A Study on the Efficacy and Pharmacological Mechanism of Liposome Complexes Containing STING Agonist and Anti-PD-L1 Nanobody in Inhibiting HCC
Abstract
1. Introduction
2. Results
2.1. The Composition and Characteristics of the Liposome Complex XA5508
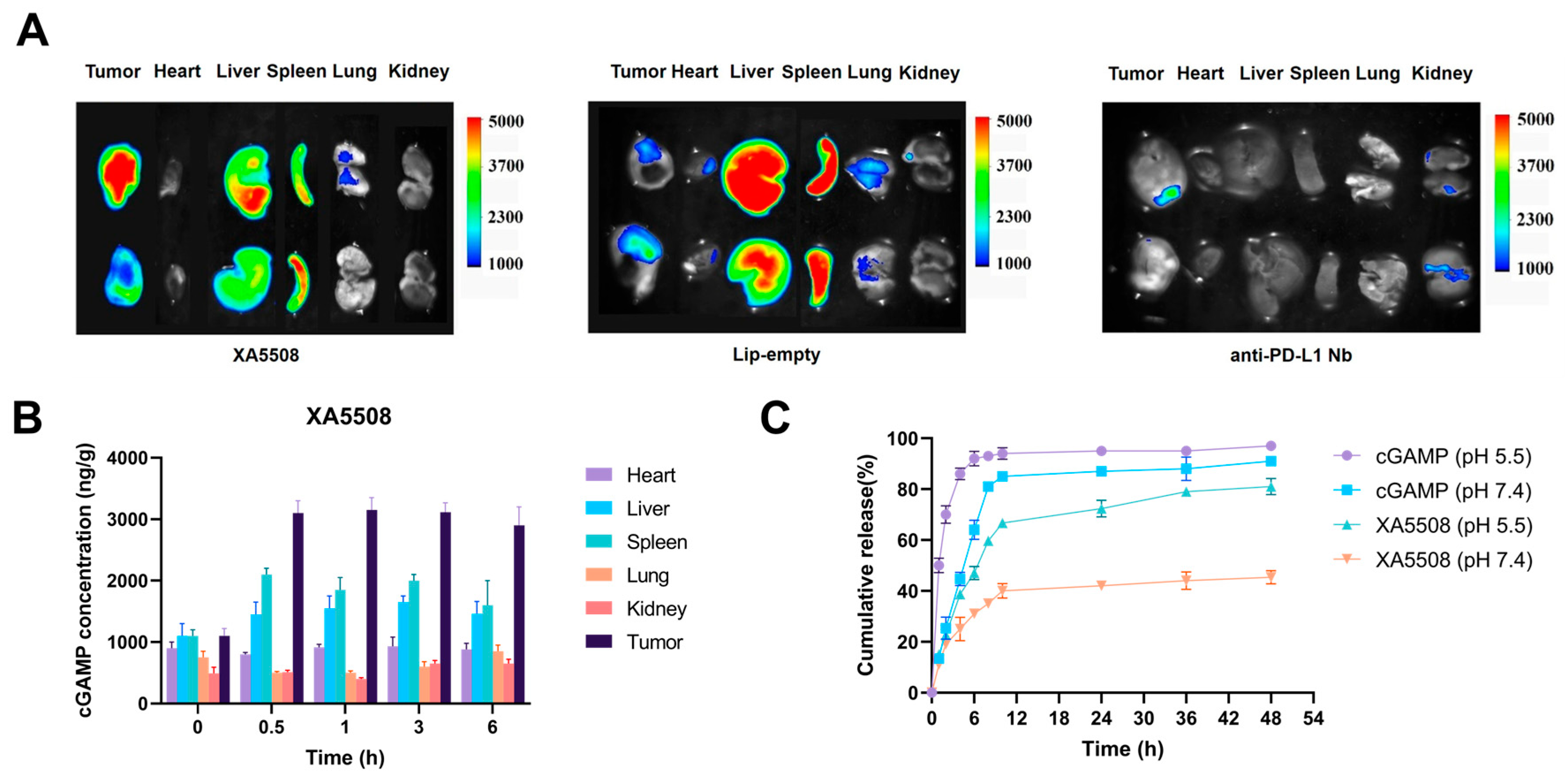
2.2. The Liposome Complex XA5508 Targets the TME for Delivery of cGAMP
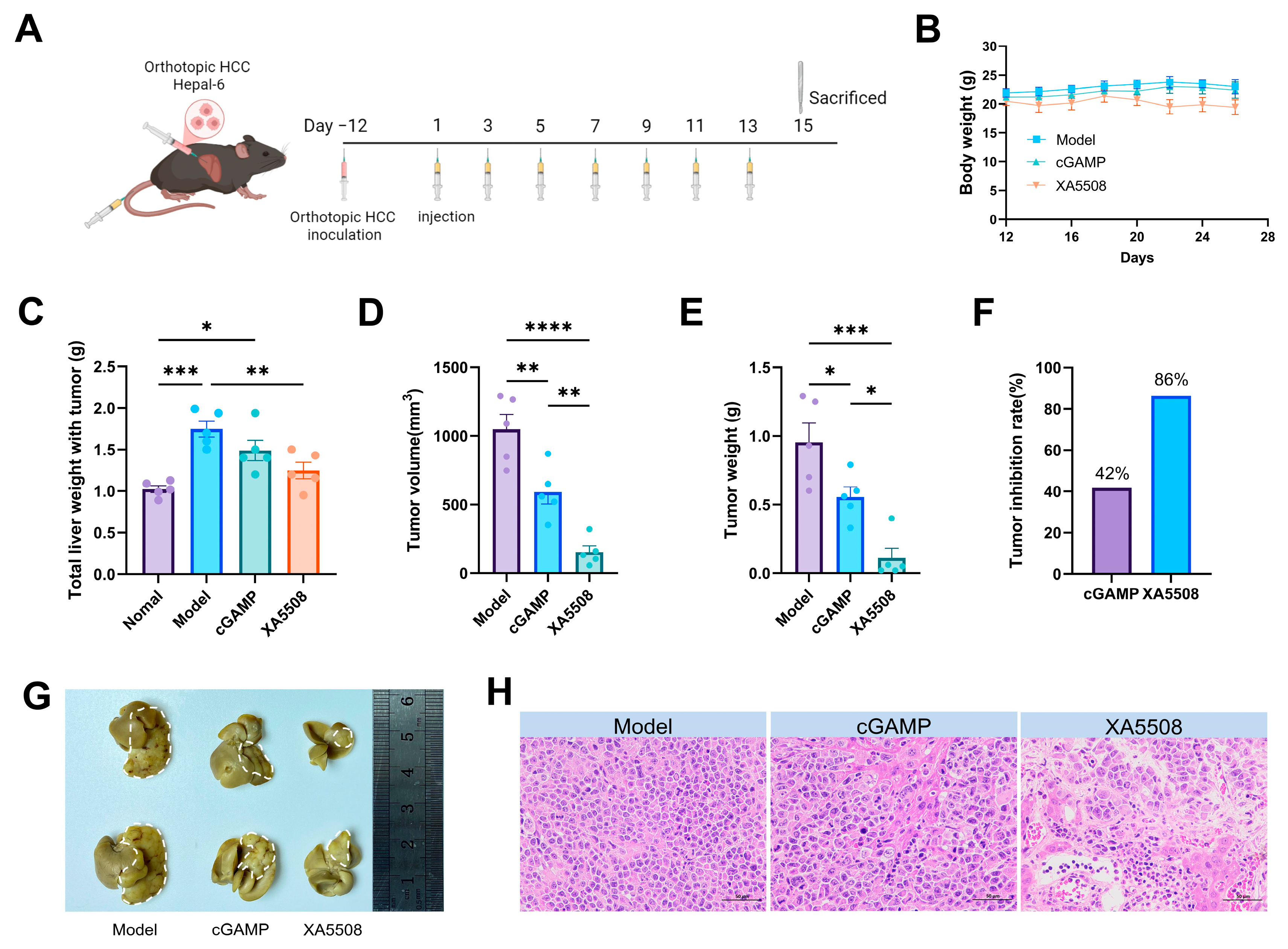
2.3. Establishment of Mouse In Situ HCC Model and In Vivo Drug Efficacy
2.4. Safety Testing of Liposome Complex XA5508
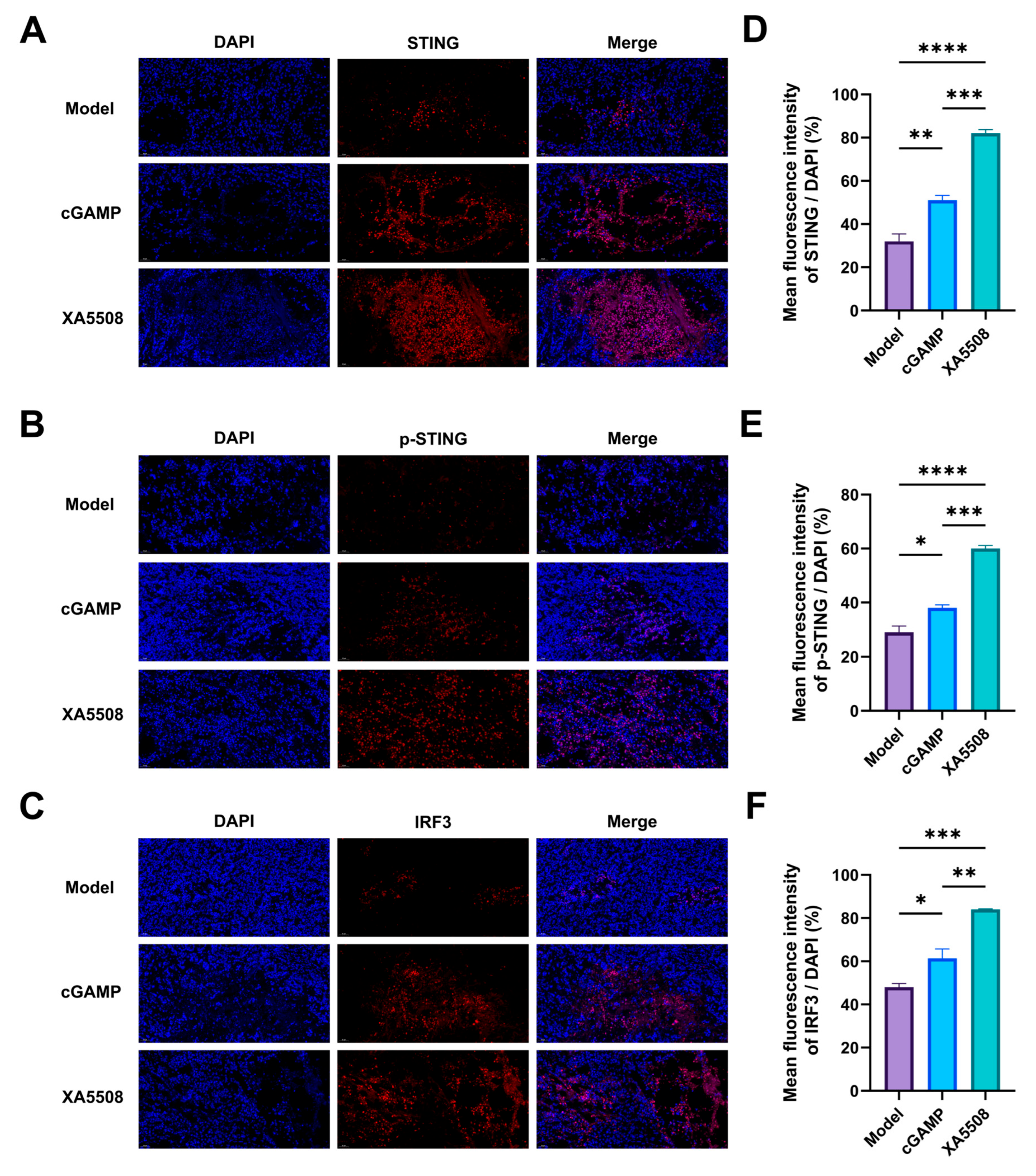
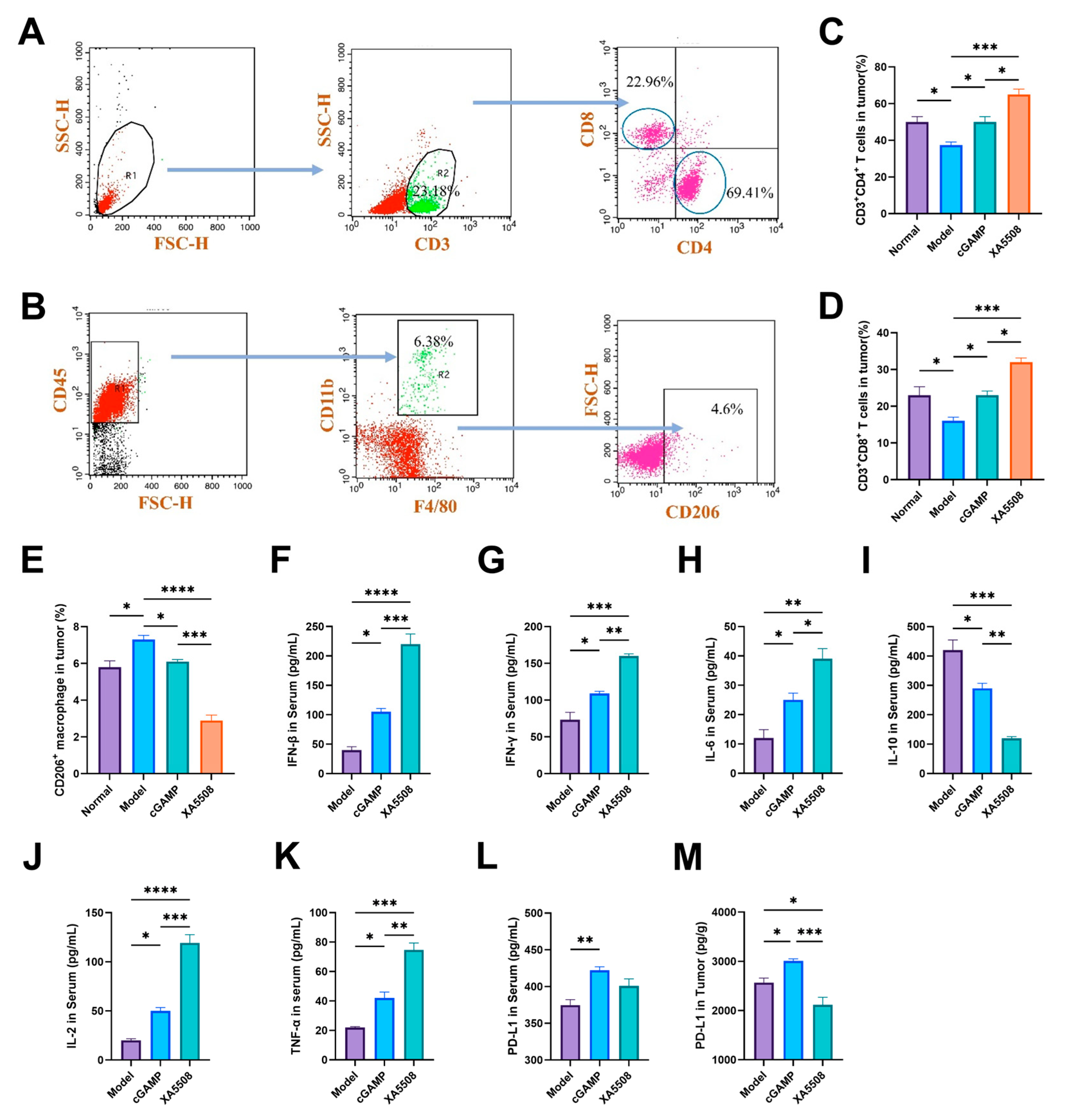
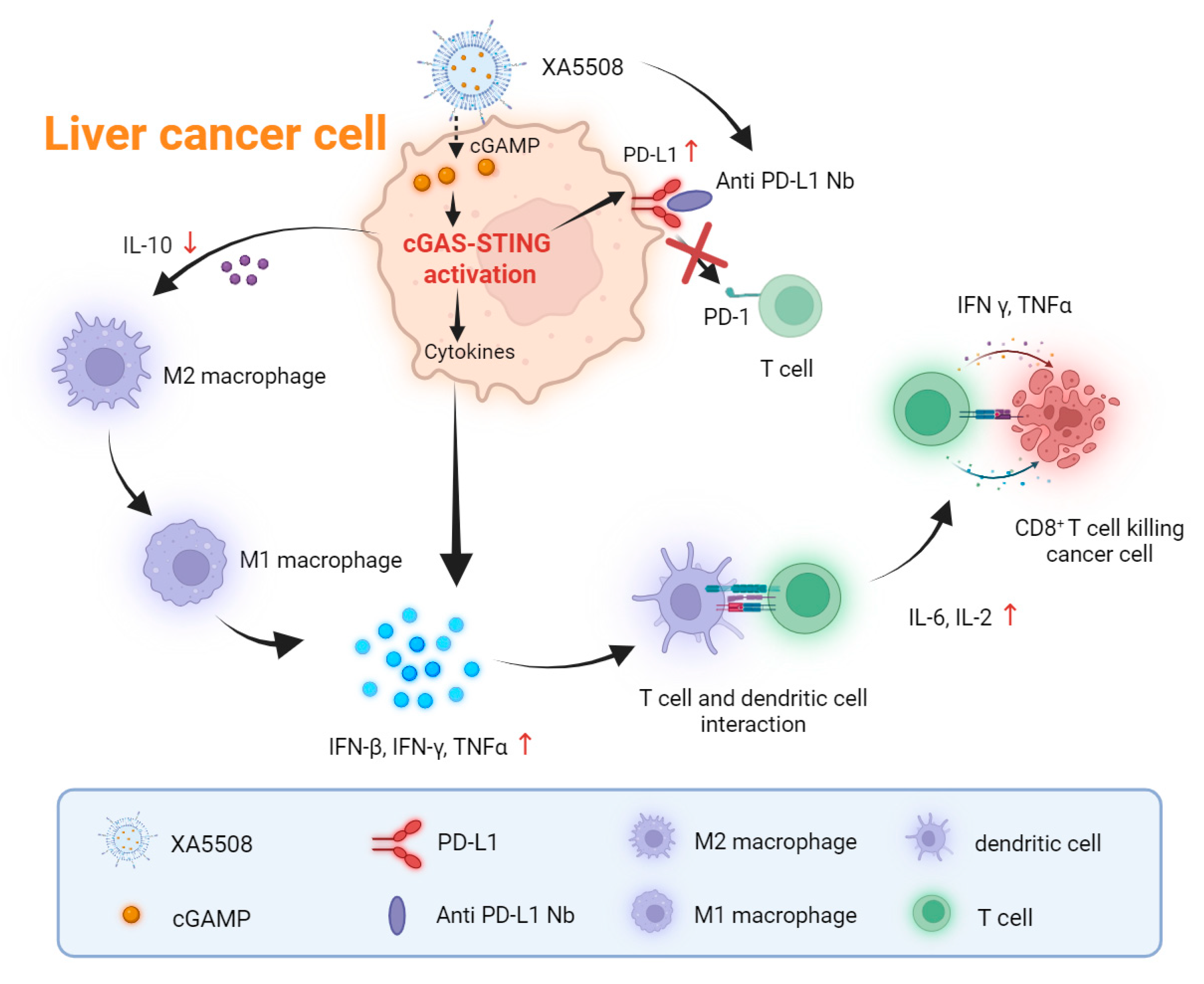
2.5. The Mechanism of Action of Anti-Tumor Immune Response
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Preparation of Nanobody
4.3. Liposomal Formulations Preparation
4.4. Characterization of cGAMP-Loaded Liposomes
4.5. In Vitro Drug Release
4.6. Cell Lines
4.7. Mice and Tumor Model Establishment
4.8. In Vivo Tumor Inhibition Study
4.9. Hematoxylin and Eosin (H&E) Staining and Blood Chemistry Analysis
4.10. Flow Cytometry Analysis of Immune Cells in the Tumor Tissues
4.11. Enzyme-Linked Immunosorbent Assay
4.12. In Vivo Imaging
4.13. Immunofluorescence
4.14. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HCC | Hepatocellular carcinoma |
| ICB | immune checkpoint blockade |
| TME | Tumor microenvironment |
| PD-1 | programmed cell death protein-1 |
| PD-L1 | programmed death ligand 1 |
| Nb | Nanobody |
References
- Biswas, S.; Samanta, A. Immune therapies in intermediate-advanced unresectable hepatocellular carcinoma: Changing the therapeutic landscape. World J. Gastroenterol. 2025, 31, 103267. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Niu, Z.S.; Wang, W.H.; Niu, X.J. Recent progress in molecular mechanisms of postoperative recurrence and metastasis of hepatocellular carcinoma. World J. Gastroenterol. 2022, 28, 6433–6477. [Google Scholar] [CrossRef]
- Forner, A.; Reig, M.; Bruix, J. Hepatocellular carcinoma. Lancet 2018, 391, 1301–1314. [Google Scholar] [CrossRef]
- Cho, J.Y.; Paik, Y.H.; Lim, H.Y.; Kim, Y.G.; Lim, H.K.; Min, Y.W.; Gwak, G.Y.; Choi, M.S.; Lee, J.H.; Koh, K.C.; et al. Clinical parameters predictive of outcomes in sorafenib-treated patients with advanced hepatocellular carcinoma. Liver Int. 2013, 33, 950–957. [Google Scholar] [CrossRef]
- Kudo, M.; Finn, R.S.; Qin, S.; Han, K.H.; Ikeda, K.; Piscaglia, F.; Baron, A.; Park, J.W.; Han, G.; Jassem, J.; et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: A randomised phase 3 non-inferiority trial. Lancet 2018, 391, 1163–1173. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Ricci, S.; Mazzaferro, V.; Hilgard, P.; Gane, E.; Blanc, J.F.; de Oliveira, A.C.; Santoro, A.; Raoul, J.L.; Forner, A.; et al. Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 2008, 359, 378–390. [Google Scholar] [CrossRef]
