Recent Insights into the Creation of Histone Deacetylase Inhibitors for the Treatment of Human Diseases
Abstract
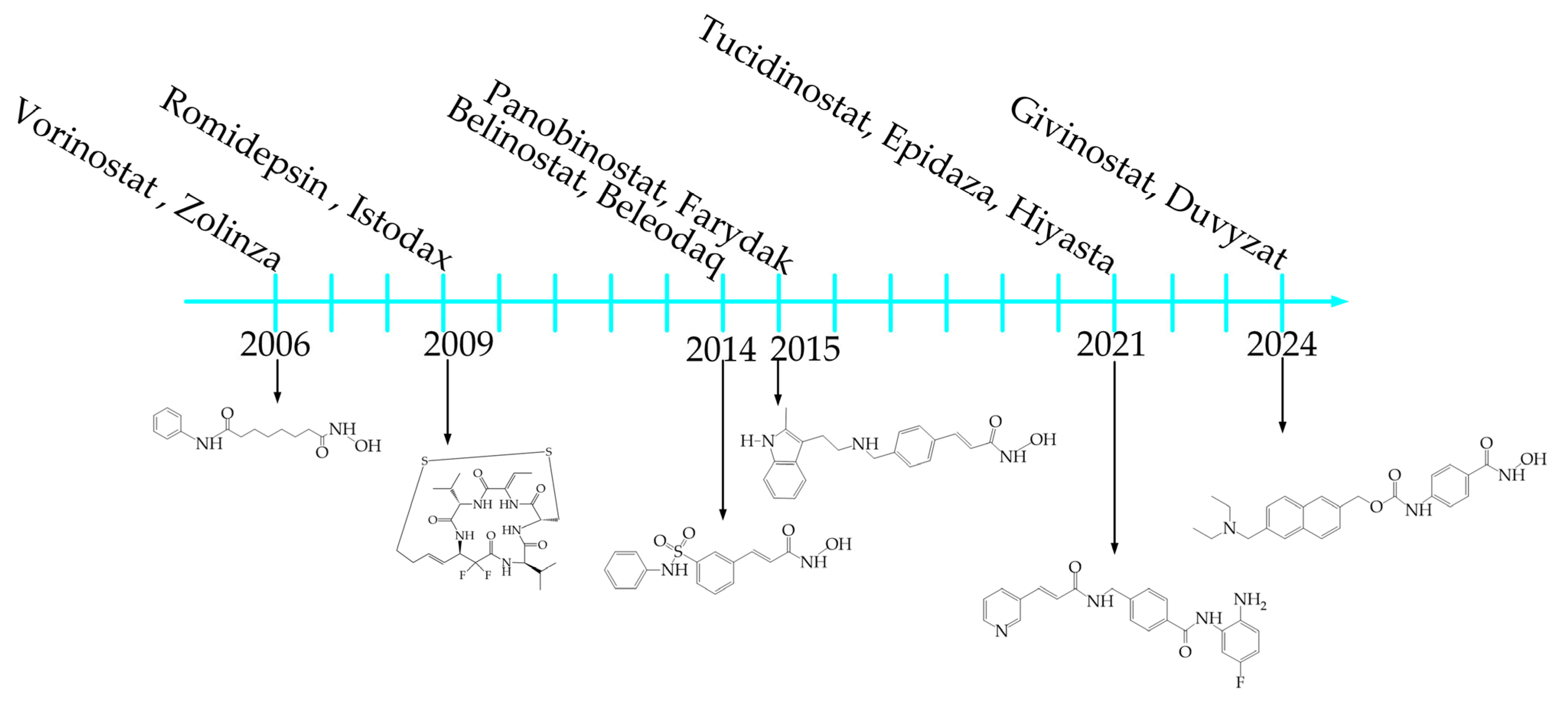
1. Introduction
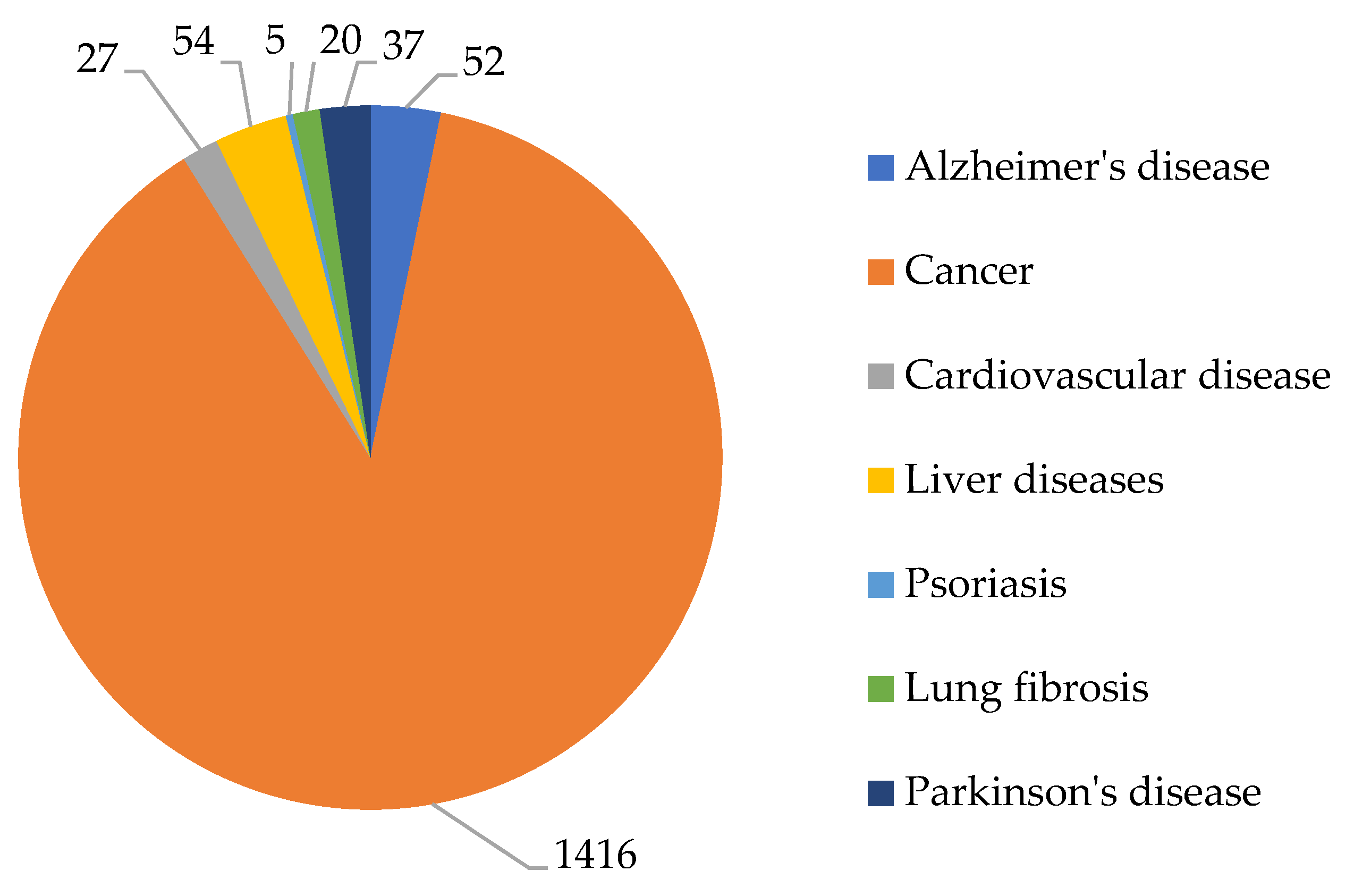
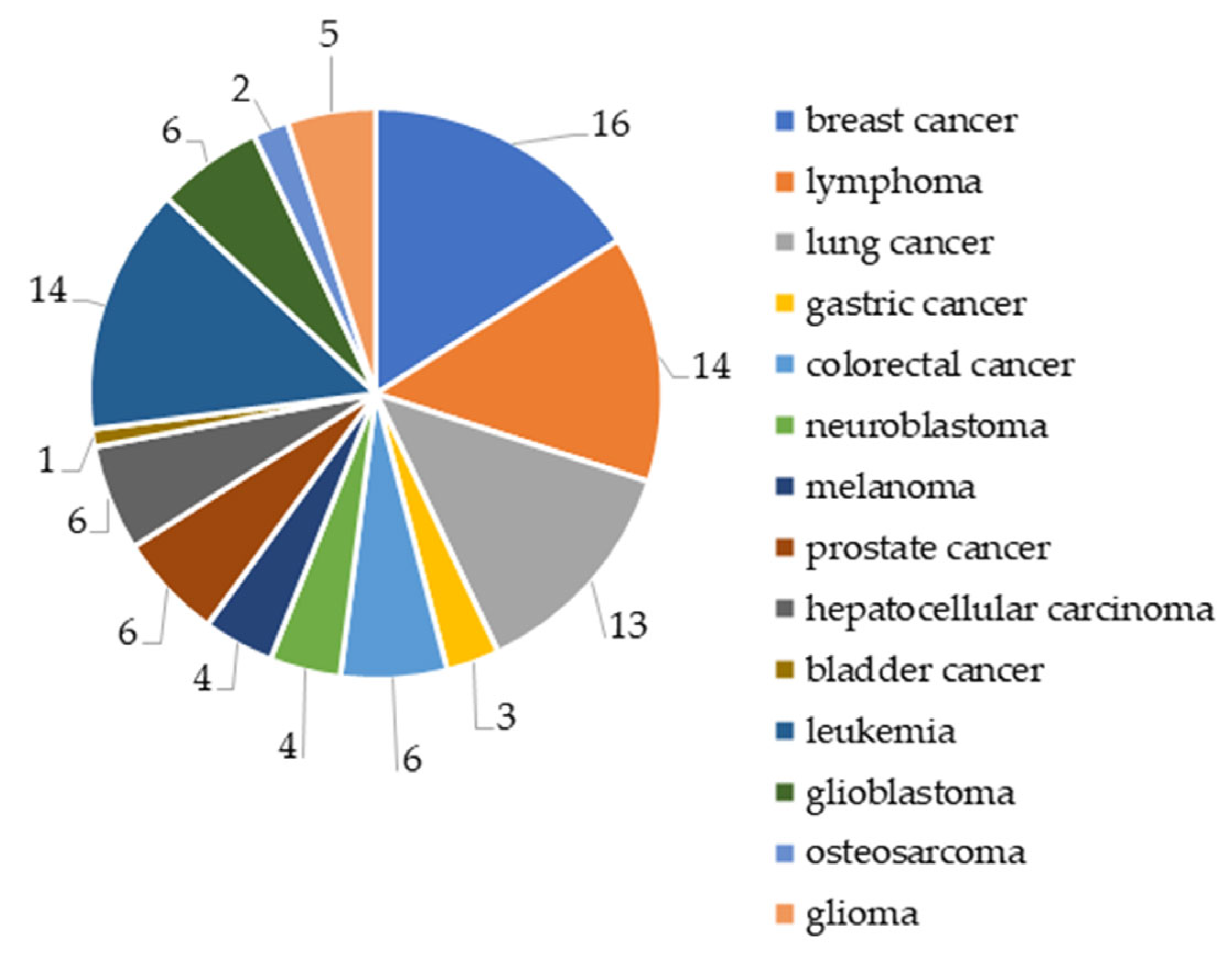
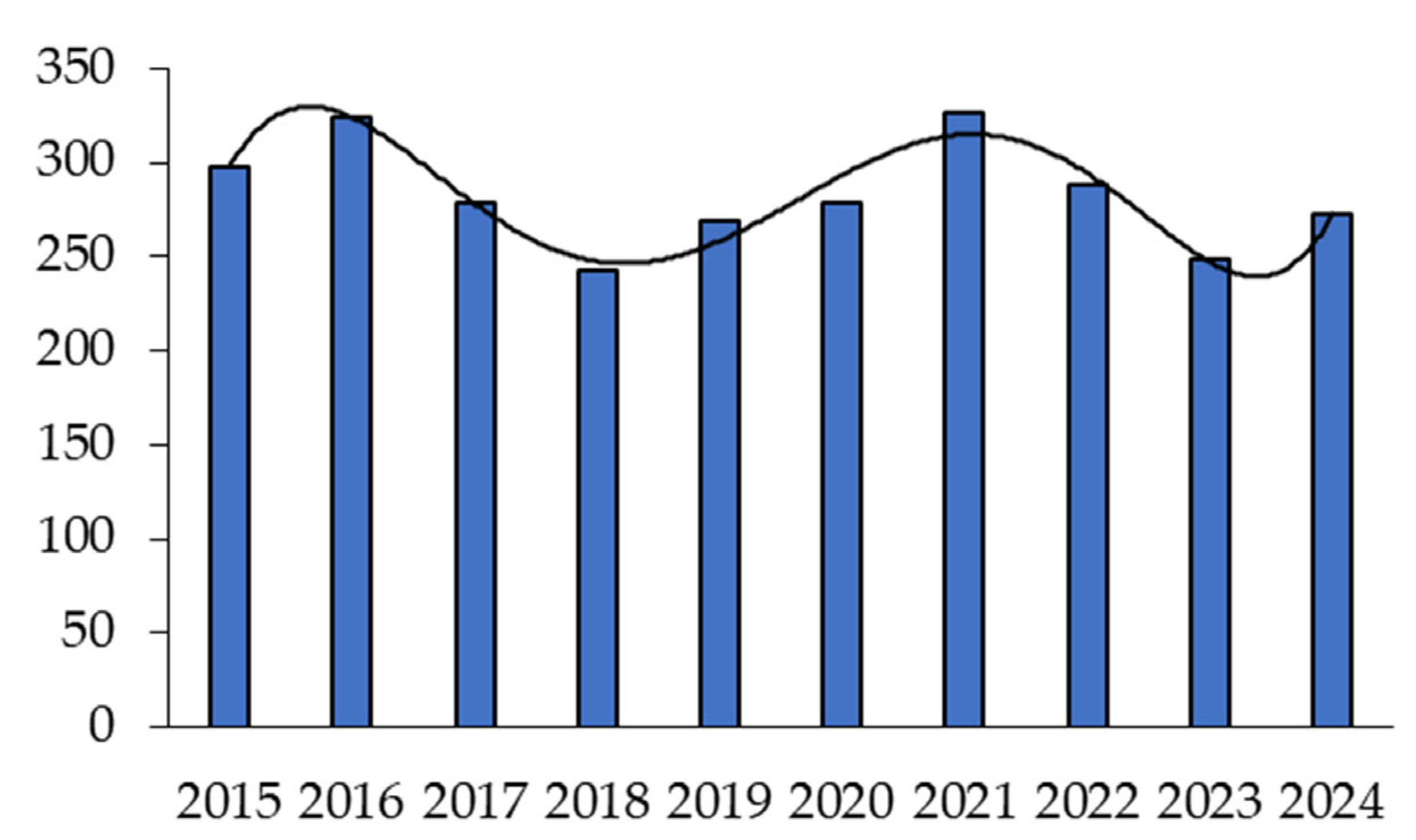
2. Analysis of Publication Activity
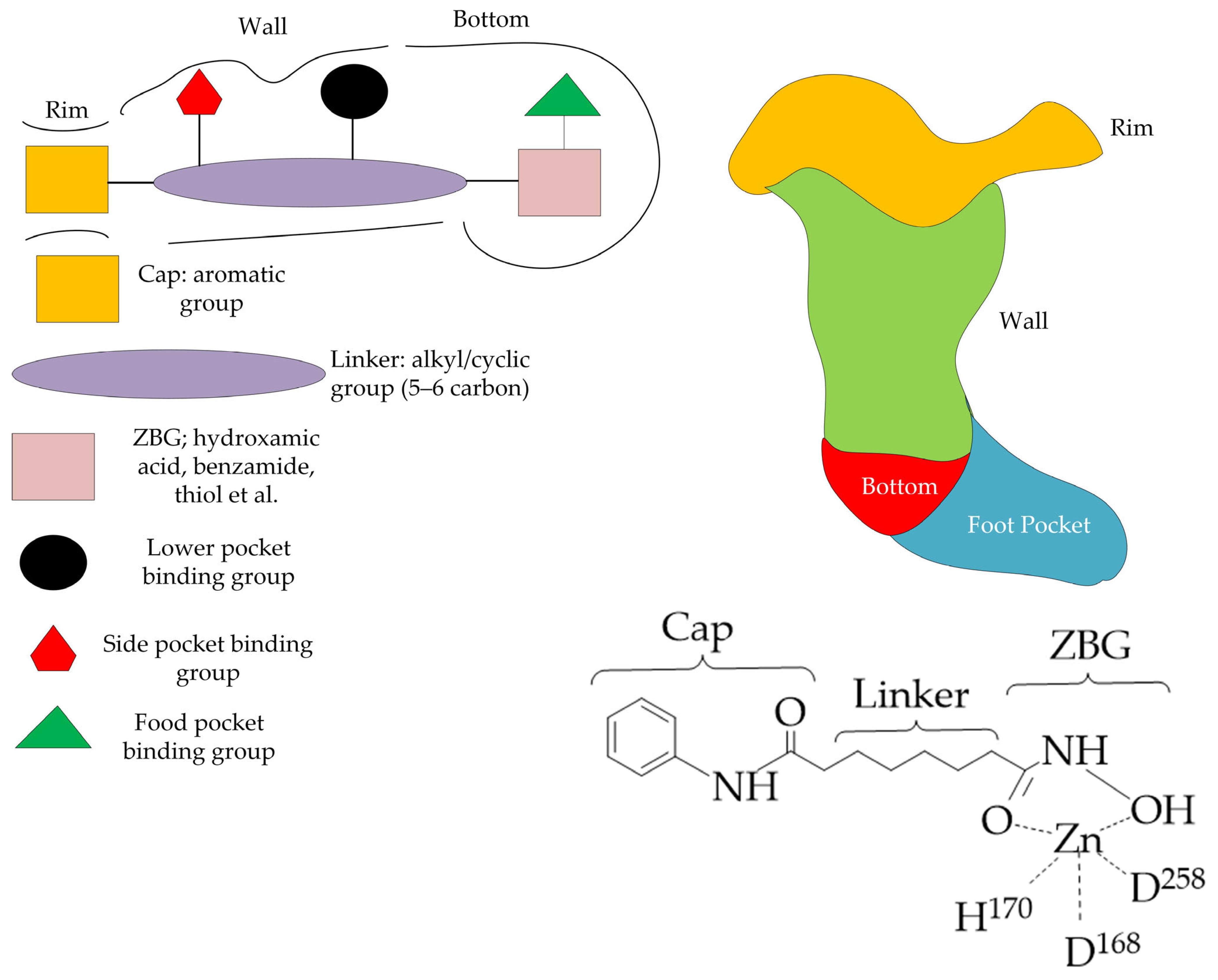
3. General Structure of HDAC Inhibitors
4. HDAC Inhibitors in Cancer Therapy
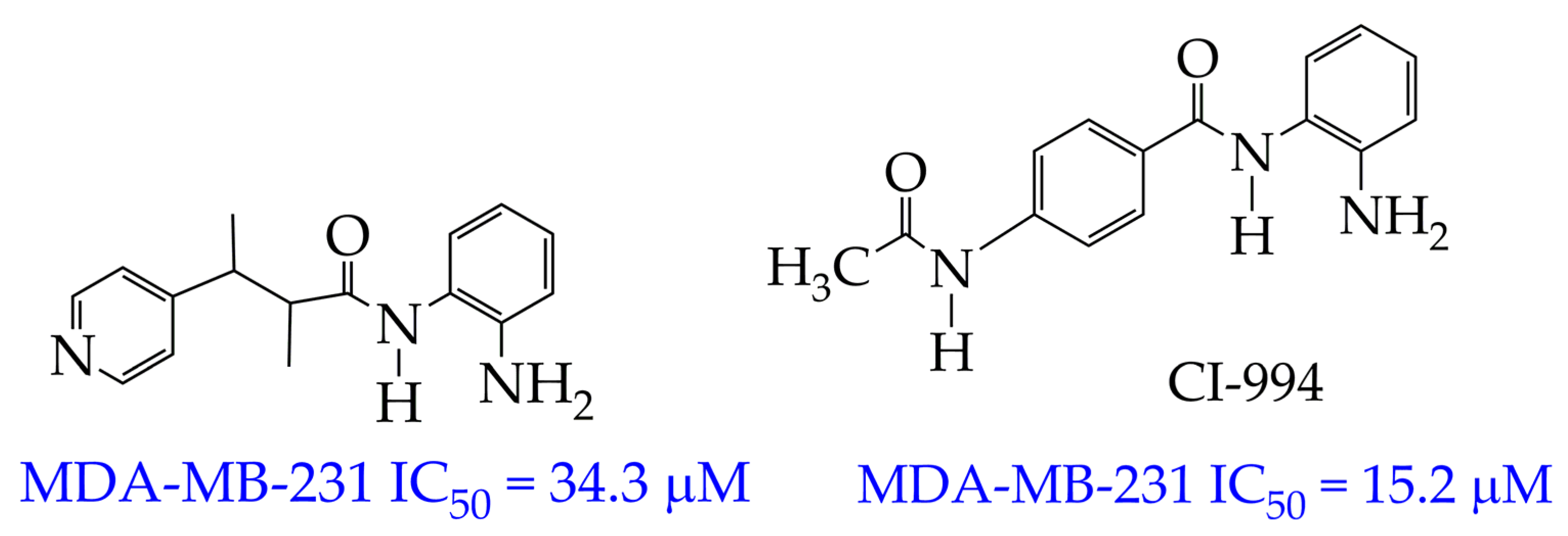
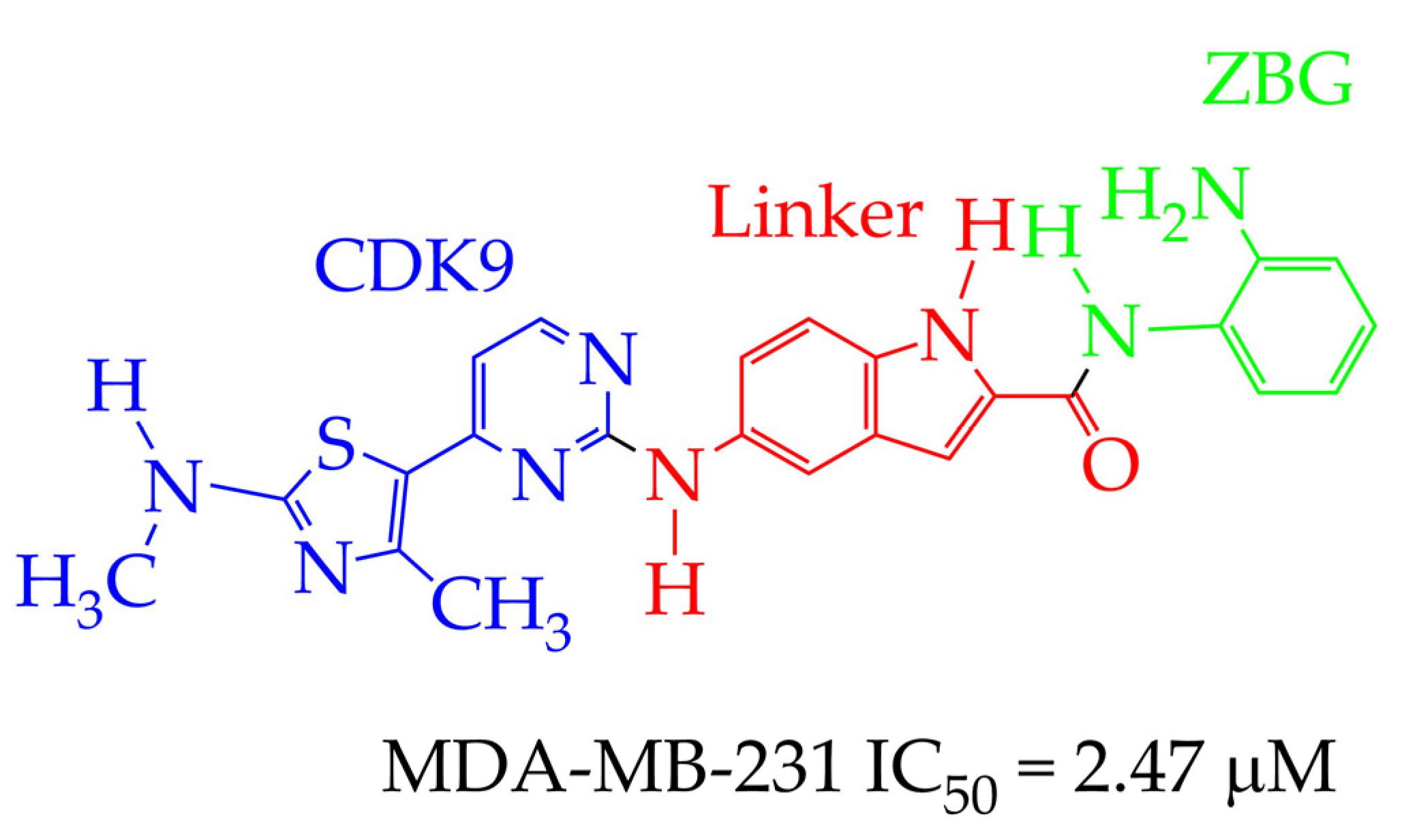
4.1. Breast Cancer
4.2. Lymphoma
4.3. HDAC Inhibitors for Other Cancers
5. HDAC Inhibitors for the Treatment of Nervous System Diseases
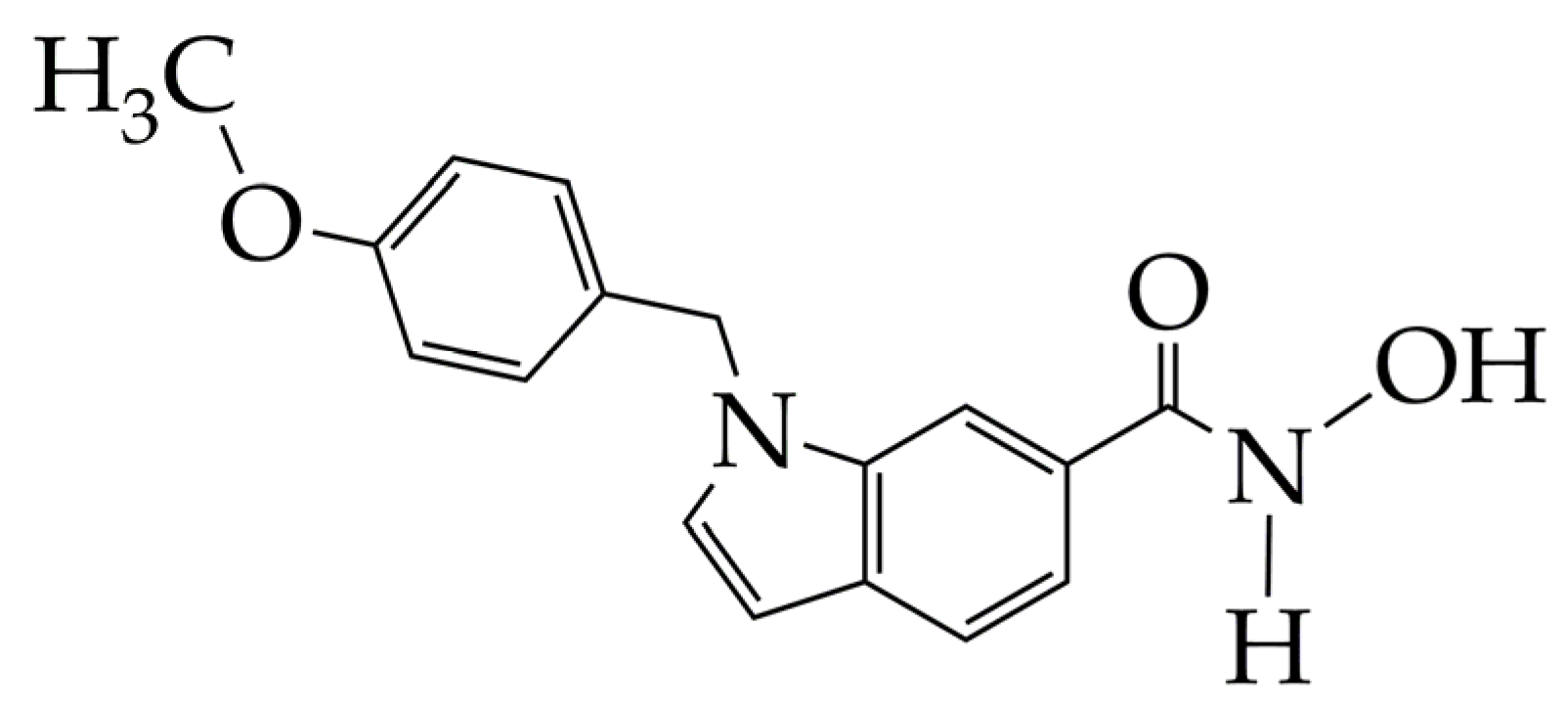
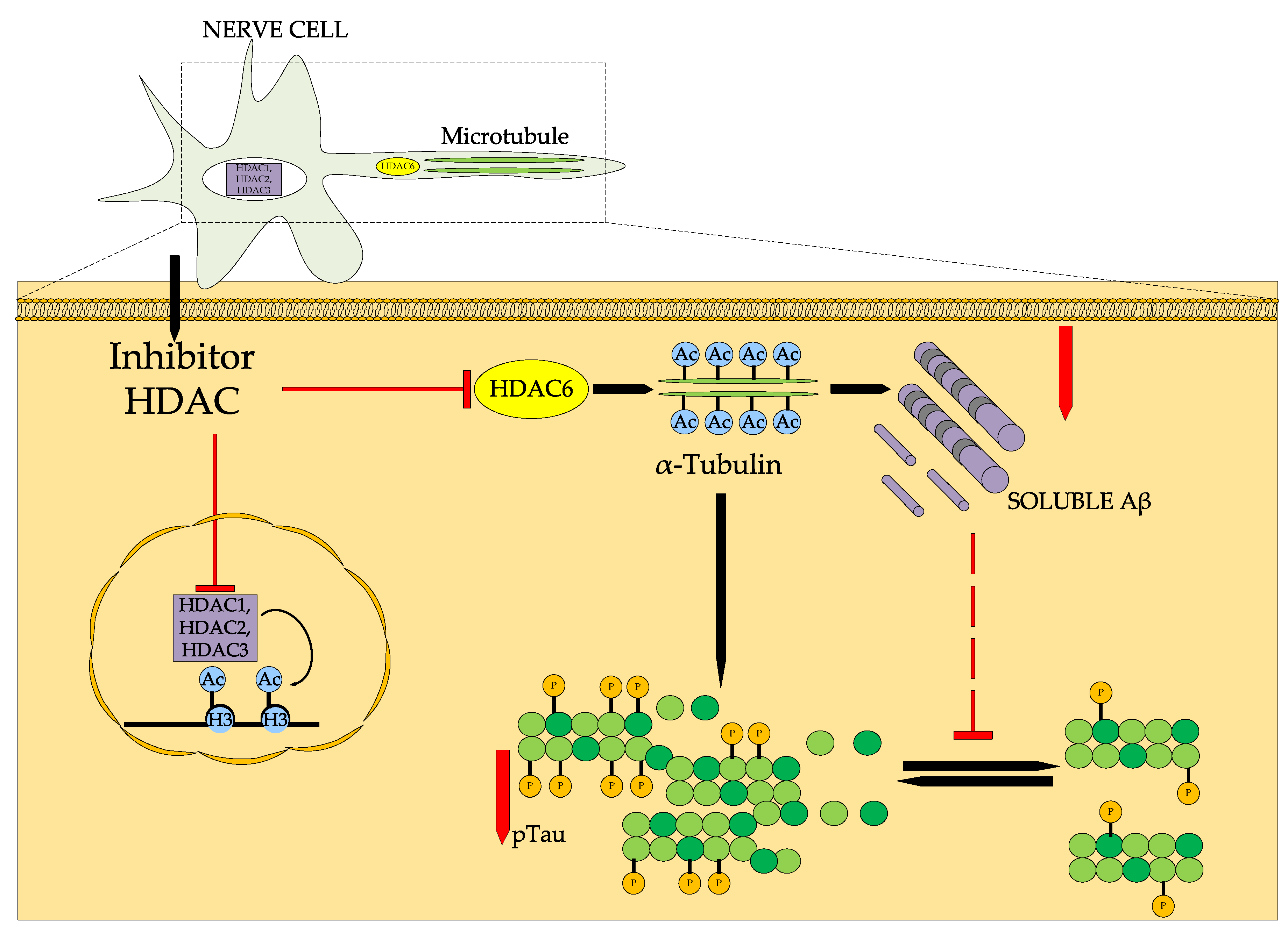
5.1. Alzheimer’s Disease
| HDAC Inhibitors | Reference |
|---|---|
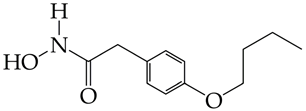 Bufexamac (DB13346), was also withdrawn by the EMA in April 2010 | [89] |
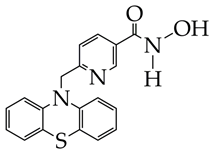 IC50 HDAC1 = 743 nM, IC50 HDAC2 = 1962 nM, IC50 HDAC6 = 2.54 nM | [90] |
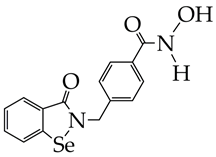 WY118 is at the preliminary preclinical studies stage. | [93] |
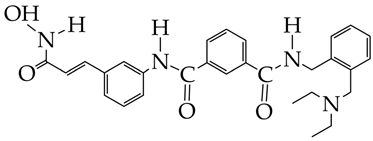 IC50 HDAC6 = 56.7 nM. | [95] |
5.2. Parkinson’s Disease
6. HDAC Inhibitors for the Treatment of Cardiovascular Diseases
7. HDAC Inhibitors for the Treatment of Liver Diseases
8. HDAC Inhibitors for the Treatment of Pulmonary Fibrosis
9. HDAC Inhibitors for Psoriasis Therapy
10. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ATP | Adenosine triphosphate |
| Bcl-2 | B-cell lymphoma 2 |
| DMSO | Dimethylsulfoxide |
| E2F1 | Transcription factor, a protein encoded in humans by the E2F1 gene |
| FDA | Food and Drug Administration |
| HDAC | Histone deacetylase |
| IPF | Idiopathic pulmonary fibrosis |
| MASLD | Metabolic dysfunction-associated steatotic liver disease |
| Mcl-1 | Myeloid cell leukemia 1 |
| NAD | Nicotinamide adenine dinucleotide |
| NF-κB | Nuclear factor κB |
| p53 | Tumor suppressor protein |
| PAK1 | P21 activated kinase 1 |
| PI3K | Phosphoinositide 3-kinase |
| RPD3 | Proteins involved in regulating gene expression in response to heme levels |
| PROTAC | Proteolysis targeting chimera |
| SAHA | Suberoylanilide hydroxamic acid |
| STAT3 | Signal transducer and activator of transcription 3 |
| TNBC | Triple negative breast cancer |
| XIAP | X-linked inhibitor of apoptosis |
| ZBG | Zinc binding group |
| ZMF-25 | Pyrido[2,3-d] pyrimidin-7(8H)-one-coupled hydroxamic acid |
| JAK | Janus kinases |
References
- Seto, E.; Yoshida, M. Erasers of Histone Acetylation: The Histone Deacetylase Enzymes. Cold Spring Harb. Perspect. Biol. 2014, 6, a018713. [Google Scholar] [CrossRef]
- Xu, X.; Wang, Q.; Guo, K.; Xu, J.; Lu, Y.; Chen, H.; Xiao, P. CD47 blockade reverses resistance to HDAC inhibitor by liberating anti-tumor capacity of macrophages. J. Experim. Clin. Cancer Res. 2025, 44, 67. [Google Scholar] [CrossRef]
- Egger, A.S.; Rauch, E.; Sharma, S.; Kipura, T.; Hotze, M.; Mair, T.; Kwiatkowski, M. Linking metabolism and histone acetylation dynamics by integrated metabolic flux analysis of Acetyl-CoA and histone acetylation sites. Mol. Metabol. 2024, 90, 102032. [Google Scholar] [CrossRef] [PubMed]
- Jia, G.; Liu, J.; Hou, X.; Jiang, Y.; Li, X. Biological function and small molecule inhibitors of histone deacetylase 11. Europ. J. Med. Chem. 2024, 276, 116634. [Google Scholar] [CrossRef] [PubMed]
- Jing, Y.; Li, X.; Liu, Z.; Li, X.D. Roles of negatively charged histone lysine acylations in regulating nucleosome structure and dynamics. Front. Mol. Biosci. 2022, 9, 899013. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, Y.; Liu, Y.; Li, B.; Tian, S.; Zhang, Z. Research Progress on the Mechanism and Function of Histone Acetylation Regulating the Interaction between Pathogenic Fungi and Plant Hosts. J. Fungi 2024, 10, 522. [Google Scholar] [CrossRef]
- Patil, R.S.; Maloney, M.E.; Lucas, R.; Fulton, D.J.R.; Patel, V.; Bagi, Z.; Kovacs-Kasa, A.; Kovacs, L.; Su, Y.; Verin, A.D. Zinc-Dependent Histone Deacetylases in Lung Endothelial Pathobiology. Biomolecules 2024, 14, 140. [Google Scholar] [CrossRef]
- Kodikara, I.K.; Nwanelo, V.O.; Belanger, A.K.; Pflum, M.K.H. HDAC7 influences ER-⍺ transcription via NCoR-HDAC3 dissociation. Biochim. Biophys. Acta (BBA)-Prot. Proteom. 2025, 1873, 141083. [Google Scholar] [CrossRef]
- Curcio, A.; Rocca, R.; Alcaro, S.; Artese, A. The histone deacetylase family: Structural features and application of combined computational methods. Pharmaceuticals 2024, 17, 620, Erratum in Pharmaceuticals 2024, 17, 1520.. [Google Scholar] [CrossRef]
- Lin, H.Y.; Chen, C.S.; Lin, S.P.; Weng, J.R.; Chen, C.S. Targeting histone deacetylase in cancer therapy. Med. Res. Rev. 2006, 26, 397–413. [Google Scholar] [CrossRef]
- Gallinari, P.; Marco, S.D.; Jones, P.; Pallaoro, M.; Steinkühler, C. HDACs, histone deacetylation and gene transcription: From molecular biology to cancer therapeutics. Cell Res. 2007, 17, 195–211. [Google Scholar] [CrossRef]
- Yadalam, P.K.; Ayyachamy, S.; Barbosa, F.T.; Natarajan, P.M. Kolmogorov-Arnold networks for predicting drug-gene associations of HDAC1 inhibitors in periodontitis. Comput. Biol. Chem. 2025, 118, 108451. [Google Scholar] [CrossRef]
- Bharadwaj, K.K.; Ahmad, I.; Pati, S.; Ghosh, A.; Rabha, B.; Sarkar, T.; Baishya, D. Screening of phytocompounds for identification of prospective histone deacetylase 1 (HDAC1) inhibitor: An in silico molecular docking, molecular dynamics simulation, and MM-GBSA approach. Appl. Biochem. Biotechnol. 2024, 196, 3747–3764. [Google Scholar] [CrossRef] [PubMed]
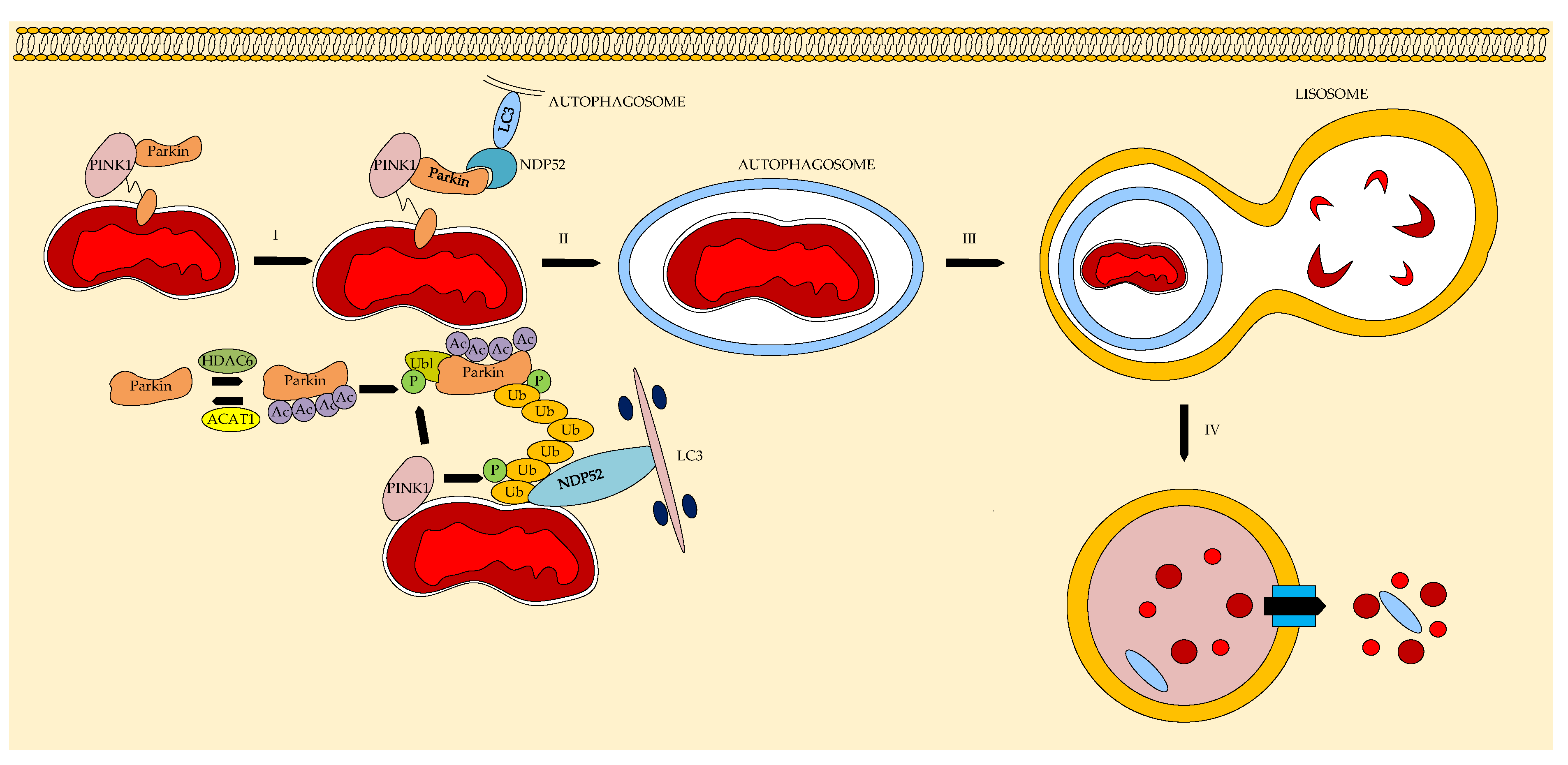
