The Involvement of RIPK1 in Alopecia Areata
Abstract
1. Introduction
2. Results
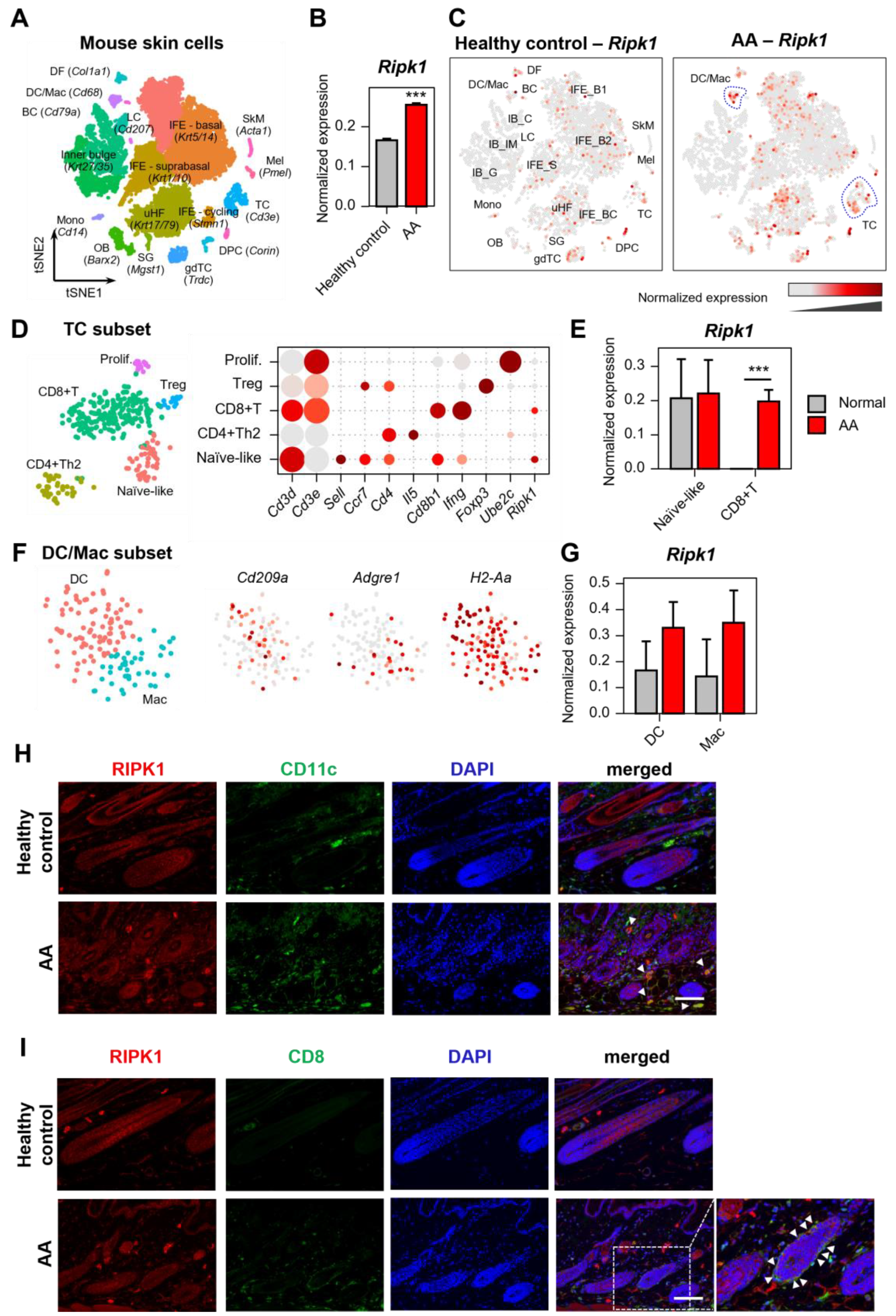
2.1. Increased Expression of Ripk1 in AA Mouse Skin
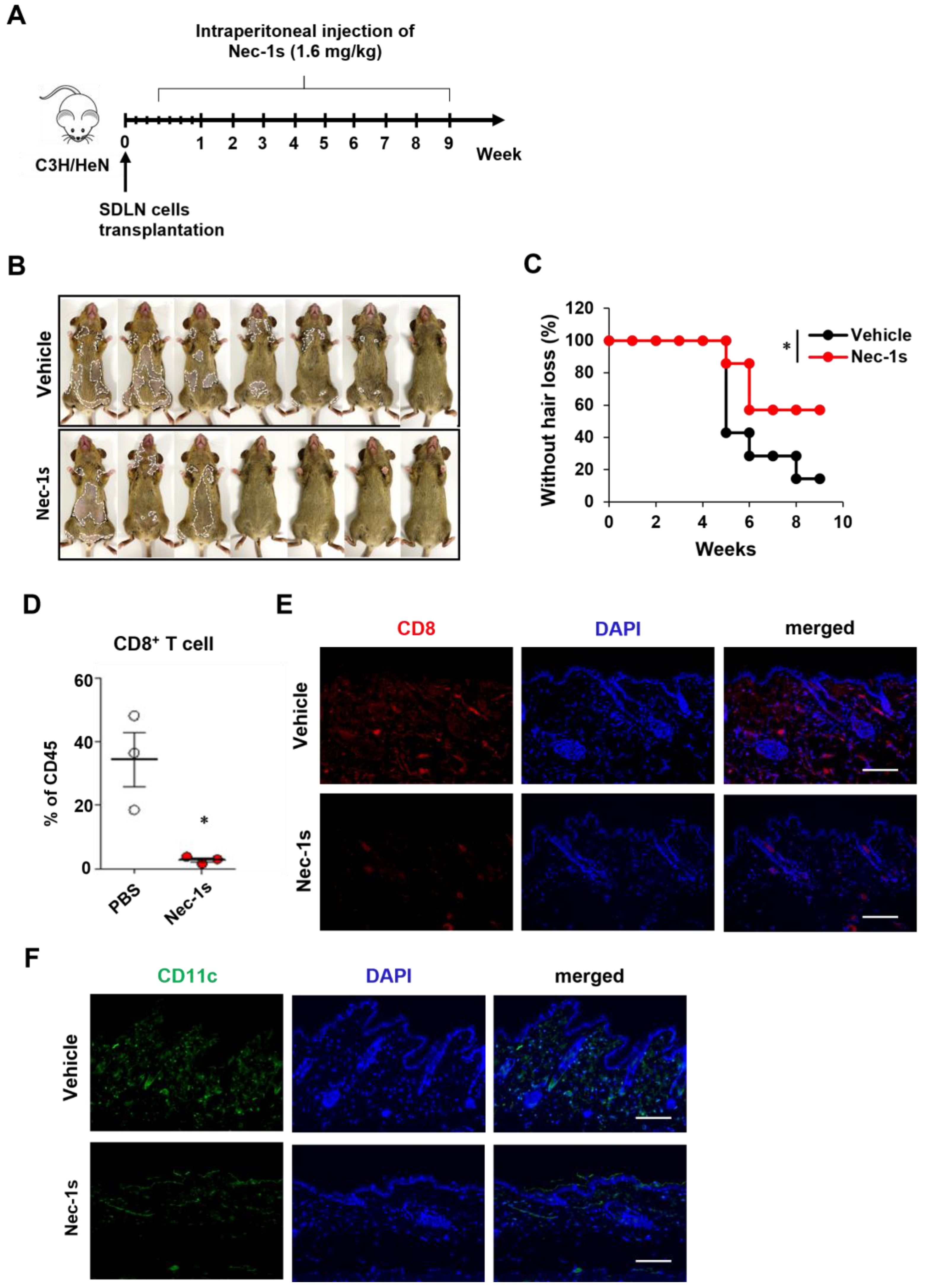
2.2. Prevention of AA Onset by Nec-1s
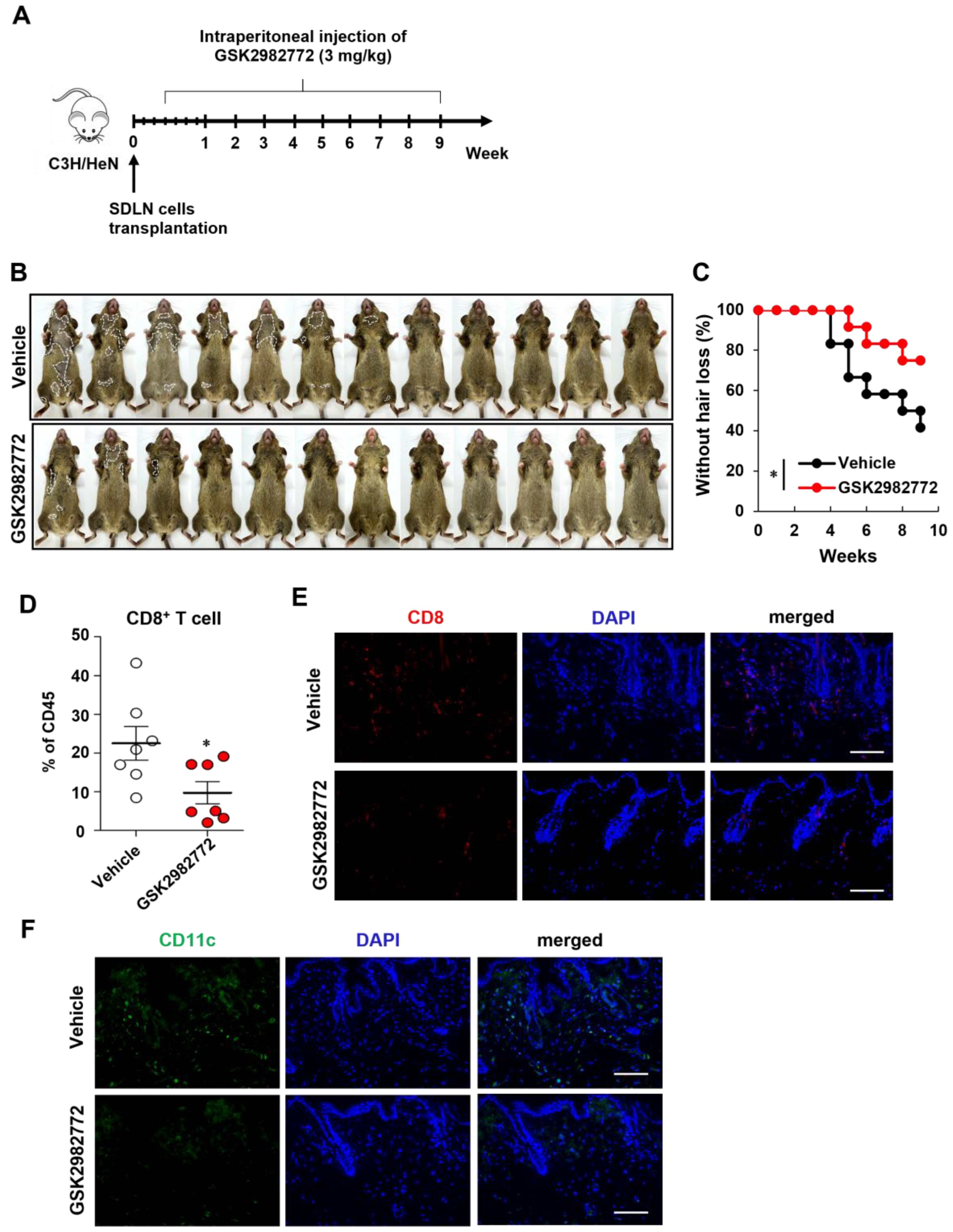
2.3. Prevention of AA Onset by GSK2982772
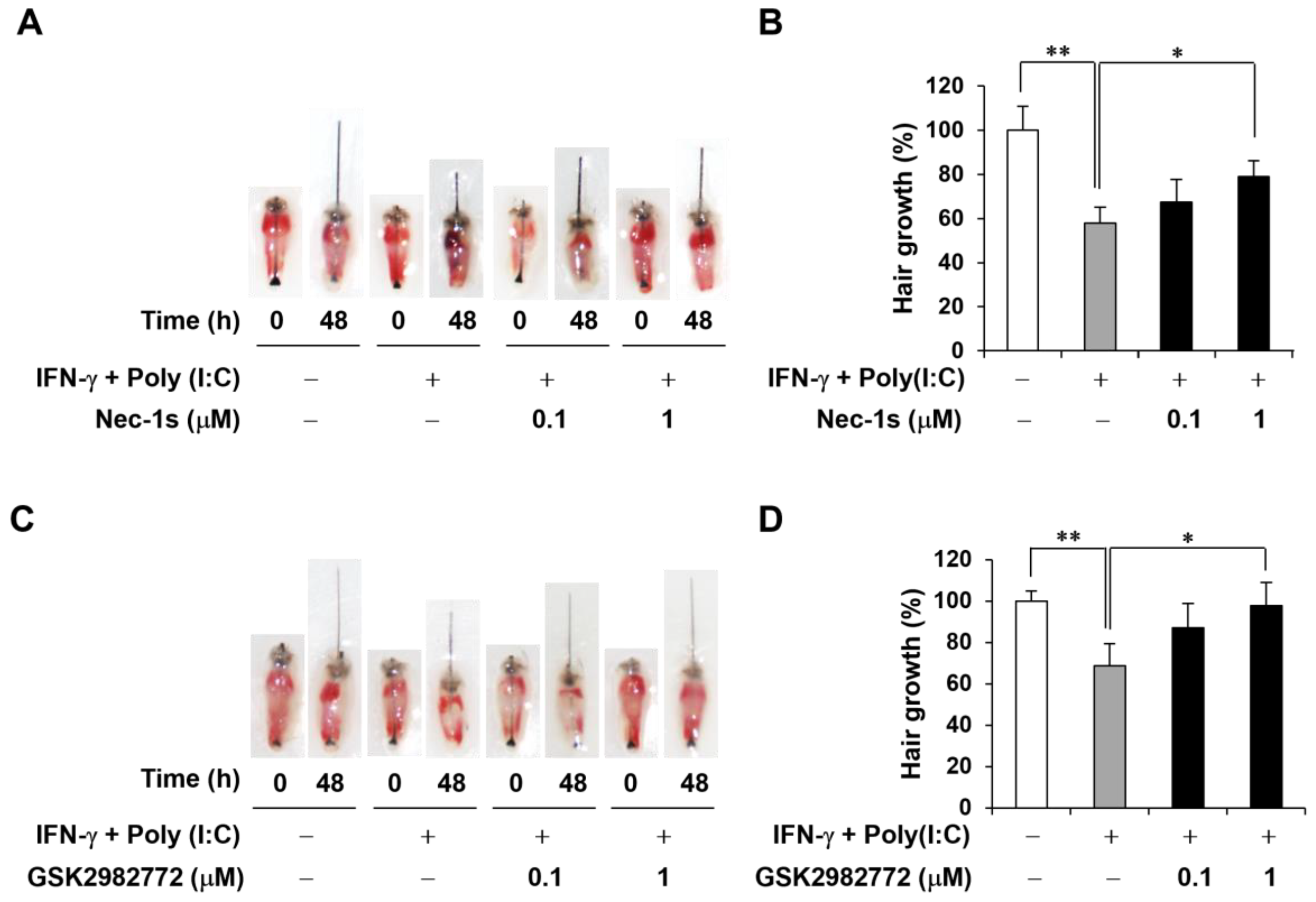
2.4. Decreased AA-like Symptoms in Mouse Vibrissa Follicles Due to RIPK1 Inhibitors
3. Discussion
4. Materials and Methods
4.1. Preparation of Single-Cell Suspension
4.2. Single-Cell RNA Sequencing
4.3. Single-Cell Transcriptome Analysis
4.4. Animals
4.5. Alopecia Areata Animal Model
4.6. Flow Cytometry Analysis
4.7. Immunostaining
4.8. Hair Organ Culture
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Degterev, A.; Ofengeim, D.; Yuan, J. Targeting RIPK1 for the treatment of human diseases. Proc. Natl. Acad. Sci. USA 2019, 116, 9714–9722. [Google Scholar] [CrossRef]
- Mifflin, L.; Ofengeim, D.; Yuan, J. Receptor-interacting protein kinase 1 (RIPK1) as a therapeutic target. Nat. Rev. Drug Discov. 2020, 19, 553–571. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Liu, P.; Yin, M.; Zhang, M.; Kuang, Y.; Zhu, W. RIPK1: A rising star in inflammatory and neoplastic skin diseases. J. Dermatol. Sci. 2020, 99, 146–151. [Google Scholar] [CrossRef]
