Melanosome Transport and Processing in Skin Pigmentation: Mechanisms and Targets for Pigmentation Modulation
Abstract
1. Introduction
2. Pre-Transport Phase: A Brief Overview of Melanogenesis
2.1. Melanin Synthesis
2.2. Melanosome Maturation
3. The Transport Phase
3.1. Intracellular Transport of Melanosomes
3.1.1. Microtubule-Based Long-Range Anterograde Transport
3.1.2. The Switch of Melanosome from Microtubule to Actin Filament Networks
3.1.3. Actin-Based Short-Range Transport
3.1.4. Microtubule-Based Long-Range Retrograde Transport
3.2. Intercellular Transfer of Melanocores or Melanosomes
4. Melanin Post-Transfer Processing in Keratinocytes
4.1. Melanin Uptake
4.2. Melanin Retention and Degradation
5. Relevance to Cosmetic Dermatology and Therapeutics: Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Taylor, A.; Pawaskar, M.; Taylor, S.L.; Balkrishnan, R.; Feldman, S.R. Prevalence of pigmentary disorders and their impact on quality of life: A prospective cohort study. J. Cosmet. Dermatol. 2008, 7, 164–168. [Google Scholar] [CrossRef] [PubMed]
- Mpofana, N.; Paulse, M.; Gqaleni, N.; Makgobole, M.U.; Pillay, P.; Hussein, A.; Dlova, N.C. The Effect of Melasma on the Quality of Life in People with Darker Skin Types Living in Durban, South Africa. Int. J. Environ. Res. Public Health 2023, 20, 7068. [Google Scholar] [CrossRef] [PubMed]
- Park, H.Y.; Kosmadaki, M.; Yaar, M.; Gilchrest, B.A. Cellular mechanisms regulating human melanogenesis. Cell Mol. Life Sci. 2009, 66, 1493–1506. [Google Scholar] [CrossRef]
- D’Mello, S.A.; Finlay, G.J.; Baguley, B.C.; Askarian-Amiri, M.E. Signaling Pathways in Melanogenesis. Int. J. Mol. Sci. 2016, 17, 1144. [Google Scholar] [CrossRef]
- Bento-Lopes, L.; Cabaço, L.C.; Charneca, J.; Neto, M.V.; Seabra, M.C.; Barral, D.C. Melanin’s Journey from Melanocytes to Keratinocytes Uncovering the Molecular Mechanisms of Melanin Transfer and Processing. Int. J. Mol. Sci. 2023, 24, 1289. [Google Scholar] [CrossRef]
- Wu, X.; Hammer, J.A. Melanosome transfer: It is best to give and receive. Curr. Opin. Cell Biol. 2014, 29, 1–7. [Google Scholar] [CrossRef]
- Kobayashi, N.; Nakagawa, A.; Muramatsu, T.; Yamashina, Y.; Shirai, T.; Hashimoto, M.W.; Ishigaki, Y.; Ohnishi, T.; Mori, T. Supranuclear melanin caps reduce ultraviolet induced DNA photoproducts in human epidermis. J. Investig. Dermatol. 1998, 110, 806–810. [Google Scholar] [CrossRef]
- Lan, Y.; Zeng, W.; Wang, Y.; Dong, X.; Shen, X.; Gu, Y.; Zhang, W.; Lu, H. Opsin 3 mediates UVA-induced keratinocyte supranuclear melanin cap formation. Commun. Biol. 2023, 6, 238. [Google Scholar] [CrossRef]
- Neto, M.V.; Hall, M.J.; Charneca, J.; Escrevente, C.; Seabra, M.C.; Barral, D.C. Photoprotective Melanin Is Maintained within Keratinocytes in Storage Lysosomes. J. Investig. Dermatol. 2025, 145, 1155–1165.e3. [Google Scholar] [CrossRef] [PubMed]
- Correia, M.S.; Moreiras, H.; Pereira, F.J.C.; Neto, M.V.; Festas, T.C.; Tarafder, A.K.; Ramalho, J.S.; Seabra, M.C.; Barral, D.C. Melanin Transferred to Keratinocytes Resides in Nondegradative Endocytic Compartments. J. Investig. Dermatol. 2018, 138, 637–646. [Google Scholar] [CrossRef]
- Murase, D.; Hachiya, A.; Takano, K.; Hicks, R.; Visscher, M.O.; Kitahara, T.; Hase, T.; Takema, Y.; Yoshimori, T. Autophagy has a significant role in determining skin color by regulating melanosome degradation in keratinocytes. J. Investig. Dermatol. 2013, 133, 2416–2424. [Google Scholar] [CrossRef] [PubMed]
- Homma, T.; Kageyama, S.; Nishikawa, A.; Nagata, K. Melanosome degradation in epidermal keratinocytes related to lysosomal protease cathepsin V. Biochem. Biophys. Res. Commun. 2018, 500, 339–343. [Google Scholar] [CrossRef]
- Marubashi, S.; Fukuda, M. Rab7B/42 Is Functionally Involved in Protein Degradation on Melanosomes in Keratinocytes. Cell Struct. Funct. 2020, 45, 45–55. [Google Scholar] [CrossRef]
- Lee, K.W.; Kim, M.; Lee, S.H.; Kim, K.D. The Function of Autophagy as a Regulator of Melanin Homeostasis. Cells 2022, 11, 2085. [Google Scholar] [CrossRef]
- Ebanks, J.P.; Koshoffer, A.; Wickett, R.R.; Schwemberger, S.; Babcock, G.; Hakozaki, T.; Boissy, R.E. Epidermal keratinocytes from light vs. dark skin exhibit differential degradation of melanosomes. J. Investig. Dermatol. 2011, 131, 1226–1233. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kim, J.; Ahn, Y.; Lee, E.J.; Hwang, S.; Almurayshid, A.; Park, K.; Chung, H.J.; Kim, H.J.; Lee, S.H.; et al. Autophagy induction can regulate skin pigmentation by causing melanosome degradation in keratinocytes and melanocytes. Pigment. Cell Melanoma Res. 2020, 33, 403–415. [Google Scholar] [CrossRef]
- Yang, Z.; Zeng, B.; Pan, Y.; Huang, P.; Wang, C. Autophagy participates in isoliquiritigenin-induced melanin degradation in human epidermal keratinocytes through PI3K/AKT/mTOR signaling. Biomed. Pharmacother. 2018, 97, 248–254. [Google Scholar] [CrossRef]
