The Impact of Lifestyle on Reproductive Health: Microbial Complexity, Hormonal Dysfunction, and Pregnancy Outcomes
Abstract
1. Introduction
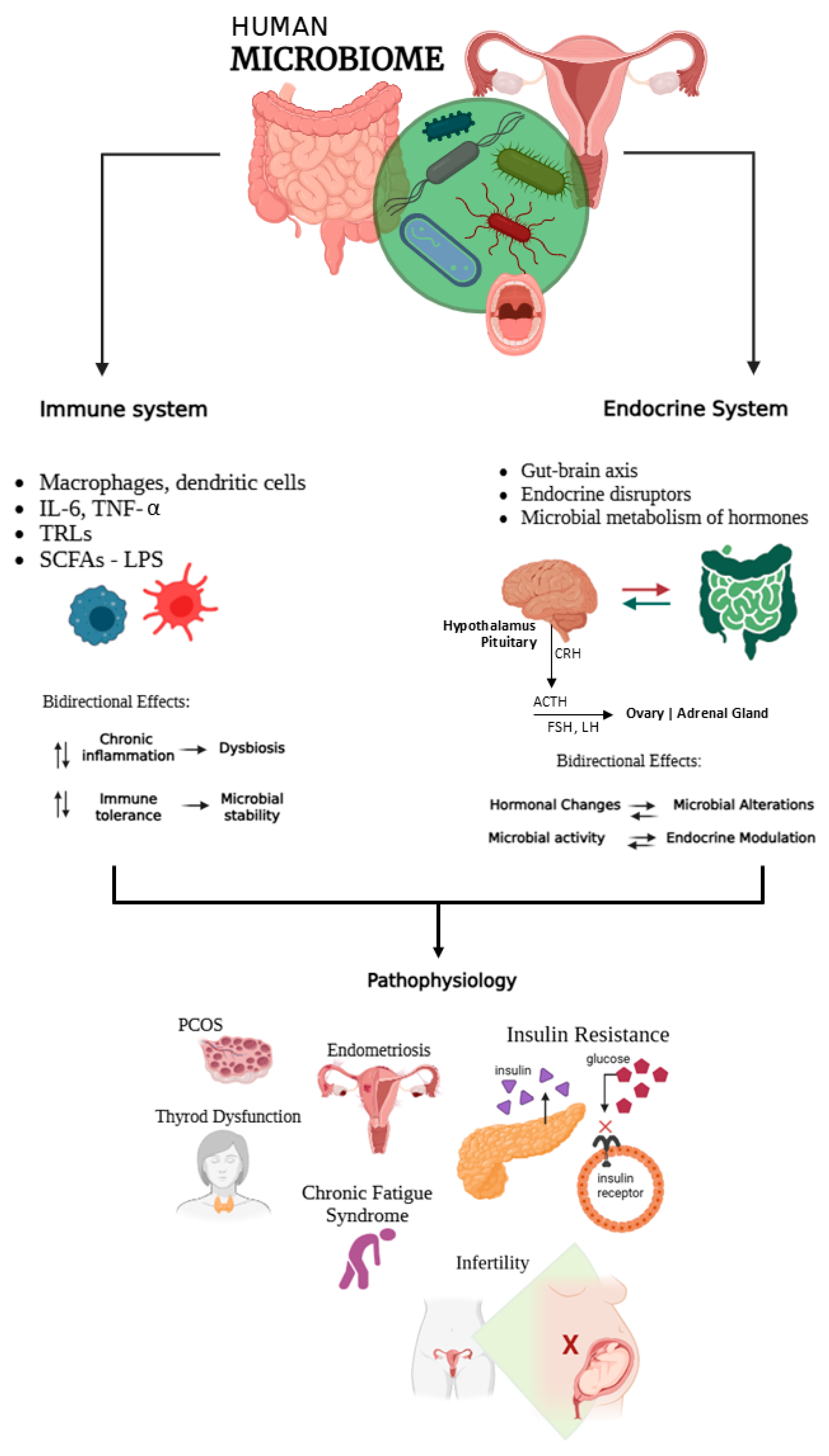
2. The Human Microbiome
2.1. Diversity of the Human Microbiome
2.2. The Microbiome and Immuno-Endocrine Interactions
3. Hormonal Regulation and Dysfunctions Related to Reproductive Health
4. The Interaction Between Hormones and Microorganisms in Fertility and Pregnancy
The Microbiome-Immune-Endocrine Axis
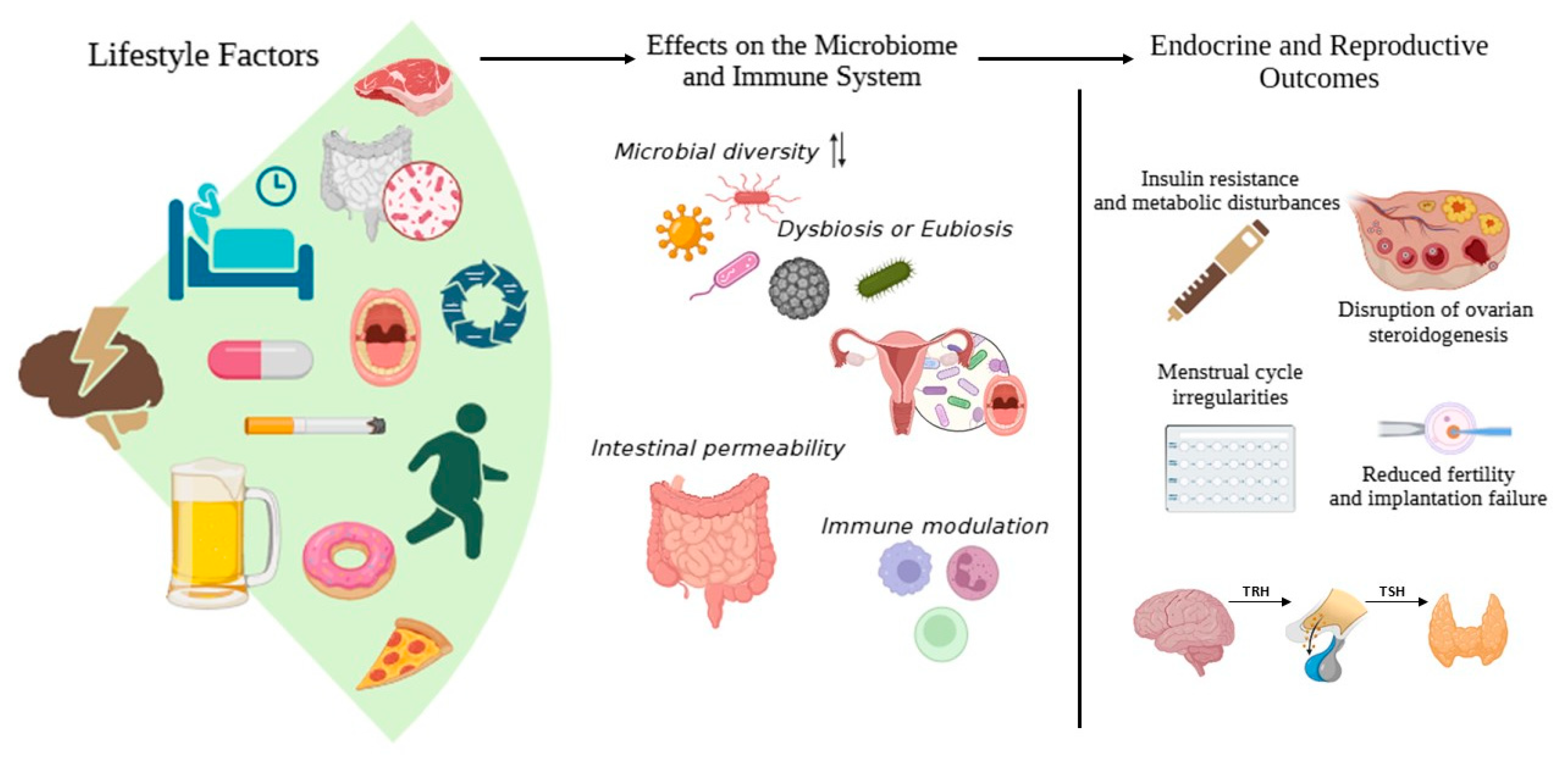
5. Influence of Lifestyle on Gut Microbiome and the Endocrine System
5.1. Diet and Exercise
5.2. Chronic Stress
5.3. Environmental Toxins
5.4. Drugs and Antibiotics
5.5. Sleep and Circadian Rhythms
5.6. Mental States (Stress, Anxiety, Depressive Symptoms)
6. Microbiome Changes During Pregnancy
6.1. Dysbiosis in Pregnancy
6.2. Role of the Maternal Microbiome in Foetal Development
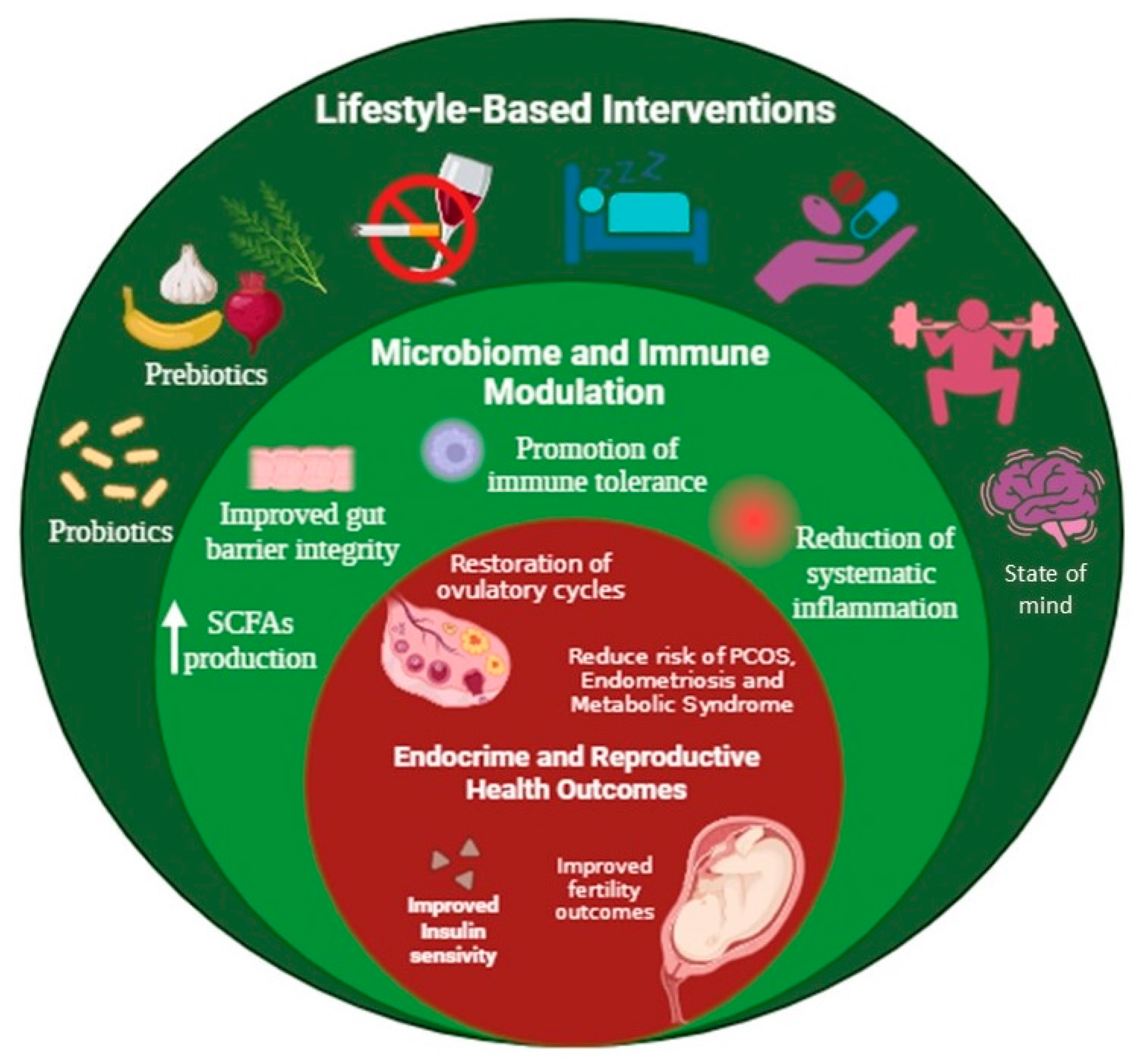
7. Preventive and Therapeutic Approaches Through Lifestyle Modifications
7.1. Diet and Exercise-Based Interventions
7.2. Personalised Strategies and Microbiome-Targeted Therapies
7.3. Male Contributions to Fertility and Pregnancy Outcomes
7.4. Clinical Assessment and Intervention of Mental States
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Teede, H.J.; Misso, M.L.; Costello, M.F.; Dokras, A.; Laven, J.; Moran, L.; Piltonen, T.; Norman, R.J. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Nat. Rev. Endocrinol. 2018, 14, 684–699. [Google Scholar] [CrossRef]
- Vander Borght, M.; Wyns, C. Fertility and infertility: Definition and epidemiology. Clin. Biochem. 2018, 62, 2–10. [Google Scholar] [CrossRef]
- Jasim, Z.A.; Al-Hakeim, H.K.; Zolghadri, S.; Stanek, A. Maternal tryptophan catabolites and insulin resistance parameters in preeclampsia. Biomolecules 2023, 13, 1447. [Google Scholar] [CrossRef]
- Toloza, F.J.K.; Derakhshan, A.; Männistö, T.; Bliddal, S.; Popova, P.V.; Carty, D.M.; Chen, L.; Taylor, P.; Mosso, L.; Oken, E.; et al. Association between maternal thyroid function and risk of gestational hypertension and pre-eclampsia: A systematic review and individual-participant data meta-analysis. Lancet Diabetes Endocrinol. 2022, 10, 243–252. [Google Scholar] [CrossRef]
- Nobles, C.J.; Mendola, P.; Mumford, S.L.; Naimi, A.I.; Yeung, E.H.; Kim, K.; Park, H.; Wilcox, B.; Silver, R.M.; Perkins, N.J.; et al. Preconception blood pressure levels and reproductive outcomes in a prospective cohort of women attempting pregnancy. Hypertension 2018, 71, 904–910. [Google Scholar] [CrossRef]
- Apicella, C.; Ruano, C.S.M.; Méhats, C.; Miralles, F.; Vaiman, D. The role of epigenetics in placental development and the etiology of preeclampsia. Int. J. Mol. Sci. 2019, 20, 2837. [Google Scholar] [CrossRef]
- Cho, I.; Blaser, M.J. The human microbiome: At the interface of health and disease. Nat. Rev. Genet. 2012, 13, 260–270. [Google Scholar] [CrossRef]
- Shreiner, A.B.; Kao, J.Y.; Young, V.B. The gut microbiome in health and in disease. Curr. Opin. Gastroenterol. 2015, 31, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Basak, S.; Mallick, R.; Navya Sree, B.; Duttaroy, A.K. Placental Epigenome Impacts Fetal Development: Effects of Maternal Nutrients and Gut Microbiota. Nutrients 2024, 16, 1860. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Cui, Z.; Yang, H. Interactions between host and gut microbiota in gestational diabetes mellitus and their impacts on offspring. BMC Microbiol. 2024, 24, 161. [Google Scholar] [CrossRef]
