Abstract
Vitamin D is increasingly recognized as a key regulator of epithelial barrier integrity and mucosal immune homeostasis, with implications extending far beyond skeletal health. Through the vitamin D receptor (VDR), vitamin D regulates epithelial cohesion, innate immune responses, and tight-junction gene expression. This review explores the multifactorial role of vitamin D in modulating inflammation and preserving tissue barriers, with particular emphasis on its effects on tight junction (TJ) regulation and disease states characterized by barrier dysfunction, namely atopic dermatitis, psoriasis, inflammatory bowel disease (IBD), and celiac disease. In these settings, vitamin D/VDR signaling exerts protective actions by enhancing barrier structure, suppressing Th1/Th17-driven inflammation, modulating the gut and skin microbiome, and promoting epithelial repair. Animal studies and clinical data suggest that vitamin D supplementation can restore TJ expression, reduce disease activity, and improve clinical outcomes in both intestinal and dermatologic diseases. In the cardiovascular system, the role of vitamin D remains complex. While vitamin D influences endothelial function, insulin sensitivity, and systemic inflammation, supplementation trials yield mixed results, indicating a need for individualized approaches. Overall, this review synthesizes mechanistic, translational, and clinical data supporting vitamin D as a crucial modulator of barrier integrity and inflammation. These findings highlight its therapeutic relevance in chronic diseases characterized by immune dysregulation and epithelial disruption.
1. Introduction
Vitamin D is a secosteroid hormone primarily synthesized in the skin upon exposure to ultraviolet B (UVB) radiation or obtained through dietary sources, including fortified foods and supplements. It undergoes hydroxylation in the liver to form 25-hydroxyvitamin D [25(OH)D], the main circulating form, followed by further conversion in the kidneys to its active form, 1,25-dihydroxyvitamin D [1,25(OH)2D] [1]. While its classical role in calcium and phosphate homeostasis and bone metabolism is well-documented, an expanding body of research has revealed its far-reaching effects on various physiological processes, including immune regulation, cardiovascular function, and epithelial barrier integrity [2]. The presence of vitamin D receptors (VDRs) in nearly all nucleated cells suggests that vitamin D functions as a crucial modulator of cellular activity across multiple organ systems [3]. VDRs are highly expressed in immune cells, endothelial tissues, and epithelial barriers, indicating a broad functional role in maintaining tissue homeostasis and regulating inflammatory responses. Vitamin D is also recognized to influence the transcription of more than 200 genes involved in cell differentiation, immune defense, and metabolism, showing its broad systemic effects [4]. Recent epidemiological studies have linked vitamin D deficiency with an increased risk of chronic diseases beyond skeletal disorders, including autoimmune diseases, cardiovascular disease (CVD), metabolic syndrome, neurodegenerative disorders, and inflammatory bowel disease (IBD) [5]. This has led to a growing interest in the potential of vitamin D supplementation as a preventive or adjunctive therapeutic strategy in non-skeletal conditions. However, the mechanisms underlying these associations remain incompletely understood, and clinical trials evaluating vitamin D supplementation have yielded mixed results, underscoring the complexity of its systemic effects [6].
A significant aspect of the non-skeletal functions of vitamin D is its role in epithelial barrier integrity. In the skin, vitamin D regulates tight junction (TJ) proteins such as occludin, claudins, and zonula occludens-1 (ZO-1), which are essential for maintaining barrier function and preventing pathogen infiltration [7] (Figure 1). Similar regulatory effects have been observed in the gut, where vitamin D deficiency has been associated with increased intestinal permeability and heightened susceptibility to inflammatory diseases such as Crohn’s disease (CD) and ulcerative colitis (UC) [8] (see Figure 1). These findings suggest that vitamin D plays a protective role in maintaining epithelial defenses across different organ systems.
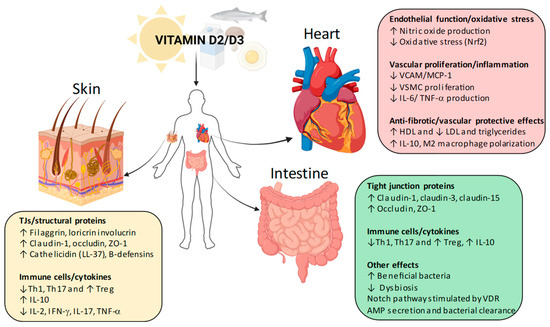
Figure 1.
Vitamin D non-skeletal effects in skin, gut, and cardiovascular system. Schematic illustration of the pleiotropic actions of vitamin D beyond bone and mineral metabolism. In the skin, vitamin D–VDR signaling strengthens the epidermal barrier by upregulating structural proteins (filaggrin, loricrin, involucrin) and tight junction proteins (claudin-1, occludin, ZO-1), while also boosting antimicrobial peptide production (cathelicidin, β-defensins). Immune modulation includes suppression of Th1 and Th17 responses, reduced pro-inflammatory cytokines (IL-2, IFN-γ, IL-17, TNF-α), and increased Treg activity and IL-10 release. In the intestine, vitamin D enhances barrier function through increased tight junction proteins (claudin-1, claudin-3, claudin-15, occludin, ZO-1), promotes a favorable microbiota balance, stimulates the Notch pathway, and augments antimicrobial peptide secretion, thereby reducing dysbiosis and supporting mucosal immunity. In the cardiovascular system, vitamin D improves endothelial function by stimulating nitric oxide production and reducing oxidative stress via Nrf2. It limits vascular inflammation and proliferation by downregulating adhesion molecules (VCAM-1, MCP-1), vascular smooth muscle cell proliferation, and pro-inflammatory cytokines such as IL-6 and TNF-α. Additionally, vitamin D exerts anti-fibrotic and vascular-protective effects by improving lipid profiles (↑ HDL, ↓ LDL and triglycerides) and promoting anti-inflammatory macrophage polarization (IL-10, M2 phenotype). AMPs, antimicrobial peptides; HDL, high-density lipoprotein; IFN-γ, interferon-gamma; IL-2, interleukin-2; IL-6, interleukin-6; IL-10, interleukin-10; IL-17, interleukin-17; LDL, low-density lipoprotein; MCP-1, monocyte chemoattractant protein-1; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; NO, nitric oxide; Nrf2, nuclear factor erythroid 2–related factor 2; PMCA, plasma membrane Ca2+ ATPase; RAAS, renin–angiotensin–aldosterone system; TJs, tight junctions; TNF-α, tumor necrosis factor-alpha; TRPV5/6, transient receptor potential vanilloid 5/6; Treg, regulatory T cell; VCAM-1, vascular cell adhesion molecule-1; VDR, vitamin D receptor; VSMC, vascular smooth muscle cell; ZO-1, zonula occludens-1.
Furthermore, vitamin D has emerged as a key modulator of immune function. It exerts immunoregulatory effects by modulating Toll-like receptor (TLR) signaling, enhancing antimicrobial peptide production (such as cathelicidin), and promoting anti-inflammatory cytokines while inhibiting pro-inflammatory pathways [9]. This has significant implications for autoimmune diseases and chronic inflammatory conditions, where vitamin D deficiency is commonly observed and may contribute to disease pathogenesis.
In the cardiovascular system, vitamin D has been implicated in endothelial function, arterial stiffness, and the regulation of blood pressure through its effects on the renin–angiotensin–aldosterone system (RAAS) [10]. Observational studies have shown an inverse relationship between serum vitamin D levels and the incidence of hypertension, myocardial infarction, and stroke, suggesting a cardioprotective role for vitamin D [11,12] (Figure 1). However, interventional trials evaluating vitamin D supplementation for cardiovascular disease prevention have yielded inconsistent findings, highlighting the need for further research to clarify optimal dosing and target populations [13]. Finally, regarding non-skeletal effects of vitamin D, it is known that it plays a pivotal role also in malignancies. Specifically, vitamin D has been increasingly recognized for its immunomodulatory and antiproliferative properties, with potential relevance in oncology. Vitamin D receptors are expressed in melanocytes and melanoma cells, indicating a direct regulatory role [14,15,16]. Nonetheless, further prospective studies are needed to clarify its therapeutic potential and optimal supplementation strategies.
This review aims to provide a comprehensive overview of the emerging non-skeletal roles of vitamin D, with particular focus on its effects on skin integrity, cardiovascular health, gut barrier function and immune regulation. By synthesizing current evidence from clinical studies, animal models, and mechanistic research, we seek to elucidate the biological pathways through which vitamin D exerts its diverse physiological effects and explore its potential as a therapeutic target in non-skeletal diseases.
2. Vitamin D and the Skin Barrier: Atopic Dermatitis and Psoriasis
The skin is not only a site of vitamin D synthesis but also a target of vitamin D’s actions. Active vitamin D (calcitriol, 1,25(OH)2D) is generated locally in the skin via keratinocyte expression of CYP27B1 (1α-hydroxylase) from circulating 25(OH)D and is inactivated by CYP24A1 (24-hydroxylase) [17]. By binding to VDR in keratinocytes, calcitriol promotes differentiation, supports stratum corneum formation, and regulates the expression of key barrier proteins [18]. TJ components in the stratum granulosum, including claudins, occludin, and zonula occludens-1 (ZO-1), form the sealing barrier between keratinocytes [19]. Calcitriol has been shown to enhance the expression and membrane localization of TJ proteins such as claudin-4, claudin-7, occludin, and ZO-1 in human keratinocyte monolayers [20]. In wounded skin, increased CYP27B1 activity enhances vitamin D activation and induces antimicrobial peptides like cathelicidin (LL-37), which contribute to both antimicrobial defense and barrier repair [21]. Conversely, vitamin D deficiency or impaired VDR function can disrupt keratinocyte maturation and compromise barrier integrity, potentially contributing to inflammatory skin conditions [17]. Through these mechanisms, promoting differentiation, tightening cell junctions, and boosting antimicrobial defenses, adequate vitamin D activity is crucial for optimal skin barrier performance (Figure 1; Table 1).

Table 1.
Extra-skeletal effects of vitamin D on skin, gut, and cardiovascular system, highlighting key molecular targets and clinical evidence from epidemiological and interventional studies.
2.1. The Regulatory Role of Vitamin D in Atopic Dermatitis
Atopic dermatitis (AD), also termed atopic eczema, represents a prevalent, chronic, and recurrent inflammatory dermatosis [22]. It is a complex disease with a spectrum of clinical presentations and combinations of symptoms [22]. A central pathogenic feature is the defective skin barrier, which leads to increased transepidermal water loss and heightened permeability to allergens, microbes, and irritants [23]. Contributing factors include altered expression of structural proteins (i.e., filaggrin, loricrin, involucrin, keratins), imbalance in protease activity, changes in pH, and ceramide loss. Together, these weaken the lipid barrier and reduce hydration [24]. Among these, filaggrin loss-of-function mutations represent the most studied genetic risk factor for AD, although their frequency varies significantly across populations, from a few percent to nearly half [25,26]. In addition, immune dysregulation plays a pivotal role, involving a disturbed balance among T helper 1 (Th1) cells, Th2, and regulatory T cells (Tregs), leading to skewed Th1/Th2 immune responses and heightened IgE-mediated allergic reactions. In AD lesions, Th2-associated cytokines, particularly interleukin (IL)-4 and IL-13, are markedly upregulated [27]. These mediators impair epidermal maturation, resulting in decreased production of filaggrin and antimicrobial peptides. Moreover, IL-31 is implicated in inducing intense itching and further suppresses keratinocyte differentiation. As the disease progresses, the immune response becomes increasingly complex, with involvement of additional cell subsets such as Th17 and Th22 cells, contributing to chronic inflammation and barrier impairment [28].
Vitamin D mediates its biological activity predominantly through the VDR, which is expressed in keratinocytes as well as in various immune cell types, including T lymphocytes and monocytes [29]. Upon binding of its active form, calcitriol, the VDR regulates the transcription of numerous genes involved in maintaining cutaneous immune balance and skin barrier integrity [30]. Among its key functions, vitamin D promotes keratinocyte maturation and enhances the expression of antimicrobial peptides (AMPs) such as cathelicidin and β-defensins, molecules that play a critical role in the innate immune defense of the skin and are often deficient in AD [30].
Vitamin D also participates in the fine-tuning of both the innate and adaptive immune response. VDR expression has been confirmed in multiple immune cell subsets, and its activation by 1,25(OH)2D has been shown to inhibit the proliferation of Th1 cells, which are typically responsible for producing cytokines like interferon-γ (IFN-γ) and IL-2, as well as for macrophage activation [31]. In parallel, vitamin D suppresses Th17 cell activity, another pro-inflammatory subset that secretes IL-17 and IL-22, both implicated in skin inflammation [29,32,33]. On the other hand, it favors the expansion and function of regulatory T cells (CD4+/CD25+), enhancing IL-10 secretion and thereby promoting an anti-inflammatory microenvironment [29]. In vitro experiments have further clarified the mechanistic role of vitamin D in skin immunity. Treatment of keratinocytes with 1,25(OH)2D has been shown to stimulate the expression of cathelicidin, an antimicrobial peptide effective against Staphylococcus aureus. This induction enhances cutaneous antimicrobial defenses and selectively downregulates cutaneous lymphocyte-associated antigen (CLA), a molecule involved in directing T lymphocytes to the skin [34,35].
Interestingly, this effect appears to be tissue-specific, as cathelicidin does not alter lymphocyte trafficking to other organs [34]. These findings provide mechanistic support for the clinical benefits of vitamin D supplementation in AD, aligning with its known roles in skin barrier regulation and immune modulation [34,35,36].
2.2. Clinical and Epidemiological Evidence Linking Vitamin D to AD
A growing body of clinical, epidemiological, and immunological research points to a significant association between vitamin D status and the development and severity of AD. Numerous observational studies and meta-analyses have consistently demonstrated that individuals with AD, both children and adults, exhibit lower circulating levels of 25(OH)D compared to healthy controls [37,38,39,40,41]. In a recent 2024 cross-sectional study including 681 participants aged 0–30 years, 84% of AD patients were found to have vitamin D deficiency or insufficiency, with significantly reduced levels among those with moderate-to-severe disease (EASI > 10) [37]. Notably, individuals with serum 25(OH)D levels below 25 nmol/L had a threefold increased risk of severe AD, and vitamin D concentrations inversely correlated with disease severity (r = −0.22; p < 0.001) [37]. This inverse relationship has been further confirmed by systematic reviews and meta-analyses.
Two reviews and a meta-analysis of 11 randomized controlled trials (RCTs, n = 686) demonstrated that vitamin D supplementation significantly reduced AD severity, as measured by SCORAD and EASI scores. Greater benefits were observed with daily doses of 1500–1600 IU administered for at least 12 weeks [40,41,42]. Additional studies support this correlation, showing symptom improvement and better disease control in patients with adequate vitamin D levels [36,43,44]. In particular, pediatric research suggests that sufficient vitamin D may be protective, helping to mitigate AD severity [44].
Maternal vitamin D intake during pregnancy has also been implicated in reducing the risk of atopic conditions in offspring, including wheezing disorders and eczema, indicating a possible immunoprotective role in early development [45]. Moreover, vitamin D deficiency at birth has been associated with a higher incidence of AD in infancy [46].
Despite these findings, some studies have yielded conflicting results, with reports of no significant association or even a paradoxical positive correlation between serum vitamin D levels and AD risk [47,48]. The variability in findings could be attributed to methodological heterogeneity across studies, such as differences in design, sample size, follow-up duration, and participant selection criteria, as well as to the lack of standardized supplementation protocols regarding dosage, duration, and timing. Moreover, since atopic dermatitis is influenced by numerous genetic and environmental factors, vitamin D likely represents only one of many contributing elements.
Genetic analyses have explored associations between vitamin D pathway polymorphisms and AD risk. Polymorphisms in the VDR gene, particularly BsmI, ApaI, TaqI, and A1012G, have been linked to increased disease susceptibility or severity [49,50,51]. The CYP24A1 rs2248359 C allele and related haplotypes have also been associated with more severe phenotypes [52]. In an Italian cohort, the heterozygous A1012G variant conferred protection against juvenile-onset AD (OR: 0.046, CI: 0.004–0.51, p = 0.012), while individuals with two or more homozygous variants in vitamin D-related genes had a significantly higher risk of early-onset severe disease and allergic sensitization (p = 0.0003) [51]. These variants were also linked to altered expression of TJ proteins: ZO-1 was associated with obesity (OR: 12.1, p = 0.045), and claudin-1 with allergic sensitization (OR: 8.23, p = 0.046) [51]. Notably, serum 25(OH)D levels inversely correlated with ZO-1 expression (ρ = −0.43, p = 0.0058) [51]. A follow-up proteomic analysis by the same authors revealed a 77.7% increase in protein content in lesional vs. perilesional biopsies (p < 0.001), including elevated expression of VDR, filaggrin, occludin, claudin-1, cingulin, and cathelicidin [7].
Patients with vitamin D levels below 30 ng/mL had increased expression of CYP24A (p = 0.054), alpha-catenin (p = 0.043), and haptoglobin (p = 0.033) [7]. In severe cases (EASI ≥ 16), higher expression levels of CYP24A, CYP27B1, filaggrin, claudin-1, and occludin were noted (all p < 0.05). Multivariate models confirmed strong associations between low vitamin D, altered TJ proteins, and allergic sensitization [7]. These results highlight the multifaceted role of vitamin D in maintaining skin integrity and regulating immune responses in AD (Table 2).

Table 2.
Key clinical and interventional evidence on the non-skeletal effects of vitamin D. This table summarizes the most relevant observational and interventional studies supporting the non-skeletal effects of vitamin D in atopic dermatitis, psoriasis, IBD, celiac disease, and cardiovascular disease.
These findings highlight vitamin D as a potentially modifiable factor in atopic dermatitis, emphasizing its translational relevance as both a preventive target and an adjunctive therapeutic strategy.
