Genome-Wide Identification of Autophagy-Related Gene Family and Gene Expression Analysis of the CmATG8 Under Heat Stress in Chrysanthemum
Abstract
1. Introduction
2. Results
2.1. Gene Family Identification and Physicochemical Property Analysis
2.2. Phylogenetic Analysis
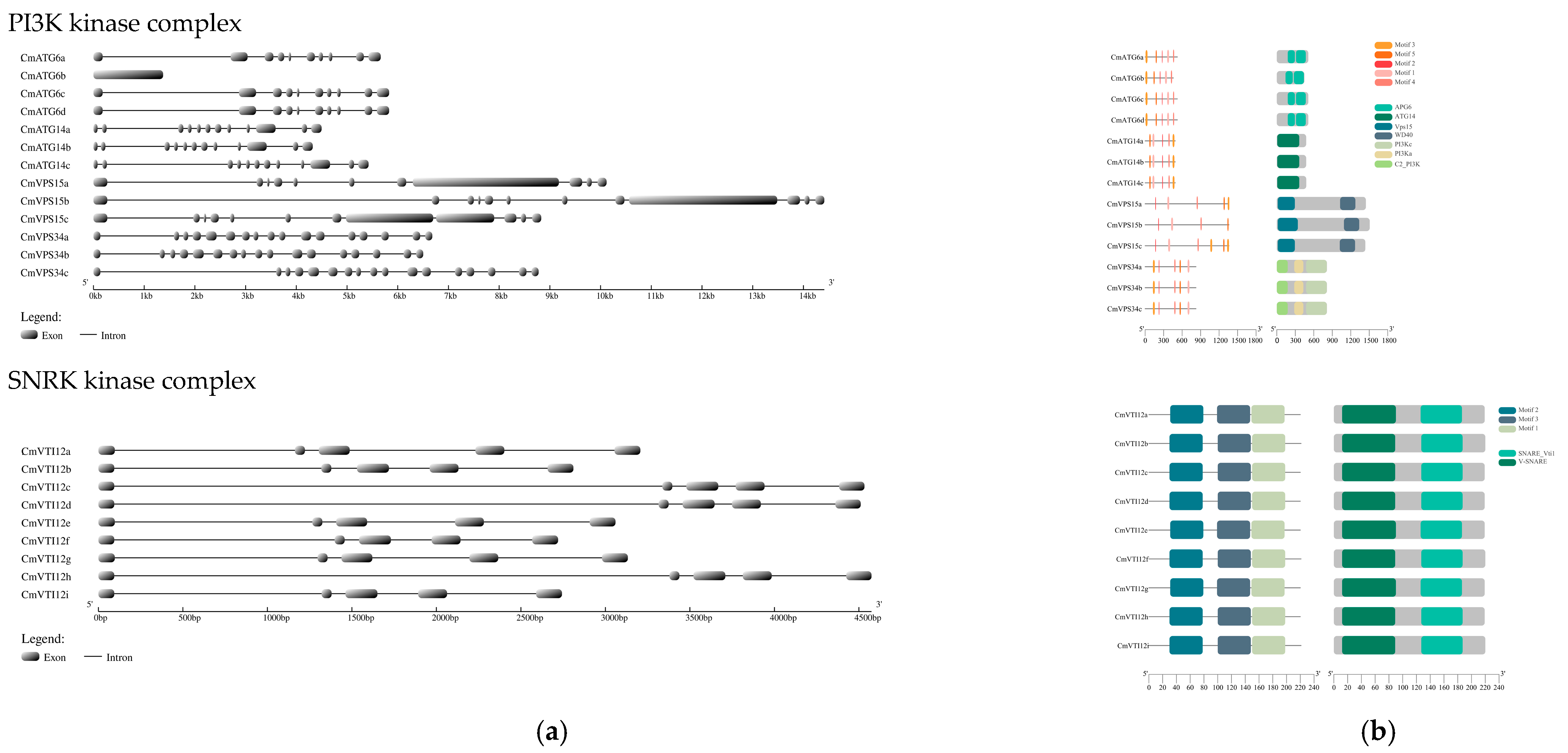
2.3. Gene Structure, Conserved Motif, and Domain Analysis of the ATG Gene Family
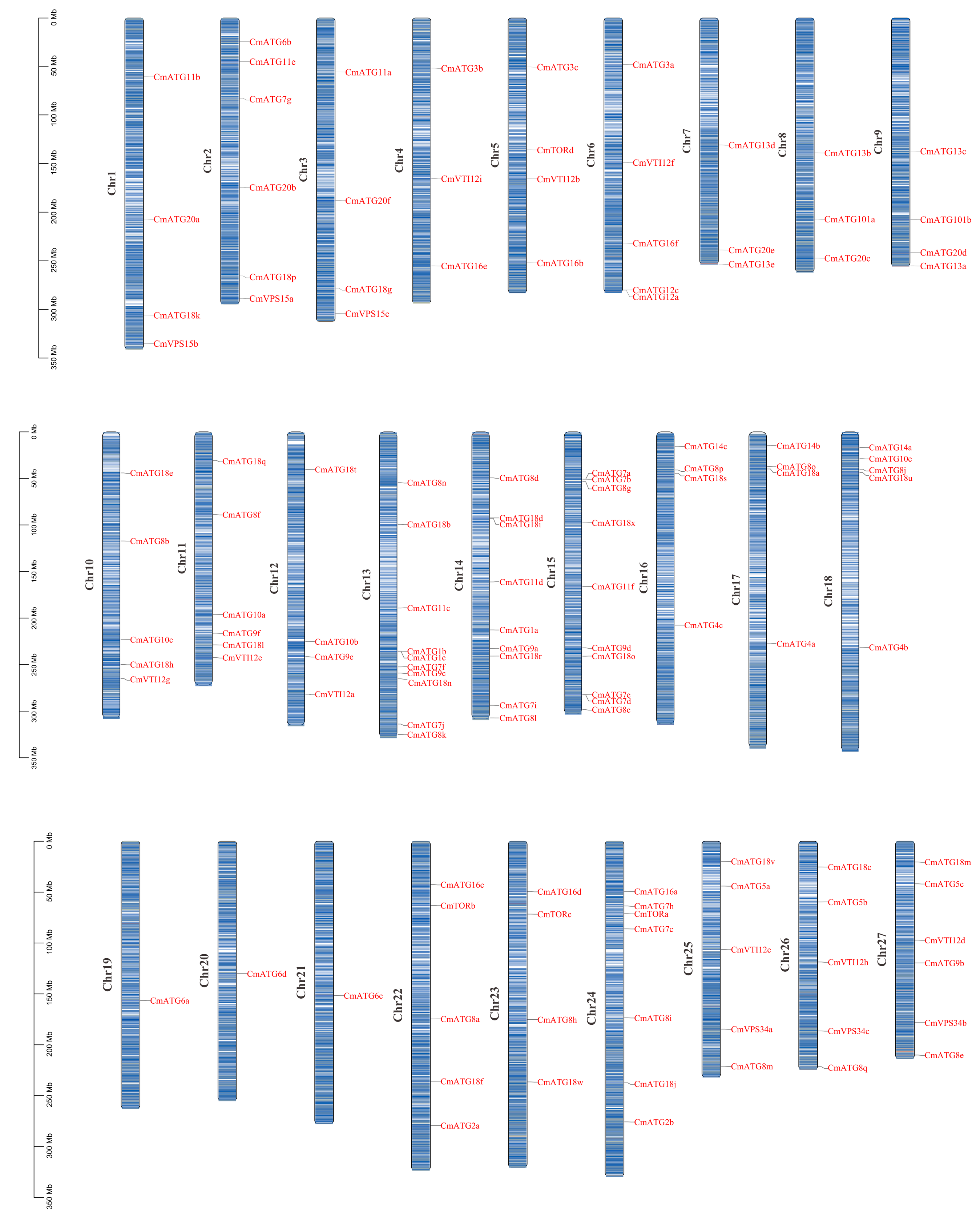
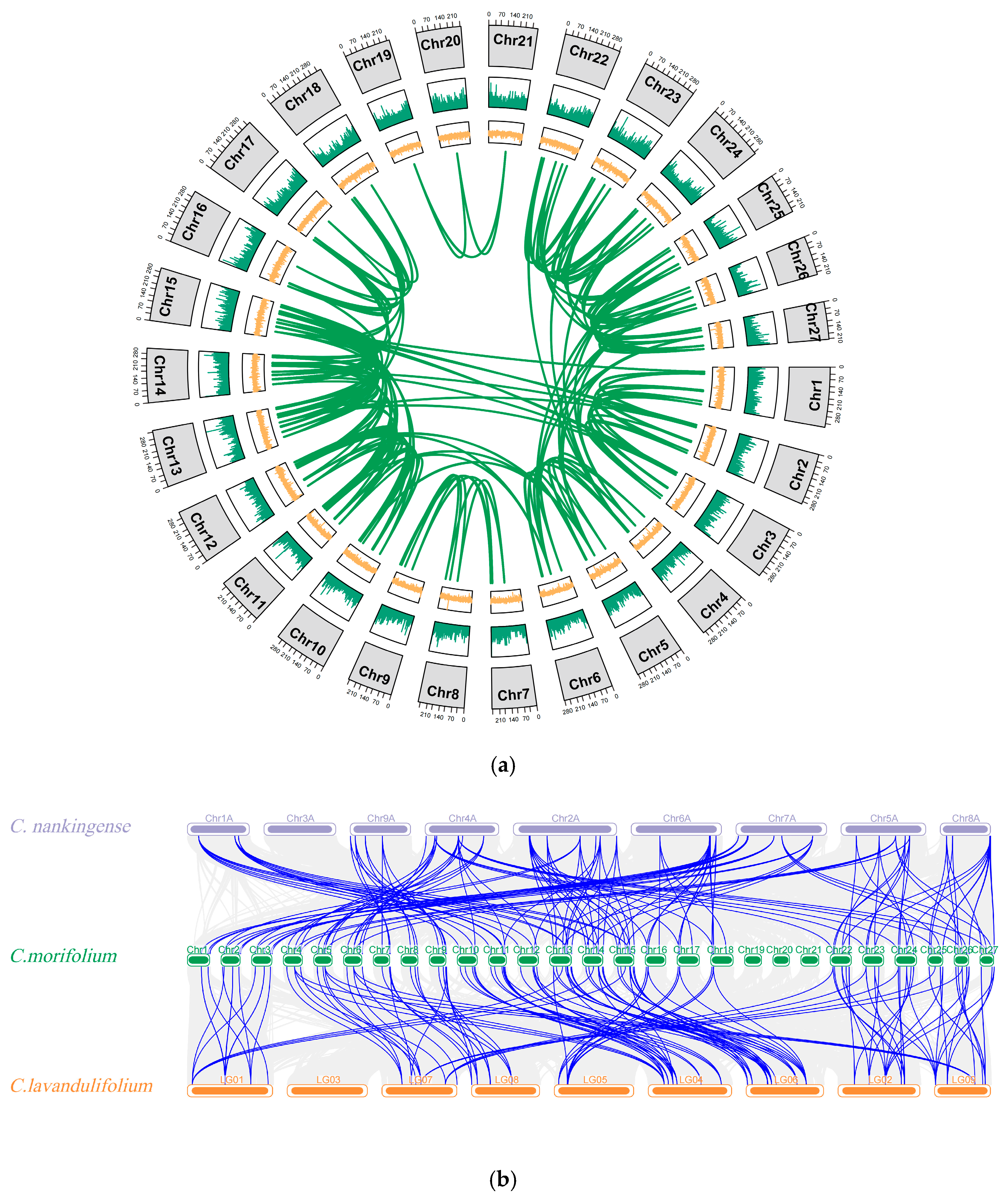
2.4. Chromosomal Localization and Synteny Analysis of ATG Gene Family Members
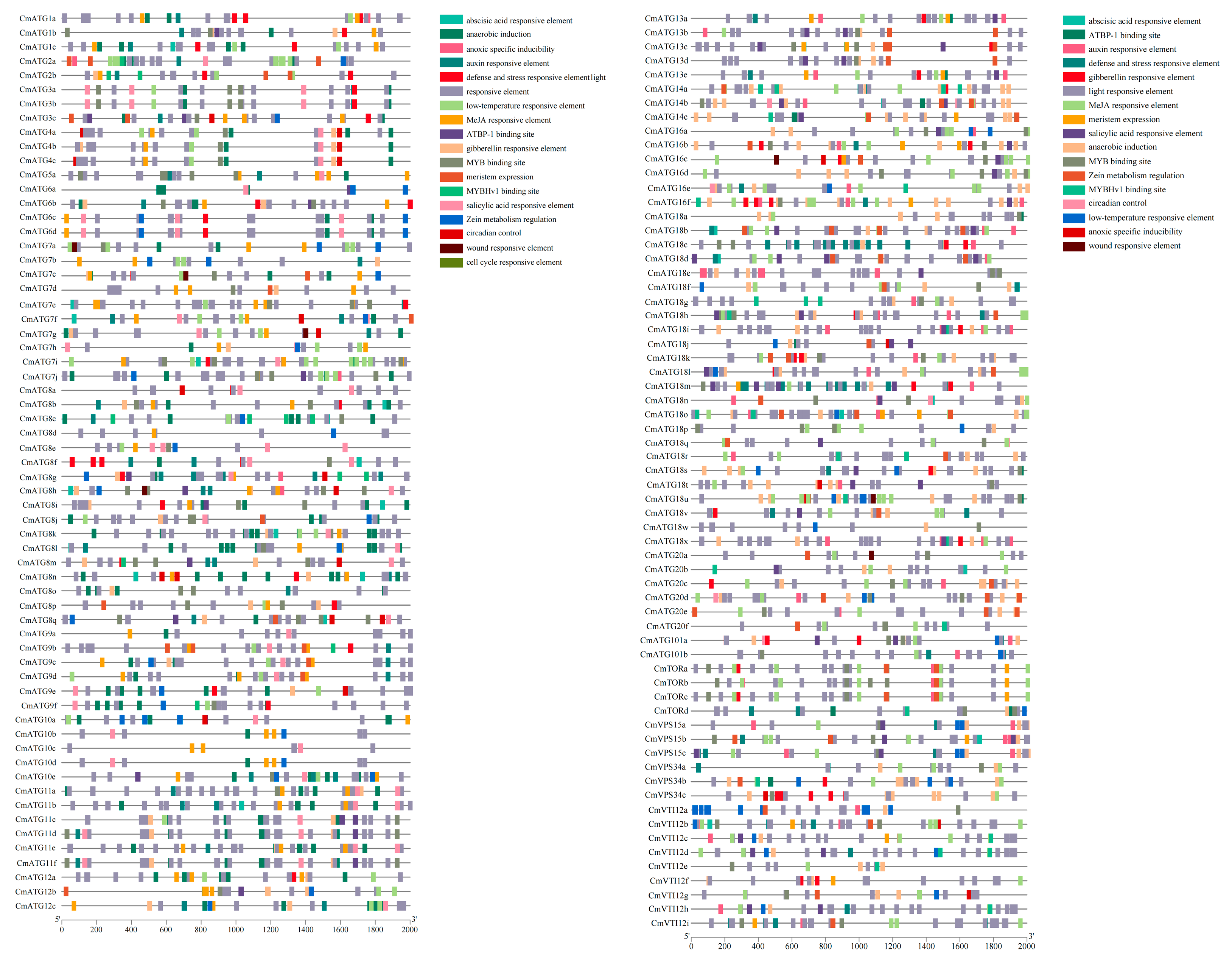
2.5. Analysis of Cis-Regulatory Elements in the Promoters of the Chrysanthemum ATG Gene Family
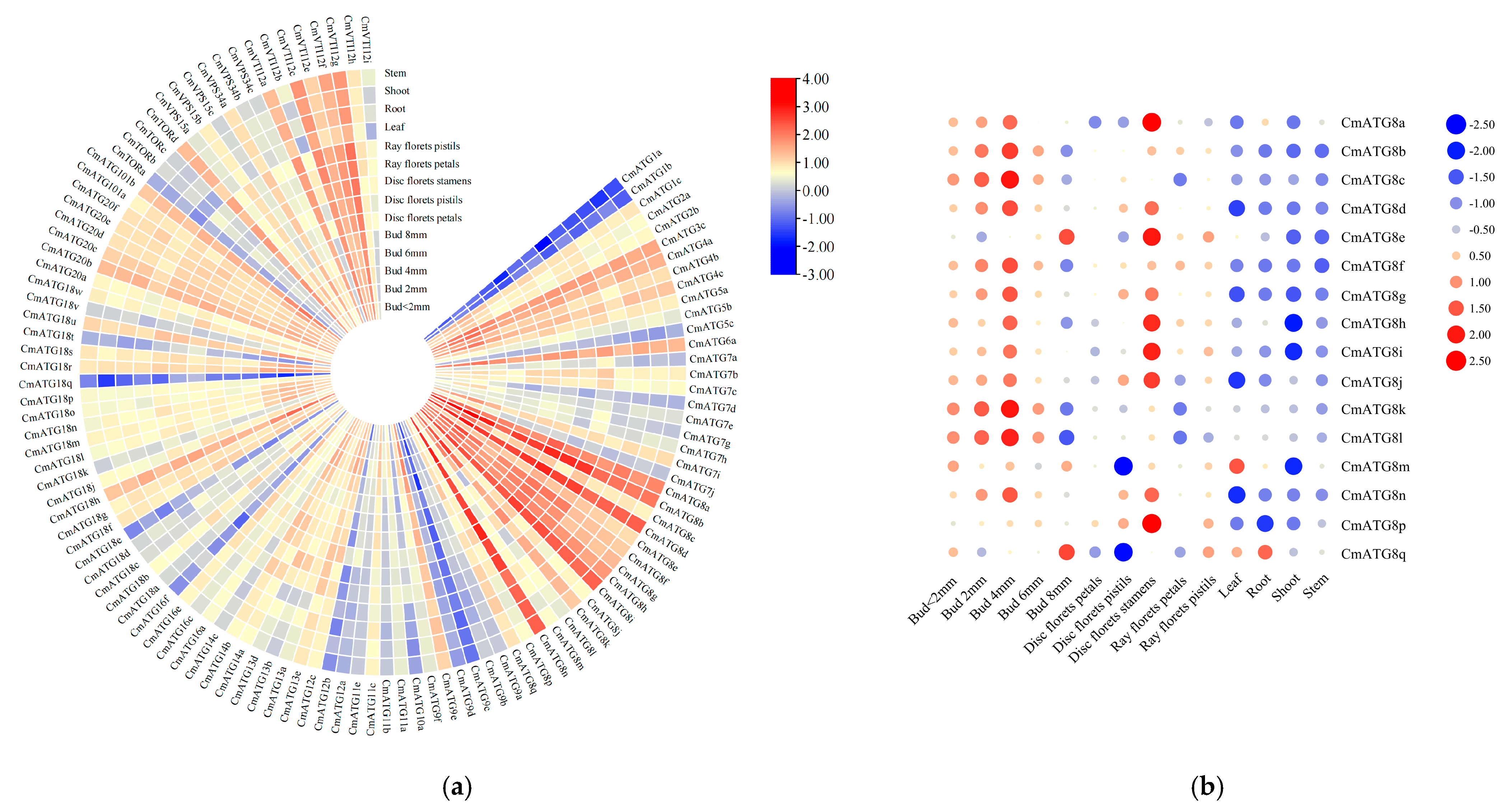
2.6. Tissue-Specific Expression of the Chrysanthemum ATG Gene Family
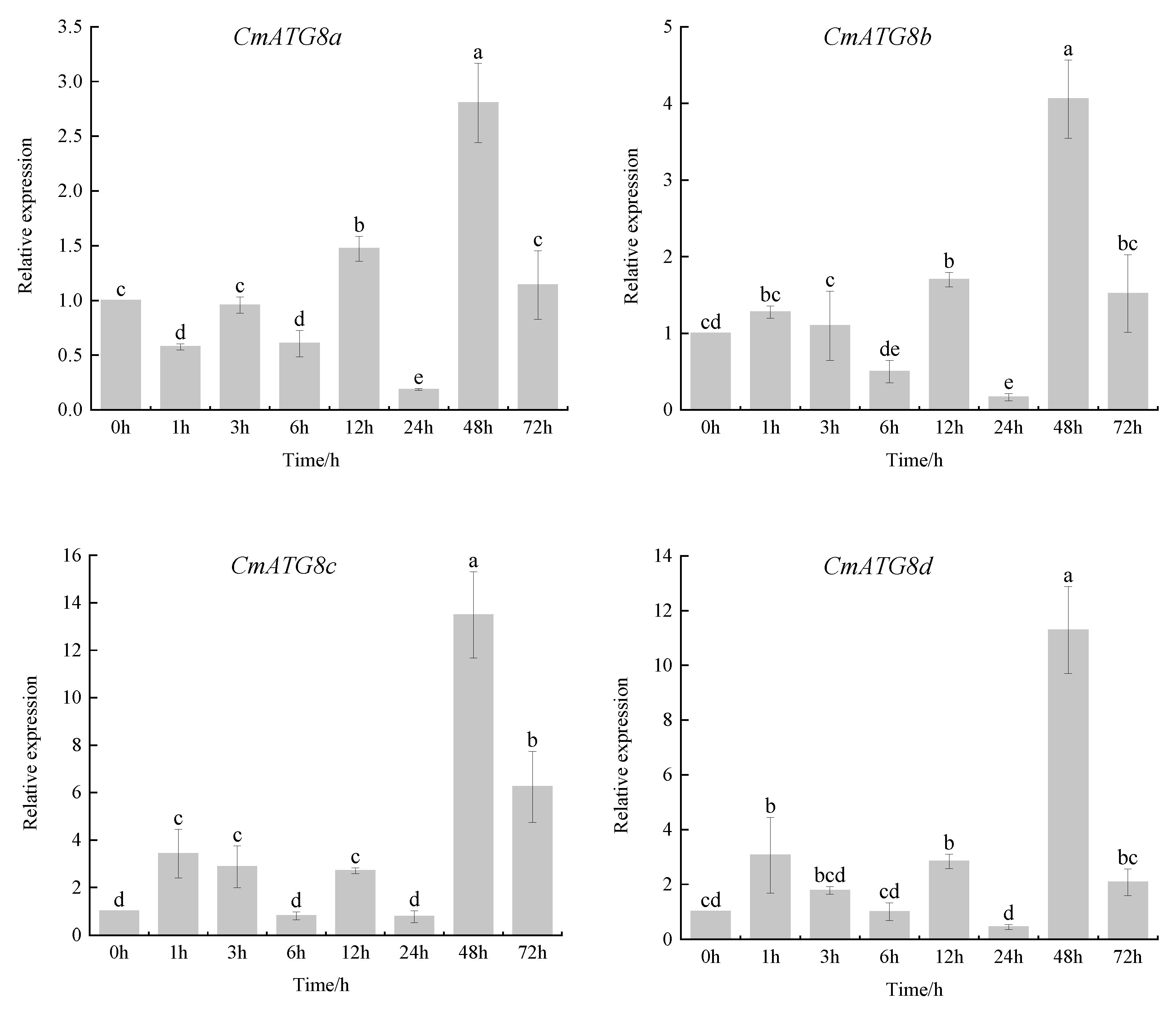
2.7. Expression Analysis of CmATG8 Subfamily in Response to Heat Stress
3. Discussion
4. Materials and Methods
4.1. Identification of ATG Gene Family Members
4.2. Phylogenetic Tree Construction of ATG Gene Family
4.3. Gene Structure, Conserved Motif, and Domain Analysis of ATG Gene Family
4.4. Chromosomal Localization and Synergy Analysis of ATG Gene Family Members
4.5. Analysis of Cis-Regulatory Elements in the Promoters of the Chrysanthemum ATG Gene Family
4.6. Tissue-Specific Expression of the Chrysanthemum ATG Gene Family
4.7. Expression Analysis of CmATG8 Subfamily in Response to Heat Stress
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yagyu, M.; Yoshimoto, K. New insights into plant autophagy: Molecular mechanisms and roles in development and stress responses. J. Exp. Bot. 2024, 75, 1234–1251. [Google Scholar] [CrossRef] [PubMed]
