Molecular Characterization of the GALC Mutation Thr112Ala Causing Krabbe Disease
Abstract
1. Introduction
2. Results
2.1. Homology Model of Human Galactocerebrosidase
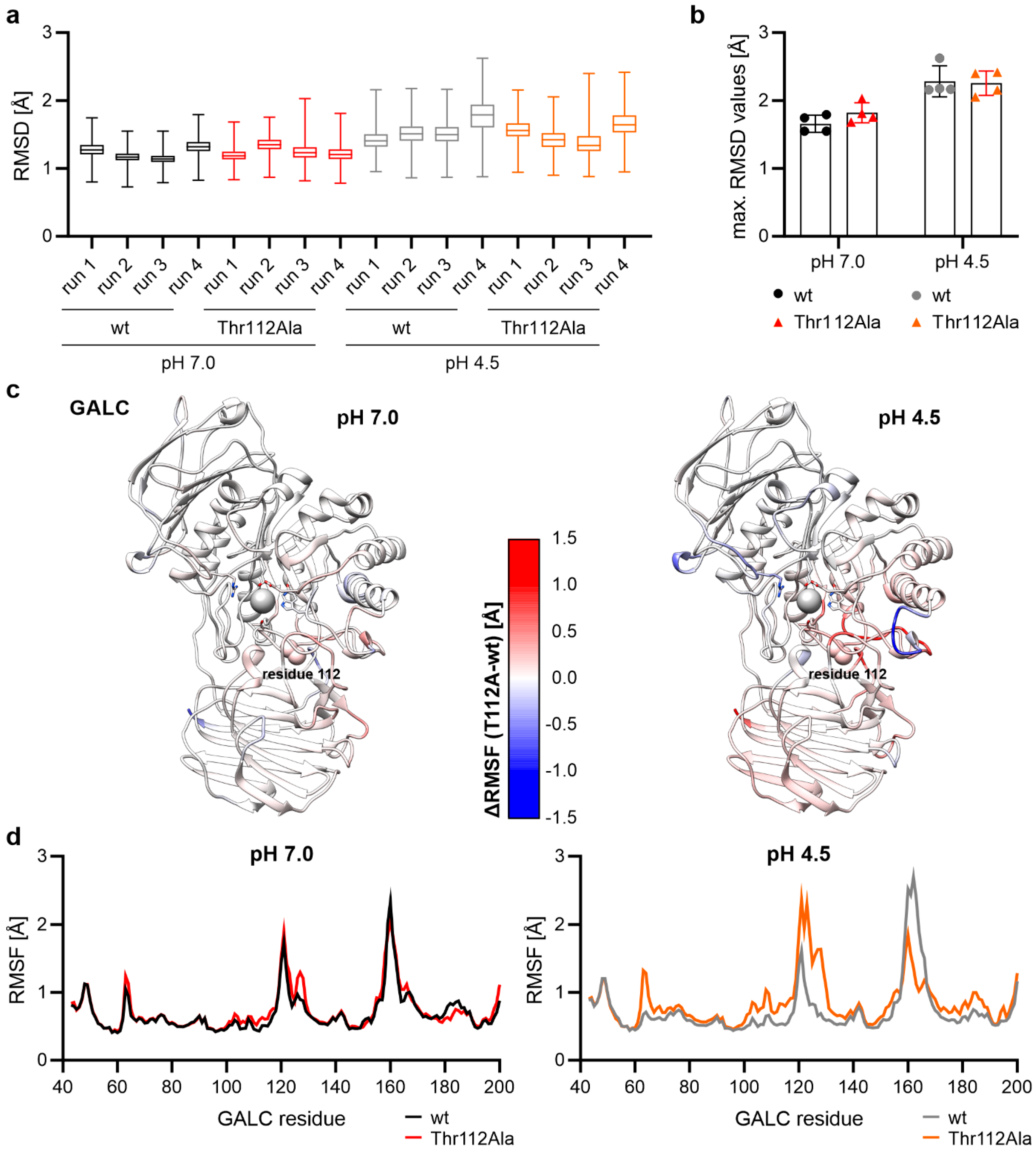
2.2. Differences in Protein Flexibility at Lysosomal pH
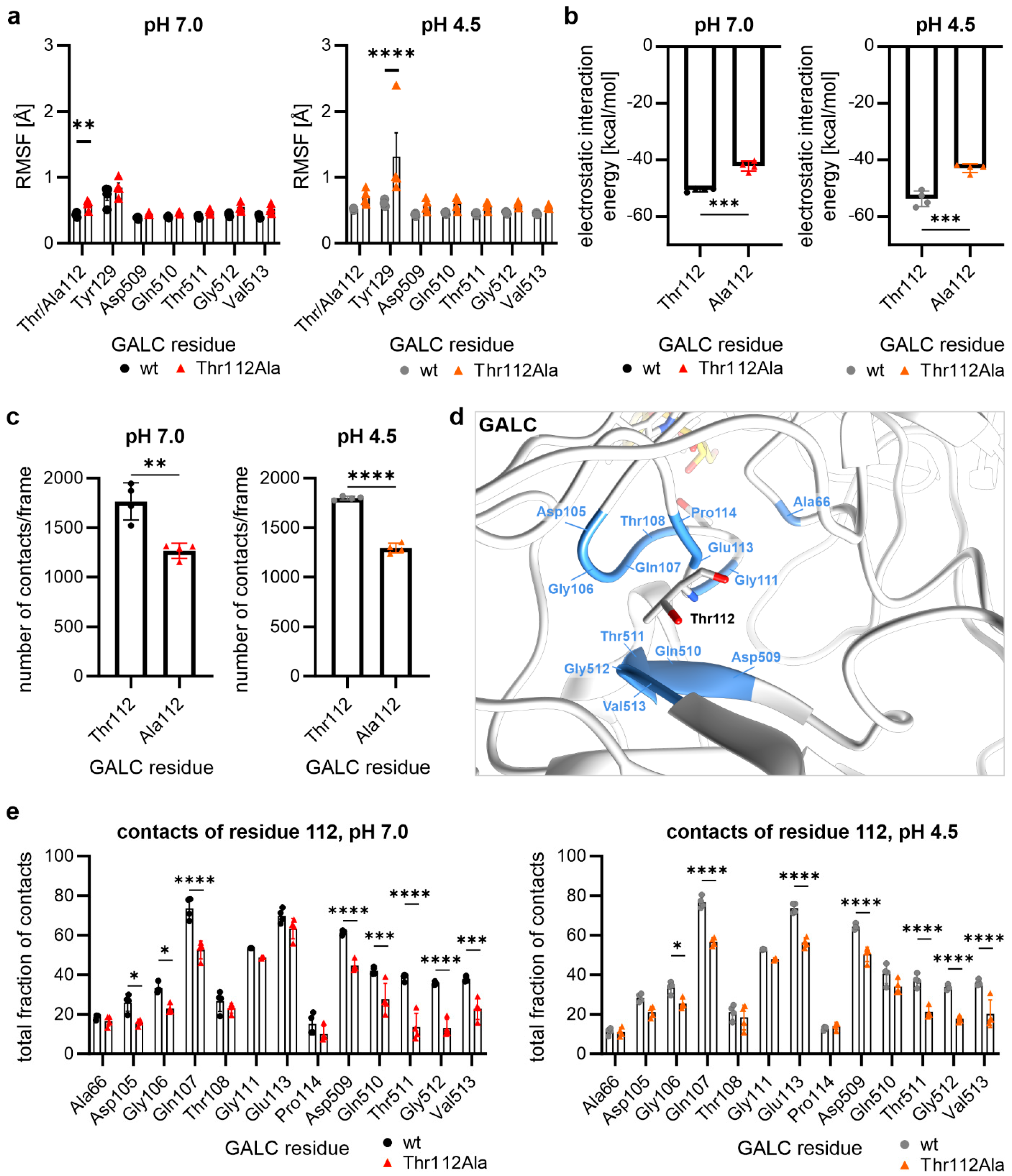
2.3. Thr112Ala: Higher Local Flexibility in the Vicinity Around Residue 112
2.4. Hydrogen Bonds of the Residue at Position 112
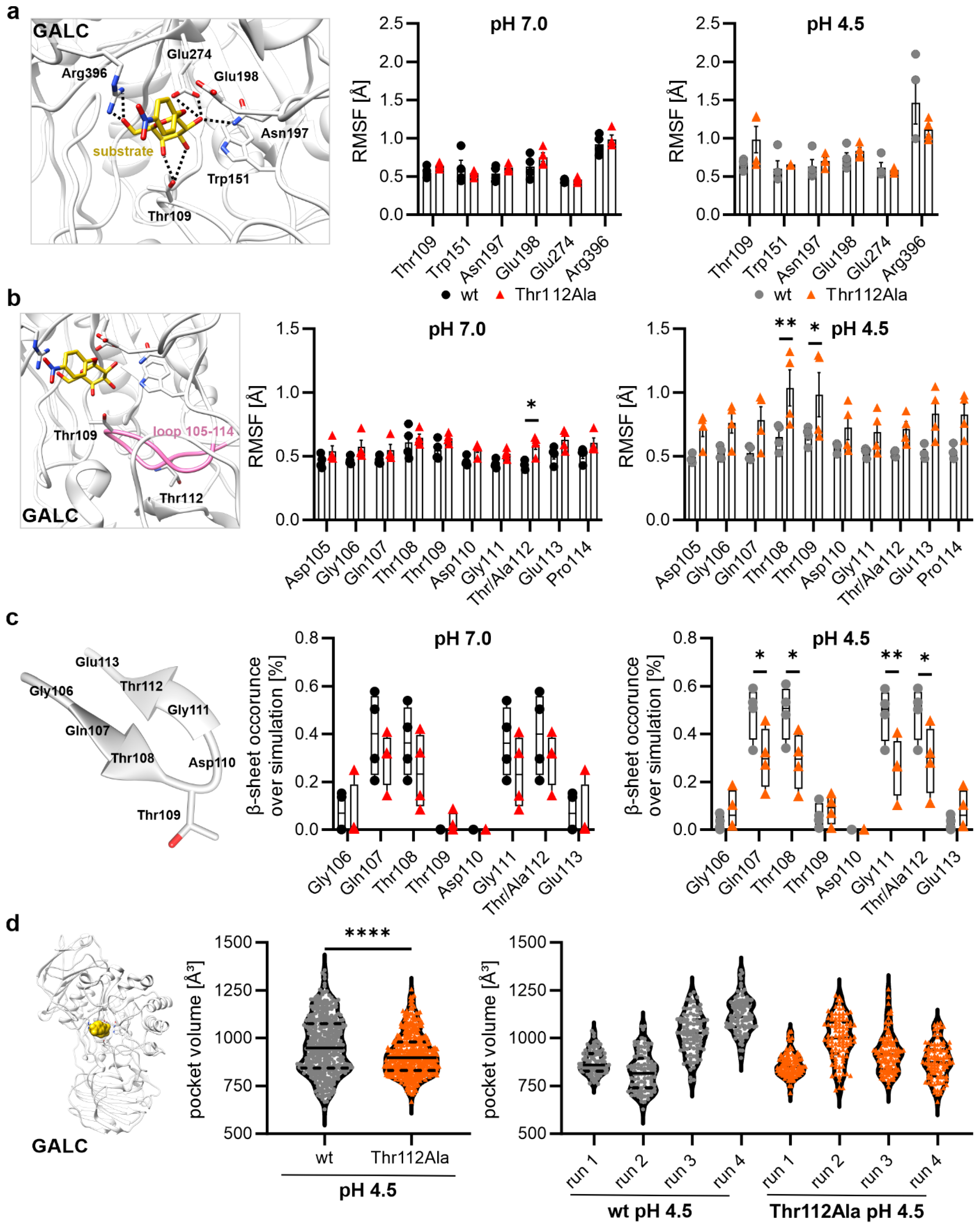
2.5. Influence of the Mutation Thr112Ala on Substrate-Binding Residues and Residues of the Active Site
2.6. Mutational Effect on the Size of the Binding Pocket at pH 4.5
3. Discussion
4. Materials and Methods
4.1. Homology Modeling of Human β-Galactocerebrosidase
4.2. Generation of the Starting Structures
4.3. Molecular Dynamics Simulations and Their Subsequent Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CPU | Central Processing Unit |
| GALC | β-galactocerebrosidase |
| GPU | Graphics Processing Unit |
| MD | Molecular Dynamics |
| RMSD | Root-Mean-Square Deviation |
| RMSF | Root-Mean-Square Fluctuation |
| SD | Standard Deviation |
| wt | Wild Type |
References
- Hill, C.H.; Graham, S.C.; Read, R.J.; Deane, J.E. Structural snapshots illustrate the catalytic cycle of β-galactocerebrosidase, the defective enzyme in Krabbe disease. Proc. Natl. Acad. Sci. USA 2013, 110, 20479–20484. [Google Scholar] [CrossRef]
- Wenger, D.A.; Rafi, M.A.; Luzi, P.; Datto, J.; Costantino-Ceccarini, E. Krabbe disease: Genetic aspects and progress toward therapy. Mol. Genet. Metab. 2000, 70, 1–9. [Google Scholar] [CrossRef]
- White, A.B.; Givogri, M.I.; Lopez-Rosas, A.; Cao, H.; van Breemen, R.; Thinakaran, G.; Bongarzone, E.R. Psychosine accumulates in membrane microdomains in the brain of krabbe patients, disrupting the raft architecture. J. Neurosci. 2009, 29, 6068–6077. [Google Scholar] [CrossRef] [PubMed]
