An Interdisciplinary Study of Lysozyme Interactions with Hexacyanoferrate(III)/(II) Ions
Abstract
1. Introduction
2. Results and Discussion
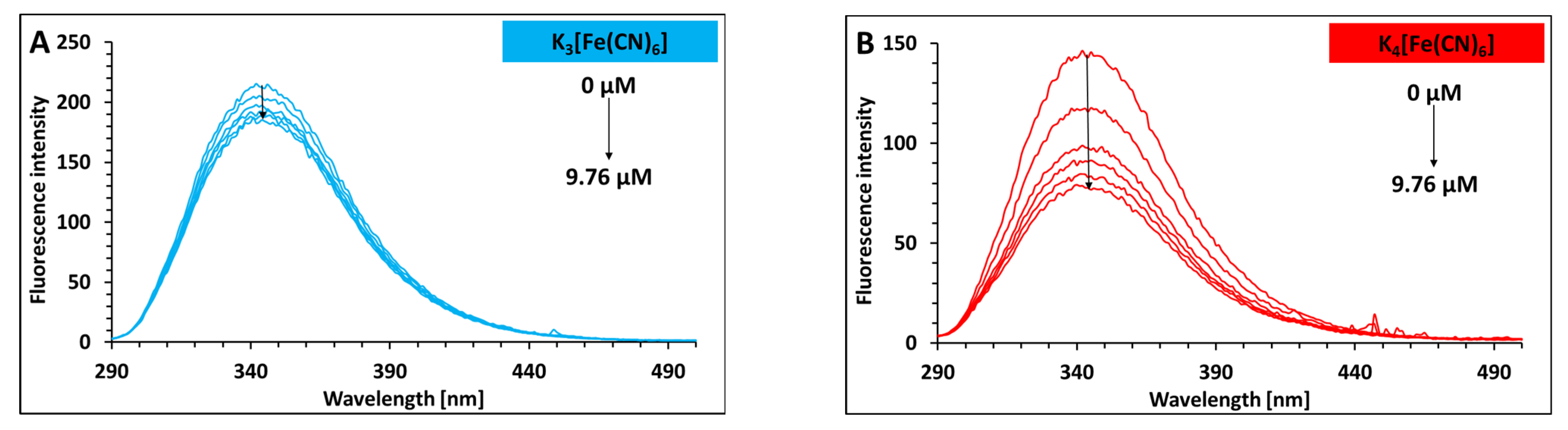
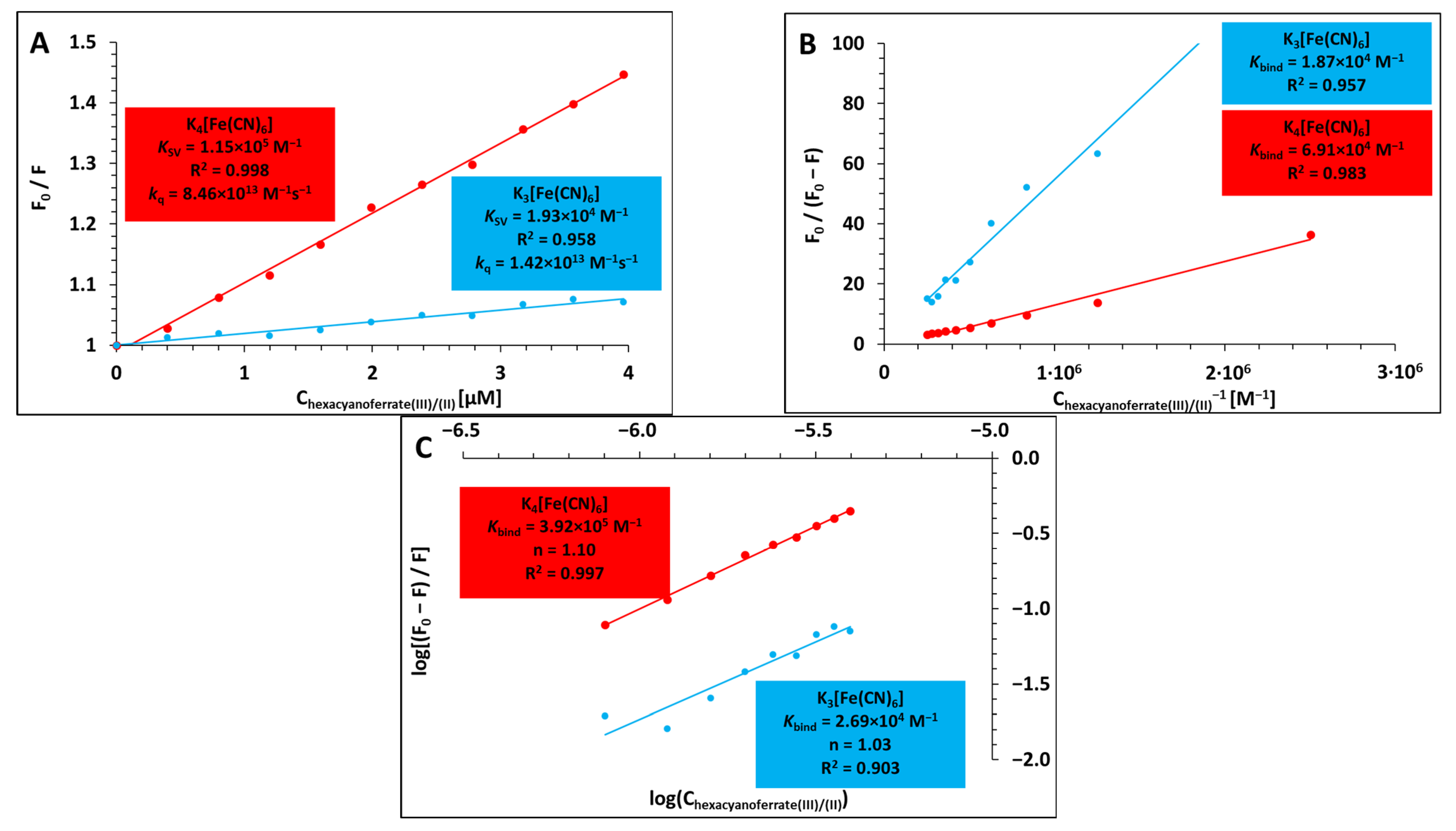
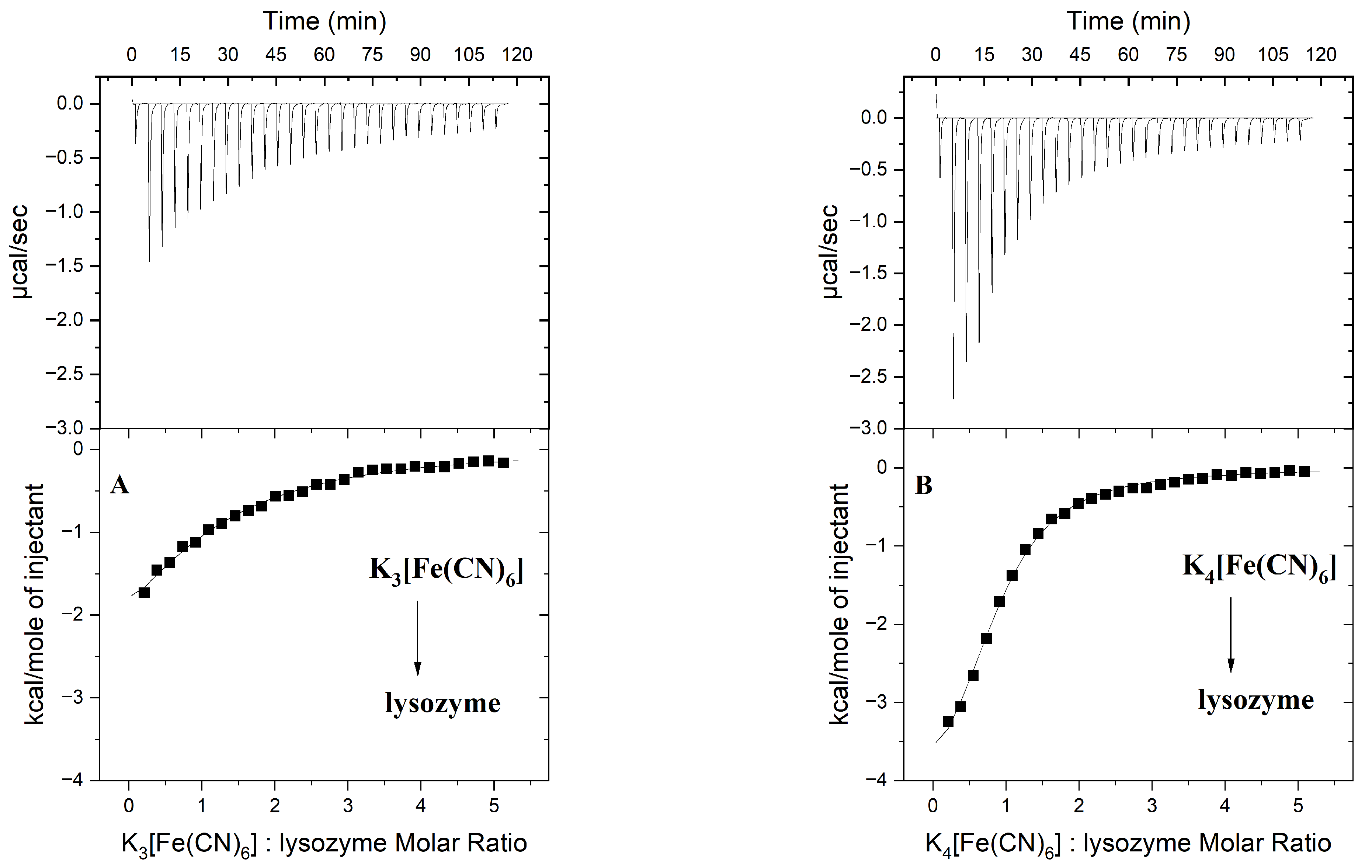
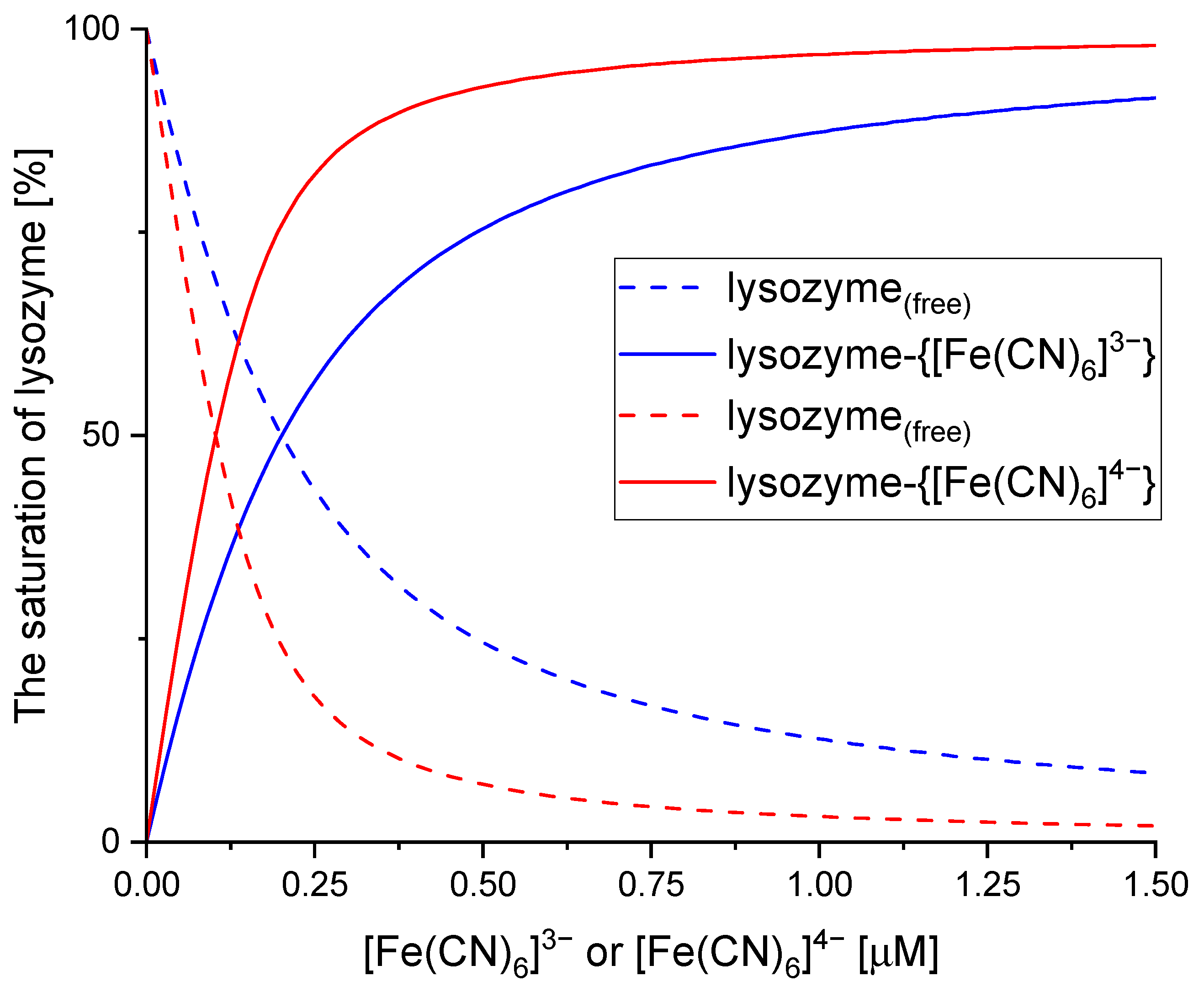
2.1. Binding Analysis of Hexacyanoferrate(III)/(II) Ions to Lysozyme
2.2. The Electrochemical Behaviour of Hexacyanoferrate(III)/(II) and Its Lysozyme Complexes
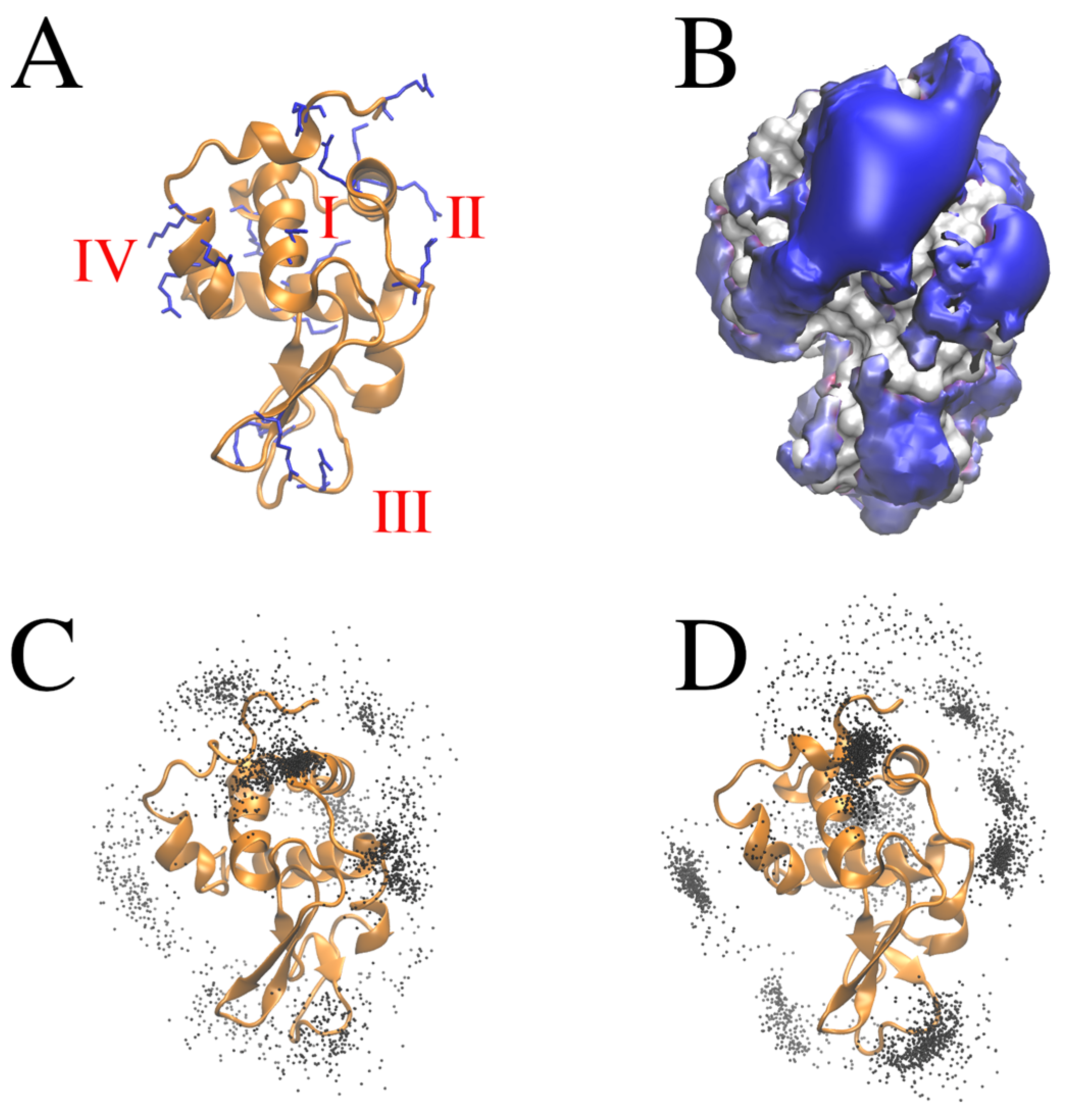
2.3. Binding Sites of Hexacyanoferrate(III)/(II) Ions to Lysozyme
2.4. Lysozyme Activity
2.4.1. Biochemical Studies
2.4.2. Biological Studies
3. Materials and Methods
3.1. Materials
3.2. Isothermal Titration Calorimetry (ITC)
3.3. Steady-State Fluorescence Spectroscopy (SF)
3.4. Circular Dichroism Spectroscopy (CD)
3.5. Molecular Dynamics (MD) Simulations
3.6. Biochemical and Biological Assays
3.7. Cyclic Voltammetry (CV)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, X.; Ren, Y.; Qu, G.; Wang, Z.; Yang, Y.; Ning, P. A review of environmental functional materials for cyanide removal by adsorption and catalysis. Inorg. Chem. Commun. 2023, 157, 111298. [Google Scholar] [CrossRef]
- Loos-Neskovic, C.; Ayrault, S.; Badillo, V.; Jimenez, B.; Garnier, E.; Fedoroff, M.; Jones, D.J.; Merinov, B. Structure of copper-potassium hexacyanoferrate (II) and sorption mechanisms of cesium. J. Solid State Chem. 2004, 177, 1817–1828. [Google Scholar] [CrossRef]
- Vincent, T.; Vincent, C.; Guibal, E. Immobilization of metal hexacyanoferrate ion-exchangers for the synthesis of metal ion sorbents—A mini-review. Molecules 2015, 20, 20582–20613. [Google Scholar] [CrossRef]