- Torres-Torres, J.; Monroy-Muñoz, I.E.; Perez-Duran, J.; Solis-Paredes, J.M.; Camacho-Martinez, Z.A.; Baca, D.; Espino-y-Sosa, S.; Martinez-Portilla, R.; Rojas-Zepeda, L.; Borboa-Olivares, H.; et al. Cellular and Molecular Pathophysiology of Gestational Diabetes. Int. J. Mol. Sci. 2024, 25, 11641. [Google Scholar] [CrossRef] [PubMed]
- Gomez de Agüero, M.; Ganal-Vonarburg, S.C.; Fuhrer, T.; Rupp, S.; Uchimura, Y.; Li, H.; Steinert, A.; Heikenwalder, M.; Hapfelmeier, S.; Sauer, U.; et al. The maternal microbiota drives early postnatal innate immune development. Science 2016, 351, 1296–1302. [Google Scholar] [CrossRef] [PubMed]
- Zhernakova, A.; Kurilshikov, A.; Bonder, M.J.; Tigchelaar, E.F.; Schirmer, M.; Vatanen, T.; Mujagic, Z.; Vich Vila, A.; Falony, G.; Vieira-Silva, S.; et al. Population-based metagenomics analysis reveals markers for gut microbiome composition and diversity. Science 2016, 352, 565–569. [Google Scholar] [CrossRef]
- Conlon, M.A.; Bird, A.R. The impact of diet and lifestyle on gut microbiota and human health. Nutrients 2015, 7, 17–44. [Google Scholar] [CrossRef]
- Gilbert, L.; Gross, J.; Lanzi, S.; Quansah, D.Y.; Puder, J.; Horsch, A. How diet, physical activity and psychosocial well-being interact in women with gestational diabetes mellitus: An integrative review. BMC Pregnancy Childbirth 2019, 19, 60. [Google Scholar] [CrossRef]
- Howard, L.M.; Khalifeh, H. Perinatal mental health: A review of progress and challenges. World Psychiatry 2020, 19, 313–327. [Google Scholar] [CrossRef]
- Rothschild, D.; Weissbrod, O.; Barkan, E.; Kurilshikov, A.; Korem, T.; Zeevi, D.; Costea, P.I.; Godneva, A.; Kalka, I.N.; Bar, N.; et al. Environment dominates over host genetics in shaping human gut microbiota composition. Nature 2018, 555, 210–215. [Google Scholar] [CrossRef]
- Koren, O.; Goodrich, J.K.; Cullender, T.C.; Spor, A.; Laitinen, K.; Kling Bäckhed, H.; González, A.; Werner, J.J.; Angenent, L.T.; Knight, R.; et al. Host remodeling of the gut microbiome and metabolic changes during pregnancy. Cell 2012, 150, 470–480. [Google Scholar] [CrossRef] [PubMed]
- Aagaard, K.; Ma, J.; Antony, K.M.; Ganu, R.; Petrosino, J.F.; Versalovic, J. The placenta harbors a unique microbiome. Sci. Transl. Med. 2014, 6, 237ra65. [Google Scholar] [CrossRef] [PubMed]
- Torres-Torres, J.; Basurto-Serrano, J.A.; Camacho-Martinez, Z.A.; Guadarrama-Sanchez, F.R.; Monroy-Muñoz, I.E.; Perez-Duran, J.; Solis-Paredes, J.M.; Martinez-Portilla, R.; Espino-y-Sosa, S.; Ramirez-Gonzalez, A.; et al. Microbiota Dysbiosis: A Key Modulator in Preeclampsia Pathogenesis and Its Therapeutic Potential. Microorganisms 2025, 13, 245. [Google Scholar] [CrossRef]
- Lloyd-Price, J.; Mahurkar, A.; Rahnavard, G.; Crabtree, J.; Orvis, J.; Hall, A.B.; Brady, A.; Creasy, H.H.; McCracken, C.; Giglio, M.G.; et al. Strains, functions and dynamics in the expanded Human Microbiome Project. Nature 2017, 550, 61–66. [Google Scholar] [CrossRef]
- Bogaert, D.; van Beveren, G.J.; de Koff, E.M.; Lusarreta Parga, P.; Balcazar Lopez, C.E.; Koppensteiner, L.; Clerc, M.; Hasrat, R.; Arp, K.; Chu, M.L.J.N.; et al. Mother-to-infant microbiota transmission and infant microbiota development across multiple body sites. Cell Host Microbe 2023, 31, 447–460.e6. [Google Scholar] [CrossRef]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef]
- Marques, P.; De Sousa Lages, A.; Skorupskaite, K.; Rozario, K.S.; Anderson, R.A.; George, J.T. Physiology of GnRH and Gonadotrophin Secretion. In Endotext; MDText.com, Inc.: Portland, OR, USA, 2000. Available online: https://www.ncbi.nlm.nih.gov/books/NBK279070/ (accessed on 10 June 2025).