- Chen, H.; Lin, Q.; Zeng, Y.; Chen, P.; Guo, P.; Feng, R.; Zhou, X. Xinyin tablets affect mitophagy and cardiomyocyte apoptosis to alleviate chronic heart failure by regulating histone deacetylase 3 (HDAC3)-mediated PTEN induced putative kinase 1 (PINK1)/Parkin signaling pathway. J. Ethnopharm. 2025, 346, 119666. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Pan, B.; Liu, L.; Xu, X.; Zhao, W.; Mou, Q.; Tian, J. Epigallocatechin-3-gallate restores mitochondrial homeostasis impairment by inhibiting HDAC1-mediated NRF1 histone deacetylation in cardiac hypertrophy. Molec. Cell. Biochem. 2024, 479, 963–973. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Xu, X.; Liu, S.; Wang, X.; Musha, J.; Li, T.; Zhan, J. Butyrate inhibits histone deacetylase 2 expression to alleviate liver fibrosis in biliary atresia. BMC Pediatrics 2025, 25, 286. [Google Scholar] [CrossRef]
- Gao, L.; Cueto, M.A.; Asselbergs, F.; Atadja, P. Cloning and functional characterization of HDAC11, a novel member of the human histone deacetylase family. J. Biol. Chem. 2002, 277, 25748–25755. [Google Scholar] [CrossRef]
- Wang, L.; Bai, Y.; Cao, Z.; Guo, Z.; Lian, Y.; Liu, P.; Chen, Q. Histone deacetylases and inhibitors in diabetes mellitus and its complications. Biomed. Pharmacother. 2024, 177, 117010. [Google Scholar] [CrossRef]
- Choi, M.A.; Park, S.Y.; Chae, H.Y.; Song, Y.; Sharma, C.; Seo, Y.H. Design, synthesis and biological evaluation of a series of CNS penetrant HDAC inhibitors structurally derived from amyloid-β probes. Sci. Rep. 2019, 9, 13187. [Google Scholar] [CrossRef]
- Lamb, Y.N. Givinostat: First approval. Drugs 2024, 84, 849–856. [Google Scholar] [CrossRef]
- Embaby, A.; Huijberts, S.C.; Wang, L.; Leite de Oliveira, R.; Rosing, H.; Nuijen, B.; Wilgenhof, S. A Proof-of-Concept Study of Sequential Treatment with the HDAC Inhibitor Vorinostat following BRAF and MEK Inhibitors in BRAF V600-Mutated Melanoma. Clin. Cancer Res. 2024, 30, 3157–3166. [Google Scholar] [CrossRef]
- Liu, W.B.; Song, J.; Zhang, S.Y. A short overview of dual targeting HDAC inhibitors. Future Med. Chem. 2025, 17, 5–7. [Google Scholar] [CrossRef]
- Foley, N.; Riedell, P.A.; Bartlett, N.L.; Cashen, A.F.; Kahl, B.S.; Fehniger, T.A.; Mehta-Shah, N. A Phase I Study of Romidepsin in Combination with Gemcitabine, Oxaliplatin, and Dexamethasone in Patients with Relapsed or Refractory Aggressive Lymphomas Enriched for T-Cell Lymphomas. Clin. Lymph. Myel. Leuk. 2025, 25, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Seane, E.N.; Nair, S.; Vandevoorde, C.; Joubert, A. Mechanistic Sequence of Histone Deacetylase Inhibitors and Radiation Treatment: An Overview. Pharmaceuticals 2024, 17, 602. [Google Scholar] [CrossRef] [PubMed]
- Shirbhate, E.; Singh, V.; Jahoriya, V.; Mishra, A.; Veerasamy, R.; Tiwari, A.K.; Rajak, H. Dual inhibitors of HDAC and other epigenetic regulators: A novel strategy for cancer treatment. Eur. J. Med. Chem. 2024, 263, 115938. [Google Scholar] [CrossRef] [PubMed]
- Aroonthongsawat, P.; Manocheewa, S.; Srisawat, C.; Punnakitikashem, P.; Suwanwong, Y. Enhancement of the in vitro anti-leukemic effect of the histone deacetylase inhibitor Romidepsin using Poly-(D, L-lactide-co-glycolide) nanoparticles as a drug carrier. Eur. J. Pharm. Sci. 2025, 207, 107043. [Google Scholar] [CrossRef]
- Han, R.; Zhou, H.; Peng, B.; Yu, S.; Zhu, J.; Chen, J. Synergistic Integration of HDAC Inhibitors and Individualized Neoantigen Therapy (INT): A Next-Generation Combinatorial Approach for Cancer Immunotherapy. Vaccines 2025, 13, 550. [Google Scholar] [CrossRef]
- Letafati, A.; Mehdigholian Chaijani, R.; Edalat, F.; Eslami, N.; Askari, H.; Askari, F.; Mozhgani, S.H. Advances in epigenetic treatment of adult T-cell leukemia/lymphoma: A comprehensive review. Clin. Epigenet 2025, 17, 39. [Google Scholar] [CrossRef]
- Tian, J.; Han, M.; Song, F.; Liu, Y.; Shen, Y.; Zhong, J. Advances of HDAC inhibitors in tumor therapy: Potential applications through immune modulation. Front. Oncol. 2025, 15, 1576781. [Google Scholar] [CrossRef]
- Alqaraleh, M.; Al-Omari, L.; Al-Rawashde, F.A.; Abu Hajleh, M.N.; Alqaraleh, S.; Abu Alsamen, D.R.; Khleifat, K.M. The Combination Effects of Vorinostat and Silver Nanoparticles Targeting Angiogenesis, Oxidative Stress, and Inflammation: An In Vitro Study. J. Med. Chem. Sci. 2024, 7, 1398–1410. [Google Scholar]
- Blumenschein, G.R.; Kies, M.S.; Papadimitrakopoulou, V.A.; Lu, C.; Kumar, A.J.; Ricker, J.L.; Chiao, J.H.; Chen, C.; Frankel, S.R. Phase II trial of the histone deacetylase inhibitor vorinostat (Zolinza™, suberoylanilide hydroxamic acid, SAHA) in patients with recurrent and/or metastatic head and neck cancer. Investig. New Drugs 2008, 26, 81–87. [Google Scholar] [CrossRef]
- Shustov, A.R.; Coiffier, B.; Horwitz, S.M.; Sokol, L.; Pro, B.; Nielsen, T.; Bates, S.E. Romidepsin Is Effective and Well-Tolerated in Patients≥ 60 Years Old with Relapsed or Refractory Peripheral T-Cell Lymphoma (PTCL): Analysis from Phase 2 Trials. Blood 2013, 122, 4385. [Google Scholar] [CrossRef]
- Athira, K.V.; Sadanandan, P.; Chakravarty, S. Repurposing vorinostat for the treatment of disorders affecting brain. Neuromol. Med. 2021, 23, 449–465. [Google Scholar] [CrossRef]
- Hong, L.; Ni, M.; Xue, F.; Jiang, T.; Wu, X.; Li, C.; Wu, Q. The Role of HDAC3 in Pulmonary Diseases. Lung 2025, 203, 1–13. [Google Scholar] [CrossRef]
- El Omari, N.; Bakrim, S.; Elhrech, H.; Aanniz, T.; Balahbib, A.; Lee, L.H.; Bouyahya, A. Clinical efficacy and mechanistic insights of FDA-approved HDAC inhibitors in the treatment of lymphoma. Eur. J. Pharm. Sci. 2025, 208, 107057. [Google Scholar] [CrossRef]
- Bakrim, S.; Atifi, F.; Omari, N.E.; Zaid, Y.; Aanniz, T.; Lee, L.H.; Bouyahya, A. Clinical Applications of HDAC Inhibitors as Anticancer Agents in Prostate, Breast, Ovarian, and Cervical Cancers. ChemistrySelect 2025, 10, e202405484. [Google Scholar] [CrossRef]
