The Role of Puroindoline, Gpc-B1, Starch Synthase Genes, and Gluten Proteins in Regulating End-Use Quality in Wheat
Abstract
1. Introduction
2. The End-Use Quality
2.1. Grain Protein Content
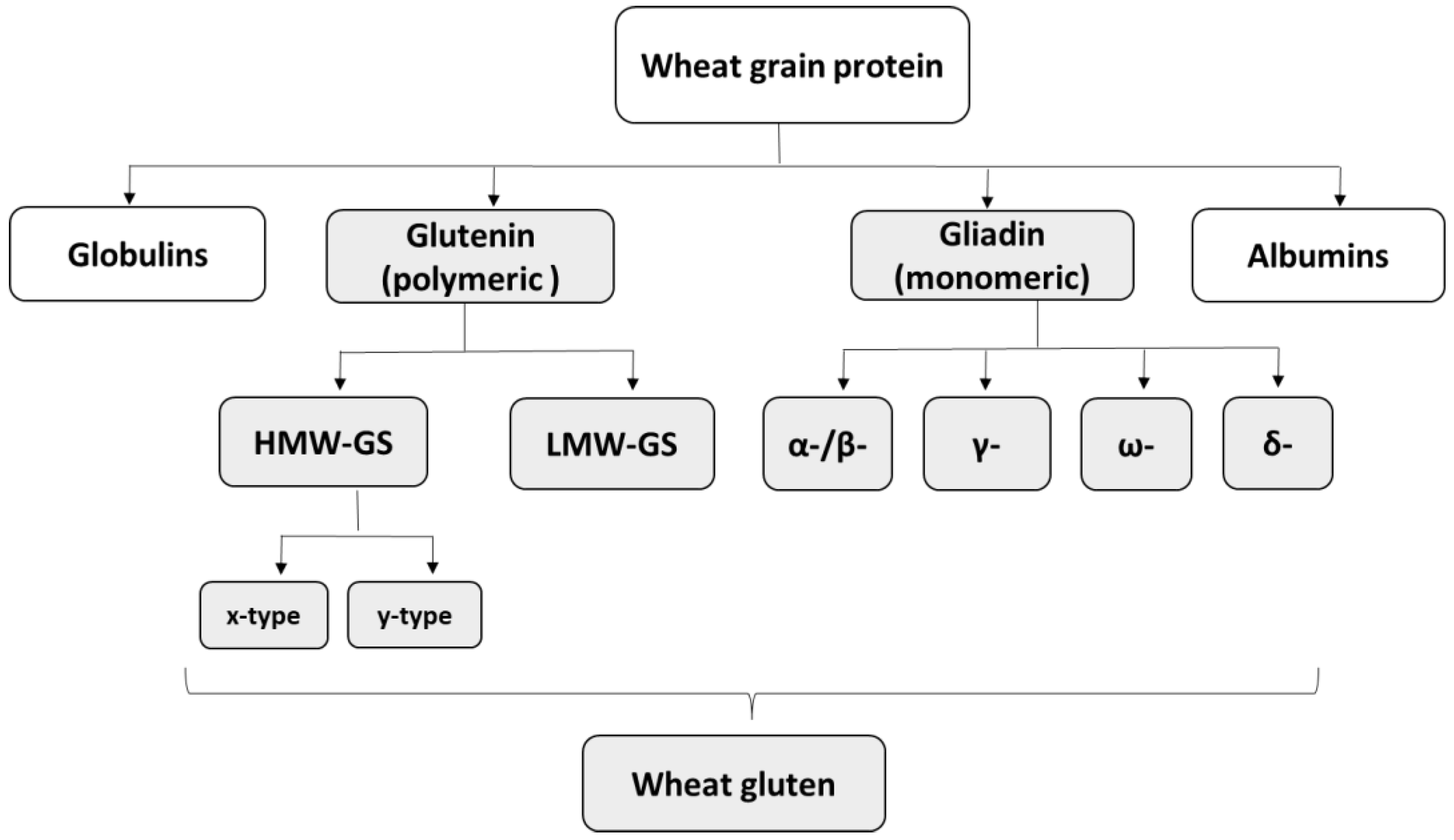
2.2. Storage Proteins
2.3. Grain Hardness
2.4. Starch Properties
3. Genetic Control of End-Use Quality Traits
3.1. The Influence of Gpc-B1 Genes on Grain Protein Content and Nutritional Quality
3.2. The Influence of Glu-1, Glu-3, and Gli-1 Genes on the End-Use Quality of Wheat
3.3. The Influence of Pina and Pinb Genes on the End-Use Quality of Wheat
3.4. The Influence of GBSSI (Waxy) Genes on the End-Use Quality of Wheat
4. Challenges in Breeding for Improved End-Use Quality
4.1. Quality Deterioration
4.2. The Effects of External Factors on Wheat Grain Quality
5. Opportunities for End-Use Quality Breeding
5.1. Genetic Variation as a Source of End-Use Quality Traits
5.2. Marker-Assisted, Genomic, and Phenomic Selection
5.3. Genome Editing Technologies
6. Outlook and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| GEI | Genotype-by-environment interaction |
| GHI | Grain hardness index |
| GPC | Grain protein content |
| GWASs | Genome-wide association studies |
| MAS | Marker-assisted selection |
| NAC | NAM, ATAF1/2, and CUC2 |
| QTL | Quantitative trait locus |
| RS | Resistant starch |
| SDS | Sodium dodecyl-sulfate |
References
- Cockx, L.; Colen, L.; De Weerdt, J.; Gomez, Y.; Paloma, S. Urbanization as a Driver of Changing Food Demand in Africa: Evidence from Rural-Urban Migration in Tanzania; Publications Office of the European Union: Luxembourg, 2019. [Google Scholar]
- de Bruin, S.; Dengerink, J.; van Vliet, J. Urbanisation as driver of food system transformation and opportunities for rural livelihoods. Food Secur. 2021, 13, 781–798. [Google Scholar] [CrossRef]
- Shewry, P.R. The contribution of wheat to human nutrition and health. A Year on the field, 2021. Available online: https://www.yearonthefield.net/post/the-contribution-of-wheat-to-human-nutrition-and-health (accessed on 7 August 2025).
- Peña, R.J. Current and future trends of wheat quality needs. In Wheat Production in Stressed Environments. Developments in Plant Breeding; Buck, H.T., Nisi, J.E., Salomón, N., Eds.; Springer: Dordrecht, The Netherlands, 2007; Chapter 12; pp. 411–424. [Google Scholar]
- Reynolds, M.P.; Hays, D.; Chapman, S. Breeding for adaptation to heat and drought stress. In Climate Change and Crop Production; CABI: Wallingford, UK, 2010; pp. 71–91. [Google Scholar]
- Helguera, M.; Abugalieva, A.; Battenfield, S.; Békés, F.; Branlard, G.; Cuniberti, M.; Hüsken, A.; Johansson, E.; Morris, C.F.; Nurit, E.; et al. Grain Quality in Breeding. In Wheat Quality for Improving Processing and Human Health; Igrejas, G., Ikeda, T.M., Guzmán, C., Eds.; Springer Nature Switzerland AG: Cham, Switzerland, 2020; pp. 273–307. [Google Scholar]
- FAOSTAT. Online Statistical Database: Food and Agriculture Organization, Rome. 2024. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 17 January 2025).
- Food and Agriculture Organization (FAO). The State of the World’s Biodiversity for Food and Agriculture; FAO: Rome, Italy, 2019; Available online: https://www.fao.org/3/CA3129EN/ca3129en.pdf (accessed on 23 November 2024).
- Feldman, M. Origin of cultivated wheat. In The World Wheat Book: A History of Wheat Breeding; Bonjean, A.P., Angus, W.J., Eds.; Lavoisier Publishing: Paris, France, 2001; pp. 3–56. [Google Scholar]
- Poudel, R.; Bhatta, M. Review of nutraceuticals and functional properties of whole wheat. J. Nutr. Food Sci. 2017, 6, 571. [Google Scholar] [CrossRef]
- Garutti, M.; Nevola, G.; Mazzeo, R.; Cucciniello, L.; Totaro, F.; Bertuzzi, C.A.; Caccialanza, R.; Pedrazzoli, P.; Puglisi, F. The impact of cereal grain composition on the health and disease outcomes. Front. Nutr. 2022, 9, 888974. [Google Scholar] [CrossRef] [PubMed]
- Peña, R.J.; Trethowan, R.; Pfeiffer, W.H.; Ginkel, M.V. Quality (end-use) improvement in wheat: Compositional, genetic, and environmental factors. J. Crop Prod. 2002, 5, 1–37. [Google Scholar] [CrossRef]
- Shewry, P.R. Wheat. J. Exp. Bot. 2009, 60, 1537–1553. [Google Scholar] [CrossRef] [PubMed]
- Clavijo, B.J.; Venturini, L.; Schudoma, C.; Accinelli, G.G.; Kaithakottil, G.; Wright, J.; Borrill, P.; Kettleborough, G.; Heavens, D.; Chapman, H.; et al. An improved assembly and annotation of the allohexaploid wheat genome identifies complete families of agronomic genes and provides genomic evidence for chromosomal translocations. Genome Res. 2017, 27, 885–896. [Google Scholar] [CrossRef]
- Khan, H. Genetic improvement for end-use quality in wheat. In Quality Breeding in Field Crops; Qureshi, A., Dar, Z., Wani, S., Eds.; Springer Nature: Berlin/Heidelberg, Germany, 2019; pp. 239–253. [Google Scholar]
- Garg, M.; Dhaliwal, H.S.; Chhuneja, P.; Kumar, D.; Dou, Q.W.; Tanaka, H.; Elamein, H.M.; Tsujimoto, H. Negative effect of chromosome 1A on dough strength shown by modification of 1D addition in durum wheat (Triticum durum). Theor. Appl. Genet. 2007, 114, 1141–1150. [Google Scholar] [CrossRef]
- Sharma, A.; Garg, S.; Sheikh, I.; Vyas, P.; Dhaliwal, H.S. Effect of wheat grain protein composition on end-use quality. J. Food Sci. Technol. 2020, 57, 2771–2785. [Google Scholar] [CrossRef]
- Kaur, A.; Shevkani, K.; Katyal, M.; Singh, N.; Ahlawat, A.K.; Singh, A.M. Physicochemical and rheological properties of starch and flour from different durum wheat varieties and their relationships with noodle quality. J. Food Sci. Technol. 2016, 4, 2127–2138. [Google Scholar] [CrossRef]
- Rai, A.; Han, S.S. Recent developments on the contribution of glutenin and puroindoline proteins to improve wheat grain quality. Cereal Chem. 2023, 100, 56–71. [Google Scholar] [CrossRef]
- Mann, G.; Diffey, S.; Cullis, B.; Azanza, F.; Martin, D.; Kelly, A.; McIntyre, L.; Schmidt, A.; Ma, W.; Nath, Z.; et al. Genetic control of wheat quality: Interactions between chromosomal regions determining protein content and composition, dough rheology, and sponge and dough baking properties. Theor. Appl. Genet. 2009, 118, 1519–1537. [Google Scholar] [CrossRef]
- Li, J.; Cui, F.; Ding, A.M.; Zhao, C.H.; Wang, X.Q.; Wang, L.; Bao, Y.G.; Qi, X.L.; Li, X.F.; Gao, J.R.; et al. QTL detection of seven quality traits in wheat using two related recombinant inbred line populations. Euphytica 2012, 183, 207–226. [Google Scholar] [CrossRef]
- Payne, P.I. Genetics of wheat storage proteins and the effect of allelic variation on bread-making quality. Annu. Rev. Plant Physiol. 1987, 38, 141–153. [Google Scholar] [CrossRef]
- Naraghi, S.M.; Simsek, S.; Kumar, A.; Al Rabbi, S.H.; Alamri, M.S.; Elias, E.M.; Mergoum, M. Deciphering the genetics of major end-use quality traits in wheat. G3 Genes Genom. Genet. 2019, 9, 1405–1427. [Google Scholar] [CrossRef]
- Subedi, M.; Ghimire, B.; Bagwell, J.W.; Buck, J.W.; Mergoum, M. Wheat end-use quality: State of art, genetics, genomics-assisted improvement, future challenges, and opportunities. Front. Genet. 2023, 13, 1032601. [Google Scholar] [CrossRef]
- Guzmán, C.; Ibba, M.I.; Álvarez, J.B.; Sissons, M.; Morris, C. Wheat Quality. In Wheat Improvement: Food Security in a Changing Climate; Reynolds, M.P., Braun, H.J., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 177–193. [Google Scholar]
- Goutam, U.; Kukreja, S.; Tiwari, R.; Chaudhury, A.; Gupta, R.K.; Dholakia, B.B.; Yadav, R. Biotechnological approaches for grain quality improvement in wheat: Present status and future possibilities. Aust. J. Crop Sci. 2013, 7, 469–483. [Google Scholar]
- Aoun, M.; Carter, A.H.; Morris, C.F.; Kiszonas, A.M. Genetic architecture of end-use quality traits in soft white winter wheat. BMC Genom. 2022, 23, 440. [Google Scholar] [CrossRef] [PubMed]
- Shewry, P.R. Improving the protein content and composition of cereal grain. J. Cereal Sci. 2007, 46, 239–250. [Google Scholar] [CrossRef]
- Zhao, L.A.; Zhang, K.P.; Liu, B.; Deng, Z.Y.; Qu, H.L.; Tian, J.C. A comparison of grain protein content QTLs and flour protein content QTLs across environments in cultivated wheat. Euphytica 2010, 174, 325–335. [Google Scholar] [CrossRef]
- Velu, G.; Singh, R.P.; Cardenas, M.E.; Wu, B.; Guzman, C.; Ortiz-Monasterio, I. Characterization of grain protein content gene (GPC-B1) introgression lines and its potential use in breeding for enhanced grain zinc and iron concentration in spring wheat. Acta Physiol. Plant. 2017, 39, 212. [Google Scholar] [CrossRef]
- Liu, J.; Huang, L.; Wang, C.; Liu, Y.; Yan, Z.; Wang, Z.; Xiang, L.; Zhong, X.; Gong, F.; Zheng, Y.; et al. Genome-wide association study reveals novel genomic regions associated with high grain protein content in wheat lines derived from wild emmer wheat. Front. Plant Sci. 2019, 10, 464. [Google Scholar] [CrossRef]
- Tabbita, F.; Pearce, S.; Barneix, A.J. Breeding for increased grain protein and micronutrient content in wheat: Ten years of the GPC-B1 gene. J. Cereal Sci. 2017, 73, 183–191. [Google Scholar] [CrossRef]
- Guzmán, C.; Crossa, J.; Mondal, S.; Govindan, V.; Huerta, J.; Crespo-Herrera, L.; Vargas, M.; Singh, R.P.; Ibba, M.I. Effects of glutenins (Glu-1 and Glu-3) allelic variation on dough properties and bread-making quality of CIMMYT bread wheat breeding lines. Field Crops Res. 2022, 284, 108585. [Google Scholar] [CrossRef]
- Avivi, L.Y. High protein content in wild tetraploid Triticum dicoccoides Korn. In Proceedings of the 5th International Wheat Genetics Symposium, New Delhi, India, 23–28 February 1978; Indian Society of Genet and Plant Breed: New Delhi, India, 1978; pp. 372–380. [Google Scholar]
- Dotlačil, L.; Hermuth, J.; Stehno, Z.; Dvořáček, V.; Bradová, J.; Leišová, L. How can wheat landraces contribute to present breeding? Czech J. Genet. Plant Breed. 2010, 46, 70–74. [Google Scholar] [CrossRef]
