Monothiooxalamide–Benzothiazole Hybrids: Predictive Docking on HDAC6, Synthesis, Molecular Structure, and Antiproliferative Activity on Breast Cancer Cells
Abstract
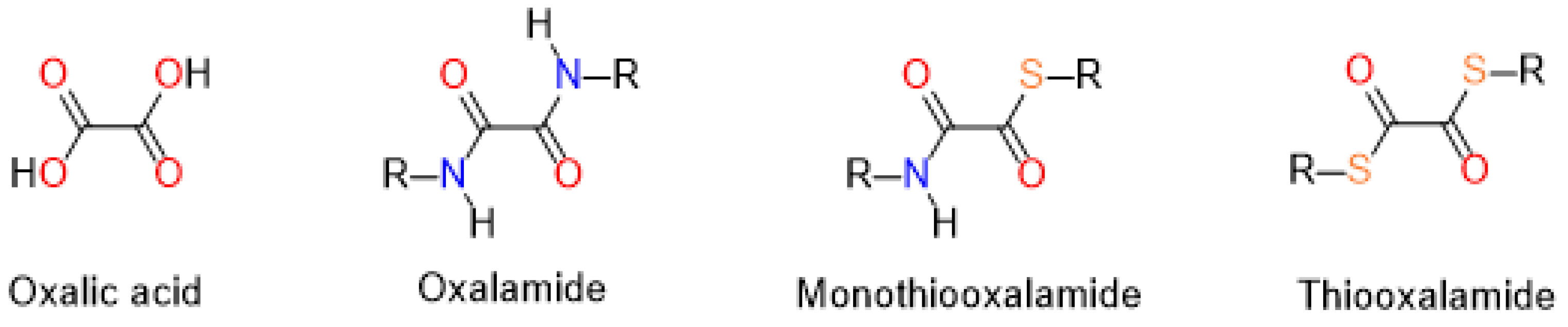
1. Introduction
2. Results and Discussion
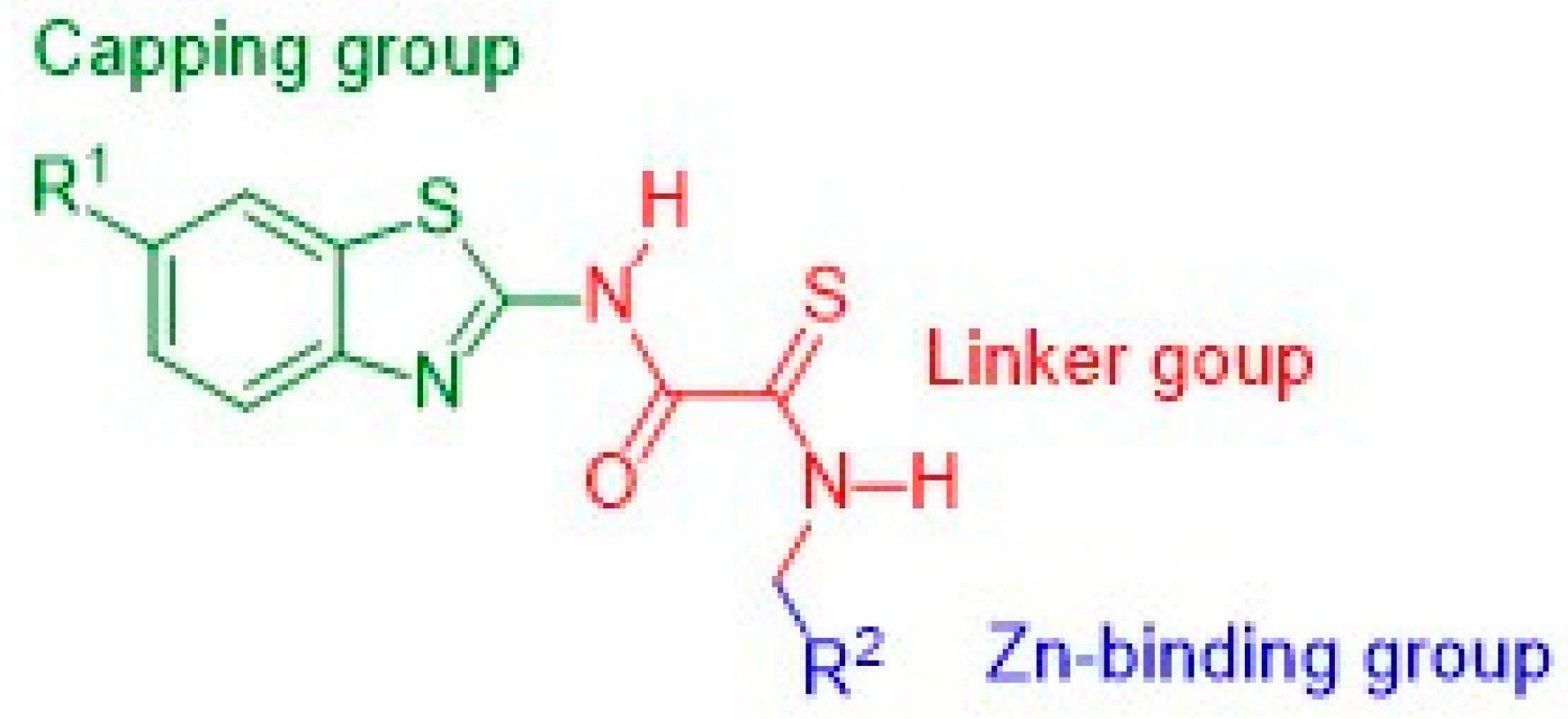
2.1. Rational Design of Compounds
2.2. Synthesis and NMR Assignments
2.3. Molecular Structure and Intramolecular Hydrogen Bonding of Compound 1c
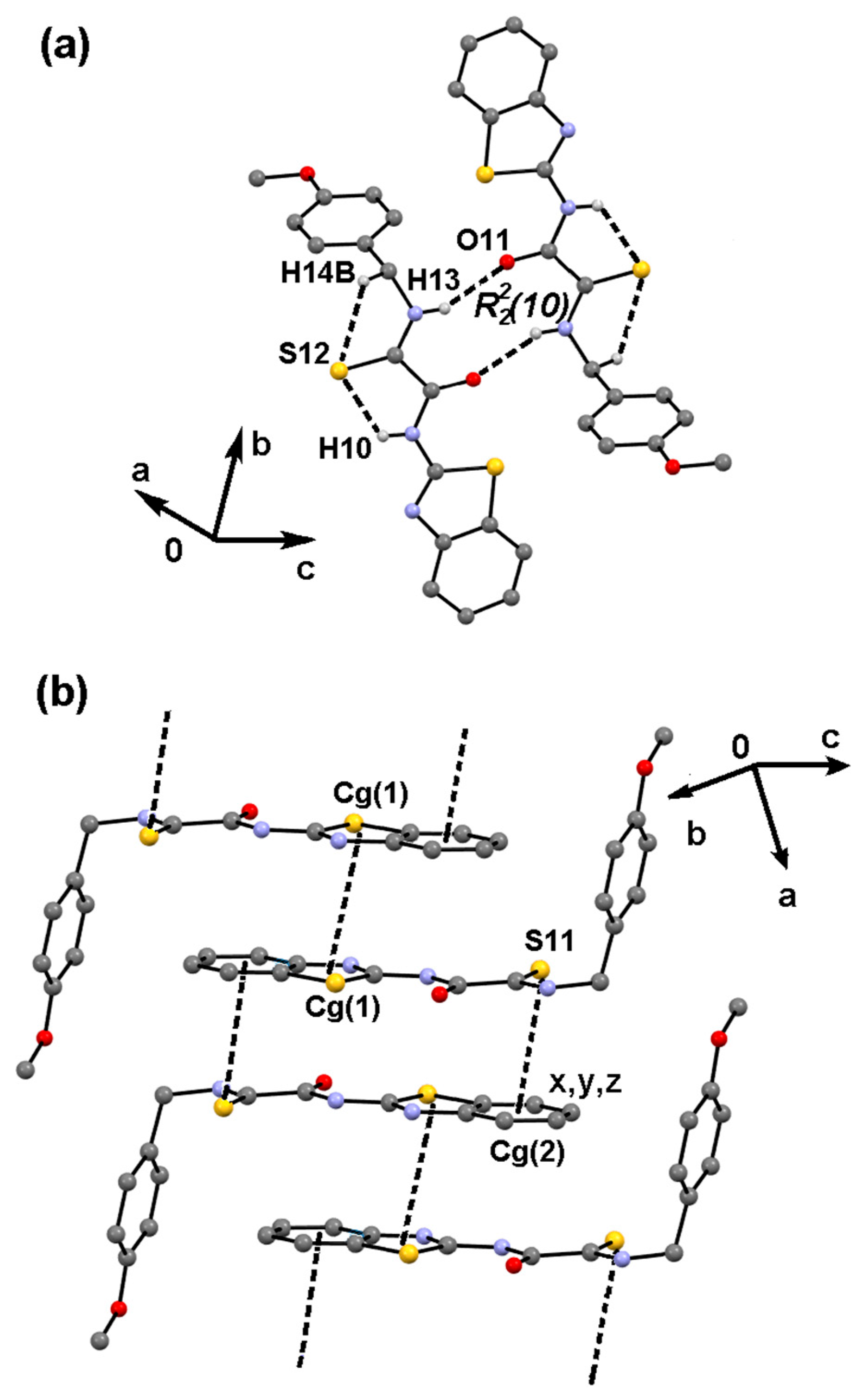
2.4. Supramolecular Structure of Compound 1c
2.5. Molecular Docking to DD2-HDAC6 Catalytic Site
2.6. Antiproliferative Activity
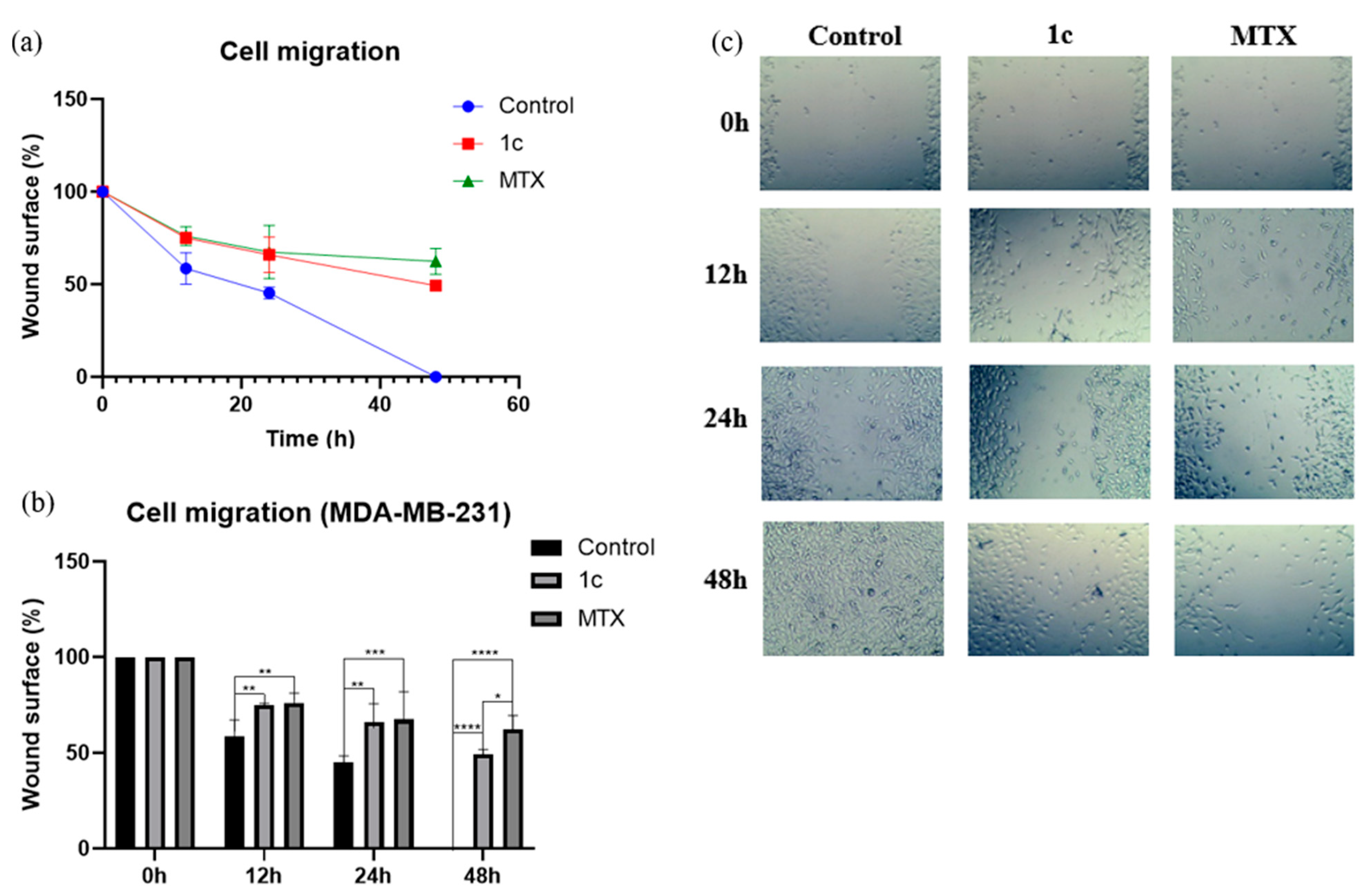
2.7. Cell Migration Assay
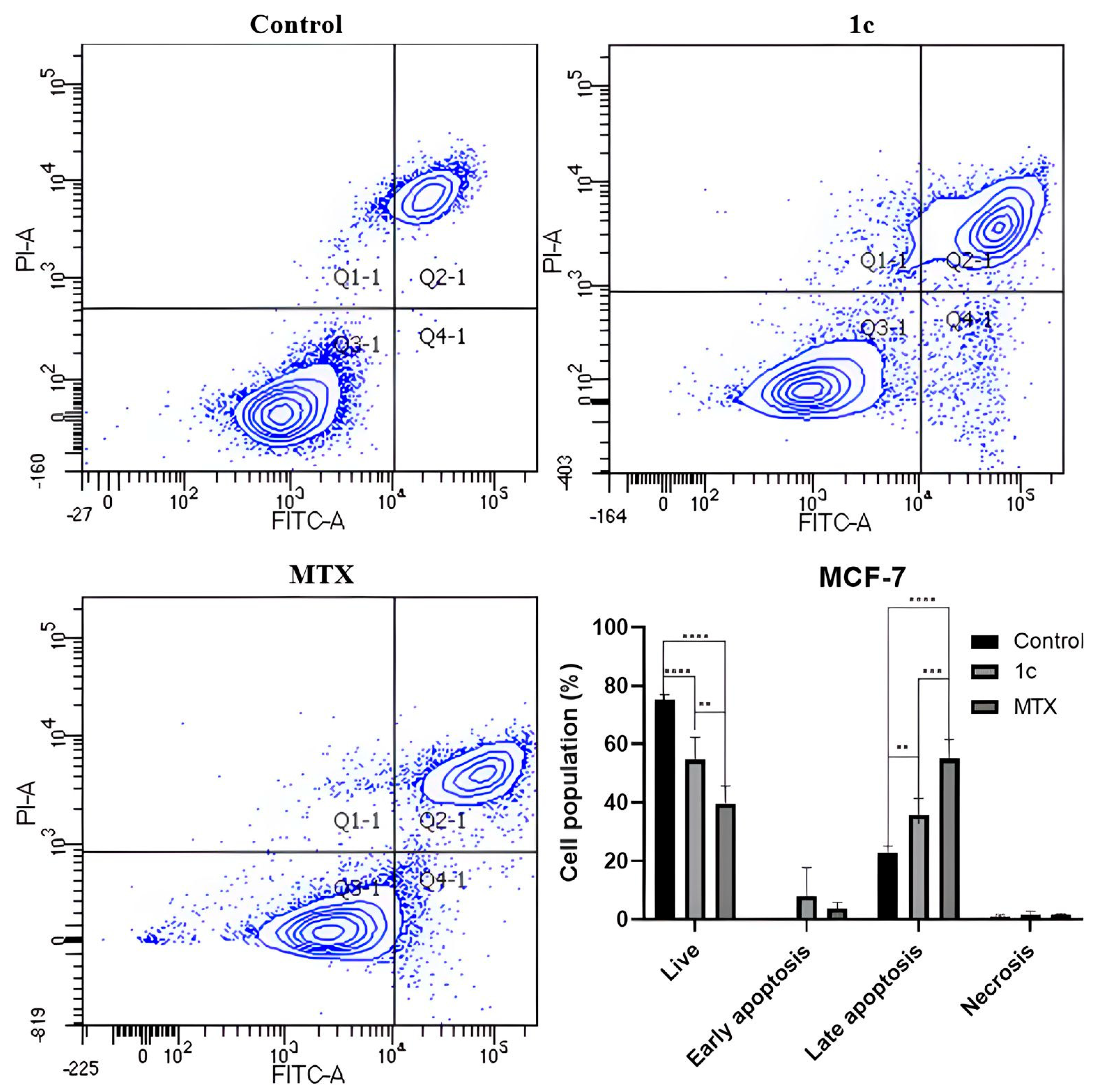
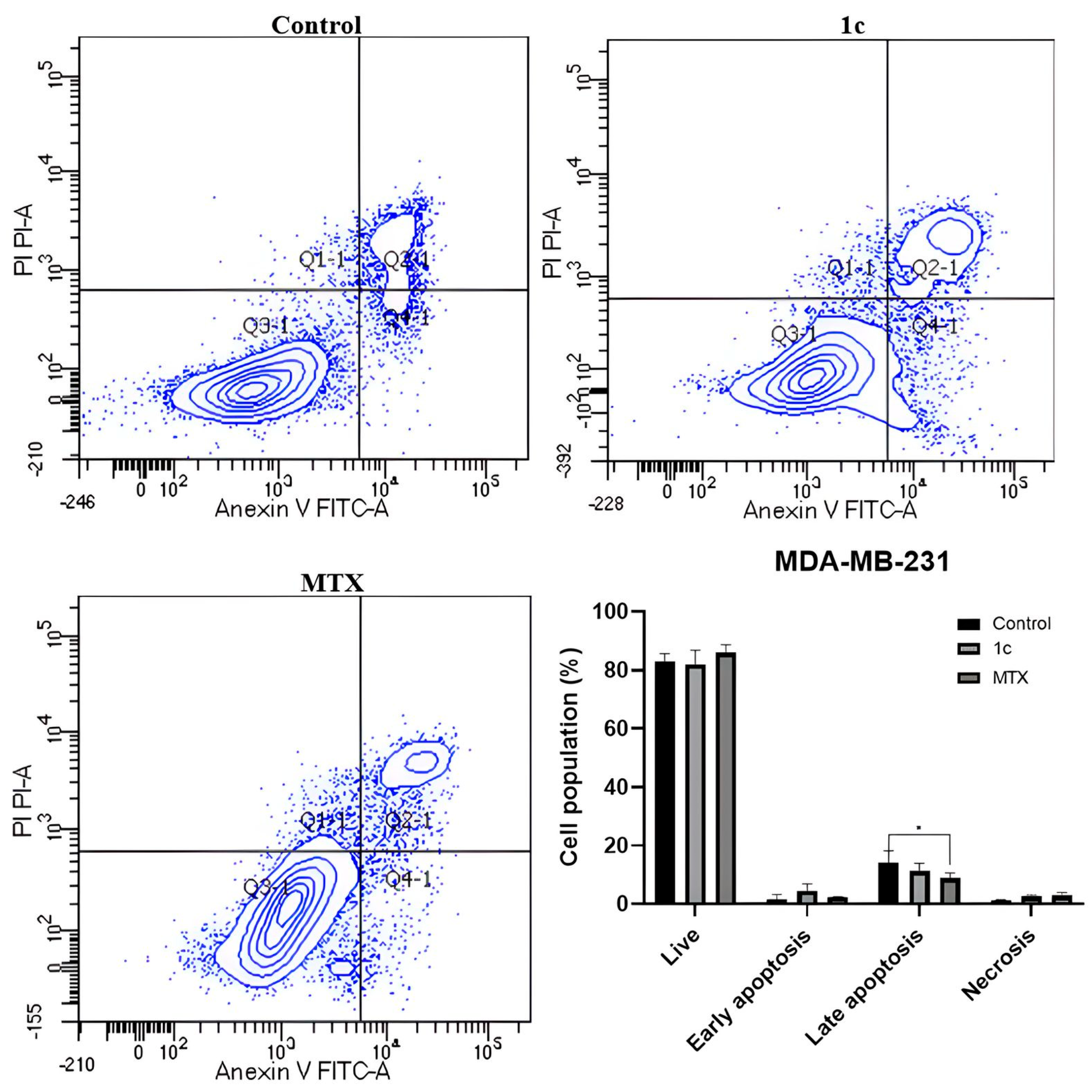
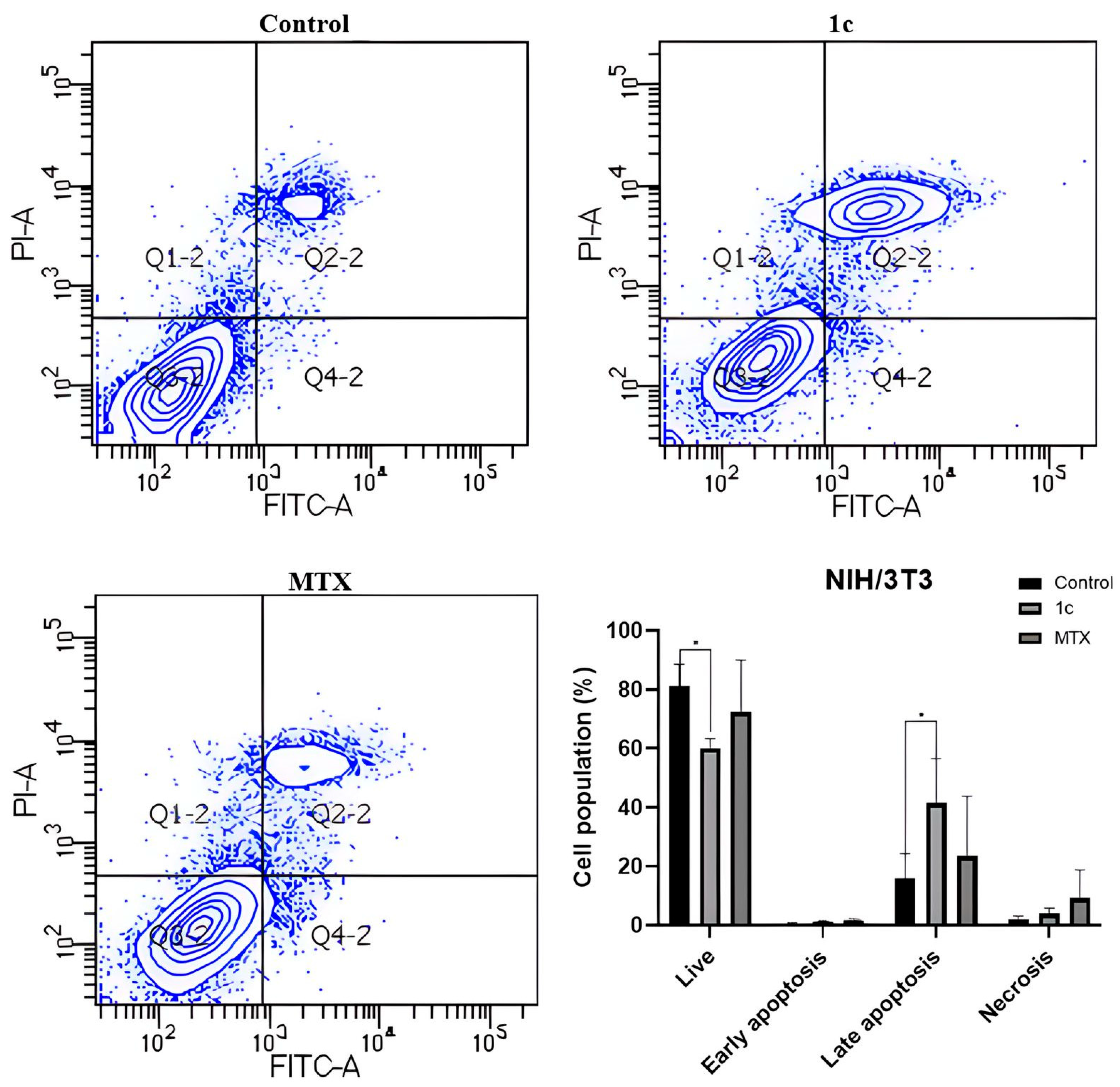
2.8. Apoptosis Induction
2.9. Physicochemical and Toxicological Properties Prediction
3. Materials and Methods
3.1. Materials and Equipment
3.2. General Procedure for the Synthesis of MTA-BTs 1a–d and 2a–d
3.3. Monocrystal X-Ray Diffraction
3.4. Docking Simulation
3.5. Antiproliferative Activity Assays
3.6. Wound Closure Assay
3.7. Apoptosis Induction Assays
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nguyen, T.L.; Fowler, F.W.; Lauher, J.W. Commensurate and Incommensurate Hydrogen Bonds. An Exercise in Crystal Engineering. J. Am. Chem. Soc. 2001, 123, 11057–11064. [Google Scholar] [CrossRef]
- Ramírez-Milanés, E.G.; Martínez-Martínez, F.J.; Magaña-Vergara, N.E.; Rojas-Lima, S.; Avendano-Jiménez, Y.A.; García-Báez, E.V.; Morín-Sánchez, L.M.; Padilla-Martínez, I.I. Positional Isomerism and Steric Effects in the Self-Assemblies of Phenylene Bis-Monothiooxalamides. Cryst. Growth Des. 2017, 17, 2513–2528. [Google Scholar] [CrossRef]
- Savchenko, R.G.; Mescheryakova, E.S.; Bikmukhametov, K.S.; Tulyabaev, A.R.; Parfenova, L.V.; Khalilov, L.M. Hydroxy Derivatives of Poststerone and Its Nontrivial 13(14→8)-Abeo-analogues: Synthesis, Crystal Packing, and Intermolecular Hydrogen Bonds. J. Mol. Struct. 2021, 1227, 129509. [Google Scholar] [CrossRef]
- Colorado-Peralta, R.; Olivares-Romero, J.L.; Rosete-Luna, S.; García-Barradas, O.; Reyes-Márquez, V.; Hernández-Romero, D.; Morales-Morales, D. Copper-Coordinated Thiazoles and Benzothiazoles: A Perfect Alliance in the Search for Compounds with Antibacterial and Antifungal Activity. Inorganics 2023, 11, 185. [Google Scholar] [CrossRef]
- Bhat, M.; Belagali, S.L. Structural Activity Relationship and Importance of Benzothiazole Derivatives in Medicinal Chemistry: A Comprehensive Review. Mini-Rev. Org. Chem. 2023, 17, 323–350. [Google Scholar] [CrossRef]
- Law, C.S.W.; Yeong, K.Y. Current trends of benzothiazoles in drug discovery: A patent review (2015–2020). Expert Opin. Ther. Pat. 2022, 32, 323–350. [Google Scholar] [CrossRef]
- Dawood, D.H.; Anwar, M.M. Recent advances in the therapeutic insights of thiazole scaffolds as acetylcholinesterase inhibitors. Eur. J. Med. Chem. 2025, 287, 117331. [Google Scholar] [CrossRef]
- Huang, G.; Cierpicki, T.; Grembecka, J. 2-Aminobenzothiazoles in anticancer drug design and discovery. Bioorg. Chem. 2023, 135, 106477. [Google Scholar] [CrossRef]
- Rouf, A.; Tanyeli, C. Bioactive thiazole and benzothiazole derivatives. Eur. J. Med. Chem. 2015, 97, 911–927. [Google Scholar] [CrossRef]
