Synergistic MDM2-STAT3 Inhibition Demonstrates Strong Anti-Leukemic Efficacy in Acute Lymphoblastic Leukemia
Abstract
1. Introduction
2. Results
2.1. The Inhibition of Acute Lymphoblastic Leukemia Cell Proliferation by Napabucasin and Idasanutlin
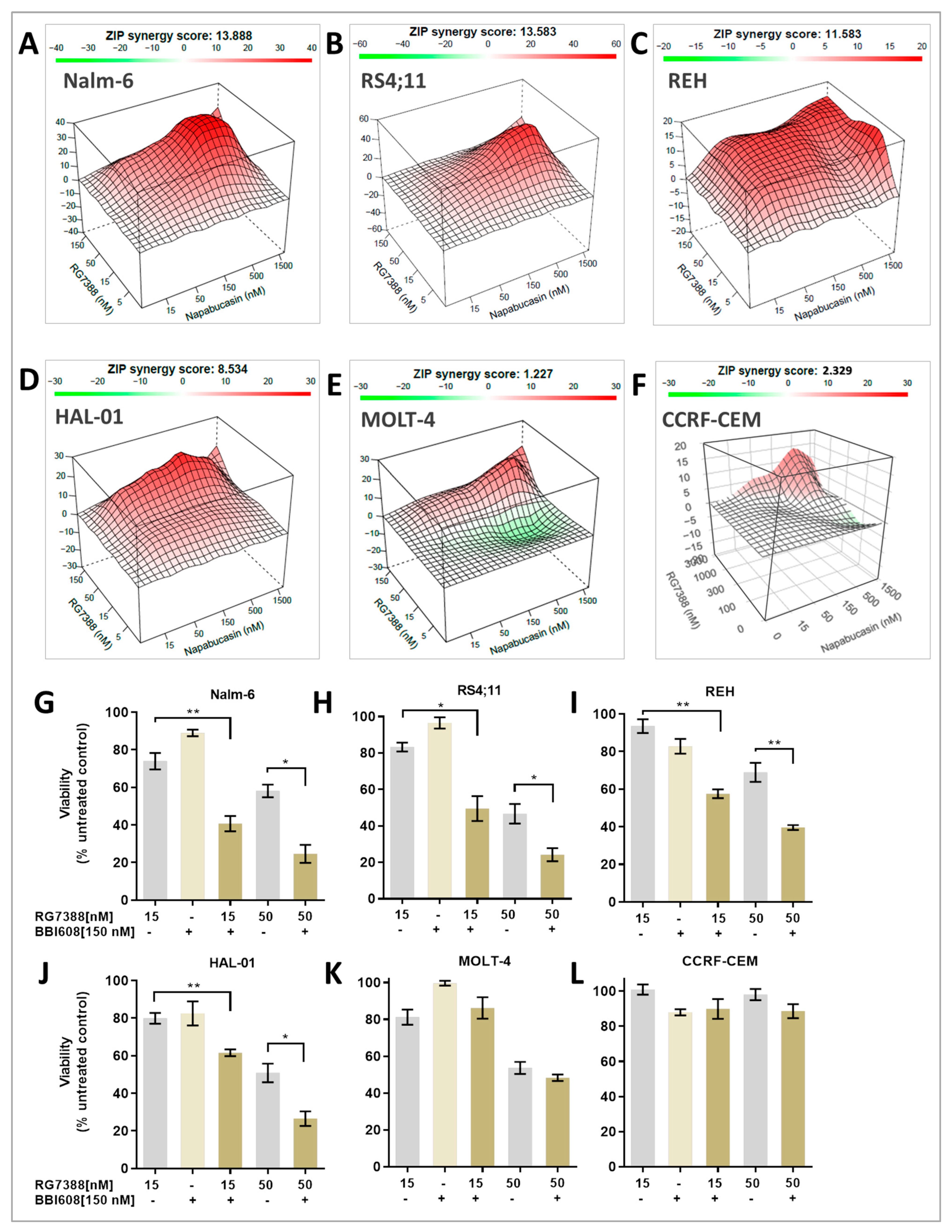
2.2. MDM2 Inhibition Exhibits Synergistic Antiproliferative Effects with STAT3 Inhibitor In Vitro
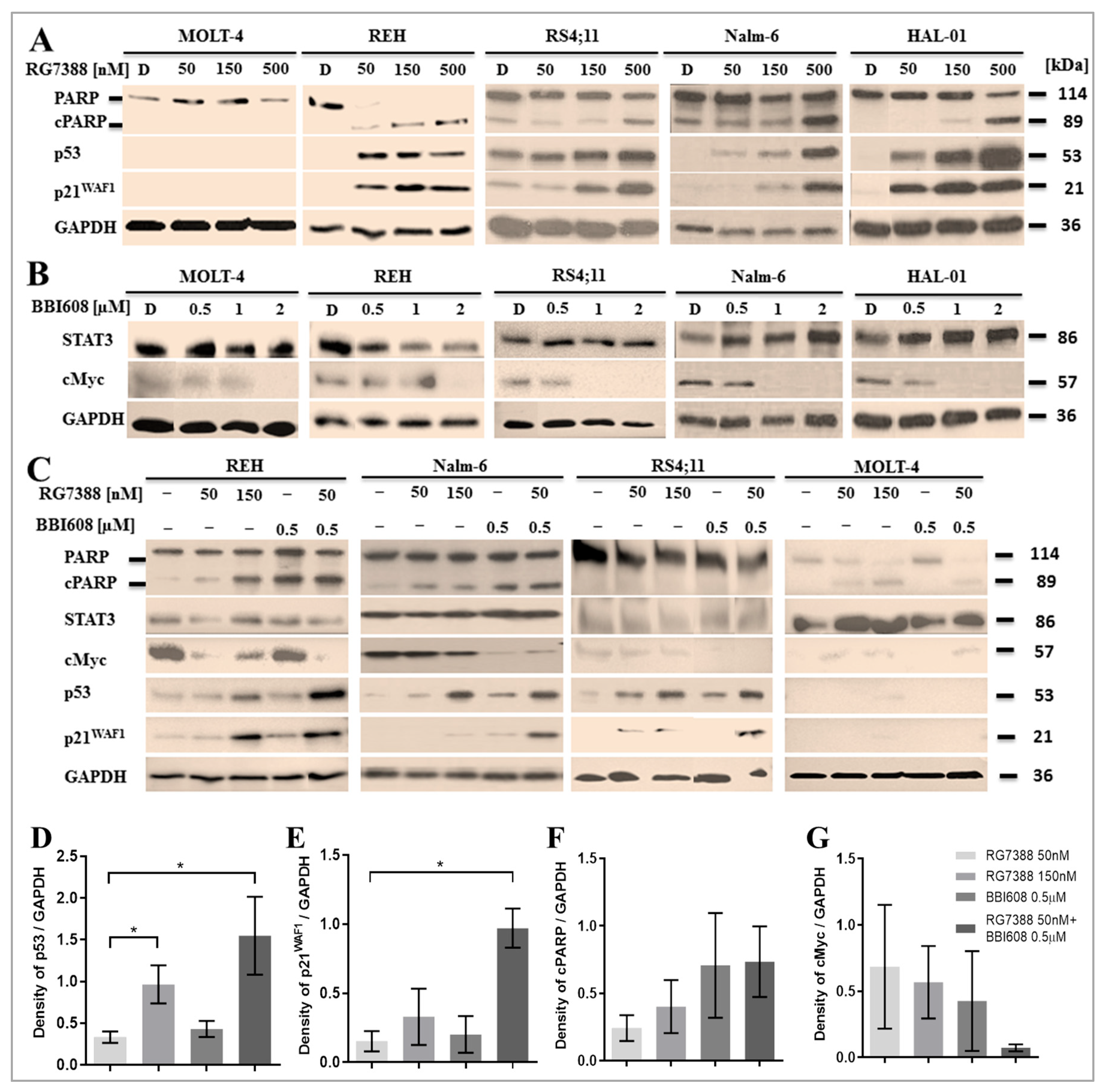
2.3. Suppression of STAT3 Activity Amplifies P53 Downstream Activation Induced by MDM2 Inhibition
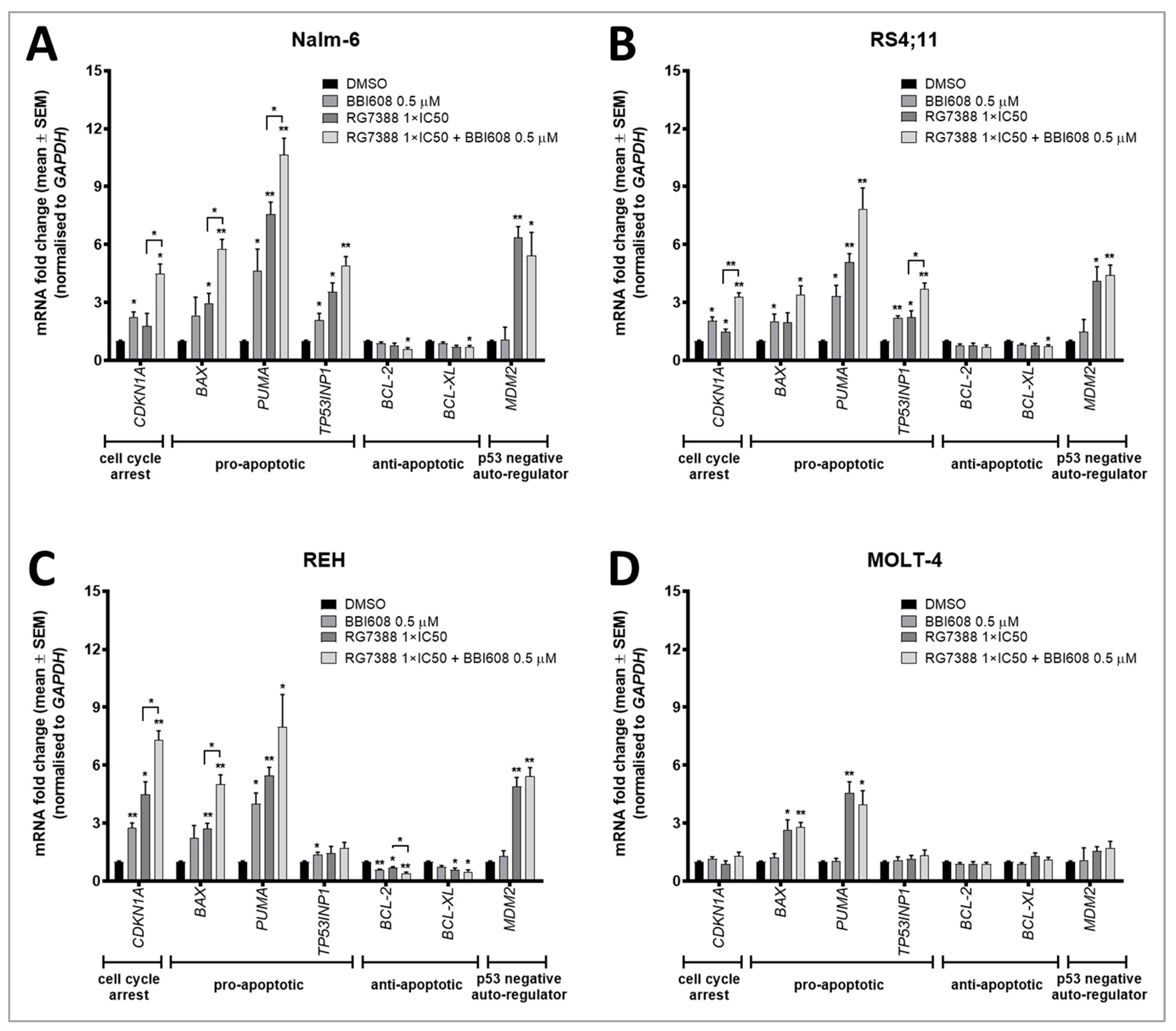
2.4. BBI608 Enhances the mRNA Expression of P53 Transcriptionally Regulated Genes When Combined with the MDM2 Inhibitor RG7388
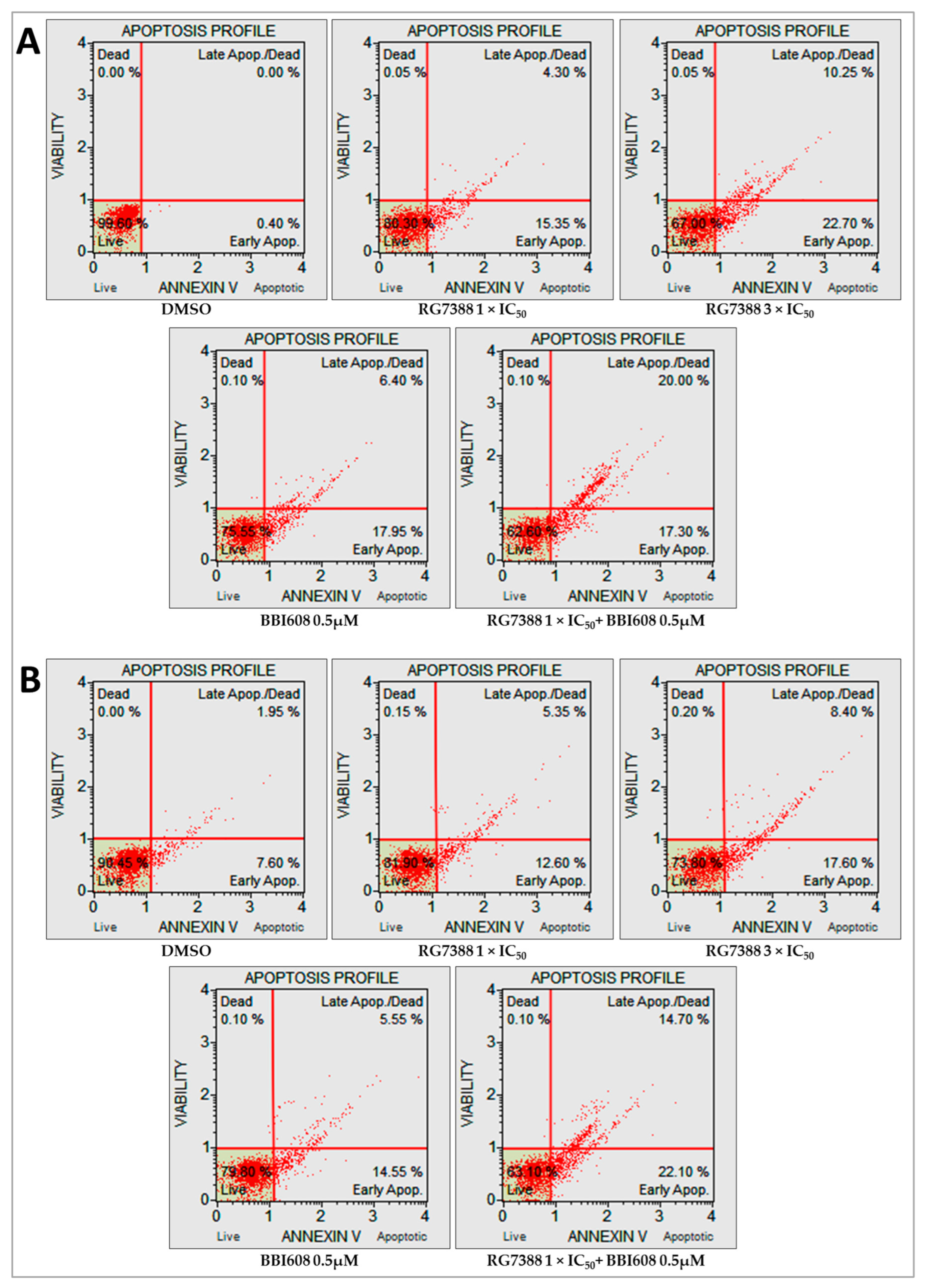
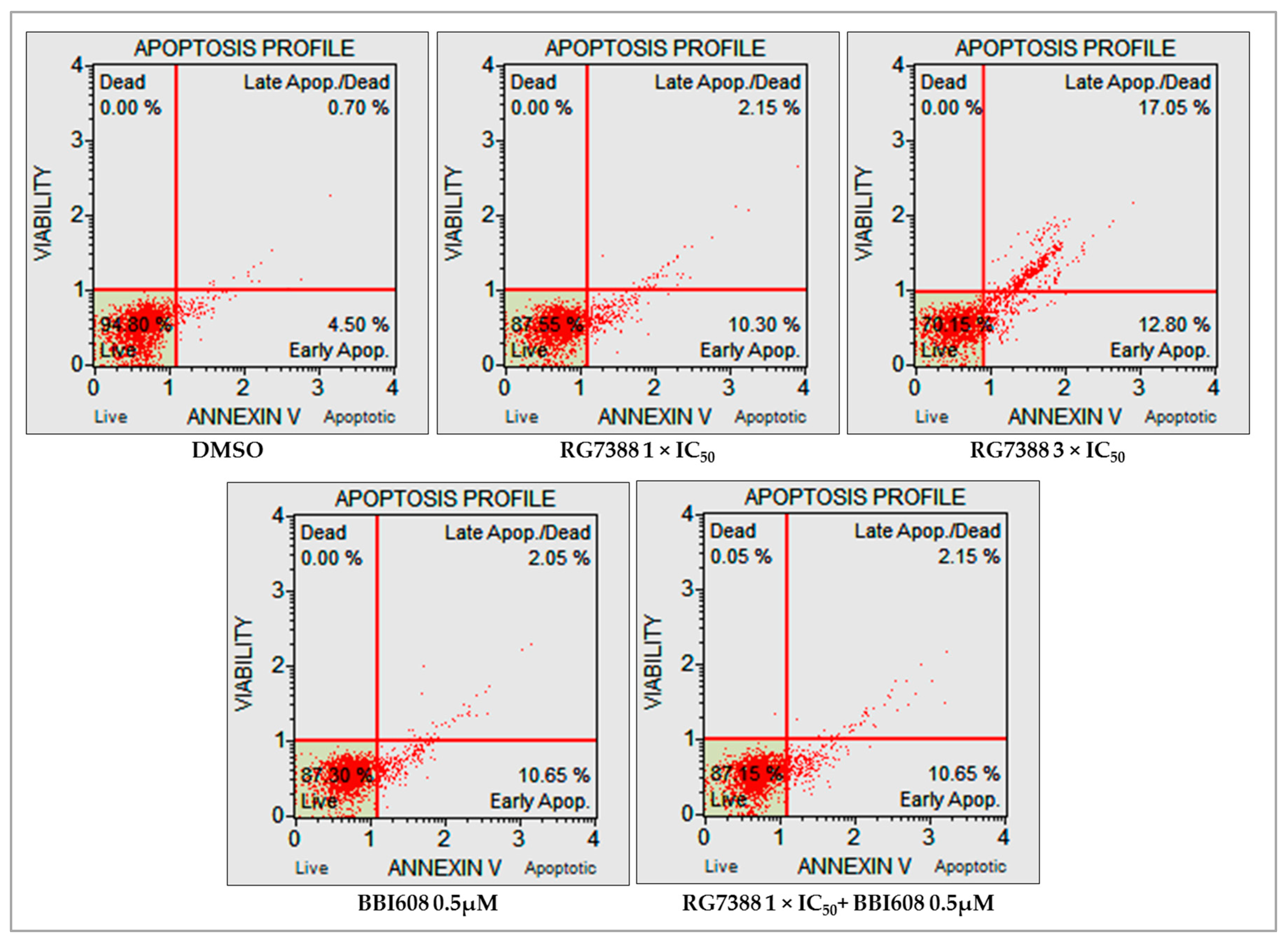
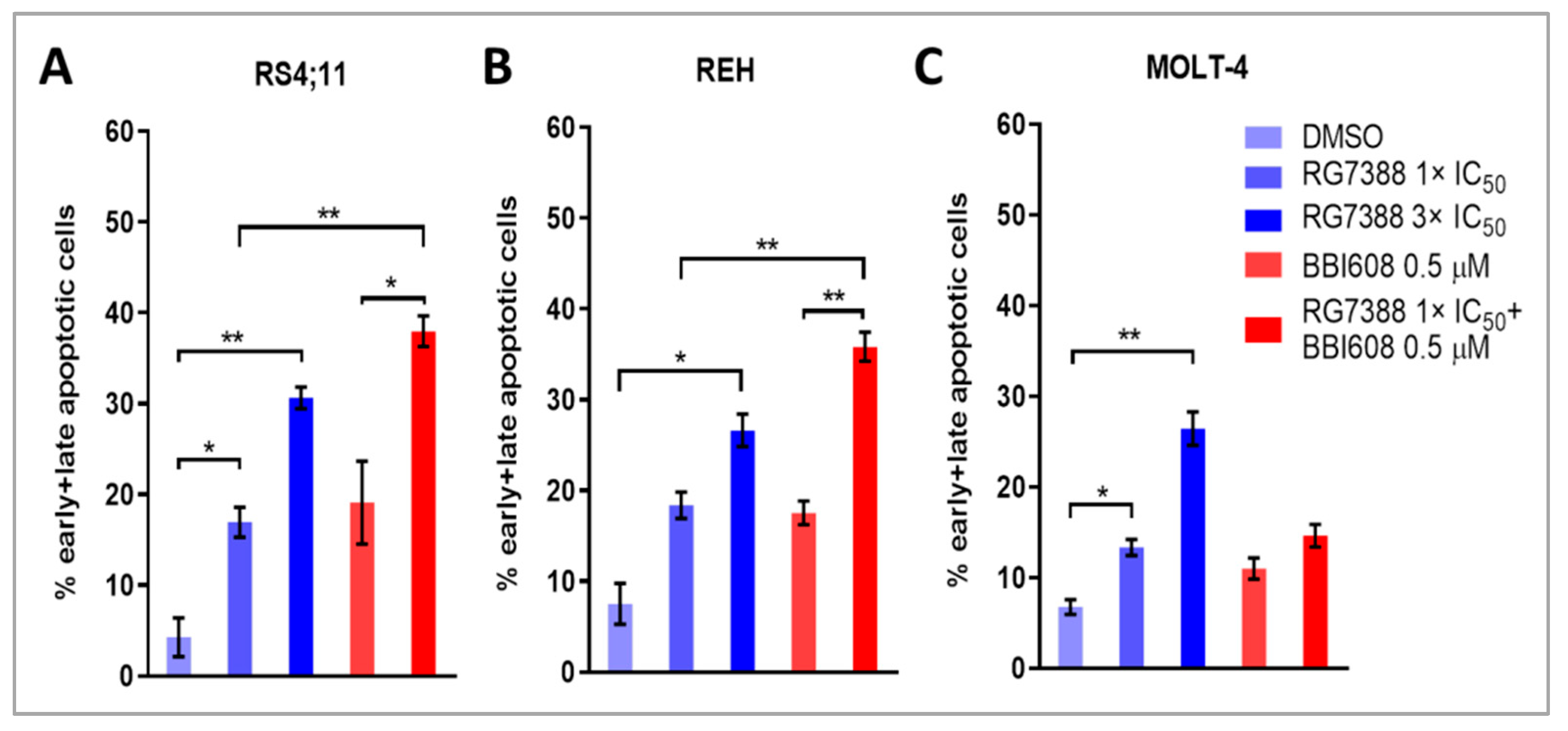
2.5. Combined Treatment with BBI608 and RG7388 Enhanced Apoptotic Responses, Demonstrated by an Increase in Annexin-Positive Cells
3. Discussion
4. Materials and Methods
4.1. Cell Lines and Culture Conditions
4.2. Compounds
4.3. Cell Viability Assay
4.4. Assessment of Combination Treatment
4.5. Immunoblotting
4.6. RNA Extraction and Quantitative Real-Time PCR (qRT-PCR) Analysis
4.7. Apoptosis Analysis by Annexin V/7-AAD Using Fluorescence-Activated Cell Sorting
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Terwilliger, T.; Abdul-Hay, M. Acute lymphoblastic leukemia: A comprehensive review and 2017 update. Blood Cancer J. 2017, 7, e577. [Google Scholar] [CrossRef]
- Saygin, C.; Zhang, P.; Stauber, J.; Aldoss, I.; Sperling, A.S.; Weeks, L.D.; Luskin, M.R.; Knepper, T.C.; Wanjari, P.; Wang, P.; et al. Acute Lymphoblastic Leukemia with Myeloid Mutations Is a High-Risk Disease Associated with Clonal Hematopoiesis. Blood Cancer Discov. 2024, 5, 164–179. [Google Scholar] [CrossRef] [PubMed]
- Wetzler, M.; Dodge, R.K.; Mrozek, K.; Carroll, A.J.; Tantravahi, R.; Block, A.W.; Pettenati, M.J.; Le Beau, M.M.; Frankel, S.R.; Stewart, C.C.; et al. Prospective karyotype analysis in adult acute lymphoblastic leukemia: The cancer and leukemia Group B experience. Blood 1999, 93, 3983–3993. [Google Scholar]
- Specchia, G.; Pastore, D.; Carluccio, P.; Liso, A.; Mestice, A.; Rizzi, R.; Ciuffreda, L.; Pietrantuono, G.; Liso, V. FLAG-IDA in the treatment of refractory/relapsed adult acute lymphoblastic leukemia. Ann. Hematol. 2005, 84, 792–795. [Google Scholar] [CrossRef]
- Farooq, M.U.; Mushtaq, F.; Farooq, A.; Khan, D.H.; Mir, M.A. FLAG vs FLAG-IDA: Outcomes in relapsed/refractory acute leukemias. Cancer Chemother. Pharmacol. 2019, 83, 1191–1193. [Google Scholar] [CrossRef]
- Yuan, J.; Zhang, Y.; Zhou, H.; Wang, S.; Liu, Q.; Gao, Y. Radiotherapy in Philadelphia chromosome-positive acute lymphoblastic leukemia with pericardial invasion after transplantation: A case report. Oncol. Lett. 2023, 26, 415. [Google Scholar] [CrossRef] [PubMed]
- Chitadze, G.; Laqua, A.; Lettau, M.; Baldus, C.D.; Bruggemann, M. Bispecific antibodies in acute lymphoblastic leukemia therapy. Expert Rev. Hematol. 2020, 13, 1211–1233. [Google Scholar] [CrossRef]
- Maude, S.L.; Laetsch, T.W.; Buechner, J.; Rives, S.; Boyer, M.; Bittencourt, H.; Bader, P.; Verneris, M.R.; Stefanski, H.E.; Myers, G.D.; et al. Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia. N. Engl. J. Med. 2018, 378, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Malagola, M.; Papayannidis, C.; Baccarani, M. Tyrosine kinase inhibitors in Ph+ acute lymphoblastic leukaemia: Facts and perspectives. Ann. Hematol. 2016, 95, 681–693. [Google Scholar] [CrossRef]
- Thomas, X.; Boiron, J.M.; Huguet, F.; Dombret, H.; Bradstock, K.; Vey, N.; Kovacsovics, T.; Delannoy, A.; Fegueux, N.; Fenaux, P.; et al. Outcome of treatment in adults with acute lymphoblastic leukemia: Analysis of the LALA-94 trial. J. Clin. Oncol. 2004, 22, 4075–4086. [Google Scholar] [CrossRef]
- Sun, W.; Huang, X. Role of allogeneic haematopoietic stem cell transplantation in the treatment of adult acute lymphoblastic leukaemia in the era of immunotherapy. Chin. Med. J. 2022, 135, 890–900. [Google Scholar] [CrossRef]
- Pulte, D.; Jansen, L.; Gondos, A.; Katalinic, A.; Barnes, B.; Ressing, M.; Holleczek, B.; Eberle, A.; Brenner, H.; Group, G.C.S.W. Survival of adults with acute lymphoblastic leukemia in Germany and the United States. PLoS ONE 2014, 9, e85554. [Google Scholar] [CrossRef]
