Biomass-Derived Magnetic Fe3O4/Biochar Nanoparticles from Baobab Seeds for Sustainable Wastewater Dye Remediation
Abstract
1. Introduction
2. Results and Discussions
2.1. Characterization
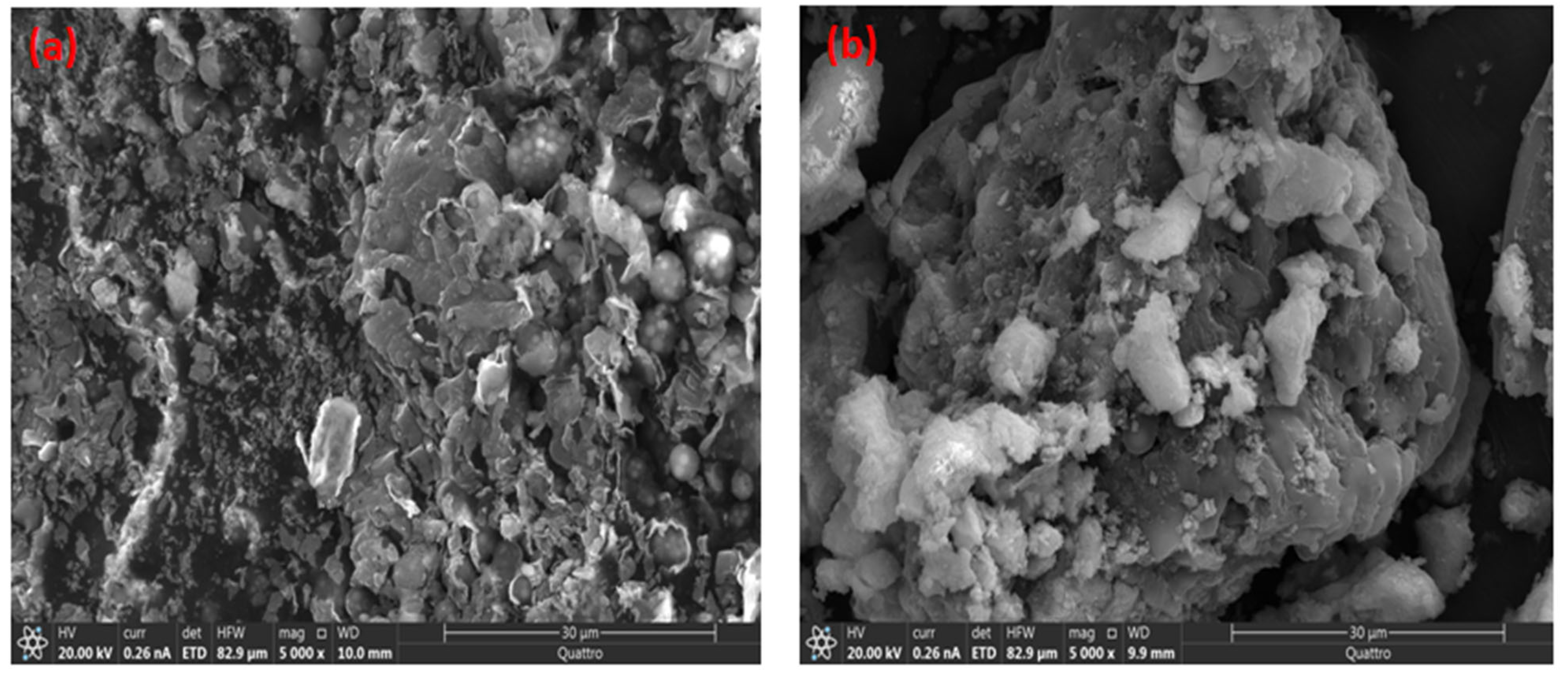
2.1.1. SEM Characterization
2.1.2. FTIR Characterization
2.1.3. TGA Analysis
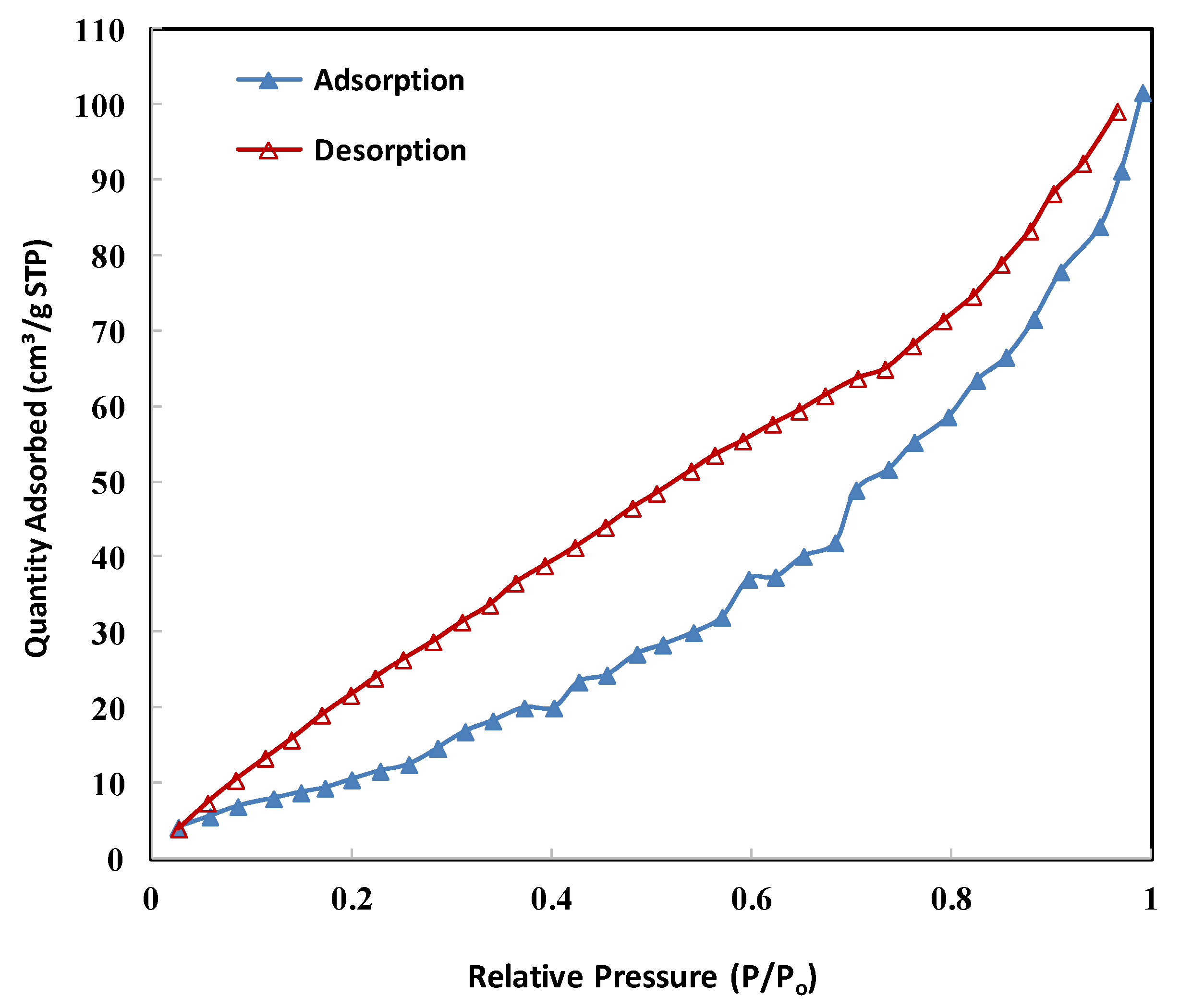
2.1.4. BET Analysis
2.2. Degradation Studies
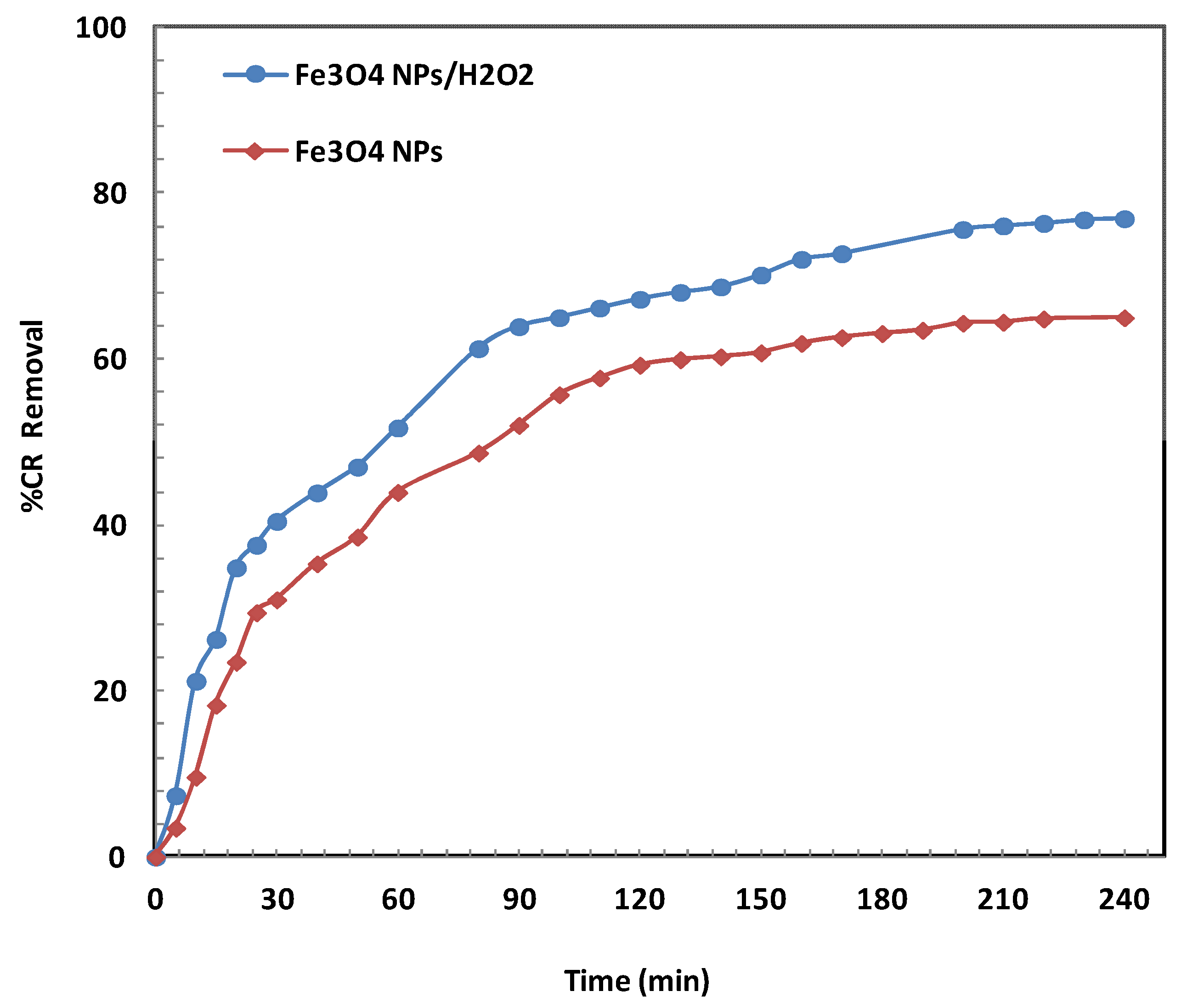
2.2.1. Effect of Contact Time
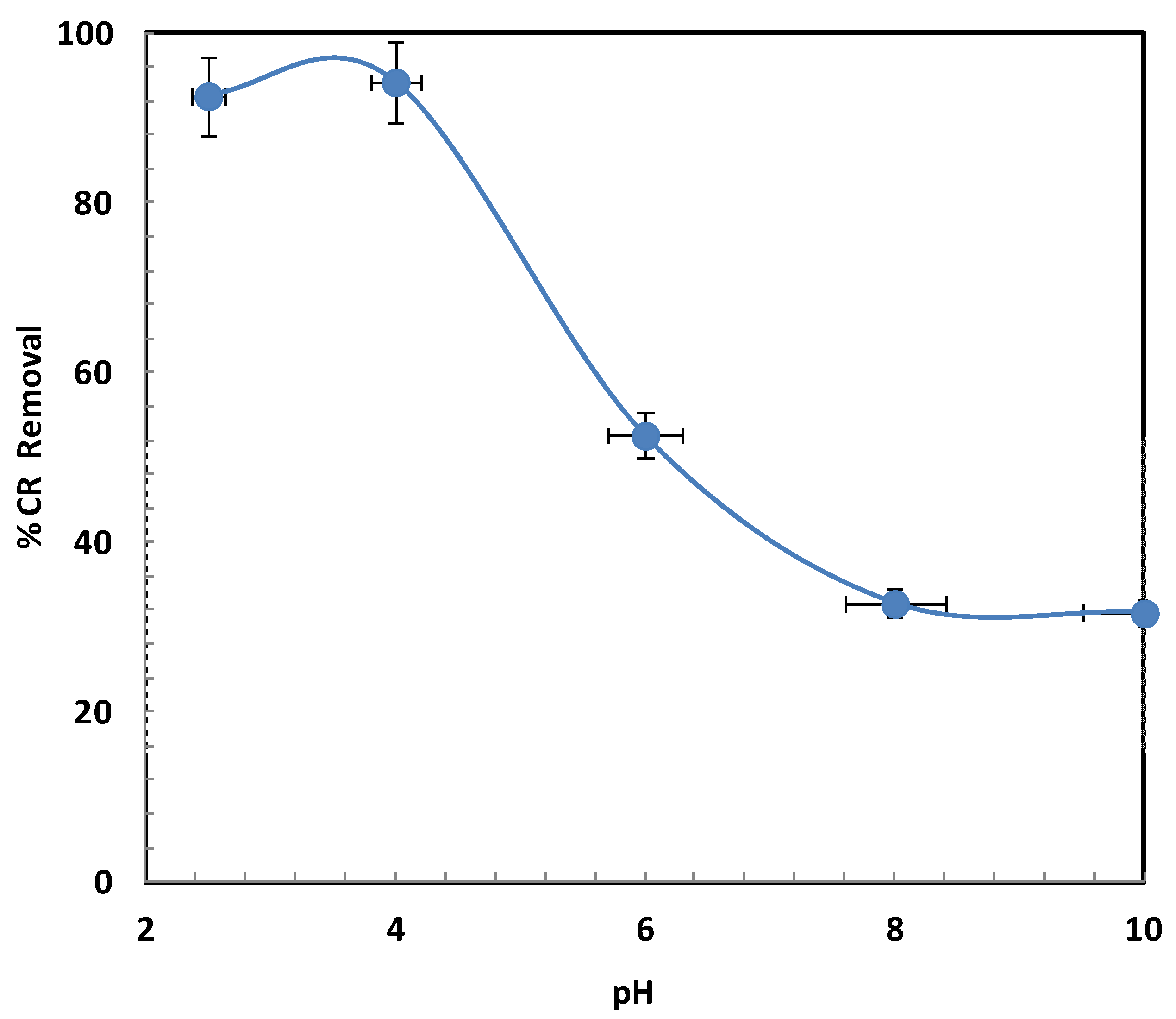
2.2.2. Influence of pH
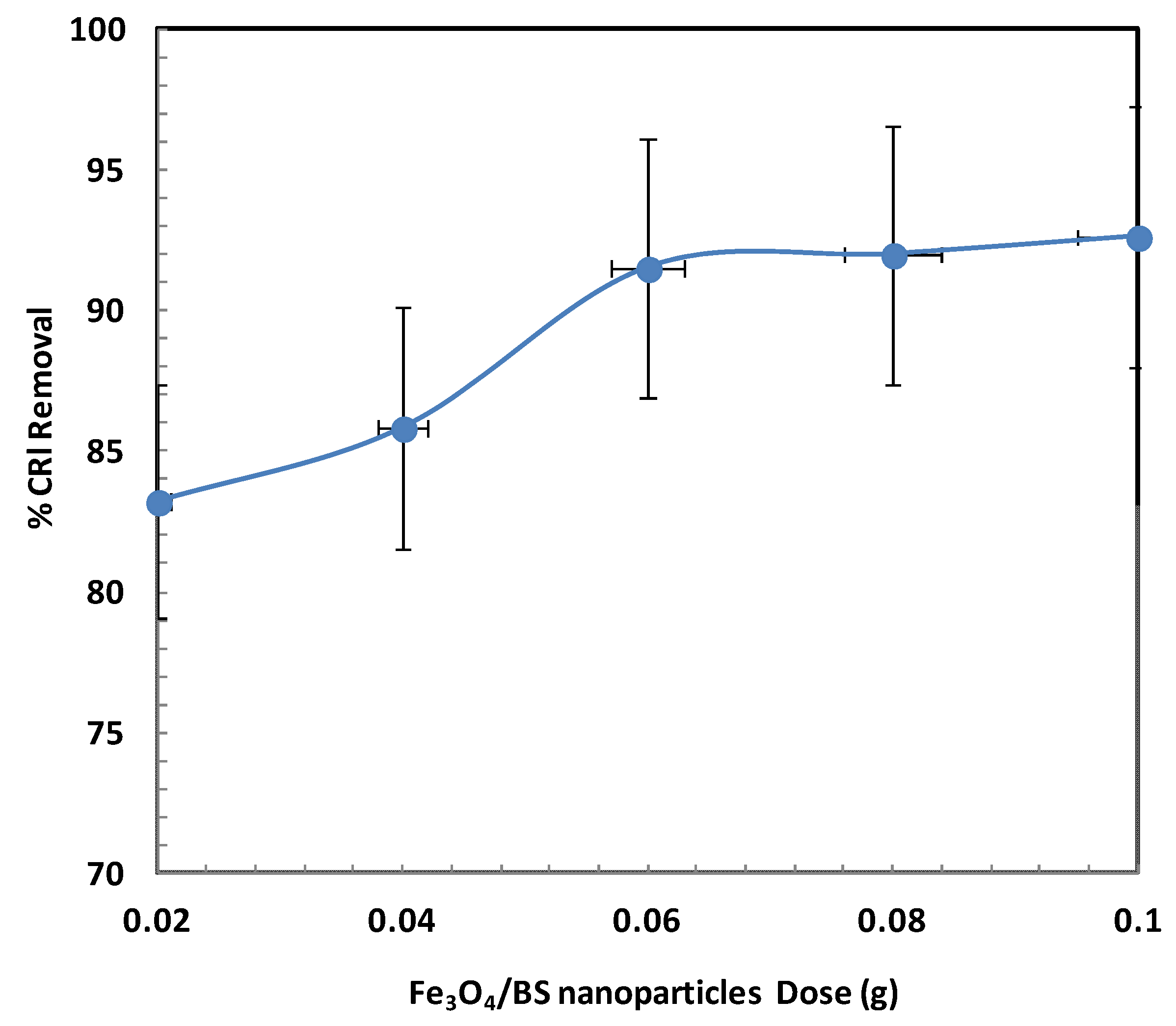
2.2.3. Influence of Adsorbent Dose
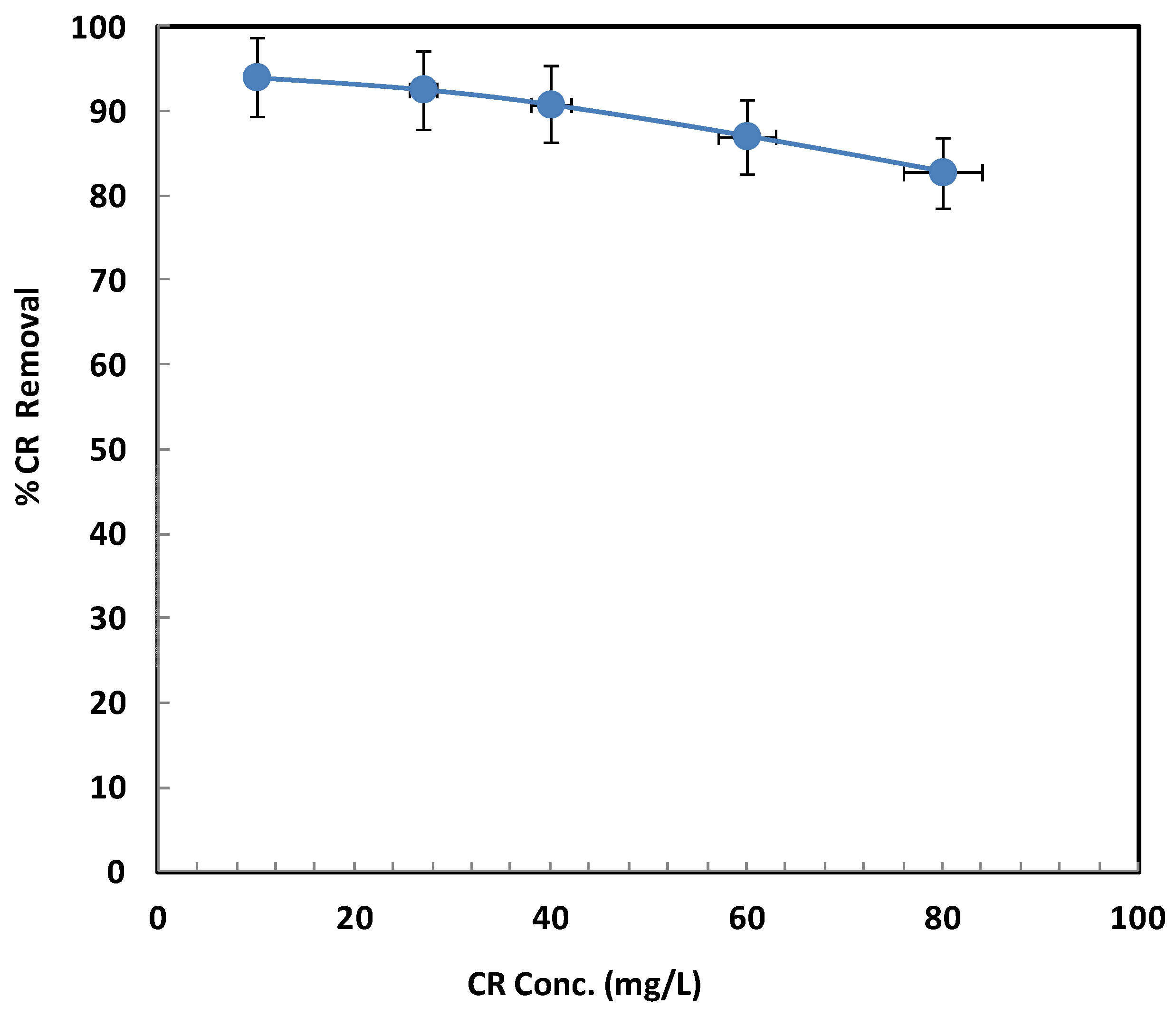
2.2.4. Influence of Initial Concentration
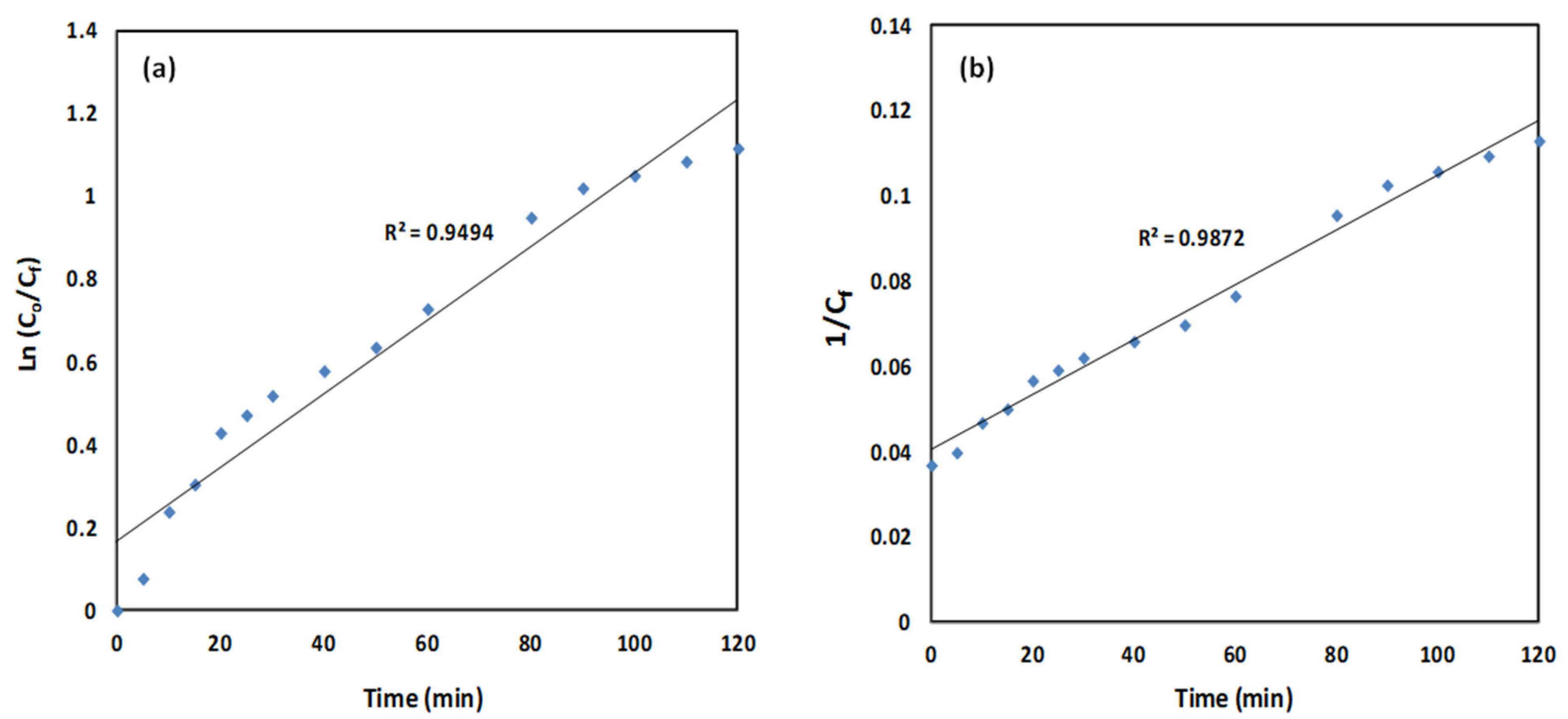
2.2.5. Adsorption Kinetics
2.2.6. Degradation Mechanism of CR Dye
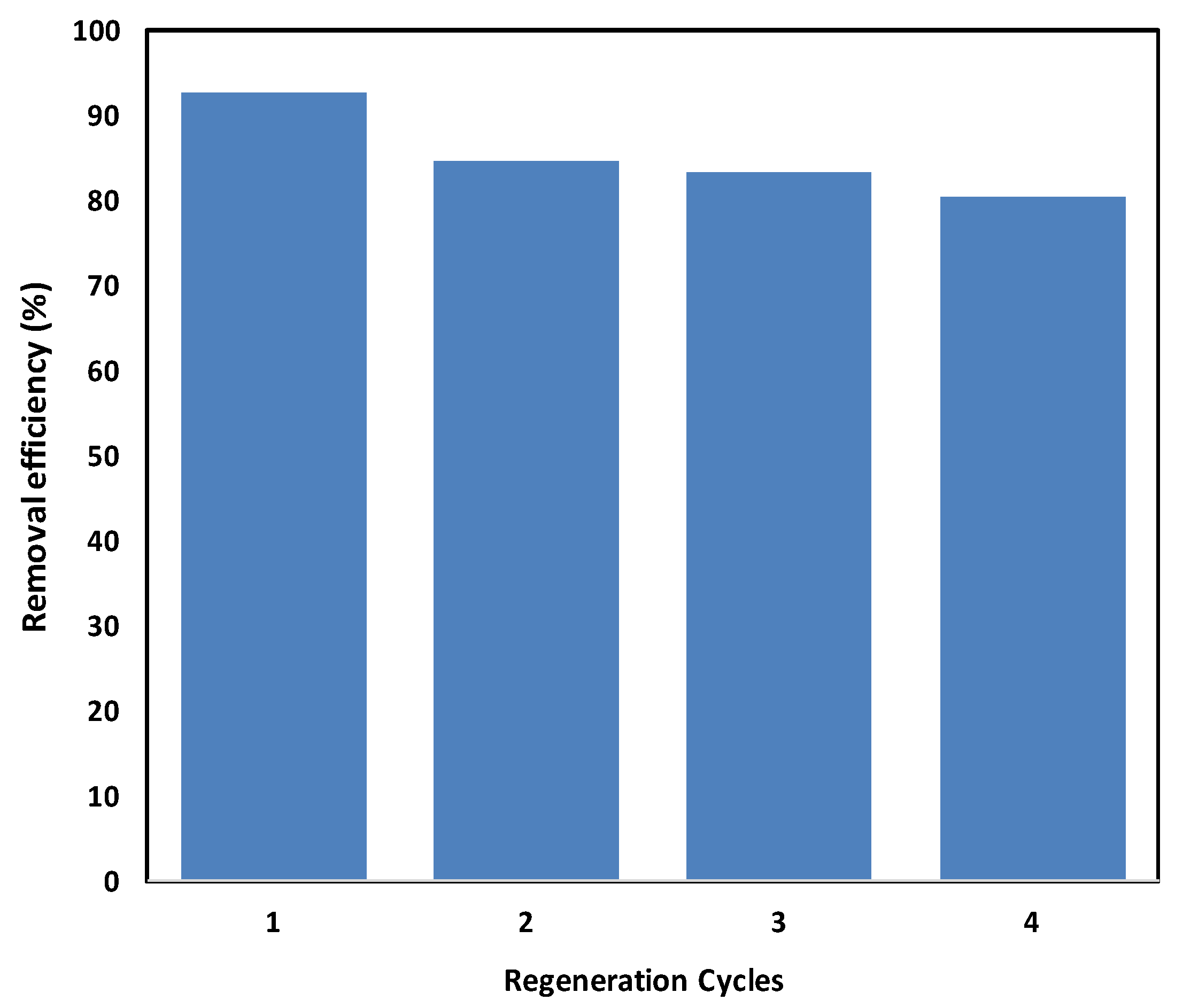
2.2.7. Recycling Test
3. Materials and Methods
3.1. Chemical
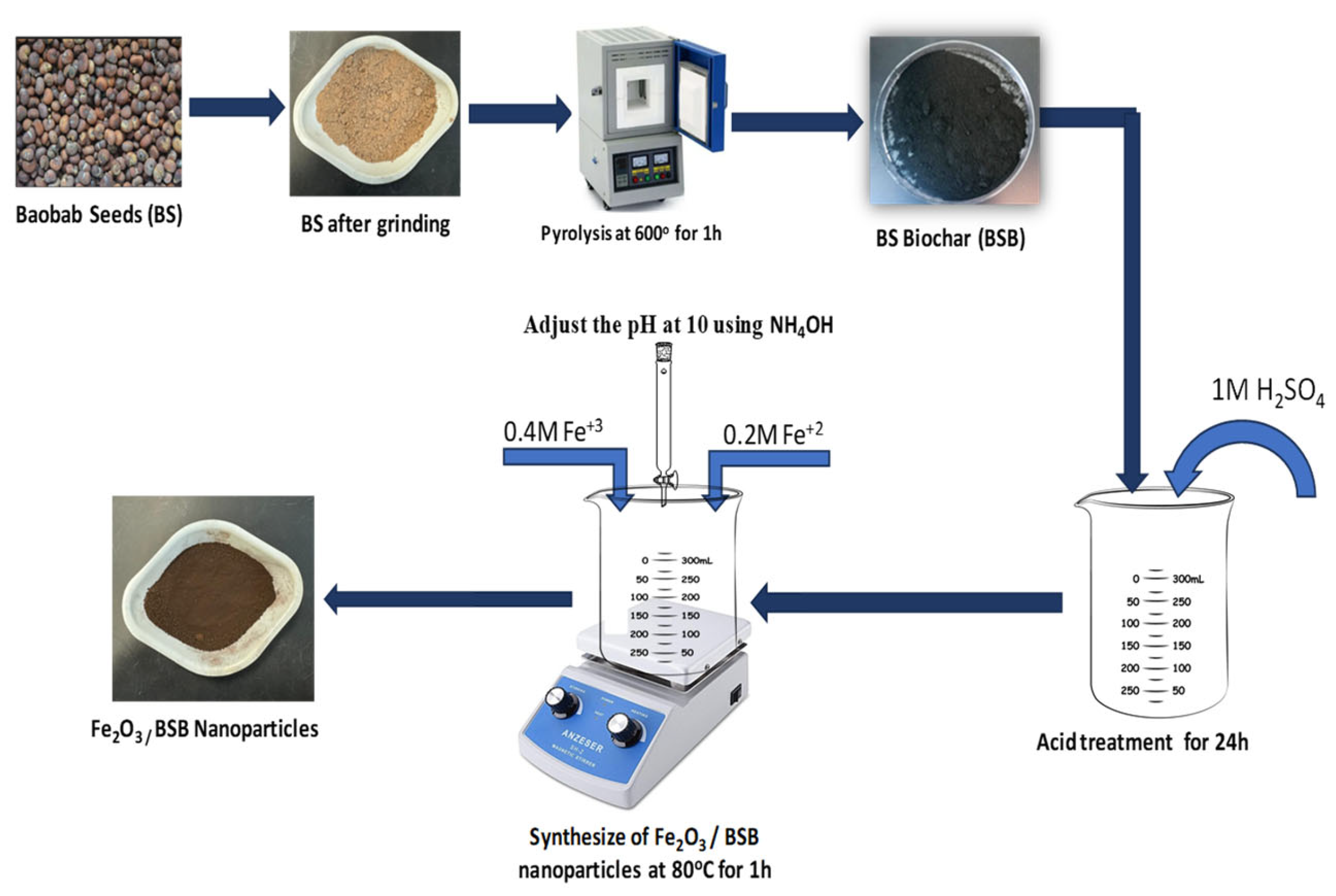
3.2. Synthesis of Fe3O4/Biochar Nanoparticles
3.3. Instrument Analysis
3.4. Experimental Procedures
4. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Elgarahy, A.M.; Elwakeel, K.Z.; Mohammad, S.H.; Elshoubaky, G.A. A critical review of biosorption of dyes, heavy metals and metalloids from wastewater as an efficient and green process. Clean. Eng. Technol. 2021, 4, 100209. [Google Scholar] [CrossRef]
- Dutta, S.; Gupta, B.; Srivastava, S.K.; Gupta, A.K. Recent advances on the removal of dyes from wastewater using various adsorbents: A critical review. Mater. Adv. 2021, 2, 4497–4531. [Google Scholar] [CrossRef]
