Frequency of Polymorphisms in SLC47A1 (rs2252281 and rs2289669) and SLC47A2 (rs34834489 and rs12943590) and the Influence of SLC22A1 (rs72552763 and rs622342) on HbA1c Levels in Mexican-Mestizo Patients with DMT2 Treated with Metformin Monotherapy
Abstract
1. Introduction
2. Results
2.1. SCL47A1 and SLC47A2 Allelic and Genotypic Frequencies
2.2. Clinical and Demographic Characteristics Across Different Genotype Carriers Undergoing Metformin Monotherapy
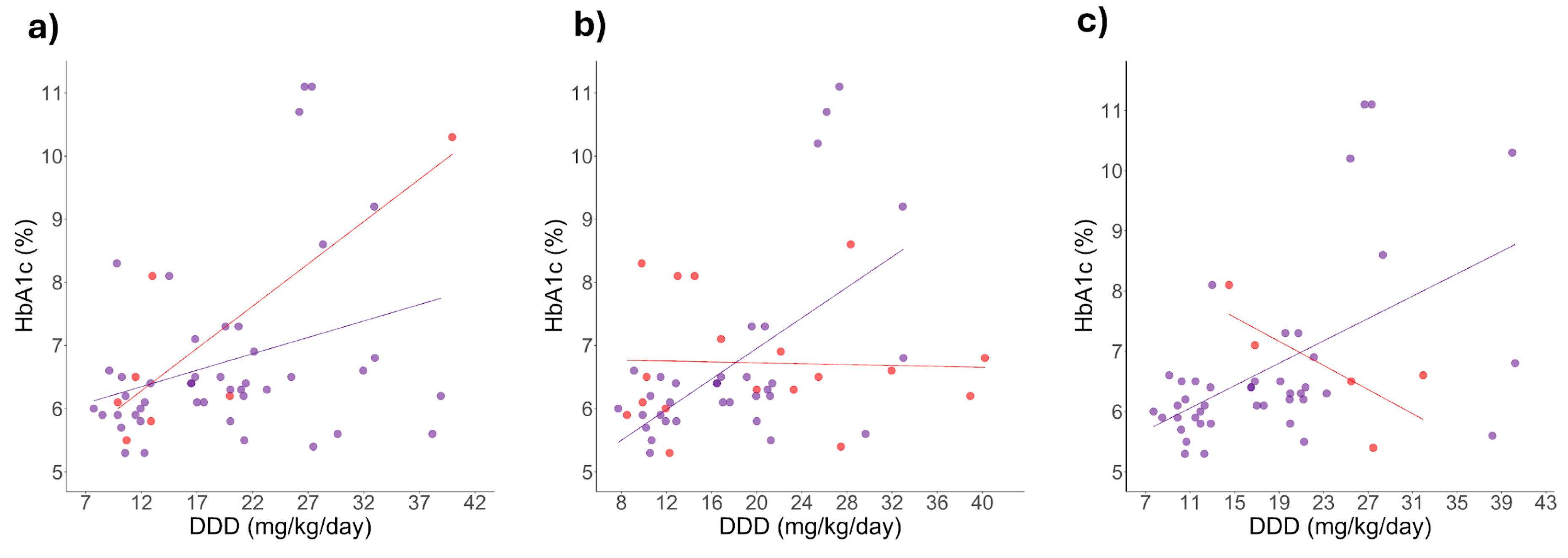
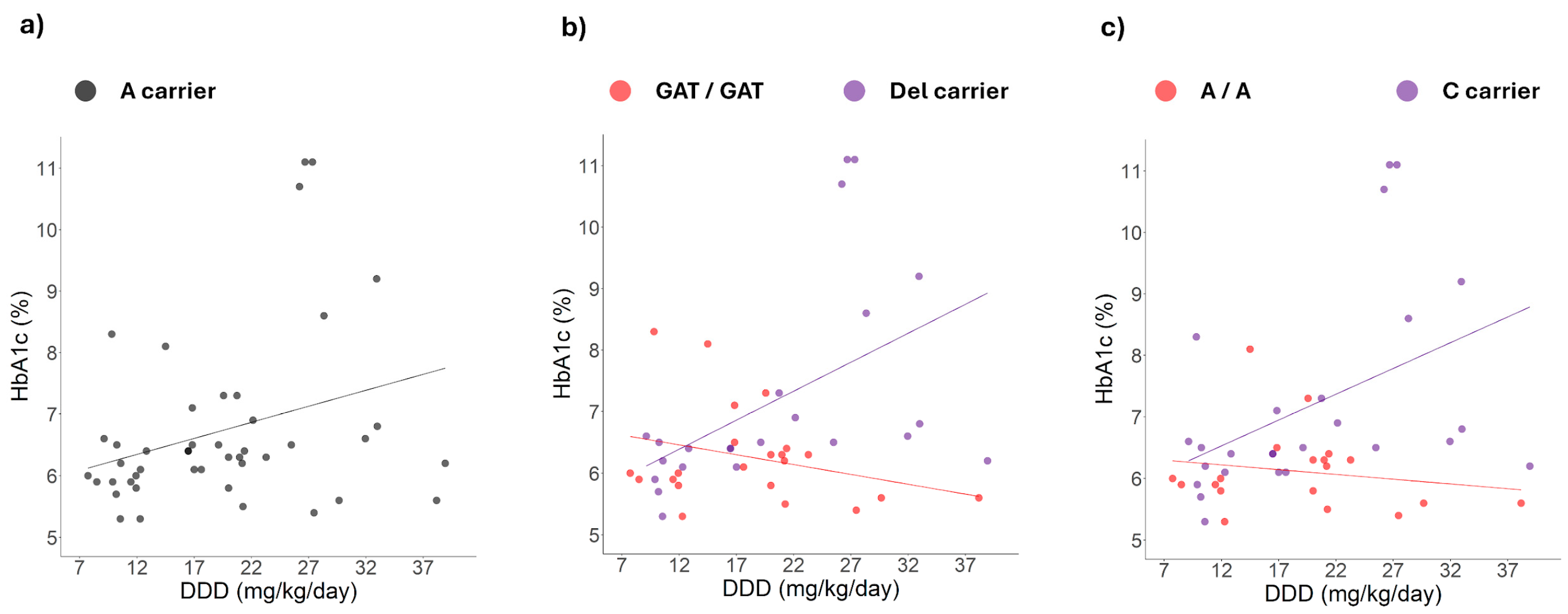
2.3. Correlation Between HbA1c Levels and Daily Dosage
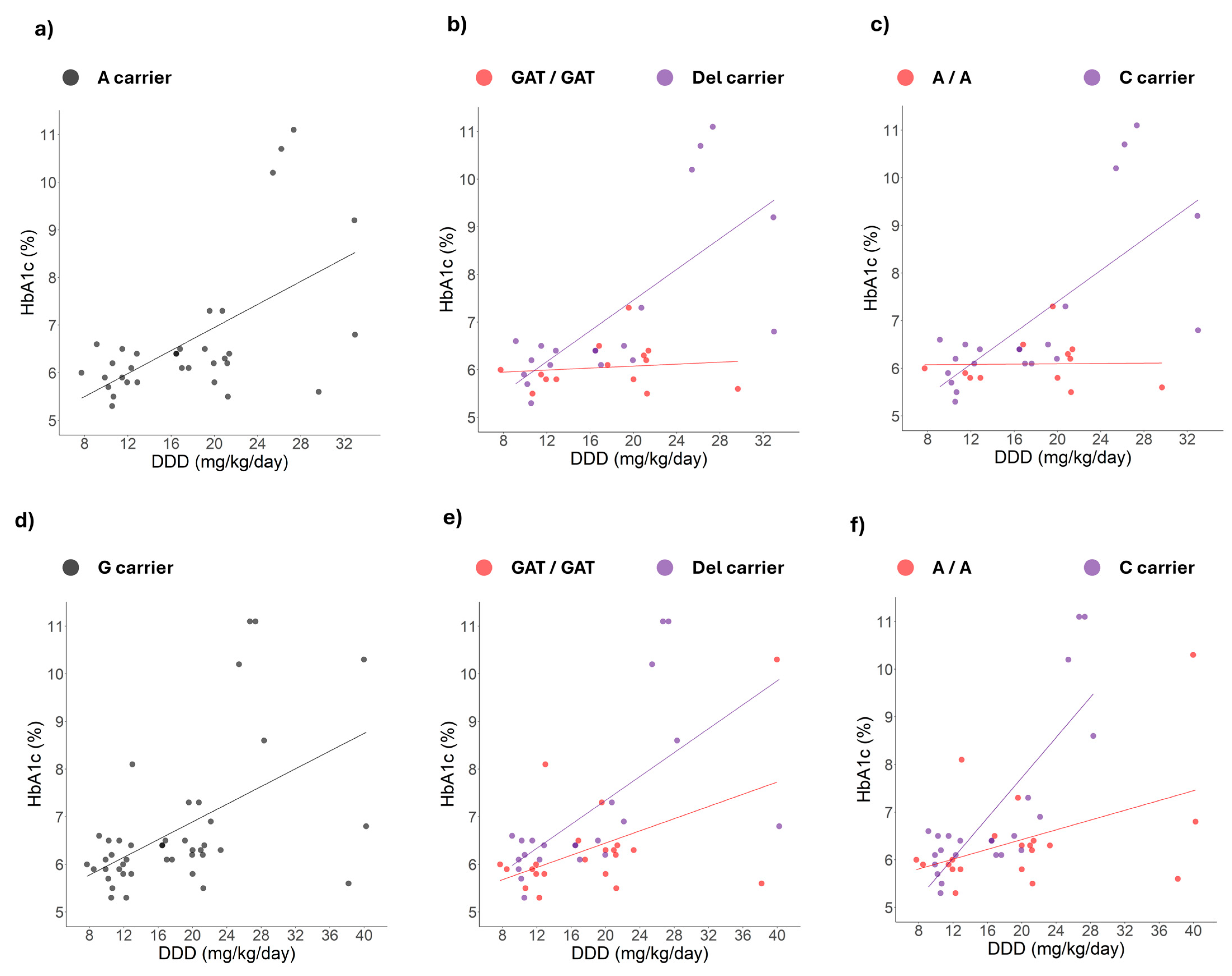
2.4. Correlation Changes Between HbA1c Levels and Daily Dose Induced by Simultaneous Genotypes
3. Discussion
3.1. Genotypic Frequency Comparison Against Other World Populations
3.2. No Association Between Polymorphisms and Metformin Therapeutic Efficacy
