Neocnidilide and 6-Gingerol as Key Bioactives in Fresh and Dried Centipeda minima: Distinct Th1/Th2 Modulation via NF-κB/JAK-STAT Pathways for Allergic Rhinitis Therapy
Abstract
1. Introduction
2. Results
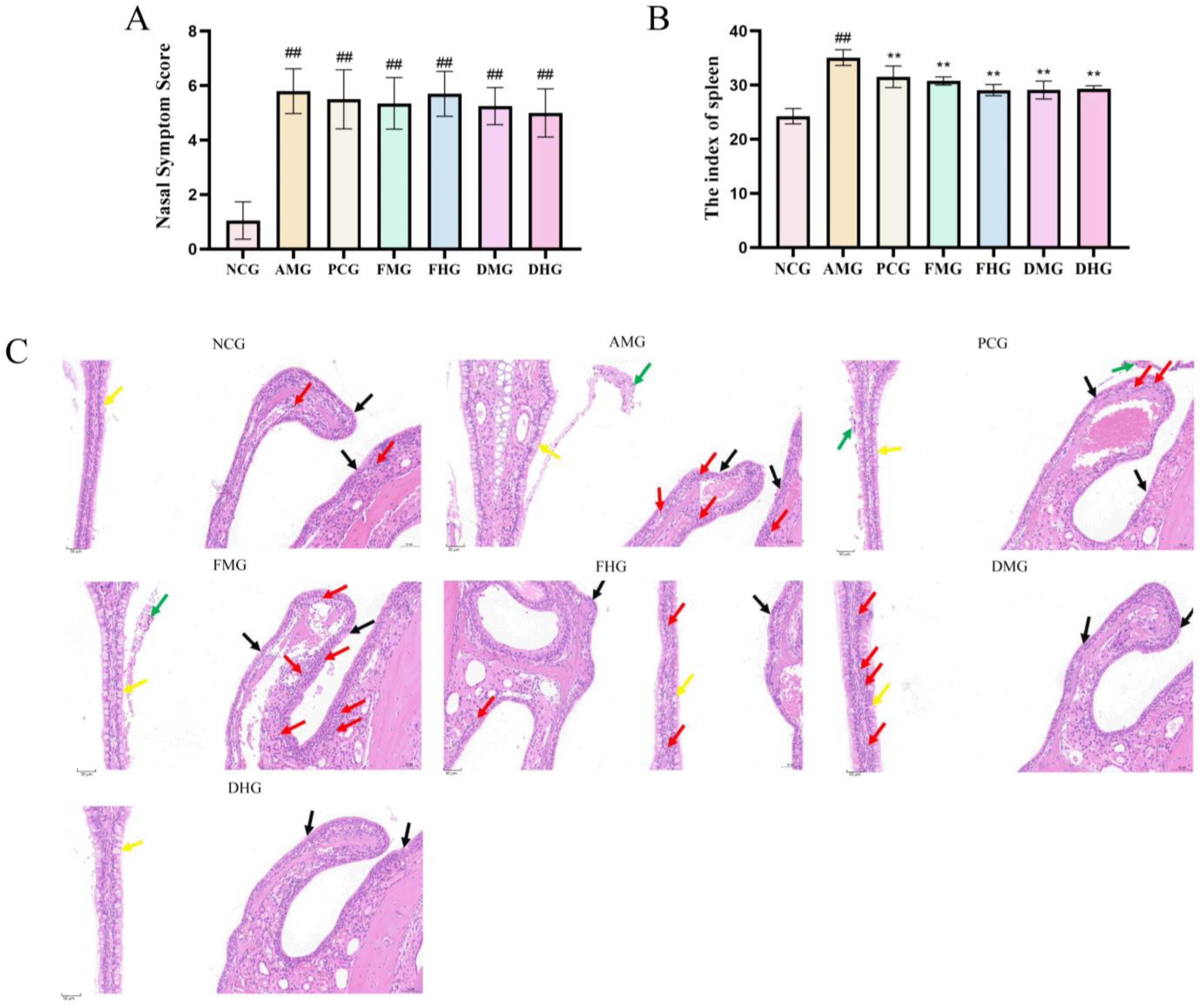
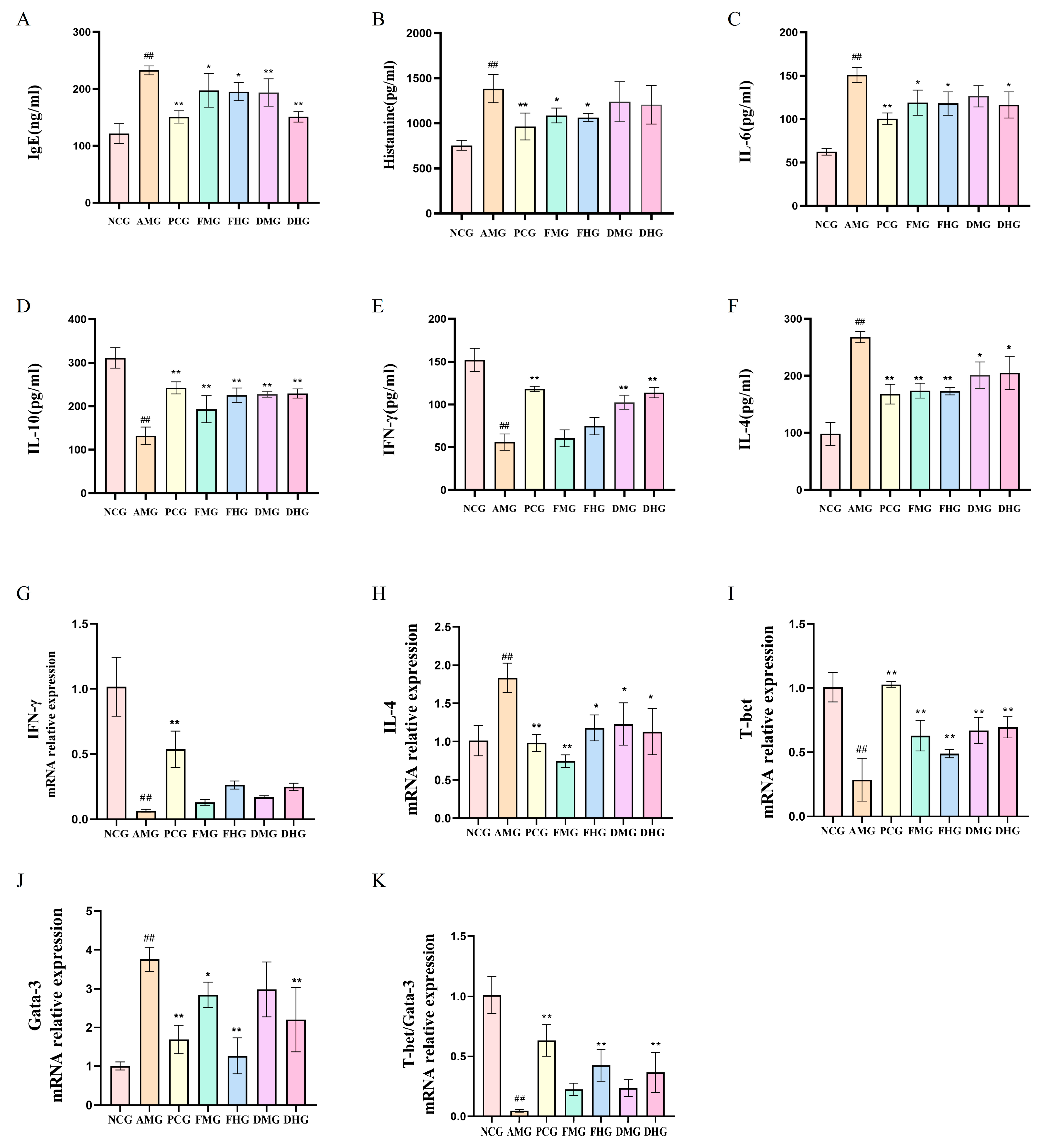
2.1. Therapeutic Effect of CMF and CMD on AR Mice
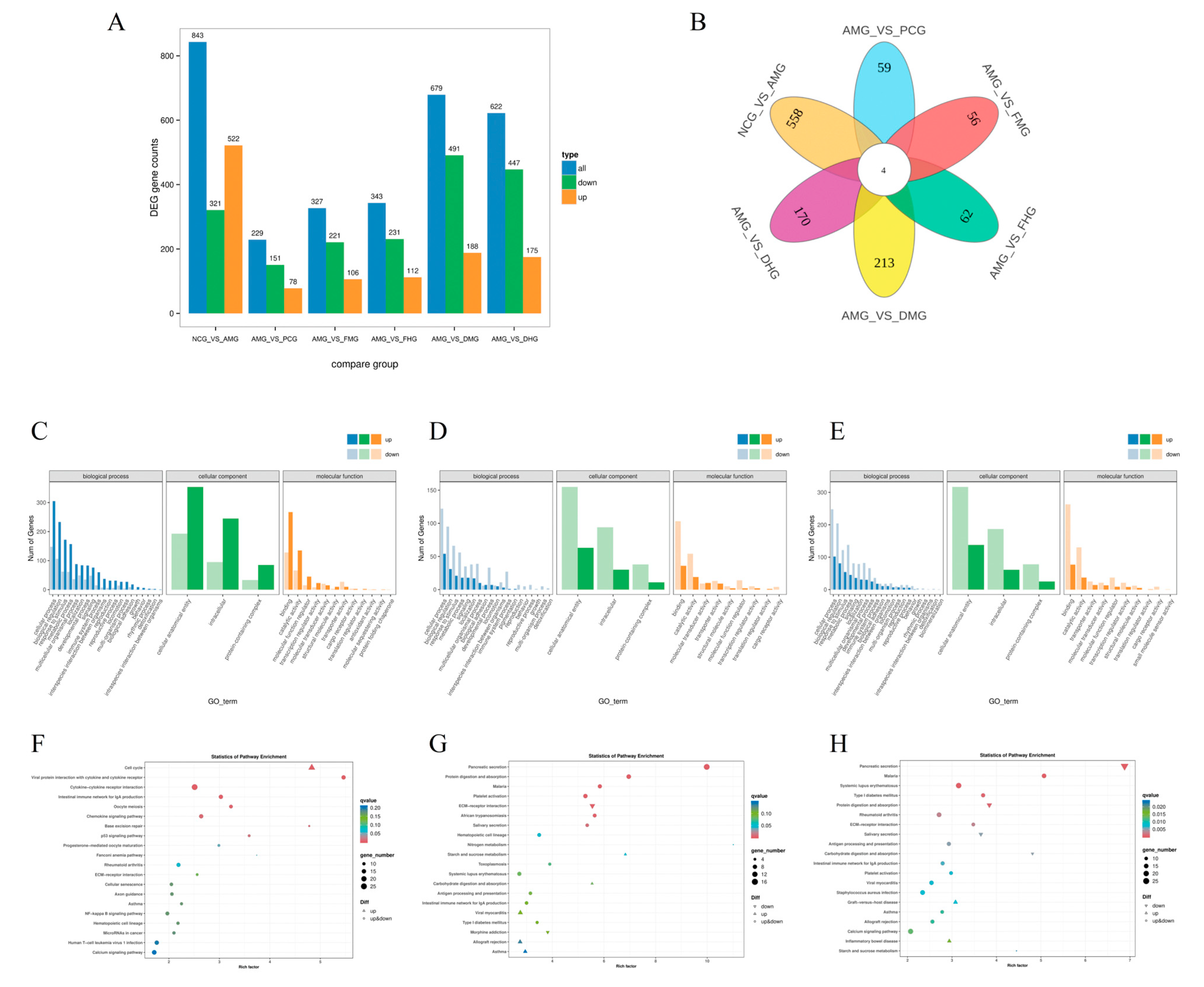
2.2. Splenic Transcriptome Profiling of Differentially Expressed Genes in AR Mice
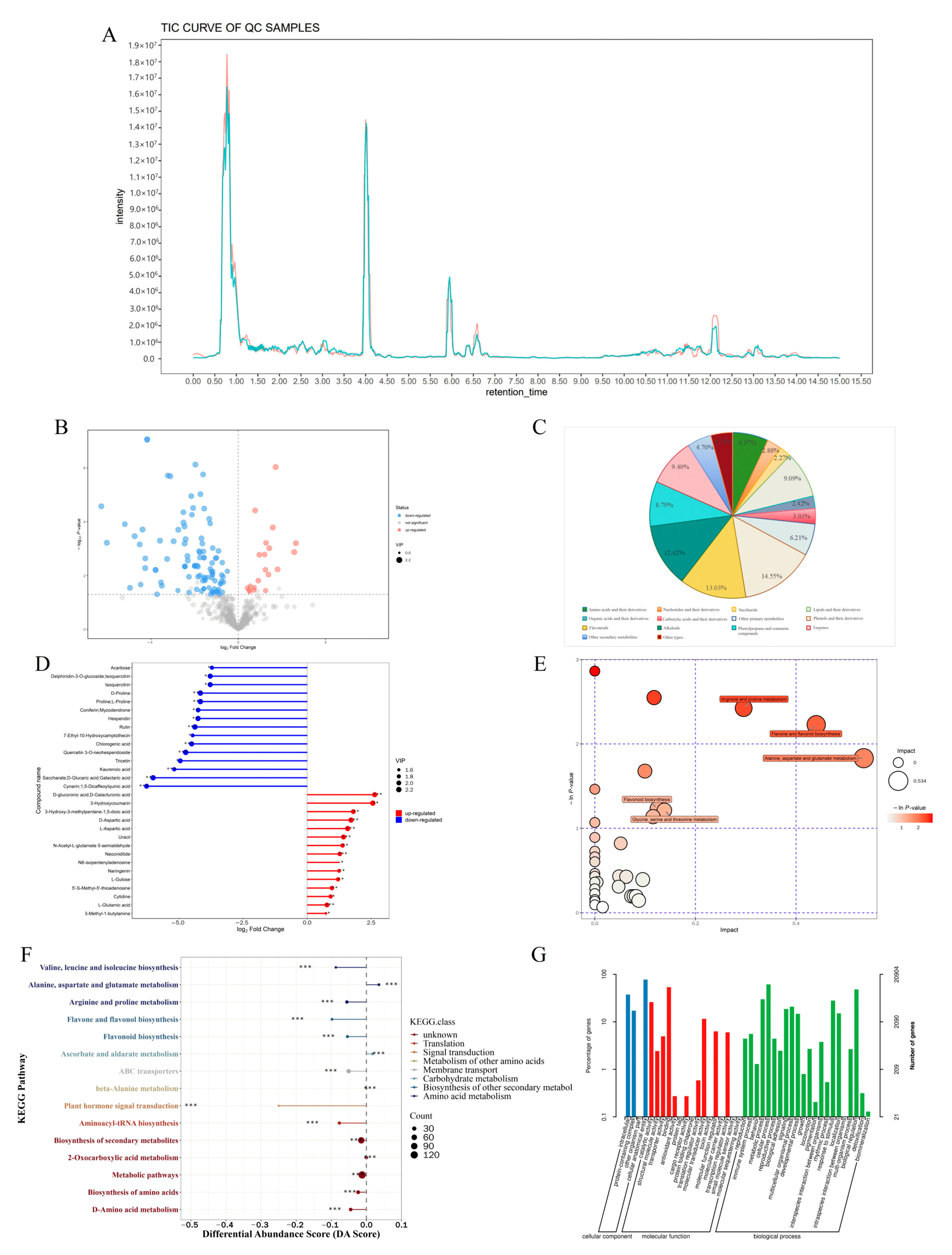
2.3. Differential Metabolites Identification in CMF and CMD
2.4. Network Pharmacological Analysis of Differential Metabolites in AR Activity
2.5. Cellular Evidence for the Anti-Allergic and Anti-Inflammatory Properties of CMF and CMD Differential Metabolites
3. Discussion
4. Material and Methods
4.1. Chemicals and Reagents
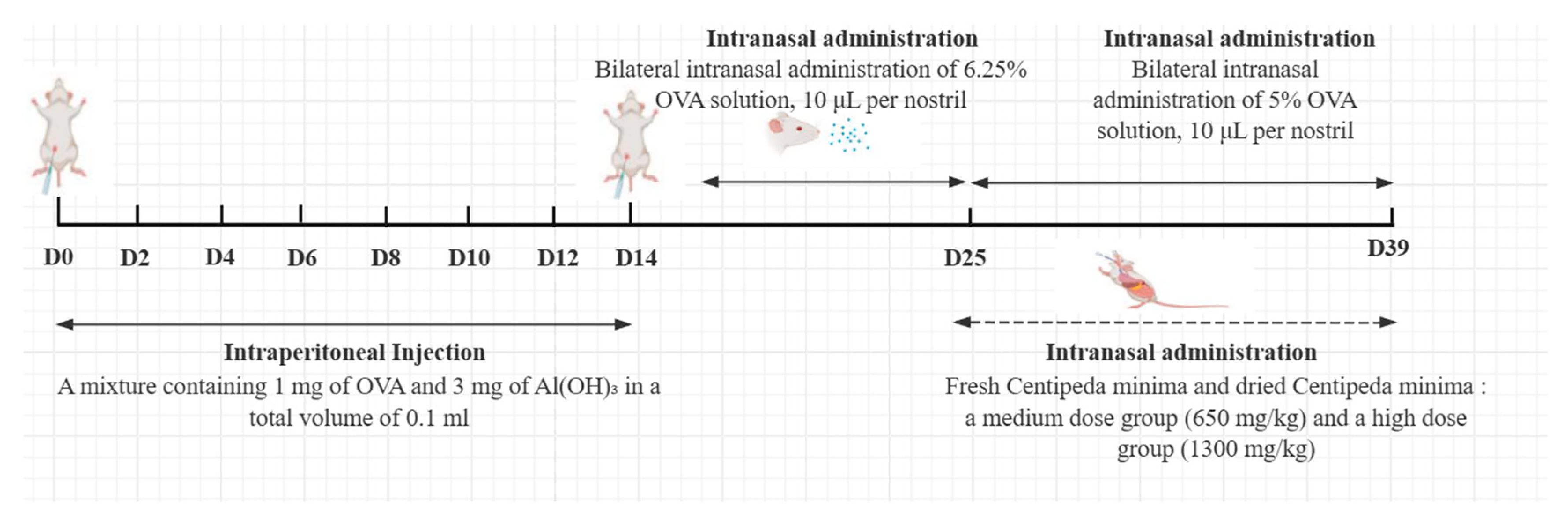
4.2. Animals and Treatment
4.3. General Behavioral Scoring
4.4. Murine Spleen Index Analysis
4.5. Serum Biochemical Analysis
4.6. Histopathological Examination
4.7. Transcriptome Analysis
4.8. Widely Targeted Metabonomic Analysis
4.9. Network Pharmacology and Molecular Docking Analysis
4.10. Cells and Treatment
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bernstein, J.A.; Bernstein, J.S.; Makol, R.; Ward, S. Allergic Rhinitis: A Review. JAMA 2024, 331, 866–877. [Google Scholar] [CrossRef]
- Bousquet, J.; Anto, J.M.; Bachert, C.; Baiardini, I.; Bosnic-Anticevich, S.; Canonica, G.W.; Melén, E.; Palomares, O.; Scadding, G.K.; Togias, A.; et al. Allergic Rhinitis. Nat. Rev. Dis. Prim. 2020, 6, 95. [Google Scholar] [CrossRef] [PubMed]
- Licari, A.; Magri, P.; De Silvestri, A.; Giannetti, A.; Indolfi, C.; Mori, F.; Marseglia, G.L.; Peroni, D. Epidemiology of Allergic Rhinitis in Children: A Systematic Review and Meta-Analysis. J. Allergy Clin. Immunol. Pract. 2023, 11, 2547–2556. [Google Scholar] [CrossRef] [PubMed]
- Pagel, J.M.L.; Mattos, J.L. Allergic Rhinitis and Its Effect on Sleep. Otolaryngol. Clin. N. Am. 2024, 57, 319–328. [Google Scholar] [CrossRef]