- Sakai, N. Pathogenesis of leukodystrophy for Krabbe disease: Molecular mechanism and clinical treatment. Brain Dev. 2009, 31, 485–487. [Google Scholar] [CrossRef] [PubMed]
- Duffner, P.K.; Caviness, V.S.; Erbe, R.W.; Patterson, M.C.; Schultz, K.R.; Wenger, D.A.; Whitley, C. The long-term outcomes of presymptomatic infants transplanted for Krabbe disease: Report of the workshop held on July 11 and 12, 2008, Holiday Valley, New York. Genet. Med. 2009, 11, 450–454. [Google Scholar] [CrossRef]
- Weinstock, N.I.; Shin, D.; Dhimal, N.; Hong, X.; Irons, E.E.; Silvestri, N.J.; Reed, C.B.; Nguyen, D.; Sampson, O.; Cheng, Y.-C.; et al. Macrophages Expressing GALC Improve Peripheral Krabbe Disease by a Mechanism Independent of Cross-Correction. Neuron 2020, 107, 65–81.e9. [Google Scholar] [CrossRef]
- Rafi, M.A.; Rao, H.Z.; Luzi, P.; Curtis, M.T.; Wenger, D.A. Extended normal life after AAVrh10-mediated gene therapy in the mouse model of Krabbe disease. Mol. Ther. 2012, 20, 2031–2042. [Google Scholar] [CrossRef]
- Wenger, D.A.; Rafi, M.A.; Luzi, P. Krabbe disease: One Hundred years from the bedside to the bench to the bedside. J. Neurosci. Res. 2016, 94, 982–989. [Google Scholar] [CrossRef]
- Orsini, J.J.; Kay, D.M.; Saavedra-Matiz, C.A.; Wenger, D.A.; Duffner, P.K.; Erbe, R.W.; Biski, C.; Martin, M.; Krein, L.M.; Nichols, M.; et al. Newborn screening for Krabbe disease in New York State: The first eight years’ experience. Genet. Med. 2016, 18, 239–248. [Google Scholar] [CrossRef]
- Su, Y.; Wei, L.; Wang, L.; Xu, P.; Mo, M. Splicing mutations of GALC in adult patient with adult-onset Krabbe disease: Case report and review of literature. Neurocase 2024, 30, 63–67. [Google Scholar] [CrossRef]
- Zerkaoui, M.; Ratbi, I.; Castellotti, B.; Gellera, C.; Lyahyai, J.; Kriouile, Y.; Sefiani, A. Clinical and molecular report of novel GALC mutations in Moroccan patient with Krabbe disease: Case report. BMC Pediatr. 2015, 15, 182. [Google Scholar] [CrossRef]
