Toxicity of Magnetic Nanoparticles in Medicine: Contributing Factors and Modern Assessment Methods
Abstract
1. Introduction
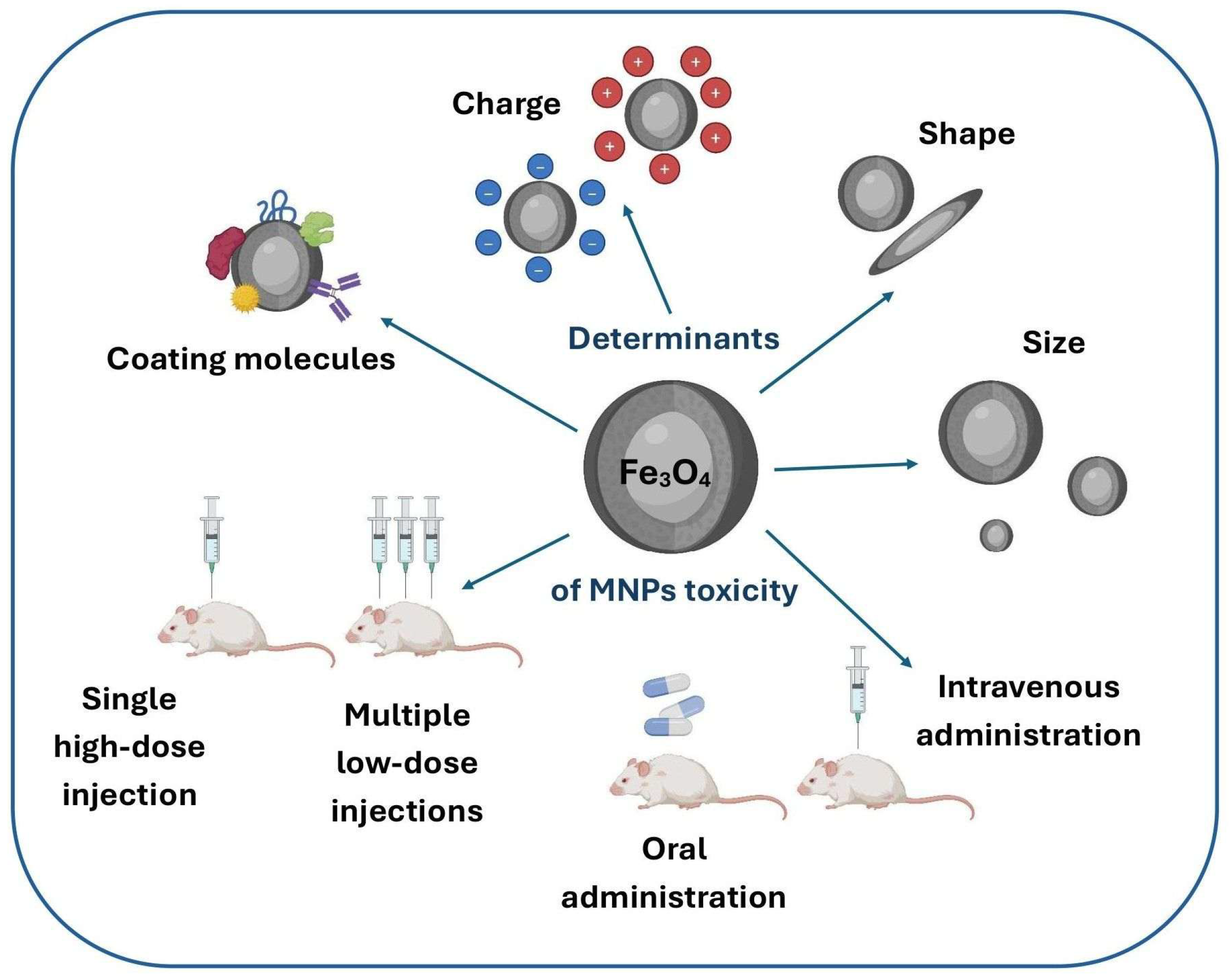
2. Factors Affecting the Potential Toxicity of MNPs
2.1. Size and Shape
2.2. Surface Chemistry and Charge
2.3. Technical Aspects
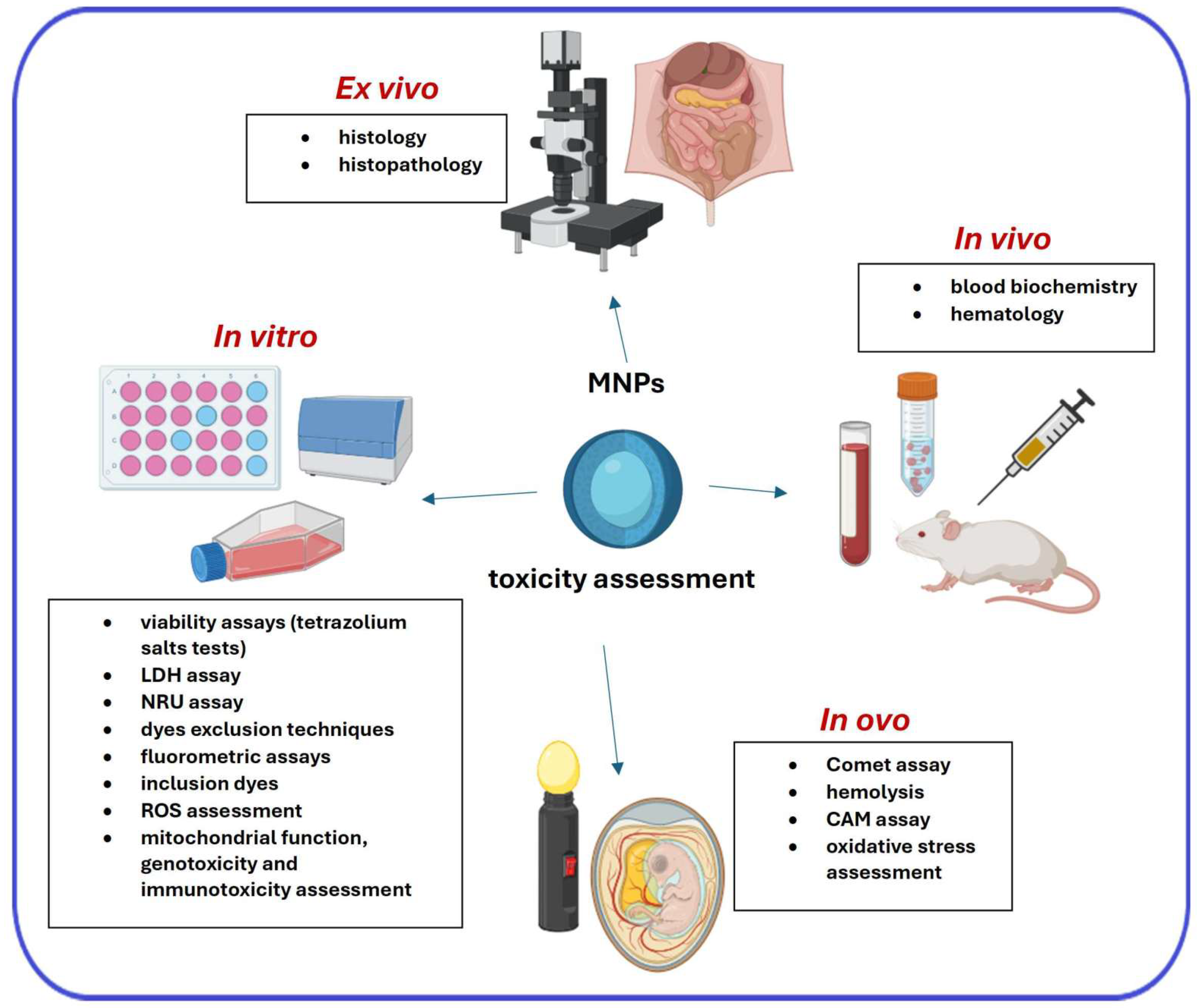
3. Primary Techniques for the Assessment of the Toxicity of MNPs
3.1. In Vitro Techniques
3.1.1. Cells Proliferation/Viability Investigation
3.1.2. Oxidative Stress Assessment
3.1.3. Genotoxicity Evaluation
3.1.4. Apoptosis Detection
3.1.5. Immunotoxicity Assessment
3.1.6. Membrane Function Detection
3.1.7. Assessing the In Vitro Toxicity of Magnetic Iron Oxide Nanoparticles: Techniques and Experimental Findings
3.2. Ex Vivo and In Vivo Techniques
4. New Approach Methods (NAMs)
4.1. Advanced 3D Models in Nanotoxicology: Organoids and Bioprinting Approaches
4.1.1. Three-Dimensional Bioprinting
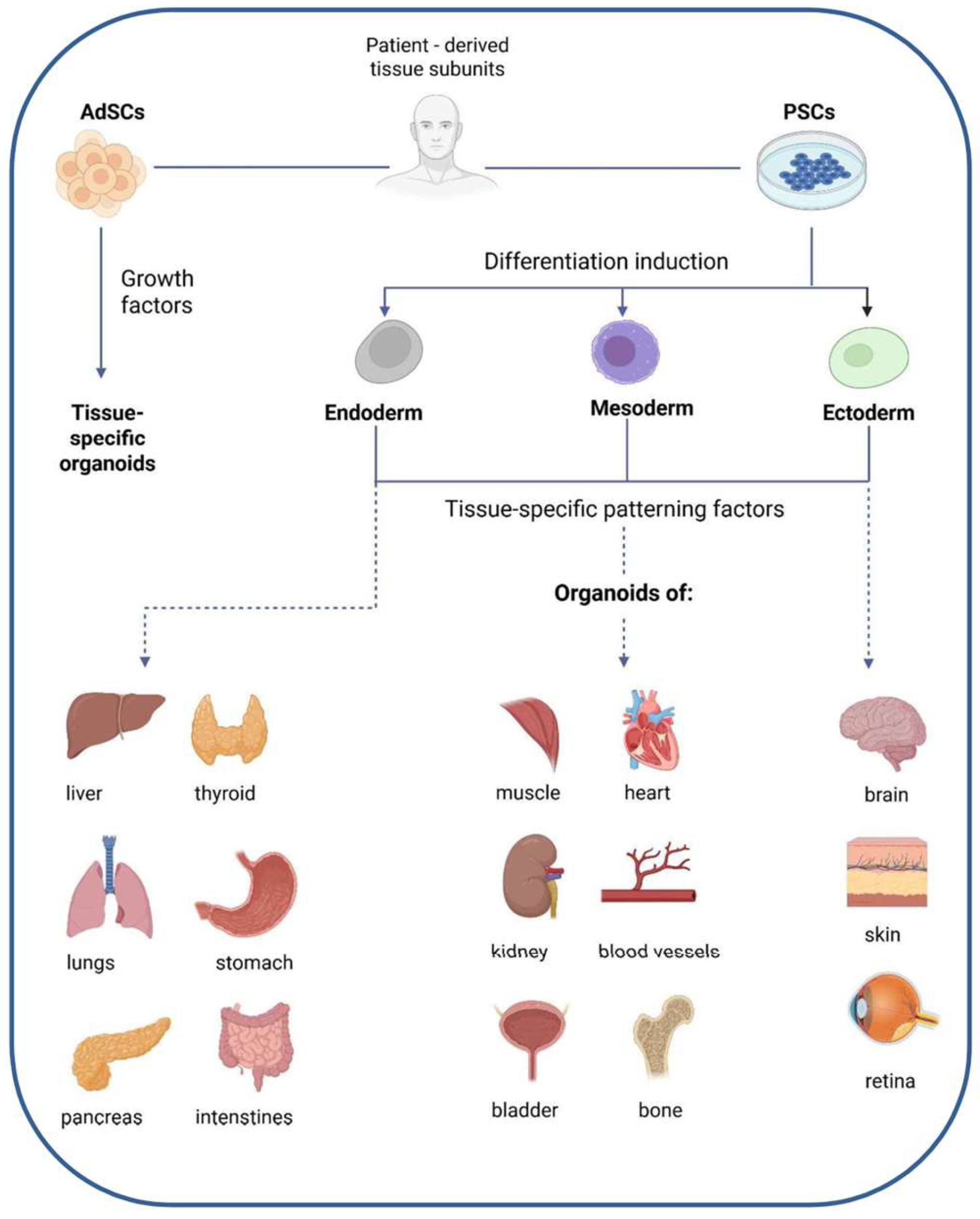
4.1.2. Organoids
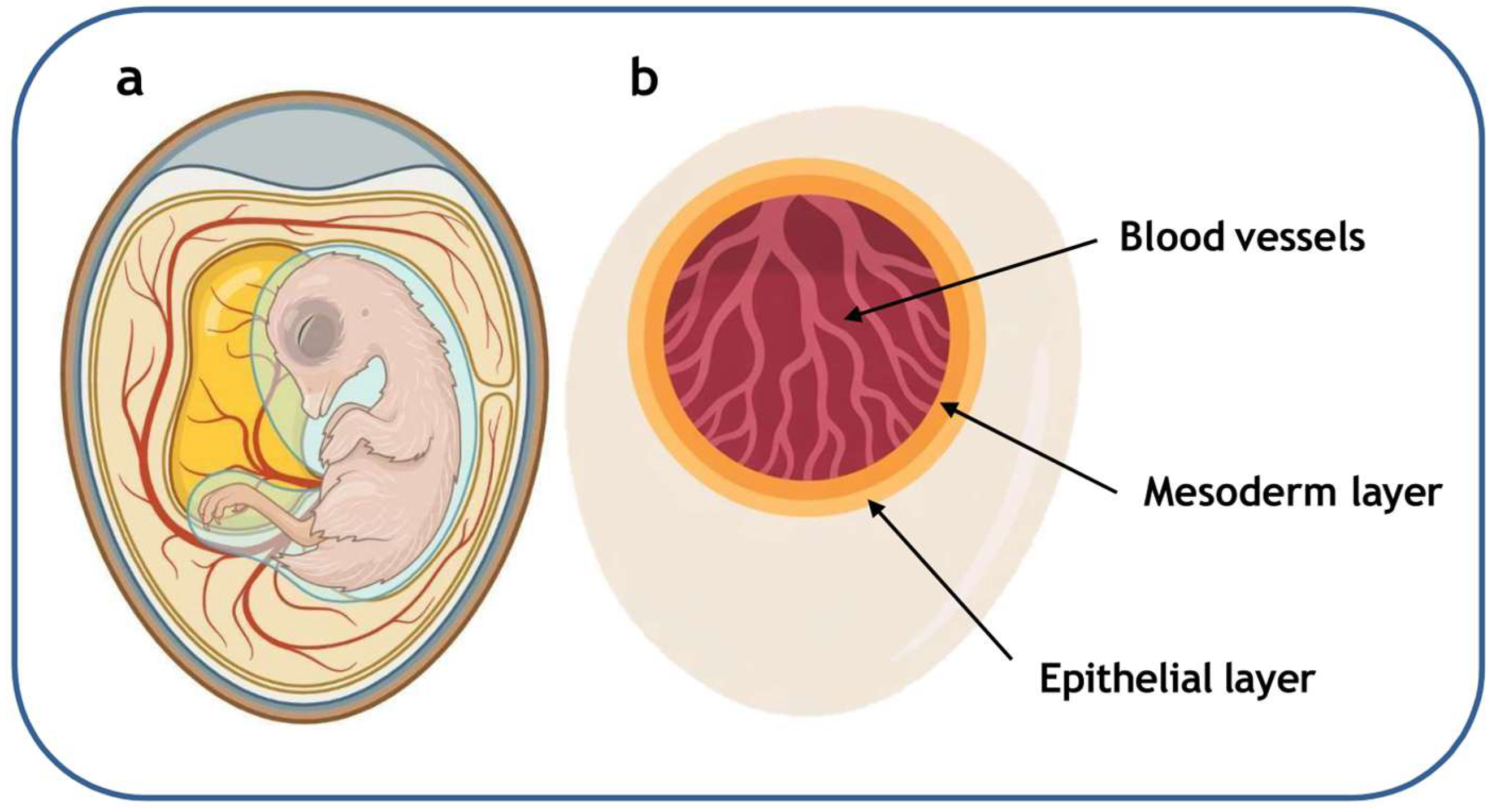
4.2. In Ovo Models
4.3. Imaging-Based Cytometry: Visualizing Nanoparticle—Cell Interactions
5. Discussion and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 5-CFDA-AM | 5-Carboxyfluorescein Diacetate Acetoxymethyl Ester (fluorescent viability dye activated by intracellular esterases) |
| 8-OHdG | 8-Hydroxy-2′-deoxyguanosine (oxidative DNA damage biomarker) |
| 8-dG | 8-deoxyguanosine (oxidative DNA lesion, precursor form measured in DNA damage studies) |
| A/G | Albumin/Globulin ratio (serum protein ratio) |
| AEAPS | N-(2-aminoethyl)-3-aminopropyl trimethoxysilane (surface coating agent for nanoparticles) |
| ALB | Albumin (major serum protein) |
| ALP | Alkaline Phosphatase (enzyme, liver/bone function marker) |
| ALT | Alanine Aminotransferase (hepatic enzyme, liver function marker) |
| AO/EB | Acridine Orange/Ethidium Bromide (dual-staining method to distinguish viable from apoptotic/necrotic cells) |
| APTMS | (3-Aminopropyl)trimethoxysilane (silane coupling agent introducing amine groups) |
| AST | Aspartate Aminotransferase (hepatic enzyme, liver function marker) |
| ATP | Adenosine Triphosphate (primary energy carrier in cells) |
| BMP6 | Bone Morphogenetic Protein 6 (regulator of systemic iron metabolism) |
| BSA | Bovine Serum Albumin (protein used as nanoparticle coating) |
| BUN | Blood Urea Nitrogen (biochemical marker of kidney function) |
| CAM assay | Chorioallantoic Membrane assay (in ovo method for testing angiogenesis, toxicity, and nanoparticle effects) |
| CCK-8 | Cell Counting Kit-8 (commercial assay kit using WST-8 for cell viability measurement) |
| CD11b | Cluster of Differentiation 11b (integrin alpha M chain, expressed on monocytes, macrophages, NK cells, granulocytes) |
| CD14 | Cluster of Differentiation 14 (co-receptor for bacterial lipopolysaccharide, monocyte/macrophage marker) |
| CD4+ | Cluster of Differentiation 4 positive (marker of helper T lymphocytes) |
| CD86 | Cluster of Differentiation 86 (co-stimulatory molecule expressed on antigen-presenting cells, involved in T-cell activation) |
| CRE | Creatinine (biochemical marker of kidney function) |
| Calcein-AM | Calcein Acetoxymethyl Ester (cell-permeant, non-fluorescent dye converted by esterases to fluorescent calcein in viable cells) |
| Ca2+-ATPase | Calcium Adenosine Triphosphatase (ATP-dependent calcium pump in membranes) |
| DBIL | Direct Bilirubin (conjugated bilirubin marker) |
| DCF | 2′,7′-Dichlorofluorescein (fluorescent oxidation product used as ROS indicator) |
| DCF assay | Dichlorofluorescein assay (fluorometric method for measuring intracellular ROS generation) |
| DCFDA | 2′,7′-Dichlorofluorescin Diacetate (fluorescent probe for intracellular ROS, also called H2DCFDA, DCFH-DA, or DCFH) |
| DCFH-DA/DCFH | 2′,7′-Dichlorodihydrofluorescein Diacetate (alternative abbreviations for DCFDA) |
| DEX | Dextran (polysaccharide used as nanoparticle coating) |
| DLP | Digital Light Processing (light-based 3D bioprinting method using projected light for layer curing) |
| DMSA | Dimercaptosuccinic Acid (chelating agent used for nanoparticle coating) |
| DMSO | Dimethyl Sulfoxide (solvent used to dissolve formazan products in MTT assay) |
| DMT1 | Divalent Metal Transporter 1 (iron transporter protein) |
| DPPC | 1,2-Dipalmitoyl-sn-glycero-3-phosphocholine (major phospholipid component of cell membranes; model bilayer system) |
| DSPE-PEG | 1,2-Distearoyl-sn-glycero-3-phosphoethanolamine-Polyethylene Glycol (lipid–PEG conjugate for nanoparticle coating) |
| Dh | Hydrodynamic diameter (effective particle size in suspension) |
| ECM | Extracellular Matrix (network of proteins and polysaccharides that provides structural and biochemical support to cells) |
| EDD | Embryonic Development Day (day of embryo development, used to standardize in ovo experiments) |
| ELISA | Enzyme-Linked Immunosorbent Assay (plate reader often used for colorimetric absorbance measurement) |
| EMA | European Medicines Agency |
| EPR/ESR | Electron Paramagnetic Resonance/Electron Spin Resonance (spectroscopic methods for detecting free radicals and NO) |
| ESR | Erythrocyte Sedimentation Rate (non-specific marker of inflammation) |
| EURL ECVAM | European Union Reference Laboratory for Alternatives to Animal Testing/European Centre for the Validation of Alternative Methods |
| FACS | Fluorescence-Activated Cell Sorting (method for multiparametric single-cell analysis and sorting) |
| FDA | U.S. Food and Drug Administration |
| FITC | Fluorescein Isothiocyanate (fluorescent dye commonly used for labeling biomolecules, here dextran-coated MNPs) |
| FMR | Ferromagnetic Resonance (spectroscopic technique for detecting magnetic nanoparticle distribution in tissues) |
| G6PD | Glucose-6-Phosphate Dehydrogenase (enzyme supporting antioxidant defenses via NADPH regeneration) |
| GGT | Gamma-Glutamyl Transferase (enzyme, liver function marker) |
| GLB | Globulin (serum protein fraction) |
| GMNPs | GoldMag Nanoparticles (superparamagnetic core/shell nanoparticles with Fe3O4 core and gold shell) |
| GPx | Glutathione Peroxidase (antioxidant enzyme) |
| GR | Glutathione Reductase (enzyme regenerating reduced glutathione) |
| GelMA | Gelatin Methacryloyl (biocompatible hydrogel commonly used as bioink in tissue engineering) |
| H&E | Hematoxylin and Eosin staining (routine histological staining method) |
| HA | Hyaluronic Acid (glycosaminoglycan used for nanoparticle coating/targeting) |
| HEC | Hydroxyethyl Cellulose (cationic biopolymer used for nanoparticle functionalization) |
| HPLC | High-Performance Liquid Chromatography (analytical separation technique) |
| H2DCFDA | 2′,7′-Dichlorodihydrofluorescein Diacetate (reduced form of DCFDA) |
| IFC | Imaging Flow Cytometry (hybrid technique combining flow cytometry with high-resolution microscopy for single-cell imaging and quantitative analysis) |
| IL-6 | Interleukin-6 |
| INT | 2-(4-Iodophenyl)-3-(4-nitrophenyl)-5-phenyl-2H-tetrazolium (iodonitrotetrazolium, tetrazolium salt used in LDH assays) |
| ISC machinery | Iron–Sulfur Cluster assembly machinery (system for Fe–S cluster biogenesis) |
| LDH | Lactate Dehydrogenase (enzyme, cytotoxicity/necrosis marker) |
| LD50 | Median Lethal Dose (dose required to kill 50% of a test population) |
| LIP | Labile Iron Pool (cytosolic pool of weakly bound iron ions) |
| LPA | Lymphocyte Proliferation Assay (in vitro immunotoxicity test measuring clonal expansion of lymphocytes) |
| MMP | Mitochondrial Membrane Potential (indicator of mitochondrial integrity and function) |
| MNP(s) | Magnetic Nanoparticle(s) |
| MPI | Magnetic Particle Imaging |
| MPT | Mitochondrial Permeability Transition (process involving opening of the mitochondrial permeability transition pore) |
| MRI | Magnetic Resonance Imaging |
| MTS | 3-(4,5-Dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (tetrazolium compound used in colorimetric assays) |
| MTT | 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (tetrazolium salt used in cell viability assays) |
| Mg2+-ATPase | Magnesium Adenosine Triphosphatase (ATP-dependent magnesium transport enzyme) |
