Molecular and Immunomodulatory Mechanisms of Statins in Inflammation and Cancer Therapeutics with Emphasis on the NF-κB, NLRP3 Inflammasome, and Cytokine Regulatory Axes
Abstract
1. Introduction
2. Statins at a Glance
2.1. Cholesterol-Lowering Mechanism of Statins
2.1.1. Inhibition of HMG-CoA Reductase
Decreased Cholesterol Synthesis in Hepatocytes
2.1.2. Upregulation of LDL Receptors
2.1.3. Reduction in VLDL Secretion
2.1.4. Inhibition of Isoprenoid Synthesis
2.1.5. Impact on Sterol Regulatory Element-Binding Proteins (SREBPs)
2.1.6. Reduction in Circulating LDL-C and Other Lipoproteins
2.1.7. Reflection from Clinical Trials
3. Anti-Inflammatory Mechanisms of Statins
3.1. Effect on NLRP3 Inflammasome
3.2. Effect on the NF-κB Pathway
3.3. Comparative Integration of NF-κB and NLRP3 Signalling Under Statin Modulation
3.4. Effect on the MAPK Pathway
3.5. Effect on T-Cell Differentiation
3.6. Effect on Leukocyte Adhesion and Migration
3.7. Effect on Cytokine Production
4. Statins in Chronic Inflammatory Conditions
4.1. Inhibition of Pro-Inflammatory Cytokines
4.2. Modulation of Immune Cell Phenotypes
4.3. Endothelial Protection and Inflammation Reduction
4.4. Inhibition of Inflammasome Activation
4.5. Modulation of Protease-Activated Receptor-2 (PAR-2) Signalling
4.6. Antioxidant and ROS Scavenging Activity
4.7. Reduction in Acute Phase Proteins
4.8. Statins and Rheumatoid Arthritis
4.9. Statins and COPD
5. Limitations
5.1. Organ Dysfunction
5.2. Hormones
5.3. Epigenetics
5.4. Socioeconomic
6. Conclusions
7. Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Randomised trial of cholestesrol lowering in 4444 patients with coronary heart disease: The Scandinavian Simvastatin Survival Study (4S). Lancet 1994, 344, 1383–1389.
- Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: A randomised placebo-controlled trial. Lancet 2002, 360, 7–22. [Google Scholar] [CrossRef]
- Sacks, F.M.; Pfeffer, M.A.; Moye, L.A.; Rouleau, J.L.; Rutherford, J.D.; Cole, T.G.; Brown, L.; Warnica, J.W.; Arnold, J.M.; Wun, C.C.; et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events Trial investigators. N. Engl. J. Med. 1996, 335, 1001–1009. [Google Scholar] [CrossRef]
- LIPID Study Group. Long-term effectiveness and safety of pravastatin in 9014 patients with coronary heart disease and average cholesterol concentrations: The LIPID trial follow-up. Lancet 2002, 359, 1379–1387, Erratum in Lancet 2002, 360, 1430. [Google Scholar] [CrossRef] [PubMed]
- Gibson, C.M.; Pride, Y.B.; Hochberg, C.P.; Sloan, S.; Sabatine, M.S.; Cannon, C.P.; Group, T.S. Effect of intensive statin therapy on clinical outcomes among patients undergoing percutaneous coronary intervention for acute coronary syndrome. PCI-PROVE IT: A PROVE IT-TIMI 22 (Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis In Myocardial Infarction 22) Substudy. J. Am. Coll. Cardiol. 2009, 54, 2290–2295. [Google Scholar] [PubMed]
- Ridker, P.M.; Danielson, E.; Fonseca, F.A.; Genest, J.; Gotto, A.M., Jr.; Kastelein, J.J.; Koenig, W.; Libby, P.; Lorenzatti, A.J.; MacFadyen, J.G.; et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N. Engl. J. Med. 2008, 359, 2195–2207. [Google Scholar] [CrossRef]
- Shepherd, J. The West of Scotland Coronary Prevention Study: A trial of cholesterol reduction in Scottish men. Am. J. Cardiol. 1995, 76, 113C–117C. [Google Scholar] [CrossRef] [PubMed]
- Kjekshus, J.; Apetrei, E.; Barrios, V.; Bohm, M.; Cleland, J.G.; Cornel, J.H.; Dunselman, P.; Fonseca, C.; Goudev, A.; Grande, P.; et al. Rosuvastatin in older patients with systolic heart failure. N. Engl. J. Med. 2007, 357, 2248–2261. [Google Scholar] [CrossRef] [PubMed]
- Sever, P.S.; Dahlof, B.; Poulter, N.R.; Wedel, H.; Beevers, G.; Caulfield, M.; Collins, R.; Kjeldsen, S.E.; Kristinsson, A.; McInnes, G.T.; et al. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial–Lipid Lowering Arm (ASCOT-LLA): A multicentre randomised controlled trial. Lancet 2003, 361, 1149–1158. [Google Scholar]
- Lietzau, G.; Sienkiewicz, W.; Karwacki, Z.; Dziewiatkowski, J.; Kaleczyc, J.; Kowianski, P. The Effect of Simvastatin on the Dynamics of NF-kappaB-Regulated Neurodegenerative and Neuroprotective Processes in the Acute Phase of Ischemic Stroke. Mol. Neurobiol. 2023, 60, 4935–4951. [Google Scholar] [CrossRef]
- Wozniak, E.; Broncel, M.; Niedzielski, M.; Wozniak, A.; Gorzelak-Pabis, P. The effect of lipid-lowering therapies on the pro-inflammatory and anti-inflammatory properties of vascular endothelial cells. PLoS ONE 2023, 18, e0280741. [Google Scholar] [CrossRef]
- Altun, I.; Oz, F.; Arkaya, S.C.; Altun, I.; Bilge, A.K.; Umman, B.; Turkoglu, U.M. Effect of statins on endothelial function in patients with acute coronary syndrome: A prospective study using adhesion molecules and flow-mediated dilatation. J. Clin. Med. Res. 2014, 6, 354–361. [Google Scholar] [CrossRef]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef]
- Michels da Silva, D.; Langer, H.; Graf, T. Inflammatory and Molecular Pathways in Heart Failure-Ischemia, HFpEF and Transthyretin Cardiac Amyloidosis. Int. J. Mol. Sci. 2019, 20, 2322. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, X.; Hu, Q.; Wu, J.; Wang, G.; Hong, Z.; Ren, J.; Lab for Trauma and Surgical Infections; Surgical, I. Mitochondrial DNA in liver inflammation and oxidative stress. Life Sci. 2019, 236, 116464. [Google Scholar] [CrossRef]
- Fritsch, J.; Abreu, M.T. The Microbiota and the Immune Response: What Is the Chicken and What Is the Egg? Gastrointest. Endosc. Clin. N. Am. 2019, 29, 381–393. [Google Scholar] [CrossRef] [PubMed]
- Greten, F.R.; Grivennikov, S.I. Inflammation and Cancer: Triggers, Mechanisms, and Consequences. Immunity 2019, 51, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Deng, Y.; Li, Z.; Chen, Y.; Zhu, X.; Tan, X.; Cao, G. Cancer Evo-Dev: A Theory of Inflammation-Induced Oncogenesis. Front. Immunol. 2021, 12, 768098. [Google Scholar] [CrossRef] [PubMed]
- Akkiz, H.; Simsek, H.; Balci, D.; Ulger, Y.; Onan, E.; Akcaer, N.; Delik, A. Inflammation and cancer: Molecular mechanisms and clinical consequences. Front. Oncol. 2025, 15, 1564572. [Google Scholar] [CrossRef]
- Yang, J.; Li, C.; Shen, Y.; Zhou, H.; Shao, Y.; Zhu, W.; Chen, Y. Impact of statin use on cancer-specific mortality and recurrence: A meta-analysis of 60 observational studies. Medicine 2020, 99, e19596. [Google Scholar] [CrossRef]
- Zaky, M.Y.; Fan, C.; Zhang, H.; Sun, X.F. Unraveling the Anticancer Potential of Statins: Mechanisms and Clinical Significance. Cancers 2023, 15, 4787. [Google Scholar] [CrossRef]
- Khatiwada, N.; Hong, Z. Potential Benefits and Risks Associated with the Use of Statins. Pharmaceutics 2024, 16, 214. [Google Scholar] [CrossRef] [PubMed]
- Mach, F.; Ray, K.K.; Wiklund, O.; Corsini, A.; Catapano, A.L.; Bruckert, E.; De Backer, G.; Hegele, R.A.; Hovingh, G.K.; Jacobson, T.A.; et al. Adverse effects of statin therapy: Perception vs. the evidence—Focus on glucose homeostasis, cognitive, renal and hepatic function, haemorrhagic stroke and cataract. Eur. Heart J. 2018, 39, 2526–2539. [Google Scholar] [CrossRef]
- Grundy, S.M.; Stone, N.J.; Bailey, A.L.; Beam, C.; Birtcher, K.K.; Blumenthal, R.S.; Braun, L.T.; de Ferranti, S.; Faiella-Tommasino, J.; Forman, D.E.; et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019, 139, e1082–e1143. [Google Scholar]
- Taylor, F.C.; Huffman, M.; Ebrahim, S. Statin therapy for primary prevention of cardiovascular disease. JAMA 2013, 310, 2451–2452. [Google Scholar] [CrossRef] [PubMed]
- Chou, R.; Cantor, A.; Dana, T.; Wagner, J.; Ahmed, A.Y.; Fu, R.; Ferencik, M. Statin Use for the Primary Prevention of Cardiovascular Disease in Adults: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA 2022, 328, 754–771. [Google Scholar] [CrossRef]
- Wiggins, B.S.; Saseen, J.J.; Page, R.L., 2nd; Reed, B.N.; Sneed, K.; Kostis, J.B.; Lanfear, D.; Virani, S.; Morris, P.B.; American Heart Association Clinical Pharmacology Committee of the Council on Clinical Cardiology. Recommendations for Management of Clinically Significant Drug-Drug Interactions with Statins and Select Agents Used in Patients With Cardiovascular Disease: A Scientific Statement From the American Heart Association. Circulation 2016, 134, e468–e495. [Google Scholar] [CrossRef]
- Newman, C.B.; Preiss, D.; Tobert, J.A.; Jacobson, T.A.; Page, R.L., 2nd; Goldstein, L.B.; Chin, C.; Tannock, L.R.; Miller, M.; Raghuveer, G.; et al. Statin Safety and Associated Adverse Events: A Scientific Statement From the American Heart Association. Arterioscler. Thromb. Vasc. Biol. 2019, 39, e38–e81, Correction in Arterioscler. Thromb. Vasc. Biol. 2019, 39, e158. [Google Scholar] [CrossRef]
- Balasubramanian, R.; Maideen, N.M.P. HMG-CoA Reductase Inhibitors (Statins) and their Drug Interactions Involving CYP Enzymes, P-glycoprotein and OATP Transporters-An Overview. Curr. Drug Metab. 2021, 22, 328–341. [Google Scholar]
- Hirota, T.; Fujita, Y.; Ieiri, I. An updated review of pharmacokinetic drug interactions and pharmacogenetics of statins. Expert Opin. Drug Metab. Toxicol. 2020, 16, 809–822. [Google Scholar] [CrossRef] [PubMed]
- Wasim, R.; Ansari, T.M.; Ahsan, F.; Siddiqui, M.H.; Singh, A.; Shariq, M.; Parveen, S. Pleiotropic Benefits of Statins in Cardiovascular Diseases. Drug Res. 2022, 72, 477–486. [Google Scholar] [CrossRef]
- Eberle, D.; Hegarty, B.; Bossard, P.; Ferre, P.; Foufelle, F. SREBP transcription factors: Master regulators of lipid homeostasis. Biochimie 2004, 86, 839–848. [Google Scholar] [CrossRef]
- Daemen, S.; Kutmon, M.; Evelo, C.T. A pathway approach to investigate the function and regulation of SREBPs. Genes Nutr. 2013, 8, 289–300. [Google Scholar] [CrossRef]
- Miyata, S.; Kodaka, M.; Kikuchi, A.; Matsunaga, Y.; Shoji, K.; Kuan, Y.C.; Iwase, M.; Takeda, K.; Katsuta, R.; Ishigami, K.; et al. Sulforaphane suppresses the activity of sterol regulatory element-binding proteins (SREBPs) by promoting SREBP precursor degradation. Sci. Rep. 2022, 12, 8715. [Google Scholar] [CrossRef] [PubMed]
- Chan, M.L.; Shiu, S.W.; Cheung, C.L.; Yu-Hung Leung, A.; Tan, K.C. Effects of statins on the inducible degrader of low-density lipoprotein receptor in familial hypercholesterolemia. Endocr. Connect. 2022, 11, e220019. [Google Scholar] [CrossRef]
- Dong, B.; Wu, M.; Li, H.; Kraemer, F.B.; Adeli, K.; Seidah, N.G.; Park, S.W.; Liu, J. Strong induction of PCSK9 gene expression through HNF1alpha and SREBP2: Mechanism for the resistance to LDL-cholesterol lowering effect of statins in dyslipidemic hamsters. J. Lipid Res. 2010, 51, 1486–1495. [Google Scholar] [CrossRef]