- Bildik, G.; Akin, N.; Esmaeilian, Y.; Yilmaz, M.; Seyhan, A.; Sezerman, O.U.; Adali, E.; Uzun, I.; Yaba, A.; Demir, N.; et al. Terminal differentiation of human granulosa cells as luteinization is reversed by activin-A through silencing of Jnk pathway. Cell Death Discov. 2020, 6, 93. [Google Scholar] [CrossRef]
- Longo, M.; Liuzzi, F.; De Carlini, S.; La Marca, A. The role of LH in follicle development: From physiology to new clinical implications. Reprod. Biol. Endocrinol. 2025, 23 (Suppl. S1), 22. [Google Scholar] [CrossRef] [PubMed]
- Bulletti, C.; Bulletti, F.M.; Sciorio, R.; Guido, M. Progesterone: The Key Factor of the Beginning of Life. Int. J. Mol. Sci. 2022, 23, 14138. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-C.; Chiang, Y.-F.; Huang, K.-C.; Wang, K.-L.; Huang, Y.-J.; Shieh, T.-M.; Ali, M.; Hsia, S.-M. The vaginal microbiome: Associations with vaginal pH, menopause and metabolic parameters. Microorganisms 2025, 13, 1317. [Google Scholar] [CrossRef] [PubMed]
- Al-Maweri, S.A.; Al-Mashraqi, A.A.; Al-Qadhi, G.; Al-Hebshi, N.; Ba-Hattab, R. The association between the oral microbiome and hypertension: A systematic review. J. Oral. Microbiol. 2025, 17, 2459919. [Google Scholar] [CrossRef]
- Dunlop, A.L.; Mulle, J.G.; Ferranti, E.P.; Edwards, S.; Dunn, A.B.; Corwin, E.J. Maternal Microbiome and Pregnancy Outcomes That Impact Infant Health: A Review. Adv. Neonatal Care 2015, 15, 377–385. [Google Scholar] [CrossRef]
- Oliphant, K.; Allen-Vercoe, E. Macronutrient metabolism by the human gut microbiome: Major fermentation by-products and their impact on host health. Microbiome 2019, 7, 91. [Google Scholar] [CrossRef]
- Senthilkumar, H.; Arumugam, M. Gut microbiota: A hidden player in polycystic ovary syndrome. J. Transl. Med. 2025, 23, 443. [Google Scholar] [CrossRef]
- Fan, Y.; Pedersen, O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 2021, 19, 55–71. [Google Scholar] [CrossRef]
- da Silva, T.R.; Marchesan, L.B.; Rampelotto, P.H.; Longo, L.; de Oliveira, T.F.; Landberg, R.; de Mello, V.; Spritzer, P.M. Gut microbiota and gut-derived metabolites are altered and associated with dietary intake in women with polycystic ovary syndrome. J. Ovarian Res. 2024, 17, 232. [Google Scholar] [CrossRef]
- Borrel, G.; Brugère, J.F.; Gribaldo, S.; Schmitz, R.A.; Moissl-Eichinger, C. The host-associated archaeome. Nat. Rev. Microbiol. 2020, 18, 622–636. [Google Scholar] [CrossRef]
- Pausan, M.R.; Csorba, C.; Singer, G.; Till, H.; Schöpf, V.; Santigli, E.; Klug, B.; Högenauer, C.; Blohs, M.; Moissl-Eichinger, C. Exploring the archaeome: Detection of archaeal signatures in the human body. Front. Microbiol. 2019, 10, 2796. [Google Scholar] [CrossRef] [PubMed]
- Koskinen, K.; Pausan, M.R.; Perras, A.K.; Beck, M.; Bang, C.; Mora, M.; Schilhabel, A.; Schmitz, R.A.; Moissl-Eichinger, C. First insights into the diverse human archaeome: Specific detection of Archaea in the gastrointestinal tract, lung, and nose and on skin. mBio 2017, 8, e00824-17. [Google Scholar] [CrossRef] [PubMed]
- Vierbuchen, T.; Bang, C.; Rosigkeit, H.; Schmitz, R.A.; Heine, H. The human-associated archaeon Methanosphaera stadtmanae is recognized through its RNA and induces TLR8-dependent NLRP3 inflammasome activation. Front. Immunol. 2017, 8, 1535. [Google Scholar] [CrossRef] [PubMed]
- Shkoporov, A.N.; Hill, C. Bacteriophages of the human gut: The “known unknown” of the microbiome. Cell Host Microbe 2019, 25, 195–209. [Google Scholar] [CrossRef]
- de Jonge, P.A.; Wortelboer, K.; Scheithauer, T.P.M.; van den Born, B.H.; Zwinderman, A.H.; Nobrega, F.L.; Dutilh, B.E.; Nieuwdorp, M.; Herrema, H. Gut virome profiling identifies a widespread bacteriophage family associated with metabolic syndrome. Nat. Commun. 2022, 13, 3594. [Google Scholar] [CrossRef]
- Norman, J.M.; Handley, S.A.; Baldridge, M.T.; Droit, L.; Liu, C.Y.; Keller, B.C.; Kambal, A.; Monaco, C.L.; Zhao, G.; Fleshner, P.; et al. Disease-specific alterations in the enteric virome in inflammatory bowel disease. Cell 2015, 160, 447–460. [Google Scholar] [CrossRef]
- Madere, F.S.; Monaco, C.L. The female reproductive tract virome: Understanding the dynamic role of viruses in gynecological health and disease. Curr. Opin. Virol. 2022, 52, 15–23. [Google Scholar] [CrossRef]
- Happel, A.-U.; Varsani, A.; Balle, C.; Passmore, J.-A.; Jaspan, H. The vaginal virome—Balancing female genital tract bacteriome, mucosal immunity, and sexual and reproductive health outcomes. Viruses 2020, 12, 832. [Google Scholar] [CrossRef]
- Niyibizi, J.; Zanré, N.; Mayrand, M.H.; Trottier, H. Association Between Maternal Human Papillomavirus Infection and Adverse Pregnancy Outcomes: Systematic Review and Meta-Analysis. J. Infect. Dis. 2020, 221, 1925–1937. [Google Scholar] [CrossRef]
- Wylie, T.N.; Wylie, K.M.; Herter, B.N.; Storch, G.A. Enhanced virome sequencing using targeted sequence capture. Genome Res. 2015, 25, 1910–1920. [Google Scholar] [CrossRef] [PubMed]
- Kyathanahalli, C.; Snedden, M.; Hirsch, E. Human Anelloviruses: Prevalence and Clinical Significance During Pregnancy. Front. Virol. 2021, 1, 782886. [Google Scholar] [CrossRef]
- Yin, G.; Chen, F.; Chen, G.; Yang, X.; Huang, Q.; Chen, L.; Chen, M.; Zhang, W.; Ou, M.; Cao, M.; et al. Alterations of bacteriome, mycobiome and metabolome characteristics in PCOS patients with normal/overweight individuals. J. Ovarian Res. 2022, 15, 117. [Google Scholar] [CrossRef] [PubMed]
- Spatz, M.; Richard, M.L. Overview of the potential role of Malassezia in gut health and disease. Front. Cell Infect. Microbiol. 2020, 10, 201. [Google Scholar] [CrossRef]
- Wu, J.; Wang, K.; Qi, X.; Zhou, S.; Zhao, S.; Lu, M.; Nie, Q.; Li, M.; Han, M.; Luo, X.; et al. The intestinal fungus Aspergillus tubingensis promotes polycystic ovary syndrome through a secondary metabolite. Cell Host Microbe 2025, 33, 119–136.e11. [Google Scholar] [CrossRef]
- Ho, J.; Camilli, G.; Griffiths, J.S.; Richardson, J.P.; Kichik, N.; Naglik, J.R. Candida albicans and candidalysin in inflammatory disorders and cancer. Immunology 2021, 162, 11–16. [Google Scholar] [CrossRef]
- Liang, Y.; Zeng, W.; Hou, T.; Pan, R.; Huang, L. Gut microbiome and reproductive endocrine diseases: A Mendelian randomization study. Front. Endocrinol. 2023, 14, 1164186. [Google Scholar] [CrossRef]
- Park, J.; Kim, M.; Kang, S.G.; Jannasch, A.H.; Cooper, B.; Patterson, J.; Kim, C.H. Short-chain fatty acids induce both effector and regulatory T cells by suppression of histone deacetylases and regulation of the mTOR-S6K pathway. Mucosal Immunol. 2015, 8, 80–93. [Google Scholar] [CrossRef]
- Zhou, L.; Ni, Z.; Cheng, W.; Yu, J.; Sun, S.; Zhai, D.; Su, Y.; Tao, Y.; Pan, W.; Zeng, X.; et al. Dysbiosis of gut microbiota associated with clinical parameters in polycystic ovary syndrome. Front. Microbiol. 2020, 11, 1462. [Google Scholar] [CrossRef]
- Plottel, C.S.; Blaser, M.J. Microbiome and Malignancy. Cell Host Microbe 2011, 10, 324–335. [Google Scholar] [CrossRef]
- Sirota, I.; Zarek, S.M.; Segars, J.H. Potential influence of the microbiome on infertility and assisted reproductive technology. Semin. Reprod. Med. 2014, 32, 35–42. [Google Scholar] [CrossRef]
- Sun, Y.; Gao, S.; Ye, C.; Zhao, W. Gut microbiota dysbiosis in polycystic ovary syndrome: Mechanisms of progression and clinical applications. Front. Cell Infect. Microbiol. 2023, 13, 1142041. [Google Scholar] [CrossRef]
- Qi, X.; Yun, C.; Sun, L.; Xia, J.; Wu, Q.; Wang, Y.; Wang, L.; Zhang, Y.; Liang, X.; Wang, L.; et al. Gut microbiota–bile acid–interleukin-22 axis orchestrates polycystic ovary syndrome. Nat. Med. 2019, 25, 1225–1233. [Google Scholar] [CrossRef]
- Anahtar, M.N.; Gootenberg, D.B.; Mitchell, C.M.; Kwon, D.S. Cervicovaginal microbiota and reproductive health: The virtue of simplicity. Cell Host Microbe 2018, 23, 159–168. [Google Scholar] [CrossRef]
- Fettweis, J.M.; Serrano, M.G.; Brooks, J.P.; Edwards, D.J.; Girerd, P.H.; Parikh, H.I.; Huang, B.; Arodz, T.J.; Edupuganti, L.; Glascock, A.L.; et al. The vaginal microbiome and preterm birth. Nat. Med. 2019, 25, 1012–1021. [Google Scholar] [CrossRef]
- Torres, P.J.; Ho, B.S.; Arroyo, P.; Sau, L.; Chen, Y.H.; Julliard, K.; Butts, C.L.; Stener-Victorin, E.; Kurland, I.J.; Kitami, T. Gut microbial diversity in women with polycystic ovary syndrome correlates with hyperandrogenism. J. Clin. Endocrinol. Metab. 2018, 103, 1502–1511. [Google Scholar] [CrossRef] [PubMed]
- Fasano, A. Zonulin and its regulation of intestinal barrier function: The biological door to inflammation, autoimmunity, and cancer. Physiol. Rev. 2011, 91, 151–175. [Google Scholar] [CrossRef] [PubMed]
- Krassas, G.E.; Poppe, K.; Glinoer, D. Thyroid function and human reproductive health. Endocr. Rev. 2010, 31, 702–755. [Google Scholar] [CrossRef] [PubMed]
- Bucci, I.; Giuliani, C.; Di Dalmazi, G.; Formoso, G.; Napolitano, G. Thyroid autoimmunity in female infertility and assisted reproductive technology outcome. Front. Endocrinol. 2022, 13, 768363. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, M.H.; Howards, P.P.; Darrow, L.A.; Meadows, J.W.; Kesner, J.S.; Spencer, J.B.; Terrell, M.L.; Marcus, M. Thyroid hormones and menstrual cycle function in a longitudinal cohort of premenopausal women. Paediatr. Perinat. Epidemiol. 2018, 32, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk-Zieba, I.; Staszkiewicz-Chodor, J.; Boruszewska, D.; Lukaszuk, K.; Jaworska, J.; Woclawek-Potocka, I. Hypothyroidism affects uterine function via the modulation of prostaglandin signaling and uterine-receptivity factors in a rat model. Animals 2021, 11, 2636. [Google Scholar] [CrossRef]