- Marshall, R.S.; Vierstra, R.D. Autophagy: The Master of Bulk and Selective Recycling. Annu. Rev. Plant Biol. 2018, 69, 173–208. [Google Scholar] [CrossRef]
- Oku, M.; Sakai, Y. Three Distinct Types of Microautophagy Based on Membrane Dynamics and Molecular Machineries. Bioessays 2018, 40, e1800008. [Google Scholar] [CrossRef]
- Antonioli, M.; Di Rienzo, M.; Piacentini, M.; Fimia, G.M. Emerging Mechanisms in Initiating and Terminating Autophagy. Trends Biochem. Sci. 2017, 42, 28–41. [Google Scholar] [CrossRef]
- Li, F.; Chung, T.; Vierstra, R.D. AUTOPHAGY-RELATED11 plays a critical role in general autophagy- and senescence-induced mitophagy in Arabidopsis. Plant Cell 2014, 26, 788–807. [Google Scholar] [CrossRef]
- Li, Y.B.; Cui, D.Z.; Sui, X.X.; Huang, C.; Huang, C.Y.; Fan, Q.Q.; Chu, X.S. Autophagic Survival Precedes Programmed Cell Death in Wheat Seedlings Exposed to Drought Stress. Int. J. Mol. Sci. 2019, 20, 5777. [Google Scholar] [CrossRef]
- Chen, X.; He, Y.; Wu, Z.; Lu, X.; Yin, Z.; Zhao, L.; Huang, H.; Meng, Y.; Fan, Y.; Guo, L.; et al. Systematic analysis and expression of Gossypium ATG8 family reveals the roles of GhATG8f responding to salt stress in cotton. Plant Cell Rep. 2024, 43, 58. [Google Scholar] [CrossRef]
- Huo, L.; Sun, X.; Guo, Z.; Jia, X.; Che, R.; Sun, Y.; Zhu, Y.; Wang, P.; Gong, X.; Ma, F. MdATG18a overexpression improves basal thermotolerance in transgenic apple by decreasing damage to chloroplasts. Hortic. Res. 2020, 7, 21. [Google Scholar] [CrossRef]
- Yu, X.Q.; Su, W.; Zhang, H.; Niu, M.; Liu, X.; Li, Z.; Liu, C.; Wang, H.L.; Yin, W.; Xia, X. Genome-wide analysis of autophagy-related gene family and PagATG18a enhances salt tolerance by regulating ROS homeostasis in poplar. Int. J. Biol. Macromol. 2023, 224, 1524–1540. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Wang, J.; Cheng, Y.; Chi, Y.J.; Fan, B.; Yu, J.Q.; Chen, Z. Correction: NBR1-Mediated Selective Autophagy Targets Insoluble Ubiquitinated Protein Aggregates in Plant Stress Responses. PLOS Genet. 2014, 10, e1004477. [Google Scholar] [CrossRef] [PubMed]