- Eroi, S.N.; Ello, A.S.; Diabaté, D.; Ossonon, D.B. Heterogeneous WO3/H2O2 system for degradation of Indigo Carmin dye from aqueous solution. South Afr. J. Chem. Eng. 2021, 37, 53–60. [Google Scholar] [CrossRef]
- Yang, H.R.; Li, S.S.; An, Q.D.; Zhai, S.R.; Xiao, Z.Y.; Zhang, L.P. Facile transformation of carboxymethyl cellulose beads into hollow composites for dye adsorption. Int. J. Biol. Macromol. 2021, 190, 919–926. [Google Scholar] [CrossRef]
- Khorramfar, S.; Mahmoodi, N.M.; Arami, M.; Bahrami, H. Oxidation of dyes from colored wastewater using activated carbon/hydrogen peroxide. Desalination 2011, 279, 183–189. [Google Scholar] [CrossRef]
- Deng, Y.; Zhao, R. Advanced oxidation processes (AOPs) in wastewater treatment. Curr. Pollut. Rep. 2015, 1, 167–176. [Google Scholar] [CrossRef]
- Bokhari, T.H.; Ahmad, N.; Jilani, M.I.; Saeed, M.; Usman, M.; Haq, A.U.; Rehman, R.; Iqbal, M.; Nazir, A.; Javed, T. UV/H2O2, UV/H2O2/SnO2 and Fe/H2O2 based advanced oxidation processes for the degradation of disperse violet 63 in aqueous medium. Mater. Res. Express 2020, 7, 015531. [Google Scholar] [CrossRef]
- Ma, D.; Yi, H.; Lai, C.; Liu, X.; Huo, X.; An, Z.; Li, L.; Fu, Y.; Li, B.; Zhang, M.; et al. Critical review of advanced oxidation processes in organic wastewater treatment. Chemosphere 2021, 275, 130104. [Google Scholar] [CrossRef]
- Karami, M.A.; Sharafi, K.; Asadi, A.; Bagheri, A.; Yosefvand, F.; Charganeh, S.S.; Mirzaei, N.; Velayati, A. Degradation of Reactive Red 198 (RR198) from aqueous solutions by advanced oxidation processes (AOPS): O3, H2O2/O3 and H2O2/ultrasonic. Bulg. Chem. Commun. 2016, 48, 43–49. [Google Scholar]