- Gellersen, B.; Brosens, J.J. Cyclic decidualization of the human endometrium in reproductive health and failure. Endocr. Rev. 2014, 35, 851–905. [Google Scholar] [CrossRef]
- Lessey, B.A. Assessment of endometrial receptivity. Fertil. Steril. 2011, 96, 522–529. [Google Scholar] [CrossRef]
- Maraka, S.; Singh Ospina, N.M.; O’Keeffe, D.T.; Rodriguez-Gutierrez, R.; Espinosa De Ycaza, A.E.; Wi, C.I.; Juhn, Y.J.; Coddington, C.C., 3rd; Montori, V.M.; Stan, M.N. Effects of Levothyroxine Therapy on Pregnancy Outcomes in Women with Subclinical Hypothyroidism. Thyroid 2016, 26, 980–986. [Google Scholar] [CrossRef]
- Alexander, E.K.; Pearce, E.N.; Brent, G.A.; Brown, R.S.; Chen, H.; Dosiou, C.; Grobman, W.A.; Laurberg, P.; Lazarus, J.H.; Mandel, S.J.; et al. 2017 Guidelines of the American Thyroid Association for the Diagnosis and Management of Thyroid Disease During Pregnancy and the Postpartum. Thyroid 2017, 27, 315–389, Correction in Thyroid, 2017, 27, 315–389.. [Google Scholar] [CrossRef]
- Stagnaro-Green, A.; Abalovich, M.; Alexander, E.; Azizi, F.; Mestman, J.; Negro, R.; Nixon, A.; Pearce, E.N.; Soldin, O.P.; Sullivan, S.; et al. Guidelines of the American Thyroid Association for the Diagnosis and Management of Thyroid Disease During Pregnancy and Postpartum. Thyroid 2011, 21, 1081–1125. [Google Scholar] [CrossRef]
- De Groot, L.; Abalovich, M.; Alexander, E.K.; Amino, N.; Barbour, L.; Cobin, R.H.; Eastman, C.J.; Lazarus, J.H.; Luton, D.; Mandel, S.J.; et al. Management of thyroid dysfunction during pregnancy and postpartum: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2012, 97, 2543–2565. [Google Scholar] [CrossRef]
- Zhang, J.; Zhu, Y.; Wang, J.; Hu, H.; Jin, Y.; Mao, X.; Zhang, H.; Ye, Y.; Xin, X.; Li, D. Global burden and epidemiological prediction of polycystic ovary syndrome from 1990 to 2019: A systematic analysis from the Global Burden of Disease Study 2019. PLoS ONE 2024, 19, e0306991. [Google Scholar] [CrossRef] [PubMed]
- Rosenfield, R.L.; Ehrmann, D.A. The Pathogenesis of Polycystic Ovary Syndrome (PCOS): The Hypothesis of PCOS as Functional Ovarian Hyperandrogenism Revisited. Endocr. Rev. 2016, 37, 467–520. [Google Scholar] [CrossRef]
- Goodarzi, M.O.; Dumesic, D.A.; Chazenbalk, G.; Azziz, R. Polycystic ovary syndrome: Etiology, pathogenesis and diagnosis. Nat. Rev. Endocrinol. 2011, 7, 219–231. [Google Scholar] [CrossRef]
- Diamanti-Kandarakis, E.; Dunaif, A. Insulin resistance and the polycystic ovary syndrome revisited: An update on mechanisms and implications. Endocr. Rev. 2012, 33, 981–1030. [Google Scholar] [CrossRef]
- Palomba, S.; Santagni, S.; Falbo, A.; La Sala, G.B. Complications and challenges associated with polycystic ovary syndrome: Current perspectives. Int. J. Womens Health 2015, 7, 745–763. [Google Scholar] [CrossRef] [PubMed]
- Legro, R.S.; Arslanian, S.A.; Ehrmann, D.A.; Hoeger, K.M.; Murad, M.H.; Pasquali, R.; Welt, C.K.; Endocrine Society. Diagnosis and treatment of polycystic ovary syndrome: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2013, 98, 4565–4592. [Google Scholar] [CrossRef]
- D’Anna, R.; Di Benedetto, A.; Scilipoti, A.; Santamaria, A.; Interdonato, M.L.; Petrella, E.; Neri, I.; Pintaudi, B.; Corrado, F.; Facchinetti, F. Myo-inositol Supplementation for Prevention of Gestational Diabetes in Obese Pregnant Women: A Randomized Controlled Trial. Obstet. Gynecol. 2015, 126, 310–315. [Google Scholar] [CrossRef]
- Critchley, H.O.D.; Maybin, J.A.; Armstrong, G.; Williams, A.R.W. Physiology of the Endometrium and Regulation of Menstruation. Physiol. Rev. 2020, 100, 1149–1179. [Google Scholar] [CrossRef]
- Mendelson, C.R. Minireview: Fetal-Maternal Hormonal Signaling in Pregnancy and Labor. Mol. Endocrinol. 2009, 23, 947–954. [Google Scholar] [CrossRef]
- Moloney, R.D.; Desbonnet, L.; Clarke, G.; Dinan, T.G.; Cryan, J.F. The microbiome: Stress, health and disease. Mamm. Genome 2014, 25, 49–74. [Google Scholar] [CrossRef] [PubMed]
- Herman, J.P.; McKlveen, J.M.; Ghosal, S.; Kopp, B.; Wulsin, A.; Makinson, R.; Scheimann, J.; Myers, B. Regulation of the Hypothalamic-Pituitary-Adrenocortical Stress Response. Compr. Physiol. 2016, 6, 603–621. [Google Scholar] [CrossRef]
- Schliep, K.C.; Mumford, S.L.; Vladutiu, C.J.; Ahrens, K.A.; Perkins, N.J.; Sjaarda, L.A.; Kissell, K.A.; Prasad, A.; Wactawski-Wende, J.; Schisterman, E.F. Perceived stress, reproductive hormones, and ovulatory function: A prospective cohort study. Epidemiology 2015, 26, 177–184. [Google Scholar] [CrossRef]
- Ferin, M. Clinical review 105: Stress and the reproductive cycle. J. Clin. Endocrinol. Metab. 1999, 84, 1768–1774. [Google Scholar] [CrossRef]
- Heintz-Buschart, A.; Wilmes, P. Human Gut Microbiome: Function Matters. Trends Microbiol. 2018, 26, 563–574. [Google Scholar] [CrossRef] [PubMed]
- Rinne, G.R.; Somers, J.A.; Ramos, I.F.; Ross, K.M.; Coussons-Read, M.; Dunkel Schetter, C. Increases in maternal depressive symptoms during pregnancy and infant cortisol reactivity: Mediation by placental corticotropin-releasing hormone. Dev. Psychopathol. 2023, 35, 1997–2010. [Google Scholar] [CrossRef]
- Bakhireva, L.N.; Ma, X.; Wiesel, A.; Lowe, J.R.; Rai, R.; Solomon, E.; Weinberg, J.; Roberts, M.H. Predictive utility of placental hypothalamic–pituitary–adrenal axis biomarkers and infant neurodevelopment. J Neuroendocr. 2025, e70071. [Google Scholar] [CrossRef]
- Simonovich, S.D.; Nidey, N.L.; Gavin, A.R.; Piñeros-Leaño, M.; Hsieh, W.-J.; Sbrilli, M.D.; Ables-Torres, L.A.; Huang, H.; Ryckman, K.; Tabb, K.M. Meta-analysis of antenatal depression and adverse birth outcomes in U.S. populations, 2010–2020. Health Aff. 2021, 40, 1560–1565. [Google Scholar] [CrossRef]
- Argaw-Denboba, A.; Schmidt, T.S.B.; Di Giacomo, M.; Zhernakova, D.V.; Li, Y.; Bonder, M.J.; Kurilshikov, A.; Sinha, T.; Lifshitz, S.; Zhiheng, P.; et al. Paternal microbiome perturbations impact offspring fitness. Nature 2024, 629, 652–659. [Google Scholar] [CrossRef] [PubMed]
- Dao, M.C.; Everard, A.; Aron-Wisnewsky, J.; Sokolovska, N.; Prifti, E.; Verger, E.O.; Kayser, B.D.; Levenez, F.; Chilloux, J.; Hoyles, L.; et al. Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity: Relationship with gut microbiome richness and ecology. Gut 2016, 65, 426–436. [Google Scholar] [CrossRef]
- Miquel, S.; Martín, R.; Rossi, O.; Bermúdez-Humarán, L.G.; Chatel, J.M.; Sokol, H.; Thomas, M.; Wells, J.M.; Langella, P. Faecalibacterium prausnitzii and human intestinal health. Curr. Opin. Microbiol. 2013, 16, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Russell, N.J.; Seale, A.C.; O’Driscoll, M.; O’Sullivan, C.; Bianchi-Jassir, F.; Gonzalez-Guarin, J.; Lawn, J.E.; Baker, C.J.; Bartlett, L.; Cutland, C.; et al. Maternal Colonization With Group B Streptococcus and Serotype Distribution Worldwide: Systematic Review and Meta-analyses. Clin. Infect. Dis. 2017, 65 (Suppl. S2), S100–S111. [Google Scholar] [CrossRef]
- Brotman, R.M.; Shardell, M.D.; Gajer, P.; Fadrosh, D.; Chang, K.; Silver, M.I.; Viscidi, R.P.; Burke, A.E.; Ravel, J.; Gravitt, P.E. Association between the vaginal microbiota, menopause status, and signs of vulvovaginal atrophy. Menopause 2014, 21, 450–458. [Google Scholar] [CrossRef]
- Kwa, M.; Plottel, C.S.; Blaser, M.J.; Adams, S. The Intestinal Microbiome and Estrogen Receptor-Positive Female Breast Cancer. J. Natl. Cancer Inst. 2016, 108, djw029. [Google Scholar] [CrossRef]
- Ait-Belgnaoui, A.; Durand, H.; Cartier, C.; Chaumaz, G.; Eutamene, H.; Ferrier, L.; Houdeau, E.; Fioramonti, J.; Bueno, L.; Theodorou, V. Prevention of gut leakiness by a probiotic treatment leads to attenuated HPA response to an acute psychological stress in rats. Psychoneuroendocrinology 2012, 37, 1885–1895. [Google Scholar] [CrossRef]
- Colldén, H.; Landin, A.; Wallenius, V.; Elebring, E.; Fändriks, L.; Nilsson, M.E.; Ryberg, H.; Poutanen, M.; Sjögren, K.; Vandenput, L.; et al. The gut microbiota is a major regulator of androgen metabolism in intestinal contents. Am. J. Physiol. Endocrinol. Metab. 2019, 317, E1182–E1192. [Google Scholar] [CrossRef]
- Nikiforou, M.; Jacobs, E.M.; Kemp, M.W.; Hornef, M.W.; Payne, M.S.; Saito, M.; Newnham, J.P.; Janssen, L.E.; Jobe, A.H.; Kallapur, S.G.; et al. Intra-amniotic Candida albicans infection induces mucosal injury and inflammation in the ovine fetal intestine. Sci. Rep. 2016, 6, 29806. [Google Scholar] [CrossRef]
- Iliev, I.D.; Funari, V.A.; Taylor, K.D.; Nguyen, Q.; Reyes, C.N.; Strom, S.P.; Brown, J.; Becker, C.A.; Fleshner, P.R.; Dubinsky, M.; et al. Interactions between commensal fungi and the C-type lectin receptor Dectin-1 influence colitis. Science 2012, 336, 1314–1317. [Google Scholar] [CrossRef]
- Ardekani, O.S.; Letafati, A.; Dehkordi, S.E.; Farahani, A.V.; Bahari, M.; Mahdavi, B.; Ariamand, N.; Taghvaei, M.; Kohkalani, M.; Pirkooh, A.A.; et al. From infection to infertility: A review of the role of human papillomavirus-induced oxidative stress on reproductive health and infertility. Eur. J. Med. Res. 2025, 30, 339. [Google Scholar] [CrossRef] [PubMed]
- da Costa, A.C.; Moron, A.F.; Forney, L.J.; Linhares, I.M.; Sabino, E.; Costa, S.F.; Mendes-Correa, M.C.; Witkin, S.S. Identification of bacteriophages in the vagina of pregnant women: A descriptive study. BJOG 2021, 128, 976–982. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Liwinski, T.; Elinav, E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020, 30, 492–506. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Huang, Y.; Cai, W.; Li, D.; Zheng, W.; Xiao, Y.; Liu, Y.; Zhao, H.; Pan, S. Effect of oral Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14 on vaginal Group B Streptococcus colonization and vaginal microbiome in late pregnancy. J. South. Med. Univ. 2020, 40, 1753–1759. [Google Scholar] [CrossRef]