- Tosca, M.A.; Trincianti, C.; Naso, M.; Nosratian, V.; Ciprandi, G. Treatment of Allergic Rhinitis in Clinical Practice. Curr. Pediatr. Rev. 2024, 20, 271–277. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, Y.; Zhang, H.; Hu, L.; Liu, J.; Wang, L.; Wang, T.; Zhang, H.; Cong, L.; Wang, Q. Pathogenesis of Allergic Diseases and Implications for Therapeutic Interventions. Signal Transduct. Target. Ther. 2023, 8, 138. [Google Scholar] [CrossRef]
- Zhang, Y.; Lan, F.; Zhang, L. Update on Pathomechanisms and Treatments in Allergic Rhinitis. Allergy 2022, 77, 3309–3319. [Google Scholar] [CrossRef] [PubMed]
- Klimek, L.; Mullol, J.; Ellis, A.K.; Izquierdo-Domínguez, A.; Hagemann, J.; Casper, I.; Davis, A.; Becker, S. Current Management of Allergic Rhinitis. J. Allergy Clin. Immunol. Pract. 2024, 12, 1399–1412. [Google Scholar] [CrossRef]
- Shao, T.; Han, L.; Xie, Y.; Shi, Z.; Yang, Q.; Liu, A.; Liu, Y.; Chen, L.; Huang, J.; Peng, B.; et al. Bilateral Synergistic Effects of Phototherapy-Based NIR-II Absorption Photosensitizer for Allergic Rhinitis. Small 2025, 21, e2412249. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, J.; Wu, L.; Zhang, Y.; Chen, H.; Zhang, Z. Efficacy of Chinese Herbal Medicine on Nasal Itching in Children with Allergic Rhinitis: A Systematic Review and Meta-Analysis. Front. Pharmacol. 2023, 14, 1240917. [Google Scholar] [CrossRef]
- Hoang, M.P.; Chitsuthipakorn, W.; Snidvongs, K. Herbal Medicines for Allergic Rhinitis: A Systematic Review and Meta-Analysis. Curr. Allergy Asthma Rep. 2021, 21, 25. [Google Scholar] [CrossRef]
- Wang, S.; He, G.; Sun, H.; Gu, M. The Application of Traditional Chinese Herbal. Medicine in the Treatment of Allergic Rhinitis: A Bibliometric Analysis (1999–2024). Asian J. Surg. 2024. [Google Scholar] [CrossRef]
- Committe National Pharmacopo. Pharmacopoeia of People’s Republic of China, 2025th ed.; Part 1; China Medical Science Press: Beijing, China, 2025. [Google Scholar]
- Ma, Y.; Kim, B.H.; Yun, S.K.; Roh, Y.-S. Centipeda Minima Extract Inhibits Inflammation and Cell Proliferation by Regulating JAK/STAT Signaling in Macrophages and Keratinocytes. Molecules 2023, 28, 1723. [Google Scholar] [CrossRef] [PubMed]
- Su, F.; Yang, G.; Hu, D.; Ruan, C.; Wang, J.; Zhang, Y.; Zhu, Q. Chemical Composition, Antibacterial and Antioxidant Activities of Essential Oil from Centipeda Minima. Molecules 2023, 28, 824. [Google Scholar] [CrossRef]