- Arshad, R.; Bokhari, T.H.; Javed, T.; Bhatti, I.A.; Rasheed, S.; Iqbal, M.; Nazir, A.; Naz, S.; Khan, M.I.; Khosa, M.K.; et al. Degradation product distribution of Reactive Red-147 dye treated by UV/H2O2/TiO2 advanced oxidation process. J. Mater. Res. Technol. 2020, 9, 3168–3178. [Google Scholar] [CrossRef]
- Ren, Z.; Chen, F.; Wang, B.; Song, Z.; Zhou, Z.; Ren, D. Magnetic biochar from alkali-activated rice straw for removal of rhodamine B from aqueous solution. Environ. Eng. Res. 2020, 25, 536–544. [Google Scholar] [CrossRef]
- Qin, Y.; Luo, J.; Zhao, Y.; Yao, C.; Li, Y.; An, Q.; Xiao, Z.; Zhai, S. Dual-wastes derived biochar with tailored surface features for highly efficient p-nitrophenol adsorption. J. Clean. Prod. 2022, 353, 131571. [Google Scholar] [CrossRef]
- Wang, F.; Liu, L.; Liu, F.; Wang, L.; Ouyang, T.; Chang, C. Facile one-step synthesis of magnetically modified biochar with enhanced removal capacity for hexavalent chromium from aqueous solution. J. Taiwan Inst. Chem. Eng. 2017, 81, 414–418. [Google Scholar] [CrossRef]
- Yap, M.; Mubarak, N.; Sahu, J.; Abdullah, E. Microwave induced synthesis of magnetic biochar from agricultural biomass for removal of lead and cadmium from wastewater. J. Ind. Eng. Chem. 2017, 45, 287–295. [Google Scholar] [CrossRef]
- Santhosh, C.; Daneshvar, E.; Tripathi, K.M.; Baltrėnas, P.; Kim, T.; Baltrėnaitė, E.; Bhatnagar, A. Synthesis and characterization of magnetic biochar adsorbents for the removal of Cr (VI) and Acid orange 7 dye from aqueous solution. Environ. Sci. Pollut. Res. 2020, 27, 32874–32887. [Google Scholar] [CrossRef]
