Stage-Specific Lipidomes of Gastrodia elata Extracellular Vesicles Modulate Fungal Symbiosis
Abstract
1. Introduction
2. Results
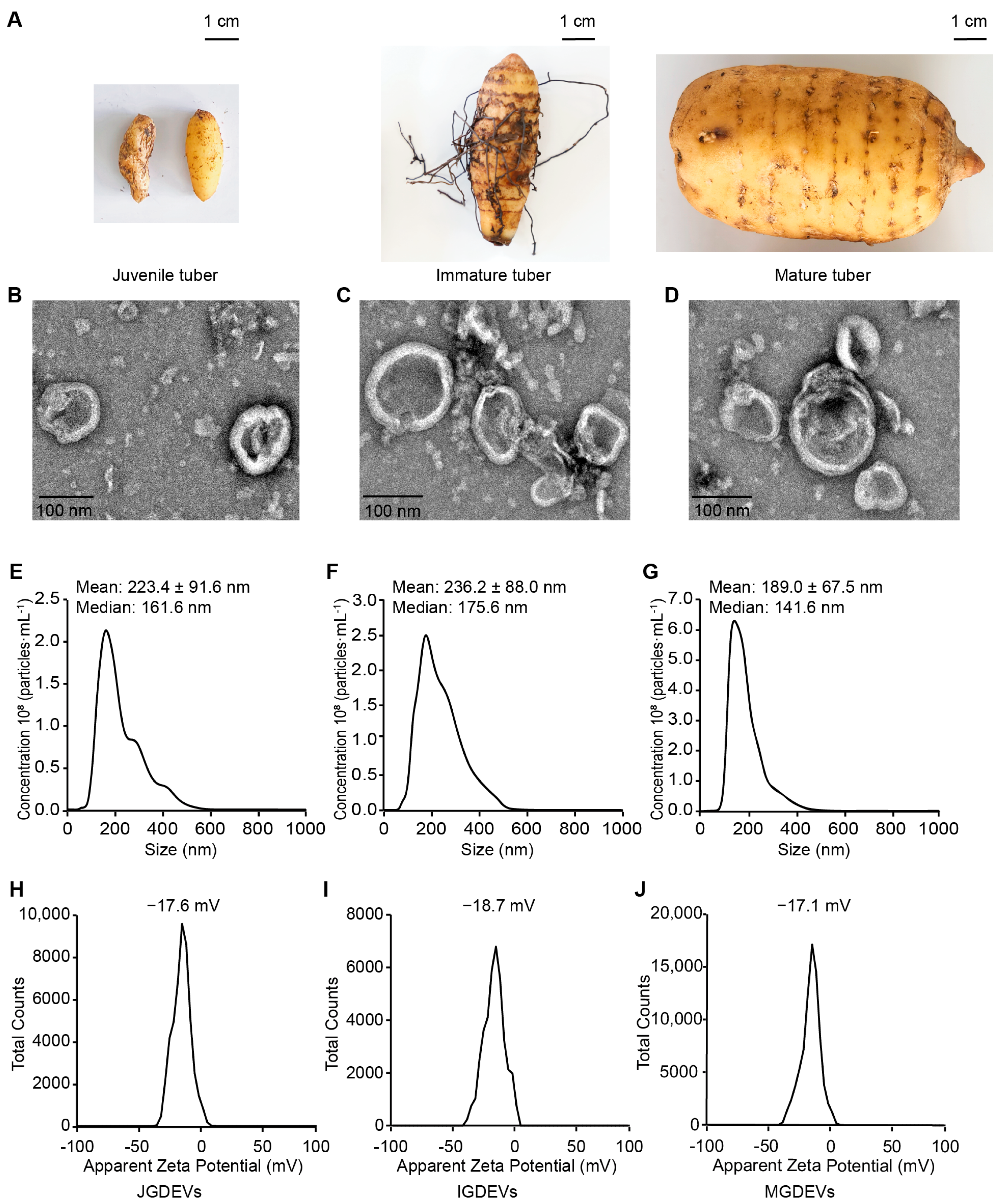
2.1. Isolation and Morphological Characterization of Extracellular Vesicles from Different Developmental Stages of G. elata
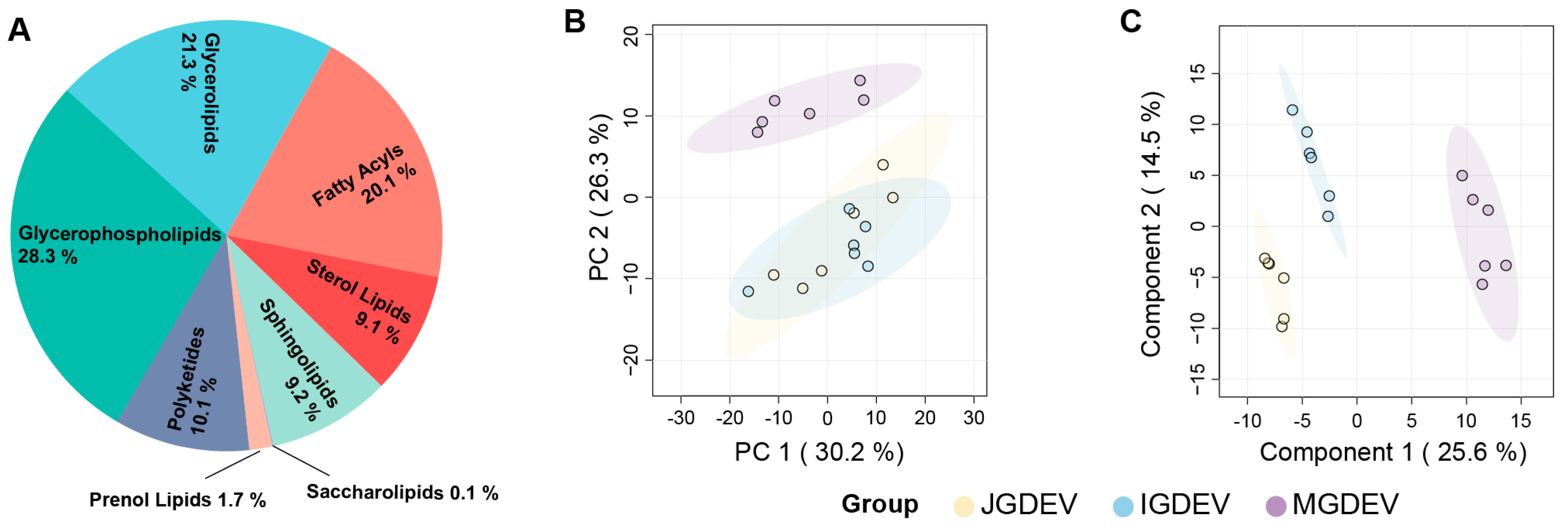
2.2. Comprehensive Lipidomic Profiling Reveals Metabolic Shifts Across G. elata Tuber Development
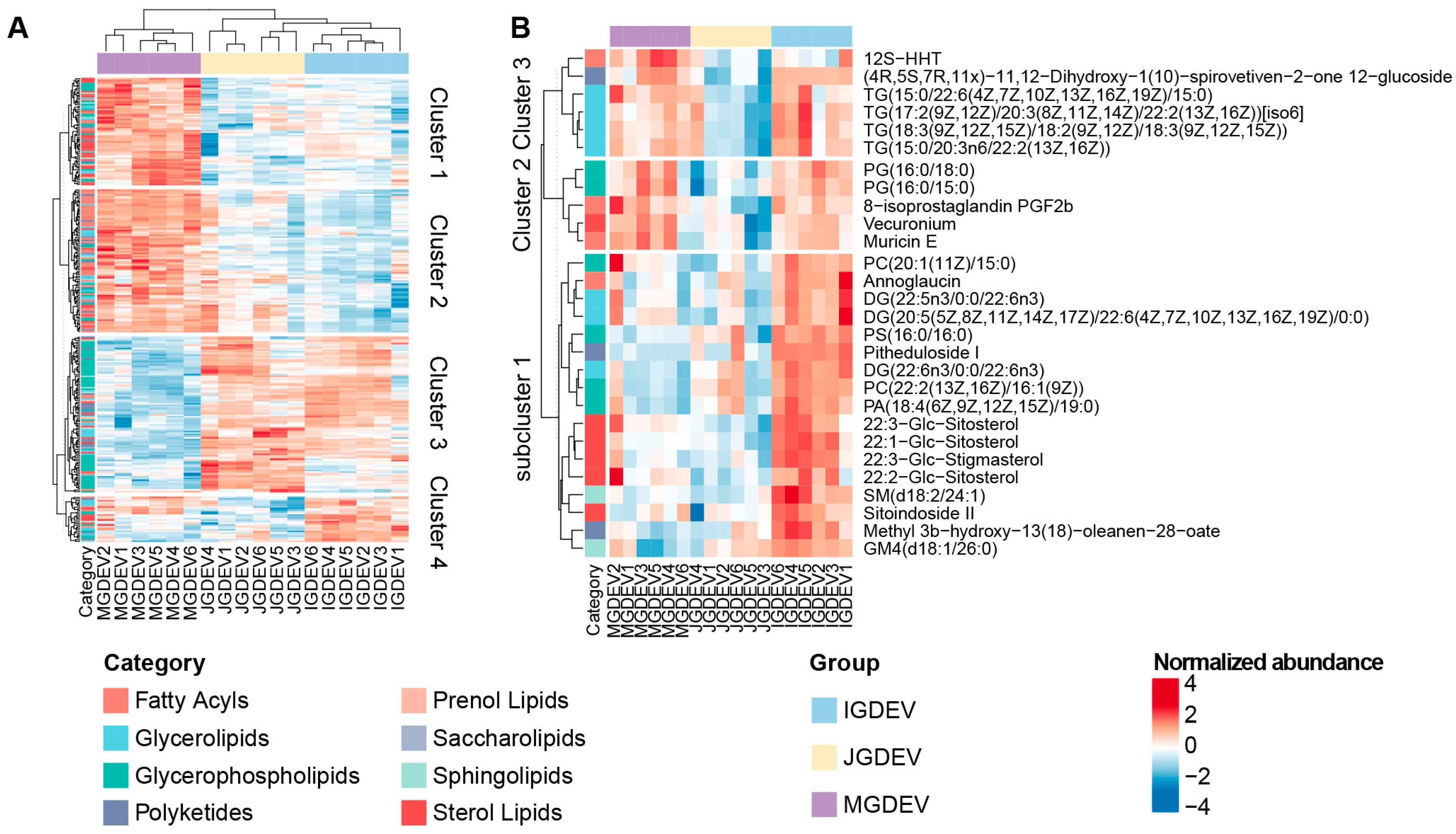
2.3. ANOVA-Based Identification and Clustering of Stage-Associated Lipids
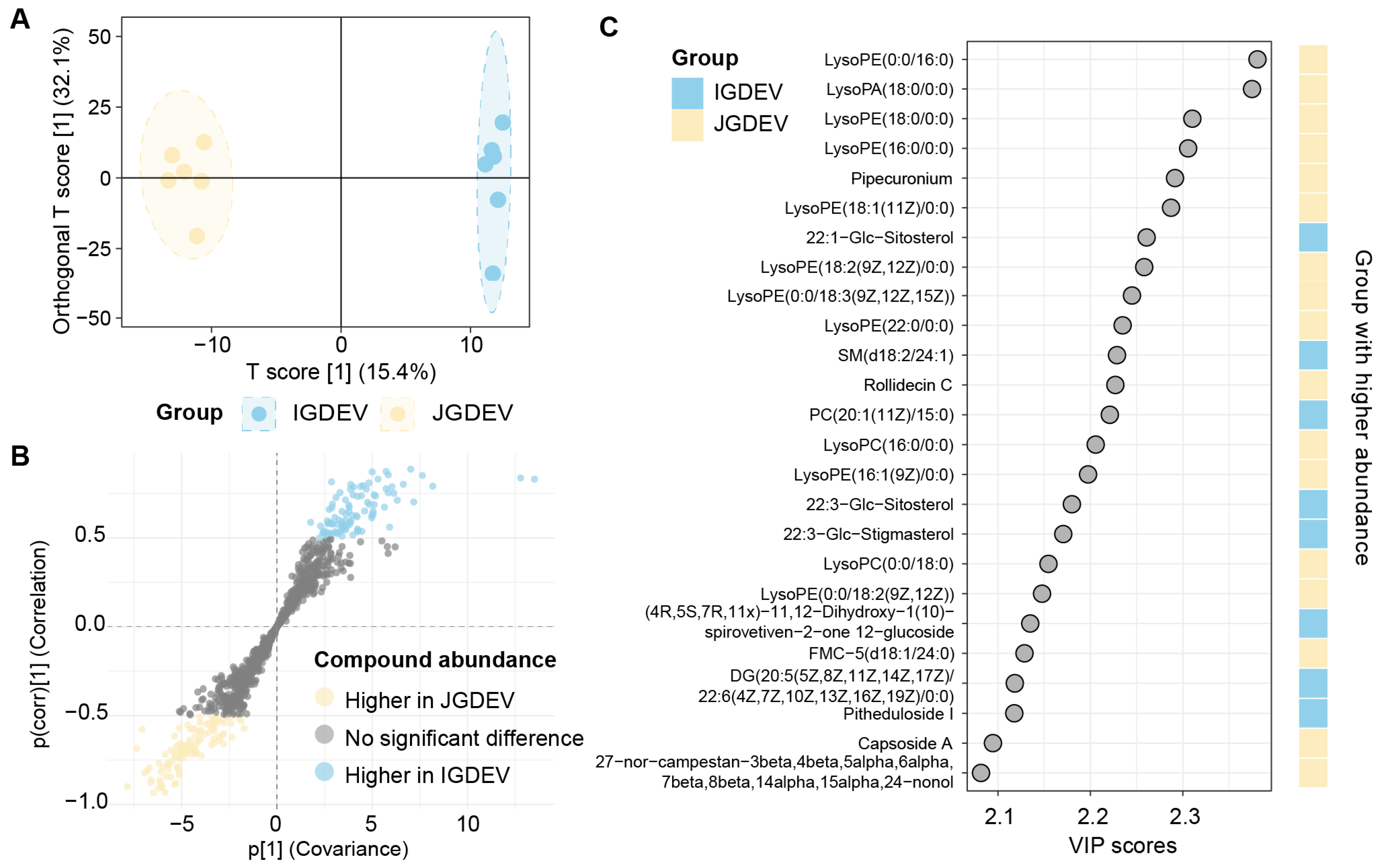
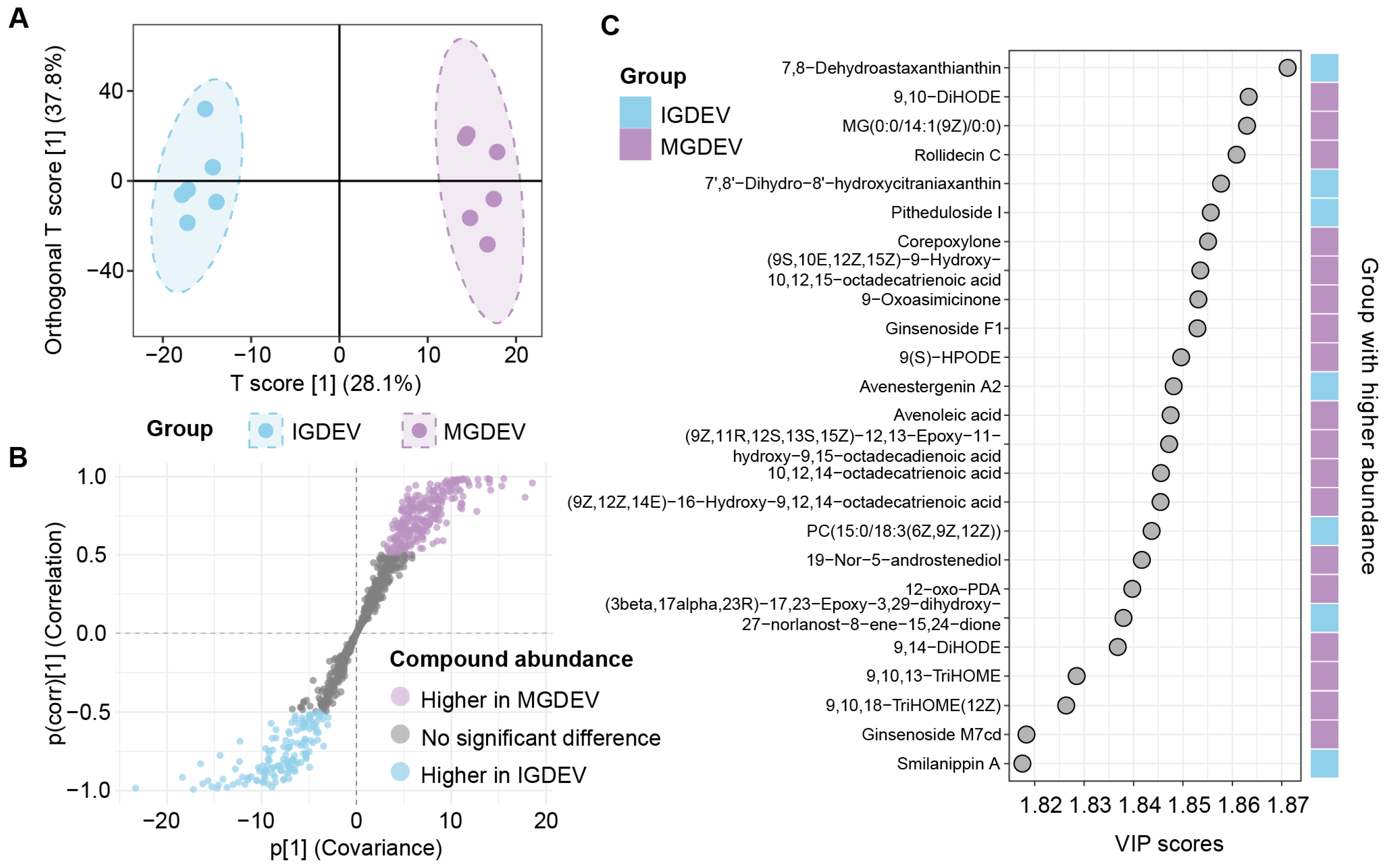
2.4. OPLS-DA Reveals Major Lipidomic Differences Across Developmental Stages
2.5. Quantitative Differential Analysis of Key Lipid Species
3. Discussion
3.1. Plant-Derived Extracellular Vesicles as Novel Mediators in Mycoheterotrophic Symbiosis
3.2. Lipidomic Remodeling Reflects Developmental Transitions and Symbiotic Interactions
3.3. Core Lipid Markers in GDEVs and Their Potential Roles in Symbiotic Signaling
3.4. Future Perspectives
4. Materials and Methods
4.1. Isolation and Characterization Purification of GDEVs from Different Developmental Phases
4.2. Transmission Electron Microscopy (TEM) Imaging
4.3. Nanoparticle Tracking Analysis (NTA)
4.4. Zeta Potential Analysis
4.5. Lipidomics Analysis
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AM | arbuscular mycorrhizal |
| EVs | extracellular vesicles |
| FAs | fatty acyls |
| GDEVs | G. elata-derived extracellular vesicles |
| GL | glycerolipid |
| GP | glycerophospholipid |
| IGDEV | immature tuber-derived extracellular vesicle |
| JGDEV | juvenile tuber-derived extracellular vesicle |
| MGDEV | mature tuber-derived extracellular vesicle |
| NTA | nanoparticle tracking analysis |
| OPLS-DA | orthogonal partial least squares discriminant analysis |