3.3. Positive Correlation Between HbA1c Levels and Metformin Dose Among Patients Carrying Specific Polymorphisms
3.4. Simultaneous Presence of Polymorphisms and DDD-HbA1c Correlation Changes
4. Materials and Methods
4.1. Study Design and Patient Recruitment
4.2. Sample Delimitation
4.3. Genotyping
4.4. Plasmatic Metformin Determination
4.5. Statistical Analysis
4.5.1. Analysis of Allelic and Genotypic Frequencies
4.5.2. Initial Inferential Analysis
4.5.3. Linear Regressions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- American Diabetes Association Professional Practice Committee. Introduction and Methodology: Standards of Care in Diabetes—2024. Diabetes Care 2024, 47, S1–S4. [Google Scholar] [CrossRef]
- Banday, M.Z.; Sameer, A.S.; Nissar, S. Pathophysiology of Diabetes: An Overview. Avicenna J. Med. 2020, 10, 174–188. [Google Scholar] [CrossRef]
- International Diabetes Federation. IDF Diabetes Atlas. Available online: https://www.diabetesatlas.org (accessed on 24 April 2025).
- WHO. The Top 10 Causes of Death. Available online: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (accessed on 2 May 2025).
- Zhu, R.; Zhou, S.; Xia, L.; Bao, X. Incidence, Morbidity and Years Lived With Disability Due to Type 2 Diabetes Mellitus in 204 Countries and Territories: Trends From 1990 to 2019. Front. Endocrinol. 2022, 13, 905538. [Google Scholar] [CrossRef] [PubMed]
- IDF. IDF Global Clinical Practice Recommendations for Managing Type 2 Diabetes—2025. Available online: https://idf.org/t2d-cpr-2025 (accessed on 4 May 2025).
- Secretaría de Salud NORMA Oficial Mexicana NOM-015-SSA2-2010, Para La Prevención, Tratamiento y Control de La Diabetes Mellitus (NOM-015-SSA2-2010). Available online: https://www.cndh.org.mx/documento/nom-015-ssa2-2010-para-la-prevencion-tratamiento-y-control-de-la-diabetes-mellitus (accessed on 1 August 2024).