- Schwartz, M.S.; Muffly, L.S. Predicting relapse in acute lymphoblastic leukemia. Leuk. Lymphoma 2024, 65, 1934–1940. [Google Scholar] [CrossRef]
- Braun, T.P.; Eide, C.A.; Druker, B.J. Response and Resistance to BCR-ABL1-Targeted Therapies. Cancer Cell 2020, 37, 530–542. [Google Scholar] [CrossRef] [PubMed]
- Hathaway, L.; Sen, J.M.; Keng, M. Impact of blinatumomab on patient outcomes in relapsed/refractory acute lymphoblastic leukemia: Evidence to date. Patient Relat. Outcome Meas. 2018, 9, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Xi, R.; Mao, D.; Zhao, X.; Wu, T. Efficacy and Safety of Blinatumomab for the Treatment of Relapsed/Refractory Acute Lymphoblastic Leukemia: A Systemic Review and Meta-Analysis. Clin. Lymphoma Myeloma Leuk. 2023, 23, e139–e149. [Google Scholar] [CrossRef]
- Al-Salama, Z.T. Inotuzumab Ozogamicin: A Review in Relapsed/Refractory B-Cell Acute Lymphoblastic Leukaemia. Target. Oncol. 2018, 13, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Uy, N.; Nadeau, M.; Stahl, M.; Zeidan, A.M. Inotuzumab ozogamicin in the treatment of relapsed/refractory acute B cell lymphoblastic leukemia. J. Blood Med. 2018, 9, 67–74. [Google Scholar] [CrossRef]
- Ojemolon, P.E.; Kalidindi, S.; Ahlborn, T.A.; Aihie, O.P.; Awoyomi, M.I. Cytokine Release Syndrome Following Blinatumomab Therapy. Cureus 2022, 14, e21583. [Google Scholar] [CrossRef]
- Jain, T.; Litzow, M.R. Management of toxicities associated with novel immunotherapy agents in acute lymphoblastic leukemia. Ther. Adv. Hematol. 2020, 11, 2040620719899897. [Google Scholar] [CrossRef]
- Kebriaei, P.; Cutler, C.; de Lima, M.; Giralt, S.; Lee, S.J.; Marks, D.; Merchant, A.; Stock, W.; van Besien, K.; Stelljes, M. Management of important adverse events associated with inotuzumab ozogamicin: Expert panel review. Bone Marrow Transplant. 2018, 53, 449–456. [Google Scholar] [CrossRef]
- Mejstrikova, E.; Hrusak, O.; Borowitz, M.J.; Whitlock, J.A.; Brethon, B.; Trippett, T.M.; Zugmaier, G.; Gore, L.; von Stackelberg, A.; Locatelli, F. CD19-negative relapse of pediatric B-cell precursor acute lymphoblastic leukemia following blinatumomab treatment. Blood Cancer J. 2017, 7, 659. [Google Scholar] [CrossRef]
- Zhao, Y.; Short, N.J.; Kantarjian, H.M.; Chang, T.C.; Ghate, P.S.; Qu, C.; Macaron, W.; Jain, N.; Thakral, B.; Phillips, A.H.; et al. Genomic determinants of response and resistance to inotuzumab ozogamicin in B-cell ALL. Blood 2024, 144, 61–73. [Google Scholar] [CrossRef]
- Kansagra, A.J.; Frey, N.V.; Bar, M.; Laetsch, T.W.; Carpenter, P.A.; Savani, B.N.; Heslop, H.E.; Bollard, C.M.; Komanduri, K.V.; Gastineau, D.A.; et al. Clinical utilization of Chimeric Antigen Receptor T-cells (CAR-T) in B-cell acute lymphoblastic leukemia (ALL)-an expert opinion from the European Society for Blood and Marrow Transplantation (EBMT) and the American Society for Blood and Marrow Transplantation (ASBMT). Bone Marrow Transplant. 2019, 54, 1868–1880. [Google Scholar] [CrossRef]
- Nie, Y.; Lu, W.; Chen, D.; Tu, H.; Guo, Z.; Zhou, X.; Li, M.; Tu, S.; Li, Y. Mechanisms underlying CD19-positive ALL relapse after anti-CD19 CAR T cell therapy and associated strategies. Biomark. Res. 2020, 8, 18. [Google Scholar] [CrossRef]
- Egler, R.A.; Ahuja, S.P.; Matloub, Y. L-asparaginase in the treatment of patients with acute lymphoblastic leukemia. J. Pharmacol. Pharmacother. 2016, 7, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Pieters, R.; Hunger, S.P.; Boos, J.; Rizzari, C.; Silverman, L.; Baruchel, A.; Goekbuget, N.; Schrappe, M.; Pui, C.H. L-asparaginase treatment in acute lymphoblastic leukemia: A focus on Erwinia asparaginase. Cancer 2011, 117, 238–249. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, T.; Nakagawara, A. Role of p53 in Cell Death and Human Cancers. Cancers 2011, 3, 994–1013. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, T.; Su, W.; Dou, Z.; Zhao, D.; Jin, X.; Lei, H.; Wang, J.; Xie, X.; Cheng, B.; et al. Mutant p53 in cancer: From molecular mechanism to therapeutic modulation. Cell Death Dis. 2022, 13, 974. [Google Scholar] [CrossRef]
- Hollstein, M.; Sidransky, D.; Vogelstein, B.; Harris, C.C. p53 mutations in human cancers. Science 1991, 253, 49–53. [Google Scholar] [CrossRef]