- Zheng, M.; Choi, N.; Jang, Y.; Kwak, D.E.; Kim, Y.; Kim, W.S.; Oh, S.H.; Sung, J.H. Hair growth promotion by necrostatin-1s. Sci. Rep. 2020, 10, 17622. [Google Scholar] [CrossRef]
- Sun, P.; Wang, Z.; Li, S.; Yin, J.; Gan, Y.; Liu, S.; Lin, Z.; Wang, H.; Fan, Z.; Qu, Q.; et al. Autophagy induces hair follicle stem cell activation and hair follicle regeneration by regulating glycolysis. Cell Biosci. 2024, 14, 6. [Google Scholar] [CrossRef]
- Chai, M.; Jiang, M.; Vergnes, L.; Fu, X.; de Barros, S.C.; Doan, N.B.; Huang, W.; Chu, J.; Jiao, J.; Herschman, H.; et al. Stimulation of Hair Growth by Small Molecules that Activate Autophagy. Cell Rep. 2019, 27, 3413–3421.e3. [Google Scholar] [CrossRef]
- Lindner, G.; Botchkarev, V.A.; Botchkareva, N.V.; Ling, G.; van der Veen, C.; Paus, R. Analysis of apoptosis during hair follicle regression (catagen). Am. J. Pathol. 1997, 151, 1601–1617. [Google Scholar]
- Botchkareva, N.V.; Ahluwalia, G.; Shander, D. Apoptosis in the hair follicle. J. Investig. Dermatol. 2006, 126, 258–264. [Google Scholar] [CrossRef]
