In Silico Analysis of Two Hard Tick P450s: Identification, Characterization, and Putative Metabolism of Cymbopogon citratus Essential Oil Constituents
Abstract
1. Introduction
2. Results
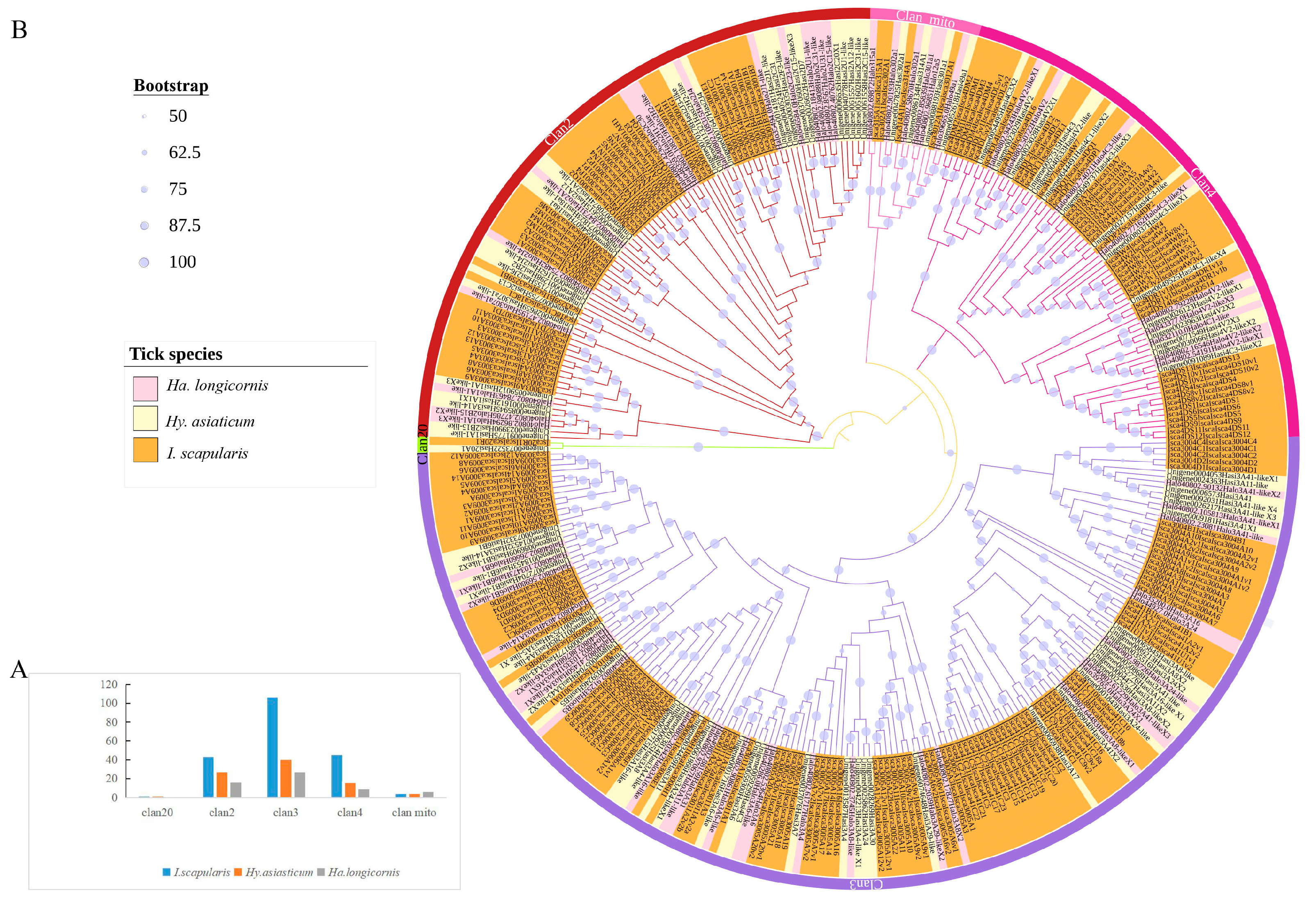
2.1. Identification and Phylogenetic Analysis of the NRNPTs
2.2. Function Annotation and Classification
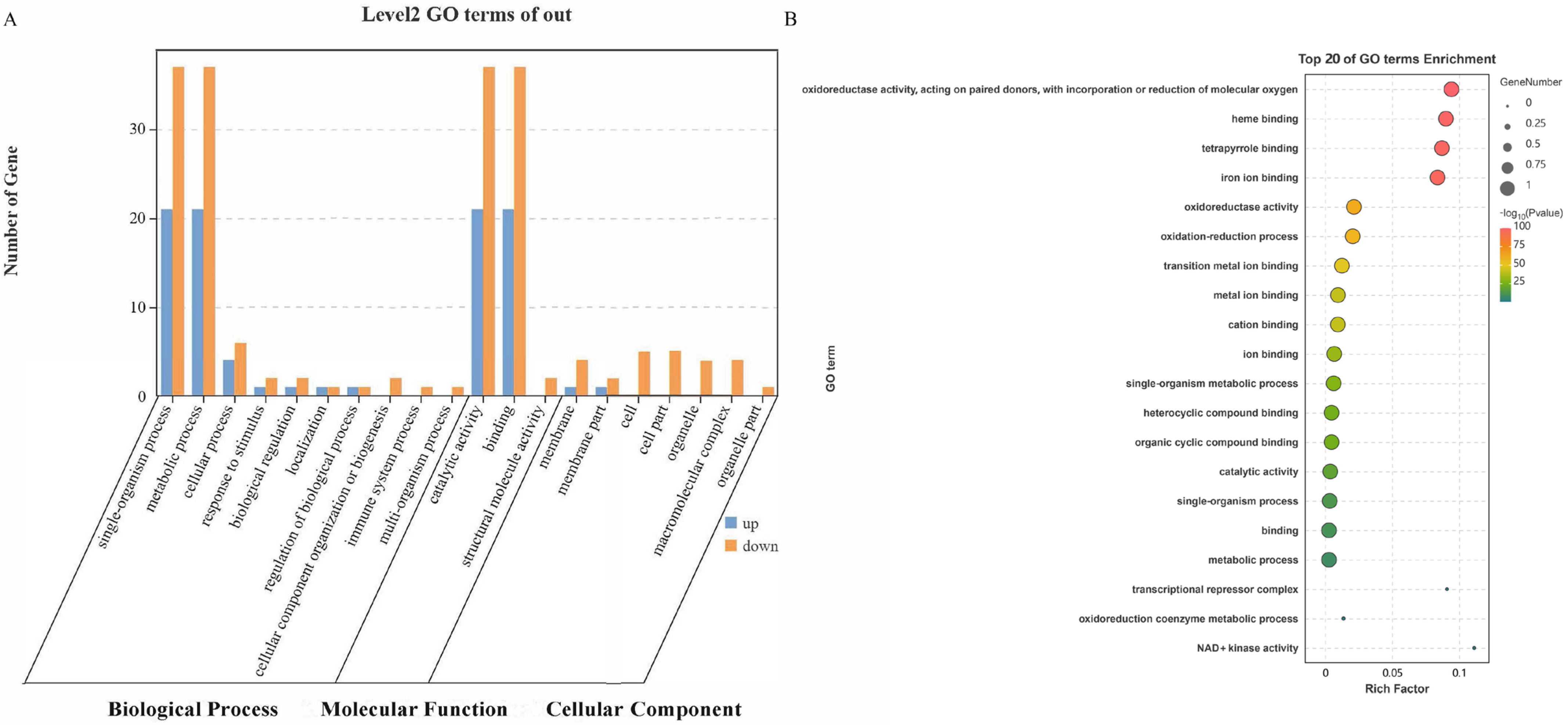
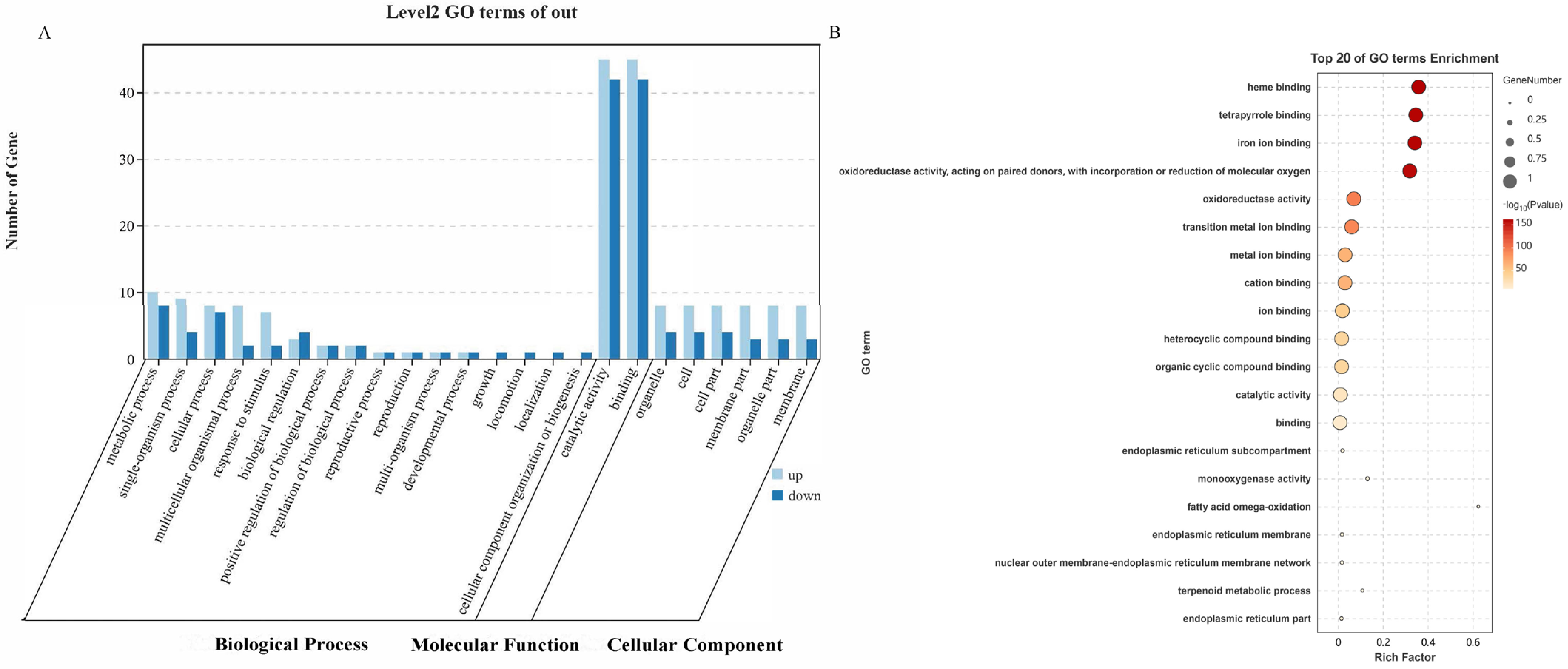
2.2.1. GO Annotations and Enrichment Analysis
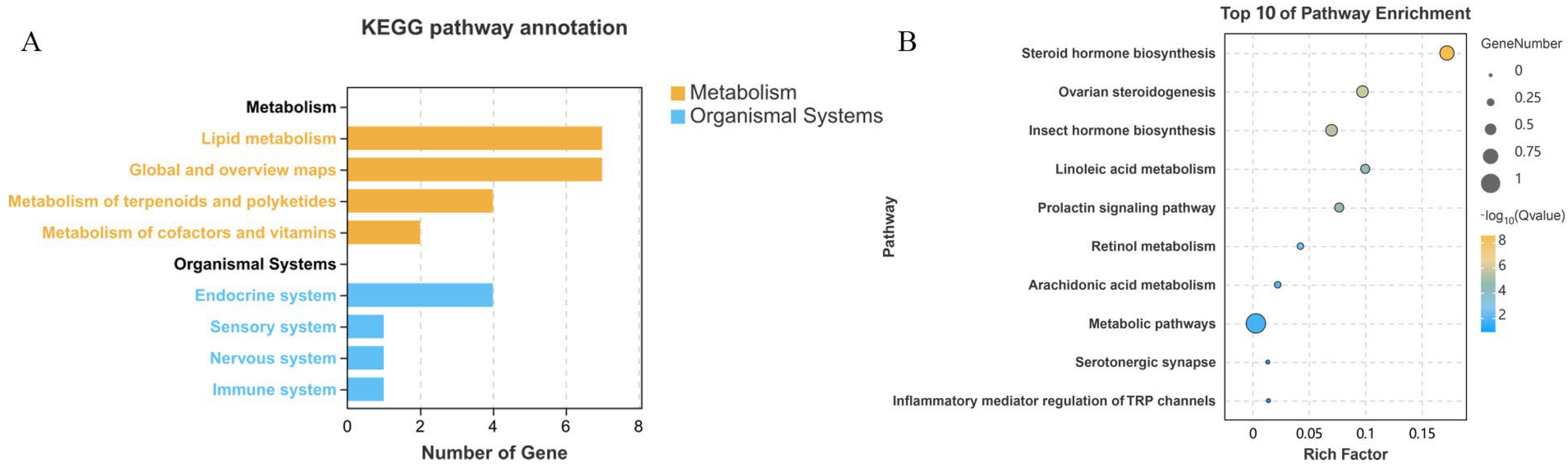
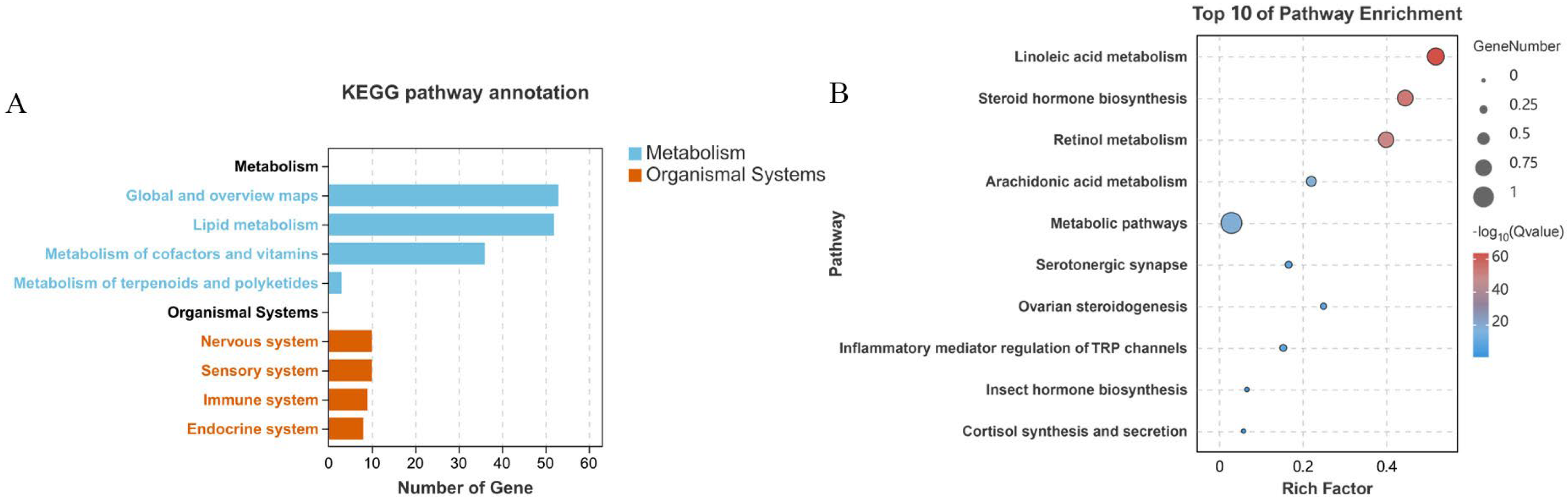
2.2.2. KEGG Annotations and Enrichment Analysis
2.3. Expression Profiles of Cytochrome p450 Genes in Response to Potential Plant-Derived Acaricides
2.4. Cloning of Hy. asiaticum p450 Genes
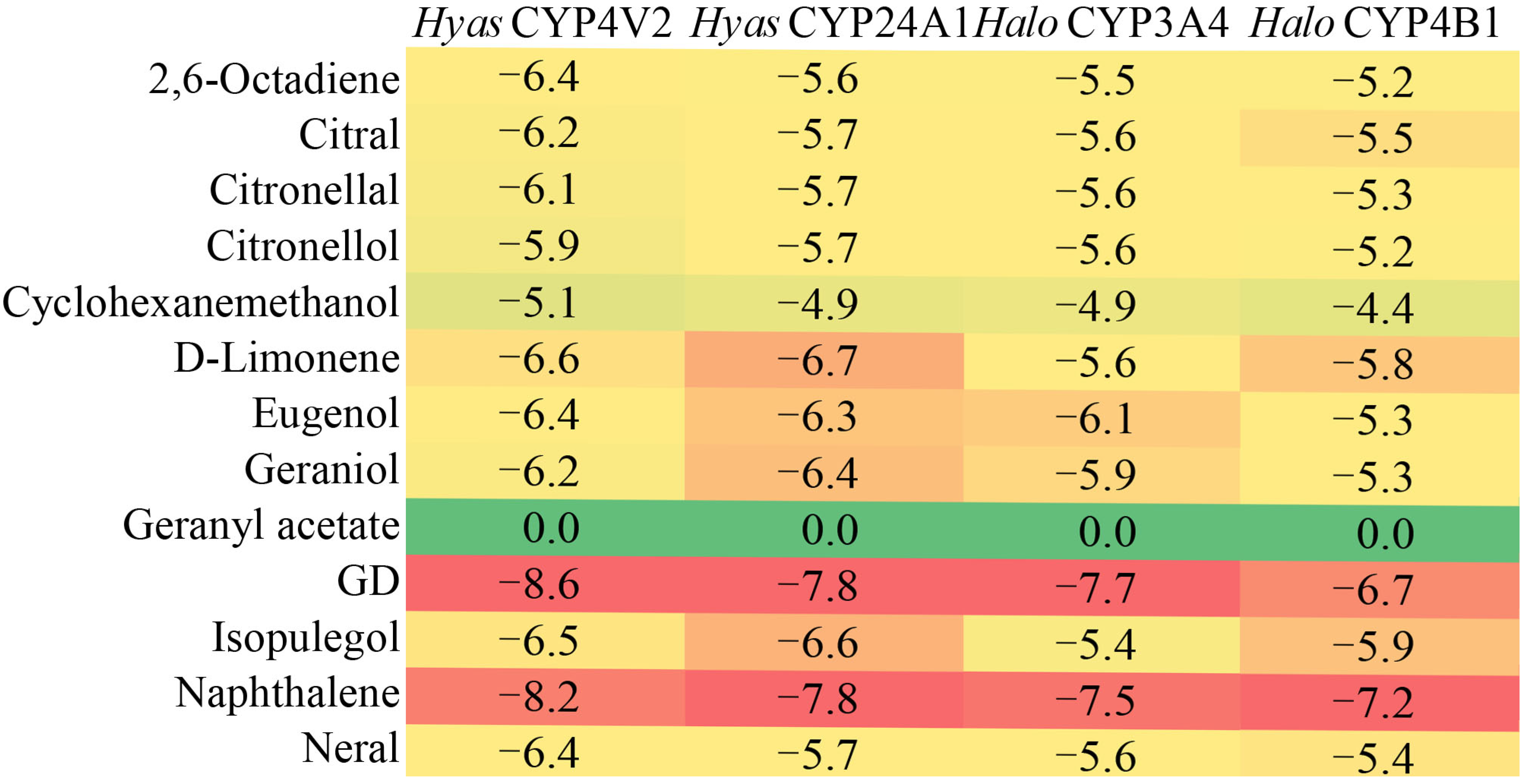
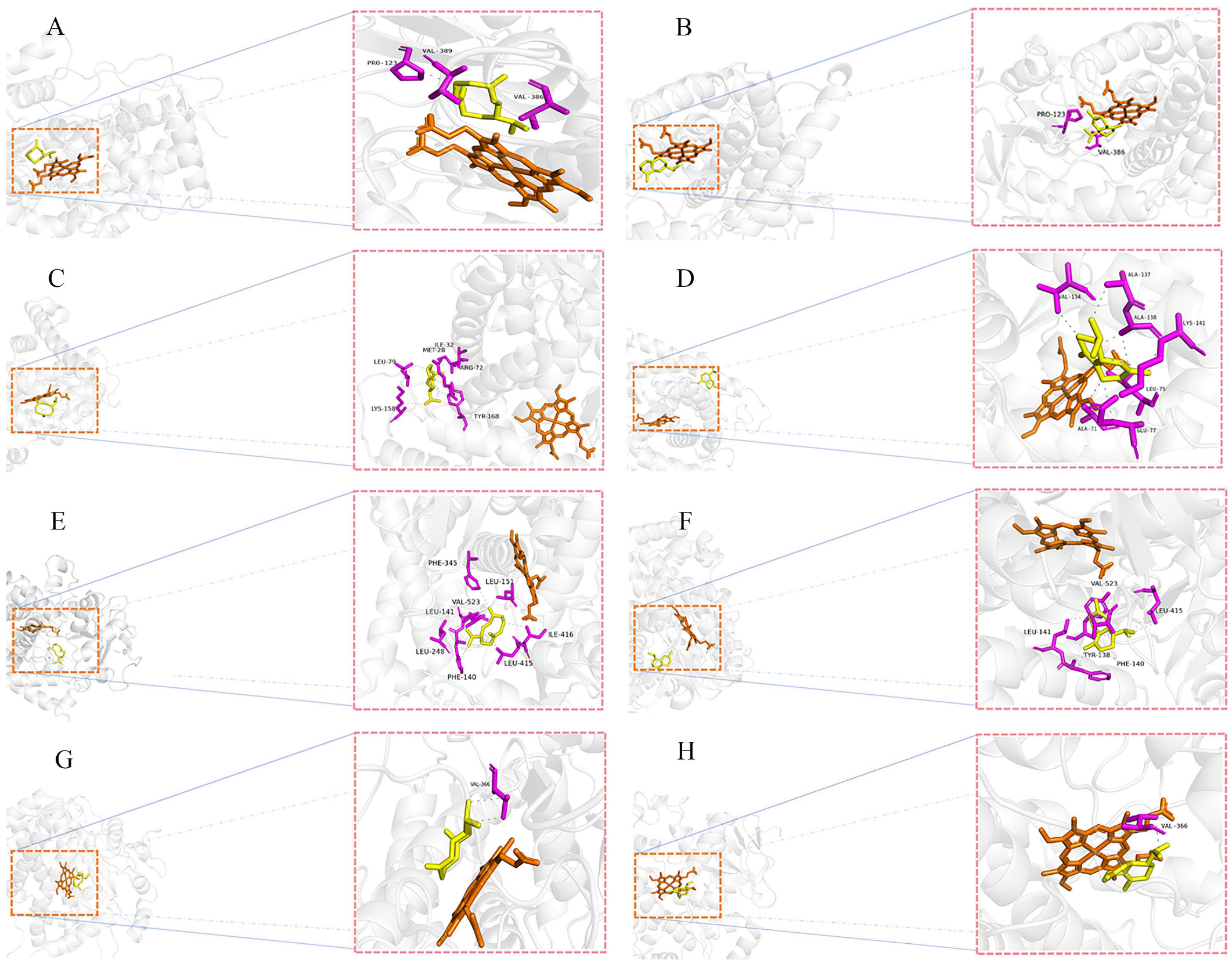
2.5. Molecular Docking Analysis
3. Discussion
4. Materials and Methods
4.1. Identification of CYP450: The Non-Redundant Number of Transcripts
4.2. p450: The Non-Redundant Number of Transcripts Phylogenetic Analysis
4.3. NRNPTs Expressive Profile Analysis Under the Abiotic Stress
4.4. GO and KEGG Functional Analysis of NRNPTs
4.5. Complete CDS Cloning for the Metabolism of CCEO Components by Hy. asiaticum
4.6. Molecular Docking
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kartashov, M.Y.; Kononova, Y.V.; Petrova, I.D.; Tupota, N.L.; Mikryukova, T.P.; Ternovoi, V.A.; Tishkova, F.H.; Loktev, V.B. Detection of Ehrlichia spp. and Theileria spp. in Hyalomma anatolicum ticks collected in Tajikistan. Vavilovskii Zhurnal Genet. I Sel. 2020, 24, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Cao, J.; Wang, Y.; Battsetseg, B.; Battur, B.; Zhang, H.; Zhou, J. Repellent effects of Chinese cinnamon oil on nymphal ticks of Haemaphysalis longicornis, Rhipicephalus haemaphysaloides, and Hyalomma asiaticum. Exp. Appl. Acarol. 2023, 91, 497–507. [Google Scholar] [CrossRef]
- Byun, H.R.; Rieu, M.S.; Ji, S.R.; Nam, H.Y.; Seo, S.; Choi, C.Y.; Linh, B.K.; Thanh, H.L.; Kaewthamasorn, M.; Sahara, A.; et al. Detection of tick-borne pathogens in blood-fed ticks from animals across nine Asian countries. Microbiol. Spectr. 2025, 13, e0244924. [Google Scholar] [CrossRef]
- Magesa, W.S.; Haji, I.; Kinimi, E.; Nzalawahe, J.S.; Kazwala, R. Distribution and molecular identification of ixodid ticks infesting cattle in Kilombero and Iringa Districts, Tanzania. BMC Vet. Res. 2023, 19, 121. [Google Scholar] [CrossRef]