- Minina, E.A.; Moschou, P.N.; Vetukuri, R.R.; Sanchez-Vera, V.; Cardoso, C.; Liu, Q.; Elander, P.H.; Dalman, K.; Beganovic, M.; Yilmaz, J.L.; et al. Transcriptional stimulation of rate-limiting components of the autophagic pathway improves plant fitness. J. Exp. Bot. 2018, 69, 1415–1432. [Google Scholar] [CrossRef]
- Xia, J.; Wang, Z.; Liu, S.; Fang, X.; Hakeem, A.; Fang, J.; Shangguan, L. VvATG6 contributes to copper stress tolerance by enhancing the antioxidant ability in transgenic grape calli. Physiol. Mol. Biol. Plants 2024, 30, 137–152. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cai, S.; Yin, L.; Shi, K.; Xia, X.; Zhou, Y.; Yu, J.; Zhou, J. Tomato HsfA1a plays a critical role in plant drought tolerance by activating ATG genes and inducing autophagy. Autophagy 2015, 11, 2033–2047. [Google Scholar] [CrossRef]
- Liu, Y.; Burgos, J.S.; Deng, Y.; Srivastava, R.; Howell, S.H.; Bassham, D.C. Degradation of the endoplasmic reticulum by autophagy during endoplasmic reticulum stress in Arabidopsis. Plant Cell 2012, 24, 4635–4651. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Srivastava, R.; Howell, S.H.; Bassham, D.C. Activation of autophagy by unfolded proteins during endoplasmic reticulum stress. Plant J. 2016, 85, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Bu, F.; Yang, M.; Guo, X.; Huang, W.; Chen, L. Multiple Functions of ATG8 Family Proteins in Plant Autophagy. Front. Cell Dev. Biol. 2020, 8, 466. [Google Scholar] [CrossRef]