- Wang, G.; Liu, H.; Zhang, Q.; Mou, X.; Zhao, Y.; Fan, H.; Xu, H.; Chen, D.; Qiu, F.; Zhao, F. Two Sesquiterpene Lactones, Arnicolide B and Arnicolide C, Isolated from Centipeda Minima, Exert Anti-Inflammatory Effects in LPS Stimulated RAW 264.7 Macrophages via Inactivation of the MAPK Pathway. Nat. Prod. Res. 2023, 37, 2969–2972. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-C.; Wu, P.-E.; He, W.-D.; Chen, C.-Y.; Zheng, R.-Q.; Pang, Y.-C.; Wu, L.-C.; Cheng, Y.-X.; Liu, Y.-Q. Centipeda Minima Extracts and the Active Sesquiterpene Lactones Have Therapeutic Efficacy in Non-Small Cell Lung Cancer by Suppressing Skp2/P27 Signaling Pathway. J. Ethnopharmacol. 2025, 340, 119277. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Zhao, X.; Wu, B.; Tan, Z.; Xie, Y.; Wei, M.; Wu, L. Centipeda Minima Extract Exhibits Anti-Liver Cancer Effects via the ER Stress/HMOX1/Fe2+/ROS Pathway. Phytomedicine 2025, 139, 156487. [Google Scholar] [CrossRef]
- Jia, Y.; Zou, J.; Wang, Y.; Zhang, X.; Shi, Y.; Liang, Y.; Guo, D.; Yang, M. Mechanism of Allergic Rhinitis Treated by Centipeda Minima from Different Geographic Areas. Pharm. Biol. 2021, 59, 606–618. [Google Scholar] [CrossRef]
- Liang, Y.; Zou, J.; Zhang, X.; Shi, Y.; Tai, J.; Wang, Y.; Guo, D.; Yang, M. Preparation and Quality Evaluation of a Volatile Oil Microemulsion from Flos Magnoliae and Centipeda Minima. Mol. Med. Rep. 2020, 22, 4531–4540. [Google Scholar] [CrossRef]
- Caprari, C.; Fantasma, F.; Divino, F.; Bucci, A.; Iorizzi, M.; Naclerio, G.; Ranalli, G.; Saviano, G. Chemical Profile, In Vitro Biological Activity and Comparison of Essential Oils from Fresh and Dried Flowers of Lavandula angustifolia L. Molecules 2021, 26, 5317. [Google Scholar] [CrossRef]
- Fahmy, N.M.; Fayez, S.; Uba, A.I.; Shariati, M.A.; Aljohani, A.S.M.; El-Ashmawy, I.M.; Batiha, G.E.-S.; Eldahshan, O.A.; Singab, A.N.; Zengin, G. Comparative GC-MS Analysis of Fresh and Dried Curcuma Essential Oils with Insights into Their Antioxidant and Enzyme Inhibitory Activities. Plants 2023, 12, 1785. [Google Scholar] [CrossRef]
- Pinto, M.; Soares, C.; Pereira, R.; Rodrigues, J.A.; Fidalgo, F.; Valente, I.M. Untargeted Metabolomic Profiling of Fresh and Dried Leaf Extracts of Young and Mature Eucalyptus Globulus Trees Indicates Differences in the Presence of Specialized Metabolites. Front. Plant Sci. 2022, 13, 986197. [Google Scholar] [CrossRef]
