CD93 in Health and Disease: Bridging Physiological Functions and Clinical Applications
Abstract
1. Introduction
2. The Expression of CD93
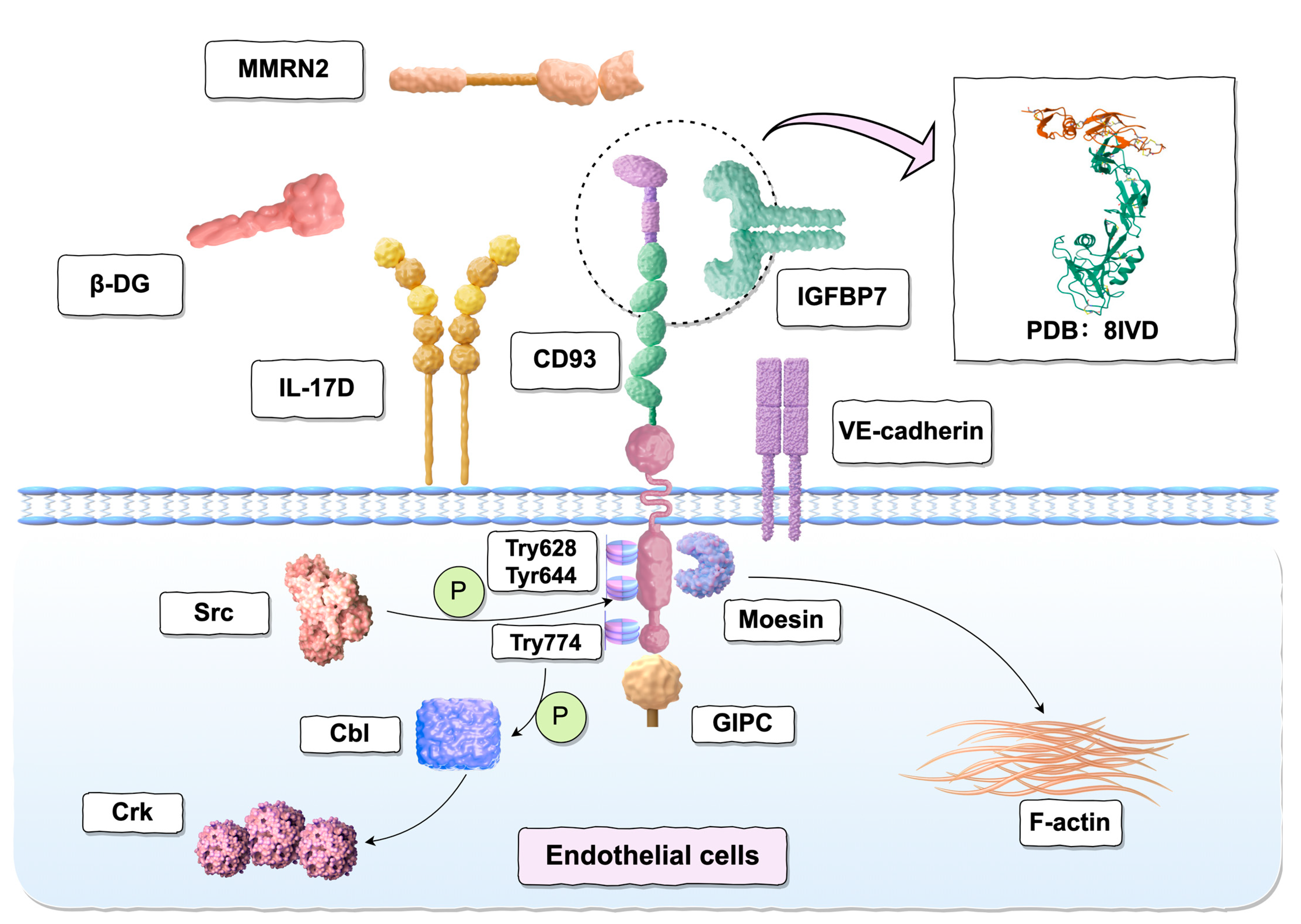
3. Binding Ligands and Interaction Proteins of CD93
3.1. Moesin
3.2. GIPC
3.3. Multimerin-2 (MMRN2)
3.4. Insulin-like Growth Factor Binding Protein 7 (IGFBP7)
3.5. β-dystroglycan (DG)
3.6. Adaptor Protein Cbl
3.7. Vascular Endothelial Cadherin (VE-Cadherin)
3.8. Interleukin-17D (IL-17D)
4. Physiological Function
4.1. Adhesion and Migration
4.2. Angiogenesis and Vascular Maturation
4.3. Clearance of Apoptotic Cells
4.4. Stabilized Thrombin Receptor
5. Soluble CD93
6. The Role of CD93 in Diseases
6.1. Inflammation
6.2. Cardiovascular Diseases (CVD)
6.3. Autoimmune Disorders
6.4. Cancer
6.5. Other Diseases
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AA | allergic asthma |
| ACC | adrenocortical carcinoma |
| ACT | adoptive cell therapy |
| ADAM | a disintegrin and metalloproteinase |
| AMD | age-related macular degeneration |
| AML | acute myeloid leukemia |
| ApoB | apolipoprotein B |
| AR | allergic rhinitis |
| ASC | antibody-secreting plasma cells |
| BCs | breast cancers |
| BE | barrett’s esophagus |
| BECs | brain endothelial cells |
| BLCA | bladder urothelial carcinoma |
| BMI | body mass index |
| BPRA | brachial plexus root avulsion |
| BRCA | breast invasive carcinoma |
| CAFs | cancer-associated fibroblasts |
| CAR | chimeric antigen receptor |
| CCM | cerebral cavernous malformation |
| CCRCCs | clear cell renal cell carcinomas |
| CESC | cervical squamous cell carcinoma and endocervical adenocarcinoma |
| CEUS | contrast-enhanced ultrasound |
| CHD | coronary heart disease |
| CHO | Chinese hamster ovary |
| CHOL | cholangiocarcinoma |
| COAD | colon adenocarcinoma |
| C1qRp | complement component C1q receptor |
| CRC | colorectal cancer |
| CSU | chronic spontaneous urticaria |
| CTLD | C-type lectin-like domain |
| CVD | cardiovascular diseases |
| Cyto | Cytoplasmic Domain |
| DG | dystroglycan |
| DLBC | diffuse large B-cell lymphoma |
| EAC | esophageal adenocarcinoma |
| ECM | extracellular matrix |
| ECs | endothelial cells |
| EGF | epidermal growth factor |
| EMILINs | elastin microfibril interface proteins |
| Erk | extracellular regulated protein kinase |
| FAK | focal adhesion kinase |
| FH | hypercholesterolemia |
| GAIP | Gα Interacting Protein |
| GBM | glioblastoma multiforme |
| GC | gastric cancer |
| GCPM | gastric cancer peritoneal metastasis |
| GPCR15 | G protein-coupled receptor 15 |
| GSEA | gene set enrichment analysis |
| GSK3β | glycogen synthase kinase 3β |
| GSRA | gene surface display library system |
| GST | glutathione S-transferase |
| HAECs | human aortic endothelial cells |
| HCC | hepatocellular carcinoma |
| HDL-C | high density lipoprotein cholesterol |
| HMGB1 | high-mobility group box 1 |
| HNSCCs | head and neck squamous cell carcinomas |
| HR | hazard ratio |
| HSPC | hematopoietic stem and progenitor cells |
| HSP70-1 | heat shock protein 70-1 |
| HUVEC | human umbilical vein endothelial cells |
| ICIs | immune checkpoint inhibitors |
| IGFs | insulin-like growth factors |
| IGFBP7 | insulin-like growth factor-binding protein 7 |
| IL-17D | Interleukin-17D |
| ILC3 | innate lymphoid cells |
| IMT | intima-media thickness |
| kDa | kilodaltons |
| KICH | kidney chromophobe |
| KIRC | kidney renal clear cell carcinoma |
| KIRP | kidney renal papillary cell carcinoma |
| LAIR1 | leukocyte-associated immunoglobulin-like receptor 1 |
| LAML | acute myeloid leukemia |
| LDL | low-density lipoprotein |
| LGG | lower grade glioma |
| LIHC | liver hepatocellular carcinoma |
| LL ASC | “long-lived” antibody-secreting plasma cells |
| LPS | lipopolysaccharide |
| LSCs | leukemia stem cells |
| LUAD | lung adenocarcinoma |
| LUSC | lung squamous cell carcinoma |
| mAb | monoclonal antibodies |
| MAdCAM-1 | mucosal addressin cell adhesion molecule-1 |
| MBs | microbubbles |
| MMPs | matrix metalloproteinases |
| MMRN2 | multimerin-2 |
| MR | Mendelian randomization |
| MSCs | mesenchymal stem cells |
| NA | non-allergic asthma |
| NB-UVB | narrow-band ultraviolet B |
| NETs | neutrophil extracellular traps |
| NK | natural killer |
| NP | normotensive pregnancies |
| NPC | nasopharyngeal carcinoma |
| NRP1 | neuropilin 1 |
| NSAIDs | non-steroidal anti-inflammatory drugs |
| NT-proBNP | N-terminal pro-B-type natriuretic peptide |
| OV | ovarian serous cystadenocarcinoma |
| PAAD | pancreatic adenocarcinoma |
| PAMP | pathogen-associated molecular pattern |
| PCOS | polycystic ovary syndrome |
| PD | Parkinson’s disease |
| PDBu | phorbol 12,13-dibutyrate |
| PD-L1 | programmed death-ligand 1 |
| PE | pre-eclampsia |
| pEVs | plasma-derived extracellular vesicles |
| PKB | protein kinase B |
| PKC | protein kinase C |
| pMCs | pleural mesothelial cells |
| PRAD | prostate adenocarcinoma |
| PSGL-1 | p-selectin glycoprotein ligand-1 |
| RA | rheumatoid arthritis |
| rCD93 | recombinant human CD93 |
| ROS | reactive oxygen species |
| sCD93 | soluble CD93 |
| SKCM | skin cutaneous melanoma |
| SLE | systemic lupus erythematosus |
| SNPs | single-nucleotide polymorphisms |
| SSc | systemic sclerosis |
| STAD | stomach adenocarcinoma |
| TACE | TNF-α converting enzyme |
| TB | tuberculosis |
| TGCT | testicular germ cell tumors |
| THCA | thyroid carcinoma |
| TM | thrombomodulin |
| TMB | tumor mutation burden |
| TME | tumor microenvironment |
| TNF-α | tumor necrosis factor-α |
| UCEC | uterine corpus endometrial carcinoma |
| UCS | uterine carcinosarcoma |
| UTR | untranslated region |
| VE-cadherin | vascular endothelial cadherin |
| VEGF | vascular endothelial growth factor |
| VEGFR2 | vascular endothelial growth factor receptor 2 |
| VM | vasculogenic mimicry |
| VMC | viral myocarditis |
| VSIR | v-set immunoregulatory receptor |
| WT | wild-type |
| 3H-2-DG | 3H-2-deoxyglucose |
References
- Nepomuceno, R.R.; Henschen-Edman, A.H.; Burgess, W.H.; Tenner, A.J. cDNA cloning and primary structure analysis of C1qR(P), the human C1q/MBL/SPA receptor that mediates enhanced phagocytosis in vitro. Immunity 1997, 6, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.A.; McMurray, J.L.; Mohammed, F.; Bicknell, R. C-type lectin domain group 14 proteins in vascular biology, cancer and inflammation. FEBS J. 2019, 286, 3299–3332. [Google Scholar] [CrossRef] [PubMed]
- Nanda, A.; Karim, B.; Peng, Z.; Liu, G.; Qiu, W.; Gan, C.; Vogelstein, B.; Croix, B.S.; Kinzler, K.W.; Huso, D.L. Tumor endothelial marker 1 (Tem1) functions in the growth and progression of abdominal tumors. Proc. Natl. Acad. Sci. USA 2006, 103, 3351–3356. [Google Scholar] [CrossRef]
- Maia, M.; DeVriese, A.; Janssens, T.; Moons, M.; Lories, R.J.; Tavernier, J.; Conway, E.M. CD248 facilitates tumor growth via its cytoplasmic domain. BMC Cancer 2011, 11, 162. [Google Scholar] [CrossRef]
- van der Net, J.B.; Oosterveer, D.M.; Versmissen, J.; Defesche, J.C.; Yazdanpanah, M.; Aouizerat, B.E.; Steyerberg, E.W.; Malloy, M.J.; Pullinger, C.R.; Kastelein, J.J.P.; et al. Replication study of 10 genetic polymorphisms associated with coronary heart disease in a specific high-risk population with familial hypercholesterolemia. Eur. Heart J. 2008, 29, 2195–2201. [Google Scholar] [CrossRef]
- Mälarstig, A.; Silveira, A.; Wågsäter, D.; Öhrvik, J.; Bäcklund, A.; Samnegård, A.; Khademi, M.; Hellenius, M.-L.; Leander, K.; Olsson, T.; et al. Plasma CD93 concentration is a potential novel biomarker for coronary artery disease. J. Intern. Med. 2011, 270, 229–236. [Google Scholar] [CrossRef]
- Olsen, R.S.; Lindh, M.; Vorkapic, E.; Andersson, R.E.; Zar, N.; Löfgren, S.; Dimberg, J.; Matussek, A.; Wågsäter, D. CD93 gene polymorphism is associated with disseminated colorectal cancer. Int. J. Color. Dis. 2015, 30, 883–890. [Google Scholar] [CrossRef][Green Version]
- McGreal, E.P.; Ikewaki, N.; Akatsu, H.; Morgan, B.P.; Gasque, P. Human C1qRp is identical with CD93 and the mNI-11 antigen but does not bind C1q. J. Immunol. 2002, 168, 5222–5232. [Google Scholar] [CrossRef]
- Borah, S.; Vasudevan, D.; Swain, R.K. C-type lectin family XIV members and angiogenesis. Oncol. Lett. 2019, 18, 3954–3962. [Google Scholar] [CrossRef]