- Sharma, P.; Allison, J.P. The future of immune checkpoint therapy. Science 2015, 348, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Yarmarkovich, M.; Farrel, A.; Sison, A.R.; di Marco, M.; Raman, P.; Parris, J.L.; Monos, D.; Lee, H.; Stevanovic, S.; Maris, J.M. Immunogenicity and Immune Silence in Human Cancer. Front. Immunol. 2020, 11, 69. [Google Scholar] [CrossRef]
- Yu, J.; Ling, S.; Hong, J.; Zhang, L.; Zhou, W.; Yin, L.; Xu, S.; Que, Q.; Wu, Y.; Zhan, Q.; et al. TP53/mTORC1-mediated bidirectional regulation of PD-L1 modulates immune evasion in hepatocellular carcinoma. J. Immunother. Cancer 2023, 11, e007479. [Google Scholar] [CrossRef]
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O.; et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N. Engl. J. Med. 2020, 382, 1894–1905. [Google Scholar] [CrossRef]
- Yau, T.; Galle, P.R.; Decaens, T.; Sangro, B.; Qin, S.; Da, F.L.; Karachiwala, H.; Blanc, J.F.; Park, J.W.; Gane, E.; et al. Nivolumab plus ipilimumab versus lenvatinib or sorafenib as first-line treatment for unresectable hepatocellular carcinoma (CheckMate 9DW): An open-label, randomised, phase 3 trial. Lancet 2025, 405, 1851–1864. [Google Scholar] [CrossRef] [PubMed]
- El-Khoueiry, A.B.; Sangro, B.; Yau, T.; Crocenzi, T.S.; Kudo, M.; Hsu, C.; Kim, T.Y.; Choo, S.P.; Trojan, J.; Welling, T.R.; et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): An open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 2017, 389, 2492–2502. [Google Scholar] [CrossRef]
- Qin, S.; Ren, Z.; Meng, Z.; Chen, Z.; Chai, X.; Xiong, J.; Bai, Y.; Yang, L.; Zhu, H.; Fang, W.; et al. Camrelizumab in patients with previously treated advanced hepatocellular carcinoma: A multicentre, open-label, parallel-group, randomised, phase 2 trial. Lancet Oncol. 2020, 21, 571–580. [Google Scholar] [CrossRef]
- Hu, B.; Yu, M.; Ma, X.; Sun, J.; Liu, C.; Wang, C.; Wu, S.; Fu, P.; Yang, Z.; He, Y.; et al. IFNalpha Potentiates Anti-PD-1 Efficacy by Remodeling Glucose Metabolism in the Hepatocellular Carcinoma Microenvironment. Cancer Discov. 2022, 12, 1718–1741. [Google Scholar] [CrossRef]
- Zhao, J.; Tong, A.; Liu, J.; Xu, M.; Mi, P. Tumor-targeting nanocarriers amplified immunotherapy of cold tumors by STING activation and inhibiting immune evasion. Sci. Adv. 2025, 11, eadr1728. [Google Scholar] [CrossRef]
- Chen, Q.; Sun, L.; Chen, Z.J. Regulation and function of the cGAS-STING pathway of cytosolic DNA sensing. Nat. Immunol. 2016, 17, 1142–1149. [Google Scholar] [CrossRef] [PubMed]
- Gajewski, T.F.; Higgs, E.F. Immunotherapy with a sting. Science 2020, 369, 921–922. [Google Scholar] [CrossRef]