- Ruiz, H.; Lacasta, D.; Villanueva-Saz, S.; González, J.M.; Ortín, A.; Ramos, J.J.; Benito, A.; Estrada-Peña, A.; Fernández, A.; Pomar, M.; et al. Tick control prevents carcass condemnations in lambs caused by Anaplasma ovis. Vet. Res. Commun. 2024, 48, 3899–3906. [Google Scholar] [CrossRef]
- Hu, E.; Hu, Z.; Mi, X.; Li, C.; He, W.; Gan, L.; Li, Y.; Zhang, W.; Meng, Y.; Gailike, B. Distribution Prediction of Hyalomma asiaticum (Acari: Ixodidae) in a Localized Region in Northwestern China. J. Parasitol. 2022, 108, 330–336. [Google Scholar] [CrossRef]
- Niu, D.; Zhao, Y.; Yang, Y.; Yang, R.; Gong, X.; Hu, L. De Novo RNA-seq and Functional Annotation of Haemaphysalis longicornis. Acta Parasitol. 2019, 64, 807–820. [Google Scholar] [CrossRef]
- Li, C.; Zhao, X.; Liu, W.; Wen, L.; Deng, Y.; Shi, W.; Zhou, N.; Song, R.; Hu, E.; Guo, Q.; et al. Biological Characteristics of the Cytochrome P 450 Family and the Mechanism of Terpinolene Metabolism in Hyalomma asiaticum (Acari: Ixodidae). Int. J. Mol. Sci. 2024, 25, 11467. [Google Scholar] [CrossRef] [PubMed]
- Hekimoğlu, O.; Sağlam, İ.K. High Crimean-Congo hemorrhagic fever incidence linked to greater genetic diversity and differentiation in Hyalomma marginatum populations in Türkiye. Parasites Vectors 2024, 17, 477. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, N.; Zheng, T.; Lu, M.; Baoli, B.; Jie, R.; Wang, X.; Li, K. Tick-borne bacterial agents in Hyalomma asiaticum ticks from Xinjiang Uygur Autonomous Region, Northwest China. Parasites Vectors 2024, 17, 167. [Google Scholar] [CrossRef] [PubMed]
- Meng, K.; Li, Z.; Wang, Y.; Jing, Z.; Zhao, X.; Liu, J.; Cai, D.; Zhang, L.; Yang, D.; Wang, S. PCR-based detection of Theileria annulata in Hyalomma asiaticum ticks in northwestern China. Ticks Tick-Borne Dis. 2014, 5, 105–106. [Google Scholar] [CrossRef]
- Lee, J.; Moon, K.; Kim, M.; Lee, W.G.; Lee, H.I.; Park, J.K.; Kim, Y.H. Seasonal distribution of Haemaphysalis longicornis (Acari: Ixodidae) and detection of SFTS virus in Gyeongbuk Province, Republic of Korea, 2018. Acta Trop. 2021, 221, 106012. [Google Scholar] [CrossRef]