- Sartore, G.; Ragazzi, E.; Caprino, R.; Lapolla, A. Long-Term HbA1c Variability and Macro-/Micro-Vascular Complications in Type 2 Diabetes Mellitus: A Meta-Analysis Update. Acta Diabetol. 2023, 60, 721–738. [Google Scholar] [CrossRef]
- Boye, K.S.; Thieu, V.T.; Lage, M.J.; Miller, H.; Paczkowski, R. The Association Between Sustained HbA1c Control and Long-Term Complications Among Individuals with Type 2 Diabetes: A Retrospective Study. Adv. Ther. 2022, 39, 2208–2221. [Google Scholar] [CrossRef]
- Wu, T.-E.; Su, Y.-W.; Chen, H.-S. Mean HbA1c and HbA1c Variability Are Associated with Differing Diabetes-Related Complications in Patients with Type 2 Diabetes Mellitus. Diabetes Res. Clin. Pract. 2022, 192, 110069. [Google Scholar] [CrossRef]
- Pan American Health Organization. Panorama of Diabetes in the Americas; Pan American Health Organization: Washington, DC, USA, 2022; ISBN 978-92-75-12633-2. [Google Scholar]
- Avilés-Santa, M.L.; Monroig-Rivera, A.; Soto-Soto, A.; Lindberg, N.M. Current State of Diabetes Mellitus Prevalence, Awareness, Treatment, and Control in Latin America: Challenges and Innovative Solutions to Improve Health Outcomes Across the Continent. Curr. Diabetes Rep. 2020, 20, 62. [Google Scholar] [CrossRef]
- Basto-Abreu, A.; Reyes-Garcia, A.; Stern, D.; Torres-Ibarra, L.; Rojas-Martínez, R.; Aguilar-Salinas, C.A.; Romero-Martínez, M.; Campos-Nonato, I.; López-Ridaura, R.; Barrientos-Gutiérrez, T. Cascadas de Tamizaje y Atención de La Diabetes Tipo 2 En México. Salud Pública México 2024, 66, 528–536. [Google Scholar] [CrossRef] [PubMed]
- Bitew, Z.W.; Alemu, A.; Jember, D.A.; Tadesse, E.; Getaneh, F.B.; Seid, A.; Weldeyonnes, M. Prevalence of Glycemic Control and Factors Associated With Poor Glycemic Control: A Systematic Review and Meta-Analysis. Inq. J. Health Care Organ. Provis. Financ. 2023, 60, 1–15. [Google Scholar] [CrossRef]
- Gaohua, L.; Miao, X.; Dou, L. Crosstalk of Physiological pH and Chemical pKa under the Umbrella of Physiologically Based Pharmacokinetic Modeling of Drug Absorption, Distribution, Metabolism, Excretion, and Toxicity. Expert Opin. Drug Metab. Toxicol. 2021, 17, 1103–1124. [Google Scholar] [CrossRef] [PubMed]
- Chan, P.; Shao, L.; Tomlinson, B.; Zhang, Y.; Liu, Z.-M. Metformin Transporter Pharmacogenomics: Insights into Drug Disposition—Where Are We Now? Expert Opin. Drug Metab. Toxicol. 2018, 14, 1149–1159. [Google Scholar] [CrossRef]
- LaMoia, T.E.; Shulman, G.I. Cellular and Molecular Mechanisms of Metformin Action. Endocr. Rev. 2021, 42, 77–96. [Google Scholar] [CrossRef]
- Menjivar, M.; Sánchez-Pozos, K.; Jaimes-Santoyo, J.; Monroy-Escutia, J.; Rivera- Santiago, C.; de los Ángeles Granados-Silvestre, M.; Ortiz-López, M.G. Pharmacogenetic Evaluation of Metformin and Sulphonylurea Response in Mexican Mestizos with Type 2 Diabetes. Curr. Drug Metab. 2020, 21, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Moreno-González, J.G.; Reza-López, S.A.; González-Rodríguez, E.; Siqueiros-Cendón, T.S.; Escareño Contreras, A.; Rascón-Cruz, Q.; Leal-Berumen, I. Genetic Variants of SLC22A1 rs628031 and rs622342 and Glycemic Control in T2DM Patients from Northern Mexico. Genes 2025, 16, 139. [Google Scholar] [CrossRef]
- Ortega-Ayala, A.; De Andrés, F.; Llerena, A.; Bartolo-Montiel, C.M.; Acosta-Altamirano, G.; Molina-Guarneros, J.A. Longitudinal Assessment of SNPs rs72552763 and rs622342 in SLC22A1 over HbA1c Control among Mexican-Mestizo Diabetic Type 2 Patients. Front. Pharmacol. 2024, 15, 1433519. [Google Scholar] [CrossRef] [PubMed]