- He, Z.; Pang, X.; Bai, J.; Wang, H.; Feng, F.; Du, R.; Huang, X. A novel GALC gene mutation associated with adult-onset Krabbe disease: A case report. Neurocase 2022, 28, 314–319. [Google Scholar] [CrossRef]
- Nicita, F.; Stregapede, F.; Deodato, F.; Pizzi, S.; Martinelli, S.; Pagliara, D.; Aiello, C.; Cumbo, F.; Piemonte, F.; D’Amico, J.; et al. “Atypical” Krabbe disease in two siblings harboring biallelic GALC mutations including a deep intronic variant. Eur. J. Hum. Genet. 2022, 30, 984–988. [Google Scholar] [CrossRef] [PubMed]
- Nashabat, M.; Al-Khenaizan, S.; Alfadhel, M. Report of a Case that Expands the Phenotype of Infantile Krabbe Disease. Am. J. Case Rep. 2019, 20, 643–646. [Google Scholar] [CrossRef] [PubMed]
- Luzi, P.; Rafi, M.A.; Wenger, D.A. Multiple mutations in the GALC gene in a patient with adult-onset Krabbe disease. Ann. Neurol. 1996, 40, 116–119. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.-H.; Choquet, K.; La Piana, R.; Tétreault, M.; Dicaire, M.-J.; Boycott, K.M.; Majewski, J.; Brais, B. Mutations in GALC cause late-onset Krabbe disease with predominant cerebellar ataxia. Neurogenetics 2016, 17, 137–141. [Google Scholar] [CrossRef]
- Tuncer, F.N.; Iseri, S.A.U.; Yapici, Z.; Demir, M.; Karaca, M.; Calik, M. A novel homozygous GALC variant has been associated with Krabbe disease in a consanguineous family. Neurol. Sci. 2018, 39, 2123–2128. [Google Scholar] [CrossRef]
- Hwang, N.; Kim, S.-M.; Kim, Y.-G.; Ha, C.; Lee, J.; Choi, B.-O.; Sung, W.J.; Kim, S.H.; Kim, Y.M.; Lee, Y.-W.; et al. Clinical feature, GALC variant spectrum, and genotype-phenotype correlation in Korean Krabbe disease patients: Multicenter experience over 13 years. Clin. Genet. 2024, 106, 150–160. [Google Scholar] [CrossRef]
- Deane, J.E.; Graham, S.C.; Kim, N.N.; Stein, P.E.; McNair, R.; Cachón-González, M.B.; Cox, T.M.; Read, R.J. Insights into Krabbe disease from structures of galactocerebrosidase. Proc. Natl. Acad. Sci. USA 2011, 108, 15169–15173. [Google Scholar] [CrossRef]