- Joppa, L.; Du, C.; Hart, G.E.; Hareland, G.A. Mapping gene(s) for grain protein in tetraploid wheat (Triticum turgidum L.) using a population of recombinant inbred chromosome lines. Crop Sci. 1997, 37, 1586–1589. [Google Scholar] [CrossRef]
- Olmos, S.; Distelfeld, A.; Chicaiza, O.; Schlatter, A.R.; Fahima, T.; Echenique, V.; Dubcovsky, J. Precise mapping of a locus affecting GPC in durum wheat. Theor. Appl. Genet. 2003, 107, 1243–1251. [Google Scholar] [CrossRef] [PubMed]
- Distelfeld, A.; Uauy, C.; Fahima, T.; Dubcovsky, J. Physical map of the wheat high-grain protein content gene Gpc-B1 and development of a high-throughput molecular marker. New Phytol. 2006, 169, 753–763. [Google Scholar] [CrossRef]
- Khan, K.; Frohberg, R.; Olson, T.; Huckle, L. Inheritance of gluten protein components of high-protein hard red spring wheat lines derived from Triticum turgidum var. dicoccoides. Cereal Chem. 1989, 66, 397–401. [Google Scholar]
- Mesfin, A.; Frohberg, R.C.; Khan, K.; Olsen, T. Increased grain protein content and its association with agronomic and end-use quality in two hard red spring wheat populations derived from Triticum turgidum L. var. dicoccoides. Euphytica 2000, 116, 237–242. [Google Scholar] [CrossRef]
- Uauy, C.; Distelfeld, A.; Fahima, T.; Blechl, A.; Dubcovsky, J. A NAC gene regulating senescence improves grain protein, zinc, and iron content in wheat. Science 2006, 314, 1298–1301. [Google Scholar] [CrossRef]
- Çakmak, İ.; Torun, A.Y.; Millet, E.; Feldman, M.; Fahima, T.; Korol, A.; Nevo, E.; Braun, H.J.; Özkan, H. Triticum dicoccoides: An important genetic resource for increasing zinc and iron concentration in modern cultivated wheat. Soil Sci. Plant Nutr. 2004, 50, 1047–1054. [Google Scholar] [CrossRef]
- Krishnappa, G.; Singh, A.M.; Chaudhary, S.; Ahlawat, A.K.; Singh, S.K.; Shukla, R.B.; Jaiswal, J.P.; Singh, G.P.; Solanki, I.S. Molecular mapping of the grain iron and zinc concentration, protein content and thousand kernel weight in wheat (Triticum aestivum L.). PLoS ONE 2017, 12, e0174972. [Google Scholar] [CrossRef] [PubMed]
- Velu, G.; Singh, R.P.; Huerta, J.; Guzmán, C. Genetic impact of Rht dwarfing genes on grain micronutrients concentration in wheat. Field Crops Res. 2017, 214, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Bihua, W.; Jeifei, L.; Ping, L. Genetic diversity and potential utilization of grain protein content and sink traits in Triticum dicoccoides. J. Sichuan Agric. Univ. 2008, 26, 221–225. [Google Scholar]
- Wang, Z.Z.; Huang, L.; Wu, B.H.; Hu, J.; Jiang, Z.; Qi, P.; Zheng, Y.; Liu, D. Characterization of an integrated active Glu-1Ay allele in common wheat from wild emmer and its potential role in flour improvement. Int. J. Mol. Sci. 2018, 19, 923. [Google Scholar] [CrossRef]
- Gao, L.; Wang, A.; Li, X.; Dong, K.; Wang, K.; Appels, R.; Ma, W.; Yan, Y. Wheat quality related differential expression of albumins and globulins revealed by two-dimensional difference gel electrophoresis (2-D DIGE). J. Proteom. 2009, 73, 279–296. [Google Scholar] [CrossRef]
- Shewry, P.R.; Halford, N.G.; Lafiandra, D. The genetics of wheat gluten proteins. In Advances in Genetics; Hall, J.C., Dunlap, J.C., Friedman, T., Eds.; Academic Press: San Diego, CA, USA, 2003; Chapter 49; pp. 111–184. [Google Scholar]
- Anjum, F.M.; Khan, M.R.; Din, A.; Saeed, M.; Pasha, I.; Arshad, M.U. Wheat gluten: High molecular weight glutenin subunits—Structure, genetics, and relation to dough elasticity. J. Food Sci. 2007, 72, R56–R63. [Google Scholar] [CrossRef]
- Goesaert, H.; Brijs, K.; Veraverbeke, W.; Courtin, C.M.; Gebruers, K.; Delcour, J.A. Wheat flour constituents: How they impact bread quality, and how to impact their functionality. Trends Food Sci. Technol. 2005, 16, 12–30. [Google Scholar] [CrossRef]
- Branković, G.; Pajić, V.; Zivanović, T.; Dodig, D.; Kandić, V.; Knežević, D.; Đurić, N. Genetic parameters of Triticum aestivum and Triticum durum for technological quality properties in Serbia. Zemdirb.-Agric. 2018, 105, 39–48. [Google Scholar] [CrossRef]
- Yu, S.; Assanga, S.O.; Awika, J.M.; Ibrahim, A.M.; Rudd, J.C.; Xue, Q.; Guttieri, M.J.; Zhang, G.; Baker, J.A.; Jessup, K.E.; et al. Genetic mapping of quantitative trait loci for end-use quality and grain minerals in hard red winter wheat. Agronomy 2021, 11, 2519. [Google Scholar] [CrossRef]
- Payne, P.I.; Nightingale, M.A.; Krattiger, A.F.; Holt, L.M. The relationship between HMW glutenin subunit composition and the bread-making quality of British-grown wheat varieties. J. Sci. Food Agric. 1987, 40, 51–65. [Google Scholar] [CrossRef]
- Wieser, H. Chemistry of gluten proteins. Food Microbiol. 2007, 24, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Yu, K.; Zhang, Y.; Islam, S.; Sun, D.; Ma, W. Two novel y-type high molecular weight glutenin genes in Chinese wheat landraces of the Yangtze-River region. PLoS ONE 2015, 10, e0142348. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Duncan, O.; Islam, S.; Zhang, J.; Ma, W.; Millar, A.H. Increased wheat protein content via introgression of an HMW glutenin selectively reshapes the grain proteome. Mol. Cell. Proteom. 2021, 20, 100097. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Shepherd, K.; Cornish, G. A simplified SDS-PAGE procedure for separating. J. Cereal Sci. 1991, 14, 203–208. [Google Scholar] [CrossRef]
- Liu, L.; Ikeda, T.M.; Branlard, G.; Peña, R.J.; Rogers, W.J.; Lerner, S.E.; Kolman, M.A.; Xia, X.; Wang, L.; Ma, W.; et al. Comparison of low molecular weight glutenin subunits identified by SDS-PAGE, 2-DE, MALDI-TOF-MS and PCR in common wheat. BMC Plant Biol. 2010, 10, 124. [Google Scholar] [CrossRef]
- Ibba, M.I.; Kiszonas, A.M.; Guzmán, C.; Morris, C.F. Definition of the low molecular weight glutenin subunit gene family members in a set of standard bread wheat (Triticum aestivum L.) varieties. J. Cereal Sci. 2017, 74, 263–271. [Google Scholar] [CrossRef]
- Dreisigacker, S.; Xiao, Y.; Sehgal, D.; Guzman, C.; He, Z.; Xia, X.; Pena, R.J. SNP markers for low molecular glutenin subunits (LMW-GSs) at the Glu-A3 and Glu-B3 loci in bread wheat. PLoS ONE 2020, 15, e0233056. [Google Scholar] [CrossRef]
- Cho, K.; Jang, Y.R.; Lim, S.H.; ltenbach, S.B.; Gu, Y.Q.; Simon-Buss, A.; Lee, J.Y. Proteomic Determination of Low-Molecular-Weight Glutenin Subunit Composition in Aroona Near-Isogenic Lines and Standard Wheat Cultivars. Int. J. Mol. Sci. 2021, 22, 7709. [Google Scholar] [CrossRef]
- Hernández-Espinosa, N.; Payne, T.; Huerta-Espino, J.; Cervantes, F.; Gonzalez-Santoyo, H.; Ammar, K.; Guzmán, C. Preliminary characterization for grain quality traits and high and low molecular weight glutenins subunits composition of durum wheat landraces from Iran and Mexico. J. Cereal Sci. 2019, 88, 47–56. [Google Scholar] [CrossRef]
- Bonafede, M.D.; Tranquilli, G.; Pflüger, L.A.; Pena, R.J.; Dubcovsky, J. Effect of allelic variation at the Glu-3/Gli-1 loci on bread-making quality parameters in hexaploid wheat (Triticum aestivum L.). J. Cereal Sci. 2015, 62, 143–150. [Google Scholar] [CrossRef]
- Lawrence, G.J.; Shepherd, K.W. Chromosomal location of genes controlling seed proteins in species related to wheat. Theor. Appl. Genet. 1981, 59, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Luo, G.; Song, S.; Zhao, L.; Shen, L.; Song, Y.; Wang, X.; Yu, K.; Liu, Z.; Li, Y.; Yang, W.; et al. Mechanisms, origin and heredity of Glu-1Ay silencing in wheat evolution and domestication. Theor. Appl. Genet. 2018, 131, 1561–1575. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.T.; Ma, J.; Wei, Y.M.; Liu, Y.X.; Lan, X.J.; Dai, S.F.; Lu, Z.X.; Zhao, S.; Zhao, Q.Z.; and Zheng, Y.L. Novel variants of HMW glutenin subunits from Aegilops section Sitopsis species in relation to evolution and wheat breeding. BMC Plant Biol. 2012, 12, 73. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Fu, J.; Shen, Q.; Yang, D. High-molecular-weight glutenin subunits: Genetics, structures, and relation to end use qualities. Int. J. Mol. Sci. 2020, 22, 184. [Google Scholar] [CrossRef]
- Pattison, A.L. Genetic Improvement of Grain Quality for Bread Making in Triticale. Ph.D. Thesis, University of Sydney, Sydney, Australia, 2014. [Google Scholar]
- Liu, D.; Yang, H.; Zhang, Z.; Chen, Q.; Guo, W.; Rossi, V.; Xin, M.; Du, J.; Hu, Z.; Liu, J.; et al. An elite γ-gliadin allele improves end-use quality in wheat. New Phytol. 2023, 239, 87–101. [Google Scholar] [CrossRef]
- Žilić, S.; Barać, M.; Pešić, M.; Dodig, D.; Ignjatović-Micić, D. Characterization of proteins from grain of different bread and durum wheat genotypes. Int. J. Mol. Sci. 2011, 12, 5878–5894. [Google Scholar] [CrossRef]
- Rasheed, A.; Xia, X.; Yan, Y.; Appels, R.; Mahmood, T.; He, Z. Wheat seed storage proteins: Advances in molecular genetics, diversity and breeding applications. J. Cereal Sci. 2014, 60, 11–24. [Google Scholar] [CrossRef]
- Barak, S.; Mudgil, D.; Khatkar, B.S. Biochemical and functional properties of wheat gliadins: A review. Crit. Rev. Food Sci. Nutr. 2015, 55, 357–368. [Google Scholar] [CrossRef]
- Ma, W.; Zhang, W.; Gale, K. Multiplex-PCR typing of high molecular weight glutenin alleles in wheat. Euphytica 2003, 134, 51–60. [Google Scholar] [CrossRef]
- Wan, Y.; Shewry, P.R.; Hawkesford, M. A novel family of γ-gliadin genes are highly regulated by nitrogen supply in developing wheat grain. J. Exp. Bot. 2012, 64, 161–168. [Google Scholar] [CrossRef]
- Guzmán, C.; Alvarez, J.B. Wheat waxy proteins: Polymorphism, molecular characterization and effects on starch properties. Theor. Appl. Genet. 2016, 129, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.Z.; Zaidi, S.S.; Amin, I.; Mansoor, S. A CRISPR way for fast-forward crop domestication. Trends Plant Sci. 2019, 24, 293–296. [Google Scholar] [CrossRef] [PubMed]