- Wang, P.; Nolan, T.M.; Yin, Y.; Bassham, D.C. Identification of transcription factors that regulate ATG8 expression and autophagy in Arabidopsis. Autophagy 2020, 16, 123–139. [Google Scholar] [CrossRef]
- Zou, Y.; Ohlsson, J.A.; Holla, S.; Sabljić, I.; Leong, J.X.; Ballhaus, F.; Krebs, M.; Schumacher, K.; Moschou, P.N.; Stael, S.; et al. ATG8 delipidation is not universally critical for autophagy in plants. Nat. Commun. 2025, 16, 403. [Google Scholar] [CrossRef]
- Chen, Q.; Soulay, F.; Saudemont, B.; Elmayan, T.; Marmagne, A.; Masclaux-Daubresse, C.L. Overexpression of ATG8 in Arabidopsis Stimulates Autophagic Activity and Increases Nitrogen Remobilization Efficiency and Grain Filling. Plant Cell Physiol. 2019, 60, 343–352. [Google Scholar] [CrossRef]
- González-Fuente, M.; Üstün, S. ATG8 keeps Golgi in shape after the heat. Nat. Plants 2023, 9, 685–686. [Google Scholar] [CrossRef]
- Zhou, J.; Ma, J.; Yang, C.; Zhu, X.; Li, J.; Zheng, X.; Li, X.; Chen, S.; Feng, L.; Wang, P.; et al. A non-canonical role of ATG8 in Golgi recovery from heat stress in plants. Nat. Plants 2023, 9, 749–765. [Google Scholar] [CrossRef]
- Jung, H.; Lee, H.N.; Marshall, R.S.; Lomax, A.W.; Yoon, M.J.; Kim, J.; Kim, J.H.; Vierstra, R.D.; Chung, T. Arabidopsis cargo receptor NBR1 mediates selective autophagy of defective proteins. J. Exp. Bot. 2020, 71, 73–89. [Google Scholar] [CrossRef]
- Zhai, Y.; Guo, M.; Wang, H.; Lu, J.; Liu, J.; Zhang, C.; Gong, Z.; Lu, M. Autophagy, a Conserved Mechanism for Protein Degradation, Responds to Heat, and Other Abiotic Stresses in Capsicum annuum L. Front. Plant Sci. 2016, 7, 131. [Google Scholar] [CrossRef]
- Song, L.; Wen, C.; He, Z.; Zha, X.; Cheng, Q.; Xu, W. Overexpression of SlATG8f gene enhanced autophagy and pollen protection in tomato under heat stress. Sci. Rep. 2024, 14, 26892. [Google Scholar] [CrossRef]