- Khramtsov, P.; Kropaneva, M.; Minin, A.; Bochkova, M.; Timganova, V.; Maximov, A.; Puzik, A.; Zamorina, S.; Rayev, M. Prussian blue nanozymes with enhanced catalytic activity: Size tuning and application in ELISA-like immunoassay. Nanomaterials 2022, 12, 1630. [Google Scholar] [CrossRef]
- Leal, J.M.; Garcia, B.; Domingo, P.L. Outer-sphere hexacyanoferrate (III) oxidation of organic substrates. Coord. Chem. Rev. 1998, 173, 79–131. [Google Scholar] [CrossRef]
- Puzanowska-Tarasiewicz, H.; Karpińska, J.; Kuźmicka, L. Analytical Applications of Reactions of Iron (III) and Hexacyanoferrate (III) with 2,10-Disubstituted Phenothiazines. Inter. J. Anal. Chem. 2009, 2009, 302696. [Google Scholar] [CrossRef]
- Ibargüen-López, H.; López-Balanta, B.; Betancourt-Buitrago, L.; Serna-Galvis, E.A.; Torres-Palma, R.A.; Machuca-Martínez, F.; Castilla-Acevedo, S.F. Degradation of hexacyanoferrate (III) ion by the coupling of the ultraviolet light and the activation of persulfate at basic pH. J. Environ. Chem. Eng. 2021, 9, 106233. [Google Scholar] [CrossRef]
- González-Ipia, N.; Bolaños-Chamorro, K.C.; Acuña-Bedoya, J.D.; Machuca-Martínez, F.; Castilla-Acevedo, S.F. Enhancement of the adsorption of hexacyanoferrate (III) ion on granular activated carbon by the addition of cations: A promissory application to mining wastewater treatment. J. Environ. Chem. Eng. 2020, 8, 104336. [Google Scholar] [CrossRef]
- Nong, C.; Li, X.; Xu, J. Systematic effect of different external metals of hexacyanoferrates on cesium adsorption behavior and mechanism. J. Radioanal. Nucl. Chem. 2023, 332, 1263–1275. [Google Scholar] [CrossRef]