| MitoSOX™ Red (Mito-HE) | Mitochondria-targeted hydroethidine probe (dihydroethidium derivative conjugated with triphenylphosphonium cation, specific for mitochondrial superoxide detection) |
| NADH | Nicotinamide Adenine Dinucleotide (reduced form; electron donor in metabolic reactions) |
| NADPH | Nicotinamide Adenine Dinucleotide Phosphate (reduced form; cofactor in biosynthetic reactions) |
| NAD+ | Nicotinamide Adenine Dinucleotide (oxidized form) |
| NAM(s) | New Approach Methodologies |
| NCC | Nanocrystalline Cellulose (anionic biopolymer, nanoparticle coating material) |
| NCOA4 | Nuclear Receptor Coactivator 4 (mediator of ferritinophagy) |
| NRU | Neutral Red Uptake assay (colorimetric cytotoxicity assay measuring lysosomal activity) |
| Na+/K+-ATPase | Sodium-Potassium Adenosine Triphosphatase (membrane ion pump maintaining Na+/K+ gradients) |
| OECD | Organization for Economic Co-operation and Development |
| PDI | Polydispersity Index (measure of particle size distribution uniformity, obtained by DLS) |
| PEG | Polyethylene Glycol (polyether polymer used for nanoparticle surface modification) |
| PEI | Polyethylenimine (cationic polymer used for nanoparticle coating/transfection) |
| PES | Phenazine Ethosulfate (alternative electron coupling reagent in tetrazolium assays) |
| PET | Positron Emission Tomography |
| PI | Propidium Iodide (fluorescent dye binding nucleic acids in non-viable cells) |
| PLGA | Poly(lactic-co-glycolic acid) (biodegradable copolymer used for nanoparticle coatings) |
| PMN | Polymorphonuclear leukocytes (granulocytes, a subtype of white blood cells) |
| PMS | Phenazine Methosulfate (electron coupling reagent in tetrazolium assays) |
| PT pore | Permeability Transition pore (non-specific mitochondrial pore enabling solute passage < 1500 Da) |
| PVA | Poly(vinyl alcohol) (synthetic polymer used as nanoparticle coating) |
| PVP | Poly(vinylpyrrolidone) (synthetic polymer used as stabilizer and nanoparticle coating) |
| Pi | Inorganic Phosphate (direct product of ATP hydrolysis, measured colorimetrically in ATPase activity assays) |
| RBCs | Red Blood Cells (erythrocytes) |
| RNA-seq | RNA sequencing (high-throughput transcriptomic profiling technique) |
| RNS | Reactive Nitrogen Species |
| ROS | Reactive Oxygen Species |
| RT-CES | Real-Time Cell Electronic Sensing (impedance-based cytotoxicity assay) |
| SCGE | Single-Cell Gel Electrophoresis (formal name of the comet assay, DNA damage detection method) |
| SEM | Scanning Electron Microscopy |
| SIM | Structured Illumination Microscopy (super-resolution fluorescence microscopy technique) |
| SLA | Stereolithography (light-based 3D bioprinting method using photopolymerization) |
| SMAD | SMAD family proteins (signal transducers in BMP/TGF-β pathways) |
| SMART | Somatic Mutation and Recombination Test |
| SOD | Superoxide Dismutase (antioxidant enzyme) |
| SOPs | Standard Operating Procedures (standardized, detailed instructions ensuring reproducibility and consistency in experimental protocols) |
| SPECT | Single Photon Emission Computed Tomography |
| STEAP3 | Six-Transmembrane Epithelial Antigen of Prostate 3 (lysosomal ferrireductase enzyme) |
| TBIL | Total Bilirubin (serum bilirubin marker) |
| TEM | Transmission Electron Microscopy |
| TEOS | Tetraethyl Orthosilicate (silica precursor used for hydroxyl surface functionalization) |
| TP | Total Protein (serum protein marker) |
| TRPML1/MCOLN1 | Transient Receptor Potential Mucolipin 1 (endolysosomal ion channel protein) |
| TUNEL | Terminal deoxynucleotidyl transferase dUTP Nick End Labeling (assay for detecting single- and double-stranded DNA breaks) |
| UA | Uric Acid (serum biochemical marker) |
| WBC | White Blood Cell count |
| WST | Water-Soluble Tetrazolium (family of tetrazolium salts used in viability assays) |
| WST-1 | 2-(4-Iodophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium (mitochondrial activity-based viability assay reagent) |
| WST-8 | 2-(2-Methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium (high-sensitivity tetrazolium salt for viability assays) |
| XTT | 2,3-Bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide (water-soluble tetrazolium salt used for viability assays) |
| cGMP | current Good Manufacturing Practice |
| qRT-PCR | Quantitative Reverse Transcription Polymerase Chain Reaction (technique for quantifying mRNA expression) |
| 3H-thymidine | Tritiated Thymidine (radioactive thymidine analog used to measure DNA synthesis and cell proliferation/apoptosis) |
| ζ (zeta potential) | Zeta Potential (indicator of surface charge and colloidal stability of nanoparticles) |
| Cell Lines and Biological Models | |
| A172 | Human glioblastoma cell line, model for brain tumor research and drug sensitivity testing |
| A549 | Human lung adenocarcinoma epithelial cell line |
| AdSCs | Adult Stem Cells (tissue-specific stem cells with limited differentiation potential) |
| Balb/c mice | Inbred mouse strain, frequently used in immunological and toxicological studies |
| C57BL/6 mice | Inbred mouse strain, most commonly used model organism in immunology, oncology, and metabolism research |
| CE | Chicken Embryo (experimental model used in in ovo studies with CAM) |
| DCs (monocyte-derived dendritic cells) | Human dendritic cells differentiated from monocytes, model for immune function and immunotoxicity |
| Fibrosarcoma cells | Human fibrosarcoma cell line (malignant connective tissue–derived) |
| HEK293 | Human embryonic kidney cell line, widely used as a model for transfection, gene expression, and toxicology assays |
| HeLa | Human cervical cancer cell line, classical model in oncology, virology, and cytotoxicity testing |
| Hep3B | Human hepatocellular carcinoma cell line |
| HepG2 | Human hepatocellular carcinoma cell line, model for hepatocyte metabolism, drug toxicity, and genotoxicity studies |
| hFOB | Human Fetal Osteoblast (cell line derived from human fetal bone tissue) |
| Human aortic endothelial cells | Primary human endothelial cells derived from the aorta |
| Human coronary artery endothelial cells | Primary human endothelial cells derived from coronary artery |
| Human monocyte-derived dendritic cells | Dendritic cells differentiated in vitro from primary human monocytes |
| Human skin fibroblasts | Primary or immortalized fibroblast cells derived from human skin |
| Human umbilical vein endothelial cells (HUVECs) | Primary human endothelial cells derived from umbilical vein |
| HUVECs | Human Umbilical Vein Endothelial Cells |
| L929 | Mouse fibroblast cell line, widely used in cytotoxicity and biocompatibility assays |
| LNCaP | Human prostate cancer cell line (lymph node metastasis of prostate adenocarcinoma), androgen-sensitive model |
| MCF-7 | Human breast cancer adenocarcinoma cell line, estrogen receptor–positive model for breast cancer studies |
| NIH3T3 | Mouse embryonic fibroblast cell line, commonly used as a model for fibroblast proliferation and transformation |
| Normal fibroblasts | Primary or immortalized fibroblast cells (normal human connective tissue–derived fibroblasts) |
| Normal fibroblasts | Human fibroblast cell line (non-transformed connective tissue cells) |
| Pancreatic islet organoids | 3D in vitro organoid model derived from pancreatic islet cells, used to study insulin secretion and metabolic function |
| Patient-derived pancreatic ductal adenocarcinoma organoids | Organoids cultured from human pancreatic ductal adenocarcinoma tissue, preserving tumor-specific features |
| Primary rat hepatocytes | Primary liver parenchymal cells isolated from rat |
| PSCs | Pluripotent Stem Cells (stem cells capable of differentiating into all three germ layers: endoderm, mesoderm, ectoderm) |
| SH-SY5Y | Human neuroblastoma cell line, model for neuronal differentiation and neurotoxicity |
| SHR (Spontaneously Hypertensive Rats) | Rat model of essential hypertension |
| SK-Hep-1 | Human liver adenocarcinoma cell line (often used as hepatoma model) |
| THP-1 | Human monocytic leukemia cell line, widely used to study monocyte/macrophage biology, immune activation, and nanoparticle uptake |
| U251 | Human glioblastoma cell line |
| WKY (Wistar–Kyoto) rats | Normotensive rat strain, standard control in cardiovascular research |