2.3. The Regulatory Role of Vitamin D in Psoriasis
Psoriasis is a chronic inflammatory skin disease [69], characterized by dysregulated immune responses that result in persistent inflammation, accelerated keratinocyte proliferation, and the development of erythematous, scaly plaques [69]. Although its precise etiology remains not fully understood, psoriasis is widely regarded as a multifactorial condition driven by the interaction between genetic predisposition and environmental factors such as infections, skin trauma (Koebner phenomenon), psychological stress, smoking, and ultraviolet radiation [70]. In genetically susceptible individuals, these external stimuli may trigger aberrant immune activation, leading to chronic cutaneous inflammation and excessive epidermal turnover [71].
At the immunological level, psoriasis mainly involves T cell activation and an imbalance that favors Th1 and Th17 responses [72]. Under normal conditions, Th1 cells release pro-inflammatory cytokines such as IL-2, IFN-γ, and IL-12, while Th2 cells produce anti-inflammatory cytokines like IL-4 and IL-10. In psoriatic patients, this balance shifts toward Th1 predominance, along with an expansion of Th17 and Th22 populations that secrete IL-6, IL-17, and IL-22, key mediators of epidermal hyperplasia and chronic inflammation [70,72].
In addition to adaptive immunity, innate immune components, including dendritic cells, macrophages, neutrophils, natural killer (NK) cells, and even keratinocytes, play a significant role in amplifying the inflammatory cascade via the release of pro-inflammatory cytokines [70]. In psoriasis, self-DNA and LL-37 can activate plasmacytoid dendritic cells, leading to type I interferon release and stimulation of Th1/Th17 cells. This inflammatory environment promotes keratinocyte hyperproliferation, leukocyte infiltration, and neoangiogenesis in affected skin areas [70].
Vitamin D, particularly in its active form, 1,25(OH)2D, plays a multifaceted role in the pathogenesis and potential treatment of psoriasis, exerting effects on both the immune system and epidermal homeostasis [18]. At the innate immune level, vitamin D enhances the function of dendritic cells, macrophages, and T lymphocytes by boosting their phagocytic activity and stimulating AMP production, notably cathelicidin. These AMPs reinforce the skin’s defense against microbial colonization, which is frequently disrupted in psoriatic plaques [73]. Specifically, plasmacytoid dendritic cells (pDCs), key initiators of psoriatic inflammation, express both VDR and vitamin D-metabolizing enzymes. Vitamin D signaling in these cells suppresses their activation, limiting T-cell stimulation and IFN-γ secretion [74]. In the adaptive immune system, vitamin D promotes immune balance by inhibiting Th1 cell proliferation and their associated cytokines IL-2 and IFN-γ, while simultaneously encouraging a Th2 cytokine profile, favoring IL-4 and IL-10 expression, which helps dampen chronic inflammation [75]. It also downregulates the Th17 axis, reducing skin infiltration and activity of Th17 cells that are responsible for producing IL-17, IL-22, and other psoriasis-related cytokines [76,77]. Furthermore, vitamin D suppresses pro-inflammatory mediators elevated in psoriatic lesions, including IL-1α, IL-1β, Tumor Necrosis Factor (TNF)-α, and the IL-12/23p40 subunit [78,79].
Beyond cytokine regulation, vitamin D modulates downstream inflammatory proteins central to psoriatic pathology. For instance, treatment with calcipotriol (a vitamin D analog) reduces the expression of Th17-induced molecules such as psoriasin (S100A7) and koebnerisin (S100A15), which act as chemoattractants and amplify skin inflammation [80]. In reconstructed psoriatic skin models, the analog 1α,25(OH)2D3–3-bromoacetate exhibited stronger antiproliferative activity than calcitriol, reversing IL-22–induced signaling pathways and downregulating AKT1, mTOR, IL-8, RANTES, and psoriasin [81].
Vitamin D also contributes to skin barrier integrity and keratinocyte homeostasis. Through VDR binding in keratinocytes, it promotes the expression of barrier proteins such as filaggrin, loricrin, and involucrin, components frequently deficient in psoriatic skin [70,82]. Hosomi et al. showed that vitamin D suppresses DNA synthesis in keratinocytes, promoting terminal differentiation and the formation of cornified envelopes, effectively counteracting keratinocyte hyperproliferation typical of psoriatic lesions [83].
Additionally, vitamin D influences TJ protein expression, with VDR activity being linked to the regulation of claudin, ZO-1, and occluding, molecules that are often downregulated in psoriatic epidermis [84]. It also modulates integrins and immune activation markers such as ICAM-1, CD26, and HLA-DR, contributing to normalization of skin structure and immune surveillance [85].
Emerging research has also explored VDR gene polymorphisms as genetic contributors to psoriasis susceptibility. Variants such as A-1012G, FokI, BsmI, ApaI, and TaqI have shown associations with disease risk in Italian and Chinese cohorts, while no significant associations were found in studies from Croatia and Egypt—suggesting that genetic effects may be population-specific [86,87,88,89,90]. Furthermore, these polymorphisms may predict treatment response: the TaqI variant has been linked to shorter remission following NB-UVB phototherapy, and wild-type alleles of A1012G, FokI, and TaqI have correlated with improved outcomes after calcipotriol treatment [91,92,93].
Taken together, these findings emphasize the multifactorial role of vitamin D in psoriasis, from immunoregulation and epidermal repair to potential pharmacogenetic implications, and support its use as both a therapeutic agent and biomarker in managing the disease.
2.4. Clinical and Epidemiological Evidence Linking Vitamin D to Psoriasis
A substantial body of clinical and epidemiological research has investigated the association between vitamin D status and psoriasis, with many studies reporting significantly reduced serum 25(OH)D levels in psoriatic patients compared to healthy controls [54,57,94,95,96]. For example, Chandrashekar et al. [55] and Maleki et al. [54] observed markedly lower serum vitamin D levels in individuals with psoriasis, with an inverse correlation to PASI scores, suggesting that more severe disease is often associated with more profound vitamin D deficiency [54,55]. Similar findings were confirmed by Bergler-Czop et al. [95] and Pokharel et al. [97], reinforcing the link between hypovitaminosis D and disease burden. However, Wilson et al. [98] reported contradictory data in a large population-based screening, finding no significant difference in serum vitamin D levels between patients with or without psoriasis. These discrepancies may be attributed to confounding variables such as sun exposure, dietary intake, seasonality, race, and baseline vitamin D status, underscoring the need for cautious interpretation.
Several clinical trials have assessed the therapeutic impact of vitamin D supplementation. Finamor et al. [56] demonstrated that oral administration of 35,000 IU/day of vitamin D3 for six months led to significant PASI improvement, alongside a robust rise in serum 25(OH)D levels [56]. Conversely, trials using high-dose monthly supplementation (e.g., 100,000 IU) failed to achieve consistent clinical benefits despite biochemical normalization, as seen in studies by Ingram and Jarrett [57,58]. Additionally, Prystowsky et al. [99] found no synergistic benefit when combining oral calcitriol with NB-UVB phototherapy [99], while Gumowski-Sunek et al. [100] reported disturbances in calcium metabolism with oral forms not observed in topical applications [100]. However, topical vitamin D analogs, such as calcipotriol, tacalcitol, and maxacalcitol, remain first-line options in mild to moderate psoriasis due to their favorable efficacy and safety profiles [101]. Calcipotriol has shown clinical superiority when combined with NB-UVB or corticosteroids and is capable of selectively reducing IL-6 expression in psoriatic lesions [102,103,104,105]. Tacalcitol and maxacalcitol also demonstrate effective PASI reduction without altering calcium homeostasis, although the latter carries a slightly higher hypercalcemia risk at systemic doses [106,107,108].
Notably, the combination of vitamin D analogs with corticosteroids has proven more effective than monotherapy, offering complementary mechanisms. In this regard, vitamin D restores epidermal differentiation, while corticosteroids mitigate inflammation and local irritation [109,110,111]. Furthermore, NB-UVB and UVA/UVB phototherapy are believed to exert part of their therapeutic efficacy via increased endogenous vitamin D synthesis [107,112].
Despite these promising findings, variability among studies remains, largely due to differences in dosage, duration, formulations, baseline vitamin D status, and patient heterogeneity. This limits definitive conclusions regarding the optimal use of systemic supplementation. Moreover, most clinical trials focus on established moderate-to-severe psoriasis, leaving open the question of whether vitamin D could play a preventive or disease-modifying role in early or subclinical stages of psoriasis (Table 2).
Taken together, the evidence supports vitamin D as a clinically relevant modulator of epidermal differentiation and inflammation in psoriasis, with potential application in therapeutic strategies pending more standardized supplementation trials.
3. Vitamin D and Cardiovascular Health
Psoriasis is not only a chronic inflammatory skin condition, but also a systemic disorder increasingly recognized for its association with cardiovascular comorbidities. Patients with psoriasis exhibit a significantly higher risk of developing cardiovascular disease (CVD), including myocardial infarction, stroke, and metabolic syndrome. This relationship is thought to be mediated by shared inflammatory pathways and altered vitamin D metabolism [113]. These findings underline the broader systemic implications of vitamin D dysregulation in psoriatic patients. Transitioning from its dermatologic roles, vitamin D is also a crucial modulator of cardiovascular health. The presence of VDRs in cardiomyocytes, endothelial and vascular smooth muscle cells (VSMCs), fibroblasts, and pericytes indicates its capacity to influence blood pressure regulation and attenuate inflammation within the cardiovascular system [114]. This intersection highlights the relevance of vitamin D not only in skin homeostasis, but also in maintaining vascular integrity and mitigating cardiovascular risk (Figure 1; Table 1).
Furthermore, considering that TJs are present in all epithelia, not only in the epidermis, and based on the observation that patients with psoriasis more frequently present with IBD, a study [115] was conducted on the serum of patients with psoriasis to assess increased epithelial permeability, particularly of the intestinal epithelium. The study revealed elevated levels of both zonulin and lipopolysaccharide (LPS). As is well known, LPS is a component of the outer membrane of Gram-negative bacteria and plays a key role in the pathogenesis of atherosclerosis [116].
3.1. Pathogenesis of Cardiovascular Disease and Molecular Actions of Vitamin D
CVDs are multifactorial conditions driven by endothelial dysfunction, vascular inflammation, oxidative stress, dysregulation of the RAAS, lipid abnormalities, and impaired glucose metabolism [117].
Vitamin D plays a crucial role in maintaining endothelial homeostasis, essential for vascular health and atherosclerosis prevention, by reducing oxidative stress, lowering reactive oxygen species (ROS), and enhancing nitric oxide (NO) bioavailability [118]. In vitro experiments using human umbilical vein endothelial cells (HUVECs) show that calcitriol downregulates leptin-induced inflammation by suppressing VCAM-1 and MCP-1 expression and inhibiting the NF-κB pathway, while concurrently promoting NO production [119,120,121]. In a controlled clinical trial, calcitriol and cholecalciferol were randomly assigned to patients undergoing elective PCI, demonstrating a lowering effect of calcitriol on hs-CRP serum levels [122]. Additionally, vitamin D enhances angiogenesis by activating endothelial colony-forming cells (ECFCs), which are key players in vascular regeneration [123].
Vitamin D also exerts antioxidant activity via two key transcriptional regulators. The first is nuclear factor erythroid 2–related factor 2 (Nrf2), which induces the transcription of antioxidant enzymes such as catalase (CAT), γ-glutamyltransferase (γ-GT), glucose-6-phosphate dehydrogenase (G6PD), glutathione peroxidases (Gpx), glutathione (GSH), thioredoxin reductase (TR), glutathione reductase (GR), superoxide dismutase 1/2 (SOD1/2), and thioredoxin (TRX) [124]. The second is Klotho, which also enhances antioxidant capacity by upregulating CAT, peroxiredoxins 2/3 (Prx-2/3), SOD2, and Trxrd-1, and contributes to calcium regulation [125,126].
Vitamin D modulates intracellular calcium signaling through proteins such as plasma membrane Ca2+-ATPase (PMCA), calbindin, Na+/Ca2+ exchanger (NCX1), parvalbumin, and transient receptor potential V members 5 and 6 (TRPV5/6), which influence vascular tone, cardiac contractility, and insulin secretion [127].
In VSMCs, 1,25(OH)2D3 suppresses the secretion of IL-6 and TNF-α, inhibits proliferation, prevents osteogenic transdifferentiation, and promotes prostacyclin synthesis [128,129]. VSMCs derived from VDR-knockout mice show increased expression of angiotensin II type 1 receptors, confirming vitamin D’s role in vascular tone regulation [130].
Hypertension is a well-established risk factor for CVD, and vitamin D deficiency has been strongly associated with its pathogenesis [131]. The RAAS is a hormone system that regulates blood pressure and fluid balance through renin, angiotensin II, and aldosterone [132]. Vitamin D inhibits renin expression, thereby downregulating the RAAS cascade and decreasing both angiotensin II and aldosterone levels. These effects ultimately lower blood pressure and reduce hypertensive damage [133]. VDR-null mice demonstrate elevated RAAS activity, increased sympathetic tone, and raised intraglomerular pressure, which together promote hypertension [134]. However, large RCTs (VitDISH, Styrian Trial) demonstrated no clinically significant effects of vitamin D supplementation [135,136]. Only the Iranian trial showed modest blood pressure reductions but its duration and sample limited applicability [137].
Vitamin D helps prevent atherogenesis by favorably modulating lipid profiles, reducing total cholesterol, LDL, and triglycerides, while increasing HDL levels [138]. It induces the expression of ATP-binding cassette transporter A1 (ABCA1), which promotes cholesterol efflux from macrophages in atherosclerotic plaques [139] and regulates lipid-metabolizing enzymes such as lipoprotein lipase and hepatic lipase, which are essential for maintaining optimal HDL and LDL levels [140]. Moreover, vitamin D deficiency contributes to LDL accumulation, oxidation, and foam cell formation, hallmarks of early atherogenesis [127]. Indeed, it has been reported that vitamin D supplementation might exert anti-inflammatory effects, by acting on IL-6 levels, VCAM and E-selectin [141]. In a meta-analysis including 3 RCTs no significant association was found between serum vitamin D levels and prevention or regression of atherogenesis, as measured by cIMT (carotid intima-media thickness) [142].
Beyond lipid metabolism, vitamin D influences glucose homeostasis and insulin responsiveness, both key to cardiovascular health. Insulin resistance (IR) contributes to endothelial dysfunction, inflammation, and accelerated atherogenesis [143]. Pancreatic β cells express both VDR and the 1α-hydroxylase enzyme (Cyp27b1), allowing local synthesis of active vitamin D [140,144]. Animal models show that vitamin D deficiency impairs insulin secretion, which is reversible upon supplementation [145,146]. These effects are mediated through VDR-dependent gene induction [147,148] and calcium-driven insulin exocytosis involving PKA signaling and endoplasmic reticulum calcium mobilization [149,150]. Vitamin D may induce improvements in insulin resistance, as measured by glycemic markers (HbA1c, fasting glucose, HOMA-IR), though effect sizes are small and incidence of diabetes remained unaffected [151,152].
Vitamin D also protects cardiomyocytes from hyperglycemia-induced stress and angiotensin II–mediated hypertrophy [114], promotes cardiomyoblast survival and proliferation [153], and modulates immune responses by decreasing pro-inflammatory cytokines (TNF-α, IL-1β, IL-6) while enhancing IL-10 production and M2 macrophage polarization [154]. Animal models with targeted deletion of VDR or 1α-hydroxylase genes demonstrate increased RAAS activity, myocardial hypertrophy, fibrosis, apoptosis, and systolic dysfunction, emphasizing the essential role of vitamin D signaling in cardiac structure and function [127]. The effect of vitamin D supplementation on left ventricular mass has been explored in CKD, hypertensive or general older adults, showing no significant regression, as showed by using cardiac magnetic resonance (36008108), except in HF populations [155,156].
Collectively, these findings suggest that vitamin D influences multiple pathogenic pathways in cardiovascular disease, acting on endothelial function, oxidative stress, lipid and glucose metabolism, and vascular inflammation, while clinical data highlight both its therapeutic promise and the need for more definitive intervention trials.
3.2. Clinical Implications of Vitamin D Deficiency in Cardiovascular Disease: Conditions and Evidence
Vitamin D deficiency has been consistently associated with several clinical conditions that predispose to CVD, including IR, metabolic syndrome (MetS), acute coronary syndromes (ACS), and heart failure (HF) [11,61]. These disorders share key pathogenic mechanisms such as chronic inflammation, oxidative stress, endothelial dysfunction, and calcium metabolism dysregulation [11,61].
Observational studies report an inverse association between serum vitamin D levels and the Homeostatic Model Assessment of Insulin Resistance (HOMA-IR), a validated marker of IR based on fasting glucose and insulin levels [157]. Vitamin D also plays a critical role in β-cell function and skeletal muscle glucose uptake, influencing whole-body energy homeostasis and insulin sensitivity [158,159,160]. Considering that skeletal muscle accounts for up to 90% of postprandial glucose disposal, this relationship has clinical relevance.
Vitamin D modulates adipose tissue metabolism, downregulates pro-inflammatory cytokines, and exerts protective anti-inflammatory effects on pancreatic β-cells. In obesity, a common condition in IR and T2DM, vitamin D sequestration in adipose tissue reduces its bioavailability, contributing to a cycle of metabolic dysfunction [11]. A growing body of evidence links low 25(OH)D levels to MetS, characterized by central obesity, IR, hypertension, and dyslipidemia. Observational studies suggest that every 10 ng/mL increase in vitamin D levels may reduce the risk of MetS by 15–20% [161,162]. Indeed, in a meta-analyses involving >80,000 individuals suggest that benefits may be confined to subgroups such as the elderly, individuals with MetS, or those with severe baseline deficiency [163,164].
