Comparison of Current International Guidelines for the Management of Alopecia Areata—Comprehensive Review
Abstract
1. Introduction
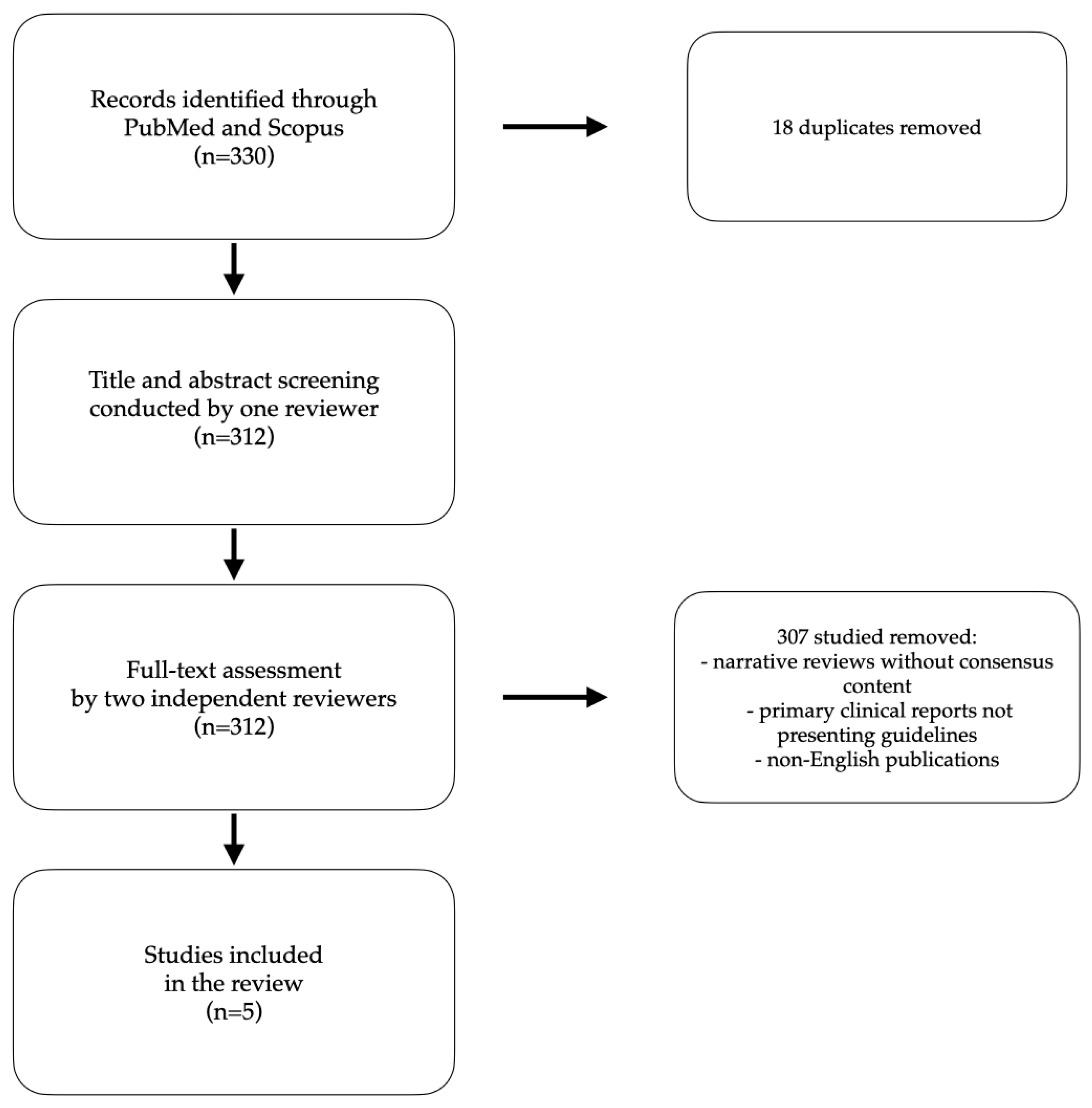
2. Materials and Methods
3. Epidemiology
4. Diagnosis
5. Pathogenesis
5.1. Genetics
5.2. Immunology
5.3. Allergy
5.4. Oxidative Stress (OS)
5.5. Microbiota
6. Comorbidities
7. Treatment
7.1. Glucocorticosteroids (GCS)
7.2. Contact Immunotherapy
7.3. Anthralin
7.4. Minoxidil
7.5. Steroid-Sparing Agents: Azathioprine, Methotrexate, Cyclosporine and Sulfasalazine
7.6. New Emerging Therapies
7.6.1. Janus Kinase (JAK) Inhibitors
7.6.2. Biologicals
7.6.3. Small-Molecule Inhibitors
7.6.4. Exosomes
7.6.5. Excimer Laser
7.6.6. UVB Phototherapy
8. Prognosis
9. Conclusions
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Hunt, N.; McHale, S. The psychological impact of alopecia. Br. Med. J. 2005, 331, 951–953. [Google Scholar] [CrossRef]
- Villasante Fricke, A.C.; Miteva, M. Epidemiology and burden of alopecia areata: A systematic review. Clin. Cosmet. Investig. Dermatol. 2015, 8, 397–403. [Google Scholar] [CrossRef]
- Rudnicka, L.; Arenbergerova, M.; Grimalt, R.; Ioannides, D.; Katoulis, A.C.; Lazaridou, E.; Olszewska, M.; Ovcharenko, Y.S.; Piraccini, B.M.; Prohic, A.; et al. European expert consensus statement on the systemic treatment of alopecia areata. J. Eur. Acad. Dermatol. Venereol. 2024, 38, 687–694. [Google Scholar] [CrossRef]
- Meah, N.; Wall, D.; York, K.; Bhoyrul, B.; Bokhari, L.; Sigall, D.A.; Bergfeld, W.F.; Betz, R.C.; Blume-Peytavi, U.; Callender, V.; et al. The Alopecia Areata Consensus of Experts (ACE) study: Results of an international expert opinion on treatments for alopecia areata. J. Am. Acad. Dermatol. 2020, 83, 123–130. [Google Scholar] [CrossRef]
- Ramos, P.M.; Anzai, A.; Duque-Estrada, B.; Melo, D.F.; Sternberg, F.; Santos, L.D.N.; Alves, L.D.; Mulinari-Brenner, F. Consensus on the treatment of alopecia areata—Brazilian Society of Dermatology. An. Bras. Dermatol. 2020, 95 (Suppl. 1), 39–52. [Google Scholar] [CrossRef]
- Cranwell, W.C.; Lai, V.W.; Photiou, L.; Meah, N.; Wall, D.; Rathnayake, D.; Joseph, S.; Chitreddy, V.; Gunatheesan, S.; Sindhu, K.; et al. Treatment of alopecia areata: An Australian expert consensus statement. Australas. J. Dermatol. 2019, 60, 163–170. [Google Scholar] [CrossRef]
- Fatani, M.I.A.; Alkhalifah, A.; Alruwaili, A.F.S.; Alharbi, A.H.S.; Alharithy, R.; Khardaly, A.M.; Almudaiheem, H.Y.; Al-Jedai, A.; Eshmawi, M.T.Y. Diagnosis and Management of Alopecia Areata: A Saudi Expert Consensus Statement (2023). Dermatol. Ther. 2023, 13, 2129–2151. [Google Scholar] [CrossRef]
- Sibbald, C. Alopecia Areata: An Updated Review for 2023. J. Cutan. Med. Surg. 2023, 27, 241–259. [Google Scholar] [CrossRef]
- Lee, H.H.; Gwillim, E.; Patel, K.R.; Hua, T.; Rastogi, S.; Ibler, E.; Silverberg, J.I. Epidemiology of alopecia areata, ophiasis, totalis, and universalis: A systematic review and meta-analysis. J. Am. Acad. Dermatol. 2020, 82, 675–682. [Google Scholar] [CrossRef]
- Jang, H.; Park, S.; Kim, M.S.; Yon, D.K.; Lee, S.W.; Koyanagi, A.; Kostev, K.; Shin, J.I.; Smith, L. Global, regional and national burden of alopecia areata and its associated diseases, 1990–2019: A systematic analysis of the Global Burden of Disease Study 2019. Eur. J. Clin. Investig. 2023, 53, e13958. [Google Scholar] [CrossRef]
- Waśkiel, A.; Rakowska, A.; Sikora, M.; Olszewska, M.; Rudnicka, L. Trichoscopy of alopecia areata: An update. J. Dermatol. 2018, 45, 692–700. [Google Scholar] [CrossRef]
- Pratt, C.H.; King, L.E.; Messenger, A.G.; Christiano, A.M.; Sundberg, J.P. Alopecia areata. Nat. Rev. Dis. Primers 2017, 3, 17011. [Google Scholar] [CrossRef]
- Anzai, A.; Wang, E.H.C.; Lee, E.Y.; Aoki, V.; Christiano, A.M. Pathomechanisms of immune-mediated alopecia. Int. Immunol. 2019, 31, 439–447. [Google Scholar] [CrossRef]
- Peckham, S.J.; Sloan, S.B.; Elston, D.M. Histologic features of alopecia areata other than peribulbar lymphocytic infiltrates. J. Am. Acad. Dermatol. 2011, 65, 615–620. [Google Scholar] [CrossRef]
