Medical Ozone Treatment Attenuates Male Reproductive Toxicity Induced by Bleomycin, Etoposide, and Cisplatin Regimen in an Experimental Animal Model
Abstract
1. Introduction
2. Results
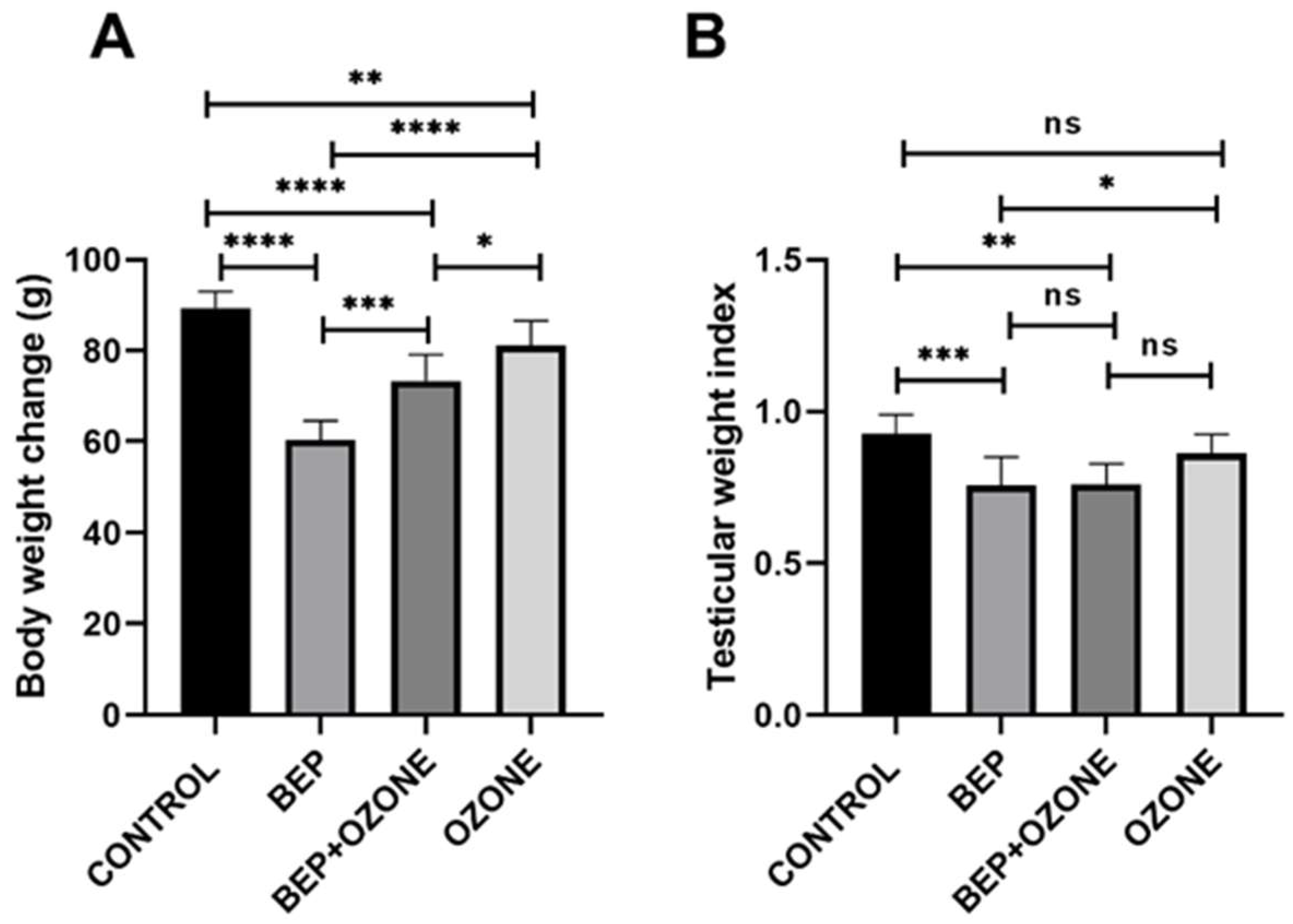
2.1. Effect of Ozone on Body Weight and Testis Weight Indices
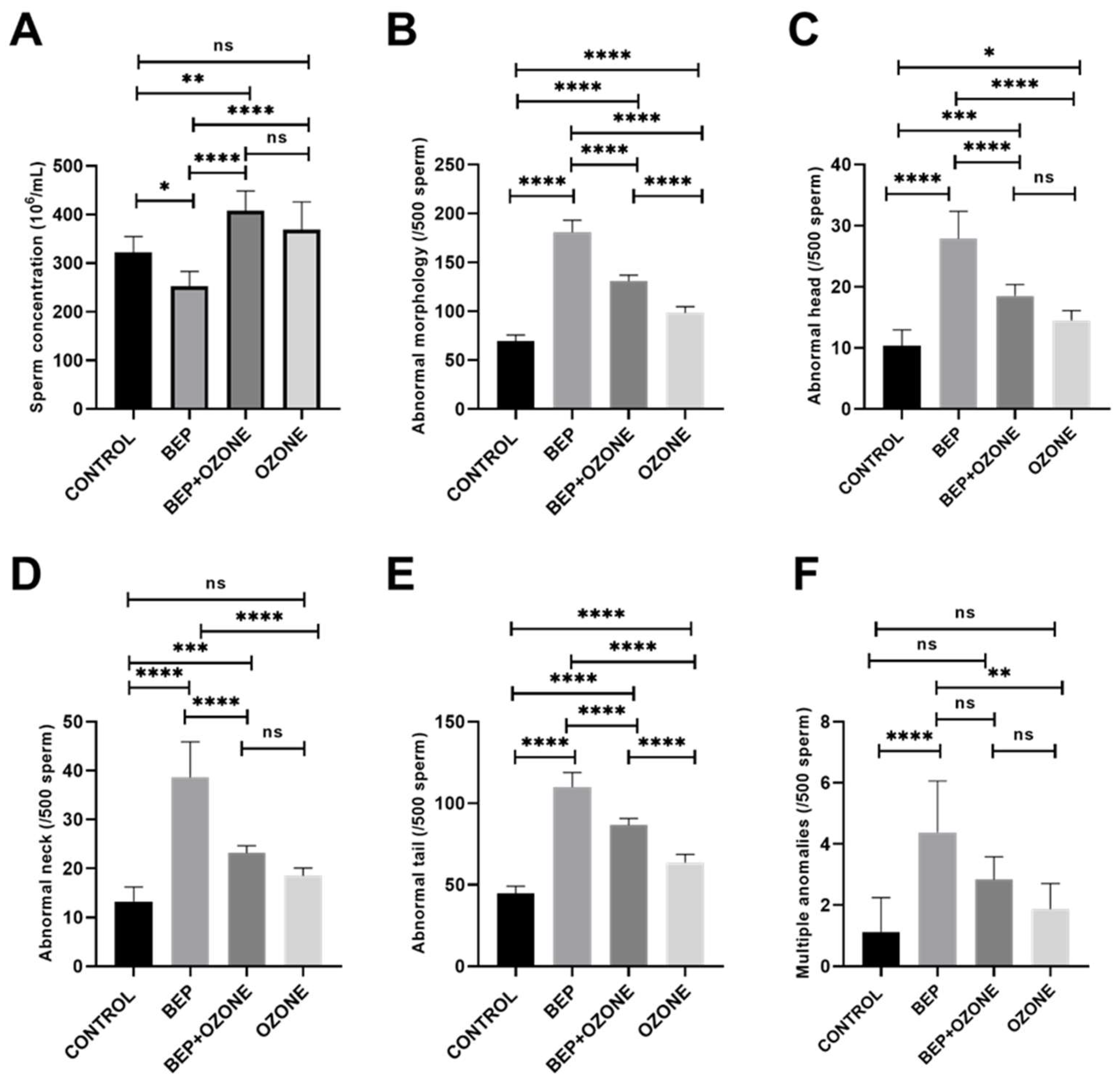
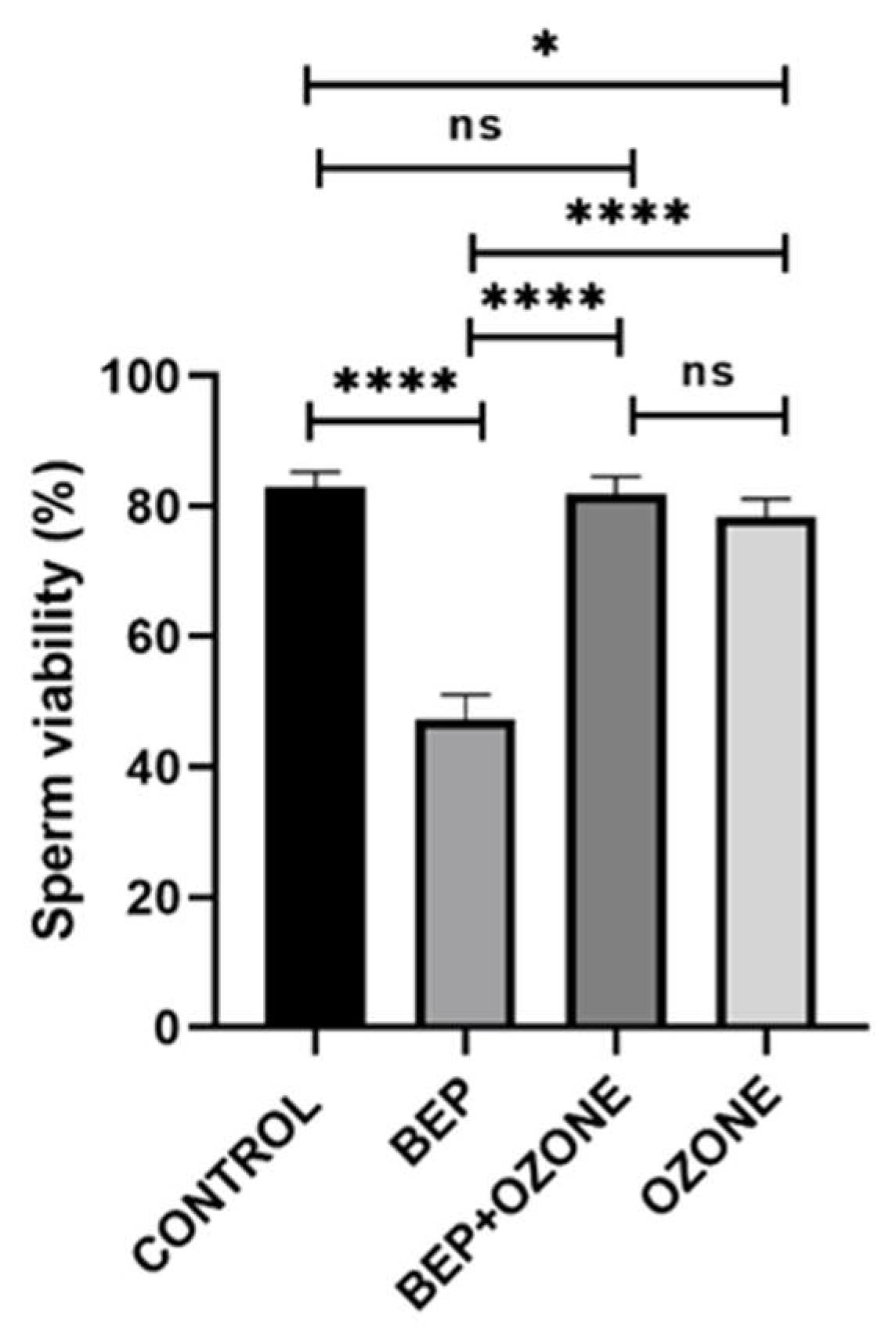
2.2. Effect of Ozone on Sperm Parameters
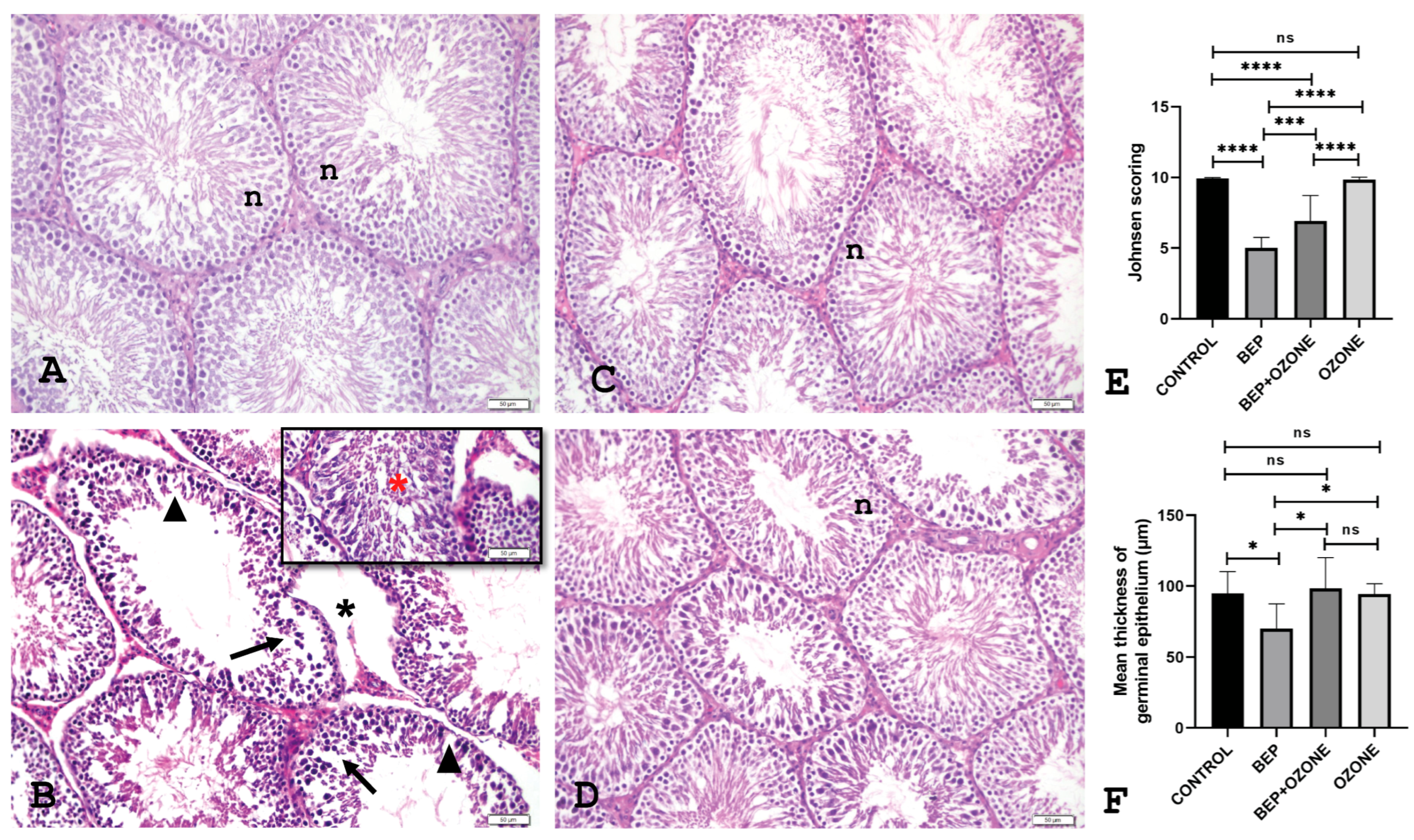
2.3. Effect of Ozone on Testicular Histopathology
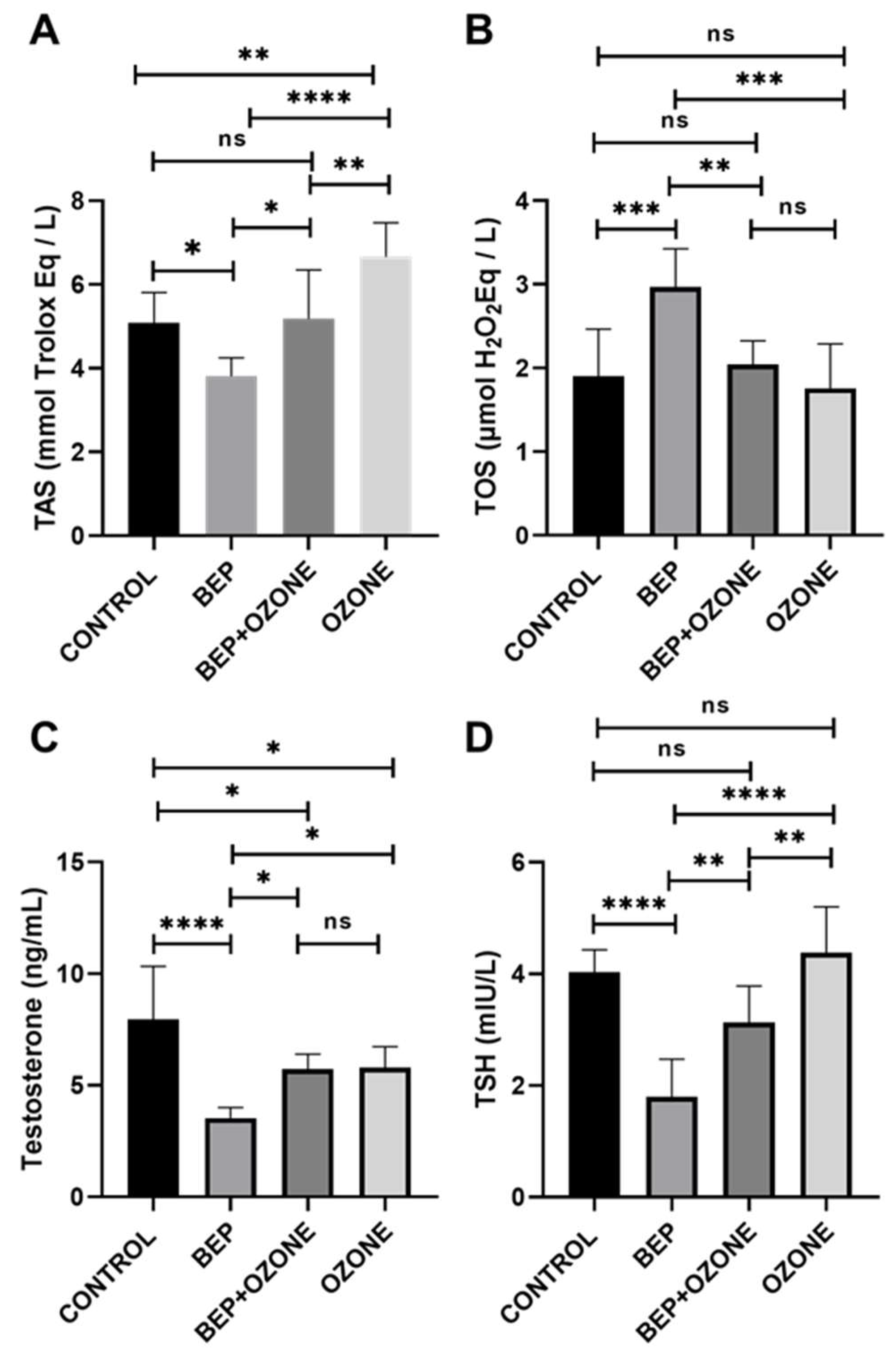
2.4. Effect of Ozone on Redox Status and Endocrine Parameters
3. Discussion
4. Materials and Methods
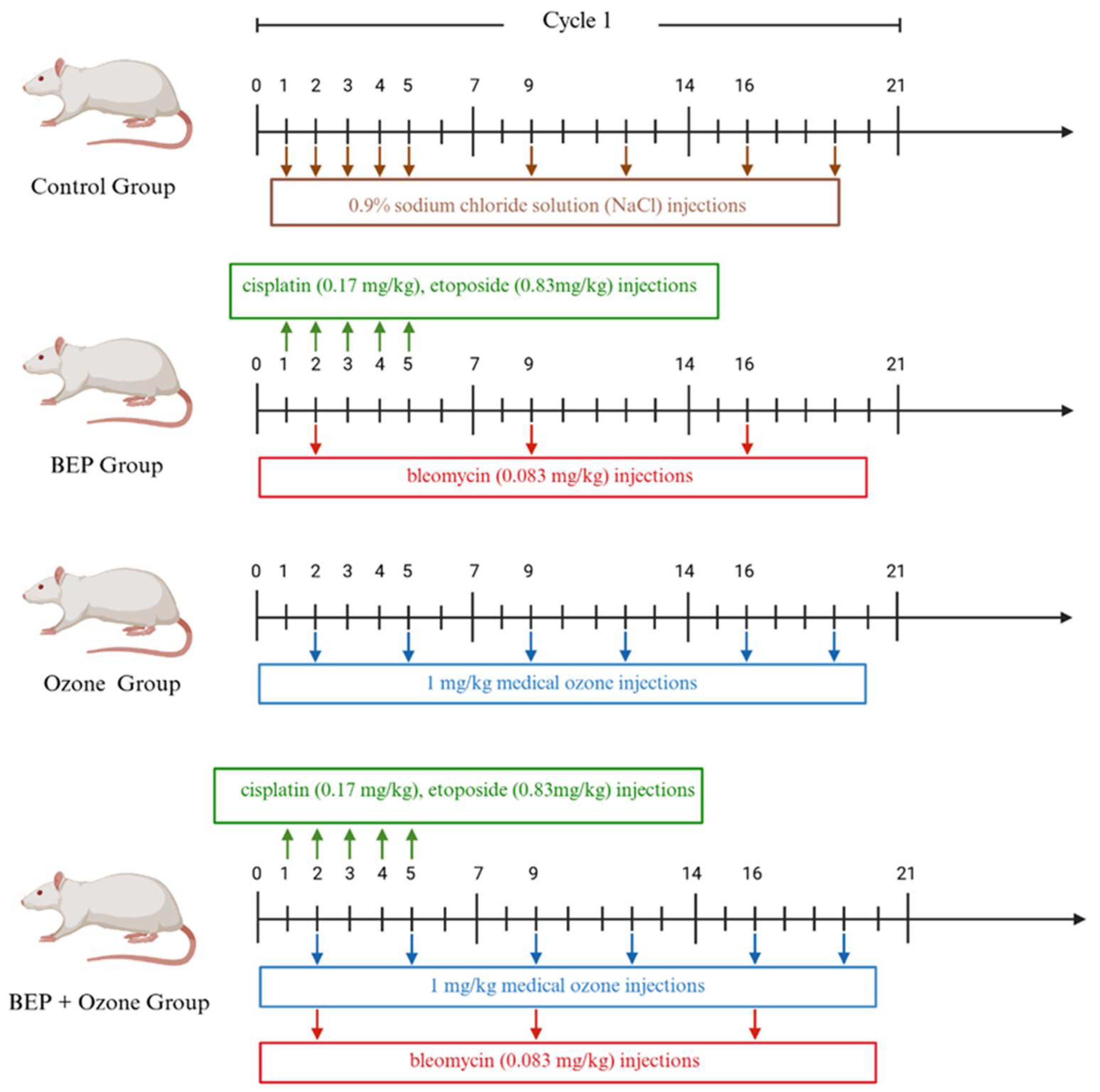
4.1. Animals and Experimental Design
4.2. Experimental Protocols for the BEP Regimen and Ozone Therapy
4.3. Collection and Preparation of Tissue and Samples
4.4. Sperm Collection and Examination
4.5. Testicular Histopathology
4.6. Endocrine and Redox Status Measurement
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ujah, G.A.; Nna, V.U.; Suleiman, J.B.; Eleazu, C.; Nwokocha, C.; Rebene, J.A.; Imowo, M.U.; Obi, E.O.; Amachree, C.; Udechukwu, E.C.; et al. Tert-butylhydroquinone attenuates doxorubicin-induced dysregulation of testicular cytoprotective and steroidogenic genes, and improves spermatogenesis in rats. Sci. Rep. 2021, 11, 5522. [Google Scholar] [CrossRef]