The cross-sectional InCHIANTI study revealed an inverse association between both 25(OH)D and 1,25(OH)2D and cardiovascular risk in 299 elderly individuals. Notably, over 60% of participants had serum levels <20 ng/mL [12]. Both serum levels of 25(OH)D and 1,25(OH)2D were negatively correlated with cardiovascular risk, as measured by the SCORE2/SCORE2-OP algorithm, a 10-year CV risk estimator endorsed by the European Society of Cardiology [12].
Interventional studies suggest that vitamin D supplementation improves surrogate markers of glucose metabolism. Significant improvements in HOMA-IR and HbA1c have been reported, particularly among insulin-resistant or obese individuals [165,166]. Randomized trials and meta-analyses also show beneficial effects on insulin sensitivity, β-cell function, and fasting glucose levels in high-risk groups [167,168,169,170,171,172]. Additionally, favorable outcomes on HDL-C and C-reactive protein (CRP) have been noted [170].
However, findings remain inconsistent. Several trials failed to show significant changes in anthropometric or lipid parameters, particularly in individuals without baseline vitamin D deficiency [173,174,175].
Vitamin D supplementation has also been linked to reductions in systolic blood pressure, possibly mediated by suppression of the RAAS and secondary hyperparathyroidism [11]. These mechanisms may offer protection against hypertension and vascular stiffness.
In individuals with type 2 diabetes mellitus T2DM, a condition marked by hyperglycemia and impaired insulin secretion, vitamin D deficiency is associated with increased risk of both microvascular and macrovascular complications [176]. Epidemiological studies link low vitamin D to diabetic retinopathy, nephropathy, neuropathy, and foot ulcers [177,178,179]. Mechanistically, vitamin D modulates nociceptor activity in neuropathy [90], regulates angiogenesis and inflammation in retinopathy [179], and reduces proteinuria in diabetic nephropathy [11].
In the macrovascular context, vitamin D deficiency is associated with endothelial dysfunction, arterial stiffness, peripheral artery disease (PAD), and carotid plaque formation [180,181,182]. The Framingham Offspring Study showed increased risk of myocardial infarction in individuals with serum 25(OH)D levels <15 ng/mL [183].
In HF patients, low vitamin D is linked to elevated BNP, cardiac remodeling, and reduced ejection fraction. Animal studies suggest a causal relationship with myocardial hypertrophy and fibrosis, although clinical trials such as VITAL-HF failed to demonstrate a reduction in hard endpoints like hospitalization or mortality [163,184]. However, a meta-analysis, considering seven RCTs (465 patients undergoing to vitamin D supplementation or placebo over 12 weeks to 9 months), reported a significant reduction in LV end-diastolic diameter (−2.31 mm) and an improvement of LVEF (+4.18%) [185].
Genetic predisposition may influence individual responses to vitamin D and explain heterogeneity across studies. A meta-analysis involving 9232 individuals revealed associations between specific VDR polymorphisms (ApaI, BsmI, TaqI) and IR-related disorders, with ethnic differences in susceptibility. For instance, the ApaI G allele was linked to MetS and PCOS in Asian and mid-latitude populations, whereas FokI showed no significant associations [163].
In HF, VDR variants may affect myocardial fibrosis and remodeling, further complicating the interpretation of vitamin D trial results [186,187]. Additionally, vitamin D deficiency promotes secondary hyperparathyroidism, leading to intracellular calcium accumulation, systemic inflammation, and worsening IR [188,189].
Despite robust observational evidence linking low vitamin D to a wide range of cardiovascular and metabolic outcomes [127], RCTs and meta-analyses have yielded mixed results.
Large primary prevention RCTs such as ViDA [190], VITAL [60], and D-Health [191] have generally reported null effects of vitamin D supplementation. A major concern is the lack of stratification by baseline status; in VITAL’s ancillary fracture analysis (LeBoff et al.), 87 percent entered with 25(OH)D above the 50 nmol/L (>20 ng/mL) threshold, limiting the chance to detect benefit [192]. While RCTs evaluating the effects of vitamin D supplementation on cardiovascular risk do offer valuable causal insights, they are often limited by relatively small sample sizes, short follow-up duration, narrow inclusion/exclusion criteria, and limited generalizability.
Another fundamental weakness of these trials relates to heterogeneity of the populations, comorbidities, and endpoints [114]. Age, gender, and physical activity influence absorption, metabolism, and responsiveness, while diabetes, obesity, hypertension, and frailty further modify outcomes. Interactions with diet, medications, and BMI complicate interpretation by altering bioavailability. Older adults, women, and deficient individuals may see greater benefit, yet inconsistent trial designs and diverse endpoints contribute to conflicting evidence, highlighting the need for personalized approaches. These and other limitations of trials to evaluate vitamin D benefit have been discussed in detail elsewhere [13,114,193,194].
Nonlinear Mendelian randomization (NMR) studies complement RCTs by evaluating long-term vitamin D exposure, deficiency, and dose–response effects on cardiovascular outcomes in real-world settings. Recent evidence from a large NMR study by Sutherland and colleagues shows mortality risk rises steeply below 50 nmol/L with a plateau at higher levels [195], implying little benefit in replete cohorts and helping explain null trial results. Supporting this hypothesis, Patriota et al. reported a significant inverse association between 25(OH)D and cardiovascular events over 14.4 years, especially in continuous analyses, with the largest effects observed at concentrations above 50 nmol/L [196].
Taken together, null RCTs likely reflect enrollment of vitamin D replete populations and design limits rather than a lack of effect. NMR and cohorts indicate benefit mainly below the threshold of 50 nmol/L. Future trials should stratify by baseline status and enrich for deficiency. These insights are summarized in Table 2. The findings also support the interpretative model proposed by Tripepi et al. [13], who argue that intervention is unlikely to yield benefit above the threshold of 20 ng/mL. Their analysis, based on NMR, identified a sharp rise in mortality below this level, thus reinforcing 20 ng/mL as a critical biological and prognostic cut-off [13]. These discrepancies highlight the need for stratified clinical trials and personalized intervention strategies.
Overall, current evidence links vitamin D deficiency to insulin resistance, metabolic syndrome, acute coronary syndromes, and heart failure, with observational data consistently supporting increased risk, while results from randomized trials highlight the need for stratified designs that target deficient populations in order to clarify true cardiovascular benefit.
4. Vitamin D and Intestinal Bowel Disease
Chronic inflammation and compromised epithelial barriers are key features in IBD, particularly CD and UC, and in celiac disease (CeD) [197]. These conditions involve disruption of TJs between enterocytes, leading to increased intestinal permeability [198]. This “leaky gut” permits the translocation of microbial products and dietary antigens into the lamina propria, fueling mucosal immune activation and sustaining a vicious cycle of inflammation and barrier breakdown [199]. In IBD, barrier dysfunction often precedes relapse and correlates with disease severity [200]; in CeD, adaptive zonulin release triggered by gluten peptides (via the CXCR3/MyD88 pathway) also disrupts TJ integrity, facilitating antigen crossing and autoimmune activation [201] (Figure 1; Table 1).
4.1. Modulation of Gut Barrier Function by Vitamin D: Epithelial, Immune, and Microbial Interactions
The intestinal barrier, comprising microbiota, mucus, epithelium, and immune cells, maintains gut and systemic homeostasis [202]. Its disruption can trigger multifactorial diseases [203]. Microbial-immune crosstalk is mediated by specialized gut structures [204,205], while the microbiota supports pathogen defense and nutrient metabolism [206,207]. The mucus layer and epithelial cells ensure selective permeability and immune protection [208,209], with immune cells in the lamina propria orchestrating surveillance and signaling [210].
Vitamin D contributes to intestinal barrier integrity through its actions on epithelial cells, immune responses, and host–microbiota interactions [211,212]. VDR signaling plays a central role in maintaining TJ integrity, as shown in models of IBD and CeD [213,214,215,216]. In VDR-deficient mice, claudin-1 and -3 expression is reduced, junctions appear disorganized, and permeability increases. VDR agonists can restore TJ protein levels and epithelial structure after inflammation [217]. Calcitriol also prevents gliadin-induced barrier disruption in CeD by inhibiting zonulin-mediated TJ disassembly [218], while cholecalciferol restores villus morphology and ZO-1 organization in gluten-sensitive mice [8].
Beyond structural effects, vitamin D also prevents epithelial cell death, strengthens adhesion, and improves resilience to microbial and toxic challenges [219]. It also modulates mucosal immunity by promoting regulatory T cell differentiation and suppressing Th1/Th17 responses [220,221].
In addition, vitamin D indirectly regulates the gut microbiota via epithelial and immune cell VDR signaling [212]. VDR deficiency leads to dysbiosis, impaired Paneth cell function, defective antimicrobial peptide secretion (e.g., lysozyme, defensin-4), and increased inflammation in mouse models [222,223,224]. On the other hand, supplementation with 1,25(OH)2D partially reverses dysbiosis [225], and higher vitamin D levels correlate with increased cathelicidin and reduced inflammation in UC patients [226].
Vitamin D also promotes epithelial regeneration. VDR-deficient mice show impaired wound healing and crypt repair in colitis models. Regenerative effects have been observed in CeD mouse models treated with high-dose vitamin D, preserving villus/crypt architecture and reducing lymphocyte infiltration [8].
In particular, oral cholecalciferol at increasing doses (5–130 µg/kg) was administered to gluten-sensitive Non-Obese Diabetic (NOD/ShiLtJ) mice fed a gluten-containing diet and receiving gliadin to induce enteropathy [8]. Compared to untreated CeD controls (gliadin + vehicle), vitamin D significantly reduced mucosal lesion severity and increased villus length in a dose-dependent manner. Only high-dose groups (50–130 µg/kg) showed near-complete restoration of villus/crypt architecture. Immunohistochemistry confirmed reduced CD3+ lymphocyte infiltration and ZO-1 expression, indicating improved epithelial integrity and barrier function [8].
Collectively, these findings support a multifaceted role of vitamin D in maintaining gut barrier function. Through enhancement of epithelial cohesion, modulation of immunity, support of microbial homeostasis, and promotion of regeneration, vitamin D/VDR signaling helps counteract the cycle of barrier dysfunction and chronic inflammation characteristic of IBD and CeD [212].
4.2. Vitamin D and Inflammatory Bowel Disease and Celiac Disease: Evidence from Clinical Trials and Meta-Analyses
Patients with IBD, have a 64% greater likelihood of vitamin D deficiency [227], with prevalence reaching 38.1% in CD and 31.6% in UC [228]. Deficiency is linked to increased disease activity, poor quality of life, and risk of relapse [62].
In particular, vitamin D deficiency is frequently observed in CeD, with pediatric prevalence ranging from 9 to 52% [229]. A meta-analysis of 24 studies (1137 CeD patients, 2613 controls) found mean serum 25(OH)D levels were 3.34 ng/mL lower in CeD patients, supporting impaired absorption due to intestinal inflammation [230]. While no human trials have tested vitamin D’s direct effect on CeD pathology, animal models show that high-dose cholecalciferol ameliorates villus atrophy, reduces CD3+ infiltration, and restores ZO-1 expression [8].
Vitamin D supplementation has demonstrated immunomodulatory benefits in both pediatric and adult IBD populations. Systematic reviews report reductions in CRP and ESR, and early signs of clinical improvement, despite suboptimal target attainment [66]. RCTs in adults confirm reduced disease activity, fecal calprotectin, and CRP at weekly doses of 2000–50,000 IU, with no major adverse events [229,231,232]. Meta-analyses show decreased relapse rates (RR 0.64), particularly in CD patients in remission (RR 0.47) [233], although effects on ESR and symptom scores remain inconsistent [234].
Microbiome studies further support vitamin D’s role in gut homeostasis. Supplementation increases microbial richness and beneficial taxa [235,236], although results vary by dose and context [237,238]. Certain microbes modulate VDR expression and vitamin D metabolism (e.g., via FGF-23, CYP27B1), and produce bioactive metabolites such as lithocholic acid, enhancing absorption [211,239]. Lactobacillus reuteri supplementation raised 25(OH)D3 levels in humans [239], while butyrate and Bifidobacteria have been positively linked to vitamin D levels [238].
Together, these findings highlight the emerging role of vitamin D as a modifiable factor in CeD and IBD management. Beyond correcting deficiency, repletion of vitamin D alongside a gluten free diet or standard therapies contributes to mucosal integrity, reduced inflammation, improvement in quality of life, and potentially enhancing pharmacological response (Table 2). The routine screening and targeted supplementation of specific patients are warranted.
5. Vitamin D and Epithelial Integrity in Chronic Respiratory Disease
Vitamin D also exerts important effects on epithelial barrier integrity and immune regulation in the respiratory system, which were not the main focus of the present review and have been discussed in detail elsewhere [240,241]. Deletion of the VDR has been shown to disrupt TJs in the lungs, leading to impaired barrier function and increased susceptibility to inflammatory damage, thereby implicating VDR signaling as a key determinant of pulmonary epithelial integrity in conditions such as pneumonia, asthma, and chronic obstructive pulmonary disease (COPD) [242].
In some specific viral infections, including COVID-19, vitamin D deficiency has been associated with dysregulated immune responses and heightened disease severity [9,240,241,243]. Mechanistic insights highlight the capacity of vitamin D to modulate innate and adaptive immunity, attenuate cytokine storm pathways, and enhance epithelial resilience [243,244]. The use of vitamin D supplementation, particularly at high doses has shown important benefit in patients with COVID-19 [9,245,246,247,248].
In addition, there is tentative evidence linking vitamin D to cancer [249,250], where it may exert antiproliferative and immunomodulatory effects [251]. For example, VDR expression in melanocytes and melanoma cells suggests a direct influence on tumor growth and immune evasion, and there is evidence from some epidemiological studies linking vitamin D deficiency to worse cancer prognosis [14,252,253].
These findings extend the role of vitamin D beyond the skin, gut, and cardiovascular system, supporting its systemic relevance in chronic inflammatory diseases characterized by barrier dysfunction.
Collectively, these data emphasize that maintaining sufficient vitamin D levels is critical for epithelial barrier protection and immune homeostasis across diverse organ systems and chronic diseases.
6. Safety of Vitamin D Supplementation
In general, there is still a lack of consensus on the recommended vitamin D supplementation regimen (doses, administration schedule, duration of treatment) [254]. This heterogeneity can be explained in part by the lack of pharmacokinetic studies evaluating different dosing schedules [255,256,257]. Safer strategies include moderate daily supplementation (800–1000 IU cholecalciferol or 10 µg calcifediol) [5,258,259,260,261,262,263].
Daily dosing is generally safer than intermittent high/megadoses, which have been linked to increased falls and fractures [264,265]. The National Academy of Medicine sets the upper tolerable intake at 4000 IU/day, although some evidence suggests risk may occur below this.
Excessive vitamin D can worsen granulomatous conditions such as sarcoidosis, where macrophage-driven calcitriol leads to hypercalcemia [266]. In addition, high-doses of vitamin D have also been associated with increased risk of hypercalciuria and kidney stones, particularly when combined with calcium [267].
In heart failure, supplementation with 4000 IU/day for 3 years did not reduce mortality [268]. Gastrointestinal implications include the use of calcifediol, which has superior absorption in liver disease and gastrointestinal malabsorption [269]. While doses up to 10,000 IU/day have been tested without acute toxicity, caution is warranted. Large boluses (≥300,000 IU intramuscularly) are discouraged due to adverse outcomes; safer intermittent boluses should not exceed 100,000 IU [260,270].
7. Conclusions
Vitamin D plays an essential role in maintaining the structural and immunological integrity of epithelial barriers, regulating innate and adaptive immune responses, and contributing to cardiovascular and gastrointestinal health. Its actions extend far beyond traditional skeletal functions, influencing pathophysiological processes in atopic dermatitis, psoriasis, metabolic syndrome, inflammatory bowel disease, and CeD. While observational and mechanistic studies provide strong support for these effects, results from supplementation trials remain variable, often depending on baseline deficiency, genetic factors, and disease state.
The cumulative evidence suggests that maintaining adequate vitamin D levels is a promising adjunctive strategy for managing chronic inflammatory conditions. However, more well-designed, stratified clinical trials are needed to clarify optimal dosing, target populations, and biomarkers of response. Future research should focus on integrating vitamin D status into personalized care approaches and elucidating its crosstalk with microbiota, epithelial regeneration pathways, and immune checkpoints in disease modulation.
8. Literature Search
In the preparation of this review, we performed a comprehensive literature search to identify relevant studies on the non-skeletal role of vitamin D. PubMed/Medline (until June 2025) was searched using the following keywords: (“vitamin D” OR cholecalciferol OR ergocalciferol OR “25-hydroxyvitamin D” OR 25OHD) AND (psoriasis OR “atopic dermatitis” or eczema OR IBD OR Crohn’s OR Celiac OR “ulcerative colitis” OR cardiovascular OR CVD OR stroke OR “acute myocardial infarction” OR diabetes or “metabolic syndrome”). We repeated this search using different Boolean variations and also with additional keywords focused on “non-skeletal” or extra-skeletal”. We also performed an additional search to add evidence on the role of vitamin D on TJ in respiratory diseases such as asthma, COVID-19 and chronic obstructive pulmonary disease, or COPD. We have included original research articles, clinical trials, meta-analyses, and high-quality reviews published in the previous 15 years. Studies that were not published in English language in addition to hand-selected case studies, abstracts, letters, and reviews were excluded. Articles not related or not relevant to the non-skeletal role of vitamin D or the topic discussed were also omitted.