- Yang, S.; Reid, G.; Challis, J.R.G.; Gloor, G.B.; Asztalos, E.; Money, D.; Seney, S.; Bocking, A.D. Effect of oral probiotic Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14 on the vaginal microbiota, cytokines and chemokines in pregnant women. Nutrients 2020, 12, 368. [Google Scholar] [CrossRef]
- Ho, M.; Chang, Y.Y.; Chang, W.C.; Lin, H.C.; Wang, M.H.; Lin, W.C.; Chiu, T.H. Oral Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14 to reduce Group B Streptococcus colonization in pregnant women: A randomized controlled trial. Taiwan. J. Obstet. Gynecol. 2016, 55, 515–518. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liang, Z. Determination of tumor necrosis factor-alpha and interleukin-6 levels of the tubal fluids in patients with infertility caused by infection of Chlamydia trachomatis. Zhonghua Fu Chan Ke Za Zhi 2000, 35, 411–412. [Google Scholar] [PubMed]
- Ikeda, R.; Ushio, N.; Abdou, A.M.; Furuoka, H.; Nishikawa, Y. Toll-Like Receptor 2 is Involved in Abnormal Pregnancy in Mice Infected with Toxoplasma gondii During Late Pregnancy. Front. Microbiol. 2021, 12, 741104. [Google Scholar] [CrossRef]
- Robert-Gangneux, F.; Dardé, M.L. Epidemiology of and Diagnostic Strategies for Toxoplasmosis. Clin. Microbiol. Rev. 2012, 25, 264–296, Correction in Clin. Microbiol. Rev., 2012, 25, 264–296.. [Google Scholar] [CrossRef]
- Taylor-Robinson, D.; Jensen, J.S. Mycoplasma genitalium: From Chrysalis to Multicolored Butterfly. Clin. Microbiol. Rev. 2011, 24, 498–514. [Google Scholar] [CrossRef] [PubMed]
- Lis, R.; Rowhani-Rahbar, A.; Manhart, L.E. Mycoplasma genitalium Infection and Female Reproductive Tract Disease: A Meta-Analysis. Clin. Infect. Dis. 2015, 61, 418–426. [Google Scholar] [CrossRef]
- Capoccia, R.; Greub, G.; Baud, D. Ureaplasma urealyticum, Mycoplasma hominis and Adverse Pregnancy Outcomes. Curr. Opin. Infect. Dis. 2013, 26, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Oh, K.J.; Lee, S.E.; Jung, H.; Kim, G.; Romero, R.; Yoon, B.H. Detection of ureaplasmas by the polymerase chain reaction in the amniotic fluid of patients with cervical insufficiency. J. Perinat. Med. 2010, 38, 261–268. [Google Scholar] [CrossRef]
- Petrova, M.I.; Lievens, E.; Verhoeven, T.L.; Macklaim, J.M.; Gloor, G.B.; Schols, D.; Vanderleyden, J.; Reid, G.; Lebeer, S. The lectin-like protein 1 in Lactobacillus rhamnosus GR-1 mediates tissue-specific adherence to vaginal epithelium and inhibits urogenital pathogens. Sci. Rep. 2016, 6, 37437. [Google Scholar] [CrossRef]
- Ravel, J.; Brotman, R.M.; Gajer, P.; Ma, B.; Nandy, M.; Fadrosh, D.W.; Sakamoto, J.; Koenig, S.S.; Fu, L.; Zhou, X.; et al. Daily temporal dynamics of vaginal microbiota before, during and after episodes of bacterial vaginosis. Microbiome 2013, 1, 29. [Google Scholar] [CrossRef]
- Romero, R.; Hassan, S.S.; Gajer, P.; Tarca, A.L.; Fadrosh, D.W.; Nikita, L.; Galuppi, M.; Lamont, R.F.; Chaemsaithong, P.; Miranda, J.; et al. The Composition and Stability of the Vaginal Microbiota of Normal Pregnant Women Is Different from That of Non-Pregnant Women. Microbiome 2014, 2, 4. [Google Scholar] [CrossRef]
- Elovitz, M.A.; Gajer, P.; Riis, V.; Brown, A.G.; Humphrys, M.S.; Holm, J.B.; Ravel, J.; Gravett, M.G.; Wadhwa, P.D.; Paquette, A.G. Cervicovaginal Microbiota and Local Immune Response Modulate the Risk of Spontaneous Preterm Delivery. Nat. Commun. 2019, 10, 1305. [Google Scholar] [CrossRef] [PubMed]
- Brotman, R.M. Vaginal Microbiome and Sexually Transmitted Infections: An Epidemiologic Perspective. J. Clin. Investig. 2011, 121, 4610–4617. [Google Scholar] [CrossRef] [PubMed]
- Lebeau, A.; Bruyere, D.; Roncarati, P.; Peixoto, P.; Hervouet, E.; Cobraiville, G.; Taminiau, B.; Masson, M.; Gallego, C.; Mazzucchelli, G.; et al. HPV infection alters vaginal microbiome through down-regulating host mucosal innate peptides used by Lactobacilli as amino acid sources. Nat. Commun. 2022, 13, 1076. [Google Scholar] [CrossRef]
- Cani, P.D.; Van Hul, M.; Lefort, C.; Depommier, C.; Rastelli, M.; Everard, A. Microbial regulation of organismal energy homeostasis. Nat. Metab. 2019, 1, 34–46. [Google Scholar] [CrossRef]
- Leeming, E.R.; Johnson, A.J.; Spector, T.D.; Le Roy, C.I. Effect of Diet on the Gut Microbiota: Rethinking Intervention Duration. Nutrients 2019, 11, 2862. [Google Scholar] [CrossRef] [PubMed]
- Chavarro, J.E.; Rich-Edwards, J.W.; Rosner, B.A.; Willett, W.C. Diet and lifestyle in the prevention of ovulatory disorder infertility. Obstet. Gynecol. 2007, 110, 1050–1058. [Google Scholar] [CrossRef]
- Chavarro, J.E.; Rich-Edwards, J.W.; Rosner, B.A.; Willett, W.C. A prospective study of dietary carbohydrate quantity and quality in relation to risk of ovulatory infertility. Eur. J. Clin. Nutr. 2009, 63, 78–86. [Google Scholar] [CrossRef]
- Martínez-González, M.A.; Gea, A.; Ruiz-Canela, M. The Mediterranean Diet and Cardiovascular Health. Circ. Res. 2019, 124, 779–798. [Google Scholar] [CrossRef]
- Xia, W.; Chiu, Y.H.; Williams, P.L.; Gaskins, A.J.; Toth, T.L.; Tanrikut, C.; Hauser, R.; Chavarro, J.E. Men’s meat intake and treatment outcomes among couples undergoing assisted reproduction. Fertil. Steril. 2015, 104, 972–979. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.M.; Mailing, L.J.; Niemiro, G.M.; Moore, R.; Cook, M.D.; White, B.A.; Holscher, H.D.; Woods, J.A. Exercise Alters Gut Microbiota Composition and Function in Lean and Obese Humans. Med. Sci. Sports Exerc. 2018, 50, 747–757. [Google Scholar] [CrossRef] [PubMed]
- Mailing, L.J.; Allen, J.M.; Buford, T.W.; Fields, C.J.; Woods, J.A. Exercise and the Gut Microbiome: A Review of the Evidence, Potential Mechanisms, and Implications for Human Health. Exerc. Sport. Sci. Rev. 2019, 47, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Deli, C.K.; Fatouros, I.G.; Poulios, A.; Liakou, C.A.; Draganidis, D.; Papanikolaou, K.; Rosvoglou, A.; Gatsas, A.; Georgakouli, K.; Tsimeas, P.; et al. Gut Microbiota in the Progression of Type 2 Diabetes and the Potential Role of Exercise: A Critical Review. Life 2024, 14, 1016. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Yang, C.; Xie, H.; Liu, Y.; Liao, Y. Effects of Aerobic Exercise on Rats with Hyperandrogenic Polycystic Ovarian Syndrome. Int. J. Endocrinol. 2021, 2021, 5561980. [Google Scholar] [CrossRef]
- Motaharinezhad, F.; Emadi, A.; Hosnian, M.; Kheirkhahan, A.; Jayedi, A.; Ehsani, F. The effects of different exercises on weight loss and hormonal changes in women with polycystic ovarian syndrome: A network meta-analysis study. BMC Womens Health 2024, 24, 512. [Google Scholar] [CrossRef]
- Joy, E.; De Souza, M.J.; Nattiv, A.; Misra, M.; Williams, N.I.; Mallinson, R.J.; Gibbs, J.C.; Olmsted, M.; Goolsby, M.; Matheson, G.; et al. 2014 female athlete triad coalition consensus statement on treatment and return to play of the female athlete triad. Curr. Sports Med. Rep. 2014, 13, 219–232. [Google Scholar] [CrossRef]
- Giallauria, F.; Palomba, S.; Maresca, L.; Vuolo, L.; Tafuri, D.; Lombardi, G.; Colao, A.; Vigorito, C.; Francesco, O. Exercise training improves autonomic function and inflammatory pattern in women with polycystic ovary syndrome (PCOS). Clin. Endocrinol. 2008, 69, 792–798. [Google Scholar] [CrossRef]
- Shele, G.; Genkil, J.; Speelman, D. A Systematic Review of the Effects of Exercise on Hormones in Women with Polycystic Ovary Syndrome. J. Funct. Morphol. Kinesiol. 2020, 5, 35. [Google Scholar] [CrossRef]
- Uchida, M.C.F.; Bacurau, R.F.P.; Navarro, F.L.P.; Pontes, F.L., Jr.; Tessuti, V.D.; Moreau, R.L.P.C.; Aoki, M.S. Alteration of testosterone:cortisol ratio induced by resistance training in women. Rev. Bras. Med. Esporte 2004, 10, 165–168. [Google Scholar] [CrossRef]
- Stepto, N.K.; Hiam, D.; Gibson-Helm, M.; Cassar, S.; Harrison, C.L.; Hutchison, S.K.; Joham, A.E.; Canny, B.J.; Moreno-Asso, A.; Strauss, B.J.; et al. Exercise and insulin resistance in PCOS: Muscle insulin signalling and fibrosis. Endocr. Connect. 2020, 9, 346–359. [Google Scholar] [CrossRef]
- Mennitti, C.; Farina, G.; Imperatore, A.; De Fonzo, G.; Gentile, A.; La Civita, E.; Carbone, G.; De Simone, R.R.; Di Iorio, M.R.; Tinto, N.; et al. How Does Physical Activity Modulate Hormone Responses? Biomolecules 2024, 14, 1418. [Google Scholar] [CrossRef]
- Clarke, S.F.; Murphy, E.F.; O’Sullivan, O.; Lucey, A.J.; Humphreys, M.; Hogan, A.; Hayes, P.; O’Reilly, M.; Jeffery, I.B.; Wood-Martin, R.; et al. Exercise and associated dietary extremes impact on gut microbial diversity. Gut 2014, 63, 1913–1920. [Google Scholar] [CrossRef]
- Varghese, S.; Rao, S.; Khattak, A.; Zamir, F.; Chaari, A. Physical Exercise and the Gut Microbiome: A Bidirectional Relationship Influencing Health and Performance. Nutrients 2024, 16, 3663. [Google Scholar] [CrossRef] [PubMed]
- Lee-Sarwar, K.A.; Ramirez, L. Diversifying your diet portfolio: Potential impacts of dietary diversity on the gut microbiome and human health. Am. J. Clin. Nutr. 2022, 116, 844–845. [Google Scholar] [CrossRef]
- Smith, P.M.; Howitt, M.R.; Panikov, N.; Michaud, M.; Gallini, C.A.; Bohlooly-Y, M.; Glickman, J.N.; Garrett, W.S. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 2013, 341, 569–573. [Google Scholar] [CrossRef] [PubMed]
- Whisner, C.M.; Maldonado, J.; Dente, B.; Krajmalnik-Brown, R.; Bruening, M. Diet, physical activity and screen time but not body mass index are associated with the gut microbiome of a diverse cohort of college students living in university housing: A cross-sectional study. BMC Microbiol. 2018, 18, 210. [Google Scholar] [CrossRef]