- Ashraf, S.; Parveen, A.; Muhammad Awais, M.; Gillani, Q.; Aktas, M.; Ozubek, S.; Iqbal, F. A report on molecular detection and phylogenetic evaluation of Anaplasma marginale in ticks and blood samples collected from cattle in district Layyah in Punjab (Pakistan). Curr. Microbiol. 2021, 78, 274–281. [Google Scholar] [CrossRef]
- Lu, M.; Qin, X.C.; Jiang, Y.Z.; Guo, Q.; Jin, X.J.; Teng, Z.Q.; Sun, X.R.; Yu, L.; Zhang, Y.F.; Wang, W.; et al. Emergence of ehrlichiosis by a new tick-borne Ehrlichia species in China. Int. J. Infect. Dis. 2023, 131, 32–39. [Google Scholar] [CrossRef]
- Mondal, D.B.; Sarma, K.; Saravanan, M. Upcoming of the integrated tick control program of ruminants with special emphasis on livestock farming system in India. Ticks Tick Borne Dis. 2013, 4, 1–10. [Google Scholar] [CrossRef]
- Ruiz-Carrascal, D.; Bastard, J.; Williams, S.C.; Diuk-Wasser, M. Modeling platform to assess the effectiveness of single and integrated Ixodes scapularis tick control methods. Parasites Vectors 2024, 17, 339. [Google Scholar] [CrossRef] [PubMed]
- Hüe, T.; Berger, A.; Wang, H.H.; Grant, W.E.; Teel, P.D.; de León, A.A.P. Integrated control of the cattle tick, Rhipicephalus australis (Acari: Ixodidae), in New Caledonia through the Pasture and Cattle Management method. Parasitol. Res. 2021, 120, 2749–2758. [Google Scholar] [CrossRef]
- Bonnet, S.I.; Vourc’h, G.; Raffetin, A.; Falchi, A.; Figoni, J.; Fite, J.; Hoch, T.; Moutailler, S.; Quillery, E. The control of Hyalomma ticks, vectors of the Crimean-Congo hemorrhagic fever virus: Where are we now and where are we going? PLoS Neglected Trop. Dis. 2022, 16, e0010846. [Google Scholar] [CrossRef]
- Agwunobi, D.O.; Yu, Z.; Liu, J. A retrospective review on ixodid tick resistance against synthetic acaricides: Implications and perspectives for future resistance prevention and mitigation. Pestic. Biochem. Physiol. 2021, 173, 104776. [Google Scholar] [CrossRef]
- Nandhini, A.R.; Harshiny, M.; Gummadi, S.N. Chlorpyrifos in environment and food: A critical review of detection methods and degradation pathways. Environ. Sci. Process. Impacts 2021, 23, 1255–1277. [Google Scholar] [CrossRef]
- Sebai, E.; Abidi, A.; Serairi, R.; Marzouki, M.; Saratsi, K.; Darghouth, M.A.; Sotiraki, S.; Akkari, H. Essential oil of Mentha pulegium induces anthelmintic effects and reduces parasite-associated oxidative stress in rodent model. Exp. Parasitol. 2021, 225, 108105. [Google Scholar] [CrossRef]
