goudsmit, C.M.; McHugh, J.B.; Tang, A.L.; Owen, J.H.; Teh, B.T.; McKean, E.; Glover, T.W.; Graham, M.P.; Prince, M.E.; et al. Integration of high-risk human papillomavirus into cellular cancer-related genes in head and neck cancer cell lines. Head. Neck J. Sci. Spec. 2017, 39, 840–852. [Google Scholar] [CrossRef]
- Olthof, N.C.; Huebbers, C.U.; Kolligs, J.; Henfling, M.; Ramaekers, F.C.; Cornet, I.; van Lent-Albrechts, J.A.; Stegmann, A.P.; Silling, S.; Wieland, U.; et al. Viral load, gene expression and mapping of viral integration sites in HPV16-associated HNSCC cell lines. Int. J. Cancer 2015, 136, E207-18. [Google Scholar] [CrossRef]
- Naito, Y.; Hino, K.; Bono, H.; Ui-Tei, K. CRISPRdirect: Software for designing CRISPR/Cas guide RNA with reduced off-target sites. Bioinformatics 2015, 31, 1120–1123. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.P.; Woodroofe, C.C.; Wood, M.G.; Que, I.; Van’t Root, M.; Ridwan, Y.; Shi, C.; Kirkland, T.A.; Encell, L.P.; Wood, K.V.; et al. Click beetle luciferase mutant and near infrared naphthyl-luciferins for improved bioluminescence imaging. Nat. Commun. 2018, 9, 132. [Google Scholar] [CrossRef]
- Wells, S.I.; Francis, D.A.; Karpova, A.Y.; Dowhanick, J.J.; Benson, J.D.; Howley, P.M. Papillomavirus E2 induces senescence in HPV-positive cells via pRB- and p21(CIP)-dependent pathways. EMBO J. 2000, 19, 5762–5771. [Google Scholar] [CrossRef]
- Jung, H.S.; Rajasekaran, N.; Ju, W.; Shin, Y.K. Human Papillomavirus: Current and Future RNAi Therapeutic Strategies for Cervical Cancer. J. Clin. Med. 2015, 4, 1126–1155. [Google Scholar] [CrossRef] [PubMed]
- Sato, N.; Saga, Y.; Uchibori, R.; Tsukahara, T.; Urabe, M.; Kume, A.; Fujiwara, H.; Suzuki, M.; Ozawa, K.; Mizukami, H. Eradication of cervical cancer in vivo by an AAV vector that encodes shRNA targeting human papillomavirus type 16 E6/E7. Int. J. Oncol. 2018, 52, 687–696. [Google Scholar]
- Cui, Z.; Liu, H.; Zhang, H.; Huang, Z.; Tian, R.; Li, L.; Fan, W.; Chen, Y.; Chen, L.; Zhang, S.; et al. The comparison of ZFNs, TALENs, and SpCas9 by GUIDE-seq in HPV-targeted gene therapy. Mol. Ther. Nucleic Acids 2021, 26, 1466–1478. [Google Scholar] [CrossRef]
- Wei, Y.; Zhao, Z.; Ma, X. Description of CRISPR-Cas9 development and its prospects in human papillomavirus-driven cancer treatment. Front. Immunol. 2022, 13, 1037124. [Google Scholar] [CrossRef]
- Kennedy, E.M.; Kornepati, A.V.; Goldstein, M.; Bogerd, H.P.; Poling, B.C.; Whisnant, A.W.; Kastan, M.B.; Cullen, B.R. Inactivation of the human papillomavirus E6 or E7 gene in cervical carcinoma cells by using a bacterial CRISPR/Cas RNA-guided endonuclease. J. Virol. 2014, 88, 11965–11972. [Google Scholar] [CrossRef]