- Hill, C.H.; Viuff, A.H.; Spratley, S.J.; Salamone, S.; Christensen, S.H.; Read, R.J.; Moriarty, N.W.; Jensen, H.H.; Deane, J.E. Azasugar inhibitors as pharmacological chaperones for Krabbe disease. Chem. Sci. 2015, 6, 3075–3086. [Google Scholar] [CrossRef]
- Hill, C.H.; Cook, G.M.; Spratley, S.J.; Fawke, S.; Graham, S.C.; Deane, J.E. The mechanism of glycosphingolipid degradation revealed by a GALC-SapA complex structure. Nat. Commun. 2018, 9, 151. [Google Scholar] [CrossRef]
- Mächtel, R.; Dobert, J.-P.; Hehr, U.; Weiss, A.; Kettwig, M.; Laugwitz, L.; Groeschel, S.; Schmidt, M.; Arnold, P.; Regensburger, M.; et al. Late-onset Krabbe disease presenting as spastic paraplegia—Implications of GCase and CTSB/D. Ann. Clin. Transl. Neurol. 2024, 11, 1715–1731. [Google Scholar] [CrossRef]
- Lee, W.C.; Kang, D.; Causevic, E.; Herdt, A.R.; Eckman, E.A.; Eckman, C.B. Molecular characterization of mutations that cause globoid cell leukodystrophy and pharmacological rescue using small molecule chemical chaperones. J. Neurosci. 2010, 30, 5489–5497. [Google Scholar] [CrossRef] [PubMed]
- Lissens, W.; Arena, A.; Seneca, S.; Rafi, M.; Sorge, G.; Liebaers, I.; Wenger, D.; Fiumara, A. A single mutation in the GALC gene is responsible for the majority of late onset Krabbe disease patients in the Catania (Sicily, Italy) region. Hum. Mutat. 2007, 28, 742. [Google Scholar] [CrossRef] [PubMed]
- Tappino, B.; Biancheri, R.; Mort, M.; Regis, S.; Corsolini, F.; Rossi, A.; Stroppiano, M.; Lualdi, S.; Fiumara, A.; Bembi, B.; et al. Identification and characterization of 15 novel GALC gene mutations causing Krabbe disease. Hum. Mutat. 2010, 31, E1894–E1914. [Google Scholar] [CrossRef] [PubMed]
- Madsen, A.M.H.; Wibrand, F.; Lund, A.M.; Ek, J.; Dunø, M.; Østergaard, E. Genotype and phenotype classification of 29 patients affected by Krabbe disease. JIMD Rep. 2019, 46, 35–45. [Google Scholar] [CrossRef]