- Eltaweil, A.S.; Mohamed, H.A.; Abd El-Monaem, E.M.; El-Subruiti, G.M. Mesoporous magnetic biochar composite for enhanced adsorption of malachite green dye: Characterization, adsorption kinetics, thermodynamics and isotherms. Adv. Powder Technol. 2020, 31, 1253–1263. [Google Scholar] [CrossRef]
- Nguyen, V.H.; Van, H.T.; Nguyen, V.Q.; Dam, X.V.; Hoang, L.P.; Ha, L.T. Magnetic Fe3O4 nanoparticle biochar derived from pomelo peel for reactive Red 21 adsorption from aqueous solution. J. Chem. 2020, 2020, 3080612. [Google Scholar] [CrossRef]
- Mkelemi, M.J.; Mwaijengo, G.N.; Rwiza, M.J. “Tree of life”: How baobab seed-derived biochar could lead to water safety for underprivileged communities through heavy metal (Fe) removal–SDG 6. Environmental Science: Advances 2024, 3, 1735–1745. [Google Scholar] [CrossRef]
- Yahaya, N.P.; Saad, Y.A.; Abubakar, A. Adsorption Studies of Pb2+, Cu2+ and Cr3+ from Aqueous Solution Using Azadirachta Indica (Neem) Seed Husk and Adansonia Digitata (Baobab) Seeds. Sci. J. Chem. 2024, 12, 73–85. [Google Scholar] [CrossRef]
- Zhang, M.; Gao, B.; Varnoosfaderani, S.; Hebard, A.; Yao, Y.; Inyang, M. Preparation and characterization of a novel magnetic biochar for arsenic removal. Bioresour. Technol. 2013, 130, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.A.; Ahmad, N.; Bello, O.S. Adsorption kinetic studies for the removal of synthetic dye using durian seed activated carbon. J. Dispers. Sci. Technol. 2015, 36, 670–684. [Google Scholar] [CrossRef]
- Urgel, J.J.; Briones, J.M.; Diaz, E.B., Jr.; Dimaculangan, K.M.; Rangel, K.L.; Lopez, E.C. Removal of diesel oil from water using biochar derived from waste banana peels as adsorbent. Carbon Res. 2024, 3, 13. [Google Scholar] [CrossRef]