- Lagace, T.A. PCSK9 and LDLR degradation: Regulatory mechanisms in circulation and in cells. Curr. Opin. Lipidol. 2014, 25, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, S.; Tsubakio-Yamamoto, K.; Ohama, T.; Nakagawa-Toyama, Y.; Nishida, M. Molecular mechanisms of HDL-cholesterol elevation by statins and its effects on HDL functions. J. Atheroscler. Thromb. 2010, 17, 436–451. [Google Scholar] [CrossRef] [PubMed]
- Colhoun, H.M.; Betteridge, D.J.; Durrington, P.N.; Hitman, G.A.; Neil, H.A.; Livingstone, S.J.; Thomason, M.J.; Mackness, M.I.; Charlton-Menys, V.; Fuller, J.H.; et al. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): Multicentre randomised placebo-controlled trial. Lancet 2004, 364, 685–696. [Google Scholar] [CrossRef]
- Farmer, J.A.; Gotto, A.M., Jr. The Heart Protection Study: Expanding the boundaries for high-risk coronary disease prevention. Am. J. Cardiol. 2003, 92, 3i–9i. [Google Scholar] [CrossRef]
- Downs, J.R.; Clearfield, M.; Weis, S.; Whitney, E.; Shapiro, D.R.; Beere, P.A.; Langendorfer, A.; Stein, E.A.; Kruyer, W.; Gotto, A.M., Jr. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: Results of AFCAPS/TexCAPS. Air Force/Texas Coronary Atherosclerosis Prevention Study. JAMA 1998, 279, 1615–1622. [Google Scholar] [CrossRef] [PubMed]
- Messerli, A.W.; Aronow, H.D.; Sprecher, D.L. The Lescol Intervention Prevention Study (LIPS): Start all patients on statins early after PCI. Clevel. Clin. J. Med. 2003, 70, 561–566. [Google Scholar] [CrossRef]
- Chan, P.; Shao, L.; Tomlinson, B.; Zhang, Y.; Liu, Z.M. An evaluation of pitavastatin for the treatment of hypercholesterolemia. Expert Opin. Pharmacother. 2019, 20, 103–113. [Google Scholar] [CrossRef]
- Adams, S.P.; Alaeiilkhchi, N.; Wright, J.M. Pitavastatin for lowering lipids. Cochrane Database Syst. Rev. 2020, 6, CD012735. [Google Scholar] [CrossRef]
- Takano, H.; Mizuma, H.; Kuwabara, Y.; Sato, Y.; Shindo, S.; Kotooka, N.; Fujimatsu, D.; Kobayashi, Y.; Inoue, T.; Node, K.; et al. Effects of pitavastatin in Japanese patients with chronic heart failure: The Pitavastatin Heart Failure Study (PEARL Study). Circ. J. 2013, 77, 917–925. [Google Scholar] [CrossRef]
- Hiro, T.; Kimura, T.; Morimoto, T.; Miyauchi, K.; Nakagawa, Y.; Yamagishi, M.; Ozaki, Y.; Kimura, K.; Saito, S.; Yamaguchi, T.; et al. Effect of intensive statin therapy on regression of coronary atherosclerosis in patients with acute coronary syndrome: A multicenter randomized trial evaluated by volumetric intravascular ultrasound using pitavastatin versus atorvastatin (JAPAN-ACS [Japan assessment of pitavastatin and atorvastatin in acute coronary syndrome] study). J. Am. Coll. Cardiol. 2009, 54, 293–302. [Google Scholar]
- Pasternak, R.C.; Smith, S.C., Jr.; Bairey-Merz, C.N.; Grundy, S.M.; Cleeman, J.I.; Lenfant, C.; American College of Cardiology; American Heart Association; National Heart, Lung and Blood Institute. ACC/AHA/NHLBI Clinical Advisory on the Use and Safety of Statins. Stroke 2002, 33, 2337–2341. [Google Scholar] [PubMed]
- Zeiser, R. Immune modulatory effects of statins. Immunology 2018, 154, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Yeganeh, B.; Wiechec, E.; Ande, S.R.; Sharma, P.; Moghadam, A.R.; Post, M.; Freed, D.H.; Hashemi, M.; Shojaei, S.; Zeki, A.A.; et al. Targeting the mevalonate cascade as a new therapeutic approach in heart disease, cancer and pulmonary disease. Pharmacol. Ther. 2014, 143, 87–110. [Google Scholar] [CrossRef]
- Arefieva, T.I.; Filatova, A.Y.; Potekhina, A.V.; Shchinova, A.M. Immunotropic Effects and Proposed Mechanism of Action for 3-Hydroxy-3-methylglutaryl-coenzyme A Reductase Inhibitors (Statins). Biochemistry 2018, 83, 874–889. [Google Scholar] [CrossRef]
- Chen, Y.R.; Xiang, X.D.; Sun, F.; Xiao, B.W.; Yan, M.Y.; Peng, B.; Liu, D. Simvastatin Reduces NETosis to Attenuate Severe Asthma by Inhibiting PAD4 Expression. Oxidative Med. Cell. Longev. 2023, 2023, 1493684. [Google Scholar] [CrossRef]
- Kim, E.K.; Cho, J.H.; Jeong, A.R.; Kim, E.J.; Park, D.K.; Kwon, K.A.; Chung, J.W.; Kim, K.O.; Kim, J.H.; Kim, J.H.; et al. Anti-inflammatory effects of simvastatin in nonsteroidal anti-inflammatory drugs-induced small bowel injury. J. Physiol. Pharmacol. 2017, 68, 69–77. [Google Scholar]
- Pathak, N.N.; Lingaraju, M.C.; Balaganur, V.; Kant, V.; More, A.S.; Kumar, D.; Kumar, D.; Tandan, S.K. Anti-inflammatory and chondroprotective effects of atorvastatin in a cartilage explant model of osteoarthritis. Inflamm. Res. 2015, 64, 161–169. [Google Scholar] [CrossRef]
- Tang, Z.; Ning, Z.; Li, Z. The beneficial effects of Rosuvastatin in inhibiting inflammation in sepsis. Aging 2024, 16, 10424–10434. [Google Scholar] [CrossRef]
- Shin, S.K.; Cho, J.H.; Kim, E.J.; Kim, E.K.; Park, D.K.; Kwon, K.A.; Chung, J.W.; Kim, K.O.; Kim, Y.J. Anti-inflammatory and anti-apoptotic effects of rosuvastatin by regulation of oxidative stress in a dextran sulfate sodium-induced colitis model. World J. Gastroenterol. 2017, 23, 4559–4568. [Google Scholar] [CrossRef]
- Xu, Y.; Du, H.P.; Li, J.; Xu, R.; Wang, Y.L.; You, S.J.; Liu, H.; Wang, F.; Cao, Y.J.; Liu, C.F.; et al. Statins upregulate cystathionine gamma-lyase transcription and H2S generation via activating Akt signaling in macrophage. Pharmacol. Res. 2014, 87, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Ni, Z.; Qian, J.; Tomino, Y. Pravastatin inhibits carboxymethyllysine-induced monocyte chemoattractant protein 1 expression in podocytes via prevention of signalling events. Nephron Exp. Nephrol. 2007, 106, e1–e10. [Google Scholar] [CrossRef]
- Li, Q.Z.; Sun, J.; Han, J.J.; Qian, Z.J. Anti-inflammation of simvastatin by polarization of murine macrophages from M1 phenotype to M2 phenotype. Zhonghua Yi Xue Za Zhi 2013, 93, 2071–2074. [Google Scholar]
- Takahashi, S.; Nakamura, H.; Seki, M.; Shiraishi, Y.; Yamamoto, M.; Furuuchi, M.; Nakajima, T.; Tsujimura, S.; Shirahata, T.; Nakamura, M.; et al. Reversal of elastase-induced pulmonary emphysema and promotion of alveolar epithelial cell proliferation by simvastatin in mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 2008, 294, L882–L890. [Google Scholar] [CrossRef] [PubMed]
- Shao, Q.; Shen, L.H.; Hu, L.H.; Pu, J.; Jing, Q.; He, B. Atorvastatin suppresses inflammatory response induced by oxLDL through inhibition of ERK phosphorylation, IkappaBalpha degradation, and COX-2 expression in murine macrophages. J. Cell. Biochem. 2012, 113, 611–618. [Google Scholar] [CrossRef] [PubMed]
- Sukhova, G.K.; Williams, J.K.; Libby, P. Statins reduce inflammation in atheroma of nonhuman primates independent of effects on serum cholesterol. Arter. Thromb. Vasc. Biol. 2002, 22, 1452–1458. [Google Scholar] [CrossRef]
- Higgins, M.J.; Prowell, T.M.; Blackford, A.L.; Byrne, C.; Khouri, N.F.; Slater, S.A.; Jeter, S.C.; Armstrong, D.K.; Davidson, N.E.; Emens, L.A.; et al. A short-term biomarker modulation study of simvastatin in women at increased risk of a new breast cancer. Breast Cancer Res. Treat. 2012, 131, 915–924. [Google Scholar] [CrossRef]
- Vinayak, S.; Schwartz, E.J.; Jensen, K.; Lipson, J.; Alli, E.; McPherson, L.; Fernandez, A.M.; Sharma, V.B.; Staton, A.; Mills, M.A.; et al. A clinical trial of lovastatin for modification of biomarkers associated with breast cancer risk. Breast Cancer Res. Treat. 2013, 142, 389–398. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Linden, K.G.; Leachman, S.A.; Zager, J.S.; Jakowatz, J.G.; Viner, J.L.; McLaren, C.E.; Barr, R.J.; Carpenter, P.M.; Chen, W.P.; Elmets, C.A.; et al. A randomized, double-blind, placebo-controlled phase II clinical trial of lovastatin for various endpoints of melanoma pathobiology. Cancer Prev. Res. 2014, 7, 496–504. [Google Scholar] [CrossRef]
- Neukamm, A.; Hoiseth, A.D.; Einvik, G.; Lehmann, S.; Hagve, T.A.; Soyseth, V.; Omland, T. Rosuvastatin treatment in stable chronic obstructive pulmonary disease (RODEO): A randomized controlled trial. J. Intern. Med. 2015, 278, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Kater, A.L.; Batista, M.C.; Ferreira, S.R. Synergistic effect of simvastatin and ezetimibe on lipid and pro-inflammatory profiles in pre-diabetic subjects. Diabetol. Metab. Syndr. 2010, 2, 34. [Google Scholar] [CrossRef]
- Pesaro, A.E.; Serrano, C.V., Jr.; Katz, M.; Marti, L.; Fernandes, J.L.; Parra, P.R.; Campos, A.H. Increasing doses of simvastatin versus combined ezetimibe/simvastatin: Effect on circulating endothelial progenitor cells. J. Cardiovasc. Pharmacol. Ther. 2013, 18, 447–452. [Google Scholar] [CrossRef]
- da Silva Pereira, E.N.G.; Franco, R.L.C.; Santos, R.; Daliry, A. Statins and non-alcoholic fatty liver disease: A concise review. Biomed. Pharmacother. 2025, 183, 117805. [Google Scholar] [CrossRef]
- Lappegard, K.T.; Pop-Purceleanu, M.; van Heerde, W.; Sexton, J.; Tendolkar, I.; Pop, G. Improved neurocognitive functions correlate with reduced inflammatory burden in atrial fibrillation patients treated with intensive cholesterol lowering therapy. J. Neuroinflammation 2013, 10, 78. [Google Scholar] [CrossRef] [PubMed]
- Ward, N.C.; Watts, G.F.; Eckel, R.H. Statin Toxicity. Circ. Res. 2019, 124, 328–350. [Google Scholar] [CrossRef]
- Methe, H.; Kim, J.O.; Kofler, S.; Nabauer, M.; Weis, M. Statins decrease Toll-like receptor 4 expression and downstream signaling in human CD14+ monocytes. Arter. Thromb. Vasc. Biol. 2005, 25, 1439–1445. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.L.; Hsiao, F.Y.; Dong, Y.H.; Shen, L.J.; Wu, F.L. Statins and the risk of liver injury: A population-based case-control study. Pharmacoepidemiol. Drug Saf. 2014, 23, 719–725. [Google Scholar] [CrossRef] [PubMed]
- Erbs, S.; Beck, E.B.; Linke, A.; Adams, V.; Gielen, S.; Krankel, N.; Mobius-Winkler, S.; Hollriegel, R.; Thiele, H.; Hambrecht, R.; et al. High-dose rosuvastatin in chronic heart failure promotes vasculogenesis, corrects endothelial function, and improves cardiac remodeling–results from a randomized, double-blind, and placebo-controlled study. Int. J. Cardiol. 2011, 146, 56–63. [Google Scholar] [CrossRef]
- Grip, O.; Janciauskiene, S. Atorvastatin reduces plasma levels of chemokine (CXCL10) in patients with Crohn’s disease. PLoS ONE 2009, 4, e5263. [Google Scholar] [CrossRef]
- Sarkar, P.; Chattopadhyay, A. Interplay of Cholesterol and Actin in Neurotransmitter GPCR Signaling: Insights from Chronic Cholesterol Depletion Using Statin. ACS Chem. Neurosci. 2023, 14, 3855–3868. [Google Scholar] [CrossRef]