- Berrettoni, M.; Mullaliu, A.; Giorgetti, M. Metal Hexacyanoferrate Absorbents for Heavy Metal Removal. In Green Adsorbents to Remove Metals, Dyes and Boron from Polluted Water; Springer International Publishing: Cham, Switzerland, 2020; pp. 171–194. [Google Scholar]
- Yang, Y.; Brownell, C.R.; Sadrieh, N.; May, J.C.; Del Grosso, A.V.; Lyon, R.C.; Faustino, P.J. Validation of an in vitro method for the determination of cyanide release from ferric-hexacyanoferrate: Prussian blue. J. Pharm. Biomed. Anal. 2007, 43, 1358–1363. [Google Scholar] [CrossRef] [PubMed]
- Stochel, G.; Martinez, P.; Van Eldik, R. Kinetics and mechanism of the oxidation of glutathione by hexacyanoferrate (III) in aqueous solution. J. Inorg. Biochem 1994, 54, 131–140. [Google Scholar] [CrossRef]
- Grabowska, O.; Samsonov, S.A.; Chmurzyński, L.; Wyrzykowski, D.; Żamojć, K. Investigation of hexacyanoferrate (II)/(III) charge-dependent interactions with bovine and human serum albumins. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2023, 293, 122505. [Google Scholar] [CrossRef]
- Lakowicz, J.R. (Ed.) Principles of Fluorescence Spectroscopy; Springer: Boston, MA, USA, 2006. [Google Scholar]
- Bergamo, A.; Sava, G. Lysozyme: A Natural Product with Multiple and Useful Antiviral Properties. Molecules 2024, 29, 652. [Google Scholar] [CrossRef]
- Nawaz, N.; Wen, S.; Wang, F.; Nawaz, S.; Raza, J.; Iftikhar, M.; Usman, M. Lysozyme and its application as antibacterial agent in food industry. Molecules 2022, 27, 6305. [Google Scholar] [CrossRef]