- Sahhaf Ebrahimi, F.; Homayouni Rad, A.; Mosen, M.; Abbasalizadeh, F.; Tabrizi, A.; Khalili, L. Effect of Lactobacillus acidophilus and Bifidobacterium lactis on blood glucose in women with gestational diabetes mellitus: A randomized placebo-controlled trial. Diabetol. Metab. Syndr. 2019, 11, 75. [Google Scholar] [CrossRef] [PubMed]
- Ward, C.P.; Perelman, D.; Durand, L.R.; Robinson, J.L.; Cunanan, K.M.; Sudakaran, S.; Sabetan, R.; Madrigal-Moeller, M.J.; Dant, C.; Sonnenburg, E.D.; et al. Effects of fermented and fiber-rich foods on maternal & offspring microbiome study (FeFiFo-MOMS)—Study design and methods. Contemp. Clin. Trials 2025, 150, 107834. [Google Scholar] [CrossRef]
- Cryan, J.F.; O’Riordan, K.J.; Sandhu, K.; Peterson, V.; Dinan, T.G. The gut microbiome in neurological disorders. Lancet Neurol. 2020, 19, 179–194. [Google Scholar] [CrossRef]
- Zijlmans, M.A.; Korpela, K.; Riksen-Walraven, J.M.; de Vos, W.M.; de Weerth, C. Maternal prenatal stress is associated with the infant intestinal microbiota. Psychoneuroendocrinology 2015, 53, 233–245. [Google Scholar] [CrossRef]
- Martin, C.R.; Osadchiy, V.; Kalani, A.; Mayer, E.A. The Brain-Gut-Microbiome Axis. Cell Mol. Gastroenterol. Hepatol. 2018, 6, 133–148. [Google Scholar] [CrossRef] [PubMed]
- Rusch, J.A.; Layden, B.T.; Dugas, L.R. Signalling cognition: The gut microbiota and hypothalamic-pituitary-adrenal axis. Front. Endocrinol. 2023, 14, 1130689. [Google Scholar] [CrossRef] [PubMed]
- Foster, J.A.; Rinaman, L.; Cryan, J.F. Stress & the gut-brain axis: Regulation by the microbiome. Neurobiol. Stress. 2017, 7, 124–136. [Google Scholar] [CrossRef] [PubMed]
- Berga, S.L.; Loucks, T.L. Use of cognitive behavior therapy for functional hypothalamic amenorrhea. Ann. N. Y. Acad. Sci. 2006, 1092, 114–129. [Google Scholar] [CrossRef]
- Charmandari, E.; Tsigos, C.; Chrousos, G. Endocrinology of the stress response. Annu. Rev. Physiol. 2005, 67, 259–284. [Google Scholar] [CrossRef]
- Jiang, H.; Ling, Z.; Zhang, Y.; Mao, H.; Ma, Z.; Yin, Y.; Wang, W.; Tang, W.; Tan, Z.; Shi, J.; et al. Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav. Immun. 2015, 48, 186–194. [Google Scholar] [CrossRef]
- Petrelli, G.; Figà-Talamanca, I.; Lauria, L.; Mantovani, A. Spontaneous abortion in spouses of greenhouse workers exposed to pesticides. Environ. Health Prev. Med. 2003, 8, 77–81. [Google Scholar] [CrossRef]
- Banerjee, B.D.; Seth, V.; Ahmed, R.S. Pesticide-induced oxidative stress: Perspectives and trends. Rev. Environ. Health 2001, 16, 1–40. [Google Scholar] [CrossRef]
- Caporossi, L.; Viganò, P.; Paci, E.; Capanna, S.; Alteri, A.; Campo, G.; Pigini, D.; De Rosa, M.; Tranfo, G.; Papaleo, B. Female Reproductive Health and Exposure to Phthalates and Bisphenol A: A Cross Sectional Study. Toxics 2021, 9, 299. [Google Scholar] [CrossRef]
- Kaltsas, A.; Zikopoulos, A.; Moustakli, E.; Zachariou, A.; Tsirka, G.; Tsiampali, C.; Palapela, N.; Sofikitis, N.; Dimitriadis, F. The Silent Threat to Women’s Fertility: Uncovering the Devastating Effects of Oxidative Stress. Antioxidants 2023, 12, 1490. [Google Scholar] [CrossRef]
- Gois, M.F.B.; Fernández-Pato, A.; Huss, A.; Gacesa, R.; Wijmenga, C.; Weersma, R.K.; Fu, J.; Vermeulen, R.C.H.; Zhernakova, A.; Lenters, V.C.; et al. Impact of Occupational Pesticide Exposure on the Human Gut Microbiome. Front. Microbiol. 2023, 14, 1223120. [Google Scholar] [CrossRef]
- Ali, A.; AlHussaini, K.I. Pesticides: Unintended Impact on the Hidden World of Gut Microbiota. Metabolites 2024, 14, 155. [Google Scholar] [CrossRef]
- Chen, L.; Yan, H.; Di, S.; Wang, X.; Liu, Y.; Zhang, Y.; Zhao, Q.; Li, J.; Sun, H.; Zhou, W.; et al. Mapping Pesticide-Induced Metabolic Alterations in Human Gut Bacteria. Nat. Commun. 2025, 16, 4355. [Google Scholar] [CrossRef]
- Aguilera, M.; Gálvez-Ontiveros, Y.; Rivas, A. Endobolome, a New Concept for Determining the Influence of Microbiota Disrupting Chemicals (MDC) in Relation to Specific Endocrine Pathogenesis. Front. Microbiol. 2020, 11, 578007. [Google Scholar] [CrossRef] [PubMed]
- Stathori, G.; Hatziagapiou, K.; Mastorakos, G.; Vlahos, N.F.; Charmandari, E.; Valsamakis, G. Endocrine-Disrupting Chemicals, Hypothalamic Inflammation and Reproductive Outcomes: A Review of the Literature. Int. J. Mol. Sci. 2021, 25, 11344. [Google Scholar] [CrossRef] [PubMed]
- Imhann, F.; Bonder, M.J.; Vich Vila, A.; Fu, J.; Mujagic, Z.; Vork, L.; Tigchelaar, E.F.; Jankipersadsing, S.A.; Cenit, M.C.; Harmsen, H.J.; et al. Proton pump inhibitors affect the gut microbiome. Gut 2016, 65, 740–748. [Google Scholar] [CrossRef] [PubMed]
- Langdon, A.; Crook, N.; Dantas, G. The effects of antibiotics on the microbiome throughout development and alternative approaches for therapeutic modulation. Genome Med. 2016, 8, 39. [Google Scholar] [CrossRef]
- Maier, L.; Pruteanu, M.; Kuhn, M.; Zeller, G.; Telzerow, A.; Anderson, E.E.; Brochado, A.R.; Fernandez, K.C.; Dose, H.; Mori, H.; et al. Extensive impact of non-antibiotic drugs on human gut bacteria. Nature 2018, 555, 623–628. [Google Scholar] [CrossRef]
- Watanabe, T.; Higuchi, K.; Kobata, A.; Nishio, H.; Tanigawa, T.; Shiba, M.; Tominaga, K.; Fujiwara, Y.; Oshitani, N.; Asahara, T.; et al. Non-steroidal anti-inflammatory drug-induced small intestinal damage is Toll-like receptor 4 dependent. Gut 2008, 57, 181–187. [Google Scholar] [CrossRef]
- Baker, J.M.; Al-Nakkash, L.; Herbst-Kralovetz, M.M. Estrogen-gut microbiome axis: Physiological and clinical implications. Maturitas 2017, 103, 45–53. [Google Scholar] [CrossRef]
- Benedict, C.; Vogel, H.; Jonas, W.; Woting, A.; Blaut, M.; Schürmann, A.; Cedernaes, J. Gut microbiota and glucometabolic alterations in response to recurrent partial sleep deprivation in normal-weight young individuals. Mol. Metab. 2016, 5, 1175–1186. [Google Scholar] [CrossRef] [PubMed]
- Spiegel, K.; Tasali, E.; Penev, P.; Van Cauter, E. Brief communication: Sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann. Intern. Med. 2004, 141, 846–850. [Google Scholar] [CrossRef]
- Taheri, S.; Lin, L.; Austin, D.; Young, T.; Mignot, E. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med. 2004, 1, e62. [Google Scholar] [CrossRef]
- Savvidis, C.; Kallistrou, E.; Kouroglou, E.; Dionysopoulou, S.; Gavriiloglou, G.; Ragia, D.; Tsiama, V.; Proikaki, S.; Belis, K.; Ilias, I. Circadian rhythm disruption and endocrine-related tumors. World J. Clin. Oncol. 2024, 15, 818–834. [Google Scholar] [CrossRef] [PubMed]
- Sanjuan, P.M.; Fokas, K.; Tonigan, J.S.; Henry, M.C.; Christian, K.; Rodriguez, A.; Larsen, J.; Yonke, N.; Leeman, L. Prenatal maternal posttraumatic stress disorder as a risk factor for adverse birth weight and gestational age outcomes: A systematic review and meta-analysis. J. Affect. Disord. 2021, 295, 530–540. [Google Scholar] [CrossRef] [PubMed]
- Jašarević, E.; Howerton, C.L.; Howard, C.D.; Bale, T.L. Alterations in the Vaginal Microbiome by Maternal Stress Are Associated with Metabolic Reprogramming of the Offspring Gut and Brain. Endocrinology 2015, 156, 3265–3276. [Google Scholar] [CrossRef]
- Wang, Z.; Hwang, S.H.; Kim, J.H.; Lim, S.S. Anti-Obesity Effect of the Above-Ground Part of Valeriana dageletiana Nakai ex F. Maek Extract in High-Fat Diet-Induced Obese C57BL/6N Mice. Nutrients 2017, 9, 689. [Google Scholar] [CrossRef]
- Wu, Y.; De Asis-Cruz, J.; Limperopoulos, C. Brain structural and functional outcomes in the offspring of women experiencing psychological distress during pregnancy. Mol. Psychiatry 2024, 29, 2223–2240. [Google Scholar] [CrossRef]
- Nuriel-Ohayon, M.; Neuman, H.; Koren, O. Microbial Changes during Pregnancy, Birth, and Infancy. Front. Microbiol. 2016, 7, 1031. [Google Scholar] [CrossRef]
- Kindinger, L.M.; Bennett, P.R.; Lee, Y.S.; Marchesi, J.R.; Smith, A.; Cacciatore, S.; Holmes, E.; Nicholson, J.K.; Teoh, T.G.; MacIntyre, D.A. The interaction between vaginal microbiota, cervical length, and vaginal progesterone treatment for preterm birth risk. Microbiome 2017, 5, 6. [Google Scholar] [CrossRef]
- Callahan, B.J.; DiGiulio, D.B.; Goltsman, D.S.A.; Sun, C.L.; Costello, E.K.; Jeganathan, P.; Biggio, J.R.; Wong, R.J.; Druzin, M.L.; Shaw, G.M.; et al. Replication and refinement of a vaginal microbial signature of preterm birth in two racially distinct cohorts of US women. Proc. Natl. Acad. Sci. USA 2017, 114, 9966–9971. [Google Scholar] [CrossRef]
- Drell, T.; Lillsaar, T.; Tummeleht, L.; Simm, J.; Aaspõllu, A.; Väin, E.; Saarma, I.; Salumets, A.; Donders, G.G.; Metsis, M. Characterization of the vaginal micro- and mycobiome in asymptomatic reproductive-age Estonian women. PLoS ONE 2013, 8, e54379. [Google Scholar] [CrossRef]
- Bradford, L.L.; Ravel, J. The vaginal mycobiome: A contemporary perspective on fungi in women’s health and diseases. Virulence 2017, 8, 342–351. [Google Scholar] [CrossRef]
- Crusell, M.K.W.; Hansen, T.H.; Nielsen, T.; Allin, K.H.; Rühlemann, M.C.; Damm, P.; Vestergaard, H.; Rørbye, C.; Jørgensen, N.R.; Christiansen, O.B.; et al. Gestational diabetes is associated with change in the gut microbiota composition in third trimester of pregnancy and postpartum. Microbiome 2018, 6, 89. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.G.; Marchesi, J.R.; Lee, Y.S.; Smith, A.; Lehne, B.; Kindinger, L.M.; Terzidou, V.; Holmes, E.; Nicholson, J.K.; Bennett, P.R.; et al. Vaginal dysbiosis increases risk of preterm fetal membrane rupture, neonatal sepsis and is exacerbated by erythromycin. BMC Med. 2018, 16, 9. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.W.; Redline, R.W.; Li, M.; Yin, L.; Hill, G.B.; McCormick, T.S. Fusobacterium nucleatum induces premature and term stillbirths in pregnant mice: Implication of oral bacteria in preterm birth. Infect. Immun. 2004, 72, 2272–2279. [Google Scholar] [CrossRef]