- Jang, Y.H.; Jin, M.; Moon, S.Y.; Eun, D.H.; Lee, W.J.; Lee, S.J.; Kim, M.K.; Kim, S.H.; Kim do, W. Investigation on the role of necroptosis in alopecia areata: A preliminary study. J. Am. Acad. Dermatol. 2016, 75, 436–439. [Google Scholar] [CrossRef]
- Xing, L.; Dai, Z.; Jabbari, A.; Cerise, J.E.; Higgins, C.A.; Gong, W.; de Jong, A.; Harel, S.; DeStefano, G.M.; Rothman, L.; et al. Alopecia areata is driven by cytotoxic T lymphocytes and is reversed by JAK inhibition. Nat. Med. 2014, 20, 1043–1049. [Google Scholar] [CrossRef]
- Dai, Z.; Chen, J.; Chang, Y.; Christiano, A.M. Selective inhibition of JAK3 signaling is sufficient to reverse alopecia areata. JCI Insight 2021, 6, e142205. [Google Scholar] [CrossRef]
- Dai, Z.; Wang, E.H.C.; Petukhova, L.; Chang, Y.; Lee, E.Y.; Christiano, A.M. Blockade of IL-7 signaling suppresses inflammatory responses and reverses alopecia areata in C3H/HeJ mice. Sci. Adv. 2021, 7, eabd1866. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Kim, M.H.; Park, S.G.; Kim, W.S.; Oh, S.H.; Sung, J.H. CXCL12 Neutralizing Antibody Promotes Hair Growth in Androgenic Alopecia and Alopecia Areata. Int. J. Mol. Sci. 2024, 25, 1705. [Google Scholar] [CrossRef]