- American Association of Cereal Chemists International. Approved Methods of Analysis, 11th ed.; Method 55-31.01 Single-Kernel Characterization Systems for Wheat Kernel Texture; AACC International: St. Paul, MN, USA, 2010. [Google Scholar]
- Guzmán, C.; Peña, R.J.; Singh, R.; Autrique, E.; Dreisigacker, S.; Crossa, J.; Rutkoski, J.; Poland, J.; Battenfield, S. Wheat quality improvement at CIMMYT and the use of genomic selection on it. Appl. Transl. Genom. 2016, 11, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Symes, K.J. The inheritance of grain hardness in wheat as measured by the particle size index. Aust. J. Agric. Res. 1965, 20, 971–979. [Google Scholar] [CrossRef]
- Greenwell, P.; Schofield, J.D. A starch granule protein associated with endosperm softness in wheat. Cereal Chem. 1986, 63, 379–380. [Google Scholar]
- Dubriel, L.; Gaborit, T.; Bouchet, B.; Gallant, D.J.; Broekaert, W.F.; Quillien, L.; Marion, D. Spatial and temporal distribution of the major isoforms of puroindolines (puroindoline-a and puroindoline-b) and non-specific lipid transfer proteins (ns-LTPs) of Triticum aestivum seeds. Relationships with their in vitro antifungal properties. Plant Sci. 1998, 138, 121–135. [Google Scholar] [CrossRef]
- Wiley, P.R.; Tosi, P.; Evrard, A.; Lovegrove, A.; Jones, H.D.; Shewry, P.R. Promoter analysis and immunolocalisation show that puroindoline genes are exclusively expressed in starchy endosperm cells of wheat grain. Plant Mol. Biol. 2007, 64, 125–136. [Google Scholar] [CrossRef]
- Anjum, F.M.; Walker, C.E. Review on the significance of starch and protein to wheat kernel hardness. J. Sci. Food Agric. 1991, 56, 1–13. [Google Scholar] [CrossRef]
- Giroux, M.J.; Morris, C.F. Wheat grain hardness results from highly conserved mutations in friabilin components puroindoline a and b. Proc. Natl. Acad. Sci. USA 1998, 95, 6262–6266. [Google Scholar] [CrossRef]
- Morris, C.F. Puroindolines: The molecular genetic basis of wheat grain hardness. Plant Mol. Biol. 2002, 48, 633–647. [Google Scholar] [CrossRef] [PubMed]
- Bhave, M.; Morris, C.F. Molecular genetics of puroindolines and related genes: Allelic diversity in wheat and other grasses. Plant Mol. Biol. 2008, 66, 205–219. [Google Scholar] [CrossRef] [PubMed]
- Morris, C.F.; Simeone, M.C.; King, G.E.; Lafiandra, D. Transfer of soft kernel texture from Triticum aestivum to durum wheat, Triticum turgidium ssp. durum. Crop Sci. 2011, 51, 114–122. [Google Scholar] [CrossRef]
- Gollan, P.; Smith, K.; Bhave, M. Gsp-1 genes comprise a multigene family in wheat that exhibits a unique combination of sequence diversity yet conservation. J. Cereal Sci. 2007, 45, 184–198. [Google Scholar] [CrossRef]
- Huang, X.Q.; Brule-Babel, A. Development of simple and co-dominant PCR markers to genotype puroindoline a and b alleles for grain hardness in bread wheat (Triticum aestivum L.). J. Cereal Sci. 2011, 53, 277–284. [Google Scholar] [CrossRef]
- Gautier, M.F.; Cosson, P.; Guirao, A.; Alary, R.; Joudrier, P. Puroindoline genes are highly conserved in diploid ancestor wheats and related species but absent in tetraploid Triticum species. Plant Sci. 2000, 153, 81–91. [Google Scholar] [CrossRef]
- Shewry, P.R.; Hawkesford, M.J.; Piironen, V.; Lampi, A.M.; Gebruers, K.; Boros, D.; Andersson, A.A.; Åman, P.; Rakszegi, M.; Bedo, Z.; et al. Natural variation in grain composition of wheat and related cereals. J. Agric. Food Chem. 2013, 61, 8295–8303. [Google Scholar] [CrossRef]
- Kim, K.H.; Kim, J.Y. Understanding wheat starch metabolism in properties, environmental stress condition, and molecular approaches for value-added utilization. Plants 2021, 10, 2282. [Google Scholar] [CrossRef]
- Shevkani, K.; Singh, N.; Bajaj, R.; Kaur, A. Wheat starch production, structure, functionality and applications—A review. Int. J. Food Sci. Technol. 2017, 52, 38–58. [Google Scholar] [CrossRef]
- Olsen, O.A.; Linnestad, C.; Nichols, S.E. Developmental biology of the cereal endosperm. Trends Plant Sci. 1999, 4, 253–257. [Google Scholar] [CrossRef]
- Olsen, O.A. Endosperm development: Cellularization and cell fate specification. Annu. Rev. Plant Physiol. Mol. Biol. 2001, 52, 233–267. [Google Scholar] [CrossRef] [PubMed]
- Begcy, K.; Sandhu, J.; Walia, H. Transient heat stress during early seed development primes germination and seedling establishment in rice. Front. Plant Sci. 2018, 9, 1768. [Google Scholar] [CrossRef] [PubMed]
- Vetrani, C.; Sestili, F.; Vitale, M.; Botticella, E.; Giacco, R.; Griffo, E.; Costabile, G.; Cipriano, P.; Tura, A.; Pacini, G.; et al. Metabolic response to amylose-rich wheat-based rusks in overweight individuals. Eur. J. Clin. Nutr. 2018, 72, 904–912. [Google Scholar] [CrossRef] [PubMed]
- Paul, C. The structure of starch. Nature 1997, 389, 338–339. [Google Scholar] [CrossRef]
- Davis, J.P.; Supatcharee, N.; Khandelwal, R.L.; Chibbar, R.N. Synthesis of novel starches in planta: Opportunities and challenges. Starch-Starke 2003, 55, 107–120. [Google Scholar] [CrossRef]
- Zhang, C.; Jiang, D.; Liu, F.; Cai, J.; Dai, T.; Cao, W. Starch granules size distribution in superior and inferior grains of wheat is related to enzyme activities and their gene expressions during grain filling. J. Cereal Sci. 2010, 51, 226–233. [Google Scholar] [CrossRef]
- Kumar, R.; Kumar, A.; Sharma, N.K.; Kaur, N.; Chunduri, V.; Chawla, M.; Sharma, S.; Singh, K.; Garg, M. Soft and Hard Textured Wheat Differ in Starch Properties as Indicated by Trimodal Distribution, Morphology, Thermal and Crystalline Properties. PLoS ONE 2016, 11, e0147622. [Google Scholar] [CrossRef]
- French, D. Fine structure of starch and its relationship to the organisation of starch granules. J. Jpn. Soc. Starch Sci. 1972, 19, 8–25. [Google Scholar] [CrossRef]
- Lineback, D.R. The starch granule: Organisation and properties. Bak. Dig. 1984, 58, 16–21. [Google Scholar]
- Swinkels, J.J.M. Composition and properties of commercial native starches. Starch-Starke 1985, 37, 1–5. [Google Scholar] [CrossRef]
- Buléon, A.; Colonna, P.; Planchot, V.; Ball, S. Starch granules: Structure and biosynthesis. Int. J. Biol. Macromol. 1998, 23, 85–112. [Google Scholar] [CrossRef]
- Guzmán, C.; Caballero, L.; Martín, L.M.; Alvarez, J.B. Waxy genes from spelt wheat: New alleles for modern wheat breeding and new phylogenetic inferences about the origin of this species. Ann. Bot. 2012, 110, 1161–1171. [Google Scholar] [CrossRef] [PubMed]
- Myers, A.M.; Morell, M.K.; James, M.G.; Ball, S.G. Recent progress toward understanding biosynthesis of the amylopectin crystal. Plant Physiol. 2000, 122, 989–997. [Google Scholar] [CrossRef]
- Jeon, J.S.; Ryoo, N.; Hahn, T.R.; Walia, H.; Nakamura, Y. Starch biosynthesis in cereal endosperm. Plant Physiol. Biochem. 2010, 48, 383–392. [Google Scholar] [CrossRef]
- Crosbie, G.B.; Lambe, W.J.; Tsutsui, H.; Gilmour, R.F. Further evaluation of the flour swelling volume test for identifying wheats potentially suitable for Japanese noodles. J. Cereal Sci. 1992, 15, 271–280. [Google Scholar] [CrossRef]
- Zeng, M.; Morris, C.F.; Batey, I.L.; Wrigley, C.W. Sources of variation for starch gelatinisation, pasting and gelation properties in wheat. Cereal Chem. 1997, 74, 63–71. [Google Scholar] [CrossRef]
- Zhao, X.C.; Sharp, P.J. Production of all eight genotypes of null alleles at ‘waxy’ loci in bread wheat Triticum aestivum L. Plant Breed. 1998, 117, 488–490. [Google Scholar] [CrossRef]
- Kiszonas, A.M.; Fuerst, E.P.; Morris, C.F. A comprehensive survey of soft wheat grain quality in U.S. germplasm. Cereal Chem. 2013, 90, 47–57. [Google Scholar] [CrossRef]
- Morris, C.F.; Engle, D.A.; Kiszonas, A.M. Breeding, selection, and quality characteristics of soft white wheat. Cereal Foods World 2020, 65, 53. [Google Scholar] [CrossRef]
- Gale, K.R. Diagnostic DNA markers for quality traits in wheat. J. Cereal Sci. 2005, 41, 181–192. [Google Scholar] [CrossRef]
- Li, X.; Li, Y.; Zhang, M.; Yu, X.; Hu, R.; Chang, J.; Yang, G.; Wang, Y.; He, G. Diversity of Puroindoline genes and their association with kernel hardness in Chinese wheat cultivars and landraces. Mol. Breed. 2019, 39, 61. [Google Scholar] [CrossRef]
- Chunduri, V.; Kaur, A.; Kaur, S.; Kumar, A.; Sharma, S.; Sharma, N.; Singh, P.; Kapoor, P.; Kaur, S.; Kumari, A.; et al. Gene expression and proteomics studies suggest an involvement of multiple pathways under day and day–night combined heat stresses during grain filling in wheat. Front. Plant Sci. 2021, 12, 660446. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Li, H.; Cui, D. Discovery, distribution and diversity of Puroindoline-D1 genes in bread wheat from five countries (Triticum aestivum L.). BMC Plant Biol. 2013, 13, 125. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.L.; Xia, X.C.; He, Z.H.; Lei, Z.S.; Appels, R.; Yang, Y.; Sun, Q.X.; Ma, W. Novel DNA variations to characterize low molecular weight glutenin Glu-D3 genes and develop STS markers in common wheat. Theor. Appl. Genet. 2007, 114, 451–460. [Google Scholar] [CrossRef]
- Sabelli, P.A.; Shewry, P.R. Characterization and organization of gene families at the Gli-1 loci of bread and durum wheats by restriction fragment analysis. Theor. Appl. Genet. 1991, 83, 209–216. [Google Scholar] [CrossRef]
- van den Broeck, H.C.; Gilissen, L.J.; Smulders, M.J.; van der Meer, I.M.; Hamer, R.J. Dough quality of bread wheat lacking α-gliadins with celiac disease epitopes and addition of celiac-safe avenins to improve dough quality. J. Cereal Sci. 2011, 53, 206–216. [Google Scholar]