- Song, Z.; Jiang, J.; Zhang, Y.; Chen, P.; Zhang, K.; Li, F.; Li, Y. Comparative transcriptomic profiling of heat-tolerant and heat-sensitive Chrysanthemum morifolium cultivars in response to heat stress. Sci. Hortic. 2025, 350, 114305. [Google Scholar] [CrossRef]
- Shi, Z.; Han, X.; Wang, G.; Qiu, J.; Zhou, L.J.; Chen, S.; Fang, W.; Chen, F.; Jiang, J. Transcriptome analysis reveals chrysanthemum flower discoloration under high-temperature stress. Front. Plant Sci. 2022, 13, 1003635. [Google Scholar] [CrossRef] [PubMed]
- Song, A.; Su, J.; Wang, H.; Zhang, Z.; Zhang, X.; Van de Peer, Y.; Chen, F.; Fang, W.; Guan, Z.; Zhang, F.; et al. Analyses of a chromosome-scale genome assembly reveal the origin and evolution of cultivated chrysanthemum. Nat. Commun. 2023, 14, 2021. [Google Scholar] [CrossRef]
- Han, S.; Yu, B.; Wang, Y.; Liu, Y. Role of plant autophagy in stress response. Protein Cell 2011, 2, 784–791. [Google Scholar] [CrossRef] [PubMed]
- Xia, K.; Liu, T.; Ouyang, J.; Wang, R.; Fan, T.; Zhang, M. Genome-wide identification, classification, and expression analysis of autophagy-associated gene homologues in rice (Oryza sativa L.). DNA Res. 2011, 18, 363–377. [Google Scholar] [CrossRef]
- Huang, W.; Ma, D.N.; Liu, H.L.; Luo, J.; Wang, P.; Wang, M.L.; Guo, F.; Wang, Y.; Zhao, H.; Ni, D.J. Genome-Wide Identification of CsATGs in Tea Plant and the Involvement of CsATG8e in Nitrogen Utilization. Int. J. Mol. Sci. 2020, 21, 7043. [Google Scholar] [CrossRef]
- Yue, W.; Nie, X.; Cui, L.; Zhi, Y.; Zhang, T.; Du, X.; Song, W. Genome-wide sequence and expressional analysis of autophagy Gene family in bread wheat (Triticum aestivum L.). J. Plant Physiol. 2018, 229, 7–21. [Google Scholar] [CrossRef]
