Decoding Thalamic Glial Interplay in Multiple Sclerosis Through Proton Magnetic Resonance Spectroscopy and Positron Emission Tomography
Abstract
1. Introduction
2. Results
2.1. Demographics
2.2. Comparison of Imaging Parameters Between pwMS and Controls
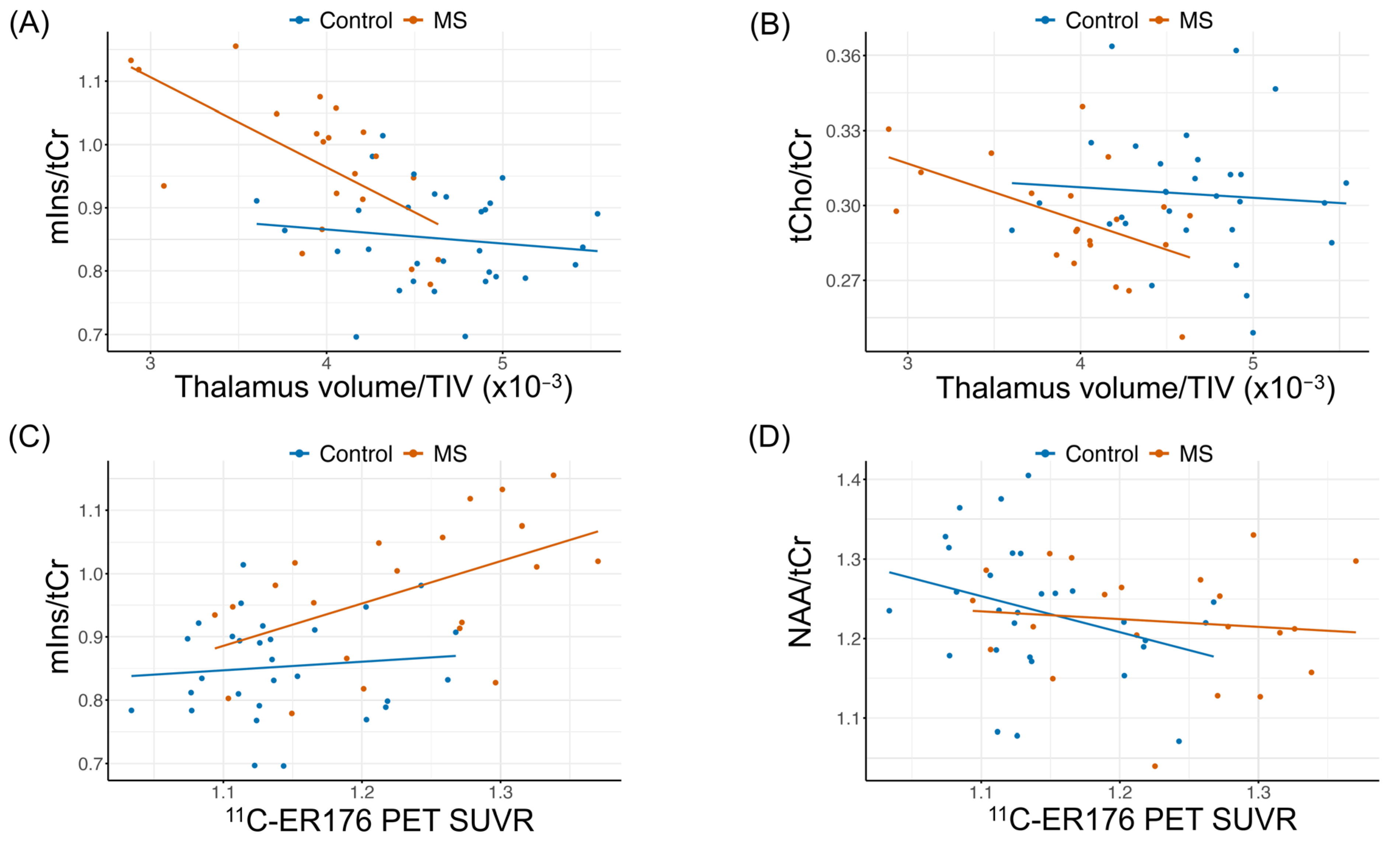
2.3. Correlation of Thalamic 1H-MRS with Normalized Thalamic Volume and Thalamic ER176 PET SUVR
2.4. Correlation of 1H-MRS Metabolites with MS-Specific Disability Metrics
3. Discussion
3.1. Smaller Normalized Thalamic Volume in pwMS
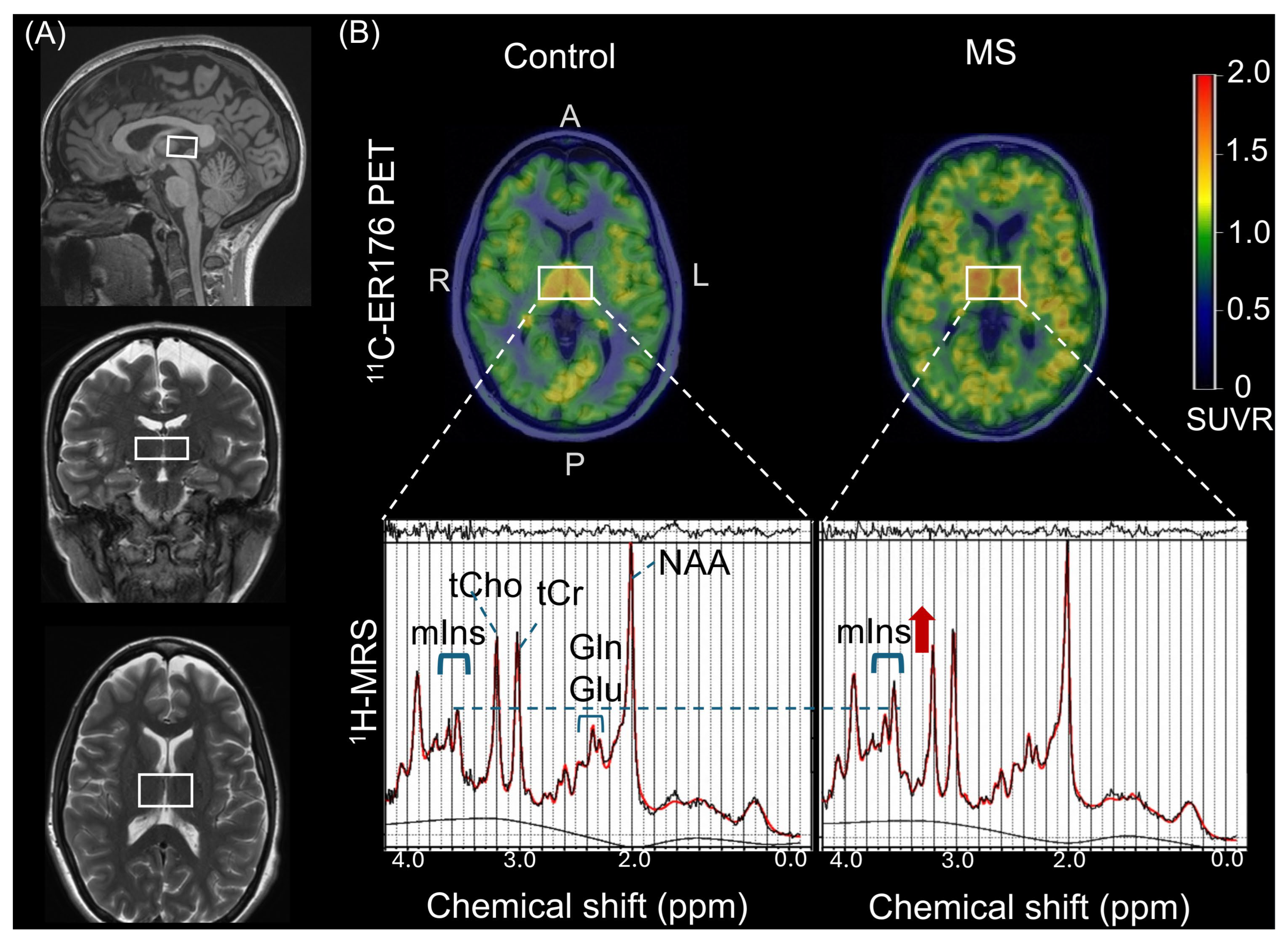
3.2. Increased Thalamic 11C-ER176 PET SUVR in PwMS
3.3. Increased Thalamic 1H-MRS [mIns/tCr] in PwMS
3.4. Increased 1H-MRS (mIns/tCr) Correlates with Higher Thalamic 11C-ER176 PET SUVR and Smaller Normalized Thalamic Volume in pwMS
3.5. Increased Thalamic mIns/tCr on 1H-MRS Correlates with Decreased Cognitive Function in PwMS
3.6. Limitations and Future Directions
4. Methods
4.1. Study Design and Participants
4.2. MRI Acquisition and Processing
4.3. 1H-MRS
4.4. 11C-ER176 PET
4.5. Clinical Assessments
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CSF | Cerebrospinal Fluid |
| CI | Confidence Interval |
| EDSS | Expanded Disability Status Scale |
| GM | Gray Matter |
| Glu | Glutamate |
| Gln | Glutamine |
| MS | Multiple Sclerosis |
| mIns | Myo-inositol |
| MRI | Magnetic Resonance Imaging |
| MPRAGE | Magnetization Prepared Rapid Acquisition Gradient Echo |
| mM: | Millimolar |
| MSFC | Multiple Sclerosis Functional Composite |
| NAA | N-acetylaspartate |
| pwMS | Patients with Multiple Sclerosis |
| PET | Positron Emission Tomography |
| PASAT | Paced Auditory Serial Addition Test |
| sLASER | Semi-Localized Adiabatic Selective Refocusing |
| SUVR | Standardized Uptake Value Ratio |
| SNR | Signal-to-Noise Ratio |
| tCr | Total Creatine |
| tCho | Total Choline |
| TSPO | Translocator Protein |
| TIV | Total Intracranial Volume |
| TR | Repetition Time |
| TE | Echo Time |
| VIF | Variance Inflation Factor |
| 9HPT | 9-Hole Peg Test |
| 25FTW | 25-Foot Timed Walk |
| 11C-ER176 | Third-generation TSPO radioligand |
| 1H-MRS | Proton Magnetic Resonance Spectroscopy |
| WM | White Matter |
References
- Azevedo, C.J.; Overton, E.; Khadka, S.; Buckley, J.; Liu, S.; Sampat, M.; Kantarci, O.; Frenay, C.L.; Siva, A.; Okuda, D.T.; et al. Early CNS neurodegeneration in radiologically isolated syndrome. Neurol. Neuroimmunol. Neuroinflamm. 2015, 2, e102. [Google Scholar] [CrossRef] [PubMed]
- Eshaghi, A.; Marinescu, R.V.; Young, A.L.; Firth, N.C.; Prados, F.; Cardoso, M.J.; Tur, C.; De Angelis, F.; Cawley, N.; Brownlee, W.J.; et al. Progression of regional grey matter atrophy in multiple sclerosis. Brain 2018, 141, 1665–1677. [Google Scholar] [CrossRef] [PubMed]
- Öz, G.; Alger, J.R.; Barker, P.B.; Bartha, R.; Bizzi, A.; Boesch, C.; Bolan, P.J.; Brindle, K.M.; Cudalbu, C.; Dinçer, A.; et al. Clinical Proton MR Spectroscopy in Central Nervous System Disorders. Radiology 2014, 270, 658–679. [Google Scholar] [CrossRef]
- Ligneul, C.; Palombo, M.; Hernández-Garzón, E.; Sauvage, M.-A.C.-D.; Flament, J.; Hantraye, P.; Brouillet, E.; Bonvento, G.; Escartin, C.; Valette, J. Diffusion-weighted magnetic resonance spectroscopy enables cell-specific monitoring of astrocyte reactivity in vivo. NeuroImage 2019, 191, 457–469. [Google Scholar] [CrossRef] [PubMed]