- Knopp, R.H.; Retzlaff, B.M.; Fish, B.; Dowdy, A.; Twaddell, B.; Nguyen, T.; Paramsothy, P. The SLIM Study: Slo-Niacin(R) and Atorvastatin Treatment of Lipoproteins and Inflammatory Markers in Combined Hyperlipidemia. J. Clin. Lipidol. 2009, 3, 167–178. [Google Scholar] [CrossRef][Green Version]
- Ljung, R.; Koster, M.; Bjorkenstam, E.; Salmi, P. Associations between statin use and suicidality, depression, anxiety, and seizures. Lancet Psychiatry 2021, 8, e2. [Google Scholar] [CrossRef] [PubMed]
- Abulhul, E.; McDonald, K.; Martos, R.; Phelan, D.; Spiers, J.P.; Hennessy, M.; Baugh, J.; Watson, C.; O’Loughlin, C.; Ledwidge, M. Long-term statin therapy in patients with systolic heart failure and normal cholesterol: Effects on elevated serum markers of collagen turnover, inflammation, and B-type natriuretic peptide. Clin. Ther. 2012, 34, 91–100. [Google Scholar] [CrossRef]
- Bielecka-Dabrowa, A.; Goch, J.H.; Mikhailidis, D.P.; Rysz, J.; Maciejewski, M.; Banach, M. The influence of atorvastatin on parameters of inflammation and function of the left ventricle in patients with dilated cardiomyopathy. Med. Sci. Monit. 2009, 15, MS12–MS23. [Google Scholar]
- Sposito, A.C.; Santos, S.N.; de Faria, E.C.; Abdalla, D.S.; da Silva, L.P.; Soares, A.A.; Japiassu, A.V.; Quinaglia e Silva, J.C.; Ramires, J.A.; Coelho, O.R. Timing and dose of statin therapy define its impact on inflammatory and endothelial responses during myocardial infarction. Arter. Thromb. Vasc. Biol. 2011, 31, 1240–1246. [Google Scholar] [CrossRef]
- Matsubara, T.; Naruse, K.; Arakawa, T.; Nakao, M.; Yokoi, K.; Oguri, M.; Marui, N.; Amano, T.; Ichimiya, S.; Ohashi, T.; et al. Impact of pitavastatin on high-sensitivity C-reactive protein and adiponectin in hypercholesterolemic patients with the metabolic syndrome: The PREMIUM Study. J. Cardiol. 2012, 60, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Puurunen, J.; Piltonen, T.; Puukka, K.; Ruokonen, A.; Savolainen, M.J.; Bloigu, R.; Morin-Papunen, L.; Tapanainen, J.S. Statin therapy worsens insulin sensitivity in women with polycystic ovary syndrome (PCOS): A prospective, randomized, double-blind, placebo-controlled study. J. Clin. Endocrinol. Metab. 2013, 98, 4798–4807. [Google Scholar] [CrossRef]
- Sena-Evangelista, K.C.; Pedrosa, L.F.; Paiva, M.S.; Dias, P.C.; Ferreira, D.Q.; Cozzolino, S.M.; Faulin, T.E.; Abdalla, D.S. The hypolipidemic and pleiotropic effects of rosuvastatin are not enhanced by its association with zinc and selenium supplementation in coronary artery disease patients: A double blind randomized controlled study. PLoS ONE 2015, 10, e0119830. [Google Scholar] [CrossRef]
- Rossi, A.; Inciardi, R.M.; Rossi, A.; Temporelli, P.L.; Lucci, D.; Gonzini, L.; Marchioli, R.; Nicolosi, G.L.; Tavazzi, L.; Investigators, G.-H. Prognostic effects of rosuvastatin in patients with co-existing chronic obstructive pulmonary disease and chronic heart failure: A sub-analysis of GISSI-HF trial. Pulm. Pharmacol. Ther. 2017, 44, 16–23. [Google Scholar] [CrossRef]
- Charles-Schoeman, C.; Khanna, D.; Furst, D.E.; McMahon, M.; Reddy, S.T.; Fogelman, A.M.; Paulus, H.E.; Park, G.S.; Gong, T.; Ansell, B.J. Effects of high-dose atorvastatin on antiinflammatory properties of high density lipoprotein in patients with rheumatoid arthritis: A pilot study. J. Rheumatol. 2007, 34, 1459–1464. [Google Scholar]
- Lee, B.J.; Tseng, Y.F.; Yen, C.H.; Lin, P.T. Effects of coenzyme Q10 supplementation (300 mg/day) on antioxidation and anti-inflammation in coronary artery disease patients during statins therapy: A randomized, placebo-controlled trial. Nutr. J. 2013, 12, 142. [Google Scholar] [CrossRef]
- Mandal, P.; Chalmers, J.D.; Graham, C.; Harley, C.; Sidhu, M.K.; Doherty, C.; Govan, J.W.; Sethi, T.; Davidson, D.J.; Rossi, A.G.; et al. Atorvastatin as a stable treatment in bronchiectasis: A randomised controlled trial. Lancet Respir. Med. 2014, 2, 455–463. [Google Scholar] [CrossRef]
- Joyeux-Faure, M.; Tamisier, R.; Baguet, J.P.; Dias-Domingos, S.; Perrig, S.; Leftheriotis, G.; Janssens, J.P.; Trzepizur, W.; Launois, S.H.; Stanke-Labesque, F.; et al. Response to statin therapy in obstructive sleep apnea syndrome: A multicenter randomized controlled trial. Mediat. Inflamm. 2014, 2014, 423120. [Google Scholar] [CrossRef] [PubMed]
- John, M.E.; Cockcroft, J.R.; McKeever, T.M.; Coward, W.R.; Shale, D.J.; Johnson, S.R.; Thornton, J.G.; Harrison, T.W.; Knox, A.J.; Bolton, C.E. Cardiovascular and inflammatory effects of simvastatin therapy in patients with COPD: A randomized controlled trial. Int. J. Chronic Obstr. Pulm. Dis. 2015, 10, 211–221. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vincenzi, B.; Stock, S.; Borba, C.P.; Cleary, S.M.; Oppenheim, C.E.; Petruzzi, L.J.; Fan, X.; Copeland, P.M.; Freudenreich, O.; Cather, C.; et al. A randomized placebo-controlled pilot study of pravastatin as an adjunctive therapy in schizophrenia patients: Effect on inflammation, psychopathology, cognition and lipid metabolism. Schizophr. Res. 2014, 159, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.L.; Bedient, T.J.; Kozlowski, J.; Rosenbluth, D.B.; Isakow, W.; Ferkol, T.W.; Thomas, B.; Mintun, M.A.; Schuster, D.P.; Walter, M.J. [18F]fluorodeoxyglucose positron emission tomography for lung antiinflammatory response evaluation. Am. J. Respir. Crit. Care Med. 2009, 180, 533–539. [Google Scholar] [CrossRef]
- Negi, S.; Shukrullah, I.; Veledar, E.; Bloom, H.L.; Jones, D.P.; Dudley, S.C. Statin therapy for the prevention of atrial fibrillation trial (SToP AF trial). J. Cardiovasc. Electrophysiol. 2011, 22, 414–419. [Google Scholar] [CrossRef]
- Pande, R.L.; Brown, J.; Buck, S.; Redline, W.; Doyle, J.; Plutzky, J.; Creager, M.A. Association of monocyte tumor necrosis factor alpha expression and serum inflammatory biomarkers with walking impairment in peripheral artery disease. J. Vasc. Surg. 2015, 61, 155–161. [Google Scholar] [CrossRef]
- Tang, T.Y.; Howarth, S.P.; Miller, S.R.; Graves, M.J.; Patterson, A.J.; U-King-Im, J.M.; Li, Z.Y.; Walsh, S.R.; Brown, A.P.; Kirkpatrick, P.J.; et al. The ATHEROMA (Atorvastatin Therapy: Effects on Reduction of Macrophage Activity) Study. Evaluation using ultrasmall superparamagnetic iron oxide-enhanced magnetic resonance imaging in carotid disease. J. Am. Coll. Cardiol. 2009, 53, 2039–2050. [Google Scholar] [CrossRef] [PubMed]
- Os, H.A.; Rollefstad, S.; Gerdts, E.; Kringeland, E.; Ikdahl, E.; Semb, A.G.; Midtbo, H. Preclinical cardiac organ damage during statin treatment in patients with inflammatory joint diseases: The RORA-AS statin intervention study. Rheumatology 2020, 59, 3700–3708. [Google Scholar] [CrossRef] [PubMed]
- Svanteson, M.; Rollefstad, S.; Klow, N.E.; Hisdal, J.; Ikdahl, E.; Semb, A.G.; Haig, Y. Associations between coronary and carotid artery atherosclerosis in patients with inflammatory joint diseases. RMD Open 2017, 3, e000544. [Google Scholar] [CrossRef]
- Ikdahl, E.; Rollefstad, S.; Hisdal, J.; Olsen, I.C.; Pedersen, T.R.; Kvien, T.K.; Semb, A.G. Sustained Improvement of Arterial Stiffness and Blood Pressure after Long-Term Rosuvastatin Treatment in Patients with Inflammatory Joint Diseases: Results from the RORA-AS Study. PLoS ONE 2016, 11, e0153440. [Google Scholar] [CrossRef]
- Ikdahl, E.; Hisdal, J.; Rollefstad, S.; Olsen, I.C.; Kvien, T.K.; Pedersen, T.R.; Semb, A.G. Rosuvastatin improves endothelial function in patients with inflammatory joint diseases, longitudinal associations with atherosclerosis and arteriosclerosis: Results from the RORA-AS statin intervention study. Arthritis Res. Ther. 2015, 17, 279. [Google Scholar] [CrossRef]
- Rollefstad, S.; Ikdahl, E.; Hisdal, J.; Olsen, I.C.; Holme, I.; Hammer, H.B.; Smerud, K.T.; Kitas, G.D.; Pedersen, T.R.; Kvien, T.K.; et al. Rosuvastatin-Induced Carotid Plaque Regression in Patients with Inflammatory Joint Diseases: The Rosuvastatin in Rheumatoid Arthritis, Ankylosing Spondylitis and Other Inflammatory Joint Diseases Study. Arthritis Rheumatol. 2015, 67, 1718–1728. [Google Scholar] [CrossRef] [PubMed]
- Pignatelli, P.; Carnevale, R.; Pastori, D.; Cangemi, R.; Napoleone, L.; Bartimoccia, S.; Nocella, C.; Basili, S.; Violi, F. Immediate antioxidant and antiplatelet effect of atorvastatin via inhibition of Nox2. Circulation 2012, 126, 92–103. [Google Scholar] [CrossRef]
- Tahara, N.; Kai, H.; Yamagishi, S.; Mizoguchi, M.; Nakaura, H.; Ishibashi, M.; Kaida, H.; Baba, K.; Hayabuchi, N.; Imaizumi, T. Vascular inflammation evaluated by [18F]-fluorodeoxyglucose positron emission tomography is associated with the metabolic syndrome. J. Am. Coll. Cardiol. 2007, 49, 1533–1539. [Google Scholar] [CrossRef]
- Tahara, N.; Kai, H.; Ishibashi, M.; Nakaura, H.; Kaida, H.; Baba, K.; Hayabuchi, N.; Imaizumi, T. Simvastatin attenuates plaque inflammation: Evaluation by fluorodeoxyglucose positron emission tomography. J. Am. Coll. Cardiol. 2006, 48, 1825–1831. [Google Scholar] [CrossRef] [PubMed]
- Emami, H.; Vucic, E.; Subramanian, S.; Abdelbaky, A.; Fayad, Z.A.; Du, S.; Roth, E.; Ballantyne, C.M.; Mohler, E.R.; Farkouh, M.E.; et al. The effect of BMS-582949, a P38 mitogen-activated protein kinase (P38 MAPK) inhibitor on arterial inflammation: A multicenter FDG-PET trial. Atherosclerosis 2015, 240, 490–496. [Google Scholar] [CrossRef]
- Singh, R.K.; Agarwal, V.; Baronia, A.K.; Kumar, S.; Poddar, B.; Azim, A. The Effects of Atorvastatin on Inflammatory Responses and Mortality in Septic Shock: A Single-center, Randomized Controlled Trial. Indian J. Crit. Care Med. 2017, 21, 646–654. [Google Scholar] [CrossRef]
- National Heart, Lung, and Blood Institute ARDS Clinical Trials Network; Truwit, J.D.; Bernard, G.R.; Steingrub, J.; Matthay, M.A.; Liu, K.D.; Albertson, T.E.; Brower, R.G.; Shanholtz, C.; Rock, P.; et al. Rosuvastatin for sepsis-associated acute respiratory distress syndrome. N. Engl. J. Med. 2014, 370, 2191–2200. [Google Scholar]
- Needham, D.M.; Colantuoni, E.; Dinglas, V.D.; Hough, C.L.; Wozniak, A.W.; Jackson, J.C.; Morris, P.E.; Mendez-Tellez, P.A.; Ely, E.W.; Hopkins, R.O. Rosuvastatin versus placebo for delirium in intensive care and subsequent cognitive impairment in patients with sepsis-associated acute respiratory distress syndrome: An ancillary study to a randomised controlled trial. Lancet Respir. Med. 2016, 4, 203–212. [Google Scholar] [CrossRef]
