Interactions of Galleria mellonella Proline-Rich Antimicrobial Peptides with Gram-Negative and Gram-Positive Bacteria
Abstract
1. Introduction
2. Results
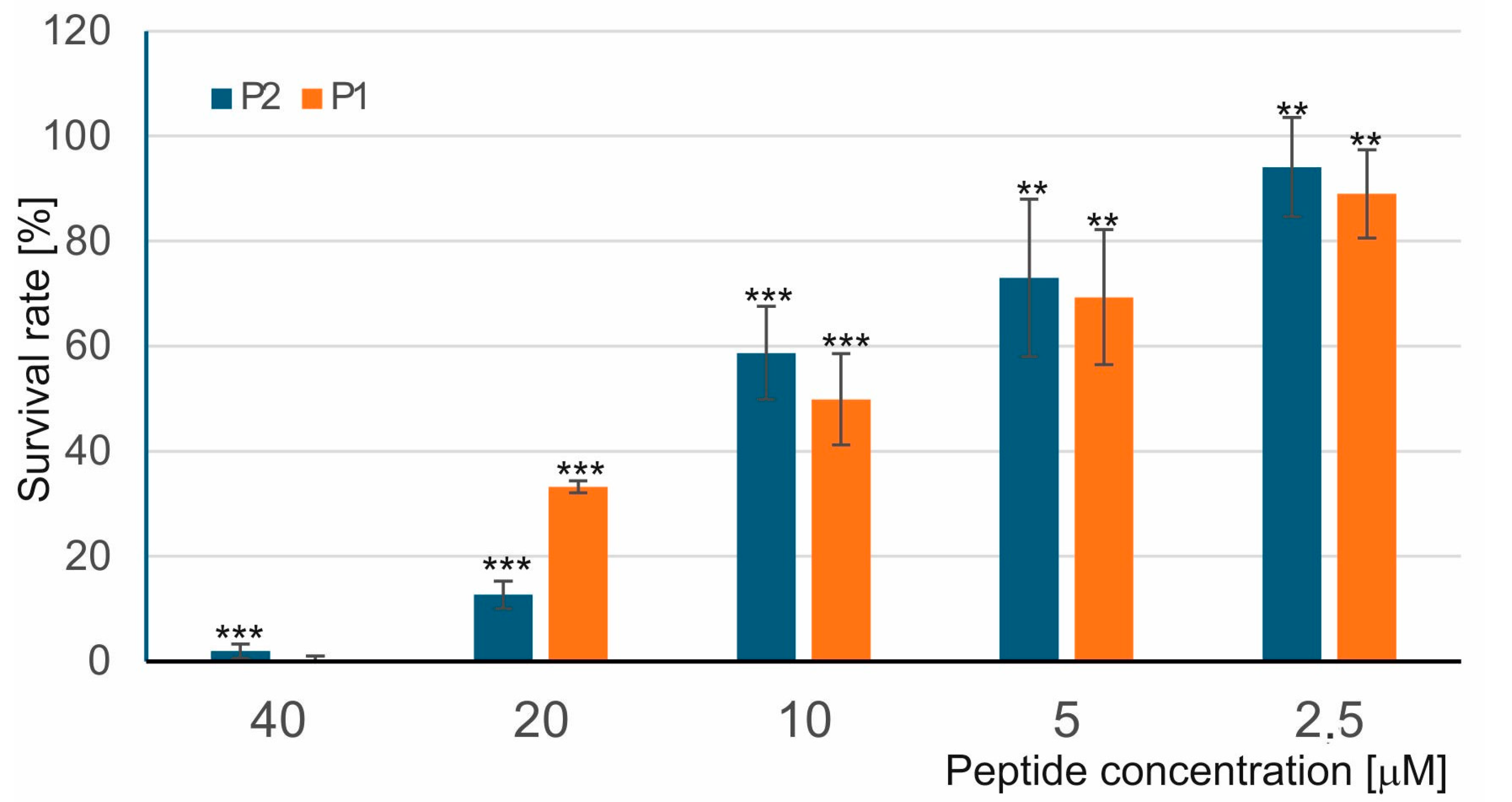
2.1. Interaction of G. mellonella PrAMPs with Micrococcus luteus Cells
2.1.1. Evaluation of Anti-M. luteus Activity
2.1.2. Morphology of M. luteus Cells After Treatment with P1 and P2 Peptides (SEM Imaging)
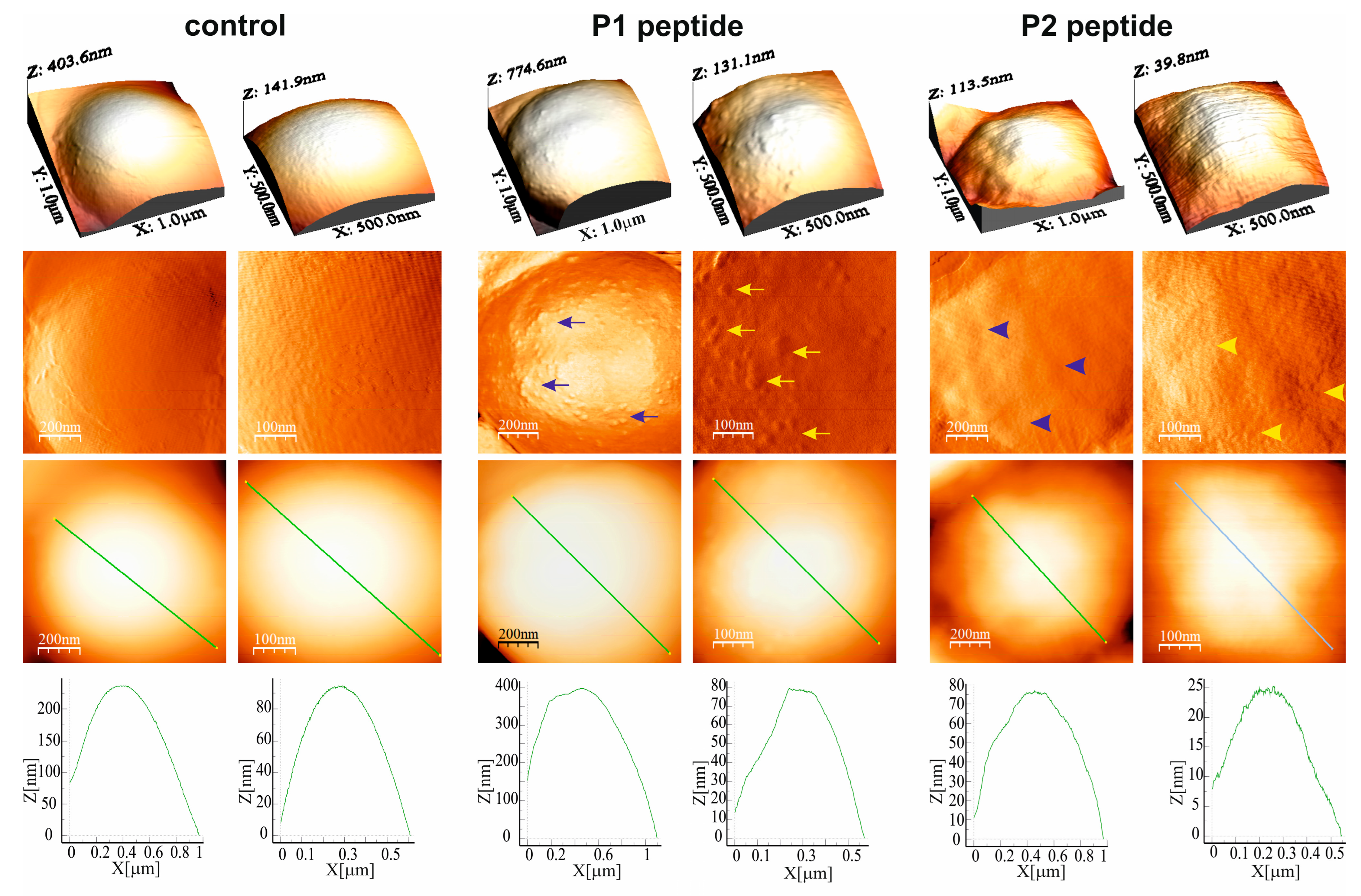
2.1.3. M. luteus Cell Surface Topography and Nanomechanical Properties After Treatment with P1 and P2 Peptides (AFM Imaging)
2.2. Interaction of G. mellonella PrAMPs with Escherichia coli Cells
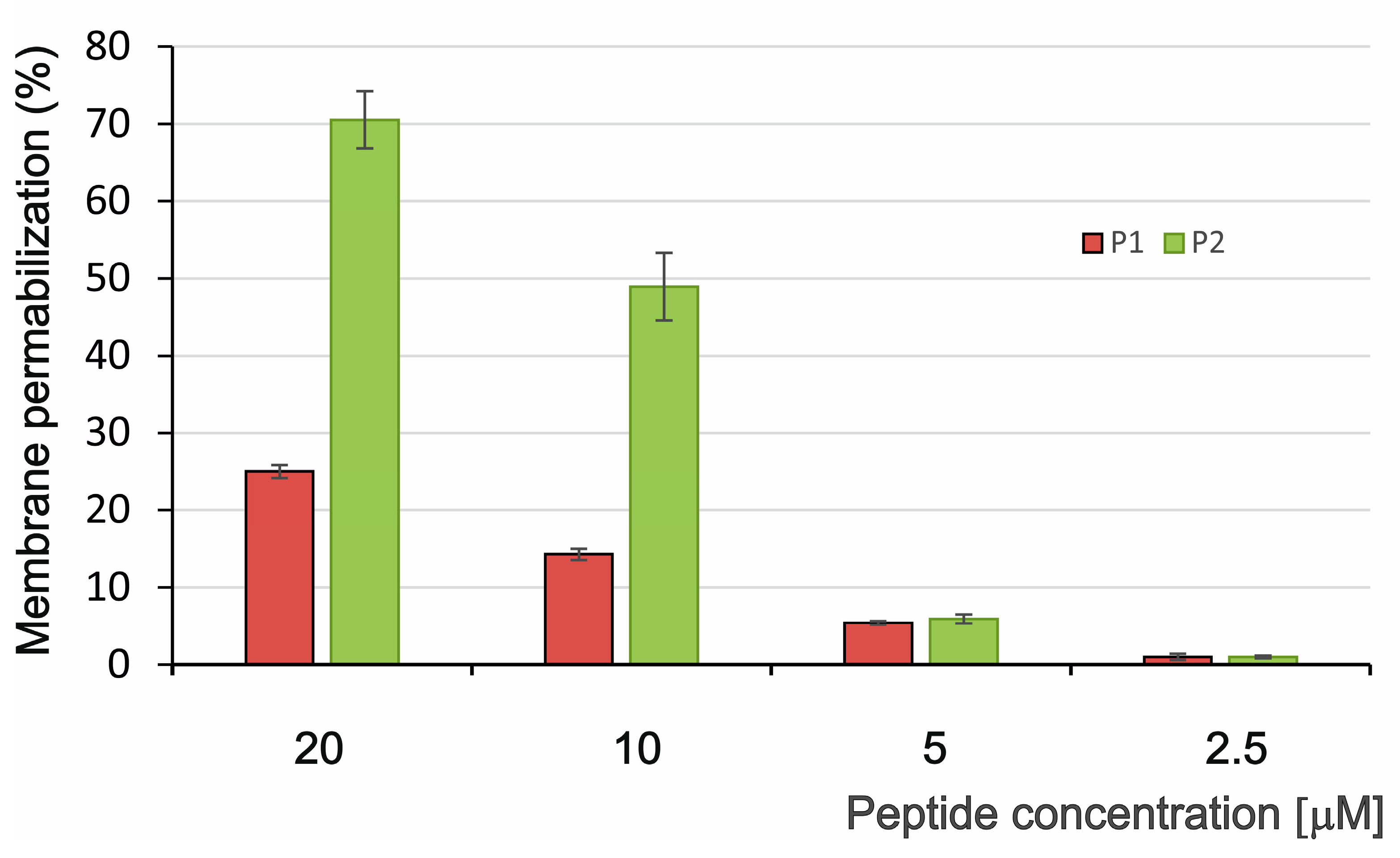
2.2.1. Permeabilization of Bacterial Membrane by P1 and P2 Peptides
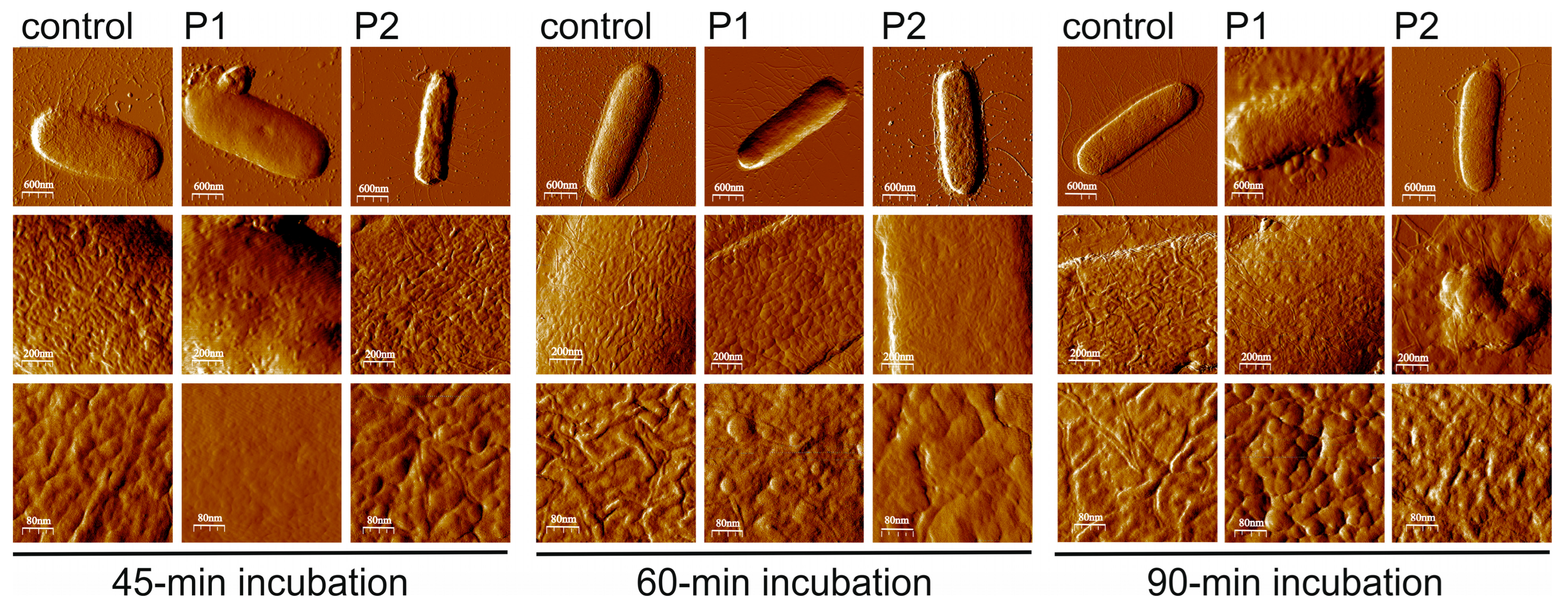
2.2.2. Morphology of E. coli Cells After Treatment with P1 and P2 Peptides (SEM Imaging)
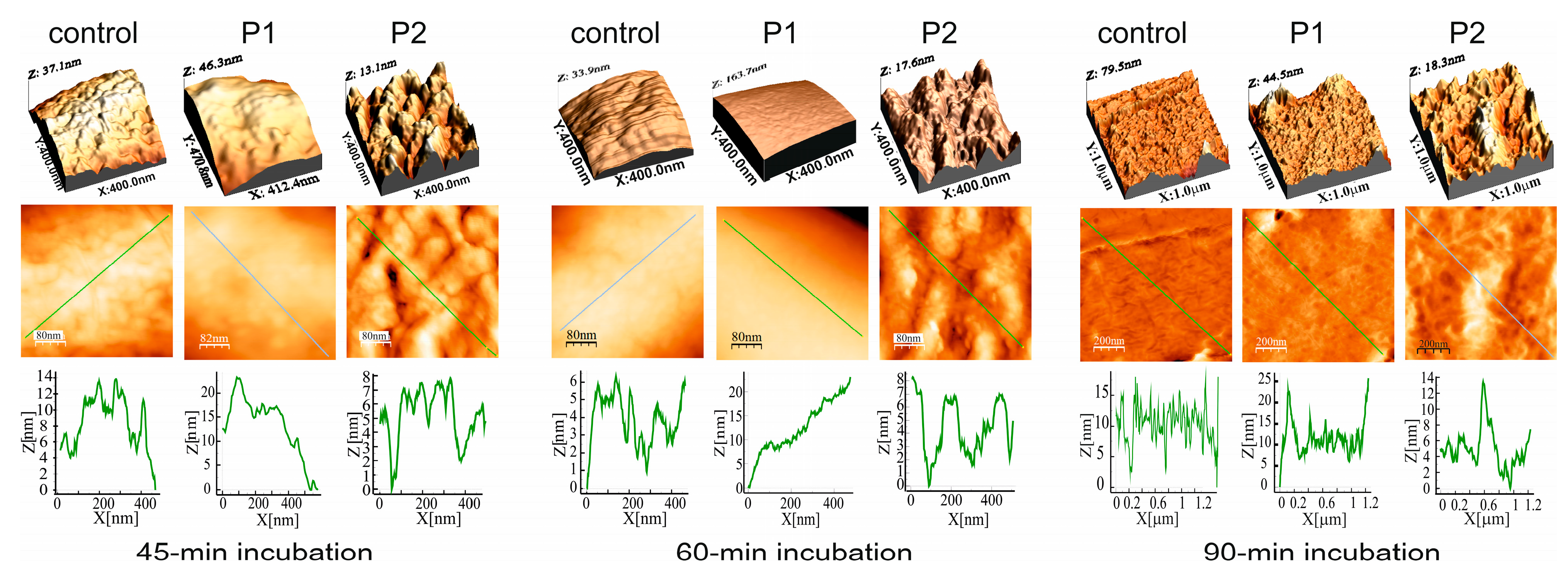
2.2.3. E. coli Cell Surface Topography and Nanomechanical Properties After Treatment with P1 and P2 Peptides (AFM Imaging)
2.3. Binding of P1 and P2 Peptides to M. luteus and E. coli Cells
2.4. FTIR Spectroscopy Investigation of P1 and P2 Effects on M. luteus and E. coli Cells
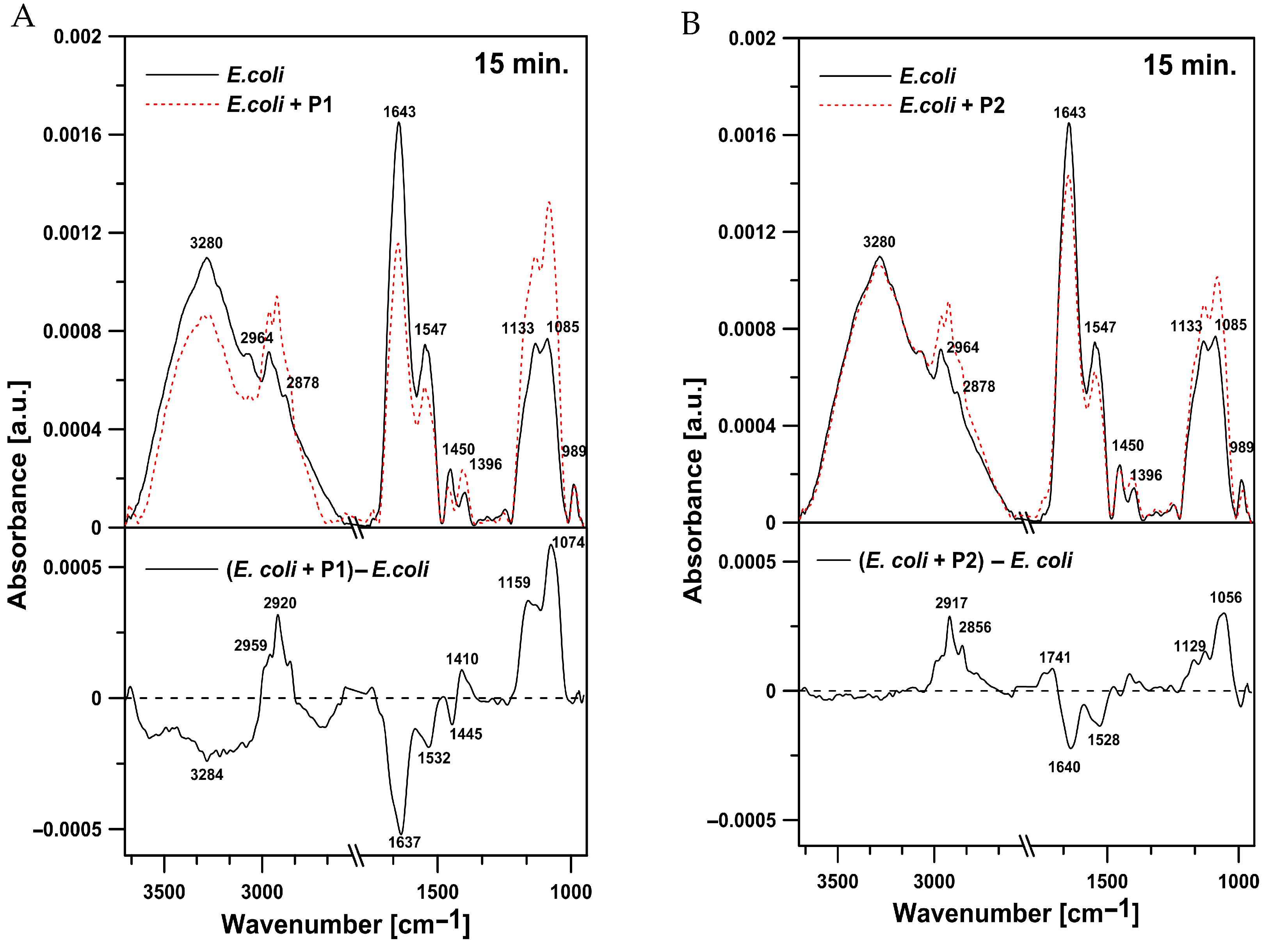
2.4.1. Effects on E. coli Cells
2.4.2. Effects on M. luteus Cells
3. Discussion
4. Materials and Methods
4.1. Insect Rearing, Immunization, Hemolymph Collection, and Preparing of Methanolic Extracts
4.2. Purification of Pro-Rich Peptides from Hemolymph of Immunized G. mellonella Larvae
4.3. Fluorescent Labeling of G. mellonella Pro-Rich Peptides
4.4. Binding of G. mellonella PrAMPs to Bacterial Cells
4.5. Bacterial Membrane Permeabilization—β-Galactosidase Assay
4.6. Antibacterial Activity Against Micrococcus luteus
4.7. Scanning Electron Microscopy (SEM) Imaging of Bacteria
4.8. Atomic Force Microscopy (AFM) Imaging and Analysis of Nanomechanical Properties of Bacterial Surface
4.9. FTIR Spectroscopy Study
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stączek, S.; Cytryńska, M.; Zdybicka-Barabas, A. Unraveling the Role of Antimicrobial Peptides in Insects. Int. J. Mol. Sci. 2023, 24, 5753. [Google Scholar] [CrossRef]
- Savitskaya, A.; Masso-Silva, J.; Haddaoui, I.; Enany, S. Exploring the arsenal of antimicrobial peptides: Mechanisms, diversity, and applications. Biochimie 2023, 214 Pt B, 216–227. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.W. Molecular mechanism of antimicrobial peptides: The origin of cooperativity. Biochim. Biophys. Acta 2006, 1758, 1292–1302. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.T.; Haney, E.F.; Vogel, H.J. The expanding scope of antimicrobial peptide structures and their modes of action. Trends Biotechnol. 2011, 29, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, K. Membrane permeabilization mechanisms. Adv. Exp. Med. Biol. 2019, 1117, 9–16. [Google Scholar] [CrossRef]