- Lopez, M.Y.; Pardon, M.-C.; Baiker, K.; Prior, M.; Yuchun, D.; Agostini, A.; Bai, L.; Auer, D.P.; Faas, H.M.; Walczak, P. Myoinositol CEST signal in animals with increased Iba-1 levels in response to an inflammatory challenge—Preliminary findings. PLoS ONE 2019, 14, e0212002. [Google Scholar] [CrossRef]
- Geurts, J.J.G.; Reuling, I.E.W.; Vrenken, H.; Uitdehaag, B.M.J.; Polman, C.H.; Castelijns, J.A.; Barkhof, F.; Pouwels, P.J.W. MR spectroscopic evidence for thalamic and hippocampal, but not cortical, damage in multiple sclerosis. Magn. Reson. Med. 2006, 55, 478–483. [Google Scholar] [CrossRef]
- Tisell, A.; Leinhard, O.D.; Warntjes, J.B.M.; Aalto, A.; Smedby, Ö.; Landtblom, A.-M.; Lundberg, P.; Smeyne, R.J. Increased Concentrations of Glutamate and Glutamine in Normal-Appearing White Matter of Patients with Multiple Sclerosis and Normal MR Imaging Brain Scans. PLoS ONE 2013, 8, e61817. [Google Scholar] [CrossRef]
- Muhlert, N.; Atzori, M.; De Vita, E.; Thomas, D.L.; Samson, R.S.; Wheeler-Kingshott, C.A.M.; Geurts, J.J.G.; Miller, D.H.; Thompson, A.J.; Ciccarelli, O. Memory in multiple sclerosis is linked to glutamate concentration in grey matter regions. J. Neurol. Neurosurg. Psychiatry 2014, 85, 833–839. [Google Scholar] [CrossRef]
- Tzanetakos, D.; Kyrozis, A.; Karavasilis, E.; Velonakis, G.; Tzartos, J.; Toulas, P.; Sotirli, S.A.; Evdokimidis, I.; Tsivgoulis, G.; Potagas, C.; et al. Early metabolic alterations in the normal-appearing grey and white matter of patients with clinically isolated syndrome suggestive of multiple sclerosis: A proton MR spectroscopic study. Exp. Ther. Med. 2023, 26, 349. [Google Scholar] [CrossRef]
- Rae, C.D. A Guide to the Metabolic Pathways and Function of Metabolites Observed in Human Brain 1H Magnetic Resonance Spectra. Neurochem. Res. 2013, 39, 1–36. [Google Scholar] [CrossRef]
- Datta, G.; Violante, I.R.; Scott, G.; Zimmerman, K.; Santos-Ribeiro, A.; A Rabiner, E.; Gunn, R.N.; Malik, O.; Ciccarelli, O.; Nicholas, R.; et al. Translocator positron-emission tomography and magnetic resonance spectroscopic imaging of brain glial cell activation in multiple sclerosis. Mult. Scler. J. 2016, 23, 1469–1478. [Google Scholar] [CrossRef]
- Sotirchos, E.S.; Gonzalez-Caldito, N.; E Dewey, B.; Fitzgerald, K.C.; Glaister, J.; Filippatou, A.; Ogbuokiri, E.; Feldman, S.; Kwakyi, O.; Risher, H.; et al. Effect of disease-modifying therapies on subcortical gray matter atrophy in multiple sclerosis. Mult. Scler. J. 2019, 26, 312–321. [Google Scholar] [CrossRef]
- Misin, O.; Matilainen, M.; Nylund, M.; Honkonen, E.; Rissanen, E.; Sucksdorff, M.; Airas, L. Innate Immune Cell–Related Pathology in the Thalamus Signals a Risk for Disability Progression in Multiple Sclerosis. Neurol. Neuroimmunol. Neuroinflamm. 2022, 9, e1182. [Google Scholar] [CrossRef]
- Inglese, M.; Liu, S.; Babb, J.S.; Mannon, L.J.; Grossman, R.I.; Gonen, O. Three-dimensional proton spectroscopy of deep gray matter nuclei in relapsing–remitting MS. Neurology 2004, 63, 170–172. [Google Scholar] [CrossRef]
- Henry, R.G.; Shieh, M.; Amirbekian, B.; Chung, S.; Okuda, D.T.; Pelletier, D. Connecting white matter injury and thalamic atrophy in clinically isolated syndromes. J. Neurol. Sci. 2009, 282, 61–66. [Google Scholar] [CrossRef]
- Steenwijk, M.D.; Daams, M.; Pouwels, P.J.; Balk, L.J.; Tewarie, P.K.; Geurts, J.J.G.; Barkhof, F.; Vrenken, H. Unraveling the relationship between regional gray matter atrophy and pathology in connected white matter tracts in long-standing multiple sclerosis. Hum. Brain Mapp. 2015, 36, 1796–1807. [Google Scholar] [CrossRef]