- Panella, N.A.; Dolan, M.C.; Karchesy, J.J.; Xiong, Y.; Peralta-Cruz, J.; Khasawneh, M.; Montenieri, J.A.; Maupin, G.O. Use of novel compounds for pest control: Insecticidal and acaricidal activity of essential oil components from heartwood of Alaska yellow cedar. J. Med. Entomol. 2005, 42, 352–358. [Google Scholar] [CrossRef]
- Adamo, S.A.; El Nabbout, A.; Ferguson, L.V.; Zbarsky, J.S.; Faraone, N. Balsam fir (Abies balsamea) needles and their essential oil kill overwintering ticks (Ixodes scapularis) at cold temperatures. Sci. Rep. 2022, 12, 12999. [Google Scholar] [CrossRef]
- Quadros, D.G.; Johnson, T.L.; Whitney, T.R.; Oliver, J.D.; Oliva Chávez, A.S. Plant-Derived Natural Compounds for Tick Pest Control in Livestock and Wildlife: Pragmatism or Utopia? Insects 2020, 11, 490. [Google Scholar] [CrossRef]
- Pavela, R.; Canale, A.; Mehlhorn, H.; Benelli, G. Application of ethnobotanical repellents and acaricides in prevention, control and management of livestock ticks: A review. Res. Vet. Sci. 2016, 109, 1–9. [Google Scholar] [CrossRef]
- Hodoșan, C.; Gîrd, C.E.; Ghica, M.V.; Dinu-Pîrvu, C.E.; Nistor, L.; Bărbuică, I.S.; Marin, Ș.C.; Mihalache, A.; Popa, L. Pyrethrins and Pyrethroids: A Comprehensive Review of Natural Occurring Compounds and Their Synthetic Derivatives. Plants 2023, 12, 4022. [Google Scholar] [CrossRef]
- Lybrand, D.B.; Xu, H.; Last, R.L.; Pichersky, E. How Plants Synthesize Pyrethrins: Safe and Biodegradable Insecticides. Trends Plant Sci. 2020, 25, 1240–1251. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Vivas, R.I.; Jonsson, N.N.; Bhushan, C. Strategies for the control of Rhipicephalus microplus ticks in a world of conventional acaricide and macrocyclic lactone resistance. Parasitol. Res. 2018, 117, 3–29. [Google Scholar] [CrossRef]
- Brügger, B.P.; Martínez, L.C.; Plata-Rueda, A.; Castro, B.; Soares, M.A.; Wilcken, C.F.; Carvalho, A.G.; Serrão, J.E.; Zanuncio, J.C. Bioactivity of the Cymbopogon citratus (Poaceae) essential oil and its terpenoid constituents on the predatory bug, Podisus nigrispinus (Heteroptera: Pentatomidae). Sci. Rep. 2019, 9, 8358. [Google Scholar] [CrossRef] [PubMed]
- Tavares, C.P.; Sousa, I.C.; Gomes, M.N.; Miró, V.; Virkel, G.; Lifschitz, A.; Costa-Junior, L.M. Combination of cypermethrin and thymol for control of Rhipicephalus microplus: Efficacy evaluation and description of an action mechanism. Ticks Tick-Borne Dis. 2022, 13, 101874. [Google Scholar] [CrossRef]