- Irfan, A.; Batool, F.; Naqvi, S.A.Z.; Islam, A.; Osman, S.M.; Nocentini, A.; Alissa, S.A.; Supuran, C.T. Benzothiazole derivatives as anticancer agents. J. Enzym. Inhib. Med. Chem. 2020, 35, 265–279. [Google Scholar] [CrossRef]
- Dhadda, S.; Raigar, A.K.; Saini, K.; Manju; Guleria, A. Benzothiazoles: From recent advances in green synthesis to anti-cancer potential. Sustain. Chem. Pharm. 2021, 24, 100521. [Google Scholar] [CrossRef]
- Paoletti, N.; Supuran, C.T. Benzothiazole derivatives in the design of antitumor agents. Arch. Pharm. 2024, 357, e2400259. [Google Scholar] [CrossRef] [PubMed]
- Tratrat, C. Benzothiazole as a Promising Scaffold for the Development of Antifungal Agents. Curr. Top. Med. Chem. 2023, 23, 491–519. [Google Scholar] [CrossRef] [PubMed]
- Vemuluri, S.P.; Somarapu, V.L.; Eppakayala, L. Design, synthesis and anticancer evaluation of various aryl amide derivatives of thiazole-benzothiazole-pyrimidines. Results Chem. 2024, 7, 101403. [Google Scholar] [CrossRef]
- Raju, V.K.; Jha, A. New benzothiazole and benzoxazole picolinamide conjugates as potential anti-cancer agents: Design, synthesis, molecular docking and anticancer studies. J. Mol. Struct. 2024, 1309, 138153. [Google Scholar] [CrossRef]
- Padalkar, V.S.; Gupta, V.D.; Phatangare, K.R.; Patil, V.S.; Umape, P.G.; Sekar, N. Synthesis of novel dipodal-benzimidazole, benzoxazole and benzothiazole from cyanuric chloride: Structural, photophysical and antimicrobial studies. J. Saudi Chem. Soc. 2014, 18, 262–268. [Google Scholar] [CrossRef]
- Haider, K.; Shrivastava, N.; Pathak, A.; Dewangan, R.P.; Yahya, S.; Yar, M.S. Recent advances and SAR study of 2-substituted benzothiazole scaffold based potent chemotherapeutic agents. Results Chem. 2022, 4, 100258. [Google Scholar] [CrossRef]
- Gschwind, A.; Fischer, O.M.; Ullrich, A. The discovery of receptor tyrosine kinases: Targets for cancer therapy. Nat. Rev. Cancer 2004, 4, 361–370. [Google Scholar] [CrossRef]
- Ropero, S.; Esteller, M. The role of histone deacetylases (HDACs) in human cancer. Mol. Oncol. 2007, 1, 19–25. [Google Scholar] [CrossRef]
- Aldana-Masangkay, G.I.; Sakamoto, K.M.; Matthias, P. The Role of HDAC6 in cancer. J. Biomed. Biotechnol. 2011, 2011, 875824. [Google Scholar] [CrossRef]
- Cheng, B.; Pan, W.; Xiao, Y.; Ding, Z.; Zhou, Y.; Fei, X.; Liu, J.; Su, Z.; Peng, X.; Chen, J. HDAC-targeting epigenetic modulators for cancer immunotherapy. Eur. J. Med. Chem. 2024, 265, 116129. [Google Scholar] [CrossRef]
- Park, C.G.; Park, S.Y.; Jun, J.A.; Jeong, K.J.; Heo, H.J.; Sohn, J.S.; Lee, H.Y.; Park, C.G.; Kang, J. Histone deacetylases 1, 6 and 8 are critical for invasion in breast cancer. Oncol. Rep. 2011, 25, 1677–1681. [Google Scholar] [CrossRef]
- Balbuena-Rebolledo, I.; Rivera-Antonio, A.M.; Sixto-López, Y.; Correa-Basurto, J.; Rosales-Hernández, M.C.; Mendieta-Wejebe, J.E.; Martínez-Martínez, F.J.; Olivares-Corichi, I.M.; García-Sánchez, J.R.; Guevara-Salazar, J.A.; et al. Dihydropyrazole-Carbohydrazide Derivatives with Dual Activity as Antioxidant and Anti-Proliferative Drugs on Breast Cancer Targeting the HDAC6. Pharmaceuticals 2022, 15, 690. [Google Scholar] [CrossRef] [PubMed]
- Macías-Hernández, C.E.; Romero-Chávez, M.M.; Mojica-Sánchez, J.P.; Pineda-Urbina, K.; Martínez, M.T.S.; Jimenez-Ruiz, E.I.; Via, L.D.; Ramos-Organillo, A. Synthesis and characterization of new monothiooxalamides containing pyridine nuclei with promising antiproliferative and antioxidant activity. J. Mol. Struct. 2022, 1265, 13336. [Google Scholar] [CrossRef]