- Cifelli, A.; Arridge, M.; Jezzard, P.; Esiri, M.M.; Palace, J.; Matthews, P.M. Thalamic neurodegeneration in multiple sclerosis. Ann. Neurol. 2002, 52, 650–653. [Google Scholar] [CrossRef]
- Cosenza-Nashat, M.; Zhao, M.; Suh, H.; Morgan, J.; Natividad, R.; Morgello, S.; Lee, S.C. Expression of the translocator protein of 18 kDa by microglia, macrophages and astrocytes based on immunohistochemical localization in abnormal human brain. Neuropathol. Appl. Neurobiol. 2009, 35, 306–328. [Google Scholar] [CrossRef]
- Venneti, S.; Lopresti, B.J.; Wang, G.; Hamilton, R.L.; Mathis, C.A.; Klunk, W.E.; Apte, U.M.; Wiley, C.A. PK11195 labels activated microglia in Alzheimer’s disease and in vivo in a mouse model using PET. Neurobiol. Aging 2009, 30, 1217–1226. [Google Scholar] [CrossRef]
- Nutma, E.; Fancy, N.; Weinert, M.; Tsartsalis, S.; Marzin, M.C.; Muirhead, R.C.J.; Falk, I.; Breur, M.; de Bruin, J.; Hollaus, D.; et al. Translocator protein is a marker of activated microglia in rodent models but not human neurodegenerative diseases. Nat. Commun. 2023, 14, 1–25. [Google Scholar] [CrossRef]
- Papadopoulos, V.; Baraldi, M.; Guilarte, T.R.; Knudsen, T.B.; Lacapère, J.-J.; Lindemann, P.; Norenberg, M.D.; Nutt, D.; Weizman, A.; Zhang, M.-R.; et al. Translocator protein (18kDa): New nomenclature for the peripheral-type benzodiazepine receptor based on its structure and molecular function. Trends Pharmacol. Sci. 2006, 27, 402–409. [Google Scholar] [CrossRef]
- Werry, E.L.; Bright, F.M.; Piguet, O.; Ittner, L.M.; Halliday, G.M.; Hodges, J.R.; Kiernan, M.C.; Loy, C.T.; Kril, J.J.; Kassiou, M. Recent Developments in TSPO PET Imaging as A Biomarker of Neuroinflammation in Neurodegenerative Disorders. Int. J. Mol. Sci. 2019, 20, 3161. [Google Scholar] [CrossRef]
- Datta, G.; Colasanti, A.; Kalk, N.; Owen, D.; Scott, G.; Rabiner, E.A.; Gunn, R.N.; Lingford-Hughes, A.; Malik, O.; Ciccarelli, O.; et al. 11C-PBR28 and 18F-PBR111 Detect White Matter Inflammatory Heterogeneity in Multiple Sclerosis. J. Nucl. Med. 2017, 58, 1477–1482. [Google Scholar] [CrossRef]
- Zeydan, B.; Neyal, N.; Son, J.; Schwarz, C.G.; Thomas, J.C.K.; A Morrison, H.; Bush, M.L.; I Reid, R.; A Przybelski, S.; Fought, A.J.; et al. Microglia positron emission tomography and progression in multiple sclerosis: Thalamus on fire. Brain Commun. 2025, 7, fcaf141. [Google Scholar] [CrossRef]
- Deelchand, D.K.; Kantarci, K.; Öz, G. Improved localization, spectral quality, and repeatability with advanced MRS methodology in the clinical setting. Magn. Reson. Med. 2017, 79, 1241–1250. [Google Scholar] [CrossRef]
- Cumbers, G.A.; Harvey-Latham, E.D.; Kassiou, M.; Werry, E.L.; Danon, J.J. Emerging TSPO-PET Radiotracers for Imaging Neuroinflammation: A Critical Analysis. Semin. Nucl. Med. 2024, 54, 856–874. [Google Scholar] [CrossRef]
- Guilarte, T.R. TSPO in diverse CNS pathologies and psychiatric disease: A critical review and a way forward. Pharmacol. Ther. 2019, 194, 44–58. [Google Scholar] [CrossRef]
- Wylezinska, M.; Cifelli, A.; Jezzard, P.; Palace, J.; Alecci, M.; Matthews, P.M. Thalamic neurodegeneration in relapsing-remitting multiple sclerosis. Neurology 2003, 60, 1949–1954. [Google Scholar] [CrossRef]
- Urenjak, J.; Williams, S.; Gadian, D.; Noble, M. Proton nuclear magnetic resonance spectroscopy unambiguously identifies different neural cell types. J. Neurosci. 1993, 13, 981–989. [Google Scholar] [CrossRef]