- Chelidze, K.; Lipner, S.R. Nail changes in alopecia areata: An update and review. Int. J. Dermatol. 2018, 57, 776–783. [Google Scholar] [CrossRef]
- Blaumeiser, B.; Van Der Goot, I.; Fimmers, R.; Hanneken, S.; Ritzmann, S.; Seymons, K.; Betz, R.C.; Ruzicka, T.; Wienker, T.F.; De Weert, J.; et al. Familial aggregation of alopecia areata. J. Am. Acad. Dermatol. 2006, 54, 627–632. [Google Scholar] [CrossRef]
- Rodriguez, T.A.; Fernandes, K.E.; Dresser, K.L.; Duvic, M. Concordance rate of alopecia areata in identical twins supports both genetic and environmental factors. J. Am. Acad. Dermatol. 2010, 62, 525–527. [Google Scholar] [CrossRef]
- Paus, R.; Slominski, A.; Czarnetzki, B.M. Is alopecia areata an autoimmune-response against melanogenesis-related proteins, exposed by abnormal MHC class I expression in the anagen hair bulb? Yale J. Biol. Med. 1993, 66, 541–554. [Google Scholar]
- Rajabi, F.; Drake, L.A.; Senna, M.M.; Rezaei, N. Alopecia areata: A review of disease pathogenesis. Br. J. Dermatol. 2018, 179, 1033–1048. [Google Scholar] [CrossRef]
- Rodriguez, R.S.; Pauli, M.L.; Neuhaus, I.M.; Yu, S.S.; Arron, S.T.; Harris, H.W.; Yang, S.H.-Y.; Anthony, B.A.; Sverdrup, F.M.; Krow-Lucal, E.; et al. Memory regulatory T cells reside in human skin. J. Clin. Investig. 2014, 124, 1027–1036. [Google Scholar] [CrossRef]
- Ali, N.; Zirak, B.; Rodriguez, R.S.; Pauli, M.L.; Truong, H.-A.; Lai, K.; Ahn, R.; Corbin, K.; Lowe, M.M.; Scharschmidt, T.C.; et al. Regulatory T Cells in Skin Facilitate Epithelial Stem Cell Differentiation. Cell 2017, 169, 1119–1129.e11. [Google Scholar] [CrossRef]
- Barahmani, N.; Schabath, M.B.; Duvic, M. History of atopy or autoimmunity increases risk of alopecia areata. J. Am. Acad. Dermatol. 2009, 61, 581–591. [Google Scholar] [CrossRef]
- Ito, T.; Kageyama, R.; Nakazawa, S.; Honda, T. Understanding the significance of cytokines and chemokines in the pathogenesis of alopecia areata. Exp. Dermatol. 2020, 29, 726–732. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, Y.; Ye, Y.; Li, S.; Qi, S.; Yang, Y.; Cao, H.; Yang, J.; Zhang, X. Lesional infiltration of mast cells, Langerhans cells, T cells and local cytokine profiles in alopecia areata. Arch. Dermatol. Res. 2015, 307, 319–331. [Google Scholar] [CrossRef]
- Yoon, T.Y.; Lee, D.Y.; Kim, Y.J.; Lee, J.Y.; Kim, M.K. Diagnostic usefulness of a peribulbar eosinophilic infiltrate in alopecia areata. JAMA Dermatol. 2014, 150, 952–956. [Google Scholar] [CrossRef]
- Zhang, X.; McElwee, K.J. Allergy promotes alopecia areata in a subset of patients. Exp. Dermatol. 2020, 29, 239–242. [Google Scholar] [CrossRef]
- Li, S.F.; Zhang, X.T.; Qi, S.L.; Ye, Y.T.; Cao, H.; Yang, Y.Q.; McElwee, K.J. Allergy to dust mites may contribute to early onset and severity of alopecia areata. Clin. Exp. Dermatol. 2015, 40, 171–176. [Google Scholar] [CrossRef]
- Uchida, H.; Kamata, M.; Watanabe, A.; Agematsu, A.; Nagata, M.; Fukaya, S.; Hayashi, K.; Fukuyasu, A.; Tanaka, T.; Ishikawa, T.; et al. Dupilumab Improved Alopecia Areata in a Patient with Atopic Dermatitis: A Case Report. Acta Derm. Venereol. 2019, 99, 675–676. [Google Scholar] [CrossRef]
- Sun, C.; Ding, H.; Zhang, L.; Wang, J.; Su, M. Co-regulatory mechanisms and potential markers of oxidative stress-related genes in vitiligo and alopecia areata. Skin Res. Technol. 2024, 30, e70001. [Google Scholar] [CrossRef]
- Acharya, P.; Mathur, M.C. Oxidative stress in alopecia areata: A systematic review and meta-analysis. Int. J. Dermatol. 2020, 59, 434–440. [Google Scholar] [CrossRef]
- Polak-Witka, K.; Rudnicka, L.; Blume-Peytavi, U.; Vogt, A. The role of the microbiome in scalp hair follicle biology and disease. Exp. Dermatol. 2020, 29, 286–294. [Google Scholar] [CrossRef]
- Lai, Y.; Di Nardo, A.; Nakatsuji, T.; Leichtle, A.; Yang, Y.; Cogen, A.L.; Wu, Z.-R.; Hooper, L.V.; Schmidt, R.R.; von Aulock, S.; et al. Commensal bacteria regulate Toll-like receptor 3-dependent inflammation after skin injury. Nat. Med. 2009, 15, 1377–1382. [Google Scholar] [CrossRef]