- Yu, L.; Bi, J.; Xu, B.; Yu, B.; Fu, Y. Clinical Significance of T Helper-1/T Helper-2 Cytokines in Peripheral Blood of Children with Otitis Media with Effusion and Allergic Rhinitis. Int. J. Pediatr. Otorhinolaryngol. 2024, 182, 111996. [Google Scholar] [CrossRef]
- Fu, S.; Ni, S.; Wang, D.; Hong, T. Coptisine Suppresses Mast Cell Degranulation and Ovalbumin-Induced Allergic Rhinitis. Molecules 2018, 23, 3039. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Li, Z.; Tan, T.; Dai, S.; Wu, Y.; Xu, F. Tanshinone IIA Improves Degranulation of Mast Cells and Allergic Rhinitis Induced by Ovalbumin by Inhibiting the PLCγ1/PKC/IP3R Pathway. Hum. Exp. Toxicol. 2021, 40, S702–S710. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Shao, L.; Li, Y.; Dai, M.; Liu, H.; Xiang, N.; Chen, H. Tanshinone IIA Alleviates Ovalbumin-Induced Allergic Rhinitis Symptoms by Inhibiting Th2 Cytokine Production and Mast Cell Histamine Release in Mice. Pharm. Biol. 2022, 60, 326–333. [Google Scholar] [CrossRef]
- Hao, Y.; Yang, Y.; Zhao, H.; Chen, Y.; Zuo, T.; Zhang, Y.; Yu, H.; Cui, L.; Song, X. Multi-Omics in Allergic Rhinitis: Mechanism Dissection and Precision Medicine. Clin. Rev. Allergy Immunol. 2025, 68, 19. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yang, N.; Yi, X.; Wang, G.; Wang, C.; Lin, H.; Sun, L.; Wang, F.; Zhu, D. Integration of Transcriptomics and Metabolomics to Reveal the Effect of Ginsenoside Rg3 on Allergic Rhinitis in Mice. Food Funct. 2023, 14, 2416–2431. [Google Scholar] [CrossRef]
- Ke, X.; Chen, Z.; Wang, X.; Kang, H.; Hong, S. Quercetin Improves the Imbalance of Th1/Th2 Cells and Treg/Th17 Cells to Attenuate Allergic Rhinitis. Autoimmunity 2023, 56, 2189133. [Google Scholar] [CrossRef]
- Wang, R.; Yang, T.; Feng, Q.; Jiang, Y.; Yuan, X.; Zhao, L.; Liu, N.; Liu, Z.; Zhang, Y.; Wang, L.; et al. Integration of Network Pharmacology and Proteomics to Elucidate the Mechanism and Targets of Traditional Chinese Medicine Biyuan Tongqiao Granule against Allergic Rhinitis in an Ovalbumin-Induced Mice Model. J. Ethnopharmacol. 2024, 318, 116816. [Google Scholar] [CrossRef]