Author Contributions
Conceptualization: E.M., E.T., F.N., G.P. (Giovanni Paolino), M.A., S.B. (Santina Battaglia), S.C., S.T. and T.G.; writing—original draft preparation: C.C. (Camilla Chello), C.G.E., E.M., G.P. (Giovanni Paolino), M.M., C.C. (Camilla Calvieri) and S.C.; writing—review and editing: all authors. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Abiogen Pharma S.p.A, Pisa, Italy.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
F.N., S.B. (Santina Battaglia), M.A., and E.T. are employed by Abiogen Pharma S.p.A. S.T and S.B. (Silvano Bonaretti) are employed by Galileo Research Srl. The funders had no role in the decision to publish, or in the preparation of the manuscript. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Holick, M.F. Vitamin D Deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef]
- Bouillon, R.; Marcocci, C.; Carmeliet, G.; Bikle, D.; White, J.H.; Dawson-Hughes, B.; Lips, P.; Munns, C.F.; Lazaretti-Castro, M.; Giustina, A.; et al. Skeletal and Extraskeletal Actions of Vitamin D: Current Evidence and Outstanding Questions. Endocr. Rev. 2019, 40, 1109–1151. [Google Scholar] [CrossRef]
- Carlberg, C.; Haq, A. The Concept of the Personal Vitamin D Response Index. J. Steroid Biochem. Mol. Biol. 2018, 175, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Pike, J.W.; Christakos, S. Biology and Mechanisms of Action of the Vitamin D Hormone. Endocrinol. Metab. Clin. N. Am. 2017, 46, 815–843. [Google Scholar] [CrossRef]
- Pludowski, P.; Holick, M.F.; Grant, W.B.; Konstantynowicz, J.; Mascarenhas, M.R.; Haq, A.; Povoroznyuk, V.; Balatska, N.; Barbosa, A.P.; Karonova, T.; et al. Vitamin D Supplementation Guidelines. J. Steroid Biochem. Mol. Biol. 2018, 175, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Scragg, R. Emerging Evidence of Thresholds for Beneficial Effects from Vitamin D Supplementation. Nutrients 2018, 10, 561. [Google Scholar] [CrossRef]
- Grieco, T.; Paolino, G.; Moliterni, E.; Chello, C.; Sernicola, A.; Egan, C.G.; Morelli, M.; Nannipieri, F.; Battaglia, S.; Accoto, M.; et al. Differential Expression of Proteins Involved in Skin Barrier Maintenance and Vitamin D Metabolism in Atopic Dermatitis: A Cross-Sectional, Exploratory Study. Int. J. Mol. Sci. 2024, 26, 211. [Google Scholar] [CrossRef]
- Trasciatti, S.; Piras, F.; Bonaretti, S.; Marini, S.; Nencioni, S.; Biasci, E.; Egan, C.G.; Nannipieri, F. Effect of Oral Cholecalciferol in a Murine Model of Celiac Disease: A Dose Ranging Study. J. Steroid Biochem. Mol. Biol. 2022, 220, 106083. [Google Scholar] [CrossRef] [PubMed]
- Giannini, S.; Giusti, A.; Minisola, S.; Napoli, N.; Passeri, G.; Rossini, M.; Sinigaglia, L. The Immunologic Profile of Vitamin D and Its Role in Different Immune-Mediated Diseases: An Expert Opinion. Nutrients 2022, 14, 473. [Google Scholar] [CrossRef]
- Vergatti, A.; Abate, V.; Iannuzzo, G.; Barbato, A.; De Filippo, G.; Rendina, D. The Bone-Heart Axis in the Pathogenesis of Cardiovascular Diseases: A Narrative Review. Nutr. Metab. Cardiovasc. Dis. 2025, 35, 103872. [Google Scholar] [CrossRef]
- Argano, C.; Mirarchi, L.; Amodeo, S.; Orlando, V.; Torres, A.; Corrao, S. The Role of Vitamin D and Its Molecular Bases in Insulin Resistance, Diabetes, Metabolic Syndrome, and Cardiovascular Disease: State of the Art. Int. J. Mol. Sci. 2023, 24, 15485. [Google Scholar] [CrossRef]
- Brandi, M.L.; Marini, F.; Parri, S.; Bandinelli, S.; Iantomasi, T.; Giusti, F.; Talluri, E.; Sini, G.; Nannipieri, F.; Battaglia, S.; et al. Association of Vitamin D and Bisphenol A Levels with Cardiovascular Risk in an Elderly Italian Population: Results from the InCHIANTI Study. GeroScience 2024, 46, 6141–6156. [Google Scholar] [CrossRef]
- Tripepi, G.; Fusaro, M.; Arcidiacono, G.; Sella, S.; Giannini, S. Evaluating Benefit from Vitamin D Supplementation: Defining the Area for Treatment. Osteoporos. Int. 2023, 34, 1531–1533. [Google Scholar] [CrossRef]
- Paolino, G.; Moliterni, E.; Didona, D.; Garelli, V.; Corsetti, P.; Lopez, T.; Richetta, A.G.; Cantisani, C.; Bottoni, U.; Calvieri, S. Clinicopathological Features, Vitamin D Serological Levels and Prognosis in Cutaneous Melanoma of Shield-Sites: An Update. Med. Oncol. 2015, 32, 451. [Google Scholar] [CrossRef]
- Paolino, G.; Panetta, C.; Cota, C.; Didona, D.; Moliterni, E.; Di Mattia, C.; De Vita, G.; Bottoni, U.; Donati, P.; Calvieri, S. Vitamin D Receptor Immunohistochemistry Variability in Sun-Exposed and Non-Sun-Exposed Melanomas. Melanoma Res. 2017, 27, 17–23. [Google Scholar] [CrossRef]
- Paolino, G.; Moliterni, E.; Corsetti, P.; Didona, D.; Bottoni, U.; Calvieri, S.; Mattozzi, C. Vitamin D and Melanoma: State of the Art and Possible Therapeutic Uses. G. Ital. Dermatol. E Venereol. Organo Uff. Soc. Ital. Dermatol. E Sifilogr. 2019, 154, 64–71. [Google Scholar] [CrossRef]
- Bikle, D.D. Vitamin D Metabolism, Mechanism of Action, and Clinical Applications. Chem. Biol. 2014, 21, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.D. Vitamin D and the Skin: Physiology and Pathophysiology. Rev. Endocr. Metab. Disord. 2012, 13, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Kirschner, N.; Houdek, P.; Fromm, M.; Moll, I.; Brandner, J.M. Tight Junctions Form a Barrier in Human Epidermis. Eur. J. Cell Biol. 2010, 89, 839–842. [Google Scholar] [CrossRef]
- Trujillo-Paez, J.V.; Peng, G.; Le Thanh Nguyen, H.; Nakamura, M.; Umehara, Y.; Yue, H.; Ikutama, R.; Takahashi, M.; Ikeda, S.; Ogawa, H.; et al. Calcitriol Modulates Epidermal Tight Junction Barrier Function in Human Keratinocytes. J. Dermatol. Sci. 2024, 114, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Segaert, S. Vitamin D Regulation of Cathelicidin in the Skin: Toward a Renaissance of Vitamin D in Dermatology? J. Investig. Dermatol. 2008, 128, 773–775. [Google Scholar] [CrossRef] [PubMed]
- Torres, T.; Ferreira, E.O.; Gonçalo, M.; Mendes-Bastos, P.; Selores, M.; Filipe, P. Update on Atopic Dermatitis. Acta Médica Port. 2019, 32, 606–613. [Google Scholar] [CrossRef]
- Umar, M.; Sastry, K.S.; Al Ali, F.; Al-Khulaifi, M.; Wang, E.; Chouchane, A.I. Vitamin D and the Pathophysiology of Inflammatory Skin Diseases. Skin Pharmacol. Physiol. 2018, 31, 74–86. [Google Scholar] [CrossRef]
- Levin, J.; Friedlander, S.F.; Del Rosso, J.Q. Atopic Dermatitis and the Stratum Corneum: Part 1: The Role of Filaggrin in the Stratum Corneum Barrier and Atopic Skin. J. Clin. Aesthetic Dermatol. 2013, 6, 16–22. [Google Scholar]
- Palmer, C.N.A.; Irvine, A.D.; Terron-Kwiatkowski, A.; Zhao, Y.; Liao, H.; Lee, S.P.; Goudie, D.R.; Sandilands, A.; Campbell, L.E.; Smith, F.J.D.; et al. Common Loss-of-Function Variants of the Epidermal Barrier Protein Filaggrin Are a Major Predisposing Factor for Atopic Dermatitis. Nat. Genet. 2006, 38, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Le Lamer, M.; Pellerin, L.; Reynier, M.; Cau, L.; Pendaries, V.; Leprince, C.; Méchin, M.-C.; Serre, G.; Paul, C.; Simon, M. Defects of Corneocyte Structural Proteins and Epidermal Barrier in Atopic Dermatitis. Biol. Chem. 2015, 396, 1163–1179. [Google Scholar] [CrossRef] [PubMed]
- Brunner, P.M.; Guttman-Yassky, E.; Leung, D.Y.M. The Immunology of Atopic Dermatitis and Its Reversibility with Broad-Spectrum and Targeted Therapies. J. Allergy Clin. Immunol. 2017, 139, S65–S76. [Google Scholar] [CrossRef]
- Borzutzky, A.; Camargo, C.A., Jr. Role of Vitamin D in the Pathogenesis and Treatment of Atopic Dermatitis. Expert Rev. Clin. Immunol. 2013, 9, 751–760. [Google Scholar] [CrossRef]
- Athanassiou, L.; Mavragani, C.P.; Koutsilieris, M. The Immunomodulatory Properties of Vitamin D. Mediterr. J. Rheumatol. 2022, 33, 7. [Google Scholar] [CrossRef]
- Lucas, R.; Mihály, J.; Gericke, J.; Törőcsik, D.; Rühl, R. Vitamin D Signaling in a Mouse Allergic Sensitization Model. Int. J. Vitam. Nutr. Res. 2020, 90, 385–388. [Google Scholar] [CrossRef]
- Kongsbak, M.; Levring, T.; Geisler, C.; von Essen, M. The Vitamin D Receptor and T Cell Function. Front. Immunol. 2013, 4, 148. [Google Scholar] [CrossRef]
- White, J.H. Vitamin D Signaling, Infectious Diseases, and Regulation of Innate Immunity. Infect. Immun. 2008, 76, 3837–3843. [Google Scholar] [CrossRef]
- Hewison, M. Vitamin D and the Immune System: New Perspectives on an Old Theme. Endocrinol. Metab. Clin. N. Am. 2010, 39, 365–379. [Google Scholar] [CrossRef]
- Schauber, J.; Dorschner, R.A.; Yamasaki, K.; Brouha, B.; Gallo, R.L. Control of the Innate Epithelial Antimicrobial Response Is Cell-Type Specific and Dependent on Relevant Microenvironmental Stimuli. Immunology 2006, 118, 509–519. [Google Scholar] [CrossRef]
- Yamanaka, K.; Dimitroff, C.J.; Fuhlbrigge, R.C.; Kakeda, M.; Kurokawa, I.; Mizutani, H.; Kupper, T.S. Vitamins A and D Are Potent Inhibitors of Cutaneous Lymphocyte-Associated Antigen Expression. J. Allergy Clin. Immunol. 2008, 121, 148–157.e3. [Google Scholar] [CrossRef]
- Peroni, D.G.; Piacentini, G.L.; Cametti, E.; Chinellato, I.; Boner, A.L. Correlation between Serum 25-Hydroxyvitamin D Levels and Severity of Atopic Dermatitis in Children. Br. J. Dermatol. 2011, 164, 1078–1082. [Google Scholar] [CrossRef]
- McCarthy, R.L.; Tawfik, S.S.; Theocharopoulos, I.; Atkar, R.; McDonald, B.; Dhoat, S.; Hughes, A.; Thomas, B.R.; O’Toole, E.A. Vitamin D Deficiency and Atopic Dermatitis Severity in a Bangladeshi Population Living in East London: A Cross-Sectional Study. Skin Health Dis. 2024, 4, e358. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Seok, J.K.; Kang, H.C.; Cho, Y.-Y.; Lee, H.S.; Lee, J.Y. Skin Barrier Abnormalities and Immune Dysfunction in Atopic Dermatitis. Int. J. Mol. Sci. 2020, 21, 2867. [Google Scholar] [CrossRef] [PubMed]
- El Taieb, M.A.; Fayed, H.M.; Aly, S.S.; Ibrahim, A.K. Assessment of Serum 25-Hydroxyvitamin d Levels in Children with Atopic Dermatitis: Correlation with SCORAD Index. Dermatitis 2013, 24, 296–301. [Google Scholar] [CrossRef]
- Kim, G.; Bae, J.-H. Vitamin D and Atopic Dermatitis: A Systematic Review and Meta-Analysis. Nutrition 2016, 32, 913–920. [Google Scholar] [CrossRef] [PubMed]
- Hattangdi-Haridas, S.R.; Lanham-New, S.A.; Wong, W.H.S.; Ho, M.H.K.; Darling, A.L. Vitamin D Deficiency and Effects of Vitamin D Supplementation on Disease Severity in Patients with Atopic Dermatitis: A Systematic Review and Meta-Analysis in Adults and Children. Nutrients 2019, 11, 1854. [Google Scholar] [CrossRef]
- Nielsen, A.Y.; Høj, S.; Thomsen, S.F.; Meteran, H. Vitamin D Supplementation for Treating Atopic Dermatitis in Children and Adults: A Systematic Review and Meta-Analysis. Nutrients 2024, 16, 4128. [Google Scholar] [CrossRef]
- Cheon, B.R.; Shin, J.E.; Kim, Y.J.; Shim, J.W.; Kim, D.S.; Jung, H.L.; Park, M.S.; Shim, J.Y. Relationship between Serum 25-Hydroxyvitamin D and Interleukin-31 Levels, and the Severity of Atopic Dermatitis in Children. Korean J. Pediatr. 2015, 58, 96–101. [Google Scholar] [CrossRef]
- Munawwarah, L.; Evalina, R.; Sofyani, S. Serum 25-Hydroxyvitamin-D Level and Atopic Dermatitis Severity in Children. Paediatr. Indones. 2017, 57, 234–238. [Google Scholar] [CrossRef][Green Version]
- Miyake, Y.; Sasaki, S.; Tanaka, K.; Hirota, Y. Dairy Food, Calcium and Vitamin D Intake in Pregnancy, and Wheeze and Eczema in Infants. Eur. Respir. J. 2010, 35, 1228–1234. [Google Scholar] [CrossRef]
- Jones, A.P.; Palmer, D.; Zhang, G.; Prescott, S.L. Cord Blood 25-Hydroxyvitamin D3 and Allergic Disease during Infancy. Pediatrics 2012, 130, e1128–e1135. [Google Scholar] [CrossRef] [PubMed]
- Bäck, O.; Blomquist, H.K.S.; Hernell, O.; Stenberg, B. Does Vitamin D Intake during Infancy Promote the Development of Atopic Allergy? Acta Derm. Venereol. 2009, 89, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Thuesen, B.H.; Heede, N.G.; Tang, L.; Skaaby, T.; Thyssen, J.P.; Friedrich, N.; Linneberg, A. No Association between Vitamin D and Atopy, Asthma, Lung Function or Atopic Dermatitis: A Prospective Study in Adults. Allergy 2015, 70, 1501–1504. [Google Scholar] [CrossRef] [PubMed]
- Heine, G.; Hoefer, N.; Franke, A.; Nöthling, U.; Schumann, R.R.; Hamann, L.; Worm, M. Association of Vitamin D Receptor Gene Polymorphisms with Severe Atopic Dermatitis in Adults. Br. J. Dermatol. 2013, 168, 855–858. [Google Scholar] [CrossRef]
- Kilic, M.; Ecin, S.; Taskin, E.; Sen, A.; Kara, M. The Vitamin D Receptor Gene Polymorphisms in Asthmatic Children: A Case-Control Study. Pediatr. Allergy Immunol. Pulmonol. 2019, 32, 63–69. [Google Scholar] [CrossRef]
- Grieco, T.; Moliterni, E.; Paolino, G.; Chello, C.; Sernicola, A.; Egan, C.G.; Nannipieri, F.; Battaglia, S.; Accoto, M.; Tirotta, E.; et al. Association between Vitamin D Receptor Polymorphisms, Tight Junction Proteins and Clinical Features of Adult Patients with Atopic Dermatitis. Dermatol. Pract. Concept. 2024, 14, e2024214. [Google Scholar] [CrossRef] [PubMed]
- Hallau, J.; Hamann, L.; Schumann, R.R.; Worm, M.; Heine, G. A Promoter Polymorphism of the Vitamin D Metabolism Gene Cyp24a1 Is Associated with Severe Atopic Dermatitis in Adults. Acta Derm. Venereol. 2016, 96, 169–172. [Google Scholar] [CrossRef]
- Camargo, C.A.; Ganmaa, D.; Sidbury, R.; Erdenedelger, K.; Radnaakhand, N.; Khandsuren, B. Randomized Trial of Vitamin D Supplementation for Winter-Related Atopic Dermatitis in Children. J. Allergy Clin. Immunol. 2014, 134, 831–835.e1. [Google Scholar] [CrossRef] [PubMed]
- Maleki, M.; Nahidi, Y.; Azizahari, S.; Meibodi, N.T.; Hadianfar, A. Serum 25-OH Vitamin D Level in Psoriatic Patients and Comparison with Control Subjects. J. Cutan. Med. Surg. 2016, 20, 207–210. [Google Scholar] [CrossRef]