- Gehlen, M.H. The centenary of the Stern-Volmer equation of fluorescence quenching: From the single line plot to the SV quenching map. J. Photochem. Photobiol. C Photochem. Rev. 2020, 42, 100338. [Google Scholar] [CrossRef]
- Geethanjali, H.S.; Nagaraja, D.; Melavanki, R.M.; Kusanur, R.A. Fluorescence quenching of boronic acid derivatives by aniline in alcohols—A negative deviation from Stern–Volmer equation. J. Lumin. 2015, 167, 216–221. [Google Scholar] [CrossRef]
- Yamashita, S.; Nishimoto, E.; Yamasaki, N. The steady state and time-resolved fluorescence studies on the lysozyme-ligand interaction. Biosci. Biotechnol. Biochem. 1995, 59, 1255–1261. [Google Scholar] [CrossRef] [PubMed]
- Ware, W.R. Oxygen quenching of fluorescence in solution: An experimental study of the diffusion process. J. Phys. Chem. 1962, 66, 455–458. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, P.; Zhou, X.; Zhang, X.; Fang, T.; Liu, P.; Min, X.; Li, X. Thermodynamic and conformational investigation of the influence of CdTe QDs size on the toxic interaction with BSA. J. Photochem. Photobiol. A Chem. 2012, 230, 23–30. [Google Scholar] [CrossRef]
- Makowska, J.; Żamojć, K.; Wyrzykowski, D.; Wiczk, W.; Chmurzyński, L. Copper (II) complexation by fragment of central part of FBP28 protein from Mus musculus. Biophys. Chem. 2018, 241, 55–60. [Google Scholar] [CrossRef]
- Makowska, J.; Żamojć, K.; Wyrzykowski, D.; Żmudzińska, W.; Uber, D.; Wierzbicka, M.; Wiczk, W.; Chmurzyński, L. Probing the binding of Cu2+ ions to a fragment of the Aβ(1–42) polypeptide using fluorescence spectroscopy, isothermal titration calorimetry and molecular dynamics simulations. Biophys. Chem. 2016, 216, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Kandagal, P.B.; Ashoka, S.; Seetharamappa, J.; Shaikh, S.M.T.; Jadegoud, Y.; Ijare, O.B. Study of the interaction of an anticancer drug with human and bovine serum albumin: Spectroscopic approach. J. Pharm. Biomed. Anal. 2006, 41, 393–399. [Google Scholar] [CrossRef]
- Liu, E.H.; Qi, L.W.; Li, P. Structural relationship and binding mechanisms of five flavonoids with bovine serum albumin. Molecules 2010, 15, 9092–9103. [Google Scholar] [CrossRef]
- Wei, X.L.; Xiao, J.B.; Wang, Y.; Bai, Y. Which model based on fluorescence quenching is suitable to study the interaction between trans-resveratrol and BSA? Spectrochim. Acta A Mol. Biomol. Spectrosc. 2010, 75, 299–304. [Google Scholar] [CrossRef]
- Kandagal, P.B.; Shaikh, S.M.T.; Manjunatha, D.H.; Seetharamappa, J.; Nagaralli, B.S. Spectroscopic studies on the binding of bioactive phenothiazine compounds to human serum albumin. J. Photochem. Photobiol. A Chem. 2007, 189, 121–127. [Google Scholar] [CrossRef]
- Khan, A.B.; Khan, J.M.; Ali, M.S.; Khan, R.H. Interaction of amphiphilic drugs with human and bovine serum albumins. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2012, 97, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Butowska, K.; Żamojć, K.; Kogut, M.; Kozak, W.; Wyrzykowski, D.; Wiczk, W.; Czub, J.; Piosik, J.; Rak, J. The product of matrix metalloproteinase cleavage of doxorubicin conjugate for anticancer drug delivery: Calorimetric, spectroscopic, and molecular dynamics studies on peptide–doxorubicin binding to DNA. Int. J. Mol. Sci. 2020, 21, 6923. [Google Scholar] [CrossRef] [PubMed]