- Hedayat, F.; Faghfuri, E. Harnessing histone deacetylase inhibitors for enhanced cancer immunotherapy. Eur. J. Pharmacol. 2025, 997, 177620. [Google Scholar] [CrossRef]
- Zhou, Y.; Luo, Q.; Gu, L.; Tian, X.; Zhao, Y.; Zhang, Y.; Wang, F. Histone Deacetylase Inhibitors Promote the Anticancer Activity of Cisplatin: Mechanisms and Potential. Pharmaceuticals 2025, 18, 563. [Google Scholar] [CrossRef] [PubMed]
- Shirbhate, E.; Singh, V.; Kore, R.; Koch, B.; Veerasamy, R.; Tiwari, A.K.; Rajak, H. Synergistic strategies: Histone deacetylase inhibitors and platinum-based drugs in cancer therapy. Exp. Rev. Antican. Ther. 2025, 25, 121–141. [Google Scholar] [CrossRef] [PubMed]
- Pu, J.; Liu, T.; Wang, X.; Sharma, A.; Schmidt-Wolf, I.G.; Jiang, L.; Hou, J. Exploring the role of histone deacetylase and histone deacetylase inhibitors in the context of multiple myeloma: Mechanisms, therapeutic implications, and future perspectives. Experim. Hemato. Oncol. 2024, 13, 45. [Google Scholar] [CrossRef]
- Pereira, M.; Cruz, M.T.; Fortuna, A.; Bicker, J. Restoring the epigenome in Alzheimer’s disease: Advancing HDAC inhibitors as therapeutic agents. Drug Discov. Today 2024, 29, 104052. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Y.; Wang, J.; Xia, Y.; Zhang, J.; Chen, L. Recent advances in Alzheimer’s disease: Mechanisms, clinical trials and new drug development strategies. Signal Transduct. Target. Ther. 2024, 9, 211. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.J.; Chen, Q.; Leung, C.H.; Sun, N.; Gao, F.; Chen, Z. Recent discoveries of the role of histone modifications and related inhibitors in pathological cardiac hypertrophy. Drug Discov. Today 2024, 29, 103878. [Google Scholar] [CrossRef] [PubMed]
- Cheshmazar, N.; Hamzeh-Mivehroud, M.; Charoudeh, H.N.; Hemmati, S.; Melesina, J.; Dastmalchi, S. Current trends in development of HDAC-based chemotherapeutics. Life Sci. 2022, 308, 120946. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, J.; Jiang, Q.; Zhang, L.; Song, W. Zinc binding groups for histone deacetylase inhibitors. J. Enz. Inhib. Med. Chem. 2018, 33, 714–721. [Google Scholar] [CrossRef]
- Wang, Y.; Stowe, R.L.; Pinello, C.E.; Tian, G.; Madoux, F.; Li, D.; Liao, D. Identification of histone deacetylase inhibitors with benzoylhydrazide scaffold that selectively inhibit class I histone deacetylases. Chem. Biol. 2015, 22, 273–284. [Google Scholar] [CrossRef]
- Melesina, J.; Simoben, C.V.; Praetorius, L.; Bülbül, E.F.; Robaa, D.; Sippl, W. Strategies to design selective histone deacetylase inhibitors. ChemMedChem 2021, 16, 1336–1359. [Google Scholar] [CrossRef]
- Luo, Y.; Yan, Z.; Chu, X.; Zhang, Y.; Qiu, Y.; Li, H. Binding mechanism and distant regulation of histone deacetylase 8 by PCI-34051. Commun. Biol. 2025, 8, 221. [Google Scholar] [CrossRef]
- Routholla, G.; Pulya, S.; Patel, T.; Amin, S.A.; Adhikari, N.; Biswas, S.; Ghosh, B. Synthesis, biological evaluation, and molecular docking analysis of novel linker-less benzamide based potent and selective HDAC3 inhibitors. Bioorg. Chem. 2021, 114, 105050. [Google Scholar] [CrossRef]
- Neganova, M.; Aleksandrova, Y.; Suslov, E.; Mozhaitsev, E.; Munkuev, A.; Tsypyshev, D.; Chicheva, M.; Rogachev, A.; Sukocheva, O.; Volcho, K.; et al. Novel Multitarget Hydroxamic Acids with a Natural Origin CAP Group against Alzheimer’s Disease: Synthesis, Docking and Biological Evaluation. Pharmaceutics 2021, 13, 1893. [Google Scholar] [CrossRef]
- Aleksandrova, Y.; Munkuev, A.; Mozhaitsev, E.; Suslov, E.; Tsypyshev, D.; Chaprov, K.; Begunov, R.; Volcho, K.; Salakhutdinov, N.; Neganova, M. Elaboration of the Effective Multi-Target Therapeutic Platform for the Treatment of Alzheimer’s Disease Based on Novel Monoterpene-Derived Hydroxamic Acids. Int. J. Mol. Sci. 2023, 24, 9743. [Google Scholar] [CrossRef] [PubMed]
- Aleksandrova, Y.; Munkuev, A.; Mozhaitsev, E.; Suslov, E.; Volcho, K.; Salakhutdinov, N.; Neganova, M. Hydroxamic Acids Containing a Bicyclic Pinane Backbone as Epigenetic and Metabolic Regulators: Synergizing Agents to Overcome Cisplatin Resistance. Cancers 2023, 15, 4985. [Google Scholar] [CrossRef] [PubMed]
- Morgan, E.; Arnold, M.; Gini, A.; Lorenzoni, V.; Cabasag, C.J.; Laversanne, M. Global burden of colorectal cancer in 2020 and 2040: Incidence and mortality estimates from GLOBOCAN. Gut 2023, 72, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Kratzer, T.B.; Giaquinto, A.N.; Sung, H.; Jemal, A. Cancer statistics. CA Cancer J. Clin. 2025, 75, 10. [Google Scholar] [CrossRef]
- Ilango, S.; Paital, B.; Jayachandran, P.; Padma, P.R.; Nirmaladevi, R. Epigenetic alterations in cancer. Front. Biosci. Land. 2020, 25, 1058–1109. [Google Scholar] [CrossRef]
- Obidiro, O.; Battogtokh, G.; Akala, E.O. Triple Negative Breast Cancer Treatment Options and Limitations: Future Outlook. Pharmaceutics 2023, 15, 1796. [Google Scholar] [CrossRef]
- Sharma, R. Global, regional, national burden of breast cancer in 185 countries: Evidence from GLOBOCAN 2018. Breast Canc. Res. Treat. 2021, 187, 557–567. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, H.; Merkher, Y.; Chen, L.; Liu, N.; Leonov, S.; Chen, Y. Recent advances in therapeutic strategies for triple-negative breast cancer. J. Hematol. Oncol. 2022, 15, 121. [Google Scholar] [CrossRef]
- Liedtke, C.; Mazouni, C.; Hess, K.R.; André, F.; Tordai, A.; Mejia, J.A.; Symmans, W.F.; Gonzalez-Angulo, A.M.; Hennessy, B.; Green, M.; et al. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J. Clin. Oncol. 2008, 26, 1275–1281. [Google Scholar] [CrossRef]