- Chandrashekar, L.; Kumarit, G.R.K.; Rajappa, M.; Revathy, G.; Munisamy, M.; Thappa, D.M. 25-Hydroxy Vitamin D and Ischaemia-Modified Albumin Levels in Psoriasis and Their Association with Disease Severity. Br. J. Biomed. Sci. 2015, 72, 56–60. [Google Scholar] [CrossRef]
- Finamor, D.C.; Sinigaglia-Coimbra, R.; Neves, L.C.M.; Gutierrez, M.; Silva, J.J.; Torres, L.D.; Surano, F.; Neto, D.J.; Novo, N.F.; Juliano, Y.; et al. A Pilot Study Assessing the Effect of Prolonged Administration of High Daily Doses of Vitamin D on the Clinical Course of Vitiligo and Psoriasis. Dermato-Endocrinology 2013, 5, 222–234. [Google Scholar] [CrossRef]
- Ingram, M.A.; Jones, M.B.; Stonehouse, W.; Jarrett, P.; Scragg, R.; Mugridge, O.; von Hurst, P.R. Oral Vitamin D3 Supplementation for Chronic Plaque Psoriasis: A Randomized, Double-Blind, Placebo-Controlled Trial. J. Dermatol. Treat. 2018, 29, 648–657. [Google Scholar] [CrossRef]
- Jarrett, P.; Camargo, C.A., Jr.; Coomarasamy, C.; Scragg, R. A Randomized, Double-Blind, Placebo-Controlled Trial of the Effect of Monthly Vitamin D Supplementation in Mild Psoriasis. J. Dermatol. Treat. 2018, 29, 324–328. [Google Scholar] [CrossRef] [PubMed]
- Upala, S.; Sanguankeo, A.; Permpalung, N. Significant Association between Vitamin D Deficiency and Sepsis: A Systematic Review and Meta-Analysis. BMC Anesthesiol. 2015, 15, 84. [Google Scholar] [CrossRef]
- Manson, J.E.; Cook, N.R.; Lee, I.-M.; Christen, W.; Bassuk, S.S.; Mora, S.; Gibson, H.; Gordon, D.; Copeland, T.; D’Agostino, D.; et al. Vitamin D Supplements and Prevention of Cancer and Cardiovascular Disease. N. Engl. J. Med. 2019, 380, 33–44. [Google Scholar] [CrossRef]
- Ahmadieh, H.; Arabi, A. Association between Vitamin D and Cardiovascular Health: Myth or Fact? A Narrative Review of the Evidence. Womens Health 2023, 19, 17455057231158222. [Google Scholar] [CrossRef] [PubMed]
- Giustina, A.; di Filippo, L.; Allora, A.; Bikle, D.D.; Cavestro, G.M.; Feldman, D.; Latella, G.; Minisola, S.; Napoli, N.; Trasciatti, S.; et al. Vitamin D and Malabsorptive Gastrointestinal Conditions: A Bidirectional Relationship? Rev. Endocr. Metab. Disord. 2023, 24, 121–138. [Google Scholar] [CrossRef] [PubMed]
- Hausmann, J.; Kubesch, A.; Amiri, M.; Filmann, N.; Blumenstein, I. Vitamin D Deficiency Is Associated with Increased Disease Activity in Patients with Inflammatory Bowel Disease. J. Clin. Med. 2019, 8, 1319. [Google Scholar] [CrossRef]
- Jørgensen, S.P.; Agnholt, J.; Glerup, H.; Lyhne, S.; Villadsen, G.E.; Hvas, C.L.; Bartels, L.E.; Kelsen, J.; Christensen, L.A.; Dahlerup, J.F. Clinical Trial: Vitamin D3 Treatment in Crohn’s Disease—A Randomized Double-Blind Placebo-Controlled Study. Aliment. Pharmacol. Ther. 2010, 32, 377–383. [Google Scholar] [CrossRef]
- Song, X.; Zhang, H.; Wang, H.; Li, Z.; Zhou, X.; Guo, H. Correlation between Treatment Outcomes and Serum Vitamin D Levels As Well As Infliximab Trough Concentration among Chinese Patients with Crohn’s Disease. Gastroenterol. Res. Pract. 2023, 2023, 6675401. [Google Scholar] [CrossRef]
- Rigterink, T.; Appleton, L.; Day, A.S. Vitamin D Therapy in Children with Inflammatory Bowel Disease: A Systematic Review. World J. Clin. Pediatr. 2019, 8, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Gubatan, J.; Chou, N.D.; Nielsen, O.H.; Moss, A.C. Systematic Review with Meta-Analysis: Association of Vitamin D Status with Clinical Outcomes in Adult Patients with Inflammatory Bowel Disease. Aliment. Pharmacol. Ther. 2019, 50, 1146–1158. [Google Scholar] [CrossRef]
- Barera, G.; Maruca, K.; Sgaramella, P.; Di Stefano, M.; Mora, S. Short-Term, Low Dose Vitamin D Supplementation in Young Patients with Celiac Disease: A Pilot Study. Eur. J. Gastroenterol. Hepatol. 2020, 32, 663–664. [Google Scholar] [CrossRef]
- Damiani, G.; Bragazzi, N.L.; Karimkhani Aksut, C.; Wu, D.; Alicandro, G.; McGonagle, D.; Guo, C.; Dellavalle, R.; Grada, A.; Wong, P.; et al. The Global, Regional, and National Burden of Psoriasis: Results and Insights From the Global Burden of Disease 2019 Study. Front. Med. 2021, 8, 743180. [Google Scholar] [CrossRef]
- Brożyna, A.A.; Slominski, R.M.; Nedoszytko, B.; Zmijewski, M.A.; Slominski, A.T. Vitamin D Signaling in Psoriasis: Pathogenesis and Therapy. Int. J. Mol. Sci. 2022, 23, 8575. [Google Scholar] [CrossRef] [PubMed]
- Benhadou, F.; Mintoff, D.; del Marmol, V. Psoriasis: Keratinocytes or Immune Cells—Which Is the Trigger? Dermatology 2018, 235, 91–100. [Google Scholar] [CrossRef]
- Priyadarssini, M.; Divya Priya, D.; Indhumathi, S.; Rajappa, M.; Chandrashekar, L.; Thappa, D.M. Immunophenotyping of T Cells in the Peripheral Circulation in Psoriasis. Br. J. Biomed. Sci. 2016, 73, 174–179. [Google Scholar] [CrossRef]
- Yamamoto, E.; Jørgensen, T.N. Immunological Effects of Vitamin D and Their Relations to Autoimmunity. J. Autoimmun. 2019, 100, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Karthaus, N.; van Spriel, A.B.; Looman, M.W.G.; Chen, S.; Spilgies, L.M.; Lieben, L.; Carmeliet, G.; Ansems, M.; Adema, G.J. Vitamin D Controls Murine and Human Plasmacytoid Dendritic Cell Function. J. Investig. Dermatol. 2014, 134, 1255–1264. [Google Scholar] [CrossRef] [PubMed]
- Wei, R.; Christakos, S. Mechanisms Underlying the Regulation of Innate and Adaptive Immunity by Vitamin D. Nutrients 2015, 7, 8251–8260. [Google Scholar] [CrossRef]
- Dyring-Andersen, B.; Bonefeld, C.M.; Bzorek, M.; Løvendorf, M.B.; Lauritsen, J.P.H.; Skov, L.; Geisler, C. The Vitamin D Analogue Calcipotriol Reduces the Frequency of CD8+ IL-17+ T Cells in Psoriasis Lesions. Scand. J. Immunol. 2015, 82, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Fujiyama, T.; Ito, T.; Umayahara, T.; Ikeya, S.; Tatsuno, K.; Funakoshi, A.; Hashizume, H.; Tokura, Y. Topical Application of a Vitamin D3 Analogue and Corticosteroid to Psoriasis Plaques Decreases Skin Infiltration of TH17 Cells and Their Ex Vivo Expansion. J. Allergy Clin. Immunol. 2016, 138, 517–528.e5. [Google Scholar] [CrossRef]
- Sato-Deguchi, E.; Imafuku, S.; Chou, B.; Ishii, K.; Hiromatsu, K.; Nakayama, J. Topical Vitamin D3 Analogues Induce Thymic Stromal Lymphopoietin and Cathelicidin in Psoriatic Skin Lesions. Br. J. Dermatol. 2012, 167, 77–84. [Google Scholar] [CrossRef]
- Balato, A.; Schiattarella, M.; Lembo, S.; Mattii, M.; Prevete, N.; Balato, N.; Ayala, F. Interleukin-1 Family Members Are Enhanced in Psoriasis and Suppressed by Vitamin D and Retinoic Acid. Arch. Dermatol. Res. 2013, 305, 255–262. [Google Scholar] [CrossRef]
- Hegyi, Z.; Zwicker, S.; Bureik, D.; Peric, M.; Koglin, S.; Batycka-Baran, A.; Prinz, J.C.; Ruzicka, T.; Schauber, J.; Wolf, R. Vitamin D Analog Calcipotriol Suppresses the Th17 Cytokine-Induced Proinflammatory S100 “Alarmins” Psoriasin (S100A7) and Koebnerisin (S100A15) in Psoriasis. J. Investig. Dermatol. 2012, 132, 1416–1424. [Google Scholar] [CrossRef]
- Datta Mitra, A.; Raychaudhuri, S.P.; Abria, C.J.; Mitra, A.; Wright, R.; Ray, R.; Kundu-Raychaudhuri, S. 1α,25-Dihydroxyvitamin-D3-3-Bromoacetate Regulates AKT/mTOR Signaling Cascades: A Therapeutic Agent for Psoriasis. J. Investig. Dermatol. 2013, 133, 1556–1564. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Komuves, L.; Yu, Q.-C.; Elalieh, H.; Ng, D.C.; Leary, C.; Chang, S.; Crumrine, D.; Yoshizawa, T.; Kato, S.; et al. Lack of the Vitamin D Receptor Is Associated with Reduced Epidermal Differentiation and Hair Follicle Growth. J. Investig. Dermatol. 2002, 118, 11–16. [Google Scholar] [CrossRef]
- Hosomi, J.; Hosoi, J.; Abe, E.; Suda, T.; Kuroki, T. Regulation of Terminal Differentiation of Cultured Mouse Epidermal Cells by 1 Alpha,25-Dihydroxyvitamin D3. Endocrinology 1983, 113, 1950–1957. [Google Scholar] [CrossRef]
- Visconti, B.; Paolino, G.; Carotti, S.; Pendolino, A.L.; Morini, S.; Richetta, A.G.; Calvieri, S. Immunohistochemical Expression of VDR Is Associated with Reduced Integrity of Tight Junction Complex in Psoriatic Skin. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 2038–2042. [Google Scholar] [CrossRef]
- Savoia, P.; Novelli, M.; De Matteis, A.; Verrone, A.; Bernengo, M.G. Effects of Topical Calcipotriol on the Expression of Adhesion Molecules in Psoriasis. J. Cutan. Pathol. 1998, 25, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Richetta, A.; Silvestri, V.; Giancristoforo, S.; Rizzolo, P.; D’Epiro, S.; Graziano, V.; Mattozzi, C.; Navazio, A.; Campoli, M.; D’Amico, C.; et al. A-1012G Promoter Polymorphism of Vitamin D Receptor Gene Is Associated with Psoriasis Risk and Lower Allele-Specific Expression. DNA Cell Biol. 2014, 33, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Xu, L.; Li, Y. The Association of Polymorphisms of the Vitamin D Receptor Gene with Psoriasis in the Han Population of Northeastern China. J. Dermatol. Sci. 2014, 73, 63–66. [Google Scholar] [CrossRef]
- Polić, M.V.; Rucević, I.; Barisić-Drusko, V.; Miskulin, M.; Glavas-Obrovac, L.; Stefanić, M.; Karner, I.; Lipozencić, J.; Bacun, T.; Mihaljević, I. Polymorphisms of Vitamin D Receptor Gene in the Population of Eastern Croatia with Psoriasis Vulgaris and Diabetes Mellitus. Coll. Antropol. 2012, 36, 451–457. [Google Scholar]
- Rucevic, I.; Stefanic, M.; Tokic, S.; Vuksic, M.; Glavas-Obrovac, L.; Barisic-Drusko, V. Lack of Association of Vitamin D Receptor Gene 3′-Haplotypes with Psoriasis in Croatian Patients. J. Dermatol. 2012, 39, 58–62. [Google Scholar] [CrossRef]
- Zuel-Fakkar, N.M.; Kamel, M.M.; Asaad, M.K.; Mahran, M.Z.; Shehab, A.A. A Study of ApaI and TaqI Genotypes of the Vitamin D Receptor in Egyptian Patients with Psoriasis. Clin. Exp. Dermatol. 2011, 36, 355–359. [Google Scholar] [CrossRef]
- Ryan, C.; Renfro, L.; Collins, P.; Kirby, B.; Rogers, S. Clinical and Genetic Predictors of Response to Narrowband Ultraviolet B for the Treatment of Chronic Plaque Psoriasis. Br. J. Dermatol. 2010, 163, 1056–1063. [Google Scholar] [CrossRef]
- Halsall, J.A.; Osborne, J.E.; Pringle, J.H.; Hutchinson, P.E. Vitamin D Receptor Gene Polymorphisms, Particularly the Novel A-1012G Promoter Polymorphism, Are Associated with Vitamin D3 Responsiveness and Non-Familial Susceptibility in Psoriasis. Pharmacogenet. Genom. 2005, 15, 349–355. [Google Scholar] [CrossRef]
- Saeki, H.; Asano, N.; Tsunemi, Y.; Takekoshi, T.; Kishimoto, M.; Mitsui, H.; Tada, Y.; Torii, H.; Komine, M.; Asahina, A.; et al. Polymorphisms of Vitamin D Receptor Gene in Japanese Patients with Psoriasis Vulgaris. J. Dermatol. Sci. 2002, 30, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Disphanurat, W.; Viarasilpa, W.; Chakkavittumrong, P.; Pongcharoen, P. The Clinical Effect of Oral Vitamin D2 Supplementation on Psoriasis: A Double-Blind, Randomized, Placebo-Controlled Study. Dermatol. Res. Pract. 2019, 2019, 5237642. [Google Scholar] [CrossRef] [PubMed]
- Bergler-Czop, B.; Brzezińska-Wcisło, L. Serum Vitamin D Level—The Effect on the Clinical Course of Psoriasis. Postepy Dermatol. Alergol. 2016, 33, 445–449. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Song, G.G. Association between Circulating 25-Hydroxyvitamin D Levels and Psoriasis, and Correlation with Disease Severity: A Meta-Analysis. Clin. Exp. Dermatol. 2018, 43, 529–535. [Google Scholar] [CrossRef]
- Pokharel, R.; Agrawal, S.; Pandey, P.; Lamsal, M. Assessment of Vitamin D Level in Patients with Psoriasis and Its Correlation with Disease Severity: A Case–Control Study. Psoriasis Targets Ther. 2022, 12, 251–258. [Google Scholar] [CrossRef]
- Wilson, P.B. Serum 25-Hydroxyvitamin D Status in Individuals with Psoriasis in the General Population. Endocrine 2013, 44, 537–539. [Google Scholar] [CrossRef]
- Prystowsky, J.H.; Muzio, P.J.; Sevran, S.; Clemens, T.L. Effect of UVB Phototherapy and Oral Calcitriol (1,25-Dihydroxyvitamin D3) on Vitamin D Photosynthesis in Patients with Psoriasis. J. Am. Acad. Dermatol. 1996, 35, 690–695. [Google Scholar] [CrossRef]
- Gumowski-Sunek, D.; Rizzoli, R.; Saurat, J.H. Effects of Topical Calcipotriol on Calcium Metabolism in Psoriatic Patients: Comparison with Oral Calcitriol. Dermatologica 1991, 183, 275–279. [Google Scholar] [CrossRef]
- Shah, K.N. Diagnosis and Treatment of Pediatric Psoriasis: Current and Future. Am. J. Clin. Dermatol. 2013, 14, 195–213. [Google Scholar] [CrossRef]
- Kokelj, F.; Lavaroni, G.; Guadagnini, A. UVB versus UVB plus Calcipotriol (MC 903) Therapy for Psoriasis Vulgaris. Acta Derm. Venereol. 1995, 75, 386–387. [Google Scholar] [CrossRef]
- Gollnick, H.; Altmeyer, P.; Kaufmann, R.; Ring, J.; Christophers, E.; Pavel, S.; Ziegler, J. Topical Calcipotriol plus Oral Fumaric Acid Is More Effective and Faster Acting than Oral Fumaric Acid Monotherapy in the Treatment of Severe Chronic Plaque Psoriasis Vulgaris. Dermatology 2002, 205, 46–53. [Google Scholar] [CrossRef]
- Kokelj, F.; Torsello, P.; Plozzer, C. Calcipotriol Improves the Efficacy of Cyclosporine in the Treatment of Psoriasis Vulgaris. J. Eur. Acad. Dermatol. Venereol. 1998, 10, 143–146. [Google Scholar] [CrossRef]
- Pinter, A.; Green, L.J.; Selmer, J.; Praestegaard, M.; Gold, L.S.; Augustin, M.; Group, T.T.I. A Pooled Analysis of Randomized, Controlled, Phase 3 Trials Investigating the Efficacy and Safety of a Novel, Fixed Dose Calcipotriene and Betamethasone Dipropionate Cream for the Topical Treatment of Plaque Psoriasis. J. Eur. Acad. Dermatol. Venereol. 2022, 36, 228–236. [Google Scholar] [CrossRef]
- Aggarwal, P.; Aggarwal, K.; Jain, V.K. Tacalcitol: A Useful Adjunct to Narrow Band Ultraviolet B Phototherapy in Psoriasis. J. Dermatol. Treat. 2016, 27, 546–551. [Google Scholar] [CrossRef]
