Serum Vitamin D Levels as Predictors of Response to Intravitreal Anti-VEGF Therapy in Diabetic Macular Edema: A Clinical Correlation Study
Abstract
1. Introduction
2. Results
2.1. Baseline Characteristics
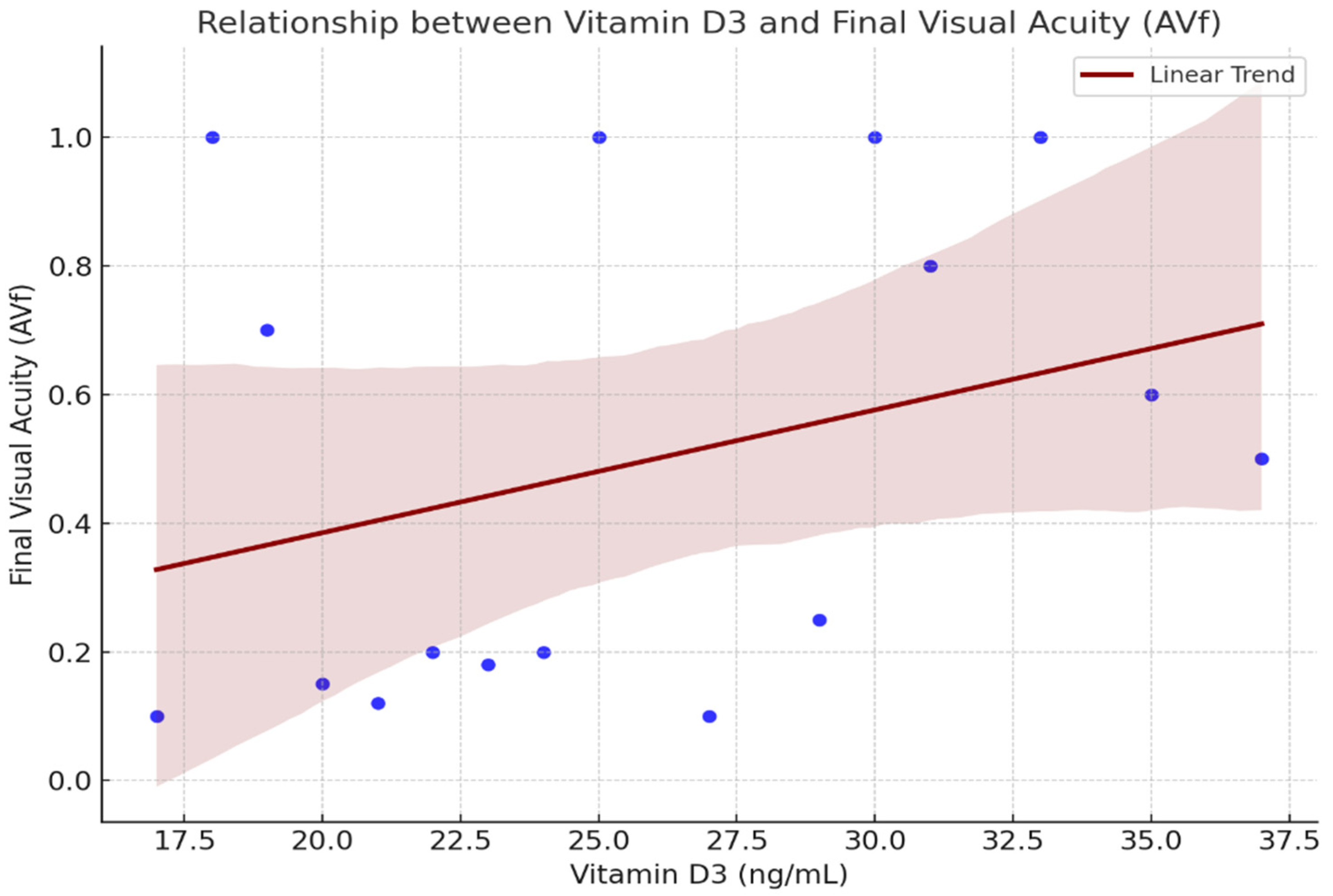
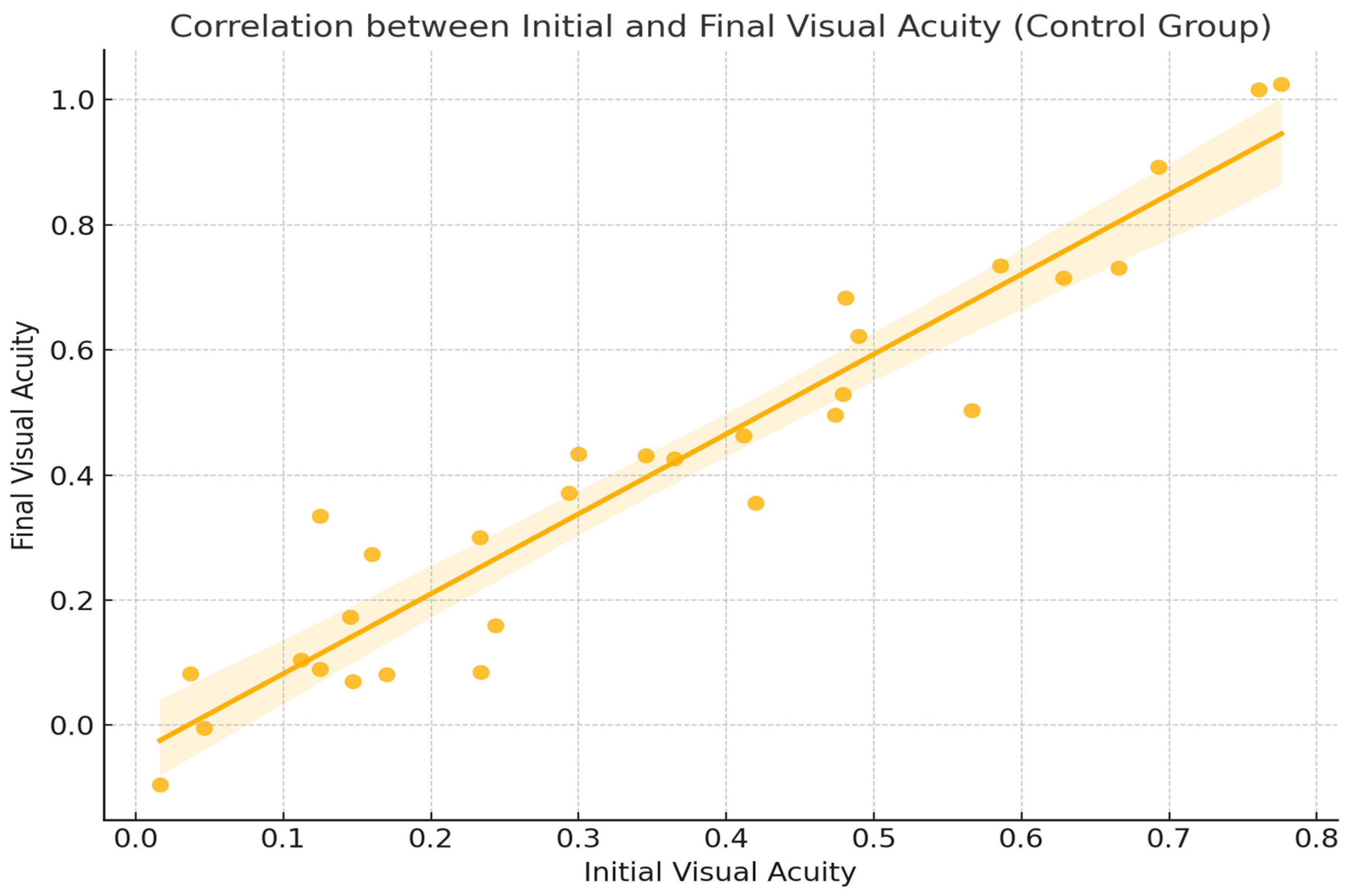
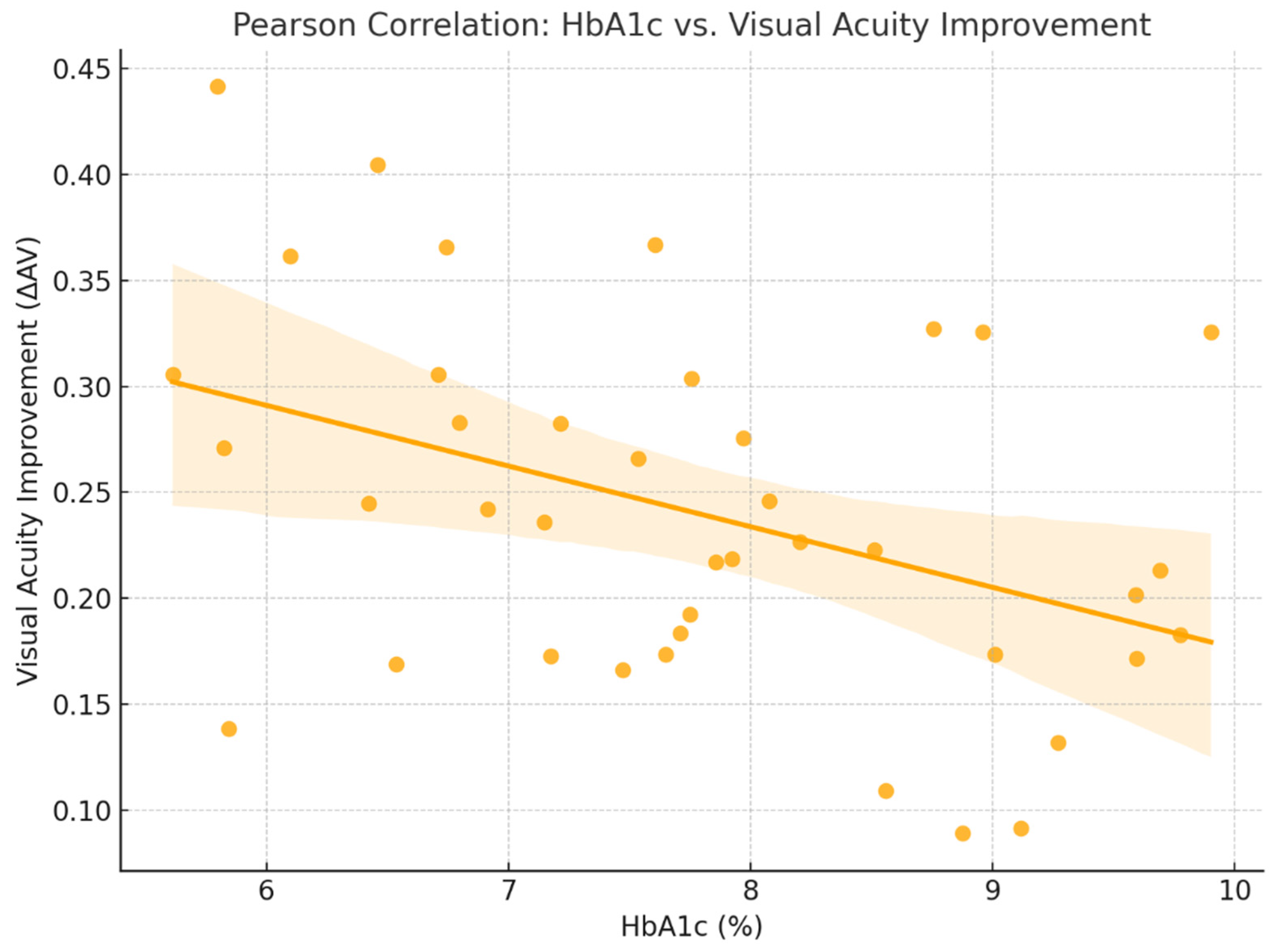
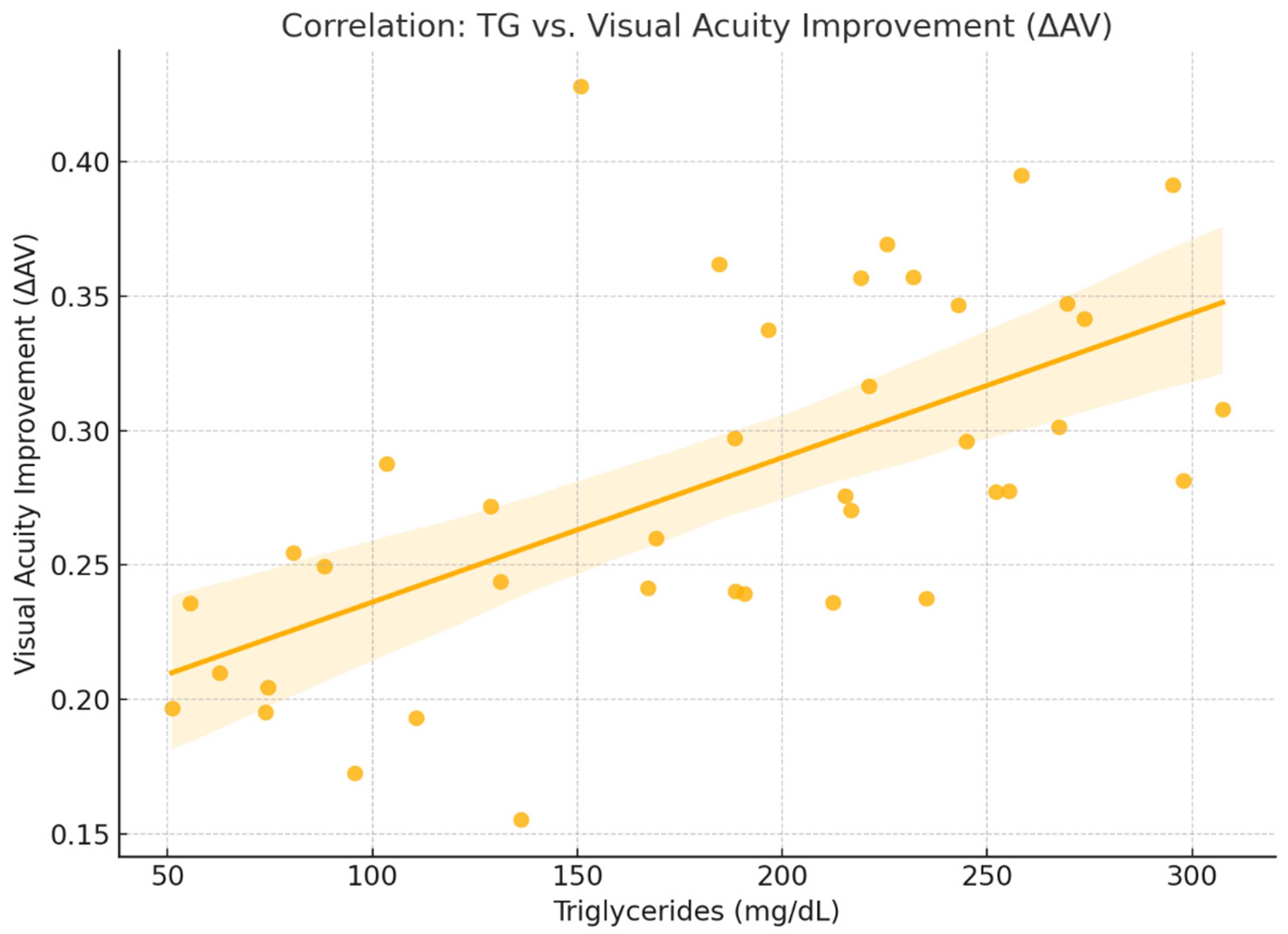
2.2. Functional Response
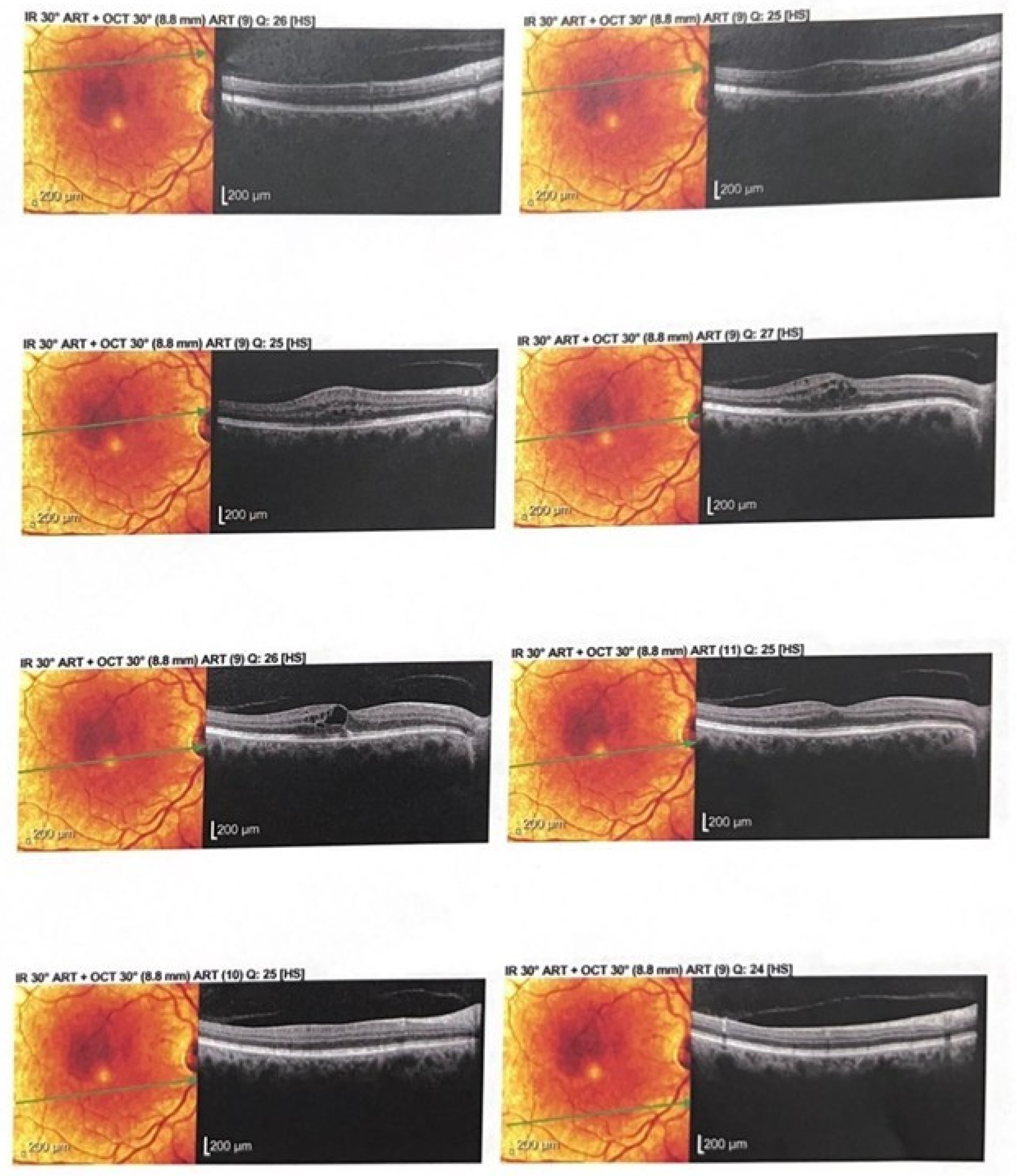
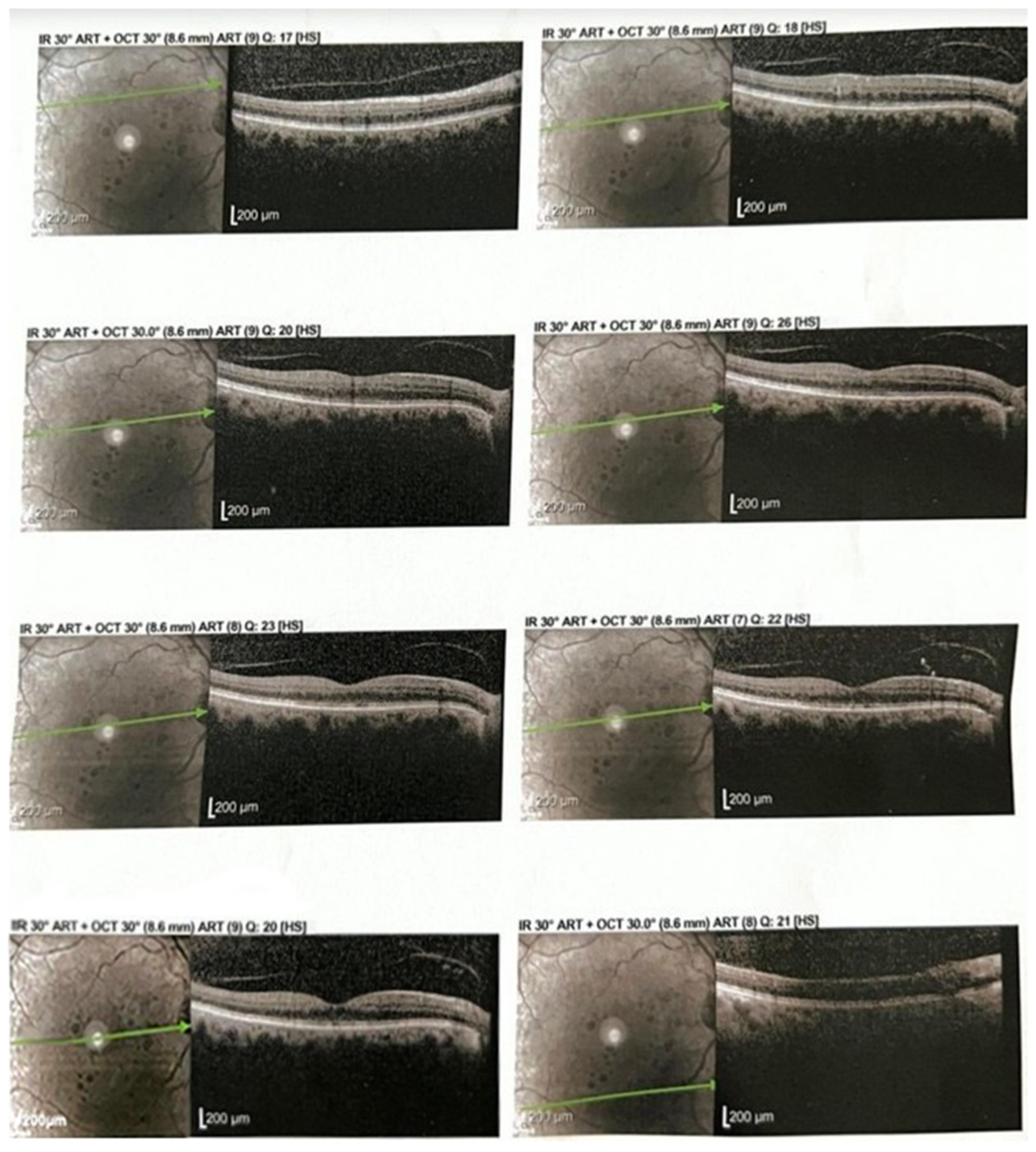
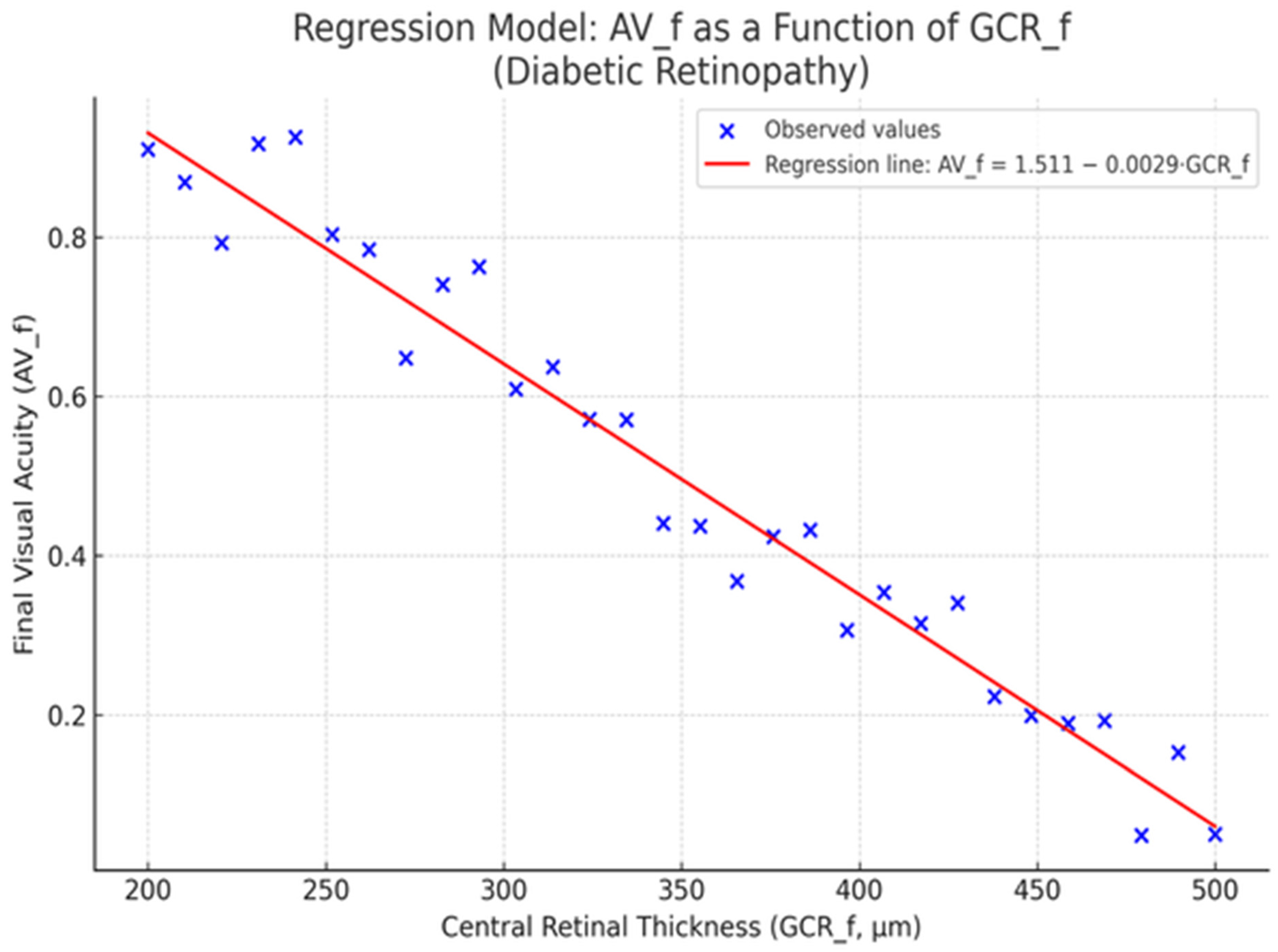
2.3. Anatomical Response
2.4. Figures
- CRT_f—Central retinal thickness (measured in microns) post-treatment
- AV_f—Final visual acuity, expressed in decimal notation
- Intercept (a): 1.444
- Slope (b): −0.0028
- Coefficient of determination (R2): 0.29
- p-value: 0.0008
3. Discussion
4. Materials and Methods
4.1. Study Design and Population