- Osko, J.D.; Christianson, D.W. Structural determinants of affinity and selectivity in the binding of inhibitors to histone deacetylase 6. Bioorg. Med. Chem. Lett. 2020, 30, 127023. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhao, C.; Zhang, G.; Xu, Q.; Liu, Q.; Zhao, W.; Chou, C.J.; Zhang, Y. Development of selective HDAC6 inhibitors with in vitro and in vivo anti-multiple myeloma activity. Bioorg. Chem. 2021, 116, 105278. [Google Scholar] [CrossRef]
- Estiu, G.; Greenberg, E.; Harrison, C.B.; Kwiatkowski, N.P.; Mazitschek, R.; Bradner, J.E.; Wiest, O. Structural Origin of Selectivity in Class II-Selective Histone Deacetylase Inhibitors Guillermina. J. Med. Chem. 2008, 51, 2898–2906. [Google Scholar] [CrossRef]
- Butler, K.V.; Kalin, J.; Brochier, C.; Vistoli, G.; Langley, B.; Kozikowski, A.P. Rational Design and Simple Chemistry Yield a Superior, Neuroprotective HDAC6 Inhibitor, Tubastatin A. J. Am. Chem. Soc. 2010, 132, 10842–10846. [Google Scholar] [CrossRef]
- Sixto-López, Y.; Gómez-Vidal, J.A.; de Pedro, N.; Bello, M.; Rosales-Hernández, M.C.; Correa-Basurto, J. Hydroxamic acid derivatives as HDAC1, HDAC6 and HDAC8 inhibitors with antiproliferative activity in cancer cell lines. Sci. Rep. 2020, 10, 10462. [Google Scholar] [CrossRef]
- Mottamal, M.; Zheng, S.; Huang, T.L.; Wang, G. Histone Deacetylase Inhibitors in Clinical Studies as Templates for New Anticancer Agents. Molecules 2015, 20, 3898–3941. [Google Scholar] [CrossRef]
- Yousefian, M.; Hashemi, M.; Eskandarpour, V.; Hadizadeh, F.; Zarghi, A.; Ghodsi, R. Design, synthesis, biological evaluation and molecular docking study of novel chalcone-based hydroxamic acids possessing a central 2, 4-dimethy pyrrole linker as potential HDAC (Histone Deacetylase) inhibitors and anticancer agents. J. Mol. Struct. 2024, 1305, 137749. [Google Scholar] [CrossRef]
- Wang, L.Z.; Ramírez, J.; Yeo, W.; Chan, M.Y.M.; Thuya, W.L.; Lau, J.Y.A.; Wan, S.C.; Wong, A.L.A.; Zee, Y.K.; Lim, R.; et al. Glucuronidation by UGT1A1 Is the Dominant Pathway of the Metabolic Disposition of Belinostat in Liver Cancer Patients. PLoS ONE 2013, 8, 354522. [Google Scholar] [CrossRef]
- Cheng, M.H.; Weng, J.Y.; Chuang, C.H.; Liao, W.T.; Lai, Y.F.; Liu, J.Y.; Fang, Y.P. Prolonging the Half-Life of Histone Deacetylase Inhibitor Belinostat via 50 nm Scale Liposomal Subcutaneous Delivery System for Peripheral T-Cell Lymphoma. Cancers 2020, 12, 2558. [Google Scholar] [CrossRef]
- Finnegan, E.; Ding, W.; Ude, Z.; Terer, S.; McGivern, T.; Blümel, A.M.; Kirwan, G.; Shao, X.; Genua, F.; Yin, X.; et al. Complexation of histone deacetylase inhibitor belinostat to Cu(II) prevents premature metabolic inactivation in vitro and demonstrates potent anti-cancer activity in vitro and ex vivo in colon cancer. Cell. Oncol. 2024, 47, 533–553. [Google Scholar] [CrossRef]
- Shen, S.; Kozikowski, A.P. Why Hydroxamates May Not Be the Best Histone Deacetylase Inhibitors—What Some May Have Forgotten or Would Rather Forget? ChemMedChem 2016, 11, 15–21. [Google Scholar] [CrossRef]
- Friedrich, A.; Assmann, A.S.; Schumacher, L.; Stuijvenberg, J.V.; Kassack, M.U.; Schulz, W.A.; Roos, W.P.; Hansen, F.K.; Pflieger, M.; Kurz, T.; et al. In Vitro Assessment of the Genotoxic Hazard of Novel Hydroxamic Acid- and Benzamide-Type Histone Deacetylase Inhibitors (HDACi). Int. J. Mol. Sci. 2020, 21, 4747. [Google Scholar] [CrossRef]