- Vander Haar, E.L.; So, J.; Gyamfi-Bannerman, C.; Han, Y.W. Fusobacterium nucleatum and adverse pregnancy outcomes: Epidemiological and mechanistic evidence. Anaerobe 2018, 50, 55–59. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lauder, A.P.; Roche, A.M.; Sherrill-Mix, S.; Bailey, A.; Laughlin, A.L.; Bittinger, K.; Leite, R.; Elovitz, M.A.; Parry, S.; Bushman, F.D. Comparison of placenta samples with contamination controls does not provide evidence for a distinct placenta microbiota. Microbiome 2016, 4, 29. [Google Scholar] [CrossRef]
- Astudillo-García, C.; Bell, J.J.; Webster, N.S.; Glasl, B.; Jompa, J.; Montoya, J.M.; Taylor, M.W. Evaluating the core microbiota in complex communities: A systematic investigation. Env. Microbiol. 2017, 19, 1450–1462. [Google Scholar] [CrossRef]
- Tulkens, J.; Vergauwen, G.; Van Deun, J.; Geeurickx, E.; Dhondt, B.; Lippens, L.; De Scheerder, M.A.; Miinalainen, I.; Rappu, P.; De Geest, B.G.; et al. Increased levels of systemic LPS-positive bacterial extracellular vesicles in patients with intestinal barrier dysfunction. Gut 2020, 69, 191–193. [Google Scholar] [CrossRef]
- Kaisanlahti, A.; Turunen, J.; Byts, N.; Petäistö, T.; Rautava, S.; Hällfors, J.; Vuolteenaho, R.; Iivanainen, A.; Välimaa, T.; Kauppila, T.; et al. Maternal microbiota communicates with the fetus through microbiota-derived extracellular vesicles. Microbiome 2023, 11, 249. [Google Scholar] [CrossRef] [PubMed]
- Walker, W.A. Initial intestinal colonization in the human infant and immune homeostasis. Ann. Nutr. Metab. 2013, 63 (Suppl. S2), 8–15. [Google Scholar] [CrossRef] [PubMed]
- Benítez-Páez, A.; Gómez Del Pulgar, E.M.; Kjølbæk, L.; Brahe, L.K.; Astrup, A.; Larsen, L.; Sanz, Y. Impact of dietary fiber and fat on gut microbiota re-modeling and metabolic health. Trends Food Sci. Technol. 2016, 57, 11–20. [Google Scholar] [CrossRef]
- Xenou, M.; Gourounti, K. Dietary Patterns and Polycystic Ovary Syndrome: A Systematic Review. Maedica 2021, 16, 516–521. [Google Scholar] [CrossRef]
- Tachedjian, G.; Aldunate, M.; Bradshaw, C.S.; Cone, R.A. The role of lactic acid production by probiotic Lactobacillus species in vaginal health. Res. Microbiol. 2017, 168, 782–792. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Wang, T.; Hu, X.; Luo, J.; Li, W.; Wu, X.; Duan, Y.; Jin, F. Administration of Lactobacillus helveticus NS8 improves behavioral, cognitive, and biochemical aberrations caused by chronic restraint stress. Neuroscience 2015, 310, 561–577. [Google Scholar] [CrossRef]
- Bressa, C.; Bailén-Andrino, M.; Pérez-Santiago, J.; González-Soltero, R.; Pérez, M.; Montalvo-Lominchar, M.G.; Maté-Muñoz, J.L.; Domínguez, R.; Moreno, D.; Larrosa, M. Differences in gut microbiota profile between women with active lifestyle and sedentary women. PLoS ONE 2017, 12, e0171352. [Google Scholar] [CrossRef]
- Integrative HMP (iHMP) Research Network Consortium. The Integrative Human Microbiome Project. Nature 2019, 569, 641–648. [Google Scholar] [CrossRef]
- Sorbara, M.T.; Pamer, E.G. Microbiome-Based Therapeutics. Nat. Rev. Microbiol. 2022, 20, 365–380. [Google Scholar] [CrossRef]
- Zeevi, D.; Korem, T.; Zmora, N.; Israeli, D.; Rothschild, D.; Weinberger, A.; Ben-Yacov, O.; Lador, D.; Avnit-Sagi, T.; Lotan-Pompan, M.; et al. Personalized Nutrition by Prediction of Glycemic Responses. Cell 2015, 163, 1079–1094. [Google Scholar] [CrossRef]
- Depommier, C.; Everard, A.; Druart, C.; Plovier, H.; Van Hul, M.; Vieira-Silva, S.; Falony, G.; Raes, J.; Maiter, D.; Delzenne, N.M.; et al. Supplementation with Akkermansia muciniphila in Overweight and Obese Human Volunteers: A Proof-of-Concept Exploratory Study. Nat. Med. 2019, 25, 1096–1103. [Google Scholar] [CrossRef]
- Ratiner, K.; Ciocan, D.; Abdeen, S.K.; Pevsner-Fischer, M.; Ben-Yacov, O.; David, E.; Leshansky, L.; David, I.; Harmelin, A.; Shapiro, H.; et al. Utilization of the Microbiome in Personalized Medicine. Nat. Rev. Microbiol. 2024, 22, 291–308. [Google Scholar] [CrossRef]
- Olofsson, L.E.; Bäckhed, F. The Metabolic Role and Therapeutic Potential of the Microbiome. Endocr. Rev. 2022, 43, 907–926. [Google Scholar] [CrossRef]
- Zmora, N.; Zilberman-Schapira, G.; Suez, J.; Mor, U.; Dori-Bachash, M.; Bashiardes, S.; Kotler, E.; Zur, M.; Regev-Lehavi, D.; Brik, R.B.; et al. Personalized gut mucosal colonization resistance to empiric probiotics is associated with unique host and microbiome features. Cell 2018, 174, 1388–1405.e21. [Google Scholar] [CrossRef] [PubMed]
- Derrien, M.; van Hylckama Vlieg, J.E. Fate, activity, and impact of ingested bacteria within the human gut microbiota. Trends Microbiol. 2015, 23, 354–366. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Liu, Y.; Li, Y.; Bai, G.; Pang, J.; Wu, M.; Li, J.; Zhao, X.; Xia, Y. Implications of Gut Microbiota-Mediated Epigenetic Modifications in Intestinal Diseases. Gut Microbes 2025, 17, 2508426. [Google Scholar] [CrossRef]
- Chee, W.J.Y.; Chew, S.Y.; Than, L.T.L. Vaginal microbiota and the potential of Lactobacillus derivatives in maintaining vaginal health. Microb. Cell Fact. 2020, 19, 203. [Google Scholar] [CrossRef]
- Escorcia Mora, P.; Valbuena, D.; Diez-Juan, A. The Role of the Gut Microbiota in Female Reproductive and Gynecological Health: Insights into Endometrial Signaling Pathways. Life 2025, 15, 762. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Chen, J.; Zhou, M.; Tang, R.; Xu, S.; Zhao, Y.; Su, M.; Zhang, X.; Zhang, P.; Chen, R. Dysbiosis of the Oral-Gut Microbiome in PCOS Patients and Its Implication for Noninvasive Diagnosis. Clin. Transl. Med. 2024, 14, e70001. [Google Scholar] [CrossRef]
- Bar, O.; Vagios, S.; Barkai, O.; Peretz, A.; Atzmony, L.; Levin, D.; Aizikovich, A.; Porat-Katz, B.S.; Dekel, N.; Shufaro, Y.; et al. Harnessing Vaginal Inflammation and Microbiome: A Machine Learning Model for Predicting IVF Success. npj Biofilms Microbiomes 2025, 11, 95. [Google Scholar] [CrossRef]
- Golob, J.L.; Oskotsky, T.T.; Tang, A.S.; Roldan, A.; Chung, V.; Ha, C.W.Y.; Wong, R.J.; Flynn, K.J.; Parraga-Leo, A.; Wibrand, C.; et al. Microbiome Preterm Birth DREAM Challenge: Crowdsourcing Machine Learning Approaches to Advance Preterm Birth Research. Cell Rep. Med. 2024, 5, 101350. [Google Scholar] [CrossRef]
- Berry, S.E.; Valdes, A.M.; Drew, D.A.; Asnicar, F.; Mazidi, M.; Wolf, J.; Capdevila, J.; Hadjigeorgiou, G.; Davies, R.; Al Khatib, H.; et al. Human Postprandial Responses to Food and Potential for Precision Nutrition. Nat. Med. 2020, 26, 964–973. [Google Scholar] [CrossRef]
- Gkouskou, K.; Vlastos, I.; Karkalousos, P.; Chaniotis, D.; Sanoudou, D.; Eliopoulos, A.G. The “Virtual Digital Twins” Concept in Precision Nutrition. Adv. Nutr. 2020, 11, 1405–1413. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Toalá, J.E.; García-Varela, R.; García, H.S.; Mata-Haro, V.; González-Córdova, A.F.; Vallejo-Cordoba, B.; Hernández-Mendoza, A. Postbiotics: An evolving term within the functional foods field. Trends Food Sci. Technol. 2018, 75, 105–114. [Google Scholar] [CrossRef]
- Abeltino, A.; Hatem, D.; Serantoni, C.; Riente, A.; De Giulio, M.M.; De Spirito, M.; De Maio, F.; Maulucci, G. Unraveling the Gut Microbiota: Implications for Precision Nutrition and Personalized Medicine. Nutrients 2024, 16, 3806. [Google Scholar] [CrossRef]
- Hood, L.; Friend, S.H. Predictive, personalized, preventive, participatory (P4) cancer medicine. Nat. Rev. Clin. Oncol. 2011, 8, 184–187. [Google Scholar] [CrossRef]
- Austin, C.; Puri, D.; Al Azzawi, S.; Hsieh, T.-C.; Patel, D.P. The impact of obesity and metabolic health on male fertility: A systematic review. Fertil. Steril. 2023, 120, 1098–1111. [Google Scholar] [CrossRef] [PubMed]
- Boxem, A.J.; Blaauwendraad, S.M.; Mulders, A.G.M.G.J.; Bekkers, E.L.; Kruithof, C.J.; Steegers, E.A.P.; Gaillard, R.; Jaddoe, V.W.V. Preconception and Early-Pregnancy Body Mass Index in Women and Men, Time to Pregnancy, and Risk of Miscarriage. JAMA Netw. Open 2024, 7, e2436157. [Google Scholar] [CrossRef]
- du Fossé, N.A.; van der Hoorn, M.-L.P.; van Lith, J.M.M.; le Cessie, S.; Lashley, E.E.L.O. Advanced paternal age is associated with an increased risk of spontaneous miscarriage: A systematic review and meta-analysis. Hum. Reprod. Update 2020, 26, 650–669. [Google Scholar] [CrossRef]
- Anelli, V.; Gatta, E.; Pirola, I.; Delbarba, A.; Rotondi, M.; Cappelli, C. Thyroid impairment and male fertility: A narrative review of literature. Aging Male 2024, 27, 1. [Google Scholar] [CrossRef] [PubMed]
- Vozdova, M.; Kubickova, S.; Kopecka, V.; Sipek, J.; Rubes, J. Effect of body mass index on semen quality, sperm chromatin integrity and sperm DNA methylation. Obes. Res. Clin. Pract. 2024, 18, 380–387. [Google Scholar] [CrossRef]
- Magill, R.G.; MacDonald, S.M. Male infertility and the human microbiome. Front. Reprod. Health 2023, 5, 1166201. [Google Scholar] [CrossRef]
- Osadchiy, V.; Belarmino, A.; Kianian, R.; Sigalos, J.T.; Ancira, J.S.; Kanie, T.; Mangum, S.F.; Tipton, C.D.; Hsieh, T.M.; Mills, J.N.; et al. Semen Microbiota Are Dramatically Altered in Men with Abnormal Sperm Parameters. Sci. Rep. 2024, 14, 1068. [Google Scholar] [CrossRef] [PubMed]
- Chatzokou, D.; Tsarna, E.; Davouti, E.; Siristatidis, C.S.; Christopoulou, S.; Spanakis, N.; Tsakris, A.; Christopoulos, P. Semen Microbiome, Male Infertility, and Reproductive Health. Int. J. Mol. Sci. 2025, 26, 1446. [Google Scholar] [CrossRef]