| PCA | principal component analysis |
| PDEVs | plant-derived extracellular vesicles |
| PK | polyketide |
| PLS-DA | partial least squares discriminant analysis |
| PR | prenol lipid |
| SL | saccharolipid |
| SP | sphingolipid |
| ST | sterol lipid |
| TEM | transmission electron microscopy |
References
- Liu, J.J.; Yang, X.Q.; Li, Z.Y.; Miao, J.Y.; Li, S.B.; Zhang, W.P.; Lin, Y.C.; Lin, L.B. The role of symbiotic fungi in the life cycle of Gastrodia elata Blume (Orchidaceae): A comprehensive review. Front. Plant Sci. 2023, 14, 1309038. [Google Scholar] [CrossRef]
- Zhou, X. The life circle of Gastrodia elata Blume. Acta Bot. Yunnan. 1981, 3, 197–202. [Google Scholar]
- Yuan, Y.; Jin, X.; Liu, J.; Zhao, X.; Zhou, J.; Wang, X.; Wang, D.; Lai, C.; Xu, W.; Huang, J.; et al. The Gastrodia elata genome provides insights into plant adaptation to heterotrophy. Nat. Commun. 2018, 9, 1615. [Google Scholar] [CrossRef]
- Alzahrani, F.A.; Khan, M.I.; Kameli, N.; Alsahafi, E.; Riza, Y.M. Plant-Derived Extracellular Vesicles and Their Exciting Potential as the Future of Next-Generation Drug Delivery. Biomolecules 2023, 13, 839. [Google Scholar] [CrossRef]
- Cui, Y.; Gao, J.; He, Y.; Jiang, L. Plant extracellular vesicles. Protoplasma 2020, 257, 3–12. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Cai, Q.; Qiao, L.; Huang, C.Y.; Wang, S.; Miao, W.; Ha, T.; Wang, Y.; Jin, H. RNA-binding proteins contribute to small RNA loading in plant extracellular vesicles. Nat. Plants 2021, 7, 342–352, Correction in Nat. Plants 2021, 7, 539. [Google Scholar] [CrossRef]
- Huang, Z.; Pych, E.; Whitehead, B.J.; Nejsum, P.L.; Bellini, I.; Drace, T.; Boesen, T.; Paponov, I.; Rasmussen, M.K. Impact of abiotic stress on miRNA profiles in tomato-derived extracellular vesicles and their biological activity. Int. J. Biol. Macromol. 2025, 319, 145260. [Google Scholar] [CrossRef]