- Chiaretti, S.; Brugnoletti, F.; Tavolaro, S.; Bonina, S.; Paoloni, F.; Marinelli, M.; Patten, N.; Bonifacio, M.; Kropp, M.G.; Sica, S.; et al. TP53 mutations are frequent in adult acute lymphoblastic leukemia cases negative for recurrent fusion genes and correlate with poor response to induction therapy. Haematologica 2013, 98, e59–e61. [Google Scholar] [CrossRef]
- Stengel, A.; Schnittger, S.; Weissmann, S.; Kuznia, S.; Kern, W.; Kohlmann, A.; Haferlach, T.; Haferlach, C. TP53 mutations occur in 15.7% of ALL and are associated with MYC-rearrangement, low hypodiploidy, and a poor prognosis. Blood 2014, 124, 251–258. [Google Scholar] [CrossRef]
- Yu, C.H.; Chang, W.T.; Jou, S.T.; Lin, T.K.; Chang, Y.H.; Lin, C.Y.; Lin, K.H.; Lu, M.Y.; Chen, S.H.; Wu, K.H.; et al. TP53 alterations in relapsed childhood acute lymphoblastic leukemia. Cancer Sci. 2020, 111, 229–238. [Google Scholar] [CrossRef]
- Irving, J.A.; Enshaei, A.; Parker, C.A.; Sutton, R.; Kuiper, R.P.; Erhorn, A.; Minto, L.; Venn, N.C.; Law, T.; Yu, J.; et al. Integration of genetic and clinical risk factors improves prognostication in relapsed childhood B-cell precursor acute lymphoblastic leukemia. Blood 2016, 128, 911–922. [Google Scholar] [CrossRef]
- Zhang, H.H.; Wang, H.S.; Qian, X.W.; Fan, C.Q.; Li, J.; Miao, H.; Zhu, X.H.; Yu, Y.; Meng, J.H.; Cao, P.; et al. Genetic variants and clinical significance of pediatric acute lymphoblastic leukemia. Ann. Transl. Med. 2019, 7, 296. [Google Scholar] [CrossRef] [PubMed]
- Oliner, J.D.; Kinzler, K.W.; Meltzer, P.S.; George, D.L.; Vogelstein, B. Amplification of a gene encoding a p53-associated protein in human sarcomas. Nature 1992, 358, 80–83. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.E.; Chen, C.P.; Huang, W.K.; Pan, Y.R.; Aptullahoglu, E.; Yeh, C.N.; Lunec, J. p53 as a biomarker and potential target in gastrointestinal stromal tumors. Front. Oncol. 2022, 12, 872202. [Google Scholar] [CrossRef] [PubMed]
- Hassin, O.; Oren, M. Drugging p53 in cancer: One protein, many targets. Nat. Rev. Drug Discov. 2023, 22, 127–144. [Google Scholar] [CrossRef]
- Moll, U.M.; Petrenko, O. The MDM2-p53 interaction. Mol. Cancer Res. 2003, 1, 1001–1008. [Google Scholar]
- Oliner, J.D.; Saiki, A.Y.; Caenepeel, S. The Role of MDM2 Amplification and Overexpression in Tumorigenesis. Cold Spring Harb. Perspect. Med. 2016, 6, a026336. [Google Scholar] [CrossRef]
- Gustafsson, B.; Stal, O.; Gustafsson, B. Overexpression of MDM2 in acute childhood lymphoblastic leukemia. Pediatr. Hematol. Oncol. 1998, 15, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Vassilev, L.T. Small-molecule antagonists of p53-MDM2 binding: Research tools and potential therapeutics. Cell Cycle 2004, 3, 419–421. [Google Scholar] [CrossRef]
- Ding, Q.; Zhang, Z.; Liu, J.J.; Jiang, N.; Zhang, J.; Ross, T.M.; Chu, X.J.; Bartkovitz, D.; Podlaski, F.; Janson, C.; et al. Discovery of RG7388, a potent and selective p53-MDM2 inhibitor in clinical development. J. Med. Chem. 2013, 56, 5979–5983. [Google Scholar] [CrossRef] [PubMed]
- Furet, P.; Masuya, K.; Kallen, J.; Stachyra-Valat, T.; Ruetz, S.; Guagnano, V.; Holzer, P.; Mah, R.; Stutz, S.; Vaupel, A.; et al. Discovery of a novel class of highly potent inhibitors of the p53-MDM2 interaction by structure-based design starting from a conformational argument. Bioorganic Med. Chem. Lett. 2016, 26, 4837–4841. [Google Scholar] [CrossRef] [PubMed]
- Rew, Y.; Sun, D. Discovery of a small molecule MDM2 inhibitor (AMG 232) for treating cancer. J. Med. Chem. 2014, 57, 6332–6341. [Google Scholar] [CrossRef]
- Willmore, E.; Ahn, M.; Kyle, S.; Zhao, Y.; Thomas, H.; Rankin, K.S.; Bevan, L.; Fazal, L.; Hearn, K.; Wilsher, N.; et al. Targeting the MDM2-p53 interaction: Time- and concentration-dependent studies in tumor and normal human bone marrow cells reveal strategies for an enhanced therapeutic index. Cancer Res. 2024, 84, 3333. [Google Scholar] [CrossRef]
- Wang, S.M.; Sun, W.; Zhao, Y.J.; McEachern, D.; Meaux, I.; Barrière, C.; Stuckey, J.A.; Meagher, J.L.; Bai, L.C.; Liu, L.; et al. SAR405838: An Optimized Inhibitor of MDM2-p53 Interaction That Induces Complete and Durable Tumor Regression. Cancer Res. 2014, 74, 5855–5865. [Google Scholar] [CrossRef]
