The Emerging Role of the Gut Microbiome in Cerebral Cavernous Malformation: A New Novel Therapeutic Strategy?
Abstract
1. Introduction
2. Gut Microbiome in CCM Pathogenesis
3. Gut–Brain Axis
4. Gut Microbiome Manipulations
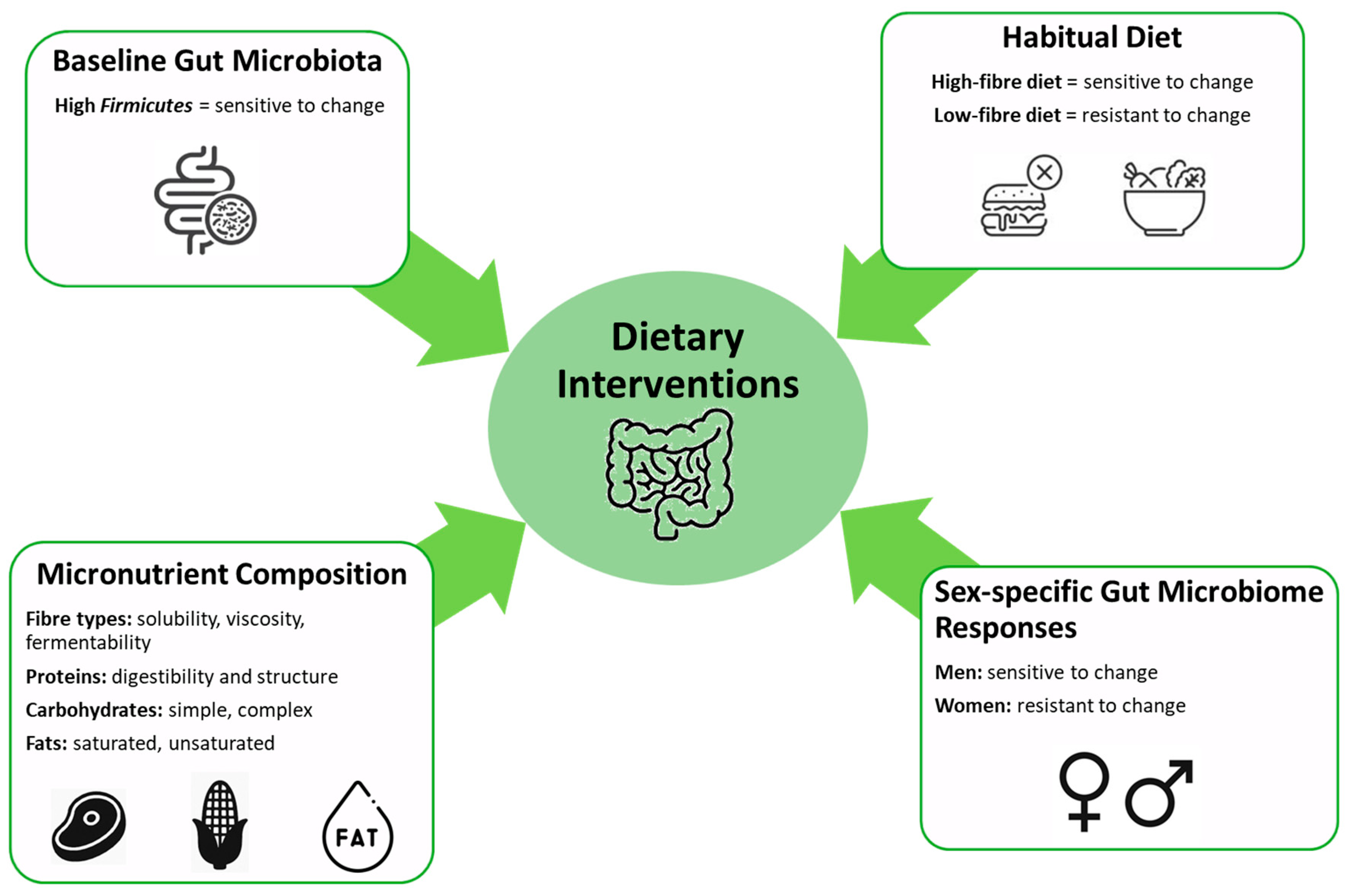
4.1. Diets
4.2. Faecal Microbiota Transplantation (FMT)
4.3. Prebiotics and Probiotics
5. Summary and Future Directions
- Characterization of baseline microbiome profiles in CCM disease: A fundamental step involves identifying and characterizing the baseline gut microbiome composition in CCM patients compared to healthy individuals. Such comparative analyses may reveal disease-specific microbial signatures or dysbiotic patterns that could serve as diagnostic or prognostic indicators and guide targeted interventions.
- Microbiome-targeted therapeutics—probiotics, synbiotics, and diets: Integrative strategies employing specific probiotic strains, rationally designed synbiotic combinations, and precision nutrition approaches should be tested for their capacity to attenuate gut-derived inflammation and modulate host immune responses. These strategies should particularly focus on reducing Gram-negative bacterial taxa and associated endotoxins, which are hypothesized to contribute to endothelial dysfunction in CCM pathogenesis.
- Bridging preclinical and clinical research—translational research: Longitudinal studies involving both well characterized animal models and patient cohorts are needed to evaluate causal links between microbiome shifts and CCM outcomes. Integration of multi-omics approaches—such as metagenomics, metabolomics, and transcriptomics—will enhance mechanistic understanding and enable the identification of therapeutic windows.