- Pinto, D.; Calabrese, F.M.; De Angelis, M.; Celano, G.; Giuliani, G.; Gobbetti, M.; Rinaldi, F. Predictive Metagenomic Profiling, Urine Metabolomics, and Human Marker Gene Expression as an Integrated Approach to Study Alopecia Areata. Front. Cell. Infect. Microbiol. 2020, 10, 146. [Google Scholar] [CrossRef]
- Fyhrquist, N.; Muirhead, G.; Prast-Nielsen, S.; Jeanmougin, M.; Olah, P.; Skoog, T.; Jules-Clement, G.; Feld, M.; Barrientos-Somarribas, M.; Sinkko, H.; et al. Microbe-host interplay in atopic dermatitis and psoriasis. Nat. Commun. 2019, 10, 4703. [Google Scholar] [CrossRef]
- Sánchez-Pellicer, P.; Navarro-Moratalla, L.; Núñez-Delegido, E.; Agüera-Santos, J.; Navarro-López, V. How Our Microbiome Influences the Pathogenesis of Alopecia Areata. Genes 2022, 13, 1860. [Google Scholar] [CrossRef]
- Seo, H.M.; Han, S.S.; Kim, J.S. Cancer risks among patients with alopecia areata: A population-based case-control study in Korea. J. Am. Acad. Dermatol. 2018, 78, 209–211. [Google Scholar] [CrossRef]
- Kamada, N.; Hatamochi, A.; Shinkai, H. Alopecia areata associated with myasthenia gravis and thymoma: A case of alopecia with marked improvement following thymectomy and high level prednisolone administration. J. Dermatol. 1997, 24, 769–772. [Google Scholar] [CrossRef]
- Richmond, H.M.; Lozano, A.; Jones, D.; Duvic, M. Primary cutaneous follicle center lymphoma associated with alopecia areata. Clin. Lymphoma Myeloma 2008, 8, 121–124. [Google Scholar] [CrossRef]
- Chan, P.D.; Berk, M.A.; Kucuk, O.; Singh, S. Simultaneously occurring alopecia areata and Hodgkin’s lymphoma: Complete remission of both diseases with MOPP/ABV chemotherapy. Med. Pediatr. Oncol. 1992, 20, 345–348. [Google Scholar] [CrossRef]
- Shin, J.W.; Kang, T.; Lee, J.S.; Kang, M.J.; Huh, C.-H.; Kim, M.-S.; Kim, H.J.; Ahn, H.S. Time-Dependent Risk of Acute Myocardial Infarction in Patients with Alopecia Areata in Korea. JAMA Dermatol. 2020, 156, 763–771. [Google Scholar] [CrossRef]
- Dai, Y.X.; Yeh, F.Y.; Shen, Y.J.; Tai, Y.-H.; Chou, Y.-J.; Chang, Y.-T.; Chen, T.-J.; Li, C.-P.; Wu, C.-Y. Cigarette Smoking, Alcohol Consumption, and Risk of Alopecia Areata: A Population-Based Cohort Study in Taiwan. Am. J. Clin. Dermatol. 2020, 21, 901–911. [Google Scholar] [CrossRef]
- Seo, H.M.; Kim, T.L.; Kim, J.S. The risk of alopecia areata and other related autoimmune diseases in patients with sleep disorders: A Korean population-based retrospective cohort study. Sleep 2018, 41, zsy111. [Google Scholar] [CrossRef]
- Dai, Y.X.; Tai, Y.H.; Chen, C.C.; Chang, Y.-T.; Chen, T.-J.; Chen, M.-H. Bidirectional association between alopecia areata and sleep disorders: A population-based cohort study in Taiwan. Sleep Med. 2020, 75, 112–116. [Google Scholar] [CrossRef]
- Ahn, D.; Kim, H.; Lee, B.; Hahm, D.H. Psychological Stress-Induced Pathogenesis of Alopecia Areata: Autoimmune and Apoptotic Pathways. Int. J. Mol. Sci. 2023, 24, 11711. [Google Scholar] [CrossRef]
- Rodriguez, T.A.; Duvic, M. Onset of alopecia areata after Epstein-Barr virus infectious mononucleosis. J. Am. Acad. Dermatol. 2008, 59, 137–139. [Google Scholar] [CrossRef]
- Xuan, L.; Baohua, Y.; Lan, B. Alopecia areata and vitiligo as primary presentations in a young male with human immunodeficiency virus. Indian J. Dermatol. 2014, 59, 209. [Google Scholar] [CrossRef]
- Tu, T.Y.; Chang, R.; Lai, J.N.; Tseng, C.-C.; Chen, M.-L.; Yip, H.-T.; Hung, Y.-M.; Wei, J.C.-C. Human papillomavirus symptomatic infection associated with increased risk of new-onset alopecia areata: A nationwide population-based cohort study. J. Autoimmun. 2021, 119, 102618. [Google Scholar] [CrossRef]
- Lai, Y.C.; Yew, Y.W. Severe Autoimmune Adverse Events Post Herpes Zoster Vaccine: A Case-Control Study of Adverse Events in a National Database. J. Drugs Dermatol. JDD 2015, 14, 681–684. Available online: https://jddonline.com/articles/severe-autoimmune-adverse-events-post-herpes-zoster-vaccine-a-case-control-study-of-adverse-events-i-S1545961615P0681X/ (accessed on 29 December 2024).