- Davie, C.A.; Hawkins, C.P.; Barker, G.J.; Brennan, A.; Tofts, P.S.; Miller, D.H.; McDonald, W.I. Serial proton magnetic resonance spectroscopy in acute multiple sclerosis lesions. Brain 1994, 117, 49–58. [Google Scholar] [CrossRef]
- Orije, J.; Kara, F.; Guglielmetti, C.; Praet, J.; Van der Linden, A.; Ponsaerts, P.; Verhoye, M. Longitudinal monitoring of metabolic alterations in cuprizone mouse model of multiple sclerosis using 1H-magnetic resonance spectroscopy. NeuroImage 2015, 114, 128–135. [Google Scholar] [CrossRef]
- Caramanos, Z.; Narayanan, S.; Arnold, D.L. 1H-MRS quantification of tNA and tCr in patients with multiple sclerosis: A meta-analytic review. Brain 2005, 128, 2483–2506. [Google Scholar] [CrossRef] [PubMed]
- Haris, M.; Singh, A.; Cai, K.; Nath, K.; Crescenzi, R.; Kogan, F.; Hariharan, H.; Reddy, R. MICEST: A potential tool for non-invasive detection of molecular changes in Alzheimer’s disease. J. Neurosci. Methods 2013, 212, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Genovese, G.; Palombo, M.; Santin, M.D.; Valette, J.; Ligneul, C.; Aigrot, M.; Abdoulkader, N.; Langui, D.; Millecamps, A.; Evercooren, A.B.; et al. Inflammation-driven glial alterations in the cuprizone mouse model probed with diffusion-weighted magnetic resonance spectroscopy at 11.7 T. NMR Biomed. 2021, 34, e4480. [Google Scholar] [CrossRef]
- Sun, Y.; Yu, H.; Guan, Y. Glia Connect Inflammation and Neurodegeneration in Multiple Sclerosis. Neurosci. Bull. 2023, 39, 466–478. [Google Scholar] [CrossRef] [PubMed]
- Solanky, B.; John, N.; DeAngelis, F.; Stutters, J.; Prados, F.; Schneider, T.; Parker, R.; Weir, C.; Monteverdi, A.; Plantone, D.; et al. NAA is a Marker of Disability in Secondary-Progressive MS: A Proton MR Spectroscopic Imaging Study. Am. J. Neuroradiol. 2020, 41, 2209–2218. [Google Scholar] [CrossRef]
- Niess, E.; Dal-Bianco, A.; Strasser, B.; Niess, F.; Hingerl, L.; Bachrata, B.; Motyka, S.; Rommer, P.; Trattnig, S.; Bogner, W. Topographical mapping of metabolic abnormalities in multiple sclerosis using rapid echo-less 3D-MR spectroscopic imaging at 7T. NeuroImage 2025, 308, 121043. [Google Scholar] [CrossRef]
- Gracien, R.M.; Jurcoane, A.; Wagner, M.; Reitz, S.C.; Mayer, C.; Volz, S.; Hof, S.M.; Fleischer, V.; Droby, A.; Steinmetz, H.; et al. The Relationship between Gray Matter Quantitative MRI and Disability in Secondary Progressive Multiple Sclerosis. PLoS One 2016, 11, e0161036. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, R.; Sailasuta, N.; Hurd, R.; Nelson, S.; Pelletier, D. Evidence of elevated glutamate in multiple sclerosis using magnetic resonance spectroscopy at 3 T. Brain 2005, 128, 1016–1025. [Google Scholar] [CrossRef] [PubMed]
- Montalban, X. Revisions of the McDonald criteria ECTRIMS: 40th Congress of the European Committee for Treatment and Research in Multiple Sclerosis. 2024. Available online: https://ectrims.eu/mcdonald-diagnostic-criteria (accessed on 19 June 2025).
- Brownlee, W.J.; Vidal-Jordana, A.; Shatila, M.; Strijbis, E.; Schoof, L.; Killestein, J.; Barkhof, F.; Bollo, L.; Rovira, A.; Sastre-Garriga, J.; et al. Towards a Unified Set of Diagnostic Criteria for Multiple Sclerosis. Ann. Neurol. 2024, 97, 571–582. [Google Scholar] [CrossRef] [PubMed]