- Wang, F.; Ma, W.; Fan, D.; Hu, J.; An, X.; Wang, Z. The biochemistry of melanogenesis: An insight into the function and mechanism of melanogenesis-related proteins. Front. Mol. Biosci. 2024, 11, 1440187. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Sun, X.; Liu, Y.; Wang, W.; Yang, H.; Ge, Y.; Yang, Y.; Chen, X.; Lin, T. Metformin inhibits melanin synthesis and melanosome transfer through the cAMP pathway. Sci. Rep. 2025, 15, 11442. [Google Scholar] [CrossRef]
- Markiewicz, E.; Karaman-Jurukovska, N.; Mammone, T.; Idowu, O.C. Post-Inflammatory Hyperpigmentation in Dark Skin: Molecular Mechanism and Skincare Implications. Clin. Cosmet. Investig. Dermatol. 2022, 15, 2555–2565. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H.; Fukuda, M. Establishment of a synchronized tyrosinase transport system revealed a role of Tyrp1 in efficient melanogenesis by promoting tyrosinase targeting to melanosomes. Sci. Rep. 2024, 14, 2529. [Google Scholar] [CrossRef]
- Hodgkinson, C.A.; Moore, K.J.; Nakayama, A.; Steingrimsson, E.; Copeland, N.G.; Jenkins, N.A.; Arnheiter, H. Mutations at the mouse microphthalmia locus are associated with defects in a gene encoding a novel basic-helix-loop-helix-zipper protein. Cell 1993, 74, 395–404. [Google Scholar] [CrossRef]
- Wolf Horrell, E.M.; Boulanger, M.C.; D’Orazio, J.A. Melanocortin 1 Receptor: Structure, Function, and Regulation. Front. Genet. 2016, 7, 95. [Google Scholar] [CrossRef]
- Kawakami, A.; Fisher, D.E. The master role of microphthalmia-associated transcription factor in melanocyte and melanoma biology. Lab. Investig. 2017, 97, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Bertolotto, C.; Abbe, P.; Hemesath, T.J.; Bille, K.; Fisher, D.E.; Ortonne, J.P.; Ballotti, R. Microphthalmia gene product as a signal transducer in cAMP-induced differentiation of melanocytes. J. Cell Biol. 1998, 142, 827–835. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Hemesath, T.J.; Takemoto, C.M.; Horstmann, M.A.; Wells, A.G.; Price, E.R.; Fisher, D.Z.; Fisher, D.E. c-Kit triggers dual phosphorylations, which couple activation and degradation of the essential melanocyte factor Mi. Genes. Dev. 2000, 14, 301–312. [Google Scholar] [CrossRef]
- Schepsky, A.; Bruser, K.; Gunnarsson, G.J.; Goodall, J.; Hallsson, J.H.; Goding, C.R.; Steingrimsson, E.; Hecht, A. The microphthalmia-associated transcription factor Mitf interacts with beta-catenin to determine target gene expression. Mol. Cell Biol. 2006, 26, 8914–8927. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Olle, L.; Proano-Perez, E.; Aparicio, C.; Guerrero, M.; Munoz-Cano, R.; Martin, M. MRGPRX2 signaling involves the Lysyl-tRNA synthetase and MITF pathway. Front. Immunol. 2023, 14, 1154108. [Google Scholar] [CrossRef]
- Yardman-Frank, J.M.; Fisher, D.E. Skin pigmentation and its control: From ultraviolet radiation to stem cells. Exp. Dermatol. 2021, 30, 560–571. [Google Scholar] [CrossRef]
- Schallreuter, K.U.; Kothari, S.; Chavan, B.; Spencer, J.D. Regulation of melanogenesis--controversies and new concepts. Exp. Dermatol. 2008, 17, 395–404. [Google Scholar] [CrossRef]
- Waku, T.; Nakada, S.; Masuda, H.; Sumi, H.; Wada, A.; Hirose, S.; Aketa, I.; Kobayashi, A. The CNC-family transcription factor Nrf3 coordinates the melanogenesis cascade through macropinocytosis and autophagy regulation. Cell Rep. 2023, 42, 111906. [Google Scholar] [CrossRef]
- Berson, J.F.; Harper, D.C.; Tenza, D.; Raposo, G.; Marks, M.S. Pmel17 initiates premelanosome morphogenesis within multivesicular bodies. Mol. Biol. Cell 2001, 12, 3451–3464. [Google Scholar] [CrossRef]
- Bissig, C.; Rochin, L.; van Niel, G. PMEL Amyloid Fibril Formation: The Bright Steps of Pigmentation. Int. J. Mol. Sci. 2016, 17, 1438. [Google Scholar] [CrossRef]
- Hoashi, T.; Watabe, H.; Muller, J.; Yamaguchi, Y.; Vieira, W.D.; Hearing, V.J. MART-1 is required for the function of the melanosomal matrix protein PMEL17/GP100 and the maturation of melanosomes. J. Biol. Chem. 2005, 280, 14006–14016. [Google Scholar] [CrossRef] [PubMed]
- Kushimoto, T.; Basrur, V.; Valencia, J.; Matsunaga, J.; Vieira, W.D.; Ferrans, V.J.; Muller, J.; Appella, E.; Hearing, V.J. A model for melanosome biogenesis based on the purification and analysis of early melanosomes. Proc. Natl. Acad. Sci. USA 2001, 98, 10698–10703. [Google Scholar] [CrossRef] [PubMed]
- Giordano, F.; Bonetti, C.; Surace, E.M.; Marigo, V.; Raposo, G. The ocular albinism type 1 (OA1) G-protein-coupled receptor functions with MART-1 at early stages of melanogenesis to control melanosome identity and composition. Hum. Mol. Genet. 2009, 18, 4530–4545. [Google Scholar] [CrossRef] [PubMed]
