The Anti-Inflammatory Actions of Soluble Klotho in Brain Aging and Its Main Associated Diseases
Abstract
1. Introduction
2. Klotho Subtypes
2.1. α-Klotho as a Hormone
2.2. α-klotho Polymorphism
2.3. α-Klotho in the Brain
3. Brain Aging and α-Klotho
3.1. Aging Brain
3.1.1. Aging-Associated Cognitive Impairment
3.1.2. Age-Related Dysfunctions of the Blood–Brain Barrier and Blood–Brain–Cerebrospinal Fluid Barrier
3.2. Neuroinflammation
3.3. Neuroinflammation in Aging (Inflammaging)
3.4. α-Klotho Effects in Brain Aging
4. Main Neurological Disorders Associated with Aging
4.1. Alzheimer’s Disease (AD)
4.1.1. Neuroinflammation in AD
4.1.2. α-Klotho Effects in AD
4.2. Parkinson’s Disease (PD)
4.2.1. Neuroinflammation in PD
4.2.2. α-Klotho Effects in PD
4.3. Focal Ischemic Stroke (FIS)
4.3.1. Neuroinflammation in FIS
4.3.2. α-Klotho Effects in FIS
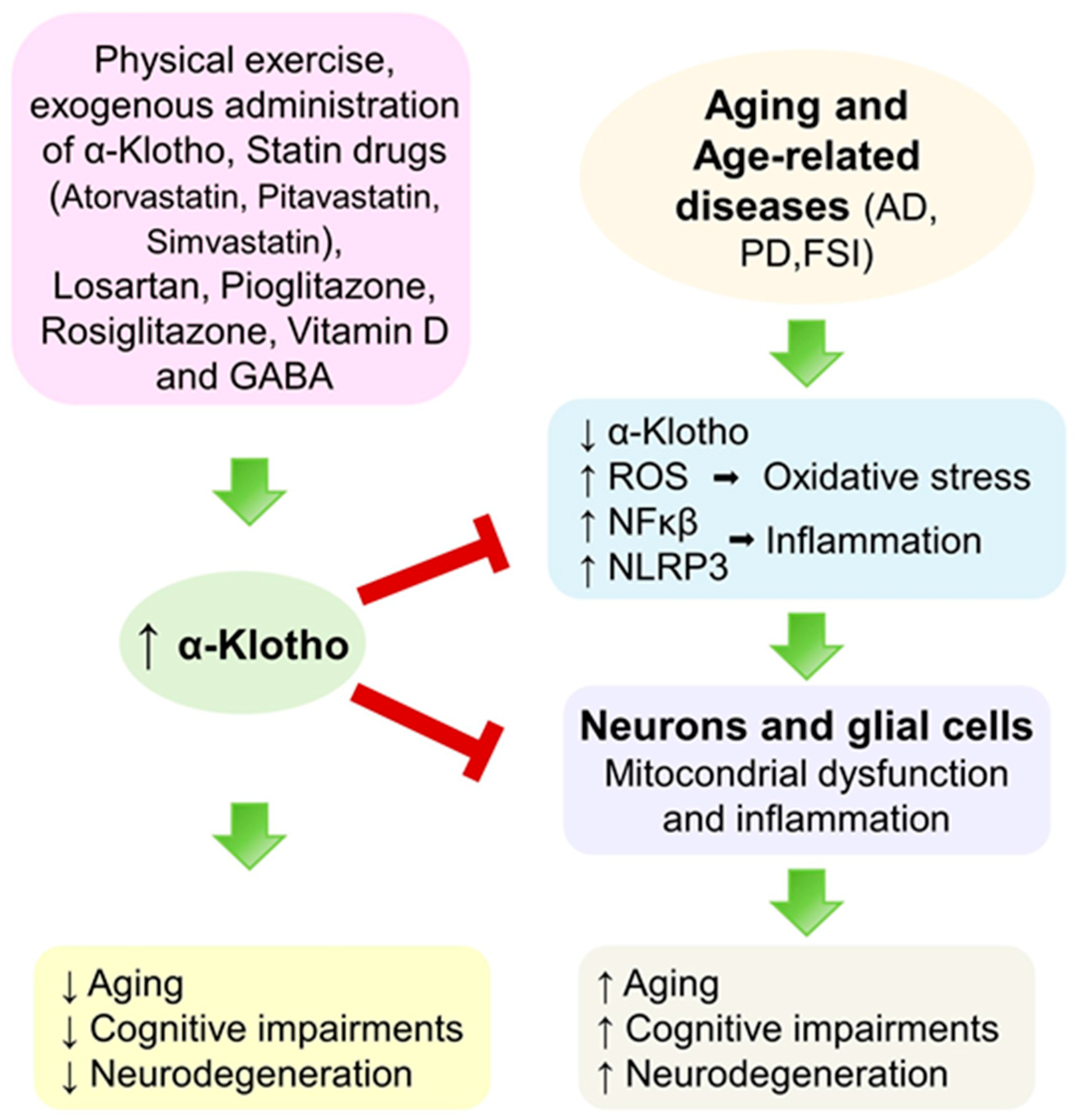
5. Discussion
5.1. Age, Gender, and Serum α-Klotho
5.2. Physical Exercise and Exogenous Administration of α-Klotho
5.3. Repurposing Approved Drugs
5.4. Other Compounds
6. Research Limitations
7. Conclusions
8. Future Directions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s disease |
| ADAM | a disintegrin and metalloproteinase |
| Apo | apolipoprotein |
| APP | amyloid precursor protein |
| BACE1 | β-site amyloid precursor protein cleaving enzyme 1 |
| BBB | blood–brain barrier |
| BCSFB | Blood–cerebrospinal fluid barrier |
| CNS | central nervous system |
| CP | choroid plexus |
| CSF | cerebrospinal fluid |
| DAMPs | damage-associated molecular patterns |
| Erk | extracellular signal-regulated kinase |
| FAD | familial Alzheimer’s disease |
| FGF | fibroblast growth factor |
| FGFR | FGF receptor |
| FoxO1 | forkhead box protein O1 |
| HEK | human embryonic kidney |
| IGF | insulin-like growth factor |
| IL | interleukin |
| KL | Klotho |
| KL-VS | Klotho with valine and serine substitutions |
| MHC II | major histocompatibility complex class II |
| MAPK | mitogen-activated protein kinase |
| MMSE | Mini-Mental Status Examination |
| NFκB | nuclear factor κ-light-chain-enhancer of activated B cells |
| NLRP3 | NACHT-, LRR- and pyrin domain-containing protein 3 |
| NMDA | N-methyl-d-aspartate |
| PERK | protein kinase R (PKR)-like endoplasmic reticulum kinase |
| PI3K | phosphoinositide 3-kinase |
| rDLPFC | right dorsolateral prefrontal cortex |
| PRRs | pattern recognition receptors |
| SAMP | senescence-accelerated prone mouse |
| SNP | single-nucleotide polymorphism |
References
- Martínez-Coria, H.; Arrieta-Cruz, I.; Gutiérrez-Juárez, R.; López-Valdés, H.E. Anti-Inflammatory Effects of Flavonoids in Common Neurological Disorders Associated with Aging. Int. J. Mol. Sci. 2023, 24, 4297. [Google Scholar] [CrossRef]
- Teissier, T.; Boulanger, E.; Cox, L.S. Interconnections between Inflammageing and Immunosenescence during Ageing. Cells 2022, 11, 359. [Google Scholar] [CrossRef]
- Martínez-Coria, H.; Arrieta-Cruz, I.; Cruz, M.-E.; López-Valdés, H.E. Physiopathology of Ischemic Stroke and Its Modulation Using Memantine: Evidence from Preclinical Stroke. Neural Regen. Res. 2021, 16, 433–439. [Google Scholar] [CrossRef]
- Liu, F.; Wu, S.; Ren, H.; Gu, J. Klotho Suppresses RIG-I-Mediated Senescence-Associated Inflammation. Nat. Cell Biol. 2011, 13, 254–262. [Google Scholar] [CrossRef]
- Zeldich, E.; Chen, C.-D.; Colvin, T.A.; Bove-Fenderson, E.A.; Liang, J.; Zhou, T.B.T.; Harris, D.A.; Abraham, C.R. The Neuroprotective Effect of Klotho Is Mediated via Regulation of Members of the Redox System*. J. Biol. Chem. 2014, 289, 24700–24715. [Google Scholar] [CrossRef]
- Kuro-o, M.; Matsumura, Y.; Aizawa, H.; Kawaguchi, H.; Suga, T.; Utsugi, T.; Ohyama, Y.; Kurabayashi, M.; Kaname, T.; Kume, E.; et al. Mutation of the Mouse Klotho Gene Leads to a Syndrome Resembling Ageing. Nature 1997, 390, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Kurosu, H.; Yamamoto, M.; Clark, J.D.; Pastor, J.V.; Nandi, A.; Gurnani, P.; McGuinness, O.P.; Chikuda, H.; Yamaguchi, M.; Kawaguchi, H.; et al. Suppression of Aging in Mice by the Hormone Klotho. Science 2005, 309, 1829–1833. [Google Scholar] [CrossRef]
- Ito, S.; Kinoshita, S.; Shiraishi, N.; Nakagawa, S.; Sekine, S.; Fujimori, T.; Nabeshima, Y. Molecular Cloning and Expression Analyses of Mouse Βklotho, Which Encodes a Novel Klotho Family Protein. Mech. Dev. 2000, 98, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Fujimori, T.; Hayashizaki, Y.; Nabeshima, Y. Identification of a Novel Mouse Membrane-Bound Family 1 Glycosidase-like Protein, Which Carries an Atypical Active Site Structure. Biochim. Biophys. Acta (BBA)—Gene Struct. Expr. 2002, 1576, 341–345. [Google Scholar] [CrossRef]
- Kuro-O, M. The Klotho Proteins in Health and Disease. Nat. Rev. Nephrol. 2019, 15, 27–44. [Google Scholar] [CrossRef]
- Fon Tacer, K.; Bookout, A.L.; Ding, X.; Kurosu, H.; John, G.B.; Wang, L.; Goetz, R.; Mohammadi, M.; Kuro-o, M.; Mangelsdorf, D.J.; et al. Research Resource: Comprehensive Expression Atlas of the Fibroblast Growth Factor System in Adult Mouse. Mol. Endocrinol. 2010, 24, 2050–2064. [Google Scholar] [CrossRef] [PubMed]
- Erben, R.G. α-Klotho’s Effects on Mineral Homeostasis Are Fibroblast Growth Factor-23 Dependent. Curr. Opin. Nephrol. Hypertens. 2018, 27, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Prié, D.; Friedlander, G. Reciprocal Control of 1,25-Dihydroxyvitamin D and FGF23 Formation Involving the FGF23/Klotho System. Clin. J. Am. Soc. Nephrol. 2010, 5, 1717–1722. [Google Scholar] [CrossRef]
- Chen, C.-D.; Tung, T.Y.; Liang, J.; Zeldich, E.; Tucker Zhou, T.B.; Turk, B.E.; Abraham, C.R. Identification of Cleavage Sites Leading to the Shed Form of the Anti-Aging Protein Klotho. Biochemistry 2014, 53, 5579–5587. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-E.; Chen, W.-L. Soluble Klotho as an Effective Biomarker to Characterize Inflammatory States. Ann. Med. 2022, 54, 1520–1529. [Google Scholar] [CrossRef]
- Chen, C.-D.; Podvin, S.; Gillespie, E.; Leeman, S.E.; Abraham, C.R. Insulin Stimulates the Cleavage and Release of the Extracellular Domain of Klotho by ADAM10 and ADAM17. Proc. Natl. Acad. Sci. USA 2007, 104, 19796–19801. [Google Scholar] [CrossRef]
- Shiraki-Iida, T.; Aizawa, H.; Matsumura, Y.; Sekine, S.; Iida, A.; Anazawa, H.; Nagai, R.; Kuro-o, M.; Nabeshima, Y. Structure of the Mouse Klotho Gene and Its Two Transcripts Encoding Membrane and Secreted Protein. FEBS Lett. 1998, 424, 6–10. [Google Scholar] [CrossRef]