- Moradi, M.; Hashemian, M.A.; Faramarzi, A.; Goodarzi, N.; Hashemian, A.H.; Cheraghi, H.; Jalili, C. Therapeutic effect of sodium alginate on bleomycin, etoposide and cisplatin (BEP)-induced reproductive toxicity by inhibiting nitro-oxidative stress, inflammation and apoptosis. Sci. Rep. 2024, 14, 1565. [Google Scholar] [CrossRef] [PubMed]
- Aydoğdu, İ.; Ekin, R.G.; Yıldız, P.; Mirapoğlu, S.L.; Çay, A.; Aydoğdu, Y.E.; Kılınçaslan, H.; Semerci, M.B.; İlbey, Y.Ö. Does Ozone Administration Have a Protective Effect Against Cisplatin induced Histological Changes in Rat Testis? J. Urol. Surg. 2019, 6, 32–37. [Google Scholar] [CrossRef]
- Einhorn, L.H.; Foster, R.S. Bleomycin, Etoposide, and Cisplatin for Three Cycles Compared With Etoposide and Cisplatin for Four Cycles in Good-Risk Germ Cell Tumors: Is There a Preferred Regimen? J. Clin. Oncol. 2006, 24, 2597–2598. [Google Scholar] [CrossRef]
- Kopp, H.G.; Kuczyk, M.; Classen, J.; Stenzl, A.; Kanz, L.; Mayer, F.; Bamberg, M.; Hartmann, J.T. Advances in the treatment of testicular cancer. Drugs 2006, 66, 641–659. [Google Scholar] [CrossRef] [PubMed]
