siRNA Therapeutics for the Treatment of Hereditary Diseases and Other Conditions: A Review
Abstract
1. Introduction
2. RNA Therapeutics
2.1. Patisiran—A First-Generation Therapeutic for Patients with Hereditary Transthyretin-Mediated Amyloidosis (hATTR)
2.2. Vutrisiran—Second-Generation Therapeutic for Patients with hATTR
2.3. Givosiran/Givlaari—A Second-Generation Therapeutic for Patients with Acute Hepatic Porphyria
2.4. Lumasiran/Oxlumo—A Second-Generation Therapeutic for Patients with Primary Hyperoxaluria Type 1 Targeting Glyoxylate Oxidase
2.5. Nedosiran—A Second-Generation Therapeutic for Patients with Primary Hyperoxaluria Type 1 Targeting Lactate Dehydrogenase
2.6. Inclisiran/Leqvio—A Second-Generation Therapeutic for Adults with Primary Hypercholesterolaemia or Mixed Dyslipidaemia
2.7. Fitusiran—A Second-Generation Therapeutic for Patients with Haemophilia A and Haemophilia B
2.8. Teprasiran—A p53-Repressing Therapeutic for Treatment of Delayed Graft Function (DGF)
2.9. Cosdosiran—A Therapeutic for Patients with Nonarteritic Anterior Ischaemic Optic Neuropathy and Primary Angle Closure Glaucoma
2.10. Tivanisiran—An siRNA-Derived Therapeutic for the Treatment of Dry Eye Disease
2.11. Cemdisiran—An siRNA-Derived Therapeutic for Adults with Immunoglobulin A Nephropathy (IgAN)
2.12. MRG110 (Anti-miR-92), for Wound Healing and Preventing Heart Failure (HF)
2.13. Remlarsen/MRG201 (miR-29 Mimetic), for Prevention of Fibrous and Keloid Scar Formation
2.14. Lademirsen/RG012 (Anti-miR-21), for Treatment of Alport Syndrome
2.15. RNA-Based Drugs and Clinical Trials
3. Discussion
Next-Generation Delivery Strategies
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Palmieri, L.; Ferrand, M.; Vu Hong, A.; Richard, I.; Albini, S. In Silico Structural Prediction for the Generation of Novel Performant Midi-Dystrophins Based on Intein-Mediated Dual AAV Approach. Int. J. Mol. Sci. 2024, 25, 10444. [Google Scholar] [CrossRef]
- Zhang, L.; Tan, F.; Qi, J.; Lu, Y.; Wang, X.; Yang, X.; Chen, X.; Zhang, X.; Fan, J.; Zhou, Y.; et al. AAV-Mediated Gene Therapy for Hereditary Deafness: Progress and Perspectives. Adv. Sci. Weinh. Baden-Wurtt. Ger. 2024, 11, e2402166. [Google Scholar] [CrossRef]
- Duan, D. Full-Length Dystrophin Gene Therapy for Duchenne Muscular Dystrophy. Mol. Ther. J. Am. Soc. Gene Ther. 2024, 32, 2817–2818. [Google Scholar] [CrossRef]
- Zhu, H.; Zhu, S.; Jiang, Q.; Pang, Y.; Huang, Y.; Chen, Y.; Hou, T.; Deng, W.; Liu, X.; Zeng, L.; et al. Vulto-van Silfhout-de Vries Syndrome Caused by de Novo Variants of DEAF1 Gene: A Case Report and Literature Review. Front. Neurol. 2023, 14, 1251467. [Google Scholar] [CrossRef]
- Fire, A.; Xu, S.; Montgomery, M.K.; Kostas, S.A.; Driver, S.E.; Mello, C.C. Potent and Specific Genetic Interference by Double-Stranded RNA in Caenorhabditis Elegans. Nature 1998, 391, 806–811. [Google Scholar] [CrossRef]
- Zhang, M.M.; Bahal, R.; Rasmussen, T.P.; Manautou, J.E.; Zhong, X. The Growth of siRNA-Based Therapeutics: Updated Clinical Studies. Biochem. Pharmacol. 2021, 189, 114432. [Google Scholar] [CrossRef]
- Adams, D.; Gonzalez-Duarte, A.; O’Riordan, W.D.; Yang, C.-C.; Ueda, M.; Kristen, A.V.; Tournev, I.; Schmidt, H.H.; Coelho, T.; Berk, J.L.; et al. Patisiran, an RNAi Therapeutic, for Hereditary Transthyretin Amyloidosis. N. Engl. J. Med. 2018, 379, 11–21. [Google Scholar] [CrossRef]