- Mencke, R.; Harms, G.; Moser, J.; van Meurs, M.; Diepstra, A.; Leuvenink, H.G.; Hillebrands, J.-L. Human Alternative Klotho mRNA Is a Nonsense-Mediated mRNA Decay Target Inefficiently Spliced in Renal Disease. JCI Insight 2017, 2, e94375. [Google Scholar] [CrossRef]
- Massó, A.; Sánchez, A.; Bosch, A.; Giménez-Llort, L.; Chillón, M. Secreted αKlotho Isoform Protects against Age-Dependent Memory Deficits. Mol. Psychiatry 2018, 23, 1937–1947. [Google Scholar] [CrossRef]
- Roig-Soriano, J.; Griñán-Ferré, C.; Espinosa-Parrilla, J.F.; Abraham, C.R.; Bosch, A.; Pallàs, M.; Chillón, M. AAV-Mediated Expression of Secreted and Transmembrane αKlotho Isoforms Rescues Relevant Aging Hallmarks in Senescent SAMP8 Mice. Aging Cell 2022, 21, e13581. [Google Scholar] [CrossRef]
- Gupta, S.; Moreno, A.J.; Wang, D.; Leon, J.; Chen, C.; Hahn, O.; Poon, Y.; Greenberg, K.; David, N.; Wyss-Coray, T.; et al. KL1 Domain of Longevity Factor Klotho Mimics the Metabolome of Cognitive Stimulation and Enhances Cognition in Young and Aging Mice. J. Neurosci. 2022, 42, 4016–4025. [Google Scholar] [CrossRef] [PubMed]
- Massó, A.; Sánchez, A.; Gimen ez-Llort, L.; Lizcano, J.M.; Cañete, M.; García, B.; Torres-Lista, V.; Puig, M.; Bosch, A.; Chillon, M. Secreted and Transmembrane αKlotho Isoforms Have Different Spatio-Temporal Profiles in the Brain during Aging and Alzheimer’s Disease Progression. PLoS ONE 2015, 10, e0143623. [Google Scholar] [CrossRef]
- Kunert, S.K.; Hartmann, H.; Haffner, D.; Leifheit-Nestler, M. Klotho and Fibroblast Growth Factor 23 in Cerebrospinal Fluid in Children. J. Bone Min. Metab. 2017, 35, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.C.; Shi, M.; Zhang, J.; Addo, T.; Cho, H.J.; Barker, S.L.; Ravikumar, P.; Gillings, N.; Bian, A.; Sidhu, S.S.; et al. Renal Production, Uptake, and Handling of Circulating αKlotho. J. Am. Soc. Nephrol. 2016, 27, 79–90. [Google Scholar] [CrossRef]
- Leon, J.; Moreno, A.J.; Garay, B.I.; Chalkley, R.J.; Burlingame, A.L.; Wang, D.; Dubal, D.B. Peripheral Elevation of a Klotho Fragment Enhances Brain Function and Resilience in Young, Aging, and α-Synuclein Transgenic Mice. Cell Rep. 2017, 20, 1360–1371. [Google Scholar] [CrossRef] [PubMed]
- Lindberg, K.; Amin, R.; Moe, O.W.; Hu, M.-C.; Erben, R.G.; Wernerson, A.Ö.; Lanske, B.; Olauson, H.; Larsson, T.E. The Kidney Is the Principal Organ Mediating Klotho Effects. J. Am. Soc. Nephrol. 2014, 25, 2169–2175. [Google Scholar] [CrossRef]
- Li, S.-A.; Watanabe, M.; Yamada, H.; Nagai, A.; Kinuta, M.; Takei, K. Immunohistochemical Localization of Klotho Protein in Brain, Kidney, and Reproductive Organs of Mice. Cell Struct. Funct. 2004, 29, 91–99. [Google Scholar] [CrossRef]
- Dalton, G.; An, S.-W.; Al-Juboori, S.I.; Nischan, N.; Yoon, J.; Dobrinskikh, E.; Hilgemann, D.W.; Xie, J.; Luby-Phelps, K.; Kohler, J.J.; et al. Soluble Klotho Binds Monosialoganglioside to Regulate Membrane Microdomains and Growth Factor Signaling. Proc. Natl. Acad. Sci. USA 2017, 114, 752–757. [Google Scholar] [CrossRef]
- Cha, S.-K.; Ortega, B.; Kurosu, H.; Rosenblatt, K.P.; Kuro-O, M.; Huang, C.-L. Removal of Sialic Acid Involving Klotho Causes Cell-Surface Retention of TRPV5 Channel via Binding to Galectin-1. Proc. Natl. Acad. Sci. USA 2008, 105, 9805–9810. [Google Scholar] [CrossRef]
- Wright, J.D.; An, S.-W.; Xie, J.; Lim, C.; Huang, C.-L. Soluble Klotho Regulates TRPC6 Calcium Signaling via Lipid Rafts, Independent of the FGFR-FGF23 Pathway. FASEB J. 2019, 33, 9182–9193. [Google Scholar] [CrossRef]
- Chang, Q.; Hoefs, S.; van der Kemp, A.W.; Topala, C.N.; Bindels, R.J.; Hoenderop, J.G. The Beta-Glucuronidase Klotho Hydrolyzes and Activates the TRPV5 Channel. Science 2005, 310, 490–493. [Google Scholar] [CrossRef]
- Cha, S.-K.; Hu, M.-C.; Kurosu, H.; Kuro-O, M.; Moe, O.; Huang, C.-L. Regulation of Renal Outer Medullary Potassium Channel and Renal K(+) Excretion by Klotho. Mol. Pharmacol. 2009, 76, 38–46. [Google Scholar] [CrossRef]
- Huang, C.-L. Regulation of Ion Channels by Secreted Klotho: Mechanisms and Implications. Kidney Int. 2010, 77, 855–860. [Google Scholar] [CrossRef]
- Landry, T.; Li, P.; Shookster, D.; Jiang, Z.; Li, H.; Laing, B.T.; Bunner, W.; Langton, T.; Tong, Q.; Huang, H. Centrally Circulating α-Klotho Inversely Correlates with Human Obesity and Modulates Arcuate Cell Populations in Mice. Mol. Metab. 2021, 44, 101136. [Google Scholar] [CrossRef]
- Semba, R.D.; Moghekar, A.R.; Hu, J.; Sun, K.; Turner, R.; Ferrucci, L.; O’Brien, R. Klotho in the Cerebrospinal Fluid of Adults with and without Alzheimer’s Disease. Neurosci. Lett. 2014, 558, 37–40. [Google Scholar] [CrossRef]
- Shardell, M.; Semba, R.D.; Rosano, C.; Kalyani, R.R.; Bandinelli, S.; Chia, C.W.; Ferrucci, L. Plasma Klotho and Cognitive Decline in Older Adults: Findings from the InCHIANTI Study. J. Gerontol. A Biol. Sci. Med. Sci. 2016, 71, 677–682. [Google Scholar] [CrossRef] [PubMed]
- Sanz, B.; Arrieta, H.; Rezola-Pardo, C.; Fernández-Atutxa, A.; Garin-Balerdi, J.; Arizaga, N.; Rodriguez-Larrad, A.; Irazusta, J. Low Serum Klotho Concentration Is Associated with Worse Cognition, Psychological Components of Frailty, Dependence, and Falls in Nursing Home Residents. Sci. Rep. 2021, 11, 9098. [Google Scholar] [CrossRef] [PubMed]
- Ge, S.; Dong, F.; Tian, C.; Yang, C.-H.; Liu, M.; Wei, J. Serum Soluble Alpha-Klotho Klotho and Cognitive Functioning in Older Adults Aged 60 and 79: An Analysis of Cross-Sectional Data of the National Health and Nutrition Examination Survey 2011 to 2014. BMC Geriatr. 2024, 24, 245. [Google Scholar] [CrossRef]
- Sun, X.; Chen, L.; He, Y.; Zheng, L. Circulating α-Klotho Levels in Relation to Cardiovascular Diseases: A Mendelian Randomization Study. Front. Endocrinol. 2022, 13, 842846. [Google Scholar] [CrossRef]
- Cheng, Y.-W.; Hung, C.-C.; Fang, W.-H.; Chen, W.-L. Association between Soluble α-Klotho Protein and Metabolic Syndrome in the Adult Population. Biomolecules 2022, 12, 70. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Su, W.; Shen, Z.; Wang, R. Correlation between Soluble α-Klotho and Renal Function in Patients with Chronic Kidney Disease: A Review and Meta-Analysis. Biomed. Res. Int. 2018, 2018, 9481475. [Google Scholar] [CrossRef]
- Cardoso, A.L.; Fernandes, A.; Aguilar-Pimentel, J.A.; de Angelis, M.H.; Guedes, J.R.; Brito, M.A.; Ortolano, S.; Pani, G.; Athanasopoulou, S.; Gonos, E.S.; et al. Towards Frailty Biomarkers: Candidates from Genes and Pathways Regulated in Aging and Age-Related Diseases. Ageing Res. Rev. 2018, 47, 214–277. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.; Liu, X.; Zhang, T. L-Shaped Association of Systemic Immune-Inflammation Index (SII) with Serum Soluble α-Klotho in the Prospective Cohort Study from the NHANES Database. Sci. Rep. 2024, 14, 13189. [Google Scholar] [CrossRef]