- Barber, G.N. STING: Infection, inflammation and cancer. Nat. Rev. Immunol. 2015, 15, 760–770. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.; Altman, M.D.; Lesburg, C.A.; Perera, S.A.; Piesvaux, J.A.; Schroeder, G.K.; Wyss, D.F.; Cemerski, S.; Chen, Y.; DiNunzio, E.; et al. Discovery of MK-1454: A Potent Cyclic Dinucleotide Stimulator of Interferon Genes Agonist for the Treatment of Cancer. J. Med. Chem. 2022, 65, 5675–5689. [Google Scholar] [CrossRef]
- Takahashi-Ruiz, L.; Fermaintt, C.S.; Wilkinson, N.J.; Chan, P.; Mooberry, S.L.; Risinger, A.L. The Microtubule Destabilizer Eribulin Synergizes with STING Agonists to Promote Antitumor Efficacy in Triple-Negative Breast Cancer Models. Cancers 2022, 14, 5962. [Google Scholar] [CrossRef]
- Zhang, X.; Bai, X.C.; Chen, Z.J. Structures and Mechanisms in the cGAS-STING Innate Immunity Pathway. Immunity 2020, 53, 43–53. [Google Scholar] [CrossRef]
- Tao, J.; Zhou, X.; Jiang, Z. cGAS-cGAMP-STING: The three musketeers of cytosolic DNA sensing and signaling. IUBMB Life 2016, 68, 858–870. [Google Scholar] [CrossRef]
- Galluzzi, L.; Vanpouille-Box, C.; Bakhoum, S.F.; Demaria, S. SnapShot: CGAS-STING Signaling. Cell 2018, 173, 276. [Google Scholar] [CrossRef]
- Vornholz, L.; Isay, S.E.; Kurgyis, Z.; Strobl, D.C.; Loll, P.; Mosa, M.H.; Luecken, M.D.; Sterr, M.; Lickert, H.; Winter, C.; et al. Synthetic enforcement of STING signaling in cancer cells appropriates the immune microenvironment for checkpoint inhibitor therapy. Sci. Adv. 2023, 9, eadd8564. [Google Scholar] [CrossRef]
- Jiang, X.; Luo, T.; Yang, K.; Lee, M.J.; Liu, J.; Tillman, L.; Zhen, W.; Weichselbaum, R.R.; Lin, W. STING activation disrupts tumor vasculature to overcome the EPR limitation and increase drug deposition. Sci. Adv. 2024, 10, eado82. [Google Scholar] [CrossRef]
- Thomsen, M.K.; Skouboe, M.K.; Boularan, C.; Vernejoul, F.; Lioux, T.; Leknes, S.L.; Berthelsen, M.F.; Riedel, M.; Cai, H.; Joseph, J.V.; et al. The cGAS-STING pathway is a therapeutic target in a preclinical model of hepatocellular carcinoma. Oncogene 2020, 39, 1652–1664. [Google Scholar] [CrossRef]
- Jiang, M.; Chen, P.; Wang, L.; Li, W.; Chen, B.; Liu, Y.; Wang, H.; Zhao, S.; Ye, L.; He, Y.; et al. cGAS-STING, an important pathway in cancer immunotherapy. J. Hematol. Oncol. 2020, 13, 81. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Wang, Y.; Miao, Q.; Chen, Y. The therapeutic potential of PD-1/PD-L1 pathway on immune-related diseases: Based on the innate and adaptive immune components. Biomed. Pharmacother. 2023, 167, 115569. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Yin, Q.; Kuss, P.; Maliga, Z.; Millan, J.L.; Wu, H.; Mitchison, T.J. Hydrolysis of 2’3’-cGAMP by ENPP1 and design of nonhydrolyzable analogs. Nat. Chem. Biol. 2014, 10, 1043–1048. [Google Scholar] [CrossRef] [PubMed]