- Savini, F.; Loffredo, M.R.; Troiano, C.; Bobone, S.; Malanovic, N.; Eichmann, T.O.; Caprio, L.; Canale, V.C.; Park, Y.; Mangoni, M.L.; et al. Binding of an antimicrobial peptide to bacterial cells: Interaction with different species, strains and cellular components. Biochim. Biophys. Acta 2020, 1862, 183291. [Google Scholar] [CrossRef]
- Malanovic, N.; Marx, L.; Blondelle, S.E.; Pabst, G.; Semeraro, E.F. Experimental concepts for linking the biological activities of antimicrobial peptides to their molecular modes of action. Biochim. Biophys. Acta 2020, 1862, 183275. [Google Scholar] [CrossRef]
- Scocchi, M.; Tossi, A.; Gennaro, R. Proline-rich antimicrobial peptides: Converging to a non-lytic mechanism of action. Cell. Mol. Life Sci. 2011, 68, 2317–2330. [Google Scholar] [CrossRef]
- Benfield, A.H.; Henriques, S.T. Mode-of-Action of Antimicrobial Peptides: Membrane Disruption vs. Intracellular Mechanisms. Front. Med. Technol. 2020, 2, 610997. [Google Scholar] [CrossRef]
- Stączek, S.; Kunat-Budzyńska, M.; Cytryńska, M.; Zdybicka-Barabas, A. Proline-Rich Antimicrobial Peptides from Invertebrates. Molecules 2024, 29, 5864. [Google Scholar] [CrossRef]
- Piatek, M.; Sheehan, G.; Kavanagh, K. Galleria mellonella: The Versatile Host for Drug Discovery, In Vivo Toxicity Testing and Characterising Host-Pathogen Interactions. Antibiotics 2021, 10, 1545. [Google Scholar] [CrossRef] [PubMed]
- Curtis, A.; Binder, U.; Kavanagh, K. Galleria mellonella Larvae as a Model for Investigating Fungal-Host Interactions. Front. Fungal Biol. 2022, 3, 893494. [Google Scholar] [CrossRef] [PubMed]
- Grzenkowicz, M.; Kaźmierczak, N.; Piechowicz, L.; Kosznik-Kwaśnicka, K. Stimulating effect of lactoferrin on antibacterial efficacy of vB_SauM-A phage against multi-drug resistant strains of Staphylococcus aureus. Microb. Pathog. 2025, 207, 107872. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Bao, F.; Dong, F.; Yu, G.; Tian, H. Virulence and biological characteristics of Talaromyces wortmannii isolated from deep-seated dermatomycosis by in vitro and in vivo evaluation. BMC Infect. Dis. 2025, 25, 884. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.; Sun, Y.; Zhang, J.; Yao, Z.; Cao, J.; Hu, P.; Qian, C.; Li, J.; Zhou, T. Outer membrane protein A mediates Klebsiella pneumoniae penetration of the blood-brain barrier and induces bacterial meningitis. Microbiol. Res. 2025, 299, 128262. [Google Scholar] [CrossRef]