- Zeien, J.; Qiu, W.; Triay, M.; Dhaibar, H.A.; Cruz-Topete, D.; Cornett, E.M.; Urits, I.; Viswanath, O.; Kaye, A.D. Clinical implications of chemotherapeutic agent organ toxicity on perioperative care. Biomed. Pharmacother. 2022, 146, 112503. [Google Scholar] [CrossRef]
- He, Y.; Zheng, J.; Ye, B.; Dai, Y.; Nie, K. Chemotherapy-induced gastrointestinal toxicity: Pathogenesis and current management. Biochem. Pharmaco. 2023, 216, 115787. [Google Scholar] [CrossRef]
- Słonimska, P.; Sachadyn, P.; Zieliński, J.; Skrzypski, M.; Pikuła, M. Chemotherapy-Mediated Complications of Wound Healing: An Understudied Side Effect. Adv. Wound Care 2024, 13, 187–199. [Google Scholar] [CrossRef]
- Felemban, S.G.; Aldubayan, M.A.; Alhowail, A.H.; Almami, I.S. Vitamin B17 ameliorates methotrexate-induced reproductive toxicity, oxidative stress, and testicular injury in male rats. Oxidative Med. Cell. Longev. 2020, 2020, 4372719. [Google Scholar] [CrossRef]
- Narayana, K.; Al-Bader, M.; Mousa, A.; Khan, K.M. Molecular effects of chemotherapeutic drugs and their modulation by antioxidants in the testis. Eur. J. Pharmacol. 2012, 674, 207–216. [Google Scholar] [CrossRef]
- Nna, V.U.; Ujah, G.A.; Suleiman, J.B.; Mohamed, M.; Nwokocha, C.; Akpan, T.J.; Ekuma, H.C.; Fubara, V.V.; Kekung-Asu, C.B.; Osim, E.E. Tert-butylhydroquinone preserve testicular steroidogenesis and spermatogenesis in cisplatin-intoxicated rats by targeting oxidative stress, inflammation and apoptosis. Toxicology 2020, 441, 152528. [Google Scholar] [CrossRef]