- Reséndiz-Abarca, C.A.; Flores-Alfaro, E.; Suárez-Sánchez, F.; Cruz, M.; Valladares-Salgado, A.; del Carmen Alarcón-Romero, L.; Vázquez-Moreno, M.A.; Wacher-Rodarte, N.A.; Gómez-Zamudio, J.H. Altered Glycemic Control Associated With Polymorphisms in the SLC22A1 (OCT1) Gene in a Mexican Population With Type 2 Diabetes Mellitus Treated With Metformin: A Cohort Study. J. Clin. Pharmacol. 2019, 59, 1384–1390. [Google Scholar] [CrossRef]
- Dyer, S.C.; Austine-Orimoloye, O.; Azov, A.G.; Barba, M.; Barnes, I.; Barrera-Enriquez, V.P.; Becker, A.; Bennett, R.; Beracochea, M.; Berry, A.; et al. Ensembl 2025. Nucleic Acids Res. 2025, 53, D948–D957. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Ayala, A.; Rodríguez-Rivera, N.S.; de Andrés, F.; Llerena, A.; Pérez-Silva, E.; Espinosa-Sánchez, A.G.; Molina-Guarneros, J.A. Pharmacogenetics of Metformin Transporters Suggests No Association with Therapeutic Inefficacy among Diabetes Type 2 Mexican Patients. Pharmaceuticals 2022, 15, 774. [Google Scholar] [CrossRef]
- Chung, J.-Y.; Cho, S.K.; Kim, T.H.; Kim, K.H.; Jang, G.H.; Kim, C.O.; Park, E.-M.; Cho, J.-Y.; Jang, I.-J.; Choi, J.H. Functional Characterization of MATE2-K Genetic Variants and Their Effects on Metformin Pharmacokinetics. Pharmacogenet. Genom. 2013, 23, 365–373. [Google Scholar] [CrossRef]
- Becker, M.L.; Visser, L.E.; van Schaik, R.H.N.; Hofman, A.; Uitterlinden, A.G.; Stricker, B.H.C. Genetic Variation in the Multidrug and Toxin Extrusion 1 Transporter Protein Influences the Glucose-Lowering Effect of Metformin in Patients with Diabetes: A Preliminary Study. Diabetes 2009, 58, 745–749. [Google Scholar] [CrossRef]
- Becker, M.L.; Visser, L.E.; van Schaik, R.H.N.; Hofman, A.; Uitterlinden, A.G.; Stricker, B.H.C. Interaction between Polymorphisms in the OCT1 and MATE1 Transporter and Metformin Response. Pharmacogenet. Genom. 2010, 20, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Stocker, S.L.; Morrissey, K.M.; Yee, S.W.; Castro, R.A.; Xu, L.; Dahlin, A.; Ramirez, A.H.; Roden, D.M.; Wilke, R.A.; McCarty, C.A.; et al. The Effect of Novel Promoter Variants in MATE1 and MATE2 on the Pharmacokinetics and Pharmacodynamics of Metformin. Clin. Pharmacol. Ther. 2013, 93, 186–194. [Google Scholar] [CrossRef]
- Chung, H.; Oh, J.; Yoon, S.H.; Yu, K.-S.; Cho, J.-Y.; Chung, J.-Y. A Non-Linear Pharmacokinetic-Pharmacodynamic Relationship of Metformin in Healthy Volunteers: An Open-Label, Parallel Group, Randomized Clinical Study. PLoS ONE 2018, 13, e0191258. [Google Scholar] [CrossRef]
- Auton, A.; Abecasis, G.R.; Altshuler, D.M.; Durbin, R.M.; Abecasis, G.R.; Bentley, D.R.; Chakravarti, A.; Clark, A.G.; Donnelly, P.; Eichler, E.E.; et al. A Global Reference for Human Genetic Variation. Nature 2015, 526, 68–74. [Google Scholar] [CrossRef]
- Sohail, M.; Palma-Martínez, M.J.; Chong, A.Y.; Quinto-Cortés, C.D.; Barberena-Jonas, C.; Medina-Muñoz, S.G.; Ragsdale, A.; Delgado-Sánchez, G.; Cruz-Hervert, L.P.; Ferreyra-Reyes, L.; et al. Mexican Biobank Advances Population and Medical Genomics of Diverse Ancestries. Nature 2023, 622, 775–783. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Ayala, A.; de la Cruz, C.G.; Dorado, P.; Rodrigues-Soares, F.; Castillo-Nájera, F.; LLerena, A.; Molina-Guarneros, J. Molecular Ancestry Across Allelic Variants of SLC22A1, SLC22A2, SLC22A3, ABCB1, CYP2C8, CYP2C9, and CYP2C19 in Mexican-Mestizo DMT2 Patients. Biomedicines 2025, 13, 1156. [Google Scholar] [CrossRef]
- Turner, B.E.; Steinberg, J.R.; Weeks, B.T.; Rodriguez, F.; Cullen, M.R. Race/Ethnicity Reporting and Representation in US Clinical Trials: A Cohort Study. Lancet Reg. Health-Am. 2022, 11, 100252. [Google Scholar] [CrossRef]