Funding
Conflicts of Interest
Abbreviations
References
- Fritschi, J.; Reulen, H.-J.; Spetzler, R.F.; Zabramski, J.M. Cavernous malformations of the brain stem. Acta Neurochir. 1994, 130, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Flemming, K.D.; Graff-Radford, J.; Aakre, J.; Kantarci, K.; Lanzino, G.; Brown, R.D.; Mielke, M.M.; Roberts, R.O.; Kremers, W.; Knopman, D.S. Population-based prevalence of cerebral cavernous malformations in older adults: Mayo Clinic Study of Aging. JAMA Neurol. 2017, 74, 801–805. [Google Scholar] [CrossRef]
- Otten, P.; Pizzolato, G.; Rilliet, B.; Berney, J. 131 cases of cavernous angioma (cavernomas) of the CNS, discovered by retrospective analysis of 24,535 autopsies. Neurochirurgie 1989, 35, 82–83, 128. [Google Scholar]
- Robinson, J.R.; Awad, I.A.; Little, J.R. Natural history of the cavernous angioma. J. Neurosurg. 1991, 75, 709–714. [Google Scholar] [CrossRef] [PubMed]
- Chohan, M.O.; Marchio, S.; Morrison, L.A.; Sidman, R.L.; Cavenee, W.K.; Dejana, E.; Yonas, H.; Pasqualini, R.; Arap, W. Emerging Pharmacologic Targets in Cerebral Cavernous Malformation and Potential Strategies to Alter the Natural History of a Difficult Disease: A Review. JAMA Neurol. 2018, 76, 492–500. [Google Scholar] [CrossRef] [PubMed]
- Al-Holou, W.N.; O’Lynnger, T.M.; Pandey, A.S.; Gemmete, J.J.; Thompson, B.G.; Muraszko, K.M.; Garton, H.J.; Maher, C.O. Natural history and imaging prevalence of cavernous malformations in children and young adults. J. Neurosurg. Pediatr. 2012, 9, 198–205. [Google Scholar] [CrossRef]
- Chan, A.C.; Drakos, S.G.; Ruiz, O.E.; Smith, A.C.; Gibson, C.C.; Ling, J.; Passi, S.F.; Stratman, A.N.; Sacharidou, A.; Revelo, M.P.; et al. Mutations in 2 distinct genetic pathways result in cerebral cavernous malformations in mice. J. Clin. Investig. 2011, 121, 1871–1881. [Google Scholar] [CrossRef]
- Wong, J.H.; Awad, I.A.; Kim, J.H. Ultrastructural pathological features of cerebrovascular malformations: A preliminary report. Neurosurgery 2000, 46, 1454–1459. [Google Scholar] [CrossRef]
- Choi, J.P.; Wang, R.; Yang, X.; Wang, X.; Wang, L.; Ting, K.K.; Foley, M.; Cogger, V.; Yang, Z.; Liu, F.; et al. Ponatinib (AP24534) inhibits MEKK3-KLF signaling and prevents formation and progression of cerebral cavernous malformations. Sci. Adv. 2018, 4, eaau0731. [Google Scholar] [CrossRef]
- Dubovsky, J.; Zabramski, J.M.; Kurth, J.; Spetzler, R.F.; Rich, S.S.; Orr, H.T.; Weber, J.L. A gene responsible for cavernous malformations of the brain maps to chromosome 7q. Hum. Mol. Genet. 1995, 4, 453–458. [Google Scholar] [CrossRef]
- Craig, H.D.; Gunel, M.; Cepeda, O.; Johnson, E.W.; Ptacek, L.; Steinberg, G.K.; Ogilvy, C.S.; Berg, M.J.; Crawford, S.C.; Scott, R.M.; et al. Multilocus linkage identifies two new loci for a mendelian form of stroke, cerebral cavernous malformation, at 7p15-13 and 3q25.2-27. Hum. Mol. Genet. 1998, 7, 1851–1858. [Google Scholar] [CrossRef]
- Zhou, Z.; Tang, A.T.; Wong, W.Y.; Bamezai, S.; Goddard, L.M.; Shenkar, R.; Zhou, S.; Yang, J.; Wright, A.C.; Foley, M.; et al. Cerebral cavernous malformations arise from endothelial gain of MEKK3-KLF2/4 signalling. Nature 2016, 532, 122–126. [Google Scholar] [CrossRef]
- Snellings, D.A.; Hong, C.C.; Ren, A.A.; Lopez-Ramirez, M.A.; Girard, R.; Srinath, A.; Marchuk, D.A.; Ginsberg, M.H.; Awad, I.A.; Kahn, M.L. Cerebral cavernous malformation: From mechanism to therapy. Circ. Res. 2021, 129, 195–215, Erratum in: Circ. Res. 2021, 129, e101. [Google Scholar] [CrossRef]
- Polster, S.P.; Sharma, A.; Tanes, C.; Tang, A.T.; Mericko, P.; Cao, Y.; Carrion-Penagos, J.; Girard, R.; Koskimaki, J.; Zhang, D.; et al. Permissive microbiome characterizes human subjects with a neurovascular disease cavernous angioma. Nat. Commun. 2020, 11, 2659. [Google Scholar] [CrossRef]
- Srinath, A.; Xie, B.; Li, Y.; Sone, J.Y.; Romanos, S.; Chen, C.; Sharma, A.; Polster, S.; Dorrestein, P.C.; Weldon, K.C.; et al. Plasma metabolites with mechanistic and clinical links to the neurovascular disease cavernous angioma. Commun. Med. 2023, 3, 35. [Google Scholar] [CrossRef] [PubMed]
- Batra, S.; Lin, D.; Recinos, P.F.; Zhang, J.; Rigamonti, D. Cavernous malformations: Natural history, diagnosis and treatment. Nat. Rev. Neurol. 2009, 5, 659–670. [Google Scholar] [CrossRef] [PubMed]
- Flemming, K.D. Clinical management of cavernous malformations. Curr. Cardiol. Rep. 2017, 19, 122. [Google Scholar] [CrossRef]
- Gault, J.; Awad, I.A.; Recksiek, P.; Shenkar, R.; Breeze, R.; Handler, M.; Kleinschmidt-DeMasters, B.K. Cerebral cavernous malformations: Somatic mutations in vascular endothelial cells. Neurosurgery 2009, 65, 138–145. [Google Scholar] [PubMed]
- Tang, A.T.; Choi, J.P.; Kotzin, J.J.; Yang, Y.; Hong, C.C.; Hobson, N.; Girard, R.; Zeineddine, H.A.; Lightle, R.; Moore, T.; et al. Endothelial TLR4 and the microbiome drive cerebral cavernous malformations. Nature 2017, 545, 305–310. [Google Scholar] [CrossRef]