- Citarella, A.; Moi, D.; Pinzi, L.; Bonanni, D.; Rastelli, G. Hydroxamic Acid Derivatives: From Synthetic Strategies to Medicinal Chemistry Applications. ACS Omega 2021, 6, 21843–21849. [Google Scholar] [CrossRef]
- Manjula, S.N.; Noolvi, N.M.; Parihar, K.V.; Reddy, S.A.M.; Ramani, V.; Gadad, A.K.; Singh, G.; Kutty, N.G.; Rao, C.M. Synthesis and antitumor acticity of optically active thiourea and their 2-aminobenzothiazole derivatives: A novel class of anticancer agents. Eur. J. Med. Chem. 2009, 44, 2923–2929. [Google Scholar] [CrossRef] [PubMed]
- Kassem, A.F.; Althomali, R.H.; Anwar, M.M.; El-Sofany, W.I. Thiazole moiety: A promising scaffold for anticancer drug discovery. J. Mol. Struct. 2024, 1303, 137510. [Google Scholar] [CrossRef]
- Oanh, D.T.K.; Hai, H.V.; Park, S.H.; Kim, H.J.; Han, B.-W.; Kim, H.-S.; Hong, J.-T.; Han, S.-B.; Hue, V.T.M.; Nam, N.-H. Benzothiazole-containing hydroxamic acids as histone deacetylase inhibitors and antitumor agents Dao. Bioorg. Med. Chem. Lett. 2011, 21, 7509–7512. [Google Scholar] [CrossRef] [PubMed]
- Romero-Chávez, M.M.; Macías-Hernández, C.E.; Ramos-Organillo, A.; Jiménez-Ruiz, E.I.; Robles-Machuca, M.; Ocaño-Higuera, V.M.; Sumaya-Martínez, M.T. Synthesis and toxicity of monothiooxalamides against human red blood cells, brine shrimp (Artemia salina), and fruit fly (Drosophila melanoganster). Heliyon 2024, 10, e36182. [Google Scholar] [CrossRef] [PubMed]
- Wiberg, K.B.; Wang, Y. A comparison of some properties of C=O and C=S bonds Kenneth. ARKIVOC 2011, 2011, 45–56. [Google Scholar] [CrossRef]
- Uhlenheuer, D.A.; Petkau, K.; Brunsveld, L. Combining supramolecular chemistry with biology. Chem. Soc. Rev. 2010, 39, 2817–2826. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Zhao, Y. Biomedical Applications of Supramolecular Systems Based on Host–Guest Interactions. Chem. Rev. 2015, 115, 7794–7839. [Google Scholar] [CrossRef]
- Lu, R.; Zhang, X.; Cheng, X.; Zhang, Y.; Zan, X.; Zhang, L. Medical Applications Based on Supramolecular Self-Assembled Materials from Tannic Acid. Front. Chem. 2020, 8, 583484. [Google Scholar] [CrossRef]
- Regen, S.L. Drug Discovery and the Supramolecular Factor. ACS Med. Chem. Lett. 2023, 14, 687–688. [Google Scholar] [CrossRef]
- Allen, F.H.; Kennard, O.; Watson, D.G.; Brammer, L.; Orpen, A.G.; Taylor, R. Tables of Bond Lengths determined by X-Ray and Neutron Diffraction. Part I. Bond Lengths in Organic Compounds. J. Chem. Soc. Perkin Trans. 2 1987, 1, 1987. [Google Scholar] [CrossRef]
- Bondi, A. Van der Waals Volumes and Radii. J. Phys. Chem. 1964, 68, 441–451. [Google Scholar] [CrossRef]
- Ho, P.C.; Wang, J.Z.; Meloni, F.; Vargas-Baca, I. Chalcogen bonding in materials chemistry. Coord. Chem. Rev. 2020, 422, 213464. [Google Scholar] [CrossRef]
- Mahmudov, K.T.; Gurbanov, A.V.; Aliyeva, V.A.; da Silva, M.F.C.G.; Resnati, G.; Pombeiro, A.J.L. Chalcogen bonding in coordination chemistry. Coord. Chem. Rev. 2022, 464, 214556. [Google Scholar] [CrossRef]
- Sixto-López, Y.; Bello, M.; Rodríguez-Fonseca, R.A.; Rosales-Hernández, M.C.; Martínez-Archundia, M.; Gómez-Vidal, J.A.; Correa-Basurto, J. Searching the Conformational complexity and binding properties of HDAC6 through docking and Molecular Dynamic simulations. J. Biomol. Struct. Dyn. 2017, 35, 2794–2814. [Google Scholar] [CrossRef]