- Opris, R.V.; Baciu, A.M.; Filip, G.A.; Florea, A.; Costache, C. The use of Galleria mellonella in metal nanoparticle development: A systematic review. Chem. Biol. Interact. 2025, 415, 111511. [Google Scholar] [CrossRef]
- Cytryńska, M.; Mak, P.; Zdybicka-Barabas, A.; Suder, P.; Jakubowicz, T. Purification and characterization of eight peptides from Galleria mellonella immune hemolymph. Peptides 2007, 28, 533–546. [Google Scholar] [CrossRef]
- Brown, S.E.; Howard, A.; Kasprzak, A.B.; Gordon, K.H.; East, P.D. The discovery and analysis of a diverged family of novel antifungal moricin-like peptides in the wax moth Galleria mellonella. Insect Biochem. Mol. Biol. 2008, 38, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.E.; Howard, A.; Kasprzak, A.B.; Gordon, K.H.; East, P.D. A peptidomics study reveals the impressive antimicrobial peptide arsenal of the wax moth Galleria mellonella. Insect Biochem. Mol. Biol. 2009, 39, 792–800. [Google Scholar] [CrossRef]
- Kordaczuk, J.; Sułek, M.; Mak, P.; Zdybicka-Barabas, A.; Śmiałek, J.; Wojda, I. Cationic protein 8 plays multiple roles in Galleria mellonella immunity. Sci. Rep. 2022, 12, 11737. [Google Scholar] [CrossRef]
- Kordaczuk, J.; Sułek, M.; Mak, P.; Pawlikowska-Pawlęga, B.; Wojda, I. Serine protease inhibitor dipetalogastin-like from Galleria mellonella is involved in insect immunity. Sci. Rep. 2025, 15, 24094. [Google Scholar] [CrossRef] [PubMed]
- Kordaczuk, J.; Sułek, M.; Mak, P.; Frączek, A.; Wojda, I. Chemosensory protein 16 has an immune function and participates in host-pathogen interaction in Galleria mellonella infected with Pseudomonas entomophila. Virulence 2025, 16, 2471367. [Google Scholar] [CrossRef]
- Sułek, M.; Kordaczuk, J.; Mak, P.; Śmiałek-Bartyzel, J.; Hułas-Stasiak, M.; Wojda, I. Immune priming modulates Galleria mellonella and Pseudomonas entomophila interaction. Antimicrobial properties of Kazal peptide Pr13a. Front. Immunol. 2024, 15, 1358247. [Google Scholar] [CrossRef] [PubMed]
- Kordaczuk, J.; Sułek, M.; Mak, P.; Śmiałek-Bartyzel, J.; Hułas-Stasiak, M.; Wojda, I. Defence response of Galleria mellonella larvae to oral and intrahemocelic infection with Pseudomonas entomophila. Dev. Comp. Immunol. 2023, 147, 104749. [Google Scholar] [CrossRef]
- Altschul, S.F.; Madden, T.L.; Schaffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef] [PubMed]