- Yang, M.; Wang, L.; Chen, C.; Guo, X.; Lin, C.; Huang, W.; Chen, L. Genome-wide analysis of autophagy-related genes in Medicago truncatula highlights their roles in seed development and response to drought stress. Sci. Rep. 2021, 11, 22933. [Google Scholar] [CrossRef]
- Zhou, X.M.; Zhao, P.; Wang, W.; Zou, J.; Cheng, T.H.; Peng, X.B.; Sun, M.X. A comprehensive, genome-wide analysis of autophagy-related genes identified in tobacco suggests a central role of autophagy in plant response to various environmental cues. DNA Res. 2015, 22, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Wang, S.; Wu, H.; Li, C.; Zhao, H.; Chen, H.; Wang, X.; Wu, Q. Genome-Wide Identification of ATG Gene Family Members in Fagopyrum tataricum and Their Expression during Stress Responses. Int. J. Mol. Sci. 2022, 23, 14845. [Google Scholar] [CrossRef] [PubMed]
- Jia, R.; Zhou, R.; Chang, Y.; Wei, L.; Yi, L.; Ma, B.; Shi, S. Genome-Wide and Transcriptome Analysis of Autophagy-Related ATG Gene Family and Their Response to Low-Nitrogen Stress in Sugar Beet. Int. J. Mol. Sci. 2024, 25, 11932. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Wu, Y.; Li, M.; Luo, N.; Li, F.; Zeng, S.; Wang, Y.; Yang, C. Genome-wide identification and analysis of autophagy-related (ATG) genes in Lycium ruthenicum Murray reveals their crucial roles in salt stress tolerance. Plant Sci. 2025, 352, 112371. [Google Scholar] [CrossRef]
- Zhu, X.; Majeed, Y.; Zhang, N.; Li, W.; Duan, H.; Dou, X.; Jin, H.; Chen, Z.; Chen, S.; Zhou, J.; et al. Identification of autophagy gene family in potato and the role of StATG8a in salt and drought stress. Physiol. Plant 2024, 176, e14584. [Google Scholar] [CrossRef]
