Clinical and Molecular Presentation of a Patient with Paternal Uniparental Isodisomy of Chromosome 16
Abstract
1. Introduction
2. Results
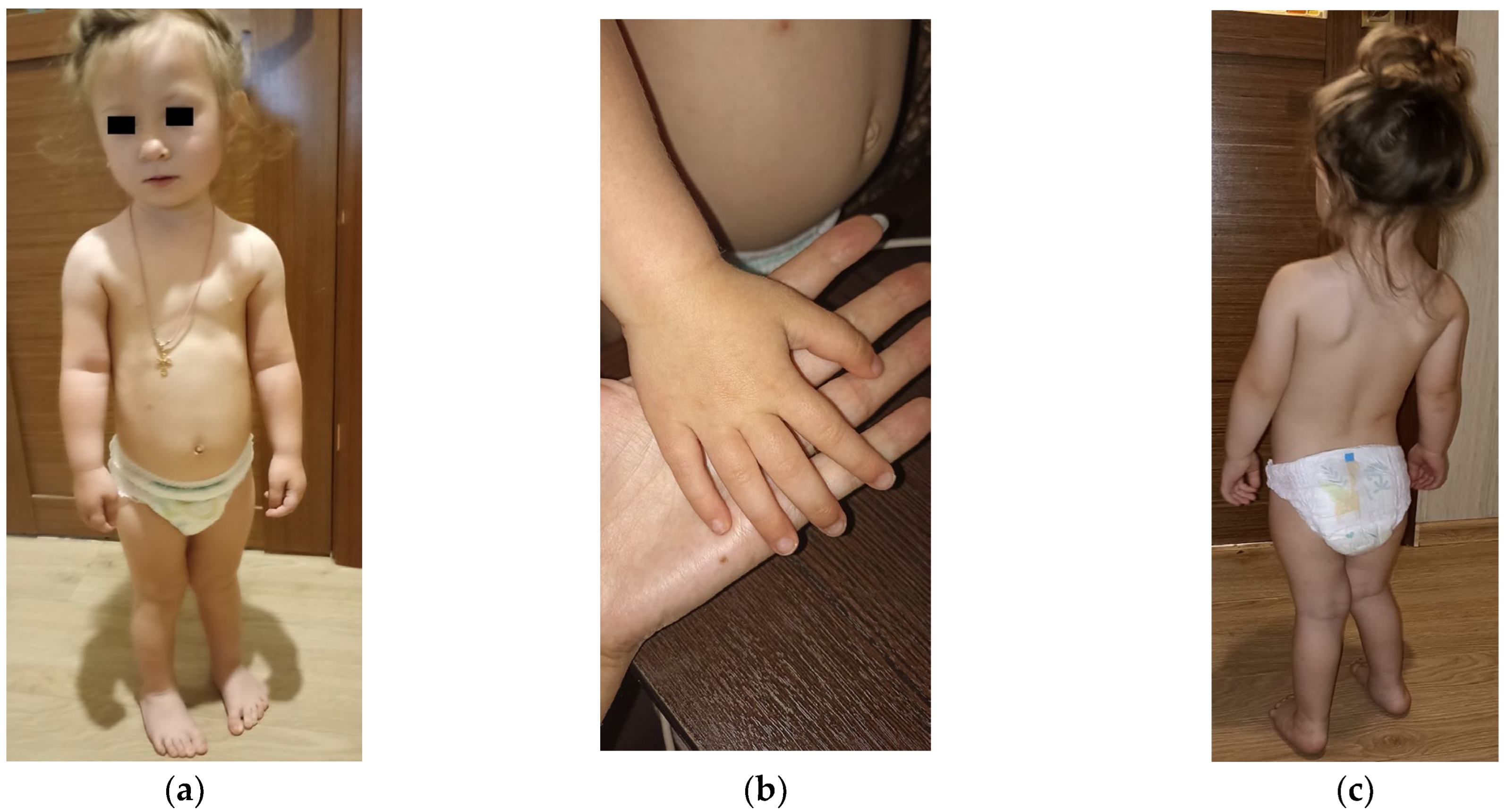
2.1. Clinical Presentation
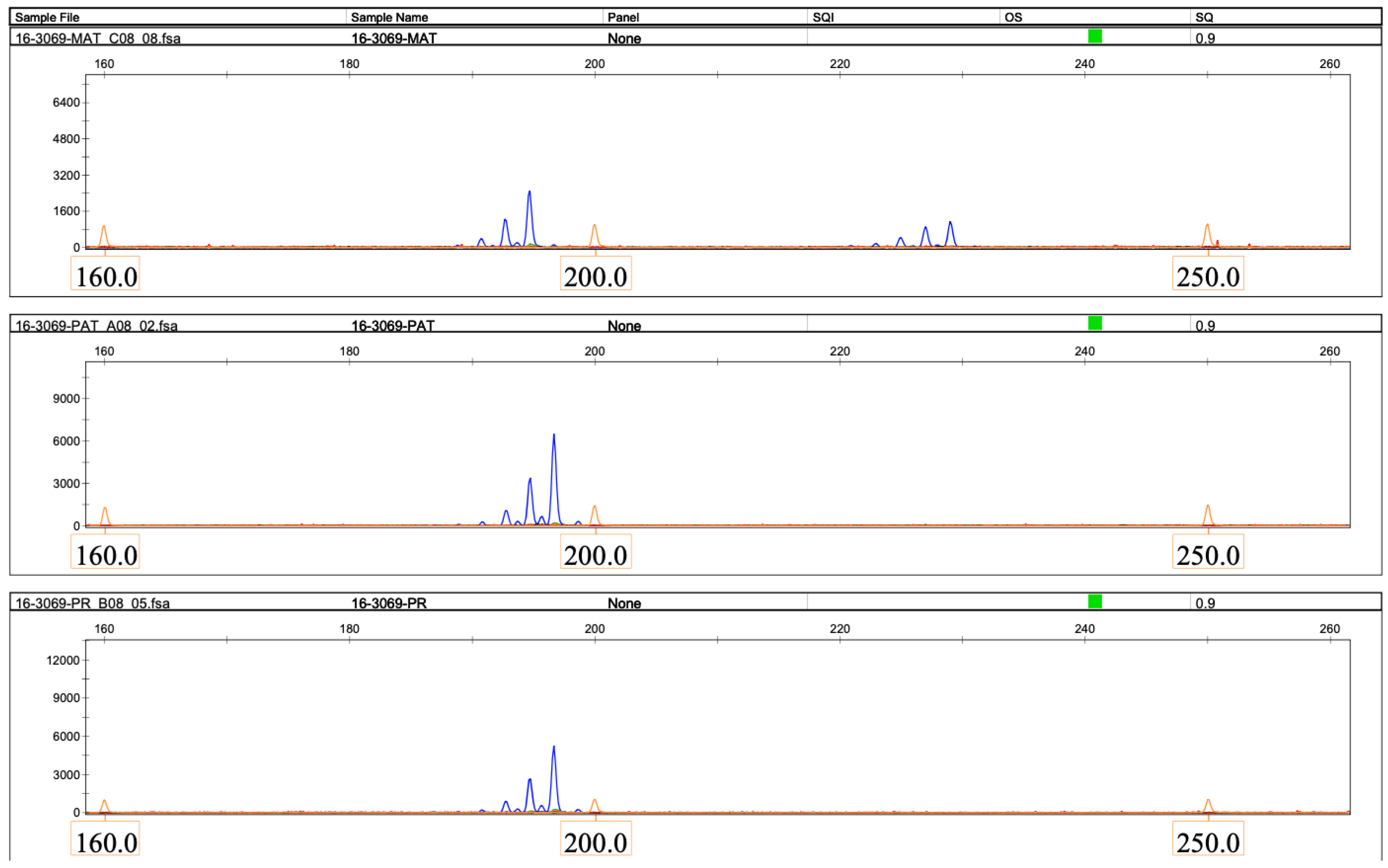
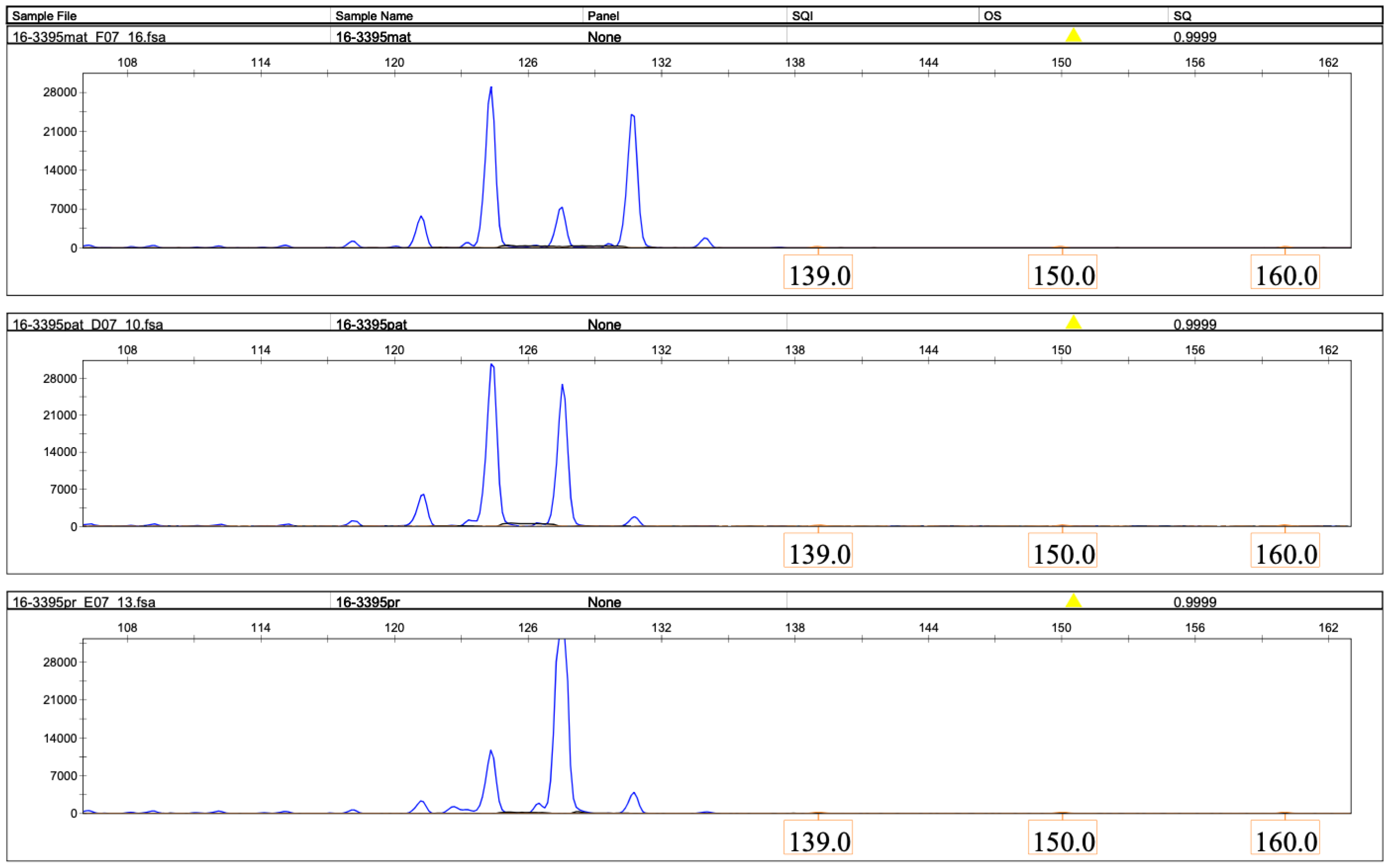
2.2. Molecular Genetic Findings
2.2.1. Loss of Heterozygosity on Chromosome 16
2.2.2. The Loss of Heterozygosity on Chromosome 16 Is Due to Paternal Uniparental Disomy
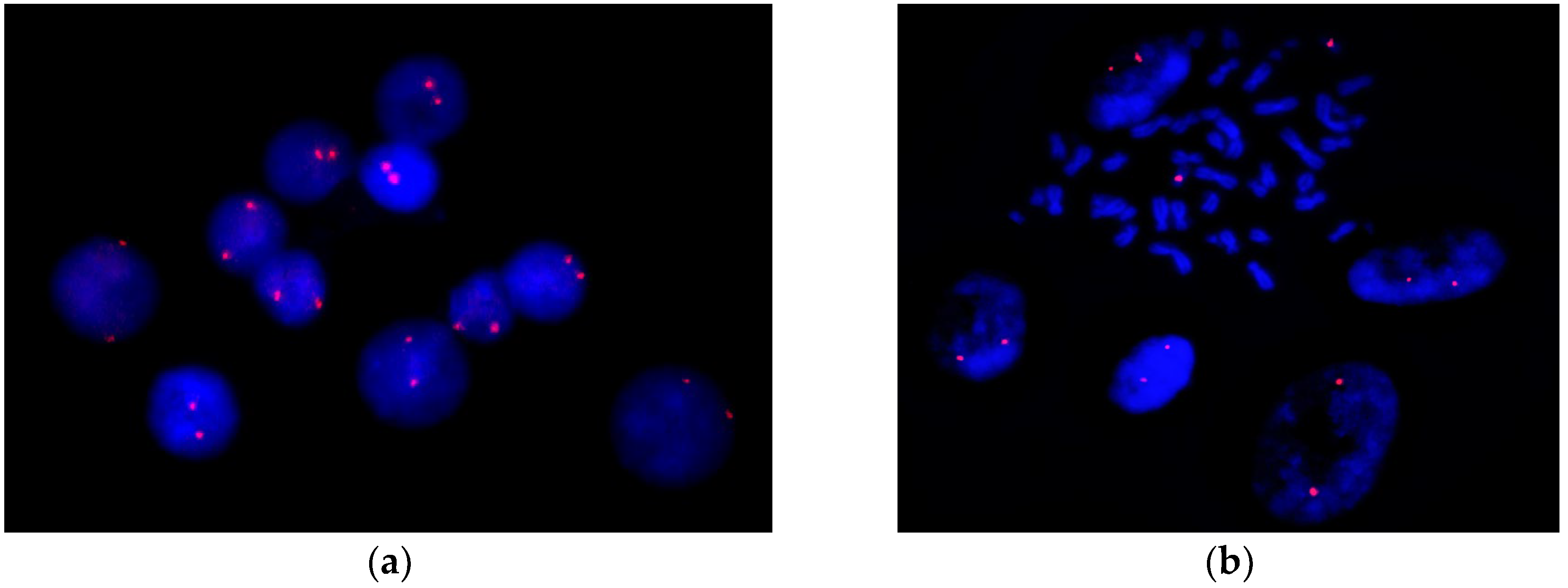
2.2.3. Low-Level Trisomy 16 Mosaicism Was Excluded by FISH
2.2.4. Absence of Detected Unmasked Pathogenic Recessive Genetic Variants on Chromosome 16
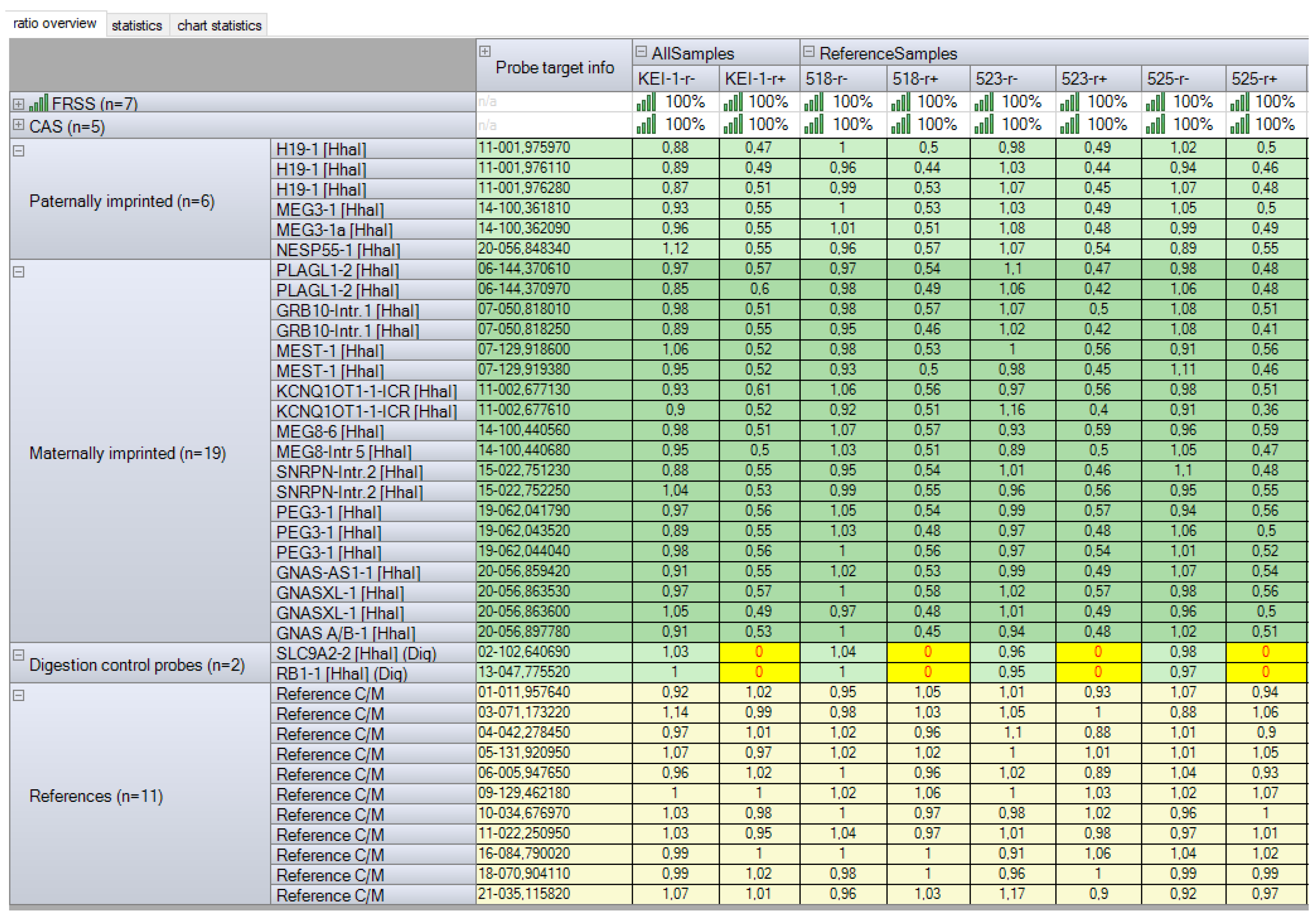
2.2.5. Imprinting Disorders and Multilocus Imprinting Disturbances Were Excluded by Methylation-Specific Multiplex Ligation-Dependent Probe Amplification
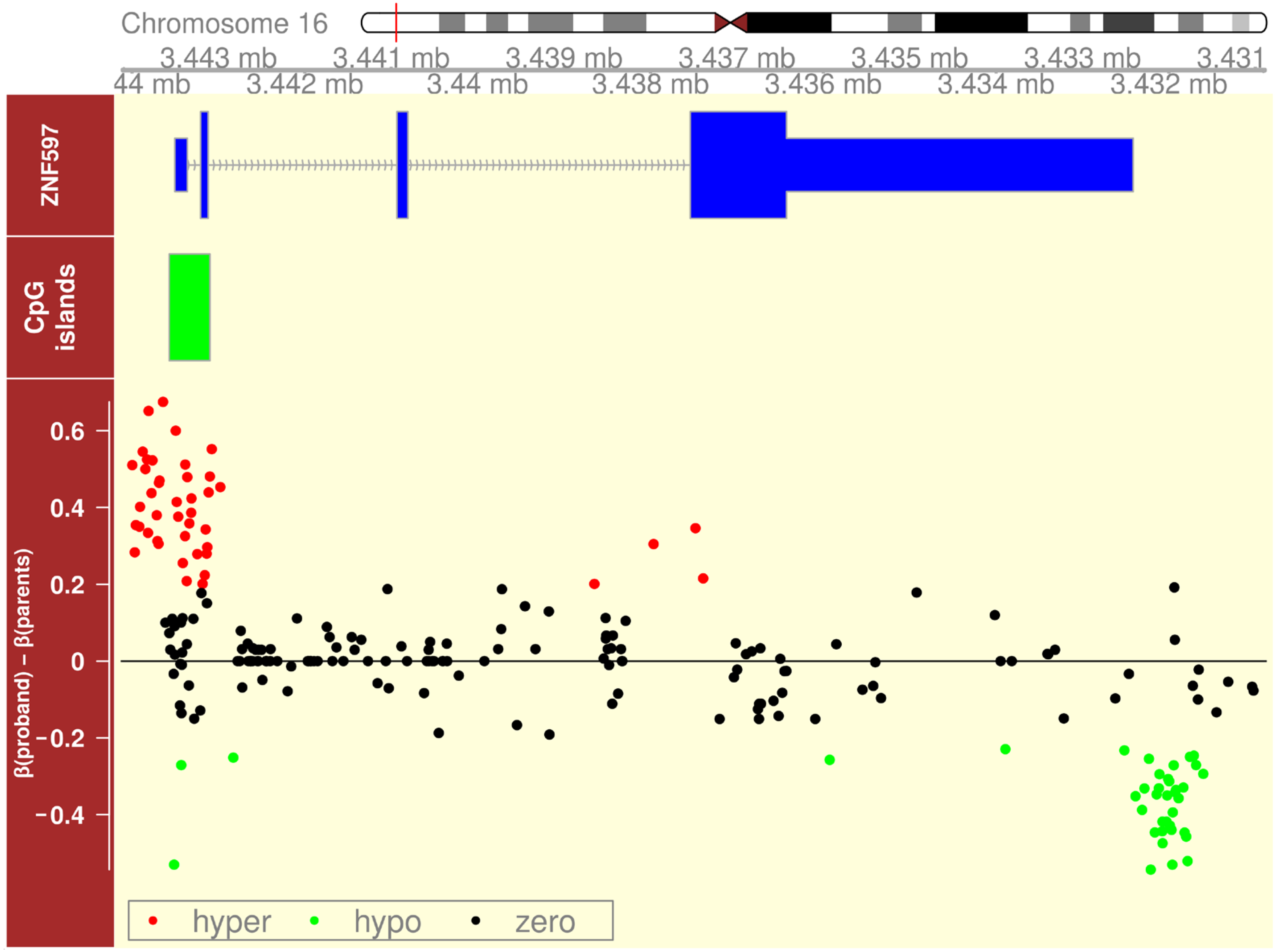
2.2.6. Imprinting Disorder on Chromosome 16 Revealed by Oxford Nanopore Sequencing
3. Discussion
4. Materials and Methods
4.1. Consent and Approval
4.2. Clinical Assessment
4.3. Molecular Genetic Testing
4.3.1. Chromosomal Microarray Analysis
4.3.2. Microsatellite Analysis
4.3.3. Fluorescence In Situ Hybridization
4.3.4. Exome Sequencing
4.3.5. Oxford Nanopore Sequencing