- Richardson, C.T.; Hayden, M.S.; Gilmore, E.S.; Poligone, B. Evaluation of the Relationship between Alopecia Areata and Viral Antigen Exposure. Am. J. Clin. Dermatol. 2018, 19, 119–126. [Google Scholar] [CrossRef]
- Chu, C.H.; Cheng, Y.P.; Chan, J.Y.L. Alopecia Areata After Vaccination: Recurrence with Rechallenge. Pediatr. Dermatol. 2016, 33, e218–e219. [Google Scholar] [CrossRef]
- Geier, D.A.; Geier, M.R. A case-control study of quadrivalent human papillomavirus vaccine-associated autoimmune adverse events. Clin. Rheumatol. 2015, 34, 1225–1231. [Google Scholar] [CrossRef]
- Zschoche, C.; Bidier, M.; Hadaschik, E. Alopecia areata during treatment with adalimumab: Therapy with an alternative TNF-alpha inhibitor is possible. J. Dtsch. Dermatol. Ges. 2013, 11, 450–453. [Google Scholar] [CrossRef]
- Le Bidre, E.; Chaby, G.; Martin, L.; Perrussel, M.; Sassolas, B.; Sigal, M.-L.; Kaassis, C.; Lespessailles, E.; Nseir, A.; Estève, E. Pelade au cours d’un traitement par anti-TNF alpha: Neuf cas. Ann. Dermatol. Venereol. 2011, 138, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Dourra, M.; Mussad, S.; Qiblawi, S.; Singer, R. Denosumab-induced alopecia areata with lichenoid eruption. JAAD Case Rep. 2021, 17, 9–11. [Google Scholar] [CrossRef]
- Flanagan, K.; Sperling, L.; Lin, J. Drug-induced alopecia after dupilumab therapy. JAAD Case Rep. 2018, 5, 54–56. [Google Scholar] [CrossRef]
- Pan, Y.; Rao, N.A. Alopecia areata during etanercept therapy. Ocul. Immunol. Inflamm. 2009, 17, 127–129. [Google Scholar] [CrossRef]
- Ettefagh, L.; Nedorost, S.; Mirmirani, P. Alopecia areata in a patient using infliximab: New insights into the role of tumor necrosis factor on human hair follicles. Arch. Dermatol. 2004, 140, 1012. [Google Scholar] [CrossRef]
- Lakhmiri, M.; Cavelier-Balloy, B.; Lacoste, C.; Cassius, C.; Baroudjian, B.; Delyon, J.; Lebbé, C.; Reygagne, P. Nivolumab-induced alopecia areata: A reversible factor of good prognosis? JAAD Case Rep. 2018, 4, 761–765. [Google Scholar] [CrossRef]
- Magen, E. Alopecia Areata after Omalizumab Treatment for Chronic Spontaneous Urticaria. Acta Derm. Venereol. 2019, 99, 919–920. [Google Scholar] [CrossRef] [PubMed]
- Guidry, J.; Brown, M.; Medina, T. PD-1 inhibitor induced alopecia areata. Dermatol. Online J. 2018, 24, 14. [Google Scholar] [CrossRef]
- Choi, E.; Thomson, O.; Smith, D. Alopecia Areata After Initiation of Secukinumab Therapy for Plaque Psoriasis. Cureus 2023, 15, e38986. [Google Scholar] [CrossRef]
- Aksu Cerman, A.; Sarikaya Solak, S.; Kivanc Altunay, I. Vitamin D deficiency in alopecia areata. Br. J. Dermatol. 2014, 170, 1299–1304. [Google Scholar] [CrossRef]
- Bhat, Y.J.; Latif, I.; Malik, R.; Hassan, I.; Sheikh, G.; Lone, K.S.; Majeed, S.; Sajad, P. Vitamin D Level in Alopecia Areata. Indian J. Dermatol. 2017, 62, 407–410. [Google Scholar] [CrossRef] [PubMed]
- Kantor, J.; Kessler, L.J.; Brooks, D.G.; Cotsarelis, G. Decreased serum ferritin is associated with alopecia in women. J. Investig. Dermatol. 2003, 121, 985–988. [Google Scholar] [CrossRef] [PubMed]
- Lalosevic, J.; Gajic-Veljic, M.; Lalosevic Misovic, J.; Nikolic, M. Serum Zinc Concentration in Patients with Alopecia Areata. Acta Derm. Venereol. 2023, 103, adv13358. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Kim, C.W.; Kim, S.S.; Park, C.W. The therapeutic effect and the changed serum zinc level after zinc supplementation in alopecia areata patients who had a low serum zinc level. Ann. Dermatol. 2009, 21, 142–146. [Google Scholar] [CrossRef]
- King, B.A.; Senna, M.M.; Ohyama, M.; Tosti, A.; Sinclair, R.D.; Ball, S.; Ko, J.M.; Glashofer, M.; Pirmez, R.; Shapiro, J. Defining Severity in Alopecia Areata: Current Perspectives and a Multidimensional Framework. Dermatol. Ther. 2022, 12, 825–834. [Google Scholar] [CrossRef]
- King, B.; Zhang, X.; Harcha, W.G.; Szepietowski, J.C.; Shapiro, J.; Lynde, C.; Mesinkovska, N.A.; Zwillich, S.H.; Napatalung, L.; Wajsbrot, D.; et al. Efficacy and safety of ritlecitinib in adults and adolescents with alopecia areata: A randomised, double-blind, multicentre, phase 2b-3 trial. Lancet 2023, 401, 1518–1529, Erratum in Lancet 2023, 401, 1928. https://doi.org/10.1016/S0140-6736(23)01078-4; Erratum in Lancet 2025, 406, 810. https://doi.org/10.1016/S0140-6736(25)01723-4. [Google Scholar] [CrossRef]
- Wambier, C.G.; Craiglow, B.G.; King, B.A. Combination tofacitinib and oral minoxidil treatment for severe alopecia areata. J. Am. Acad. Dermatol. 2021, 85, 743–745. [Google Scholar] [CrossRef]
- Freire, P.C.B.; Riera, R.; Martimbianco, A.L.C.; Petri, V.; Atallah, A. Minoxidil for patchy alopecia areata: Systematic review and meta-analysis. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 1792–1799. [Google Scholar] [CrossRef]
- El Taieb, M.A.; Ibrahim, H.; Nada, E.A.; Seif Al-Din, M. Platelets rich plasma versus minoxidil 5% in treatment of alopecia areata: A trichoscopic evaluation. Dermatol. Ther. 2017, 30, e12437. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Lin, P.; Lin, H.; Ma, C.; Hu, Y.; Wang, Y.; Zhang, Y. Laser and light therapy combined with topical minoxidil for alopecia areata: A systematic review and meta-analysis of randomized controlled trials. Lasers Med. Sci. 2023, 38, 74. [Google Scholar] [CrossRef] [PubMed]