- Miranda, F.R.; Avelar, B.R.; de Jesus, I.L.R.; Guimarães, B.G.; Bonfim, I.V.; Alves, M.C.C.; Ferreira, T.P.; Azevedo, T.R.C.; Cid, Y.P.; Scott, F.B. Do combinations of fipronil, eugenol and carvacrol have synergistic effects against Rhipicephalus sanguineus? Parasitol. Res. 2023, 123, 48. [Google Scholar] [CrossRef]
- Tavares, C.P.; Sabadin, G.A.; Sousa, I.C.; Gomes, M.N.; Soares, A.M.S.; Monteiro, C.M.O.; Vaz, I.S., Jr.; Costa-Junior, L.M. Effects of carvacrol and thymol on the antioxidant and detoxifying enzymes of Rhipicephalus microplus (Acari: Ixodidae). Ticks Tick-Borne Dis. 2022, 13, 101929. [Google Scholar] [CrossRef]
- Scott, J.G.; Liu, N.; Wen, Z. Insect cytochromes P450: Diversity, insecticide resistance and tolerance to plant toxins. Comp. Biochem. Physiol. Part C Pharmacol. Toxicol. Endocrinol. 1998, 121, 147–155. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Li, G.Y.; Li, L.; Song, Q.S.; Stanley, D.; Wei, S.J.; Zhu, J.Y. Genome-wide and expression-profiling analyses of the cytochrome P450 genes in Tenebrionidea. Arch. Insect Biochem. Physiol. 2022, 111, e21954. [Google Scholar] [CrossRef]
- Moustafa, M.A.M.; Hassan, N.N.; Alfuhaid, N.A.; Amer, A.; Awad, M. Insights into the toxicity, biochemical activity, and molecular docking of Cymbopogon citratus essential oils and citral on Spodoptera littoralis (Lepidoptera: Noctuidae). J. Econ. Entomol. 2023, 116, 1185–1195. [Google Scholar] [CrossRef]
- Lee, N.H.; Lee, S.; Chung, N.; Lee, H.S. Haemaphysalis longicornis and Carvacrol as Acaricide: Efficacy and Mechanism of Action. Molecules 2025, 30, 1518. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, A.; Satta, Y. Substrate-dependent evolution of cytochrome P450: Rapid turnover of the detoxification-type and conservation of the biosynthesis-type. PLoS ONE 2014, 9, e100059. [Google Scholar] [CrossRef]
- Dermauw, W.; Van Leeuwen, T.; Feyereisen, R. Diversity and evolution of the P450 family in arthropods. Insect Biochem. Mol. Biol. 2020, 127, 103490. [Google Scholar] [CrossRef]