4.2. Baseline Evaluation and Data Collection
4.3. Treatment Protocol
4.4. Outcome Measures
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- International Diabetes Federation. IDF Diabetes Atlas, 10th ed.; International Diabetes Federation: Brussels, Belgium, 2021. [Google Scholar]
- Simó, R.; Hernández, C. Neurodegeneration in the diabetic eye: New insights and therapeutic perspectives. Trends Endocrinol. Metab. 2014, 25, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Cheung, N.; Mitchell, P.; Wong, T.Y. Diabetic retinopathy. Lancet 2010, 376, 124–136. [Google Scholar] [CrossRef]
- Nguyen, Q.D.; Brown, D.M.; Marcus, D.M.; Boyer, D.S.; Patel, S.; Feiner, L.; Gibson, A.; Sy, J.; Rundle, A.C.; Hopkins, J.J.; et al. Ranibizumab for diabetic macular edema: Results from two phase III randomized trials: RISE and RIDE. Ophthalmology 2012, 119, 789–801. [Google Scholar] [CrossRef]
- Korobelnik, J.F.; Do, D.V.; Schmidt-Erfurth, U.; Boyer, D.S.; Holz, F.G.; Heier, J.S.; Midena, E.; Kaiser, P.K.; Terasaki, H.; Marcus, D.M.; et al. Intravitreal aflibercept for diabetic macular edema. Ophthalmology 2014, 121, 2247–2254. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.D. Vitamin D: Newly discovered actions require reconsideration of physiologic requirements. Trends Endocrinol. Metab. 2010, 21, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Payne, J.F.; Ray, R.; Watson, D.G.; Delille, C.; Rimler, E.; Cleveland, J.; Lynn, M.J.; Tangpricha, V.; Srivastava, S.K. Vitamin D insufficiency in diabetic retinopathy. Endocr. Pract. 2012, 18, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Donaghue, K.C.; Chan, A.K.; Benitez-Aguirre, P.; Hing, S.; Lloyd, M.; Cusumano, J.; Pryke, A.; Craig, M.E. Vitamin