- van den Broeck, H.C.; van Herpen, T.W.; Schuit, C.; Salentijn, E.M.; Dekking, L.; Bosch, D.; Hamer, R.J.; Smulders, M.J.; Gilissen, L.J.; van der Meer, I.M. Removing celiac disease-related gluten proteins from bread wheat while retaining technological properties: A study with Chinese Spring deletion lines. BMC Plant Biol. 2009, 9, 41. [Google Scholar] [CrossRef]
- Nakamura, T.; Yamamori, M.; Hirano, H.; Hidaka, S. Identification of three Wx proteins in wheat (Triticum aestivum L.). Biochem. Genet. 1993, 31, 75–86. [Google Scholar] [CrossRef]
- Vrinten, P.; Nakamura, T.; Yamamori, M. Molecular characterization of waxy mutations in wheat. Mol. Gen. Genet. 1999, 261, 463–471. [Google Scholar] [CrossRef]
- Nakamura, T.; Vrinten, P.; Saito, M.; Konda, M. Rapid classification of partial waxy wheats using PCR-based markers. Genome 2002, 45, 1150–1156. [Google Scholar] [CrossRef]
- Li, Z.; Rahman, S.; Kosar-Hashemi, B.; Mouille, G.; Appels, R.; Morell, M.K. Cloning and characterization of a gene encoding wheat starch synthase I. Theor. Appl. Genet. 1999, 98, 1208–1216. [Google Scholar] [CrossRef]
- Peng, M.; Hucl, P.; Chibbar, R.N. Isolation, characterization and expression analysis of starch synthase I from wheat (Triticum aestivum L.). Plant Sci. 2001, 161, 1055–1062. [Google Scholar] [CrossRef]
- Botticella, E.; Sestili, F.; Sparla, F.; Moscatello, S.; Marri, L.; Cuesta-Seijo, J.A.; Falini, G.; Battistelli, A.; Trost, P.; Lafiandra, D. Combining mutations at genes encoding key enzymes involved in starch synthesis affects the amylose content, carbohydrate allocation and hardness in the wheat grain. Plant Biotechnol. J. 2018, 16, 1723–1734. [Google Scholar] [CrossRef] [PubMed]
- Schoen, A.; Joshi, A.; Tiwari, V.; Gill, B.S.; Rawat, N. Triple null mutations in starch synthase SSIIa gene homoeologs lead to high amylose and resistant starch in hexaploid wheat. BMC Plant Biol. 2021, 21, 74. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Mouille, G.; Kosar-Hashemi, B.; Rahman, S.; Clarke, B.; Gale, K.R.; Appels, R.; Morell, M.K. The structure and expression of the wheat starch synthase III gene. Motifs in the expressed gene define the lineage of the starch synthase III gene family. Plant Physiol. 2000, 123, 613–624. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Nameirakpam, B.; Murugesh, T.; Pragya, P.; Mishra, A.; Panigrahi, S.; Pankaj, Y.K.; Kumar, R. Comprehensive analysis of wheat starch synthase III revealed existence of two copies differentially expressed under heat stress. Cereal Res. Commun. 2025, 53, 193–209. [Google Scholar] [CrossRef]
- Huang, X.Q.; Cloutier, S.; Lycar, L.; Radovanovic, N.; Humphreys, D.G.; Noll, J.S.; Somers, D.J.; Brown, P.D. Molecular detection of QTLs for agronomic and quality traits in a doubled haploid population derived from two Canadian wheats (Triticum aestivum L.). Theor. Appl. Genet. 2006, 113, 753–766. [Google Scholar] [CrossRef]
- Tsilo, T.J.; Hareland, G.A.; Simsek, S.; Chao, S.; Anderson, J.A. Genome mapping of kernel characteristics in hard red spring wheat breeding lines. Theor. Appl. Genet. 2010, 121, 717–730. [Google Scholar] [CrossRef]
- Tiwari, C.; Wallwork, H.; Arun, B.; Mishra, V.K.; Velu, G.; Stangoulis, J.; Kumar, U.; Joshi, A.K. Molecular mapping of quantitative trait loci for zinc, iron and protein content in the grains of hexaploid wheat. Euphytica 2016, 207, 563–570. [Google Scholar] [CrossRef]
- Boehm, J.D.; Mit, I.; Kiszonas, A.M.; See, D.R.; Skinner, D.Z.; Morris, C.F. Identification of genotyping-by-sequencing sequence tags associated with milling performance and end-use quality traits in hard red spring wheat (Triticum aestivum L.). J. Cereal Sci. 2017, 77, 73–83. [Google Scholar] [CrossRef]
- Brevis, J.; Morris, C.F.; Manthey, F.; Dubcovsky, J. Effect of the grain protein content locus Gpc-B1 on bread and pasta quality. J. Cereal Sci. 2010, 51, 357–365. [Google Scholar] [CrossRef]
- Kumar, J.; Jaiswal, V.; Kumar, A.; Kumar, N.; Mir, R.R.; Kumar, S.; Dhariwal, R.; Tyagi, S.; Khandelwal, M.; Prabhu, K.V.; et al. Introgression of a major gene for high grain protein content in some Indian bread wheat cultivars. Field Crops Res. 2011, 123, 226–233. [Google Scholar] [CrossRef]
- Kiszonas, A.M.; Ibba, M.I.; Boehm Jr, J.D.; Morris, C.F. Effects of the functional Gpc-B1 allele on soft durum wheat grain, milling, flour, dough, and breadmaking quality. Cereal Chem. 2021, 98, 1250–1258. [Google Scholar] [CrossRef]
- Ohm, J.B.; Klindworth, D.L.; Hareland, G.A.; Faris, J.D.; Elias, E.M.; Xu, S.S. Variation in kernel characteristics and protein molecular weight distribution of Langdon durum-wild emmer wheat chromosome substitution lines. J. Cereal Sci. 2010, 52, 207–214. [Google Scholar] [CrossRef]
- Salmanowicz, B.P.; Langner, M.; Mrugalska, B.; Ratajczak, D.; Górny, A.G. Grain quality characteristics and dough rheology properties in Langdon durum—Wild emmer wheat chromosome substitution lines under nitrogen and water deficits. J. Sci. Food Agric. 2017, 97, 2030–2041, Erratum in J. Sci. Food Agric. 2021, 101, 2168. [Google Scholar] [CrossRef] [PubMed]
- Payne, P.I.; Holt, L.M.; Jackson, E.A.; Law, C.N. Wheat storage proteins: Their genetics and their potential for manipulation by plant breeding. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1984, 304, 359–371. [Google Scholar]
- Gupta, R.B.; Singh, N.K.; Shepherd, K.W. The cumulative effect of allelic variation in LMW and HMW glutenin subunits on dough properties in the progeny of two bread wheats. Theor. Appl. Genet. 1989, 77, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Payne, P.I.; Lawrence, G.J. Catalogue of alleles for the complex gene loci, Glu-A1, Glu-B1 and Glu-D1, which code for high molecular weight subunits of glutenin in hexaploid wheat. Cereal Res. Commun. 1983, 11, 29–35. [Google Scholar]
- Guo, J.; Khan, J.; Pradhan, S.; Shahi, D.; Khan, N.; Avci, M.; Mcbreen, J.; Harrison, S.; Brown-Guedira, G.; Murphy, J.P.; et al. Multi-trait genomic prediction of yield-related traits in US soft wheat under variable water regimes. Genes 2020, 11, 1270. [Google Scholar] [CrossRef]
- Kuchel, H.; Langridge, P.; Mosionek, L.; Williams, K.; Jefferies, S.P. The genetic control of milling yield, dough rheology and baking quality of wheat. Theor. Appl. Genet. 2006, 112, 1487–1495. [Google Scholar] [CrossRef]
- Mao, X.; Li, Y.; Zhao, S.; Zhang, J.; Lei, Q.; Meng, D.; Ma, F.; Hu, W.; Chen, M.; Chang, J.; et al. The interactive effects of transgenically overexpressed 1Ax1 with various HMW-GS combinations on dough quality by introgression of exogenous subunits into an elite Chinese wheat variety. PLoS ONE 2013, 8, e78451. [Google Scholar] [CrossRef]
- Li, X.; Liu, T.; Song, L.; Zhang, H.; Li, L.; Gao, X. Influence of high-molecular-weight glutenin subunit composition at Glu-A1 and Glu-D1 loci on secondary and micro structures of gluten in wheat (Triticum aestivum L.). Food Chem. 2016, 213, 728–734. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Liu, T.; Ding, M.; Wang, J.; Li, C.; Wang, Z.; Li, X. Effects of HMW-GS Ax1 or Dx2 absence on the glutenin polymerization and gluten micro structure of wheat (Triticum aestivum L.). Food Chem. 2018, 240, 626–633. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Li, Y.; Sun, F.; Li, X.; Wang, P.; Chang, J.; Wang, Y.; Yang, G.; He, G. Co-expression of high-molecular-weight glutenin subunit 1Ax1 and Puroindoline a (Pina) genes in transgenic durum wheat (Triticum turgidum ssp. durum) improves milling and pasting quality. BMC Plant Biol. 2019, 19, 126. [Google Scholar]
- Roy, N.; Islam, S.; Ma, J.; Lu, M.; Torok, K.; Tomoskozi, S.; Bekes, F.; Lafiandra, D.; Appels, R.; Ma, W. Expressed Ay HMW glutenin subunit in Australian wheat cultivars indicates a positive effect on wheat quality. J. Cereal Sci. 2018, 79, 494–500. [Google Scholar] [CrossRef]
- Ammar, K.; Kronstad, W.E.; Morris, C.F. Bread-making quality of selected durum wheat genotypes and its relationship with high molecular weight glutenin subunits allelic variation and gluten protein polymeric composition. Cereal Chem. 2000, 77, 230–236. [Google Scholar] [CrossRef]
- Morris, C.F. Bread—Baking quality and the effects of Glu-D1 gene introgressions in durum wheat (Triticum turgidum ssp. durum). Cereal Chem. 2021, 98, 1151–1158. [Google Scholar] [CrossRef]
- Wesley, A.S.; Lukow, O.M.; Ames, N.; Kovacs, M.I.P.; McKenzie, R.I.H.; Brown, D. Effect of single substitution of glutenin or gliadin proteins on flour quality of alpha 16, a Canada prairie spring wheat breeders’ line. Cereal Chem. 1999, 76, 743–747. [Google Scholar] [CrossRef]
- Chugh, V.; Kaur, K.; Singh, D.; Kumar, V.; Kaur, H.; Dhaliwal, H.S. Molecular characterization of diverse wheat germplasm for puroindoline proteins and their antimicrobial activity. Turk. J. Biol. 2015, 39, 359–369. [Google Scholar] [CrossRef]
- Khurshid, M.; Ahmad, M. Prevalence of puroindoline genes and their impact on quality traits in a diverse germplasm of wheat genotypes. Agrivita 2021, 43, 454. [Google Scholar] [CrossRef]
- Cane, K.; Spackman, M.; Eagles, H.A. Puroindoline genes and their effects on grain quality traits in southern Australian wheat cultivars. Aust. J. Agric. Res. 2004, 55, 89–95. [Google Scholar] [CrossRef]
- Eagles, H.A.; Cane, K.; Eastwood, R.F.; Hollamby, G.J.; Kuchel, H.; Martin, P.J.; Cornish, G.B. Contributions of glutenin and puroindoline genes to grain quality traits in southern Australian wheat breeding programs. Aust. J. Agric. Res. 2006, 57, 179–186. [Google Scholar] [CrossRef]
- Krishnamurthy, K.; Balconi, C.; Sherwood, E.J.; Giroux, M.J. Wheat puroindolines enhance fungal disease resistance in transgenic rice. Mol. Plant Microbe Interact. 2001, 14, 1255–1260. [Google Scholar] [CrossRef] [PubMed]
- Giroux, M.J.; Sripo, T.; Gerhardt, S. Puroindolines: Their role in grain hardness and plant defence. Biotechnol. Genet. Eng. Rev. 2003, 20, 277–290. [Google Scholar] [CrossRef][Green Version]