- Al-Bader, M.; Kilarkaje, N. Effects of bleomycin, etoposide and cisplatin treatment on Leydig cell structure and transcription of steroidogenic enzymes in rat testis. Eur. J. Pharmacol. 2015, 747, 150–159. [Google Scholar] [CrossRef]
- Bayram, H.; Donmez Cakil, Y.; Sitar, M.E.; Demirel, G.; Selam, B.; Cincik, M. The Effects of Glutathione on Clinically Essential Fertility Parameters in a Bleomycin Etoposide Cisplatin Chemotherapy Model. Life 2023, 13, 815. [Google Scholar] [CrossRef]
- Kilarkaje, N.; Mousa, A.M.; Al-Bader, M.M.; Khan, K.M. Antioxidants enhance the recovery of three cycles of bleomycin, etoposide, and cisplatin-induced testicular dysfunction, pituitary-testicular axis, and fertility in rats. Fertil. Steril. 2013, 100, 1151–1159. [Google Scholar] [CrossRef] [PubMed]
- Moradi, M.; Goodarzi, N.; Faramarzi, A.; Cheraghi, H.; Hashemian, A.H.; Jalili, C. Melatonin protects rats testes against bleomycin, etoposide, and cisplatin-induced toxicity via mitigating nitro-oxidative stress and apoptosis. Biomed. Pharmacother. 2021, 138, 111481. [Google Scholar] [CrossRef] [PubMed]