- Stotz, H.U.; Brotherton, D.; Inal, J. Communication is key: Extracellular vesicles as mediators of infection and defence during host-microbe interactions in animals and plants. FEMS Microbiol. Rev. 2022, 46, fuab044. [Google Scholar] [CrossRef]
- Roth, R.; Hillmer, S.; Funaya, C.; Chiapello, M.; Schumacher, K.; Lo Presti, L.; Kahmann, R.; Paszkowski, U. Arbuscular cell invasion coincides with extracellular vesicles and membrane tubules. Nat. Plants 2019, 5, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, S.; Austin, J., 2nd; Berg, R.H.; Harrison, M.J. Extensive membrane systems at the host-arbuscular mycorrhizal fungus interface. Nat. Plants 2019, 5, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Koch, B.L.; Rutter, B.D.; Borniego, M.L.; Singla-Rastogi, M.; Gardner, D.M.; Innes, R.W. Arabidopsis Produces Distinct Subpopulations of Extracellular Vesicles That Respond Differentially to Biotic Stress, Altering Growth and Infectivity of a Fungal Pathogen. J. Extracell. Vesicles 2025, 14, e70090. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.; Ma, J.; Zhou, Y.; Lu, R. Focusing on Future Applications and Current Challenges of Plant Derived Extracellular Vesicles. Pharmaceuticals 2022, 15, 708. [Google Scholar] [CrossRef] [PubMed]
- Rutter, B.D.; Innes, R.W. Extracellular Vesicles Isolated from the Leaf Apoplast Carry Stress-Response Proteins. Plant Physiol. 2017, 173, 728–741. [Google Scholar] [CrossRef]
- Regente, M.; Pinedo, M.; San Clemente, H.; Balliau, T.; Jamet, E.; de la Canal, L. Plant extracellular vesicles are incorporated by a fungal pathogen and inhibit its growth. J. Exp. Bot. 2017, 68, 5485–5495. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Xu, J.; Gao, T.; Huang, D.; Jin, W. Molecular mechanism of modulating miR482b level in tomato with botrytis cinerea infection. BMC Plant Biol. 2021, 21, 496. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.-Y.; Yao, N. Sphingolipids in plant immunity. Phytopathol. Res. 2022, 4, 20. [Google Scholar] [CrossRef]
- Maillet, F.; Poinsot, V.; Andre, O.; Puech-Pages, V.; Haouy, A.; Gueunier, M.; Cromer, L.; Giraudet, D.; Formey, D.; Niebel, A.; et al. Fungal lipochitooligosaccharide symbiotic signals in arbuscular mycorrhiza. Nature 2011, 469, 58–63. [Google Scholar] [CrossRef]
- Fahy, E.; Subramaniam, S.; Brown, H.A.; Glass, C.K.; Merrill, A.H., Jr.; Murphy, R.C.; Raetz, C.R.; Russell, D.W.; Seyama, Y.; Shaw, W.; et al. A comprehensive classification system for lipids. J. Lipid Res. 2005, 46, 839–861. [Google Scholar] [CrossRef]
- Liu, N.J.; Wang, N.; Bao, J.J.; Zhu, H.X.; Wang, L.J.; Chen, X.Y. Lipidomic Analysis Reveals the Importance of GIPCs in Arabidopsis Leaf Extracellular Vesicles. Mol. Plant 2020, 13, 1523–1532. [Google Scholar] [CrossRef]
- Wang, Q.; Zhuang, X.; Mu, J.; Deng, Z.B.; Jiang, H.; Zhang, L.; Xiang, X.; Wang, B.; Yan, J.; Miller, D.; et al. Delivery of therapeutic agents by nanoparticles made of grapefruit-derived lipids. Nat. Commun. 2013, 4, 1867, Correction in Nat. Commun. 2013, 4, 2358; Correction in Nat. Commun. 2016, 7, 11347. [Google Scholar] [CrossRef]
- Zhuang, X.; Deng, Z.B.; Mu, J.; Zhang, L.; Yan, J.; Miller, D.; Feng, W.; McClain, C.J.; Zhang, H.G. Ginger-derived nanoparticles protect against alcohol-induced liver damage. J. Extracell. Vesicles 2015, 4, 28713. [Google Scholar] [CrossRef]
- Wang, F.; Li, L.; Deng, J.; Ai, J.; Mo, S.; Ding, D.; Xiao, Y.; Hu, S.; Zhu, D.; Li, Q.; et al. Lipidomic analysis of plant-derived extracellular vesicles for guidance of potential anti-cancer therapy. Bioact. Mater. 2025, 46, 82–96. [Google Scholar] [CrossRef]