- Zhong, J.; Jiang, F.; Yang, H.; Li, J.; Cheng, J.; Zeng, Q.; Xu, Q. Novel GALC Mutations Cause Adult-Onset Krabbe Disease With Myelopathy in Two Chinese Families: Case Reports and Literature Review. Front. Neurol. 2020, 11, 830. [Google Scholar] [CrossRef]
- Feo, F.; Tramacere, L.; Ramat, S.; Govoni, A.; Caremani, L.; Grigioni, G.; Mei, D.; Falliano, S.; Marin, F.; Ferri, L.; et al. High Prevalence of GALC Gene Variants in Adults With Neurodegenerative Conditions. Eur. J. Neurol. 2025, 32, e70206. [Google Scholar] [CrossRef]
- Anania, M.; Giacomarra, M.; D’Errico, A.; Marano, M.; Tartaglione, I.; Spada, M.; Pagliardini, V.; de Paolis, M.R.; Giuffrida, G.; Duro, G.; et al. Identification of Novel Mutations in Patients Affected by Gaucher Disease. Int. J. Mol. Sci. 2025, 26, 3918. [Google Scholar] [CrossRef]
- La Russa, A.; Siniscalchi, A.; Bonaventura, A.; Di Noia, D.; Valsania, T.; Stallone, G.; Tartaglia, L.; Chiapparino, C.; Di Rienzo, G.; Coppolino, G.; et al. Potential Pathogenetic Role of the D313Y Mutation in the GLA Gene in Anderson Fabry Disease: Two Case Reports. Int. J. Mol. Sci. 2025, 26, 4400. [Google Scholar] [CrossRef]
- Aghamahdi, F.; Nirouei, M.; Savad, S. Niemann-Pick type A disease with new mutation: A case report. J. Med. Case Rep. 2022, 16, 288. [Google Scholar] [CrossRef]
- Zhou, Z.-W.; Wang, S.-H.; Xu, C.-A.; Wu, W.-H.; Hui, T.-C.; Yin, Q.-Q.; Zheng, W.; Pan, H.-Y. Three-years misdiagnosis of Niemann Pick disease type B with novel mutations in SMPD1 gene as Budd-Chiari syndrome. BMC Med. Genom. 2022, 15, 196. [Google Scholar] [CrossRef]
- Viuff, A.; Salamone, S.; McLoughlin, J.; Deane, J.E.; Jensen, H.H. The Bicyclic Form of galacto-Noeurostegine Is a Potent Inhibitor of β-Galactocerebrosidase. ACS Med. Chem. Lett. 2021, 12, 56–59. [Google Scholar] [CrossRef] [PubMed]