- Huang, Z.; Li, L.; Cheng, B.; Li, D. Small molecules targeting HDAC6 for cancer treatment: Current progress and novel strategies. Biomed. Pharmacother. 2024, 178, 117218. [Google Scholar] [CrossRef]
- Singh, A.; Patel, V.K.; Rajak, H. Appraisal of pyrrole as connecting unit in hydroxamic acid based histone deacetylase inhibitors: Synthesis, anticancer evaluation and molecular docking studies Avineesh. J. Mol. Struct. 2021, 1240, 130590. [Google Scholar] [CrossRef]
- Kumar, K.; Das, R.; Thapa, B.; Rakhecha, B.; Srivastava, S.; Savita, K.; Israr, M.; Chanda, D.; Banerjee, D.; Shanker, K.; et al. Dual targeted 2-Benzylideneindanone pendant hydroxamic acid group exhibits selective HDAC6 inhibition along with tubulin stabilization effect. Bioorg. Med. Chem. 2023, 86, 117300. [Google Scholar] [CrossRef] [PubMed]
- Porter, N.J.; Mahendran, A.; Breslow, R.; Christianson, D.W. Unusual zinc-binding mode of HDAC6-selective hydroxamate inhibitors. Proc. Natl. Acad. Sci. USA 2017, 114, 13459–13464. [Google Scholar] [CrossRef] [PubMed]
- Pflieger, M.; Sönnichsen, M.; Horstick-Muche, N.; Yang, J.; Schliehe-Diecks, J.; Schöler, A.; Borkhardt, A.; Hamacher, A.; Kassack, M.U.; Hansen, F.K.; et al. Oxa Analogues of Nexturastat A Demonstrate Improved HDAC6 Selectivity and Superior Antileukaemia Activity. ChemMedChem 2021, 16, 1798–1803. [Google Scholar] [CrossRef]
- Kotte, R.; Vedula, G.S. Design, synthesis and anticancer activity of benzothiazole hybrids: Insights from molecular docking studies. Asian J. Chem. 2025, 37, 521–528. [Google Scholar] [CrossRef]
- Aleksanyan, D.V.; Churusova, S.G.; Brunova, V.V.; Rybalkina, E.Y.; Susova, O.Y.; Peregudov, A.S.; Klemenkova, Z.S.; Denisov, G.L.; Kozlov, V.A. Synthesis, characterization, and cytotoxic activity of N-metallated rhenium(I) pincer complexes with (thio)phosphoryl pendant arms. J. Organomet. Chem. 2020, 926, 121498. [Google Scholar] [CrossRef]
- Aljbr, B.A.; Zihlif, M.; Abu-Dagab, R.; Zalloum, H. Effect of quercetin on doxorubicin cytotoxicity in sensitive and resistant human MCF7 breast cancer cell lines. Biomed. Rep. 2024, 20, 53. [Google Scholar] [CrossRef]
- Zou, J.; Zhu, L.; Jiang, X.; Wang, Y.; Wang, Y.; Wang, X.; Chen, B. Curcumin increases breast cancer cell sensitivity to cisplatin by decreasing FEN1 expression. Oncotarget 2018, 9, 11268–11278. [Google Scholar] [CrossRef]
- Leon-Galicia, I.; Diaz-Chavez, J.; Albino-Sanchez, M.E.; Garcia-Villa, E.; Bermudez-Cruz, R.; Garcia-Mena, J.; Herrera, L.A.; García-Carrancá, A.; Gariglio, P. Resveratrol decreases Rad51 expression and sensitizes cisplatin-resistant MCF-7 breast cancer cells. Oncol. Rep. 2018, 39, 3025–3033. [Google Scholar] [CrossRef]
- Yang, P.-M.; Lin, J.-H.; Huang, W.-Y.; Lin, Y.-C.; Yeh, S.-H.; Chen, C.-C. Inhibition of histone deacetylase activity is a novel function of the antifolate drug methotrexate. Biochem. Biophys. Res. Commun. 2010, 391, 1396–1399. [Google Scholar] [CrossRef]
- Huang, W.-Y.; Yang, P.-M.; Chang, Y.-F.; Marquez, V.E.; Chen, C.-C. Methotrexate induces apoptosis through p53/p21-dependent pathway and increases E-cadherin expression through downregulation of HDAC/EZH2. Biochem. Pharmacol. 2011, 81, 510–517. [Google Scholar] [CrossRef]
- Wu, B.; Fathi, S.; Mortley, M.; Mohiuddin, M.; Jang, Y.C.; Oyelere, A.K. Pyrimethamine conjugated histone deacetylase inhibitors: Design, synthesis and evidence for triple negative breast cancer selective cytotoxicity. Bioorg. Med. Chem. 2020, 28, 115345. [Google Scholar] [CrossRef]