- Bisht, A.; Avinash, D.; Sahu, K.K.; Patel, P.; Das Gupta, G.; Kurmi, B.D. A comprehensive review on doxorubicin: Mechanisms, toxicity, clinical trials, combination therapies and nanoformulations in breast cancer. Drug Deliv. Trans. Res. 2025, 15, 102–133. [Google Scholar] [CrossRef]
- Yudaev, P.; Tupikov, A.; Chistyakov, E. Organocyclophosphazenes and Materials Based on Them for Pharmaceuticals and Biomedicine. Biomolecules 2025, 15, 262. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, U. Epigenetic Therapies in Triple-Negative Breast Cancer: Concepts, Visions, and Challenges. Cancers 2024, 16, 2164. [Google Scholar] [CrossRef] [PubMed]
- Alothaim, T.; Charbonneau, M.; Tang, X. HDAC6 inhibitors sensitize non-mesenchymal triple-negative breast cancer cells to cysteine deprivation. Sci. Rep. 2021, 11, 10956. [Google Scholar] [CrossRef] [PubMed]
- Barone, S.; Bello, I.; Guadagni, A.; Cerchia, C.; Filocamo, G.; Cassese, E.; Brindisi, M. Challenging Triple Negative Breast Cancer through HDAC6 selective inhibition: Novel cap-group identification, structure-activity relationships, computational and biological studies. Eur. J. Med. Chem. 2025, 292, 117634. [Google Scholar] [CrossRef]
- Himaja, A.; Routholla, G.; Patel, T.; Banerjee, S.; Begum, D.; Regula, S.; Ghosh, B. Design and synthesis of pyridine-based benzamides as potent HDAC3 inhibitors as an armament against breast cancer with in vivo validation. Eur. J. Med. Chem. 2025, 291, 117636. [Google Scholar] [CrossRef]
- Chen, X.; Li, R.; Qiu, Y.; Lin, F.; Chen, S.; Li, X.; Fang, M. Design, synthesis, and biological evaluation of N-(2-amino-phenyl)-5-(4-aryl-pyrimidin-2-yl) amino)-1H-indole-2-carboxamide derivatives as novel inhibitors of CDK9 and class I HDACs for cancer treatment. Bioorg. Chem. 2025, 162, 108577, Erratum in Bioorg. Chem. 2025, 163, 108657.. [Google Scholar] [CrossRef]
- Lin, C.Y.; Lovén, J.; Rahl, P.B.; Paranal, R.M.; Burge, C.B.; Bradner, J.E.; Young, R.A. Transcriptional amplification in tumor cells with elevated c-Myc. Cell 2012, 151, 56–67. [Google Scholar] [CrossRef]
- Zhang, J.; Cheng, X.; Chen, G.; Chen, X.; Zhao, X.; Chen, W.; Yao, D. Discovery of a Novel Selective PAK1/HDAC6/HDAC10 Inhibitor ZMF-25 that Induces Mitochondrial Metabolic Breakdown and Autophagy-Related Cell Death in Triple-Negative Breast Cancer. Research 2025, 8, 0670. [Google Scholar] [CrossRef]
- Silkenstedt, E.; Salles, G.; Campo, E.; Dreyling, M. B-cell non-Hodgkin lymphomas. Lancet 2024, 403, 1791–1807. [Google Scholar] [CrossRef]
- El Omari, N.; Khalid, A.; Makeen, H.A.; Alhazmi, H.A.; Albratty, M.; Mohan, S.; Bouyahya, A. Stochasticity of anticancer mechanisms underlying clinical effectiveness of vorinostat. Heliyon 2024, 10, e33052. [Google Scholar] [CrossRef]
- Westin, J.R. Status of PI3K/Akt/mTOR pathway inhibitors in lymphoma. Clin. Lymph. Myel. Leuk. 2014, 14, 335–342. [Google Scholar] [CrossRef]
- Hou, B.; Jia, G.; Li, Z.; Jiang, Y.; Chen, Y.; Li, X. Discovery of hydrazide-based PI3K/HDAC dual inhibitors with enhanced pro-apoptotic activity in lymphoma cells. Eur. J. Med. Chem. 2025, 292, 117658. [Google Scholar] [CrossRef]
- Yaguchi, S.I.; Fukui, Y.; Koshimizu, I.; Yoshimi, H.; Matsuno, T.; Gouda, H.; Yamori, T. Antitumor activity of ZSTK474, a new phosphatidylinositol 3-kinase inhibitor. J. Nat. Canc. Inst. 2006, 98, 545–556. [Google Scholar] [CrossRef]
- Zuo, Y.; Zhu, Y.; Luo, P.; Huang, N.; Yao, K.; Zhang, K.; Gou, X. Design, synthesis, and biological evaluation of novel PI3Kδ/HDAC6 dual inhibitors for the treatment of non-Hodgkin’s lymphoma. Eur. J. Med. Chem. 2025, 296, 117852. [Google Scholar] [CrossRef] [PubMed]
- Oliveira-Silva, J.M.; Oliveira, L.S.; Chiminazo, C.B.; Fonseca, R.; de Souza, C.V.E.; Aissa, A.F.; Castro-Gamero, A.M. WT161, a selective HDAC6 inhibitor, decreases growth, enhances chemosensitivity, promotes apoptosis, and suppresses motility of melanoma cells. Cancer Chem. Pharmacol. 2025, 95, 1–16. [Google Scholar] [CrossRef]
- Hu, Z.; Li, S.; Pan, W.; Wu, H.; Peng, X. Design, synthesis and bioevaluation of novel hydrazide derivatives as enhancers of immunotherapy and DNA-damage response in antitumor therapy. Eur. J. Med. Chem. 2025, 291, 117601. [Google Scholar] [CrossRef]
- Yang, M.; Zhao, W.; Zhang, J.; Liu, L.; Tian, S.; Miao, Y.; You, X. HDAC11 Inhibition as a Potential Therapeutic Strategy for AML: Target Identification, Lead Discovery, Antitumor Potency, and Mechanism Investigation. J. Med. Chem. 2025, 68, 8124–8142. [Google Scholar] [CrossRef]
- Onuscakova, M.; Kauerova, T.; Fialova, E.; Pizova, H.; Garaj, V.; Kemka, M.; Bobal, P. New potent N-hydroxycinnamamide-based histone deacetylase inhibitors suppress proliferation and trigger apoptosis in THP-1 leukaemia cells. Archiv. Pharmaz. 2025, 358, e2400889. [Google Scholar] [CrossRef]
- Tretbar, M.; Schliehe-Diecks, J.; von Bredow, L.; Tan, K.; Roatsch, M.; Tu, J.W.; Hansen, F.K. Preferential HDAC6 inhibitors derived from HPOB exhibit synergistic antileukemia activity in combination with decitabine. Eur. J. Med. Chem. 2025, 272, 116447. [Google Scholar] [CrossRef]
- Hemida, N.; ElGamil, D.S.; ElHady, A.K.; Lin, K.C.; Chang, Y.H.; Hilscher, S.; Abdel-Halim, M. Unlocking the potential of novel tetrahydro-β-carboline-based HDAC6 inhibitors for colorectal cancer therapy: Design, synthesis and biological evaluation. Bioorg. Chem. 2025, 160, 108454. [Google Scholar] [CrossRef]
- Zhong, R.; Qiu, C.; Chan, S.; Wang, Y.; Liu, K.; Xia, Y.; Zou, B. TDH-11 inhibits the proliferation and colonization of colorectal cancer by reducing the activity of HDAC. Cell. Signal. 2025, 132, 111817. [Google Scholar] [CrossRef] [PubMed]
- Hsu, K.C.; Huang, Y.Y.; Chu, J.C.; Huang, Y.W.; Hu, J.L.; Lin, T.E.; Huang, W.J. Synthesis and biological evaluation of ortho-phenyl phenylhydroxamic acids containing phenothiazine with improved selectivity for class IIa histone deacetylases. J. Enz. Inhibit. Med. Chem. 2024, 39, 2406025. [Google Scholar] [CrossRef] [PubMed]