- Liu, Y.; Kong, Y.; Zhou, X. Screening and Analysis for Potential Clinical Diagnostic and Prognostic Markers in Allergic Rhinitis. Am. J. Transl. Res. 2024, 16, 2670–2682. [Google Scholar] [CrossRef] [PubMed]
- Husna, S.M.N.; Tan, H.-T.T.; Shukri, N.M.; Ashari, N.S.M.; Wong, K.K. Allergic Rhinitis: A Clinical and Pathophysiological Overview. Front. Med. 2022, 9, 874114. [Google Scholar] [CrossRef]
- Sarah, C.O.S.; Ashari, N.S.M. Exploration of Allergic Rhinitis: Epidemiology, Predisposing Factors, Clinical Manifestations, Laboratory Characteristics, and Emerging Pathogenic Mechanisms. Cureus 2024, 16, e71409. [Google Scholar] [CrossRef]
- Yan, Z.; Liu, L.; Yuan, J.; Jiao, L.; Zhou, M.; Liu, J.; Wen, X.; Liu, S.; Hao, P.; Liu, J.; et al. Yiqi Jiemin Decoction Alleviates Allergic Rhinitis in a Guinea Pig Model by Suppressing Inflammation, Restoring Th1/Th2 Balance, and Improving Cellular Metabolism. Aging 2021, 13, 18423–18441. [Google Scholar] [CrossRef]
- Han, J.; Zhang, S.; Jiang, B.; Wang, J.; Ge, X.; Wu, B.; Zhang, S.; Wang, D. Sesquiterpene Lactones from Xanthium Sibiricum Patrin Alleviate Asthma by Modulating the Th1/Th2 Balance in a Murine Model. Phytomedicine 2022, 99, 154032. [Google Scholar] [CrossRef]
- Han, L.; Zhang, L. CCL21/CCR7 Axis as a Therapeutic Target for Autoimmune Diseases. Int. Immunopharmacol. 2023, 121, 110431. [Google Scholar] [CrossRef]
- Van Raemdonck, K.; Umar, S.; Shahrara, S. The Pathogenic Importance of CCL21 and CCR7 in Rheumatoid Arthritis. Cytokine Growth Factor. Rev. 2020, 55, 86–93. [Google Scholar] [CrossRef]
- Beesetti, S. Ubiquitin Ligases in Control: Regulating NLRP3 Inflammasome Activation. Front. Biosci. 2025, 30, 25970. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wang, Y.; Liao, W.; Liang, T.; Liu, W.; Xie, J.; Wang, X.; Yang, P.; Lu, W.; Zhang, X. MUC1 Deficiency Induces the Nasal Epithelial Barrier Dysfunction via RBFOX3 Shortage Augment Ubiquitin-Proteasomal Degradation in Allergic Rhinitis Pathogenesis. Allergy 2022, 77, 1596–1599. [Google Scholar] [CrossRef]
- Ayustaningwarno, F.; Anjani, G.; Ayu, A.M.; Fogliano, V. A Critical Review of Ginger’s (Zingiber officinale) Antioxidant, Anti-Inflammatory, and Immunomodulatory Activities. Front. Nutr. 2024, 11, 1364836. [Google Scholar] [CrossRef] [PubMed]