- Hu, Z.; Yu, L.; Zhu, D.; Ding, W.; Wang, X.; Zhang, C.; Wang, L.; Jiang, X.; Shen, H.; He, D.; et al. Disruption of HPV16-E7 by CRISPR/Cas system induces apoptosis and growth inhibition in HPV16 positive human cervical cancer cells. BioMed Res. Int. 2014, 2014, 612823. [Google Scholar] [CrossRef] [PubMed]
- Ehrke-Schulz, E.; Heinemann, S.; Schulte, L.; Schiwon, M.; Ehrhardt, A. Adenoviral Vectors Armed with PAPILLOMAVIRUs Oncogene Specific CRISPR/Cas9 Kill Human-Papillomavirus-Induced Cervical Cancer Cells. Cancers 2020, 12, 1934. [Google Scholar] [CrossRef] [PubMed]
- Shankar, S.; Prasad, D.; Sanawar, R.; Das, A.V.; Pillai, M.R. TALEN based HPV-E7 editing triggers necrotic cell death in cervical cancer cells. Sci. Rep. 2017, 7, 5500. [Google Scholar] [CrossRef]
- Xiong, J.; Tan, S.; Yu, L.; Shen, H.; Qu, S.; Zhang, C.; Ren, C.; Zhu, D.; Wang, H. E7-Targeted Nanotherapeutics for Key HPV Afflicted Cervical Lesions by Employing CRISPR/Cas9 and Poly (Beta-Amino Ester). Int. J. Nanomed. 2021, 16, 7609–7622. [Google Scholar] [CrossRef]
- Li, X.; Guo, M.; Hou, B.; Zheng, B.; Wang, Z.; Huang, M.; Xu, Y.; Chang, J.; Wang, T. CRISPR/Cas9 nanoeditor of double knockout large fragments of E6 and E7 oncogenes for reversing drugs resistance in cervical cancer. J. Nanobiotechnol. 2021, 19, 231. [Google Scholar] [CrossRef]
- Jubair, L.; Fallaha, S.; McMillan, N.A.J. Systemic Delivery of CRISPR/Cas9 Targeting HPV Oncogenes Is Effective at Eliminating Established Tumors. Mol. Ther. 2019, 27, 2091–2099. [Google Scholar] [CrossRef]
- Hsu, D.S.; Kornepati, A.V.; Glover, W.; Kennedy, E.M.; Cullen, B.R. Targeting HPV16 DNA using CRISPR/Cas inhibits anal cancer growth in vivo. Future Virol. 2018, 13, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Guo, M.; Wang, Q.; Dang, J.; Liu, X.; Jin, Z. Blocking activity of the HPV18 virus in cervical cancer cells using the CRISPR/Cas9 system. Int. J. Clin. Exp. Pathol. 2018, 11, 4230–4235. [Google Scholar] [PubMed]
- Zhen, S.; Lu, J.J.; Wang, L.J.; Sun, X.M.; Zhang, J.Q.; Li, X.; Luo, W.J.; Zhao, L. In Vitro and In Vivo Synergistic Therapeutic Effect of Cisplatin with Human Papillomavirus16 E6/E7 CRISPR/Cas9 on Cervical Cancer Cell Line. Transl. Oncol. 2016, 9, 498–504. [Google Scholar] [CrossRef]
- Tian, R.; Liu, J.; Fan, W.; Li, R.; Cui, Z.; Jin, Z.; Huang, Z.; Xie, H.; Li, L.; Huang, Z.; et al. Gene knock-out chain reaction enables high disruption efficiency of HPV18 E6/E7 genes in cervical cancer cells. Mol. Ther. Oncolytics 2022, 24, 171–179. [Google Scholar] [CrossRef]
- Hu, Z.; Ding, W.; Zhu, D.; Yu, L.; Jiang, X.; Wang, X.; Zhang, C.; Wang, L.; Ji, T.; Liu, D.; et al. TALEN-mediated targeting of HPV oncogenes ameliorates HPV-related cervical malignancy. J. Clin. Investig. 2015, 125, 425–436. [Google Scholar] [CrossRef]