- Shae, D.; Becker, K.W.; Christov, P.; Yun, D.S.; Lytton-Jean, A.; Sevimli, S.; Ascano, M.; Kelley, M.; Johnson, D.B.; Balko, J.M.; et al. Endosomolytic polymersomes increase the activity of cyclic dinucleotide STING agonists to enhance cancer immunotherapy. Nat. Nanotechnol. 2019, 14, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Dubensky, T.J.; Kanne, D.B.; Leong, M.L. Rationale, progress and development of vaccines utilizing STING-activating cyclic dinucleotide adjuvants. Ther. Adv. Vaccines 2013, 1, 131–143. [Google Scholar] [CrossRef]
- Liu, Y.; Crowe, W.N.; Wang, L.; Lu, Y.; Petty, W.J.; Habib, A.A.; Zhao, D. An inhalable nanoparticulate STING agonist synergizes with radiotherapy to confer long-term control of lung metastases. Nat. Commun. 2019, 10, 5108. [Google Scholar] [CrossRef]
- Zhou, L.; Hou, B.; Wang, D.; Sun, F.; Song, R.; Shao, Q.; Wang, H.; Yu, H.; Li, Y. Engineering Polymeric Prodrug Nanoplatform for Vaccination Immunotherapy of Cancer. Nano Lett. 2020, 20, 4393–4402. [Google Scholar] [CrossRef]
- Borden, E.C. Interferons alpha and beta in cancer: Therapeutic opportunities from new insights. Nat. Rev. Drug Discov. 2019, 18, 219–234. [Google Scholar] [CrossRef]
- Parkes, E.E.; Walker, S.M.; Taggart, L.E.; McCabe, N.; Knight, L.A.; Wilkinson, R.; McCloskey, K.D.; Buckley, N.E.; Savage, K.I.; Salto-Tellez, M.; et al. Activation of STING-Dependent Innate Immune Signaling By S-Phase-Specific DNA Damage in Breast Cancer. J. Natl. Cancer Inst. 2017, 109, djw199. [Google Scholar] [CrossRef]
- Snell, L.M.; McGaha, T.L.; Brooks, D.G. Type I Interferon in Chronic Virus Infection and Cancer. Trends Immunol. 2017, 38, 542–557. [Google Scholar] [CrossRef] [PubMed]
- Moore, E.; Clavijo, P.E.; Davis, R.; Cash, H.; Van Waes, C.; Kim, Y.; Allen, C. Established T Cell-Inflamed Tumors Rejected after Adaptive Resistance Was Reversed by Combination STING Activation and PD-1 Pathway Blockade. Cancer Immunol. Res. 2016, 4, 1061–1071. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Zeng, Z.; Li, Y.; Li, Z.; Jin, L.; Zhang, Z.; Wang, L.; Wang, F.S. Impaired function of CD4+ T follicular helper (Tfh) cells associated with hepatocellular carcinoma progression. PLoS ONE 2015, 10, e117458. [Google Scholar] [CrossRef]
- Chen, T.; Li, Q.; Liu, Z.; Chen, Y.; Feng, F.; Sun, H. Peptide-based and small synthetic molecule inhibitors on PD-1/PD-L1 pathway: A new choice for immunotherapy? Eur. J. Med. Chem. 2019, 161, 378–398. [Google Scholar] [CrossRef]