- Mandeville, J.S.; Froehlich, E.; Tajmir-Riahi, H.A. Study of curcumin and genistein interactions with human serum albumin. J. Pharm. Biomed. Anal. 2009, 49, 468–474. [Google Scholar] [CrossRef] [PubMed]
- Kumari, M.; Maurya, J.K.; Singh, U.K.; Khan, A.B.; Ali, M.; Singh, P.; Patel, R. Spectroscopic and docking studies on the interaction between pyrrolidinium based ionic liquid and bovine serum albumin. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 124, 349–356. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, X.; Zhou, L.; Yang, L.; Xia, G.; Chen, Z.; Duan, M. Synthesis and binding with BSA of a new gemini surfactant. Colloids Surf. A Physicochem. Eng. Asp. 2013, 436, 1159–1169. [Google Scholar] [CrossRef]
- Cheah, M.H.; Chernev, P. Electrochemical oxidation of ferricyanide. Sci. Rep. 2021, 11, 23058. [Google Scholar] [CrossRef]
- Vaney, M.C.; Broutin, I.; Retailleau, P.; Douangamath, A.; Lafont, S.; Hamiaux, C.; Prangé, T.; Ducruix, A.; Ries-Kautt, M. Structural effects of monovalent anions on polymorphic lysozyme crystals. Acta Crystallogr. D Biol. Crystallogr. 2001, 57, 929–940. [Google Scholar] [CrossRef]
- Lin, K.C.; Wey, M.T.; Kan, L.S.; Shiuan, D. Characterization of the interactions of lysozyme with DNA by surface plasmon resonance and circular dichroism spectroscopy. Appl. Biochem. Biotechnol. 2009, 158, 631–641. [Google Scholar] [CrossRef]
- Zalar, M.; Bye, J.; Curtis, R. Nonspecific binding of adenosine tripolyphosphate and tripolyphosphate modulates the phase behavior of lysozyme. J. Am. Chem. Soc. 2023, 145, 929–943. [Google Scholar] [CrossRef] [PubMed]
- Held, J.; van Smaalen, S. The active site of hen egg-white lysozyme: Flexibility and chemical bonding. Acta Crystallogr. Sec. D Biol. Crystallogr. 2014, 70, 1136–1146. [Google Scholar] [CrossRef] [PubMed]