- Barker, J.N.; Ashton, R.E.; Marks, R.; Harris, R.I.; Berth-Jones, J. Topical Maxacalcitol for the Treatment of Psoriasis Vulgaris: A Placebo-Controlled, Double-Blind, Dose-Finding Study with Active Comparator. Br. J. Dermatol. 1999, 141, 274–278. [Google Scholar] [CrossRef]
- Yamamoto, A.; Furuhashi, T.; Matsumoto, K.; Morita, A. Safety Profiles of Topical Vitamin D3 in Psoriasis Patients: A Retrospective Large-Scale Study. Psoriasis Targets Ther. 2012, 2, 81–88. [Google Scholar] [CrossRef]
- Segaert, S.; Ropke, M. The Biological Rationale for Use of Vitamin d Analogs in Combination with Corticosteroids for the Topical Treatment of Plaque Psoriasis. J. Drugs Dermatol. 2013, 12, e129–e137. [Google Scholar] [PubMed]
- Bagel, J.; Levi, E.; Tyring, S.; Knuckles, M.L.F. Real-Life Treatment Profile of Calcipotriene and Betamethasone Dipropionate Topical Suspension in Patients with Psoriasis Vulgaris. J. Drugs Dermatol. 2014, 13, 1374–1379. [Google Scholar] [PubMed]
- Eichenfield, L.F.; Ganslandt, C.; Kurvits, M.; Schlessinger, J. Safety and Efficacy of Calcipotriene plus Betamethasone Dipropionate Topical Suspension in the Treatment of Extensive Scalp Psoriasis in Adolescents Ages 12 to 17 Years. Pediatr. Dermatol. 2015, 32, 28–35. [Google Scholar] [CrossRef]
- Franken, S.M.; Witte, B.; Pavel, S.; Rustemeyer, T. Psoriasis and Daily Low-Emission Phototherapy: Effects on Disease and Vitamin D Level. Photodermatol. Photoimmunol. Photomed. 2015, 31, 83–89. [Google Scholar] [CrossRef]
- Takeshita, J.; Grewal, S.; Langan, S.M.; Mehta, N.N.; Ogdie, A.; Van Voorhees, A.S.; Gelfand, J.M. Psoriasis and Comorbid Diseases: Implications for Management. J. Am. Acad. Dermatol. 2017, 76, 393–403. [Google Scholar] [CrossRef]
- Mattioli, A.V.; Coppi, F.; Severino, P.; Penna, C.; Pagliaro, P.; Dei Cas, A.; Bucciarelli, V.; Madonna, R.; Tarperi, C.; Schena, F.; et al. A Personalized Approach to Vitamin D Supplementation in Cardiovascular Health Beyond the Bone: An Expert Consensus by the Italian National Institute for Cardiovascular Research. Nutrients 2025, 17, 115. [Google Scholar] [CrossRef] [PubMed]
- Richetta, A.G.; Grassi, S.; Moliterni, E.; Chello, C.; Calvieri, C.; Carnevale, R.; Peruzzi, M.; Violi, F.; Calvieri, S. Increased Intestinal Barrier Permeability in Patients with Moderate to Severe Plaque-Type Psoriasis. J. Dermatol. 2020, 47, e366–e368. [Google Scholar] [CrossRef] [PubMed]
- Violi, F.; Cammisotto, V.; Bartimoccia, S.; Pignatelli, P.; Carnevale, R.; Nocella, C. Gut-Derived Low-Grade Endotoxaemia, Atherothrombosis and Cardiovascular Disease. Nat. Rev. Cardiol. 2023, 20, 24–37. [Google Scholar] [CrossRef]
- Penna, C.; Pagliaro, P. Endothelial Dysfunction: Redox Imbalance, NLRP3 Inflammasome, and Inflammatory Responses in Cardiovascular Diseases. Antioxidants 2025, 14, 256. [Google Scholar] [CrossRef] [PubMed]
- Janubová, M.; Žitňanová, I. The Effects of Vitamin D on Different Types of Cells. Steroids 2024, 202, 109350. [Google Scholar] [CrossRef]
- Uberti, F.; Lattuada, D.; Morsanuto, V.; Nava, U.; Bolis, G.; Vacca, G.; Squarzanti, D.F.; Cisari, C.; Molinari, C. Vitamin D Protects Human Endothelial Cells from Oxidative Stress through the Autophagic and Survival Pathways. J. Clin. Endocrinol. Metab. 2014, 99, 1367–1374. [Google Scholar] [CrossRef]
- Molinari, C.; Rizzi, M.; Squarzanti, D.F.; Pittarella, P.; Vacca, G.; Renò, F. 1α,25-Dihydroxycholecalciferol (Vitamin D3) Induces NO-Dependent Endothelial Cell Proliferation and Migration in a Three-Dimensional Matrix. Cell. Physiol. Biochem. 2013, 31, 815–822. [Google Scholar] [CrossRef]
- Laera, N.; Malerba, P.; Vacanti, G.; Nardin, S.; Pagnesi, M.; Nardin, M. Impact of Immunity on Coronary Artery Disease: An Updated Pathogenic Interplay and Potential Therapeutic Strategies. Life 2023, 13, 2128. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, L.; Izadpanah, P.; Asadi, S. The Effect of Calcitriol and Cholecalciferol on Inflammatory Markers in Periprocedural Myocardial Injury: A Randomized Controlled Trial. Medicine 2025, 104, e42103. [Google Scholar] [CrossRef]
- Liu, Y.; Lyons, C.J.; Ayu, C.; O’Brien, T. Recent Advances in Endothelial Colony-Forming Cells: From the Transcriptomic Perspective. J. Transl. Med. 2024, 22, 313. [Google Scholar] [CrossRef]
- Berridge, M.J. Vitamin D Cell Signalling in Health and Disease. Biochem. Biophys. Res. Commun. 2015, 460, 53–71. [Google Scholar] [CrossRef]
- Zeldich, E.; Chen, C.-D.; Colvin, T.A.; Bove-Fenderson, E.A.; Liang, J.; Zhou, T.B.T.; Harris, D.A.; Abraham, C.R. The Neuroprotective Effect of Klotho Is Mediated via Regulation of Members of the Redox System. J. Biol. Chem. 2014, 289, 24700–24715. [Google Scholar] [CrossRef]
- Kuro-o, M. Klotho, Phosphate and FGF-23 in Ageing and Disturbed Mineral Metabolism. Nat. Rev. Nephrol. 2013, 9, 650–660. [Google Scholar] [CrossRef]
- Al-Oanzi, Z.H.; Alenazy, F.O.; Alhassan, H.H.; Alruwaili, Y.; Alessa, A.I.; Alfarm, N.B.; Alanazi, M.O.; Alghofaili, S.I. The Role of Vitamin D in Reducing the Risk of Metabolic Disturbances That Cause Cardiovascular Diseases. J. Cardiovasc. Dev. Dis. 2023, 10, 209. [Google Scholar] [CrossRef]
- Izzo, M.; Carrizzo, A.; Izzo, C.; Cappello, E.; Cecere, D.; Ciccarelli, M.; Iannece, P.; Damato, A.; Vecchione, C.; Pompeo, F. Vitamin D: Not Just Bone Metabolism but a Key Player in Cardiovascular Diseases. Life 2021, 11, 452. [Google Scholar] [CrossRef] [PubMed]
- de la Guía-Galipienso, F.; Martínez-Ferran, M.; Vallecillo, N.; Lavie, C.J.; Sanchis-Gomar, F.; Pareja-Galeano, H. Vitamin D and Cardiovascular Health. Clin. Nutr. 2021, 40, 2946–2957. [Google Scholar] [CrossRef] [PubMed]
- Kassi, E.; Adamopoulos, C.; Basdra, E.K.; Papavassiliou, A.G. Role of Vitamin D in Atherosclerosis. Circulation 2013, 128, 2517–2531. [Google Scholar] [CrossRef]
- Pilz, S.; Tomaschitz, A.; Ritz, E.; Pieber, T.R. Vitamin D Status and Arterial Hypertension: A Systematic Review. Nat. Rev. Cardiol. 2009, 6, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.C.; Qiao, G.; Uskokovic, M.; Xiang, W.; Zheng, W.; Kong, J. Vitamin D: A Negative Endocrine Regulator of the Renin-Angiotensin System and Blood Pressure. J. Steroid Biochem. Mol. Biol. 2004, 89–90, 387–392. [Google Scholar] [CrossRef]
- Ajabshir, S.; Asif, A.; Nayer, A. The Effects of Vitamin D on the Renin-Angiotensin System. J. Nephropathol. 2014, 3, 41–43. [Google Scholar] [CrossRef]
- Han, L.; Xu, X.-J.; Zhang, J.-S.; Liu, H.-M. Association between Vitamin D Deficiency and Levels of Renin and Angiotensin in Essential Hypertension. Int. J. Clin. Pract. 2022, 2022, 8975396. [Google Scholar] [CrossRef]
- Witham, M.D.; Price, R.J.G.; Struthers, A.D.; Donnan, P.T.; Messow, C.-M.; Ford, I.; McMurdo, M.E.T. Cholecalciferol Treatment to Reduce Blood Pressure in Older Patients with Isolated Systolic Hypertension: The VitDISH Randomized Controlled Trial. JAMA Intern. Med. 2013, 173, 1672–1679. [Google Scholar] [CrossRef]
- Theiler-Schwetz, V.; Trummer, C.; Grübler, M.R.; Keppel, M.H.; Zittermann, A.; Tomaschitz, A.; Karras, S.N.; März, W.; Pilz, S.; Gängler, S. Effects of Vitamin D Supplementation on 24-Hour Blood Pressure in Patients with Low 25-Hydroxyvitamin D Levels: A Randomized Controlled Trial. Nutrients 2022, 14, 1360. [Google Scholar] [CrossRef]
- Panahi, Y.; Namazi, S.; Rostami-Yalmeh, J.; Sahebi, E.; Khalili, N.; Jamialahmadi, T.; Sahebkar, A. Effect of Vitamin D Supplementation on the Regulation of Blood Pressure in Iranian Patients with Essential Hypertension: A Clinical Trial. Adv. Exp. Med. Biol. 2021, 1328, 501–511. [Google Scholar] [CrossRef]
- Nardin, M.; Verdoia, M.; Nardin, S.; Cao, D.; Chiarito, M.; Kedhi, E.; Galasso, G.; Condorelli, G.; De Luca, G. Vitamin D and Cardiovascular Diseases: From Physiology to Pathophysiology and Outcomes. Biomedicines 2024, 12, 768. [Google Scholar] [CrossRef] [PubMed]
- Khanolkar, S.; Hirani, S.; Mishra, A.; Vardhan, S.; Hirani, S.; Prasad, R.; Wanjari, M. Exploring the Role of Vitamin D in Atherosclerosis and Its Impact on Cardiovascular Events: A Comprehensive Review. Cureus 2023, 15, e42470. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Li, X.; Wang, M.; Ning, H.; A, L.; Li, Y.; Sun, C. Lipoprotein Lipase Links Vitamin D, Insulin Resistance, and Type 2 Diabetes: A Cross-Sectional Epidemiological Study. Cardiovasc. Diabetol. 2013, 12, 17. [Google Scholar] [CrossRef]
- Salekzamani, S.; Bavil, A.S.; Mehralizadeh, H.; Jafarabadi, M.A.; Ghezel, A.; Gargari, B.P. The Effects of Vitamin D Supplementation on Proatherogenic Inflammatory Markers and Carotid Intima Media Thickness in Subjects with Metabolic Syndrome: A Randomized Double-Blind Placebo-Controlled Clinical Trial. Endocrine 2017, 57, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Säidifard, N.; Tangestani, H.; Djafarian, K.; Shab-Bidar, S. Serum Vitamin D Level and Carotid Intima-Media Thickness: A Systematic Review and Meta-Analysis of Observational Studies and Randomized Control Trials. Horm. Metab. Res. 2020, 52, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Reaven, G.M. Insulin Resistance: The Link Between Obesity and Cardiovascular Disease. Med. Clin. N. Am. 2011, 95, 875–892. [Google Scholar] [CrossRef]
- Inomata, S.; Kadowaki, S.; Yamatani, T.; Fukase, M.; Fujita, T. Effect of 1 Alpha (OH)-Vitamin D3 on Insulin Secretion in Diabetes Mellitus. Bone Miner. 1986, 1, 187–192. [Google Scholar]
- Wu, J.; Atkins, A.; Downes, M.; Wei, Z. Vitamin D in Diabetes: Uncovering the Sunshine Hormone’s Role in Glucose Metabolism and Beyond. Nutrients 2023, 15, 1997. [Google Scholar] [CrossRef]
- Zeitz, U.; Weber, K.; Soegiarto, D.W.; Wolf, E.; Balling, R.; Erben, R.G. Impaired Insulin Secretory Capacity in Mice Lacking a Functional Vitamin D Receptor. FASEB J. 2003, 17, 509–511. [Google Scholar] [CrossRef]
- Wolden-Kirk, H.; Overbergh, L.; Gysemans, C.; Brusgaard, K.; Naamane, N.; Van Lommel, L.; Schuit, F.; Eizirik, D.L.; Christesen, H.; Mathieu, C. Unraveling the Effects of 1,25OH2D3 on Global Gene Expression in Pancreatic Islets. J. Steroid Biochem. Mol. Biol. 2013, 136, 68–79. [Google Scholar] [CrossRef]
- Maestro, B.; Dávila, N.; Carranza, M.C.; Calle, C. Identification of a Vitamin D Response Element in the Human Insulin Receptor Gene Promoter. J. Steroid Biochem. Mol. Biol. 2003, 84, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Sergeev, I.N.; Rhoten, W.B. 1,25-Dihydroxyvitamin D3 Evokes Oscillations of Intracellular Calcium in a Pancreatic Beta-Cell Line. Endocrinology 1995, 136, 2852–2861. [Google Scholar] [CrossRef]
- Altieri, B.; Grant, W.B.; Della Casa, S.; Orio, F.; Pontecorvi, A.; Colao, A.; Sarno, G.; Muscogiuri, G. Vitamin D and Pancreas: The Role of Sunshine Vitamin in the Pathogenesis of Diabetes Mellitus and Pancreatic Cancer. Crit. Rev. Food Sci. Nutr. 2017, 57, 3472–3488. [Google Scholar] [CrossRef]
- Pramono, A.; Jocken, J.W.E.; Blaak, E.E.; van Baak, M.A. The Effect of Vitamin D Supplementation on Insulin Sensitivity: A Systematic Review and Meta-Analysis. Diabetes Care 2020, 43, 1659–1669. [Google Scholar] [CrossRef]
- Mirhosseini, N.; Vatanparast, H.; Mazidi, M.; Kimball, S.M. Vitamin D Supplementation, Glycemic Control, and Insulin Resistance in Prediabetics: A Meta-Analysis. J. Endocr. Soc. 2018, 2, 687–709. [Google Scholar] [CrossRef] [PubMed]
- Pacini, S.; Morucci, G.; Branca, J.J.V.; Aterini, S.; Amato, M.; Gulisano, M.; Ruggiero, M. Effects of Vitamin D3 and Paricalcitol on Immature Cardiomyocytes: A Novel Role for Vitamin D Analogs in the Prevention of Cardiovascular Diseases. Nutrients 2013, 5, 2076–2092. [Google Scholar] [CrossRef] [PubMed]
- Moslemi, E.; Musazadeh, V.; Kavyani, Z.; Naghsh, N.; Shoura, S.M.S.; Dehghan, P. Efficacy of Vitamin D Supplementation as an Adjunct Therapy for Improving Inflammatory and Oxidative Stress Biomarkers: An Umbrella Meta-Analysis. Pharmacol. Res. 2022, 186, 106484. [Google Scholar] [CrossRef]
- Thadhani, R.; Appelbaum, E.; Pritchett, Y.; Chang, Y.; Wenger, J.; Tamez, H.; Bhan, I.; Agarwal, R.; Zoccali, C.; Wanner, C.; et al. Vitamin D Therapy and Cardiac Structure and Function in Patients with Chronic Kidney Disease: The PRIMO Randomized Controlled Trial. JAMA 2012, 307, 674–684. [Google Scholar] [CrossRef]
- Gnudi, L.; Fountoulakis, N.; Panagiotou, A.; Corcillo, A.; Maltese, G.; Rife, M.F.; Ntalas, I.; Franks, R.; Chiribiri, A.; Ayis, S.; et al. Effect of Active Vitamin-D on Left Ventricular Mass Index: Results of a Randomized Controlled Trial in Type 2 Diabetes and Chronic Kidney Disease. Am. Heart J. 2023, 261, 1–9. [Google Scholar] [CrossRef]
- Rafiq, S.; Jeppesen, P.B. Vitamin D Deficiency Is Inversely Associated with Homeostatic Model Assessment of Insulin Resistance. Nutrients 2021, 13, 4358. [Google Scholar] [CrossRef]
- Ryan, Z.C.; Craig, T.A.; Folmes, C.D.; Wang, X.; Lanza, I.R.; Schaible, N.S.; Salisbury, J.L.; Nair, K.S.; Terzic, A.; Sieck, G.C.; et al. 1α,25-Dihydroxyvitamin D3 Regulates Mitochondrial Oxygen Consumption and Dynamics in Human Skeletal Muscle Cells. J. Biol. Chem. 2016, 291, 1514–1528. [Google Scholar] [CrossRef] [PubMed]
- Jefferson, G.E.; Schnell, D.M.; Thomas, D.T.; Bollinger, L.M. Calcitriol Concomitantly Enhances Insulin Sensitivity and Alters Myocellular Lipid Partitioning in High Fat-Treated Skeletal Muscle Cells. J. Physiol. Biochem. 2017, 73, 613–621. [Google Scholar] [CrossRef]
- Krul-Poel, Y.H.M.; Ter Wee, M.M.; Lips, P.; Simsek, S. MANAGEMENT OF ENDOCRINE DISEASE: The Effect of Vitamin D Supplementation on Glycaemic Control in Patients with Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis. Eur. J. Endocrinol. 2017, 176, R1–R14. [Google Scholar] [CrossRef]