D deficiency is associated with retinopathy in children and adolescents with type 1 diabetes. Diabetes Care 2011, 34, 1400–1402. [Google Scholar] [CrossRef] [PubMed]
- Reins, R.Y.; McDermott, A.M. Vitamin D: Implications for ocular disease and therapeutic potential. Exp. Eye Res. 2015, 134, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Albert, D.M.; Scheef, E.A.; Wang, S.; Mehraein, F.; Darjatmoko, S.R.; Sorenson, C.M.; Sheibani, N. Calcitriol is a potent inhibitor of retinal neovascularization. Investig. Ophthalmol. Vis. Sci. 2007, 48, 2327–2334. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef] [PubMed]
- Beck, R.W.; Moke, P.S.; Turpin, A.H.; Ferris, F.L.; SanGiovanni, J.P.; A Johnson, C.; E Birch, E.; Chandler, D.L.; A Cox, T.; Blair, R.C.; et al. A computerized method of visual acuity testing: Adaptation of the early treatment of diabetic retinopathy study testing protocol. Am. J. Ophthalmol. 2003, 135, 194–205. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xu, X.; Elliott, M.H.; Zhu, M.; Le, Y.Z. Müller cell–derived VEGF is essential for diabetes-induced retinal inflammation and vascular leakage. Diabetes 2010, 59, 2297–2305. [Google Scholar] [CrossRef] [PubMed]
- Yau, J.W.Y.; Rogers, S.L.; Kawasaki, R.; Lamoureux, E.L.; Kowalski, J.W.; Bek, T.; Chen, S.-J.; Dekker, J.M.; Fletcher, A.; Grauslund, J.; et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care 2012, 35, 556–564. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Chen, Z.; Watsky, M.A. Effects of 1,25 and 24,25 Vitamin D on Corneal Fibroblast VDR and Vitamin D Metabolizing and Catabolizing Enzymes. Current Eye Research 2021, 46, 1271–1282. [Google Scholar] [CrossRef] [PubMed]