- Capparelli, R.; Amoroso, M.G.; Palumbo, D.; Iannaccone, M.; Faleri, C.; Cresti, M. Two plant puroindolines colocalize in wheat seed and in vitro synergistically fight against pathogens. Plant Mol. Biol. 2005, 58, 857–867. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Q.; Li, X.Y.; Xiao, X.; Sun, F.; Wang, C.; Hu, W.; Feng, Z.; Chang, J.; Chen, M.; et al. Coexpression of the high molecular weight glutenin subunit 1Ax1 and puroindoline improves dough mixing properties in durum wheat (Triticum turgidum L. ssp. durum). PLoS ONE 2012, 7, e50057. [Google Scholar] [CrossRef] [PubMed]
- Lesage, V.; Rhazi, L.; Aussenac, T.; Meleard, B.; Branlard, G. Effects of HMW- and LMW-glutenins and grain hardness on size of gluten polymers. In Proceedings of the 11th International Gluten Workshop, Beijing, China, 12–15 August 2012; He, Z., Wang, D., Eds.; CIMMYT: El Batán, Mexico, 2012; pp. 200–205. [Google Scholar]
- Denyer, K.; Clarke, B.; Hylton, C.; Tatge, H.; Smith, A.M. The elongation of amylase and amylopectin chains in isolated starch granules. Plant J. 1996, 10, 1135–1143. [Google Scholar] [CrossRef]
- Vrinten, P.; Nakamura, T. Wheat granule-bound starch synthase I and II are encoded by separate genes that are expressed in different tissues. Plant Physiol. 2000, 122, 255–263. [Google Scholar] [CrossRef]
- Geera, B.P.; Nelson, J.E.; Souza, E.; Huber, K.C. Granule bound starch synthase I (GBSSI) gene effects related to soft wheat flour/starch characteristics and properties. Cereal Chem. 2006, 83, 544–550. [Google Scholar] [CrossRef]
- Ball, S.; Guan, H.-P.; James, M.; Myers, A.; Keeling, P.; Mouille, G.; Buléon, A.; Colonna, P.; Preiss, J. From glycogen to amylopectin: A model explaining the biogenesis of the plant starch granule. Cell 1996, 86, 349–352. [Google Scholar]
- Mason-Gamer, R.J.; Weil, C.F.; Kellogg, E.A. Granule-bound starch synthase: Structure, function, and phylogenetic utility. Mol. Biol. Evol. 1998, 15, 1658–1673. [Google Scholar] [CrossRef]
- Hanashiro, I.; Itoh, K.; Kuratomi, Y.; Yamazaki, M.; Igarashi, T.; Matsugasako, J.I.; Takeda, Y. Granule-bound starch synthase I is responsible for biosynthesis of extra-long unit chains of amylopectin in rice. Plant Cell Physiol. 2008, 49, 925–933. [Google Scholar] [CrossRef]
- Ainsworth, C.; Clark, J.; Balsdon, J. Expression, organisation and structure of the genes encoding the waxy protein (granule-bound starch synthase) in wheat. Plant Mol. Biol. 1993, 22, 67–82. [Google Scholar] [CrossRef]
- Chao, S.; Sharp, P.J.; Worland, A.J.; Warham, E.J.; Koebner, R.M.D.; Gale, M.D. RFLP-based genetic maps of wheat homoeologous group 7 chromosomes. Theor. Appl. Genet. 1989, 78, 495–504. [Google Scholar] [CrossRef]
- Yamamori, M.; Nakamura, T.; Endo, T.R.; Nagamine, T. Waxy protein deficiency and chromosomal location of coding genes in common wheat. Theor. Appl. Genet. 1994, 89, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Graybosch, R.A. Waxy wheats: Origin properties, and prospects. Trends Food Sci. Technol. 1998, 9, 135–142. [Google Scholar] [CrossRef]
- Nakamura, T.; Yamamori, M.; Hirano, H.; Hidaka, S.; Nagamine, T. Production of waxy (amylose-free) wheats. Mol. Gen. Genet. 1995, 248, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Filip, E.; Woronko, K.; Stępień, E.; Czarniecka, N. An overview of factors affecting the functional quality of common wheat (Triticum aestivum L.). Int. J. Mol. Sci. 2023, 24, 7524. [Google Scholar] [CrossRef]
- Landjeva, S.; Lohwasser, U.; Börner, A. Genetic mapping within the wheat D genome reveals QTL for germination, seed vigour and longevity, and early seedling growth. Euphytica 2010, 171, 129–143. [Google Scholar] [CrossRef]
- Arif, M.A.R.; Nagel, M.; Neumann, K.; Kobiljski, B.; Lohwasser, U.; Börner, A. Genetic studies of seed longevity in hexaploid wheat using segregation and association mapping approaches. Euphytica 2012, 186, 1–13. [Google Scholar] [CrossRef]
- Han, Z.; Ku, L.; Zhang, Z.; Zhang, J.; Guo, S.; Liu, H.; Zhao, R.; Ren, Z.; Zhang, L.; Su, H.; et al. QTLs for seed vigor-related traits identified in maize seeds germinated under artificial aging conditions. PLoS ONE 2014, 9, e92535. [Google Scholar] [CrossRef]
- Chen, L.; Sun, A.; Yang, M.; Chen, L.L.; Ma, X.L.; Li, M.L.; Yin, Y.P. Seed vigor evaluation based on adversity resistance index of wheat seed germination under stress conditions. Ying Yong Sheng Tai Xue Bao J. Appl. Ecol. 2016, 27, 2968–2974. [Google Scholar]
- Morgounov, A.; Akin, B.; Demir, L.; Keser, M.; Kokhmetova, A.; Martynov, S.; Orhan, Ş.; Özdemir, F.; Özseven, İ.; Sapakhova, Z.; et al. Yield gain due to fungicide application in varieties of winter wheat (Triticum aestivum) resistant and susceptible to leaf rust. Crop Pasture Sci. 2015, 66, 649–659. [Google Scholar] [CrossRef]
- Castro, A.C.; Simón, M.R. Effect of tolerance to Septoria tritici blotch on grain yield, yield components and grain quality in Argentinean wheat cultivars. Crop Prot. 2016, 90, 66–76. [Google Scholar] [CrossRef]
- Castro, A.C.; Simón, M.R. The impact of Septoria tritici blotch in bread making quality among argentinean wheat cultivars. J. Cereal Sci. 2017, 77, 259–265. [Google Scholar] [CrossRef]
- Fleitas, M.C.; Schierenbeck, M.; Gerard, G.S.; Dietz, J.I.; Golik, S.I.; Campos, P.E.; Simón, M.R. How leaf rust disease and its control with fungicides affect dough properties, gluten quality and loaf volume under different N rates in wheat. J. Cereal Sci. 2018, 80, 119–127. [Google Scholar] [CrossRef]
- Dziki, D.; Laskowski, J. Influence of kernel size on grinding process of wheat at respective grinding stages. Pol. J. Food Nutr. Sci. 2004, 13, 29–34. [Google Scholar]
- Buendía-Ayala, B.L.; Martínez-Cruz, E.; Villaseñor, H.E.; Hortelano Santa Rosa, R.; Espitia-Rangel, E.; Buendía-González, M.O. The incidence of yellow rust and the industrial quality of the grain and the dough in bread wheat. Rev. Mex. De Cienc. Agrícolas 2019, 10, 143–154. [Google Scholar]
- Esmail, S.M.; Omar, G.E.; El-Orabey, W.M.; Börner, A.; Mourad, A.M. Exploring the genetic variation of Stripe Rust foliar and head infection in Egyptian wheat as an effect of climate change. Agronomy 2023, 13, 1509. [Google Scholar] [CrossRef]
- Zhang, G.; Chen, R.Y.; Shao, M.; Bai, G.; Seabourn, B.W. Genetic analysis of end-use quality traits in wheat. Crop Sci. 2021, 61, 1709–1723. [Google Scholar] [CrossRef]
- Johansson, E.; Henriksson, T.; Prieto-Linde, M.L.; Andersson, S.; Ashraf, R.; Rahmatov, M. Diverse wheat-alien introgression lines as a basis for durable resistance and quality characteristics in bread wheat. Front. Plant Sci. 2020, 11, 1067. [Google Scholar] [CrossRef]
- Koo, D.H.; Friebe, B.; Gill, B.S. Homoeologous recombination: A novel and efficient system for broadening the genetic variability in wheat. Agronomy 2020, 10, 1059. [Google Scholar] [CrossRef]
- Riley, R.; Chapman, V. Genetic control of the cytologically diploid behavior of hexaploid wheat. Nature 1958, 182, 713–715. [Google Scholar] [CrossRef]
- Sears, E.R.; Okamoto, M. Intergenomic chromosome relationship in hexaploid wheat. In Proceedings of the 10th International Congress of Genetics, Montreal, QC, Canada, 20–27 August 1958; pp. 258–259. [Google Scholar]
- Sears, E.R. Genetics society of Canada award of excellence lecture an induced mutant with homoeologous pairing in common wheat. Can. J. Genet. Cytol. 1977, 19, 585–593. [Google Scholar] [CrossRef]
- Friebe, B.; Jiang, J.; Raupp, W.J.; McIntosh, R.A.; Gill, B.S. Characterization of wheat-alien translocations conferring resistance to diseases and pests: Current status. Euphytica 1996, 91, 59–87. [Google Scholar] [CrossRef]
- Friebe, B.; Qi, L.L.; Wilson, D.L.; Chang, Z.J.; Seifers, D.L.; Martin, T.J.; Fritz, A.K.; Gill, B.S. Wheat-Thinopyrum intermedium recombinants resistant to wheat streak mosaic virus and Triticum mosaic virus. Crop Sci. 2009, 49, 1221–1226. [Google Scholar] [CrossRef]
- Gill, B.S.; Friebe, B.; Raupp, W.J.; Wilson, D.L.; Cox, T.S.; Sears, R.G.; Brown-Guedira, G.L.; Fritz, A.K. Wheat Genetics Resource Center: The first 25 years. Adv. Agron. 2006, 85, 73–135. [Google Scholar]
- Qi, L.L.; Friebe, B.; Gill, B.S. Homoeologous recombination, chromosome engineering and crop improvement. Chromosome Res. 2007, 15, 3–19. [Google Scholar] [CrossRef]
- Lukaszewski, A.J. Manipulation of homologous and homoeologous chromosome recombination in wheat. Methods Mol. Biol. 2016, 1429, 77–89. [Google Scholar]
- Ehdaie, B.; Layne, A.P.; Waines, J.G. Root system plasticity to drought influences grain yield in bread wheat. Euphytica 2011, 186, 219–232. [Google Scholar] [CrossRef]
- Mohler, V.; Hsam, S.; Zeller, F.; Wenzel, G. An STS marker distinguishing the rye-derived powdery mildew resistance alleles at the Pm8/Pm17 locus of common wheat. Plant Breed. 2001, 120, 448–450. [Google Scholar] [CrossRef]
- Mago, R.; Zhang, P.; Vautrin, S.; Šimková, H.; Bansal, U.; Luo, M.C.; Rouse, M.; Karaoglu, H.; Periyannan, S.; Kolmer, J.; et al. The wheat Sr50 gene reveals rich diversity at a cereal disease resistance locus. Nat. Plants 2015, 1, 15186. [Google Scholar] [CrossRef]
- Crespo-Herrera, L.A.; Garkava-Gustavsson, L.; Åhman, I. A systematic review of rye (Secale cereale L.) as a source of resistance to pathogens and pests in wheat (Triticum aestivum L.). Hereditas 2017, 154, 14. [Google Scholar] [CrossRef]
- Dong, Z.; Ma, C.; Tian, X.; Zhu, C.; Wang, G.; Lv, Y.; Friebe, B.; Li, H.; Liu, W. Genome-wide impacts of alien chromatin introgression on wheat gene transcriptions. Sci. Rep. 2020, 10, 4801. [Google Scholar] [CrossRef] [PubMed]