- van Niel, G.; Charrin, S.; Simoes, S.; Romao, M.; Rochin, L.; Saftig, P.; Marks, M.S.; Rubinstein, E.; Raposo, G. The tetraspanin CD63 regulates ESCRT-independent and -dependent endosomal sorting during melanogenesis. Dev. Cell 2011, 21, 708–721. [Google Scholar] [CrossRef]
- Cortese, K.; Giordano, F.; Surace, E.M.; Venturi, C.; Ballabio, A.; Tacchetti, C.; Marigo, V. The ocular albinism type 1 (OA1) gene controls melanosome maturation and size. Investig. Ophthalmol. Vis. Sci. 2005, 46, 4358–4364. [Google Scholar] [CrossRef]
- Giordano, F.; Simoes, S.; Raposo, G. The ocular albinism type 1 (OA1) GPCR is ubiquitinated and its traffic requires endosomal sorting complex responsible for transport (ESCRT) function. Proc. Natl. Acad. Sci. USA 2011, 108, 11906–11911. [Google Scholar] [CrossRef]
- Lamason, R.L.; Mohideen, M.A.; Mest, J.R.; Wong, A.C.; Norton, H.L.; Aros, M.C.; Jurynec, M.J.; Mao, X.; Humphreville, V.R.; Humbert, J.E.; et al. SLC24A5, a putative cation exchanger, affects pigmentation in zebrafish and humans. Science 2005, 310, 1782–1786. [Google Scholar] [CrossRef]
- Ginger, R.S.; Askew, S.E.; Ogborne, R.M.; Wilson, S.; Ferdinando, D.; Dadd, T.; Smith, A.M.; Kazi, S.; Szerencsei, R.T.; Winkfein, R.J.; et al. SLC24A5 encodes a trans-Golgi network protein with potassium-dependent sodium-calcium exchange activity that regulates human epidermal melanogenesis. J. Biol. Chem. 2008, 283, 5486–5495. [Google Scholar] [CrossRef]
- Sitaram, A.; Marks, M.S. Mechanisms of protein delivery to melanosomes in pigment cells. Physiology 2012, 27, 85–99. [Google Scholar] [CrossRef]
- Bellono, N.W.; Escobar, I.E.; Lefkovith, A.J.; Marks, M.S.; Oancea, E. An intracellular anion channel critical for pigmentation. eLife 2014, 3, e04543. [Google Scholar] [CrossRef] [PubMed]
- Ancans, J.; Tobin, D.J.; Hoogduijn, M.J.; Smit, N.P.; Wakamatsu, K.; Thody, A.J. Melanosomal pH controls rate of melanogenesis, eumelanin/phaeomelanin ratio and melanosome maturation in melanocytes and melanoma cells. Exp. Cell Res. 2001, 268, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Bin, B.H.; Bhin, J.; Yang, S.H.; Shin, M.; Nam, Y.J.; Choi, D.H.; Shin, D.W.; Lee, A.Y.; Hwang, D.; Cho, E.G.; et al. Membrane-Associated Transporter Protein (MATP) Regulates Melanosomal pH and Influences Tyrosinase Activity. PLoS ONE 2015, 10, e0129273. [Google Scholar] [CrossRef] [PubMed]
- Le, L.; Escobar, I.E.; Ho, T.; Lefkovith, A.J.; Latteri, E.; Haltaufderhyde, K.D.; Dennis, M.K.; Plowright, L.; Sviderskaya, E.V.; Bennett, D.C.; et al. SLC45A2 protein stability and regulation of melanosome pH determine melanocyte pigmentation. Mol. Biol. Cell 2020, 31, 2687–2702. [Google Scholar] [CrossRef] [PubMed]
- Bajpai, V.K.; Swigut, T.; Mohammed, J.; Naqvi, S.; Arreola, M.; Tycko, J.; Kim, T.C.; Pritchard, J.K.; Bassik, M.C.; Wysocka, J. A genome-wide genetic screen uncovers determinants of human pigmentation. Science 2023, 381, eade6289. [Google Scholar] [CrossRef]
- Setty, S.R.; Tenza, D.; Sviderskaya, E.V.; Bennett, D.C.; Raposo, G.; Marks, M.S. Cell-specific ATP7A transport sustains copper-dependent tyrosinase activity in melanosomes. Nature 2008, 454, 1142–1146. [Google Scholar] [CrossRef]
- Ishida, M.; Ohbayashi, N.; Maruta, Y.; Ebata, Y.; Fukuda, M. Functional involvement of Rab1A in microtubule-dependent anterograde melanosome transport in melanocytes. J. Cell Sci. 2012, 125, 5177–5187. [Google Scholar] [CrossRef]
- Moreiras, H.; Seabra, M.C.; Barral, D.C. Melanin Transfer in the Epidermis: The Pursuit of Skin Pigmentation Control Mechanisms. Int. J. Mol. Sci. 2021, 22, 4466. [Google Scholar] [CrossRef]
- Ishida, M.; Ohbayashi, N.; Fukuda, M. Rab1A regulates anterograde melanosome transport by recruiting kinesin-1 to melanosomes through interaction with SKIP. Sci. Rep. 2015, 5, 8238. [Google Scholar] [CrossRef]
- Robinson, C.L.; Evans, R.D.; Briggs, D.A.; Ramalho, J.S.; Hume, A.N. Inefficient recruitment of kinesin-1 to melanosomes precludes it from facilitating their transport. J. Cell Sci. 2017, 130, 2056–2065. [Google Scholar] [CrossRef]
- Jiang, M.; Paniagua, A.E.; Volland, S.; Wang, H.; Balaji, A.; Li, D.G.; Lopes, V.S.; Burgess, B.L.; Williams, D.S. Microtubule motor transport in the delivery of melanosomes to the actin-rich apical domain of the retinal pigment epithelium. J. Cell Sci. 2020, 133, 242214. [Google Scholar] [CrossRef]
- Rogers, S.L.; Gelfand, V.I. Myosin cooperates with microtubule motors during organelle transport in melanophores. Curr. Biol. 1998, 8, 161–164. [Google Scholar] [CrossRef] [PubMed]
- Provance, D.W., Jr.; Wei, M.; Ipe, V.; Mercer, J.A. Cultured melanocytes from dilute mutant mice exhibit dendritic morphology and altered melanosome distribution. Proc. Natl. Acad. Sci. USA 1996, 93, 14554–14558. [Google Scholar] [CrossRef] [PubMed]