| Zebrafish embryos | Widely used vertebrate model in developmental biology and nanotoxicology, transparent and genetically tractable |
References
- Stanicki, D.; Vangijzegem, T.; Ternad, I.; Laurent, S. An update on the applications and characteristics of magnetic iron oxide nanoparticles for drug delivery. Expert Opin. Drug Deliv. 2023, 19, 321–335. [Google Scholar] [CrossRef]
- Avval, Z.M.; Malekpour, L.; Raeisi, F.; Babapoor, A.; Mousavi, S.M.; Hashemi, S.A.; Salari, M. Introduction of magnetic and supermagnetic nanoparticles in new approach of targeting drug delivery and cancer therapy application. Drug Metab. Rev. 2020, 52, 157–184. [Google Scholar] [CrossRef]
- Wang, X.; Bai, R. Advances in smart delivery of magnetic field-targeted drugs in cardiovascular diseases. Drug Deliv. 2023, 30, 2256495. [Google Scholar] [CrossRef] [PubMed]
- Ulbrich, K.; Holá, K.; Šubr, V.; Bakandritsos, A.; Tuček, J.; Zbořil, R. Targeted drug delivery with polymers and magnetic nanoparticles: Covalent and noncovalent approaches, release control, and clinical studies. Chem. Rev. 2016, 116, 5338–5431. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Sherwood, J.A.; Sun, Z. Magnetic iron oxide nanoparticles as T1 c ontrast agents for magnetic resonance imaging. J. Mater. Chem. C 2018, 6, 1280–1290. [Google Scholar] [CrossRef]
- Palzer, J.; Eckstein, L.; Slabu, I.; Reisen, O.; Neumann, U.P.; Roeth, A.A. Iron oxide nanoparticle-based hyperthermia as a treatment option in various gastrointestinal malignancies. Nanomaterials 2021, 11, 3013. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M. Magnetic resonance imaging and iron-oxide nanoparticles in the era of personalized medicine. Nanotheranostics 2023, 7, 424–449. [Google Scholar] [CrossRef]
- Mahmoudi, K.; Bouras, A.; Bozec, D.; Ivkov, R.; Hadjipanayis, C. Magnetic hyperthermia therapy for the treatment of glioblastoma: A review of the therapy’s history, efficacy and application in humans. Int. J. Hyperth. 2018, 34, 1316–1328. [Google Scholar] [CrossRef]
- Mu, X.; Li, J.; Yan, S.; Zhang, H.; Zhang, W.; Zhang, F.; Jiang, J. siRNA delivery with stem cell membrane-coated magnetic nanoparticles for imaging-guided photothermal therapy and gene therapy. ACS Biomater. Sci. Eng. 2018, 4, 3895–3905. [Google Scholar] [CrossRef]
- Russell, E.; Dunne, V.; Russell, B.; Mohamud, H.; Ghita, M.; McMahon, S.J.; Butterworth, K.T.; Schettino, G.; Mc Garry, C.K.; Prise, K.M. Impact of superparamagnetic iron oxide nanoparticles on in vitro and in vivo radiosensitisation of cancer cells. Radiat. Oncol. 2021, 16, 104. [Google Scholar] [CrossRef]
- Sizikov, A.A.; Nikitin, P.I.; Nikitin, M.P. Magnetofection in vivo by nanomagnetic carriers systemically administered into the bloodstream. Pharmaceutics 2021, 13, 1927. [Google Scholar] [CrossRef] [PubMed]
- Pellico, J.; Gawne, P.J.; De Rosales, R.T.M. Radiolabelling of nanomaterials for medical imaging and therapy. Chem. Soc. Rev. 2021, 50, 3355–3423. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, G.R.; López-Abarrategui, C.; De La Serna Gómez, I.; Dias, S.C.; Otero-González, A.J.; Franco, O.L. Antimicrobial magnetic nanoparticle-based therapies for controlling infectious diseases. Int. J. Pharm. 2019, 555, 356–367. [Google Scholar] [CrossRef] [PubMed]
- Velazquez-Albino, A.C.; Imhoff, E.D.; Rinaldi-Ramos, C.M. Advances in engineering nanoparticles for magnetic particle imaging (MPI). Sci. Adv. 2025, 11, eado7356. [Google Scholar] [CrossRef]
- Li, Z.; Xue, L.; Wang, P.; Ren, X.; Zhang, Y.; Wang, C.; Sun, J. Biological scaffolds assembled with magnetic nanoparticles for bone tissue engineering: A review. Materials 2023, 16, 1429. [Google Scholar] [CrossRef]
- Carvalho, T.S.S.; Torres, P.M.C.; Belo, J.H.; Mano, J.; Olhero, S.M. Bioactive magnetic materials in bone tissue engineering: A review of recent findings in CaP-based particles and 3D-printed scaffolds. Adv. NanoBiomed Res. 2023, 3, 2300035. [Google Scholar] [CrossRef]
- Bauso, L.V.; La Fauci, V.; Longo, C.; Calabrese, G. Bone tissue engineering and nanotechnology: A promising combination for bone regeneration. Biology 2024, 13, 237. [Google Scholar] [CrossRef]
- Waalders, N.; Van Lier, D.; Gerretsen, J.; Moran, L.; Stegmann, K.A.; Twigger, W.; Blanco-Andujar, C.; Frodsham, G.; Kox, M.; Pickkers, P. Preclinical and first-in-human safety studies on a novel magnetism-based haemofiltration method. Sci. Rep. 2024, 14, 14077. [Google Scholar] [CrossRef]
- Wallace, D.F. The regulation of iron absorption and homeostasis. Clin. Biochem. Rev. 2016, 37, 51–62. [Google Scholar]
- Meng, F.; Fleming, B.A.; Jia, X.; Rousek, A.A.; Mulvey, M.A.; Ward, D.M. Lysosomal iron recycling in mouse macrophages is dependent upon both LcytB and Steap3 reductases. Blood Adv. 2022, 6, 1692–1707. [Google Scholar] [CrossRef]
- Yambire, K.F.; Rostosky, C.; Watanabe, T.; Pacheu-Grau, D.; Torres-Odio, S.; Sanchez-Guerrero, A.; Sendorovich, O.; Meyron-Holtz, E.G.; Milosevic, J.; Frahm, J.; et al. Impaired lysosomal acidification triggers iron deficiency and inflammation in vivo. eLife 2019, 8, e51031. [Google Scholar] [CrossRef]
- Kautz, L.; Jung, G.; Valore, E.V.; Rivella, S.; Nemeth, E.; Ganz, T. Identification of erythroferrone as an erythroid regulator of iron metabolism. Nat. Genet. 2014, 46, 678–684. [Google Scholar] [CrossRef]
- Arosio, P.; Cairo, G.; Bou-Abdallah, F. A brief history of ferritin, an ancient and versatile protein. Int. J. Mol. Sci. 2024, 26, 206. [Google Scholar] [CrossRef]
- Bou-Abdallah, F. The iron redox and hydrolysis chemistry of the ferritins. Biochim. Biophys. Acta Gen. Subj. 2010, 1800, 719–731. [Google Scholar] [CrossRef] [PubMed]
- Obi, C.D.; Bhuiyan, T.; Dailey, H.A.; Medlock, A.E. Ferrochelatase: Mapping the intersection of iron and porphyrin metabolism in the mitochondria. Front. Cell Dev. Biol. 2022, 10, 894591. [Google Scholar] [CrossRef] [PubMed]
- Maio, N.; Jain, A.; Rouault, T.A. Mammalian iron–sulfur cluster biogenesis: Recent insights into the roles of frataxin, acyl carrier protein and ATPase-mediated transfer to recipient proteins. Curr. Opin. Chem. Biol. 2020, 55, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Pearson, S.A.; Cowan, J.A. Evolution of the human mitochondrial ABCB7 2Fe–2S4 cluster exporter and the molecular mechanism of an E433K disease-causing mutation. Arch. Biochem. Biophys. 2021, 697, 108661. [Google Scholar] [CrossRef]
- Li, P.; Hendricks, A.L.; Wang, Y.; Villones, R.L.E.; Lindkvist-Petersson, K.; Meloni, G.; Cowan, J.A.; Wang, K.; Gourdon, P. Structures of Atm1 provide insight into [2Fe-2S] cluster export from mitochondria. Nat. Commun. 2022, 13, 4339. [Google Scholar] [CrossRef]
- White, C.; Yuan, X.; Schmidt, P.J.; Bresciani, E.; Samuel, T.K.; Campagna, D.; Hal, C.; Bishop, K.; Calicchio, M.L.; Lapierre, A.; et al. HRG1 is essential for heme transport from the phagolysosome of macrophages during erythrophagocytosis. Cell Metab. 2013, 17, 261–270. [Google Scholar] [CrossRef]
- Helman, S.L.; Zhou, J.; Fuqua, B.K.; Lu, Y.; Collins, J.F.; Chen, H.; Vulpe, C.D.; Anderson, G.J.; Frazer, D.M. The biology of mammalian multi-copper ferroxidases. BioMetals 2023, 36, 263–281. [Google Scholar] [CrossRef]
- Wang, J.; Wu, N.; Peng, M.; Oyang, L.; Jiang, X.; Peng, Q.; Zhou, Y.; He, Z.; Liao, Q. Ferritinophagy: Research advance and clinical significance in cancers. Cell Death Discov. 2023, 9, 463. [Google Scholar] [CrossRef]
- Colucci, S.; Altamura, S.; Marques, O.; Müdder, K.; Agarvas, A.R.; Hentze, M.W.; Muckenthaler, M.U. Iron-dependent BMP6 regulation in liver sinusoidal endothelial cells is instructed by hepatocyte-derived secretory signals. HemaSphere 2022, 6, e773. [Google Scholar] [CrossRef]
- Camaschella, C.; Nai, A.; Silvestri, L. Iron metabolism and iron disorders revisited in the hepcidin era. Haematologica 2020, 105, 260–272. [Google Scholar] [CrossRef]
- El-Boubbou, K. Magnetic iron oxide nanoparticles as drug carriers: Clinical relevance. Nanomedicine 2018, 13, 953–971. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration (FDA). Considerations for Drug Products That Contain Nanomaterials. 2022. Available online: https://www.fda.gov/drugs/cder-small-business-industry-assistance-sbia/considerations-drug-products-contain-nanomaterials (accessed on 23 July 2025).