- Arnhold, V.; Schmelz, K.; Proba, J.; Winkler, A.; Wunschel, J.; Toedling, J.; Deubzer, H.E.; Kunkele, A.; Eggert, A.; Schulte, J.H.; et al. Reactivating TP53 signaling by the novel MDM2 inhibitor DS-3032b as a therapeutic option for high-risk neuroblastoma. Oncotarget 2018, 9, 2304–2319. [Google Scholar] [CrossRef]
- Ciardullo, C.; Aptullahoglu, E.; Woodhouse, L.; Lin, W.Y.; Wallis, J.P.; Marr, H.; Marshall, S.; Bown, N.; Willmore, E.; Lunec, J. Non-genotoxic MDM2 inhibition selectively induces a pro-apoptotic p53 gene signature in chronic lymphocytic leukemia cells. Haematologica 2019, 104, 2429–2442. [Google Scholar] [CrossRef]
- Aptullahoglu, E.; Ciardullo, C.; Wallis, J.P.; Marr, H.; Marshall, S.; Bown, N.; Willmore, E.; Lunec, J. Splicing Modulation Results in Aberrant Isoforms and Protein Products of p53 Pathway Genes and the Sensitization of B Cells to Non-Genotoxic MDM2 Inhibition. Int. J. Mol. Sci. 2023, 24, 2410. [Google Scholar] [CrossRef]
- Gungordu, S.; Aptullahoglu, E. Targeting MDM2-mediated suppression of p53 with idasanutlin: A promising therapeutic approach for acute lymphoblastic leukemia. Investig. New Drugs 2024, 42, 603–611. [Google Scholar] [CrossRef]
- Johansson, K.B.; Zimmerman, M.S.; Dmytrenko, I.V.; Gao, F.; Link, D.C. Idasanutlin and navitoclax induce synergistic apoptotic cell death in T-cell acute lymphoblastic leukemia. Leukemia 2023, 37, 2356–2366. [Google Scholar] [CrossRef] [PubMed]
- Ghotaslou, A.; Samii, A.; Boustani, H.; Kiani Ghalesardi, O.; Shahidi, M. AMG-232, a New Inhibitor of MDM-2, Enhance Doxorubicin Efficiency in Pre-B Acute Lymphoblastic Leukemia Cells. Rep. Biochem. Mol. Biol. 2022, 11, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Aptullahoglu, E.; Howladar, M.; Wallis, J.P.; Marr, H.; Marshall, S.; Irving, J.; Willmore, E.; Lunec, J. Targeting the MDM2-p53 Interaction with Siremadlin: A Promising Therapeutic Strategy for Treating TP53 Wild-Type Chronic Lymphocytic Leukemia. Cancers 2025, 17, 274. [Google Scholar] [CrossRef]
- Bell, H.L.; Blair, H.J.; Jepson Gosling, S.J.; Galler, M.; Astley, D.; Moorman, A.V.; Heidenreich, O.; Veal, G.J.; van Delft, F.W.; Lunec, J.; et al. Combination p53 activation and BCL-x(L)/BCL-2 inhibition as a therapeutic strategy in high-risk and relapsed acute lymphoblastic leukemia. Leukemia 2024, 38, 1223–1235. [Google Scholar] [CrossRef]
- Wu, C.E.; Esfandiari, A.; Ho, Y.H.; Wang, N.; Mahdi, A.K.; Aptullahoglu, E.; Lovat, P.; Lunec, J. Targeting negative regulation of p53 by MDM2 and WIP1 as a therapeutic strategy in cutaneous melanoma. Brit J. Cancer 2018, 118, 495–508. [Google Scholar] [CrossRef]
- Ciardullo, C.; Woodhouse, L.; Aptullahoglu, E.; Wallis, J.P.; Marr, H.J.; Marshall, S.R.; Bown, N.; Willmore, E.; Lunec, J. The p53-MDM2 Antagonist RG7388 Activates p53 and Induces a Predominantly Pro-Apoptotic Gene Expression Signature in Chronic Lymphocytic Leukemia. Blood 2016, 128, 893. [Google Scholar] [CrossRef]
- Stein, E.M.; DeAngelo, D.J.; Chromik, J.; Chatterjee, M.; Bauer, S.; Lin, C.C.; Suarez, C.; de Vos, F.; Steeghs, N.; Cassier, P.A.; et al. Results from a First-in-Human Phase I Study of Siremadlin (HDM201) in Patients with Advanced Wild-Type TP53 Solid Tumors and Acute Leukemia. Clin. Cancer Res. 2022, 28, 870–881. [Google Scholar] [CrossRef] [PubMed]
- Koyama, T.; Shimizu, T.; Kojima, Y.; Sudo, K.; Okuma, H.S.; Shimoi, T.; Ichikawa, H.; Kohsaka, S.; Sadachi, R.; Hirakawa, A.; et al. Clinical Activity and Exploratory Resistance Mechanism of Milademetan, an MDM2 Inhibitor, in Intimal Sarcoma with MDM2 Amplification: An Open-Label Phase Ib/II Study. Cancer Discov. 2023, 13, 1814–1825. [Google Scholar] [CrossRef]
- Daver, N.G.; Dail, M.; Garcia, J.S.; Jonas, B.A.; Yee, K.W.L.; Kelly, K.R.; Vey, N.; Assouline, S.; Roboz, G.J.; Paolini, S.; et al. Venetoclax and idasanutlin in relapsed/refractory AML: A non-randomized, open-label phase 1b trial. Blood 2022, 141, 1265–1276. [Google Scholar] [CrossRef]
- Konopleva, M.Y.; Rollig, C.; Cavenagh, J.; Deeren, D.; Girshova, L.; Krauter, J.; Martinelli, G.; Montesinos, P.; Schafer, J.A.; Ottmann, O.; et al. Idasanutlin plus cytarabine in relapsed or refractory acute myeloid leukemia: Results of the MIRROS trial. Blood Adv. 2022, 6, 4147–4156. [Google Scholar] [CrossRef]