- Lapitan, N.L.; Peng, J.; Sharma, V. A high-density map and PCR markers for Russian wheat aphid resistance gene Dn7 on chromosome 1RS/1BL. Crop Sci. 2007, 47, 811–818. [Google Scholar] [CrossRef]
- Tolmay, V.L.; Sydenham, S.L.; Sikhakhane, T.N.; Nhlapho, B.N.; Tsilo, T.J. Elusive diagnostic markers for Russian wheat aphid resistance in bread wheat: Deliberating and reviewing the status quo. Int. J. Mol. Sci. 2020, 21, 8271. [Google Scholar] [CrossRef]
- Schlegel, R.; Meinel, A. A quantitative trait locus (QTL) on chromosome arm 1RS of rye and its effect on yield performance of hexaploid wheat. Cereal Res. Commun. 1994, 22, 7–13. [Google Scholar]
- Hoffmann, B. Alteration of drought tolerance of winter wheat caused by translocation of rye chromosome segment 1RS. Cereal Res. Commun. 2008, 36, 269–278. [Google Scholar] [CrossRef]
- Tahmasebi, S.; Heidari, B.; Pakniyat, H.; Dadkhodaie, A. Consequences of 1BL/1RS Translocation on Agronomic and Physiological Traits in Wheat. Cereal Res. Commun. 2015, 43, 554–566. [Google Scholar] [CrossRef]
- Kim, W.O.; Johnson, J.W.; Baenziger, P.S.; Lukaszewski, A.J.; Gaines, C.S. Agronomic effect of wheat-rye translocation carrying rye chromatin (1R) from different sources. Crop Sci. 2004, 44, 1254–1258. [Google Scholar] [CrossRef]
- Karki, D.; Wyant, W.; Berzonsky, W.A.; Glover, K.D. Investigating physiological and morphological mechanisms of drought tolerance in wheat (Triticum aestivum L.) lines with 1RS translocation. Am. J. Plant Sci. 2014, 5, 1936–1944. [Google Scholar] [CrossRef][Green Version]
- Graybosch, R.A. Mini review: Uneasy unions: Quality effects of rye chromatin transfers to wheat. J. Cereal Sci. 2001, 33, 3–16. [Google Scholar] [CrossRef]
- Howell, T.; Hale, I.; Jankuloski, L.; Bonafede, M.; Gilbert, M.; Dubcovsky, J. Mapping a region within the 1RS. 1BL translocation in common wheat affecting grain yield and canopy water status. Theor. Appl. Genet. 2014, 127, 2695–2709. [Google Scholar] [CrossRef] [PubMed]
- Kiszonas, A.M.; Morris, C.F. Wheat breeding for quality: A historical review. Cereal Chem. 2018, 95, 17–34. [Google Scholar] [CrossRef]
- Kim, W.; Johnson, J.W.; Baenziger, P.S.; Lukaszewski, A.J.; Gaines, C.S. Quality effect of wheat-rye (1R) translocation in ‘Pavon 76’. Plant Breed. 2005, 124, 334–337. [Google Scholar] [CrossRef]
- Jouanin, A.; Gilissen, L.J.; Schaart, J.G.; Leigh, F.J.; Cockram, J.; Wallington, E.J.; Boyd, L.A.; van den Broeck, H.C.; van der Meer, I.M.; America, A.H.P.; et al. CRISPR/Cas9 gene editing of gluten in wheat to reduce gluten content and exposure—Reviewing methods to screen for coeliac safety. Front. Nutr. 2020, 7, 00051. [Google Scholar] [CrossRef]
- Liu, L.; He, Z.; Yan, J.; Zhang, Y.; Xia, X.; Pena, R.J. Allelic variation at the Glu-1 and Glu-3 loci, presence of the 1B. 1R translocation, and their effects on mixographic properties in Chinese bread wheats. Euphytica 2005, 142, 197–204. [Google Scholar] [CrossRef]
- Lukaszewski, A.J. Manipulation of the 1RS. 1BL translocation in wheat by induced homoeologous recombination. Crop Sci. 2000, 40, 216–225. [Google Scholar] [CrossRef]
- Hysing, S.C.; Hsam, S.L.K.; Singh, R.P.; Huerta-Espino, J.; Boyd, L.A.; Koebner, R.M.; Cambron, S.; Johnson, J.W.; Bland, D.E.; Liljeroth, E.; et al. Agronomic performance and multiple disease resistance in T2BS.2RL wheat-rye translocation lines. Crop Sci. 2007, 47, 254–260. [Google Scholar] [CrossRef]
- Roy, N.; Islam, S.; Al-Habbar, Z.; Yu, Z.; Liu, H.; Lafiandra, D.; Masci, S.; Lu, M.; Sultana, N.; Ma, W. Contribution to bread-making performance of two different HMW glutenin 1Ay alleles expressed in hexaploid wheat. J. Agric. Food Chem. 2020, 69, 36–44. [Google Scholar] [CrossRef]
- Gobaa, S.; Brabant, C.; Kleijer, G.; Stamp, P. Effect of the 1BL.1RS translocation and of the Glu-B3 variation on fifteen quality tests in a doubled haploid population of wheat (Triticum aestivum L.). J. Cereal Sci. 2008, 48, 598–603. [Google Scholar] [CrossRef]
- Garg, M.; Tanaka, H.; Tsujimoto, H. Exploration of Triticeae seed storage proteins for improvement of wheat end-product quality. Breed. Sci. 2009, 59, 519–528. [Google Scholar] [CrossRef]
- Lukaszewski, A.J. Registration of six germplasms of durum wheat with introgressions of the Glu-D1 locus. Crop Sci. 2003, 43, 1138. [Google Scholar] [CrossRef]
- Boehm Jr, J.D.; Ibba, M.I.; Kiszonas, A.M.; Morris, C.F. End-use quality of CIMMYT-derived soft-kernel durum wheat germplasm: I. grain, milling, and soft wheat quality. Crop Sci. 2017, 57, 1–10. [Google Scholar] [CrossRef]
- Boehm Jr, J.D.; Ibba, M.I.; Kiszonas, A.M.; Morris, C.F. End-use quality of CIMMYT-derived soft-kernel durum wheat germplasm: II. dough strength and pan bread quality. Crop Sci. 2017, 57, 1485–1494. [Google Scholar] [CrossRef]
- Dai, Y.; Li, J.; Shi, J.; Gao, Y.; Ma, H.; Wang, Y.; Ma, H. Molecular Characterization and Marker Development of the HMW-GS Gene from Thinopyrum elongatum for Improving Wheat Quality. Int. J. Mol. Sci. 2023, 24, 11072. [Google Scholar] [CrossRef] [PubMed]
- Halford, N.G.; Curtis, T.Y.; Chen, Z.; Huang, J. Effects of abiotic stress and crop management on cereal grain composition: Implications for food quality and safety. J. Exp. Bot. 2015, 66, 1145–1156. [Google Scholar] [CrossRef]
- Kumar, R.R.; Sharma, S.K.; Goswami, S.; Singh, G.P.; Singh, R.; Singh, K.; Pathak, H.; Rai, R.D. Characterization of differentially expressed stress-associated proteins in starch granule development under heat stress in wheat (Triticum aestivum L.). Indian J. Biochem. Biophys. 2013, 50, 126–138. [Google Scholar]
- Wang, D.; Li, F.; Cao, S.; Zhang, K. Genomic and functional genomics analyses of gluten proteins and prospect for simultaneous improvement of end-use and health-related traits in wheat. Theor. Appl. Genet. 2020, 133, 1521–1539. [Google Scholar] [CrossRef]
- Pearce, S.; Tabbita, F.; Cantu, D.; Buffalo, V.; Avni, R.; Vazquez-Gross, H.; Zhao, R.; Conley, C.J.; Distelfeld, A.; Dubcovksy, J. Regulation of Zn and Fe transporters by the GPC1 gene during early wheat monocarpic senescence. BMC Plant Biol. 2014, 14, 368. [Google Scholar] [CrossRef]
- Lephuthing, M.C.; Tolmay, V.L.; Baloyi, T.A.; Hlongoane, T.; Oliphant, T.A.; Tsilo, T.J. Relationship of grain micronutrient concentrations and grain yield components in a doubled haploid bread wheat (Triticum aestivum) population. Crop Pasture Sci. 2021, 73, 116–126. [Google Scholar] [CrossRef]
- Yang, F.; Jørgensen, A.D.; Li, H.; Søndergaard, I.; Finnie, C.; Svensson, B.; Jiang, D.; Wollenweber, B.; Jacobsen, S. Implications of high-temperature events and water deficits on protein profiles in wheat (Triticum aestivum L. cv. Vinjett) grain. Proteomics 2011, 11, 1684–1695. [Google Scholar] [CrossRef]
- Hurkman, W.J.; Tanaka, C.K.; Vensel, W.H.; Thilmony, R.; Altenbach, S.B. Comparative proteomic analysis of the effect of temperature and fertilizer on gliadin and glutenin accumulation in the developing endosperm and flour from Triticum aestivum L. cv. Butte 86. Proteome Sci. 2013, 11, 8. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Hu, X.; Islam, S.; She, M.; Peng, Y.; Yu, Z.; Wylie, S.; Juhasz, A.; Dowla, M.; Yang, R.; et al. New insights into the evolution of wheat avenin-like proteins in wild emmer wheat (Triticum dicoccoides). Proc. Natl. Acad. Sci. USA 2018, 115, 13312–13317. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lou, H.; Guo, D.; Zhang, R.; Su, M.; Hou, Z.; Zhou, H.; Liang, R.; Xie, C.; You, M.; et al. Identifying changes in the wheat kernel proteome under heat stress using iTRAQ. Crop J. 2018, 6, 600–610. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, D.; Deng, X.; Zhen, S.; Wang, Z.; Yan, Y. Effects of water deficit on bread-making quality and storage protein compositions in bread wheat (Triticum aestivum L.). J. Sci. Food Agric. 2018, 98, 4357–4368. [Google Scholar] [CrossRef]
- Majoul, T.; Bancel, E.; Triboï, E.; Ben Hamida, J.; Branlard, G. Proteomic analysis of the effect of heat stress on hexaploid wheat grain: Characterization of heat--responsive proteins from total endosperm. Proteomics 2003, 3, 175–183. [Google Scholar] [CrossRef]
- Mazzeo, M.F.; Di Stasio, L.; D’Ambrosio, C.; Arena, S.; Scaloni, A.; Corneti, S.; Ceriotti, A.; Tuberosa, R.; Siciliano, R.A.; Picariello, G.; et al. Identification of early represented gluten proteins during durum wheat grain development. J. Agric. Food Chem. 2017, 65, 3242–3250. [Google Scholar] [CrossRef]
- Garg, M.; Kumar, R.; Singh, R.P. Development of an Aegilops longissima substitution line with improved bread-making quality. J. Cereal Sci. 2014, 60, 389–396. [Google Scholar] [CrossRef]
- Bhullar, S.S.; Jenner, C.F. Differential responses to high temperatures of starch and nitrogen accumulation in the grain of four cultivars of wheat. Aust. J. Plant Physiol. 2005, 12, 363–375. [Google Scholar] [CrossRef]
- Farooq, M.; Bramley, H.; Palta, J.A.; Siddique, K.H. Heat stress in wheat during reproductive and grain-filling phases. Crit. Rev. Plant Sci. 2011, 30, 491–507. [Google Scholar] [CrossRef]
- Dupont, F.M.; Altenbach, S.B. Molecular and biochemical impacts of environmental factors on wheat grain development and protein synthesis. J. Cereal Sci. 2003, 38, 133–146. [Google Scholar] [CrossRef]
- Wilhelm, E.P.; Mullen, R.E.; Keeling, P.L.; Singletary, G.W. Heat stress during grain filling in maize: Effects on kernel growth and metabolism. Crop Sci. 1999, 39, 1733–1741. [Google Scholar] [CrossRef]
- Hurkman, W.; McCue, K.F.; Altenbach, S.; Korn, A.; Tanaka, C.K.; Kothari, K.M.; Johnson, E.L.; Bechtel, D.B.; Wilson, J.D.; Anderson, O.D.; et al. Effect of temperature on expression of genes encoding enzymes for starch biosynthesis in developing wheat endosperm. Plant Sci. 2003, 164, 873–881. [Google Scholar] [CrossRef]