- Kara, F.; Joers, J.M.; Deelchand, D.K.; Park, Y.W.; Przybelski, S.A.; Lesnick, T.G.; Senjem, M.L.; Zeydan, B.; Knopman, D.S.; Lowe, V.J.; et al. 1H MR spectroscopy biomarkers of neuronal and synaptic function are associated with tau deposition in cognitively unimpaired older adults. Neurobiol. Aging 2022, 112, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, C.G.; Gunter, J.L.; Wiste, H.J.; Przybelski, S.A.; Weigand, S.D.; Ward, C.P.; Senjem, M.L.; Vemuri, P.; Murray, M.E.; Dickson, D.W.; et al. A large-scale comparison of cortical thickness and volume methods for measuring Alzheimer’s disease severity. NeuroImage Clin. 2016, 11, 802–812. [Google Scholar] [CrossRef]
- Raz, L.; Jayachandran, M.; Tosakulwong, N.; Lesnick, T.G.; Wille, S.M.; Murphy, M.C.; Senjem, M.L.; Gunter, J.L.; Vemuri, P.; Jr, C.R.J.; et al. Thrombogenic microvesicles and white matter hyperintensities in postmenopausal women. Neurology 2013, 80, 911–918. [Google Scholar] [CrossRef]
- Öz, G.; Tkáč, I. Short-echo, single-shot, full-intensity proton magnetic resonance spectroscopy for neurochemical profiling at 4 T: Validation in the cerebellum and brainstem. Magn. Reson. Med. 2010, 65, 901–910. [Google Scholar] [CrossRef]
- Tkáč, I.; Starčuk, Z.; Choi, I.; Gruetter, R. In vivo1H NMR spectroscopy of rat brain at 1 ms echo time. Magn. Reson. Med. 1999, 41, 649–656. [Google Scholar] [CrossRef]
- Deelchand, D.K.; Adanyeguh, I.M.; Emir, U.E.; Nguyen, T.-M.; Valabregue, R.; Henry, P.-G.; Mochel, F.; Öz, G. Two-site reproducibility of cerebellar and brainstem neurochemical profiles with short-echo, single-voxel MRS at 3T. Magn. Reson. Med. 2014, 73, 1718–1725. [Google Scholar] [CrossRef]
- Gruetter, R.; Tkáč, I. Field mapping without reference scan using asymmetric echo-planar techniques. Magn. Reson. Med. 2000, 43, 319–323. [Google Scholar] [CrossRef]
- Provencher, S.W. Automatic quantitation of localized in vivo1H spectra with LCModel. NMR Biomed. 2001, 14, 260–264. [Google Scholar] [CrossRef]
- Deelchand, D.K.; Henry, P.; Uǧurbil, K.; Marjańska, M. Measurement of transverse relaxation times of J-coupled metabolites in the human visual cortex at 4 T. Magn. Reson. Med. 2011, 67, 891–897. [Google Scholar] [CrossRef] [PubMed]
- Quadrelli, S.; Mountford, C.; Ramadan, S. Hitchhiker’S Guide to Voxel Segmentation for Partial Volume Correction of in Vivo Magnetic Resonance Spectroscopy. Magn. Reson. Insights 2016, 9, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Near, J.; Harris, A.D.; Juchem, C.; Kreis, R.; Marjańska, M.; Öz, G.; Slotboom, J.; Wilson, M.; Gasparovic, C. Preprocessing, analysis and quantification in single-voxel magnetic resonance spectroscopy: Experts’ consensus recommendations. NMR Biomed. 2020, 34, e4257. [Google Scholar] [CrossRef] [PubMed]
- Randall, L.O. CHEMICAL TOPOGRAPHY OF THE BRAIN. J. Biol. Chem. 1938, 124, 481–488. [Google Scholar] [CrossRef]
- Norton, W.T.; Poduslo, S.E.; Suzuki, K. Subacute Sclerosing Leukoencephalitis*†‡. J. Neuropathol. Exp. Neurol. 1966, 25, 582–597. [Google Scholar] [CrossRef]
- Bednařík, P.; Henry, P.-G.; Khowaja, A.; Rubin, N.; Kumar, A.; Deelchand, D.; E Eberly, L.; Seaquist, E.; Öz, G.; Moheet, A. Hippocampal Neurochemical Profile and Glucose Transport Kinetics in Patients With Type 1 Diabetes. J. Clin. Endocrinol. Metab. 2019, 105, 479–491, Correction in J. Clin. Endocrinol. Metab. 2020, 105, e1935.. [Google Scholar] [CrossRef]
- Sedlacik, J.; Boelmans, K.; Löbel, U.; Holst, B.; Siemonsen, S.; Fiehler, J. Reversible, irreversible and effective transverse relaxation rates in normal aging brain at 3T. NeuroImage 2014, 84, 1032–1041. [Google Scholar] [CrossRef]
- Schwarz, C.G.; Gunter, J.L.; Ward, C.P.; Kantarci, K.; Senjem, M.L.; Petersen, R.C.; Knopman, D.S.; Jack, C.R., Jr. P3-382: Methods to improve SPM12 tissue segmentations of older adults brains. Alzheimer’s & Dementia 2018, 14, 1240–1241. [Google Scholar] [CrossRef]