- Abdul Razak, A.R.; Bauer, S.; Suarez, C.; Lin, C.C.; Quek, R.; Hutter-Kronke, M.L.; Cubedo, R.; Ferretti, S.; Guerreiro, N.; Jullion, A.; et al. Co-Targeting of MDM2 and CDK4/6 with Siremadlin and Ribociclib for the Treatment of Patients with Well-Differentiated or Dedifferentiated Liposarcoma: Results from a Proof-of-Concept, Phase Ib Study. Clin. Cancer Res. 2022, 28, 1087–1097. [Google Scholar] [CrossRef] [PubMed]
- Haronikova, L.; Bonczek, O.; Zatloukalova, P.; Kokas-Zavadil, F.; Kucerikova, M.; Coates, P.J.; Fahraeus, R.; Vojtesek, B. Resistance mechanisms to inhibitors of p53-MDM2 interactions in cancer therapy: Can we overcome them? Cell Mol. Biol. Lett. 2021, 26, 53. [Google Scholar] [CrossRef] [PubMed]
- Aptullahoglu, E.; Wallis, J.P.; Marr, H.; Marshall, S.; Bown, N.; Willmore, E.; Lunec, J. SF3B1 Mutations Are Associated with Resistance to Non-Genotoxic MDM2 Inhibition in Chronic Lymphocytic Leukemia. Int. J. Mol. Sci. 2023, 24, 11335. [Google Scholar] [CrossRef] [PubMed]
- Savoy, L.; Bottomly, D.; Chen, R.; Allen, B.; Tyner, J.W.; McWeeney, S.K.; Zhang, H.J. Characterize Biomarkers and Mechanisms of Resistance for MDM2 Inhibitors in AML. Blood 2022, 140, 8778–8779. [Google Scholar] [CrossRef]
- Aptullahoglu, E.; Nakjang, S.; Wallis, J.P.; Marr, H.; Marshall, S.; Willmore, E.; Lunec, J. RNA Sequencing Reveals Candidate Genes and Pathways Associated with Resistance to MDM2 Antagonist Idasanutlin in TP53 Wild-Type Chronic Lymphocytic Leukemia. Biomedicines 2024, 12, 1388. [Google Scholar] [CrossRef]
- Ihle, J.N. The Stat family in cytokine signaling. Curr. Opin. Cell Biol. 2001, 13, 211–217. [Google Scholar] [CrossRef]
- Hirano, T.; Ishihara, K.; Hibi, M. Roles of STAT3 in mediating the cell growth, differentiation and survival signals relayed through the IL-6 family of cytokine receptors. Oncogene 2000, 19, 2548–2556. [Google Scholar] [CrossRef]
- Gouilleux-Gruart, V.; Gouilleux, F.; Desaint, C.; Claisse, J.F.; Capiod, J.C.; Delobel, J.; Weber-Nordt, R.; Dusanter-Fourt, I.; Dreyfus, F.; Groner, B.; et al. STAT-related transcription factors are constitutively activated in peripheral blood cells from acute leukemia patients. Blood 1996, 87, 1692–1697. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.E.; O’Keefe, R.A.; Grandis, J.R. Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat. Rev. Clin. Oncol. 2018, 15, 234–248. [Google Scholar] [CrossRef]
- Shi, Y.; Zhang, Z.; Qu, X.; Zhu, X.; Zhao, L.; Wei, R.; Guo, Q.; Sun, L.; Yin, X.; Zhang, Y.; et al. Roles of STAT3 in leukemia (Review). Int. J. Oncol. 2018, 53, 7–20. [Google Scholar] [CrossRef] [PubMed]
- Zou, S.; Tong, Q.; Liu, B.; Huang, W.; Tian, Y.; Fu, X. Targeting STAT3 in Cancer Immunotherapy. Mol. Cancer 2020, 19, 145. [Google Scholar] [CrossRef]
- Aptullahoglu, E. Brigimadlin (BI-907828) and napabucasin (BBI608) cooperatively trigger apoptosis in chronic lymphocytic leukemia cells by simultaneous iInhibition of MDM2 and STAT3. Mol. Biol. Rep. 2025, 52, 731. [Google Scholar] [CrossRef]
- Niu, G.; Wright, K.L.; Ma, Y.; Wright, G.M.; Huang, M.; Irby, R.; Briggs, J.; Karras, J.; Cress, W.D.; Pardoll, D.; et al. Role of Stat3 in regulating p53 expression and function. Mol. Cell Biol. 2005, 25, 7432–7440. [Google Scholar] [CrossRef]
- Pathania, A.S.; Kumar, S.; Guru, S.K.; Bhushan, S.; Sharma, P.R.; Aithagani, S.K.; Singh, P.P.; Vishwakarma, R.A.; Kumar, A.; Malik, F. The synthetic tryptanthrin analogue suppresses STAT3 signaling and induces caspase dependent apoptosis via ERK up regulation in human leukemia HL-60 cells. PLoS ONE 2014, 9, e110411. [Google Scholar] [CrossRef]
- Redell, M.S.; Ruiz, M.J.; Alonzo, T.A.; Gerbing, R.B.; Tweardy, D.J. Stat3 signaling in acute myeloid leukemia: Ligand-dependent and -independent activation and induction of apoptosis by a novel small-molecule Stat3 inhibitor. Blood 2011, 117, 5701–5709. [Google Scholar] [CrossRef]
- Huang, C.; Cao, J.; Huang, K.J.; Zhang, F.; Jiang, T.; Zhu, L.; Qiu, Z.J. Inhibition of STAT3 activity with AG490 decreases the invasion of human pancreatic cancer cells in vitro. Cancer Sci. 2006, 97, 1417–1423. [Google Scholar] [CrossRef] [PubMed]
- Konikova, E.; Kusenda, J. Altered expression of p53 and MDM2 proteins in hematological malignancies. Neoplasma 2003, 50, 31–40. [Google Scholar] [PubMed]