- Tang, A.T.; Sullivan, K.R.; Hong, C.C.; Goddard, L.M.; Mahadevan, A.; Ren, A.; Pardo, H.; Peiper, A.; Griffin, E.; Tanes, C.; et al. Distinct cellular roles for PDCD10 define a gut-brain axis in cerebral cavernous malformation. Sci. Transl. Med. 2019, 11, eaaw3521. [Google Scholar] [CrossRef]
- Ressler, A.K.; Snellings, D.A.; Girard, R.; Gallione, C.J.; Lightle, R.; Allen, A.S.; Awad, I.A.; Marchuk, D.A. Single-nucleus DNA sequencing reveals hidden somatic loss-of-heterozygosity in Cerebral Cavernous Malformations. Nat. Commun. 2023, 14, 7009. [Google Scholar] [CrossRef]
- Ren, A.A.; Snellings, D.A.; Su, Y.S.; Hong, C.C.; Castro, M.; Tang, A.T.; Detter, M.R.; Hobson, N.; Girard, R.; Romanos, S.; et al. PIK3CA and CCM mutations fuel cavernomas through a cancer-like mechanism. Nature 2021, 594, 271–276. [Google Scholar] [CrossRef]
- Abdelilah-Seyfried, S.; Tournier-Lasserve, E.; Derry, W.B. Blocking Signalopathic Events to Treat Cerebral Cavernous Malformations. Trends Mol. Med. 2020, 26, 874–887. [Google Scholar] [CrossRef]
- Gilbert, J.A.; Quinn, R.A.; Debelius, J.; Xu, Z.Z.; Morton, J.; Garg, N.; Jansson, J.K.; Dorrestein, P.C.; Knight, R. Microbiome-wide association studies link dynamic microbial consortia to disease. Nature 2016, 535, 94–103. [Google Scholar] [CrossRef]
- Mineharu, Y.; Nakamura, Y.; Sato, N.; Kamata, T.; Oichi, Y.; Fujitani, T.; Funaki, T.; Okuno, Y.; Miyamoto, S.; Koizumi, A. Increased abundance of Ruminococcus gnavus in gut microbiota is associated with moyamoya disease and non-moyamoya intracranial large artery disease. Sci. Rep. 2022, 12, 20244. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Qin, Q.; Yan, S.; Yang, Y.; Yan, H.; Li, T.; Wang, L.; Gao, X.; Li, A.; Ding, S. Gut microbiome alterations in patients with carotid atherosclerosis. Front. Cardiovasc. Med. 2021, 8, 739093. [Google Scholar] [CrossRef]
- Kawabata, S.; Takagaki, M.; Nakamura, H.; Oki, H.; Motooka, D.; Nakamura, S.; Nishida, T.; Terada, E.; Izutsu, N.; Takenaka, T. Dysbiosis of gut microbiome is associated with rupture of cerebral aneurysms. Stroke 2022, 53, 895–903. [Google Scholar] [CrossRef] [PubMed]
- Murgiano, M.; Bartocci, B.; Puca, P.; Di Vincenzo, F.; Del Gaudio, A.; Papa, A.; Cammarota, G.; Gasbarrini, A.; Scaldaferri, F.; Lopetuso, L.R. Gut microbiota modulation in IBD: From the old paradigm to revolutionary tools. Int. J. Mol. Sci. 2025, 26, 3059. [Google Scholar] [CrossRef] [PubMed]
- Khosravi, A.; Yáñez, A.; Price, J.G.; Chow, A.; Merad, M.; Goodridge, H.S.; Mazmanian, S.K. Gut microbiota promote hematopoiesis to control bacterial infection. Cell Host Microbe 2014, 15, 374–381. [Google Scholar] [CrossRef]
- Wu, S.; Liu, X.; Jiang, R.; Yan, X.; Ling, Z. Roles and mechanisms of gut microbiota in patients with Alzheimer’s disease. Front. Aging Neurosci. 2021, 13, 650047. [Google Scholar] [CrossRef]
- Polster, S.P.; Shen, L.; Sharma, A.; Stadnik, A.; Carrion-Penagos, J.; Girard, R.; Koskimäki, J.; Romanos, S.; Lyne, S.; Shenkar, R. Abstract TMP107: Microbiome Signature of Cerebral Cavernous Malformation Patients. Stroke 2019, 50 (Suppl. 1), ATMP107. [Google Scholar] [CrossRef]
- Pols, T.W.; Noriega, L.G.; Nomura, M.; Auwerx, J.; Schoonjans, K. The bile acid membrane receptor TGR5 as an emerging target in metabolism and inflammation. J. Hepatol. 2011, 54, 1263–1272. [Google Scholar] [CrossRef] [PubMed]
- Weiman, S. Harnessing the Power of Microbes as Therapeutics: Bugs as Drugs: Report on an American Academy of Microbiology Colloquium Held in San Diego, CA, in April 2014; Fox, J., Ed.; American Society for Microbiology: Washington, DC, USA, 2015. [Google Scholar]
- Seguella, L.; Gulbransen, B.D. Enteric glial biology, intercellular signalling and roles in gastrointestinal disease. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 571–587. [Google Scholar] [CrossRef]
- Yu, L.; Li, Y. Involvement of intestinal enteroendocrine cells in neurological and psychiatric disorders. Biomedicines 2022, 10, 2577. [Google Scholar] [CrossRef]
- Morais, L.H.; Schreiber, H.L.t.; Mazmanian, S.K. The gut microbiota-brain axis in behaviour and brain disorders. Nat. Rev. Microbiol. 2021, 19, 241–255. [Google Scholar] [CrossRef]
- Sherwin, E.; Dinan, T.G.; Cryan, J.F. Recent developments in understanding the role of the gut microbiota in brain health and disease. Ann. N. Y. Acad. Sci. 2018, 1420, 5–25. [Google Scholar] [CrossRef]
- Forsythe, P.; Bienenstock, J.; Kunze, W.A. Vagal pathways for microbiome-brain-gut axis communication. In Microbial Endocrinology: The Microbiota-Gut-Brain Axis in Health and Disease; Springer: New York, NY, USA, 2014; pp. 115–133. [Google Scholar]
- Bauer, K.C.; Huus, K.E.; Finlay, B.B. Microbes and the mind: Emerging hallmarks of the gut microbiota–brain axis. Cell. Microbiol. 2016, 18, 632–644. [Google Scholar] [CrossRef]