- Ye, C.; Yuan, L.; Wu, K.; Shen, B.; Zhu, C. Association between Systemic Immune-Inflammation Index and Chronic Obstructive Pulmonary Disease: A Population-Based Study. BMC Pulm. Med. 2023, 23, 295. [Google Scholar] [CrossRef]
- Revelas, M.; Thalamuthu, A.; Oldmeadow, C.; Evans, T.-J.; Armstrong, N.J.; Kwok, J.B.; Brodaty, H.; Schofield, P.R.; Scott, R.J.; Sachdev, P.S.; et al. Review and Meta-Analysis of Genetic Polymorphisms Associated with Exceptional Human Longevity. Mech. Ageing Dev. 2018, 175, 24–34. [Google Scholar] [CrossRef]
- Zhu, Z.; Xia, W.; Cui, Y.; Zeng, F.; Li, Y.; Yang, Z.; Hequn, C. Klotho Gene Polymorphisms Are Associated with Healthy Aging and Longevity: Evidence from a Meta-Analysis. Mech. Ageing Dev. 2019, 178, 33–40. [Google Scholar] [CrossRef]
- Almeida, O.P.; Morar, B.; Hankey, G.J.; Yeap, B.B.; Golledge, J.; Jablensky, A.; Flicker, L. Longevity Klotho Gene Polymorphism and the Risk of Dementia in Older Men. Maturitas 2017, 101, 1–5. [Google Scholar] [CrossRef]
- Arking, D.E.; Atzmon, G.; Arking, A.; Barzilai, N.; Dietz, H.C. Association between a Functional Variant of the KLOTHO Gene and High-Density Lipoprotein Cholesterol, Blood Pressure, Stroke, and Longevity. Circ. Res. 2005, 96, 412–418. [Google Scholar] [CrossRef] [PubMed]
- Clinton, S.M.; Glover, M.E.; Maltare, A.; Laszczyk, A.M.; Mehi, S.J.; Simmons, R.K.; King, G.D. Expression of Klotho mRNA and Protein in Rat Brain Parenchyma from Early Postnatal Development into Adulthood. Brain Res. 2013, 1527, 1–14. [Google Scholar] [CrossRef]
- German, D.C.; Khobahy, I.; Pastor, J.; Kuro-O, M.; Liu, X. Nuclear Localization of Klotho in Brain: An Anti-Aging Protein. Neurobiol. Aging 2012, 33, 1483.e25–1483.e30. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Jing, D.; Liu, Z.; Chen, Y.; Huang, F.; Behnisch, T. Enhanced Expression of Secreted α-Klotho in the Hippocampus Alters Nesting Behavior and Memory Formation in Mice. Front. Cell Neurosci. 2019, 13, 133. [Google Scholar] [CrossRef] [PubMed]
- Shiozaki, M.; Yoshimura, K.; Shibata, M.; Koike, M.; Matsuura, N.; Uchiyama, Y.; Gotow, T. Morphological and Biochemical Signs of Age-Related Neurodegenerative Changes in Klotho Mutant Mice. Neuroscience 2008, 152, 924–941. [Google Scholar] [CrossRef] [PubMed]
- Dubal, D.B.; Yokoyama, J.S.; Zhu, L.; Broestl, L.; Worden, K.; Wang, D.; Sturm, V.E.; Kim, D.; Klein, E.; Yu, G.-Q.; et al. Life Extension Factor Klotho Enhances Cognition. Cell Rep. 2014, 7, 1065–1076. [Google Scholar] [CrossRef]
- Kuriyama, N.; Ozaki, E.; Mizuno, T.; Ihara, M.; Mizuno, S.; Koyama, T.; Matsui, D.; Watanabe, I.; Akazawa, K.; Takeda, K.; et al. Association between α-Klotho and Deep White Matter Lesions in the Brain: A Pilot Case Control Study Using Brain MRI. J. Alzheimers Dis. 2018, 61, 145–155. [Google Scholar] [CrossRef]
- Chen, C.-D.; Li, H.; Liang, J.; Hixson, K.; Zeldich, E.; Abraham, C.R. The Anti-Aging and Tumor Suppressor Protein Klotho Enhances Differentiation of a Human Oligodendrocytic Hybrid Cell Line. J. Mol. Neurosci. 2015, 55, 76–90. [Google Scholar] [CrossRef]
- Zeldich, E.; Chen, C.-D.; Avila, R.; Medicetty, S.; Abraham, C.R. The Anti-Aging Protein Klotho Enhances Remyelination Following Cuprizone-Induced Demyelination. J. Mol. Neurosci. 2015, 57, 185–196. [Google Scholar] [CrossRef]
- MacNee, W.; Rabinovich, R.A.; Choudhury, G. Ageing and the Border between Health and Disease. Eur. Respir. J. 2014, 44, 1332–1352. [Google Scholar] [CrossRef]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The Hallmarks of Aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. Hallmarks of Aging: An Expanding Universe. Cell 2023, 186, 243–278. [Google Scholar] [CrossRef]
- Lee, J.; Kim, H.-J. Normal Aging Induces Changes in the Brain and Neurodegeneration Progress: Review of the Structural, Biochemical, Metabolic, Cellular, and Molecular Changes. Front. Aging Neurosci. 2022, 14, 931536. [Google Scholar] [CrossRef] [PubMed]
- Blinkouskaya, Y.; Caçoilo, A.; Gollamudi, T.; Jalalian, S.; Weickenmeier, J. Brain Aging Mechanisms with Mechanical Manifestations. Mech. Ageing Dev. 2021, 200, 111575. [Google Scholar] [CrossRef]
- Mishra, A.; Bandopadhyay, R.; Singh, P.K.; Mishra, P.S.; Sharma, N.; Khurana, N. Neuroinflammation in Neurological Disorders: Pharmacotherapeutic Targets from Bench to Bedside. Metab. Brain Dis. 2021, 36, 1591–1626. [Google Scholar] [CrossRef] [PubMed]
- Ownby, R.L. Neuroinflammation and Cognitive Aging. Curr. Psychiatry Rep. 2010, 12, 39–45. [Google Scholar] [CrossRef] [PubMed]
- National Institute on Aging What Are the Signs of Alzheimer’s Disease? Available online: https://www.nia.nih.gov/health/alzheimers-symptoms-and-diagnosis/what-are-signs-alzheimers-disease (accessed on 30 July 2025).
- Baghel, M.S.; Singh, P.; Srivas, S.; Thakur, M.K. Cognitive Changes with Aging. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2019, 89, 765–773. [Google Scholar] [CrossRef]
- Mekhora, C.; Lamport, D.J.; Spencer, J.P.E. An Overview of the Relationship between Inflammation and Cognitive Function in Humans, Molecular Pathways and the Impact of Nutraceuticals. Neurochem. Int. 2024, 181, 105900. [Google Scholar] [CrossRef]
- Janelidze, S.; Mattsson, N.; Stomrud, E.; Lindberg, O.; Palmqvist, S.; Zetterberg, H.; Blennow, K.; Hansson, O. CSF Biomarkers of Neuroinflammation and Cerebrovascular Dysfunction in Early Alzheimer Disease. Neurology 2018, 91, e867–e877. [Google Scholar] [CrossRef]
- Alley, D.E.; Crimmins, E.M.; Karlamangla, A.; Hu, P.; Seeman, T.E. Inflammation and Rate of Cognitive Change in High-Functioning Older Adults. J. Gerontol. A Biol. Sci. Med. Sci. 2008, 63, 50–55. [Google Scholar] [CrossRef]
- Yaffe, K.; Lindquist, K.; Penninx, B.W.; Simonsick, E.M.; Pahor, M.; Kritchevsky, S.; Launer, L.; Kuller, L.; Rubin, S.; Harris, T. Inflammatory Markers and Cognition in Well-Functioning African-American and White Elders. Neurology 2003, 61, 76–80. [Google Scholar] [CrossRef] [PubMed]
- d’Avila, J.C.; Siqueira, L.D.; Mazeraud, A.; Azevedo, E.P.; Foguel, D.; Castro-Faria-Neto, H.C.; Sharshar, T.; Chrétien, F.; Bozza, F.A. Age-Related Cognitive Impairment Is Associated with Long-Term Neuroinflammation and Oxidative Stress in a Mouse Model of Episodic Systemic Inflammation. J. Neuroinflamm. 2018, 15, 28. [Google Scholar] [CrossRef]
- IL-17A Is Implicated in Lipopolysaccharide-Induced Neuroinflammation and Cognitive Impairment in Aged Rats Via Microglial Activation-PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/26373740/ (accessed on 30 July 2025).