- Dinglas, V.D.; Hopkins, R.O.; Wozniak, A.W.; Hough, C.L.; Morris, P.E.; Jackson, J.C.; Mendez-Tellez, P.A.; Bienvenu, O.J.; Ely, E.W.; Colantuoni, E.; et al. One-year outcomes of rosuvastatin versus placebo in sepsis-associated acute respiratory distress syndrome: Prospective follow-up of SAILS randomised trial. Thorax 2016, 71, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.Y.; Ge, Z.Z.; Xiang, J.; Gao, Y.X.; Lu, X.; Walline, J.H.; Qin, M.B.; Zhu, H.D.; Li, Y. Is rosuvastatin protective against sepsis-associated encephalopathy? A secondary analysis of the SAILS trial. World J. Emerg. Med. 2022, 13, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Hoppe, C.; Kuypers, F.; Larkin, S.; Hagar, W.; Vichinsky, E.; Styles, L. A pilot study of the short-term use of simvastatin in sickle cell disease: Effects on markers of vascular dysfunction. Br. J. Haematol. 2011, 153, 655–663. [Google Scholar] [CrossRef]
- Braganza, G.; Chaudhuri, R.; McSharry, C.; Weir, C.J.; Donnelly, I.; Jolly, L.; Lafferty, J.; Lloyd, S.M.; Spears, M.; Mair, F.; et al. Effects of short-term treatment with atorvastatin in smokers with asthma—A randomized controlled trial. BMC Pulm. Med. 2011, 11, 16. [Google Scholar] [CrossRef]
- Bedi, P.; Chalmers, J.D.; Graham, C.; Clarke, A.; Donaldson, S.; Doherty, C.; Govan, J.R.W.; Davidson, D.J.; Rossi, A.G.; Hill, A.T. A Randomized Controlled Trial of Atorvastatin in Patients with Bronchiectasis Infected With Pseudomonas Aeruginosa: A Proof of Concept Study. Chest 2017, 152, 368–378. [Google Scholar] [CrossRef]
- Velarde, G.P.; Choudhary, N.; Bravo-Jaimes, K.; Smotherman, C.; Sherazi, S.; Kraemer, D.F. Effect of atorvastatin on lipogenic, inflammatory and thrombogenic markers in women with the metabolic syndrome. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 634–640. [Google Scholar] [CrossRef] [PubMed]
- Donnino, M.W.; Cocchi, M.N.; Salciccioli, J.D.; Kim, D.; Naini, A.B.; Buettner, C.; Akuthota, P. Coenzyme Q10 levels are low and may be associated with the inflammatory cascade in septic shock. Crit. Care 2011, 15, R189. [Google Scholar] [CrossRef]
- Bass, A.R.; Szymonifka, J.D.; Rondina, M.T.; Bogardus, M.; Scott, M.G.; Woller, S.C.; Stevens, S.M.; Eby, C.; Merritt, K.; Valle, A.G.D.; et al. Postoperative Myocardial Injury and Inflammation Is Not Blunted by a Trial of Atorvastatin in Orthopedic Surgery Patients. HSS J. 2018, 14, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, I.; Agarwal, S.; Gautam, S.; Desai, M.; Steen, H.; Warren, W.P.; Xavier, S.S.; Lima, J.A. Aortic plaque regression as determined by magnetic resonance imaging with high-dose and low-dose statin therapy. J. Cardiovasc. Med. 2008, 9, 700–706. [Google Scholar] [CrossRef] [PubMed]
- Lo, J.; Lu, M.T.; Ihenachor, E.J.; Wei, J.; Looby, S.E.; Fitch, K.V.; Oh, J.; Zimmerman, C.O.; Hwang, J.; Abbara, S.; et al. Effects of statin therapy on coronary artery plaque volume and high-risk plaque morphology in HIV-infected patients with subclinical atherosclerosis: A randomised, double-blind, placebo-controlled trial. Lancet HIV 2015, 2, e52–e63. [Google Scholar] [CrossRef]
- deFilippi, C.; Christenson, R.; Joyce, J.; Park, E.A.; Wu, A.; Fitch, K.V.; Looby, S.E.; Lu, M.T.; Hoffmann, U.; Grinspoon, S.K.; et al. Brief Report: Statin Effects on Myocardial Fibrosis Markers in People Living with HIV. J. Acquir. Immune Defic. Syndr. 2018, 78, 105–110. [Google Scholar] [CrossRef]
- Kini, A.S.; Vengrenyuk, Y.; Shameer, K.; Maehara, A.; Purushothaman, M.; Yoshimura, T.; Matsumura, M.; Aquino, M.; Haider, N.; Johnson, K.W.; et al. Intracoronary Imaging, Cholesterol Efflux, and Transcriptomes After Intensive Statin Treatment: The YELLOW II Study. J. Am. Coll. Cardiol. 2017, 69, 628–640. [Google Scholar] [CrossRef]
- Neilipovitz, D.T.; Bryson, G.L.; Taljaard, M. STAR VaS–Short Term Atorvastatin Regime for Vasculopathic Subjects: A randomized placebo-controlled trial evaluating perioperative atorvastatin therapy in noncardiac surgery. Can. J. Anaesth. 2012, 59, 527–537. [Google Scholar] [CrossRef]
- Kolovou, G.; Giannakopoulou, V.; Kalogeropoulos, P.; Anagnostopoulou, K.; Goumas, G.; Kazianis, G.; Limberi, S.; Perrea, D.; Mihas, C.; Kolovou, V.; et al. Hellenic Postprandial Lipemia Study (HPLS): Rationale and design of a prospective, open-label trial to determinate the prevalence of abnormal postprandial lipemia as well as its interaction with statins in patients at high- and very high-risk for cardiovascular disease. Contemp. Clin. Trials 2019, 82, 101–105. [Google Scholar]
- Negredo, E.; Jimenez, M.; Puig, J.; Loste, C.; Perez-Alvarez, N.; Urrea, V.; Echeverria, P.; Bonjoch, A.; Clotet, B.; Blanco, J.; et al. A randomized pilot trial to evaluate the benefit of the concomitant use of atorvastatin and Raltegravir on immunological markers in protease-inhibitor-treated subjects living with HIV. PLoS ONE 2020, 15, e0238575. [Google Scholar] [CrossRef]
- Negredo, E.; Estrada, V.; Domingo, P.; Gutierrez, M.D.; Mateo, G.M.; Puig, J.; Bonjoch, A.; Ornelas, A.; Echeverria, P.; Estany, C.; et al. Switching from a ritonavir-boosted PI to dolutegravir as an alternative strategy in virologically suppressed HIV-infected individuals. J. Antimicrob. Chemother. 2017, 72, 844–849. [Google Scholar] [CrossRef]
- De Giorgi, R.; Quinton, A.M.G.; Waters, S.; Cowen, P.J.; Harmer, C.J. An experimental medicine study of the effects of simvastatin on emotional processing, reward learning, verbal memory, and inflammation in healthy volunteers. Psychopharmacology 2022, 239, 2635–2645. [Google Scholar] [CrossRef]
- Swanson, K.V.; Deng, M.; Ting, J.P. The NLRP3 inflammasome: Molecular activation and regulation to therapeutics. Nat. Rev. Immunol. 2019, 19, 477–489. [Google Scholar] [CrossRef]
- Weber, A.N.R.; Bittner, Z.A.; Shankar, S.; Liu, X.; Chang, T.H.; Jin, T.; Tapia-Abellan, A. Recent insights into the regulatory networks of NLRP3 inflammasome activation. J. Cell Sci. 2020, 133, jcs248344. [Google Scholar] [CrossRef] [PubMed]
- Kelley, N.; Jeltema, D.; Duan, Y.; He, Y. The NLRP3 Inflammasome: An Overview of Mechanisms of Activation and Regulation. Int. J. Mol. Sci. 2019, 20, 3328. [Google Scholar] [CrossRef] [PubMed]
- Pfeiler, S.; Winkels, H.; Kelm, M.; Gerdes, N. IL-1 family cytokines in cardiovascular disease. Cytokine 2019, 122, 154215. [Google Scholar] [CrossRef] [PubMed]
- Kaplanski, G. Interleukin-18: Biological properties and role in disease pathogenesis. Immunol. Rev. 2018, 281, 138–153. [Google Scholar] [CrossRef]
- Holschermann, H.; Schuster, D.; Parviz, B.; Haberbosch, W.; Tillmanns, H.; Muth, H. Statins prevent NF-kappaB transactivation independently of the IKK-pathway in human endothelial cells. Atherosclerosis 2006, 185, 240–245. [Google Scholar] [CrossRef]
- Tousoulis, D.; Psarros, C.; Demosthenous, M.; Patel, R.; Antoniades, C.; Stefanadis, C. Innate and adaptive inflammation as a therapeutic target in vascular disease: The emerging role of statins. J. Am. Coll. Cardiol. 2014, 63, 2491–2502. [Google Scholar] [CrossRef] [PubMed]
- Koushki, K.; Shahbaz, S.K.; Mashayekhi, K.; Sadeghi, M.; Zayeri, Z.D.; Taba, M.Y.; Banach, M.; Al-Rasadi, K.; Johnston, T.P.; Sahebkar, A. Anti-inflammatory Action of Statins in Cardiovascular Disease: The Role of Inflammasome and Toll-Like Receptor Pathways. Clin. Rev. Allergy Immunol. 2021, 60, 175–199. [Google Scholar] [CrossRef]
- Liao, Y.H.; Lin, Y.C.; Tsao, S.T.; Lin, Y.C.; Yang, A.J.; Huang, C.T.; Huang, K.C.; Lin, W.W. HMG-CoA reductase inhibitors activate caspase-1 in human monocytes depending on ATP release and P2X7 activation. J. Leukoc. Biol. 2013, 93, 289–299. [Google Scholar] [CrossRef]
- Antonello, J.; Roy, P. Damage-associated molecular patterns (DAMPs) in vascular diseases. J. Biol. Chem. 2025, 301, 110241. [Google Scholar] [CrossRef]
- Falck-Hansen, M.; Kassiteridi, C.; Monaco, C. Toll-like receptors in atherosclerosis. Int. J. Mol. Sci. 2013, 14, 14008–14023. [Google Scholar] [CrossRef] [PubMed]
- Jannati, S.; Patel, A.; Patnaik, R.; Banerjee, Y. Oleocanthal as a Multifunctional Anti-Cancer Agent: Mechanistic Insights, Advanced Delivery Strategies, and Synergies for Precision Oncology. Int. J. Mol. Sci. 2025, 26, 5521. [Google Scholar] [CrossRef]
- Kusiak, A.; Brady, G. Bifurcation of signalling in human innate immune pathways to NF-kB and IRF family activation. Biochem. Pharmacol. 2022, 205, 115246. [Google Scholar] [CrossRef] [PubMed]
- Ortego, M.; Gomez-Hernandez, A.; Vidal, C.; Sanchez-Galan, E.; Blanco-Colio, L.M.; Martin-Ventura, J.L.; Tunon, J.; Diaz, C.; Hernandez, G.; Egido, J. HMG-CoA reductase inhibitors reduce I kappa B kinase activity induced by oxidative stress in monocytes and vascular smooth muscle cells. J. Cardiovasc. Pharmacol. 2005, 45, 468–475. [Google Scholar] [CrossRef] [PubMed]
- Mylonas, K.S.; Peroulis, M.; Schizas, D.; Kapelouzou, A. MYD88 and Proinflammatory Chemokines in Aortic Atheromatosis: Exploring Novel Statin Effects. Int. J. Mol. Sci. 2023, 24, 9248. [Google Scholar] [CrossRef]
- Wang, S.; Xie, X.; Lei, T.; Zhang, K.; Lai, B.; Zhang, Z.; Guan, Y.; Mao, G.; Xiao, L.; Wang, N. Statins Attenuate Activation of the NLRP3 Inflammasome by Oxidized LDL or TNFalpha in Vascular Endothelial Cells through a PXR-Dependent Mechanism. Mol. Pharmacol. 2017, 92, 256–264. [Google Scholar] [CrossRef]
- Liao, J.K. Isoprenoids as mediators of the biological effects of statins. J. Clin. Investig. 2002, 110, 285–288. [Google Scholar] [CrossRef][Green Version]
- Sadeghi, M.; Khayati, S.; Dehnavi, S.; Almahmeed, W.; Sukhorukovi, V.N.; Sahebkar, A. Regulatory impact of statins on macrophage polarization: Mechanistic and therapeutic implications. J. Pharm. Pharmacol. 2024, 76, 763–775. [Google Scholar] [CrossRef]
- Menini, S.; Iacobini, C.; Vitale, M.; Pugliese, G. The Inflammasome in Chronic Complications of Diabetes and Related Metabolic Disorders. Cells 2020, 9, 1812. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; Alabdullah, M.; Grossmann, J.; Spieler, F.; Abdosh, R.; Lutz, V.; Kalies, K.; Knopp, K.; Rieckmann, M.; Koch, S.; et al. The differential statin effect on cytokine production of monocytes or macrophages is mediated by differential geranylgeranylation-dependent Rac1 activation. Cell Death Dis. 2019, 10, 880. [Google Scholar] [CrossRef] [PubMed]
- Bordbar, M.; de Mutsert, R.; Cevval, M.; Rosendaal, F.R.; Jukema, J.W.; Lijfering, W.M. Differential effect of statin use on coagulation markers: An active comparative analysis in the NEO study. Thromb. J. 2021, 19, 45. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Chen, Z.; Zhou, Y.; Wu, Y.; Xia, Y.; Lu, D.; Fan, M.; Li, S.; Chen, J.; Sun, A.; et al. Rosuvastatin protects against coronary microembolization-induced cardiac injury via inhibiting NLRP3 inflammasome activation. Cell Death Dis. 2021, 12, 78. [Google Scholar] [CrossRef]