- Oberhofer, A.; Spieler, P.; Rosenfeld, Y.; Stepp, W.L.; Cleetus, A.; Hume, A.N.; Mueller-Planitz, F.; Okten, Z. Myosin Va’s adaptor protein melanophilin enforces track selection on the microtubule and actin networks in vitro. Proc. Natl. Acad. Sci. USA 2017, 114, E4714–E4723. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Sakamoto, T.; Zhang, F.; Sellers, J.R.; Hammer, J.A., 3rd. In vitro reconstitution of a transport complex containing Rab27a, melanophilin and myosin Va. FEBS Lett. 2006, 580, 5863–5868. [Google Scholar] [CrossRef] [PubMed]
- Ramkumar, A.; Murthy, D.; Raja, D.A.; Singh, A.; Krishnan, A.; Khanna, S.; Vats, A.; Thukral, L.; Sharma, P.; Sivasubbu, S.; et al. Classical autophagy proteins LC3B and ATG4B facilitate melanosome movement on cytoskeletal tracks. Autophagy 2017, 13, 1331–1347. [Google Scholar] [CrossRef]
- Zhang, J.; Yue, J.; Wu, X. Spectraplakin family proteins—Cytoskeletal crosslinkers with versatile roles. J. Cell Sci. 2017, 130, 2447–2457. [Google Scholar] [CrossRef]
- Leung, C.L.; Sun, D.; Zheng, M.; Knowles, D.R.; Liem, R.K. Microtubule actin cross-linking factor (MACF): A hybrid of dystonin and dystrophin that can interact with the actin and microtubule cytoskeletons. J. Cell Biol. 1999, 147, 1275–1286. [Google Scholar] [CrossRef]
- Cusseddu, R.; Robert, A.; Cote, J.F. Strength Through Unity: The Power of the Mega-Scaffold MACF1. Front. Cell Dev. Biol. 2021, 9, 641727. [Google Scholar] [CrossRef]
- Li, X.; Goult, B.T.; Ballestrem, C.; Zacharchenko, T. The structural basis of the talin-KANK1 interaction that coordinates the actin and microtubule cytoskeletons at focal adhesions. Open Biol. 2023, 13, 230058. [Google Scholar] [CrossRef] [PubMed]
- Bahadoran, P.; Aberdam, E.; Mantoux, F.; Busca, R.; Bille, K.; Yalman, N.; de Saint-Basile, G.; Casaroli-Marano, R.; Ortonne, J.P.; Ballotti, R. Rab27a: A key to melanosome transport in human melanocytes. J. Cell Biol. 2001, 152, 843–850. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, T.S.; Ariga, H.; Fukuda, M. The actin-binding domain of Slac2-a/melanophilin is required for melanosome distribution in melanocytes. Mol. Cell Biol. 2003, 23, 5245–5255. [Google Scholar] [CrossRef] [PubMed]
- Strom, M.; Hume, A.N.; Tarafder, A.K.; Barkagianni, E.; Seabra, M.C. A family of Rab27-binding proteins. Melanophilin links Rab27a and myosin Va function in melanosome transport. J. Biol. Chem. 2002, 277, 25423–25430. [Google Scholar] [CrossRef]
- Lambert, J.; Onderwater, J.; Vander Haeghen, Y.; Vancoillie, G.; Koerten, H.K.; Mommaas, A.M.; Naeyaert, J.M. Myosin V colocalizes with melanosomes and subcortical actin bundles not associated with stress fibers in human epidermal melanocytes. J. Investig. Dermatol. 1998, 111, 835–840. [Google Scholar] [CrossRef]
- Jo, C.S.; Park, H.I.; Jung, H.J.; Park, J.I.; Lee, J.E.; Myung, C.H.; Hwang, J.S. A novel function of Prohibitin on melanosome transport in melanocytes. Theranostics 2020, 10, 3880–3891. [Google Scholar] [CrossRef]
- Ohbayashi, N.; Maruta, Y.; Ishida, M.; Fukuda, M. Melanoregulin regulates retrograde melanosome transport through interaction with the RILP-p150Glued complex in melanocytes. J. Cell Sci. 2012, 125, 1508–1518. [Google Scholar] [CrossRef]
- Aktary, Z.; Conde-Perez, A.; Rambow, F.; Di Marco, M.; Amblard, F.; Hurbain, I.; Raposo, G.; Delevoye, C.; Coscoy, S.; Larue, L. A role for Dynlt3 in melanosome movement, distribution, acidity and transfer. Commun. Biol. 2021, 4, 423. [Google Scholar] [CrossRef]
- Maruta, Y.; Fukuda, M. Large Rab GTPase Rab44 regulates microtubule-dependent retrograde melanosome transport in melanocytes. J. Biol. Chem. 2022, 298, 102508. [Google Scholar] [CrossRef]
- Matsui, T.; Ohbayashi, N.; Fukuda, M. The Rab interacting lysosomal protein (RILP) homology domain functions as a novel effector domain for small GTPase Rab36: Rab36 regulates retrograde melanosome transport in melanocytes. J. Biol. Chem. 2012, 287, 28619–28631. [Google Scholar] [CrossRef] [PubMed]
- Jordens, I.; Westbroek, W.; Marsman, M.; Rocha, N.; Mommaas, M.; Huizing, M.; Lambert, J.; Naeyaert, J.M.; Neefjes, J. Rab7 and Rab27a control two motor protein activities involved in melanosomal transport. Pigment. Cell Res. 2006, 19, 412–423. [Google Scholar] [CrossRef] [PubMed]
- Van Den Bossche, K.; Naeyaert, J.M.; Lambert, J. The quest for the mechanism of melanin transfer. Traffic 2006, 7, 769–778. [Google Scholar] [CrossRef]
- Okazaki, K.; Uzuka, M.; Morikawa, F.; Toda, K.; Seiji, M. Transfer mechanism of melanosomes in epidermal cell culture. J. Investig. Dermatol. 1976, 67, 541–547. [Google Scholar] [CrossRef]
- Yamamoto, O.; Bhawan, J. Three modes of melanosome transfers in Caucasian facial skin: Hypothesis based on an ultrastructural study. Pigment. Cell Res. 1994, 7, 158–169. [Google Scholar] [CrossRef]
- Mottaz, J.H.; Zelickson, A.S. Melanin transfer: A possible phagocytic process. J. Investig. Dermatol. 1967, 49, 605–610. [Google Scholar] [CrossRef]