- Lammers, T.; Kiessling, F.; Hennink, W.E.; Storm, G. Drug targeting to tumors: Principles, pitfalls and (pre-) clinical progress. J. Control Release 2012, 161, 175–187. [Google Scholar] [CrossRef]
- Demaria, O.; Cornen, S.; Daeron, M.; Morel, Y.; Medzhitov, R.; Vivier, E. Harnessing innate immunity in cancer therapy. Nature 2019, 574, 45–56. [Google Scholar] [CrossRef]
- Kim, D.; Matsuoka, H.; Yusa, S.I.; Saruwatari, Y. Collapse Behavior of Polyion Complex (PIC) Micelles upon Salt Addition and Reforming Behavior by Dialysis and Its Temperature Responsivity. Langmuir 2020, 36, 15485–15492. [Google Scholar] [CrossRef] [PubMed]
- Mi, P.; Cabral, H.; Kataoka, K. Ligand-Installed Nanocarriers toward Precision Therapy. Adv. Mater. 2020, 32, e1902604. [Google Scholar] [CrossRef] [PubMed]
- Piotrowski-Daspit, A.S.; Kauffman, A.C.; Bracaglia, L.G.; Saltzman, W.M. Polymeric vehicles for nucleic acid delivery. Adv. Drug Deliv. Rev. 2020, 156, 119–132. [Google Scholar] [CrossRef] [PubMed]
- Chuprin, J.; Buettner, H.; Seedhom, M.O.; Greiner, D.L.; Keck, J.G.; Ishikawa, F.; Shultz, L.D.; Brehm, M.A. Humanized mouse models for immuno-oncology research. Nat. Rev. Clin. Oncol. 2023, 20, 192–206. [Google Scholar] [CrossRef]
- Li, X.; Yao, W.; Yuan, Y.; Chen, P.; Li, B.; Li, J.; Chu, R.; Song, H.; Xie, D.; Jiang, X.; et al. Targeting of tumour-infiltrating macrophages via CCL2/CCR2 signalling as a therapeutic strategy against hepatocellular carcinoma. Gut 2017, 66, 157–167. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Y.; Chen, S.; Zhang, L.; Rao, J.; Lu, X.; Ma, Y. Liver organoids: A promising three-dimensional model for insights and innovations in tumor progression and precision medicine of liver cancer. Front. Immunol. 2023, 14, 1180184. [Google Scholar] [CrossRef]
- Reiberger, T.; Chen, Y.; Ramjiawan, R.R.; Hato, T.; Fan, C.; Samuel, R.; Roberge, S.; Huang, P.; Lauwers, G.Y.; Zhu, A.X.; et al. An orthotopic mouse model of hepatocellular carcinoma with underlying liver cirrhosis. Nat. Protoc. 2015, 10, 1264–1274. [Google Scholar] [CrossRef]
- Dettorre, G.M.; Patel, M.; Gennari, A.; Pentheroudakis, G.; Romano, E.; Cortellini, A.; Pinato, D.J. The systemic pro-inflammatory response: Targeting the dangerous liaison between COVID-19 and cancer. ESMO Open 2021, 6, 100123. [Google Scholar] [CrossRef]
- Aroca-Crevillen, A.; Vicanolo, T.; Ovadia, S.; Hidalgo, A. Neutrophils in Physiology and Pathology. Annu. Rev. Pathol. 2024, 19, 227–259. [Google Scholar] [CrossRef]
- Liu, J.; Qiu, P.; Qin, J.; Wu, X.; Wang, X.; Yang, X.; Li, B.; Zhang, W.; Ye, K.; Peng, Z.; et al. Allogeneic adipose-derived stem cells promote ischemic muscle repair by inducing M2 macrophage polarization via the HIF-1alpha/IL-10 pathway. Stem Cells 2020, 38, 1307–1320. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.W.; Hsu, F.F.; Qiu, J.T.; Chern, G.J.; Lee, Y.A.; Chang, C.C.; Huang, Y.T.; Sung, Y.C.; Chiang, C.C.; Huang, R.L.; et al. Highly efficient and tumor-selective nanoparticles for dual-targeted immunogene therapy against cancer. Sci. Adv. 2020, 6, eaax5032. [Google Scholar] [CrossRef]