- Shukla, A.K.; Hamidullah; Shrivash, M.K.; Tripathi, V.D.; Konwar, R.; Pandey, J. Identification of N-Hydroxycinnamamide analogues and their bio-evaluation against breast cancer cell lines. Biomed. Pharmacother. 2018, 107, 475–483. [Google Scholar] [CrossRef]
- Madrigal-Angulo, J.L.; Hernández-Fuentes, G.A.; Parra-Delgado, H.; Olvera-Valdéz, M.; Padilla-Martínez, I.I.; Cabrera-Licona, A.; Espinosa-Gil, A.S.; Delgado-Enciso, I.; Martínez-Martínez, F.J. Design, synthesis, biological and in silico evaluation of 3-carboxy-coumarin sulfonamides as potential antiproliferative agents targeting HDAC6. Biomed. Rep. 2025, 22, 6. [Google Scholar] [CrossRef]
- Hiyoshi, H.; Goto, N.; Tsuchiya, M.; Iida, K.; Nakajima, Y.; Hirata, N.; Kanda, Y.; Nagasawa, K.; Yanagisawa, J. 2-(4-Hydroxy-3-methoxyphenyl)-benzothiazole suppresses tumor progression and metastatic potential of breast cancer cells by inducing ubiquitin ligase CHIP. Sci. Rep. 2014, 4, 7095. [Google Scholar] [CrossRef] [PubMed]
- Uremis, M.M.; Ceylan, M.; Turkoz, Y. Investigation of Apoptotic and Anticancer Effects of 2-substituted Benzothiazoles in Breast Cancer Cell Lines: EGFR Modulation and Mechanistic Insights. Anti-Cancer Agents Med. Chem. 2025, 25, 433–445. [Google Scholar] [CrossRef] [PubMed]
- Geng, S.; Zuo, D.; Song, A.; Huang, B.; Wang, H.; Yu, S. RNAs associated with oxidative stress and apoptosis show anticancer effects of Flos Sophorae flavonoids extract on human hepatoma cells. Int. J. Biol. Macromol. 2024, 282, 136750. [Google Scholar] [CrossRef]
- Salvador, D.; Bastos, V.; Oliveira, H. Hyperthermia Enhances Doxorubicin Therapeutic Efficacy against A375 and MNT-1 Melanoma Cells. Int. J. Mol. Sci. 2022, 23, 35. [Google Scholar] [CrossRef] [PubMed]
- Escobedo-González, R.; Vargas-Requena, C.L.; Moyers-Montoya, E.; Aceves-Hernández, J.M.; Nicolás-Vázquez, M.I.; Miranda-Ruvalcaba, R. In silico Study of the Pharmacologic Properties and Cytotoxicity Pathways in Cancer Cells of Various Indolylquinone Analogues of Perezone. Molecules 2017, 22, 1060. [Google Scholar] [CrossRef] [PubMed]
- Ayati, A.; Falahati, M.; Irannejad, H.; Emami, S. Synthesis, in vitro antifungal evaluation and in silico study of 3-azolyl-4-chromanone phenylhydrazones. DARU J. Pharm. Sci. 2012, 20, 46. [Google Scholar] [CrossRef]
- Uysal, U.D.; Ercengiz, D.; Karaosmanoğlu, O.; Berber, B.; Sivas, H.; Berber, H. Theoretical and experimental electronic transition behaviour study of 2-((4-(dimethylamino)benzylidene) amino)-4-methylphenol and its cytotoxicity. J. Mol. Struct. 2021, 1227, 129370. [Google Scholar] [CrossRef]
- Li, W.; Si, H.; Li, Y.; Ge, C.; Song, F.; Ma, X.; Duan, Y.; Zhai, H. 3D-QSAR and molecular docking studies on designing inhibitors of the hepatitis C virus NS5B polymerase. J. Mol. Struct. 2016, 1117, 227–239. [Google Scholar] [CrossRef]
- Bechlem, K.; Aissaoui, M.; Belhani, B.; Rachedi, K.O.; Bouacida, S.; Bahadi, R.; Djouad, S.-E.; Ben Mansour, R.; Bouaziz, M.; Almalki, F.; et al. Synthesis, X-ray crystallographic study and molecular docking of new α-sulfamidophosphonates: POM analyses of their cytotoxic activity. J. Mol. Struct. 2020, 1210, 127990. [Google Scholar] [CrossRef]