- Talmor-Barkan, Y.; Bernheim, J.; Green, J.; Benchetrit, S.; Rashid, G. Calcitriol counteracts endothelial cell pro-inflammatory processes in a chronic kidney disease-like environment. J. Steroid Biochem. Mol. Biol. 2011, 124, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Romila, A.; Potop, V.; Ciuluvica, R.; Monica, B.; Mehedinti Hincu, M.C.; Jurja, S. Correlation between metabolic status and diabetic retinopathy evolution in type 1 diabetes. Exp. Ther. Med. 2021, 22, 1214. [Google Scholar] [CrossRef] [PubMed]
- Dumitru, A.; Matei, E.; Cozaru, G.C.; Chisoi, A.; Alexandrescu, L.; Popescu, R.C.; Butcaru, M.P.; Dumitru, E.; Rugină, S.; Tocia, C. Endotoxin Inflammatory Action on Cells by Dysregulated-Immunological-Barrier-Linked ROS-Apoptosis Mechanisms in Gut-Liver Axis. Int. J. Mol. Sci. 2024, 25, 2472. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Luo, B.A.; Gao, F.; Qin, L.L. The Association between Vitamin D Deficiency and Diabetic Retinopathy in Type 2 Diabetes: A Meta-Analysis of Observational Studies. Nutrients 2017, 9, 307. [Google Scholar] [CrossRef] [PubMed]
- Karimi, S.; Movafaghi, V.; Arabi, A.; Shahraki, T.; Safi, S. Effects of Oral Vitamin D Supplement Therapy on Clinical Outcomes of Intravitreal Bevacizumab in Diabetic Macular Edema. J. Ophthalmic. Vis. Res. 2021, 16, 34–41. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dervis, N.; Jurja, S.; Chisnoiu, T.; Mihai, C.M.; Stoica, A.M. Serum Vitamin D Levels as Predictors of Response to Intravitreal Anti-VEGF Therapy in Diabetic Macular Edema: A Clinical Correlation Study. Int. J. Mol. Sci. 2025, 26, 8481. https://doi.org/10.3390/ijms26178481
Dervis N, Jurja S, Chisnoiu T, Mihai CM, Stoica AM. Serum Vitamin D Levels as Predictors of Response to Intravitreal Anti-VEGF Therapy in Diabetic Macular Edema: A Clinical Correlation Study. International Journal of Molecular Sciences. 2025; 26(17):8481. https://doi.org/10.3390/ijms26178481
Chicago/Turabian StyleDervis, Nejla, Sanda Jurja, Tatiana Chisnoiu, Cristina Maria Mihai, and Ana Maria Stoica. 2025. "Serum Vitamin D Levels as Predictors of Response to Intravitreal Anti-VEGF Therapy in Diabetic Macular Edema: A Clinical Correlation Study" International Journal of Molecular Sciences 26, no. 17: 8481. https://doi.org/10.3390/ijms26178481
APA StyleDervis, N., Jurja, S., Chisnoiu, T., Mihai, C. M., & Stoica, A. M. (2025). Serum Vitamin D Levels as Predictors of Response to Intravitreal Anti-VEGF Therapy in Diabetic Macular Edema: A Clinical Correlation Study. International Journal of Molecular Sciences, 26(17), 8481. https://doi.org/10.3390/ijms26178481