- Marcon, L.; Zhang, X.; Hales, B.F.; Robaire, B.; Nagano, M.C. Effects of chemotherapeutic agents for testicular cancer on rat spermatogonial stem/progenitor cells. J. Androl. 2011, 32, 432–443. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.R.; Sheehan, P.K.; Bazin, A.; Leonard, C.; Aleem, U.; Corrigan, L.; McDermott, R. Late side effects of testicular cancer and treatment: A comprehensive review. Discov. Oncol. 2024, 15, 646. [Google Scholar] [CrossRef]
- Khadivi, F.; Razavi, S.; Hashemi, F. Protective effects of zinc on rat sperm chromatin integrity involvement: DNA methylation, DNA fragmentation, ubiquitination and protamination after bleomycin etoposide and cis-platin treatment. Theriogenology 2020, 142, 177–183. [Google Scholar] [CrossRef]
- Salem, E.A.; Salem, N.A.; Hellstrom, W.J. Therapeutic effect of ozone and rutin on adriamycin-induced testicular toxicity in an experimental rat model. Andrologia 2017, 49, e12603. [Google Scholar] [CrossRef]
- Sagai, M.; Bocci, V. Mechanisms of Action Involved in Ozone Therapy: Is Healing Induced via a Mild Oxidative Stress? Med. Gas Res. 2011, 1, 29. [Google Scholar] [CrossRef]
- Franzini, M.; Valdenassi, L.; Pandolfi, S.; Tirelli, U.; Ricevuti, G.; Simonetti, V.; Berretta, M.; Vaiano, F.; Chirumbolo, S. The biological activity of medical ozone in the hormetic range and the role of full expertise professionals. Front. Public Health 2022, 10, 979076. [Google Scholar] [CrossRef]