- Silhavy, T.J.; Kahne, D.; Walker, S. The bacterial cell envelope. Cold Spring Harb. Perspect. Biol. 2010, 2, a000414. [Google Scholar] [CrossRef] [PubMed]
- Ragland, S.A.; Criss, A.K. From bacterial killing to immune modulation: Recent insights into the functions of lysozyme. PLoS Pathog. 2017, 13, e1006512. [Google Scholar] [CrossRef]
- Gill, S.C.; Von Hippel, P.H. Calculation of protein extinction coefficients from amino acid sequence data. Anal. Biochem. 1989, 182, 319–326. [Google Scholar] [CrossRef]
- Grabowska, O.; Samsonov, S.A.; Kogut-Günthel, M.M.; Żamojć, K.; Wyrzykowski, D. Elucidation of binding mechanisms of bovine serum albumin and 1-alkylsulfonates with different hydrophobic chain lengths. Int. J. Biol. Macromol. 2024, 266, 131134. [Google Scholar] [CrossRef]
- Case, D.A.; Belfon, K.; Ben-Shalom, I.Y.; Brozell, S.R.; Cerutti, D.S.; Cheatham, T.E., III; Cruzeiro, V.W.D.; Darden, T.A.; Duke, R.E.; Giambasu, G.; et al. AMBER 2020; University of California, San Francisco: San Francisco, CA, USA, 2020. [Google Scholar]
- Maier, J.A.; Martinez, C.; Kasavajhala, K.; Wickstrom, L.; Hauser, K.E.; Simmerling, C. ff14SB: Improving the Accuracy of Protein Side Chain and Backbone Parameters from ff99SB. J. Chem. Theory Comput. 2015, 11, 3696–3713. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wolf, R.M.; Caldwell, J.W.; Kollman, P.A.; Case, D.A. Development and testing of a general Amber force field. J. Comput. Chem. 2004, 25, 1157–1174. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org/ (accessed on 17 May 2025).