- Jubair, L.; Lam, A.K.; Fallaha, S.; McMillan, N.A.J. CRISPR/Cas9-loaded stealth liposomes effectively cleared established HPV16-driven tumours in syngeneic mice. PLoS ONE 2021, 16, e0223288. [Google Scholar] [CrossRef] [PubMed]
- Zhen, S.; Qiang, R.; Lu, J.; Tuo, X.; Yang, X.; Li, X. CRISPR/Cas9-HPV-liposome enhances antitumor immunity and treatment of HPV infection-associated cervical cancer. J. Med. Virol. 2023, 95, e28144. [Google Scholar] [CrossRef] [PubMed]
- Iyer, V.; Boroviak, K.; Thomas, M.; Doe, B.; Riva, L.; Ryder, E.; Adams, D.J. No unexpected CRISPR-Cas9 off-target activity revealed by trio sequencing of gene-edited mice. PLoS Genet. 2018, 14, e1007503. [Google Scholar] [CrossRef]
- Lyle, C.; McCormick, F. Integrin alphavbeta5 is a primary receptor for adenovirus in CAR-negative cells. Virol. J. 2010, 7, 148. [Google Scholar] [CrossRef] [PubMed]
- Stichling, N.; Suomalainen, M.; Flatt, J.W.; Schmid, M.; Pacesa, M.; Hemmi, S.; Jungraithmayr, W.; Maler, M.D.; Freudenberg, M.A.; Pluckthun, A.; et al. Lung macrophage scavenger receptor SR-A6 (MARCO) is an adenovirus type-specific virus entry receptor. PLoS Pathog. 2018, 14, e1006914. [Google Scholar] [CrossRef] [PubMed]
- Reeh, M.; Bockhorn, M.; Gorgens, D.; Vieth, M.; Hoffmann, T.; Simon, R.; Izbicki, J.R.; Sauter, G.; Schumacher, U.; Anders, M. Presence of the coxsackievirus and adenovirus receptor (CAR) in human neoplasms: A multitumour array analysis. Br. J. Cancer 2013, 109, 1848–1858. [Google Scholar] [CrossRef]
- Kim, J.S.; Lee, S.H.; Cho, Y.S.; Kim, Y.H.; Lee, J.H. Ectopic expression of the coxsackievirus and adenovirus receptor increases susceptibility to adenoviral infection in the human cervical cancer cell line, SiHa. Biochem. Biophys. Res. Commun. 2001, 288, 240–244. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Nagasato, M.; Yoshida, T.; Aoki, K. Recent advances in genetic modification of adenovirus vectors for cancer treatment. Cancer Sci. 2017, 108, 831–837. [Google Scholar] [CrossRef]
- Mali, P.; Yang, L.; Esvelt, K.M.; Aach, J.; Guell, M.; DiCarlo, J.E.; Norville, J.E.; Church, G.M. RNA-guided human genome engineering via Cas9. Science 2013, 339, 823–826. [Google Scholar] [CrossRef]
- Saito, I.; Stark, G.R. Charomids: Cosmid vectors for efficient cloning and mapping of large or small restriction fragments. Proc. Natl. Acad. Sci. USA 1986, 83, 8664–8668. [Google Scholar] [CrossRef]
- Yugawa, T.; Nishino, K.; Ohno, S.; Nakahara, T.; Fujita, M.; Goshima, N.; Umezawa, A.; Kiyono, T. Noncanonical NOTCH signaling limits self-renewal of human epithelial and induced pluripotent stem cells through ROCK activation. Mol. Cell. Biol. 2013, 33, 4434–4447. [Google Scholar] [CrossRef]
- Skehan, P.; Storeng, R.; Scudiero, D.; Monks, A.; McMahon, J.; Vistica, D.; Warren, J.T.; Bokesch, H.; Kenney, S.; Boyd, M.R. New colorimetric cytotoxicity assay for anticancer-drug screening. J. Natl. Cancer Inst. 1990, 82, 1107–1112. [Google Scholar] [CrossRef]