- Usmani, K.; Jain, S.K.; Yadav, S. Mechanism of Action of Certain Medicinal Plants for the Treatment of Asthma. J. Ethnopharmacol. 2023, 317, 116828. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Li, Z.; Wang, J.; Lin, Y.; Yu, Y. 6-Gingerol and Its Derivatives Inhibit Helicobacter Pylori-Induced Gastric Mucosal Inflammation and Improve Gastrin and Somatostatin Secretion. Front. Microbiol. 2024, 15, 1451563. [Google Scholar] [CrossRef]
- Han, M.; Lee, D.; Lee, S.H.; Kim, T.H. Oxidative Stress and Antioxidant Pathway in Allergic Rhinitis. Antioxidants 2021, 10, 1266. [Google Scholar] [CrossRef]
- Choi, Y.Y.; Jin, S.C.; Yi, S.; Yang, W.M. The Essential Oils from Asarum Sieboldii Miq. Alleviate Allergic Rhinitis by Regulating Tight Junction and Inflammation; Network Analysis and Preclinical Validation. J. Ethnopharmacol. 2025, 338, 119032. [Google Scholar] [CrossRef]
- Hua, Y.; Tan, X.; Zhang, J.; Xu, N.; Chen, R.; Zhou, S.; Liu, S.; Li, K.; Chen, W.; Luo, Q.; et al. Deciphering the Pharmacological Mechanism of Radix Astragali for Allergic Rhinitis through Network Pharmacology and Experimental Validation. Sci. Rep. 2024, 14, 29873. [Google Scholar] [CrossRef] [PubMed]
- Mao, W.; Wang, B.; Chen, F.; Luo, D.; Li, Y.; Liu, Y.; Liu, Y.; Dong, P.; Huang, R. Trans-Resveratrol Mitigates miR-204-3p Mediated Progression of Allergic Rhinitis by Regulating the EGLN3/HIF-1α/IL33/ST2 Signalling Pathway. Phytomedicine 2024, 134, 155967. [Google Scholar] [CrossRef]
- Xiong, J.; Wu, Y.; Luo, L.; Shen, X.; Zeng, Y.; Meng, X.; Zhang, H. Network Pharmacology Integrated with Experimental Validation Reveals the Mechanism of Xanthii Fructus against Allergic Rhinitis via JAK2/STAT3/HIF-1α Signaling Pathway. J. Ethnopharmacol. 2025, 343, 119461. [Google Scholar] [CrossRef]
- Mirzakhani, H.; Wang, A.L.; Sharma, R.; Sun, M.; Panganiban, R.; Lu, Q.; McGeachie, M.; Lu, Z.; Litonjua, A.A.; Tantisira, K.G.; et al. Early-Life microRNA Signatures in Cord Blood Associated with Allergic Rhinitis and Asthma Development. J. Allergy Clin. Immunol. 2024, 156, 129–138. [Google Scholar] [CrossRef]