4.3.6. Methylation-Specific Multiplex Ligation-Dependent Probe Amplification
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Carli, D.; Riberi, E.; Ferrero, G.B.; Mussa, A. Syndromic Disorders Caused by Disturbed Human Imprinting. J. Clin. Res. Pediatr. Endocrinol. 2020, 12, 1–16. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dufke, A.; Eggermann, T.; Kagan, K.O.; Hoopmann, M.; Elbracht, M. Prenatal testing for Imprinting Disorders: A clinical perspective. Prenat. Diagn. 2023, 43, 983–992. [Google Scholar] [CrossRef] [PubMed]
- Spence, J.E.; Perciaccante, R.G.; Greig, G.M.; Willard, H.F.; Ledbetter, D.H.; Hejtmancik, J.F.; Pollack, M.S.; O’Brien, W.E.; Beaudet, A.L. Uniparental disomy as a mechanism for human genetic disease. Am. J. Hum. Genet. 1988, 42, 217–226. [Google Scholar] [PubMed] [PubMed Central]
- ACMG. ACMG Updates Chromosomal Microarray Analysis Guidelines. Am. J. Med. Genet Part A 2022, 188, 392–393. [Google Scholar] [CrossRef] [PubMed]
- Kohlhase, J.; Janssen, B.; Weidenauer, K.; Harms, K.; Bartels, I. First confirmed case with paternal uniparental disomy of chromosome 16. Am. J. Med. Genet. 2000, 91, 190–191. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, L.; Liu, Y.; Pan, X. Case Report: Paternal Uniparental Isodisomy and Heterodisomy of Chromosome 16 With a Normal Phenotype. Front. Pediatr. 