- Harris, P.A.; Berger, S.B.; Jeong, J.U.; Nagilla, R.; Bandyopadhyay, D.; Campobasso, N.; Capriotti, C.A.; Cox, J.A.; Dare, L.; Dong, X.; et al. Discovery of a First-in-Class Receptor Interacting Protein 1 (RIP1) Kinase Specific Clinical Candidate (GSK2982772) for the Treatment of Inflammatory Diseases. J. Med. Chem. 2017, 60, 1247–1261. [Google Scholar] [CrossRef]
- Degterev, A.; Huang, Z.; Boyce, M.; Li, Y.; Jagtap, P.; Mizushima, N.; Cuny, G.D.; Mitchison, T.J.; Moskowitz, M.A.; Yuan, J. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat. Chem. Biol. 2005, 1, 112–119. [Google Scholar] [CrossRef]
- Yamaguchi, H.L.; Yamaguchi, Y.; Peeva, E. Pathogenesis of Alopecia Areata and Vitiligo: Commonalities and Differences. Int. J. Mol. Sci. 2024, 25, 4409. [Google Scholar] [CrossRef]
- Shin, J.M.; Choi, D.K.; Sohn, K.C.; Koh, J.W.; Lee, Y.H.; Seo, Y.J.; Kim, C.D.; Lee, J.H.; Lee, Y. Induction of alopecia areata in C3H/HeJ mice using polyinosinic-polycytidylic acid (poly[I:C]) and interferon-gamma. Sci. Rep. 2018, 8, 12518. [Google Scholar] [CrossRef] [PubMed]
- Morgun, E.I.; Pozdniakova, E.D.; Vorotelyak, E.A. Expression of Protein Kinases RIPK-1 and RIPK-3 in Mouse and Human Hair Follicle. Dokl. Biochem. Biophys. 2020, 494, 252–255. [Google Scholar] [CrossRef]