- Bohlson, S.S.; Greenlee, M.C.; Sullivan, S.A. CD93 and related family members: Their role in innate immunity. Curr. Drug Targets 2008, 9, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.D.; Willment, J.A.; Whitehead, L. C-type lectins in immunity and homeostasis. Nat. Rev. Immunol. 2018, 18, 374–389. [Google Scholar] [CrossRef]
- Zelensky, A.N.; Gready, J.E. The C-type lectin-like domain superfamily. FEBS J. 2005, 272, 6179–6217. [Google Scholar]
- Yee, E.J.; Vigil, I.; Sun, Y.; Torphy, R.J.; Schulick, R.D.; Zhu, Y. Group XIV C-type lectins: Emerging targets in tumor angiogenesis. Angiogenesis 2024, 27, 173–192. [Google Scholar] [CrossRef] [PubMed]
- Conway, E.M. The type XIV family of C-type lectin-like domain (CTLD) containing proteins. Curr. Drug Targets 2012, 13, 409–410. [Google Scholar]
- Blackburn, J.W.D.; Lau, D.H.C.; Liu, E.Y.; Ellins, J.; Vrieze, A.M.; Pawlak, E.N.; Dikeakos, J.D.; Heit, B. Soluble CD93 is an apoptotic cell opsonin recognized by α(x) β(2). Eur. J. Immunol. 2019, 49, 600–610. [Google Scholar] [CrossRef]
- McMahon, S.A.; Miller, J.L.; ALawton, J.; EKerkow, D.; Hodes, A.; AMarti-Renom, M.; Doulatov, S.; Narayanan, E.; Sali, A.; Miller, J.F.; et al. The C-type lectin fold as an evolutionary solution for massive sequence variation. Nat. Struct. Mol. Biol. 2005, 12, 886–892. [Google Scholar] [CrossRef]
- Norman, D.; Barlow, P.; Baron, M.; Day, A.; Sim, R.; Campbell, I. Three-dimensional structure of a complement control protein module in solution. J. Mol. Biol. 1991, 219, 717–725. [Google Scholar] [CrossRef]
- Barbera, S.; Raucci, L.; Tassone, G.; Tinti, L.; Prischi, F.; Santucci, A.; Mongiat, M.; Tosi, G.M.; Galvagni, F.; Dimberg, A.; et al. Dimerization of the C-type lectin-like receptor CD93 promotes its binding to Multimerin-2 in endothelial cells. Int. J. Biol. Macromol 2023, 224, 453–464. [Google Scholar] [PubMed]
- Wouters, M.A.; Rigoutsos, I.; Chu, C.K.; Feng, L.L.; Sparrow, D.B.; Dunwoodie, S.L. Evolution of distinct EGF domains with specific functions. Protein Sci. 2005, 14, 1091–1103. [Google Scholar] [CrossRef]
- Jentoft, N. Why are proteins O-glycosylated? Trends Biochem. Sci. 1990, 15, 291–294. [Google Scholar] [CrossRef] [PubMed]
- Doyonnas, R.; Chan, J.Y.-H.; Butler, L.H.; Rappold, I.; Lee-Prudhoe, J.E.; Zannettino, A.C.W.; Simmons, P.J.; BühRing, H.-J.; Levesque, J.-P.; Watt, S.M. CD164 monoclonal antibodies that block hemopoietic progenitor cell adhesion and proliferation interact with the first mucin domain of the CD164 receptor. J. Immunol. 2000, 165, 840–851. [Google Scholar] [CrossRef]
- Briskin, M.J.; McEvoy, L.M.; Butcher, E.C. MAdCAM-1 has homology to immunoglobulin and mucin-like adhesion receptors and to IgA1. Nature 1993, 363, 461–464. [Google Scholar] [PubMed]
- Nepomuceno, R.R.; Ruiz, S.; Park, M.; Tenner, A.J. C1qRP is a heavily O-glycosylated cell surface protein involved in the regulation of phagocytic activity. J. Immunol. 1999, 162, 3583–3589. [Google Scholar] [CrossRef]
- Park, M.; Tenner, A.J. Cell surface expression of C1qRP/CD93 is stabilized by O-glycosylation. J. Cell. Physiol. 2003, 196, 512–522. [Google Scholar]
- Nepomuceno, R.R.; Tenner, A.J. C1qRP, the C1q receptor that enhances phagocytosis, is detected specifically in human cells of myeloid lineage, endothelial cells, and platelets. J. Immunol. 1998, 160, 1929–1935. [Google Scholar]
- Liu, C.; Cui, Z.; Wang, S.; Zhang, D. CD93 and GIPC expression and localization during central nervous system inflammation. Neural Regen. Res. 2014, 9, 1995–2001. [Google Scholar] [CrossRef]
- Løvik, G.; Sand, K.L.; Iversen, J.G.; Rolstad, B. C1qRp elicits a Ca++ response in rat NK cells but does not influence NK-mediated cytotoxicity. Scand. J. Immunol. 2001, 53, 410–415. [Google Scholar]
- Danet, G.H.; Luongo, J.L.; Butler, G.; Lu, M.M.; Tenner, A.J.; Simon, M.C.; Bonnet, D.A. C1qRp defines a new human stem cell population with hematopoietic and hepatic potential. Proc. Natl. Acad. Sci. USA 2002, 99, 10441–10445. [Google Scholar] [CrossRef] [PubMed]
- Ikewaki, N.; Yamao, H.; Kulski, J.K.; Inoko, H. Flow cytometric identification of CD93 expression on naive T lymphocytes (CD4(+)CD45RA (+) cells) in human neonatal umbilical cord blood. J. Clin. Immunol. 2010, 30, 723–733. [Google Scholar] [PubMed]
- Chevrier, S.; Genton, C.; Kallies, A.; Karnowski, A.; Otten, L.A.; Malissen, B.; Malissen, M.; Botto, M.; Corcoran, L.M.; Nutt, S.L.; et al. CD93 is required for maintenance of antibody secretion and persistence of plasma cells in the bone marrow niche. Proc. Natl. Acad. Sci. USA 2009, 106, 3895–3900. [Google Scholar] [CrossRef] [PubMed]
- Petrenko, O.; Beavis, A.; Klaine, M.; Kittappa, R.; Godin, I.; Lemischka, I.R. The molecular characterization of the fetal stem cell marker AA4. Immunity 1999, 10, 691–700. [Google Scholar] [CrossRef]
- Dean, Y.D.; McGreal, E.P.; Akatsu, H.; Gasque, P. Molecular and cellular properties of the rat AA4 antigen, a C-type lectin-like receptor with structural homology to thrombomodulin. J. Biol. Chem. 2000, 275, 34382–34392. [Google Scholar] [CrossRef]
- Fonseca, M.I.; Carpenter, P.M.; Park, M.; Palmarini, G.; Nelson, E.L.; Tenner, A.J. C1qR(P), a myeloid cell receptor in blood, is predominantly expressed on endothelial cells in human tissue. J. Leukoc. Biol. 2001, 70, 793–800. [Google Scholar] [CrossRef]
- Tossetta, G.; Piani, F.; Borghi, C.; Marzioni, D. Role of CD93 in Health and Disease. Cells 2023, 12, 1778. [Google Scholar] [CrossRef]
- Rho, S.-S.; Choi, H.-J.; Min, J.-K.; Lee, H.-W.; Park, H.; Park, H.; Kim, Y.-M.; Kwon, Y.-G. Clec14a is specifically expressed in endothelial cells and mediates cell to cell adhesion. Biochem. Biophys. Res. Commun. 2011, 404, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Teicher, B.A. CD248: A therapeutic target in cancer and fibrotic diseases. Oncotarget 2019, 10, 993–1009. [Google Scholar] [CrossRef]
- Huang, H.; Auerbach, R. Identification and characterization of hematopoietic stem cells from the yolk sac of the early mouse embryo. Proc. Natl. Acad. Sci. USA 1993, 90, 10110–10114. [Google Scholar] [CrossRef]
- Marcos, M.A.; Morales-Alcelay, S.; Godin, I.E.; Dieterlen-Lievre, F.; Copin, S.G.; Gaspar, M.L. Antigenic phenotype and gene expression pattern of lymphohemopoietic progenitors during early mouse ontogeny. J. Immunol. 1997, 158, 2627–2637. [Google Scholar] [CrossRef] [PubMed]
- Yoder, M.C.; Hiatt, K.; Dutt, P.; Mukherjee, P.; Bodine, D.M.; Orlic, D. Characterization of definitive lymphohematopoietic stem cells in the day 9 murine yolk sac. Immunity 1997, 7, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Greenlee-Wacker, M.C.; Galvan, M.D.; Bohlson, S.S. CD93: Recent advances and implications in disease. Curr. Drug Targets 2012, 13, 411–420. [Google Scholar] [CrossRef]
- Orlic, D.; Fischer, R.; Nishikawa, S.; Nienhuis, A.W.; Bodine, D.M. Purification and characterization of heterogeneous pluripotent hematopoietic stem cell populations expressing high levels of c-kit receptor. Blood 1993, 82, 762–770. [Google Scholar] [CrossRef][Green Version]
- Løvik, G.; Vaage, J.T.; Dissen, E.; Szpirer, C.; Ryan, J.C.; Rolstad, B. Characterization and molecular cloning of rat C1qRp, a receptor on NK cells. Eur. J. Immunol. 2000, 30, 3355–3362. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.J.; Ding, Z.; Dowling, M.R.; Hill, D.L.; Webster, R.H.; McKenzie, C.; Pitt, C.; O’dOnnell, K.; Mulder, J.; Brodie, E.; et al. Intrinsically determined turnover underlies broad heterogeneity in plasma-cell lifespan. Immunity 2023, 56, 1596–1612e4. [Google Scholar] [CrossRef]
- Norsworthy, P.J.; Fossati-jimack, L.; Taylor, P.R.; Bygrave, A.E.; Thompson, R.D.; Nourshargh, S.; Walport, M.J.; Botto, M.; Norsworthy, P.J.; Fossati-jimack, L.; et al. Murine CD93 (C1qRp) contributes to the removal of apoptotic cells in vivo but is not required for C1q-mediated enhancement of phagocytosis. J. Immunol. 2004, 172, 3406–3414. [Google Scholar] [CrossRef] [PubMed]
- Greenlee-Wacker, M.C.; Briseño, C.; Galvan, M.; Moriel, G.; Velázquez, P.; Bohlson, S.S. Membrane-associated CD93 regulates leukocyte migration and C1q-hemolytic activity during murine peritonitis. J. Immunol. 2011, 187, 3353–3361. [Google Scholar] [CrossRef]