- Wendel, J.F. The wondrous cycles of polyploidy in plants. Am. J. Bot. 2015, 102, 1753–1756. [Google Scholar] [CrossRef]
- Yue, W.; Zhang, H.; Sun, X.; Su, N.; Zhao, Q.; Yan, Z.; Weining, S.; Yue, H. The Landscape of Autophagy-Related (ATG) Genes and Functional Characterization of TaVAMP727 to Autophagy in Wheat. Int. J. Mol. Sci. 2022, 23, 891. [Google Scholar] [CrossRef]
- Cao, J.; Zheng, X.; Xie, D.; Zhou, H.; Shao, S.; Zhou, J. Autophagic pathway contributes to low-nitrogen tolerance by optimizing nitrogen uptake and utilization in tomato. Hortic. Res. 2022, 9, uhac068. [Google Scholar] [CrossRef]
- Zeng, Z.-X.; Wang, C.-M.; Zhao, Y.-T.; Yang, Y.-Y.; Shan, W.; Kuang, J.-F.; Lu, W.-J.; Fan, Z.-Q.; Su, X.-G.; Lin, H.-T.; et al. Molecular characterization of leaf senescence-associated autophagy genes in postharvest Chinese flowering cabbage and identifying their transcriptional activator BrMYB108. Postharvest Biol. Technol. 2022, 185, 111785. [Google Scholar] [CrossRef]
- Lai, Z.; Wang, F.; Zheng, Z.; Fan, B.; Chen, Z. A critical role of autophagy in plant resistance to necrotrophic fungal pathogens. Plant J. 2011, 66, 953–968. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Wang, J.; Yu, J.Q.; Chen, Z. Role and regulation of autophagy in heat stress responses of tomato plants. Front. Plant Sci. 2014, 5, 174. [Google Scholar] [CrossRef]
- Zhu, T.; Zou, L.; Li, Y.; Yao, X.; Xu, F.; Deng, X.; Zhang, D.; Lin, H. Mitochondrial alternative oxidase-dependent autophagy involved in ethylene-mediated drought tolerance in Solanum lycopersicum. Plant Biotechnol. J. 2018, 16, 2063–2076. [Google Scholar] [CrossRef]
- Cannon, S.B.; Mitra, A.; Baumgarten, A.; Young, N.D.; May, G. The roles of segmental and tandem gene duplication in the evolution of large gene families in Arabidopsis thaliana. BMC Plant Biol. 2004, 4, 10. [Google Scholar] [CrossRef] [PubMed]