- Lu, H.; Wang, C.; Guo, T.; Xie, Y.; Feng, W.; Li, S. Starch composition and its granules distribution in wheat grains in relation to post-anthesis high temperature and drought stress treatments. Starch-Stärke 2014, 66, 419–428. [Google Scholar] [CrossRef]
- Lu, H.; Hu, Y.; Wang, C.; Liu, W.; Ma, G.; Han, Q.; Ma, D. Effects of high temperature and drought stress on the expression of gene encoding enzymes and the activity of key enzymes involved in starch biosynthesis in wheat grains. Front. Plant Sci. 2019, 10, 1414. [Google Scholar] [CrossRef]
- Chen, F.; Zhang, F.Y.; Xia, X.C.; Dong, Z.D.; Cui, D.Q. Distribution of puroindoline alleles in bread wheat cultivars of the Yellow and Huai valley of China and discovery of a novel puroindoline a allele without PINA protein. Mol. Breed. 2012, 29, 371–378. [Google Scholar] [CrossRef]
- Brown, W.L. Genetic diversity and genetic vulnerability–An appraisal. Econ. Bot. 1983, 37, 4–12. [Google Scholar] [CrossRef]
- Salimath, P.M.; Toker, C.; Sandhu, J.S.; Kumar, J.; Suma, B.; Yadav, S.S.; Bahl, P.N. Conventional breeding methods. In Chickpea Breeding and Management; CABI: Wallingford, UK, 2007; pp. 369–390. [Google Scholar]
- Dwivedi, N.; Kumar, R.; Paliwal, R.; Kumar, U.; Kumar, S.; Singh, M.; Singh, R.K. QTL mapping for important horticultural traits in pepper (Capsicum annuum L.). J. Plant Biochem. Biotechnol. 2015, 24, 154–160. [Google Scholar] [CrossRef]
- Saini, P.; Kaur, H.; Tyagi, V.; Yadav, A.N.; Saini, P.; Sharma, V.; Singh, C.; Dhaliwal, H.S.; Sheikh, I. Genetic enhancement of nutritional and end-use quality in bread wheat through alien introgressions from wild relatives. Cereal Res. Commun. 2023, 51, 295–314. [Google Scholar] [CrossRef]
- Lillemo, M.; Morris, C.F. A leucine to proline mutation in puroindoline b is frequently present in hard wheats from Northern Europe. Theor. Appl. Genet. 2000, 100, 1100–1107. [Google Scholar] [CrossRef]
- Vikram, P.; Franco, J.; Burgueño-Ferreira, J.; Li, H.; Sehgal, D.; Saint Pierre, C.; Ortiz, C.; Sneller, C.; Tattaris, M.; Guzman, C.; et al. Corrigendum: Unlocking the genetic diversity of Creole wheats. Sci. Rep. 2016, 6, 23092, Erratum in Sci. Rep. 2016, 6, 26216. [Google Scholar] [CrossRef]
- Huertas-García, A.B.; Guzmán, C.; Tabbita, F.; Alvarez, J.B. Allelic variation of puroindolines genes in Iranian common wheat landraces. Agriculture 2022, 12, 1196. [Google Scholar] [CrossRef]
- Margiotta, B.; Urbano, M.; Colaprico, G.; Johansson, E.; Buonocore, F.; D’Ovidio, R.; Lafiandra, D. Detection of y-type subunit at the Glu-A1 locus in some Swedish bread wheat lines. J. Cereal Sci. 1996, 23, 203–212. [Google Scholar] [CrossRef]
- Rogers, W.J.; Miller, T.E.; Payne, P.I.; Seekings, J.A.; Sayers, E.J.; Holt, L.M.; Law, C.N. Introduction to bread wheat (Triticum aestivum L.) and assessment for bread-making quality of alleles from T. boeoticum Boiss. ssp. thaoudar at Glu-A1 encoding two high-molecular-weight subunits of glutenin. Euphytica 1997, 93, 19–29. [Google Scholar]
- Morris, C.F.; Casper, J.; Kiszonas, A.M.; Fuerst, E.P.; Murray, J.; Simeone, M.C.; Lafiandra, D. Soft kernel durum wheat: A new bakery ingredient? Cereal Foods World 2015, 60, 76–83. [Google Scholar] [CrossRef]
- Heinze, K.; Kiszonas, A.M.; Murray, J.C.; Morris, C.F.; Lullien-Pellerin, V. Puroindoline genes introduced into durum wheat reduce milling energy and change milling behavior similar to soft common wheats. J. Cereal Sci. 2016, 71, 183–189. [Google Scholar] [CrossRef]
- Sorrells, M.E. Application of new knowledge, technologies, and strategies to wheat improvement. Euphytica 2007, 157, 299–306. [Google Scholar] [CrossRef]
- Meuwissen, T.H.; Hayes, B.J.; Goddard, M.E. Prediction of Total Genetic Value Using Genome-wide Dense Marker Maps. Genetics 2001, 157, 1819–1829. [Google Scholar] [CrossRef] [PubMed]
- Gaspa, G.; Veerkamp, R.F.; Calus, M.P.; Windig, J.J. Assessment of genomic selection for introgression of polledness into Holstein Friesian cattle by simulation. Livest. Sci. 2015, 179, 86–95. [Google Scholar] [CrossRef]
- Yuan, Y.; Cairns, J.E.; Babu, R.; Gowda, M.; Makumbi, D.; Magorokosho, C.; Zhang, A.; Liu, Y.; Wang, N.; Hao, Z.; et al. Genome-wide association mapping and genomic prediction analyses reveal the genetic architecture of grain yield and flowering time under drought and heat stress conditions in maize. Front. Plant Sci. 2019, 9, 1919. [Google Scholar] [CrossRef] [PubMed]
- Budhlakoti, N.; Kushwaha, A.K.; Rai, A.; Chaturvedi, K.K.; Kumar, A.; Pradhan, A.K.; Kumar, U.; Kumar, R.R.; Juliana, P.; Mishra, D.C.; et al. Genomic selection: A tool for accelerating the efficiency of molecular breeding for development of climate-resilient crops. Front. Genet. 2022, 13, 832153. [Google Scholar] [CrossRef] [PubMed]
- Poland, J.; Endelman, J.; Dawson, J.; Rutkoski, J.; Wu, S.; Manes, Y.; Dreisigacker, S.; Crossa, J.; Sánchez-Villeda, H.; Sorrells, M.; et al. Genomic selection in wheat breeding using genotyping-by-sequencing. Plant Genome 2012, 5, 103. [Google Scholar] [CrossRef]
- Crossa, J.; Perez, P.; Hickey, J.; Burgueno, J.; Ornella, L.; Cerón-Rojas, J.; Zhang, X.; Dreisigacker, S.; Babu, R.; Li, Y.; et al. Genomic prediction in CIMMYT maize and wheat breeding programs. Heredity 2014, 112, 48–60. [Google Scholar] [CrossRef]
- Arruda, M.P.; Brown, P.J.; Lipka, A.E.; Krill, A.M.; Thurber, C.; Kolb, F.L. Genomic selection for predicting head blight resistance in a wheat breeding program. Plant Genome 2015, 8, plantgenome2015.01.0003. [Google Scholar] [CrossRef]
- Battenfield, S.D.; Guzmán, C.; Gaynor, R.C.; Singh, R.P.; Peña, R.J.; Dreisigacker, S.; Fritz, A.K.; Poland, J.A. Genomic selection for processing and end--use quality traits in the CIMMYT spring bread wheat breeding program. Plant Genome 2016, 9, plantgenome2016.01.0005. [Google Scholar] [CrossRef]
- Sandhu, K.S.; Aoun, M.; Morris, C.F.; Carter, A.H. Genomic selection for end-use quality and processing traits in soft white winter wheat breeding program with machine and deep learning models. Biology 2021, 10, 689. [Google Scholar] [CrossRef]
- Gill, H.S.; Brar, N.; Halder, J.; Hall, C.; Seabourn, B.W.; Chen, Y.R.; St. Amand, P.; Bernardo, A.; Bai, G.; Glover, K.; et al. Multi-trait genomic selection improves the prediction accuracy of end-use quality traits in hard winter wheat. Plant Genome 2023, 16, e20331. [Google Scholar] [CrossRef]
- Collard, B.C.; Jahufer, M.Z.Z.; Brouwer, J.B.; Pang, E.C.K. An introduction to markers, quantitative trait loci (QTL) mapping and marker-assisted selection for crop improvement: The basic concepts. Euphytica 2005, 142, 169–196. [Google Scholar] [CrossRef]
- Grewal, S.; Hubbart-Edwards, S.; Yang, C.; Devi, U.; Baker, L.; Heath, J.; Ashling, S.; Scholefield, D.; Howells, C.; Yarde, J.; et al. Rapid identification of homozygosity and site of wild relative introgressions in wheat through chromosome-specific KASP genotyping assays. Plant Biotechnol. J. 2020, 18, 743–755. [Google Scholar] [CrossRef]
- Krishnappa, G.; Khan, H.; Krishna, H.; Devate, N.B.; Kumar, S.; Mishra, C.N.; Parkash, O.; Kumar, S.; Kumar, M.; Mamrutha, H.M.; et al. Genome-wide association study for grain protein, thousand kernel weight, and normalized difference vegetation index in bread wheat (Triticum aestivum L.). Genes 2023, 14, 637. [Google Scholar] [CrossRef]
- Campbell, K.G.; Finney, P.L.; Bergman, C.J.; Gualberto, D.G.; Anderson, J.A.; Giroux, M.J.; Siritunga, D.; Zhu, J.; Gendre, F.; Roué, C.; et al. Quantitative trait loci associated with milling and baking quality in a soft x hard wheat cross. Crop Sci. 2001, 41, 1275–1285. [Google Scholar] [CrossRef]
- Breseghello, F.; Sorrells, M.E. Association mapping of kernel size and milling quality in wheat (Triticum aestivum L.) cultivars. Genetics 2006, 172, 1165–1177. [Google Scholar] [CrossRef] [PubMed]
- El-Feki, W.M.; Byrne, P.F.; Reid, S.D.; Haley, S.D. Registration of CO940610/‘Platte’ wheat doubled haploid mapping population. J. Plant Regist. 2015, 9, 419–423. [Google Scholar] [CrossRef]
- Terasawa, Y.; Ito, M.; Tabiki, T.; Nagasawa, K.; Hatta, K.; Nishio, Z. Mapping of a major QTL associated with protein content on chromosome 2B in hard red winter wheat (Triticum aestivum L.). Breed. Sci. 2016, 66, 471–480. [Google Scholar] [CrossRef]
- Singh, K.; Batra, R.; Sharma, S.; Saripalli, G.; Gautam, T.; Singh, R.; Pal, S.; Malik, P.; Kumar, M.; Jan, I.; et al. WheatQTLdb: A QTL database for wheat. Mol. Genet. Genom. 2021, 296, 1051–1056, Erratum in Mol. Genet. Genom. 2021, 296, 1359–1360. [Google Scholar] [CrossRef]
- Giroux, M.J.; Morris, C.F. A glycine to serine change in puroindoline b is associated with wheat grain hardness and low levels of starch-surface friabilin. Theor. Appl. Genet. 1997, 95, 857–864. [Google Scholar] [CrossRef]
- Chen, F.; Beecher, B.; Morris, C. Physical mapping and a new variant of Puroindoline b-2 genes in wheat. Theor. Appl. Genet. 2010, 120, 745–751. [Google Scholar] [CrossRef]
- Liu, S.; Chao, S.; Anderson, J.A. New DNA markers for high molecular weight glutenin subunits in wheat. Theor. Appl. Genet. 2008, 118, 177–183. [Google Scholar] [CrossRef]
- Ravel, C.; Faye, A.; Ben-Sadoun, S.; Ranoux, M.; Dardevet, M.; Dupuits, C.; Exbrayat, F.; Poncet, C.; Sourdille, P.; Branlard, G. SNP markers for early identification of high molecular weight glutenin subunits (HMWGSs) in bread wheat. Theor. Appl. Genet. 2020, 133, 751–770. [Google Scholar] [CrossRef]
- Wang, L.; Li, G.; Peña, R.J.; Xia, X.; He, Z. Development of STS markers and establishment of multiplex PCR for Glu-A3 alleles in common wheat (Triticum aestivum L.). J. Cereal Sci. 2010, 51, 305–312. [Google Scholar] [CrossRef]