- Zeberkiewicz, M.; Rudnicka, L.; Malejczyk, J. Immunology of alopecia areata. Cent. Eur. J. Immunol. 2020, 45, 325–333. [Google Scholar] [CrossRef]
- Lee, E.Y.; Dai, Z.; Jaiswal, A.; Wang, E.H.C.; Anandasabapathy, N.; Christiano, A.M. Functional interrogation of lymphocyte subsets in alopecia areata using single-cell RNA sequencing. Proc. Natl. Acad. Sci. USA 2023, 120, e2305764120. [Google Scholar] [CrossRef]
- Speir, M.; Djajawi, T.M.; Conos, S.A.; Tye, H.; Lawlor, K.E. Targeting RIP Kinases in Chronic Inflammatory Disease. Biomolecules 2021, 11, 646. [Google Scholar] [CrossRef] [PubMed]
- Jhun, J.; Lee, S.H.; Kim, S.Y.; Ryu, J.; Kwon, J.Y.; Na, H.S.; Jung, K.; Moon, S.J.; Cho, M.L.; Min, J.K. RIPK1 inhibition attenuates experimental autoimmune arthritis via suppression of osteoclastogenesis. J. Transl. Med. 2019, 17, 84. [Google Scholar] [CrossRef]
- Duan, X.; Liu, X.; Liu, N.; Huang, Y.; Jin, Z.; Zhang, S.; Ming, Z.; Chen, H. Inhibition of keratinocyte necroptosis mediated by RIPK1/RIPK3/MLKL provides a protective effect against psoriatic inflammation. Cell Death Dis. 2020, 11, 134. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Carbonell, R.; Yao, S.J.; Das, S.; Guma, M. Dysregulation of Intestinal Epithelial Cell RIPK Pathways Promotes Chronic Inflammation in the IBD Gut. Front. Immunol. 2019, 10, 1094. [Google Scholar] [CrossRef] [PubMed]