- Breeze, E.; Harrison, E.; McHattie, S.; Hughes, L.; Hickman, R.; Hill, C.; Kiddle, S.; Kim, Y.S.; Penfold, C.A.; Jenkins, D.; et al. High-resolution temporal profiling of transcripts during Arabidopsis leaf senescence reveals a distinct chronology of processes and regulation. Plant Cell 2011, 23, 873–894. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, B.-Y.; Meng, H.; Lu, Z.-Y.; Wang, P.; Zheng, C.-S.; Wu, X.-Y.; Kang, D.-R.; Wang, W.-L. Genome-Wide Identification of Autophagy-Related Gene Family and Gene Expression Analysis of the CmATG8 Under Heat Stress in Chrysanthemum. Int. J. Mol. Sci. 2025, 26, 8642. https://doi.org/10.3390/ijms26178642
Luo B-Y, Meng H, Lu Z-Y, Wang P, Zheng C-S, Wu X-Y, Kang D-R, Wang W-L. Genome-Wide Identification of Autophagy-Related Gene Family and Gene Expression Analysis of the CmATG8 Under Heat Stress in Chrysanthemum. International Journal of Molecular Sciences. 2025; 26(17):8642. https://doi.org/10.3390/ijms26178642
Chicago/Turabian StyleLuo, Bing-Yu, Hui Meng, Zheng-Yu Lu, Peng Wang, Cheng-Shu Zheng, Xiao-Yun Wu, Dong-Ru Kang, and Wen-Li Wang. 2025. "Genome-Wide Identification of Autophagy-Related Gene Family and Gene Expression Analysis of the CmATG8 Under Heat Stress in Chrysanthemum" International Journal of Molecular Sciences 26, no. 17: 8642. https://doi.org/10.3390/ijms26178642
APA StyleLuo, B.-Y., Meng, H., Lu, Z.-Y., Wang, P., Zheng, C.-S., Wu, X.-Y., Kang, D.-R., & Wang, W.-L. (2025). Genome-Wide Identification of Autophagy-Related Gene Family and Gene Expression Analysis of the CmATG8 Under Heat Stress in Chrysanthemum. International Journal of Molecular Sciences, 26(17), 8642. https://doi.org/10.3390/ijms26178642