- Cinatl, J.; Speidel, D.; Hardcastle, I.; Michaelis, M. Resistance acquisition to MDM2 inhibitors. Biochem. Soc. Trans. 2014, 42, 752–757. [Google Scholar] [CrossRef]
- Ame, J.C.; Spenlehauer, C.; de Murcia, G. The PARP superfamily. Bioessays 2004, 26, 882–893. [Google Scholar] [CrossRef]
- Petsri, K.; Thongsom, S.; Racha, S.; Chamni, S.; Jindapol, S.; Kaekratoke, N.; Zou, H.; Chanvorachote, P. Novel mechanism of napabucasin, a naturally derived furanonaphthoquinone: Apoptosis and autophagy induction in lung cancer cells through direct targeting on Akt/mTOR proteins. BMC Complement. Med. Ther. 2022, 22, 250. [Google Scholar] [CrossRef]
- Langleben, A.; Supko, J.G.; Hotte, S.J.; Batist, G.; Hirte, H.W.; Rogoff, H.; Li, Y.Z.; Li, W.; Kerstein, D.; Leggett, D.; et al. A dose-escalation phase I study of a first-in-class cancer stemness inhibitor in patients with advanced malignancies. J. Clin. Oncol. 2013, 31, 2542. [Google Scholar] [CrossRef]
- Bekaii-Saab, T.S.; Starodub, A.; El-Rayes, B.F.; O’Neil, B.H.; Shanda, S.; Ciombor, K.K.; Noonan, A.M.; Hanna, W.T.; Sehdev, A.; Shaib, W.L.; et al. A phase Ib/II study of cancer sternness inhibitor napabucasin (BBI-608) in combination with gemcitabine (gem) and nab-paclitaxel (nabPTX) in metastatic pancreatic adenocarcinoma (mPDAC) patients (pts). J. Clin. Oncol. 2017, 35, 4106. [Google Scholar] [CrossRef]
- Kawazoe, A.; Kuboki, Y.; Shinozaki, E.; Hara, H.; Nishina, T.; Komatsu, Y.; Yuki, S.; Wakabayashi, M.; Nomura, S.; Sato, A.; et al. Multicenter Phase I/II Trial of Napabucasin and Pembrolizumab in Patients with Metastatic Colorectal Cancer (EPOC1503/SCOOP Trial). Clin. Cancer Res. 2020, 26, 5887–5894. [Google Scholar] [CrossRef]
- Yu, H.Y.; Yue, X.T.; Zhao, Y.H.; Li, X.Y.; Wu, L.H.; Zhang, C.; Liu, Z.; Lin, K.V.; Xu-Monette, Z.Y.; Young, K.H.; et al. LIF negatively regulates tumour-suppressor p53 through Stat3/ID1/MDM2 in colorectal cancers. Nat. Commun. 2014, 5, 5218. [Google Scholar] [CrossRef] [PubMed]
- Gasparoli, L.; Virely, C.; Tsakaneli, A.; Che, N.; Edwards, D.; Bartram, J.; Hubank, M.; Pal, D.; Heidenreich, O.; Martens, J.H.A.; et al. Susceptibility of pediatric acute lymphoblastic leukemia to STAT3 inhibition depends on p53 induction. Haematologica 2024, 109, 1069–1081. [Google Scholar] [CrossRef]
- Jasek-Gajda, E.; Jurkowska, H.; JasiNska, M.; Litwin, J.A.; Lis, G.J. Combination of ERK2 and STAT3 Inhibitors Promotes Anticancer Effects on Acute Lymphoblastic Leukemia Cells. Cancer Genom. Proteom. 2020, 17, 517–527. [Google Scholar] [CrossRef]
- Timofeev, O.; Schlereth, K.; Wanzel, M.; Braun, A.; Nieswandt, B.; Pagenstecher, A.; Rosenwald, A.; Elsässer, H.P.; Stiewe, T. p53 DNA Binding Cooperativity Is Essential for Apoptosis and Tumor Suppression In Vivo. Cell Rep. 2013, 3, 1512–1525. [Google Scholar] [CrossRef]
- Shin, D.Y. TP53 Mutation in Acute Myeloid Leukemia: An Old Foe Revisited. Cancers 2023, 15, 4816. [Google Scholar] [CrossRef]
- Monti, P.; Menichini, P.; Speciale, A.; Cutrona, G.; Fais, F.; Taiana, E.; Neri, A.; Bomben, R.; Gentile, M.; Gattei, V.; et al. Heterogeneity of TP53 Mutations and P53 Protein Residual Function in Cancer: Does It Matter? Front. Oncol. 2020, 10, 593383. [Google Scholar] [CrossRef] [PubMed]
- Muller, P.A.; Vousden, K.H. Mutant p53 in cancer: New functions and therapeutic opportunities. Cancer Cell 2014, 25, 304–317. [Google Scholar] [CrossRef]
- Epling-Burnette, P.K.; Liu, J.H.; Catlett-Falcone, R.; Turkson, J.; Oshiro, M.; Kothapalli, R.; Li, Y.; Wang, J.M.; Yang-Yen, H.F.; Karras, J.; et al. Inhibition of STAT3 signaling leads to apoptosis of leukemic large granular lymphocytes and decreased Mcl-1 expression. J. Clin. Investig. 2001, 107, 351–362. [Google Scholar] [CrossRef]
- Ishdorj, G.; Johnston, J.B.; Gibson, S.B. Inhibition of constitutive activation of STAT3 by curcurbitacin-I (JSI-124) sensitized human B-leukemia cells to apoptosis. Mol. Cancer Ther. 2010, 9, 3302–3314. [Google Scholar] [CrossRef]
- Kida, H.; Ihara, S.; Kumanogoh, A. Involvement of STAT3 in immune evasion during lung tumorigenesis. Oncoimmunology 2013, 2, e22653. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hou, D.; Wang, B.; You, R.; Wang, X.; Liu, J.; Zhan, W.; Chen, P.; Qin, T.; Zhang, X.; Huang, H. Stromal cells promote chemoresistance of acute myeloid leukemia cells via activation of the IL-6/STAT3/OXPHOS axis. Ann. Transl. Med. 2020, 8, 1346. [Google Scholar] [CrossRef] [PubMed]