- Liu, J.C.; Lei, S.Y.; Zhang, D.H.; He, Q.Y.; Sun, Y.Y.; Zhu, H.J.; Qu, Y.; Zhou, S.Y.; Yang, Y.; Li, C.; et al. The pleiotropic effects of statins: A comprehensive exploration of neurovascular unit modulation and blood-brain barrier protection. Mol. Med. 2024, 30, 256. [Google Scholar] [CrossRef]
- German, C.A.; Liao, J.K. Understanding the molecular mechanisms of statin pleiotropic effects. Arch. Toxicol. 2023, 97, 1529–1545. [Google Scholar] [CrossRef]
- Arthur, J.S.; Ley, S.C. Mitogen-activated protein kinases in innate immunity. Nat. Rev. Immunol. 2013, 13, 679–692. [Google Scholar] [CrossRef]
- Costa-Pereira, A.P. Regulation of IL-6-type cytokine responses by MAPKs. Biochem. Soc. Trans. 2014, 42, 59–62. [Google Scholar] [CrossRef]
- Hazeldine, J.; Hampson, P.; Opoku, F.A.; Foster, M.; Lord, J.M. N-Formyl peptides drive mitochondrial damage associated molecular pattern induced neutrophil activation through ERK1/2 and P38 MAP kinase signalling pathways. Injury 2015, 46, 975–984. [Google Scholar] [CrossRef]
- Henriksbo, B.D.; Tamrakar, A.K.; Phulka, J.S.; Barra, N.G.; Schertzer, J.D. Statins activate the NLRP3 inflammasome and impair insulin signaling via p38 and mTOR. Am. J. Physiol. Endocrinol. Metab. 2020, 319, E110–E116. [Google Scholar] [CrossRef] [PubMed]
- Vogiatzi, G.; Oikonomou, E.; Siasos, G.; Tsalamandris, S.; Briasoulis, A.; Androulakis, E.; Latsios, G.; Papaioannou, S.; Tsioufis, K.; Tousoulis, D. Statins and inflammation in cardiovascular disease. Curr. Pharm. Des. 2017, 23, 7027–7039. [Google Scholar] [CrossRef]
- Satny, M.; Hubacek, J.A.; Vrablik, M. Statins and Inflammation. Curr. Atheroscler. Rep. 2021, 23, 80. [Google Scholar] [CrossRef]
- Anannya, O.; Huang, W.; August, A. The kinase ITK controls a Ca2+-mediated switch that balances T(H)17 and T(reg) cell differentiation. Sci. Signal. 2024, 17, eadh2381. [Google Scholar] [CrossRef]
- Prado, D.S.; Cattley, R.T.; Shipman, C.W.; Happe, C.; Lee, M.; Boggess, W.C.; MacDonald, M.L.; Hawse, W.F. Synergistic and additive interactions between receptor signaling networks drive the regulatory T cell versus T helper 17 cell fate choice. J. Biol. Chem. 2021, 297, 101330. [Google Scholar] [CrossRef]
- Li, P.; Spolski, R.; Liao, W.; Leonard, W.J. Complex interactions of transcription factors in mediating cytokine biology in T cells. Immunol. Rev. 2014, 261, 141–156. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, X.; Zhu, Y.; Liu, X.; Gu, Y.; Dai, X.; Li, B. Transcriptional and posttranslational regulation of Th17/Treg balance in health and disease. Eur. J. Immunol. 2021, 51, 2137–2150. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, X.; Wan, Y.Y. Intricacies of TGF-beta signaling in Treg and Th17 cell biology. Cell. Mol. Immunol. 2023, 20, 1002–1022. [Google Scholar] [CrossRef] [PubMed]
- Diller, M.L.; Kudchadkar, R.R.; Delman, K.A.; Lawson, D.H.; Ford, M.L. Balancing Inflammation: The Link between Th17 and Regulatory T Cells. Mediat. Inflamm. 2016, 2016, 6309219. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, A.; Kuchroo, V.K. Th17 cells: From precursors to players in inflammation and infection. Int. Immunol. 2009, 21, 489–498. [Google Scholar] [CrossRef]
- Miller, S.; Eizenberg-Magar, I.; Reich-Zeliger, S.; Rimer, J.; Zaretsky, I.; Reshef, D.; Kopitman, E.; Friedman, N.; Antebi, Y.E. Independent and temporally separated dynamics for RORgammat and Foxp3 during Th17 differentiation. Front. Immunol. 2025, 16, 1462045. [Google Scholar] [CrossRef]
- Le Menn, G.; Jablonska, A.; Chen, Z. The effects of post-translational modifications on Th17/Treg cell differentiation. Biochim. Biophys. Acta Mol. Cell Res. 2022, 1869, 119223. [Google Scholar] [CrossRef]
- Mira, E.; Leon, B.; Barber, D.F.; Jimenez-Baranda, S.; Goya, I.; Almonacid, L.; Marquez, G.; Zaballos, A.; Martinez, A.C.; Stein, J.V.; et al. Statins induce regulatory T cell recruitment via a CCL1 dependent pathway. J. Immunol. 2008, 181, 3524–3534. [Google Scholar] [CrossRef] [PubMed]
- Ntolkeras, G.; Barba, C.; Mavropoulos, A.; Vasileiadis, G.K.; Dardiotis, E.; Sakkas, L.I.; Hadjigeorgiou, G.; Bogdanos, D.P. On the immunoregulatory role of statins in multiple sclerosis: The effects on Th17 cells. Immunol. Res. 2019, 67, 310–324. [Google Scholar] [CrossRef]
- Ulivieri, C.; Baldari, C.T. Statins: From cholesterol-lowering drugs to novel immunomodulators for the treatment of Th17-mediated autoimmune diseases. Pharmacol. Res. 2014, 88, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Rose, D.M.; Alon, R.; Ginsberg, M.H. Integrin modulation and signaling in leukocyte adhesion and migration. Immunol. Rev. 2007, 218, 126–134. [Google Scholar] [CrossRef]
- Ley, K.; Laudanna, C.; Cybulsky, M.I.; Nourshargh, S. Getting to the site of inflammation: The leukocyte adhesion cascade updated. Nat. Rev. Immunol. 2007, 7, 678–689. [Google Scholar] [CrossRef]
- Muller, W.A. How endothelial cells regulate transmigration of leukocytes in the inflammatory response. Am. J. Pathol. 2014, 184, 886–896. [Google Scholar] [CrossRef]
- Dehnavi, S.; Sohrabi, N.; Sadeghi, M.; Lansberg, P.; Banach, M.; Al-Rasadi, K.; Johnston, T.P.; Sahebkar, A. Statins and autoimmunity: State-of-the-art. Pharmacol. Ther. 2020, 214, 107614. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Garlanda, C. Humoral Innate Immunity and Acute-Phase Proteins. N. Engl. J. Med. 2023, 388, 439–452. [Google Scholar] [CrossRef]
- Yazdi, A.S.; Ghoreschi, K. The Interleukin-1 Family. Adv. Exp. Med. Biol. 2016, 941, 21–29. [Google Scholar]
- Pyrillou, K.; Burzynski, L.C.; Clarke, M.C.H. Alternative Pathways of IL-1 Activation, and Its Role in Health and Disease. Front. Immunol. 2020, 11, 613170. [Google Scholar] [CrossRef]
- Chan, A.H.; Schroder, K. Inflammasome signaling and regulation of interleukin-1 family cytokines. J. Exp. Med. 2020, 217, e20190314. [Google Scholar] [CrossRef] [PubMed]
- Dinarello, C.A. Overview of the IL-1 family in innate inflammation and acquired immunity. Immunol. Rev. 2018, 281, 8–27. [Google Scholar] [CrossRef] [PubMed]
- Akdis, M.; Aab, A.; Altunbulakli, C.; Azkur, K.; Costa, R.A.; Crameri, R.; Duan, S.; Eiwegger, T.; Eljaszewicz, A.; Ferstl, R.; et al. Interleukins (from IL-1 to IL-38), interferons, transforming growth factor beta, and TNF-alpha: Receptors, functions, and roles in diseases. J. Allergy Clin. Immunol. 2016, 138, 984–1010. [Google Scholar] [CrossRef]
- Kany, S.; Vollrath, J.T.; Relja, B. Cytokines in Inflammatory Disease. Int. J. Mol. Sci. 2019, 20, 6008. [Google Scholar] [CrossRef]
- Kramer, F.; Torzewski, J.; Kamenz, J.; Veit, K.; Hombach, V.; Dedio, J.; Ivashchenko, Y. Interleukin-1beta stimulates acute phase response and C-reactive protein synthesis by inducing an NFkappaB- and C/EBPbeta-dependent autocrine interleukin-6 loop. Mol. Immunol. 2008, 45, 2678–2689. [Google Scholar] [CrossRef]
- Jeong, A.; Suazo, K.F.; Wood, W.G.; Distefano, M.D.; Li, L. Isoprenoids and protein prenylation: Implications in the pathogenesis and therapeutic intervention of Alzheimer’s disease. Crit. Rev. Biochem. Mol. Biol. 2018, 53, 279–310. [Google Scholar] [CrossRef] [PubMed]
- Hameed, I.; Masoodi, S.R.; Mir, S.A.; Nabi, M.; Ghazanfar, K.; Ganai, B.A. Type 2 diabetes mellitus: From a metabolic disorder to an inflammatory condition. World J. Diabetes 2015, 6, 598–612. [Google Scholar] [CrossRef]
- Gonzalez, L.L.; Garrie, K.; Turner, M.D. Type 2 diabetes—An autoinflammatory disease driven by metabolic stress. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 3805–3823. [Google Scholar] [CrossRef]
- Li, Z.; Cheng, L.; Liang, H.; Duan, W.; Hu, J.; Zhi, W.; Yang, J.; Liu, Z.; Zhao, M.; Liu, J. GPER inhibits diabetes-mediated RhoA activation to prevent vascular endothelial dysfunction. Eur. J. Cell Biol. 2016, 95, 100–113. [Google Scholar] [CrossRef] [PubMed]
- Nunes, K.P.; Rigsby, C.S.; Webb, R.C. RhoA/Rho-kinase and vascular diseases: What is the link? Cell. Mol. Life Sci. 2010, 67, 3823–3836. [Google Scholar] [CrossRef]
- Nakayama, Y.; Komuro, R.; Yamamoto, A.; Miyata, Y.; Tanaka, M.; Matsuda, M.; Fukuhara, A.; Shimomura, I. RhoA induces expression of inflammatory cytokine in adipocytes. Biochem. Biophys. Res. Commun. 2009, 379, 288–292. [Google Scholar] [CrossRef]
- Sylow, L.; Jensen, T.E.; Kleinert, M.; Hojlund, K.; Kiens, B.; Wojtaszewski, J.; Prats, C.; Schjerling, P.; Richter, E.A. Rac1 signaling is required for insulin-stimulated glucose uptake and is dysregulated in insulin-resistant murine and human skeletal muscle. Diabetes 2013, 62, 1865–1875. [Google Scholar] [CrossRef]
- Tanaka, S.; Fukumoto, Y.; Nochioka, K.; Minami, T.; Kudo, S.; Shiba, N.; Takai, Y.; Williams, C.L.; Liao, J.K.; Shimokawa, H. Statins exert the pleiotropic effects through small GTP-binding protein dissociation stimulator upregulation with a resultant Rac1 degradation. Arter. Thromb. Vasc. Biol. 2013, 33, 1591–1600. [Google Scholar] [CrossRef]
- Vecchione, C.; Gentile, M.T.; Aretini, A.; Marino, G.; Poulet, R.; Maffei, A.; Passarelli, F.; Landolfi, A.; Vasta, A.; Lembo, G. A novel mechanism of action for statins against diabetes-induced oxidative stress. Diabetologia 2007, 50, 874–880. [Google Scholar] [CrossRef]
- Bruder-Nascimento, T.; Callera, G.; Montezano, A.C.; Antunes, T.T.; He, Y.; Cat, A.N.; Ferreira, N.S.; Barreto, P.A.; Olivon, V.C.; Tostes, R.C.; et al. Renoprotective Effects of Atorvastatin in Diabetic Mice: Downregulation of RhoA and Upregulation of Akt/GSK3. PLoS ONE 2016, 11, e0162731. [Google Scholar] [CrossRef] [PubMed]
- Donath, M.Y.; Shoelson, S.E. Type 2 diabetes as an inflammatory disease. Nat. Rev. Immunol. 2011, 11, 98–107. [Google Scholar] [CrossRef]
- Cnop, M.; Welsh, N.; Jonas, J.C.; Jorns, A.; Lenzen, S.; Eizirik, D.L. Mechanisms of pancreatic beta-cell death in type 1 and type 2 diabetes: Many differences, few similarities. Diabetes 2005, 54 (Suppl. S2), S97–S107. [Google Scholar] [CrossRef] [PubMed]
- Mao, H.; Zhao, X.; Sun, S.C. NF-kappaB in inflammation and cancer. Cell. Mol. Immunol. 2025, 22, 811–839. [Google Scholar] [CrossRef]
- Eizirik, D.L.; Cardozo, A.K.; Cnop, M. The role for endoplasmic reticulum stress in diabetes mellitus. Endocr. Rev. 2008, 29, 42–61. [Google Scholar] [CrossRef]
- Suryavanshi, S.V.; Kulkarni, Y.A. NF-kappabeta: A Potential Target in the Management of Vascular Complications of Diabetes. Front. Pharmacol. 2017, 8, 798. [Google Scholar] [CrossRef]