- Fahed, G.; Aoun, L.; Bou Zerdan, M.; Allam, S.; Bou Zerdan, M.; Bouferraa, Y.; Assi, H.I. Metabolic Syndrome: Updates on Pathophysiology and Management in 2021. Int. J. Mol. Sci. 2022, 23, 786. [Google Scholar] [CrossRef]
- Zhu, Y.; Xian, X.; Wang, Z.; Bi, Y.; Chen, Q.; Han, X.; Tang, D.; Chen, R. Research Progress on the Relationship between Atherosclerosis and Inflammation. Biomolecules 2018, 8, 80. [Google Scholar] [CrossRef]
- Beierwaltes, W.H. The Role of Calcium in the Regulation of Renin Secretion. Am. J. Physiol. Renal Physiol. 2010, 298, F1–F11. [Google Scholar] [CrossRef] [PubMed]
- Asbaghi, O.; Khosroshahi, M.Z.; Kashkooli, S.; Abbasnezhad, A. Effect of Calcium-Vitamin D Co-Supplementation on Insulin, Insulin Sensitivity, and Glycemia: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Horm. Metab. Res. 2019, 51, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Martins, D.; Wolf, M.; Pan, D.; Zadshir, A.; Tareen, N.; Thadhani, R.; Felsenfeld, A.; Levine, B.; Mehrotra, R.; Norris, K. Prevalence of Cardiovascular Risk Factors and the Serum Levels of 25-Hydroxyvitamin D in the United States: Data from the Third National Health and Nutrition Examination Survey. Arch. Intern. Med. 2007, 167, 1159–1165. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Chen, J.; Sun, X.; Wang, L.; Wang, A. Efficacy of Vitamin D Supplementation on Glycemic Control in Type 2 Diabetes Patients: A Meta-Analysis of Interventional Studies. Medicine 2019, 98, e14970. [Google Scholar] [CrossRef]
- Gagnon, C.; Lu, Z.X.; Magliano, D.J.; Dunstan, D.W.; Shaw, J.E.; Zimmet, P.Z.; Sikaris, K.; Grantham, N.; Ebeling, P.R.; Daly, R.M. Serum 25-Hydroxyvitamin D, Calcium Intake, and Risk of Type 2 Diabetes After 5 Years: Results from a National, Population-Based Prospective Study (the Australian Diabetes, Obesity and Lifestyle Study). Diabetes Care 2011, 34, 1133–1138. [Google Scholar] [CrossRef]
- Nazarian, S.; St Peter, J.V.; Boston, R.C.; Jones, S.A.; Mariash, C.N. Vitamin D3 Supplementation Improves Insulin Sensitivity in Subjects with Impaired Fasting Glucose. Transl. Res. 2011, 158, 276–281. [Google Scholar] [CrossRef]
- Alvarez, J.A.; Ashraf, A. Role of Vitamin D in Insulin Secretion and Insulin Sensitivity for Glucose Homeostasis. Int. J. Endocrinol. 2010, 2010, 351385. [Google Scholar] [CrossRef]
- Jafari, T.; Fallah, A.A.; Barani, A. Effects of Vitamin D on Serum Lipid Profile in Patients with Type 2 Diabetes: A Meta-Analysis of Randomized Controlled Trials. Clin. Nutr. 2016, 35, 1259–1268. [Google Scholar] [CrossRef]
- Ostadmohammadi, V.; Milajerdi, A.; Ghayour-Mobarhan, M.; Ferns, G.; Taghizadeh, M.; Badehnoosh, B.; Mirzaei, H.; Asemi, Z. The Effects of Vitamin D Supplementation on Glycemic Control, Lipid Profiles and C-Reactive Protein Among Patients with Cardiovascular Disease: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Curr. Pharm. Des. 2019, 25, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Qi, K.-J.; Zhao, Z.-T.; Zhang, W.; Yang, F. The Impacts of Vitamin D Supplementation in Adults with Metabolic Syndrome: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Front. Pharmacol. 2022, 13, 1033026. [Google Scholar] [CrossRef]
- Ganji, V.; Sukik, A.; Alaayesh, H.; Rasoulinejad, H.; Shraim, M. Serum Vitamin D Concentrations Are Inversely Related to Prevalence of Metabolic Syndrome in Qatari Women. BioFactors 2020, 46, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Wang, C.; Chen, X.; Wan, H.; Chen, Y.; Chen, C.; Han, B.; Lu, Y. Vitamin D, Prediabetes and Type 2 Diabetes: Bidirectional Mendelian Randomization Analysis. Eur. J. Nutr. 2020, 59, 1379–1388. [Google Scholar] [CrossRef]
- Zheng, J.-S.; Luan, J.; Sofianopoulou, E.; Sharp, S.J.; Day, F.R.; Imamura, F.; Gundersen, T.E.; Lotta, L.A.; Sluijs, I.; Stewart, I.D.; et al. The Association between Circulating 25-Hydroxyvitamin D Metabolites and Type 2 Diabetes in European Populations: A Meta-Analysis and Mendelian Randomisation Analysis. PLoS MED. 2020, 17, e1003394. [Google Scholar] [CrossRef]
- Lotfy, M.; Adeghate, J.; Kalasz, H.; Singh, J.; Adeghate, E. Chronic Complications of Diabetes Mellitus: A Mini Review. Curr. Diabetes Rev. 2017, 13, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, S.; Singh, R.P.; Dwivedi, N.C.; Singh, K.; Gupta, A.; Mathur, M. Vitamin D Levels and Microvascular Complications in Type 2 Diabetes. Indian J. Endocrinol. Metab. 2014, 18, 537. [Google Scholar] [CrossRef]
- Shehab, D.; Al-Jarallah, K.; Mojiminiyi, O.A.; Al Mohamedy, H.; Abdella, N.A. Does Vitamin D Deficiency Play a Role in Peripheral Neuropathy in Type 2 Diabetes? Diabet. Med. 2012, 29, 43–49. [Google Scholar] [CrossRef]
- Assy, M.H.; Draz, N.A.; Fathy, S.E.; Hamed, M.G. Impact of Vitamin D Level in Diabetic People with Peripheral Neuropathy. Egypt. J. Neurol. Psychiatry Neurosurg. 2021, 57, 117. [Google Scholar] [CrossRef]
- Parker, J.; Hashmi, O.; Dutton, D.; Mavrodaris, A.; Stranges, S.; Kandala, N.-B.; Clarke, A.; Franco, O.H. Levels of Vitamin D and Cardiometabolic Disorders: Systematic Review and Meta-Analysis. Maturitas 2010, 65, 225–236. [Google Scholar] [CrossRef]
- Lee, K.; Kim, J. Serum Vitamin D Status and Metabolic Syndrome: A Systematic Review and Dose-Response Meta-Analysis. Nutr. Res. Pract. 2021, 15, 329–345. [Google Scholar] [CrossRef] [PubMed]
- Song, H.R.; Park, C.H. Low Serum Vitamin D Level Is Associated with High Risk of Metabolic Syndrome in Post-Menopausal Women. J. Endocrinol. Investig. 2013, 36, 791–796. [Google Scholar] [CrossRef] [PubMed]
- Tomaschitz, A.; Pilz, S.; Ritz, E.; Grammer, T.; Drechsler, C.; Boehm, B.O.; März, W. Independent Association between 1,25-Dihydroxyvitamin D, 25-Hydroxyvitamin D and the Renin-Angiotensin System: The Ludwigshafen Risk and Cardiovascular Health (LURIC) Study. Clin. Chim. Acta 2010, 411, 1354–1360. [Google Scholar] [CrossRef] [PubMed]
- Forman, J.P.; Williams, J.S.; Fisher, N.D.L. Plasma 25-Hydroxyvitamin D and Regulation of the Renin-Angiotensin System in Humans. Hypertension 2010, 55, 1283–1288. [Google Scholar] [CrossRef]
- Zhao, J.-D.; Jia, J.-J.; Dong, P.-S.; Zhao, D.; Yang, X.-M.; Li, D.-L.; Zhang, H.-F. Effect of Vitamin D on Ventricular Remodelling in Heart Failure: A Meta-Analysis of Randomised Controlled Trials. BMJ Open 2018, 8, e020545. [Google Scholar] [CrossRef]
- Dorsch, M.P.; Nemerovski, C.W.; Ellingrod, V.L.; Cowger, J.A.; Dyke, D.B.; Koelling, T.M.; Wu, A.H.; Aaronson, K.D.; Simpson, R.U.; Bleske, B.E. Vitamin D Receptor Genetics on Extracellular Matrix Biomarkers and Hemodynamics in Systolic Heart Failure. J. Cardiovasc. Pharmacol. Ther. 2014, 19, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Janjusevic, M.; Gagno, G.; Fluca, A.L.; Padoan, L.; Beltrami, A.P.; Sinagra, G.; Moretti, R.; Aleksova, A. The Peculiar Role of Vitamin D in the Pathophysiology of Cardiovascular and Neurodegenerative Diseases. Life Sci. 2022, 289, 120193. [Google Scholar] [CrossRef]
- Vaidya, A.; Brown, J.M.; Williams, J.S. The Renin–Angiotensin–Aldosterone System and Calcium-Regulatory Hormones. J. Hum. Hypertens. 2015, 29, 515–521. [Google Scholar] [CrossRef]
- Williams, A.; Zhao, S.; Brock, G.; Kline, D.; Echouffo-Tcheugui, J.B.; Effoe, V.S.; Bertoni, A.G.; Michos, E.D.; de Boer, I.H.; Kestenbaum, B.; et al. Vitamin D, Parathyroid Hormone, Glucose Metabolism and Incident Diabetes in the Multiethnic Study of Atherosclerosis. BMJ Open Diabetes Res. Care 2022, 10, e002931. [Google Scholar] [CrossRef]
- Scragg, R.; Waayer, D.; Stewart, A.W.; Lawes, C.M.M.; Toop, L.; Murphy, J.; Khaw, K.-T.; Camargo, C.A. The Vitamin D Assessment (ViDA) Study: Design of a Randomized Controlled Trial of Vitamin D Supplementation for the Prevention of Cardiovascular Disease, Acute Respiratory Infection, Falls and Non-Vertebral Fractures. J. Steroid Biochem. Mol. Biol. 2016, 164, 318–325. [Google Scholar] [CrossRef]
- Thompson, B.; Waterhouse, M.; English, D.R.; McLeod, D.S.; Armstrong, B.K.; Baxter, C.; Romero, B.D.; Ebeling, P.R.; Hartel, G.; Kimlin, M.G.; et al. Vitamin D Supplementation and Major Cardiovascular Events: D-Health Randomised Controlled Trial. BMJ 2023, 381, e075230. [Google Scholar] [CrossRef]
- LeBoff, M.S.; Chou, S.H.; Ratliff, K.A.; Cook, N.R.; Khurana, B.; Kim, E.; Cawthon, P.M.; Bauer, D.C.; Black, D.; Gallagher, J.C.; et al. Supplemental Vitamin D and Incident Fractures in Midlife and Older Adults. N. Engl. J. Med. 2022, 387, 299–309. [Google Scholar] [CrossRef]
- Fassio, A.; Rossini, M.; Gatti, D. Vitamin D: No Efficacy without Deficiency. What’s New? Reumatismo 2019, 71, 57–61. [Google Scholar] [CrossRef]
- Scragg, R. Clinical Trials of Vitamin D Supplementation and Cardiovascular Disease: A Synthesis of the Evidence. J. Steroid Biochem. Mol. Biol. 2025, 250, 106733. [Google Scholar] [CrossRef]
- Sutherland, J.P.; Zhou, A.; Hyppönen, E. Vitamin D Deficiency Increases Mortality Risk in the UK Biobank: A Nonlinear Mendelian Randomization Study. Ann. Intern. Med. 2022, 175, 1552–1559. [Google Scholar] [CrossRef]
- Patriota, P.; Guessous, I.; Rezzi, S.; Marques-Vidal, P. Vitamin D Levels Are Associated with Cardiovascular Disease Events but Not with Cardiovascular Disease or Overall Mortality: A Prospective Population-Based Study. Nutrients 2023, 15, 4046. [Google Scholar] [CrossRef]
- Chelakkot, C.; Ghim, J.; Ryu, S.H. Mechanisms Regulating Intestinal Barrier Integrity and Its Pathological Implications. Exp. Mol. Med. 2018, 50, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Visser, J.; Rozing, J.; Sapone, A.; Lammers, K.; Fasano, A. Tight Junctions, Intestinal Permeability, and Autoimmunity Celiac Disease and Type 1 Diabetes Paradigms. Ann. N. Y. Acad. Sci. 2009, 1165, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Rath, T.; Atreya, R.; Neurath, M.F. A Spotlight on Intestinal Permeability and Inflammatory Bowel Diseases. Expert Rev. Gastroenterol. Hepatol. 2023, 17, 893–902. [Google Scholar] [CrossRef] [PubMed]
- Schoultz, I.; Keita, Å.V. Cellular and Molecular Therapeutic Targets in Inflammatory Bowel Disease—Focusing on Intestinal Barrier Function. Cells 2019, 8, 193. [Google Scholar] [CrossRef]
- Fasano, A. Zonulin and Its Regulation of Intestinal Barrier Function: The Biological Door to Inflammation, Autoimmunity, and Cancer. Physiol. Rev. 2011, 91, 151–175. [Google Scholar] [CrossRef]
- Neurath, M.F.; Artis, D.; Becker, C. The Intestinal Barrier: A Pivotal Role in Health, Inflammation, and Cancer. Lancet Gastroenterol. Hepatol. 2025, 10, 573–592. [Google Scholar] [CrossRef]
- Untersmayr, E.; Brandt, A.; Koidl, L.; Bergheim, I. The Intestinal Barrier Dysfunction as Driving Factor of Inflammaging. Nutrients 2022, 14, 949. [Google Scholar] [CrossRef]
- Liu, Q.; Yu, Z.; Tian, F.; Zhao, J.; Zhang, H.; Zhai, Q.; Chen, W. Surface Components and Metabolites of Probiotics for Regulation of Intestinal Epithelial Barrier. Microb. Cell Factories 2020, 19, 23. [Google Scholar] [CrossRef]
- Hiippala, K.; Jouhten, H.; Ronkainen, A.; Hartikainen, A.; Kainulainen, V.; Jalanka, J.; Satokari, R. The Potential of Gut Commensals in Reinforcing Intestinal Barrier Function and Alleviating Inflammation. Nutrients 2018, 10, 988. [Google Scholar] [CrossRef] [PubMed]
- Moens, E.; Veldhoen, M. Epithelial Barrier Biology: Good Fences Make Good Neighbours. Immunology 2012, 135, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hooper, L.V.; Gordon, J.I. Commensal Host-Bacterial Relationships in the Gut. Science 2001, 292, 1115–1118. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.R. Intestinal Mucosal Barrier Function in Health and Disease. Nat. Rev. Immunol. 2009, 9, 799–809. [Google Scholar] [CrossRef]
- König, J.; Wells, J.; Cani, P.D.; García-Ródenas, C.L.; MacDonald, T.; Mercenier, A.; Whyte, J.; Troost, F.; Brummer, R.-J. Human Intestinal Barrier Function in Health and Disease. Clin. Transl. Gastroenterol. 2016, 7, e196. [Google Scholar] [CrossRef]
- Mowat, A.M.; Agace, W.W. Regional Specialization within the Intestinal Immune System. Nat. Rev. Immunol. 2014, 14, 667–685. [Google Scholar] [CrossRef]
- Wang, J.; Mei, L.; Hao, Y.; Xu, Y.; Yang, Q.; Dai, Z.; Yang, Y.; Wu, Z.; Ji, Y. Contemporary Perspectives on the Role of Vitamin D in Enhancing Gut Health and Its Implications for Preventing and Managing Intestinal Diseases. Nutrients 2024, 16, 2352. [Google Scholar] [CrossRef]
- Vernia, F.; Valvano, M.; Longo, S.; Cesaro, N.; Viscido, A.; Latella, G. Vitamin D in Inflammatory Bowel Diseases. Mechanisms of Action and Therapeutic Implications. Nutrients 2022, 14, 269. [Google Scholar] [CrossRef]
- Campbell, H.K.; Maiers, J.L.; DeMali, K.A. Interplay between Tight Junctions & Adherens Junctions. Exp. Cell Res. 2017, 358, 39–44. [Google Scholar] [CrossRef]
- Odenwald, M.A.; Turner, J.R. The Intestinal Epithelial Barrier: A Therapeutic Target? Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 9–21. [Google Scholar] [CrossRef]
- Al-Sadi, R.; Boivin, M.; Ma, T. Mechanism of Cytokine Modulation of Epithelial Tight Junction Barrier. Front. Biosci. 2009, 14, 2765–2778. [Google Scholar] [CrossRef] [PubMed]
- Trasciatti, S.; Grizzi, F. Vitamin D and Celiac Disease. Adv. Food Nutr. Res. 2024, 109, 249–270. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Guo, Y.; Geng, C.; Song, S.; Yang, W.; Li, X.; Wang, C. Vitamin D/Vitamin D Receptor Protects Intestinal Barrier against Colitis by Positively Regulating Notch Pathway. Front. Pharmacol. 2024, 15, 1421577. [Google Scholar] [CrossRef]
- Dong, S.; Singh, T.P.; Wei, X.; Yao, H.; Wang, H. Protective Effect of 1,25-Dihydroxy Vitamin D3 on Pepsin-Trypsin-Resistant Gliadin-Induced Tight Junction Injuries. Dig. Dis. Sci. 2018, 63, 92–104. [Google Scholar] [CrossRef]