- Chaukura, N.; Murimba, E.C.; Gwenzi, W. Synthesis, characterisation and methyl orange adsorption capacity of ferric oxide–biochar nano-composites derived from pulp and paper sludge. Appl. Water Sci. 2017, 7, 2175–2186. [Google Scholar] [CrossRef]
- Peršić, A.; Popov, N.; Kratofil Krehula, L.; Krehula, S. The influence of different hematite (α-Fe2O3) particles on the thermal, optical, mechanical, and barrier properties of LDPE/hematite composites. Materials 2023, 16, 706. [Google Scholar] [CrossRef]
- Hossain, N.; Nizamuddin, S.; Griffin, G.; Selvakannan, P.; Mubarak, N.M.; Mahlia, T.M. Synthesis and characterization of rice husk biochar via hydrothermal carbonization for wastewater treatment and biofuel production. Sci. Rep. 2020, 10, 18851. [Google Scholar] [CrossRef] [PubMed]
- Alsaiari, N.S.; Alsaiari, M.S.; Alzahrani, F.M.; Amari, A.; Tahoon, M.A. Synthesis, characterization, and application of the novel nanomagnet adsorbent for the removal of Cr (vi) ions. Rev. Adv. Mater. Sci. 2023, 62, 20230145. [Google Scholar] [CrossRef]
- Shahwan, T.; Abu Sirriah, S.; Nairat, M.; Boyaci, E.; Eroglu, A.E.; Scott, T.B.; Hallam, K.R. Green synthesis of iron nanoparticles and their application as a Fenton-like catalyst for the degradation of aqueous cationic and anionic dyes. Chem. Eng. J. 2011, 172, 258–266. [Google Scholar] [CrossRef]
- Van, H.T.; Nguyen, T.M.; Thao, V.T.; Vu, X.H.; Nguyen, T.V.; Nguyen, L.H. Applying activated carbon derived from coconut shell loaded by silver nanoparticles to remove methylene blue in aqueous solution. Water Air Soil Pollut. 2018, 229, 393. [Google Scholar] [CrossRef]