2021, 9, 732645. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, J.; Chen, B.; Liu, Y.; Kong, J.; Zhang, B.; Han, L.; Mei, D.; Ma, C.Y.; Shang, Q.; Xie, Z.; et al. Paternal uniparental disomy of chromosome 16 resulting in homozygosity of a GPT2 mutation causes intellectual and developmental disability. Eur. J. Med. Genet. 2022, 65, 104554. [Google Scholar] [CrossRef] [PubMed]
- Soehn, A.S.; Rattay, T.W.; Beck-Wödl, S.; Schäferhoff, K.; Monk, D.; Döbler-Neumann, M.; Hörtnagel, K.; Schlüter, A.; Ruiz, M.; Pujol, A.; et al. Uniparental disomy of chromosome 16 unmasks recessive mutations of FA2H/SPG35 in 4 families. Neurology 2016, 87, 186–191. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rattay, T.W.; Soehn, A.S.; Karle, K.N.; Wiethoff, S.; Reichbauer, J.; Döbler-Neumann, M.; Gaiser, U.; Bauer, P.; Züchner, S.; Schüle, R.; et al. Uniparental disomy causing hereditary spastic paraplegia: Paternal disomy in FA2H causing homozygous SPG35 in two non-consanguine families. Eur. J. Hum. Genet. 2015, 23 (Suppl. S1), 180. [Google Scholar]
- Hamvas, A.; Nogee, L.M.; Wegner, D.J.; Depass, K.; Christodoulou, J.; Bennetts, B.; McQuade, L.R.; Gray, P.H.; Deterding, R.R.; Carroll, T.R.; et al. Inherited surfactant deficiency caused by uniparental disomy of rare mutations in the surfactant protein-B and ATP binding cassette, subfamily a, member 3 genes. J. Pediatr. 