- Zhang, Y.; Qu, Y.; Cai, R.; Gao, J.; Xu, Q.; Zhang, L.; Kang, M.; Jia, H.; Chen, Q.; Liu, Y.; et al. Atorvastatin ameliorates diabetic nephropathy through inhibiting oxidative stress and ferroptosis signaling. Eur. J. Pharmacol. 2024, 976, 176699. [Google Scholar] [CrossRef]
- Han, Q.; Liu, Q.; Zhang, H.; Lu, M.; Wang, H.; Tang, F.; Zhang, Y. Simvastatin Improves Cardiac Hypertrophy in Diabetic Rats by Attenuation of Oxidative Stress and Inflammation Induced by Calpain-1-Mediated Activation of Nuclear Factor-kappaB (NF-kappaB). Med. Sci. Monit. 2019, 25, 1232–1241. [Google Scholar] [CrossRef]
- Lin, C.P.; Huang, P.H.; Lai, C.F.; Chen, J.W.; Lin, S.J.; Chen, J.S. Simvastatin Attenuates Oxidative Stress, NF-kappaB Activation, and Artery Calcification in LDLR-/- Mice Fed with High Fat Diet via Down-regulation of Tumor Necrosis Factor-alpha and TNF Receptor 1. PLoS ONE 2015, 10, e0143686. [Google Scholar] [CrossRef]
- Di Giacomo Barbagallo, F.; Bosco, G.; Di Marco, M.; Scilletta, S.; Miano, N.; Musmeci, M.; Martedi, M.; Gonzalez-Lleo, A.M.; Ibarretxe, D.; De Francesco, E.M.; et al. Evaluation of glycemic status and subclinical atherosclerosis in familial hypercholesterolemia subjects with or without LDL receptor mutation. Cardiovasc. Diabetol. 2025, 24, 126. [Google Scholar] [CrossRef] [PubMed]
- Scicali, R.; Di Pino, A.; Urbano, F.; Ferrara, V.; Marchisello, S.; Di Mauro, S.; Scamporrino, A.; Filippello, A.; Rabuazzo, A.M.; Purrello, F.; et al. Analysis of steatosis biomarkers and inflammatory profile after adding on PCSK9 inhibitor treatment in familial hypercholesterolemia subjects with nonalcoholic fatty liver disease: A single lipid center real-world experience. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 869–879. [Google Scholar] [CrossRef]
- Yu, K.; Li, Z.; Shi, W.; Zhao, Z.; Yang, L. Causal impact of statins on susceptibility to osteoarthritis: Insights from a two-sample Mendelian randomization analysis. Int. J. Clin. Pharm. 2024, 46, 1208–1214. [Google Scholar] [CrossRef]
- Chen, W.; Sun, Z.; Xiong, X.; Tan, H.; Hu, J.; Liu, C.; Chen, C. Exploring the causal link among statin drugs and the osteoarthritis risk based on Mendelian randomization research. Front. Genet. 2024, 15, 1390387. [Google Scholar] [CrossRef] [PubMed]
- Heidari, B.; Babaei, M.; Yosefghahri, B. Prevention of Osteoarthritis Progression by Statins, Targeting Metabolic and Inflammatory Aspects: A Review. Mediterr. J. Rheumatol. 2021, 32, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Dinc, M.; Bilgen, M.S.; Kucukalp, A.; Bilgen, O.F. An assessment of the chondroprotective effects of intra-articular application of statin and tetracycline on early-stage experimental osteoarthritis. ISRN Orthop. 2012, 2012, 182097. [Google Scholar] [CrossRef][Green Version]
- van der Kraan, P.M.; van den Berg, W.B. Anabolic and destructive mediators in osteoarthritis. Curr. Opin. Clin. Nutr. Metab. Care 2000, 3, 205–211. [Google Scholar] [CrossRef]
- Yokota, K.; Miyazaki, T.; Hirano, M.; Akiyama, Y.; Mimura, T. Simvastatin inhibits production of interleukin 6 (IL-6) and IL-8 and cell proliferation induced by tumor necrosis factor-alpha in fibroblast-like synoviocytes from patients with rheumatoid arthritis. J. Rheumatol. 2006, 33, 463–471. [Google Scholar] [PubMed]
- Lazzerini, P.E.; Lorenzini, S.; Selvi, E.; Capecchi, P.L.; Chindamo, D.; Bisogno, S.; Ghittoni, R.; Natale, M.R.; Caporali, F.; Giuntini, S.; et al. Simvastatin inhibits cytokine production and nuclear factor-kB activation in interleukin 1beta-stimulated synoviocytes from rheumatoid arthritis patients. Clin. Exp. Rheumatol. 2007, 25, 696–700. [Google Scholar] [PubMed]
- Ridker, P.M.; MacFadyen, J.G.; Thuren, T.; Everett, B.M.; Libby, P.; Glynn, R.J.; Group, C.T. Effect of interleukin-1beta inhibition with canakinumab on incident lung cancer in patients with atherosclerosis: Exploratory results from a randomised, double-blind, placebo-controlled trial. Lancet 2017, 390, 1833–1842. [Google Scholar] [CrossRef]
- Sun, D.; Gong, L.; Wang, X.; Chen, S.; Yi, J.; Liu, X. Pro-inflammatory Cytokines Promote the Occurrence and Development of Colitis-associated Colorectal Cancer by Inhibiting miR-615-5p. Inflamm. Bowel Dis. 2023, 29, 1854–1864. [Google Scholar] [CrossRef]
- Florescu, D.N.; Boldeanu, M.V.; Serban, R.E.; Florescu, L.M.; Serbanescu, M.S.; Ionescu, M.; Streba, L.; Constantin, C.; Vere, C.C. Correlation of the Pro-Inflammatory Cytokines IL-1beta, IL-6, and TNF-alpha, Inflammatory Markers, and Tumor Markers with the Diagnosis and Prognosis of Colorectal Cancer. Life 2023, 13, 2261. [Google Scholar] [CrossRef]
- Klampfer, L. Cytokines, inflammation and colon cancer. Curr. Cancer Drug Targets 2011, 11, 451–464. [Google Scholar] [CrossRef]
- Shibabaw, T.; Teferi, B.; Ayelign, B. The role of Th-17 cells and IL-17 in the metastatic spread of breast cancer: As a means of prognosis and therapeutic target. Front. Immunol. 2023, 14, 1094823. [Google Scholar] [CrossRef] [PubMed]
- Habanjar, O.; Bingula, R.; Decombat, C.; Diab-Assaf, M.; Caldefie-Chezet, F.; Delort, L. Crosstalk of Inflammatory Cytokines within the Breast Tumor Microenvironment. Int. J. Mol. Sci. 2023, 24, 4002. [Google Scholar] [CrossRef]
- Liu, F.; Li, L.; Lan, M.; Zou, T.; Kong, Z.; Cai, T.; Wu, X.Y.; Cai, Y. Key Factor Regulating Inflammatory Microenvironment, Metastasis, and Resistance in Breast Cancer: Interleukin-1 Signaling. Mediat. Inflamm. 2021, 2021, 7785890. [Google Scholar] [CrossRef] [PubMed]
- Diep, S.; Maddukuri, M.; Yamauchi, S.; Geshow, G.; Delk, N.A. Interleukin-1 and Nuclear Factor Kappa B Signaling Promote Breast Cancer Progression and Treatment Resistance. Cells 2022, 11, 1673. [Google Scholar] [CrossRef] [PubMed]
- Matanic, D.; Beg-Zec, Z.; Stojanovic, D.; Matakoric, N.; Flego, V.; Milevoj-Ribic, F. Cytokines in patients with lung cancer. Scand. J. Immunol. 2003, 57, 173–178. [Google Scholar] [CrossRef]
- Silva, E.M.; Mariano, V.S.; Pastrez, P.R.A.; Pinto, M.C.; Castro, A.G.; Syrjanen, K.J.; Longatto-Filho, A. High systemic IL-6 is associated with worse prognosis in patients with non-small cell lung cancer. PLoS ONE 2017, 12, e0181125. [Google Scholar] [CrossRef]
- Song, X.Y.; Zhou, S.J.; Xiao, N.; Li, Y.S.; Zhen, D.Z.; Su, C.Y.; Liu, Z.D. Research on the relationship between serum levels of inflammatory cytokines and non-small cell lung cancer. Asian Pac. J. Cancer Prev. 2013, 14, 4765–4768. [Google Scholar] [CrossRef]
- Xiao, M.; Xu, J.; Wang, W.; Zhang, B.; Liu, J.; Li, J.; Xu, H.; Zhao, Y.; Yu, X.; Shi, S. Functional significance of cholesterol metabolism in cancer: From threat to treatment. Exp. Mol. Med. 2023, 55, 1982–1995. [Google Scholar] [CrossRef]
- Bergman, M.; Salman, H.; Djaldetti, M.; Bessler, H. Statins as modulators of colon cancer cells induced cytokine secretion by human PBMC. Vasc. Pharmacol. 2011, 54, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Malicki, S.; Winiarski, M.; Matlok, M.; Kostarczyk, W.; Guzdek, A.; Konturek, P.C. IL-6 and IL-8 responses of colorectal cancer in vivo and in vitro cancer cells subjected to simvastatin. J. Physiol. Pharmacol. 2009, 60, 141–146. [Google Scholar]
- Dang, Y.; Zhang, Y.; Wang, Z. The role of statins in the regulation of breast and colorectal cancer and future directions. Front. Pharmacol. 2025, 16, 1578345. [Google Scholar] [CrossRef]
- Liu, S.; Uppal, H.; Demaria, M.; Desprez, P.Y.; Campisi, J.; Kapahi, P. Simvastatin suppresses breast cancer cell proliferation induced by senescent cells. Sci. Rep. 2015, 5, 17895. [Google Scholar] [CrossRef] [PubMed]
- Galland, S.; Martin, P.; Fregni, G.; Letovanec, I.; Stamenkovic, I. Attenuation of the pro-inflammatory signature of lung cancer-derived mesenchymal stromal cells by statins. Cancer Lett. 2020, 484, 50–64. [Google Scholar] [CrossRef]
- Gallelli, L.; Falcone, D.; Scaramuzzino, M.; Pelaia, G.; D’Agostino, B.; Mesuraca, M.; Terracciano, R.; Spaziano, G.; Maselli, R.; Navarra, M.; et al. Effects of simvastatin on cell viability and proinflammatory pathways in lung adenocarcinoma cells exposed to hydrogen peroxide. BMC Pharmacol. Toxicol. 2014, 15, 67. [Google Scholar] [CrossRef]
- Iwata, A.; Shirai, R.; Ishii, H.; Kushima, H.; Otani, S.; Hashinaga, K.; Umeki, K.; Kishi, K.; Tokimatsu, I.; Hiramatsu, K.; et al. Inhibitory effect of statins on inflammatory cytokine production from human bronchial epithelial cells. Clin. Exp. Immunol. 2012, 168, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Sabeel, S.; Motaung, B.; Nguyen, K.A.; Ozturk, M.; Mukasa, S.L.; Wolmarans, K.; Blom, D.J.; Sliwa, K.; Nepolo, E.; Gunther, G.; et al. Impact of statins as immune-modulatory agents on inflammatory markers in adults with chronic diseases: A systematic review and meta-analysis. PLoS ONE 2025, 20, e0323749. [Google Scholar] [CrossRef] [PubMed]
- Jeong, G.H.; Lee, K.H.; Kim, J.Y.; Eisenhut, M.; Kronbichler, A.; van der Vliet, H.J.; Hong, S.H.; Shin, J.I.; Gamerith, G. Effect of Statin on Cancer Incidence: An Umbrella Systematic Review and Meta-Analysis. J. Clin. Med. 2019, 8, 819. [Google Scholar] [CrossRef]
- Dale, K.M.; Coleman, C.I.; Henyan, N.N.; Kluger, J.; White, C.M. Statins and cancer risk: A meta-analysis. JAMA 2006, 295, 74–80. [Google Scholar] [CrossRef]
- Zhou, Q.; Jiao, Z.; Liu, Y.; Devreotes, P.N.; Zhang, Z. The effects of statins in patients with advanced-stage cancers—A systematic review and meta-analysis. Front. Oncol. 2023, 13, 1234713. [Google Scholar] [CrossRef]
- Ridker, P.M. From C-Reactive Protein to Interleukin-6 to Interleukin-1: Moving Upstream To Identify Novel Targets for Atheroprotection. Circ. Res. 2016, 118, 145–156. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, X.; Tian, W.; Wang, T.; Jia, J.; Lai, R.; Wang, T.; Zhang, Z.; Song, L.; Ju, J.; et al. The effect of various types and doses of statins on C-reactive protein levels in patients with dyslipidemia or coronary heart disease: A systematic review and network meta-analysis. Front. Cardiovasc. Med. 2022, 9, 936817. [Google Scholar] [CrossRef] [PubMed]
- Ostadal, P.; Alan, D.; Hajek, P.; Vejvoda, J.; Mates, M.; Blasko, P.; Veselka, J.; Kvapil, M.; Kettner, J.; Wiendl, M.; et al. Fluvastatin in the therapy of acute coronary syndrome: Rationale and design of a multicenter, randomized, double-blind, placebo-controlled trial (The FACS Trial)[ISRCTN81331696]. CuZrr. Control Trials Cardiovasc. Med. 2005, 6, 4. [Google Scholar] [CrossRef]