- Rossano, S.; Talmasov, D.; Johnson, A.S.; Smith, A.C.; Guzmán, D.S.; Okafor, A.; Kreisl, W.C.; De Jager, P.L.; Lao, P.J. Validating noninvasive techniques for 11C-ER176 PET quantification in controls and Alzheimer’s disease patients. Alzheimer’s Dement. 2023, 19, e081801. [Google Scholar] [CrossRef]
- Schwarz, C.G.; Senjem, M.L.; Gunter, J.L.; Tosakulwong, N.; Weigand, S.D.; Kemp, B.J.; Spychalla, A.J.; Vemuri, P.; Petersen, R.C.; Lowe, V.J.; et al. Optimizing PiB-PET SUVR change-over-time measurement by a large-scale analysis of longitudinal reliability, plausibility, separability, and correlation with MMSE. NeuroImage 2017, 144, 113–127. [Google Scholar] [CrossRef]
- Kreisl, W.C.; Fujita, M.; Fujimura, Y.; Kimura, N.; Jenko, K.J.; Kannan, P.; Hong, J.; Morse, C.L.; Zoghbi, S.S.; Gladding, R.L.; et al. Comparison of [11C]-(R)-PK 11195 and [11C]PBR28, two radioligands for translocator protein (18 kDa) in human and monkey: Implications for positron emission tomographic imaging of this inflammation biomarker. NeuroImage 2010, 49, 2924–2932. [Google Scholar] [CrossRef] [PubMed]
- Ikawa, M.; Lohith, T.G.; Shrestha, S.; Telu, S.; Zoghbi, S.S.; Castellano, S.; Taliani, S.; Da Settimo, F.; Fujita, M.; Pike, V.W.; et al. 11C-ER176, a Radioligand for 18-kDa Translocator Protein, Has Adequate Sensitivity to Robustly Image All Three Affinity Genotypes in Human Brain. J. Nucl. Med. 2017, 58, 320–325. [Google Scholar] [CrossRef]
- Kreisl, W.C.; Kim, M.-J.; Coughlin, J.M.; Henter, I.D.; Owen, D.R.; Innis, R.B. PET imaging of neuroinflammation in neurological disorders. Lancet Neurol. 2020, 19, 940–950. [Google Scholar] [CrossRef]
- Viviano, M.; Barresi, E.; Siméon, F.G.; Costa, B.; Taliani, S.; Da Settimo, F.; Pike, V.W.; Castellano, S. Essential Principles and Recent Progress in the Development of TSPO PET Ligands for Neuroinflammation Imaging. Curr. Med. Chem. 2022, 29, 4862–4890. [Google Scholar] [CrossRef]
- Kurtzke, J.F. Rating neurologic impairment in multiple sclerosis: An expanded disability status scale (EDSS). Neurology 1983, 33, 1444–1452. [Google Scholar] [CrossRef]
- Cutter, G.R.; Baier, M.L.; Rudick, R.A.; Cookfair, D.L.; Fischer, J.S.; Petkau, J.; Syndulko, K.; Weinshenker, B.G.; Antel, J.P.; Confavreux, C.; et al. Development of a multiple sclerosis functional composite as a clinical trial outcome measure. Brain 1999, 122, 871–882. [Google Scholar] [CrossRef] [PubMed]
- Goodkin, D.E.; Hertsgaard, D.; Seminary, J. Upper extremity function in multiple sclerosis: Improving assessment sensitivity with box-and-block and nine-hole peg tests. Arch. Phys. Med. Rehabil. 1988, 69, 850–854. [Google Scholar]
- Gronwall, D.M.A. Paced Auditory Serial-Addition Task: A Measure of Recovery from Concussion. Percept. Mot. Ski. 1977, 44, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Fischer, J.S.; Rudick, R.A.; Cutter, G.R.; Reingold, S.C. The Multiple Sclerosis Functional Composite measure (MSFC): An integrated approach to MS clinical outcome assessment. Mult. Scler. J. 1999, 5, 244–250. [Google Scholar] [CrossRef]
- Fischer, J.S.; Jak, A.; Kniker, J.; Rudick, R.; Cutter, G.; Multiple Sclerosis Functional Composite (MSFC): Administration and Scoring Manual. New York: National Multiple Sclerosis Society 2001; pp. 1–2. Available online: http://main.nationalmssociety.org/docs/HOM/MSFC_Manual_and_Forms.pdf (accessed on 26 August 2025).