- Nowaczyk, J.; Makowska, K.; Rakowska, A.; Sikora, M.; Rudnicka, L. Cyclosporine with and Without Systemic Corticosteroids in Treatment of Alopecia Areata: A Systematic Review. Dermatol. Ther. 2020, 10, 387–399. [Google Scholar] [CrossRef] [PubMed]
- Mateos-Haro, M.; Novoa-Candia, M.; Sánchez Vanegas, G.; Correa-Pérez, A.; Gil, A.G.; Fernández-García, S.; Ortega-Quijano, D.; Rodriguez, M.G.U.; Saceda-Corralo, D.; Bennouna-Dalero, T.; et al. Treatments for alopecia areata: A network meta-analysis. Cochrane Database Syst. Rev. 2023, 2023, CD013719. [Google Scholar] [CrossRef]
- Ali, E.; Owais, R.; Sheikh, A.; Shaikh, A. Olumniant (Baricitinib) oral tablets: An insight into FDA-approved systemic treatment for Alopecia Areata. Ann. Med. Surg. 2022, 80, 104157. [Google Scholar] [CrossRef]
- FDA Approves Pfizer’s LITFULOTM (Ritlecitinib) for Adults and Adolescents with Severe Alopecia Areata|Business Wire [WWW Document]. Available online: https://www.businesswire.com/news/home/20230623087591/en/FDA-Approves-Pfizer%E2%80%99s-LITFULO%E2%84%A2-Ritlecitinib-for-Adults-and-Adolescents-With-Severe-Alopecia-Areata (accessed on 29 December 2024).
- FDA and EMA Accept Regulatory Submission for Pfizer’s Ritlecitinib for Individuals 12 Years and Older with Alopecia Areata |Pfizer [WWW Document]. Available online: https://www.pfizer.com/news/press-release/press-release-detail/fda-and-ema-accept-regulatory-submission-pfizers (accessed on 29 December 2024).
- Wei, D.; Chen, Y.; Shen, Y.; Xie, B.; Song, X. Efficacy and safety of different JAK inhibitors in the treatment of alopecia areata: A network meta-analysis. Front. Immunol. 2023, 14, 1152513. [Google Scholar] [CrossRef]
- Husein-ElAhmed, H.; Husein-ElAhmed, S. Comparative efficacy of oral Janus kinase inhibitors and biologics in adult alopecia areata: A systematic review and Bayesian network meta-analysis. J. Eur. Acad. Dermatol. Venereol. 2024, 38, 835–843. [Google Scholar] [CrossRef]
- Olsen, E.A.; Hordinsky, M.K.; Price, V.H.; Roberts, J.L.; Shapiro, J.; Canfield, D.; Duvic, M.; King, L.E.; McMichael, A.J.; Randall, V.A.; et al. Alopecia areata investigational assessment guidelines-Part II. J. Am. Acad. Dermatol. 2004, 51, 440–447. [Google Scholar] [CrossRef]
- Hordinsky, M.; Donati, A. Alopecia areata: An evidence-based treatment update. Am. J. Clin. Dermatol. 2014, 15, 231–246. [Google Scholar] [CrossRef]
- Guttman-Yassky, E.; Renert-Yuval, Y.; Bares, J.; Chima, M.; Hawkes, J.E.; Gilleaudeau, P.; Sullivan-Whalen, M.; Singer, G.K.; Garcet, S.; Pavel, A.B.; et al. Phase 2a randomized clinical trial of dupilumab (anti-IL-4Rα) for alopecia areata patients. Allergy 2022, 77, 897–906. [Google Scholar] [CrossRef]
- Harada, K.; Irisawa, R.; Ito, T.; Uchiyama, M.; Tsuboi, R. The effectiveness of dupilumab in patients with alopecia areata who have atopic dermatitis: A case series of seven patients. Br. J. Dermatol. 2020, 183, 396–397. [Google Scholar] [CrossRef]
- Kulkarni, M.; Rohan, C.A.; Travers, J.B.; Serrao, R. Long-Term Efficacy of Dupilumab in Alopecia Areata. Am. J. Case Rep. 2022, 23, e936488. [Google Scholar] [CrossRef] [PubMed]
- Magdaleno-Tapial, J.; Valenzuela-Oñate, C.; García-Legaz-Martínez, M.; Martínez-Domenech, Á.; Pérez-Ferriols, A. Improvement of alopecia areata with Dupilumab in a patient with severe atopic dermatitis and review the literature. Australas. J. Dermatol. 2020, 61, e223–e225. [Google Scholar] [CrossRef] [PubMed]
- Carnicle, J.M.; Hendricks, A.J.; Shi, V.Y. Reactivation of Alopecia Areata After Dupilumab Therapy for Atopic Dermatitis. Dermatitis 2021, 32, E80–E82. [Google Scholar] [CrossRef] [PubMed]
- Mackay-Wiggan, J.; Sallee, B.N.; Wang, E.H.C.; Sansaricq, F.; Nguyen, N.; Kim, C.; Chen, J.C.; Christiano, A.M.; Clynes, R. An open-label study evaluating the efficacy of abatacept in alopecia areata. J. Am. Acad. Dermatol. 2021, 84, 841–844. [Google Scholar] [CrossRef]
- Guttman-Yassky, E.; Ungar, B.; Noda, S.; Suprun, M.; Shroff, A.; Dutt, R.; Khattri, S.; Min, M.; Mansouri, Y.; Zheng, X.; et al. Extensive alopecia areata is reversed by IL-12/IL-23p40 cytokine antagonism. J. Allergy Clin. Immunol. 2016, 137, 301–304. [Google Scholar] [CrossRef]
- Słowińska, M.; Kardynal, A.; Warszawik, O.; Czuwara, J.; Rudnicka, L. Alopecia areata developing paralell to improvement of psoriasis during ustekinumab therapy. J. Dermatol. Case Rep. 2010, 4, 15–17. [Google Scholar] [CrossRef]
- Verros, C.; Rallis, E.; Crowe, M. Letter: Alopecia areata during ustekinumab administration: Co-existence or an adverse reaction? Dermatol. Online J. 2012, 18, 14. [Google Scholar] [CrossRef]
- Stępień, M.; Anczyk, S. Cytokine-targeted treatment in alopecia areata—New possibilities? Prospect. Pharm. Sci. 2023, 21, 22–29. [Google Scholar] [CrossRef]
- Estébanez, A.; Estébanez, N.; Martín, J.; Montesinos, E. Apremilast in Refractory Alopecia Areata. Int. J. Trichology 2019, 11, 213–215. [Google Scholar] [CrossRef]
- Mikhaylov, D.; Pavel, A.; Yao, C.; Kimmel, G.; Nia, J.; Hashim, P.; Vekaria, A.S.; Taliercio, M.; Singer, G.; Karalekas, R.; et al. A randomized placebo-controlled single-center pilot study of the safety and efficacy of apremilast in subjects with moderate-to-severe alopecia areata. Arch. Dermatol. Res. 2019, 311, 29–36. [Google Scholar] [CrossRef]