- Raj, G.M.; Mathaiyan, J.; Wyawahare, M.; Priyadarshini, R. Lack of Effect of the SLC47A1 and SLC47A2 Gene Polymorphisms on the Glycemic Response to Metformin in Type 2 Diabetes Mellitus Patients. Drug Metab. Pers. Ther. 2018, 33, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, A.J.T.; Stage, T.B.; Glintborg, D.; Andersen, M.; Christensen, M.M.H. The Pharmacogenetics of Metformin in Women with Polycystic Ovary Syndrome: A Randomized Trial. Basic Clin. Pharmacol. Toxicol. 2018, 122, 239–244. [Google Scholar] [CrossRef]
- He, R.; Zhang, D.; Lu, W.; Zheng, T.; Wan, L.; Liu, F.; Jia, W. SLC47A1 Gene rs2289669 G> A Variants Enhance the Glucose-Lowering Effect of Metformin via Delaying Its Excretion in Chinese Type 2 Diabetes Patients. Diabetes Res. Clin. Pract. 2015, 109, 57–63. [Google Scholar] [CrossRef]
- Chen, P.; Cao, Y.; Chen, S.; Liu, Z.; Chen, S.; Guo, Y. Association of SLC22A1, SLC22A2, SLC47A1, and SLC47A2 Polymorphisms with Metformin Efficacy in Type 2 Diabetic Patients. Biomedicines 2022, 10, 2546. [Google Scholar] [CrossRef] [PubMed]
- Christensen, M.M.H.; Pedersen, R.S.; Stage, T.B.; Brasch-Andersen, C.; Nielsen, F.; Damkier, P.; Beck-Nielsen, H.; Brøsen, K. A Gene–Gene Interaction between Polymorphisms in the OCT2 and MATE1 Genes Influences the Renal Clearance of Metformin. Pharmacogenet. Genom. 2013, 23, 526–534. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.J.; Oh, J.; Lee, S.H.; Choi, Y.; Yu, K.-S.; Chung, J.-Y. Effect of Plasma Membrane Monoamine Transporter Genetic Variants on Pharmacokinetics of Metformin in Humans. Transl. Clin. Pharmacol. 2018, 26, 79. [Google Scholar] [CrossRef]
- Instituto Mexicano del Seguro Social. Diagnóstico y Tratamiento Farmacológico de la Diabetes Mellitus Tipo 2 en el Primer Nivel de Atención. Guía de Evidencias y Recomendaciones: Guía de Práctica Clínica; Instituto Mexicano del Seguro Social: Mexico City, Mexico, 2018; pp. 1–55. [Google Scholar]
- Zake, D.M.; Kurlovics, J.; Zaharenko, L.; Komasilovs, V.; Klovins, J.; Stalidzans, E. Physiologically Based Metformin Pharmacokinetics Model of Mice and Scale-up to Humans for the Estimation of Concentrations in Various Tissues. PLoS ONE 2021, 16, e0249594. [Google Scholar] [CrossRef]
- Lalau, J.-D.; Lemaire-Hurtel, A.-S.; Lacroix, C. Establishment of a Database of Metformin Plasma Concentrations and Erythrocyte Levels in Normal and Emergency Situations. Clin. Drug Investig. 2011, 31, 435–438. [Google Scholar] [CrossRef]
- Choi, J.H.; Yee, S.W.; Ramirez, A.H.; Morrissey, K.M.; Jang, G.H.; Joski, P.J.; Mefford, J.A.; Hesselson, S.E.; Schlessinger, A.; Jenkins, G.; et al. A Common 5′-UTR Variant in MATE2-K Is Associated With Poor Response to Metformin. Clin. Pharmacol. Ther. 2011, 90, 674–684. [Google Scholar] [CrossRef] [PubMed]
- Sundelin, E.; Gormsen, L.; Jensen, J.; Vendelbo, M.; Jakobsen, S.; Munk, O.; Christensen, M.; Brøsen, K.; Frøkiær, J.; Jessen, N. Genetic Polymorphisms in Organic Cation Transporter 1 Attenuates Hepatic Metformin Exposure in Humans. Clin. Pharmacol. Ther. 2017, 102, 841–848. [Google Scholar] [CrossRef]
- Bland, M. An Introduction to Medical Statistics; Oxford University Press: Oxford, UK, 2015. [Google Scholar]
- Althubaiti, A. Sample Size Determination: A Practical Guide for Health Researchers. J. Gen. Fam. Med. 2023, 24, 72–78. [Google Scholar] [CrossRef]
- Secretaría de Salud NORMA Oficial Mexicana NOM-177-SSA1-2013, Que Establece Las Pruebas y Procedimientos Para Demostrar Que Un Medicamento Es Intercambiable. Diario Oficial de La Federación. 2013. Available online: https://www.gob.mx/cms/uploads/attachment/file/745138/NOM-177-SSA1-2013.pdf (accessed on 19 May 2025).