- Mowla, S.; Farahani, L.; Tharakan, T.; Davies, R.; Correia, G.D.S.; Lee, Y.S.; Kundu, S.; Khanjani, S.; Sindi, E.; Rai, R.; et al. Characterisation and comparison of semen microbiota and bacterial load in men with infertility, recurrent miscarriage, or proven fertility. eLife 2025, 13, RP96090. [Google Scholar] [CrossRef]
- Lv, S.; Huang, J.; Luo, Y.; Wen, Y.; Chen, B.; Qiu, H.; Chen, H.; Yue, T.; He, L.; Feng, B.; et al. Gut microbiota is involved in male reproductive function: A review. Front. Microbiol. 2024, 15, 1371667. [Google Scholar] [CrossRef]
- Chen, W.; Zou, H.; Xu, H.; Cao, R.; Zhang, H.; Zhang, Y.; Zhao, J. The potential influence and intervention measures of gut microbiota on sperm: It is time to focus on testis-gut microbiota axis. Front. Microbiol. 2024, 15, 1478082. [Google Scholar] [CrossRef] [PubMed]
- Alhaj, H.W.; Li, Z.; Shan, T.; Dai, P.; Zhu, P.; Li, Y.; Alsiddig, M.A.; Abdelghani, E.; Li, C. Effects of Dietary Sodium Butyrate on Reproduction in Adult Breeder Roosters. Anim. Reprod. Sci. 2018, 196, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Yang, Y.; Cao, Y.; Wen, Q.; Xi, Y.; Cheng, J.; Zhao, Q.; Weng, J.; Hong, K.; Jiang, H.; et al. The Gut Metabolite 3-Hydroxyphenylacetic Acid Rejuvenates Spermatogenic Dysfunction in Aged Mice through GPX4-Mediated Ferroptosis. Microbiome 2023, 11, 212. [Google Scholar] [CrossRef]
- Han, X.; Tian, H.; Yang, L.; Wang, Y.; Li, J.; Zhang, Q.; Liu, Z.; Chen, Y.; Zhao, W.; Xu, H.; et al. Bidirectional Mendelian Randomization to Explore the Causal Relationships between the Gut Microbiota and Male Reproductive Diseases. Sci. Rep. 2024, 14, 18306. [Google Scholar] [CrossRef]
- Zhang, F.; Xiong, Y.; Wu, K.; Wang, L.; Ji, Y.; Zhang, B. Genetic Insights into Intestinal Microbiota and Risk of Infertility: A Mendelian Randomization Study. Microorganisms 2023, 11, 2319. [Google Scholar] [CrossRef]
- Brannigan, R.E.; Hermanson, L.; Kaczmarek, J.; Kim, S.K.; Kirkby, E.; Tanrikut, C. Updates to male infertility: AUA/ASRM guideline (2024). J. Urol. 2024, 212, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Bocu, K.; Boeri, L.; Mahmutoglu, A.M.; Vogiatzi, P. Can lifestyle changes significantly improve male fertility: A narrative review? Arab. J. Urol. 2024, 23, 190–200. [Google Scholar] [CrossRef]
- Lo Giudice, A.; Asmundo, M.G.; Cimino, S.; Morgia, G.; Cocci, A.; Falcone, M.; Sokolakis, I.; Capogrosso, P.; Morgado, A.; Russo, G.I.; et al. Effects of physical activity on fertility parameters: A meta-analysis of randomized controlled trials. World J. Mens. Health 2024, 42, 555–562. [Google Scholar] [CrossRef]
- Simas, T.A.M.; Hoffman, M.C.; Miller, E.S.; Metz, T.; Byatt, N.; Roussos-Ross, K. Screening and Diagnosis of Mental Health Conditions During Pregnancy and Postpartum: ACOG Clinical Practice Guideline No. 4. Obstet. Gynecol. 2023, 141, 1232–1261. [Google Scholar] [CrossRef]
- Li, X.; Laplante, D.P.; Paquin, V.; Lafortune, S.; Elgbeili, G.; King, S. Effectiveness of cognitive behavioral therapy for perinatal maternal depression, anxiety and stress: A systematic review and meta-analysis of randomized controlled trials. Clin. Psychol. Rev. 2022, 92, 102129. [Google Scholar] [CrossRef]
- García-Gómez, E.; González-Pedrajo, B.; Camacho-Arroyo, I. Role of sex steroid hormones in bacterial-host interactions. Biomed. Res. Int. 2013, 2013, 928290. [Google Scholar] [CrossRef]
- Bunesova, V.; Lacroix, C.; Schwab, C. Mucin cross-feeding of infant Bifidobacteria and Eubacterium hallii. Microb. Ecol. 2018, 75, 228–238. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Geng, H.; Ye, C.; Liu, J. Dysbiotic alteration in the fecal microbiota of patients with polycystic ovary syndrome. Microbiol. Spectr. 2024, 12, e0429123. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhai, Q.; Wang, J.; Ma, X.; Xing, B.; Fan, H.; Gao, Z.; Zhao, F.; Liu, W. Variation of the vaginal microbiome during and after pregnancy in Chinese women. Genom. Proteom. Bioinform. 2022, 20, 322–333. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.; Jung, S.C.; Kwak, K.; Kim, J.S. The role of prebiotics in modulating gut microbiota: Implications for human health. Int. J. Mol. Sci. 2024, 25, 4834. [Google Scholar] [CrossRef] [PubMed]
| Microorganisms | Affected Hormones | Effects | Reproductive Health | References |
|---|---|---|---|---|
| Akkermansia muciniphila | Insulin/Leptin | Energetic metabolism and inflammation | Improves insulin sensitivity and reduces inflammation; important in PCOS and metabolic infertility | [90] |
| Prevotella spp. | Oestrogens and cytokines | Vaginal immunoregulation | Associated with vaginal dysbiosis and premature births if overexpressed | [59] |
| Faecalibacterium prausnitzii | Cortisol/IL-10 | Systemic inflammation, gut–brain axis | Anti-inflammatory; improves mood and HPA axis. Indirectly promotes fertility by reducing stress | [91] |
| Streptococcus agalactiae | Does not directly affect hormones | Vaginal colonisation, neonatal infection | Increased risk of perinatal infection, chorioamnionitis and premature birth if not controlled | [92] |
| Gardnerella vaginalis | Oestrogen | Cervical mucus, vaginal environment | Associated with bacterial vaginosis; may alter embryo implantation and increase risk of miscarriage | [93] |
| Clostridium spp. | Oestrogens (oestradiol) | Oestrogen enterohepatic circulation | Increases circulating oestrogen levels; risk of hypoestrogenism, menstrual disorders, endometriosis | [94] |
| Lactobacillus spp. | Oestrogen and progesterone | Modulation of the HPG axis | Prevention of vaginal infections and premature birth; improved embryo implantation | [58] |
| Bifidobacterium longum | Cortisol | Modulation of the HPA axis | Stress reduction; promotes ovulation and success in fertility treatments | [95] |
| Bacteroides spp. | Testosterone | Hepatic metabolism of androgens | It can influence PCOS or hypogonadism | [96] |
| Candida albicans | Oestradiol modulation; inflammatory cytokines | Vaginal immunity; intestinal permeability | Associated with vaginal dysbiosis, infertility, and pregnancy complications | [97,98] |
| Malassezia spp. | Inflammatory cytokines (IL-1β, TNF-α) | Gut–immune interaction | Emerging role in mucosal inflammation and possible endocrine-metabolic dysregulation | [48] |
| Human papillomavirus | Immune evasion and of hormonal signalling | Cervical epithelial changes | Linked to cervical dysplasia, persistent inflammation, infertility, risk of miscarriage | [99] |
| Anelloviruses | Indirect influence via immune modulation | Vaginal virome stability; immune tolerance | Associated with Lactobacillus- depleted microbiota and local inflammation in CST-IV profiles; potential marker of dysbiosis | [43] |
| Bacteriophages | Indirect via hormone-sensitive bacteria | Vaginal microbial homeostasis | Dysregulation may reduce Lactobacillus, impair mucosal immunity, increase BV, infertility and risk of preterm birth | [100,101] |
| Lactobacillus rhamnosus | Oestrogens | Vaginal and endometrial microbiota | Promotes a Lactobacillus-dominant microbiome; enhances endometrial immune modulation and supports implantation | [102,103] |
| Lactobacillus reuteri | Oestrogens | Vaginal health and immune signalling | Contributes to urogenital health by reducing Group B Streptococcus colonisation and modulating vaginal cytokine profiles during pregnancy | [103,104] |
| Chlamydia trachomatis | Inflammatory cytokines (IL-6, TNF-α) | Fallopian tube mucosa | Promotes chronic inflammation leading to tubal factor infertility and ectopic pregnancy | [105] |
| Toxoplasma gondii | Th1/Th2 immune shift | Placental and foetal tissues | Acute infection during gestation disrupts placental Th1/Th2 balance → miscarriage, intrauterine growth restriction, preterm birth | [106,107] |
| Mycoplasma genitalium | Mucosal immunity | Endometrium, fallopian tubes | Linked to pelvic inflammatory disease, endometritis, infertility, tubal damage, infertility, ectopic pregnancy; ↑ risk preterm birth | [108,109] |
| Ureaplasma urealyticum | Pro-inflammatory cytokines | Amniotic cavity and cervicovaginal area | Implicated in chorioamnionitis, preterm prelabour rupture of membranes recurrent miscarriage, neonatal morbidity, and increased risk of preterm labour | [110] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barraza-Ortega, E.; Gómez-Gil, B.; García-Gasca, T.; Lizárraga, D.; Díaz, N.; García-Gasca, A. The Impact of Lifestyle on Reproductive Health: Microbial Complexity, Hormonal Dysfunction, and Pregnancy Outcomes. Int. J. Mol. Sci. 2025, 26, 8574. https://doi.org/10.3390/ijms26178574
Barraza-Ortega E, Gómez-Gil B, García-Gasca T, Lizárraga D, Díaz N, García-Gasca A. The Impact of Lifestyle on Reproductive Health: Microbial Complexity, Hormonal Dysfunction, and Pregnancy Outcomes. International Journal of Molecular Sciences. 2025; 26(17):8574. https://doi.org/10.3390/ijms26178574
Chicago/Turabian StyleBarraza-Ortega, Eunice, Bruno Gómez-Gil, Teresa García-Gasca, Dennise Lizárraga, Natalia Díaz, and Alejandra García-Gasca. 2025. "The Impact of Lifestyle on Reproductive Health: Microbial Complexity, Hormonal Dysfunction, and Pregnancy Outcomes" International Journal of Molecular Sciences 26, no. 17: 8574. https://doi.org/10.3390/ijms26178574
APA StyleBarraza-Ortega, E., Gómez-Gil, B., García-Gasca, T., Lizárraga, D., Díaz, N., & García-Gasca, A. (2025). The Impact of Lifestyle on Reproductive Health: Microbial Complexity, Hormonal Dysfunction, and Pregnancy Outcomes. International Journal of Molecular Sciences, 26(17), 8574. https://doi.org/10.3390/ijms26178574