- Lee, E.; Choi, M.S.; Cho, B.S.; Won, Y.J.; Lee, J.H.; Kim, S.R.; Kim, M.H.; Jeon, J.H.; Park, G.H.; Kwon, H.H.; et al. The efficacy of adipose stem cell-derived exosomes in hair regeneration based on a preclinical and clinical study. Int. J. Dermatol. 2024, 63, 1212–1220. [Google Scholar] [CrossRef]
- Rudnicka, L.; Olszewska, M. The emerging role of exosomes in the treatment of hair loss. Int. J. Dermatol. 2024, 63, 1124–1125. [Google Scholar] [CrossRef]
- Esen Salman, K.; Kivanç Altunay, İ.; Salman, A. The efficacy and safety of targeted narrowband UVB therapy: A retrospective cohort study. Turk. J. Med. Sci. 2019, 49, 595–603. [Google Scholar] [CrossRef] [PubMed]
- Messenger, A.G.; McKillop, J.; Farrant, P.; McDonagh, A.; Sladden, M.; Hughes, J.; McLelland, J.; Punjabi, S.; Buckley, D.; Nasr, I.; et al. British Association of Dermatologists’ guidelines for the management of alopecia areata 2012. Br. J. Dermatol. 2012, 166, 916–926. [Google Scholar] [CrossRef]
- Tosti, A.; Bellavista, S.; Iorizzo, M. Alopecia areata: A long term follow-up study of 191 patients. J. Am. Acad. Dermatol. 2006, 55, 438–441. [Google Scholar] [CrossRef] [PubMed]
- Rossi, A.; Magri, F.; Michelini, S.; Caro, G.; Di Fraia, M.; Fortuna, M.C.; Pellacani, G.; Carlesimo, M. Recurrence of alopecia areata after COVID-19 vaccination: A report of three cases in Italy. J. Cosmet. Dermatol. 2021, 20, 3753–3757. [Google Scholar] [CrossRef]
- Islam, N.; Leung, P.S.C.; Huntley, A.C.; Eric Gershwin, M. The autoimmune basis of alopecia areata: A comprehensive review. Autoimmun. Rev. 2015, 14, 81–89. [Google Scholar] [CrossRef]
- Alkhalifah, A.; Alsantali, A.; Wang, E.; McElwee, K.J.; Shapiro, J. Alopecia areata update: Part II. Treatment. J. Am. Acad. Dermatol. 2010, 62, 191–202, quiz 203–204. [Google Scholar] [CrossRef]
- Ait Ourhroui, M.; Hassam, B.; Khoudri, I. Treatment of alopecia areata with prednisone in a once-monthly oral pulse. Ann. Dermatol. Venereol. 2010, 137, 514–518. [Google Scholar] [CrossRef]
- Rakowska, A.; Rudnicka, L.; Olszewska, M.; Bergler-Czop, B.; Czuwara, J.; Brzezińska-Wcisło, L.; Narbutt, J.; Placek, W.; Zegarska, B. Alopecia areata. Diagnostic and therapeutic recommendations of the Polish Society of Dermatology. Part 2: Treatment. Dermatol. Rev. Przegląd Dermatol. 2023, 110, 101–120. [Google Scholar]
- Strazzulla, L.C.; Wang, E.H.C.; Avila, L.; Sicco, K.L.; Brinster, N.; Christiano, A.M.; Shapiro, J. Alopecia areata: An appraisal of new treatment approaches and overview of current therapies. J. Am. Acad. Dermatol. 2018, 78, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Orecchia, G.; Perfetti, L. Alopecia areata and topical sensitizers: Allergic response is necessary, but irritation is not. Br. J. Dermatol. 1991, 124, 509. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.Z.; Wang, S.; Ratnaparkhi, R.; Bergfeld, W.F. Treatment of pediatric alopecia areata with anthralin: A retrospective study of 37 patients. Pediatr. Dermatol. 2018, 35, 817–820. [Google Scholar] [CrossRef] [PubMed]
- Özdemir, M.; Balevi, A. Bilateral Half-Head Comparison of 1% Anthralin Ointment in Children with Alopecia Areata. Pediatr. Dermatol. 2017, 34, 128–132. [Google Scholar] [CrossRef]
- Vañó-Galván, S.; Fernández-Crehuet, P.; Grimalt, R.; Garcia-Hernandez, M.; Rodrigues-Barata, R.; Arias-Santiago, S.; Molina-Ruiz, A.; Garcia-Lora, E.; Dominguez-Cruz, J.; Brugues, A.; et al. Alopecia areata totalis and universalis: A multicenter review of 132 patients in Spain. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 550–556. [Google Scholar] [CrossRef]
- Stoehr, J.R.; Choi, J.N.; Colavincenzo, M.; Vanderweil, S. Off-Label Use of Topical Minoxidil in Alopecia: A Review. Am. J. Clin. Dermatol. 2019, 20, 237–250. [Google Scholar] [CrossRef]
- White, S.I.; Friedmann, P.S. Topical Minoxidil Lacks Efficacy in Alopecia Areata. Arch. Dermatol. 1985, 121, 591. [Google Scholar] [CrossRef]
- Carey, R.M.; Calhoun, D.A.; Bakris, G.L.; Brook, R.D.; Daugherty, S.L.; Dennison-Himmelfarb, C.R.; Egan, B.M.; Flack, J.M.; Gidding, S.S.; Judd, E.; et al. Resistant Hypertension: Detection, Evaluation, and Management: A Scientific Statement from the American Heart Association. Hypertension 2018, 72, E53–E90. [Google Scholar] [CrossRef]
- Browne, R.; Stewart, L.; Williams, H.C. Is methotrexate an effective and safe treatment for maintaining hair regrowth in people with alopecia totalis? A Critically Appraised Topic. Br. J. Dermatol. 2018, 179, 609–614. [Google Scholar] [CrossRef]
- Rashidi, T.; Mahd, A.A. Treatment of persistent alopecia areata with sulfasalazine. Int. J. Dermatol. 2008, 47, 850–852. [Google Scholar] [CrossRef]
- Aghaei, S. An uncontrolled, open label study of sulfasalazine in severe alopecia areata. Indian J. Dermatol. Venereol. Leprol. 2008, 74, 611–613. [Google Scholar] [CrossRef] [PubMed]
- Taylor, C.R.; Hawk, J.L.M. PUVA treatment of alopecia areata partialis, totalis and universalis: Audit of 10 years’ experience at St John’s Institute of Dermatology. Br. J. Dermatol. 1995, 133, 914–918. [Google Scholar] [CrossRef] [PubMed]