- Yu, Q.; Yang, X.; Duan, W.; Li, C.; Luo, Y.; Lu, S. miRNA-346 Promotes Proliferation, Migration and Invasion in Liver Cancer. Oncol. Lett. 2017, 14, 3255–3260. [Google Scholar] [CrossRef] [PubMed]

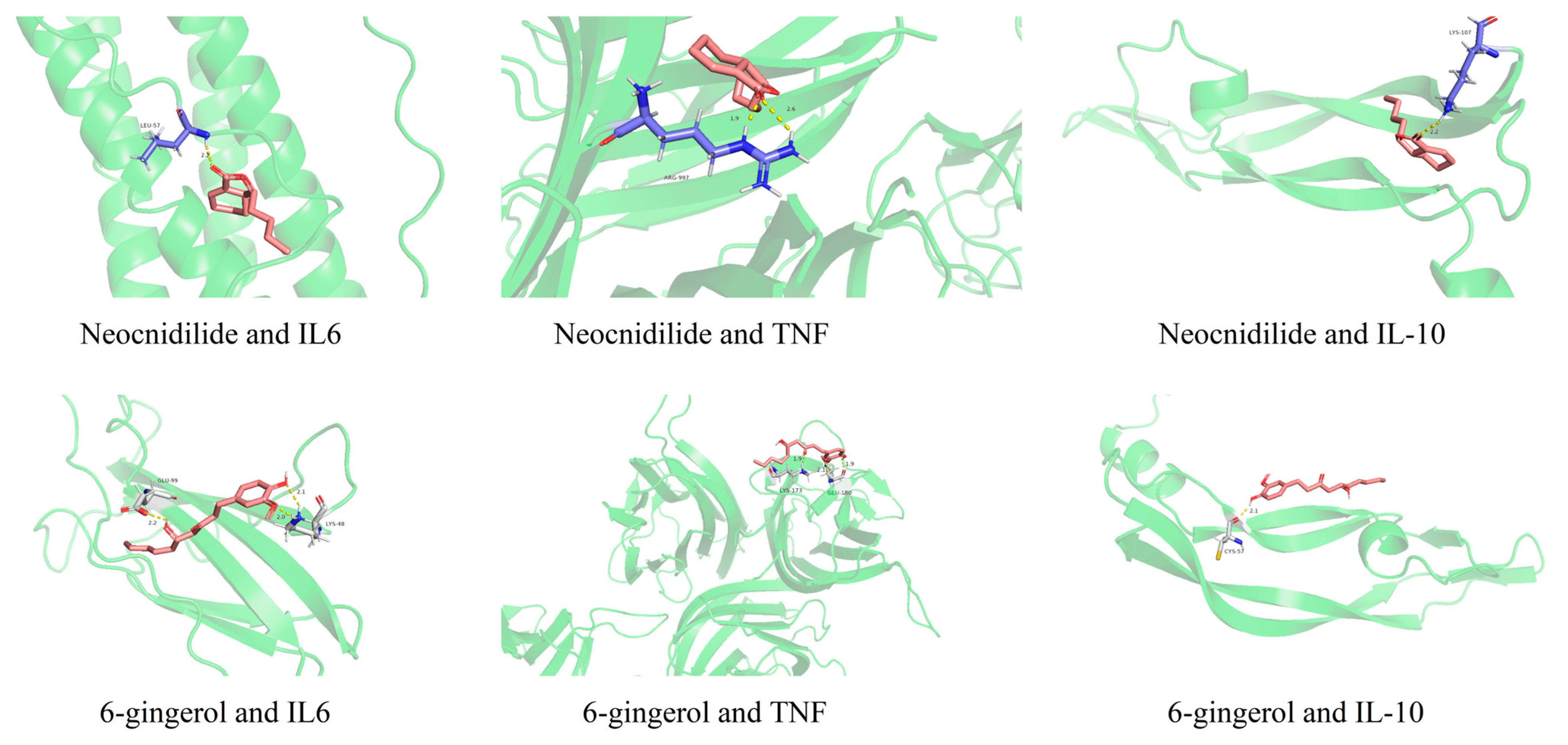


| NO. | Compound | Category | Formula | VIP | Trend |
|---|---|---|---|---|---|
| 1 | D-Aspartic acid | Alkaloids | C4H7NO4 | 2.1657 | ↑ |
| 2 | 3-Hydroxycoumarin | Coumarins | C9H6O3 | 2.1513 | ↑ |
| 3 | L-Aspartic acid | Amino acids | C4H7NO4 | 2.1313 | ↑ |
| 4 | D-glucoronic acid | Carbohydrates | C6H10O7 | 2.0943 | ↑ |
| 5 | Uracil | Nucleotide | C4H4N2O2 | 2.0516 | ↑ |
| 6 | 3-Hydroxy-3-methylpentane-1,5-dioic acid | Amino acid and derivatives | C6H10O5 | 1.9844 | ↑ |
| 7 | Neocnidilide | Lactones | C12H18O2 | 1.8927 | ↑ |
| 8 | N-Acetyl-L-glutamate 5-semialdehyde | Carboxylic acids | C7H11NO4 | 1.8650 | ↑ |
| 9 | L-Gulose | Carbohydrates | C6H12O6 | 1.8193 | ↑ |
| 10 | Naringenin | Flavonoids | C15H12O5 | 1.6946 | ↑ |
| 11 | N6-isopentenyladenosine | Phytohormone | C15H21N5O4 | 1.4313 | ↑ |
| 12 | Quercetin 3-O-neohesperidoside | Flavonoids | C27H30O16 | 2.2128 | ↓ |
| 13 | Rutin | Flavonoids | C27H30O16 | 2.2006 | ↓ |
| 14 | Saccharate | Organooxygen compounds | C6H10O8 | 2.1704 | ↓ |
| 15 | 6-Gingerol | Phenols | C17H26O4 | 2.1281 | ↓ |
| 16 | Cleomiscosin A | Coumarins | C20H18O8 | 2.1241 | ↓ |
| 17 | Gentisic acid | Phenols | C7H6O4 | 2.1208 | ↓ |
| 18 | Isoquercitrin | Flavonoids | C21H20O12 | 2.0966 | ↓ |
| 19 | Chlorogenic acid | Phenylpropanoids | C16H18O9 | 2.0955 | ↓ |
| 20 | Cynarin; 1,5-Dicaffeoylquinic acid | Organic acids | C25H24O12 | 2.0374 | ↓ |
| 21 | Tricetin | Flavonoids | C15H10O7 | 2.0285 | ↓ |
| 22 | Kaempferol-3-O-rutinoside | Flavonoids | C27H30O15 | 1.9922 | ↓ |
| 23 | Esculin | Coumarins | C15H16O9 | 1.9670 | ↓ |
| 24 | Kaurenoic acid | Diterpenes | C20H30O2 | 1.8277 | ↓ |
| 25 | Ponasterone A | Steroids | C27H44O6 | 1.7986 | ↓ |
| 26 | Hyperoside | Flavonoids | C21H20O12 | 1.7832 | ↓ |
| 27 | 7-Ethyl-10-Hydroxycamptothecin | Alkaloids | C22H20N2O5 | 1.7391 | ↓ |
| 28 | Homoeriodictyol | Flavonoids | C16H14O6 | 1.5835 | ↓ |
| 29 | 3-Ethoxy-4-hydroxybenzaldehyde | Phenols | C9H10O3 | 1.5441 | ↓ |
| 30 | 2′,3,5,7-Tetrahydroxyflavone | Flavonoids | C15H10O6 | 1.4038 | ↓ |
| Gene Name | Degree | Ingredient Name | Category |