- European Medicines Agency (EMA). Nanotechnology-Based Medicinal Products for Human Use: EU-IN Horizon Scanning Report. 2020. Available online: https://www.ema.europa.eu/en/documents/report/nanotechnology-based-medicinal-products-human-use-eu-horizon-scanning-report_en.pdf (accessed on 23 July 2025).
- Weissleder, R.; Stark, D.; Engelstad, B.; Bacon, B.; Compton, C.; White, D.; Jacobs, P.; Lewis, J. Superparamagnetic iron oxide: Pharmacokinetics and toxicity. Am. J. Roentgenol. 1989, 152, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Bulte, J.W.M.; Kraitchman, D.L. Iron oxide MR contrast agents for molecular and cellular imaging. NMR Biomed. 2004, 17, 484–499. [Google Scholar] [CrossRef] [PubMed]
- McCullough, B.J.; Kolokythas, O.; Maki, J.H.; Green, D.E. Ferumoxytol in clinical practice: Implications for MRI. J. Magn. Reson. Imaging 2013, 37, 1476–1479. [Google Scholar] [CrossRef]
- Maier-Hauff, K.; Ulrich, F.; Nestler, D.; Niehoff, H.; Wust, P.; Thiesen, B.; Orawa, H.; Budach, V.; Jordan, A. Efficacy and safety of intratumoral thermotherapy using magnetic iron-oxide nanoparticles combined with external beam radiotherapy on patients with recurrent glioblastoma multiforme. J. Neurooncol. 2011, 103, 317–324. [Google Scholar] [CrossRef]
- Johannsen, M.; Gneveckow, U.; Thiesen, B.; Taymoorian, K.; Cho, C.H.; Waldöfner, N.; Sholtz, R.; Jordan, A.; Loening, S.A.; Wust, P. Thermotherapy of prostate cancer using magnetic nanoparticles: Feasibility, imaging, and three-dimensional temperature distribution. Eur. Urol. 2007, 52, 1653–1662. [Google Scholar] [CrossRef]
- Đorđević, S.; Gonzalez, M.M.; Conejos-Sánchez, I.; Carreira, B.; Pozzi, S.; Acúrcio, R.C.; Satchi-Fainaro, R.; Florindo, H.F.; Vicent, M.J. Current hurdles to the translation of nanomedicines from bench to the clinic. Drug Deliv. Transl. Res. 2022, 12, 500–525. [Google Scholar] [CrossRef]
- Mai, T.; Hilt, J.Z. Magnetic nanoparticles: Reactive oxygen species generation and potential therapeutic applications. J. Nanopart Res. 2017, 19, 253. [Google Scholar] [CrossRef]
- Abakumov, M.A.; Semkina, A.S.; Skorikov, A.S.; Vishnevskiy, D.A.; Ivanova, A.V.; Mironova, E.; Davydova, G.A.; Majouga, A.G.; Chekhonin, V.P. Toxicity of iron oxide nanoparticles: Size and coating effects. J. Biochem. Mol. Toxicol. 2018, 32, e22225. [Google Scholar] [CrossRef] [PubMed]
- Nowak-Jary, J.; Machnicka, B. In vivo biodistribution and clearance of magnetic iron oxide nanoparticles for medical applications. Int. J. Nanomed. 2023, 18, 4067–4100. [Google Scholar] [CrossRef] [PubMed]
- Frantellizzi, V.; Conte, M.; Pontico, M.; Pani, A.; Pani, R.; De Vincentis, G. New frontiers in molecular imaging with superparamagnetic iron oxide nanoparticles (SPIONs): Efficacy, toxicity, and future applications. Nucl. Med. Mol. Imaging 2020, 54, 65–80. [Google Scholar] [CrossRef]
- Yang, L.; Kuang, H.; Zhang, W.; Aguilar, Z.P.; Xiong, Y.; Lai, W.; Xu, H.; Wei, H. Size dependent biodistribution and toxicokinetics of iron oxide magnetic nanoparticles in mice. Nanoscale 2015, 7, 625–636. [Google Scholar] [CrossRef]
- Li, J.; Yuan, Z.; Liu, H.; Feng, J.; Chen, Z. Size-dependent tissue-specific biological effects of core–shell structured Fe3O4@SiO2–NH2 nanoparticles. J. Nanobiotechnol. 2019, 17, 124. [Google Scholar] [CrossRef]
- Wu, L.; Wen, W.; Wang, X.; Huang, D.; Cao, J.; Qi, X.; Shen, S. Ultrasmall iron oxide nanoparticles cause significant toxicity by specifically inducing acute oxidative stress to multiple organs. Part. Fibre Toxicol. 2022, 19, 24. [Google Scholar] [CrossRef]
- Xie, Y.; Liu, D.; Cai, C.; Chen, X.; Zhou, Y.; Wu, L.; Sun, Y.; Dai, H.; Kong, X.; Liu, P. Size-dependent cytotoxicity of Fe3O4 nanoparticles induced by biphasic regulation of oxidative stress in different human hepatoma cells. Int. J. Nanomed. 2016, 11, 3557–3570. [Google Scholar]
- Kaygisiz, Ş.Y.; Ciğerci, İ.H. Genotoxic evaluation of different sizes of iron oxide nanoparticles and ionic form by SMART, Allium and comet assay. Toxicol. Ind. Health 2017, 33, 802–809. [Google Scholar] [CrossRef]
- Lee, J.H.; Ju, J.E.; Kim, B.I.; Pak, P.J.; Choi, E.; Lee, H.; Chung, N. Rod-shaped iron oxide nanoparticles are more toxic than sphere-shaped nanoparticles to murine macrophage cells. Environ. Toxicol. Chem. 2014, 33, 2759–2766. [Google Scholar] [CrossRef]
- Hu, B.; Zeng, M.; Chen, J.; Zhang, Z.; Zhang, X.; Fan, Z.; Zhang, X. External magnetic field-induced targeted delivery of highly sensitive iron oxide nanocubes for MRI of myocardial infarction. Small 2016, 12, 4707–4712. [Google Scholar] [CrossRef] [PubMed]
- Hossaini Nasr, S.; Tonson, A.; El-Dakdouki, M.H.; Zhu, D.C.; Agnew, D.; Wiseman, R.; Qian, C.; Huang, X. Effects of nanoprobe morphology on cellular binding and inflammatory responses: Hyaluronan-conjugated magnetic nanoworms for magnetic resonance imaging of atherosclerotic plaques. ACS Appl. Mater. Interfaces 2018, 10, 11495–11507. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, M.; Simchi, A.; Milani, A.S.; Stroeve, P. Cell toxicity of superparamagnetic iron oxide nanoparticles. J. Colloid Interface Sci. 2009, 336, 510–518. [Google Scholar] [CrossRef]
- Bourquin, J.; Milosevic, A.; Hauser, D.; Lehner, R.; Blank, F.; Petri-Fink, A.; Rothen-Rutishauser, B. Biodistribution, clearance, and long-term fate of clinically relevant nanomaterials. Adv. Mater. 2018, 30, 1704307. [Google Scholar] [CrossRef]
- Turrina, C.; Klassen, A.; Milani, D.; Rojas-González, D.M.; Ledinski, G.; Auer, D.; Sartori, B.; Cvirn, G.; Mela, P.; Berensmeier, S.; et al. Superparamagnetic iron oxide nanoparticles for their application in the human body: Influence of the surface. Heliyon 2023, 9, e16487. [Google Scholar] [CrossRef]
- Rabel, M.; Warncke, P.; Grüttner, C.; Bergemann, C.; Kurland, H.D.; Müller, R.; Dungandžić, V.; Thamm, J.; Müller, F.A.; Popp, J.; et al. Simulation of the long-term fate of superparamagnetic iron oxide-based nanoparticles using simulated biological fluids. Nanomedicine 2019, 14, 1681–1706. [Google Scholar] [CrossRef]
- Ajdary, M.; Keyhanfar, F.; Moosavi, M.A.; Shabani, R.; Mehdizadeh, M.; Varma, R.S. Potential toxicity of nanoparticles on the reproductive system in animal models: A review. J. Reprod. Immunol. 2021, 148, 103384. [Google Scholar] [CrossRef]
- Zavisova, V.; Koneracka, M.; Gabelova, A.; Svitkova, B.; Ursinyova, M.; Kubovcikova, M.; Antal, I.; Khmara, I.; Jurikova, A.; Molcan, M.; et al. Effect of magnetic nanoparticles coating on cell proliferation and uptake. J. Magn. Magn. Mater. 2019, 472, 66–73. [Google Scholar] [CrossRef]
- Bekaroğlu, M.G.; Alemdar, A.; İşçi, S. Comparison of ionic polymers in the targeted drug delivery applications as the coating materials on the Fe3O4 nanoparticles. Mater. Sci. Eng. C 2019, 103, 109838. [Google Scholar] [CrossRef]
- Zheng, M.; Lu, J.; Zhao, D. Effects of starch-coating of magnetite nanoparticles on cellular uptake, toxicity and gene expression profiles in adult zebrafish. Sci. Total Environ. 2018, 622–623, 930–941. [Google Scholar] [CrossRef]
- Liu, Y.; Xia, Q.; Liu, Y.; Zhang, S.; Cheng, F.; Zhong, Z.; Wang, L.; Li, H.; Xiao, K. Genotoxicity assessment of magnetic iron oxide nanoparticles with different particle sizes and surface coatings. Nanotechnology 2014, 25, 425101. [Google Scholar] [CrossRef]
- Lazaratos, M.; Karathanou, K.; Mainas, E.; Chatzigoulas, A.; Pippa, N.; Demetzos, C.; Cournia, Z. Coating of magnetic nanoparticles affects their interactions with model cell membranes. Biochim. Biophys. Acta Gen. Subj. 2020, 1864, 129671. [Google Scholar] [CrossRef]
- Yang, W.; Lee, J.; Hong, S.; Lee, J.; Lee, J.; Han, D.W. Difference between toxicities of iron oxide magnetic nanoparticles with various surface-functional groups against human normal fibroblasts and fibrosarcoma cells. Materials 2013, 6, 4689–4706. [Google Scholar] [CrossRef]
- Blank, F.; Gerber, P.; Rothen-Rutishauser, B.; Sakulkhu, U.; Salaklang, J.; De Peyer, K.; Gehr, P.; Nicod, L.P.; Hofmann, H.; Geiser, T.; et al. Biomedical nanoparticles modulate specific CD4+ T cell stimulation by inhibition of antigen processing in dendritic cells. Nanotoxicology 2011, 5, 606–621. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, S.; De Haan, L.H.; Evers, N.M.; Jiang, X.; Marcelis, A.T.; Zuilhof, H.; Rietjens, J.M.C.M.; Alink, G.M. Role of surface charge and oxidative stress in cytotoxicity of organic monolayer-coated silicon nanoparticles towards macrophage NR8383 cells. Part. Fibre Toxicol. 2010, 7, 25. [Google Scholar] [CrossRef] [PubMed]
- Gokduman, K.; Bestepe, F.; Li, L.; Yarmush, M.L.; Usta, O.B. Dose-, treatment- and time-dependent toxicity of superparamagnetic iron oxide nanoparticles on primary rat hepatocytes. Nanomedicine 2018, 13, 1267–1284. [Google Scholar] [CrossRef] [PubMed]
- Gong, M.; Yang, H.; Zhang, S.; Yang, Y.; Zhang, D.; Qi, Y.; Zou, L. Superparamagnetic core/shell GoldMag nanoparticles: Size-, concentration- and time-dependent cellular nanotoxicity on human umbilical vein endothelial cells and the suitable conditions for magnetic resonance imaging. J. Nanobiotechnol. 2015, 13, 24. [Google Scholar] [CrossRef]
- Palacios-Hernandez, T.; Diaz-Diestra, D.M.; Nguyen, A.K.; Skoog, S.A.; Vijaya Chikkaveeraiah, B.; Tang, X.; Wu, Y.; Petrochenko, P.E.; Sussman, E.M.; Goering, P.L. Cytotoxicity, cellular uptake and apoptotic responses in human coronary artery endothelial cells exposed to ultrasmall superparamagnetic iron oxide nanoparticles. J. Appl. Toxicol. 2020, 40, 918–930. [Google Scholar] [CrossRef]
- Lomphithak, T.; Helvacioglu, S.; Armenia, I.; Keshavan, S.; Ovejero, J.G.; Baldi, G.; Ravagli, C.; Grazú, V.; Fadeel, B. High-dose exposure to polymer-coated iron oxide nanoparticles elicits autophagy-dependent ferroptosis in susceptible cancer cells. Nanomaterials 2023, 13, 1719. [Google Scholar] [CrossRef]
- Sadeghi, L.; Babadi, V.Y.; Espanani, H.R. Toxic effects of the Fe2O3 nanoparticles on the liver and lung tissue. Bratisl. Med. J. 2015, 116, 373–378. [Google Scholar] [CrossRef]