- Nagar, G.; Upadhaya, D.; Sharma, A.K.; Kumar, R.; Fular, A.; Ghosh, S. Association between overexpression of cytochrome P450 genes and deltamethrin resistance in Rhipicephalus microplus. Ticks Tick-Borne Dis. 2021, 12, 101610. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, N.; Osakabe, M. Cross-resistance between cyenopyrafen and pyridaben in the twospotted spider mite Tetranychus urticae (Acari: Tetranychidae). Pest Manag. Sci. 2014, 70, 1090–1096. [Google Scholar] [CrossRef]
- Huang, Y.; Liao, M.; Yang, Q.; Xiao, J.; Hu, Z.; Zhou, L.; Cao, H. Transcriptome profiling reveals differential gene expression of detoxification enzymes in Sitophilus zeamais responding to terpinen-4-ol fumigation. Pestic. Biochem. Physiol. 2018, 149, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Liao, M.; Yang, Q.; Shi, S.; Xiao, J.; Cao, H. Knockdown of NADPH-cytochrome P450 reductase and CYP6MS1 increases the susceptibility of Sitophilus zeamais to terpinen-4-ol. Pestic. Biochem. Physiol. 2020, 162, 15–22. [Google Scholar] [CrossRef]
- Agwunobi, D.O.; Zhang, M.; Zhang, X.; Wang, T.; Yu, Z.; Liu, J. Transcriptome profile of Haemaphysalis longicornis (Acari: Ixodidae) exposed to Cymbopogon citratus essential oil and citronellal suggest a cytotoxic mode of action involving mitochondrial Ca(2+) overload and depolarization. Pestic. Biochem. Physiol. 2021, 179, 104971. [Google Scholar] [CrossRef]
- Gulia-Nuss, M.; Nuss, A.B.; Meyer, J.M.; Sonenshine, D.E.; Roe, R.M.; Waterhouse, R.M.; Sattelle, D.B.; de la Fuente, J.; Ribeiro, J.M.; Megy, K.; et al. Genomic insights into the Ixodes scapularis tick vector of Lyme disease. Nat. Commun. 2016, 7, 10507. [Google Scholar] [CrossRef]
- Nauen, R.; Bass, C.; Feyereisen, R.; Vontas, J. The Role of Cytochrome P450s in Insect Toxicology and Resistance. Annu. Rev. Entomol. 2022, 67, 105–124. [Google Scholar] [CrossRef]
- Xiong, Y.S.; Cui, L.L.; Hu, G.L.; Jiang, Y.T.; Lv, Y.P.; Zhang, P.; Zheng, J.S.; Zhang, B.Z.; Liu, R.Q. Overexpression of CYP6CY1 is Involved in Imidacloprid Resistance in Sitobion miscanthi (Takahashi) (Homoptera: Aphidae). Neotrop. Entomol. 2025, 54, 23. [Google Scholar] [CrossRef]
- Lin, R.; Yang, M.; Yao, B. The phylogenetic and evolutionary analyses of detoxification gene families in Aphidinae species. PLoS ONE 2022, 17, e0263462. [Google Scholar] [CrossRef] [PubMed]
- Feyereisen, R. Insect CYP genes and P450 enzymes. In Insect Molecular Biology and Biochemistry; Elsevier: Amsterdam, The Netherlands, 2012; pp. 236–316. [Google Scholar]