- Stuart, T.; Butler, A.; Hoffman, P.; Hafemeister, C.; Papalexi, E.; Mauck, W.M., 3rd; Hao, Y.; Stoeckius, M.; Smibert, P.; Satija, R. Comprehensive Integration of Single-Cell Data. Cell 2019, 177, 1888–1902.e21. [Google Scholar] [CrossRef]
- Lee, K.J.; An, S.; Kim, M.Y.; Kim, S.M.; Jeong, W.I.; Ko, H.J.; Yang, Y.M.; Noh, M.; Han, Y.H. Hepatic TREM2(+) macrophages express matrix metalloproteinases to control fibrotic scar formation. Immunol. Cell Biol. 2023, 101, 216–230. [Google Scholar] [CrossRef]
- McGinnis, C.S.; Murrow, L.M.; Gartner, Z.J. DoubletFinder: Doublet Detection in Single-Cell RNA Sequencing Data Using Artificial Nearest Neighbors. Cell Syst. 2019, 8, 329–337.e4. [Google Scholar] [CrossRef]
- Wang, E.H.C.; Khosravi-Maharlooei, M.; Jalili, R.B.; Yu, R.; Ghahary, A.; Shapiro, J.; McElwee, K.J. Transfer of Alopecia Areata to C3H/HeJ Mice Using Cultured Lymph Node-Derived Cells. J. Investig. Dermatol. 2015, 135, 2530–2532. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.H.C.; McElwee, K.J. Nonsurgical Induction of Alopecia Areata in C3H/HeJ Mice via Adoptive Transfer of Cultured Lymphoid Cells. Methods Mol. Biol. 2020, 2154, 121–131. [Google Scholar]