- Zhang, X.; Goel, V.; Robbie, G.J. Pharmacokinetics of Patisiran, the First Approved RNA Interference Therapy in Patients with Hereditary Transthyretin-Mediated Amyloidosis. J. Clin. Pharmacol. 2020, 60, 573–585. [Google Scholar] [CrossRef]
- Butler, J.S.; Chan, A.; Costelha, S.; Fishman, S.; Willoughby, J.L.; Borland, T.D.; Milstein, S.; Foster, D.J.; Gonçalves, P.; Chen, Q.; et al. Preclinical Evaluation of RNAi as a Treatment for Transthyretin-mediated Amyloidosis. Amyloid 2016, 23, 109–118. [Google Scholar] [CrossRef]
- Kristen, A.V.; Ajroud-Driss, S.; Conceição, I.; Gorevic, P.; Kyriakides, T.; Obici, L. Patisiran, an RNAi Therapeutic for the Treatment of Hereditary Transthyretin-Mediated Amyloidosis. Neurodegener. Dis. Manag. 2019, 9, 5–23. [Google Scholar] [CrossRef]
- Janas, M.M.; Zlatev, I.; Liu, J.; Jiang, Y.; Barros, S.A.; Sutherland, J.E.; Davis, W.P.; Liu, J.; Brown, C.R.; Liu, X.; et al. Safety Evaluation of 2′-Deoxy-2′-Fluoro Nucleotides in GalNAc-siRNA Conjugates. Nucleic Acids Res. 2019, 47, 3306–3320. [Google Scholar] [CrossRef]
- Foster, D.J.; Brown, C.R.; Shaikh, S.; Trapp, C.; Schlegel, M.K.; Qian, K.; Sehgal, A.; Rajeev, K.G.; Jadhav, V.; Manoharan, M.; et al. Advanced siRNA Designs Further Improve In Vivo Performance of GalNAc-siRNA Conjugates. Mol. Ther. 2018, 26, 708–717. [Google Scholar] [CrossRef]
- Planté-Bordeneuve, V.; Perrain, V. Vutrisiran: A New Drug in the Treatment Landscape of Hereditary Transthyretin Amyloid Polyneuropathy. Expert Opin. Drug Discov. 2024, 19, 393–402. [Google Scholar] [CrossRef]
- Habtemariam, B.A.; Karsten, V.; Attarwala, H.; Goel, V.; Melch, M.; Clausen, V.A.; Garg, P.; Vaishnaw, A.K.; Sweetser, M.T.; Robbie, G.J.; et al. Single-Dose Pharmacokinetics and Pharmacodynamics of Transthyretin Targeting N-acetylgalactosamine–Small Interfering Ribonucleic Acid Conjugate, Vutrisiran, in Healthy Subjects. Clin. Pharmacol. Ther. 2021, 109, 372–382. [Google Scholar] [CrossRef]
- Sardh, E.; Barbaro, M. Acute Intermittent Porphyria. In GeneReviews®; Adam, M.P., Feldman, J., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 2005. [Google Scholar]
- Anderson, K.E. Acute Hepatic Porphyrias: Current Diagnosis & Management. Mol. Genet. Metab. 2019, 128, 219–227. [Google Scholar] [CrossRef]
- Besur, S.; Hou, W.; Schmeltzer, P.; Bonkovsky, H.L. Clinically Important Features of Porphyrin and Heme Metabolism and the Porphyrias. Metabolites 2014, 4, 977–1006. [Google Scholar] [CrossRef]
- Pallet, N.; Karras, A.; Thervet, E.; Gouya, L.; Karim, Z.; Puy, H. Porphyria and Kidney Diseases. Clin. Kidney J. 2018, 11, 191–197. [Google Scholar] [CrossRef]
- Balwani, M.; Sardh, E.; Ventura, P.; Peiró, P.A.; Rees, D.C.; Stölzel, U.; Bissell, D.M.; Bonkovsky, H.L.; Windyga, J.; Anderson, K.E.; et al. Phase 3 Trial of RNAi Therapeutic Givosiran for Acute Intermittent Porphyria. N. Engl. J. Med. 2020, 382, 2289–2301. [Google Scholar] [CrossRef]
- Sardh, E.; Harper, P.; Balwani, M.; Stein, P.; Rees, D.; Bissell, D.M.; Desnick, R.; Parker, C.; Phillips, J.; Bonkovsky, H.L.; et al. Phase 1 Trial of an RNA Interference Therapy for Acute Intermittent Porphyria. N. Engl. J. Med. 2019, 380, 549–558. [Google Scholar] [CrossRef]