- Akçan, R.; Aydogan, H.C.; Yildirim, M.Ş.; Taştekin, B.; Sağlam, N. Nanotoxicity: A challenge for future medicine. Turk. J. Med. Sci. 2020, 50, 1180–1196. [Google Scholar] [CrossRef]
- Arami, H.; Khandhar, A.; Liggitt, D.; Krishnan, K.M. In vivo delivery, pharmacokinetics, biodistribution and toxicity of iron oxide nanoparticles. Chem. Soc. Rev. 2015, 44, 8576–8607. [Google Scholar] [CrossRef]
- Fakhardo, A.F.; Anastasova, E.I.; Gabdullina, S.R.; Solovyeva, A.S.; Saparova, V.B.; Chrishtop, V.V.; Koshevaya, E.D.; Krivoshapkina, E.F.; Krivoshapkin, P.V.; Kiselev, G.O.; et al. Toxicity patterns of clinically relevant metal oxide nanoparticles. ACS Appl. Bio Mater. 2019, 2, 4427–4435. [Google Scholar] [CrossRef] [PubMed]
- Yousefi Babadi, V.; Amraeai, E.; Salehh, H.; Sadeghi, L.; Najafi, L.; Fazilati, M. Evaluation of iron oxide nanoparticles effects on tissue and enzymes of thyroid in rats. Int. Res. J. Biol. Sci. 2013, 2, 67–69. [Google Scholar]
- Nemmar, A.; Beegam, S.; Yuvaraju, P.; Yasin, J.; Tariq, S.; Attoub, S.; Ali, B.H. Ultrasmall superparamagnetic iron oxide nanoparticles acutely promote thrombosis and cardiac oxidative stress and DNA damage in mice. Part. Fibre Toxicol. 2016, 13, 22. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, C.; Wang, L.; Yang, Q.; Tang, W.; She, Z.; Deng, Y. A frustrating problem: Accelerated blood clearance of PEGylated solid lipid nanoparticles following subcutaneous injection in rats. Eur. J. Pharm. Biopharm. 2012, 81, 506–513. [Google Scholar] [CrossRef]
- Feng, Q.; Liu, Y.; Huang, J.; Chen, K.; Huang, J.; Xiao, K. Uptake, distribution, clearance, and toxicity of iron oxide nanoparticles with different sizes and coatings. Sci. Rep. 2018, 8, 2082. [Google Scholar] [CrossRef]
- Amin, R.M.; Abdelmonem, A.; Verwanger, T.; Elsherbini, E.; Krammer, B. Cytotoxicity of magnetic nanoparticles on normal and malignant human skin cells. Nano LIFE 2014, 4, 1440002. [Google Scholar] [CrossRef]
- Yu, M.; Huang, S.; Yu, K.J.; Clyne, A.M. Dextran and polymer polyethylene glycol (PEG) coating reduce both 5 and 30 nm iron oxide nanoparticle cytotoxicity in 2D and 3D cell culture. Int. J. Mol. Sci. 2012, 13, 5554–5570. [Google Scholar] [CrossRef]
- Jurewicz, A.; Ilyas, S.; Uppal, J.K.; Ivandic, I.; Korsching, S.; Mathur, S. Evaluation of magnetite nanoparticle-based toxicity on embryo–larvae stages of zebrafish (Danio rerio). ACS Appl. Nano Mater. 2020, 3, 1621–1629. [Google Scholar] [CrossRef]
- Kamiloglu, S.; Sari, G.; Ozdal, T.; Capanoglu, E. Guidelines for cell viability assays. Food Front. 2020, 1, 332–349. [Google Scholar] [CrossRef]
- Shukla, S.; Jadaun, A.; Arora, V.; Sinha, R.K.; Biyani, N.; Jain, V.K. In vitro toxicity assessment of chitosan oligosaccharide coated iron oxide nanoparticles. Toxicol. Rep. 2015, 2, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Agotegaray, M.; Campelo, A.; Zysler, R.; Gumilar, F.; Bras, C.; Minetti, A.; Massheimer, V.; Lassalle, V. Influence of chitosan coating on magnetic nanoparticles in endothelial cells and acute tissue biodistribution. J. Biomater. Sci. Polym. Ed. 2016, 27, 1069–1085. [Google Scholar] [CrossRef] [PubMed]
- Uggeri, J.; Gatti, R.; Belletti, S.; Scandroglio, R.; Corradini, R.; Rotoli, B.M.; Orlandini, G. Calcein-AM is a detector of intracellular oxidative activity. Histochem. Cell Biol. 2004, 122, 499–505. [Google Scholar] [CrossRef]
- Remya, N.S.; Syama, S.; Sabareeswaran, A.; Mohanan, P.V. Toxicity, toxicokinetics and biodistribution of dextran stabilized iron oxide nanoparticles for biomedical applications. Int. J. Pharm. 2016, 511, 586–598. [Google Scholar] [CrossRef]
- Caro, C.; Egea-Benavente, D.; Polvillo, R.; Royo, J.L.; Pernia Leal, M.; García-Martín, M.L. Comprehensive toxicity assessment of PEGylated magnetic nanoparticles for in vivo applications. Colloids Surf. B Biointerfaces 2019, 177, 253–259. [Google Scholar] [CrossRef]
- Nosrati, H.; Tarantash, M.; Bochani, S.; Charmi, J.; Bagheri, Z.; Fridoni, M.; Abdollahifar, M.-A.; Davaran, S.; Danafar, H.; Manjili, K.K. Glutathione (GSH) peptide conjugated magnetic nanoparticles as blood–brain barrier shuttle for MRI-monitored brain delivery of paclitaxel. ACS Biomater. Sci. Eng. 2019, 5, 1677–1685. [Google Scholar] [CrossRef]
- Chandekar, K.V.; Shkir, M.; Alshahrani, T.; Ibrahim, E.H.; Kilany, M.; Ahmad, Z.; Manthrammel, M.A.; AlFaify, S.; Kateb, B.; Kaushik, A. One-spot fabrication and in vivo toxicity evaluation of core–shell magnetic nanoparticles. Mater. Sci. Eng. C 2021, 122, 111898. [Google Scholar] [CrossRef]
- Obireddy, S.R.; Lai, W.F. ROS-generating amine-functionalized magnetic nanoparticles coupled with carboxymethyl chitosan for pH-responsive release of doxorubicin. Int. J. Nanomed. 2022, 17, 589–601. [Google Scholar] [CrossRef]
- Do, X.H.; Nguyen, T.D.; Le, T.T.H.; To, T.T.; Bui, T.V.K.; Pham, N.H.; Lam, K.; Hoang, T.M.N.; Ha, P.T. High biocompatibility, MRI enhancement, and dual chemo- and thermal-therapy of curcumin-encapsulated alginate/Fe3O4 nanoparticles. Pharmaceutics 2023, 15, 1523. [Google Scholar] [CrossRef]
- Jiang, Y.; Xu, X.; Lu, J.; Yin, C.; Li, G.; Bai, L.; Zhang, T.; Mo, J.; Wang, X.; Shi, Q.; et al. Development of ε-poly(L-lysine) carbon dots-modified magnetic nanoparticles and their applications as novel antibacterial agents. Front. Chem. 2023, 11, 1184592. [Google Scholar] [CrossRef]
- Berridge, M.V.; Herst, P.M.; Tan, A.S. Tetrazolium dyes as tools in cell biology: New insights into their cellular reduction. Biotechnol. Annu. Rev. 2005, 11, 127–152. [Google Scholar] [CrossRef]
- Kumar, P.; Nagarajan, A.; Uchil, P.D. Analysis of cell viability by the lactate dehydrogenase assay. Cold Spring Harb. Protoc. 2018, 2018, 469–471. [Google Scholar] [CrossRef]
- Repetto, G.; Del Peso, A.; Zurita, J.L. Neutral red uptake assay for the estimation of cell viability/cytotoxicity. Nat. Protoc. 2008, 3, 1125–1131. [Google Scholar] [CrossRef] [PubMed]
- Rozhina, E.; Danilushkina, A.; Akhatova, F.; Fakhrullin, R.; Rozhin, A.; Batasheva, S. Biocompatibility of magnetic nanoparticles coating with polycations using A549 cells. J. Biotechnol. 2021, 325, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Gillissen, M.A.; Yasuda, E.; de Jong, G.; Levie, S.E.; Go, D.; Spits, H.; van Helden, P.M.; Hazenberg, M.D. The modified FACS calcein AM retention assay: A high throughput flow cytometer based method to measure cytotoxicity. J. Immunol. Methods 2016, 434, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Mieloch, A.A.; Żurawek, M.; Giersig, M.; Rozwadowska, N.; Rybka, J.D. Bioevaluation of superparamagnetic iron oxide nanoparticles (SPIONs) functionalized with dihexadecyl phosphate (DHP). Sci. Rep. 2020, 10, 2725. [Google Scholar] [CrossRef]
- Weydert, C.J.; Cullen, J.J. Measurement of superoxide dismutase, catalase and glutathione peroxidase in cultured cells and tissue. Nat. Protoc. 2010, 5, 51–66. [Google Scholar] [CrossRef]
- Csonka, C.; Páli, T.; Bencsik, P.; Görbe, A.; Ferdinandy, P.; Csont, T. Measurement of NO in biological samples. Br. J. Pharmacol. 2015, 172, 1620–1632. [Google Scholar] [CrossRef]
- Cheng, G.; Zielonka, M.; Dranka, B.; Kumar, S.N.; Myers, C.R.; Bennett, B.; Garces, A.M.; Machado, L.G.D.D.; Thiebaut, D.; Quari, O.; et al. Detection of mitochondria-generated reactive oxygen species in cells using multiple probes and methods: Potentials, pitfalls, and the future. J. Biol. Chem. 2018, 293, 10363–10380. [Google Scholar] [CrossRef]
- Nakkala, J.R.; Mata, R.; Raja, K.; Khub Chandra, V.; Sadras, S.R. Green synthesized silver nanoparticles: Catalytic dye degradation, in vitro anticancer activity and in vivo toxicity in rats. Mater. Sci. Eng. C 2018, 91, 372–381. [Google Scholar] [CrossRef]
- Liao, W.; McNutt, M.A.; Zhu, W.G. The comet assay: A sensitive method for detecting DNA damage in individual cells. Methods 2009, 48, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Omari Shekaftik, S.; Nasirzadeh, N. 8-Hydroxy-2′-deoxyguanosine (8-OHdG) as a biomarker of oxidative DNA damage induced by occupational exposure to nanomaterials: A systematic review. Nanotoxicology 2021, 15, 850–864. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, M.A.; Wahab, R.; Saquib, Q.; Ahmad, J.; Farshori, N.N.; Al-Sheddi, E.S.; Al-Oqail, M.M.; Al-Massarani, S.M.; Al-Khedhairy, A.A. Iron oxide nanoparticles induced cytotoxicity, oxidative stress, cell cycle arrest, and DNA damage in human umbilical vein endothelial cells. J. Trace Elem Med. Biol. 2023, 76, 127302. [Google Scholar] [CrossRef] [PubMed]
- Fontaine, M.; Bartolami, E.; Prono, M.; Béal, D.; Blosi, M.; Costa, A.L.; Ravagli, C.; Baldi, G.; Sprio, S.; Tampieri, A.; et al. Nanomaterial genotoxicity evaluation using the high-throughput p53-binding protein 1 (53BP1) assay. PLoS ONE 2023, 18, e0288737. [Google Scholar] [CrossRef]
- Sharma, R.; Ahmad, G.; Esteves, S.C.; Agarwal, A. Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay using bench top flow cytometer for evaluation of sperm DNA fragmentation in fertility laboratories: Protocol, reference values, and quality control. J. Assist. Reprod. Genet. 2016, 33, 291–300. [Google Scholar] [CrossRef]
- Patsula, V.; Tulinska, J.; Trachtová, Š.; Kuricova, M.; Liskova, A.; Španová, A.; Ciampor, F.; Vavra, I.; Rittich, B.; Ursinyova, M. Toxicity evaluation of monodisperse PEGylated magnetic nanoparticles for nanomedicine. Nanotoxicology 2019, 13, 510–526. [Google Scholar] [CrossRef]
- Niles, A.L.; Moravec, R.A.; Riss, T.L. Caspase activity assays. In Apoptosis and Cancer; Mor, G., Alvero, A.B., Eds.; Humana Press: Totowa, NJ, USA, 2008; pp. 137–150. [Google Scholar] [CrossRef]
- Hofer, S.; Hofstätter, N.; Punz, B.; Hasenkopf, I.; Johnson, L.; Himly, M. Immunotoxicity of nanomaterials in health and disease: Current challenges and emerging approaches for identifying immune modifiers in susceptible populations. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2022, 14, e1804. [Google Scholar] [CrossRef]