- Zhang, D.; Li, S.; Hou, L.; Jing, L.; Ruan, Z.; Peng, B.; Zhang, X.; Hong, J.-S.; Zhao, J.; Wang, Q. Microglial Activation Contributes to Cognitive Impairments in Rotenone-Induced Mouse Parkinson’s Disease Model. J. Neuroinflamm. 2021, 18, 4. [Google Scholar] [CrossRef]
- Gelb, S.; Lehtinen, M.K. Snapshot: Choroid Plexus Brain Barrier. Cell 2023, 186, 3522–3522.e1. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Chen, Q.; Chen, X.; Han, F.; Chen, Z.; Wang, Y. The Blood–Brain Barrier: Structure, Regulation and Drug Delivery. Sig. Transduct. Target. Ther. 2023, 8, 1–27. [Google Scholar] [CrossRef]
- Knox, E.G.; Aburto, M.R.; Clarke, G.; Cryan, J.F.; O’Driscoll, C.M. The Blood-Brain Barrier in Aging and Neurodegeneration. Mol. Psychiatry 2022, 27, 2659–2673. [Google Scholar] [CrossRef]
- Vandenbroucke, R.E. A Hidden Epithelial Barrier in the Brain with a Central Role in Regulating Brain Homeostasis. Implications for Aging. Ann. ATS 2016, 13, S407–S410. [Google Scholar] [CrossRef]
- Xu, H.; Hehnly, C.; Lehtinen, M.K. The Choroid Plexus: A Command Center for Brain–Body Communication during Inflammation. Curr. Opin. Immunol. 2025, 93, 102540. [Google Scholar] [CrossRef]
- Chung, C.-P.; Chang, Y.-C.; Ding, Y.; Lim, K.; Liu, Q.; Zhu, L.; Zhang, W.; Lu, T.-S.; Molostvov, G.; Zehnder, D.; et al. α-Klotho Expression Determines Nitric Oxide Synthesis in Response to FGF-23 in Human Aortic Endothelial Cells. PLoS ONE 2017, 12, e0176817. [Google Scholar] [CrossRef]
- Zhu, L.; Stein, L.R.; Kim, D.; Ho, K.; Yu, G.-Q.; Zhan, L.; Larsson, T.E.; Mucke, L. Klotho Controls the Brain–Immune System Interface in the Choroid Plexus. Proc. Natl. Acad. Sci. USA 2018, 115, E11388–E11396. [Google Scholar] [CrossRef]
- López-Valdés, H.E.; Martínez-Coria, H. The Role of Neuroinflammation in Age-Related Dementias. Rev. Investig. Clin. 2016, 68, 40–48. [Google Scholar] [CrossRef]
- Fulop, T.; Larbi, A.; Pawelec, G.; Khalil, A.; Cohen, A.A.; Hirokawa, K.; Witkowski, J.M.; Franceschi, C. Immunology of Aging: The Birth of Inflammaging. Clin. Rev. Allergy Immunol. 2023, 64, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wu, M. Pattern Recognition Receptors in Health and Diseases. Signal Transduct. Target. Ther. 2021, 6, 291. [Google Scholar] [CrossRef] [PubMed]
- Rea, I.M.; Gibson, D.S.; McGilligan, V.; McNerlan, S.E.; Alexander, H.D.; Ross, O.A. Age and Age-Related Diseases: Role of Inflammation Triggers and Cytokines. Front. Immunol. 2018, 9, 586. [Google Scholar] [CrossRef]
- Kumar, V. Toll-like Receptors in the Pathogenesis of Neuroinflammation. J. Neuroimmunol. 2019, 332, 16–30. [Google Scholar] [CrossRef]
- Pascual, M.; Calvo-Rodriguez, M.; Núñez, L.; Villalobos, C.; Ureña, J.; Guerri, C. Toll-like Receptors in Neuroinflammation, Neurodegeneration, and Alcohol-Induced Brain Damage. IUBMB Life 2021, 73, 900–915. [Google Scholar] [CrossRef]
- Vidal-Itriago, A.; Radford, R.A.W.; Aramideh, J.A.; Maurel, C.; Scherer, N.M.; Don, E.K.; Lee, A.; Chung, R.S.; Graeber, M.B.; Morsch, M. Microglia Morphophysiological Diversity and Its Implications for the CNS. Front. Immunol. 2022, 13, 997786. [Google Scholar] [CrossRef]
- Paolicelli, R.C.; Sierra, A.; Stevens, B.; Tremblay, M.-E.; Aguzzi, A.; Ajami, B.; Amit, I.; Audinat, E.; Bechmann, I.; Bennett, M.; et al. Microglia States and Nomenclature: A Field at Its Crossroads. Neuron 2022, 110, 3458–3483. [Google Scholar] [CrossRef] [PubMed]
- Escartin, C.; Galea, E.; Lakatos, A.; O’Callaghan, J.P.; Petzold, G.C.; Serrano-Pozo, A.; Steinhäuser, C.; Volterra, A.; Carmignoto, G.; Agarwal, A.; et al. Reactive Astrocyte Nomenclature, Definitions, and Future Directions. Nat. Neurosci. 2021, 24, 312–325. [Google Scholar] [CrossRef] [PubMed]
- Chou, S.-M.; Yen, Y.-H.; Yuan, F.; Zhang, S.-C.; Chong, C.-M. Neuronal Senescence in the Aged Brain. Aging Dis. 2023, 14, 1618–1632. [Google Scholar] [CrossRef]
- Martínez-Cué, C.; Rueda, N. Cellular Senescence in Neurodegenerative Diseases. Front. Cell Neurosci. 2020, 14, 16. [Google Scholar] [CrossRef] [PubMed]
- Godbout, J.P.; Chen, J.; Abraham, J.; Richwine, A.F.; Berg, B.M.; Kelley, K.W.; Johnson, R.W. Exaggerated Neuroinflammation and Sickness Behavior in Aged Mice Following Activation of the Peripheral Innate Immune System. FASEB J. 2005, 19, 1329–1331. [Google Scholar] [CrossRef]
- Caldeira, C.; Oliveira, A.F.; Cunha, C.; Vaz, A.R.; Falcão, A.S.; Fernandes, A.; Brites, D. Microglia Change from a Reactive to an Age-like Phenotype with the Time in Culture. Front. Cell Neurosci. 2014, 8, 152. [Google Scholar] [CrossRef]
- Njie, E.G.; Boelen, E.; Stassen, F.R.; Steinbusch, H.W.M.; Borchelt, D.R.; Streit, W.J. Ex Vivo Cultures of Microglia from Young and Aged Rodent Brain Reveal Age-Related Changes in Microglial Function. Neurobiol. Aging 2012, 33, 195.e1–195.e12. [Google Scholar] [CrossRef] [PubMed]
- Sierra, A.; Encinas, J.M.; Deudero, J.J.P.; Chancey, J.H.; Enikolopov, G.; Overstreet-Wadiche, L.S.; Tsirka, S.E.; Maletic-Savatic, M. Microglia Shape Adult Hippocampal Neurogenesis through Apoptosis-Coupled Phagocytosis. Cell Stem Cell 2010, 7, 483–495. [Google Scholar] [CrossRef]
- Mou, Y.; Du, Y.; Zhou, L.; Yue, J.; Hu, X.; Liu, Y.; Chen, S.; Lin, X.; Zhang, G.; Xiao, H.; et al. Gut Microbiota Interact with the Brain Through Systemic Chronic Inflammation: Implications on Neuroinflammation, Neurodegeneration, and Aging. Front. Immunol. 2022, 13, 796288. [Google Scholar] [CrossRef]
- Colombo, A.V.; Sadler, R.K.; Llovera, G.; Singh, V.; Roth, S.; Heindl, S.; Sebastian Monasor, L.; Verhoeven, A.; Peters, F.; Parhizkar, S.; et al. Microbiota-Derived Short Chain Fatty Acids Modulate Microglia and Promote Aβ Plaque Deposition. eLife 2021, 10, e59826. [Google Scholar] [CrossRef]
- Sun, M.-F.; Zhu, Y.-L.; Zhou, Z.-L.; Jia, X.-B.; Xu, Y.-D.; Yang, Q.; Cui, C.; Shen, Y.-Q. Neuroprotective Effects of Fecal Microbiota Transplantation on MPTP-Induced Parkinson’s Disease Mice: Gut Microbiota, Glial Reaction and TLR4/TNF-α Signaling Pathway. Brain Behav. Immun. 2018, 70, 48–60. [Google Scholar] [CrossRef]
- Sampson, T.R.; Debelius, J.W.; Thron, T.; Janssen, S.; Shastri, G.G.; Ilhan, Z.E.; Challis, C.; Schretter, C.E.; Rocha, S.; Gradinaru, V.; et al. Gut Microbiota Regulate Motor Deficits and Neuroinflammation in a Model of Parkinson’s Disease. Cell 2016, 167, 1469–1480.e12. [Google Scholar] [CrossRef]