- Grib, I.; Berredjem, M.; Rachedi, K.O.; Djouad, S.-E.; Bouacida, S.; Bahadi, R.; Ouk, T.-S.; Kadri, M.; Ben Hadda, T.; Belhani, B. Novel N-sulfonylphthalimides: Efficient synthesis, X-ray characterization, spectral investigations, POM analyses, DFT computations and antibacterial activity. J. Mol. Struct. 2020, 1217, 128423. [Google Scholar] [CrossRef]
- CrysAlis PRO, (v 17136.20); Agilent Technologies Ltd.: Oxfordshire, UK, 2012.
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Cryst. Struct. Commun. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Farrugia, L.J. WinGX and ORTEP for Windows: An update. J. Appl. Crystallogr. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Spek, A.L. Structure validation in chemical crystallography. Biol. Crystallogr. 2009, 65, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Macrae, C.F.; Bruno, I.J.; Chisholm, J.A.; Edgington, P.R.; Mccabe, P.; Pidcock, E.; Rodriguez-monge, L.; Taylor, R.; Van De Streek, J.; Wood, P.A. Mercury CSD 2.0—New features for the visualization and investigation of crystal structures. J. Appl. Crystallogr. 2008, 41, 466–470. [Google Scholar] [CrossRef]
- Bernstein, J.; Davis, R.E.; Shimoni, L.; Chang, N. Patterns in Hydrogen Bonding: Functionality and Graph Set Analysis in Crystals. Angew. Chem. Int. Ed. Engl. 1995, 34, 1555–1573. [Google Scholar] [CrossRef]
- Spackman, M.A.; Jayatilaka, D. Hirshfeld surface analysis. CrystEngComm 2009, 11, 19–32. [Google Scholar] [CrossRef]
- Mckinnon, J.J.; Jayatilaka, D.; Spackman, M.A. Towards quantitative analysis of intermolecular interactions with Hirshfeld surfaces. Chem. Commun. 2007, 7, 3814–3816. [Google Scholar] [CrossRef]
- Spackman, M.A.; Mckinnon, J.J. Fingerprinting intermolecular interactions in molecular crystals. CrystEngComm 2002, 4, 378–392. [Google Scholar] [CrossRef]
- Morales-Santana, M.; Chong-Canto, S.; Santiago-Quintana, J.M.; Martínez-Martínez, F.J.; García-Báez, E.V.; Cruz, A.; Rojas-Lima, S.; Padilla-Martínez, I.I. Microcrystalline solid–solid transformations of conformationally-responsive solvates, desolvates and a salt of N,N′-(1,4-phenylene)dioxalamic acid: The energetics of hydrogen bonding and n/π → π* interactions. CrystEngComm 2022, 24, 1017–1034. [Google Scholar] [CrossRef]
- Luna-Martínez, M.M.; Morales-Santana, M.; Santiago-Quintana, J.M.; García-Báez, E.V.; Narayanan, J.; Rosales-Hoz, M.J.; Padilla-Martínez, I.I. Crystal Structure, Supramolecular Organization, Hirshfeld Analysis, Interaction Energy, and Spectroscopy of Two Tris(4-aminophenyl)amine-Based Derivatives Mayra. Crystals 2024, 14, 855. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09; Revision A.02; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Mackenzie, C.F.; Spackman, P.R.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer model energies and energy frame- works: Extension to metal coordination compounds, organic salts, solvates and open-shell systems. IUCrJ 2017, 4, 575–587. [Google Scholar] [CrossRef] [PubMed]
- Turner, M.J.; Thomas, S.P.; Shi, M.W.; Jayatilaka, D.; Spackman, M.A. Energy frameworks: Insights into interaction anisotropy and the mechanical properties of molecular crystals. Chem. Commun. 2015, 51, 3735–3738. [Google Scholar] [CrossRef]
- Eberhardt, J.; Santos-Martins, D.; Tillack, A.F.; Forli, S. AutoDock Vina 1.2.0: New Docking Methods, Expanded Force Field, and Python Bindings. J. Chem. Inf. Model. 2021, 61, 3891–3898. [Google Scholar] [CrossRef]
- Allouche, A. Software News and Updates Gabedit—A Graphical User Interface for Computational Chemistry Softwares. J. Comput. Chem. 2012, 32, 174–182. [Google Scholar] [CrossRef]