- Wewer, V.; Brands, M.; Dörmann, P. Fatty acid synthesis and lipid metabolism in the obligate biotrophic fungus Rhizophagus irregularis during mycorrhization of Lotus japonicus. Plant J. 2014, 79, 398–412. [Google Scholar] [CrossRef]
- Reszczynska, E.; Hanaka, A. Lipids Composition in Plant Membranes. Cell Biochem. Biophys. 2020, 78, 401–414. [Google Scholar] [CrossRef]
- Shen, M.; Yuan, L.; Zhang, J.; Wang, X.; Zhang, M.; Li, H.; Jing, Y.; Zeng, F.; Xie, J. Phytosterols: Physiological Functions and Potential Application. Foods 2024, 13, 1754. [Google Scholar] [CrossRef]
- Macabuhay, A.; Arsova, B.; Walker, R.; Johnson, A.; Watt, M.; Roessner, U. Modulators or facilitators? Roles of lipids in plant root-microbe interactions. Trends Plant Sci. 2022, 27, 180–190. [Google Scholar] [CrossRef]
- Griebel, T.; Zeier, J. A role for beta-sitosterol to stigmasterol conversion in plant-pathogen interactions. Plant J. 2010, 63, 254–268. [Google Scholar] [CrossRef]
- Hertweck, C. The biosynthetic logic of polyketide diversity. Angew. Chem. Int. Ed. Engl. 2009, 48, 4688–4716. [Google Scholar] [CrossRef] [PubMed]
- Risdian, C.; Mozef, T.; Wink, J. Biosynthesis of Polyketides in Streptomyces. Microorganisms 2019, 7, 124. [Google Scholar] [CrossRef] [PubMed]
- Furlanetto, V.; Kalyani, D.C.; Kostelac, A.; Puc, J.; Haltrich, D.; Hallberg, B.M.; Divne, C. Structural and Functional Characterization of a Gene Cluster Responsible for Deglycosylation of C-glucosyl Flavonoids and Xanthonoids by Deinococcus aerius. J. Mol. Biol. 2024, 436, 168547. [Google Scholar] [CrossRef] [PubMed]
- Debnath, T.; Bandyopadhyay, T.K.; Vanitha, K.; Bobby, M.N.; Nath Tiwari, O.; Bhunia, B.; Muthuraj, M. Astaxanthin from microalgae: A review on structure, biosynthesis, production strategies and application. Food Res. Int. 2024, 176, 113841, Correction in Food Res. Int. 2025, 200, 115519. [Google Scholar] [CrossRef]