- Gonzalez-Aseguinolaza, G. Givosiran—Running RNA Interference to Fight Porphyria Attacks. N. Engl. J. Med. 2020, 382, 2366–2367. [Google Scholar] [CrossRef]
- Dindo, M.; Conter, C.; Oppici, E.; Ceccarelli, V.; Marinucci, L.; Cellini, B. Molecular Basis of Primary Hyperoxaluria: Clues to Innovative Treatments. Urolithiasis 2019, 47, 67–78. [Google Scholar] [CrossRef]
- Kletzmayr, A.; Ivarsson, M.E.; Leroux, J.-C. Investigational Therapies for Primary Hyperoxaluria. Bioconjug. Chem. 2020, 31, 1696–1707. [Google Scholar] [CrossRef]
- Goldfarb, D.S.; Lieske, J.C.; Groothoff, J.; Schalk, G.; Russell, K.; Yu, S.; Vrhnjak, B. Nedosiran in Primary Hyperoxaluria Subtype 3: Results from a Phase I, Single-Dose Study (PHYOX4). Urolithiasis 2023, 51, 80, Erratum in: Urolithiasis 2023, 51, 85. [Google Scholar] [CrossRef]
- Hu, B.; Zhong, L.; Weng, Y.; Peng, L.; Huang, Y.; Zhao, Y.; Liang, X.-J. Therapeutic siRNA: State of the Art. Signal Transduct. Target. Ther. 2020, 5, 101. [Google Scholar] [CrossRef]
- Fitzgerald, K.; White, S.; Borodovsky, A.; Bettencourt, B.R.; Strahs, A.; Clausen, V.; Wijngaard, P.; Horton, J.D.; Taubel, J.; Brooks, A.; et al. A Highly Durable RNAi Therapeutic Inhibitor of PCSK9. N. Engl. J. Med. 2017, 376, 41–51. [Google Scholar] [CrossRef]
- Maningat, P.; Gordon, B.R.; Breslow, J.L. How Do We Improve Patient Compliance and Adherence to Long-Term Statin Therapy? Curr. Atheroscler. Rep. 2013, 15, 291. [Google Scholar] [CrossRef]
- Dyrbuś, K.; Gąsior, M.; Penson, P.; Ray, K.K.; Banach, M. Inclisiran-New Hope in the Management of Lipid Disorders? J. Clin. Lipidol. 2020, 14, 16–27. [Google Scholar] [CrossRef]
- Khvorova, A. Oligonucleotide Therapeutics—A New Class of Cholesterol-Lowering Drugs. N. Engl. J. Med. 2017, 376, 4–7. [Google Scholar] [CrossRef]
- Hovingh, G.K.; Lepor, N.E.; Kallend, D.; Stoekenbroek, R.M.; Wijngaard, P.L.J.; Raal, F.J. Inclisiran Durably Lowers Low-Density Lipoprotein Cholesterol and Proprotein Convertase Subtilisin/Kexin Type 9 Expression in Homozygous Familial Hypercholesterolemia: The ORION-2 Pilot Study. Circulation 2020, 141, 1829–1831. [Google Scholar] [CrossRef]
- Sheridan, C. PCSK9-Gene-Silencing, Cholesterol-Lowering Drug Impresses. Nat. Biotechnol. 2019, 37, 1385–1387. [Google Scholar] [CrossRef]
- Bamji, A.N. Do PCSK9 Inhibitors Do Anything More than Reduce LDL Cholesterol? BMJ 2020, 368, m1159. [Google Scholar] [CrossRef]
- Castaman, G.; Matino, D. Hemophilia A and B: Molecular and Clinical Similarities and Differences. Haematologica 2019, 104, 1702–1709. [Google Scholar] [CrossRef]
- Rezende, S.M.; Neumann, I.; Angchaisuksiri, P.; Awodu, O.; Boban, A.; Cuker, A.; Curtin, J.A.; Fijnvandraat, K.; Gouw, S.C.; Gualtierotti, R.; et al. International Society on Thrombosis and Haemostasis Clinical Practice Guideline for Treatment of Congenital Hemophilia A and B Based on the Grading of Recommendations Assessment, Development, and Evaluation Methodology. J. Thromb. Haemost. JTH 2024, 22, 2629–2652. [Google Scholar] [CrossRef]
- Berk, C.; Civenni, G.; Wang, Y.; Steuer, C.; Catapano, C.V.; Hall, J. Pharmacodynamic and Pharmacokinetic Properties of Full Phosphorothioate Small Interfering RNAs for Gene Silencing In Vivo. Nucleic Acid Ther. 2021, 31, 237–244. [Google Scholar] [CrossRef]
- Davidson, B.L.; McCray, P.B. Current Prospects for RNA Interference-Based Therapies. Nat. Rev. Genet. 2011, 12, 329–340. [Google Scholar] [CrossRef]