- Krstić, D.; Krinulović, K.; Vasić, V. Inhibition of Na+/K+-ATPase and Mg2+-ATPase by metal ions and prevention and recovery of inhibited activities by chelators. J. Enzyme Inhib. Med. Chem. 2005, 20, 469–476. [Google Scholar] [CrossRef]
- Yoloğlu, E. Assessment of Na+/K+-ATPase, Mg2+-ATPase, Ca2+-ATPase, and total-ATPase activities in gills of freshwater mussels exposed to penconazole. Commagene J. Biol. 2019, 3, 125–132. [Google Scholar] [CrossRef]
- Anuje, M.; Pawaskar, P.N.; Khot, V.; Sivan, A.; Jadhav, S.; Meshram, J.; Thombare, B. Synthesis, characterization, and cytotoxicity evaluation of polyethylene glycol-coated iron oxide nanoparticles for radiotherapy application. J. Med. Phys. 2021, 46, 154–161. [Google Scholar] [CrossRef]
- Prodan, A.M.; Iconaru, S.L.; Ciobanu, C.S.; Chifiriuc, M.C.; Stoicea, M.; Predoi, D. Iron oxide magnetic nanoparticles: Characterization and toxicity evaluation by in vitro and in vivo assays. J. Nanomater. 2013, 2013, 587021. [Google Scholar] [CrossRef]
- Jarockyte, G.; Daugelaite, E.; Stasys, M.; Statkute, U.; Poderys, V.; Tseng, T.C.; Hsu, S.-H.; Karabanovas, V.; Rotomskis, R. Accumulation and toxicity of superparamagnetic iron oxide nanoparticles in cells and experimental animals. Int. J. Mol. Sci. 2016, 17, 1193. [Google Scholar] [CrossRef] [PubMed]
- Malvindi, M.A.; De Matteis, V.; Galeone, A.; Brunetti, V.; Anyfantis, G.C.; Athanassiou, A.; Cingolani, R.; Pompa, P.P. Toxicity assessment of silica coated iron oxide nanoparticles and biocompatibility improvement by surface engineering. PLoS ONE 2014, 9, e85835. [Google Scholar] [CrossRef] [PubMed]
- Marcos-Campos, I.; Asín, L.; Torres, T.E.; Marquina, C.; Tres, A.; Ibarra, M.R.; Goya, G.F. Cell death induced by the application of alternating magnetic fields to nanoparticle-loaded dendritic cells. Nanotechnology 2011, 22, 205101. [Google Scholar] [CrossRef]
- Costa, C.; Brandão, F.; Bessa, M.J.; Costa, S.; Valdiglesias, V.; Kiliç, G.; Fernández-Bertólez, N.; Quaresma, P.; Pereira, E.; Pásaro, E. In vitro cytotoxicity of superparamagnetic iron oxide nanoparticles on neuronal and glial cells: Evaluation of nanoparticle interference with viability tests. J. Appl. Toxicol. 2016, 36, 361–372. [Google Scholar] [CrossRef]
- Hoskins, C.; Wang, L.; Cheng, W.P.; Cuschieri, A. Dilemmas in the reliable estimation of the in vitro cell viability in magnetic nanoparticle engineering: Which tests and what protocols? Nanoscale Res. Lett. 2012, 7, 77. [Google Scholar] [CrossRef]
- Han, X.; Gelein, R.; Corson, N.; Wade-Mercer, P.; Jiang, J.; Biswas, P.; Biswas, P.; Finkelstein, J.N.; Elder, A.; Oberdörster, G. Validation of an LDH assay for assessing nanoparticle toxicity. Toxicology 2011, 287, 99–104. [Google Scholar] [CrossRef]
- Reddy, U.A.; Prabhakar, P.V.; Mahboob, M. Biomarkers of oxidative stress for in vivo assessment of toxicological effects of iron oxide nanoparticles. Saudi J. Biol. Sci. 2017, 24, 1172–1180. [Google Scholar] [CrossRef]
- Pongrac, I.M.; Pavičić, I.; Milić, M.; Brkić Ahmed, L.; Babič, M.; Horák, D.; Vinković Vrček, J.; Gajović, S. Oxidative stress response in neural stem cells exposed to different superparamagnetic iron oxide nanoparticles. Int. J. Nanomed. 2016, 11, 1701–1715. [Google Scholar] [CrossRef]
- Radu, M.; Dinu, D.; Sima, C.; Burlacu, R.; Hermenean, A.; Ardelean, A.; Dinischiotu, A. Magnetite nanoparticles induced adaptive mechanisms counteract cell death in human pulmonary fibroblasts. Toxicol. Vitr. 2015, 29, 1492–1502. [Google Scholar] [CrossRef]
- Gaharwar, U.S.; Meena, R.; Rajamani, P. Iron oxide nanoparticles induced cytotoxicity, oxidative stress and DNA damage in lymphocytes. J. Appl. Toxicol. 2017, 37, 1232–1244. [Google Scholar] [CrossRef] [PubMed]
- Seo, D.Y.; Jin, M.; Ryu, J.C.; Kim, Y.J. Investigation of the genetic toxicity by dextran-coated superparamagnetic iron oxide nanoparticles in HepG2 cells using the comet assay and cytokinesis-block micronucleus assay. Toxicol. Environ. Health Sci. 2017, 9, 23–29. [Google Scholar] [CrossRef]
- Jacewicz, D.; Siedlecka-Kroplewska, K.; Drzeżdżon, J.; Piotrowska, A.; Wyrzykowski, D.; Tesmar, A.; Żamojć, K.; Chmurzyński, L. Method for detection of hydrogen peroxide in HT22 cells. Sci. Rep. 2017, 7, 45673. [Google Scholar] [CrossRef] [PubMed]
- Erofeev, A.; Gorelkin, P.; Garanina, A.; Alova, A.; Efremova, M.; Vorobyeva, N.; Edwards, C.; Korchev, Y.; Majouga, A. Novel method for rapid toxicity screening of magnetic nanoparticles. Sci. Rep. 2018, 8, 7462. [Google Scholar] [CrossRef]
- Cellai, F.; Munnia, A.; Viti, J.; Doumett, S.; Ravagli, C.; Ceni, E.; Mello, T.; Polvani, S.; Giese, R.W.; Baldi, G.; et al. Magnetic hyperthermia and oxidative damage to DNA of human hepatocarcinoma cells. Int. J. Mol. Sci. 2017, 18, 939. [Google Scholar] [CrossRef]
- Woo, S.; Kim, S.; Kim, H.; Cheon, Y.W.; Yoon, S.; Oh, J.H.; Park, J. Charge-modulated synthesis of highly stable iron oxide nanoparticles for in vitro and in vivo toxicity evaluation. Nanomaterials 2021, 11, 3068. [Google Scholar] [CrossRef]
- Radosinska, J.; Jasenovec, T.; Radosinska, D.; Balis, P.; Puzserova, A.; Skratek, M.; Manka, J.; Bernatova, I. Ultra-small superparamagnetic iron-oxide nanoparticles exert different effects on erythrocytes in normotensive and hypertensive rats. Biomedicines 2021, 9, 377. [Google Scholar] [CrossRef]
- Ruíz-Baltazar, Á.D.J.; Reyes-López, S.Y.; Méndez-Lozano, N.; Juárez-Moreno, K. Evaluation of superparamagnetic Fe3O4-Ag decorated nanoparticles: Cytotoxicity studies in human fibroblasts (HFF-1) and breast cancer cells (MCF-7). Appl. Sci. 2024, 14, 6750. [Google Scholar] [CrossRef]
- Paulini, F.; Marangon, A.R.M.; Azevedo, C.L.; Brito, J.L.M.; Lemos, M.S.; Sousa, M.H.; Veiga-Sonza, F.H.; Sonza, P.E.N.; Lucci, C.M.; Azevedo, R.B. In vivo evaluation of DMSA-coated magnetic nanoparticle toxicity and biodistribution in rats: A long-term follow-up. Nanomaterials 2022, 12, 3513. [Google Scholar] [CrossRef]
- Mîndrilă, B.; Rogoveanu, I. Liver histopathological changes related to intraperitoneal administration of salicylic acid/Fe3O4 nanoparticles to C57BL/6 mice. Curr. Health Sci. J. 2022, 48, 146–154. [Google Scholar] [CrossRef]
- Sewell, F.; Alexander-White, C.; Brescia, S.; Currie, R.A.; Roberts, R.; Roper, C.; Vickers, C.; Westmoreland, C.; Kimber, I. New approach methodologies (NAMs): Identifying and overcoming hurdles to accelerated adoption. Toxicol. Res. 2024, 13, tfae044. [Google Scholar] [CrossRef] [PubMed]
- Usmani, S.M.; Bremer-Hoffmann, S.; Cheyns, K.; Cubadda, F.; Dumit, V.I.; Escher, S.E.; Fessard, V.; Gutleb, A.C.; Léger, T.; Liu, Y.-C.; et al. Review of new approach methodologies for application in risk assessment of nanoparticles in the food and feed sector: Status and challenges. EFSA Support. Publ. 2024, 21, 8826E. [Google Scholar] [CrossRef]
- Yang, H.; Niu, S.; Guo, M.; Xue, Y. Applications of 3D organoids in toxicological studies: A comprehensive analysis based on bibliometrics and advances in toxicological mechanisms. Arch. Toxicol. 2024, 98, 2309–2330. [Google Scholar] [CrossRef] [PubMed]
- Gerbolés, A.G.; Galetti, M.; Rossi, S.; Lo Muzio, F.P.; Pinelli, S.; Delmonte, N.; Malvezzi, C.C.; Macaluso, C.; Miragoli, M.; Foresti, R. Three-dimensional bioprinting of organoid-based scaffolds (OBST) for long-term nanoparticle toxicology investigation. Int. J. Mol. Sci. 2023, 24, 6595. [Google Scholar] [CrossRef]
- Groll, J.; Boland, T.; Blunk, T.; Burdick, J.A.; Cho, D.W.; Dalton, P.D.; Derby, B.; Forgacs, G.; Li, Q.; Mironov, V.A.; et al. Biofabrication: Reappraising the definition of an evolving field. Biofabrication 2016, 8, 013001. [Google Scholar] [CrossRef]
- Li, X.; Liu, B.; Pei, B.; Chen, J.; Zhou, D.; Peng, J.; Zhang, X.; Jia, W.; Xu, T. Inkjet bioprinting of biomaterials. Chem. Rev. 2020, 120, 10793–10833. [Google Scholar] [CrossRef]
- Mandrycky, C.; Wang, Z.; Kim, K.; Kim, D.H. 3D bioprinting for engineering complex tissues. Biotechnol. Adv. 2016, 34, 422–434. [Google Scholar] [CrossRef]
- Li, J.; Chen, M.; Fan, X.; Zhou, H. Recent advances in bioprinting techniques: Approaches, applications and future prospects. J. Transl. Med. 2016, 14, 271. [Google Scholar] [CrossRef]
- Daly, A.C.; Prendergast, M.E.; Hughes, A.J.; Burdick, J.A. Bioprinting for the biologist. Cell 2021, 184, 18–32. [Google Scholar] [CrossRef]
- Theus, A.S.; Ning, L.; Kabboul, G.; Hwang, B.; Tomov, M.L.; LaRock, C.N.; Bauser-Heaton, H.; Mahmoudi, M.; Serpooshan, V. 3D bioprinting of nanoparticle-laden hydrogel scaffolds with enhanced antibacterial and imaging properties. iScience 2022, 25, 104947. [Google Scholar] [CrossRef]
- Ning, L.; Zanella, S.; Tomov, M.L.; Amoli, M.S.; Jin, L.; Hwang, B.; Saadeh, M.; Chen, H.; Neelakantan, S.; Dasi, L.P. Targeted rapamycin delivery via magnetic nanoparticles to address stenosis in a 3D bioprinted in vitro model of pulmonary veins. Adv. Sci. 2024, 11, 2400476. [Google Scholar] [CrossRef] [PubMed]
- Fatehullah, A.; Tan, S.H.; Barker, N. Organoids as an in vitro model of human development and disease. Nat. Cell Biol. 2016, 18, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Palzer, J.; Mues, B.; Goerg, R.; Aberle, M.; Rensen, S.S.; Olde Damink, S.W.; Vaes, R.D.W.; Cramer, T.; Schmitz-Rode, T.; Neumann, U.P. Magnetic fluid hyperthermia as treatment option for pancreatic cancer cells and pancreatic cancer organoids. Int. J. Nanomed. 2021, 16, 2965–2981. [Google Scholar] [CrossRef]
- Sun, A.; Hayat, H.; Liu, S.; Tull, E.; Bishop, J.O.; Dwan, B.F.; Gudi, M.; Talebloo, N.; Dizon, J.R.; Li, W.; et al. 3D in vivo magnetic particle imaging of human stem cell-derived islet organoid transplantation using a machine learning algorithm. Front. Cell Dev. Biol. 2021, 9, 704483. [Google Scholar] [CrossRef]
- Yazdani, M. In Vitro, In Ovo and In Vivo Models for Cytotoxicity, Oxidative Stress, Neurotoxicity and DNA Damage. Ph.D. Thesis, Oslo University Hospital, Oslo, Norway, 2014. Available online: https://www.researchgate.net/publication/342241588 (accessed on 20 July 2025).