- Da’na, E.; Taha, A.; Afkar, E. Green synthesis of iron nanoparticles by Acacia nilotica pods extract and its catalytic, adsorption, and antibacterial activities. Appl. Sci. 2018, 8, 1922. [Google Scholar] [CrossRef]
- Jain, S.N.; Tamboli, S.R.; Sutar, D.S.; Jadhav, S.R.; Marathe, J.V.; Shaikh, A.A.; Prajapati, A.A. Batch and continuous studies for adsorption of anionic dye onto waste tea residue: Kinetic, equilibrium, breakthrough and reusability studies. J. Clean. Prod. 2020, 252, 119778. [Google Scholar] [CrossRef]
- Daffalla, S.; Taha, A.; Da’na, E.; El-Aassar, M.R. Sustainable Banana-Waste-Derived Biosorbent for Congo Red Removal from Aqueous Solutions: Kinetics, Equilibrium, and Breakthrough Studies. Water 2024, 16, 1449. [Google Scholar] [CrossRef]
- Abbas, M.; Trari, M. Kinetic, equilibrium and thermodynamic study on the removal of Congo Red from aqueous solutions by adsorption onto apricot stone. Process Saf. Environ. Prot. 2015, 98, 424–436. [Google Scholar] [CrossRef]
- Nasser, A.O.; Kareem, S.L. Removal of Congo red from aqueous solution using lemon peel-Fe3O4 nanocomposite adsorbent. Biomass Convers. Biorefinery 2024, 14, 23183–23193. [Google Scholar] [CrossRef]
- Mahmud, N.; Yasser, L.; Mahmoud, R.B.; Benamor, A. Adsorption of Congo Red Dye Using Activated Carbon-Fe3O4 Composite. In Proceedings of the 2nd International Conference on Civil Infrastructure and Construction, Doha, Qatar, 5–8 February 2023. [Google Scholar]
- Mansour, D.; Alblawi, E.; Alsukaibi, A.K.; Al Shammari, B. Removal of Congo red dye by electrochemical advanced oxidation process: Optimization, degradation pathways, and mineralization. Sustain. Water Resour. Manag. 2024, 10, 41. [Google Scholar] [CrossRef]