- Makris, K.; Spanou, L. Acute Kidney Injury: Definition, Pathophysiology and Clinical Phenotypes. Clin. Biochem. Rev. 2016, 37, 85–98. [Google Scholar]
- Molitoris, B.A.; Dagher, P.C.; Sandoval, R.M.; Campos, S.B.; Ashush, H.; Fridman, E.; Brafman, A.; Faerman, A.; Atkinson, S.J.; Thompson, J.D.; et al. siRNA Targeted to P53 Attenuates Ischemic and Cisplatin-Induced Acute Kidney Injury. J. Am. Soc. Nephrol. JASN 2009, 20, 1754–1764. [Google Scholar] [CrossRef]
- Thielmann, M.; Corteville, D.; Szabo, G.; Swaminathan, M.; Lamy, A.; Lehner, L.J.; Brown, C.D.; Noiseux, N.; Atta, M.G.; Squiers, E.C.; et al. Teprasiran, a Small Interfering RNA, for the Prevention of Acute Kidney Injury in High-Risk Patients Undergoing Cardiac Surgery: A Randomized Clinical Study. Circulation 2021, 144, 1133–1144. [Google Scholar] [CrossRef]
- Gaier, E.D.; Torun, N. The Enigma of Nonarteritic Anterior Ischemic Optic Neuropathy: An Update for the Comprehensive Ophthalmologist. Curr. Opin. Ophthalmol. 2016, 27, 498–504. [Google Scholar] [CrossRef]
- Ahmed, Z.; Kalinski, H.; Berry, M.; Almasieh, M.; Ashush, H.; Slager, N.; Brafman, A.; Spivak, I.; Prasad, N.; Mett, I.; et al. Ocular Neuroprotection by siRNA Targeting Caspase-2. Cell Death Dis. 2011, 2, e173. [Google Scholar] [CrossRef]
- Solano, E.C.R.; Kornbrust, D.J.; Beaudry, A.; Foy, J.W.-D.; Schneider, D.J.; Thompson, J.D. Toxicological and Pharmacokinetic Properties of QPI-1007, a Chemically Modified Synthetic siRNA Targeting Caspase 2 mRNA, Following Intravitreal Injection. Nucleic Acid Ther. 2014, 24, 258–266. [Google Scholar] [CrossRef]
- Jiang, J.; Zhang, X.; Tang, Y.; Li, S.; Chen, J. Progress on Ocular siRNA Gene-Silencing Therapy and Drug Delivery Systems. Fundam. Clin. Pharmacol. 2021, 35, 4–24. [Google Scholar] [CrossRef]
- Moreno-Montañés, J.; Bleau, A.-M.; Jimenez, A.I. Tivanisiran, a Novel siRNA for the Treatment of Dry Eye Disease. Expert Opin. Investig. Drugs 2018, 27, 421–426. [Google Scholar] [CrossRef]
- Bereiter, D.A.; Rahman, M.; Thompson, R.; Stephenson, P.; Saito, H. TRPV1 and TRPM8 Channels and Nocifensive Behavior in a Rat Model for Dry Eye. Investig. Ophthalmol. Vis. Sci. 2018, 59, 3739–3746. [Google Scholar] [CrossRef]
- Gaya, A.; Munir, T.; Urbano-Ispizua, A.; Griffin, M.; Taubel, J.; Bush, J.; Bhan, I.; Borodovsky, A.; Wang, Y.; Badri, P.; et al. Results of a Phase 1/2 Study of Cemdisiran in Healthy Subjects and Patients with Paroxysmal Nocturnal Hemoglobinuria. EJHaem 2023, 4, 612–624. [Google Scholar] [CrossRef]
- Gallant-Behm, C.L.; Piper, J.; Dickinson, B.A.; Dalby, C.M.; Pestano, L.A.; Jackson, A.L. A Synthetic microRNA-92a Inhibitor (MRG-110) Accelerates Angiogenesis and Wound Healing in Diabetic and Nondiabetic Wounds. Wound Repair Regen. 2018, 26, 311–323. [Google Scholar] [CrossRef]
- Bansal, R.; Prakash, J.; De Ruiter, M.; Poelstra, K. Targeted Recombinant Fusion Proteins of IFNγ and Mimetic IFNγ with PDGFβR Bicyclic Peptide Inhibits Liver Fibrogenesis In Vivo. PLoS ONE 2014, 9, e89878. [Google Scholar] [CrossRef]
- Chioccioli, M.; Roy, S.; Newell, R.; Pestano, L.; Dickinson, B.; Rigby, K.; Herazo-Maya, J.; Jenkins, G.; Ian, S.; Saini, G.; et al. A Lung Targeted miR-29 Mimic as a Therapy for Pulmonary Fibrosis. eBioMedicine 2022, 85, 104304. [Google Scholar] [CrossRef]