- Abraham, C.R.; Li, A. Aging-Suppressor Klotho: Prospects in Diagnostics and Therapeutics. Ageing Res. Rev. 2022, 82, 101766. [Google Scholar] [CrossRef] [PubMed]
- Kurosu, H.; Ogawa, Y.; Miyoshi, M.; Yamamoto, M.; Nandi, A.; Rosenblatt, K.P.; Baum, M.G.; Schiavi, S.; Hu, M.-C.; Moe, O.W.; et al. Regulation of Fibroblast Growth Factor-23 Signaling by Klotho. J. Biol. Chem. 2006, 281, 6120–6123. [Google Scholar] [CrossRef]
- Ikushima, M.; Rakugi, H.; Ishikawa, K.; Maekawa, Y.; Yamamoto, K.; Ohta, J.; Chihara, Y.; Kida, I.; Ogihara, T. Anti-Apoptotic and Anti-Senescence Effects of Klotho on Vascular Endothelial Cells. Biochem. Biophys. Res. Commun. 2006, 339, 827–832. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Zhao, Y.; Sarkar, P.S.; Rosenblatt, K.P.; Tilton, R.G.; Choudhary, S. Klotho Ameliorates Chemically Induced Endoplasmic Reticulum (ER) Stress Signaling. Cell. Physiol. Biochem. 2013, 31, 659–672. [Google Scholar] [CrossRef]
- Yamamoto, M.; Clark, J.D.; Pastor, J.V.; Gurnani, P.; Nandi, A.; Kurosu, H.; Miyoshi, M.; Ogawa, Y.; Castrillon, D.H.; Rosenblatt, K.P.; et al. Regulation of Oxidative Stress by the Anti-Aging Hormone Klotho*,♦. J. Biol. Chem. 2005, 280, 38029–38034. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Pu, S.; Zhou, H.; Guo, Y. Klotho as Potential Autophagy Regulator and Therapeutic Target. Front. Pharmacol. 2021, 12, 755366. [Google Scholar] [CrossRef]
- Chen, C.-D.; Sloane, J.A.; Li, H.; Aytan, N.; Giannaris, E.L.; Zeldich, E.; Hinman, J.D.; Dedeoglu, A.; Rosene, D.L.; Bansal, R.; et al. The Antiaging Protein Klotho Enhances Oligodendrocyte Maturation and Myelination of the CNS. J. Neurosci. 2013, 33, 1927–1939. [Google Scholar] [CrossRef]
- Baluchnejadmojarad, T.; Eftekhari, S.-M.; Jamali-Raeufy, N.; Haghani, S.; Zeinali, H.; Roghani, M. The Anti-Aging Protein Klotho Alleviates Injury of Nigrostriatal Dopaminergic Pathway in 6-Hydroxydopamine Rat Model of Parkinson’s Disease: Involvement of PKA/CaMKII/CREB Signaling. Exp. Gerontol. 2017, 100, 70–76. [Google Scholar] [CrossRef]
- Sedighi, M.; Baluchnejadmojarad, T.; Fallah, S.; Moradi, N.; Afshin-Majdd, S.; Roghani, M. Klotho Ameliorates Cellular Inflammation via Suppression of Cytokine Release and Upregulation of miR-29a in the PBMCs of Diagnosed Alzheimer’s Disease Patients. J. Mol. Neurosci. 2019, 69, 157–165. [Google Scholar] [CrossRef]
- Zhao, Y.; Zeng, C.-Y.; Li, X.-H.; Yang, T.-T.; Kuang, X.; Du, J.-R. Klotho Overexpression Improves Amyloid-β Clearance and Cognition in the APP/PS1 Mouse Model of Alzheimer’s Disease. Aging Cell 2020, 19, e13239. [Google Scholar] [CrossRef]
- Xiang, T.; Luo, X.; Ye, L.; Huang, H.; Wu, Y. Klotho Alleviates NLRP3 Inflammasome-Mediated Neuroinflammation in a Temporal Lobe Epilepsy Rat Model by Activating the Nrf2 Signaling Pathway. Epilepsy Behav. 2022, 128, 108509. [Google Scholar] [CrossRef]
- Lehmann, M.; Hu, Q.; Hu, Y.; Costa, R.; Ansari, M.; Pineda, R.; Janssen, W.; Theis, F.; Schiller, H.; Königshoff, M. Prolonged WNT/ß-Catenin Signaling Induces Cellular Senescence in Aging and Pulmonary Fibrosis. ERJ Open Res. 2020, 6, 81. [Google Scholar] [CrossRef]
- Zhou, L.; Li, Y.; Zhou, D.; Tan, R.J.; Liu, Y. Loss of Klotho Contributes to Kidney Injury by Derepression of Wnt/β-Catenin Signaling. J. Am. Soc. Nephrol. 2013, 24, 771–785. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Fergusson, M.M.; Castilho, R.M.; Liu, J.; Cao, L.; Chen, J.; Malide, D.; Rovira, I.I.; Schimel, D.; Kuo, C.J.; et al. Augmented Wnt Signaling in a Mammalian Model of Accelerated Aging. Science 2007, 317, 803–806. [Google Scholar] [CrossRef]
- Zhou, H.-J.; Zeng, C.-Y.; Yang, T.-T.; Long, F.-Y.; Kuang, X.; Du, J.-R. Lentivirus-Mediated Klotho up-Regulation Improves Aging-Related Memory Deficits and Oxidative Stress in Senescence-Accelerated Mouse Prone-8 Mice. Life Sci. 2018, 200, 56–62. [Google Scholar] [CrossRef]
- Castner, S.A.; Gupta, S.; Wang, D.; Moreno, A.J.; Park, C.; Chen, C.; Poon, Y.; Groen, A.; Greenberg, K.; David, N.; et al. Longevity Factor Klotho Enhances Cognition in Aged Nonhuman Primates. Nat. Aging 2023, 3, 931–937. [Google Scholar] [CrossRef]
- Zhu, Y.; Prata, L.G.P.L.; Gerdes, E.O.W.; Netto, J.M.E.; Pirtskhalava, T.; Giorgadze, N.; Tripathi, U.; Inman, C.L.; Johnson, K.O.; Xue, A.; et al. Orally-Active, Clinically-Translatable Senolytics Restore α-Klotho in Mice and Humans. EBioMedicine 2022, 77, 103912. [Google Scholar] [CrossRef] [PubMed]
- Nakao, V.W.; Mazucanti, C.H.Y.; de Sá Lima, L.; de Mello, P.S.; de Souza Port’s, N.M.; Kinoshita, P.F.; Leite, J.A.; Kawamoto, E.M.; Scavone, C. Neuroprotective Action of α-Klotho against LPS-Activated Glia Conditioned Medium in Primary Neuronal Culture. Sci. Rep. 2022, 12, 18884. [Google Scholar] [CrossRef]
- Mayne, K.; White, J.A.; McMurran, C.E.; Rivera, F.J.; de la Fuente, A.G. Aging and Neurodegenerative Disease: Is the Adaptive Immune System a Friend or Foe? Front. Aging Neurosci. 2020, 12, 572090. [Google Scholar] [CrossRef] [PubMed]
- Pons, V.; Rivest, S. Targeting Systemic Innate Immune Cells as a Therapeutic Avenue for Alzheimer Disease. Pharmacol. Rev. 2022, 74, 1–17. [Google Scholar] [CrossRef]
- Alzheimer’s Association. 2019 Alzheimer’s Disease Facts and Figures. Alzheimer’s Dement. 2019, 15, 321–387. [Google Scholar] [CrossRef]
- Breijyeh, Z.; Karaman, R. Comprehensive Review on Alzheimer’s Disease: Causes and Treatment. Molecules 2020, 25, 5789. [Google Scholar] [CrossRef] [PubMed]
- Andrade-Guerrero, J.; Santiago-Balmaseda, A.; Jeronimo-Aguilar, P.; Vargas-Rodríguez, I.; Cadena-Suárez, A.R.; Sánchez-Garibay, C.; Pozo-Molina, G.; Méndez-Catalá, C.F.; Cardenas-Aguayo, M.-C.; Diaz-Cintra, S.; et al. Alzheimer’s Disease: An Updated Overview of Its Genetics. Int. J. Mol. Sci. 2023, 24, 3754. [Google Scholar] [CrossRef]
- Hampel, H.; Caraci, F.; Cuello, A.C.; Caruso, G.; Nisticò, R.; Corbo, M.; Baldacci, F.; Toschi, N.; Garaci, F.; Chiesa, P.A.; et al. A Path Toward Precision Medicine for Neuroinflammatory Mechanisms in Alzheimer’s Disease. Front. Immunol. 2020, 11, 456. [Google Scholar] [CrossRef]
- Sosna, J.; Philipp, S.; Albay, R.; Reyes-Ruiz, J.M.; Baglietto-Vargas, D.; LaFerla, F.M.; Glabe, C.G. Early Long-Term Administration of the CSF1R Inhibitor PLX3397 Ablates Microglia and Reduces Accumulation of Intraneuronal Amyloid, Neuritic Plaque Deposition and Pre-Fibrillar Oligomers in 5XFAD Mouse Model of Alzheimer’s Disease. Mol. Neurodegener. 2018, 13, 11. [Google Scholar] [CrossRef]