- Jain, S.N.; Gogate, P.R. Efficient removal of Acid Green 25 dye from wastewater using activated Prunus Dulcis as biosorbent: Batch and column studies. J. Environ. Manag. 2018, 210, 226–238. [Google Scholar] [CrossRef]
- Shaban, M.; Abukhadra, M.R.; Khan, A.A.; Jibali, B.M. Removal of Congo red, methylene blue and Cr (VI) ions from water using natural serpentine. J. Taiwan Inst. Chem. Eng. 2018, 82, 102–116. [Google Scholar] [CrossRef]
- Zhao, F.; Shan, R.; Li, S.; Yuan, H.; Chen, Y. Characterization and Co-adsorption mechanism of magnetic clay-biochar composite for de-risking Cd (II) and methyl orange contaminated water. Int. J. Mol. Sci. 2023, 24, 5755. [Google Scholar] [CrossRef]
- Muhammad, H.; Tuzen, M.; Siddiqui, A.; Umar, A.R. Effective adsorption of Congo red azo dye from different water and wastewater by using porous Fe3O4-bentonite@ chitosan nanocomposite: A multivariate optimization. Int. J. Biol. Macromol. 2025, 23, 143439. [Google Scholar] [CrossRef]
- Cao, V.; Cao, P.A.; Han, D.L.; Ngo, M.T.; Vuong, T.X.; Manh, H.N. The suitability of Fe3O4/graphene oxide nanocomposite for adsorptive removal of methylene blue and congo red. Nat. Environ. Pollut. Technol. 2024, 23, 255–263. [Google Scholar] [CrossRef]
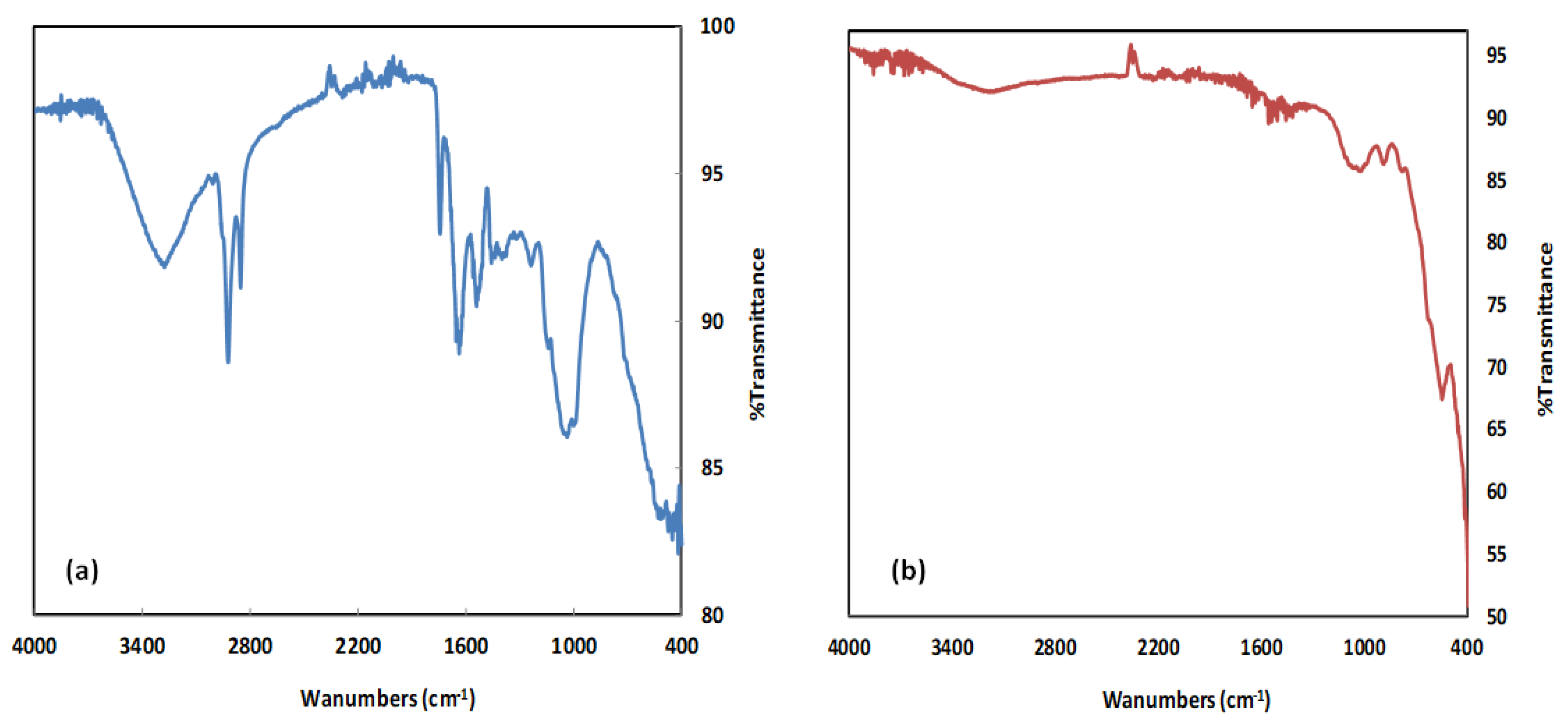
| Functional Groups | Wavenumber (cm−1) | Reference | |
|---|---|---|---|
| Fe3O4/BSB NPs | BS | ||
| O–H stretching vibrations of alcohols and carboxylic groups. | 3128.53 | 3259 | 3600–3400 |
| C–H stretching vibrations of alkanes. | - | 2852.72, 2922.16 | 3000–2800 |