- Gomez, I.G.; MacKenna, D.A.; Johnson, B.G.; Kaimal, V.; Roach, A.M.; Ren, S.; Nakagawa, N.; Xin, C.; Newitt, R.; Pandya, S.; et al. Anti-microRNA-21 Oligonucleotides Prevent Alport Nephropathy Progression by Stimulating Metabolic Pathways. J. Clin. Investig. 2015, 125, 141–156. [Google Scholar] [CrossRef]
- Rheault, M.N.; Savige, J.; Randles, M.J.; Weinstock, A.; Stepney, M.; Turner, A.N.; Parziale, G.; Gross, O.; Flinter, F.A.; Miner, J.H.; et al. The Importance of Clinician, Patient and Researcher Collaborations in Alport Syndrome. Pediatr. Nephrol. 2020, 35, 733–742. [Google Scholar] [CrossRef]
- Kashtan, C. Alport Syndrome: Facts and Opinions. F1000Research 2017, 6, 50. [Google Scholar] [CrossRef]
- Clark, S.D.; Song, W.; Cianciolo, R.; Lees, G.; Nabity, M.; Liu, S. Abnormal Expression of miR-21 in Kidney Tissue of Dogs With X-Linked Hereditary Nephropathy: A Canine Model of Chronic Kidney Disease. Vet. Pathol. 2019, 56, 93–105. [Google Scholar] [CrossRef]
- Rubel, D.; Boulanger, J.; Craciun, F.; Xu, E.Y.; Zhang, Y.; Phillips, L.; Callahan, M.; Weber, W.; Song, W.; Ngai, N.; et al. Anti-microRNA-21 Therapy on Top of ACE Inhibition Delays Renal Failure in Alport Syndrome Mouse Models. Cells 2022, 11, 594. [Google Scholar] [CrossRef]
- Gale, D.P.; Gross, O.; Wang, F.; Esteban De La Rosa, R.J.; Hall, M.; Sayer, J.A.; Appel, G.; Hariri, A.; Liu, S.; Maski, M.; et al. A Randomized Controlled Clinical Trial Testing Effects of Lademirsen on Kidney Function Decline in Adults with Alport Syndrome. Clin. J. Am. Soc. Nephrol. 2024, 19, 995–1004. [Google Scholar] [CrossRef]
- Weinstock, B.A. Lessons Learned from HERA: The First Alport Syndrome Therapeutic Clinical Trial. Clin. J. Am. Soc. Nephrol. 2024, 19, 946–948. [Google Scholar] [CrossRef]
- Sahraei, M.; Chaube, B.; Liu, Y.; Sun, J.; Kaplan, A.; Price, N.L.; Ding, W.; Oyaghire, S.; García-Milian, R.; Mehta, S.; et al. Suppressing miR-21 Activity in Tumor-Associated Macrophages Promotes an Antitumor Immune Response. J. Clin. Investig. 2019, 129, 5518–5536. [Google Scholar] [CrossRef]
- Chen, Z.; Song, S.; Zhu, J.; Lai, X. Regulatory Mechanism of MiR-21 in Formation and Rupture of Intracranial Aneurysm through JNK Signaling Pathway-Mediated Inflammatory Response. Int. J. Clin. Exp. Pathol. 2020, 13, 1834–1841. [Google Scholar]
- Wu, X.; Liu, S.; Liu, D.; Li, X.; Wang, H.; Han, X. Global and Chinese Trends in Oligonucleotide Drug Clinical Development: A Comparative Analysis. Pharmacol. Res. 2024, 210, 107487. [Google Scholar] [CrossRef]
- Dykxhoorn, D.M.; Schlehuber, L.D.; London, I.M.; Lieberman, J. Determinants of Specific RNA Interference-Mediated Silencing of Human Beta-Globin Alleles Differing by a Single Nucleotide Polymorphism. Proc. Natl. Acad. Sci. USA 2006, 103, 5953–5958. [Google Scholar] [CrossRef]
- Ahmed, F.; Raghava, G.P.S. Designing of Highly Effective Complementary and Mismatch siRNAs for Silencing a Gene. PLoS ONE 2011, 6, e23443. [Google Scholar] [CrossRef]
- Townsend, R.R.; Wall, D.A.; Hubbard, A.L.; Lee, Y.C. Rapid Release of Galactose-Terminated Ligands after Endocytosis by Hepatic Parenchymal Cells: Evidence for a Role of Carbohydrate Structure in the Release of Internalized Ligand from Receptor. Proc. Natl. Acad. Sci. USA 1984, 81, 466–470. [Google Scholar] [CrossRef] [PubMed]