- Wolff, K. Melanocyte-keratinocyte interactions in vivo: The fate of melanosomes. Yale J. Biol. Med. 1973, 46, 384–396. [Google Scholar]
- Scott, G.; Leopardi, S.; Printup, S.; Madden, B.C. Filopodia are conduits for melanosome transfer to keratinocytes. J. Cell Sci. 2002, 115, 1441–1451. [Google Scholar] [CrossRef]
- Singh, S.K.; Kurfurst, R.; Nizard, C.; Schnebert, S.; Perrier, E.; Tobin, D.J. Melanin transfer in human skin cells is mediated by filopodia--a model for homotypic and heterotypic lysosome-related organelle transfer. FASEB J. 2010, 24, 3756–3769. [Google Scholar] [CrossRef]
- Domingues, L.; Hurbain, I.; Gilles-Marsens, F.; Sires-Campos, J.; Andre, N.; Dewulf, M.; Romao, M.; Viaris de Lesegno, C.; Mace, A.S.; Blouin, C.; et al. Coupling of melanocyte signaling and mechanics by caveolae is required for human skin pigmentation. Nat. Commun. 2020, 11, 2988. [Google Scholar] [CrossRef]
- Singh, S.K.; Baker, R.; Sikkink, S.K.; Nizard, C.; Schnebert, S.; Kurfurst, R.; Tobin, D.J. E-cadherin mediates ultraviolet radiation- and calcium-induced melanin transfer in human skin cells. Exp. Dermatol. 2017, 26, 1125–1133. [Google Scholar] [CrossRef]
- Beaumont, K.A.; Hamilton, N.A.; Moores, M.T.; Brown, D.L.; Ohbayashi, N.; Cairncross, O.; Cook, A.L.; Smith, A.G.; Misaki, R.; Fukuda, M.; et al. The recycling endosome protein Rab17 regulates melanocytic filopodia formation and melanosome trafficking. Traffic 2011, 12, 627–643. [Google Scholar] [CrossRef] [PubMed]
- Swift, J.A. Transfer of Melanin Granules from Melanocytes to the Cortical Cells of Human Hair. Nature 1964, 203, 976–977. [Google Scholar] [CrossRef] [PubMed]
- Tarafder, A.K.; Bolasco, G.; Correia, M.S.; Pereira, F.J.C.; Iannone, L.; Hume, A.N.; Kirkpatrick, N.; Picardo, M.; Torrisi, M.R.; Rodrigues, I.P.; et al. Rab11b mediates melanin transfer between donor melanocytes and acceptor keratinocytes via coupled exo/endocytosis. J. Investig. Dermatol. 2014, 134, 1056–1066. [Google Scholar] [CrossRef] [PubMed]
- Moreiras, H.; Pereira, F.J.C.; Neto, M.V.; Bento-Lopes, L.; Festas, T.C.; Seabra, M.C.; Barral, D.C. The exocyst is required for melanin exocytosis from melanocytes and transfer to keratinocytes. Pigment. Cell Melanoma Res. 2020, 33, 366–371. [Google Scholar] [CrossRef]
- Cabaco, L.C.; Bento-Lopes, L.; Neto, M.V.; Ferreira, A.; Staubli, W.B.L.; Ramalho, J.S.; Seabra, M.C.; Barral, D.C. RAB3A Regulates Melanin Exocytosis and Transfer Induced by Keratinocyte-Conditioned Medium. JID Innov. 2022, 2, 100139. [Google Scholar] [CrossRef]
- Moreiras, H.; Bento-Lopes, L.; Neto, M.V.; Escrevente, C.; Cabaco, L.C.; Hall, M.J.; Ramalho, J.S.; Seabra, M.C.; Barral, D.C. Melanocore uptake by keratinocytes occurs through phagocytosis and involves protease-activated receptor-2 internalization. Traffic 2022, 23, 331–345. [Google Scholar] [CrossRef]
- Ando, H.; Niki, Y.; Ito, M.; Akiyama, K.; Matsui, M.S.; Yarosh, D.B.; Ichihashi, M. Melanosomes are transferred from melanocytes to keratinocytes through the processes of packaging, release, uptake, and dispersion. J. Investig. Dermatol. 2012, 132, 1222–1229. [Google Scholar] [CrossRef]
- Ando, H.; Niki, Y.; Yoshida, M.; Ito, M.; Akiyama, K.; Kim, J.H.; Yoon, T.J.; Matsui, M.S.; Yarosh, D.B.; Ichihashi, M. Involvement of pigment globules containing multiple melanosomes in the transfer of melanosomes from melanocytes to keratinocytes. Cell Logist. 2011, 1, 12–20. [Google Scholar] [CrossRef]
- Tadokoro, R.; Murai, H.; Sakai, K.I.; Okui, T.; Yokota, Y.; Takahashi, Y. Melanosome transfer to keratinocyte in the chicken embryonic skin is mediated by vesicle release associated with Rho-regulated membrane blebbing. Sci. Rep. 2016, 6, 38277. [Google Scholar] [CrossRef]
- Halprin, K.M. Epidermal "turnover time"--a re-examination. Br. J. Dermatol. 1972, 86, 14–19. [Google Scholar] [CrossRef]
- Ebanks, J.P.; Wickett, R.R.; Boissy, R.E. Mechanisms regulating skin pigmentation: The rise and fall of complexion coloration. Int. J. Mol. Sci. 2009, 10, 4066–4087. [Google Scholar] [CrossRef] [PubMed]
- Seiberg, M.; Paine, C.; Sharlow, E.; Andrade-Gordon, P.; Costanzo, M.; Eisinger, M.; Shapiro, S.S. The protease-activated receptor 2 regulates pigmentation via keratinocyte-melanocyte interactions. Exp. Cell Res. 2000, 254, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Ando, H.; Niki, Y.; Yoshida, M.; Ito, M.; Akiyama, K.; Kim, J.H.; Yoon, T.J.; Lee, J.H.; Matsui, M.S.; Ichihashi, M. Keratinocytes in culture accumulate phagocytosed melanosomes in the perinuclear area. Pigment. Cell Melanoma Res. 2010, 23, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Belleudi, F.; Purpura, V.; Scrofani, C.; Persechino, F.; Leone, L.; Torrisi, M.R. Expression and signaling of the tyrosine kinase FGFR2b/KGFR regulates phagocytosis and melanosome uptake in human keratinocytes. FASEB J. 2011, 25, 170–181. [Google Scholar] [CrossRef]