- Nair, R.R.; Tolentino, J.H.; Hazlehurst, L.A. Role of STAT3 in Transformation and Drug Resistance in CML. Front. Oncol. 2012, 2, 30. [Google Scholar] [CrossRef] [PubMed]
- Mo, J.; Deng, L.; Peng, K.; Ouyang, S.; Ding, W.; Lou, L.; Lin, Z.; Zhu, J.; Li, J.; Zhang, Q.; et al. Targeting STAT3-VISTA axis to suppress tumor aggression and burden in acute myeloid leukemia. J. Hematol. Oncol. 2023, 16, 15. [Google Scholar] [CrossRef]
- Tesoriere, A.; Dinarello, A.; Argenton, F. The Roles of Post-Translational Modifications in STAT3 Biological Activities and Functions. Biomedicines 2021, 9, 956. [Google Scholar] [CrossRef]
- Lim, C.P.; Cao, X. Regulation of Stat3 activation by MEK kinase 1. J. Biol. Chem. 2001, 276, 21004–21011. [Google Scholar] [CrossRef]
- Jain, N.; Zhang, T.; Fong, S.L.; Lim, C.P.; Cao, X. Repression of Stat3 activity by activation of mitogen-activated protein kinase (MAPK). Oncogene 1998, 17, 3157–3167. [Google Scholar] [CrossRef][Green Version]
- Chang, W.L.; Yang, K.C.; Peng, J.Y.; Hong, C.L.; Li, P.C.; Chye, S.M.; Lu, F.J.; Shih, C.W.; Chen, C.H. Parecoxib Enhances Resveratrol against Human Colorectal Cancer Cells through Akt and TXNDC5 Inhibition and MAPK Regulation. Nutrients 2024, 16, 3020. [Google Scholar] [CrossRef]
- Yee, K.; Martinelli, G.; Vey, N.; Dickinson, M.J.; Seiter, K.; Assouline, S.; Drummond, M.; Yoon, S.S.; Kasner, M.; Lee, J.H.; et al. Phase 1/1b Study of RG7388, a Potent MDM2 Antagonist, in Acute Myelogenous Leukemia (AML) Patients (Pts). Blood 2014, 124, 116. [Google Scholar] [CrossRef]
- Italiano, A.; Miller, W.H., Jr.; Blay, J.Y.; Gietema, J.A.; Bang, Y.J.; Mileshkin, L.R.; Hirte, H.W.; Higgins, B.; Blotner, S.; Nichols, G.L.; et al. Phase I study of daily and weekly regimens of the orally administered MDM2 antagonist idasanutlin in patients with advanced tumors. Investig. New Drugs 2021, 39, 1587–1597. [Google Scholar] [CrossRef]
- Shah, M.A.; Shitara, K.; Lordick, F.; Bang, Y.J.; Tebbutt, N.C.; Metges, J.P.; Muro, K.; Lee, K.W.; Shen, L.; Tjulandin, S.; et al. Randomized, Double-Blind, Placebo-Controlled Phase III Study of Paclitaxel +/- Napabucasin in Pretreated Advanced Gastric or Gastroesophageal Junction Adenocarcinoma. Clin. Cancer Res. 2022, 28, OF1–OF9. [Google Scholar] [CrossRef] [PubMed]
- Umehara, K.; Cleary, Y.; Fowler, S.; Parrott, N.; Tuerck, D. Accelerating Clinical Development of Idasanutlin through a Physiologically Based Pharmacokinetic Modeling Risk Assessment for CYP450 Isoenzyme-Related Drug-Drug Interactions. Drug Metab. Dispos. 2022, 50, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Karol, M.D.; Hitron, M.; Hard, M.L.; Blanchard, J.E.; Eraut, N.; Rich, N.; Gufford, B.T. Mass balance and pharmacokinetics of an oral dose of (14) C-napabucasin in healthy adult male subjects. Pharmacol. Res. Perspect. 2021, 9, e00722. [Google Scholar] [CrossRef] [PubMed]
- Pourhassan, H.; Murphy, L.; Aldoss, I. Glucocorticoid Therapy in Acute Lymphoblastic Leukemia: Navigating Short-Term and Long-Term Effects and Optimal Regimen Selection. Curr. Hematol. Malig. Rep. 2024, 19, 175–185. [Google Scholar] [CrossRef]
- Abaan, O.D.; Polley, E.C.; Davis, S.R.; Zhu, Y.J.; Bilke, S.; Walker, R.L.; Pineda, M.; Gindin, Y.; Jiang, Y.; Reinhold, W.C.; et al. The exomes of the NCI-60 panel: A genomic resource for cancer biology and systems pharmacology. Cancer Res. 2013, 73, 4372–4382. [Google Scholar] [CrossRef]
- Ikediobi, O.N.; Davies, H.; Bignell, G.; Edkins, S.; Stevens, C.; O’Meara, S.; Santarius, T.; Avis, T.; Barthorpe, S.; Brackenbury, L.; et al. Mutation analysis of 24 known cancer genes in the NCI-60 cell line set. Mol. Cancer Ther. 2006, 5, 2606–2612. [Google Scholar] [CrossRef]
- Olivier, M.; Hollstein, M.; Hainaut, P. TP53 mutations in human cancers: Origins, consequences, and clinical use. Cold Spring Harb. Perspect. Biol. 2010, 2, a001008. [Google Scholar] [CrossRef]
- Scudiero, D.A.; Shoemaker, R.H.; Paull, K.D.; Monks, A.; Tierney, S.; Nofziger, T.H.; Currens, M.J.; Seniff, D.; Boyd, M.R. Evaluation of a soluble tetrazolium/formazan assay for cell growth and drug sensitivity in culture using human and other tumor cell lines. Cancer Res. 1988, 48, 4827–4833. [Google Scholar] [PubMed]