- Dinan, T.G.; Cryan, J.F. Gut instincts: Microbiota as a key regulator of brain development, ageing and neurodegeneration. J. Physiol. 2017, 595, 489–503. [Google Scholar] [CrossRef]
- Dinan, T.G.; Cryan, J.F. The microbiome-gut-brain axis in health and disease. Gastroenterol. Clin. 2017, 46, 77–89. [Google Scholar] [CrossRef]
- Sampson, T.R.; Debelius, J.W.; Thron, T.; Janssen, S.; Shastri, G.G.; Ilhan, Z.E.; Challis, C.; Schretter, C.E.; Rocha, S.; Gradinaru, V.; et al. Gut microbiota regulate motor deficits and neuroinflammation in a model of Parkinson’s disease. Cell 2016, 167, 1469–1480.e12. [Google Scholar] [CrossRef] [PubMed]
- Paton, S.E.; Solano, J.L.; Coulombe-Rozon, F.; Lebel, M.; Menard, C. Barrier–environment interactions along the gut–brain axis and their influence on cognition and behaviour throughout the lifespan. J. Psychiatry Neurosci. 2023, 48, E190–E208. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, Q.; Ren, J.; Zhu, Q.; Wang, L.; Zhang, Y.; Geng, Z. Evolution of brain morphology in spontaneously hypertensive and Wistar-Kyoto rats from early adulthood to aging: A longitudinal magnetic resonance imaging study. Front. Aging Neurosci. 2021, 13, 757808. [Google Scholar] [CrossRef]
- Berer, K.; Gerdes, L.A.; Cekanaviciute, E.; Jia, X.; Xiao, L.; Xia, Z.; Liu, C.; Klotz, L.; Stauffer, U.; Baranzini, S.E. Gut microbiota from multiple sclerosis patients enables spontaneous autoimmune encephalomyelitis in mice. Proc. Natl. Acad. Sci. USA 2017, 114, 10719–10724. [Google Scholar] [CrossRef]
- Singh, V.; Roth, S.; Llovera, G.; Sadler, R.; Garzetti, D.; Stecher, B.; Dichgans, M.; Liesz, A. Microbiota dysbiosis controls the neuroinflammatory response after stroke. J. Neurosci. 2016, 36, 7428–7440. [Google Scholar] [CrossRef]
- Fowlie, G.; Cohen, N.; Ming, X. The Perturbance of Microbiome and Gut-Brain Axis in Autism Spectrum Disorders. Int. J. Mol. Sci. 2018, 19, 2251. [Google Scholar] [CrossRef] [PubMed]
- Strati, F.; Cavalieri, D.; Albanese, D.; De Felice, C.; Donati, C.; Hayek, J.; Jousson, O.; Leoncini, S.; Renzi, D.; Calabrò, A.; et al. New evidences on the altered gut microbiota in autism spectrum disorders. Microbiome 2017, 5, 24. [Google Scholar] [CrossRef] [PubMed]
- Brigida, A.L.; Schultz, S.; Cascone, M.; Antonucci, N.; Siniscalco, D. Endocannabinod Signal Dysregulation in Autism Spectrum Disorders: A Correlation Link between Inflammatory State and Neuro-Immune Alterations. Int. J. Mol. Sci. 2017, 18, 1425. [Google Scholar] [CrossRef]
- Wong, C.G.; Bottiglieri, T.; Snead, O.C., 3rd. GABA, gamma-hydroxybutyric acid, and neurological disease. Ann. Neurol. 2003, 54 (Suppl. 6), S3–S12. [Google Scholar] [CrossRef] [PubMed]
- Valles-Colomer, M.; Falony, G.; Darzi, Y.; Tigchelaar, E.F.; Wang, J.; Tito, R.Y.; Schiweck, C.; Kurilshikov, A.; Joossens, M.; Wijmenga, C.; et al. The neuroactive potential of the human gut microbiota in quality of life and depression. Nat. Microbiol. 2019, 4, 623–632. [Google Scholar] [CrossRef]
- Torres, N.; Guevara-Cruz, M.; Velázquez-Villegas, L.A.; Tovar, A.R. Nutrition and Atherosclerosis. Arch. Med. Res. 2015, 46, 408–426. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, Q.; Jiang, H. Gut microbiota in atherosclerosis: Focus on trimethylamine N-oxide. Apmis 2020, 128, 353–366. [Google Scholar] [CrossRef]
- Wang, Z.; Klipfell, E.; Bennett, B.J.; Koeth, R.; Levison, B.S.; Dugar, B.; Feldstein, A.E.; Britt, E.B.; Fu, X.; Chung, Y.M.; et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 2011, 472, 57–63. [Google Scholar] [CrossRef]
- Chen, K.; Zheng, X.; Feng, M.; Li, D.; Zhang, H. Gut Microbiota-Dependent Metabolite Trimethylamine N-Oxide Contributes to Cardiac Dysfunction in Western Diet-Induced Obese Mice. Front. Physiol. 2017, 8, 139. [Google Scholar] [CrossRef]
- Miao, J.; Ling, A.V.; Manthena, P.V.; Gearing, M.E.; Graham, M.J.; Crooke, R.M.; Croce, K.J.; Esquejo, R.M.; Clish, C.B.; Morbid Obesity Study Group; et al. Flavin-containing monooxygenase 3 as a potential player in diabetes-associated atherosclerosis. Nat. Commun. 2015, 6, 6498. [Google Scholar] [CrossRef]
- Neal, E.G.; Chaffe, H.; Schwartz, R.H.; Lawson, M.S.; Edwards, N.; Fitzsimmons, G.; Whitney, A.; Cross, J.H. The ketogenic diet for the treatment of childhood epilepsy: A randomised controlled trial. Lancet Neurol. 2008, 7, 500–506. [Google Scholar] [CrossRef] [PubMed]
- Reddel, S.; Putignani, L.; Del Chierico, F. The Impact of Low-FODMAPs, Gluten-Free, and Ketogenic Diets on Gut Microbiota Modulation in Pathological Conditions. Nutrients 2019, 11, 373. [Google Scholar] [CrossRef] [PubMed]
- Wheless, J.W. History of the ketogenic diet. Epilepsia 2008, 49 (Suppl. 8), 3–5. [Google Scholar] [CrossRef]
- Stafstrom, C.E.; Rho, J.M. The ketogenic diet as a treatment paradigm for diverse neurological disorders. Front. Pharmacol. 2012, 3, 59. [Google Scholar] [CrossRef]