- Schaffer, A.A.; Aravind, L.; Madden, T.L.; Shavirin, S.; Spouge, J.L.; Wolf, Y.I.; Koonin, E.V.; Altschul, S.F. Improving the accuracy of PSI-BLAST protein database searches with composition-based statistics and other refinements. Nucleic Acids Res. 2001, 29, 2994–3005. [Google Scholar] [CrossRef]
- Andrejko, M.; Mak, P.; Siemińska-Kuczer, A.; Iwański, B.; Wojda, I.; Suder, P.; Kuleta, P.; Regucka, K.; Cytryńska, M. A comparison of the production of antimicrobial peptides and proteins by Galleria mellonella larvae in response to infection with two Pseudomonas aeruginosa strains differing in the profile of secreted proteases. J. Insect Physiol. 2021, 131, 104239. [Google Scholar] [CrossRef]
- Stączek, S.; Zdybicka-Barabas, A.; Wojda, I.; Wiater, A.; Mak, P.; Suder, P.; Skrzypiec, K.; Cytryńska, M. Fungal α-1,3-Glucan as a New Pathogen-Associated Molecular Pattern in the Insect Model Host Galleria mellonella. Molecules 2021, 26, 5097. [Google Scholar] [CrossRef]
- Mak, P.; Zdybicka-Barabas, A.; Cytryńska, M. A different repertoire of Galleria mellonella antimicrobial peptides in larvae challenged with bacteria and fungi. Dev. Comp. Immunol. 2010, 34, 1129–1136. [Google Scholar] [CrossRef]
- Tamm, L.K.; Tatulian, S.A. Infrared spectroscopy of proteins and peptides in lipid bilayers. Quart. Rev. Biophys. 1997, 30, 365–429. [Google Scholar] [CrossRef]
- Otvos, L., Jr. The short proline-rich antibacterial peptide family. Cell. Mol. Life Sci. 2002, 59, 1138–1150. [Google Scholar] [CrossRef] [PubMed]
- Eleftherianos, I.; Zhang, W.; Heryanto, C.; Mohamed, A.; Contreras, G.; Tettamanti, G.; Wink, M.; Bassal, T. Diversity of insect antimicrobial peptides and proteins—A functional perspective: A review. Int. J. Biol. Macromol. 2021, 191, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Welch, N.G.; Li, W.; Hossain, M.A.; Separovic, F.; O’Brien-Simpson, N.M.; Wade, J.D. (Re)Defining the proline-rich antimicrobial peptide family and the identification of putative new members. Front. Chem. 2020, 8, 607769. [Google Scholar] [CrossRef] [PubMed]
- Handley, T.N.G.; Li, W.; Welch, N.G.; O’Brien-Simpson, N.M.; Hossain, M.A.; Wade, J.D. Evaluation of potential DnaK modulating proline-rich antimicrobial peptides identified by computational screening. Front. Chem. 2022, 10, 875233. [Google Scholar] [CrossRef]
- Cytryńska, M.; Rahnamaeian, M.; Zdybicka-Barabas, A.; Dobslaff, K.; Züchner, T.; Sacheau, G.; Innis, C.A.; Vilcinskas, A. Proline-Rich Antimicrobial Peptides in Medicinal Maggots of Lucilia sericata Interact with Bacterial DnaK but Do Not Inhibit Protein Synthesis. Front. Pharmacol. 2020, 11, 532. [Google Scholar] [CrossRef]