- Kamloon, T.; Senawong, T.; Senawong, G.; Namwan, N.; Kumboonma, P.; Somsakeesit, L.O.; Phaosiri, C. Exploring putative histone deacetylase inhibitors with antiproliferative activity of chrysin derivatives. Med. Chem. Res. 2025, 34, 1308–1320. [Google Scholar] [CrossRef]
- Niotis, K.; Saperia, C.; Saif, N.; Carlton, C.; Isaacson, R.S. Alzheimer’s disease risk reduction in clinical practice: A priority in the emerging field of preventive neurology. Nat. Ment. Health 2024, 2, 25–40. [Google Scholar] [CrossRef]
- World Health Organization. Global action plan on the public health response to dementia 2017–2025. In Global Action Plan on the Public Health Response to Dementia 2017–2025; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Gupta, R.; Ambasta, R.K.; Kumar, P. Identification of novel class I and class IIb histone deacetylase inhibitor for Alzheimer’s disease therapeutics. Life Sci. 2020, 256, 117912. [Google Scholar] [CrossRef]
- Lu, X.; Wang, L.; Yu, C.; Yu, D.; Yu, G. Histone acetylation modifiers in the pathogenesis of Alzheimer’s disease. Front. Cell. Neurosci. 2015, 9, 226. [Google Scholar] [CrossRef]
- Cuadrado-Tejedor, M.; Garcia-Barroso, C.; Sánchez-Arias, J.A.; Rabal, O.; Pérez-González, M.; Mederos, S.; Garcia-Osta, A. A first-in-class small-molecule that acts as a dual inhibitor of HDAC and PDE5 and that rescues hippocampal synaptic impairment in Alzheimer’s disease mice. Neuropsychopharmacology 2017, 42, 524–539. [Google Scholar] [CrossRef]
- Mirehei, M.; Motamedi, F.; Maghsoudi, N.; Mansouri, Z.; Naderi, S.; Khodagholi, F.; Abbaszadeh, F. Effects of Bufexamac, a class IIb HDAC inhibitor, on behavior and neuropathological features in an Aβ-induced rat model of Alzheimer’s disease. Experim. Gerontol. 2025, 204, 112746. [Google Scholar] [CrossRef]
- Wang, X.X.; Xie, F.; Jia, C.C.; Yan, N.; Zeng, Y.L.; Wu, J.D.; Liu, Z.P. Synthesis and biological evaluation of selective histone deacetylase 6 inhibitors as multifunctional agents against Alzheimer’s disease. Eur. J. Med. Chem. 2021, 225, 113821. [Google Scholar] [CrossRef]
- Li, Z.; Yin, B.; Zhang, S.; Lan, Z.; Zhang, L. Targeting protein kinases for the treatment of Alzheimer’s disease: Recent progress and future perspectives. Eur. J. Med. Chem. 2023, 261, 115817. [Google Scholar]
- Haussmann, R.; Noppes, F.; Brandt, M.D.; Bauer, M.; Donix, M. Minireview: Lithium: A therapeutic option in Alzheimer’s disease and its prodromal stages? Neurosci. Lett. 2021, 760, 136044. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Jiang, Z.; Huang, X.; Chen, Y.; Feng, L.; Mai, J.; Hu, J. Anti-Alzheimer effects of an HDAC6 inhibitor, WY118, alone and in combination of lithium chloride: Synergistic suppression of ferroptosis via the modulation of tau phosphorylation and MAPK signaling. Eur. J. Pharmacol. 2025, 997, 177605. [Google Scholar] [CrossRef] [PubMed]
- Rejc, L.; Gómez-Vallejo, V.; Joya, A.; Moreno, O.; Egimendia, A.; Castellnou, P.; Llop, J. Longitudinal evaluation of a novel BChE PET tracer as an early in vivo biomarker in the brain of a mouse model for Alzheimer disease. Theranos. 2021, 11, 6542. [Google Scholar] [CrossRef] [PubMed]
- Lv, B.; Wang, Z.; Wang, Q.; Xu, Z.; Tang, J.; Pei, Y.; Chen, Y. Dual inhibitors of butyrylcholinesterase and histone deacetylase 6 for the treatment of Alzheimer’s disease: Design, synthesis, and biological evaluation. Bioorg. Med. Chem. 2025, 127, 118219. [Google Scholar] [CrossRef]
- Cheng, G.; Liu, Y.; Ma, R.; Cheng, G.; Guan, Y.; Chen, X.; Chen, T. Anti-Parkinsonian therapy: Strategies for crossing the blood–brain barrier and nano-biological effects of nanomaterials. Nano-Micro Lett. 2022, 14, 105. [Google Scholar] [CrossRef]
- Cui, W.; Yang, X.; Chen, X.; Xiao, D.; Zhu, J.; Zhang, M.; Lin, Y. Treating LRRK2-related Parkinson’s disease by inhibiting the mTOR signaling pathway to restore autophagy. Adv. Func. Mater. 2021, 31, 2105152. [Google Scholar] [CrossRef]
- O’Mahony, A.G.; Mazzocchi, M.; Morris, A.; Morales-Prieto, N.; Guinane, C.; Wyatt, S.L.; O’Keeffe, G.W. The class-IIa HDAC inhibitor TMP269 promotes BMP-Smad signalling and is neuroprotective in in vitro and in vivo 6-hydroxydopamine models of Parkinson’s disease. Neuropharmacol. 2025, 268, 110319. [Google Scholar] [CrossRef]
- Lee, H.; Kim, H.J.; Min, J.S.; Lee, E.; Choi, D.K.; Choi, J.H.; Kwon, O.B. HDAC4/5 Inhibitor, LMK-235 Improves Animal Voluntary Movement in MPTP-Induced Parkinson’s Disease Model. Pharmacol. Res. Perspect. 2025, 13, e70057. [Google Scholar] [CrossRef]
- Pham, K.Y.; Khanal, S.; Bohara, G.; Rimal, N.; Song, S.H.; Nguyen, T.T.K.; Yook, S. HDAC6 inhibitor-loaded brain-targeted nanocarrier-mediated neuroprotection in methamphetamine-driven Parkinson’s disease. Redox Biol. 2025, 79, 103457. [Google Scholar] [CrossRef]
- Hausenloy, D.J.; Botker, H.E.; Engstrom, T.; Erlinge, D.; Heusch, G.; Ibanez, B.; Garcia-Dorado, D. Targeting reperfusion injury in patients with ST-segment elevation myocardial infarction: Trials and tribulations. Eur. Heart J. 2017, 38, 935–941. [Google Scholar] [CrossRef]
- Nagata, S.; Marunouchi, T.; Tanonaka, K. Histone deacetylase inhibitor SAHA treatment prevents the development of heart failure after myocardial infarction via an induction of heat-shock proteins in rats. Biol. Pharm. Bull. 2019, 42, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhao, Y.; Yan, L.; Tan, M.; Jin, Y.; Yin, Y.; Li, H. Corosolic acid attenuates cardiac ischemia/reperfusion injury through the PHB2/PINK1/parkin/mitophagy pathway. Iscience 2024, 27, 110448. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wang, Z.; Wang, L.; Jiang, T.; Dong, D.; Sun, M. The PINK1/Parkin signaling pathway-mediated mitophagy: A forgotten protagonist in myocardial ischemia/reperfusion injury. Pharmacol. Res. 2024, 209, 107466. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Shu, Y.; Ye, G.; Wu, C.; Xu, M.; Gao, R.; Zhang, J. Histone deacetylase inhibitors inhibit cervical cancer growth through Parkin acetylation-mediated mitophagy. Acta Pharmaceut. Sin. 2022, 12, 838–852. [Google Scholar] [CrossRef]