- Kim, S.T.; Kang, J.H.; Lee, J.; Park, S.H.; Park, J.O.; Park, Y.S.; Lim, H.Y.; Hwang, I.G.; Lee, S.C.; Park, K.W.; et al. Simvastatin plus capecitabine-cisplatin versus placebo plus capecitabine-cisplatin in patients with previously untreated advanced gastric cancer: A double-blind randomised phase 3 study. Eur. J. Cancer 2014, 50, 2822–2830. [Google Scholar] [CrossRef]
- Han, J.Y.; Lee, S.H.; Yoo, N.J.; Hyung, L.S.; Moon, Y.J.; Yun, T.; Kim, H.T.; Lee, J.S. A randomized phase II study of gefitinib plus simvastatin versus gefitinib alone in previously treated patients with advanced non-small cell lung cancer. Clin. Cancer Res. 2011, 17, 1553–1560. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Lee, K.H.; Lee, G.K.; Lee, S.H.; Lim, K.Y.; Joo, J.; Go, Y.J.; Lee, J.S.; Han, J.Y. Randomized Phase II Study of Afatinib Plus Simvastatin Versus Afatinib Alone in Previously Treated Patients with Advanced Nonadenocarcinomatous Non-small Cell Lung Cancer. Cancer Res. Treat. 2017, 49, 1001–1011. [Google Scholar] [CrossRef]
- Alarfi, H.; Youssef, L.A.; Salamoon, M. A Prospective, Randomized, Placebo-Controlled Study of a Combination of Simvastatin and Chemotherapy in Metastatic Breast Cancer. J. Oncol. 2020, 2020, 4174395. [Google Scholar] [CrossRef]
- Park, J.H.; Mortaja, M.; Son, H.G.; Zhao, X.; Sloat, L.M.; Azin, M.; Wang, J.; Collier, M.R.; Tummala, K.S.; Mandinova, A.; et al. Statin prevents cancer development in chronic inflammation by blocking interleukin 33 expression. Nat. Commun. 2024, 15, 4099. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, Y.; Pantea Stoian, A.; Cicero, A.F.G.; Fogacci, F.; Nikolic, D.; Sachinidis, A.; Rizvi, A.A.; Janez, A.; Rizzo, M. Inclisiran: A small interfering RNA strategy targeting PCSK9 to treat hypercholesterolemia. Expert Opin. Drug Saf. 2022, 21, 9–20. [Google Scholar] [CrossRef]
- Di Giacomo-Barbagallo, F.; Andreychuk, N.; Scicali, R.; Gonzalez-Lleo, A.; Piro, S.; Masana, L.; Ibarretxe, D. Inclisiran, Reasons for a Novel Agent in a Crowded Therapeutic Field. Curr. Atheroscler. Rep. 2025, 27, 25. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Niu, Q.; Wu, A.; Zhang, Y.; Hong, L.; Wang, H. Causal relationship between circulating immune cells and the risk of type 2 diabetes: A Mendelian randomization study. Front. Endocrinol. 2023, 14, 1210415. [Google Scholar] [CrossRef]
- Britt, R.D., Jr.; Thompson, M.A.; Sasse, S.; Pabelick, C.M.; Gerber, A.N.; Prakash, Y.S. Th1 cytokines TNF-alpha and IFN-gamma promote corticosteroid resistance in developing human airway smooth muscle. Am. J. Physiol. Lung Cell. Mol. Physiol. 2019, 316, L71–L81. [Google Scholar] [CrossRef]
- Dludla, P.V.; Mabhida, S.E.; Ziqubu, K.; Nkambule, B.B.; Mazibuko-Mbeje, S.E.; Hanser, S.; Basson, A.K.; Pheiffer, C.; Kengne, A.P. Pancreatic beta-cell dysfunction in type 2 diabetes: Implications of inflammation and oxidative stress. World J. Diabetes 2023, 14, 130–146. [Google Scholar] [CrossRef]
- Madhumitha, H.; Mohan, V.; Deepa, M.; Babu, S.; Aravindhan, V. Increased Th1 and suppressed Th2 serum cytokine levels in subjects with diabetic coronary artery disease. Cardiovasc. Diabetol. 2014, 13, 1. [Google Scholar] [CrossRef] [PubMed]
- Coward, W.; Chow, S.C. Effect of atorvastatin on TH1 and TH2 cytokine secreting cells during T cell activation and differentiation. Atherosclerosis 2006, 186, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Hakamada-Taguchi, R.; Uehara, Y.; Kuribayashi, K.; Numabe, A.; Saito, K.; Negoro, H.; Fujita, T.; Toyo-oka, T.; Kato, T. Inhibition of hydroxymethylglutaryl-coenzyme a reductase reduces Th1 development and promotes Th2 development. Circ. Res. 2003, 93, 948–956. [Google Scholar] [CrossRef]
- Brumeanu, T.D.; Goldstein, R.; Casares, S. Down-regulation of autoreactive T-cells by HMG CoA reductase inhibitors. Clin. Immunol. 2006, 119, 1–12. [Google Scholar] [CrossRef]
- Espinosa-Carrasco, G.; Le Saout, C.; Fontanaud, P.; Stratmann, T.; Mollard, P.; Schaeffer, M.; Hernandez, J. CD4(+) T Helper Cells Play a Key Role in Maintaining Diabetogenic CD8(+) T Cell Function in the Pancreas. Front. Immunol. 2017, 8, 2001. [Google Scholar] [CrossRef]
- Takeda, Y.; Matoba, K.; Sekiguchi, K.; Nagai, Y.; Yokota, T.; Utsunomiya, K.; Nishimura, R. Endothelial Dysfunction in Diabetes. Biomedicines 2020, 8, 182. [Google Scholar] [CrossRef]
- Muffova, B.; Kauerova, S.; Paukner, K.; Bartuskova, H.; Poledne, R.; Kralova Lesna, I. Anti-inflammatory effect of fluvastatin on polarized macrophages and its dependence on the mevalonate pathway. Sci. Rep. 2025, 15, 19237. [Google Scholar] [CrossRef]
- Meng, X.; Zhang, K.; Li, J.; Dong, M.; Yang, J.; An, G.; Qin, W.; Gao, F.; Zhang, C.; Zhang, Y. Statins induce the accumulation of regulatory T cells in atherosclerotic plaque. Mol. Med. 2012, 18, 598–605. [Google Scholar] [CrossRef] [PubMed]
- Mausner-Fainberg, K.; Luboshits, G.; Mor, A.; Maysel-Auslender, S.; Rubinstein, A.; Keren, G.; George, J. The effect of HMG-CoA reductase inhibitors on naturally occurring CD4+CD25+ T cells. Atherosclerosis 2008, 197, 829–839. [Google Scholar] [CrossRef]
- Liberale, L.; Carbone, F.; Montecucco, F.; Sahebkar, A. Statins reduce vascular inflammation in atherogenesis: A review of underlying molecular mechanisms. Int. J. Biochem. Cell Biol. 2020, 122, 105735. [Google Scholar] [CrossRef]
- Rodriguez-Perea, A.L.; Montoya, C.J.; Olek, S.; Chougnet, C.A.; Velilla, P.A. Statins increase the frequency of circulating CD4+ FOXP3+ regulatory T cells in healthy individuals. J. Immunol. Res. 2015, 2015, 762506. [Google Scholar] [CrossRef]
- Zou, X.; Xu, H.; Qian, W. Macrophage Polarization in the Osteoarthritis Pathogenesis and Treatment. Orthop. Surg. 2025, 17, 22–35. [Google Scholar] [CrossRef]
- Wen, Z.; Qiu, L.; Ye, Z.; Tan, X.; Xu, X.; Lu, M.; Kuang, G. The role of Th/Treg immune cells in osteoarthritis. Front. Immunol. 2024, 15, 1393418. [Google Scholar] [CrossRef]
- Greenwood, J.; Steinman, L.; Zamvil, S.S. Statin therapy and autoimmune disease: From protein prenylation to immunomodulation. Nat. Rev. Immunol. 2006, 6, 358–370. [Google Scholar] [CrossRef]
- Fujita, E.; Shimizu, A.; Masuda, Y.; Kuwahara, N.; Arai, T.; Nagasaka, S.; Aki, K.; Mii, A.; Natori, Y.; Iino, Y.; et al. Statin attenuates experimental anti-glomerular basement membrane glomerulonephritis together with the augmentation of alternatively activated macrophages. Am. J. Pathol. 2010, 177, 1143–1154. [Google Scholar] [CrossRef]
- Alkakhan, W.; Farrar, N.; Sikora, V.; Emecen-Huja, P.; Huja, S.S.; Yilmaz, O.; Pandruvada, S.N. Statins Modulate Microenvironmental Cues Driving Macrophage Polarization in Simulated Periodontal Inflammation. Cells 2023, 12, 1961. [Google Scholar] [CrossRef] [PubMed]
- Forero-Pena, D.A.; Gutierrez, F.R. Statins as modulators of regulatory T-cell biology. Mediat. Inflamm. 2013, 2013, 167086. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Rajput, A.; Jin, N.; Wang, J. Mechanisms of Immunosuppression in Colorectal Cancer. Cancers 2020, 12, 3850. [Google Scholar] [CrossRef] [PubMed]
- He, R.; Huang, S.; Lu, J.; Su, L.; Gao, X.; Chi, H. Unveiling the immune symphony: Decoding colorectal cancer metastasis through immune interactions. Front. Immunol. 2024, 15, 1362709. [Google Scholar] [CrossRef]
- Nie, S.C.; Jing, Y.H.; Lu, L.; Ren, S.S.; Ji, G.; Xu, H.C. Mechanisms of myeloid-derived suppressor cell-mediated immunosuppression in colorectal cancer and related therapies. World J. Gastrointest. Oncol. 2024, 16, 1690–1704. [Google Scholar] [CrossRef]
- Zhang, X.F.; Zhang, X.L.; Wang, Y.J.; Fang, Y.; Li, M.L.; Liu, X.Y.; Luo, H.Y.; Tian, Y. The regulatory network of the chemokine CCL5 in colorectal cancer. Ann. Med. 2023, 55, 2205168. [Google Scholar] [CrossRef]
- Kundu, M.; Butti, R.; Panda, V.K.; Malhotra, D.; Das, S.; Mitra, T.; Kapse, P.; Gosavi, S.W.; Kundu, G.C. Modulation of the tumor microenvironment and mechanism of immunotherapy-based drug resistance in breast cancer. Mol. Cancer 2024, 23, 92. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wang, S.; Zhou, Q. The Resistance Mechanisms of Lung Cancer Immunotherapy. Front. Oncol. 2020, 10, 568059. [Google Scholar] [CrossRef]
- Al-Husein, B.A.; Dawah, B.; Bani-Hani, S.; Al Bashir, S.M.; Al-Sawalmeh, K.M.; Ayoub, N.M. Immunomodulatory effect of statins on Regulatory T Lymphocytes in human colorectal cancer is determined by the stage of disease. Oncotarget 2018, 9, 35752–35761. [Google Scholar] [CrossRef]
- Williams, M. The Role of Cholesterol Absorption in Modulating the Immune Response in Colorectal Cancer. Immunome Res. 2024, 20, 1–2. [Google Scholar]
- Al Dujaily, E.; Baena, J.; Das, M.; Sereno, M.; Smith, C.; Kamata, T.; Officer, L.; Pritchard, C.; Le Quesne, J. Reduced Protumorigenic Tumor-Associated Macrophages With Statin Use in Premalignant Human Lung Adenocarcinoma. JNCI Cancer Spectr. 2020, 4, pkz101. [Google Scholar] [CrossRef] [PubMed]
- Mao, W.; Cai, Y.; Chen, D.; Jiang, G.; Xu, Y.; Chen, R.; Wang, F.; Wang, X.; Zheng, M.; Zhao, X.; et al. Statin shapes inflamed tumor microenvironment and enhances immune checkpoint blockade in non-small cell lung cancer. JCI Insight 2022, 7, e161940. [Google Scholar] [CrossRef]
- Qiao, X.; Hu, Z.; Xiong, F.; Yang, Y.; Peng, C.; Wang, D.; Li, X. Lipid metabolism reprogramming in tumor-associated macrophages and implications for therapy. Lipids Health Dis. 2023, 22, 45. [Google Scholar] [CrossRef]
- Li, L.; Wang, H.; Zhang, S.; Gao, S.; Lu, X.; Pan, Y.; Tang, W.; Huang, R.; Qiao, K.; Ning, S. Statins inhibit paclitaxel-induced PD-L1 expression and increase CD8+ T cytotoxicity for better prognosis in breast cancer. Int. J. Surg. 2024, 110, 4716–4726. [Google Scholar] [CrossRef]
- Cade, W.T. Diabetes-related microvascular and macrovascular diseases in the physical therapy setting. Phys. Ther. 2008, 88, 1322–1335. [Google Scholar] [CrossRef] [PubMed]
- Plowman, T.J.; Shah, M.H.; Fernandez, E.; Christensen, H.; Aiges, M.; Ramana, K.V. Role of Innate Immune and Inflammatory Responses in the Development of Secondary Diabetic Complications. Curr. Mol. Med. 2023, 23, 901–920. [Google Scholar] [CrossRef]
- Meza, C.A.; La Favor, J.D.; Kim, D.H.; Hickner, R.C. Endothelial Dysfunction: Is There a Hyperglycemia-Induced Imbalance of NOX and NOS? Int. J. Mol. Sci. 2019, 20, 3775. [Google Scholar] [CrossRef] [PubMed]
- Cyr, A.R.; Huckaby, L.V.; Shiva, S.S.; Zuckerbraun, B.S. Nitric Oxide and Endothelial Dysfunction. Crit. Care Clin. 2020, 36, 307–321. [Google Scholar] [CrossRef]