- Hossam Abdelmonem, B.; Abdelaal, N.M.; Anwer, E.K.E.; Rashwan, A.A.; Hussein, M.A.; Ahmed, Y.F.; Khashana, R.; Hanna, M.M.; Abdelnaser, A. Decoding the Role of CYP450 Enzymes in Metabolism and Disease: A Comprehensive Review. Biomedicines 2024, 12, 1467. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Fu, D.; Ning, H.; Tang, M.; Chen, H. Knockdown of CYP6CR2 and CYP6DE5 reduces tolerance to host plant allelochemicals in the Chinese white pine beetle Dendroctonus armandi. Pestic. Biochem. Physiol. 2022, 187, 105180. [Google Scholar] [CrossRef]
- Huang, M.; Gong, P.; Yin, C.; Yang, J.; Liu, S.; Fu, B.; Wei, X.; Liang, J.; Xue, H.; He, C.; et al. Cytochrome P450 CYP6EM1 confers resistance to thiamethoxam in the whitefly Bemisia tabaci (Hemiptera: Aleyrodidae) via detoxification metabolism. Pestic. Biochem. Physiol. 2025, 208, 106272. [Google Scholar] [CrossRef]
- Gao, S.; Guo, X.; Liu, S.; Li, S.; Zhang, J.; Xue, S.; Tang, Q.; Zhang, K.; Li, R. Cytochrome P450 gene CYP6BQ8 mediates terpinen-4-ol susceptibility in the red flour beetle, Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae). Bull. Entomol. Res. 2023, 113, 271–281. [Google Scholar] [CrossRef]
- Liu, N.; Li, M.; Gong, Y.; Liu, F.; Li, T. Cytochrome P450s--Their expression, regulation, and role in insecticide resistance. Pestic. Biochem. Physiol. 2015, 120, 77–81. [Google Scholar] [CrossRef]
- Bissantz, C.; Kuhn, B.; Stahl, M. A medicinal chemist’s guide to molecular interactions. J. Med. Chem. 2010, 53, 5061–5084. [Google Scholar] [CrossRef]
- Anighoro, A. Underappreciated Chemical Interactions in Protein-Ligand Complexes. Methods Mol. Biol. 2020, 2114, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Dyson, H.J.; Wright, P.E.; Scheraga, H.A. The role of hydrophobic interactions in initiation and propagation of protein folding. Proc. Natl. Acad. Sci. USA 2006, 103, 13057–13061. [Google Scholar] [CrossRef]
- Sobolev, V.; Wade, R.C.; Vriend, G.; Edelman, M. Molecular docking using surface complementarity. Proteins 1996, 25, 120–129. [Google Scholar] [CrossRef]