- Wang, L.H.; Zhao, X.L.; He, Z.H.; Ma, W.; Appels, R.; Peña, R.J.; Xia, X.C. Characterization of low-molecular-weight glutenin subunit Glu-B3 genes and development of STS markers in common wheat (Triticum aestivum L.). Theor. Appl. Genet. 2009, 118, 525–539. [Google Scholar] [CrossRef]
- Zhang, X.; Karim, H.; Feng, X.; Lan, J.; Tang, H.; Guzmán, C.; Xu, Q.; Zhang, Y.; Qi, P.; Deng, M.; et al. A single base change at exon of Wx-A1 caused gene inactivation and starch properties modified in a wheat EMS mutant line. J. Sci. Food Agric. 2022, 102, 2012–2022. [Google Scholar] [CrossRef] [PubMed]
- Kaya, C. Optimizing crop production with plant phenomics through high-throughput phenotyping and AI in controlled environments. Food Energy Secur. 2025, 14, e70050. [Google Scholar] [CrossRef]
- Kaushal, S.; Gill, H.S.; Billah, M.M.; Khan, S.N.; Halder, J.; Bernardo, A.; Amand, P.S.; Bai, G.; Glover, K.; Maimaitijiang, M.; et al. Enhancing the potential of phenomic and genomic prediction in winter wheat breeding using high-throughput phenotyping and deep learning. Front. Plant Sci. 2024, 15, 1410249. [Google Scholar] [CrossRef] [PubMed]
- Carroll, D. Focus: Genome editing: Genome editing: Past, present and future. Yale J. Biol. Med. 2017, 90, 653–659. [Google Scholar]
- Zhan, X.; Lu, Y.; Zhu, J.K. Genome editing for plant research and crop improvement. J. Integr. Plant Biol. 2021, 63, 3–33. [Google Scholar] [CrossRef]
- Ma, X.; Zhang, Q.; Zhu, Q.; Liu, W.; Chen, Y.; Qiu, R.; Wang, B.; Yang, Z.; Li, H.; Lin, Y.; et al. A robust CRISPR/Cas9 system for convenient, high efficiency multiplex genome editing in monocot and dicot plants. Mol. Plant 2015, 8, 1274–1284. [Google Scholar] [CrossRef]
- Fernie, A.R.; Yan, J. De novo domestication: An alternative route toward new crops for the future. Mol. Plant 2019, 12, 615–631. [Google Scholar] [CrossRef]
- Wang, X.; Lv, S.; Liu, T.; Wei, J.; Qu, S.; Lu, Y.; Zhang, J.; Oo, S.; Zhang, B.; Pan, X.; et al. CRISPR/Cas9 genome editing shows the important role of AZC_2928 gene in nitrogen-fixing bacteria of plants. Funct. Integr. Genom. 2020, 20, 657–668. [Google Scholar] [CrossRef]
- Krishna, T.P.A.; Maharajan, T.; Ceasar, S.A. Application of CRISPR/Cas9 genome editing system to reduce the pre- and postharvest yield losses in cereals. Open Biotechnol. J. 2022, 16, e2205190. [Google Scholar] [CrossRef]
- Svitashev, S.; Young, J.K.; Schwartz, C.; Gao, H.; Falco, S.C.; Cigan, A.M. Targeted mutagenesis, precise gene editing, and site-specific gene insertion in maize using Cas9 and guide RNA. Plant Physiol. 2015, 169, 931–945. [Google Scholar] [CrossRef] [PubMed]
- Ali, Z.; Eid, A.; Ali, S.; Mahfouz, M.M. Pea early-browning virus-mediated genome editing via the CRISPR/Cas9 system in Nicotiana benthamiana and Arabidopsis. Virus Res. 2018, 244, 333–337. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cheng, X.; Shan, Q.; Zhang, Y.; Liu, J.; Gao, C.; Qiu, J.L. Simultaneous editing of three homoeoalleles in hexaploid bread wheat confers heritable resistance to powdery mildew. Nat. Biotechnol. 2014, 32, 947–951. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Simmonds, J.; Pan, Q.; Davidson, D.; He, F.; Battal, A.; Akhunova, A.; Trick, H.N.; Uauy, C.; Akhunov, E. Gene editing and mutagenesis reveal inter-cultivar differences and additivity in the contribution of TaGW2 homoeologues to grain size and weight in wheat. Theor. Appl. Genet. 2018, 131, 2463–2475. [Google Scholar] [CrossRef]
- Wang, W.; Pan, Q.; Tian, B.; He, F.; Chen, Y.; Bai, G.; Akhunova, A.; Trick, H.N.; Akhunov, E. Gene editing of the wheat homologs of TONNEAU1-recruiting motif encoding gene affects grain shape and weight in wheat. Plant J. 2019, 100, 251–264. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, D.; Zhang, D.; Zhao, X.; Cao, X.; Dong, L.; Liu, J.; Chen, K.; Zhang, H.; Gao, C.; et al. Analysis of the functions of TaGW2 homoeologs in wheat grain weight and protein content traits. Plant J. 2018, 94, 857–866. [Google Scholar] [CrossRef]
- Zhang, Z.; Hua, L.; Gupta, A.; Tricoli, D.; Edwards, K.J.; Yang, B.; Li, W. Development of an Agrobacterium-delivered CRISPR/Cas9 system for wheat genome editing. Plant Biotechnol. J. 2019, 17, 1623–1635. [Google Scholar] [CrossRef]
- Abe, F.; Haque, E.; Hisano, H.; Tanaka, T.; Kamiya, Y.; Mikami, M.; Kawaura, K.; Endo, M.; Onishi, K.; Hayashi, T.; et al. Genome-edited triple-recessive mutation alters seed dormancy in wheat. Cell Rep. 2019, 28, 1362–1369. [Google Scholar] [CrossRef]
- Abdallah, N.A.; Elsharawy, H.; Abulela, H.A.; Thilmony, R.; Abdelhadi, A.A.; Elarabi, N.I. Multiplex CRISPR/Cas9-mediated genome editing to address drought tolerance in wheat. GM Crops Food 2022, 16, 1–17. [Google Scholar] [CrossRef]
- Sanchez-Leon, S.; Gil-Humanes, J.; Ozuna, C.V.; Giménez, M.J.; Sousa, C.; Voytas, D.F.; Barro, F. Low-gluten, nontransgenic wheat engineered with CRISPR/Cas9. Plant Biotechnol. J. 2018, 16, 902–910. [Google Scholar] [CrossRef]
- Li, J.; Jiao, G.; Sun, Y.; Chen, J.; Zhong, Y.; Yan, L.; Jiang, D.; Ma, Y.; Xia, L. Modification of starch composition, structure and properties through editing of TaSBEIIa in both winter and spring wheat varieties by CRISPR/Cas9. Plant Biotechnol. J. 2021, 19, 937–951. [Google Scholar] [CrossRef]
- Karimi, M.A.; Paryan, M.; Behrouzian Fard, G.; Sadeghian, H.; Zarrinfar, H.; Hosseini Bafghi, M. Challenges and Opportunities in the Application of CRISPR-Cas9: A Review on Genomic Editing and Therapeutic Potentials. Med. Princ. Pract. 2025, 1–30. [Google Scholar] [CrossRef]
- Elsharawy, H.; Refat, M. CRISPR/Cas9 genome editing in wheat: Enhancing quality and productivity for global food security–a review. Funct. Integr. Genom. 2023, 23, 265. [Google Scholar] [CrossRef]
| Trait | Protein/Enzyme | Genes | Chromosomes | References |
|---|---|---|---|---|
| Grain protein content | Protein | Gpc-B1 | 6BS | [38,41] |
| Grain hardness | Puroindoline a | Pina-D1 | 5DS | [85] |
| Puroindoline b | Pinb-D1 | 5DS | [117] | |
| Gluten quality | HMW-GS | Glu-A1 | 1AL | [22] |
| Glu-B1 | 1BL | [73] | ||
| Glu-D1 | 1DL | [73] | ||
| LMW-GS | Glu-A3 | 1AS | [22,60] | |
| Glu-B3 | 1BS | [60] | ||
| Glu-D3 | 1DS | [118] | ||
| γ and ω-gliadins | Gli-1 | 1AS, 1BS, 1DS | [69,119] | |
| α/β-gliadins | Gli-2 | 6AL, 6BL, 6DL | [120,121] | |
| Starch properties | GBSSI or waxy | Wx-A1 | 7AS | [106,122,123] |
| GBSSI or waxy | Wx-B1 | 4AL | [123,124] | |
| GBSSI or waxy | Wx-D1 | 7DS | [123,124] | |
| Starch synthase I | SSI | 7AS, 7BS, 7DS | [125,126] | |
| Starch synthase IIa | SSIIa | 7AS, 7BS, 7DS | [127,128] | |
| Starch synthase III | SSIII | 1AS, 1BS, 1DS | [129,130] |
| Wheat Type | Introgressed Allele/Genes/Protein/Locus | Source | Protein Type | Characteristics | Reference |
|---|---|---|---|---|---|
| Bread | 1Ay21* | T. dicoccoides or T. urartu | HMW-GS | Improved protein content, storage protein composition, and bread-making quality | [149,216] |
| Bread | 1RS.1BL | Secale cereale | Storage protein secalin | Deteriorates bread-making quality | [208,210,217] |
| Bread | 1E | Agropyron elongatum | Storage protein | Enhanced bread-making quality | [218] |
| Durum | Glu-1D locus | T. aestivum | HMW-GS | Positive effect on bread-making quality | [219] |
| Durum | Ha locus (Pin-D1 genes) | T. aestivum | Hardness | Improved the grain texture in durum wheat | [220,221] |
| Bread | Glu-1Ey | Thinopyrum elongatum | HMW-GS | Improve grain protein content, wet-gluten content, flour, and bread volume value | [222] |
| Trait | Gene/Locus | Marker | Allele | Cultivar/ Accession | Reference |
|---|---|---|---|---|---|
| Protein content | Gpc-B1 | SSR | Gene specific | Langdon | [38,41] |
| Grain hardness | Pina-D1 | STS | Pina-D1a, b | Chinese Spring, Zhongyou 9507 | [243] |
| Pinb-D1 | STS, CAPS | Pinb-D1a, b, c, d, e, p | Chinese Spring, Lorvin10 | [248,274] | |
| Pinb-B2 | Pinb-B2v2 (Pinb2_IND) | Pinb-B2a, b | Chinese Spring, Zhongmai 175 | [275] | |
| HMW-GS | Glu-A1 | KASP | Ax1, Ax2 a, AxNull | Chinese Spring (CS), Opata 85 | [276,277] |
| Glu-B1 | STS, KASP | Bx7, 8, 9, 13, 14, 15, 16, 17, 20, 23 | Various markers | [277] | |
| Glu-D1 | AS-PCR, KASP | Dx2, 3, 5, Dy10, 12 | Various markers | [277] | |
| LMW-GS | Glu-A3 | STS, KASP | a, b, c, d, e, f, g | Various markers | [60,278] |
| Glu-B3 | AS-PCR, STS, KASP | a, b, c, d, e, f, g, h, i | Various markers | [60,279] | |
| Glu-D3 | STS | a, b, c, d, e, g, h, i, j, k | Various markers | [118] | |
| Starch properties | Wx-A1 | SSR, RFLP, KASP, STS | Wx-A1a, b, c, d, e, f, g, h, i | SM126 | [124,280] |
| Wx-B1 | SSR, STS | Wx-B1a, b, e | Various markers | [123] | |
| Wx-D1 | SSR | Wx-D1a, b | F2-lines | [124] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lephuthing, M.C.; Khumalo-Mthembu, T.P.; Tsilo, T.J. The Role of Puroindoline, Gpc-B1, Starch Synthase Genes, and Gluten Proteins in Regulating End-Use Quality in Wheat. Int. J. Mol. Sci. 2025, 26, 8565. https://doi.org/10.3390/ijms26178565
Lephuthing MC, Khumalo-Mthembu TP, Tsilo TJ. The Role of Puroindoline, Gpc-B1, Starch Synthase Genes, and Gluten Proteins in Regulating End-Use Quality in Wheat. International Journal of Molecular Sciences. 2025; 26(17):8565. https://doi.org/10.3390/ijms26178565
Chicago/Turabian StyleLephuthing, Mantshiuwa C., Thobeka Philile Khumalo-Mthembu, and Toi John Tsilo. 2025. "The Role of Puroindoline, Gpc-B1, Starch Synthase Genes, and Gluten Proteins in Regulating End-Use Quality in Wheat" International Journal of Molecular Sciences 26, no. 17: 8565. https://doi.org/10.3390/ijms26178565
APA StyleLephuthing, M. C., Khumalo-Mthembu, T. P., & Tsilo, T. J. (2025). The Role of Puroindoline, Gpc-B1, Starch Synthase Genes, and Gluten Proteins in Regulating End-Use Quality in Wheat. International Journal of Molecular Sciences, 26(17), 8565. https://doi.org/10.3390/ijms26178565