- Wright, R.S.; Collins, M.G.; Stoekenbroek, R.M.; Robson, R.; Wijngaard, P.L.J.; Landmesser, U.; Leiter, L.A.; Kastelein, J.J.P.; Ray, K.K.; Kallend, D. Effects of Renal Impairment on the Pharmacokinetics, Efficacy, and Safety of Inclisiran: An Analysis of the ORION-7 and ORION-1 Studies. Mayo Clin. Proc. 2020, 95, 77–89. [Google Scholar] [CrossRef]
- Dirisala, A.; Uchida, S.; Toh, K.; Li, J.; Osawa, S.; Tockary, T.A.; Liu, X.; Abbasi, S.; Hayashi, K.; Mochida, Y.; et al. Transient Stealth Coating of Liver Sinusoidal Wall by Anchoring Two-Armed PEG for Retargeting Nanomedicines. Sci. Adv. 2020, 6, eabb8133. [Google Scholar] [CrossRef]
- Ge, P.; Dong, C.; Ren, X.; Weiderpass, E.; Zhang, C.; Fan, H.; Zhang, J.; Zhang, Y.; Xi, J. The High Prevalence of Low HDL-Cholesterol Levels and Dyslipidemia in Rural Populations in Northwestern China. PLoS ONE 2015, 10, e0144104. [Google Scholar] [CrossRef]
- Cheng, Q.; Wei, T.; Farbiak, L.; Johnson, L.T.; Dilliard, S.A.; Siegwart, D.J. Selective Organ Targeting (SORT) Nanoparticles for Tissue-Specific mRNA Delivery and CRISPR–Cas Gene Editing. Nat. Nanotechnol. 2020, 15, 313–320. [Google Scholar] [CrossRef]
- Reilley, M.J.; McCoon, P.; Cook, C.; Lyne, P.; Kurzrock, R.; Kim, Y.; Woessner, R.; Younes, A.; Nemunaitis, J.; Fowler, N.; et al. STAT3 Antisense Oligonucleotide AZD9150 in a Subset of Patients with Heavily Pretreated Lymphoma: Results of a Phase 1b Trial. J. Immunother. Cancer 2018, 6, 119. [Google Scholar] [CrossRef]
- Dooley, K.; McConnell, R.E.; Xu, K.; Lewis, N.D.; Haupt, S.; Youniss, M.R.; Martin, S.; Sia, C.L.; McCoy, C.; Moniz, R.J.; et al. A Versatile Platform for Generating Engineered Extracellular Vesicles with Defined Therapeutic Properties. Mol. Ther. 2021, 29, 1729–1743. [Google Scholar] [CrossRef]
- Komiya, R. Biogenesis of Diverse Plant phasiRNAs Involves an miRNA-Trigger and Dicer-Processing. J. Plant Res. 2017, 130, 17–23. [Google Scholar] [CrossRef]
- Verlaan, M.G.; Hutton, S.F.; Ibrahem, R.M.; Kormelink, R.; Visser, R.G.F.; Scott, J.W.; Edwards, J.D.; Bai, Y. The Tomato Yellow Leaf Curl Virus Resistance Genes Ty-1 and Ty-3 Are Allelic and Code for DFDGD-Class RNA–Dependent RNA Polymerases. PLoS Genet. 2013, 9, e1003399. [Google Scholar] [CrossRef]
- Wang, X.-B.; Wu, Q.; Ito, T.; Cillo, F.; Li, W.-X.; Chen, X.; Yu, J.-L.; Ding, S.-W. RNAi-Mediated Viral Immunity Requires Amplification of Virus-Derived siRNAs in Arabidopsis Thaliana. Proc. Natl. Acad. Sci. USA 2010, 107, 484–489. [Google Scholar] [CrossRef]
- Zhang, F.; Parayath, N.N.; Ene, C.I.; Stephan, S.B.; Koehne, A.L.; Coon, M.E.; Holland, E.C.; Stephan, M.T. Genetic Programming of Macrophages to Perform Anti-Tumor Functions Using Targeted mRNA Nanocarriers. Nat. Commun. 2019, 10, 3974. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, J.; Rhodes, K.R.; Green, J.J.; Tzeng, S.Y. Poly(Beta-Amino Ester)s as Gene Delivery Vehicles: Challenges and Opportunities. Expert Opin. Drug Deliv. 2020, 17, 1395–1410. [Google Scholar] [CrossRef] [PubMed]
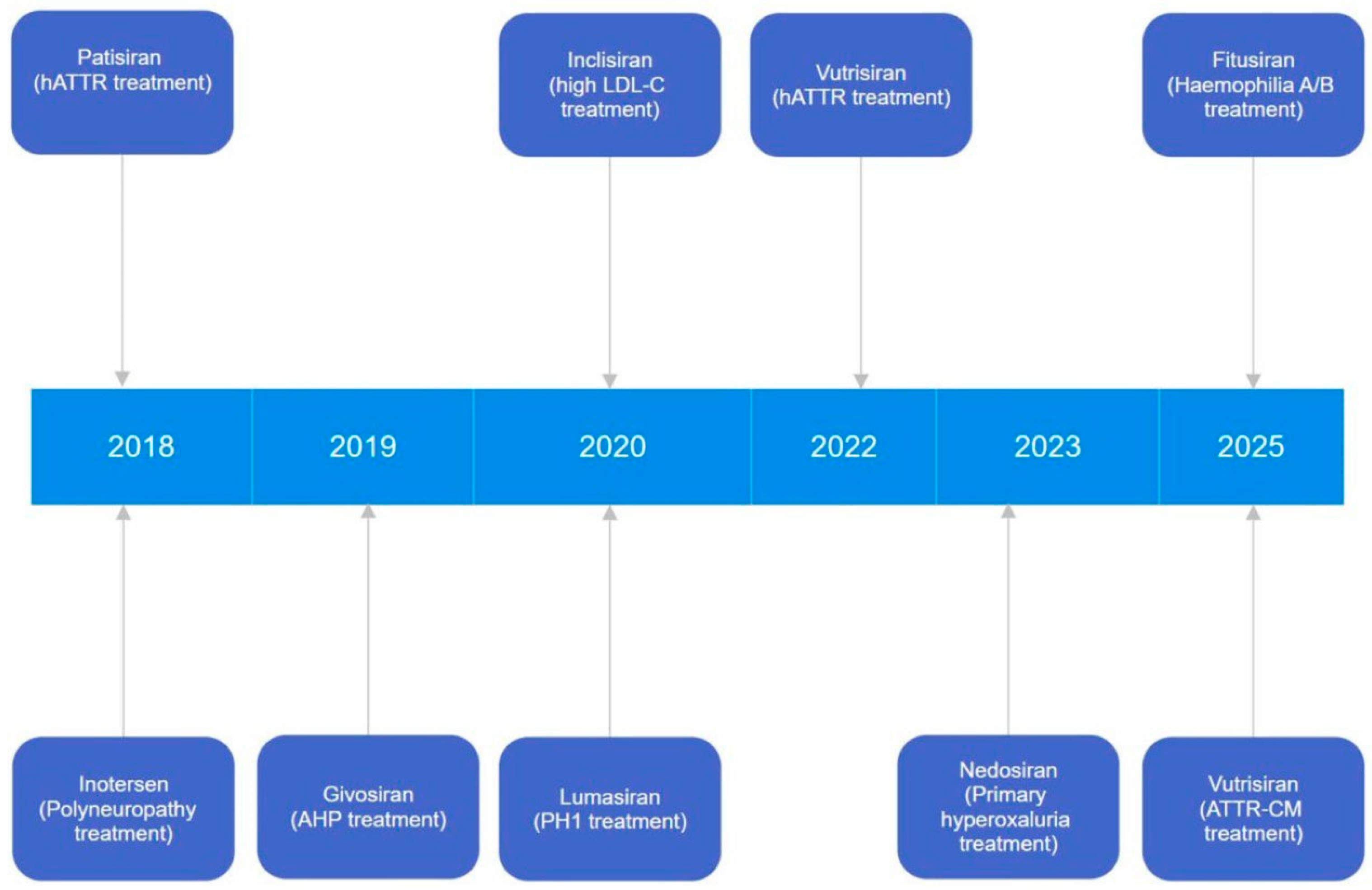

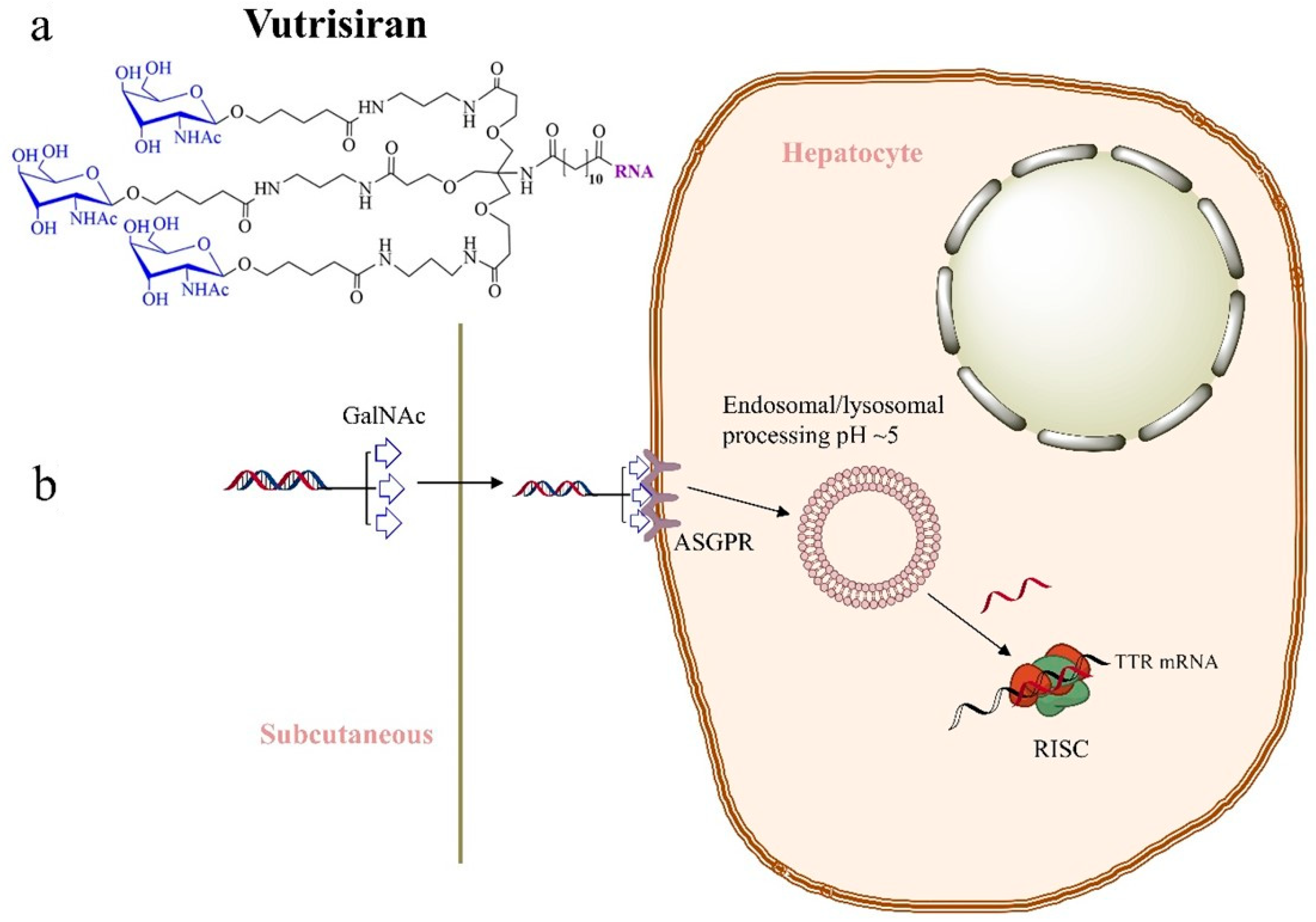
| Drug Name | Therapeutic Application | Molecular Target | RNA Type | Delivery System |
|---|---|---|---|---|
| Approved | ||||