- Healy, E.; Rogers, S. PUVA treatment for alopecia areata--does it work? A retrospective review of 102 cases. Br. J. Dermatol. 1993, 129, 42–44. [Google Scholar] [CrossRef]
- Mlacker, S.; Aldahan, A.S.; Simmons, B.J.; Shah, V.; McNamara, C.A.; Samarkandy, S.; Nouri, K. A review on laser and light-based therapies for alopecia areata. J. Cosmet. Laser Ther. 2017, 19, 93–99. [Google Scholar] [CrossRef]
- Tkachenko, E.; Okhovat, J.P.; Manjaly, P.; Huang, K.P.; Senna, M.M.; Mostaghimi, A. Complementary and alternative medicine for alopecia areata: A systematic review. J. Am. Acad. Dermatol. 2023, 88, 131–143. [Google Scholar] [CrossRef]
- Sterkens, A.; Lambert, J.; Bervoets, A. Alopecia areata: A review on diagnosis, immunological etiopathogenesis and treatment options. Clin. Exp. Med. 2021, 21, 215–230. [Google Scholar] [CrossRef]
- Jiménez-Herrera, E.A.; Rios-Garza, Z.; Peralta-Pedrero, M.L.; Cruz, F.J.-S.; Morales-Sánchez, M.A. Prognostic Factors in Mexican Patients with Patchy and Other Types of Alopecia Areata. Skin Appendage Disord. 2020, 6, 296–303. [Google Scholar] [CrossRef]
- Lyakhovitsky, A.; Aronovich, A.; Gilboa, S.; Barzilai, B.A. Alopecia areata: A long-term follow-up study of 104 patients. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 1602–1609. [Google Scholar] [CrossRef]
- Burroway, B.; Griggs, J.; Tosti, A. Alopecia totalis and universalis long-term outcomes: A review. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 709–715. [Google Scholar] [CrossRef]
- Sharma, V.K.; Dawn, G.; Kumar, B. Profile of alopecia areata in Northern India. Int. J. Dermatol. 1996, 35, 22–27. [Google Scholar] [CrossRef]
- Lew, B.L.; Shin, M.K.; Sim, W.Y. Acute diffuse and total alopecia: A new subtype of alopecia areata with a favorable prognosis. J. Am. Acad. Dermatol. 2009, 60, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, T. A new classification of alopecia areata. Dermatologica 1965, 131, 421–445. [Google Scholar] [CrossRef] [PubMed]
- Chanprapaph, K.; Mahasaksiri, T.; Kositkuljorn, C.; Leerunyakul, K.; Suchonwanit, P. Prevalence and Risk Factors Associated with the Occurrence of Autoimmune Diseases in Patients with Alopecia Areata. J. Inflamm. Res. 2021, 14, 4881–4891. [Google Scholar] [CrossRef]
- Kutlubay, Z.; Sevim Keçici, A.; Aydin, Ö.; Vehid, S.; Serdaroğlu, S. Assessment of treatment efficacy of diphenylcyclopropenone (DPCP) for alopecia areata. Turk. J. Med. Sci. 2020, 50, 1817–1824. [Google Scholar] [CrossRef]
| Most common co-morbidities: thyroid disease, diabetes mellitus, inflammatory bowel disease, systemic lupus erythematosus, rheumatoid arthritis, psoriasis with psoriatic arthritis, allergic rhinitis, asthma, eczema, contact dermatitis, depression, anxiety. Malignancies: thyroid cancer, myasthenia gravis and thymoma, primary cutaneous follicle center lymphoma, Hodgkin’s lymphoma [36,37,38,39]. Cardiovascular diseases: acute myocardial infarction [40]. |
| Triggers: anxiety, smoking, sleeping disorders, stress [8,41,42,43,44]. Infections: Epstein–Barr infectious mononucleosis, human immunodeficiency virus (HIV) infection, Human Papillomavirus Infection [45,46,47]. Vaccinations: herpes zoster, hepatitis B, Japanese encephalitis, influenza, quadrivalent HPV, COVID-19 [48,49,50,51]. Drugs: Adalimumab, Denosumab, Dupilumab, Etanercept, Infliximab, Nivolumab, Omalizumab, Pembrolizumab, Secukinumab [52,53,54,55,56,57,58,59,60,61]. Other: vitamin D deficiency, decreased serum ferritin, low serum zinc [62,63,64,65,66]. |
| Group of Drugs | Agent | Limitations | Adverse Side Effects |
|---|---|---|---|
| Glucocorticosteroids (GCS) [4,5,8,97,98,99,100,101,102,103,104] | Intralesional GCS | Require multiple treatments and cause patient discomfort and pain | Possible systemic effects Dyspigmentation Skin atrophy Anaphylaxis (rarely) |
| Topical GCS | Limited benefit in patchy AA++ Patients commonly suffer relapses Ultrapoent GCS, e.g., clobetasole, should be avoided in the eyebrow area | Folliculitis Local skin atrophy Striae Acne Teleangiectasia Dyschromia Adrenal suppression (rarely) | |
| Systemic GCS | Not favored for chronic AA due to long-term therapy safety concerns that are associated with prolonged therapy | Weight gain Cushing’s disease Hypertension Osteoporosis Acne | |
| Contact immunotherapy [103,105,106,107] | DPCP SADBE | Immunotherapy is an absolute contraindication due to opposing mechanism of action Desensitization and allergic reactions may occur in the clinicians who apply the preparation to patient’s scalp | Contact eczema Urticaria Lymphadenopathy |
| Anthralin | High relapse rate (up to 64%) Low effectiveness limits its use in clinical practice Based on the available data, the estimated efficacy is 32–33% of partial hair regrowth with the maximum effect occurring 9–15 months of therapy | Skin irritation Lymphadenopathy | |
| Hair growth stimulants [4,70,103,108,109,110,111] | Topical Minoxidil | Its efficacy as adjuvant drug is still under investigation, with some data indicating that it could potentially accelerate hair regrowth in hairless patches; however, numerous data have proven that it is ineffective as monotherapy Some clinicians recommend regular monitoring of blood pressure, heart rate and electrocardiographic changes, fundoscopic examination and renal function | Hypertrichosis Contact dermatitis Transient shedding Sparse vellus hair on various body parts Tachycardia |