- Jindo, T.; Imai, R.; Takamori, K.; Ogawa, H. Organ culture of mouse vibrissal hair follicles in serum-free medium. J. Dermatol. 1993, 20, 756–762. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.; Zheng, M.; An, S.; Park, I.G.; Song, L.; Noh, M.; Sung, J.-H. The Involvement of RIPK1 in Alopecia Areata. Int. J. Mol. Sci. 2025, 26, 1565. https://doi.org/10.3390/ijms26041565
Kim H, Zheng M, An S, Park IG, Song L, Noh M, Sung J-H. The Involvement of RIPK1 in Alopecia Areata. International Journal of Molecular Sciences. 2025; 26(4):1565. https://doi.org/10.3390/ijms26041565
Chicago/Turabian StyleKim, Hyunju, Mei Zheng, Seungchan An, In Guk Park, Leegu Song, Minsoo Noh, and Jong-Hyuk Sung. 2025. "The Involvement of RIPK1 in Alopecia Areata" International Journal of Molecular Sciences 26, no. 4: 1565. https://doi.org/10.3390/ijms26041565
APA StyleKim, H., Zheng, M., An, S., Park, I. G., Song, L., Noh, M., & Sung, J.-H. (2025). The Involvement of RIPK1 in Alopecia Areata. International Journal of Molecular Sciences, 26(4), 1565. https://doi.org/10.3390/ijms26041565