- Engqvist, L. The mistreatment of covariate interaction terms in linear model analyses of behavioural and evolutionary ecology studies. Anim. Behav. 2005, 70, 967–971. [Google Scholar] [CrossRef]
- Cohen, J.; Cohen, P.; West, S.G.; Aiken, L.S. Applied Multiple Regression/Correlation Analysis for the Behavioral Sciences, 3rd ed.; Routledge: New York, NY, USA, 2003; ISBN 9780805822236. [Google Scholar]
- Rothman, K.J. No adjustments are needed for multiple comparisons. Epidemiology 1990, 1, 43–46. [Google Scholar] [CrossRef] [PubMed]
| MS (N = 21) | |
|---|---|
| Sex | |
| F | 15 (71%) |
| M | 6 (29%) |
| MS phase | |
| Relapsing | 14 (67%) |
| Progressive | 7 (33%) |
| Disease duration | 14.8 [6.3, 20.3]/14.9 (±9.9) |
| Age at MS onset (years) | 31.9 [26.7, 34.6]/33.1 (±10.3) |
| Age at progressive MS onset (years) | 46.0 [34.3, 51.5]/43.7 (±10.6) |
| Age at imaging (years) | 48.0 [40.0, 54.0]/47.3 (±12.1) |
| DMT | |
| No | 7 (33%) |
| Yes | 14 (67%) |
| EDSS score | 3 [1, 4]/2.81 (±2.12) |
| EDSS status | |
| Mild | 10 (48%) |
| Moderate | 7 (33%) |
| Severe | 4 (19%) |
| MSFC z-score | −0.19 [−0.32, 0.31]/−0.04 (±0.56) |
| PASAT score | 45.00 [38.75, 52.00]/44.25 (±9.66) |
| 9HPT score (s) | 22.35 [20.04, 26.98]/28.74 (±19.43) |
| 25FTW (s) | 4.90 [4.25, 6.40]/8.04 (±8.66) |
| Control (N = 30) | MS (N = 21) | p-Value (Age-Adjusted) | |
|---|---|---|---|
| Sex | 0.76 a | ||
| F | 19 (63%) | 15 (71%) | |
| M | 11 (37%) | 6 (29%) | |
| Age at imaging (years) | 0.017 b | ||
| IQR (Q1, Q3) | 38.5 [29.2, 47.5] | 48.0 [40.0, 54.0] | |
| Mean (±SD) | 39.0 (±11.1) | 47.3 (±12.1) | |
| Thalamus volume (mL) | 1 × 10−5 | ||
| IQR (Q1, Q3) | 6.70 [6.34, 7.17] | 5.60 [5.35, 6.05] | |
| Mean (±SD) | 6.72 (±0.72) | 5.58 (±0.76) | |
| Total intracranial volume (mL) | 0.52 | ||
| IQR (Q1, Q3) | 1463.15 [1348.13, 1558.50] | 1383.83 [1339.77, 1492.54] | |
| Mean (±SD) | 1454.57 (±144.92) | 1413.36 (±110.85) | |
| Thalamus volume/TIV ×10−3 | 1 × 10−5 | ||
| IQR (Q1, Q3) | 4.64 [4.34, 4.92] | 4.01 [3.86, 4.21] | |
| Mean (±SD) | 4.64 (±0.46) | 3.95 (±0.50) | |
| Thalamus 11C-ER176 PET SUVR | 2.7 × 10−4 | ||
| IQR (Q1, Q3) | 1.13 [1.11, 1.17] | 1.23 [1.15, 1.30] | |
| Mean (±SD) | 1.14 (±0.06) | 1.23 (±0.08) | |
| Thalamus 1H-MRS metabolites | |||
| tCho/tCr | 0.20 | ||
| IQR (Q1, Q3) | 0.3 [0.29, 0.32] | 0.29 [0.28, 0.3] | |
| Mean (±SD) | 0.30 (±0.03) | 0.29 (±0.02) | |
| NAA/tCr | 0.95 | ||
| IQR (Q1, Q3) | 1.24 [1.19, 1.30] | 1.22 [1.19, 1.27] | |
| Mean (±SD) | 1.24 (±0.09) | 1.22 (±0.07) | |
| Glu/tCr | 0.20 | ||
| IQR (Q1, Q3) | 1.14 [1.05, 1.24] | 1.15 [1.11, 1.22] | |
| Mean (±SD) | 1.14 (±0.14) | 1.18 (±0.09) | |
| Gln/tCr | 0.48 | ||
| IQR (Q1, Q3) | 0.41 [0.36, 0.49] | 0.45 [0.42, 0.52] | |
| Mean (±SD) | 0.42 (±0.10) | 0.45 (±0.08) | |
| mIns/tCr | 5.5 × 10−4 | ||
| IQR (Q1, Q3) | 0.84 [0.79, 0.91] | 0.98 [0.91, 1.05] | |
| Mean (±SD) | 0.85 (±0.08) | 0.97 (±0.11) |
| MS (N = 21) | Control (N = 30) | |||||
|---|---|---|---|---|---|---|
| 1H-MRS Metabolites | r | 95% CI | p-Value | r | 95% CI | p-Value |
| tCho/tCr | −0.52 | [−0.9, −0.14] | 0.019 | −0.08 | [−0.45, 0.29] | 0.678 |
| NAA/tCr | 0.32 | [−0.11, 0.75] | 0.175 | −0.11 | [−0.48, 0.26] | 0.564 |