- Włodarek, D. Role of ketogenic diets in neurodegenerative diseases (Alzheimer’s disease and Parkinson’s disease). Nutrients 2019, 11, 169. [Google Scholar] [CrossRef] [PubMed]
- Skonieczna-Żydecka, K.; Marlicz, W.; Misera, A.; Koulaouzidis, A.; Łoniewski, I. Microbiome-The Missing Link in the Gut-Brain Axis: Focus on Its Role in Gastrointestinal and Mental Health. J. Clin. Med. 2018, 7, 521. [Google Scholar] [CrossRef]
- Sarkar, A.; Lehto, S.M.; Harty, S.; Dinan, T.G.; Cryan, J.F.; Burnet, P.W.J. Psychobiotics and the Manipulation of Bacteria-Gut-Brain Signals. Trends Neurosci. 2016, 39, 763–781. [Google Scholar] [CrossRef]
- Doenyas, C. Dietary interventions for autism spectrum disorder: New perspectives from the gut-brain axis. Physiol. Behav. 2018, 194, 577–582. [Google Scholar] [CrossRef] [PubMed]
- Conlon, M.A.; Bird, A.R. The impact of diet and lifestyle on gut microbiota and human health. Nutrients 2014, 7, 17–44. [Google Scholar] [CrossRef] [PubMed]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What is the healthy gut microbiota composition? A changing ecosystem across age, environment, diet, and diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- Deschasaux, M.; Bouter, K.E.; Prodan, A.; Levin, E.; Groen, A.K.; Herrema, H.; Tremaroli, V.; Bakker, G.J.; Attaye, I.; Pinto-Sietsma, S.-J. Depicting the composition of gut microbiota in a population with varied ethnic origins but shared geography. Nat. Med. 2018, 24, 1526–1531. [Google Scholar] [CrossRef]
- Gupta, V.K.; Paul, S.; Dutta, C. Geography, ethnicity or subsistence-specific variations in human microbiome composition and diversity. Front. Microbiol. 2017, 8, 1162. [Google Scholar] [CrossRef]
- Koliada, A.; Moseiko, V.; Romanenko, M.; Lushchak, O.; Kryzhanovska, N.; Guryanov, V.; Vaiserman, A. Sex differences in the phylum-level human gut microbiota composition. BMC Microbiol. 2021, 21, 131. [Google Scholar] [CrossRef]
- Zhu, B.; Wang, X.; Li, L. Human gut microbiome: The second genome of human body. Protein Cell 2010, 1, 718–725. [Google Scholar] [CrossRef]
- Chong, P.P.; Chin, V.K.; Looi, C.Y.; Wong, W.F.; Madhavan, P.; Yong, V.C. The microbiome and irritable bowel syndrome–a review on the pathophysiology, current research and future therapy. Front. Microbiol. 2019, 10, 424646, Erratum in: Front. Microbiol. 2019, 10, 1870. [Google Scholar] [CrossRef]
- Manrique, P.; Bolduc, B.; Walk, S.T.; van der Oost, J.; de Vos, W.M.; Young, M.J. Healthy human gut phageome. Proc. Natl. Acad. Sci. USA 2016, 113, 10400–10405. [Google Scholar] [CrossRef]
- Gomaa, E.Z. Human gut microbiota/microbiome in health and diseases: A review. Antonie Van. Leeuwenhoek 2020, 113, 2019–2040. [Google Scholar] [CrossRef]
- Qin, J.; Li, R.; Raes, J.; Arumugam, M.; Burgdorf, K.S.; Manichanh, C.; Nielsen, T.; Pons, N.; Levenez, F.; Yamada, T. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010, 464, 59–65. [Google Scholar] [CrossRef]
- Valdes, A.M.; Walter, J.; Segal, E.; Spector, T.D. Role of the gut microbiota in nutrition and health. BMJ 2018, 361, k2179. [Google Scholar] [CrossRef]
- Salonen, A.; Lahti, L.; Salojärvi, J.; Holtrop, G.; Korpela, K.; Duncan, S.H.; Date, P.; Farquharson, F.; Johnstone, A.M.; Lobley, G.E. Impact of diet and individual variation on intestinal microbiota composition and fermentation products in obese men. ISME J. 2014, 8, 2218–2230. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.J.; Vangay, P.; Al-Ghalith, G.A.; Hillmann, B.M.; Ward, T.L.; Shields-Cutler, R.R.; Kim, A.D.; Shmagel, A.K.; Syed, A.N.; Walter, J. Daily sampling reveals personalized diet-microbiome associations in humans. Cell Host Microbe 2019, 25, 789–802.e5. [Google Scholar] [CrossRef]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Valeri, F.; Endres, K. How biological sex of the host shapes its gut microbiota. Front. Neuroendocrinol. 2021, 61, 100912. [Google Scholar] [CrossRef] [PubMed]
- Rothschild, D.; Weissbrod, O.; Barkan, E.; Kurilshikov, A.; Korem, T.; Zeevi, D.; Costea, P.I.; Godneva, A.; Kalka, I.N.; Bar, N. Environment dominates over host genetics in shaping human gut microbiota. Nature 2018, 555, 210–215. [Google Scholar] [CrossRef]
- Black, R.E.; Victora, C.G.; Walker, S.P.; Bhutta, Z.A.; Christian, P.; De Onis, M.; Ezzati, M.; Grantham-McGregor, S.; Katz, J.; Martorell, R. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet 2013, 382, 427–451. [Google Scholar] [CrossRef]
- Kau, A.L.; Ahern, P.P.; Griffin, N.W.; Goodman, A.L.; Gordon, J.I. Human nutrition, the gut microbiome and the immune system. Nature 2011, 474, 327–336. [Google Scholar] [CrossRef]
- Blanton, L.V.; Barratt, M.J.; Charbonneau, M.R.; Ahmed, T.; Gordon, J.I. Childhood undernutrition, the gut microbiota, and microbiota-directed therapeutics. Science 2016, 352, 1533. [Google Scholar] [CrossRef]