|---|---|---|---|
| TNF | 310 | Quercetin 3-O-neohesperidoside | Flavonoids |
| Rutin | Flavonoids | ||
| Isoquercitrin | Flavonoids | ||
| Kaempferol-3-O-rutinoside | Flavonoids | ||
| Hyperoside | Flavonoids | ||
| Esculin | Coumarins | ||
| IL6 | 294 | Ponasterone A | Steroids |
| 6-Gingerol | Phenols | ||
| ALB | 272 | Gentisic acid | Phenols |
| AKT1 | 270 | Tricetin | Flavonoids |
| Quercetin | Flavonoids | ||
| 3-Hydroxycoumarin | Coumarins | ||
| 2′,3,5,7-Tetrahydroxyflavone | Flavonoids | ||
| IL1B | 258 | Neocnidilide | Lactones |
| IL-10 | 45 | Naringenin | Flavonoids |
| SRC | 226 | Tricetin | Flavonoids |
| 3-Hydroxycoumarin | Coumarins | ||
| Homoeriodictyol | Flavonoids | ||
| TP53 | 224 | Kaurenoic acid | Diterpenes |
| EGFR | 212 | Tricetin | Flavonoids |
| CTNNB1 | 206 | (+)-Abscisic acid | Phytohormone |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Lin, J.; Xu, X.; Lu, X.; Li, L.; Yang, Y.; Lin, W. Neocnidilide and 6-Gingerol as Key Bioactives in Fresh and Dried Centipeda minima: Distinct Th1/Th2 Modulation via NF-κB/JAK-STAT Pathways for Allergic Rhinitis Therapy. Int. J. Mol. Sci. 2025, 26, 8678. https://doi.org/10.3390/ijms26178678
Zhang Y, Lin J, Xu X, Lu X, Li L, Yang Y, Lin W. Neocnidilide and 6-Gingerol as Key Bioactives in Fresh and Dried Centipeda minima: Distinct Th1/Th2 Modulation via NF-κB/JAK-STAT Pathways for Allergic Rhinitis Therapy. International Journal of Molecular Sciences. 2025; 26(17):8678. https://doi.org/10.3390/ijms26178678
Chicago/Turabian StyleZhang, Yamin, Jiajia Lin, Xiaomei Xu, Xuehua Lu, Lisha Li, Yuezhen Yang, and Wenjin Lin. 2025. "Neocnidilide and 6-Gingerol as Key Bioactives in Fresh and Dried Centipeda minima: Distinct Th1/Th2 Modulation via NF-κB/JAK-STAT Pathways for Allergic Rhinitis Therapy" International Journal of Molecular Sciences 26, no. 17: 8678. https://doi.org/10.3390/ijms26178678
APA StyleZhang, Y., Lin, J., Xu, X., Lu, X., Li, L., Yang, Y., & Lin, W. (2025). Neocnidilide and 6-Gingerol as Key Bioactives in Fresh and Dried Centipeda minima: Distinct Th1/Th2 Modulation via NF-κB/JAK-STAT Pathways for Allergic Rhinitis Therapy. International Journal of Molecular Sciences, 26(17), 8678. https://doi.org/10.3390/ijms26178678