- Huang, W.; Baliga, C.; Aleksandrova, E.V.; Atkinson, G.; Polikanov, Y.S.; Vázquez-Laslop, N.; Mankin, A.S. Activity, structure, and diversity of Type II proline-rich antimicrobial peptides from insects. EMBO Rep. 2024, 25, 5194–5211. [Google Scholar] [CrossRef]
- Graf, M.; Mardirossian, M.; Nguyen, F.; Seefeldt, A.C.; Guichard, G.; Scocchi, M.; Innis, C.A.; Wilson, D.N. Proline-rich antimicrobial peptides targeting protein synthesis. Nat. Prod. Rep. 2017, 34, 702–711. [Google Scholar] [CrossRef]
- Rahnamaeian, M.; Cytryńska, M.; Zdybicka-Barabas, A.; Vilcinskas, A. The functional interaction between abaecin and pore-forming peptides indicates a general mechanism of antibacterial potentiation. Peptides 2016, 78, 17–23. [Google Scholar] [CrossRef]
- Rahnamaeian, M.; Cytryńska, M.; Zdybicka-Barabas, A.; Dobslaff, K.; Wiesner, J.; Twyman, R.M.; Zuchner, T.; Sadd, B.M.; Regoes, R.R.; Schmid-Hempel, P.; et al. Insect antimicrobial peptides show potentiating functional interactions against Gram-negative bacteria. Proc. Biol. Sci. B 2015, 282, 20150293. [Google Scholar] [CrossRef]
- Van Durme, J.; Maurer-Stroh, S.; Gallardo, R.; Wilkinson, H.; Rousseau, F.; Schymkowitz, J. Accurate prediction of DnaK-peptide binding via homology modelling and experimental data. PLoS Comput. Biol. 2009, 5, e1000475. [Google Scholar] [CrossRef]
- Mardirossian, M.; Sola, R.; Beckert, B.; Valencic, E.; Collis, D.W.P.; Borišek, J.; Armas, F.; Di Stasi, A.; Buchmann, J.; Syroegin, E.A.; et al. Peptide Inhibitors of Bacterial Protein Synthesis with Broad Spectrum and SbmA-Independent Bactericidal Activity against Clinical Pathogens. J. Med. Chem. 2020, 63, 9590–9602. [Google Scholar] [CrossRef]
- Pacor, S.; Benincasa, M.; Musso, M.V.; Krce, L.; Aviani, I.; Pallavicini, A.; Scocchi, M.; Gerdol, M.; Mardirossian, M. The proline-rich myticalins from Mytilus galloprovincialis display a membrane-permeabilizing antimicrobial mode of action. Peptides 2021, 143, 170594. [Google Scholar] [CrossRef]
- Destoumieux-Garzón, D.; Rosa, R.D.; Schmitt, P.; Barreto, C.; Vidal-Dupiol, J.; Mitta, G.; Gueguen, Y.; Bachère, E. Antimicrobial peptides in marine invertebrate health and disease. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2016, 371, 20150300. [Google Scholar] [CrossRef] [PubMed]
- Paulsen, V.S.; Blencke, H.M.; Benincasa, M.; Haug, T.; Eksteen, J.J.; Styrvold, O.B.; Scocchi, M.; Stensvåg, K. Structure-activity relationships of the antimicrobial peptide arasin 1—And mode of action studies of the N-terminal, proline-rich region. PLoS ONE 2013, 8, e53326. [Google Scholar] [CrossRef] [PubMed]