- Cardinali, G.; Bolasco, G.; Aspite, N.; Lucania, G.; Lotti, L.V.; Torrisi, M.R.; Picardo, M. Melanosome transfer promoted by keratinocyte growth factor in light and dark skin-derived keratinocytes. J. Investig. Dermatol. 2008, 128, 558–567. [Google Scholar] [CrossRef]
- Nanni, M.; Ranieri, D.; Raffa, S.; Torrisi, M.R.; Belleudi, F. Interplay between FGFR2b-induced autophagy and phagocytosis: Role of PLCgamma-mediated signalling. J. Cell Mol. Med. 2018, 22, 668–683. [Google Scholar] [CrossRef]
- Koike, S.; Yamasaki, K.; Yamauchi, T.; Shimada-Omori, R.; Tsuchiyama, K.; Ando, H.; Aiba, S. TLR3 stimulation induces melanosome endo/phagocytosis through RHOA and CDC42 in human epidermal keratinocyte. J. Dermatol. Sci. 2019, 96, 168–177. [Google Scholar] [CrossRef]
- Zhou, B.K.; Boissy, R.E.; Pifko-Hirst, S.; Moran, D.J.; Orlow, S.J. Lysosome-associated membrane protein-1 (LAMP-1) is the melanocyte vesicular membrane glycoprotein band II. J. Investig. Dermatol. 1993, 100, 110–114. [Google Scholar] [CrossRef]
- Yun, C.Y.; Choi, N.; Lee, J.U.; Lee, E.J.; Kim, J.Y.; Choi, W.J.; Oh, S.H.; Sung, J.H. Marliolide Derivative Induces Melanosome Degradation via Nrf2/p62-Mediated Autophagy. Int. J. Mol. Sci. 2021, 22, 3995. [Google Scholar] [CrossRef]
- Jager, S.; Bucci, C.; Tanida, I.; Ueno, T.; Kominami, E.; Saftig, P.; Eskelinen, E.L. Role for Rab7 in maturation of late autophagic vacuoles. J. Cell Sci. 2004, 117, 4837–4848. [Google Scholar] [CrossRef]
- Pillaiyar, T.; Manickam, M.; Namasivayam, V. Skin whitening agents: Medicinal chemistry perspective of tyrosinase inhibitors. J. Enzyme Inhib. Med. Chem. 2017, 32, 403–425. [Google Scholar] [CrossRef] [PubMed]
- Qian, W.; Liu, W.; Zhu, D.; Cao, Y.; Tang, A.; Gong, G.; Su, H. Natural skin-whitening compounds for the treatment of melanogenesis (Review). Exp. Ther. Med. 2020, 20, 173–185. [Google Scholar] [CrossRef]
- Zolghadri, S.; Bahrami, A.; Hassan Khan, M.T.; Munoz-Munoz, J.; Garcia-Molina, F.; Garcia-Canovas, F.; Saboury, A.A. A comprehensive review on tyrosinase inhibitors. J. Enzyme Inhib. Med. Chem. 2019, 34, 279–309. [Google Scholar] [CrossRef] [PubMed]
- Saeedi, M.; Eslamifar, M.; Khezri, K. Kojic acid applications in cosmetic and pharmaceutical preparations. Biomed. Pharmacother. 2019, 110, 582–593. [Google Scholar] [CrossRef] [PubMed]
- Maeda, K.; Fukuda, M. Arbutin: Mechanism of its depigmenting action in human melanocyte culture. J. Pharmacol. Exp. Ther. 1996, 276, 765–769. [Google Scholar] [CrossRef]
- Saeedi, M.; Khezri, K.; Seyed Zakaryaei, A.; Mohammadamini, H. A comprehensive review of the therapeutic potential of alpha-arbutin. Phytother. Res. 2021, 35, 4136–4154. [Google Scholar] [CrossRef]
- Fitton, A.; Goa, K.L. Azelaic acid. A review of its pharmacological properties and therapeutic efficacy in acne and hyperpigmentary skin disorders. Drugs 1991, 41, 780–798. [Google Scholar] [CrossRef]
- Nazzaro-Porro, M.; Passi, S. Identification of tyrosinase inhibitors in cultures of Pityrosporum. J. Investig. Dermatol. 1978, 71, 205–208. [Google Scholar] [CrossRef]
- Pinnell, S.R. Cutaneous photodamage, oxidative stress, and topical antioxidant protection. J. Am. Acad. Dermatol. 2003, 48, 1–22. [Google Scholar] [CrossRef]
- Hakozaki, T.; Minwalla, L.; Zhuang, J.; Chhoa, M.; Matsubara, A.; Miyamoto, K.; Greatens, A.; Hillebrand, G.G.; Bissett, D.L.; Boissy, R.E. The effect of niacinamide on reducing cutaneous pigmentation and suppression of melanosome transfer. Br. J. Dermatol. 2002, 147, 20–31. [Google Scholar] [CrossRef]
- Cabanes, J.; Chazarra, S.; Garcia-Carmona, F. Kojic acid, a cosmetic skin whitening agent, is a slow-binding inhibitor of catecholase activity of tyrosinase. J. Pharm. Pharmacol. 1994, 46, 982–985. [Google Scholar] [CrossRef]
- Kameyama, K.; Sakai, C.; Kondoh, S.; Yonemoto, K.; Nishiyama, S.; Tagawa, M.; Murata, T.; Ohnuma, T.; Quigley, J.; Dorsky, A.; et al. Inhibitory effect of magnesium L-ascorbyl-2-phosphate (VC-PMG) on melanogenesis in vitro and in vivo. J. Am. Acad. Dermatol. 1996, 34, 29–33. [Google Scholar] [CrossRef]
- Tantanasrigul, P.; Sripha, A.; Chongmelaxme, B. The Efficacy of Topical Cosmetic Containing Alpha-Arbutin 5% and Kojic Acid 2% Compared With Triple Combination Cream for the Treatment of Melasma: A Split-Face, Evaluator-Blinded Randomized Pilot Study. J. Cosmet. Dermatol. 2025, 24, e16562. [Google Scholar] [CrossRef]
- Park, J.I.; Lee, H.Y.; Lee, J.E.; Myung, C.H.; Hwang, J.S. Inhibitory effect of 2-methyl-naphtho[1,2,3-de]quinolin-8-one on melanosome transport and skin pigmentation. Sci. Rep. 2016, 6, 29189. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Kanamaru, A.; Tada, A. Centaureidin promotes dendrite retraction of melanocytes by activating Rho. Biochim. Biophys. Acta 2006, 1760, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.G.; Kim, J.H.; Hong, S.H.; Lee, O.Y.; Kang, N.G. Exogenous pyruvate alleviates UV-induced hyperpigmentation via restraining dendrite outgrowth and Rac1 GTPase activity. J. Dermatol. Sci. 2021, 101, 101–106. [Google Scholar] [CrossRef]