- Mosqueda-Martinez, E.; Chiquete-Felix, N.; Castaneda-Tamez, P.; Ricardez-Garcia, C.; Gutierrez-Aguilar, M.; Uribe-Carvajal, S.; Mendez-Romero, O. In Rhodotorula mucilaginosa, active oxidative metabolism increases carotenoids to inactivate excess reactive oxygen species. Front. Fungal Biol. 2024, 5, 1378590. [Google Scholar] [CrossRef] [PubMed]
- Hua, Z.; Teng, X.; Huang, J.; Zhou, J.; Zhao, Y.; Huang, L.; Yuan, Y. The Armillaria response to Gastrodia elata is partially mediated by strigolactone-induced changes in reactive oxygen species. Microbiol. Res. 2023, 278, 127536. [Google Scholar] [CrossRef]
- Gavrin, A.; Chiasson, D.; Ovchinnikova, E.; Kaiser, B.N.; Bisseling, T.; Fedorova, E.E. VAMP721a and VAMP721d are important for pectin dynamics and release of bacteria in soybean nodules. New Phytol. 2016, 210, 1011–1021. [Google Scholar] [CrossRef]
- Guo, T.; Wang, H.C.; Xue, W.Q.; Zhao, J.; Yang, Z.L. Phylogenetic Analyses of Armillaria Reveal at Least 15 Phylogenetic Lineages in China, Seven of Which Are Associated with Cultivated Gastrodia elata. PLoS ONE 2016, 11, e0154794. [Google Scholar] [CrossRef]
- Hu, X.L.; Zhang, J.; Kaundal, R.; Kataria, R.; Labbe, J.L.; Mitchell, J.C.; Tschaplinski, T.J.; Tuskan, G.A.; Cheng, Z.M.; Yang, X. Diversity and conservation of plant small secreted proteins associated with arbuscular mycorrhizal symbiosis. Hortic. Res. 2022, 9, uhac043. [Google Scholar] [CrossRef]
- Hou, Y.; Zhai, Y.; Feng, L.; Karimi, H.Z.; Rutter, B.D.; Zeng, L.; Choi, D.S.; Zhang, B.; Gu, W.; Chen, X.; et al. A Phytophthora Effector Suppresses Trans-Kingdom RNAi to Promote Disease Susceptibility. Cell Host Microbe 2019, 25, 153–165.e5. [Google Scholar] [CrossRef]