- Rončević, T.; Čikeš-Čulić, V.; Maravić, A.; Capanni, F.; Gerdol, M.; Pacor, S.; Tossi, A.; Giulianini, P.G.; Pallavicini, A.; Manfrin, C. Identification and functional characterization of the astacidin family of proline-rich host defence peptides (PcAst) from the red swamp crayfish (Procambarus clarkii, Girard 1852). Dev. Comp. Immunol. 2020, 105, 103574. [Google Scholar] [CrossRef] [PubMed]
- Rattanadilog Na Phuket, T.; Charoensapsri, W.; Amparyup, P.; Imjongjirak, C. Antibacterial activity and immunomodulatory role of a proline-rich antimicrobial peptide SpPR-AMP1 against Vibrio campbellii infection in shrimp Litopenaeus vannamei. Fish Shellfish Immunol. 2023, 132, 108479. [Google Scholar] [CrossRef]
- Yang, Q.; Li, H.; Hou, L.; Zhu, L.; Kong, X. Antimicrobial peptides in crustacean, especially in Procambarus clarkii (crayfish). Fish Shellfish Immunol. 2025, 166, 110646. [Google Scholar] [CrossRef]
- Yang, L.L.; Zhan, M.Y.; Zhuo, Y.L.; Pan, Y.M.; Xu, Y.; Zhou, X.H.; Yang, P.J.; Liu, H.L.; Liang, Z.H.; Huang, X.D.; et al. Antimicrobial activities of a proline-rich proprotein from Spodoptera litura. Dev. Comp. Immunol. 2018, 87, 137–146. [Google Scholar] [CrossRef]
- Hara, S.; Yamakawa, M. A novel antibacterial peptide family isolated from the silkworm, Bombyx mori. Biochem. J. 1995, 310 Pt 2, 651–656. [Google Scholar] [CrossRef]
- Sola, R.; Mardirossian, M.; Beckert, B.; Sanghez De Luna, L.; Prickett, D.; Tossi, A.; Wilson, D.N.; Scocchi, M. Characterization of Cetacean Proline-Rich Antimicrobial Peptides Displaying Activity against ESKAPE Pathogens. Int. J. Mol. Sci. 2020, 21, 7367. [Google Scholar] [CrossRef]
- Panteleev, P.V.; Safronova, V.N.; Kruglikov, R.N.; Bolosov, I.A.; Bogdanov, I.V.; Ovchinnikova, T.V. A Novel Proline-Rich Cathelicidin from the Alpaca Vicugna pacos with Potency to Combat Antibiotic-Resistant Bacteria: Mechanism of Action and the Functional Role of the C-Terminal Region. Membranes 2022, 12, 515. [Google Scholar] [CrossRef]
- Runti, G.; Benincasa, M.; Giuffrida, G.; Devescovi, G.; Venturi, V.; Gennaro, R.; Scocchi, M. The Mechanism of Killing by the Proline-Rich Peptide Bac7(1-35) against Clinical Strains of Pseudomonas aeruginosa Differs from That against Other Gram-Negative Bacteria. Antimicrob. Agents Chemother. 2017, 61, e01660-16. [Google Scholar] [CrossRef]