- Seiberg, M.; Paine, C.; Sharlow, E.; Andrade-Gordon, P.; Costanzo, M.; Eisinger, M.; Shapiro, S.S. Inhibition of melanosome transfer results in skin lightening. J. Investig. Dermatol. 2000, 115, 162–167. [Google Scholar] [CrossRef]
- Kudo, M.; Kobayashi-Nakamura, K.; Tsuji-Naito, K. Bifunctional effects of O-methylated flavones from Scutellaria baicalensis Georgi on melanocytes: Inhibition of melanin production and intracellular melanosome transport. PLoS ONE 2017, 12, e0171513. [Google Scholar] [CrossRef]
- Gillbro, J.M.; Olsson, M.J. The melanogenesis and mechanisms of skin-lightening agents--existing and new approaches. Int. J. Cosmet. Sci. 2011, 33, 210–221. [Google Scholar] [CrossRef]
- Kumari, S.; Tien Guan Thng, S.; Kumar Verma, N.; Gautam, H.K. Melanogenesis Inhibitors. Acta Derm. Venereol. 2018, 98, 924–931. [Google Scholar] [CrossRef]
- Greatens, A.; Hakozaki, T.; Koshoffer, A.; Epstein, H.; Schwemberger, S.; Babcock, G.; Bissett, D.; Takiwaki, H.; Arase, S.; Wickett, R.R.; et al. Effective inhibition of melanosome transfer to keratinocytes by lectins and niacinamide is reversible. Exp. Dermatol. 2005, 14, 498–508. [Google Scholar] [CrossRef]
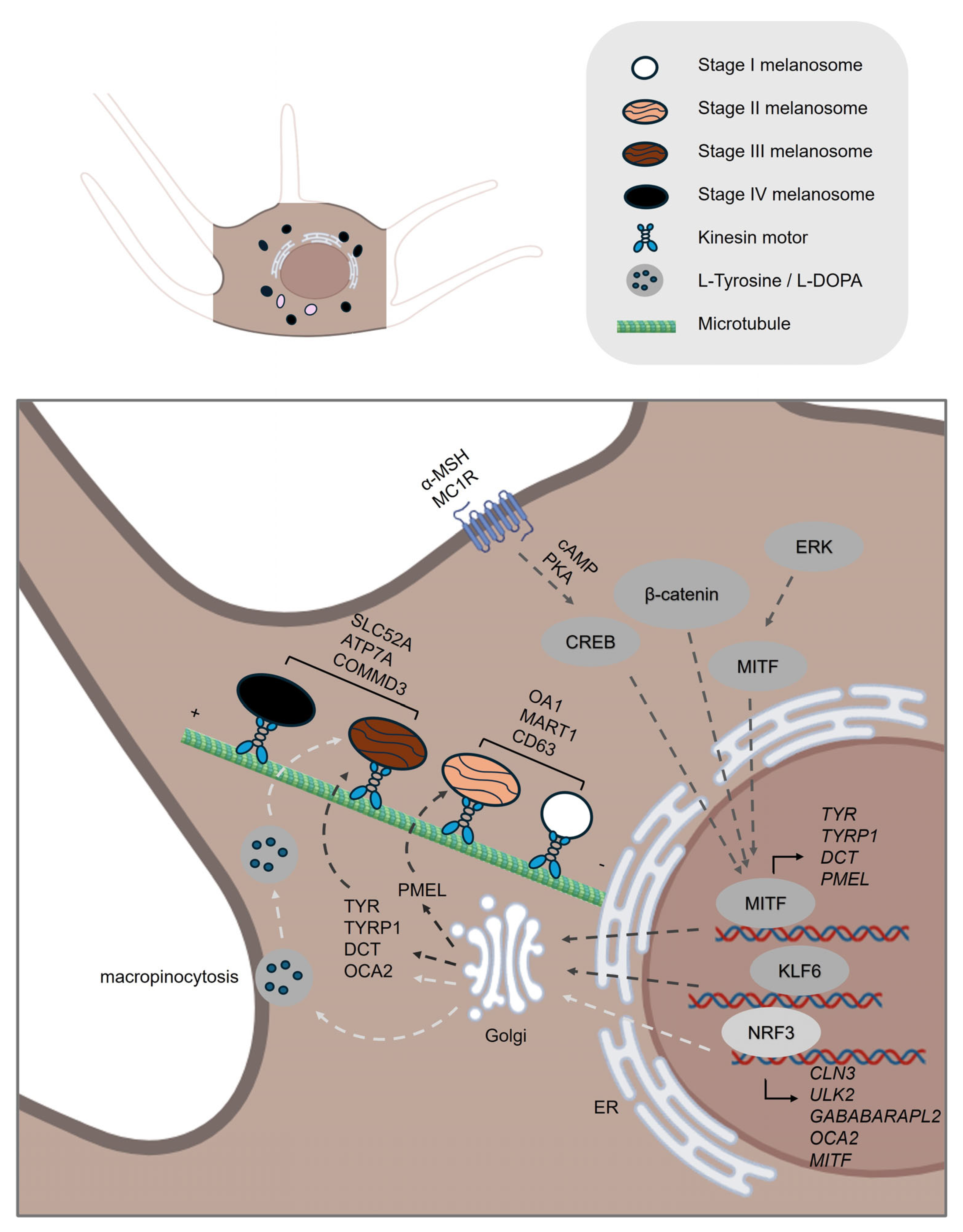
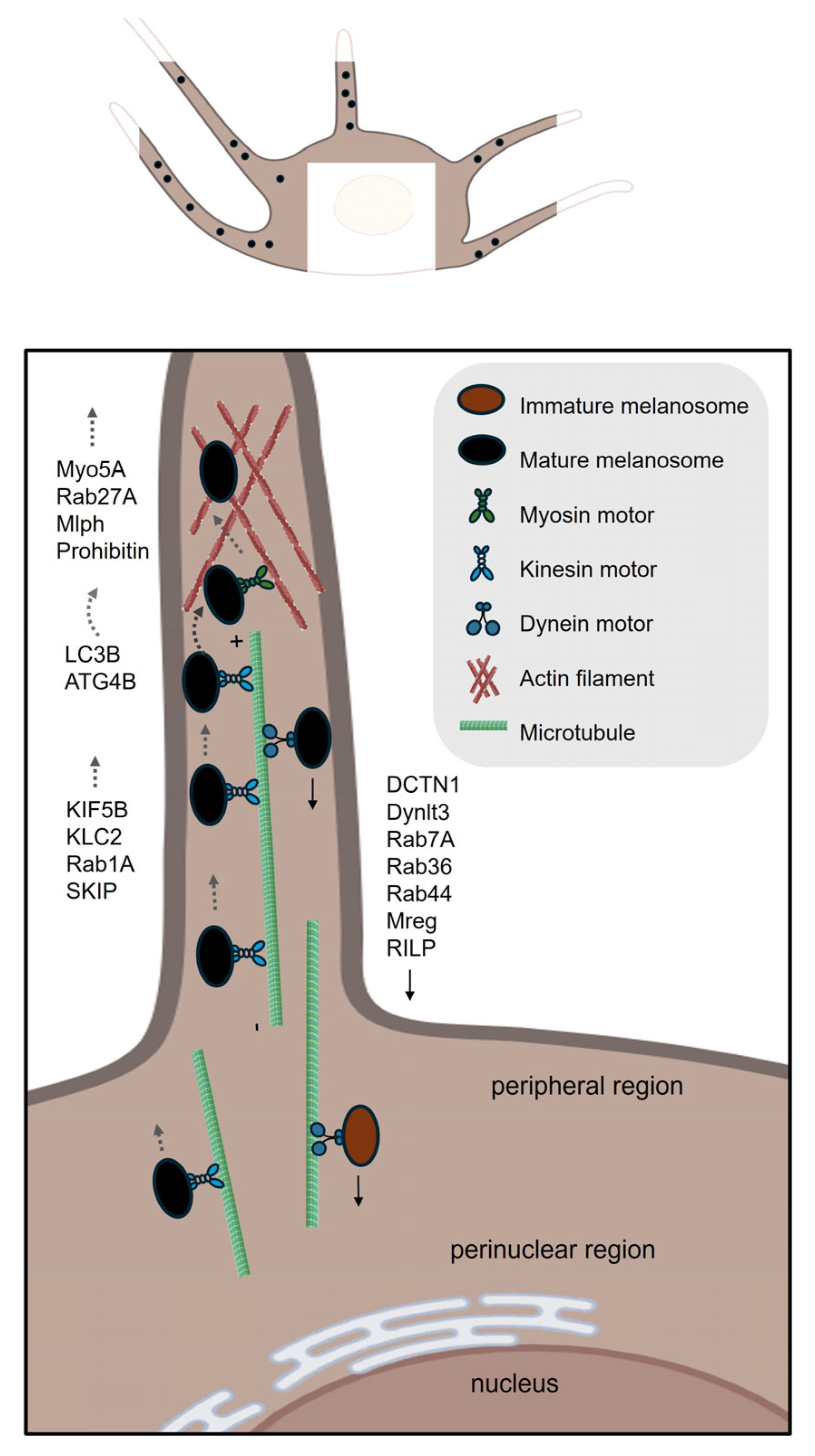
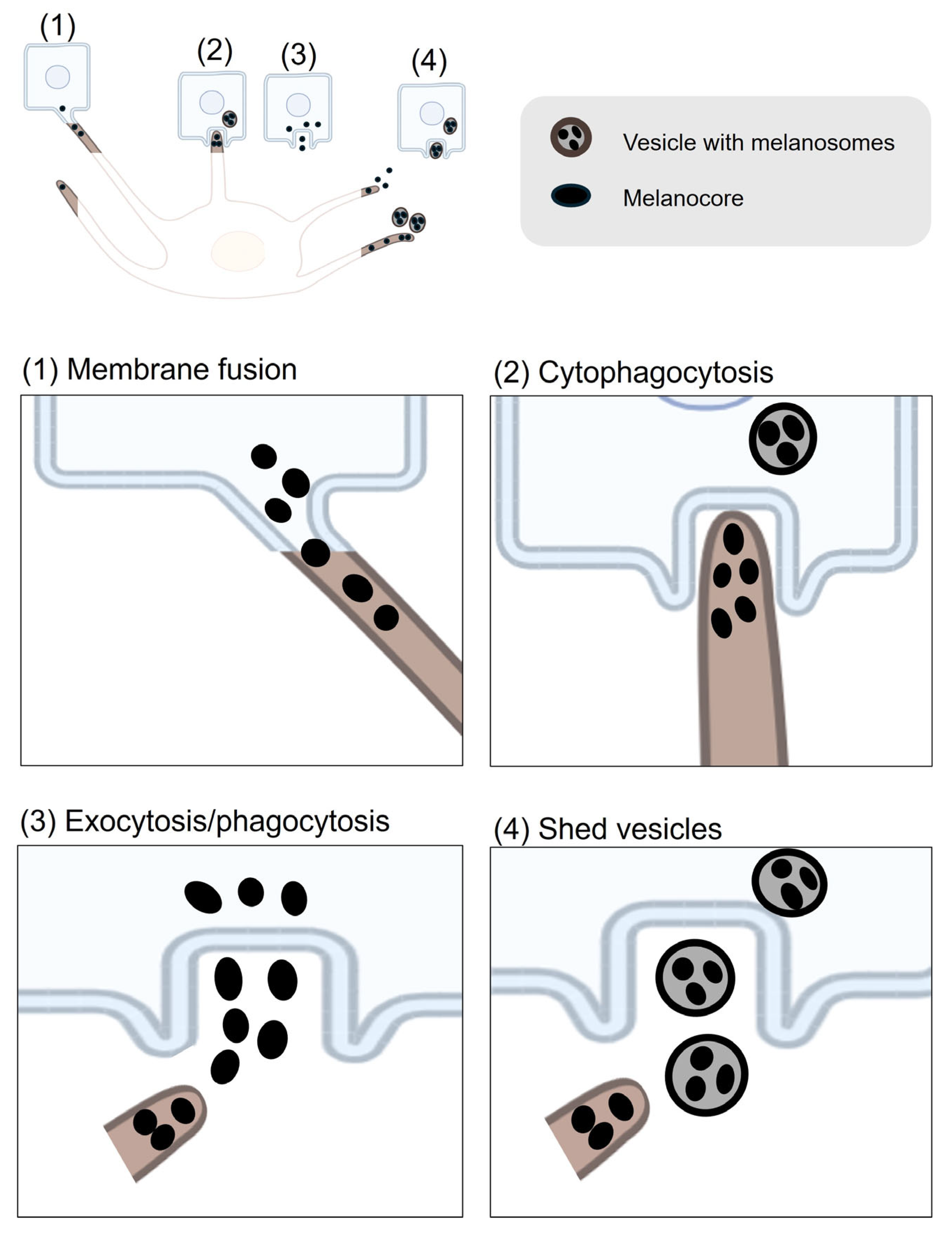
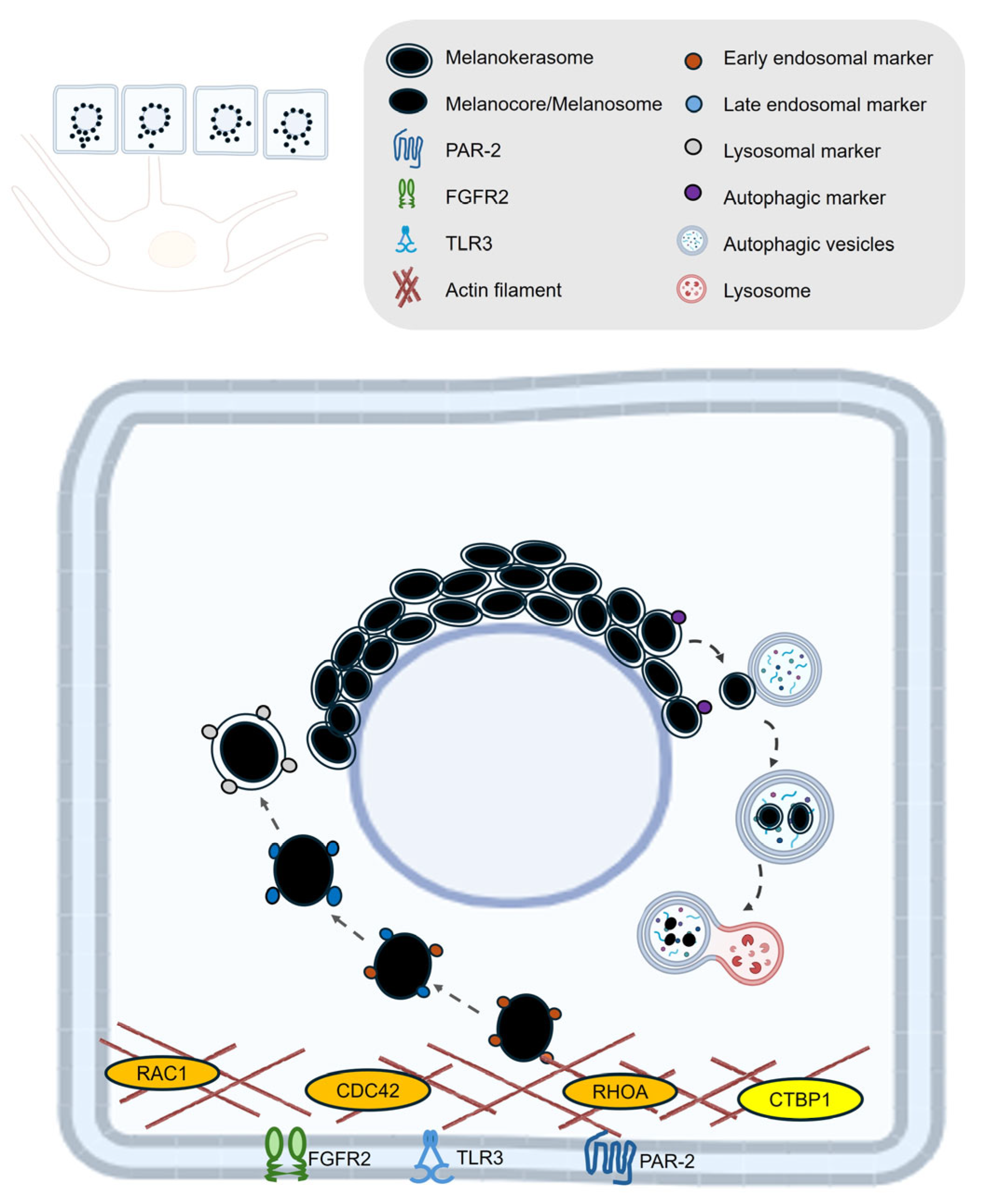
| Process | Gene ID | Function |
|---|---|---|
| (a) | ||
| Melanogenesis | TYR | Catalyzes the conversion of the amino acid tyrosine into melanin through a series of biochemical reactions [4]. |
| TYRP1 | Catalyzes the oxidation of 5,6-dihydroxyindole-2-carboxylic acid (DHICA) to indole-5,6-quinone-2-carboxylic acid in the melanin biosynthesis pathway [4]. | |
| DCT | Catalyzes the conversion of DHICA during melanin synthesis [4]. | |
| MITF | A transcription factor that controls the expression of numerous genes involved in melanin synthesis and pigmentation [24]. | |
| MC1R | A receptor activated by α-MSH that activates the cAMP signaling pathway, crucial for stimulating melanin production [23]. | |
| NRF3 | A transcription factor that regulates the uptake of melanin precursors, such as L-tyrosine and L-DOPA, through macropinocytosis and also controls the expression of autophagy-related genes involved in melanosome formation and degradation [31]. | |
| PMEL | Initiates the formation of melanosome [32]. | |
| MART1 | Forms a complex with PMEL, thereby regulating PMEL’s expression, stability, trafficking, and proteolytic processing [34]. | |
| OA1 | Functions as a key regulator of melanosome maturation by controlling melanosome biogenesis and size at distinct stages [36,38]. | |
| OCA2 | Encodes a melanosomal membrane protein that contributes to a chloride ion current, which is essential for regulating melanosomal pH [43] | |
| SLC45A2 | Encodes a melanosomal membrane transporter that functions at the late stages of melanosome maturation to maintain a neutral pH within mature melanosomes [46]. | |
| ATP7A | A copper transporter that localizes to melanosomes in a BLOC-1–dependent manner, where it supplies copper directly to TYR [48]. | |
| Transport and transfer | RAB1A | A small GTPase that promotes melanosome microtubule anterograde transport [51]. |
| SKIP (PLEKHM2) | An adaptor protein that forms a transport complex with Rab1A and kinesin-1 to facilitate melanosome microtubule anterograde transport [51]. | |
| KIF5B | The kinesin-1 heavy chain that regulates melanosome microtubule anterograde transport [51]. | |
| KCL2 | The kinesin-1 light chain that regulates melanosome microtubule anterograde transport [51]. | |
| MAP1LC3B | Induces MITF expression, mediates melanosome-microtubule interactions to facilitate melanosome trafficking on microtubule and helps to translocate melanosome from microtubule to actin [14,58]. | |
| ATG4B | Removes LC3B from microtubule and further mediates melanosome trafficking on actin [14,58]. | |
| MACF1 | Functions as a cytoskeletal crosslinker that coordinates the interaction between microtubules and actin filaments [60,61]. | |
| RAB27A | A small GTPase that promotes melanosome actin transport [63,65]. | |
| Melanophilin | An adaptor protein that bridges Rab27A/Myo5A and promotes melanosome actin transport [64,65]. | |
| MYO5A | Functions as a processive actin-based motor protein that is essential for the short-range transport and peripheral capture of melanosomes in melanocytes [65,66]. | |
| RAB36 | Promotes melanosome microtubule retrograde transport [71]. | |
| RILP | Interacts with Rab36 and promotes melanosome microtubule retrograde transport [71]. | |