- Zhang, M.; Bohlson, S.S.; Dy, M.; Tenner, A.J. Modulated interaction of the ERM protein, moesin, with CD93. Immunology 2005, 115, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Galvagni, F.; Baldari, C.T.; Oliviero, S.; Orlandini, M. An apical actin-rich domain drives the establishment of cell polarity during cell adhesion. Histochem. Cell Biol. 2012, 138, 419–433. [Google Scholar] [CrossRef]
- Langenkamp, E.; Zhang, L.; Lugano, R.; Huang, H.; Elhassan, T.E.A.; Georganaki, M.; Bazzar, W.; Lööf, J.; Trendelenburg, G.; Essand, M. Elevated expression of the C-type lectin CD93 in the glioblastoma vasculature regulates cytoskeletal rearrangements that enhance vessel function and reduce host survival. Cancer Res. 2015, 75, 4504–4516. [Google Scholar] [CrossRef]
- Bohlson, S.S.; Zhang, M.; Ortiz, C.E.; Tenner, A.J. CD93 interacts with the PDZ domain-containing adaptor protein GIPC: Implications in the modulation of phagocytosis. J. Leukoc. Biol. 2005, 77, 80–89. [Google Scholar] [CrossRef]
- Ligensa, T.; Krauss, S.; Demuth, D.; Schumacher, R.; Camonis, J.; Jaques, G.; Weidner, K.M. A PDZ domain protein interacts with the C-terminal tail of the insulin-like growth factor-1 receptor but not with the insulin receptor. J. Biol. Chem. 2001, 276, 33419–33427. [Google Scholar] [CrossRef]
- Gao, Y.; Li, M.; Chen, W.; Simons, M. Synectin, syndecan-4 cytoplasmic domain binding PDZ protein, inhibits cell migration. J. Cell. Physiol. 2000, 184, 373–379. [Google Scholar] [CrossRef]
- Galvagni, F.; Nardi, F.; Spiga, O.; Trezza, A.; Tarticchio, G.; Pellicani, R.; Andreuzzi, E.; Caldi, E.; Toti, P.; Tosi, G.M.; et al. Dissecting the CD93-Multimerin 2 interaction involved in cell adhesion and migration of the activated endothelium. Matrix Biol. 2017, 64, 112–127. [Google Scholar] [CrossRef]
- Colombatti, A.; Spessotto, P.; Doliana, R.; Mongiat, M.; Bressan, G.M.; Esposito, G. The EMILIN/Multimerin family. Front. Immunol. 2012, 2, 93. [Google Scholar] [CrossRef] [PubMed]
- Orlandini, M.; Galvagni, F.; Bardelli, M.; Rocchigiani, M.; Lentucci, C.; Anselmi, F.; Zippo, A.; Bini, L.; Oliviero, S. The characterization of a novel monoclonal antibody against CD93 unveils a new antiangiogenic target. Oncotarget 2014, 5, 2750–2760. [Google Scholar] [CrossRef]
- Khan, K.A.; Naylor, A.J.; Khan, A.; Noy, P.J.; Mambretti, M.; Lodhia, P.; Athwal, J.; Korzystka, A.; Buckley, C.D.; Willcox, B.E.; et al. Multimerin-2 is a ligand for group 14 family C-type lectins CLEC14A, CD93 and CD248 spanning the endothelial pericyte interface. Oncogene 2017, 36, 6097–6108. [Google Scholar] [CrossRef]
- Lugano, R.; Vemuri, K.; Yu, D.; Bergqvist, M.; Smits, A.; Essand, M.; Johansson, S.; Dejana, E.; Dimberg, A. CD93 promotes β1 integrin activation and fibronectin fibrillogenesis during tumor angiogenesis. J. Clin. Investig. 2018, 128, 3280–3297. [Google Scholar] [CrossRef] [PubMed]
- Lit, K.K.; Zhirenova, Z.; Blocki, A. Insulin-like growth factor-binding protein 7 (IGFBP7): A microenvironment-dependent regulator of angiogenesis and vascular remodeling. Front. Cell Dev. Biol. 2024, 12, 1421438. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Hui, L.; Li, J. The multifaceted role of insulin-like growth factor binding protein 7. Front. Cell Dev. Biol. 2024, 12, 1420862. [Google Scholar] [CrossRef]
- Komiya, E.; Furuya, M.; Watanabe, N.; Miyagi, Y.; Higashi, S.; Miyazaki, K. Elevated expression of angiomodulin (AGM/IGFBP-rP1) in tumor stroma and its roles in fibroblast activation. Cancer Sci. 2012, 103, 691–699. [Google Scholar] [CrossRef]
- Komiya, E.; Sato, H.; Watanabe, N.; Ise, M.; Higashi, S.; Miyagi, Y.; Miyazaki, K. Angiomodulin, a marker of cancer vasculature, is upregulated by vascular endothelial growth factor and increases vascular permeability as a ligand of integrin αvβ3. Cancer Med. 2014, 3, 537–549. [Google Scholar] [CrossRef]
- Buga, A.M.; Margaritescu, C.; Scholz, C.J.; Radu, E.; Zelenak, C.; Popa-Wagner, A. Transcriptomics of post-stroke angiogenesis in the aged brain. Front. Aging Neurosci. 2014, 6, 44. [Google Scholar] [CrossRef]
- Wang, J.; Deng, X.; Xie, Y.; Tang, J.; Zhou, Z.; Yang, F.; He, Q.; Cao, Q.; Zhang, L.; He, L. An Integrated Transcriptome Analysis Reveals IGFBP7 Upregulation in Vasculature in Traumatic Brain Injury. Front. Genet. 2021, 11, 599834. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, W.; Torphy, R.J.; Yao, S.; Zhu, G.; Lin, R.; Lugano, R.; Miller, E.N.; Fujiwara, Y.; Bian, L.; et al. Blockade of the CD93 pathway normalizes tumor vasculature to facilitate drug delivery and immunotherapy. Sci. Transl. Med. 2021, 13, eabc8922. [Google Scholar] [CrossRef]
- Xu, Y.; Sun, Y.; Zhu, Y.; Song, G. Structural insight into CD93 recognition by IGFBP7. Structure 2024, 32, 282–291.e4. [Google Scholar] [CrossRef]
- Sun, J.; Zheng, Q.; Wu, K. IGFBP7 mediates oxLDL-induced human vascular endothelial cell injury by suppressing the expression of SIRT1. Heliyon 2024, 10, e35359. [Google Scholar] [CrossRef]
- Galvagni, F.; Nardi, F.; Maida, M.; Bernardini, G.; Vannuccini, S.; Petraglia, F.; Santucci, A.; Orlandini, M. CD93 and dystroglycan cooperation in human endothelial cell adhesion and migration adhesion and migration. Oncotarget 2016, 7, 10090–10103. [Google Scholar] [CrossRef] [PubMed]
- Barbera, S.; Raucci, L.; Lugano, R.; Tosi, G.M.; Dimberg, A.; Santucci, A.; Galvagni, F.; Orlandini, M. CD93 Signaling via Rho Proteins Drives Cytoskeletal Remodeling in Spreading Endothelial Cells. Int. J. Mol. Sci. 2021, 22, 12417. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Hubbard, S.R. Structural characterization of a novel Cbl phosphotyrosine recognition motif in the APS family of adapter proteins. J. Biol. Chem. 2005, 280, 18943–18949. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, N.; Rose, D.W.; Xiao, S.; Egawa, K.; Martin, S.S.; Haruta, T.; Saltiel, A.R.; Olefsky, J.M. The functional role of CrkII in actin cytoskeleton organization and mitogenesis. J. Biol. Chem. 1999, 274, 3001–3008. [Google Scholar] [CrossRef]
- Liu, S.; Kang, M.; Ren, Y.; Zhang, Y.; Ba, Y.; Deng, J.; Luo, P.; Cheng, Q.; Xu, H.; Weng, S.; et al. The Interaction Between Vasculogenic Mimicry and the Immune System: Mechanistic Insights and Dual Exploration in Cancer Therapy. Cell Prolif. 2025, 58, e13814. [Google Scholar] [CrossRef]
- Lugano, R.; Vemuri, K.; Barbera, S.; Orlandini, M.; Dejana, E.; Claesson-Welsh, L.; Dimberg, A. CD93 maintains endothelial barrier function by limiting the phosphorylation and turnover of VE-cadherin. FASEB J. 2023, 37, e22894. [Google Scholar] [CrossRef]
- Luo, Q.; Wang, J.; Zhao, W.; Peng, Z.; Liu, X.; Li, B.; Zhang, H.; Shan, B.; Zhang, C.; Duan, C. Vasculogenic mimicry in carcinogenesis and clinical applications. J. Hematol. Oncol. 2020, 13, 19. [Google Scholar] [CrossRef] [PubMed]
- Maniotis, A.J.; Folberg, R.; Hess, A.; Seftor, E.A.; Gardner, L.M.; Pe’ER, J.; Trent, J.M.; Meltzer, P.S.; Hendrix, M.J. Vascular channel formation by human melanoma cells in vivo and in vitro: Vasculogenic mimicry. Am. J. Pathol. 1999, 155, 739–752. [Google Scholar] [CrossRef]
- Hendrix, M.J.C.; Seftor, E.A.; Meltzer, P.S.; Gardner, L.M.G.; Hess, A.R.; Kirschmann, D.A.; Schatteman, G.C.; Seftor, R.E.B. Expression and functional significance of VE-cadherin in aggressive human melanoma cells: Role in vasculogenic mimicry. Proc. Natl. Acad. Sci. USA 2001, 98, 8018–8023. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.-Q.; Zheng, Q.-H.; Chen, H.; Chen, L.; Xu, J.-B.; Chen, M.-Y.; Lu, D.; Wang, Z.-H.; Tong, H.-F.; Lin, S. Ginsenoside Rg3 inhibition of vasculogenic mimicry in pancreatic cancer through downregulation of VE-cadherin/EphA2/MMP9/MMP2 expression. Int. J. Oncol. 2014, 45, 1065–1072. [Google Scholar] [CrossRef]
- Zhang, R.; Yao, Y.; Gao, H.; Hu, X. Mechanisms of angiogenesis in tumour. Front Oncol. 2024, 14, 1359069. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, J.; Zhao, Y.; Fan, Z.; Yang, Y.; Mao, Y.; Yang, J.; Ma, S. CD93 regulates breast cancer growth and vasculogenic mimicry through the PI3K/AKT/SP2 signaling pathway activated by integrin β1. J. Biochem. Mol. Toxicol. 2024, 38, e23688. [Google Scholar] [CrossRef]