| Patisiran/Onpattro/ALN-TTR02 (FDA, 2018) | Treatment of hATTR (familial amyloid polyneuropathy) | TTR | siRNA | 100 nm LNP: cholesterol, DSPC, PEG2000-C-DMG, DLin-MC3 DMA |
| Givosiran/Givlaari (FDA, 2019) | Treatment of the AHP | ALAS1 | siRNA | Conjugated with triantennary GalNAc |
| Inclisiran/Leqvio (EP, 2020) | Therapy of high LDL-C and an increased risk of premature atherosclerotic CV disease | PCSK9 | siRNA | Conjugated with triantennary GalNAc |
| Lumasiran/Oxlumo (FDA, 2020) | Therapy of PH1 | GO | siRNA | Conjugated with triantennary GalNAc |
| Vutrisiran/Amvuttra (FDA, 2022, 2025) | Treatment of hATTR (familial amyloid polyneuropathy) | TTR | siRNA | Conjugated with triantennary GalNAc |
| Nedosiran/Rivfloza (FDA, 2023) | Therapy for all types of primary hyperoxaluria | LDH | siRNA | Conjugated with triantennary GalNAc |
| Fitusiran/Qfitlia (FDA, 2025) | Therapy of haemophilia A/B | Antithrombin | siRNA | Conjugated with triantennary GalNAc |
| Not approved | ||||
| Cemdisiran | Therapy of IgAN, paroxysmal nocturnal haemoglobinuria, myasthenia gravis, atypical haemolytic uremic syndrome | C5 | siRNA | Conjugated with triantennary GalNAc |
| Cosdosiran | Therapeutic for NAION and primary angle closure glaucoma | Caspase 2 | siRNA | No |
| Lademirsen/RG012 | Treatment of Alport syndrome | miR-21 | ss miR-capturer | No data |
| MRG110 | Wound healing and preventing HF | miR-92 | ss miR-capturer | No data |
| Remlarsen/MRG201 | Prevention of fibrous and keloid scar formation | miR-29 (collagens) | ds miR mimetic | No |
| Teprasiran | Therapy of DGF | p53 anti-oncogene | siRNA | No |
| Tivanisiran | Treatment of dry eye disease | TRPV1 receptor | siRNA | No |
| Drug Name | Administration Route | Efficacy | Side Effects | Reference |
|---|---|---|---|---|
| Approved | ||||
| Fitusiran/Qfitlia (FDA, 2025) | Subcutaneous | Reduction in bleeding rate. Maintained effective bleed protection over a 6-year period. Improved health-related quality of life | Viral or bacterial infection, nasopharyngitis, abnormal blood clotting, gallbladder disease symptoms, elevated transaminases | NCT03417102 NCT03417245 NCT05662319 |
| Givosiran/Givlaari (FDA, 2019) | Subcutaneous | Reduction in urinary aminolevulinic acid (ALA) and porphobilinogen (PBG). Reduction in annualised attack rate of acute hepatic porphyria (AHP) attacks vs. placebo | Nausea, injection site reactions, rash, fatigue, elevated transaminases, renal toxicity | NCT03338816 |
| Inclisiran/Leqvio (EP, 2020) | Subcutaneous | Reduction in LDL-C from baseline, on top of statin therapy. Effects sustained with biannual dosing | Injection site reaction, arthralgia, fatigue, myalgia | NCT03397121, NCT03399370, NCT03400800 |
| Lumasiran/Oxlumo (FDA, 2020) | Subcutaneous | Reduction in urinary oxalate levels from baseline in adults | Injection site reactions, abdominal pain | NCT03681184 NCT03905694 |
| Nedosiran/Rivfloza (FDA, 2023) | Subcutaneous | Reduction in oxalate production in children aged ≥ 9 years and adults with PH1 | Injection site reactions, severe and fluctuating tachycardia | NCT04555486 NCT05001269 NCT03847909 |
| Patisiran/Onpattro/ALN-TTR02 (FDA, 2018) | Intravenous | Improved mNIS+7 neuropathy score vs. placebo at 18 months. Improved quality of life, mobility, and nutritional status | Infusion-related reactions, upper respiratory tract infections | NCT01960348 |