- McQuade, A.; Blurton-Jones, M. Microglia in Alzheimer’s Disease: Exploring How Genetics and Phenotype Influence Risk. J. Mol. Biol. 2019, 431, 1805–1817. [Google Scholar] [CrossRef]
- Davies, D.S.; Ma, J.; Jegathees, T.; Goldsbury, C. Microglia Show Altered Morphology and Reduced Arborization in Human Brain during Aging and Alzheimer’s Disease. Brain Pathol. 2017, 27, 795–808. [Google Scholar] [CrossRef]
- Cagnin, A.; Brooks, D.J.; Kennedy, A.M.; Gunn, R.N.; Myers, R.; Turkheimer, F.E.; Jones, T.; Banati, R.B. In-Vivo Measurement of Activated Microglia in Dementia. Lancet 2001, 358, 461–467. [Google Scholar] [CrossRef]
- Edison, P.; Archer, H.A.; Gerhard, A.; Hinz, R.; Pavese, N.; Turkheimer, F.E.; Hammers, A.; Tai, Y.F.; Fox, N.; Kennedy, A.; et al. Microglia, Amyloid, and Cognition in Alzheimer’s Disease: An [11C](R)PK11195-PET and [11C]PIB-PET Study. Neurobiol. Dis. 2008, 32, 412–419. [Google Scholar] [CrossRef]
- Schuitemaker, A.; Kropholler, M.A.; Boellaard, R.; van der Flier, W.M.; Kloet, R.W.; van der Doef, T.F.; Knol, D.L.; Windhorst, A.D.; Luurtsema, G.; Barkhof, F.; et al. Microglial Activation in Alzheimer’s Disease: An (R)-[11C]PK11195 Positron Emission Tomography Study. Neurobiol. Aging 2013, 34, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Dani, M.; Wood, M.; Mizoguchi, R.; Fan, Z.; Walker, Z.; Morgan, R.; Hinz, R.; Biju, M.; Kuruvilla, T.; Brooks, D.J.; et al. Microglial Activation Correlates in Vivo with Both Tau and Amyloid in Alzheimer’s Disease. Brain 2018, 141, 2740–2754. [Google Scholar] [CrossRef] [PubMed]
- Zeng, C.-Y.; Yang, T.-T.; Zhou, H.-J.; Zhao, Y.; Kuang, X.; Duan, W.; Du, J.-R. Lentiviral Vector–Mediated Overexpression of Klotho in the Brain Improves Alzheimer’s Disease–like Pathology and Cognitive Deficits in Mice. Neurobiol. Aging 2019, 78, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Kuang, X.; Chen, Y.-S.; Wang, L.-F.; Li, Y.-J.; Liu, K.; Zhang, M.-X.; Li, L.-J.; Chen, C.; He, Q.; Wang, Y.; et al. Klotho Upregulation Contributes to the Neuroprotection of Ligustilide in an Alzheimer’s Disease Mouse Model. Neurobiol. Aging 2014, 35, 169–178. [Google Scholar] [CrossRef]
- Kuang, X.; Zhou, H.-J.; Thorne, A.H.; Chen, X.-N.; Li, L.-J.; Du, J.-R. Neuroprotective Effect of Ligustilide through Induction of α-Secretase Processing of Both APP and Klotho in a Mouse Model of Alzheimer’s Disease. Front. Aging Neurosci. 2017, 9, 353. [Google Scholar] [CrossRef]
- Kundu, P.; Zimmerman, B.; Quinn, J.F.; Kaye, J.; Mattek, N.; Westaway, S.K.; Raber, J. Serum Levels of α-Klotho Are Correlated with Cerebrospinal Fluid Levels and Predict Measures of Cognitive Function. J. Alzheimers Dis. 2022, 86, 1471–1481. [Google Scholar] [CrossRef] [PubMed]
- Bologna, M.; Truong, D.; Jankovic, J. The Etiopathogenetic and Pathophysiological Spectrum of Parkinsonism. J. Neurol. Sci. 2022, 433, 120012. [Google Scholar] [CrossRef]
- Bloem, B.R.; Okun, M.S.; Klein, C. Parkinson’s Disease. Lancet 2021, 397, 2284–2303. [Google Scholar] [CrossRef] [PubMed]
- Simon, D.K.; Tanner, C.M.; Brundin, P. Parkinson Disease Epidemiology, Pathology, Genetics, and Pathophysiology. Clin. Geriatr. Med. 2020, 36, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Dickson, D.W. Neuropathology of Parkinson Disease. Park. Relat. Disord. 2018, 46 (Suppl. S1), S30–S33. [Google Scholar] [CrossRef]
- Banati, R.B.; Daniel, S.E.; Blunt, S.B. Glial Pathology but Absence of Apoptotic Nigral Neurons in Long-Standing Parkinson’s Disease. Mov. Disord. 1998, 13, 221–227. [Google Scholar] [CrossRef]
- Vawter, M.P.; Dillon-Carter, O.; Tourtellotte, W.W.; Carvey, P.; Freed, W.J. TGFbeta1 and TGFbeta2 Concentrations Are Elevated in Parkinson’s Disease in Ventricular Cerebrospinal Fluid. Exp. Neurol. 1996, 142, 313–322. [Google Scholar] [CrossRef]
- Boka, G.; Anglade, P.; Wallach, D.; Javoy-Agid, F.; Agid, Y.; Hirsch, E.C. Immunocytochemical Analysis of Tumor Necrosis Factor and Its Receptors in Parkinson’s Disease. Neurosci. Lett. 1994, 172, 151–154. [Google Scholar] [CrossRef]
- Gerhard, A.; Watts, J.; Trender-Gerhard, I.; Turkheimer, F.; Banati, R.B.; Bhatia, K.; Brooks, D.J. In Vivo Imaging of Microglial Activation with [11C](R)-PK11195 PET in Corticobasal Degeneration. Mov. Disord. 2004, 19, 1221–1226. [Google Scholar] [CrossRef]
- Brochard, V.; Combadière, B.; Prigent, A.; Laouar, Y.; Perrin, A.; Beray-Berthat, V.; Bonduelle, O.; Alvarez-Fischer, D.; Callebert, J.; Launay, J.-M.; et al. Infiltration of CD4+ Lymphocytes into the Brain Contributes to Neurodegeneration in a Mouse Model of Parkinson Disease. J. Clin. Investig. 2009, 119, 182–192. [Google Scholar] [CrossRef]
- Klegeris, A.; Pelech, S.; Giasson, B.I.; Maguire, J.; Zhang, H.; McGeer, E.G.; McGeer, P.L. Alpha-Synuclein Activates Stress Signaling Protein Kinases in THP-1 Cells and Microglia. Neurobiol. Aging 2008, 29, 739–752. [Google Scholar] [CrossRef]
- Li, Y.; Xia, Y.; Yin, S.; Wan, F.; Hu, J.; Kou, L.; Sun, Y.; Wu, J.; Zhou, Q.; Huang, J.; et al. Targeting Microglial α-Synuclein/TLRs/NF-kappaB/NLRP3 Inflammasome Axis in Parkinson’s Disease. Front. Immunol. 2021, 12, 719807. [Google Scholar] [CrossRef]
- Kosakai, A.; Ito, D.; Nihei, Y.; Yamashita, S.; Okada, Y.; Takahashi, K.; Suzuki, N. Degeneration of Mesencephalic Dopaminergic Neurons in Klotho Mouse Related to Vitamin D Exposure. Brain Res. 2011, 1382, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, M.; Fandrich, M.; Jakobi, M.; Röben, B.; Wurster, I.; Lerche, S.; Schulte, C.; Zimmermann, S.; Deuschle, C.; Schneiderhan-Marra, N.; et al. Association of Elevated Cerebrospinal Fluid Levels of the Longevity Protein α-Klotho with a Delayed Onset of Cognitive Impairment in Parkinson’s Disease Patients. Eur. J. Neurol. 2024, 31, e16388. [Google Scholar] [CrossRef] [PubMed]
- Sancesario, G.M.; Di Lazzaro, G.; Grillo, P.; Biticchi, B.; Giannella, E.; Alwardat, M.; Pieri, M.; Bernardini, S.; Mercuri, N.B.; Pisani, A.; et al. Biofluids Profile of α-Klotho in Patients with Parkinson’s Disease. Park. Relat. Disord. 2021, 90, 62–64. [Google Scholar] [CrossRef]
- World Stroke Organization Impact of Stroke. Available online: https://www.world-stroke.org/world-stroke-day-campaign/about-stroke/impact-of-stroke (accessed on 29 January 2025).