- Baskova, I.P.; Kharitonova, O.V.; Zavalova, L.L. Lysozyme activity of the salivary gland secretion of the medicinal leeches H. verbana, H. medicinalis, and H. orientalis. Biochem. Suppl. Ser. B Biomed. Chem. 2012, 6, 138–143. [Google Scholar] [CrossRef]
- Yang, Y.; Hamaguchi, K. Hydrolysis of 4-methylumbelliferyl N-acetyl-chitotetraoside catalyzed by hen lysozyme. J. Biochem. 1980, 88, 829–836. [Google Scholar] [CrossRef]
- Olp, M.D.; Kalous, K.S.; Smith, B.C. ICEKAT: An interactive online tool for calculating initial rates from continuous enzyme kinetic traces. BMC Bioinform. 2020, 21, 186. [Google Scholar] [CrossRef]
- Bursch, K.L.; Olp, M.D.; Smith, B.C. Analysis of continuous enzyme kinetic data using ICEKAT. Methods Enzymol. 2023, 690, 109–129. [Google Scholar] [CrossRef] [PubMed]
| Parameter | Lysozyme | |
|---|---|---|
| K3[Fe(CN)6] | K4[Fe(CN)6] | |
| n (number of binding sites) | 1.03 | 1.10 |
| N ** (stoichiometry) | 1.01 (±0.01) | 0.86 (±0.02) |
| log(KSV) *1 | 4.29 | 5.06 |
| log(K) *2 | 4.27 | 4.84 |
| log(K) *3 | 4.43 | 5.59 |
| log(K) ** | 3.90 (±0.02) | 4.56 (±0.03) |
| ΔG ** [kJ mol−1] | −22.30 (±0.13) | −26.02 (±0.17) |
| ΔH ** [kJ mol−1] | −16.61 (±0.29) | −19.62 (±0.59) |
| TΔS ** [kJ mol−1] | 5.69 | 6.40 |
| Reagent | N | Activity (Moles min−1 mL−1) | Activity (% Vs. Lysozyme) |
|---|---|---|---|
| lysozyme | 5 | 21.9 (±1.20) a | 100 (±5.5) |
| lysozyme-[Fe(CN)6]3− | 4 | 22.0 (±1.59) b | 101 (±7.3) |
| lysozyme-[Fe(CN)6]4− | 4 | 17.8 (±3.24) ab | 82 (±14.8) |
| [Fe(CN)6]3− | 3 | 0.3 (±0.13) | 1 (±0.6) |
| [Fe(CN)6]4− | 3 | 0.2 (±0.19) | 1 (±0.9) |
| Caco buffer | 3 | 0.0 (±0.00) | 0 (±0.0) |
| Coefficient | Unit | Lysozyme | Lysozyme- [Fe(CN)6]3− | Lysozyme-[Fe(CN)6]4− |
|---|---|---|---|---|
| [E] | µM | 20 | 20 | 20 |
| Km | µM | 19.12 (±0.40) | 18.57 (±3.29) | 19.04 (±2.15) |
| Vmax | nM min−1 | 5.39 (±0.23) | 5.89 (±0.37) | 5.28 (±0.21) |
| kcat | min−1 | 0.270 | 0.295 | 0.264 |
| kcat/Km | nM−1 min−1 | 0.014 | 0.016 | 0.014 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grabowska, O.; Żamojć, K.; Kloska, A.; Niedziałkowski, P.; Samsonov, S.A.; Wyrzykowski, D. An Interdisciplinary Study of Lysozyme Interactions with Hexacyanoferrate(III)/(II) Ions. Int. J. Mol. Sci. 2025, 26, 8511. https://doi.org/10.3390/ijms26178511
Grabowska O, Żamojć K, Kloska A, Niedziałkowski P, Samsonov SA, Wyrzykowski D. An Interdisciplinary Study of Lysozyme Interactions with Hexacyanoferrate(III)/(II) Ions. International Journal of Molecular Sciences. 2025; 26(17):8511. https://doi.org/10.3390/ijms26178511
Chicago/Turabian StyleGrabowska, Ola, Krzysztof Żamojć, Anna Kloska, Paweł Niedziałkowski, Sergey A. Samsonov, and Dariusz Wyrzykowski. 2025. "An Interdisciplinary Study of Lysozyme Interactions with Hexacyanoferrate(III)/(II) Ions" International Journal of Molecular Sciences 26, no. 17: 8511. https://doi.org/10.3390/ijms26178511
APA StyleGrabowska, O., Żamojć, K., Kloska, A., Niedziałkowski, P., Samsonov, S. A., & Wyrzykowski, D. (2025). An Interdisciplinary Study of Lysozyme Interactions with Hexacyanoferrate(III)/(II) Ions. International Journal of Molecular Sciences, 26(17), 8511. https://doi.org/10.3390/ijms26178511