2009, 155, 854–859.e1. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Donovan, F.X.; Kimble, D.C.; Kim, Y.; Lach, F.P.; Harper, U.; Kamat, A.; Jones, M.; Sanborn, E.M.; Tryon, R.; Wagner, J.E.; et al. Paternal or maternal uniparental disomy of chromosome 16 resulting in homozygosity of a mutant allele causes Fanconi anemia. Hum. Mutat. 2016, 37, 465–468. [Google Scholar] [CrossRef][Green Version]
- Bis, D.M.; Schüle, R.; Reichbauer, J.; Synofzik, M.; Rattay, T.W.; Soehn, A.; de Jonghe, P.; Schöls, L.; Züchner, S. Uniparental disomy determined by whole-exome sequencing in a spectrum of rare motoneuron diseases and ataxias. Mol. Genet. Genom. Med. 2017, 5, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Vaes, L.; Tiller, G.E.; Pérez, B.; Boyer, S.W.; Berry, S.A.; Sarafoglou, K.; Morava, E. PMM2-CDG caused by uniparental disomy: Case report and literature review. JIMD Rep. 2020, 54, 16–21. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lei, Y.L.; Zhen, L.; Xu, L.L.; Yang, Y.D.; Li, D.Z. Foetal phenotype of ALG1-CDG caused by paternal uniparental disomy 16. J. Obstet. Gynaecol. 2021, 41, 828–830. [Google Scholar] [CrossRef]