- Terbuch, A.; Lopez, J. Next Generation Cancer Vaccines-Make It Personal! Vaccines 2018, 6, 52. [Google Scholar] [CrossRef]
- Li, K.; Ye, Y.; Liu, L.; Sha, Q.; Wang, X.; Jiao, T.; Zhang, L.; Wang, J. The lipid platform increases the activity of STING agonists to synergize checkpoint blockade therapy against melanoma. Biomater. Sci. 2021, 9, 765–773. [Google Scholar] [CrossRef] [PubMed]
- Sokolowska, O.; Rodziewicz-Lurzynska, A.; Pilch, Z.; Kedzierska, H.; Chlebowska-Tuz, J.; Sosnowska, A.; Szumera-Cieckiewicz, A.; Sokol, K.; Barankiewicz, J.; Salomon-Perzynski, A.; et al. Immune checkpoint inhibition improves antimyeloma activity of bortezomib and STING agonist combination in Vk*MYC preclinical model. Clin. Exp. Med. 2023, 23, 1563–1572. [Google Scholar] [CrossRef] [PubMed]
- Du, S.S.; Chen, G.W.; Yang, P.; Chen, Y.X.; Hu, Y.; Zhao, Q.Q.; Zhang, Y.; Liu, R.; Zheng, D.X.; Zhou, J.; et al. Radiation Therapy Promotes Hepatocellular Carcinoma Immune Cloaking via PD-L1 Upregulation Induced by cGAS-STING Activation. Int. J. Radiat. Oncol. Biol. Phys. 2022, 112, 1243–1255. [Google Scholar] [CrossRef]
- Chin, E.N.; Yu, C.; Vartabedian, V.F.; Jia, Y.; Kumar, M.; Gamo, A.M.; Vernier, W.; Ali, S.H.; Kissai, M.; Lazar, D.C.; et al. Antitumor activity of a systemic STING-activating non-nucleotide cGAMP mimetic. Science 2020, 369, 993–999. [Google Scholar] [CrossRef]
- Ma, H.; Kang, Z.; Foo, T.K.; Shen, Z.; Xia, B. Disrupted BRCA1-PALB2 interaction induces tumor immunosuppression and T-lymphocyte infiltration in HCC through cGAS-STING pathway. Hepatology 2023, 77, 33–47. [Google Scholar] [CrossRef]
- Wang, Y.; Luo, J.; Alu, A.; Han, X.; Wei, Y.; Wei, X. cGAS-STING pathway in cancer biotherapy. Mol. Cancer 2020, 19, 136. [Google Scholar] [CrossRef]
- Lee, S.E.; Jang, G.Y.; Lee, J.W.; Park, S.H.; Han, H.D.; Park, Y.M.; Kang, T.H. Improvement of STING-mediated cancer immunotherapy using immune checkpoint inhibitors as a game-changer. Cancer Immunol. Immunother. 2022, 71, 3029–3042. [Google Scholar] [CrossRef]
- Song, H.; Chen, L.; Pan, X.; Shen, Y.; Ye, M.; Wang, G.; Cui, C.; Zhou, Q.; Tseng, Y.; Gong, Z.; et al. Targeting tumor monocyte-intrinsic PD-L1 by rewiring STING signaling and enhancing STING agonist therapy. Cancer Cell 2025, 43, 503–518. [Google Scholar] [CrossRef]
- Wang, H.; Hu, S.; Chen, X.; Shi, H.; Chen, C.; Sun, L.; Chen, Z.J. cGAS is essential for the antitumor effect of immune checkpoint blockade. Proc. Natl. Acad. Sci. USA 2017, 114, 1637–1642. [Google Scholar] [CrossRef]