- Ghimire, S.; Zhang, X.; Zhang, J.; Wu, C. Use of chicken embryo model in toxicity studies of endocrine-disrupting chemicals and nanoparticles. Chem. Res. Toxicol. 2022, 35, 550–568. [Google Scholar] [CrossRef]
- Sarnella, A.; Ferrara, Y.; Terlizzi, C.; Albanese, S.; Monti, S.; Licenziato, L.; Mancini, M. The chicken embryo: An old but promising model for in vivo preclinical research. Biomedicines 2024, 12, 2835. [Google Scholar] [CrossRef]
- Ribatti, D. Chapter 5: Chick embryo chorioallantoic membrane as a useful tool to study angiogenesis. Int. Rev. Cell Mol. Biol. 2008, 266, 181–224. [Google Scholar] [CrossRef]
- Ribatti, D. The chick embryo chorioallantoic membrane as a model for tumor biology. Exp. Cell Res. 2014, 328, 314–324. [Google Scholar] [CrossRef]
- Auerbach, R.; Akhtar, N.; Lewis, R.L.; Shinners, B.L. Angiogenesis assays: Problems and pitfalls. Cancer Metastasis Rev. 2000, 19, 167–172. [Google Scholar] [CrossRef]
- Tufan, A.; Satiroglu-Tufan, N. The chick embryo chorioallantoic membrane as a model system for the study of tumor angiogenesis, invasion and development of anti-angiogenic agents. Curr. Cancer Drug Targets 2005, 5, 249–266. [Google Scholar] [CrossRef]
- Patel, S.; Jana, S.; Chetty, R.; Thakore, S.; Singh, M.; Devkar, R. Toxicity evaluation of magnetic iron oxide nanoparticles reveals neuronal loss in chicken embryo. Drug Chem. Toxicol. 2019, 42, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Akbari, G.; Basaki, M.; Hejazi, M.; Rezaei, H.; Babaei, M. Morphological assessment of iron oxide nanoparticles effects on the chicken embryo: Teratogenic aspect. ASJ 2022, 19, 89–100. Available online: http://anatomyjournal.ir/article-1-587-en.html (accessed on 25 July 2025).
- Scheschenja, M.; Jedelská, J.; Juchems, E.; Weinmann, M.; Pagenstecher, A.; Helmprobst, F.; Buchholz, M.; Tatura, M.; Schaefer, J.; Bakowsky, U.; et al. Chick chorioallantoic membrane model as a preclinical platform for cryoablation studies. Eur. Radiol. Exp. 2025, 9, 56. [Google Scholar] [CrossRef] [PubMed]
- Tkachenko, A.; Onishchenko, A.; Butov, D.; Tkachenko, M. Flow cytometry in nanotoxicology: Brief overview. Inter Collegas 2022, 8, 278–289. [Google Scholar] [CrossRef]
- Monteiro-Riviere, N.A.; Tran, C.L. Advanced methodologies and techniques for assessing nanomaterial toxicity. In Nanotoxicology; CRC Press: Boca Raton, FL, USA, 2014; pp. 175–196. [Google Scholar] [CrossRef]
- Gupta, A.; Harrison, P.J.; Wieslander, H.; Pielawski, N.; Kartasalo, K.; Partel, G.; Solorzano, L.; Suveer, A.; Klemm, A.H.; Spjuth, O.; et al. Deep learning in image cytometry: A review. Cytom. A 2019, 95, 366–380. [Google Scholar] [CrossRef]
- Rees, P.; Summers, H.D.; Filby, A.; Carpenter, A.E.; Doan, M. Imaging flow cytometry. Nat. Rev. Methods Primers 2022, 2, 67. [Google Scholar] [CrossRef]
- Eustaquio, T.; Leary, J.F. Single-cell nanotoxicity assays of superparamagnetic iron oxide nanoparticles. In Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2012; pp. 69–85. [Google Scholar] [CrossRef]
- Eustaquio, T.; Leary, J.F. Nanobarcoded superparamagnetic iron oxide nanoparticles for nanomedicine: Quantitative studies of cell–nanoparticle interactions by scanning image cytometry. Cytom. A 2016, 89, 207–216. [Google Scholar] [CrossRef]
- Friedrich, R.P.; Janko, C.; Pöttler, M.; Tripal, P.; Zaloga, J.; Cicha, I.; Dürr, S.; Nowak, J.; Odenbach, S.; Slabu, I.; et al. Flow cytometry for intracellular SPION quantification: Specificity and sensitivity in comparison with spectroscopic methods. Int. J. Nanomed. 2015, 10, 4185–4201. [Google Scholar] [CrossRef]
- Polasky, C.; Studt, T.; Steuer, A.K.; Loyal, K.; Lüdtke-Buzug, K.; Bruchhage, K.L.; Pries, R. Impact of superparamagnetic iron oxide nanoparticles on THP-1 monocytes and monocyte-derived macrophages. Front. Mol. Biosci. 2022, 9, 811116. [Google Scholar] [CrossRef]
- Jiang, X.; Stockwell, B.R.; Conrad, M. Ferroptosis: Mechanisms, biology and role in disease. Nat. Rev. Mol. Cell Biol. 2021, 22, 266–282. [Google Scholar] [CrossRef]
- Ru, Q.; Li, Y.; Chen, L.; Wu, Y.; Min, J.; Wang, F. Iron homeostasis and ferroptosis in human diseases: Mechanisms and therapeutic prospects. Signal Transduct. Target. Ther. 2024, 9, 271. [Google Scholar] [CrossRef]
- Quiles Del Rey, M.; Mancias, J.D. NCOA4-mediated ferritinophagy: A potential link to neurodegeneration. Front. Neurosci. 2019, 13, 238. [Google Scholar] [CrossRef]
- Kuno, S.; Fujita, H.; Tanaka, Y.; Ogra, Y.; Iwai, K. Iron-induced NCOA4 condensation regulates ferritin fate and iron homeostasis. Embo Rep. 2022, 23, e54278. [Google Scholar] [CrossRef]
- Ucar, A.; Parlak, V.; Ozgeris, F.B.; Yeltekin, A.C.; Arslan, M.E.; Alak, G.; Turkez, H.; Kocaman, E.M.; Atamanalp, M. Magnetic nanoparticles-induced neurotoxicity and oxidative stress in brain of rainbow trout: Mitigation by ulexite through modulation of antioxidant, anti-inflammatory, and antiapoptotic activities. Sci. Total Environ. 2022, 838, 155718. [Google Scholar] [CrossRef]
- Bardestani, A.; Ebrahimpour, S.; Esmaeili, A.; Esmaeili, A. Quercetin attenuates neurotoxicity induced by iron oxide nanoparticles. J. Nanobiotechnology 2021, 19, 327. [Google Scholar] [CrossRef] [PubMed]
- Bouwmeester, M.C.; Bernal, P.N.; Oosterhoff, L.A.; Van Wolferen, M.E.; Lehmann, V.; Vermaas, M.; Bucholz, M.-B.; Peiffer, Q.C.; Malda, J.; van der Laan, L.J.W.; et al. Bioprinting of human liver-derived epithelial organoids for toxicity studies. Macromol. Biosci. 2021, 21, e2100327. [Google Scholar] [CrossRef] [PubMed]
- Krewski, D.; Acosta, D.; Andersen, M.; Anderson, H.; Bailar, J.C.; Boekelheide, K.; Brent, R.; Charnley, G.; Cheung, V.G.; Green, S., Jr.; et al. Toxicity testing in the 21st century: A vision and a strategy. J. Toxicol. Environ. Health B Crit. Rev. 2010, 13, 51–138. [Google Scholar] [CrossRef] [PubMed]
- Hartung, T. The state of the scientific revolution in toxicology. ALTEX 2021, 38, 3–14. [Google Scholar] [CrossRef]
- Cossarizza, A.; Chang, H.; Radbruch, A.; Abrignani, S.; Addo, R.; Akdis, M.; Andrä, J.; Andreata, F.; Annuziato, F.; Arranz, E.; et al. Guidelines for the use of flow cytometry and cell sorting in immunological studies (third edition). Eur. J. Immunol. 2021, 51, 2708–3145. [Google Scholar] [CrossRef]
- DeLoid, G.M.; Cohen, J.M.; Pyrgiotakis, G.; Demokritou, P. Preparation, characterization, and in vitro dosimetry of dispersed, engineered nanomaterials. Nat. Protoc. 2017, 12, 355–371. [Google Scholar] [CrossRef] [PubMed]
- Nelissen, I.; Haase, A.; Anguissola, S.; Rocks, L.; Jacobs, A.; Willems, H.; Riebeling, C.; Luch, A.; Piret, J.-P.; Toussaint, O.; et al. Improving quality in nanoparticle-induced cytotoxicity testing by a tiered inter-laboratory comparison study. Nanomaterials 2020, 10, 1430. [Google Scholar] [CrossRef] [PubMed]
| Core | Shape | Core Size (nm) | Coating | Model | Key Outcomes | Ref |
|---|---|---|---|---|---|---|
| Fe3O4 | Spherical | 10, 20, 30, 40 | Amphiphilic polymers with carboxylic acid | Female KuMing mice | No significant change in 7 hepatic markers across sizes; 10 nm MNPs increased TBIL/DBIL, and lowered ALP; smaller MNPs persisted longer in blood; WBC/PMN changes; size-dependent hepatic gene expression (oxidative stress, immune, iron transport, metabolism, apoptosis) without overt toxicity | [47] |
| Fe3O4 | Spherical | 10, 20, 40 | AEAPS 1 | Male Sprague Dawley rats | Distinct biochemical responses by size; LDH (partial negative) and urea (partial positive) correlations with size | [48] |
| Fe3O4 | Spherical | 40, 80 | BSA 2; PEG 3 derivatives | Human fibroblasts and U251 glioblastoma cells | Toxicity driven by surface chemistry, dose, exposure time; variable diameters of MNPs did not cause significant changes (except proliferation assay) | [44] |
| Fe3O4 | Spherical | 2.3, 4.2, 9.3 | No coating molecules | Male ICR mice | 2.3/4.2 nm highly toxic/lethal; 9.3 nm no apparent toxicity; mechanism attributed to oxidative stress | [49] |
| Fe3O4 | Spherical | 6, 9, 14 | No coating molecules | Human hepatoma (SH-Hep-1, Hep3B) cells | 6 nm least cytotoxic; 9 nm caused mitochondrial ROS and necrosis; 14 nm caused membrane damage (LDH release) | [50] |
| γ-Fe2O3 | Spherical | <50, <100 (hydrodynamic diameter) | No coating molecules | Transheterozygous larvae of Drosophila melanogaster | MNPs < 100 nm: no genotoxicity; <50 nm: genotoxicity at 1 and 10 mM | [51] |
| γ-Fe2O3 | Rod-shaped vs. spherical | Nanorodes: 50–100 (diameter), >500 (length) Spheres: <5000 and <50 | No coating molecules | Murine macrophage cel line RAW 264.7 | Nanorods: higher uptake than spheres; higher accumulation led to necrosis in non-tumorigenic cells | [52] |
| Not specified | Nanocubes | 40 | DSPE 4-PEG | Mouse monocyte macrophage RAW264.7 cell line | Low cytotoxicity up to 0.5 mg Fe/mL | [53] |
| Fe3O4 | Nanoworms | Nanoworms: 65 (length) Spheres: 6 | HA 5 (hyaluronian) | CD44 expressing mouse macrophage RAW264.7 cells | Nanoworms elicited lower inflammatory response than spherical nanoparticles | [54] |
| Fe3O4 | Spheres, nanoworms, nanorodes, magnetic beads | 2.8–21.6 | PVA 6 Polyvinyl alcohol | L929 fibroblasts | Abnormal morphology (cell shrinkage); toxicity order: beads < nanoworms < nanospheres | [55] |
| Category | Factor | Why It Matters | Representative Examples and Models | Typical Direction of Effect | Controls/Standardization |
|---|---|---|---|---|---|
| MNPs | Core size | Alters surface-to-volume ratio, dissolution kinetics, biodistribution, and intracellular processing. | BSA 1-coated Fe3O4 40 vs. 80 nm largely driven by surface chemistry/dose/time rather than diameter (human fibroblasts and U251 glioblastosoma cells) [44]; PEG 2-coated 10–40 nm spheres in female KuMing mice showed no size-dependent shifts in seven standard hepatic markers versus controls, but 10 nm particles increased TBIL/DBIL and lowered ALP; smaller particles also persisted longer in blood and modulated WBC/PMN without overt toxicity [47]; 10/20/40 nm silica-coated MNPs in male Sprague Dawley rats (LDH/urea correlations)—the smallest particles showed the highest toxicity [48]; ultrasmall Fe3O4 (2.3–4.2 nm) were lethal in male ICR mice vs. 9.3 nm without acute toxicity [49]; Fe3O4 6/9/14 nm showed model-specific mechanisms in human hepatoma SK-Hep-1/Hep3B (mitochondrial ROS vs. membrane damage) [50]. | Smaller cores can increase reactivity and toxicity in vivo; in vitro relationships may invert across endpoints and lines. | Report core size (TEM/XRD), Dh 15 and PDI 16; compare within a single core chemistry under matched media and exposure. |
| Particle shape/aspect ratio | Modulates membrane contact area, uptake pathways, and intracellular fate. | Nanorods showed higher uptake and accumulation than spheres in mouse monocyte–macrophage RAW 264.7 line [52]; DSPE 3-PEG-coated nanocubes showed low cytotoxicity (≤0.5 mg Fe/mL) in mouse monocyte–macrophage RAW 264.7 line [53]; HA 4-nanoworms (mouse monocyte–macrophage RAW 264.7 line) elicited lower inflammatory response than spherical HA-MNPs (CD44) [54]; L929 fibroblasts: MNPs (with the same molarity) presented toxicity order: nanospheres.beads < nanoworms < nanospheres [55]. | Higher aspect ratio often increases uptake and injury; effects remain formulation- and model-dependent. | Compare only within the same core/coating; quantify aspect ratio; keep iron dose constant across shapes. | |
| Surface coating (chemistry) | Governs iron release in acidic compartments, protein corona, membrane interactions, biodistribution. | Acidic dissolution hierarchy: PLGA 5 slower (~9.56%) vs. bare (~15.3%) vs. DEX 6 (20.4%) and PVA 7 (21.9%) over 72 h in endosomal-like media [57]; bare MNPs exerted greater toxicity than starch-coated MNPs in adult Zebrafish gill; in contrast, starch-MNPs triggered more severe damage on liver [62]; PVA and poly(arabic acid) coatings did not penetrate/destabilize DPPC bilayer [64]; PVA coating-MNPs reduced antigen processing/CD4+ T-cell stimulation in dendric cells (DCs) [66]; DEX/PEG 6 were less cytotoxic to aortic endothelium than uncoated (lower ROS formation) [81]. | Coatings can attenuate or exacerbate apparent toxicity depending on dissolution kinetics and interfacial behavior. | Specify polymer identity/MW 17 and grafting density; characterize corona; interpret within one coating class. | |