- Bharucha-Goebel, D.X.; Norato, G.; Saade, D.; Paredes, E.; Biancavilla, V.; Donkervoort, S.; Kaur, R.; Lehky, T.; Fink, M.; Armao, D.; et al. Giant axonal neuropathy: Cross-sectional analysis of a large natural history cohort. Brain 2021, 144, 3239–3250. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nishino, M.; Tanaka, M.; Imagawa, K.; Yaita, K.; Enokizono, T.; Ohto, T.; Suzuki, H.; Yamada, M.; Takenouchi, T.; Kosaki, K.; et al. Identification of a novel splice-site WWOX variant with paternal uniparental isodisomy in a patient with infantile epileptic encephalopathy. Am. J. Med. Genet. Part A 2024, 194, e63575. [Google Scholar] [CrossRef] [PubMed]
- Yamazawa, K.; Inoue, T.; Sakemi, Y.; Nakashima, T.; Yamashita, H.; Khono, K.; Fujita, H.; Enomoto, K.; Nakabayashi, K.; Hata, K.; et al. Loss of imprinting of the human-specific imprinted gene ZNF597 causes prenatal growth retardation and dysmorphic features: Implications for phenotypic overlap with Silver-Russell syndrome. J. Med. Genet. 2021, 58, 427–432. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Product Description SALSA® MLPA® Probemix ME034-D1 Multi-Locus Imprinting. Available online: https://www.mrcholland.com/products/37165/Product%20description%20ME034-D1%20Multi-locus%20Imprinting-v02.pdf (accessed on 6 July 2025).