- Sharma, P.; Vaiwala, R.; Gopinath, A.K.; Chockalingam, R.; Ayappa, K.G. Structure of the Bacterial Cell Envelope and Interactions with Antimicrobials: Insights from Molecular Dynamics Simulations. Langmuir 2024, 40, 7791–7811. [Google Scholar] [CrossRef]
- Fivenson, E.M.; Dubois, L.; Bernhardt, T.G. Co-ordinated assembly of the multilayered cell envelope of Gram-negative bacteria. Curr. Opin. Microbiol. 2024, 79, 102479. [Google Scholar] [CrossRef]
- Maher, C.; Hassan, K.A. The Gram-negative permeability barrier: Tipping the balance of the in and the out. Mbio 2023, 14, e0120523. [Google Scholar] [CrossRef]
- Fleitas, O.; Rebollar, E.A.; Bustamante, V.H. Extracellular defense of bacteria against antimicrobial peptides. J. Bacteriol. 2025, 207, e0016625. [Google Scholar] [CrossRef]
- Rossetti, P.; Trollmann, M.F.W.; Wichmann, C.; Gutsmann, T.; Eggeling, C.; Böckmann, R.A. From Membrane Composition to Antimicrobial Strategies: Experimental and Computational Approaches to AMP Design and Selectivity. Small 2025, e2411476. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, B.; Munoz-Garay, C. Unlocking the power of membrane biophysics: Enhancing the study of antimicrobial peptides activity and selectivity. Biophys. Rev. 2025, 17, 605–625. [Google Scholar] [CrossRef] [PubMed]
- Wladyka, B.; Wielebska, K.; Wloka, M.; Bochenska, O.; Dubin, G.; Dubin, A.; Mak, P. Isolation, biochemical characterization, and cloning of a bacteriocin from the poultry-associated Staphylococcus aureus strain CH-91. Appl. Microbiol. Biotechnol. 2013, 97, 7229–7239. [Google Scholar] [CrossRef]
- Schägger, H.; von Jagow, G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal. Biochem. 1987, 166, 368–379. [Google Scholar] [CrossRef] [PubMed]
- Zdybicka-Barabas, A.; Palusińska-Szysz, M.; Gruszecki, W.I.; Mak, P.; Cytryńska, M. Galleria mellonella apolipophorin III—An apolipoprotein with anti-Legionella pneumophila activity. Biochim. Biophys. Acta 2014, 1838, 2689–2697. [Google Scholar] [CrossRef]
- Zdybicka-Barabas, A.; Stączek, S.; Pawlikowska-Pawlęga, B.; Mak, P.; Luchowski, R.; Skrzypiec, K.; Mendyk, E.; Wydrych, J.; Gruszecki, W.I.; Cytryńska, M. Studies on the interactions of neutral Galleria mellonella cecropin D with living bacterial cells. Amino Acids 2019, 51, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Zdybicka-Barabas, A.; Mak, P.; Klys, A.; Skrzypiec, K.; Mendyk, E.; Fiołka, M.J.; Cytryńska, M. Synergistic action of Galleria mellonella anionic peptide 2 and lysozyme against Gram-negative bacteria. Biochim. Biophys. Acta 2012, 1818, 2623–2635. [Google Scholar] [CrossRef] [PubMed]