| Surface charge (ζ-potential) | Electrostatics control adhesion to negatively charged membranes, uptake, and intracellular trafficking. | Amine (APTMS 8) and TEOS 9/APTMS-coated MNPs (+) increased membrane attachment and induced dose-dependent DNA damage in fibroblasts and fibrosarcoma normal cells; bare/TEOS-coated MNPs (−) did not in the same assays [65]; amine-Si-coated MNPs (+) were more cytotoxic than neutral azide-Si-coated, while carboxyl-Si-coated MNPs (−) were minimally cytotoxic (rat alveolar macrophage and human colonic adenocarcinoma cells) [67]. | Positive charge often increases cytotoxicity and genotoxicity; neutral/negative usually milder (toxicity system- and exposure-dependent) | Report ζ-potential in the exposure medium; maintain ionic strength/serum constant; compare within a given charge class. | |
| Colloidal behavior (Dh, aggregation, sedimentation) | Delivered dose and cell contact depend on Dh/agglomeration; can invert apparent toxicity rankings. | PLGA-PEG-coated MNPs caused sedimentation effect—after 24 h viability loss in human lung adenocarcinoma epithelial cell line A549 due to slow sedimentation/film over cells [60]; general note that aggregation depends on charge/coating/medium composition [59]. | Large/agglomerated Dh can increase apparent toxicity via sedimentation artifacts rather than intrinsic chemistry. | Measure Dh/PDI in relevant media; pre-disperse consistently; control plate geometry and mixing; normalize to delivered dose. | |
| Experimental models | Cell lineage/phenotype | Different uptake, antioxidant capacity, and membrane properties drive model-specific responses. | Human lung adenocarcinoma epithelial A549 epithelial cells: PLGA-PEG-coated MNPs—sedimentation effect [60]; human fetal osteoblast (hFOB) and human breast adenocarcinoma (MCF-7) cell line: bare most cytotoxic; HEC 10/PVP 11-coated mild toxicity; partial NCC 12 coverage increased toxicity [61]; human umbicilal artery smooth muscle cells (HUASMCs): ~10 nm cores with DEX/PVA/PLGA showed >70% viability without morphology changes [57]; DCs showed reduced antigen processing with PVA-MNPs [66]; porcine aortic endothelial cells (PAEC) less sensitive to DEX/PEG-coated vs. uncoated [81]. | Phagocytic/immune cells often show stronger responses; endothelial/mesenchymal responses vary by coating. | Use panels spanning epithelial, endothelial, immune, and stromal lines; report passage, origin, and culture conditions. |
| Organ/tissue context and species/stage | Organ physiology and developmental stage alter exposure routes, clearance, and pathway activation. | Adult zebrafish: bare MNPs more potent in gill; starch-coated more hepatic; overlapping pathways by RNA-seq [62]; zebrafish embryos: CR 13-MNPs non-teratogenic at tested levels; higher toxic effect on zebrafish larvae [82]. | Organ- and stage-specific effects; embryo assays may reveal developmental hazards not seen in adults. | State species, stage, organ; align exposure metrics; avoid cross-species generalization without caveats. | |
| Technical factors | Dose metrics and exposure duration | Apparent toxicity scales with concentration/time but nonlinearly across endpoints and models. | All formulations produced a concentration- and time-dependent decrease in viability of human lung adenocarcinoma epithelial A549 cells [60]; concentration- and time-dependent toxicity of core/shell GoldMag nanoparticles in human umbilical vein endothelial cells (HUVEs) [69]; concentration- and time-dependent toxicity of PVP-MNPs in human coronary artery endothelial cells (HCAEs) [70]; zebrafish embryo assays: minimal toxic effect at 200 μg/mL of CR-MNPs; toxic effects at high concentration (800 μg/mL) [82]. | Longer exposure and higher dose usually increase toxic effects; kinetics and accumulation modulate outcomes. | Report iron mass (µg Fe/mL), molarity (mM Fe), and surface area where possible; include multiple time points. |
| Medium composition/assay system | Ions, proteins, and buffer components shape aggregation, corona, and membrane interaction; assay readouts vary by platform. | Aggregation/agglomeration depends on i.a. medium composition and controls delivered dose [59]; bare (44 nm) and PEG-coated MNPs (76 nm) were ≥2× less cytotoxic than PEG-PLGA-MNPs under matched conditions [60]; DPPC bilayer studies showed that PVA/PAA 14-coated MNPs are non-disruptive at early contact [63]; genotoxicity outcomes differed across assays (Ames vs. mammalian panels) [62]. | Serum and ionic strength can mask or enhance effects; different assays capture different hazard facets. | Fix serum %, ionic strength, and pH; run orthogonal assays (e.g., viability + LDH + genotoxicity); document media and buffers. | |
| Rout of administration | Pulmonary, oral, intravenous injection | Pulmonary administration of MNPs in Wistar rats induce lung inflammations [72]; Oral: no observable changes in the digestive system [74]; possible hormonal imbalances [78]; intravenous injection of MNPs associated with adverse effects: oxidative stress and DNA damage in the heart [79]; necrosis of cardiac muscle tissue [80]; hemolysis and elevated AST and ALT levels [83]; apoptosis in human skin fibroblasts [84]. | Pulmonary: acute lung inflammation. increase in BALF LDH/total protein; oxidative stress in lung; histologic inflammatory changes; Oral: generally low overt GI pathology; Intravenous injection: Rapid RES sequestration (liver/spleen/lymph nodes); potential hemolysis/coagulation activation; transient hemodynamic effects; increase in liver enzymes; cardiac oxidative stress/DNA damage for certain coatings/doses | Inhalation: OECD TG 412/413, NP characterization, sham/positive controls. Instillation: standardized dose/dispersion, endotoxin-free (LAL). Oral: OECD TG 420/407/408, vehicle control, GI monitoring. Intravenous: sterile, endotoxin-free, hemocompatibility tests, saline/iron controls. |
| Purpose | Toxicity Test/Principle of the Method | Ref |
|---|---|---|
| Cells proliferation/viability investigation | Colorimetric tetrazolium salts: MTT 1, XTT 2, MTS 3, and WSTs (i.a., WST-1 4) assays. In contrast to dead cells, tetrazolium salts are reduced in viable, metabolically active cells to intensely colored formazan products. | [83,94] |
| LDH 5 assay. The enzyme is released from the cytoplasm into the cell culture medium following loss of membrane integrity, serving as an indicator of apoptosis, necrosis, or other forms of cellular damage. | [44,83,95] | |
| NRU 6 colorimetric assay. Viable cells incorporate neutral red into lysosomes, whereas dead cells fail to do so. The bound dye is subsequently extracted from the cytoplasm for quantification. | [96] | |
| Trypan blue stain assay (dye exclusion assay). Non-viable cells take up the dye and appear blue under light microscopy, whereas viable cells exclude it. | [90] | |
| Almar blue (resazurin) assay: A fluorometric method based on the enzymatic reduction of resazurin by viable cells into resorufin, a pink, fluorescent product that diffuses into the culture medium. | [97] | |
| Inclusion dyes such as calcein-AM 7 and 5-CFDA-AM 8 require intracellular enzymatic activity and membrane integrity. In viable cells, esterases cleave non-fluorescent precursors, producing fluorescent compounds retained within the cytoplasm. | [86] | |
| Oxidative stress assessment | ROS 9 production by DCFDA 10 assay. DCFDA diffuses into the cells and is deacylated by esterases to a non-fluorescent compound, which is oxidized by ROS into the highly fluorescent DCF 11. | [44,99] |
| Measurement of GPx 12, SOD 13, and catalase levels. The enzymes convert superoxide radicals into H2O2 and O2 (SOD), H2O2 into water (GPx), or H2O2 into O2 and water (catalase). The antioxidant protein expression levels can be detected using immunohistochemistry, immunofluorescence, or immunogold labeling. | [100] | |
| Estimation of NO level. Direct methods include EPR/ESR 14 and electrochemical assays, while indirect methods involve the determination of nitrate and nitrite concentrations. | [85,101] | |
| Measurement of disruption of mitochondrial functions. Such dysfunctions include changes in the mitochondrial membrane potential (MMP), which can be assessed by monitoring the uptake of rhodamine 123. | [102,103] | |
| Genotoxicity evaluation | Comet assay. The negatively charged, low-molecular-weight DNA fragments generated by damage migrate towards the anode during electrophoresis, producing a comet-like structure. The measurement of its “tail” length and intensity reflects the extent of DNA damage. | [104] |
| 8-OHdG 15 as a biomarker for oxidative DNA damage. ELISA or HPLC are commonly employed to detect 8-OHdG at oxidized DNA sites, predominantly within guanine bases. | [105] | |
| PI 16 or Hoechst dyes. PI stains DNA and RNA in non-viable cells or cells with reversibly damaged membranes. Hoechst dyes, such as bisbenzimide (Hoechst 33342) trihydrochloride, bind to the minor groove of double-stranded DNA—preferentially at A/T-rich regions—resulting in high fluorescence intensity. | [106,107] | |
| TUNEL assay. Detects both single- and double-stranded DNA breaks by enzymatic incorporation of modified nucleotides at sites of damage. | [108] | |
| Apoptosis detection | Measurement of the Caspase-3 activity. Since caspase-3 is a key executioner protease in apoptosis, its activity can be quantified using pro-fluorescent peptide substrates. Upon caspase-mediated cleavage, the fluorophore is released from the peptide backbone, yielding a measurable fluorescence signal. | [110] |
| AO/EB 17 staining. Acridine orange (AO) readily diffuses into viable cells, whereas ethidium bromide (EB) penetrates only cells with compromised membranes, as in apoptosis or necrosis. Cells stained solely with AO fluoresce green, while apoptotic or membrane-compromised cells preferentially incorporate EB, yielding red fluorescence. | [84] | |
| Immunotoxicity assessment | LPA 18 assay. LPA measures the ability of lymphocytes placed in a short-time tissue culture to undergo clonal proliferation when stimulated in vitro by an antigen, mitogen, or foreign molecules such as MNPs. | [111] |
| Membrane function assessment | ATPase assay. The ATPase assay determines the activity of membrane-bound ATPases based on the enzymatic hydrolysis of ATP to ADP and inorganic phosphate (Pi). The amount of Pi released is quantified colorimetrically, providing an indicator of membrane integrity and ion transport efficiency. | [112,113] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nowak-Jary, J.; Machnicka, B. Toxicity of Magnetic Nanoparticles in Medicine: Contributing Factors and Modern Assessment Methods. Int. J. Mol. Sci. 2025, 26, 8586. https://doi.org/10.3390/ijms26178586
Nowak-Jary J, Machnicka B. Toxicity of Magnetic Nanoparticles in Medicine: Contributing Factors and Modern Assessment Methods. International Journal of Molecular Sciences. 2025; 26(17):8586. https://doi.org/10.3390/ijms26178586
Chicago/Turabian StyleNowak-Jary, Julia, and Beata Machnicka. 2025. "Toxicity of Magnetic Nanoparticles in Medicine: Contributing Factors and Modern Assessment Methods" International Journal of Molecular Sciences 26, no. 17: 8586. https://doi.org/10.3390/ijms26178586
APA StyleNowak-Jary, J., & Machnicka, B. (2025). Toxicity of Magnetic Nanoparticles in Medicine: Contributing Factors and Modern Assessment Methods. International Journal of Molecular Sciences, 26(17), 8586. https://doi.org/10.3390/ijms26178586