| Glu/tCr | 0.22 | [−0.22, 0.66] | 0.347 | −0.10 | [−0.47, 0.27] | 0.617 |
| Gln/tCr | −0.07 | [−0.52, 0.38] | 0.782 | 0.13 | [−0.24, 0.5] | 0.498 |
| mIns/tCr | −0.67 | [−1, −0.34] | 0.001 | −0.19 | [−0.55, 0.17] | 0.32 |
| MS | Control | |||||
|---|---|---|---|---|---|---|
| 1H-MRS Metabolites | r | 95% CI | p-Value | r | 95% CI | p-Value |
| tCho/tCr | 0.24 | [−0.2, 0.68] | 0.319 | −0.12 | [−0.49, 0.25] | 0.528 |
| NAA/tCr | −0.04 | [−0.49, 0.41] | 0.876 | −0.41 | [−0.75, −0.07] | 0.031 |
| Glu/tCr | −0.13 | [−0.58, 0.32] | 0.578 | −0.28 | [−0.64, 0.08] | 0.151 |
| Gln/tCr | 0.01 | [−0.44, 0.46] | 0.972 | −0.19 | [−0.56, 0.18] | 0.327 |
| mIns/tCr | 0.48 | [0.09, 0.87] | 0.034 | 0.19 | [−0.18, 0.56] | 0.338 |
| MSFC z-Score (N = 21) | PASAT z-Score (N = 21) | EDSS Score (N = 21) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 1H-MRS Metabolites | Rho | 95% CI | p-Value | Rho | 95% CI | p-Value | Rho | 95% CI | p-Value |
| tCho/tCr | 0.26 | [−0.19, 0.71] | 0.275 | 0.13 | [−0.33, 0.59] | 0.604 | 0.13 | [−0.32, 0.58] | 0.580 |
| NAA/tCr | 0.15 | [−0.31, 0.61] | 0.546 | 0.03 | [−0.43, 0.49] | 0.907 | −0.4 | [−0.81, 0.01] | 0.084 |
| Glu/tCr | 0.18 | [−0.27, 0.63] | 0.466 | 0.31 | [−0.13, 0.75] | 0.198 | −0.12 | [−0.57, 0.33] | 0.627 |
| Gln/tCr | 0.12 | [−0.34, 0.58] | 0.628 | 0.23 | [−0.22, 0.68] | 0.340 | 0.04 | [−0.41, 0.49] | 0.868 |
| mIns/tCr | −0.22 | [−0.67, 0.23] | 0.363 | −0.48 | [−0.89, −0.07] | 0.036 | 0.13 | [−0.32, 0.58] | 0.588 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kara, F.; Neyal, N.; Kamykowski, M.G.; Schwarz, C.G.; Kendall-Thomas, J.; Morrison, H.A.; Senjem, M.L.; Przybelski, S.A.; Fought, A.J.; Port, J.D.; et al. Decoding Thalamic Glial Interplay in Multiple Sclerosis Through Proton Magnetic Resonance Spectroscopy and Positron Emission Tomography. Int. J. Mol. Sci. 2025, 26, 8656. https://doi.org/10.3390/ijms26178656
Kara F, Neyal N, Kamykowski MG, Schwarz CG, Kendall-Thomas J, Morrison HA, Senjem ML, Przybelski SA, Fought AJ, Port JD, et al. Decoding Thalamic Glial Interplay in Multiple Sclerosis Through Proton Magnetic Resonance Spectroscopy and Positron Emission Tomography. International Journal of Molecular Sciences. 2025; 26(17):8656. https://doi.org/10.3390/ijms26178656
Chicago/Turabian StyleKara, Firat, Nur Neyal, Michael G. Kamykowski, Christopher G. Schwarz, June Kendall-Thomas, Holly A. Morrison, Matthew L. Senjem, Scott A. Przybelski, Angela J. Fought, John D. Port, and et al. 2025. "Decoding Thalamic Glial Interplay in Multiple Sclerosis Through Proton Magnetic Resonance Spectroscopy and Positron Emission Tomography" International Journal of Molecular Sciences 26, no. 17: 8656. https://doi.org/10.3390/ijms26178656
APA StyleKara, F., Neyal, N., Kamykowski, M. G., Schwarz, C. G., Kendall-Thomas, J., Morrison, H. A., Senjem, M. L., Przybelski, S. A., Fought, A. J., Port, J. D., Deelchand, D. K., Lowe, V. J., Öz, G., Kantarci, K., Kantarci, O. H., & Zeydan, B. (2025). Decoding Thalamic Glial Interplay in Multiple Sclerosis Through Proton Magnetic Resonance Spectroscopy and Positron Emission Tomography. International Journal of Molecular Sciences, 26(17), 8656. https://doi.org/10.3390/ijms26178656