- Cai, Q.; Halilovic, L.; Shi, T.; Chen, A.; He, B.; Wu, H.; Jin, H. Extracellular vesicles: Cross-organismal RNA trafficking in plants, microbes, and mammalian cells. Extracell. Vesicles Circ. Nucleic Acids 2023, 4, 262–282. [Google Scholar] [CrossRef] [PubMed]
- Ledford, W.C.; Silvestri, A.; Fiorilli, V.; Roth, R.; Rubio-Somoza, I.; Lanfranco, L. A journey into the world of small RNAs in the arbuscular mycorrhizal symbiosis. New Phytol. 2024, 242, 1534–1544. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Hua, Z.; Han, P.; Zhao, Y.; Zhou, J.; Jin, Y.; Li, X.; Huang, L.; Yuan, Y. Mycorrhizosphere Bacteria, Rahnella sp. HPDA25, Promotes the Growth of Armillaria gallica and Its Parasitic Host Gastrodia elata. Front. Microbiol. 2022, 13, 842893. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Y.; Nan, T.; Yuan, Y. GC-MS analysis of volatile organic compounds in Gastrodia elata during Growth: Characterization of odor-active compounds associated with horse urine odor. Food Biosci. 2025, 69, 106958. [Google Scholar] [CrossRef]
- Chen, Q.; Zu, M.; Gong, H.; Ma, Y.; Sun, J.; Ran, S.; Shi, X.; Zhang, J.; Xiao, B. Tea leaf-derived exosome-like nanotherapeutics retard breast tumor growth by pro-apoptosis and microbiota modulation. J. Nanobiotechnol. 2023, 21, 6. [Google Scholar] [CrossRef] [PubMed]
- Conroy, M.J.; Andrews, R.M.; Andrews, S.; Cockayne, L.; Dennis, E.A.; Fahy, E.; Gaud, C.; Griffiths, W.J.; Jukes, G.; Kolchin, M.; et al. LIPID MAPS: Update to databases and tools for the lipidomics community. Nucleic Acids Res. 2023, 52, D1677–D1682. [Google Scholar] [CrossRef] [PubMed]
- Pang, Z.; Lu, Y.; Zhou, G.; Hui, F.; Xu, L.; Viau, C.; Spigelman, A.F.; MacDonald, P.E.; Wishart, D.S.; Li, S.; et al. MetaboAnalyst 6.0: Towards a unified platform for metabolomics data processing, analysis and interpretation. Nucleic Acids Res. 2024, 52, W398–W406. [Google Scholar] [CrossRef] [PubMed]