- O’sUllivan, T.; Saddawi-Konefka, R.; Gross, E.; Tran, M.; Mayfield, S.P.; Ikeda, H.; Bui, J.D. Interleukin-17D mediates tumor rejection through recruitment of natural killer cells. Cell Rep. 2014, 7, 989–998. [Google Scholar] [CrossRef]
- Johansen, C.; Usher, P.; Kjellerup, R.; Lundsgaard, D.; Iversen, L.; Kragballe, K. Characterization of the interleukin-17 isoforms and receptors in lesional psoriatic skin. Br. J. Dermatol. 2009, 160, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Lee, H.-Y.; Zhao, X.; Han, J.; Su, Y.; Sun, Q.; Shao, J.; Ge, J.; Zhao, Y.; Bai, X.; et al. Interleukin-17D regulates group 3 innate lymphoid cell function through its receptor CD93. Immunity 2021, 54, 673–686.e4. [Google Scholar] [CrossRef]
- Wang, P.; Li, T.; Niu, C.; Sun, S.; Liu, D. ROS-activated MAPK/ERK pathway regulates crosstalk between Nrf2 and Hif-1α to promote IL-17D expression protecting the intestinal epithelial barrier under hyperoxia. Int. Immunopharmacol. 2023, 116, 109763. [Google Scholar] [CrossRef]
- Ni, X.; Xu, Y.; Wang, W.; Kong, B.; Ouyang, J.; Chen, J.; Yan, M.; Wu, Y.; Chen, Q.; Wang, X.; et al. IL-17D-induced inhibition of DDX5 expression in keratinocytes amplifies IL-36R-mediated skin inflammation. Nat. Immunol. 2022, 23, 1577–1587. [Google Scholar] [CrossRef]
- Qiao, N.; Zhang, J.; Zhang, Y.; Liu, X. Synergistic regulation of microglia differentiation by CD93 and integrin β1 in the rat pneumococcal meningitis model. Immunol. Lett. 2022, 251–252, 63–74. [Google Scholar] [CrossRef]
- Tian, Y.; Gong, X.; Qin, D.; Cao, Y.; Zhang, S.; Xia, L.; Liu, F.; Su, Z. S1PR1-dependent migration of ILC3s from intestinal tissue to the heart in a mouse model of viral myocarditis. J. Leukoc. Biol. 2023, 114, 154–163. [Google Scholar] [CrossRef]
- Gu, X.; Weng, R.; Deng, Q.; Rao, J.; Zhao, J.; Hou, J.; Liu, S. Interleukin-17D accelerates atherosclerosis through promoting endothelial cells ferroptosis via CD93/miR-181a-5p/SLC7A11 signaling. Int. Immunopharmacol. 2024, 143 Pt 3, 113558. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.-W.; Jung, J.-G.; Shin, E.-C.; Choi, H.I.; Kim, H.Y.; Cho, M.-L.; Kim, S.-W.; Jang, Y.-S.; Sohn, M.-H.; Moon, J.-H.; et al. Soluble CD93 induces differentiation of monocytes and enhances TLR responses. J. Immunol. 2010, 185, 4921–4927. [Google Scholar] [CrossRef] [PubMed]
- Hynes, R.O. Integrins: Bidirectional, allosteric signaling machines. Cell 2002, 110, 673–687. [Google Scholar] [CrossRef] [PubMed]
- Pugacheva, E.N.; Roegiers, F.; Golemis, E.A. Interdependence of cell attachment and cell cycle signaling. Curr. Opin. Cell Biol. 2006, 18, 507–515. [Google Scholar] [CrossRef]
- Barbera, S.; Lugano, R.; Pedalina, A.; Mongiat, M.; Santucci, A.; Tosi, G.M.; Dimberg, A.; Galvagni, F.; Orlandini, M. The C-type lectin CD93 controls endothelial cell migration via activation of the Rho family of small GTPases. Matrix Biol. 2021, 99, 1–17. [Google Scholar] [CrossRef]
- Ridley, A.J. Rho GTPase signalling in cell migration. Curr. Opin. Cell Biol. 2015, 36, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Barresi, R.; Campbell, K.P. Dystroglycan: From biosynthesis to pathogenesis of human disease. J. Cell Sci. 2006, 119 Pt 2, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Ikewaki, N.; Tamauchi, H.; Yamada, A.; Mori, N.; Yamao, H.; Inoue, H.; Inoko, H. A unique monoclonal antibody mNI-11 rapidly enhances spread formation in human umbilical vein endothelial cells. J. Clin. Immunol. 2000, 20, 317–324. [Google Scholar] [CrossRef]
- Barbera, S.; Nardi, F.; Elia, I.; Realini, G.; Lugano, R.; Santucci, A.; Tosi, G.M.; Dimberg, A.; Galvagni, F.; Orlandini, M. The small GTPase Rab5c is a key regulator of trafficking of the CD93/Multimerin-2/β1 integrin complex in endothelial cell adhesion and migration. Cell Commun. Signal. 2019, 17, 55. [Google Scholar] [CrossRef]
- Lamalice, L.; Le Boeuf, F.; Huot, J. Endothelial cell migration during angiogenesis. Circ. Res. 2007, 100, 782–794. [Google Scholar] [CrossRef]
- Ayers, M.; Fargnoli, J.; Lewin, A.; Wu, Q.; Platero, J.S. Discovery and validation of biomarkers that respond to treatment with brivanib alaninate, a small-molecule VEGFR-2/FGFR-1 antagonist. Cancer Res. 2007, 67, 6899–6906. [Google Scholar] [CrossRef]
- Xu, Y.; Jia, Y.; Wu, N.; Wang, J.; He, L.; Yang, D. CD93 Ameliorates Diabetic Wounds by Promoting Angiogenesis via the p38MAPK/MK2/HSP27 Axis. Eur. J. Vasc. Endovasc. Surg. 2023, 66, 707–721. [Google Scholar] [CrossRef]
- Kao, Y.-C.; Jiang, S.-J.; Pan, W.-A.; Wang, K.-C.; Chen, P.-K.; Wei, H.-J.; Chen, W.-S.; Chang, B.-I.; Shi, G.-Y.; Wu, H.-L.; et al. The epidermal growth factor-like domain of CD93 is a potent angiogenic factor. PLoS ONE 2012, 7, e51647. [Google Scholar] [CrossRef] [PubMed]
- Claesson-Welsh, L.; Dejana, E.; McDonald, D.M. McDonald, Permeability of the Endothelial Barrier: Identifying and Reconciling Controversies. Trends Mol. Med. 2021, 27, 314–331. [Google Scholar] [CrossRef] [PubMed]
- Trivigno, S.M.G.; Vismara, M.; Canobbio, I.; Rustichelli, S.; Galvagni, F.; Orlandini, M.; Torti, M.; Guidetti, G.F. The C-Type Lectin Receptor CD93 Regulates Platelet Activation and Surface Expression of the Protease Activated Receptor 4. Thromb. Haemost. 2024, 124, 122–134. [Google Scholar] [CrossRef]
- Lee, M.; Park, H.S.; Choi, M.Y.; Kim, H.Z.; Moon, S.J.; Ha, J.Y.; Choi, A.; Park, Y.W.; Park, J.S.; Shin, E.-C.; et al. Significance of Soluble CD93 in Type 2 Diabetes as a Biomarker for Diabetic Nephropathy: Integrated Results from Human and Rodent Studies. J. Clin. Med. 2020, 9, 1394. [Google Scholar] [CrossRef]
- Yanaba, K.; Asano, Y.; Noda, S.; Akamata, K.; Aozasa, N.; Taniguchi, T.; Takahashi, T.; Ichimura, Y.; Toyama, T.; Sumida, H.; et al. Augmented production of soluble CD93 in patients with systemic sclerosis and clinical association with severity of skin sclerosis. Br. J. Dermatol. 2012, 167, 542–547. [Google Scholar] [CrossRef]
- Linton, M.F.; Babaev, V.R.; Huang, J.; Linton, E.F.; Tao, H.; Yancey, P.G. Macrophage Apoptosis and Efferocytosis in the Pathogenesis of Atherosclerosis. Circ. J. 2016, 80, 2259–2268. [Google Scholar] [CrossRef] [PubMed]
- Bohlson, S.S.; Silva, R.; Fonseca, M.I.; Tenner, A.J. CD93 is rapidly shed from the surface of human myeloid cells and the soluble form is detected in human plasma. J. Immunol. 2005, 175, 1239–1247. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.-H.; Chen, P.-K.; Chang, B.-I.; Sung, M.-C.; Shi, C.-S.; Lee, J.-S.; Chang, C.-F.; Shi, G.-Y.; Wu, H.-L. The recombinant lectin-like domain of thrombomodulin inhibits angiogenesis through interaction with Lewis Y antigen. Blood 2012, 119, 1302–1313. [Google Scholar] [CrossRef] [PubMed]
- Garton, K.J.; Gough, P.J.; Raines, E.W. Emerging roles for ectodomain shedding in the regulation of inflammatory responses. J. Leukoc. Biol. 2006, 79, 1105–1116. [Google Scholar] [CrossRef] [PubMed]
- Greenlee, M.C.; Sullivan, S.A.; Bohlson, S.S. Detection and characterization of soluble CD93 released during inflammation. Inflamm. Res. 2009, 58, 909–919. [Google Scholar] [CrossRef]
- Park, H.J.; Oh, E.-Y.; Han, H.-J.; Park, K.H.; Jeong, K.-Y.; Park, J.-W.; Lee, J.-H. Soluble CD93 in allergic asthma. Sci. Rep. 2020, 10, 323. [Google Scholar] [CrossRef]
- Strawbridge, R.J.; Hilding, A.; Silveira, A.; Österholm, C.; Sennblad, B.; McLeod, O.; Tsikrika, P.; Foroogh, F.; Tremoli, E.; Baldassarre, D.; et al. Soluble CD93 Is Involved in Metabolic Dysregulation but Does Not Influence Carotid Intima-Media Thickness. Diabetes 2016, 65, 2888–2899. [Google Scholar] [CrossRef]
- Hawiger, J.; Zienkiewicz, J. Decoding inflammation, its causes, genomic responses, and emerging countermeasures. Scand. J. Immunol. 2019, 90, e12812. [Google Scholar] [CrossRef]
- Ley, K.; Laudanna, C.; Cybulsky, M.I.; Nourshargh, S. Getting to the site of inflammation: The leukocyte adhesion cascade updated. Nat. Rev. Immunol. 2007, 7, 678–689. [Google Scholar] [CrossRef]
- Medzhitov, R. The spectrum of inflammatory responses. Science 2021, 374, 1070–1075. [Google Scholar] [CrossRef]