- Burova, E.; Ioffe, E. Chromatographic purification of recombinant adenoviral and adeno-associated viral vectors: Methods and implications. Gene Ther. 2005, 12 (Suppl. S1), S5–S17. [Google Scholar] [CrossRef]
- Pei, Z.; Kondo, S.; Kanegae, Y.; Saito, I. Copy number of adenoviral vector genome transduced into target cells can be measured using quantitative PCR: Application to vector titration. Biochem. Biophys. Res. Commun. 2012, 417, 945–950. [Google Scholar] [CrossRef] [PubMed]
- Yagishita, S.; Kato, K.; Takahashi, M.; Imai, T.; Yatabe, Y.; Kuwata, T.; Suzuki, M.; Ochiai, A.; Ohtsu, A.; Shimada, K.; et al. Characterization of the large-scale Japanese patient-derived xenograft (J-PDX) library. Cancer Sci. 2021, 112, 2454–2466. [Google Scholar] [CrossRef] [PubMed]
- Azuma, Y.; Kusumoto-Matsuo, R.; Takeuchi, F.; Uenoyama, A.; Kondo, K.; Tsunoda, H.; Nagasaka, K.; Kawana, K.; Morisada, T.; Iwata, T.; et al. Human papillomavirus genotype distribution in cervical intraepithelial neoplasia grade 2/3 and invasive cervical cancer in Japanese women. Jpn. J. Clin. Oncol. 2014, 44, 910–917. [Google Scholar] [CrossRef]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R.; Genome Project Data Processing, S. The Sequence Alignment/Map format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef] [PubMed]
- Lanfear, R.; Schalamun, M.; Kainer, D.; Wang, W.; Schwessinger, B. MinIONQC: Fast and simple quality control for MinION sequencing data. Bioinformatics 2019, 35, 523–525. [Google Scholar] [CrossRef]
- Li, H. Minimap2: Pairwise alignment for nucleotide sequences. Bioinformatics 2018, 34, 3094–3100. [Google Scholar] [CrossRef]
- Robinson, J.T.; Thorvaldsdottir, H.; Winckler, W.; Guttman, M.; Lander, E.S.; Getz, G.; Mesirov, J.P. Integrative genomics viewer. Nat. Biotechnol. 2011, 29, 24–26. [Google Scholar] [CrossRef]
- Kyo, S.; Nakamura, M.; Kiyono, T.; Maida, Y.; Kanaya, T.; Tanaka, M.; Yatabe, N.; Inoue, M. Successful immortalization of endometrial glandular cells with normal structural and functional characteristics. Am. J. Pathol. 2003, 163, 2259–2269. [Google Scholar] [CrossRef]
- Haga, K.; Ohno, S.-i.; Yugawa, T.; Narisawa-Saito, M.; Fujita, M.; Sakamato, M.; Galloway, D.A.; Kiyono, T. Efficient immortalization of primary human cells by p16INK4a-specific short hairpin RNA or Bmi-1, combined with the introduction of hTERT. Cancer Sci. 2007, 98, 147–154. [Google Scholar] [CrossRef] [PubMed]

| Name | Sense | Target Sequence (5′ to 3′) | Anti-Sense | Target Sequence (5′ to 3′) | SNV |
|---|---|---|---|---|---|
| N1 | 16_S107gA | gAAAAGAGAACTGCAATGTTTC | 16_AS57G | GCTTTTATACTAACCGGTTT | C |
| N2 | 16_S107g+ | gAAAGAGAACTGCAATGTTTC | 16_AS57G | GCTTTTATACTAACCGGTTT | C |