- Zdybicka-Barabas, A.; Cytryńska, M. Involvement of apolipophorin III in antibacterial defense of Galleria mellonella larvae. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2011, 158, 90–98. [Google Scholar] [CrossRef]
- Palusińska-Szysz, M.; Zdybicka-Barabas, A.; Luchowski, R.; Reszczyńska, E.; Śmiałek, J.; Mak, P.; Gruszecki, W.I.; Cytryńska, M. Choline Supplementation Sensitizes Legionella dumoffii to Galleria mellonella Apolipophorin III. Int. J. Mol. Sci. 2020, 21, 5818. [Google Scholar] [CrossRef] [PubMed]
- Horcas, I.; Fernández, R.; Gómez-Rodríguez, J.M.; Colchero, J.; Gómez-Herrero, J.; Baro, A.M. WSxM: A software for scanning probe microscopy and a tool for nanotechnology. Rev. Sci. Instrum. 2007, 78, 013705. [Google Scholar] [CrossRef]
- Di Stasi, A.; de Pascale, L.; Morici, M.; Wilson, D.N.; Scocchi, M.; Mardirossian, M. Designing New Chimeric Proline-Rich Antimicrobial Peptides to Enhance Efficacy Toward the ESKAPE+E: Beyond Sequence Extension. Biomolecules 2025, 15, 776. [Google Scholar] [CrossRef]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zdybicka-Barabas, A.; Stączek, S.; Mak, P.; Kapral-Piotrowska, J.; Skrzypiec, K.; Wydrych, J.; Pawlikowska-Pawlęga, B.; Gruszecki, W.I.; Cytryńska, M. Interactions of Galleria mellonella Proline-Rich Antimicrobial Peptides with Gram-Negative and Gram-Positive Bacteria. Int. J. Mol. Sci. 2025, 26, 8438. https://doi.org/10.3390/ijms26178438
Zdybicka-Barabas A, Stączek S, Mak P, Kapral-Piotrowska J, Skrzypiec K, Wydrych J, Pawlikowska-Pawlęga B, Gruszecki WI, Cytryńska M. Interactions of Galleria mellonella Proline-Rich Antimicrobial Peptides with Gram-Negative and Gram-Positive Bacteria. International Journal of Molecular Sciences. 2025; 26(17):8438. https://doi.org/10.3390/ijms26178438
Chicago/Turabian StyleZdybicka-Barabas, Agnieszka, Sylwia Stączek, Paweł Mak, Justyna Kapral-Piotrowska, Krzysztof Skrzypiec, Jerzy Wydrych, Bożena Pawlikowska-Pawlęga, Wiesław I. Gruszecki, and Małgorzata Cytryńska. 2025. "Interactions of Galleria mellonella Proline-Rich Antimicrobial Peptides with Gram-Negative and Gram-Positive Bacteria" International Journal of Molecular Sciences 26, no. 17: 8438. https://doi.org/10.3390/ijms26178438
APA StyleZdybicka-Barabas, A., Stączek, S., Mak, P., Kapral-Piotrowska, J., Skrzypiec, K., Wydrych, J., Pawlikowska-Pawlęga, B., Gruszecki, W. I., & Cytryńska, M. (2025). Interactions of Galleria mellonella Proline-Rich Antimicrobial Peptides with Gram-Negative and Gram-Positive Bacteria. International Journal of Molecular Sciences, 26(17), 8438. https://doi.org/10.3390/ijms26178438