- Munem, F.; Thianhlun, P.C.K.; Anderson, P.H.; Stringer, A.M. Vitamin D Is a Potential Treatment for the Management of Gastrointestinal Mucositis. Curr. Opin. Support. Palliat. Care 2023, 17, 247. [Google Scholar] [CrossRef] [PubMed]
- Piemonti, L.; Monti, P.; Sironi, M.; Fraticelli, P.; Leone, B.E.; Dal Cin, E.; Allavena, P.; Di Carlo, V. Vitamin D3 Affects Differentiation, Maturation, and Function of Human Monocyte-Derived Dendritic Cells1. J. Immunol. 2000, 164, 4443–4451. [Google Scholar] [CrossRef]
- White, J.H. Vitamin D Metabolism and Signaling in the Immune System. Rev. Endocr. Metab. Disord. 2012, 13, 21–29. [Google Scholar] [CrossRef]
- Wu, S.; Zhang, Y.; Lu, R.; Xia, Y.; Zhou, D.; Petrof, E.O.; Claud, E.C.; Chen, D.; Chang, E.B.; Carmeliet, G.; et al. Intestinal Epithelial Vitamin D Receptor Deletion Leads to Defective Autophagy in Colitis. Gut 2015, 64, 1082–1094. [Google Scholar] [CrossRef]
- Wang, J.; Thingholm, L.B.; Skiecevičienė, J.; Rausch, P.; Kummen, M.; Hov, J.R.; Degenhardt, F.; Heinsen, F.-A.; Rühlemann, M.C.; Szymczak, S.; et al. Genome-Wide Association Analysis Identifies Variation in Vitamin D Receptor and Other Host Factors Influencing the Gut Microbiota. Nat. Genet. 2016, 48, 1396–1406. [Google Scholar] [CrossRef]
- Lu, R.; Zhang, Y.; Xia, Y.; Zhang, J.; Kaser, A.; Blumberg, R.; Sun, J. Paneth Cell Alertness to Pathogens Maintained by Vitamin D Receptors. Gastroenterology 2021, 160, 1269–1283. [Google Scholar] [CrossRef]
- Ooi, J.H.; Li, Y.; Rogers, C.J.; Cantorna, M.T. Vitamin D Regulates the Gut Microbiome and Protects Mice from Dextran Sodium Sulfate-Induced Colitis. J. Nutr. 2013, 143, 1679–1686. [Google Scholar] [CrossRef]
- Gubatan, J.; Mehigan, G.A.; Villegas, F.; Mitsuhashi, S.; Longhi, M.S.; Malvar, G.; Csizmadia, E.; Robson, S.; Moss, A.C. Cathelicidin Mediates a Protective Role of Vitamin D in Ulcerative Colitis and Human Colonic Epithelial Cells. Inflamm. Bowel Dis. 2020, 26, 885–897. [Google Scholar] [CrossRef]
- Del Pinto, R.; Pietropaoli, D.; Chandar, A.K.; Ferri, C.; Cominelli, F. Association Between Inflammatory Bowel Disease and Vitamin D Deficiency: A Systematic Review and Meta-Analysis. Inflamm. Bowel Dis. 2015, 21, 2708–2717. [Google Scholar] [CrossRef]
- Parizadeh, S.M.; Jafarzadeh-Esfehani, R.; Hassanian, S.M.; Mottaghi-Moghaddam, A.; Ghazaghi, A.; Ghandehari, M.; Alizade-Noghani, M.; Khazaei, M.; Ghayour-Mobarhan, M.; Ferns, G.A.; et al. Vitamin D in Inflammatory Bowel Disease: From Biology to Clinical Implications. Complement. Ther. Med. 2019, 47, 102189. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, J.; Cooper, S.C.; Ghosh, S.; Hewison, M. The Role of Vitamin D in Inflammatory Bowel Disease: Mechanism to Management. Nutrients 2019, 11, 1019. [Google Scholar] [CrossRef]
- Lu, C.; Zhou, W.; He, X.; Zhou, X.; Yu, C. Vitamin D Status and Vitamin D Receptor Genotypes in Celiac Disease: A Meta-Analysis. Crit. Rev. Food Sci. Nutr. 2021, 61, 2098–2106. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Lu, L.; Chen, Y.; Zeng, Y.; Xu, C. The Efficacy of Vitamin D Supplementation for Irritable Bowel Syndrome: A Systematic Review with Meta-Analysis. Nutr. J. 2022, 21, 24. [Google Scholar] [CrossRef] [PubMed]
- Garg, M.; Hendy, P.; Ding, J.N.; Shaw, S.; Hold, G.; Hart, A. The Effect of Vitamin D on Intestinal Inflammation and Faecal Microbiota in Patients with Ulcerative Colitis. J. Crohns Colitis 2018, 12, 963–972. [Google Scholar] [CrossRef] [PubMed]
- Valvano, M.; Magistroni, M.; Cesaro, N.; Carlino, G.; Monaco, S.; Fabiani, S.; Vinci, A.; Vernia, F.; Viscido, A.; Latella, G. Effectiveness of Vitamin D Supplementation on Disease Course in Inflammatory Bowel Disease Patients: Systematic Review with Meta-Analysis. Inflamm. Bowel Dis. 2024, 30, 281–291. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, T.; Wang, Y.; Liu, R.; Chang, M.; Wang, X. Effects of Oral Vitamin D Supplementation on Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis. Food Funct. 2021, 12, 7588–7606. [Google Scholar] [CrossRef]
- Singh, P.; Rawat, A.; Alwakeel, M.; Sharif, E.; Al Khodor, S. The Potential Role of Vitamin D Supplementation as a Gut Microbiota Modifier in Healthy Individuals. Sci. Rep. 2020, 10, 21641. [Google Scholar] [CrossRef]
- Boughanem, H.; Ruiz-Limón, P.; Pilo, J.; Lisbona-Montañez, J.M.; Tinahones, F.J.; Moreno Indias, I.; Macías-González, M. Linking Serum Vitamin D Levels with Gut Microbiota after 1-Year Lifestyle Intervention with Mediterranean Diet in Patients with Obesity and Metabolic Syndrome: A Nested Cross-Sectional and Prospective Study. Gut Microbes 2023, 15, 2249150. [Google Scholar] [CrossRef] [PubMed]
- Bellerba, F.; Muzio, V.; Gnagnarella, P.; Facciotti, F.; Chiocca, S.; Bossi, P.; Cortinovis, D.; Chiaradonna, F.; Serrano, D.; Raimondi, S.; et al. The Association between Vitamin D and Gut Microbiota: A Systematic Review of Human Studies. Nutrients 2021, 13, 3378. [Google Scholar] [CrossRef] [PubMed]
- Naderpoor, N.; Mousa, A.; Fernanda Gomez Arango, L.; Barrett, H.L.; Dekker Nitert, M.; de Courten, B. Effect of Vitamin D Supplementation on Faecal Microbiota: A Randomised Clinical Trial. Nutrients 2019, 11, 2888. [Google Scholar] [CrossRef]
- Jones, M.L.; Martoni, C.J.; Prakash, S. Oral Supplementation with Probiotic L. Reuteri NCIMB 30242 Increases Mean Circulating 25-Hydroxyvitamin D: A Post Hoc Analysis of a Randomized Controlled Trial. J. Clin. Endocrinol. Metab. 2013, 98, 2944–2951. [Google Scholar] [CrossRef]
- Hewison, M. COVID-19 and Our Understanding of Vitamin D and Immune Function. J. Steroid Biochem. Mol. Biol. 2025, 249, 106710. [Google Scholar] [CrossRef]
- Rizzi, M.; Sainaghi, P.P. Vitamin D: A Nutraceutical Supplement at the Crossroad Between Respiratory Infections and COVID-19. Int. J. Mol. Sci. 2025, 26, 2550. [Google Scholar] [CrossRef]
- Chen, H.; Lu, R.; Zhang, Y.-G.; Sun, J. Vitamin D Receptor Deletion Leads to the Destruction of Tight and Adherens Junctions in Lungs. Tissue Barriers 2018, 6, 1–13. [Google Scholar] [CrossRef]
- Bikle, D.D. Vitamin D Regulation of Immune Function during COVID-19. Rev. Endocr. Metab. Disord. 2022, 23, 279–285. [Google Scholar] [CrossRef]
- Ahsan, N.; Imran, M.; Mohammed, Y.; Al Anouti, F.; Khan, M.I.; Banerjee, T.; Adnan, M.; Ashfaq, F.; Kieliszek, M.; Ashraf, S.A.; et al. Mechanistic Insight into the Role of Vitamin D and Zinc in Modulating Immunity Against COVID-19: A View from an Immunological Standpoint. Biol. Trace Elem. Res. 2023, 201, 5546–5560. [Google Scholar] [CrossRef]
- Giannini, S.; Passeri, G.; Tripepi, G.; Sella, S.; Fusaro, M.; Arcidiacono, G.; Torres, M.O.; Michielin, A.; Prandini, T.; Baffa, V.; et al. Effectiveness of In-Hospital Cholecalciferol Use on Clinical Outcomes in Comorbid COVID-19 Patients: A Hypothesis-Generating Study. Nutrients 2021, 13, 219. [Google Scholar] [CrossRef]
- Adami, G.; Giollo, A.; Fassio, A.; Benini, C.; Bertoldo, E.; Bertoldo, F.; Orsolini, G.; Idolazzi, L.; Viapiana, O.; Giannini, S.; et al. Vitamin D and Disease Severity in Coronavirus Disease 19 (COVID-19). Reumatismo 2021, 72, 189–196. [Google Scholar] [CrossRef]
- Annweiler, G.; Corvaisier, M.; Gautier, J.; Dubée, V.; Legrand, E.; Sacco, G.; Annweiler, C. Vitamin D Supplementation Associated to Better Survival in Hospitalized Frail Elderly COVID-19 Patients: The GERIA-COVID Quasi-Experimental Study. Nutrients 2020, 12, 3377. [Google Scholar] [CrossRef] [PubMed]
- Brenner, H. Vitamin D Supplementation to Prevent COVID-19 Infections and Deaths-Accumulating Evidence from Epidemiological and Intervention Studies Calls for Immediate Action. Nutrients 2021, 13, 411. [Google Scholar] [CrossRef] [PubMed]
- Petrelli, F.; Deda, R.; Borgonovo, K.; Dognini, G.; Ghilardi, M.; Parati, M.C.; Petrò, D.; Lonati, V.; Dottorini, L.; Ghidini, A. Vitamin D3 and Cancer Risk in Healthy Subjects: An Umbrella Review of Systematic Review and Meta-Analysis. Clin. Nutr. ESPEN 2024, 63, 776–786. [Google Scholar] [CrossRef]
- Feldman, D.; Krishnan, A.V.; Swami, S.; Giovannucci, E.; Feldman, B.J. The Role of Vitamin D in Reducing Cancer Risk and Progression. Nat. Rev. Cancer 2014, 14, 342–357. [Google Scholar] [CrossRef] [PubMed]
- Munteanu, C.; Mârza, S.M.; Papuc, I. The Immunomodulatory Effects of Vitamins in Cancer. Front. Immunol. 2024, 15, 1464329. [Google Scholar] [CrossRef] [PubMed]
- Becker, A.L.; Carpenter, E.L.; Slominski, A.T.; Indra, A.K. The Role of the Vitamin D Receptor in the Pathogenesis, Prognosis, and Treatment of Cutaneous Melanoma. Front. Oncol. 2021, 11, 743667. [Google Scholar] [CrossRef]
- Slominski, R.M.; Kim, T.-K.; Janjetovic, Z.; Brożyna, A.A.; Podgorska, E.; Dixon, K.M.; Mason, R.S.; Tuckey, R.C.; Sharma, R.; Crossman, D.K.; et al. Malignant Melanoma: An Overview, New Perspectives, and Vitamin D Signaling. Cancers 2024, 16, 2262. [Google Scholar] [CrossRef] [PubMed]
- Bouillon, R. Comparative Analysis of Nutritional Guidelines for Vitamin D. Nat. Rev. Endocrinol. 2017, 13, 466–479. [Google Scholar] [CrossRef]
- Wylon, K.; Drozdenko, G.; Krannich, A.; Heine, G.; Dölle, S.; Worm, M. Pharmacokinetic Evaluation of a Single Intramuscular High Dose versus an Oral Long-Term Supplementation of Cholecalciferol. PLoS ONE 2017, 12, e0169620. [Google Scholar] [CrossRef]
- Meekins, M.E.; Oberhelman, S.S.; Lee, B.R.; Gardner, B.M.; Cha, S.S.; Singh, R.J.; Pettifor, J.M.; Fischer, P.R.; Thacher, T.D. Pharmacokinetics of Daily versus Monthly Vitamin D3 Supplementation in Non-Lactating Women. Eur. J. Clin. Nutr. 2014, 68, 632–634. [Google Scholar] [CrossRef] [PubMed]
- Rahme, M.; Sharara, S.L.; Baddoura, R.; Habib, R.H.; Halaby, G.; Arabi, A.; Singh, R.J.; Kassem, M.; Mahfoud, Z.; Hoteit, M.; et al. Impact of Calcium and Two Doses of Vitamin D on Bone Metabolism in the Elderly: A Randomized Controlled Trial. J. Bone Miner. Res. 2017, 32, 1486–1495. [Google Scholar] [CrossRef]
- Ross, A.C.; Manson, J.E.; Abrams, S.A.; Aloia, J.F.; Brannon, P.M.; Clinton, S.K.; Durazo-Arvizu, R.A.; Gallagher, J.C.; Gallo, R.L.; Jones, G.; et al. The 2011 Report on Dietary Reference Intakes for Calcium and Vitamin D from the Institute of Medicine: What Clinicians Need to Know. J. Clin. Endocrinol. Metab. 2011, 96, 53–58. [Google Scholar] [CrossRef]
- Rizzoli, R. Vitamin D Supplementation: Upper Limit for Safety Revisited? Aging Clin. Exp. Res. 2021, 33, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Giustina, A.; Bilezikian, J.P.; Adler, R.A.; Banfi, G.; Bikle, D.D.; Binkley, N.C.; Bollerslev, J.; Bouillon, R.; Brandi, M.L.; Casanueva, F.F.; et al. Consensus Statement on Vitamin D Status Assessment and Supplementation: Whys, Whens, and Hows. Endocr. Rev. 2024, 45, 625–654. [Google Scholar] [CrossRef] [PubMed]
- Bilezikian, J.P.; Formenti, A.M.; Adler, R.A.; Binkley, N.; Bouillon, R.; Lazaretti-Castro, M.; Marcocci, C.; Napoli, N.; Rizzoli, R.; Giustina, A. Vitamin D: Dosing, Levels, Form, and Route of Administration: Does One Approach Fit All? Rev. Endocr. Metab. Disord. 2021, 22, 1201–1218. [Google Scholar] [CrossRef]
- National Institute of Health. Vitamin D: Fact Sheet for Health Professionals; National Institute of Health: Bethesda, MD, USA, 2025. [Google Scholar]
- Holick, M.F. Calcium and Vitamin D. Diagnostics and Therapeutics. Clin. Lab. Med. 2000, 20, 569–590. [Google Scholar] [CrossRef]
- Sanders, K.M.; Stuart, A.L.; Williamson, E.J.; Simpson, J.A.; Kotowicz, M.A.; Young, D.; Nicholson, G.C. Annual High-Dose Oral Vitamin D and Falls and Fractures in Older Women: A Randomized Controlled Trial. JAMA 2010, 303, 1815–1822. [Google Scholar] [CrossRef] [PubMed]
- Smith, H.; Anderson, F.; Raphael, H.; Maslin, P.; Crozier, S.; Cooper, C. Effect of Annual Intramuscular Vitamin D on Fracture Risk in Elderly Men and Women--a Population-Based, Randomized, Double-Blind, Placebo-Controlled Trial. Rheumatology 2007, 46, 1852–1857. [Google Scholar] [CrossRef] [PubMed]
- Kavathia, D.; Buckley, J.D.; Rao, D.; Rybicki, B.; Burke, R. Elevated 1, 25-Dihydroxyvitamin D Levels Are Associated with Protracted Treatment in Sarcoidosis. Respir. Med. 2010, 104, 564–570. [Google Scholar] [CrossRef]
- Jackson, R.D.; LaCroix, A.Z.; Gass, M.; Wallace, R.B.; Robbins, J.; Lewis, C.E.; Bassford, T.; Beresford, S.A.A.; Black, H.R.; Blanchette, P.; et al. Calcium plus Vitamin D Supplementation and the Risk of Fractures. N. Engl. J. Med. 2006, 354, 669–683, Correction in: N. Engl. J. Med. 2006, 354, 1102. [Google Scholar] [CrossRef] [PubMed]
- Zittermann, A.; Ernst, J.B.; Prokop, S.; Fuchs, U.; Dreier, J.; Kuhn, J.; Knabbe, C.; Birschmann, I.; Schulz, U.; Berthold, H.K.; et al. Effect of Vitamin D on All-Cause Mortality in Heart Failure (EVITA): A 3-Year Randomized Clinical Trial with 4000 IU Vitamin D Daily. Eur. Heart J. 2017, 38, 2279–2286. [Google Scholar] [CrossRef]
- Cianferotti, L.; Cricelli, C.; Kanis, J.A.; Nuti, R.; Reginster, J.-Y.; Ringe, J.D.; Rizzoli, R.; Brandi, M.L. The Clinical Use of Vitamin D Metabolites and Their Potential Developments: A Position Statement from the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO) and the International Osteoporosis Foundation (IOF). Endocrine 2015, 50, 12–26. [Google Scholar] [CrossRef]
- Ducharme, F.M.; Jensen, M.; Mailhot, G.; Alos, N.; White, J.; Rousseau, E.; Tse, S.M.; Khamessan, A.; Vinet, B. Impact of Two Oral Doses of 100,000 IU of Vitamin D3 in Preschoolers with Viral-Induced Asthma: A Pilot Randomised Controlled Trial. Trials 2019, 20, 138. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).