- Schulze, M.-S.E.D.; Scholz, D.; Jnoff, E.; Hall, A.; Melin, J.; Sands, Z.A.; Rodriguez, E.; Andre, V.M. Identification of ß-Glucocerebrosidase Activators for Glucosylceramide hydrolysis. ChemMedChem 2024, 19, e202300548. [Google Scholar] [CrossRef] [PubMed]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
- Guex, N.; Peitsch, M.C. SWISS-MODEL and the Swiss-PdbViewer: An environment for comparative protein modeling. Electrophoresis 1997, 18, 2714–2723. [Google Scholar] [CrossRef]
- Jurrus, E.; Engel, D.; Star, K.; Monson, K.; Brandi, J.; Felberg, L.E.; Brookes, D.H.; Wilson, L.; Chen, J.; Liles, K.; et al. Improvements to the APBS biomolecular solvation software suite. Protein Sci. 2018, 27, 112–128. [Google Scholar] [CrossRef]
- Socher, E.; Sticht, H.; Horn, A.H.C. The conformational stability of nonfibrillar amyloid-β peptide oligomers critically depends on the C-terminal peptide length. ACS Chem. Neurosci. 2014, 5, 161–167. [Google Scholar] [CrossRef]
- Socher, E.; Conrad, M.; Heger, L.; Paulsen, F.; Sticht, H.; Zunke, F.; Arnold, P. Mutations in the B.1.1.7 SARS-CoV-2 Spike Protein Reduce Receptor-Binding Affinity and Induce a Flexible Link to the Fusion Peptide. Biomedicines 2021, 9, 525. [Google Scholar] [CrossRef]
- Socher, E.; Conrad, M.; Heger, L.; Paulsen, F.; Sticht, H.; Zunke, F.; Arnold, P. Computational decomposition reveals reshaping of the SARS-CoV-2-ACE2 interface among viral variants expressing the N501Y mutation. J. Cell. Biochem. 2021, 122, 1863–1872. [Google Scholar] [CrossRef]
- Socher, E.; Heger, L.; Paulsen, F.; Zunke, F.; Arnold, P. Molecular dynamics simulations of the delta and omicron SARS-CoV-2 spike—ACE2 complexes reveal distinct changes between both variants. Comput. Struct. Biotechnol. J. 2022, 20, 1168–1176. [Google Scholar] [CrossRef] [PubMed]
- Case, D.A.; Belfon, K.; Ben-Shalom, I.Y.; Brozell, S.R.; Cerutti, D.S.; Cheatham, T.E., III; Cruzeiro, V.W.D.; Darden, T.A.; Duke, R.E.; Giambasu, G.; et al. AMBER 2020. Available online: https://ambermd.org/doc12/Amber20.pdf (accessed on 15 January 2022).