- Zheng, S.; Wang, W.; Aldahdooh, J.; Malyutina, A.; Shadbahr, T.; Tanoli, Z.; Pessia, A.; Tang, J. SynergyFinder Plus: Toward Better Interpretation and Annotation of Drug Combination Screening Datasets. Genom. Proteom. Bioinform. 2022, 20, 587–596. [Google Scholar] [CrossRef]
- Yadav, B.; Wennerberg, K.; Aittokallio, T.; Tang, J. Searching for Drug Synergy in Complex Dose-Response Landscapes Using an Interaction Potency Model. Comput. Struct. Biotechnol. J. 2015, 13, 504–513. [Google Scholar] [CrossRef]
- Boulares, A.H.; Yakovlev, A.G.; Ivanova, V.; Stoica, B.A.; Wang, G.; Iyer, S.; Smulson, M. Role of poly(ADP-ribose) polymerase (PARP) cleavage in apoptosis. Caspase 3-resistant PARP mutant increases rates of apoptosis in transfected cells. J. Biol. Chem. 1999, 274, 22932–22940. [Google Scholar] [CrossRef]
- Vassilev, L.T.; Vu, B.T.; Graves, B.; Carvajal, D.; Podlaski, F.; Filipovic, Z.; Kong, N.; Kammlott, U.; Lukacs, C.; Klein, C.; et al. In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science 2004, 303, 844–848. [Google Scholar] [CrossRef]
- Yu, H.; Lee, H.; Herrmann, A.; Buettner, R.; Jove, R. Revisiting STAT3 signalling in cancer: New and unexpected biological functions. Nat. Rev. Cancer 2014, 14, 736–746. [Google Scholar] [CrossRef] [PubMed]

| Cell Lines | Cell Type | * TP53 Status | * STAT3 Status | ** RG7388 (nM) | ** BBI608 (nM) |
|---|---|---|---|---|---|
| Nalm-6 | B cell ALL | WT | WT | 82 ± 6 | 562 ± 43 |
| RS4;11 | B cell ALL | WT | WT | 47 ± 8 | 659 ± 79 |
| HAL-01 | B cell ALL | WT | WT | 50 ± 11 | 702 ± 18 |
| MOLT-4 | T cell ALL | Mutant (Heterozygous) c.916C>T; p.R306 * | Mutant (Heterozygous) c.2186G>A; p.R729H c.1217C>T; p.A406V | 52 ± 11 | 1114 ± 226 |
| REH | B cell ALL | Mutant (Heterozygous) c.541C>T; p.R181C | WT | 289 ± 9 | 811 ± 154 |
| CCRF-CEM | T cell ALL | Mutant (Heterozygous) c.524G>A; p.R175H c.743G>A; p.R248Q | WT | >3000 | 681 ± 45 |
| Cell Lines | * Global Synergy Score | Most Synergistic Area Score | ** Multi-Dimensional Synergy of Combinations (MuSyC) Reference Model |
|---|---|---|---|
| Nalm-6 | 13.9 | 24.4 | There is a synergistic potency shift induced by RG7388. |
| RS4;11 | 13.6 | 25.7 | There is a synergistic potency shift induced by RG7388. |
| REH | 11.6 | 13.0 | There is a synergistic potency shift induced by BBI608. There is a positive cooperativity induced by RG7388. |
| HAL-01 | 8.5 | 13.5 | There is a synergistic potency shift induced by BBI608. |
| MOLT-4 | 1.2 | 9.9 | No synergy or antagonism detected with 95 percent confidence interval. |
| CCRF-CEM | 2.3 | 14.6 | No synergy or antagonism detected with 95 percent confidence interval. |
| Genes | Forward Primers (5′-3′) | Reverse Primers (5′-3′) |
|---|---|---|
| GAPDH | CGACCACTTTGTCAAGCTCA | GGGTCTTACTCCTTGGAGGC |
| PUMA (BBC3) | ACCTCAACGCACAGTACGA | CTGGGTAAGGGCAGGAGTC |
| MDM2 | AGTAGCAGTGAATCTACAGGGA | CTGATCCAACCAATCACCTGAAT |
| CDKN1A | TGTCCGCAGAACCCATGC | AAAGTCGAAGTTCCTCGCTC |
| TP53INP1 | TCTTGAGTGCTTGGCTGATACA | GGTGGGGTGATAAACCAGCTC |
| BAX | CCCGAGAGGTCTTTTTCCGAG | CCAGCCCATGATGGTTCTGAT |
| BCL-2 | GGTGGGGTCATGTGTGTGG | CGGTTCAGGTACTCAGTCATCC |
| BCL2L1 (BCL-XL) | GCAGGCGACGAGTTTGAACT | CTCGGCTGCTGCATTGTT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aptullahoglu, E.; Kaygusuz, E. Synergistic MDM2-STAT3 Inhibition Demonstrates Strong Anti-Leukemic Efficacy in Acute Lymphoblastic Leukemia. Int. J. Mol. Sci. 2025, 26, 8648. https://doi.org/10.3390/ijms26178648
Aptullahoglu E, Kaygusuz E. Synergistic MDM2-STAT3 Inhibition Demonstrates Strong Anti-Leukemic Efficacy in Acute Lymphoblastic Leukemia. International Journal of Molecular Sciences. 2025; 26(17):8648. https://doi.org/10.3390/ijms26178648
Chicago/Turabian StyleAptullahoglu, Erhan, and Emrah Kaygusuz. 2025. "Synergistic MDM2-STAT3 Inhibition Demonstrates Strong Anti-Leukemic Efficacy in Acute Lymphoblastic Leukemia" International Journal of Molecular Sciences 26, no. 17: 8648. https://doi.org/10.3390/ijms26178648
APA StyleAptullahoglu, E., & Kaygusuz, E. (2025). Synergistic MDM2-STAT3 Inhibition Demonstrates Strong Anti-Leukemic Efficacy in Acute Lymphoblastic Leukemia. International Journal of Molecular Sciences, 26(17), 8648. https://doi.org/10.3390/ijms26178648