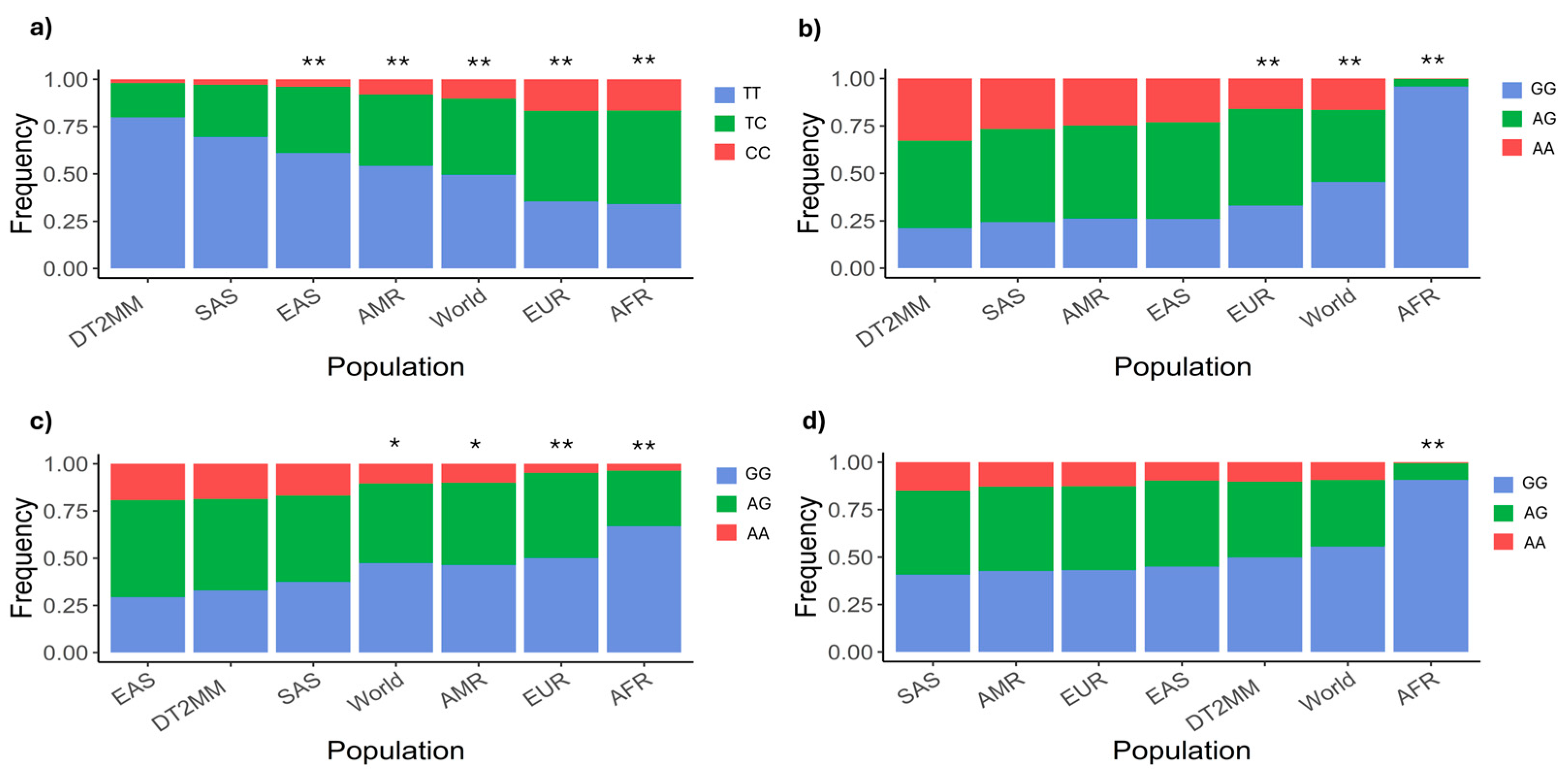

| Gene | Polymorphisms | Allele | n | Frequency | Genotype | n | Frequency | 1 p Value for HWE |
|---|---|---|---|---|---|---|---|---|
| SLC47A1 | rs2252281 n = 204 | T | 363 | 0.890 | TT | 163 | 0.799 | 0.277 |
| C | 45 | 0.110 | TC | 37 | 0.181 | |||
| CC | 4 | 0.020 | ||||||
| rs2289669 n = 204 | G | 180 | 0.441 | GG | 43 | 0.211 | 0.349 | |
| A | 228 | 0.559 | AG | 94 | 0.460 | |||
| AA | 67 | 0.328 | ||||||
| SLC47A2 | rs12943590 n = 204 | G | 175 | 0.429 | GG | 67 | 0.329 | 0.893 |
| A | 233 | 0.57 | AG | 99 | 0.485 | |||
| AA | 38 | 0.186 | ||||||
| rs34834489 n = 203 | G | 283 | 0.697 | GG | 101 | 0.498 | 0.431 | |
| A | 123 | 0.303 | AG | 81 | 0.399 | |||
| AA | 21 | 0.103 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez-Hernández, M.A.; Ortega-Ayala, A.; Rodríguez-Lima, O.; Landa, A.; Acosta-Altamirano, G.; Molina-Guarneros, J.A. Frequency of Polymorphisms in SLC47A1 (rs2252281 and rs2289669) and SLC47A2 (rs34834489 and rs12943590) and the Influence of SLC22A1 (rs72552763 and rs622342) on HbA1c Levels in Mexican-Mestizo Patients with DMT2 Treated with Metformin Monotherapy. Int. J. Mol. Sci. 2025, 26, 8652. https://doi.org/10.3390/ijms26178652