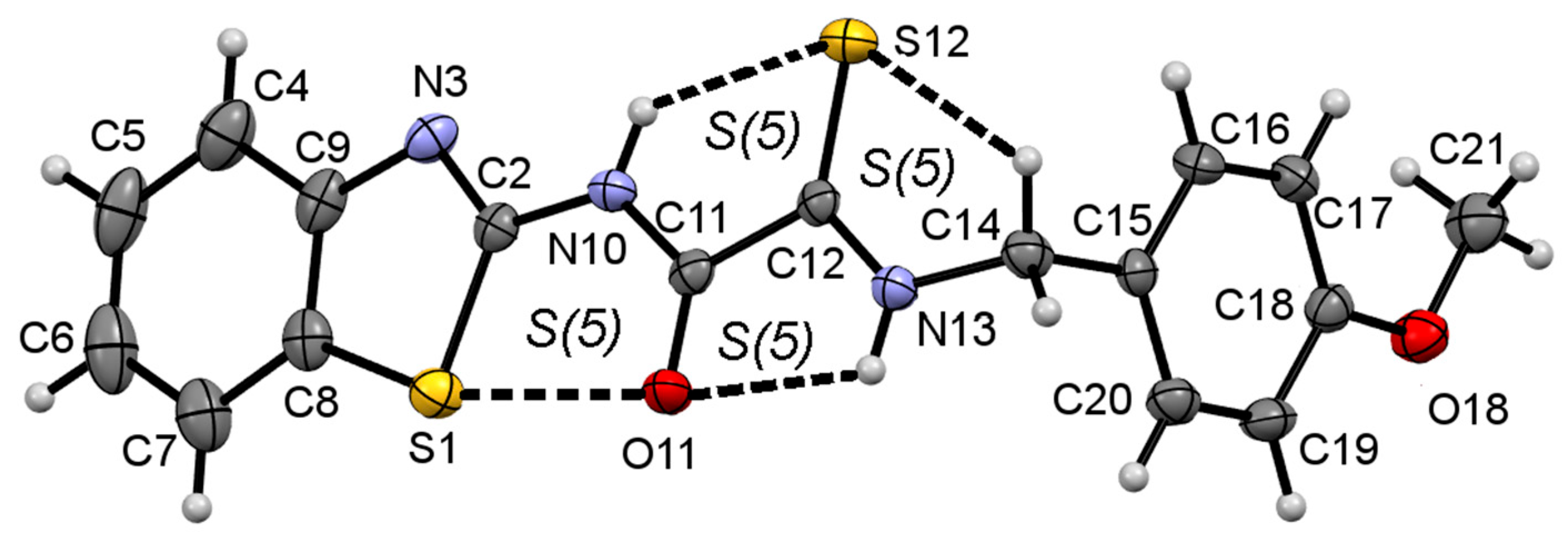

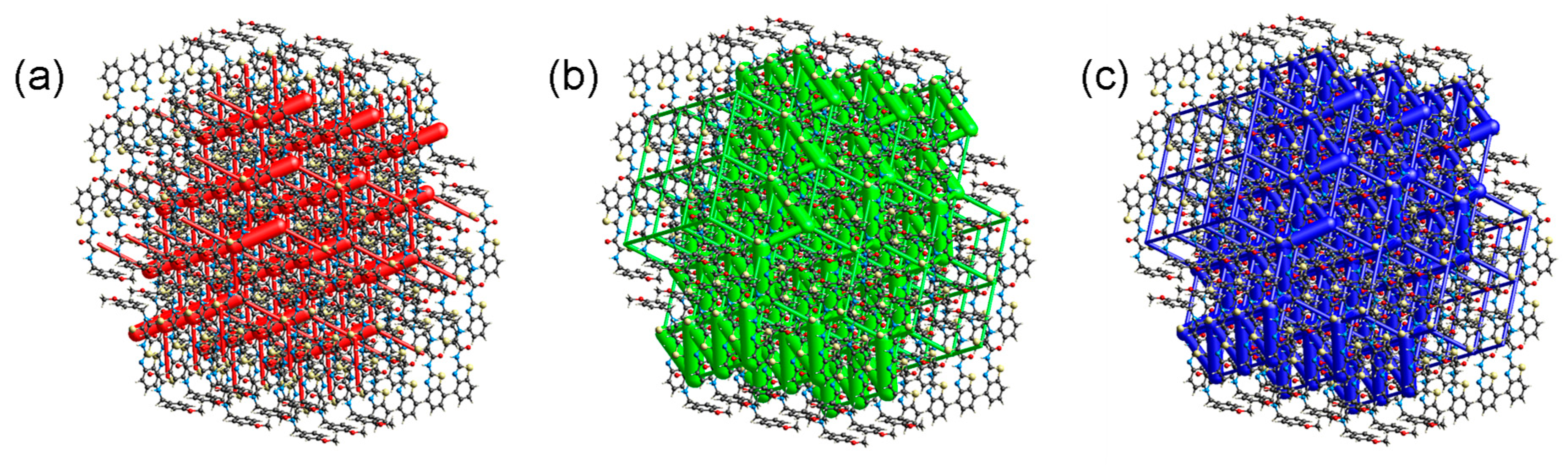

| Interaction Y—X⋯Z a | Symmetry Code | Y—X | X⋯Z/Å | Y⋯Z/Å | Y—X⋯Z/° |
|---|---|---|---|---|---|
| N10—H10⋯S12 | 0.84(3) | 2.42(3) | 2.955(3) | 122(2) | |
| N13—H13⋯O11 | 0.85(3) | 2.31(3) | 2.668(3) | 106(2) | |
| C14—H14B⋯S12 | 0.97 | 2.76 | 3.113(3) | 102 | |
| N13—H13⋯O11 | -x,2-y,1-z | 0.85(3) | 2.15(3) | 2.914(3) | 150(3) |
| C12—S12⋯Cg2 b | -x,1-y,1-z | 1.645(3) | 3.582(3) | 3.6996(18) | 72.98(11) |
| Motif | Interaction | −Eele | −Epol | −Edis | Erep | −Etot | %Eele a | %Edis a | R b/Å |
|---|---|---|---|---|---|---|---|---|---|
| R22(10) | N—H13⋯O11 | 64.3 | 9.5 | 32.7 | 43.2 | 63.3 | 60.4 | 30.8 | 6.32 |
| D | Cg1⋯Cg1 c | 17.2 | 1.9 | 76.2 | 36.3 | 59.0 | 18.1 | 80.0 | 8.36 |
| D | C8 —S12⋯Cg2 d | 5.6 | 1.9 | 60.6 | 28.1 | 40.1 | 8.2 | 89.0 | 8.48 |
| Compound | ΔGob (kcal/mol) | Kd (µM) |
|---|---|---|
| 1a | −7.4 | 3.76 |
| 1b | −7.3 | 4.45 |
| 1c | −8.3 | 0.82 |
| 1d | −8.1 | 1.15 |
| 2a | −8.2 | 0.98 |
| 2b | −7.8 | 1.91 |
| 2c | −8.6 | 0.50 |
| 2d | −8.0 | 1.37 |
| NA | −7.8 | 1.91 |
| Compound | IC50 (μM) | |||
|---|---|---|---|---|
| MCF-7 | MDA-MB-231 | MCF-10A | NIH/3T3 | |
| 1a | 95 ± 13 | 72 ± 7 | 44 ± 6 | 29 ± 6 |
| 1b | >100 | >100 | ND * | ND * |
| 1c | 32 ± 5 | 52 ± 8 | 55 ± 6 | 41 ± 5 |
| 1d | >100 | >100 | ND * | ND * |
| 2a | 72 ± 9 | >100 | 43 ± 6 | 22 ± 4 |
| 2b | >100 | >100 | 42 ± 5 | ND |
| 2c | 88 ± 6 | >100 | 74 ± 12 | 31 ± 6 |
| 2d | >100 | >100 | ND * | ND * |
| MTX | 0.8 ± 0.1 | 28 ± 3 | 14 ± 3 | 26 ± 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Macías-Hernández, C.E.; Balbuena-Rebolledo, I.; García-Báez, E.V.; Cabrera-Pérez, L.C.; Godínez-Victoria, M.; Rosales-Hernández, M.C.; Padilla-Martínez, I.I. Monothiooxalamide–Benzothiazole Hybrids: Predictive Docking on HDAC6, Synthesis, Molecular Structure, and Antiproliferative Activity on Breast Cancer Cells. Int. J. Mol. Sci. 2025, 26, 8684. https://doi.org/10.3390/ijms26178684
Macías-Hernández CE, Balbuena-Rebolledo I, García-Báez EV, Cabrera-Pérez LC, Godínez-Victoria M, Rosales-Hernández MC, Padilla-Martínez II. Monothiooxalamide–Benzothiazole Hybrids: Predictive Docking on HDAC6, Synthesis, Molecular Structure, and Antiproliferative Activity on Breast Cancer Cells. International Journal of Molecular Sciences. 2025; 26(17):8684. https://doi.org/10.3390/ijms26178684
Chicago/Turabian StyleMacías-Hernández, Carlos Eduardo, Irving Balbuena-Rebolledo, Efrén V. García-Báez, Laura C. Cabrera-Pérez, Marycarmen Godínez-Victoria, Martha C. Rosales-Hernández, and Itzia I. Padilla-Martínez. 2025. "Monothiooxalamide–Benzothiazole Hybrids: Predictive Docking on HDAC6, Synthesis, Molecular Structure, and Antiproliferative Activity on Breast Cancer Cells" International Journal of Molecular Sciences 26, no. 17: 8684. https://doi.org/10.3390/ijms26178684
APA StyleMacías-Hernández, C. E., Balbuena-Rebolledo, I., García-Báez, E. V., Cabrera-Pérez, L. C., Godínez-Victoria, M., Rosales-Hernández, M. C., & Padilla-Martínez, I. I. (2025). Monothiooxalamide–Benzothiazole Hybrids: Predictive Docking on HDAC6, Synthesis, Molecular Structure, and Antiproliferative Activity on Breast Cancer Cells. International Journal of Molecular Sciences, 26(17), 8684. https://doi.org/10.3390/ijms26178684