- Maier, J.A.; Martinez, C.; Kasavajhala, K.; Wickstrom, L.; Hauser, K.E.; Simmerling, C. ff14SB: Improving the Accuracy of Protein Side Chain and Backbone Parameters from ff99SB. J. Chem. Theory Comput. 2015, 11, 3696–3713. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Chandrasekhar, J.; Madura, J.D.; Impey, R.W.; Klein, M.L. Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 1983, 79, 926–935. [Google Scholar] [CrossRef]
- Götz, A.W.; Williamson, M.J.; Xu, D.; Poole, D.; Le Grand, S.; Walker, R.C. Routine Microsecond Molecular Dynamics Simulations with AMBER on GPUs. 1. Generalized Born. J. Chem. Theory Comput. 2012, 8, 1542–1555. [Google Scholar] [CrossRef]
- Salomon-Ferrer, R.; Götz, A.W.; Poole, D.; Le Grand, S.; Walker, R.C. Routine Microsecond Molecular Dynamics Simulations with AMBER on GPUs. 2. Explicit Solvent Particle Mesh Ewald. J. Chem. Theory Comput. 2013, 9, 3878–3888. [Google Scholar] [CrossRef]
- Le Grand, S.; Götz, A.W.; Walker, R.C. SPFP: Speed without compromise—A mixed precision model for GPU accelerated molecular dynamics simulations. Comput. Phys. Commun. 2013, 184, 374–380. [Google Scholar] [CrossRef]
- Berendsen, H.J.C.; Postma, J.P.M.; van Gunsteren, W.F.; DiNola, A.; Haak, J.R. Molecular dynamics with coupling to an external bath. J. Chem. Phys. 1984, 81, 3684–3690. [Google Scholar] [CrossRef]
- Ryckaert, J.-P.; Ciccotti, G.; Berendsen, H.J. Numerical integration of the cartesian equations of motion of a system with constraints: Molecular dynamics of n-alkanes. J. Comput. Phys. 1977, 23, 327–341. [Google Scholar] [CrossRef]
- Roe, D.R.; Cheatham, T.E. PTRAJ and CPPTRAJ: Software for Processing and Analysis of Molecular Dynamics Trajectory Data. J. Chem. Theory Comput. 2013, 9, 3084–3095. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heger, L.; Ankermann, P.; Socher, E. Molecular Characterization of the GALC Mutation Thr112Ala Causing Krabbe Disease. Int. J. Mol. Sci. 2025, 26, 8647. https://doi.org/10.3390/ijms26178647
Heger L, Ankermann P, Socher E. Molecular Characterization of the GALC Mutation Thr112Ala Causing Krabbe Disease. International Journal of Molecular Sciences. 2025; 26(17):8647. https://doi.org/10.3390/ijms26178647
Chicago/Turabian StyleHeger, Lukas, Piet Ankermann, and Eileen Socher. 2025. "Molecular Characterization of the GALC Mutation Thr112Ala Causing Krabbe Disease" International Journal of Molecular Sciences 26, no. 17: 8647. https://doi.org/10.3390/ijms26178647
APA StyleHeger, L., Ankermann, P., & Socher, E. (2025). Molecular Characterization of the GALC Mutation Thr112Ala Causing Krabbe Disease. International Journal of Molecular Sciences, 26(17), 8647. https://doi.org/10.3390/ijms26178647