| Melanoregulin | Interacts with RILP and DCTN1 and mediates melanosome microtubule retrograde transport [68]. | |
| DYNLT3 | A regulatory subunit of the cytoplasmic dynein motor complex, specifically influencing melanosome retrograde transport in melanocytes [68,69]. | |
| RAB7A | Promotes early-stage melanosome microtubule retrograde transport [72]. | |
| RAB44 | Promotes mature melanosome microtubule retrograde transport [70]. | |
| MYO10 | Is upregulated by ultraviolet radiation and Ca2+ stimulation and is important for filopodia formation and melanin transfer [81]. | |
| RAB17 | Is required for melanocyte filopodia formation and thereby facilitates pigment transfer [82]. | |
| RAB3A | Regulates melanin exocytosis, particularly under stimulation by soluble factors from differentiated keratinocytes [86]. | |
| RAB11B | Regulates keratinocytes induced melanin exocytosis and transfer [84,85]. | |
| EXOC7 | The subunits of the exocyst complex and is involved in melanin exocytosis and transfer [85]. | |
| EXOC4 | The subunits of the exocyst complex and is involved in melanin exocytosis and transfer [85]. | |
| CAV1 | Forms caveolae structures that facilitate melanocyte–keratinocyte interactions necessary for melanin transfer [80]. | |
| Process | Gene ID | Function |
| (b) | ||
| Uptake | PAR-2 | Activates phagocytic capacity of keratinocytes, receptor, promotes melanocore and melanosome uptake [10,87,88,93,94]. |
| TLR3 | UV-responsive regulator of melanin internalization. Enhances melanosome and melanocore uptake in keratinocytes via actin-dependent endocytosis, primarily by activating RhoA and Cdc42 [98]. | |
| FGFR2 | Promotes melanosome uptake through phagocytosis and links this process to autophagy, controlling both the internalization and degradation of melanosomes in keratinocytes [95,97]. | |
| RAC1 | A Rho GTPase that mainly promotes melanocore uptake [87]. | |
| CDC42 | A Rho GTPase that mainly promotes melanocore uptake [87]. | |
| RHOA | A Rho GTPase that mainly promotes melanosome uptake [87]. | |
| CTBP1 | Encodes a protein involved in membrane fission events necessary for endocytosis, particularly affecting melanosome uptake [87]. | |
| Retention and degradation | LAMP1 | Regulates lysosomal exocytosis, a process critical for melanosome transport and integration into keratinocytes. Maintains lysosomal membrane integrity, protecting against enzymatic degradation and enabling melanin’s long-term photoprotective storage in keratinocytes [5,9,13,99]. |
| EEA1 | Early endosomal marker that surrounds melanocores in keratinocytes [5,10]. | |
| RAB5 | Early endosomal marker that surrounds melanocores in keratinocytes [5,10]. | |
| P62 | Functions as an autophagy adaptor protein in keratinocytes, mediating the selective degradation of melanosomes by linking them to the autophagy machinery and facilitating their clearance through the autophagy–lysosome pathway [11,100]. | |
| ATG7 | Essential for autophagy-dependent melanosome degradation in keratinocytes by enabling the formation of autophagosomes that engulf and facilitate the lysosomal breakdown of melanin-containing compartments [11]. | |
| MAP1LC3B | LC3 (specifically LC3-II, the lipidated form of MAP1LC3B) functions in melanosome degradation in keratinocytes by marking autophagosomes that engulf melanin-containing compartments, thereby facilitating their autophagic clearance through the lysosomal pathway [11]. | |
| RAB7B | Facilitates lysosomal fusion and protein degradation on melanosomes [13]. | |
| CTSV | Lysosomal protease plays a critical role in breaking down melanosome-associated proteins and melanosome integrity, indirectly influencing melanin persistence in keratinocytes [12]. | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bao, M.; Gempeler, M.; Campiche, R. Melanosome Transport and Processing in Skin Pigmentation: Mechanisms and Targets for Pigmentation Modulation. Int. J. Mol. Sci. 2025, 26, 8630. https://doi.org/10.3390/ijms26178630
Bao M, Gempeler M, Campiche R. Melanosome Transport and Processing in Skin Pigmentation: Mechanisms and Targets for Pigmentation Modulation. International Journal of Molecular Sciences. 2025; 26(17):8630. https://doi.org/10.3390/ijms26178630
Chicago/Turabian StyleBao, Mengjing, Mathias Gempeler, and Remo Campiche. 2025. "Melanosome Transport and Processing in Skin Pigmentation: Mechanisms and Targets for Pigmentation Modulation" International Journal of Molecular Sciences 26, no. 17: 8630. https://doi.org/10.3390/ijms26178630
APA StyleBao, M., Gempeler, M., & Campiche, R. (2025). Melanosome Transport and Processing in Skin Pigmentation: Mechanisms and Targets for Pigmentation Modulation. International Journal of Molecular Sciences, 26(17), 8630. https://doi.org/10.3390/ijms26178630