| C≡C stretching vibrations of alkynes. | 2349.34 | 2032.97 | 2260–2100 |
| C=O stretching vibrations (lactones, ketones, anhydrides). | - | 1743.65 | 1740–1730 |
| C=C stretching vibrations of alkenes. | 1653.70 | 1637.56 | 1650–1600 |
| C=C aromatic ring vibrations (lignin). | 1537.26 | 1541.12 | 1600–1500 |
| C–O stretching vibrations of ether/ester. | 1026.17 | 1056.99, 1238.30 | 1300–1000 |
| C=C–H bending vibrations. | 871.82 | - | 1000–675 |
| Fe–O vibrations. | 551.64 | - | 600–500 |
| Initial CR Con. (mg/L) | Pseudo First-Order Model | Pseudo Second-Order Model | ||
|---|---|---|---|---|
| k1 (min−1) | R2 | k2 (L/mg min) | R2 | |
| 30 | 0.0089 | 0.9494 | 0.0006 | 0.9872 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daffalla, S. Biomass-Derived Magnetic Fe3O4/Biochar Nanoparticles from Baobab Seeds for Sustainable Wastewater Dye Remediation. Int. J. Mol. Sci. 2025, 26, 8499. https://doi.org/10.3390/ijms26178499
Daffalla S. Biomass-Derived Magnetic Fe3O4/Biochar Nanoparticles from Baobab Seeds for Sustainable Wastewater Dye Remediation. International Journal of Molecular Sciences. 2025; 26(17):8499. https://doi.org/10.3390/ijms26178499
Chicago/Turabian StyleDaffalla, Samah. 2025. "Biomass-Derived Magnetic Fe3O4/Biochar Nanoparticles from Baobab Seeds for Sustainable Wastewater Dye Remediation" International Journal of Molecular Sciences 26, no. 17: 8499. https://doi.org/10.3390/ijms26178499
APA StyleDaffalla, S. (2025). Biomass-Derived Magnetic Fe3O4/Biochar Nanoparticles from Baobab Seeds for Sustainable Wastewater Dye Remediation. International Journal of Molecular Sciences, 26(17), 8499. https://doi.org/10.3390/ijms26178499