- Smith, M.I.; Yatsunenko, T.; Manary, M.J.; Trehan, I.; Mkakosya, R.; Cheng, J.; Kau, A.L.; Rich, S.S.; Concannon, P.; Mychaleckyj, J.C. Gut microbiomes of Malawian twin pairs discordant for kwashiorkor. Science 2013, 339, 548–554. [Google Scholar] [CrossRef]
- Davis, J.C.; Lewis, Z.T.; Krishnan, S.; Bernstein, R.M.; Moore, S.E.; Prentice, A.M.; Mills, D.A.; Lebrilla, C.B.; Zivkovic, A.M. Growth and morbidity of Gambian infants are influenced by maternal milk oligosaccharides and infant gut microbiota. Sci. Rep. 2017, 7, 40466. [Google Scholar] [CrossRef]
- Valls-Pedret, C.; Sala-Vila, A.; Serra-Mir, M.; Corella, D.; de la Torre, R.; Martínez-González, M.Á.; Martínez-Lapiscina, E.H.; Fitó, M.; Pérez-Heras, A.; Salas-Salvadó, J. Mediterranean diet and age-related cognitive decline: A randomized clinical trial. JAMA Intern. Med. 2015, 175, 1094–1103. [Google Scholar] [CrossRef]
- Korpela, K.; Flint, H.J.; Johnstone, A.M.; Lappi, J.; Poutanen, K.; Dewulf, E.; Delzenne, N.; De Vos, W.M.; Salonen, A. Gut microbiota signatures predict host and microbiota responses to dietary interventions in obese individuals. PLoS ONE 2014, 9, e90702. [Google Scholar] [CrossRef]
- Healey, G.; Murphy, R.; Butts, C.; Brough, L.; Whelan, K.; Coad, J. Habitual dietary fibre intake influences gut microbiota response to an inulin-type fructan prebiotic: A randomised, double-blind, placebo-controlled, cross-over, human intervention study. Br. J. Nutr. 2018, 119, 176–189. [Google Scholar] [CrossRef]
- Slavin, J. Fiber and prebiotics: Mechanisms and health benefits. Nutrients 2013, 5, 1417–1435. [Google Scholar] [CrossRef]
- Fogelholm, M.; Anderssen, S.; Gunnarsdottir, I.; Lahti-Koski, M. Dietary macronutrients and food consumption as determinants of long-term weight change in adult populations: A systematic literature review. Food Nutr. Res. 2012, 56, 19103. [Google Scholar] [CrossRef] [PubMed]
- Müller, M.; Hermes, G.D.; Emanuel, E.C.; Holst, J.J.; Zoetendal, E.G.; Smidt, H.; Troost, F.; Schaap, F.G.; Damink, S.O.; Jocken, J.W. Effect of wheat bran derived prebiotic supplementation on gastrointestinal transit, gut microbiota, and metabolic health: A randomized controlled trial in healthy adults with a slow gut transit. Gut Microbes 2020, 12, 1704141. [Google Scholar] [CrossRef] [PubMed]
- Most, J.; Penders, J.; Lucchesi, M.; Goossens, G.H.; Blaak, E.E. Gut microbiota composition in relation to the metabolic response to 12-week combined polyphenol supplementation in overweight men and women. Eur. J. Clin. Nutr. 2017, 71, 1040–1045. [Google Scholar] [CrossRef] [PubMed]
- Khoruts, A.; Staley, C.; Sadowsky, M.J. Faecal microbiota transplantation for Clostridioides difficile: Mechanisms and pharmacology. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 67–80. [Google Scholar] [CrossRef]
- Lindefeldt, M.; Eng, A.; Darban, H.; Bjerkner, A.; Zetterström, C.K.; Allander, T.; Andersson, B.; Borenstein, E.; Dahlin, M.; Prast-Nielsen, S. The ketogenic diet influences taxonomic and functional composition of the gut microbiota in children with severe epilepsy. NPJ Biofilms Microbiomes 2019, 5, 5. [Google Scholar] [CrossRef] [PubMed]
- Mazzini, L.; Mogna, L.; De Marchi, F.; Amoruso, A.; Pane, M.; Aloisio, I.; Cionci, N.B.; Gaggìa, F.; Lucenti, A.; Bersano, E.; et al. Potential Role of Gut Microbiota in ALS Pathogenesis and Possible Novel Therapeutic Strategies. J. Clin. Gastroenterol. 2018, 52 (Suppl. 1), S68–S70. [Google Scholar] [CrossRef] [PubMed]
- Scheperjans, F.; Aho, V.; Pereira, P.A.; Koskinen, K.; Paulin, L.; Pekkonen, E.; Haapaniemi, E.; Kaakkola, S.; Eerola-Rautio, J.; Pohja, M.; et al. Gut microbiota are related to Parkinson’s disease and clinical phenotype. Mov. Disord. 2015, 30, 350–358. [Google Scholar] [CrossRef]
- Cree, B.A.; Spencer, C.M.; Varrin-Doyer, M.; Baranzini, S.E.; Zamvil, S.S. Gut microbiome analysis in neuromyelitis optica reveals overabundance of Clostridium perfringens. Ann. Neurol. 2016, 80, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Vogt, N.M.; Kerby, R.L.; Dill-McFarland, K.A.; Harding, S.J.; Merluzzi, A.P.; Johnson, S.C.; Carlsson, C.M.; Asthana, S.; Zetterberg, H.; Blennow, K.; et al. Gut microbiome alterations in Alzheimer’s disease. Sci. Rep. 2017, 7, 13537. [Google Scholar] [CrossRef]
- Ma, B.; Liang, J.; Dai, M.; Wang, J.; Luo, J.; Zhang, Z.; Jing, J. Altered Gut Microbiota in Chinese Children With Autism Spectrum Disorders. Front. Cell Infect. Microbiol. 2019, 9, 40. [Google Scholar] [CrossRef]
- Cosorich, I.; Dalla-Costa, G.; Sorini, C.; Ferrarese, R.; Messina, M.J.; Dolpady, J.; Radice, E.; Mariani, A.; Testoni, P.A.; Canducci, F.; et al. High frequency of intestinal T(H)17 cells correlates with microbiota alterations and disease activity in multiple sclerosis. Sci. Adv. 2017, 3, e1700492. [Google Scholar] [CrossRef]