- Face2Gene Service. Available online: https://www.face2gene.com/ (accessed on 10 May 2024).
- McGowan-Jordan, J.; Simons, A.; Schmid, M. (Eds.) ISCN2016: An International System for Human Cytogenomic Nomenclature (2016); S.Karger AG: Basel, Switzerland, 2016; ISBN 3-8055-7667-6. [Google Scholar] [CrossRef]
- Sigurpalsdottir, B.D.; Stefansson, O.A.; Holley, G.; Beyter, D.; Zink, F.; Hardarson, M.Þ.; Sverrisson, S.Þ.; Kristinsdottir, N.; Magnusdottir, D.N.; Magnusson, O.Þ.; et al. A comparison of methods for detecting DNA methylation from long-read sequencing of human genomes. Genome Biol. 2024, 25, 69. [Google Scholar] [CrossRef]
- Gug, C.; Rațiu, A.; Navolan, D.; Drăgan, I.; Groza, I.M.; Păpurică, M.; Vaida, M.A.; Mozoș, I.; Jurcă, M.C. Incidence and Spectrum of Chromosome Abnormalities in Miscarriage Samples: A Retrospective Study of 330 Cases. Cytogenet. Genome Res. 2019, 158, 171–183. [Google Scholar] [CrossRef] [PubMed]
- Nakka, P.; Pattillo Smith, S.; O’Donnell-Luria, A.H.; McManus, K.F.; 23andMe Research Team; Mountain, J.L.; Ramachandran, S.; Sathirapongsasuti, J.F. Characterization of Prevalence and Health Consequences of Uniparental Disomy in Four Million Individuals from the General Population. Am. J. Hum. Genet. 2019, 105, 921–932. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bruce, S. Genomic and Epigenetic Investigations of Silver-Russell Syndrome and Growth Restriction. Ph.D. Thesis, University of Helsinki, Helsinki, Finland, 2009. [Google Scholar]
- Ponzi, E.; Alesi, V.; Lepri, F.R.; Genovese, S.; Loddo, S.; Mucciolo, M.; Novelli, A.; Dionisi-Vici, C.; Maiorana, A. Uniparental isodisomy of chromosome 1 results in glycogen storage disease type III with profound growth retardation. Mol. Genet. Genom. Med. 2019, 7, e634. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- ChromosOmics—Database. Available online: https://cs-tl.de/DB/CA/UPD/16-UPDm.html (accessed on 30 June 2025).
- Scheuvens, R.; Begemann, M.; Soellner, L.; Meschede, D.; Raabe-Meyer, G.; Elbracht, M.; Schubert, R.; Eggermann, T. Maternal uniparental disomy of chromosome 16 [upd(16)mat]: Clinical features are rather caused by (hidden) trisomy 16 mosaicism than by upd(16)mat itself. Clin. Genet. 2017, 92, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Inoue, T.; Yagasaki, H.; Nishioka, J.; Nakamura, A.; Matsubara, K.; Narumi, S.; Nakabayashi, K.; Yamazawa, K.; Fuke, T.; Oka, A.; et al. Molecular and clinical analyses of two patients with UPD(16)mat detected by screening 94 patients with Silver-Russell syndrome phenotype of unknown aetiology. J. Med. Genet. 2019, 56, 413–418. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Whiteford, M.L.; Coutts, J.; al-Roomi, L.; Mather, A.; Lowther, G.; Cooke, A.; Vaughan, J.I.; Moore, G.E.; Tolmie, J.L. Uniparental isodisomy for chromosome 16 in a growth-retarded infant with congenital heart disease. Prenat. Diagn. 1995, 15, 579–584. [Google Scholar] [CrossRef] [PubMed]
- Schneider, A.S.; Bischoff, F.Z.; McCaskill, C.; Coady, M.L.; Stopfer, J.E.; Shaffer, L.G. Comprehensive 4-year follow-up on a case of maternal heterodisomy for chromosome 16. Am. J. Med. Genet. 1996, 66, 204–208. [Google Scholar] [CrossRef] [PubMed]
- Dworniczak, B.; Koppers, B.; Kurlemann, G.; Holzgreve, W.; Horst, J.; Miny, P. Uniparental disomy with normal phenotype. Lancet 1992, 340, 1285. [Google Scholar] [CrossRef] [PubMed]
- O’Riordan, S.; Greenough, A.; Moore, G.E.; Bennett, P.; Nicolaides, K.H. Case report: Uniparental disomy 16 in association with congenital heart disease. Prenat. Diagn. 1996, 16, 963–965. [Google Scholar] [CrossRef] [PubMed]
- Woo, V.; Bridge, P.J.; Bamforth, J.S. Maternal uniparental heterodisomy for chromosome 16: Case report. Am. J. Med. Genet. 1997, 70, 387–390. [Google Scholar] [CrossRef] [PubMed]
- Azzi, S.; Salem, J.; Thibaud, N.; Chantot-Bastaraud, S.; Lieber, E.; Netchine, I.; Harbison, M.D. A prospective study validating a clinical scoring system and demonstrating phenotypical-genotypical correlations in Silver-Russell syndrome. J. Med. Genet. 2015, 52, 446–453. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fuke, T.; Nakamura, A.; Inoue, T.; Kawashima, S.; Hara, K.I.; Matsubara, K.; Sano, S.; Yamazawa, K.; Fukami, M.; Ogata, T.; et al. Role of Imprinting Disorders in Short Children Born SGA and Silver-Russell Syndrome Spectrum. J. Clin. Endocrinol. Metab. 2021, 106, 802–813. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yauy, K.; de Leeuw, N.; Yntema, H.G.; Pfundt, R.; Gilissen, C. Accurate detection of clinically relevant uniparental disomy from exome sequencing data. Genet. Med. 2020, 22, 803–808. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cai, M.; Guo, C.; Wang, X.; Lin, M.; Xu, S.; Huang, H.; Lin, N.; Xu, L. Classifying and evaluating fetuses with multicystic dysplastic kidney in etiologic studies. Exp. Biol. Med. 2023, 248, 858–865. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Abu-Amero, S.N.; Ali, Z.; Abu-Amero, K.K.; Stanier, P.; Moore, G.E. An analysis of common isodisomic regions in five mUPD 16 probands. J. Med. Genet. 1999, 36, 204–207. [Google Scholar] [PubMed] [PubMed Central]
- Urakawa, T.; Soejima, H.; Yamoto, K.; Hara-Isono, K.; Nakamura, A.; Kawashima, S.; Narusawa, H.; Kosaki, R.; Nishimura, Y.; Yamazawa, K.; et al. Comprehensive molecular and clinical findings in 29 patients with multi-locus imprinting disturbance. Clin. Epigenetics 2024, 16, 138. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schulze, K.V.; Szafranski, P.; Lesmana, H.; Hopkin, R.J.; Hamvas, A.; Wambach, J.A.; Shinawi, M.; Zapata, G.; Carvalho, C.M.B.; Liu, Q.; et al. Novel parent-of-origin-specific differentially methylated loci on chromosome 16. Clin. Epigenetics 2019, 11, 60. [Google Scholar] [CrossRef]
- Eggermann, T. Human Reproduction and Disturbed Genomic Imprinting. Genes 2024, 15, 163. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Javadi, A.; Shamaei, M.; Mohammadi Ziazi, L.; Pourabdollah, M.; Dorudinia, A.; Seyedmehdi, S.M.; Karimi, S. Qualification study of two genomic DNA extraction methods in different clinical samples. Tanaffos 2014, 13, 41–47. [Google Scholar] [PubMed] [PubMed Central]
- Quick Guide Isis 6.3. Available online: https://metasystems-international.com/site/assets/files/24323/2024-04-23_quickguide-isis_6_3_rev1_na.pdf (accessed on 6 July 2025).