- Danese, S.; Dejana, E.; Fiocchi, C. Immune regulation by microvascular endothelial cells: Directing innate and adaptive immunity, coagulation, and inflammation. J. Immunol. 2007, 178, 6017–6022. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Xu, C.; Jarvis, J.N. C1q-bearing immune complexes induce IL-8 secretion in human umbilical vein endothelial cells (HUVEC) through protein tyrosine kinase- and mitogen-activated protein kinase-dependent mechanisms: Evidence that the 126 kD phagocytic C1q receptor mediates immune complex activation of HUVEC. Clin. Exp. Immunol. 2001, 125, 360–367. [Google Scholar]
- Wang, J.-S.; Liu, H.-C. Systemic hypoxia enhances bactericidal activities of human polymorphonuclear leuocytes. Clin. Sci. 2009, 116, 805–817. [Google Scholar] [CrossRef][Green Version]
- Adamczyk, A.M.; Leicaj, M.L.; Fabiano, M.P.; Cabrerizo, G.; Bannoud, N.; Croci, D.O.; Witwer, K.W.; Lenicov, F.R.; Ostrowski, M.; Pérez, P.S. Extracellular vesicles from human plasma dampen inflammation and promote tissue repair functions in macrophages. J. Extracell. Vesicles 2023, 12, e12331. [Google Scholar] [CrossRef]
- Huang, S.-E.; Kuo, C.-H.; Shiao, S.-Y.; Shen, C.-R.; Lee, F.-T.; Chang, B.-I.; Hsu, J.-H.; Wu, H.-L.; Yeh, J.-L.; Lai, C.-H. Soluble CD93 lectin-like domain sequesters HMGB1 to ameliorate inflammatory diseases. Theranostics 2023, 13, 4059–4078. [Google Scholar] [CrossRef] [PubMed]
- Baines, K.J.; Wood, L.G.; Gibson, P.G. The nutrigenomics of asthma: Molecular mechanisms of airway neutrophilia following dietary antioxidant withdrawal. Omics 2009, 13, 355–365. [Google Scholar] [CrossRef]
- Romanet-Manent, S.; Charpin, D.; Magnan, A.; Lanteaume, A.; Vervloet, D.; the EGEA Cooperative Group. Allergic vs nonallergic asthma: What makes the difference? Allergy 2002, 57, 607–613. [Google Scholar] [CrossRef]
- Raedler, D.; Ballenberger, N.; Klucker, E.; Böck, A.; Otto, R.; da Costa, O.P.; Holst, O.; Illig, T.; Buch, T.; von Mutius, E.; et al. Identification of novel immune phenotypes for allergic and nonallergic childhood asthma. J. Allergy Clin. Immunol. 2015, 135, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Sigari, N.; Jalili, A.; Mahdawi, L.; Ghaderi, E.; Shilan, M. Soluble CD93 as a Novel Biomarker in Asthma Exacerbation. Allergy Asthma Immunol. Res. 2016, 8, 461–465. [Google Scholar] [CrossRef]
- Park, H.J.; Han, H.; Lee, S.C.; Son, Y.W.; Sim, D.W.; Park, K.H.; Park, Y.H.; Jeong, K.Y.; Park, J.-W.; Lee, J.-H. Soluble CD93 in Serum A as a Marker of Allergic Inflammation. Yonsei Med. J. 2017, 58, 598–603. [Google Scholar] [CrossRef]
- Park, H.J.; Oh, E.-Y.; Park, Y.H.; Yang, M.; Park, K.H.; Park, J.-W.; Lee, J.-H. Potential of serum soluble CD93 as a biomarker for asthma in an ovalbumin-induced asthma murine model. Biomarkers 2018, 23, 446–452. [Google Scholar] [CrossRef]
- Harhausen, D.; Prinz, V.; Ziegler, G.; Gertz, K.; Endres, M.; Lehrach, H.; Gasque, P.; Botto, M.; Stahel, P.F.; Dirnagl, U.; et al. CD93/AA4.1: A novel regulator of inflammation in murine focal cerebral ischemia. J. Immunol. 2010, 184, 6407–6417. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, M.R.; Botto, M.; Morgan, B.P.; Neal, J.W.; Gasque, P. CD93 regulates central nervous system inflammation in two mouse models of autoimmune encephalomyelitis. Immunology 2018, 155, 346–355. [Google Scholar] [CrossRef] [PubMed]
- Zhong, K.; Li, Y.; Tang, Y.; Yu, G.; Zilundu, P.L.M.; Wang, Y.; Zhou, Y.; Xu, X.; Fu, R.; Zhou, L. Cytokine profile and glial activation following brachial plexus roots avulsion injury in mice. J. Neuroimmunol. 2021, 353, 577517. [Google Scholar] [CrossRef] [PubMed]
- Jauhiainen, S.; Onyeogaziri, F.C.; Lazzaroni, F.; Conze, L.L.; Laakkonen, J.P.; Laham-Karam, N.; Laakso, A.; Niemelä, M.; Jahromi, B.R.; Magnusson, P.U. Proteomics on human cerebral cavernous malformations reveals novel biomarkers in neurovascular dysfunction for the disease pathology. Biochim. Biophys. Acta Mol. Basis Dis. 2024, 1870, 167139. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, B.; Li, D.; Zhang, Y.; Xue, Y.; Hu, K.; Tsuji, F. Machine Learning and Mendelian Randomization Reveal Molecular Mechanisms and Causal Relationships of Immune-Related Biomarkers in Periodontitis. Mediat. Inflamm. 2024, 2024, 9983323. [Google Scholar] [CrossRef]
- Libby, P.; Ridker, P.M.; Maseri, A. Inflammation and atherosclerosis. Circ. Circulat. 2002, 105, 1135–1143. [Google Scholar] [CrossRef]
- Pearson, T.A.; Mensah, G.A.; Alexander, R.W.; Anderson, J.L.; Cannon, R.O., 3rd; Criqui, M.; Fadl, Y.Y.; Fortmann, S.P.; Hong, Y.; Myers, G.L.; et al. Markers of inflammation and cardiovascular disease: Application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation 2003, 107, 499–511. [Google Scholar] [CrossRef]
- Piani, F.; Tossetta, G.; Cara-Fuentes, G.; Agnoletti, D.; Marzioni, D.; Borghi, C. Diagnostic and Prognostic Role of CD93 in Cardiovascular Disease: A Systematic Review. Biomolecules 2023, 13, 910. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.H.K.; Huang, Y.-T.; Meng, Q.; Wu, C.; Reiner, A.; Sobel, E.M.; Tinker, L.; Lusis, A.J.; Yang, X.; Liu, S. Shared molecular pathways and gene networks for cardiovascular disease and type 2 diabetes mellitus in women across diverse ethnicities. Circ. Cardiovasc. Genet. 2014, 7, 911–919. [Google Scholar] [CrossRef]
- Zafari, F.; Shirian, S.; Sadeghi, M.; Teimourian, S.; Bakhtiyari, M. CD93 hematopoietic stem cells improve diabetic wound healing by VEGF activation and downregulation of DAPK-1. J. Cell. Physiol. 2020, 235, 2366–2376. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsova, T.; Prange, K.H.M.; Glass, C.K.; de Winther, M.P.J. Transcriptional and epigenetic regulation of macrophages in atherosclerosis. Nat. Rev. Cardiol. 2020, 17, 216–228. [Google Scholar] [CrossRef]
- Reiss, A.B.; Awadallah, N.W.; Malhotra, S.; Montesinos, M.C.; Chan, E.S.; Javitt, N.B.; Cronstein, B.N. Immune complexes and IFN-gamma decrease cholesterol 27-hydroxylase in human arterial endothelium and macrophages. J. Lipid Res. 2001, 42, 1913–1922. [Google Scholar] [CrossRef]
- Su, C.; Han, Y.; Qu, B.; Zhang, C.; Liang, T.; Gao, F.; Hou, G. CD93 in macrophages: A novel target for atherosclerotic plaque imaging? J. Cell. Mol. Med. 2022, 26, 2152–2162. [Google Scholar] [CrossRef]
- Machiraju, P.; Srinivas, R.; Kannan, R.; George, R.; Heymans, S.; Mukhopadhyay, R.; Ghosh, A. Paired Transcriptomic Analyses of Atheromatous and Control Vessels Reveal Novel Autophagy and Immunoregulatory Genes in Peripheral Artery Disease. Cells 2024, 13, 1269. [Google Scholar] [CrossRef] [PubMed]
- Patel, V.K.; Williams, H.; Li, S.C.; Fletcher, J.P.; Medbury, H.J. Monocyte inflammatory profile is specific for individuals and associated with altered blood lipid levels. Atherosclerosis 2017, 263, 15–23. [Google Scholar] [CrossRef]
- Snelder, S.M.; Pouw, N.; Aga, Y.; Cabezas, M.C.; Biter, L.U.; Zijlstra, F.; Kardys, I.; van Dalen, B.M. Cardiovascular Biomarker Profiles in Obesity and Relation to Normalization of Subclinical Cardiac Dysfunction after Bariatric Surgery. Cells 2022, 11, 422. [Google Scholar] [CrossRef]
- Alehagen, U.; Shamoun, L.; Wågsäter, D. Genetic variance and plasma concentration of CD93 is associated with cardiovascular mortality: Results from a 6.7-year follow-up of a healthy community-living elderly population. Mol. Med. Rep. 2020, 22, 4629–4636. [Google Scholar] [CrossRef]
- Sharma, K.; Chanana, N.; Mohammad, G.; Thinlas, T.; Gupta, M.; Syed, M.A.; Das, R.S.; Pasha, Q.; Mishra, A. Hypertensive Patients Exhibit Enhanced Thrombospondin-1 Levels at High-Altitude. Life 2021, 11, 893. [Google Scholar] [CrossRef] [PubMed]
- Chandramouli, C.; Ting, T.W.; Tromp, J.; Agarwal, A.; Svedlund, S.; Saraste, A.; Hage, C.; Tan, R.; Beussink-Nelson, L.; Fermer, M.L.; et al. Erratum in Sex differences in proteomic correlates of coronary microvascular dysfunction among patients with heart failure and preserved ejection fraction. Eur. J. Heart Fail. 2022, 24, 681–684. [Google Scholar] [CrossRef]
- Youn, J.-C.; Yu, H.T.; Jeon, J.-W.; Lee, H.S.; Jang, Y.; Park, Y.W.; Park, Y.-B.; Shin, E.-C.; Ha, J.-W.; Guo, Y. Soluble CD93 levels in patients with acute myocardial infarction and its implication on clinical outcome. PLoS ONE 2014, 9, e96538. [Google Scholar] [CrossRef]