- Yao, J.; Sun, X.; Chen, Y.; Xu, X.; Feng, J.; Zhang, M.; Shi, X. Histone deacetylase 6 inhibition attenuates pathological cardiac hypertrophy by promoting autophagy through MAP1LC3B ubiquitination. J. Pathol. 2025, 266, 217–229. [Google Scholar] [CrossRef]
- Zhou, H.; Kee, H.J.; Wan, L.; Asfaha, Y.; Fischer, F.; Kassack, M.U.; Jeong, M.H. YAK577 Attenuates Cardiac Remodeling and Fibrosis in Isoproterenol-Infused Heart Failure Mice by Downregulating MMP12. Korean Circul. J. 2024, 55, 231–247. [Google Scholar] [CrossRef]
- Miksiunas, R.; Rucinskas, K.; Janusauskas, V.; Labeit, S.; Bironaite, D. Histone deacetylase inhibitor suberoylanilide hydroxamic acid improves energetic status and cardiomyogenic differentiation of human dilated myocardium-derived primary mesenchymal cells. Inter. J. Mol. Sci. 2020, 21, 4845. [Google Scholar] [CrossRef]
- Ghanghas, P.; Sharma, M.; Desai, D.; Raza, K.; Bhalla, A.; Kumar, P.; Kaushal, N. Selenium-based novel epigenetic regulators offer effective chemotherapeutic alternative with wider safety margins in experimental colorectal cancer. Biol. Trace Elem. Res. 2022, 200, 635–646. [Google Scholar] [CrossRef]
- Cheng, T.; Liu, C.; Wang, Y.; Li, G.; Feng, L.; Zhang, S.; Yang, L. A novel histone deacetylase inhibitor Se-SAHA attenuates isoproterenol-induced heart failure via antioxidative stress and autophagy inhibition. Toxicol. Appl. Pharmacol. 2024, 487, 116957. [Google Scholar] [CrossRef]
- Evans, L.W.; Bender, A.; Burnett, L.; Godoy, L.; Shen, Y.; Staten, D.; Ferguson, B.S. Emodin and emodin-rich rhubarb inhibits histone deacetylase (HDAC) activity and cardiac myocyte hypertrophy. J. Nutrit. Biochem. 2020, 79, 108339. [Google Scholar] [CrossRef]
- Zhang, F.; Yue, K.; Sun, S.; Lu, S.; Jia, G.; Zha, Y.; Duan, Y. Targeting Histone Deacetylase 11 with a Highly Selective Inhibitor for the Treatment of MASLD. Adv. Sci. 2025, 12, 2412903. [Google Scholar] [CrossRef]
- Zeigerer, A. NAFLD-a rising metabolic disease. Mol. Met. 2021, 50, 101274. [Google Scholar] [CrossRef] [PubMed]
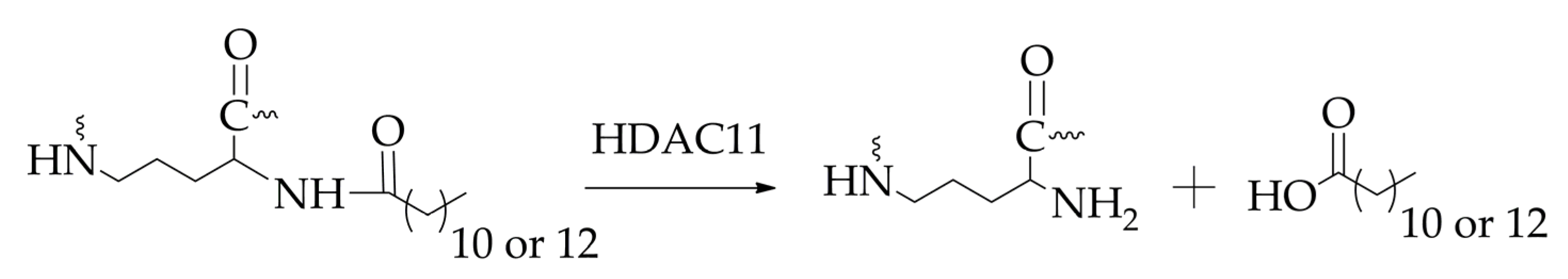
- Kutil, Z.; Novakova, Z.; Meleshin, M.; Mikesova, J.; Schutkowski, M.; Barinka, C. Histone deacetylase 11 is a fatty-acid deacylase. ACS Chem. Biol. 2018, 13, 685–693. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.T.; Peng, C.; Seto, E.; Lin, H. Trapoxin A analogue as a selective nanomolar inhibitor of HDAC11. ACS Chem. Biol. 2023, 18, 803–809. [Google Scholar] [CrossRef] [PubMed]
- Bai, P.; Liu, Y.; Yang, L.; Ding, W.; Mondal, P.; Sang, N.; Liu, G.; Lu, X.; Ho, T.T.; Zhou, Y.; et al. Development and pharmacochemical characterization discover a novel brain-permeable HDAC11-selective inhibitor with therapeutic potential by regulating neuroinflammation in mice. J. Med. Chem. 2023, 66, 16075–16090. [Google Scholar] [CrossRef]
- Lahm, A.; Paolini, C.; Pallaoro, M.; Nardi, M.C.; Jones, P.; Neddermann, P.; Sambucini, S.; Bottomley, M.J.; Lo Surdo, P.; Carfı, A.; et al. Unraveling the hidden catalytic activity of vertebrate class IIa histone deacetylases. Proc. Natl. Acad. Sci. USA 2007, 104, 17335–17340. [Google Scholar] [CrossRef]
- Kolanko, E.; Cargnoni, A.; Papait, A.; Silini, A.R.; Czekaj, P.; Parolini, O. The evolution of in vitro models of lung fibrosis: Promising prospects for drug discovery. Eur. Resp. Rev. 2024, 33, 230127. [Google Scholar] [CrossRef]
- Bouros, E.; Filidou, E.; Arvanitidis, K.; Mikroulis, D.; Steiropoulos, P.; Bamias, G.; Kolios, G. Lung fibrosis-associated soluble mediators and bronchoalveolar lavage from idiopathic pulmonary fibrosis patients promote the expression of fibrogenic factors in subepithelial lung myofibroblasts. Pulm. Pharmacol. Therapeut. 2017, 46, 78–87. [Google Scholar] [CrossRef]
- Jeong, Y.; Du, R.; Zhu, X.; Yin, S.; Wang, J.; Cui, H.; Lowenstein, C.J. Histone deacetylase isoforms regulate innate immune responses by deacetylating mitogen-activated protein kinase phosphatase-1. J. Leukoc. Biol. 2014, 95, 651–659. [Google Scholar] [CrossRef]
- Liu, C.H.; Lee, H.S.; Liou, J.P.; Hua, H.S.; Cheng, W.H.; Yuliani, F.S.; Lin, C.H. MPT0E028, a novel pan-HDAC inhibitor, prevents pulmonary fibrosis through inhibition of TGF-β-induced CTGF expression in human lung fibroblasts: Involvement of MKP-1 activation. Eur. J. Pharmacol. 2024, 977, 176711. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, P.; Hu, Z.; Cui, H.; Chen, X.; Wang, L.; Yao, Y. Design, synthesis, and evaluation of a novel TRAIL-activated HDAC6 inhibitor for the treatment of pulmonary fibrosis. Bioorg. Med. Chem. 2024, 113, 117924. [Google Scholar] [CrossRef]
- Li, Y.; Quan, J.; Song, H.; Li, D.; Ma, E.; Wang, Y.; Ma, C. Novel pyrrolo [2, 1-c][1, 4] benzodiazepine-3, 11-dione (PBD) derivatives as selective HDAC6 inhibitors to suppress tumor metastasis and invasion in vitro and in vivo. Bioorg. Chem. 2021, 114, 105081. [Google Scholar] [CrossRef] [PubMed]