| N3 | 16_S116g+ | gTGCAATGTTTCAGGACCCAC | 16_AS57G | GCTTTTATACTAACCGGTTT | C |
| N4 | 16_S107g+ | gAAAGAGAACTGCAATGTTTC | 16_AS63g+ | gATGTCTGCTTTTATACTAAC | C |
| N5 | 16_S490G | GATTCCATAATATAAGGGGT | 16_AS443g+ | gAGATGTCTTTGCTTTTCTTC | A5 |
| N6 | 16_S485G | GCAAAGATTCCATAATATAA | 16_AS443g+ | gAGATGTCTTTGCTTTTCTTC | A5 |
| N7 | 16_S486G | GCAAAGATTCCATAATATAAG | 16_AS443g+ | gAGATGTCTTTGCTTTTCTTC | A5 |
| B1 | 16_S563g+ | gAACCCAGCTGTAATCATGCA | 16_AS507G | GCAACAAGACATACATCGAC | - |
| B2 | 16_S563g | gACCCAGCTGTAATCATGCA | 16_AS507G | GCAACAAGACATACATCGAC | - |
| P1 | 16_S753G | GCAAGTGTGACTCTACGCTT | 16_AS706g+ | gATATTGTAATGGGCTCTGTC | - |
| P2 | 16_S753G | GCAAGTGTGACTCTACGCTT | 16_AS706g | gTATTGTAATGGGCTCTGTC | - |
| P3 | 16_S753G | GCAAGTGTGACTCTACGCTT | 16_AS717g+ | gAAAAGGTTACAATATTGTAA | D2, D3 |
| H1 | 16_S893G | GCAGGTACCAATGGGGAAGA | 16_AS859G | GGATCAGCCATGGTAGATTA | - |
| H2 | 16_S884G | GCTGATCCTGCAGGTACCAA | 16_AS840g+ | gATGGTTTCTGAGAACAGATG | A4 |
| Z1 | 16_S1471g+ | gACTAAAAACTAGTAATGCAA | 16_AS1438g+ | gACATTTAAAATATTTGTAAG | C |
| Z2 | 16_S1583G | GATTGGTGTATTGCTGCATT | 16_AS1543G | GTTGATTTATTACTTTTAAA | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamaji, M.; Nakahara, T.; Nakanishi, T.; Aoyama-Kikawa, S.; Yamaguchi, K.; Furukawa, Y.; Nakamura, M.; Okada, T.; Tabata, H.; Fuse, R.; et al. Disruption of Human Papillomavirus 16 E6/E7 Genes Using All-in-One Adenovirus Vectors Expressing Eight Double-Nicking Guide RNAs. Int. J. Mol. Sci. 2025, 26, 8685. https://doi.org/10.3390/ijms26178685
Yamaji M, Nakahara T, Nakanishi T, Aoyama-Kikawa S, Yamaguchi K, Furukawa Y, Nakamura M, Okada T, Tabata H, Fuse R, et al. Disruption of Human Papillomavirus 16 E6/E7 Genes Using All-in-One Adenovirus Vectors Expressing Eight Double-Nicking Guide RNAs. International Journal of Molecular Sciences. 2025; 26(17):8685. https://doi.org/10.3390/ijms26178685
Chicago/Turabian StyleYamaji, Megumi, Tomomi Nakahara, Tomoko Nakanishi, Satomi Aoyama-Kikawa, Kiyoshi Yamaguchi, Yoichi Furukawa, Mariko Nakamura, Tadashi Okada, Hirotaka Tabata, Ryoko Fuse, and et al. 2025. "Disruption of Human Papillomavirus 16 E6/E7 Genes Using All-in-One Adenovirus Vectors Expressing Eight Double-Nicking Guide RNAs" International Journal of Molecular Sciences 26, no. 17: 8685. https://doi.org/10.3390/ijms26178685
APA StyleYamaji, M., Nakahara, T., Nakanishi, T., Aoyama-Kikawa, S., Yamaguchi, K., Furukawa, Y., Nakamura, M., Okada, T., Tabata, H., Fuse, R., Shimizu, E., Kasajima, R., Imoto, S., Kukimoto, I., Saito, I., & Kiyono, T. (2025). Disruption of Human Papillomavirus 16 E6/E7 Genes Using All-in-One Adenovirus Vectors Expressing Eight Double-Nicking Guide RNAs. International Journal of Molecular Sciences, 26(17), 8685. https://doi.org/10.3390/ijms26178685