- Fantatto, R.R.; Constantini, J.V.C.; Politi, F.A.S.; Sorrechia, R.; Medeiros, C.C.B.; Luiz, M.T.; Bechara, G.H.; de Souza Chagas, A.C.; Chorilli, M.; Pietro, R. Current Tick Control Strategies and Prospects for Using Nanotechnology as an Efficient Alternative-A Review. Vet. Sci. 2025, 12, 163. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, Q.; Bai, X.; Men, L.; Ma, J.; Li, D.; Xu, M.; Wei, Q.; Chen, R.; Wang, D.; et al. Screening and modification of (+)-germacrene A synthase for the production of the anti-tumor drug (-)-β-elemene in engineered Saccharomyces cerevisiae. Int. J. Biol. Macromol. 2024, 279 Pt 4, 135455. [Google Scholar] [CrossRef]
- Daisy, B.H.; Strobel, G.A.; Castillo, U.; Ezra, D.; Sears, J.; Weaver, D.K.; Runyon, J.B. Naphthalene, an insect repellent, is produced by Muscodor vitigenus, a novel endophytic fungus. Microbiology 2002, 148 Pt 11, 3737–3741. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, X.; Gan, J.; Chen, S.; Xiao, Z.X.; Cao, Y. CB-Dock2: Improved protein-ligand blind docking by integrating cavity detection, docking and homologous template fitting. Nucleic Acids Res. 2022, 50, W159–W164. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Wen, L.; Shi, W.; Deng, Y.; Zhou, N.; Zhao, X.; Guo, Q.; Gailike, B. In Silico Analysis of Two Hard Tick P450s: Identification, Characterization, and Putative Metabolism of Cymbopogon citratus Essential Oil Constituents. Int. J. Mol. Sci. 2025, 26, 8489. https://doi.org/10.3390/ijms26178489
Li C, Wen L, Shi W, Deng Y, Zhou N, Zhao X, Guo Q, Gailike B. In Silico Analysis of Two Hard Tick P450s: Identification, Characterization, and Putative Metabolism of Cymbopogon citratus Essential Oil Constituents. International Journal of Molecular Sciences. 2025; 26(17):8489. https://doi.org/10.3390/ijms26178489
Chicago/Turabian StyleLi, Caishan, Licui Wen, Wenyu Shi, Yuqian Deng, Na Zhou, Xueqing Zhao, Qingyong Guo, and Bayinchahan Gailike. 2025. "In Silico Analysis of Two Hard Tick P450s: Identification, Characterization, and Putative Metabolism of Cymbopogon citratus Essential Oil Constituents" International Journal of Molecular Sciences 26, no. 17: 8489. https://doi.org/10.3390/ijms26178489
APA StyleLi, C., Wen, L., Shi, W., Deng, Y., Zhou, N., Zhao, X., Guo, Q., & Gailike, B. (2025). In Silico Analysis of Two Hard Tick P450s: Identification, Characterization, and Putative Metabolism of Cymbopogon citratus Essential Oil Constituents. International Journal of Molecular Sciences, 26(17), 8489. https://doi.org/10.3390/ijms26178489