- Murphy, S.J.X.; Werring, D.J. Stroke: Causes and Clinical Features. Medicine 2020, 48, 561–566. [Google Scholar] [CrossRef]
- Möhlenbruch, M.A.; Bendszus, M. Technical standards for the interventional treatment of acute ischemic stroke. Nervenarzt 2015, 86, 1209–1216. [Google Scholar] [CrossRef]
- Asadi, H.; Dowling, R.; Yan, B.; Wong, S.; Mitchell, P. Advances in Endovascular Treatment of Acute Ischaemic Stroke. Intern. Med. J. 2015, 45, 798–805. [Google Scholar] [CrossRef] [PubMed]
- Alsbrook, D.L.; Di Napoli, M.; Bhatia, K.; Biller, J.; Andalib, S.; Hinduja, A.; Rodrigues, R.; Rodriguez, M.; Sabbagh, S.Y.; Selim, M.; et al. Neuroinflammation in Acute Ischemic and Hemorrhagic Stroke. Curr. Neurol. Neurosci. Rep. 2023, 23, 407–431. [Google Scholar] [CrossRef]
- Sanchez-Bezanilla, S.; Hood, R.J.; Collins-Praino, L.E.; Turner, R.J.; Walker, F.R.; Nilsson, M.; Ong, L.K. More than Motor Impairment: A Spatiotemporal Analysis of Cognitive Impairment and Associated Neuropathological Changes Following Cortical Photothrombotic Stroke. J. Cereb. Blood Flow. Metab. 2021, 41, 2439–2455. [Google Scholar] [CrossRef]
- Walberer, M.; Jantzen, S.U.; Backes, H.; Rueger, M.A.; Keuters, M.H.; Neumaier, B.; Hoehn, M.; Fink, G.R.; Graf, R.; Schroeter, M. In-Vivo Detection of Inflammation and Neurodegeneration in the Chronic Phase after Permanent Embolic Stroke in Rats. Brain Res. 2014, 1581, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Grande, B.; Blackabey, V.; Gittens, B.; Pinteaux, E.; Denes, A. Loss of Substance P and Inflammation Precede Delayed Neurodegeneration in the Substantia Nigra after Cerebral Ischemia. Brain Behav. Immun. 2013, 29, 51–61. [Google Scholar] [CrossRef]
- Pappata, S.; Levasseur, M.; Gunn, R.N.; Myers, R.; Crouzel, C.; Syrota, A.; Jones, T.; Kreutzberg, G.W.; Banati, R.B. Thalamic Microglial Activation in Ischemic Stroke Detected In Vivo by PET and [11C]PK1195. Neurology 2000, 55, 1052–1054. [Google Scholar] [CrossRef]
- Kulesh, A.; Drobakha, V.; Kuklina, E.; Nekrasova, I.; Shestakov, V. Cytokine Response, Tract-Specific Fractional Anisotropy, and Brain Morphometry in Post-Stroke Cognitive Impairment. J. Stroke Cerebrovasc. Dis. 2018, 27, 1752–1759. [Google Scholar] [CrossRef]
- Pendlebury, S.T.; Rothwell, P.M. Oxford Vascular Study Incidence and Prevalence of Dementia Associated with Transient Ischaemic Attack and Stroke: Analysis of the Population-Based Oxford Vascular Study. Lancet Neurol. 2019, 18, 248–258. [Google Scholar] [CrossRef]
- Sexton, E.; McLoughlin, A.; Williams, D.J.; Merriman, N.A.; Donnelly, N.; Rohde, D.; Hickey, A.; Wren, M.-A.; Bennett, K. Systematic Review and Meta-Analysis of the Prevalence of Cognitive Impairment No Dementia in the First Year Post-Stroke. Eur. Stroke J. 2019, 4, 160–171. [Google Scholar] [CrossRef]
- Rost, N.S.; Brodtmann, A.; Pase, M.P.; van Veluw, S.J.; Biffi, A.; Duering, M.; Hinman, J.D.; Dichgans, M. Post-Stroke Cognitive Impairment and Dementia. Circ. Res. 2022, 130, 1252–1271. [Google Scholar] [CrossRef]
- El Husseini, N.; Katzan, I.L.; Rost, N.S.; Blake, M.L.; Byun, E.; Pendlebury, S.T.; Aparicio, H.J.; Marquine, M.J.; Gottesman, R.F.; Smith, E.E.; et al. Cognitive Impairment After Ischemic and Hemorrhagic Stroke: A Scientific Statement from the American Heart Association/American Stroke Association. Stroke 2023, 54, e272–e291. [Google Scholar] [CrossRef] [PubMed]
- Gallucci, L.; Sperber, C.; Guggisberg, A.G.; Kaller, C.P.; Heldner, M.R.; Monsch, A.U.; Hakim, A.; Silimon, N.; Fischer, U.; Arnold, M.; et al. Post-Stroke Cognitive Impairment Remains Highly Prevalent and Disabling despite State-of-the-Art Stroke Treatment. Int. J. Stroke 2024, 19, 888–897. [Google Scholar] [CrossRef] [PubMed]
- Donate-Correa, J.; Martín-Núñez, E.; Mora-Fernández, C.; Muros-de-Fuentes, M.; Pérez-Delgado, N.; Navarro-González, J.F. Klotho in Cardiovascular Disease: Current and Future Perspectives. World J. Biol. Chem. 2015, 6, 351–357. [Google Scholar] [CrossRef]
- Mencke, R.; Hillebrands, J.-L. NIGRAM consortium The Role of the Anti-Ageing Protein Klotho in Vascular Physiology and Pathophysiology. Ageing Res. Rev. 2017, 35, 124–146. [Google Scholar] [CrossRef] [PubMed]
- Woo, H.G.; Chang, Y.; Ryu, D.-R.; Song, T.-J. Plasma Klotho Concentration Is Associated with the Presence, Burden and Progression of Cerebral Small Vessel Disease in Patients with Acute Ischaemic Stroke. PLoS ONE 2019, 14, e0220796. [Google Scholar] [CrossRef] [PubMed]
- Brombo, G.; Bonetti, F.; Ortolani, B.; Morieri, M.L.; Bosi, C.; Passaro, A.; Vigna, G.B.; Borgna, C.; Arcidicono, M.V.; Tisato, V.; et al. Lower Plasma Klotho Concentrations Are Associated with Vascular Dementia but Not Late-Onset Alzheimer’s Disease. Gerontology 2018, 64, 414–421. [Google Scholar] [CrossRef]
- Lee, J.-B.; Woo, H.G.; Chang, Y.; Jin, Y.M.; Jo, I.; Kim, J.; Song, T.-J. Plasma Klotho Concentrations Predict Functional Outcome at Three Months after Acute Ischemic Stroke Patients. Ann. Med. 2019, 51, 262–269. [Google Scholar] [CrossRef]
- Majumdar, V.; Nagaraja, D.; Christopher, R. Association of the Functional KL-VS Variant of Klotho Gene with Early-Onset Ischemic Stroke. Biochem. Biophys. Res. Commun. 2010, 403, 412–416. [Google Scholar] [CrossRef]
- Zhu, G.; Xiang, T.; Liang, S.; Liu, K.; Xiao, Z.; Ye, Q. Klotho Gene Might Antagonize Ischemic Injury in Stroke Rats by Reducing the Expression of AQP4 via P38MAPK Pathway. J. Stroke Cerebrovasc. Dis. 2023, 32, 107205. [Google Scholar] [CrossRef]
- Zhou, H.-J.; Li, H.; Shi, M.-Q.; Mao, X.-N.; Liu, D.-L.; Chang, Y.-R.; Gan, Y.-M.; Kuang, X.; Du, J.-R. Protective Effect of Klotho against Ischemic Brain Injury Is Associated with Inhibition of RIG-I/NF-κB Signaling. Front. Pharmacol. 2017, 8, 950. [Google Scholar] [CrossRef]
- Liu, X.-Y.; Zhang, L.-Y.; Wang, X.-Y.; Li, S.-C.; Hu, Y.-Y.; Zhang, J.-G.; Xian, X.-H.; Li, W.-B.; Zhang, M. STAT4-Mediated Klotho Up-Regulation Contributes to the Brain Ischemic Tolerance by Cerebral Ischemic Preconditioning via Inhibiting Neuronal Pyroptosis. Mol. Neurobiol. 2024, 61, 2336–2356. [Google Scholar] [CrossRef]
- Saito, Y.; Yamagishi, T.; Nakamura, T.; Ohyama, Y.; Aizawa, H.; Suga, T.; Matsumura, Y.; Masuda, H.; Kurabayashi, M.; Kuro-o, M.; et al. Klotho Protein Protects against Endothelial Dysfunction. Biochem. Biophys. Res. Commun. 1998, 248, 324–329. [Google Scholar] [CrossRef]
- Rakugi, H.; Matsukawa, N.; Ishikawa, K.; Yang, J.; Imai, M.; Ikushima, M.; Maekawa, Y.; Kida, I.; Miyazaki, J.; Ogihara, T. Anti-Oxidative Effect of Klotho on Endothelial Cells through cAMP Activation. Endocrine 2007, 31, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Maekawa, Y.; Ishikawa, K.; Yasuda, O.; Oguro, R.; Hanasaki, H.; Kida, I.; Takemura, Y.; Ohishi, M.; Katsuya, T.; Rakugi, H. Klotho Suppresses TNF-Alpha-Induced Expression of Adhesion Molecules in the Endothelium and Attenuates NF-kappaB Activation. Endocrine 2009, 35, 341–346. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, K.; Bao, Y.; Zhang, T.; Ainiwaer, D.; Xiong, X.; Wang, G.; Sun, Z. The Serum Soluble Klotho Alleviates Cardiac Aging and Regulates M2a/M2c Macrophage Polarization via Inhibiting TLR4/Myd88/NF-κB Pathway. Tissue Cell 2022, 76, 101812. [Google Scholar] [CrossRef]
- He, J.; Cui, J.; Shi, Y.; Wang, T.; Xin, J.; Li, Y.; Shan, X.; Zhu, Z.; Gao, Y. Astragaloside IV Attenuates High-Glucose-Induced Impairment in Diabetic Nephropathy by Increasing Klotho Expression via the NF-κB/NLRP3 Axis. J. Diabetes Res. 2023, 2023, 7423661. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-κB Signaling in Inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- Romero, A.; Dongil, P.; Valencia, I.; Vallejo, S.; Hipólito-Luengo, Á.S.; Díaz-Araya, G.; Bartha, J.L.; González-Arlanzón, M.M.; Rivilla, F.; de la Cuesta, F.; et al. Pharmacological Blockade of NLRP3 Inflammasome/IL-1β-Positive Loop Mitigates Endothelial Cell Senescence and Dysfunction. Aging Dis. 2022, 13, 284–297. [Google Scholar] [CrossRef] [PubMed]