- Galiè, M.; Covi, V.; Tabaracci, G.; Malatesta, M. The Role of Nrf2 in the Antioxidant Cellular Response to Medical Ozone Exposure. Int. J. Mol. Sci. 2019, 20, 4009. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bocci, V.A. Scientific and medical aspects of ozone therapy. State of the art. Arch. Med. Res. 2006, 37, 425–435. [Google Scholar] [CrossRef]
- Bocci, V.A. Can ozonetherapy be performed if the biochemistry of the process cannot be controlled? Arch. Med. Res. 2007, 38, 584–585. [Google Scholar] [CrossRef] [PubMed]
- Khorsandi, L.; Varaa, N.; Dadfar, R.; Vastegani, S.M.; Asadi-Fard, Y.; Ahangarpour, A.; Keshavarz-Zarjani, A. The protective effect of Ozone on the mice testicular damage induced by methotrexate. JBRA Assist. Reprod. 2024, 28, 464–470. [Google Scholar] [CrossRef] [PubMed]
- Moghadam, M.T.; Dadfar, R.; Khorsandi, L. The effects of ozone and melatonin on busulfan-induced testicular damage in mice. JBRA Assist. Reprod. 2021, 25, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Madrid Declaration on Ozone Therapy. Available online: https://ozonesociety.org/wp-content/uploads/2016/06/Ozone-Therapy-Madrid_declaration.pdf (accessed on 18 August 2025).