- Adamski, M.G.; Li, Y.; Wagner, E.; Yu, H.; Seales-Bailey, C.; Soper, S.A.; Murphy, M.; Baird, A.E. Expression profile based gene clusters for ischemic stroke detection. Genomics 2014, 104, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Bicvic, A.; Scherrer, N.; Schweizer, J.; Fluri, F.; Christ-Crain, M.; De Marchis, G.M.; Luft, A.R.; Katan, M. Erratum in A novel biomarker panel index improves risk stratification after ischemic stroke. Eur. Stroke J. 2022, 7, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Chu, Y.; You, Y.; Dong, M.; Pang, X.; Chen, L.; Zhu, L.; Yang, S.; Zhou, L.; Shang, K.; et al. Systematic Druggable Genome-Wide Mendelian Randomization Identifies Therapeutic Targets for Functional Outcome After Ischemic Stroke. J. Am. Heart Assoc. 2024, 13, e034749. [Google Scholar] [CrossRef] [PubMed]
- Bouwens, E.; Berg, V.J.v.D.; Akkerhuis, K.M.; Baart, S.J.; Caliskan, K.; Brugts, J.J.; Mouthaan, H.; van Ramshorst, J.; Germans, T.; Umans, V.A.W.M.; et al. Circulating Biomarkers of Cell Adhesion Predict Clinical Outcome in Patients with Chronic Heart Failure. J. Clin. Med. 2020, 9, 195. [Google Scholar] [CrossRef]
- Payet, M.; Ah-Pine, F.; Guillot, X.; Gasque, P. Inflammatory Mesenchymal Stem Cells Express Abundant Membrane-Bound and Soluble Forms of C-Type Lectin-like CD248. Int. J. Mol. Sci. 2023, 24, 9546. [Google Scholar] [CrossRef]
- Duvetorp, A.; Olsen, R.; Skarstedt, M.; Söderman, J.; Seifert, O. Psoriasis and Pro-angiogenetic Factor CD93: Gene Expression and Association with Gene Polymorphism Suggests a Role in Disease Pathogenesis. Acta Derm. Venereol. 2017, 97, 916–921. [Google Scholar] [CrossRef]
- Shehata, W.A.; Maraee, A.H.; Tayel, N.; Mohamed, A.S.; El Gayed, E.M.A.; Elsayed, N.B.A.E.; Mostafa, M.I.; Bazid, H.A.S. CD93 has a crucial role in pathogenesis of psoriasis. J. Cosmet. Dermatol. 2022, 21, 1616–1624. [Google Scholar] [CrossRef]
- Liu, S.; Liu, F.; Zhang, Z.; Zhuang, Z.; Yuan, X.; Chen, Y. The SELP, CD93, IL2RG, and VAV1 Genes Associated with Atherosclerosis May Be Potential Diagnostic Biomarkers for Psoriasis. J. Inflamm. Res. 2023, 16, 827–843. [Google Scholar] [CrossRef]
- Moosig, F.; Fähndrich, E.; Knorr-Spahr, A.; Böttcher, S.; Ritgen, M.; Zeuner, R.; Kneba, M.; Schröder, J.O. C1qRP (CD93) expression on peripheral blood monocytes in patients with systemic lupus erythematosus. Rheumatol. Int. 2006, 26, 1109–1112. [Google Scholar] [CrossRef]
- Volkmann, E.R.; Andréasson, K.; Smith, V. Systemic sclerosis. Lancet 2023, 401, 304–318. [Google Scholar] [CrossRef]
- Siemann, D.W.; Horsman, M.R. Modulation of the tumor vasculature and oxygenation to improve therapy. Pharmacol. Ther. 2015, 153, 107–124. [Google Scholar] [CrossRef] [PubMed]
- Folkman, J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat. Med. 1995, 1, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Dianat-Moghadam, H.; Nedaeinia, R.; Keshavarz, M.; Azizi, M.; Kazemi, M.; Salehi, R. Immunotherapies targeting tumor vasculature: Challenges and opportunities. Front. Immunol. 2023, 14, 1226360. [Google Scholar] [CrossRef]
- Masiero, M.; Simões, F.C.; Han, H.D.; Snell, C.; Peterkin, T.; Bridges, E.; Mangala, L.S.; Wu, S.Y.-Y.; Pradeep, S.; Li, D.; et al. A core human primary tumor angiogenesis signature identifies the endothelial orphan receptor ELTD1 as a key regulator of angiogenesis. Cancer Cell 2013, 24, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Maas, S.L.N.; Abels, E.R.; Van De Haar, L.L.; Zhang, X.; Morsett, L.; Sil, S.; Guedes, J.; Sen, P.; Prabhakar, S.; Hickman, S.E.; et al. Glioblastoma hijacks microglial gene expression to support tumor growth. J. Neuroinflamm. 2020, 17, 120. [Google Scholar] [CrossRef]
- Riether, C.; Radpour, R.; Kallen, N.M.; Bürgin, D.T.; Bachmann, C.; Schürch, C.M.; Lüthi, U.; Arambasic, M.; Hoppe, S.; Albers, C.E.; et al. Metoclopramide treatment blocks CD93-signaling-mediated self-renewal of chronic myeloid leukemia stem cells. Cell Rep. 2021, 34, 108663. [Google Scholar] [CrossRef]
- Guo, A.; Zhang, J.; Tian, Y.; Peng, Y.; Luo, P.; Zhang, J.; Liu, Z.; Wu, W.; Zhang, H.; Cheng, Q. Identify the immune characteristics and immunotherapy value of CD93 in the pan-cancer based on the public data sets. Front. Immunol. 2022, 13, 907182. [Google Scholar] [CrossRef]
- Tong, W.; Wang, G.; Zhu, L.; Bai, Y.; Liu, Z.; Yang, L.; Wu, H.; Cui, T.; Zhang, Y. Pan-Cancer Analysis Identified CD93 as a Valuable Biomarker for Predicting Patient Prognosis and Immunotherapy Response. Front. Mol. Biosci. 2022, 8, 793445. [Google Scholar] [CrossRef]
- Zhang, Z.; Zheng, M.; Ding, Q.; Liu, M. CD93 Correlates with Immune Infiltration and Impacts Patient Immunotherapy Efficacy: A Pan-Cancer Analysis. Front. Cell Dev. Biol. 2022, 10, 817965. [Google Scholar] [CrossRef]
- Jiang, Q.; Kuai, J.; Jiang, Z.; Que, W.; Wang, P.; Huang, W.; Ding, W.; Zhong, L. CD93 overexpresses in liver hepatocellular carcinoma and represents a potential immunotherapy target. Front. Immunol. 2023, 14, 1158360. [Google Scholar] [CrossRef]
- Zhu, W.; Yi, Q.; Wang, J.; Ouyang, X.; Yang, K.; Jiang, B.; Huang, B.; Liu, J.; Zhao, L.; Liu, X.; et al. Comprehensive analysis of CLEC family genes in gastric cancer prognosis immune response and treatment. Sci. Rep. 2025, 15, 5956. [Google Scholar] [CrossRef]
- Ma, Q.; Gao, J.; Hui, Y.; Zhang, Z.-M.; Qiao, Y.-J.; Yang, B.-F.; Gong, T.; Zhao, D.-M.; Huang, B.-R. Single-cell RNA-sequencing and genome-wide Mendelian randomisation along with abundant machine learning methods identify a novel B cells signature in gastric cancer. Discov. Oncol. 2025, 16, 11. [Google Scholar] [CrossRef]
- Shen, Y.; Wu, Y.; Hao, M.; Fu, M.; Zhu, K.; Luo, P.; Wang, J. Clinicopathological association of CD93 expression in gastric adenocarcinoma. J. Cancer Res. Clin. Oncol. 2024, 150, 400. [Google Scholar] [CrossRef]
- Wu, B.; Fu, L.; Guo, X.; Hu, H.; Li, Y.; Shi, Y.; Zhang, Y.; Han, S.; Lv, C.; Tian, Y. Multi-omics profiling and digital image analysis reveal the potential prognostic and immunotherapeutic properties of CD93 in stomach adenocarcinoma. Front. Immunol. 2023, 14, 984816. [Google Scholar] [CrossRef]
- Qu, Z.; Han, Y.; Zhu, Q.; Ding, W.; Wang, Y.; Zhang, Y.; Wei, W.; Lei, Y.; Li, M.; Jiao, Y.; et al. A Novel Neutrophil Extracellular Traps Signature for Overall Survival Prediction and Tumor Microenvironment Identification in Gastric Cancer. J. Inflamm. Res. 2023, 16, 3419–3436. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Guo, H.; Wu, L.; Yuan, J. Prognostic analysis and validation of lncRNAs in bladder cancer on the basis of neutrophil extracellular traps. J. Gene Med. 2023, 25, e3525. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Lin, L.; Fu, G.; Zheng, M.; Geng, J.; Sun, X.; Xing, L. The analysis of multiple omics and examination of pathological images revealed the prognostic and therapeutic significances of CD93 in lung squamous cell carcinoma. Life Sci. 2024, 339, 122422. [Google Scholar] [CrossRef] [PubMed]
- Stabenau, K.A.; Samuels, T.L.; Lam, T.K.; Mathison, A.J.; Wells, C.; Altman, K.W.; Battle, M.A.; Johnston, N. Pepsinogen/Proton Pump Co-Expression in Barrett’s Esophageal Cells Induces Cancer-Associated Changes. Laryngoscope 2023, 133, 59–69. [Google Scholar] [CrossRef]
- Prasad, R.R.; Mishra, N.; Kant, R.; Fox, J.T.; Shoemaker, R.H.; Agarwal, C.; Raina, K.; Agarwal, R. Effect of nonsteroidal anti-inflammatory drugs (aspirin and naproxen) on inflammation-associated proteomic profiles in mouse plasma and prostate during TMPRSS2-ERG (fusion)-driven prostate carcinogenesis. Mol. Carcinog. 2024, 63, 1188–1204. [Google Scholar] [CrossRef]
- Chamo, M.; Koren, O.; Goldstein, O.; Bujanover, N.; Keinan, N.; Scharff, Y.; Gazit, R. Molecular Mechanisms in Murine Syngeneic Leukemia Stem Cells. Cancers 2023, 15, 720. [Google Scholar] [CrossRef]
- Bao, L.; Tang, M.; Zhang, Q.; You, B.; Shan, Y.; Shi, S.; Li, L.; Hu, S.; You, Y. Elevated expression of CD93 promotes angiogenesis and tumor growth in nasopharyngeal carcinoma. Biochem. Biophys. Res. Commun. 2016, 476, 467–474. [Google Scholar] [CrossRef]