- Fontana, A.; Bergantini, L.; Carullo, G.; Scalvini, L.; D’Alessandro, M.; Papulino, C.; Campiani, G. Spirotetrahydroisoquinoline-Based Histone Deacetylase Inhibitors as New Antifibrotic Agents: Biological Evaluation in Human Fibroblasts from Bronchoalveolar Lavages of Idiopathic Pulmonary Fibrosis Patients. ACS Pharmacol. Transl. Sci. 2024, 8, 380–393. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Kuai, L.; Huang, F.; Jiang, J.; Song, J.; Liu, Y.; Chen, S.; Mao, L.; Peng, W.; Luo, Y. Single-atom catalysts-based catalytic ROS clearance for efficient psoriasis treatment and relapse prevention via restoring ESR1. Nat. Commun. 2023, 14, 6767. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.M.; Jin, H.Z. Biologics in the treatment of pustular psoriasis. Exp. Opin. Drug Safety 2020, 19, 969–980. [Google Scholar] [CrossRef]
- Gavra, D.I.; Kósa, D.; Pető, Á.; Józsa, L.; Ujhelyi, Z.; Fehér, P.; Bácskay, I. Exploring the Correlation between the PASI and DLQI Scores in Psoriasis Treatment with Topical Ointments Containing Rosa× damascena Mill. Extract. Pharmaceuticals 2024, 17, 1092. [Google Scholar] [CrossRef]
- Asmamaw, M.D.; He, A.; Zhang, L.R.; Liu, H.M.; Gao, Y. Histone deacetylase complexes: Structure, regulation and function. Biochim. Biophys. Acta Rev. Cancer. 2024, 1879, 189150. [Google Scholar] [CrossRef]
- Jiang, Y.; Lu, S.; Lai, Y.; Wang, L. Topical histone deacetylase 1 inhibitor entinostat ameliorates psoriasiform dermatitis through suppression of IL-17A response. J. Dermatol. Sci. 2023, 110, 89–98. [Google Scholar] [CrossRef]
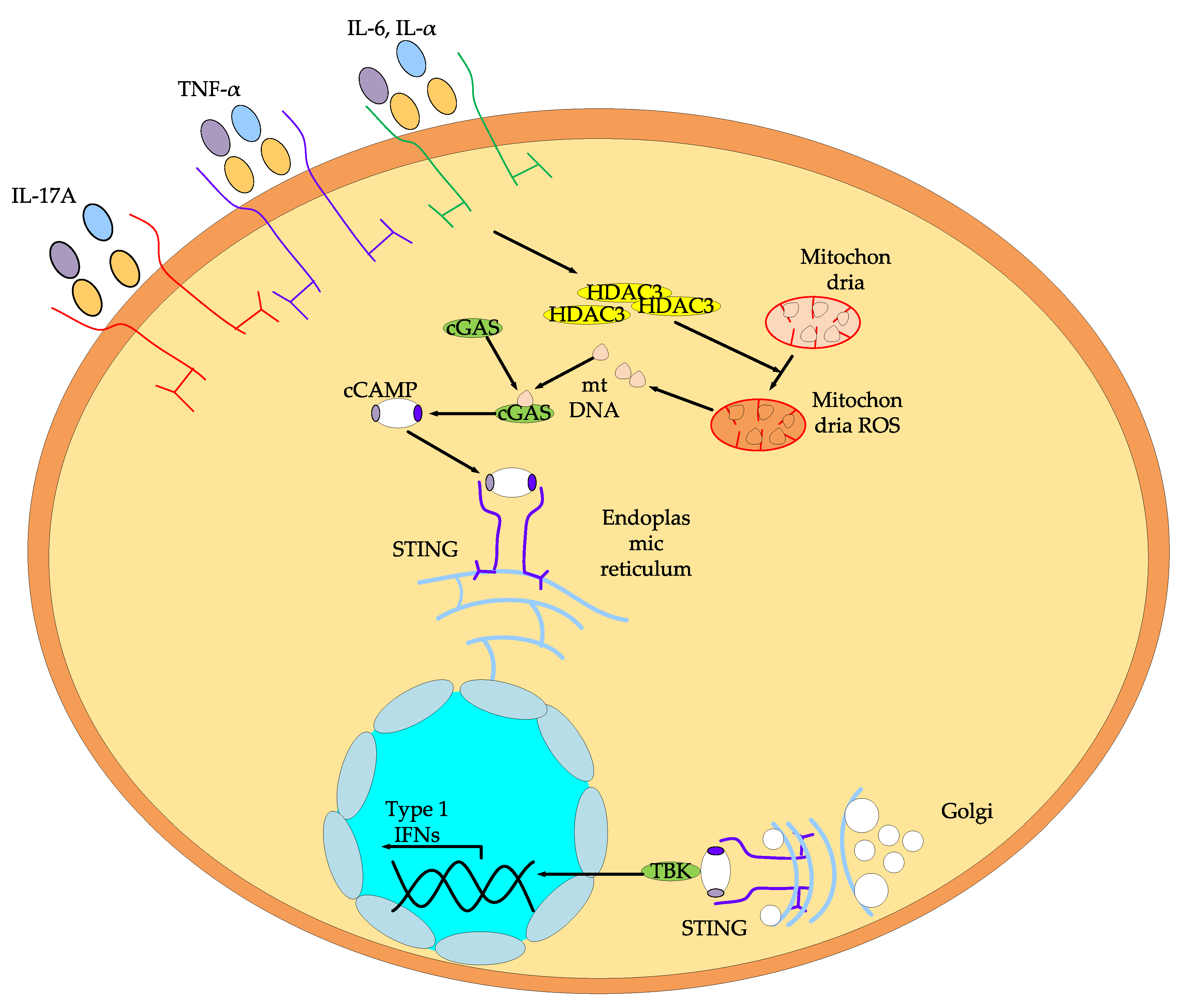
- Zeng, C.; Wen, X.; Wei, Z.; Dong, X. Inhibition of histone deacetylases 3 attenuates imiquimod-induced psoriatic dermatitis via targeting cGAS-STING signaling in keratinocytes. J. Trans. Med. 2025, 23, 1–20. [Google Scholar] [CrossRef]
- Hu, W.; Shen, J.; Zhou, C.; Tai, Z.; Zhu, Q.; Chen, Z.; Sheng, C. Discovery of Janus Kinase and Histone Deacetylase Dual Inhibitors as a New Strategy to Treat Psoriasis. J. Med. Chem. 2024, 67, 19267–19281. [Google Scholar] [CrossRef]
- Li, X.; Song, Y. Proteolysis-targeting chimera (PROTAC) for targeted protein degradation and cancer therapy. J. Hematol. Oncol. 2020, 13, 50. [Google Scholar] [CrossRef]
- Hussain, M.S.; Zhang, L.; Rana, A.J.; Maqbool, M.; Ashique, S.; Khan, Y.; Khan, G. Epigenetic therapy meets targeted protein degradation: HDAC-PROTACs in cancer treatment. Future Med. Chem. 2025, 17, 1–13. [Google Scholar] [CrossRef]
- Chen, S.; Zheng, Y.; Liang, B.; Yin, Y.; Yao, J.; Wang, Q.; Neamati, N. The application of PROTAC in HDAC. Eur. J. Med. Chem. 2023, 260, 115746. [Google Scholar] [CrossRef]

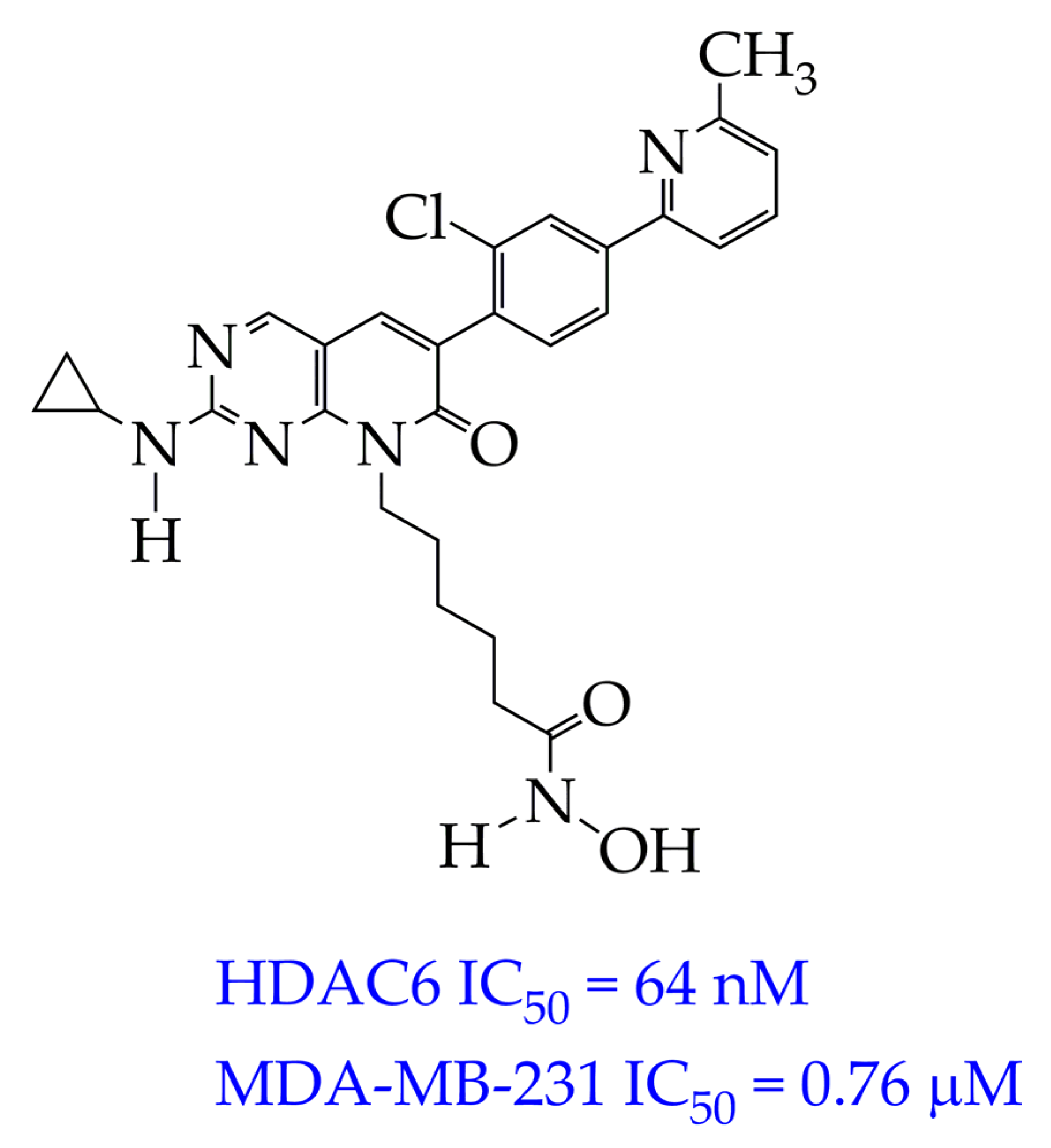
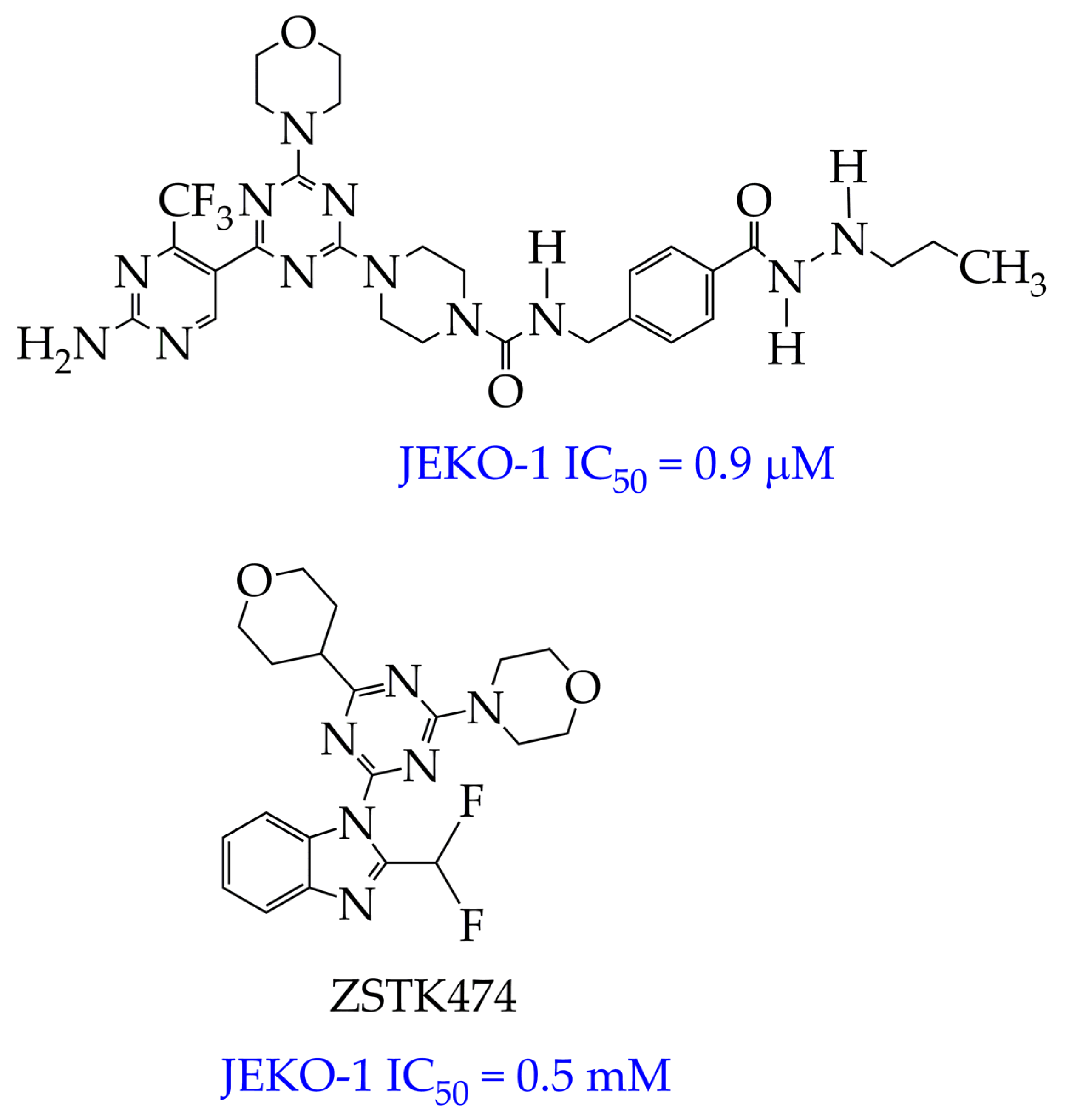
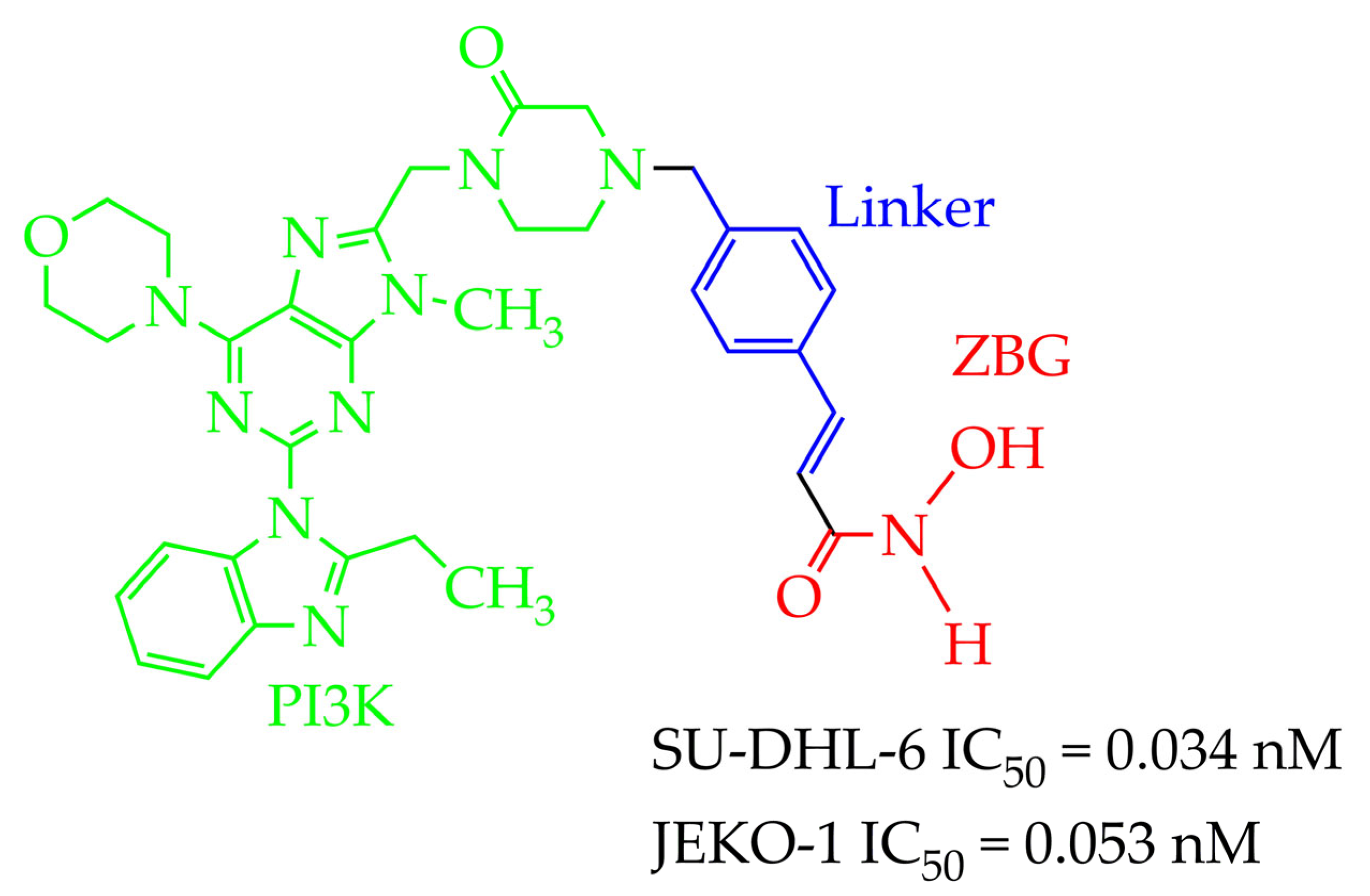

| Chemical Structure | IC50 HDAC6 (nM) | IC50 HDAC1 (nM) |
|---|---|---|
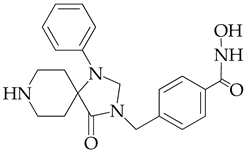 | 23.3 | 1230 |
 | 13.3 | 832 |
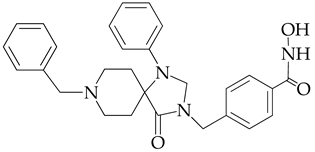 | 12.9 | 1370 |
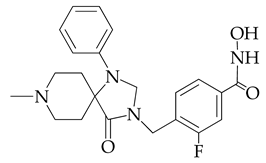 | 7.7 | 2118 |
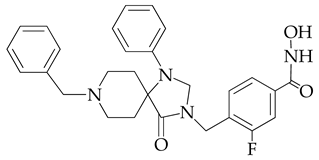 | 17.6 | 7373.2 |
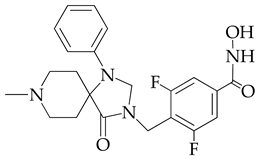 | 10.4 | 6101.3 |
 | 9.5 | 7374.5 |
| Structure | Cancer | Results | Reference |
|---|---|---|---|
 WT161 is at the preliminary preclinical studies stage. | Melanoma | 1. Increased expression of Lys40-acetylated α-tubulin, confirming selective inhibition of HDAC6. 2. Cytotoxicity towards melanoma cell lines CHL-1 (IC50 5.94 μM), SK-MEL-147 (IC50 11.75 μM), and WM1366 (IC50 26.38 μM), after 48 h of observation in the MTT test. 3. Disruption of the growth of multicellular tumor spheroids of melanoma cells, with a decrease in their size by the end of the ninth day. 4. A decrease in the clonogenic capacity of melanoma cells. 5. Enhancement of chemo-induced apoptosis with a combination of 180 μM temozolomide and 2 μM HDAC inhibitor. 6. Inhibition of the migratory capacity of melanoma cells at 6 μM. | [75] |
 | 1. Inhibitory activity against HDAC3 (IC50 311 nM), with a selectivity index against HDAC6 and HDAC8 greater than 32. 2. Antiproliferative activity against mouse skin melanoma B16-F10 cells (IC50 5.22 μM). 3. Inhibition of migration and invasion of B16-F10 cells at a concentration of 5 μM (inhibition degree 70%). 4. Induction of tumor cell apoptosis (46% at 5 μM). 5. Induction of G2/M phase arrest (regulator of DNA replication in tumor cells). 6. Increased Ac-H3 expression according to Western blotting data. 7. Low oral exposure and low oral bioavailability in an in vivo study in Sprague–Dawley (SD) rats. 8. Inhibition in melanoma growth at a dose of 50 mg/kg (in vivo study with inoculation of B16–F10 cells into the inguinal region of mice). | [76] | |
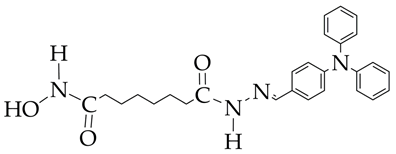
 | Acute myeloid leukemia | 1. Significant inhibitory activity against the most active isoform of HDAC11 (IC50 47 nM). 2. Suppression of growth of U937 and OCI-AML2 cell lines, with an IC50 value of about 10 μM. 3. Ferroptosis-inducing ability of the inhibitor to increase the level of lipid peroxidation and ROS was established. 4. Synergistic antitumor effects were established in combination with daunorubicin and doxorubicin. 5. Acceptable metabolic stability and bioavailability when administered intraperitoneally in vivo to ICR mice. 6. More powerful antitumor effect in vivo in combination with cytarabine compared to monotherapy. | [77] |
 | Leukemia | 1. Inhibition of proliferation of human leukemia monocytic Tohoku Hospital Pediatrics-1 (THP-1) cell line (IC50 1.6–2.2 μM at 48 h). 2. Inhibition of class I and II HDAC activity and increase in acetylation of histones H2a, H2b, H3, and H4. | [78] |
 | 1. HDAC6 inhibition (IC50 9 nM), with a selectivity coefficient relative to HDAC1 of 25. 2. Antiproliferative activity against the three leukemia cell lines HAL01 (B-cell acute lymphoblastic leukemia or B-ALL), HL60 (acute myeloid leukemia or AML), and Jurkat (T-cell acute lymphoblastic leukemia or T-ALL). IC50 values were 2.04–3.08 μM. 3. No cytotoxic effect on normal fibroblasts F107 and F188. 4. Synergistic effect of combination with decitabine (ZIP synergy index about 60) against AML cell lines. | [79] | |