- Serra, M.E.G.; Baeza-Noci, J.; Mendes Abdala, C.V.; Luvisotto, M.M.; Bertol, C.D.; Anzolin, A.P. The role of ozone treatment as integrative medicine. An evidence and gap map. Front. Public Health 2023, 10, 1112296. [Google Scholar] [CrossRef]
- Ghezzi, M.; Berretta, M.; Bottacin, A.; Palego, P.; Sartini, B.; Cosci, I.; Finos, L.; Selice, R.; Foresta, C.; Garolla, A. Impact of Bep or Carboplatin Chemotherapy on Testicular Function and Sperm Nucleus of Subjects with Testicular Germ Cell Tumor. Front. Pharmacol. 2016, 7, 122. [Google Scholar] [CrossRef]
- Favareto, A.P.A.; Fernandez, C.D.B.; Da Silva, D.A.F.; Anselmo-Franci, J.A.; Kempinas, W.D.G. Persistent Impairment of Testicular Histology and Sperm Motility in Adult Rats Treated with Cisplatin at Peri-Puberty: Cisplatin Effects In Post-Pubertal and Adult Rats. Basic Clin. Pharmacol. Toxicol. 2011, 109, 85–96. [Google Scholar] [CrossRef]
- Soni, K.K.; Kim, H.K.; Choi, B.R.; Karna, K.K.; You, J.H.; Cha, J.S.; Shin, Y.S.; Lee, S.W.; Kim, C.Y.; Park, J.K. Dose-dependent effects of cisplatin on the severity of testicular injury in Sprague Dawley rats: Reactive oxygen species and endoplasmic reticulum stress. Drug Des. Devel. Ther. 2016, 10, 3959–3968. [Google Scholar] [CrossRef]
- Treatment-Related Toxicity in Testicular Germ Cell Tumors—UpToDate. 2024. Available online: https://www.uptodate.com/contents/treatment-related-toxicity-in-testicular-germ-cell-tumors#H12 (accessed on 21 July 2025).
- Sriram, S.; Macedo, T.; Mavinkurve-Groothuis, A.; van de Wetering, M.; Looijenga, L.H.J. Alkylating agents-induced gonadotoxicity in prepubertal males: Insights on the clinical and preclinical front. Clin. Transl. Sci. 2024, 17, e13866. [Google Scholar] [CrossRef]
- Amirshahi, T.; Najafi, G.; Nejati, V. Protective effect of royal jelly on fertility and biochemical parameters in bleomycin-induced male rats. Iran. J. Reprod. Med. 2014, 12, 209–216. [Google Scholar] [PubMed]
- Chan, D.; Delbès, G.; Landry, M.; Robaire, B.; Trasler, J.M. Epigenetic Alterations in Sperm DNA Associated with Testicular Cancer Treatment. Toxicol. Sci. 2012, 125, 532–543. [Google Scholar] [PubMed]
- Maselli, J.; Hales, B.F.; Chan, P.; Robaire, B. Exposure to Bleomycin, Etoposide, and Cis-Platinum Alters Rat Sperm Chromatin Integrity and Sperm Head Protein Profile1. Biol. Reprod. 2012, 86, 166. [Google Scholar] [CrossRef] [PubMed]
- Potiris, A.; Moustakli, E.; Trismpioti, E.; Drakaki, E.; Mavrogianni, D.; Matsas, A.; Zikopoulos, A.; Sfakianakis, A.; Tsakiridis, I.; Dagklis, T.; et al. From Inflammation to Infertility: How Oxidative Stress and Infections Disrupt Male Reproductive Health. Metabolites 2025, 15, 267. [Google Scholar] [CrossRef]
- Viebahn-Haensler, R.; León Fernández, O.S. Ozone as Redox Bioregulator in Preventive Medicine: The Molecular and Pharmacological Basis of the Low-Dose Ozone Concept—A Review. Int. J. Mol. Sci. 2023, 24, 15747. [Google Scholar] [CrossRef]
- Franzini, M.; Valdenassi, L.; Pandolfi, S.; Tirelli, U.; Ricevuti, G.; Chirumbolo, S. The Role of Ozone as an Nrf2-Keap1-ARE Activator in the Anti-Microbial Activity and Immunity Modulation of Infected Wounds. Antioxidants 2023, 12, 1985. [Google Scholar] [CrossRef]
- Jeyaraman, M.; Jeyaraman, N.; Ramasubramanian, S.; Balaji, S.; Nallakumarasamy, A.; Patro, B.P.; Migliorini, F. Ozone therapy in musculoskeletal medicine: A comprehensive review. Eur. J. Med. Res. 2024, 29, 398. [Google Scholar] [CrossRef]
- Malatesta, M.; Tabaracci, G.; Pellicciari, C. Low-Dose Ozone as a Eustress Inducer: Experimental Evidence of the Molecular Mechanisms Accounting for Its Therapeutic Action. Int. J. Mol. Sci. 2024, 25, 12657. [Google Scholar] [CrossRef]
- Erginel, B.; Yanar, F.; Ilhan, B.; Yüksel, S.; Mi, P.; Berker, N.; Keskin, E.; Soysal, F.G. Is the increased ozone dosage key factor for its anti-inflammatory effect in an experimental model of mesenteric ischemia? Turk. J. Trauma Emerg. Surg. 2023, 29, 1069–1074. [Google Scholar] [CrossRef]
- Simsek, E.K.; Sahinturk, F.; Gul, E.; Tepeoglu, M.; Araz, C.; Haberal, B. Effect of Ozone Therapy on Epidural Fibrosis in Rats. World Neurosurg. 2023, 175, e296–e302. [Google Scholar] [CrossRef]