- Lin, N.Y.; Fukuoka, S.; Koyama, S.; Motooka, D.; Tourlousse, D.M.; Shigeno, Y.; Matsumoto, Y.; Yamano, H.; Murotomi, K.; Tamaki, H.; et al. Microbiota-driven antitumour immunity mediated by dendritic cell migration. Nature 2025, 644, 1058–1068. [Google Scholar] [CrossRef] [PubMed]
- Xin, Q.; Wang, D.; Wang, S.; Zhang, L.; Liang, Q.; Yan, X.; Fan, K.; Jiang, B. Tackling Esophageal Squamous Cell Carcinoma with ITFn-Pt(IV): A Novel Fusion of PD-L1 Blockade, Chemotherapy, and T-cell Activation. Adv. Heal. Healthc. Mater. 2024, 13, e2303623. [Google Scholar] [CrossRef]
- Chen, X.; Xu, Z.; Li, T.; Thakur, A.; Wen, Y.; Zhang, K.; Liu, Y.; Liang, Q.; Liu, W.; Qin, J.J.; et al. Nanomaterial-encapsulated STING agonists for immune modulation in cancer therapy. Biomark. Res. 2024, 12, 2. [Google Scholar] [CrossRef]
- Kong, X.; Zuo, H.; Huang, H.D.; Zhang, Q.; Chen, J.; He, C.; Hu, Y. STING as an emerging therapeutic target for drug discovery: Perspectives from the global patent landscape. J. Adv. Res. 2023, 44, 119–133. [Google Scholar] [CrossRef] [PubMed]
- Corrales, L.; Glickman, L.H.; McWhirter, S.M.; Kanne, D.B.; Sivick, K.E.; Katibah, G.E.; Woo, S.R.; Lemmens, E.; Banda, T.; Leong, J.J.; et al. Direct Activation of STING in the Tumor Microenvironment Leads to Potent and Systemic Tumor Regression and Immunity. Cell Rep. 2015, 11, 1018–1030. [Google Scholar] [CrossRef]
- Bilusic, M.; Gulley, J.L. Editorial: Local Immunotherapy: A Way to Convert Tumors From “Cold” to “Hot”. J. Natl. Cancer Inst. 2017, 109, djx132. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Broos, K.; Keyaerts, M.; Lecocq, Q.; Renmans, D.; Nguyen, T.; Escors, D.; Liston, A.; Raes, G.; Breckpot, K.; Devoogdt, N. Non-invasive assessment of murine PD-L1 levels in syngeneic tumor models by nuclear imaging with nanobody tracers. Oncotarget 2017, 8, 41932–41946. [Google Scholar] [CrossRef]
- Petrilli, R.; Eloy, J.O.; Lee, R.J.; Lopez, R. Preparation of Immunoliposomes by Direct Coupling of Antibodies Based on a Thioether Bond. Methods Mol. Biol. 2018, 1674, 229–237. [Google Scholar] [CrossRef] [PubMed]
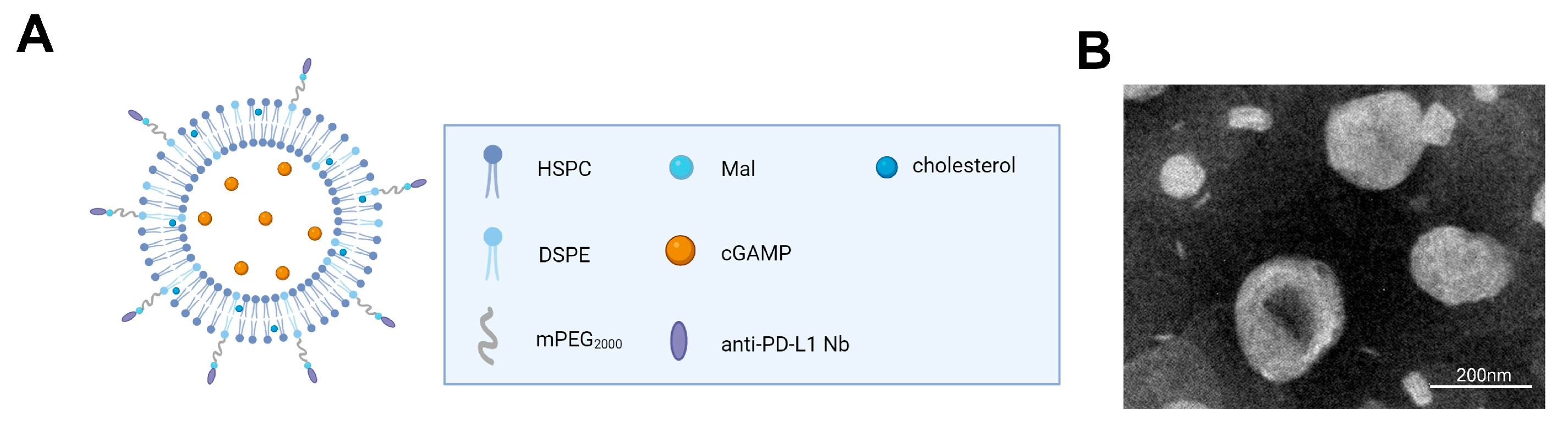
| XA5508 | |
| Particle size (nm) | 202.06 ± 3.81 |
| Zeta potential (mV) | −14.30 ± 1.30 |
| PDI | 0.182 ± 0.08 |
| EE (%) | 83.06 ± 2.68 |
| LE (%) | 8.31 ± 0.17 |
| Protein concentration (mg/mL) | 256.40 ± 33.49 |
| Phospholipid concentration (mg/mL) | 12.57 ± 0.45 |
| Normal Reference Range | Normal | Model | Model + XA5508 | |
|---|---|---|---|---|
| WBC | 0.8–10.6 (109/L) | 6.8 ± 1.2 | 3.3 ± 0.1 | 12.7 ± 1.8 |
| Gran | 0.23–3.6 (109/L) | 2.4 ± 0.6 | 1.7 ± 0.2 | 9.5 ± 1.6 |