- Al-Akl, N.S.; Khalifa, O.; Ponirakis, G.; Parray, A.; Ramadan, M.; Khan, S.; Chandran, M.; Ayadathil, R.; Elsotouhy, A.; Own, A.; et al. Untargeted Metabolomic Profiling Reveals Differentially Expressed Serum Metabolites and Pathways in Type 2 Diabetes Patients with and without Cognitive Decline: A Cross-Sectional Study. Int. J. Mol. Sci. 2024, 25, 2247. [Google Scholar] [CrossRef]
- Li, Q.; Li, B.; Zhang, R.; Liu, S.; Yang, S.; Li, Y.; Li, J. Flavoromics Approach in Critical Aroma Compounds Exploration of Peach: Correlation to Origin Based on OAV Combined with Chemometrics. Foods 2023, 12, 837. [Google Scholar] [CrossRef]

| Model | R2X (cum) | R2Y (cum) | Q2 (cum) |
|---|---|---|---|
| IGDEV–JGDEV | 0.893 | 0.995 | 0.919 |
| IGDEV–MGDEV | 0.991 | 0.991 | 0.927 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hao, S.; Hua, Z.; Yuan, Y. Stage-Specific Lipidomes of Gastrodia elata Extracellular Vesicles Modulate Fungal Symbiosis. Int. J. Mol. Sci. 2025, 26, 8611. https://doi.org/10.3390/ijms26178611
Hao S, Hua Z, Yuan Y. Stage-Specific Lipidomes of Gastrodia elata Extracellular Vesicles Modulate Fungal Symbiosis. International Journal of Molecular Sciences. 2025; 26(17):8611. https://doi.org/10.3390/ijms26178611
Chicago/Turabian StyleHao, Siyu, Zhongyi Hua, and Yuan Yuan. 2025. "Stage-Specific Lipidomes of Gastrodia elata Extracellular Vesicles Modulate Fungal Symbiosis" International Journal of Molecular Sciences 26, no. 17: 8611. https://doi.org/10.3390/ijms26178611
APA StyleHao, S., Hua, Z., & Yuan, Y. (2025). Stage-Specific Lipidomes of Gastrodia elata Extracellular Vesicles Modulate Fungal Symbiosis. International Journal of Molecular Sciences, 26(17), 8611. https://doi.org/10.3390/ijms26178611