- Sokol, R.Z.; Kraft, P.; Fowler, I.M.; Mamet, R.; Kim, E.; Berhane, K.T. Exposure to environmental ozone alters semen quality. Environ. Health Perspect. 2006, 114, 360–365. [Google Scholar] [CrossRef]
- Jedlinska-Krakowska, M.; Bomba, G.; Jakubowski, K.; Rotkiewicz, T.; Jana, B.; Penkowski, A. Impact of oxidative stress and supplementation with vitamins E and C on testes morphology in rats. J. Reprod. Dev. 2006, 52, 203–209. [Google Scholar] [CrossRef]
- Tusat, M.; Mentese, A.; Demir, S.; Alver, A.; Imamoglu, M. Medical ozone therapy reduces oxidative stress and testicular damage in an experimental model of testicular torsion in rats. Int. Braz. J. Urol. 2017, 43, 1160–1166. [Google Scholar] [CrossRef] [PubMed]
- Kheirouri, S.; Alipour, M.R.; Panahi, F.; Ghobadi, H. Effects of sulfur dioxide, ozone, and ambient air pollution on lung histopathology, oxidative-stress biomarkers, and apoptosis-related gene expressions in rats. Exp. Lung Res. 2022, 48, 137–148. [Google Scholar] [CrossRef]
- Gülcan, M.; Demirtaş, H.; Özer, A.; Yığman, Z.; Dursun, A.; Arslan, M.; Oktar, G.L. Ozone Administration Reduces Myocardial Ischemia Reperfusion Injury in Streptozotocin Induced Diabetes Mellitus Rat Model. Drug Des. Dev. Ther. 2024, 18, 4203–4213. [Google Scholar] [CrossRef] [PubMed]
- Sahinturk, V.; Guclu, C.; Baycu, C. Protective effects of vitamin E on ethane dimethane sulfonate-induced testicular toxicity in rats. Asian J. Androl. 2007, 9, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Rat Sperm Morphological Assessment, Edition 1. 2000. Available online: http://www.irdg.co.uk/Sperm_morphology.pdf (accessed on 16 April 2022).
- Jeyendran, R.S.; Van der Ven, H.H.; Perez-Pelaez, M.; Crabo, B.G.; Zaneveld, L.J. Development of an assay to assess the functional integrity of the human sperm membrane and its relationship to other semen characteristics. J. Reprod. Fertil. 1984, 70, 219–228. [Google Scholar] [CrossRef]
- Bjorndahl, L. Evaluation of the one-step eosin-nigrosin staining technique for human sperm vitality assessment. Hum. Reprod. 2003, 18, 813–816. [Google Scholar] [CrossRef]
- Mortimer, D. Practical Laboratory Andrology; Oxford University Press: Oxford, UK, 1994; pp. 66–69. [Google Scholar]
- Arıcan, E.Y.; Gökçeoğlu Kayalı, D.; Ulus Karaca, B.; Boran, T.; Öztürk, N.; Okyar, A.; Ercan, F.; Özhan, G. Reproductive effects of subchronic exposure to acetamiprid in male rats. Sci. Rep. 2020, 10, 8985. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Altıner, N.; Dönmez Çakıl, Y.; Duran, İ.; Kayalı, D.G.; Bayram, H.; Pehlivan, A.; Tombul, O.K.; Selam, B.; Cıncık, M.; Sitar, M.E. Medical Ozone Treatment Attenuates Male Reproductive Toxicity Induced by Bleomycin, Etoposide, and Cisplatin Regimen in an Experimental Animal Model. Int. J. Mol. Sci. 2025, 26, 8547. https://doi.org/10.3390/ijms26178547
Altıner N, Dönmez Çakıl Y, Duran İ, Kayalı DG, Bayram H, Pehlivan A, Tombul OK, Selam B, Cıncık M, Sitar ME. Medical Ozone Treatment Attenuates Male Reproductive Toxicity Induced by Bleomycin, Etoposide, and Cisplatin Regimen in an Experimental Animal Model. International Journal of Molecular Sciences. 2025; 26(17):8547. https://doi.org/10.3390/ijms26178547
Chicago/Turabian StyleAltıner, Necdet, Yaprak Dönmez Çakıl, İdil Duran, Damla Gökçeoğlu Kayalı, Hale Bayram, Abdullah Pehlivan, Oğuz Kaan Tombul, Belgin Selam, Mehmet Cıncık, and Mustafa Erinç Sitar. 2025. "Medical Ozone Treatment Attenuates Male Reproductive Toxicity Induced by Bleomycin, Etoposide, and Cisplatin Regimen in an Experimental Animal Model" International Journal of Molecular Sciences 26, no. 17: 8547. https://doi.org/10.3390/ijms26178547
APA StyleAltıner, N., Dönmez Çakıl, Y., Duran, İ., Kayalı, D. G., Bayram, H., Pehlivan, A., Tombul, O. K., Selam, B., Cıncık, M., & Sitar, M. E. (2025). Medical Ozone Treatment Attenuates Male Reproductive Toxicity Induced by Bleomycin, Etoposide, and Cisplatin Regimen in an Experimental Animal Model. International Journal of Molecular Sciences, 26(17), 8547. https://doi.org/10.3390/ijms26178547