- Unger, M.M.; Spiegel, J.; Dillmann, K.U.; Grundmann, D.; Philippeit, H.; Bürmann, J.; Faßbender, K.; Schwiertz, A.; Schäfer, K.H. Short chain fatty acids and gut microbiota differ between patients with Parkinson’s disease and age-matched controls. Park. Relat. Disord. 2016, 32, 66–72. [Google Scholar] [CrossRef]
- Hasegawa, S.; Goto, S.; Tsuji, H.; Okuno, T.; Asahara, T.; Nomoto, K.; Shibata, A.; Fujisawa, Y.; Minato, T.; Okamoto, A.; et al. Intestinal Dysbiosis and Lowered Serum Lipopolysaccharide-Binding Protein in Parkinson’s Disease. PLoS ONE 2015, 10, e0142164. [Google Scholar] [CrossRef]
- Willison, H.J.; Jacobs, B.C.; van Doorn, P.A. Guillain-Barré syndrome. Lancet 2016, 388, 717–727. [Google Scholar] [CrossRef]
- McElhanon, B.O.; McCracken, C.; Karpen, S.; Sharp, W.G. Gastrointestinal symptoms in autism spectrum disorder: A meta-analysis. Pediatrics 2014, 133, 872–883. [Google Scholar] [CrossRef]
- Shaaban, S.Y.; El Gendy, Y.G.; Mehanna, N.S.; El-Senousy, W.M.; El-Feki, H.S.A.; Saad, K.; El-Asheer, O.M. The role of probiotics in children with autism spectrum disorder: A prospective, open-label study. Nutr. Neurosci. 2018, 21, 676–681. [Google Scholar] [CrossRef]
- Pärtty, A.; Kalliomäki, M.; Wacklin, P.; Salminen, S.; Isolauri, E. A possible link between early probiotic intervention and the risk of neuropsychiatric disorders later in childhood: A randomized trial. Pediatr. Res. 2015, 77, 823–828. [Google Scholar] [CrossRef]
- Kałużna-Czaplińska, J.; Błaszczyk, S. The level of arabinitol in autistic children after probiotic therapy. Nutrition 2012, 28, 124–126. [Google Scholar] [CrossRef]
- Aabed, K.; Bhat, R.S.; Moubayed, N.; Al-Mutiri, M.; Al-Marshoud, M.; Al-Qahtani, A.; Ansary, A. Ameliorative effect of probiotics (Lactobacillus paracaseii and Protein®) and prebiotics (propolis and bee pollen) on clindamycin and propionic acid-induced oxidative stress and altered gut microbiota in a rodent model of autism. Cell. Mol. Biol. 2019, 65, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Sharon, G.; Cruz, N.J.; Kang, D.W.; Gandal, M.J.; Wang, B.; Kim, Y.M.; Zink, E.M.; Casey, C.P.; Taylor, B.C.; Lane, C.J.; et al. Human Gut Microbiota from Autism Spectrum Disorder Promote Behavioral Symptoms in Mice. Cell 2019, 177, 1600–1618.e1617. [Google Scholar] [CrossRef]
- Chen, R.; Wu, P.; Cai, Z.; Fang, Y.; Zhou, H.; Lasanajak, Y.; Tang, L.; Ye, L.; Hou, C.; Zhao, J. Puerariae Lobatae Radix with chuanxiong Rhizoma for treatment of cerebral ischemic stroke by remodeling gut microbiota to regulate the brain-gut barriers. J. Nutr. Biochem. 2019, 65, 101–114. [Google Scholar] [CrossRef]
- Winek, K.; Engel, O.; Koduah, P.; Heimesaat, M.M.; Fischer, A.; Bereswill, S.; Dames, C.; Kershaw, O.; Gruber, A.D.; Curato, C.; et al. Depletion of Cultivatable Gut Microbiota by Broad-Spectrum Antibiotic Pretreatment Worsens Outcome After Murine Stroke. Stroke 2016, 47, 1354–1363. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Wu, L.; Peng, G.; Han, Y.; Tang, R.; Ge, J.; Zhang, L.; Jia, L.; Yue, S.; Zhou, K.; et al. Altered microbiomes distinguish Alzheimer’s disease from amnestic mild cognitive impairment and health in a Chinese cohort. Brain Behav. Immun. 2019, 80, 633–643. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; He, Y.; Ma, J.; Huang, P.; Du, J.; Cao, L.; Wang, Y.; Xiao, Q.; Tang, H.; Chen, S. Mild cognitive impairment has similar alterations as Alzheimer’s disease in gut microbiota. Alzheimers Dement. 2019, 15, 1357–1366. [Google Scholar] [CrossRef]
- Haran, J.P.; Bhattarai, S.K.; Foley, S.E.; Dutta, P.; Ward, D.V.; Bucci, V.; McCormick, B.A. Alzheimer’s Disease Microbiome Is Associated with Dysregulation of the Anti-Inflammatory P-Glycoprotein Pathway. mBio 2019, 10, 10–1128. [Google Scholar] [CrossRef]
- Zhuang, Z.Q.; Shen, L.L.; Li, W.W.; Fu, X.; Zeng, F.; Gui, L.; Lü, Y.; Cai, M.; Zhu, C.; Tan, Y.L.; et al. Gut Microbiota is Altered in Patients with Alzheimer’s Disease. J. Alzheimers Dis. 2018, 63, 1337–1346. [Google Scholar] [CrossRef]
- Dominy, S.S.; Lynch, C.; Ermini, F.; Benedyk, M.; Marczyk, A.; Konradi, A.; Nguyen, M.; Haditsch, U.; Raha, D.; Griffin, C.; et al. Porphyromonas gingivalis in Alzheimer’s disease brains: Evidence for disease causation and treatment with small-molecule inhibitors. Sci. Adv. 2019, 5, eaau3333. [Google Scholar] [CrossRef]
- Zhao, Y.; Jaber, V.; Lukiw, W.J. Secretory Products of the Human GI Tract Microbiome and Their Potential Impact on Alzheimer’s Disease (AD): Detection of Lipopolysaccharide (LPS) in AD Hippocampus. Front. Cell Infect. Microbiol. 2017, 7, 318. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Dua, P.; Lukiw, W.J. Microbial Sources of Amyloid and Relevance to Amyloidogenesis and Alzheimer’s Disease (AD). J. Alzheimers Dis. Park. 2015, 5, 177. [Google Scholar] [CrossRef]
- Cai, Z.; Hussain, M.D.; Yan, L.J. Microglia, neuroinflammation, and beta-amyloid protein in Alzheimer’s disease. Int. J. Neurosci. 2014, 124, 307–321. [Google Scholar] [CrossRef] [PubMed]