Gómez-Hernández MA, Ortega-Ayala A, Rodríguez-Lima O, Landa A, Acosta-Altamirano G, Molina-Guarneros JA. Frequency of Polymorphisms in SLC47A1 (rs2252281 and rs2289669) and SLC47A2 (rs34834489 and rs12943590) and the Influence of SLC22A1 (rs72552763 and rs622342) on HbA1c Levels in Mexican-Mestizo Patients with DMT2 Treated with Metformin Monotherapy. International Journal of Molecular Sciences. 2025; 26(17):8652. https://doi.org/10.3390/ijms26178652
Chicago/Turabian StyleGómez-Hernández, Milton Abraham, Adiel Ortega-Ayala, Oscar Rodríguez-Lima, Abraham Landa, Gustavo Acosta-Altamirano, and Juan A. Molina-Guarneros. 2025. "Frequency of Polymorphisms in SLC47A1 (rs2252281 and rs2289669) and SLC47A2 (rs34834489 and rs12943590) and the Influence of SLC22A1 (rs72552763 and rs622342) on HbA1c Levels in Mexican-Mestizo Patients with DMT2 Treated with Metformin Monotherapy" International Journal of Molecular Sciences 26, no. 17: 8652. https://doi.org/10.3390/ijms26178652
APA StyleGómez-Hernández, M. A., Ortega-Ayala, A., Rodríguez-Lima, O., Landa, A., Acosta-Altamirano, G., & Molina-Guarneros, J. A. (2025). Frequency of Polymorphisms in SLC47A1 (rs2252281 and rs2289669) and SLC47A2 (rs34834489 and rs12943590) and the Influence of SLC22A1 (rs72552763 and rs622342) on HbA1c Levels in Mexican-Mestizo Patients with DMT2 Treated with Metformin Monotherapy. International Journal of Molecular Sciences, 26(17), 8652. https://doi.org/10.3390/ijms26178652