- Swanson, K.V.; Deng, M.; Ting, J.P.-Y. The NLRP3 Inflammasome: Molecular Activation and Regulation to Therapeutics. Nat. Rev. Immunol. 2019, 19, 477–489. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, H.; Zheng, G.; Wang, Z.; Shi, L. Gender-Specific Association between Circulating Serum Klotho and Metabolic Components in Adults. BMC Endocr. Disord. 2024, 24, 198. [Google Scholar] [CrossRef] [PubMed]
- Schweizer, J.R.d.O.L.; Schilbach, K.; Haenelt, M.; Gagliardo, A.; Peters, A.; Thorand, B.; Störmann, S.; Schopohl, J.; Reincke, M.; Lauseker, M.; et al. Soluble Alpha Klotho—Impact of Biological Variables and Reference Intervals for Adults. Eur. J. Endocrinol. 2025, 192, 631–640. [Google Scholar] [CrossRef]
- Yu, L.-X.; Li, S.-S.; Sha, M.-Y.; Kong, J.-W.; Ye, J.-M.; Liu, Q.-F. The Controversy of Klotho as a Potential Biomarker in Chronic Kidney Disease. Front. Pharmacol. 2022, 13, 931746. [Google Scholar] [CrossRef]
- Gaitán, J.M.; Moon, H.Y.; Stremlau, M.; Dubal, D.B.; Cook, D.B.; Okonkwo, O.C.; van Praag, H. Effects of Aerobic Exercise Training on Systemic Biomarkers and Cognition in Late Middle-Aged Adults at Risk for Alzheimer’s Disease. Front. Endocrinol 2021, 12, 660181. [Google Scholar] [CrossRef]
- Corrêa, H.d.L.; Raab, A.T.O.; Araújo, T.M.; Deus, L.A.; Reis, A.L.; Honorato, F.S.; Rodrigues-Silva, P.L.; Neves, R.V.P.; Brunetta, H.S.; Mori, M.A.d.S.; et al. A Systematic Review and Meta-Analysis Demonstrating Klotho as an Emerging Exerkine. Sci. Rep. 2022, 12, 17587. [Google Scholar] [CrossRef] [PubMed]
- Amaro-Gahete, F.J.; De-la-O, A.; Jurado-Fasoli, L.; Espuch-Oliver, A.; de Haro, T.; Gutierrez, A.; Ruiz, J.R.; Castillo, M.J. Exercise Training Increases the S-Klotho Plasma Levels in Sedentary Middle-Aged Adults: A Randomised Controlled Trial. The FIT-AGEING Study. J. Sports Sci. 2019, 37, 2175–2183. [Google Scholar] [CrossRef]
- Tan, S.-J.; Chu, M.M.; Toussaint, N.D.; Cai, M.M.; Hewitson, T.D.; Holt, S.G. High-Intensity Physical Exercise Increases Serum α-Klotho Levels in Healthy Volunteers. J. Circ. Biomark. 2018, 7, 1849454418794582. [Google Scholar] [CrossRef] [PubMed]
- Santos-Dias, A.; MacKenzie, B.; Oliveira-Junior, M.C.; Moyses, R.M.; Consolim-Colombo, F.M.; Vieira, R.P. Longevity Protein Klotho Is Induced by a Single Bout of Exercise. Br. J. Sports Med. 2017, 51, 549–550. [Google Scholar] [CrossRef]
- Avin, K.G.; Coen, P.M.; Huang, W.; Stolz, D.B.; Sowa, G.A.; Dubé, J.J.; Goodpaster, B.H.; O’Doherty, R.M.; Ambrosio, F. Skeletal Muscle as a Regulator of the Longevity Protein, Klotho. Front. Physiol. 2014, 5, 189. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, T.; Miyaki, A.; Akazawa, N.; Choi, Y.; Ra, S.-G.; Tanahashi, K.; Kumagai, H.; Oikawa, S.; Maeda, S. Aerobic Exercise Training Increases Plasma Klotho Levels and Reduces Arterial Stiffness in Postmenopausal Women. Am. J. Physiol. Heart Circ. Physiol. 2014, 306, H348–H355. [Google Scholar] [CrossRef]
- Becke, A.; Maass, A.; Kreutz, M.R.; Duezel, E. Serum α-Klotho Levels Correlate with Episodic Memory Changes Related to Cardiovascular Exercise in Older Adults. bioRxiv 2020. [Google Scholar] [CrossRef]
- Yoon, H.E.; Lim, S.W.; Piao, S.G.; Song, J.-H.; Kim, J.; Yang, C.W. Statin Upregulates the Expression of Klotho, an Anti-Aging Gene, in Experimental Cyclosporine Nephropathy. Nephron Exp. Nephrol. 2012, 120, e123–e133. [Google Scholar] [CrossRef]
- Kuwahara, N.; Sasaki, S.; Kobara, M.; Nakata, T.; Tatsumi, T.; Irie, H.; Narumiya, H.; Hatta, T.; Takeda, K.; Matsubara, H.; et al. HMG-CoA Reductase Inhibition Improves Anti-Aging Klotho Protein Expression and Arteriosclerosis in Rats with Chronic Inhibition of Nitric Oxide Synthesis. Int. J. Cardiol. 2008, 123, 84–90. [Google Scholar] [CrossRef]
- Narumiya, H.; Sasaki, S.; Kuwahara, N.; Irie, H.; Kusaba, T.; Kameyama, H.; Tamagaki, K.; Hatta, T.; Takeda, K.; Matsubara, H. HMG-CoA Reductase Inhibitors up-Regulate Anti-Aging Klotho mRNA via RhoA Inactivation in IMCD3 Cells. Cardiovasc. Res. 2004, 64, 331–336. [Google Scholar] [CrossRef]
- Janić, M.; Lunder, M.; Novaković, S.; Škerl, P.; Šabovič, M. Expression of Longevity Genes Induced by a Low-Dose Fluvastatin and Valsartan Combination with the Potential to Prevent/Treat “Aging-Related Disorders”. Int. J. Mol. Sci. 2019, 20, 1844. [Google Scholar] [CrossRef]
- Lim, S.C.; Liu, J.-J.; Subramaniam, T.; Sum, C.F. Elevated Circulating Alpha-Klotho by Angiotensin II Receptor Blocker Losartan Is Associated with Reduction of Albuminuria in Type 2 Diabetic Patients. J. Renin Angiotensin Aldosterone Syst. 2014, 15, 487–490. [Google Scholar] [CrossRef] [PubMed]
- Karalliedde, J.; Maltese, G.; Hill, B.; Viberti, G.; Gnudi, L. Effect of Renin-Angiotensin System Blockade on Soluble Klotho in Patients with Type 2 Diabetes, Systolic Hypertension, and Albuminuria. Clin. J. Am. Soc. Nephrol. 2013, 8, 1899–1905. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Zhang, L.; Yang, J.; Hao, L. Activation of Peroxisome Proliferator-Activated Receptor γ Inhibits Vascular Calcification by Upregulating Klotho. Exp. Ther. Med. 2017, 13, 467–474. [Google Scholar] [CrossRef]
- Chen, L.-J.; Cheng, M.-F.; Ku, P.-M.; Lin, J.-W. Rosiglitazone Increases Cerebral Klotho Expression to Reverse Baroreflex in Type 1-like Diabetic Rats. Biomed. Res. Int. 2014, 2014, 309151. [Google Scholar] [CrossRef]
- Haussler, M.R.; Livingston, S.; Sabir, Z.L.; Haussler, C.A.; Jurutka, P.W. Vitamin D Receptor Mediates a Myriad of Biological Actions Dependent on Its 1,25-Dihydroxyvitamin D Ligand: Distinct Regulatory Themes Revealed by Induction of Klotho and Fibroblast Growth Factor-23. JBMR Plus 2021, 5, e10432. [Google Scholar] [CrossRef]
- Prud’homme, G.J.; Glinka, Y.; Kurt, M.; Liu, W.; Wang, Q. The Anti-Aging Protein Klotho Is Induced by GABA Therapy and Exerts Protective and Stimulatory Effects on Pancreatic Beta Cells. Biochem. Biophys. Res. Commun. 2017, 493, 1542–1547. [Google Scholar] [CrossRef]
- Razzaque, M.S. FGF23, Klotho and Vitamin D Interactions: What Have We Learned from in Vivo Mouse Genetics Studies? Adv. Exp. Med. Biol. 2012, 728, 84–91. [Google Scholar] [CrossRef]
- Wang, Q.; Ren, L.; Wan, Y.; Prud’homme, G.J. GABAergic Regulation of Pancreatic Islet Cells: Physiology and Antidiabetic Effects. J. Cell Physiol. 2019, 234, 14432–14444. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Valdés, H.E.; Hernández-Lucas, M.; Rodríguez-Fabián, G.D.J.; Esteban-Román, N.F.; Gutiérrez-Juárez, R.; Arrieta-Cruz, I.; Martínez-Coria, H. The Anti-Inflammatory Actions of Soluble Klotho in Brain Aging and Its Main Associated Diseases. Int. J. Mol. Sci. 2025, 26, 8551. https://doi.org/10.3390/ijms26178551
López-Valdés HE, Hernández-Lucas M, Rodríguez-Fabián GDJ, Esteban-Román NF, Gutiérrez-Juárez R, Arrieta-Cruz I, Martínez-Coria H. The Anti-Inflammatory Actions of Soluble Klotho in Brain Aging and Its Main Associated Diseases. International Journal of Molecular Sciences. 2025; 26(17):8551. https://doi.org/10.3390/ijms26178551
Chicago/Turabian StyleLópez-Valdés, Héctor E., Martín Hernández-Lucas, Gustavo D. J. Rodríguez-Fabián, Nadia F. Esteban-Román, Roger Gutiérrez-Juárez, Isabel Arrieta-Cruz, and Hilda Martínez-Coria. 2025. "The Anti-Inflammatory Actions of Soluble Klotho in Brain Aging and Its Main Associated Diseases" International Journal of Molecular Sciences 26, no. 17: 8551. https://doi.org/10.3390/ijms26178551
APA StyleLópez-Valdés, H. E., Hernández-Lucas, M., Rodríguez-Fabián, G. D. J., Esteban-Román, N. F., Gutiérrez-Juárez, R., Arrieta-Cruz, I., & Martínez-Coria, H. (2025). The Anti-Inflammatory Actions of Soluble Klotho in Brain Aging and Its Main Associated Diseases. International Journal of Molecular Sciences, 26(17), 8551. https://doi.org/10.3390/ijms26178551