- Dodiya, H.B.; Kuntz, T.; Shaik, S.M.; Baufeld, C.; Leibowitz, J.; Zhang, X.; Gottel, N.; Zhang, X.; Butovsky, O.; Gilbert, J.A.; et al. Sex-specific effects of microbiome perturbations on cerebral Aβ amyloidosis and microglia phenotypes. J. Exp. Med. 2019, 216, 1542–1560. [Google Scholar] [CrossRef]
- Fu, X.; Liu, Z.; Zhu, C.; Mou, H.; Kong, Q. Nondigestible carbohydrates, butyrate, and butyrate-producing bacteria. Crit. Rev. Food Sci. Nutr. 2019, 59, S130–S152. [Google Scholar] [CrossRef]
- Collado, M.C.; Isolauri, E.; Salminen, S.; Sanz, Y. The impact of probiotic on gut health. Curr. Drug Metab. 2009, 10, 68–78. [Google Scholar] [CrossRef]
- Miles, M.P. Probiotics and gut health in athletes. Curr. Nutr. Rep. 2020, 9, 129–136. [Google Scholar] [CrossRef]
- Cencic, A.; Chingwaru, W. The role of functional foods, nutraceuticals, and food supplements in intestinal health. Nutrients 2010, 2, 611–625. [Google Scholar] [CrossRef]
- Asghari, K.M.; Dolatkhah, N.; Ayromlou, H.; Mirnasiri, F.; Dadfar, T.; Hashemian, M. The effect of probiotic supplementation on the clinical and para-clinical findings of multiple sclerosis: A randomized clinical trial. Sci. Rep. 2023, 13, 18577. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Martínez, Y.; Aguilar-Ponce, L.; Romo-Araiza, A.; Chávez-Guerra, A.; Martiñón, S.; Ibarra-García, A.P.; Arias-Santiago, S.; Gálvez-Susano, V.; Ibarra, A. Supplementation with a Symbiotic Induced Neuroprotection and Improved Memory in Rats with Ischemic Stroke. Biomedicines 2024, 12, 209. [Google Scholar] [CrossRef] [PubMed]
- Savigamin, C.; Samuthpongtorn, C.; Mahakit, N.; Nopsopon, T.; Heath, J.; Pongpirul, K. Probiotic as a potential gut microbiome modifier for stroke treatment: A systematic scoping review of in vitro and in vivo studies. Nutrients 2022, 14, 3661. [Google Scholar] [CrossRef] [PubMed]
- Olas, B. Probiotics, prebiotics and synbiotics—A promising strategy in prevention and treatment of cardiovascular diseases? Int. J. Mol. Sci. 2020, 21, 9737. [Google Scholar] [CrossRef]
- Miarons, M.; Roca, M.; Salvà, F. The role of pro-, pre-and symbiotics in cancer: A systematic review. J. Clin. Pharm. Ther. 2021, 46, 50–65. [Google Scholar] [CrossRef]
- Tomar, S.K.; Anand, S.; Sharma, P.; Sangwan, V.; Mandal, S. Role of probiotics, prebiotics, synbiotics and postbiotics in inhibition of pathogens. In The Battle Against Microbial Pathogens: Basic Science, Technological Advances and Educational Programs; Formatex Research Center S.L.: Badajoz, Spain, 2015; pp. 717–732. [Google Scholar]
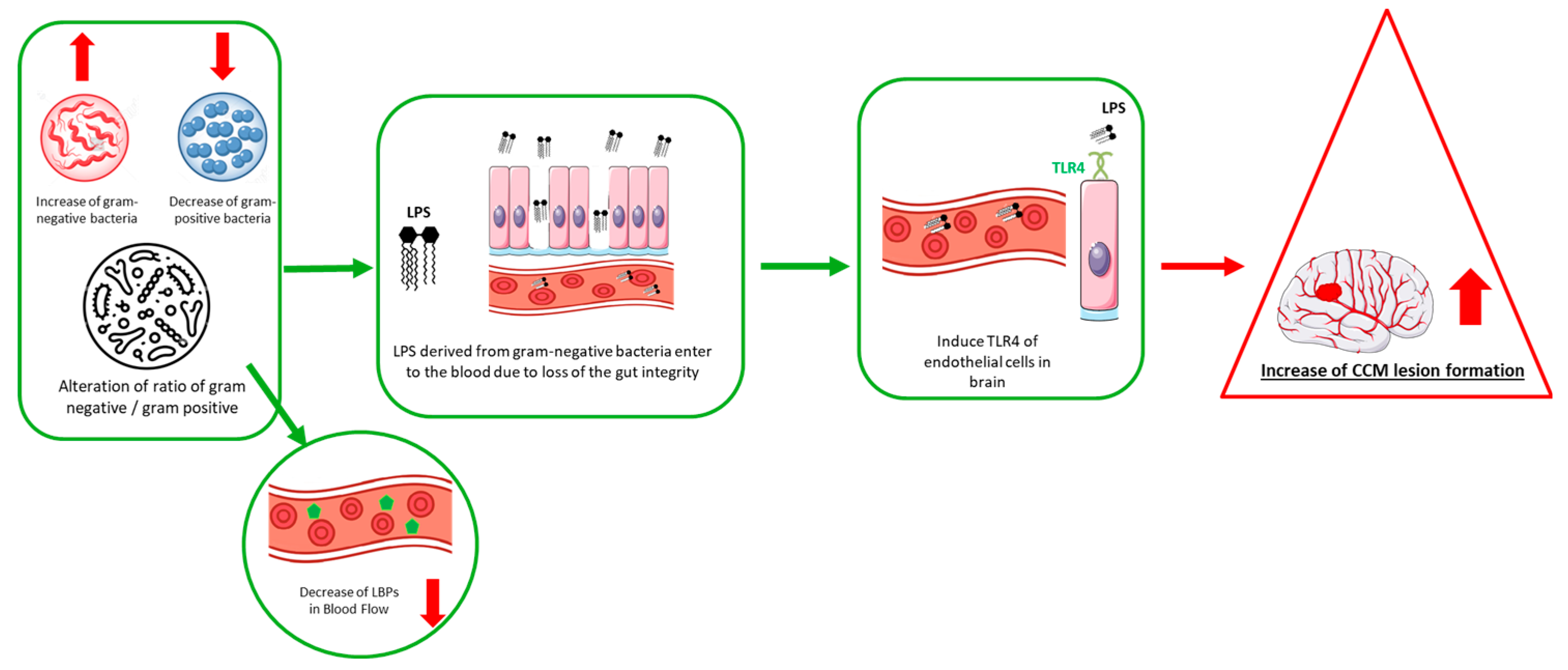
| Model/Population | Key Alterations in Gut Microbiota | Implications | Reference |
|---|---|---|---|
| Murine Models | ↑ Gram-negative Muribaculaceae (Bacteroidetes s24-7) | Activates endothelial TLR4 signaling, promoting CCM lesion formation. Antibiotic-treated or germ-free mice show reduced lesion burden. | [19] |
| Human CCM Patients | ↑ Gram-negative Odoribacter splanchnicus ↓ Gram-positive Faecalibacterium prausnitzii ↓ Gram-positive Bifidobacterium adolescentis | Correlates with disease severity and genetic mutations; suggests gut microbiome may influence CCM progression and provide diagnostic biomarkers. | [14,31] |
| Gut Microbiome Influencer | Factors |
|---|---|
| Lifestyle | - Environment - Dietary changes - Physical activity - Smoking - Drug use (e.g., antibiotics) - Stress - Shared lifestyles and environments resulting in similar gut enterotypes |
| Biological Factors | - Age - Individual uniqueness in bacterial diversity and abundance - Sex differences (men vs. women) |
| Microbiome Profile | - Determines response to interventions and microbial shifts |
| Manipulation Strategies | - Fecal microbiota transplantation (FMT) - Prebiotics/Probiotics - Dietary interventions (fibers, macronutrient ratios) - Disease-specific microbiome modulation - Precision nutrition approaches |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sadegh, H.; Choi, J.P. The Emerging Role of the Gut Microbiome in Cerebral Cavernous Malformation: A New Novel Therapeutic Strategy? Int. J. Mol. Sci. 2025, 26, 8622. https://doi.org/10.3390/ijms26178622
Sadegh H, Choi JP. The Emerging Role of the Gut Microbiome in Cerebral Cavernous Malformation: A New Novel Therapeutic Strategy? International Journal of Molecular Sciences. 2025; 26(17):8622. https://doi.org/10.3390/ijms26178622
Chicago/Turabian StyleSadegh, Hamidreza, and Jaesung P. Choi. 2025. "The Emerging Role of the Gut Microbiome in Cerebral Cavernous Malformation: A New Novel Therapeutic Strategy?" International Journal of Molecular Sciences 26, no. 17: 8622. https://doi.org/10.3390/ijms26178622
APA StyleSadegh, H., & Choi, J. P. (2025). The Emerging Role of the Gut Microbiome in Cerebral Cavernous Malformation: A New Novel Therapeutic Strategy? International Journal of Molecular Sciences, 26(17), 8622. https://doi.org/10.3390/ijms26178622