- Ni, Y.; Liu, X.; Simeneh, Z.M.; Yang, M.; Li, R. Benchmarking of Nanopore R10.4 and R9.4.1 flow cells in single-cell whole-genome amplification and whole-genome shotgun sequencing. Comput. Struct. Biotechnol. J. 2023, 21, 2352–2364. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, Z.; Liu, Z.; Fang, Y.; Zhang, H.H.; Sun, X.; Hao, N.; Que, J.; Ding, H. Training data diversity enhances the basecalling of novel RNA modification-induced nanopore sequencing readouts. Nat. Commun. 2025, 16, 679. [Google Scholar] [CrossRef]
- Li, H. Minimap2: Pairwise alignment for nucleotide sequences. Bioinformatics 2018, 34, 3094–3100. [Google Scholar] [CrossRef]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R.; 1000 Genome Project Data Processing Subgroup. The Sequence Alignment/Map format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- McKenna, A.; Hanna, M.; Banks, E.; Sivachenko, A.; Cibulskis, K.; Kernytsky, A.; Garimella, K.; Altshuler, D.; Gabriel, S.; Daly, M.; et al. The Genome Analysis Toolkit: A MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010, 20, 1297–1303. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zheng, Z.; Li, S.; Su, J.; Leung, A.W.; Lam, T.W.; Luo, R. Symphonizing pileup and full-alignment for deep learning-based long-read variant calling. Nat. Comput. Sci. 2022, 2, 797–803. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.; Ebert, P.; Marschall, T. Read-Based Phasing and Analysis of Phased Variants with WhatsHap. In Haplotyping; Methods in Molecular Biology; Humana: New York, NY, USA, 2023; Volume 2590, pp. 127–138. [Google Scholar] [CrossRef] [PubMed]
- Ahsan, M.U.; Gouru, A.; Chan, J.; Zhou, W.; Wang, K. A signal processing and deep learning framework for methylation detection using Oxford Nanopore sequencing. Nat. Commun. 2024, 15, 1448. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024. [Google Scholar]
- Hester, J.; Wickham, H.; Bryan, J. Vroom: Read and Write Rectangular Text Data Quickly. 2023. Available online: https://vroom.r-lib.org/ (accessed on 10 July 2025).
- Wickham, H.; François, R.; Henry, L.; Müller, K.; Vaughan, D. Dplyr: A Grammar of Data Manipulation. 2023. Available online: https://dplyr.tidyverse.org/ (accessed on 10 July 2025).
- Hahne, F.; Ivanek, R. Visualizing Genomic Data Using Gviz and Bioconductor. In Statistical Genomics; Mathé, E., Davis, S., Eds.; Springer: New York, NY, USA, 2016; Volume 1418, pp. 335–351. [Google Scholar]
- Lawrence, M.; Huber, W.; Pagès, H.; Aboyoun, P.; Carlson, M.; Gentleman, R.; Morgan, M.T.; Carey, V.J. Software for Computing and Annotating Genomic Ranges. PLoS Comput. Biol. 2013, 9, e1003118. [Google Scholar] [CrossRef]
- Jima, D.D.; Skaar, D.A.; Planchart, A.; Motsinger-Reif, A.; Cevik, S.E.; Park, S.S.; Cowley, M.; Wright, F.; House, J.; Liu, A.; et al. Genomic map of candidate human imprint control regions: The imprintome. Epigenetics 2022, 17, 1920–1943. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]


| Features | Patients | ||
|---|---|---|---|
| UPD (16) Mat 15 Patients * | UPD (16) Pat 2 Patients ** | UPD (16) Pat (Our Patient) | |
| Prenatal | |||
| short femora | 1/15 | − | − |
| abnormal echogenicity of the fetal left lower lung (possible isolated lung) | − | 1/2 | − |
| slight polyhydramnios | − | 1/2 | − |
| reverse flow in the ductus venosus | 1/15 | − | − |
| abnormal results of the maternal serum screen | 3/15 | 1/2 | − |
| abnormal results for the chromosome 16 number in maternal NIPT | N/S | 1/2 | N/S |
| chorionic villus sampling (CVS) karyotyping: trisomy 16 in all analyzed cells | 7/15 | − | N/S |
| amniotic fluid (AF) karyotyping: trisomy 16 mosaicism | 3/15 | 1/2 | N/S |
| placental mosaicism (CPM) for trisomy 16 | − | 1/2 | N/S |
| amniotic fluid (AF) CMA: chromosome 16 LOH | N/S | 1/2 | N/S |
| maternal anemia | − | − | + |
| pre-eclampsia | 2/15 | − | + |
| maternal hematuria | 1/15 | − | − |
| maternal hepato-renal disfunction | 1/15 | − | − |
| two-vessel placenta | 1/15 | − | − |
| Infancy and childhood | |||
| general | |||
| PBL karyotyping: no trisomy 16 mosaicism or other chromosomal abnormalities | 15/15 | 2/2 | + |
| premature birth | 8/15 | 1/2 | + |
| intrauterine growth restriction | 11/15 | 1/2 | + |
| postnatal growth failure | 9/15 | 1/2 | + |
| feeding difficulties | 6/15 | − | − |