- Yang, S.; Bai, Z.; Zhang, F.; Cui, W.; Bu, P.; Bai, W.; Xi, Y. Expression and prognostic significance of CD93 in blood vessels in colorectal cancer: An immunohistochemical analysis of 134 cases. BMC Gastroenterol. 2025, 25, 84. [Google Scholar] [CrossRef]
- Ma, K.; Chen, S.; Chen, X.; Zhao, X.; Yang, J. CD93 is Associated with Glioma-related Malignant Processes and Immunosuppressive Cell Infiltration as an Inspiring Biomarker of Survivance. J. Mol. Neurosci. 2022, 72, 2106–2124. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Park, A.K.; Lee, K.-M.; Park, S.K.; Han, S.; Han, W.; Noh, D.-Y.; Yoo, K.-Y.; Kim, H.; Chanock, S.J.; et al. Candidate gene approach evaluates association between innate immunity genes and breast cancer risk in Korean women. Carcinogenesis 2009, 30, 1528–1531. [Google Scholar] [CrossRef]
- Dong, C.; Luan, F.; Tian, W.; Duan, K.; Chen, T.; Ren, J.; Li, W.; Li, D.; Zhi, Q.; Zhou, J. Identification and validation of crucial lnc-TRIM28-14 and hub genes promoting gastric cancer peritoneal metastasis. BMC Cancer 2023, 23, 76. [Google Scholar] [CrossRef] [PubMed]
- Lai, Z.; Bai, Z.; Yang, S.; Zhang, R.; Xi, Y.; Xu, J. Hub genes in adenocarcinoma of the esophagogastric junction based on weighted gene co-expression network analysis and immunohistochemistry. Transl. Oncol. 2023, 37, 101781. [Google Scholar] [CrossRef] [PubMed]
- Dieterich, L.C.; Mellberg, S.; Langenkamp, E.; Zhang, L.; Zieba, A.; Salomäki, H.; Teichert, M.; Huang, H.; Edqvist, P.; Kraus, T.; et al. Transcriptional profiling of human glioblastoma vessels indicates a key role of VEGF-A and TGFβ2 in vascular abnormalization. J. Pathol. 2012, 228, 378–390. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Bader, J.E.; Voss, K.; Rathmell, J.C. Targeting Metabolism to Improve the Tumor Microenvironment for Cancer Immunotherapy. Mol. Cell 2020, 78, 1019–1033. [Google Scholar] [CrossRef]
- De Palma, M.; Biziato, D.; Petrova, T.V. Microenvironmental regulation of tumour angiogenesis. Nat. Rev. Cancer 2017, 17, 457–474. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.K. Normalization of tumor vasculature: An emerging concept in antiangiogenic therapy. Science 2005, 307, 58–62. [Google Scholar] [CrossRef]
- Hamzah, J.; Jugold, M.; Kiessling, F.; Rigby, P.; Manzur, M.; Marti, H.H.; Rabie, T.; Kaden, S.; Gröne, H.-J.; Hämmerling, G.J.; et al. Vascular normalization in Rgs5-deficient tumours promotes immune destruction. Nature 2008, 453, 410–414. [Google Scholar] [CrossRef]
- Huang, Y.; Goel, S.; Duda, D.G.; Fukumura, D.; Jain, R.K. Vascular normalization as an emerging strategy to enhance cancer immunotherapy. Cancer Res. 2013, 73, 2943–2948. [Google Scholar] [CrossRef]
- Vemuri, K.; Pereira, B.d.A.; Fuenzalida, P.; Subashi, Y.; Barbera, S.; van Hooren, L.; Hedlund, M.; Pontén, F.; Lindskog, C.; Olsson, A.-K.; et al. CD93 maintains endothelial barrier function and limits metastatic dissemination. J. Clin. Investig. 2024, 9, e169830. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Huang, A.; Zhu, B.-X.; Gong, J.; Ruan, Y.-H.; Liu, X.-C.; Zheng, L.; Wu, Y. Erratum in Targeting CD93 on monocytes revitalizes antitumor immunity by enhancing the function and infiltration of CD8(+) T cells. J. Immunother. Cancer 2024, 12, e010148. [Google Scholar] [CrossRef]
- Zhang, C.; Nan, X.; Zhang, B.; Wu, H.; Zeng, X.; Song, Z.; Li, S.; Xie, S.; Zhang, G.; Xiu, H.; et al. Blockade of CD93 in pleural mesothelial cells fuels anti-lung tumor immune responses. Theranostics 2024, 14, 1010–1028. [Google Scholar] [CrossRef]
- Pan, H.; Jing, C. Immune cells mediate the causal pathway linking circulating complements to cancer: A Mendelian randomization study. Inflamm. Res. 2024, 73, 2141–2152. [Google Scholar] [CrossRef]
- Sun, Y.; Yee, E.; Fujiwara, Y.; Dickinson, K.; Guo, Y.; Sun, Z.; Hu, J.; Davila, E.; Schulick, R.D.; Zhu, Y. CD93 blockade promotes effector T-cell infiltration and facilitates adoptive cell therapy in solid tumors. J. Immunother. Cancer 2025, 13, e010554. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Feng, J.; Li, Z.; Yang, S.; Qiu, X.; Xu, Y.; Shen, Z. Improved cancer immunotherapy strategies by nanomedicine. WIREs Nanomed. Nanobiotechnol. 2023, 15, e1873. [Google Scholar] [CrossRef]
- Passaro, A.; Stenzinger, A.; Peters, S. Tumor Mutational Burden as a Pan-cancer Biomarker for Immunotherapy: The Limits and Potential for Convergence. Cancer Cell 2020, 38, 624–625. [Google Scholar] [CrossRef]
- Miao, H.; Wu, Y.; Ouyang, H.; Zhang, P.; Zheng, W.; Ma, X. Screening and construction of nanobodies against human CD93 using phage libraries and study of their antiangiogenic effects. Front. Bioeng. Biotechnol. 2024, 12, 1372245. [Google Scholar] [CrossRef]
- Liang, Y.; Zhang, S.; Wang, D.; Ji, P.; Zhang, B.; Wu, P.; Wang, L.; Liu, Z.; Wang, J.; Duan, Y.; et al. Dual-Functional Nanodroplet for Tumor Vasculature Ultrasound Imaging and Tumor Immunosuppressive Microenvironment Remodeling. Adv. Healthc. Mater. 2024, 13, e2401274. [Google Scholar] [CrossRef]
- Wang, D.; Xing, C.; Liang, Y.; Wang, C.; Zhao, P.; Liang, X.; Li, Q.; Yuan, L. Ultrasound Imaging of Tumor Vascular CD93 with MMRN2 Modified Microbubbles for Immune Microenvironment Prediction. Adv. Mater. 2024, 36, e2310421. [Google Scholar] [CrossRef]
- Richards, R.M.; Zhao, F.; Freitas, K.A.; Parker, K.R.; Xu, P.; Fan, A.; Sotillo, E.; Daugaard, M.; Oo, H.Z.; Liu, J.; et al. NOT-Gated CD93 CAR T Cells Effectively Target AML with Minimized Endothelial Cross-Reactivity. Blood Cancer Discov. 2021, 2, 648–665. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Qiu, H.; Han, Z.; Ma, Y.; Hou, J.; Yuan, J.; Jia, H.; Zhou, M.; Lu, H.; Wu, Y. Topical transdermal administration of lenalidomide nanosuspensions-based hydrogels against melanoma: In vitro and in vivo studies. Int. J. Pharm. X 2025, 9, 100316. [Google Scholar] [CrossRef] [PubMed]
- Piani, F.; Tossetta, G.; Fantone, S.; Agostinis, C.; Di Simone, N.; Mandalà, M.; Bulla, R.; Marzioni, D.; Borghi, C. First Trimester CD93 as a Novel Marker of Preeclampsia and Its Complications: A Pilot Study. High. Blood Press. Cardiovasc. Prev. 2023, 30, 591–594. [Google Scholar] [CrossRef]
- Wong, W.L.; Su, X.; Li, X.; Cheung, C.M.G.; Klein, R.; Cheng, C.Y.; Wong, T.Y. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: A systematic review and meta-analysis. Lancet Glob. Health 2014, 2, e106–e116. [Google Scholar] [CrossRef] [PubMed]
- Handa, J.T.; Rickman, C.B.; Dick, A.D.; Gorin, M.B.; Miller, J.W.; Toth, C.A.; Ueffing, M.; Zarbin, M.; Farrer, L.A. A systems biology approach towards understanding and treating non-neovascular age-related macular degeneration. Nat. Commun. 2019, 10, 3347. [Google Scholar] [CrossRef]
- Tosi, G.M.; Caldi, E.; Parolini, B.; Toti, P.; Neri, G.; Nardi, F.; Traversi, C.; Cevenini, G.; Marigliani, D.; Nuti, E.; et al. CD93 as a Potential Target in Neovascular Age-Related Macular Degeneration. J. Cell Physiol. 2017, 232, 1767–1773. [Google Scholar] [CrossRef] [PubMed]
- Tosi, G.M.; Neri, G.; Barbera, S.; Mundo, L.; Parolini, B.; Lazzi, S.; Lugano, R.; Poletto, E.; Leoncini, L.; Pertile, G.; et al. The Binding of CD93 to Multimerin-2 Promotes Choroidal Neovascularization. Investig. Opthalmol. Vis. Sci. 2020, 61, 30. [Google Scholar] [CrossRef]
- Raucci, L.; Perrone, C.D.; Barbera, S.; de Boer, L.J.; Tosi, G.M.; Brunetti, J.; Bracci, L.; Pozzi, C.; Galvagni, F.; Orlandini, M. Structural and antigen-binding surface definition of an anti-CD93 monoclonal antibody for the treatment of degenerative vascular eye diseases. Int. J. Biol. Macromol. 2025, 309 Pt 4, 143118. [Google Scholar] [CrossRef]
- Cordon, J.; Duggan, M.R.; Gomez, G.T.; Pucha, K.A.; Peng, Z.; Dark, H.E.; Davatzikos, C.; Erus, G.; Lewis, A.; Moghekar, A.; et al. Identification of Clinically Relevant Brain Endothelial Cell Biomarkers in Plasma. Stroke 2023, 54, 2853–2863. [Google Scholar] [CrossRef]
- Liang, Q.; Su, L.; Zhang, D.; Jiao, J. CD93 negatively regulates astrogenesis in response to MMRN2 through the transcriptional repressor ZFP503 in the developing brain. Proc. Natl. Acad. Sci. USA 2020, 117, 9413–9422. [Google Scholar] [CrossRef]