| Vutrisiran/Amvuttra (FDA, 2022, 2025) | Subcutaneous | Reduction in urgent HF visits, risk of CV events, hospitalisations, and mortality through 36 months. Improved mNIS+7 neuropathy score vs. placebo at 9 months | Injection site reactions, pain in the limbs and joints, shortness of breath, and low vitamin A levels | NCT05635045 NCT03759379 |
| Not approved | ||||
| Cemdisiran | Subcutaneous | Substantial reduction in proteinuria in patients with IgA nephropathy and PNH. Used with complement inhibitor pozelimab for PNH | Headache, cough, fatigue, nausea | NCT04888507 NCT03841448 (Phase 2) |
| Cosdosiran | Directly into the eye | The Phase 3 trial did not meet the primary endpoint | Intraocular inflammation, increased intraocular pressure, conjunctival hyperaemia | NCT03913130 (Phase 3) |
| Fitusiran | Subcutaneous | Reduction in annualised bleeding rate (ABR) in haemophilia A and B patients (with/without inhibitors) | Injection site reactions, transaminase elevations, thrombotic events | NCT03417102, NCT03417245 |
| Lademirsen/RG012 | Subcutaneous | The Phase 2 study was completed but did not show significant efficacy on the primary endpoint | - | NCT02855268 |
| MRG110 | Intradermal injection | Targets miR-92 to promote angiogenesis and healing, as shown in Phase 2 | - | NCT03603431 (Phase 1) |
| Nedosiran/Rivfloza | Subcutaneous | Reduction in urinary oxalate from baseline in PH1 patients. A significant proportion reached normal/near-normal levels | Injection site reactions, abdominal pain, fatigue, headache | NCT03847909 |
| Remlarsen/MRG201 | Intradermal injection | Mimics miR-29 to suppress fibrosis | Injection site reactions (discomfort, discolouration, swelling) | NCT03601052 |
| Teprasiran | Single intravenous infusion | The Phase 3 trial results do not meet primary endpoint criteria | A higher incidence of hypotension was observed in the teprasiran group compared to placebo | NCT03510897 |
| Tivanisiran | Ophthalmic eye drops | Topical siRNA targeting the nerve growth factor receptor (NGFR) | Instillation site pain, irritation, blurred vision | NCT03108664 |
| Vutrisiran/Amvuttra | Intravenous | Reversing disease progression at 9 months | Injection site reactions | NCT03759379 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shevelev, A.; Pozdniakova, N.; Generalov, E.; Tarasova, O. siRNA Therapeutics for the Treatment of Hereditary Diseases and Other Conditions: A Review. Int. J. Mol. Sci. 2025, 26, 8651. https://doi.org/10.3390/ijms26178651
Shevelev A, Pozdniakova N, Generalov E, Tarasova O. siRNA Therapeutics for the Treatment of Hereditary Diseases and Other Conditions: A Review. International Journal of Molecular Sciences. 2025; 26(17):8651. https://doi.org/10.3390/ijms26178651
Chicago/Turabian StyleShevelev, Alexei, Natalia Pozdniakova, Evgenii Generalov, and Olga Tarasova. 2025. "siRNA Therapeutics for the Treatment of Hereditary Diseases and Other Conditions: A Review" International Journal of Molecular Sciences 26, no. 17: 8651. https://doi.org/10.3390/ijms26178651
APA StyleShevelev, A., Pozdniakova, N., Generalov, E., & Tarasova, O. (2025). siRNA Therapeutics for the Treatment of Hereditary Diseases and Other Conditions: A Review. International Journal of Molecular Sciences, 26(17), 8651. https://doi.org/10.3390/ijms26178651