| muscular hypotonia | 1/15 | − | − |
| abnormalities of the facial phenotype | |||
| protruding forehead | 6/15 | − | − |
| relative macrocephaly | 4/15 | − | − |
| microcephaly | 1/15 | − | + |
| triangular face | 4/15 | − | − |
| high anterior hairline | − | − | + |
| arched eyebrows | − | − | + |
| hypotelorism | − | − | + |
| epicanthus | 1/15 | − | + |
| almond-shaped palpebral fissures | 1/15 | − | + |
| slightly flatter face profile | 1/15 | − | − |
| wide nasal bridge | − | − | + |
| depressed nasal ridge | − | − | + |
| wide base of the nose with a broad tip | − | − | + |
| smoothed filter | − | − | + |
| full cheeks | − | − | + |
| downturned corners of the mouth | − | − | + |
| short chin | − | − | + |
| dysplastic ears | 1/15 | − | + |
| abnormalities of the cardiovascular system | |||
| atrial septal defect | 1/15 | − | + |
| atrioventricular defect | 4/15 | − | − |
| ventricular septal defect (VSD) | 2/15 | − | − |
| hypertrophied, dilated right ventricle suggesting pulmonary hypertension | 1/15 | − | − |
| aortic stenosis | 1/15 | − | − |
| pulmonary valve stenosis | 1/15 | − | − |
| aortarctia | 1/15 | − | − |
| congenital heart disease | 1/15 | − | − |
| abnormalities of the musculoskeletal system | |||
| scoliosis | 2/15 | − | − |
| body asymmetry | 1/15 | − | − |
| dislocation of the radio-humeral articulation | 2/15 | − | − |
| clinodactyly of the fifth fingers | 7/15 | − | − |
| assimilation of the atlas | − | − | + |
| spina bifida posterior C1 | − | − | + |
| hypoplasia of the axial atlas | − | − | + |
| bilateral pes calcaneus | − | 1/2 | − |
| an additional rudimentary mandibular dental arch | − | 1/2 | − |
| narrow funnel-shaped chest | − | − | + |
| cone-shaped fingers of the hands | − | − | + |
| proximal displacement of thumbs | − | − | + |
| hyperlordosis | − | − | + |
| valgus installation of knees and feet | − | − | + |
| rocker-bottom foot | − | − | + |
| right talipes equinovarus | 1/15 | − | − |
| abnormalities of the genitourinary system | |||
| hypospadias | 3/15 | − | − |
| pelvic dystopia | − | − | + |
| rotation of the left kidney | − | − | + |
| left hydronephrosis | 1/15 | − | − |
| left multi-cystic kidney | 1/15 | − | − |
| genitourinary anomalies | 1/15 | − | − |
| left renal agenesis | 1/15 | − | − |
| abnormalities of the digestive system | |||
| esophageal atresia | 1/15 | − | − |
| tracheoesophageal fistula | 1/15 | − | − |
| giant cell hepatitis | 1/15 | − | − |
| inguinal hernia | 1/15 | − | − |
| abnormalities of the respiratory system | |||
| pulmonary cystic changes | 2/15 | − | − |
| rudimentary bronchus on the right side | 2/15 | − | − |
| abnormalities of the nervous system | |||
| delayed speech development | 3/15 | − | + |
| delayed motor development | 1/15 | − | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panchenko, E.; Semenova, N.; Sereda, O.; Guseva, D.; Markova, Z.; Shilova, N.; Simonova, O.; Smirnov, A.; Pustoshilov, D.; Khalilova, A.; et al. Clinical and Molecular Presentation of a Patient with Paternal Uniparental Isodisomy of Chromosome 16. Int. J. Mol. Sci. 2025, 26, 8521. https://doi.org/10.3390/ijms26178521
Panchenko E, Semenova N, Sereda O, Guseva D, Markova Z, Shilova N, Simonova O, Smirnov A, Pustoshilov D, Khalilova A, et al. Clinical and Molecular Presentation of a Patient with Paternal Uniparental Isodisomy of Chromosome 16. International Journal of Molecular Sciences. 2025; 26(17):8521. https://doi.org/10.3390/ijms26178521
Chicago/Turabian StylePanchenko, Elizaveta, Natalia Semenova, Olga Sereda, Daria Guseva, Zhanna Markova, Nadezhda Shilova, Olga Simonova, Anton Smirnov, Dmitry Pustoshilov, Arina Khalilova, and et al. 2025. "Clinical and Molecular Presentation of a Patient with Paternal Uniparental Isodisomy of Chromosome 16" International Journal of Molecular Sciences 26, no. 17: 8521. https://doi.org/10.3390/ijms26178521
APA StylePanchenko, E., Semenova, N., Sereda, O., Guseva, D., Markova, Z., Shilova, N., Simonova, O., Smirnov, A., Pustoshilov, D., Khalilova, A., Udalova, V., Kanivets, I., Zaletaev, D., Strelnikov, V., & Kutsev, S. (2025). Clinical and Molecular Presentation of a Patient with Paternal Uniparental Isodisomy of Chromosome 16. International Journal of Molecular Sciences, 26(17), 8521. https://doi.org/10.3390/ijms26178521