- Wang, X.; Yang, G.; Lai, Y.; Li, Y.; Liu, X. Exploring the hub Genes and Potential Mechanisms of Complement system-related Genes in Parkinson Disease: Based on Transcriptome Sequencing and Mendelian Randomization. J. Mol. Neurosci. 2024, 74, 95. [Google Scholar] [CrossRef]
- Corleis, B.; Tzouanas, C.N.; Wadsworth, M.H.; Cho, J.L.; Linder, A.H.; Schiff, A.E.; Zessin, B.; Stei, F.; Dorhoi, A.; Dickey, A.K.; et al. Tobacco smoke exposure recruits inflammatory airspace monocytes that establish permissive lung niches for Mycobacterium tuberculosis. Sci. Transl. Med. 2023, 15, eadg3451. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Wang, Y.; Wang, X.; Deng, S.; Wang, D.; Huang, Q.; Lyu, G. Unveiling the molecular landscape of PCOS: Identifying hub genes and causal relationships through bioinformatics and Mendelian randomization. Front. Endocrinol. 2024, 15, 1431200. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Moro, Á.; González-Brusi, L.; Lamas-Toranzo, I.; González-Dosal, P.; Rodríguez-Juárez, F.; Bermejo-Álvarez, P. The human cumulus cell transcriptome provides poor predictive value for embryo transfer outcome. Reprod. Biomed. 2023, 46, 783–791. [Google Scholar] [CrossRef]
- Araujo, T.L.S.; Venturini, G.; Moretti, A.I.S.; Tanaka, L.Y.; Pereira, A.C.; Laurindo, F.R.M. Cell-surface HSP70 associates with thrombomodulin in endothelial cells. Cell Stress. Chaperones 2019, 24, 273–282. [Google Scholar] [CrossRef]
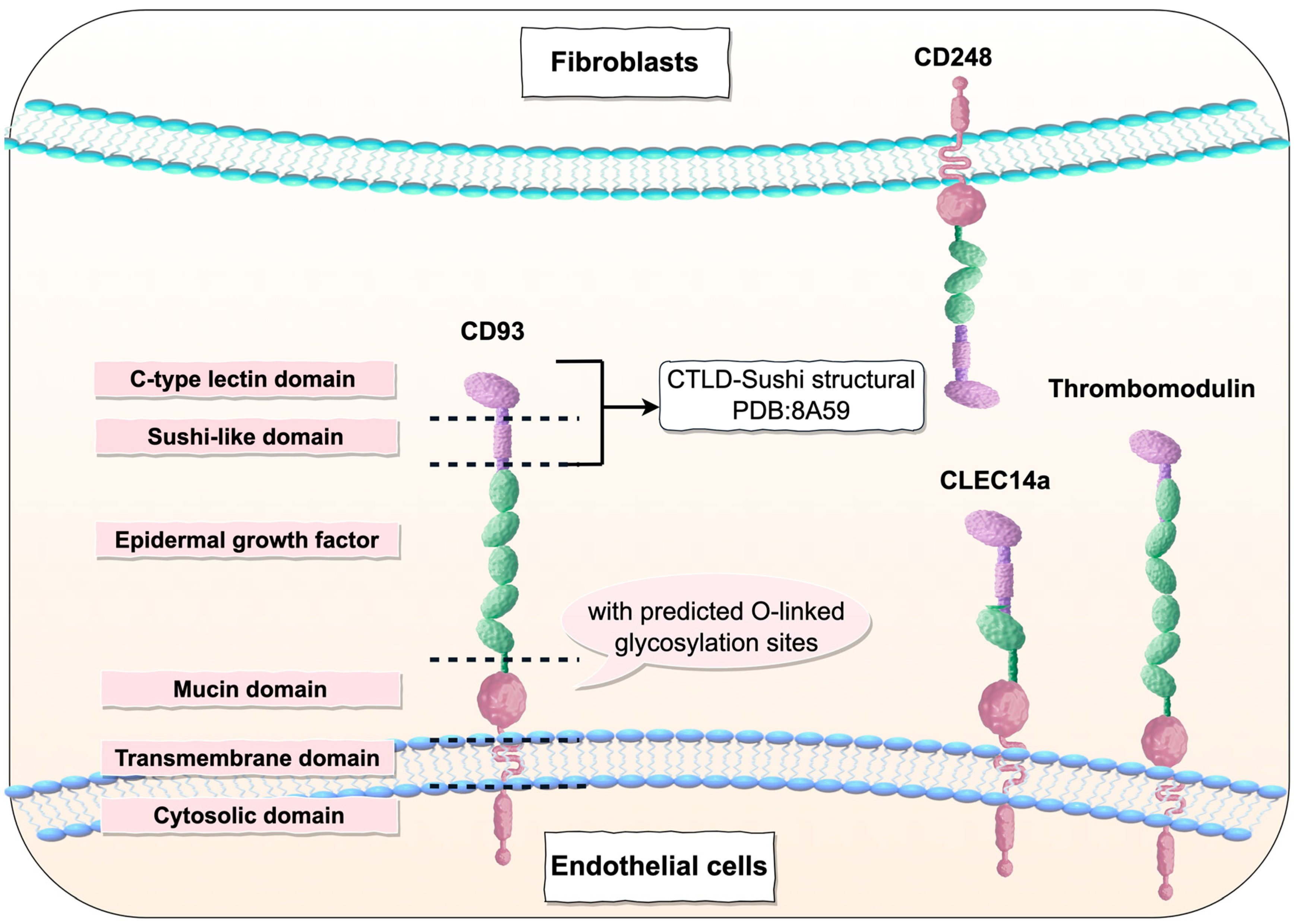

| CD93 | TM | CD248 | CLEC14a | |
|---|---|---|---|---|
| Alternative name | C1qR1, C1qRp, AA4 | CD141, BDCA3 | Endosialin, TEM1 | / |
| Structural composition | CTLD, sushi-like domain, five EGF-like domains, mucin domain, transmembrane domain, cytosolic domain | CTLD, six EGF-like domains, mucin domain, transmembrane domain, cytosolic domain | CTLD, sushi-like domain, three EGF-like domains, mucin domain, transmembrane domain, cytosolic domain | CTLD, sushi-like domain, one EGF-like domains, mucin domain, transmembrane domain, cytosolic domain |
| Mainly represent cell types | ECs, neurons, and various myeloid cells (such as macrophages, monocytes, and stem cells) | Vascular endothelial cells, lymphatic endothelial cells, mesothelial cells, astrocytes, keratinocytes, osteoblasts, chondrocytes, alveolar epithelial cells, and various hematopoietic cells | Fibroblasts, smooth muscle cells, pericytes, mesenchymal stem cells, and naive T cells | ECs |
| Normal adult tissues | Extremely low expression or complete absence | Moderate to high expression | Absence (e.g., in the brain, stomach, skin, ovaries) or low expression | Absent or minimally expressed |
| Combining ligands and interacting proteins | MMRN2, β-dystroglycan, Cbl, IGFBP7, Moesin, GIPC, VE-cadherin, IL-17D | Thrombin, protein C, fibronectin, Lewis Y antigen, GPCR15, ezrin | Extracellular matrix proteins, including collagen type I, collagen type IV, and fibronectin | MMRN2 and HSP70-1A |
| Physiological function | Clearance of apoptotic cells, endothelial cell maturation and migration, intercellular adhesion, promotion of angiogenesis | Promote embryonic development, mediate cell adhesion and inflammation regulation, anticoagulate, regulate angiogenesis | Reshape the developing vascular system, mediate the migration, activation and proliferation of stromal cells, promote inflammation | Endothelial cell migration and adhesion, regulation of lumen formation |
| Association with inflammatory diseases | It is upregulated in various inflammatory diseases, such as asthma and peritonitis. | Exerts anti—inflammatory effects by inactivating pro—inflammatory cytokines and inhibiting the inflammatory complement proteins C3a and C5a. | Promote inflammation and upregulate in fibroblasts and pericytes of synovial tissue as well as mesenchymal cells of the skin. | Undefined |
| Relationship with tumors | It is highly expressed in various cancers, and its expression level is positively correlated with tumor malignancy and poor prognosis, and negatively correlated with survival rate. | In various cancers, high expression of membrane-bound TM is negatively correlated with tumor aggressiveness and positively correlated with survival rate; in contrast, soluble TM shows the opposite pattern. | High expression levels are positively correlated with tumor invasion and metastasis and negatively correlated with survival rate. | Highly expressed in various cancers. |
| Preclinical/preclinical therapies | Monoclonal antibodies, therapeutic vaccines, small-molecule chemical drugs, antibody-conjugated radionuclides, dendritic cell vaccines | Monoclonal antibodies, protein-based drugs | Antibody-conjugated radionuclides, small-molecule chemical drugs, Monoclonal antibodies, therapeutic radiopharmaceuticals, bispecific antibodies, CAR T therapy, therapeutic vaccines, mRNA vaccines | Monoclonal antibodies, CAR T therapy |
| Clinical trial status | 1. Drug discovery (WO2024115637, CN116271112) 2. Preclinical (SY2053, CD34-Derived Allogeneic Dendritic Cell Cancer Vaccine, Anti CD93 antibody) 3. Phase 1 clinical trial (DCSZ-11) 4. Termination (DCBY020) 5. No progression (JS013) | 1. Drug discovery (CN114686444, CN118599002, CN114686443) 2. Preclinical (AD-010) | 1. Preclinical ([Ac-225]-FPI-1848, ANP-021, [177 Lu]-FPI-1835, Endosialin-directed E3K CAR-T cells) 2. Termination (Ontuxizumab, MP-ENDOS-1959, AVS300) | 1. Preclinical (Anti-clec14a antibodies) 2. No progression (Anti CLEC14a-CTLD antibody, CLEC14A-specific CAR T cells) |
| Drug Name | Mechanism of Action | Drug Type | Indications Under Research | Original Research Institution | Research Institutions Under Development | The Highest Research and Development Stage |
|---|---|---|---|---|---|---|
| DCSZ-11 (DCSZ11) | CD93 inhibitor | Monoclonal antibodies | Advanced malignant solid tumors | DynamiCure Biotechnology LLC | DynamiCure Biotechnology LLC | Phase 2 clinical trial (2025-07-15) |
| SY2053 | CD93 inhibitor | Antibodies | Tumors | Shanghai Symray Biopharma Co., Ltd. | Shanghai Symray Biopharma Co., Ltd. | Preclinical |
| CD34-Derived Allogeneic Dendritic Cell Cancer Vaccine | CD40L regulator CD93 modulators CXCL13 modulators | Therapeutic vaccines, dendritic cell vaccines | Pancreatic cancer | Renovaro, Inc. | Renovaro, Inc. | Preclinical |
| Anti CD93 antibody (China Resources Biopharmaceutical) | CD93 inhibitor | Monoclonal antibodies | Tumors | China Resources Biopharmaceutical Co., Ltd. | China Resources Biopharmaceutical Co., Ltd. | Preclinical |
| WO2024115637 | CD93 inhibitor | Small-molecule chemical drugs | Tumors | University of Bern | University of Bern | Drug discovery |
| CN116271112 | / | Antibody-conjugated radionuclides | Tumors | The Fourth Military Medical University | The Fourth Military Medical University | Drug discovery |
| DCBY0 (DCBY 02, DCB7-02) | CD93 inhibitor | Monoclonal antibodies | / | DynamiCure Biotechnology LLC | / | Termination (Phase 1 clinical trial) |
| JS013 | CD93 inhibitor | Monoclonal antibodies | / | Shanghai Junshi Biosciences Co., Ltd. | / | No progression (Preclinical) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, M.; Zhou, X.; Wang, S.; Huang, X.; Chen, W.; Chen, Y.; Huang, L.; Yan, Y.; Zhu, Y.; Ye, L. CD93 in Health and Disease: Bridging Physiological Functions and Clinical Applications. Int. J. Mol. Sci. 2025, 26, 8617. https://doi.org/10.3390/ijms26178617
Cai M, Zhou X, Wang S, Huang X, Chen W, Chen Y, Huang L, Yan Y, Zhu Y, Ye L. CD93 in Health and Disease: Bridging Physiological Functions and Clinical Applications. International Journal of Molecular Sciences. 2025; 26(17):8617. https://doi.org/10.3390/ijms26178617
Chicago/Turabian StyleCai, Menghan, Xiaoxi Zhou, Songna Wang, Xuan Huang, Wei Chen, Yiling Chen, Litao Huang, Yan Yan, Yizhun Zhu, and Li Ye. 2025. "CD93 in Health and Disease: Bridging Physiological Functions and Clinical Applications" International Journal of Molecular Sciences 26, no. 17: 8617. https://doi.org/10.3390/ijms26178617
APA StyleCai, M., Zhou, X., Wang, S., Huang, X., Chen, W., Chen, Y., Huang, L., Yan, Y., Zhu, Y., & Ye, L. (2025). CD93 in Health and Disease: Bridging Physiological Functions and Clinical Applications. International Journal of Molecular Sciences, 26(17), 8617. https://doi.org/10.3390/ijms26178617