| RBC | 6.5–11.5 (1012/L) | 8.4 ± 1.9 | 8.0 ± 1.3 | 7.3 ± 1.3 |
| HCT | 35–55 % | 44.2 ± 1.8 | 33.8 ± 2.7 | 34.5 ± 3.7 |
| PLT | 400–1600 (109/L) | 1030.0 ± 134.0 | 720.5 ± 58.7 | 1107.5 ± 64.3 |
| Normal Reference Range | Normal | Model | Model + XA5508 | |
|---|---|---|---|---|
| ALT | 10.1–96.5 (U/L) | 31.6 ± 3.9 | 29.0 ± 4.6 | 33.7 ± 7.8 |
| TBIL | 6.1–53.1 (μmol/L) | 37.5 ± 2.4 | 35.1 ± 4.2 | 42.7 ± 4.7 |
| CREA | 10.9–85.1 (μmol/L) | 32.7 ± 3.7 | 24.3 ± 4.8 | 27.6 ± 4.5 |
| CKMB | 0–2070.6 (U/L) | 220.5 ± 30.3 | 140.0 ± 20.1 | 150.8 ± 39.6 |
| LDH1 | 0–37.1 (U/L) | 14.8 ± 5.2 | 15.3 ± 1.0 | 17.6 ± 1.5 |
| Key Findings | Highlight Results | Figures to Display the Results |
|---|---|---|
| Tumor-targeted delivery | Achieved effective targeted delivery of cGAMP to tumor tissues. | Figure 2A,B |
| pH-dependent drug release | Effectively released cGAMP under acidic conditions simulating the tumor microenvironment. | Figure 2C |
| In vivo therapeutic efficacy | The tumor suppression rate of in situ HCC reached 86% after 2 weeks of treatment. | Figure 3F |
| STING pathway activation | The expression levels of STING protein, phosphorylated STING protein, IRF3 protein, and pro-inflammatory cytokines increased. | Figure 4 and Figure 5F–H,J,K |
| Enhance biological effects of innate immune activation while blocking immune escape | The infiltration of CD4+ and CD8+T cells in the tumor tissue increased. XA5508 inhibits the upregulation of PD-L1 in tumor tissues caused by cGAMP. | Figure 5C,D,M |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Lu, X.; Liu, C.; Cheng, H.; Tan, X. A Study on the Efficacy and Pharmacological Mechanism of Liposome Complexes Containing STING Agonist and Anti-PD-L1 Nanobody in Inhibiting HCC. Int. J. Mol. Sci. 2025, 26, 8649. https://doi.org/10.3390/ijms26178649
Wang X, Lu X, Liu C, Cheng H, Tan X. A Study on the Efficacy and Pharmacological Mechanism of Liposome Complexes Containing STING Agonist and Anti-PD-L1 Nanobody in Inhibiting HCC. International Journal of Molecular Sciences. 2025; 26(17):8649. https://doi.org/10.3390/ijms26178649
Chicago/Turabian StyleWang, Xiaoqing, Xing Lu, Chang Liu, Hao Cheng, and Xiangshi Tan. 2025. "A Study on the Efficacy and Pharmacological Mechanism of Liposome Complexes Containing STING Agonist and Anti-PD-L1 Nanobody in Inhibiting HCC" International Journal of Molecular Sciences 26, no. 17: 8649. https://doi.org/10.3390/ijms26178649
APA StyleWang, X., Lu, X., Liu, C., Cheng, H., & Tan, X. (2025). A Study on the Efficacy and Pharmacological Mechanism of Liposome Complexes Containing STING Agonist and Anti-PD-L1 Nanobody in Inhibiting HCC. International Journal of Molecular Sciences, 26(17), 8649. https://doi.org/10.3390/ijms26178649