| Systemic Minoxidil | Limited data on its efficacy in inducing hair regrowth; hence, it should not be used in monotherapy | Lightheadedness Fluid retention Tachycardia Headache Periorbital edema Insomnia | |
| Prostaglandin analogs [3,5,8,103] | Latanoprost Bimatoprost | Latanoprost has been associated with irreversible iridial pigmentation | Transient mild eye irritation Hyperemia Conjunctivitis |
| Steroid sparing agents [3,5,10,103,112] | Azathioprine | The literature data are insufficient to recommend this agent Thiopurine methyltransferase (TPMT) activity should be monitored prior to treatment and the dose should be modified according to TPMT activity | Gastrointestinal upset Altered thiopurine methyltransferase (TPMT) activity Elevated liver enzymes Pancreatitis Bone marrow suppression |
| Methotrexate | Currently, there are insufficient data to accurately estimate the therapeutic benefit of methotrexate as a monotherapy Its efficacy ranges vary widely in the literature data with 2.2–50% of patients achieving a therapeutic response. Better documented is the efficacy of methotrexate used in combination with GCS | Nausea Leukopenia Transient elevation of hepatic enzymes Teratogenic Nephrotoxic Hepatotoxic | |
| Ciclosporin | The high rates of recurrence after discontinuation of the drug and its adverse side effects limits its use | Nephrotoxicity Immunosuppression Arterial hypertension | |
| Miscellaneous [113,114] | Sulfasalazine | Close follow-up consisting of monitoring G6PD, blood count, biochemistry and hepatogram assesmnet is essential Discontinuation of the drug have displayed high occurrence of relapses | GI-tract distress Rashes Headaches Laboratory anomalies |
| Complementary and alternative medicine (CAM) [97,103,115,116,117,118] | Photochemotherapy. Psoralen plus ultraviolet A (PUVA) | Continued therapy is needed to maintain hair growth, which may lead to unacceptable high cumulative UVA dose High relapse rate and low response rate | Acute phototoxic reactions Hyperpigmentation Increased risk for long-term skin damage, e.g., photoaging Increased risk of skin cancer with prolonged PUVA use Nausea and gastrointestinal discomfort from oral psoralen |
| Laser therapy | Can be applied as an alternative therapy only in selected cases and as an adjuvant therapy in acutely resistant AA patches High cost with limited efficacy | Mild erythema Contact eczema Blistering Pruritus Hyperpigmentation Mild peeling of skin | |
| Aromatherapy | Results of the studies must be interpreted with caution given small study size and variable quality of study design. Disease length and severity for participants studied is unknown; consequently, generalizability to patients with varied degrees and duration of hair loss remains unclear. | Irritation at the application site Essential oils influence the skin barrier function and may induce contact dermatitis | |
| Hypnotherapy | Non-randomized trial failed to show any difference in hair growth in the treated group compared to the placebo group Effectiveness remains unclear due to small study samples in the current evidence | Headache Dizziness Anxiety Creation of false memories |
| Clinical Characteristics of Poor Prognostic Factors |
|---|
| Body hair involvement [12,120] Young age at disease onset [5,120,121,122] Extensive hair loss [5,12,120,123] Ophiasis pattern of hair loss [5,124] Family history [5,8,108,125] Severity of AA at first consultation [6] Concurrent autoimmune or atopic disease [5,122] Thyroid disease [122] Nail findings [5,126,127] Smoking [41] Episode duration longer than 1 year [5] Genetic disease association, for example, Down syndrome [5] |
| Clinical Features Reported to Increase Disease Severity |
| Extensive hair loss [12] Concomitant autoimmune disease [12] Atopic dermatitis [127] Nail findings [126] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kropidłowska, J.; Kvinen, A.; Lewandowski, M.; Nowicki, R.J.; Barańska-Rybak, W. Comparison of Current International Guidelines for the Management of Alopecia Areata—Comprehensive Review. Int. J. Mol. Sci. 2025, 26, 8632. https://doi.org/10.3390/ijms26178632
Kropidłowska J, Kvinen A, Lewandowski M, Nowicki RJ, Barańska-Rybak W. Comparison of Current International Guidelines for the Management of Alopecia Areata—Comprehensive Review. International Journal of Molecular Sciences. 2025; 26(17):8632. https://doi.org/10.3390/ijms26178632
Chicago/Turabian StyleKropidłowska, Julia, Alexandra Kvinen, Miłosz Lewandowski, Roman J. Nowicki, and Wioletta Barańska-Rybak. 2025. "Comparison of Current International Guidelines for the Management of Alopecia Areata—Comprehensive Review" International Journal of Molecular Sciences 26, no. 17: 8632. https://doi.org/10.3390/ijms26178632
APA StyleKropidłowska, J., Kvinen, A., Lewandowski, M., Nowicki, R. J., & Barańska-Rybak, W. (2025). Comparison of Current International Guidelines for the Management of Alopecia Areata—Comprehensive Review. International Journal of Molecular Sciences, 26(17), 8632. https://doi.org/10.3390/ijms26178632