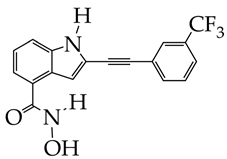
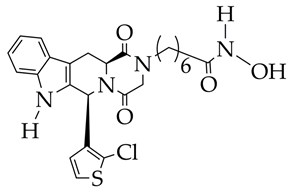 | Colorectal cancer | 1. Inhibitory activity against HDAC6 (IC50 1.3 nM) with selectivity index against HDAC1 1009. 2. Antiproliferative effect against COLO 205, HCC-2998, HCT-116, HT29, KM12, and SW-620 cells (IC50 4.3–6.47 μM). 3. Minimal cytotoxic effects against non-cancerous HaCaT (human keratinocytes) and 3 T3-L1 (mouse embryo fibroblasts) cells with viability of 87.8–88.3%. 4. Induction of apoptosis and suppression of migration in HCT-116 cells. 5. Reduction in phosphorylated ERK1/2 levels according to Western blotting. | [80] |
 TDH-11 is at the preliminary preclinical studies stage. | 1. Inhibits tumor behavior of HCT116, SW480, HT-29, and RKO cells (IC50 100, 115, 169, 201 nM, respectively) and promotes apoptosis and cell cycle arrest. 2. Low cytotoxicity to normal colon epithelial cells NCM460. 3. Inhibition of HDAC1 and HDAC8 activity. 4. Inhibition of migration and invasion of HCT116 and SW480 cells. 5. Slowing down tumor growth in vivo in a mouse xenograft model (HCT-116 cells). 6. Reduction in the number of pulmonary metastatic nodes compared to the control group (no treatment). | [81] | |
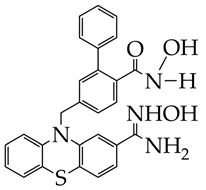 | Gastric cancer | 1. Inhibition of HDAC9 activity (IC50 40 nM). 2. Antiproliferative effect against SC-M1 (IC50 5.8 μM), induction of apoptosis, and DNA damage. | [82] |
| HDAC Inhibitors | Reference |
|---|---|
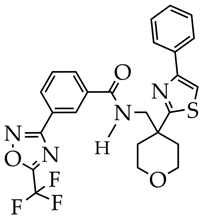 TMP269 is at the preliminary preclinical studies stage. | [98] |
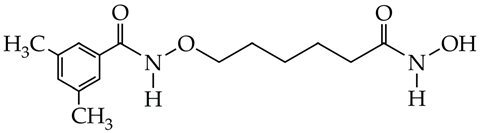 LMK-235 is at the preliminary preclinical studies stage. | [99] |
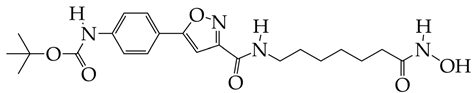 CAY is at the preliminary preclinical studies stage. | [100] |
| HDAC Inhibitors | Pathology | Reference |
|---|---|---|
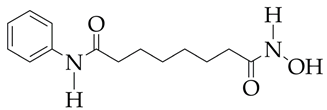
 YAK577 is at the preliminary preclinical studies stage. | Heart failure | [107] |
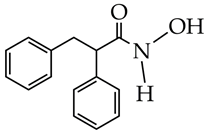
 SAHA, vorinostat (is at the preliminary preclinical studies stage for cardiovascular diseases). | Dilated cardiomyopathy | [108] |
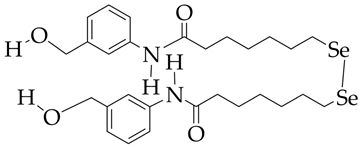 Se-SAHA is at the preliminary preclinical studies stage. | Heart failure, cardiomyopathy | [110] |
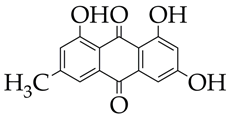 Emodin is at the preliminary preclinical studies stage. | Pathological cardiac hypertrophy | [111] |
| Chemical Structure | IC50 HDAC11, nM | Reference |
|---|---|---|
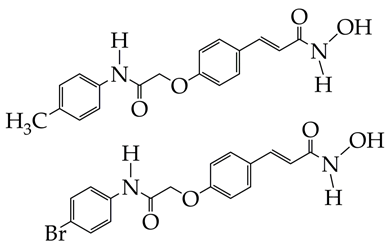
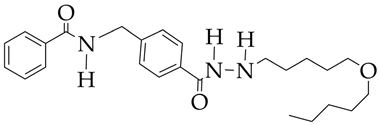 | 51.1 | [112] |
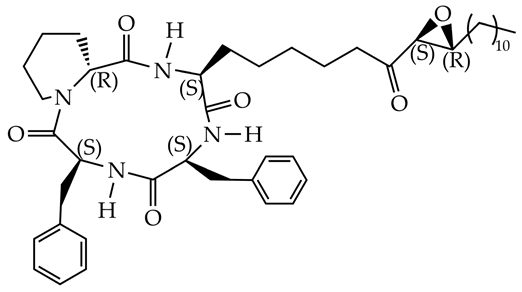 TD034 | 5.1 | [115] |
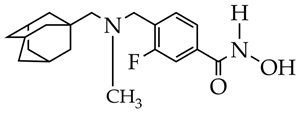 PB94 | 108 | [116] |
| Structure | Results | Reference |
|---|---|---|
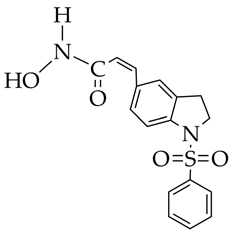 MPTOE028 | 1. Suppression of CTGF expression in human lung fibroblasts (WI-38 cell line). 2. Inhibition of JNK, p38, and ERK phosphorylation. 3. Reduction in α-SMA, fibronectin, and collagen production. 4. Attenuation of bleomycin-induced pulmonary fibrosis in C57BL/6 mice. | [121] |
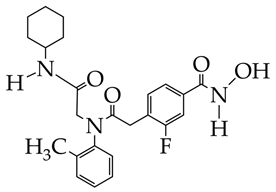
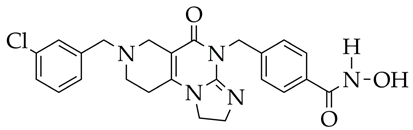 | 1. Inhibition of HDAC6 activity (94.8% at 1 μM, IC50 42.9 nM). 2. Increase in Ac-α-tubulin and Ac-H4 levels. 3. Slowing down of weight loss in a mouse model of pulmonary fibrosis. | [122] |
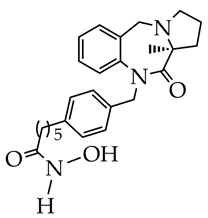 | 1. Inhibition of HDAC6 activity induced by TGF-β1 in HELF cells (IC50 8.45 nM). 2. Stability in plasma with a half-life of 289 min and in human liver microsome (145 min). 3. Inhibition of HDAC6 activity in lung tissue induced by pulmonary fibrosis. 4. Decreased collagen fiber deposition compared to the control group according to histology. 5. Dose-dependent inhibition of α-SMA and p-Smad2/3 expression in mice according to immunohistochemistry. | [123] |
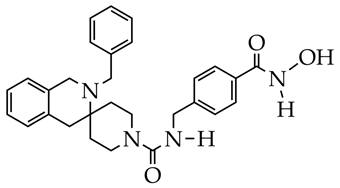 | 1. Selective inhibition of HDAC6 (IC50 63 nM) compared to HDAC1, HDAC3, HDAC5, HDAC8, HDAC10, and HDAC11. 2. Decreased fibronectin and collagen expression in an ex vivo IPF model. | [124] |
| Chemical Structure | Studies | Reference |
|---|---|---|
RGFP966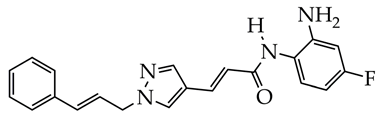 | Psoriasis-like inflammatory cell model established, intracellular ROS measurement, mitochondrial membrane potential assay, immunofluorescence staining, histological analysis, enzyme-linked immunosorbent assay, and Western blot analysis | [130] |
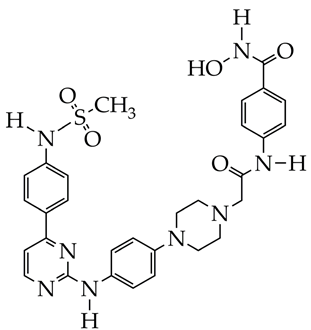 | In vitro cell viability assay, NO determination assay, Western blot analysis, imiquimod-induced psoriasis mice model | [131] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yudaev, P.; Aleksandrova, Y.; Neganova, M. Recent Insights into the Creation of Histone Deacetylase Inhibitors for the Treatment of Human Diseases. Int. J. Mol. Sci. 2025, 26, 8629. https://doi.org/10.3390/ijms26178629
Yudaev P, Aleksandrova Y, Neganova M. Recent Insights into the Creation of Histone Deacetylase Inhibitors for the Treatment of Human Diseases. International Journal of Molecular Sciences. 2025; 26(17):8629. https://doi.org/10.3390/ijms26178629
Chicago/Turabian StyleYudaev, Pavel, Yulia Aleksandrova, and Margarita Neganova. 2025. "Recent Insights into the Creation of Histone Deacetylase Inhibitors for the Treatment of Human Diseases" International Journal of Molecular Sciences 26, no. 17: 8629. https://doi.org/10.3390/ijms26178629
APA StyleYudaev, P., Aleksandrova, Y., & Neganova, M. (2025). Recent Insights into the Creation of Histone Deacetylase Inhibitors for the Treatment of Human Diseases. International Journal of Molecular Sciences, 26(17), 8629. https://doi.org/10.3390/ijms26178629