- Du, X.L.; Edelstein, D.; Dimmeler, S.; Ju, Q.; Sui, C.; Brownlee, M. Hyperglycemia inhibits endothelial nitric oxide synthase activity by posttranslational modification at the Akt site. J. Clin. Investig. 2001, 108, 1341–1348. [Google Scholar] [CrossRef]
- Giri, B.; Dey, S.; Das, T.; Sarkar, M.; Banerjee, J.; Dash, S.K. Chronic hyperglycemia mediated physiological alteration and metabolic distortion leads to organ dysfunction, infection, cancer progression and other pathophysiological consequences: An update on glucose toxicity. Biomed. Pharmacother. 2018, 107, 306–328. [Google Scholar] [CrossRef]
- Luo, J.Y.; Cheng, C.K.; Gou, L.; He, L.; Zhao, L.; Zhang, Y.; Wang, L.; Lau, C.W.; Xu, A.; Chen, A.F.; et al. Induction of KLF2 by Exercise Activates eNOS to Improve Vasodilatation in Diabetic Mice. Diabetes 2023, 72, 1330–1342. [Google Scholar] [CrossRef]
- Ota, H.; Eto, M.; Kano, M.R.; Kahyo, T.; Setou, M.; Ogawa, S.; Iijima, K.; Akishita, M.; Ouchi, Y. Induction of endothelial nitric oxide synthase, SIRT1, and catalase by statins inhibits endothelial senescence through the Akt pathway. Arter. Thromb. Vasc. Biol. 2010, 30, 2205–2211. [Google Scholar] [CrossRef]
- Ii, M.; Nishimura, H.; Kusano, K.F.; Qin, G.; Yoon, Y.S.; Wecker, A.; Asahara, T.; Losordo, D.W. Neuronal nitric oxide synthase mediates statin-induced restoration of vasa nervorum and reversal of diabetic neuropathy. Circulation 2005, 112, 93–102. [Google Scholar] [CrossRef]
- Mason, R.P.; Corbalan, J.J.; Jacob, R.F.; Dawoud, H.; Malinski, T. Atorvastatin enhanced nitric oxide release and reduced blood pressure, nitroxidative stress and rantes levels in hypertensive rats with diabetes. J. Physiol. Pharmacol. 2015, 66, 65–72. [Google Scholar] [PubMed]
- Tian, X.Y.; Wong, W.T.; Xu, A.; Chen, Z.Y.; Lu, Y.; Liu, L.M.; Lee, V.W.; Lau, C.W.; Yao, X.; Huang, Y. Rosuvastatin improves endothelial function in db/db mice: Role of angiotensin II type 1 receptors and oxidative stress. Br. J. Pharmacol. 2011, 164, 598–606. [Google Scholar] [CrossRef] [PubMed]
- Min, J.K.; Kim, Y.M.; Kim, S.W.; Kwon, M.C.; Kong, Y.Y.; Hwang, I.K.; Won, M.H.; Rho, J.; Kwon, Y.G. TNF-related activation-induced cytokine enhances leukocyte adhesiveness: Induction of ICAM-1 and VCAM-1 via TNF receptor-associated factor and protein kinase C-dependent NF-kappaB activation in endothelial cells. J. Immunol. 2005, 175, 531–540. [Google Scholar] [CrossRef]
- Etzioni, A. Adhesion molecules–their role in health and disease. Pediatr. Res. 1996, 39, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Manson, J.E.; Tinker, L.; Rifai, N.; Cook, N.R.; Hu, F.B.; Hotamisligil, G.S.; Ridker, P.M.; Rodriguez, B.L.; Margolis, K.L.; et al. Circulating levels of endothelial adhesion molecules and risk of diabetes in an ethnically diverse cohort of women. Diabetes 2007, 56, 1898–1904. [Google Scholar] [CrossRef]
- Rezaie-Majd, A.; Prager, G.W.; Bucek, R.A.; Schernthaner, G.H.; Maca, T.; Kress, H.G.; Valent, P.; Binder, B.R.; Minar, E.; Baghestanian, M. Simvastatin reduces the expression of adhesion molecules in circulating monocytes from hypercholesterolemic patients. Arter. Thromb. Vasc. Biol. 2003, 23, 397–403. [Google Scholar] [CrossRef]
- Nomura, S.; Shouzu, A.; Omoto, S.; Inami, N.; Tanaka, A.; Nanba, M.; Shouda, Y.; Takahashi, N.; Kimura, Y.; Iwasaka, T. Correlation between adiponectin and reduction of cell adhesion molecules after pitavastatin treatment in hyperlipidemic patients with type 2 diabetes mellitus. Thromb. Res. 2008, 122, 39–45. [Google Scholar] [CrossRef]
- Dogra, G.K.; Watts, G.F.; Chan, D.C.; Stanton, K. Statin therapy improves brachial artery vasodilator function in patients with Type 1 diabetes and microalbuminuria. Diabet. Med. 2005, 22, 239–242. [Google Scholar] [CrossRef]
- Hattori, Y.; Nakanishi, N.; Akimoto, K.; Yoshida, M.; Kasai, K. HMG-CoA reductase inhibitor increases GTP cyclohydrolase I mRNA and tetrahydrobiopterin in vascular endothelial cells. Arter. Thromb. Vasc. Biol. 2003, 23, 176–182. [Google Scholar] [CrossRef]
- Wenzel, P.; Daiber, A.; Oelze, M.; Brandt, M.; Closs, E.; Xu, J.; Thum, T.; Bauersachs, J.; Ertl, G.; Zou, M.H.; et al. Mechanisms underlying recoupling of eNOS by HMG-CoA reductase inhibition in a rat model of streptozotocin-induced diabetes mellitus. Atherosclerosis 2008, 198, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Landmesser, U.; Bahlmann, F.; Mueller, M.; Spiekermann, S.; Kirchhoff, N.; Schulz, S.; Manes, C.; Fischer, D.; de Groot, K.; Fliser, D.; et al. Simvastatin versus ezetimibe: Pleiotropic and lipid-lowering effects on endothelial function in humans. Circulation 2005, 111, 2356–2363. [Google Scholar] [CrossRef]
- Sallam, H.S.; Tuvdendorj, D.R.; Jialal, I.; Chandalia, M.; Abate, N. Therapeutic lifestyle change intervention improved metabolic syndrome criteria and is complementary to amlodipine/atorvastatin. J. Diabetes Complicat. 2020, 34, 107480. [Google Scholar] [CrossRef] [PubMed]
- Tsimikas, S.; Witztum, J.L.; Miller, E.R.; Sasiela, W.J.; Szarek, M.; Olsson, A.G.; Schwartz, G.G.; Myocardial Ischemia Reduction with Aggressive Cholesterol Lowering (MIRACL) Study Investigators. High-dose atorvastatin reduces total plasma levels of oxidized phospholipids and immune complexes present on apolipoprotein B-100 in patients with acute coronary syndromes in the MIRACL trial. Circulation 2004, 110, 1406–1412. [Google Scholar] [CrossRef]
- Moulin, D.; Sellam, J.; Berenbaum, F.; Guicheux, J.; Boutet, M.A. The role of the immune system in osteoarthritis: Mechanisms, challenges and future directions. Nat. Rev. Rheumatol. 2025, 21, 221–236. [Google Scholar] [CrossRef]
- de Munter, W.; van der Kraan, P.M.; van den Berg, W.B.; van Lent, P.L. High systemic levels of low-density lipoprotein cholesterol: Fuel to the flames in inflammatory osteoarthritis? Rheumatology 2016, 55, 16–24. [Google Scholar] [CrossRef]
- Meroni, P.L.; Raschi, E.; Testoni, C.; Tincani, A.; Balestrieri, G.; Molteni, R.; Khamashta, M.A.; Tremoli, E.; Camera, M. Statins prevent endothelial cell activation induced by antiphospholipid (anti-beta2-glycoprotein I) antibodies: Effect on the proadhesive and proinflammatory phenotype. Arthritis Rheum. 2001, 44, 2870–2878. [Google Scholar] [CrossRef]
- Fearon, U.; Canavan, M.; Biniecka, M.; Veale, D.J. Hypoxia, mitochondrial dysfunction and synovial invasiveness in rheumatoid arthritis. Nat. Rev. Rheumatol. 2016, 12, 385–397. [Google Scholar] [CrossRef] [PubMed]
- Fearon, U.; Hanlon, M.M.; Floudas, A.; Veale, D.J. Cellular metabolic adaptations in rheumatoid arthritis and their therapeutic implications. Nat. Rev. Rheumatol. 2022, 18, 398–414. [Google Scholar] [CrossRef] [PubMed]
- Aleksandrowicz, M.; Konop, M.; Rybka, M.; Mazurek, L.; Stradczuk-Mazurek, M.; Kciuk, M.; Badzynska, B.; Dobrowolski, L.; Kuczeriszka, M. Dysfunction of Microcirculation in Atherosclerosis: Implications of Nitric Oxide, Oxidative Stress, and Inflammation. Int. J. Mol. Sci. 2025, 26, 6467. [Google Scholar] [CrossRef] [PubMed]
- Kinderlerer, A.R.; Steinberg, R.; Johns, M.; Harten, S.K.; Lidington, E.A.; Haskard, D.O.; Maxwell, P.H.; Mason, J.C. Statin-induced expression of CD59 on vascular endothelium in hypoxia: A potential mechanism for the anti-inflammatory actions of statins in rheumatoid arthritis. Arthritis Res. Ther. 2006, 8, R130. [Google Scholar] [CrossRef]
- Weis, M.; Heeschen, C.; Glassford, A.J.; Cooke, J.P. Statins have biphasic effects on angiogenesis. Circulation 2002, 105, 739–745. [Google Scholar] [CrossRef]
- Malhab, L.J.B.; Saber-Ayad, M.M.; Al-Hakm, R.; Nair, V.A.; Paliogiannis, P.; Pintus, G.; Abdel-Rahman, W.M. Chronic Inflammation and Cancer: The Role of Endothelial Dysfunction and Vascular Inflammation. Curr. Pharm. Des. 2021, 27, 2156–2169. [Google Scholar] [CrossRef]
- Chen, W.Z.; Jiang, J.X.; Yu, X.Y.; Xia, W.J.; Yu, P.X.; Wang, K.; Zhao, Z.Y.; Chen, Z.G. Endothelial cells in colorectal cancer. World J. Gastrointest. Oncol. 2019, 11, 946–956. [Google Scholar] [CrossRef]
- Ghiabi, P.; Jiang, J.; Pasquier, J.; Maleki, M.; Abu-Kaoud, N.; Rafii, S.; Rafii, A. Endothelial cells provide a notch-dependent pro-tumoral niche for enhancing breast cancer survival, stemness and pro-metastatic properties. PLoS ONE 2014, 9, e112424. [Google Scholar] [CrossRef]
- Xu, C.; Zou, J.; Li, L.; Yuan, Q.; Wang, W. Elevated serum Cripto-1 and VEGF levels in patients with non-small cell lung cancer. FASEB BioAdvances 2022, 4, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Dulak, J.; Jozkowicz, A. Anti-angiogenic and anti-inflammatory effects of statins: Relevance to anti-cancer therapy. Curr. Cancer Drug Targets 2005, 5, 579–594. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Lee, I.; Lee, J.; Park, C.; Kang, W.K. Statins, 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors, potentiate the anti-angiogenic effects of bevacizumab by suppressing angiopoietin2, BiP, and Hsp90alpha in human colorectal cancer. Br. J. Cancer 2014, 111, 497–505. [Google Scholar] [CrossRef]
- Huang, Y.J.; Lin, J.A.; Chen, W.M.; Shia, B.C.; Wu, S.Y. Statin Therapy Reduces Radiation-Induced Cardiotoxicity in Patients With Breast Cancer Receiving Adjuvant Radiotherapy. J. Am. Heart Assoc. 2024, 13, e036411. [Google Scholar] [CrossRef]
- Chen, J.; Liu, B.; Yuan, J.; Yang, J.; Zhang, J.; An, Y.; Tie, L.; Pan, Y.; Li, X. Atorvastatin reduces vascular endothelial growth factor (VEGF) expression in human non-small cell lung carcinomas (NSCLCs) via inhibition of reactive oxygen species (ROS) production. Mol. Oncol. 2012, 6, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Meyers, A.K.; Zhu, X. The NLRP3 Inflammasome: Metabolic Regulation and Contribution to Inflammaging. Cells 2020, 9, 1808. [Google Scholar] [CrossRef]
- Meier, D.T.; de Paula Souza, J.; Donath, M.Y. Targeting the NLRP3 inflammasome-IL-1beta pathway in type 2 diabetes and obesity. Diabetologia 2025, 68, 3–16. [Google Scholar] [CrossRef]
- Karmakar, V.; Chain, M.; Majie, A.; Ghosh, A.; Sengupta, P.; Dutta, S.; Mazumder, P.M.; Gorain, B. Targeting the NLRP3 inflammasome as a novel therapeutic target for osteoarthritis. Inflammopharmacology 2025, 33, 461–484. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Y.; Liu, X.; Zhang, Y. NLRP3 Inflammasome Activation in MPhis-CRC Crosstalk Promotes Colorectal Cancer Metastasis. Ann. Clin. Lab. Sci. 2022, 52, 571–579. [Google Scholar]
- Wang, Y.; Kong, H.; Zeng, X.; Liu, W.; Wang, Z.; Yan, X.; Wang, H.; Xie, W. Activation of NLRP3 inflammasome enhances the proliferation and migration of A549 lung cancer cells. Oncol. Rep. 2016, 35, 2053–2064. [Google Scholar] [CrossRef]
- Li, Z.; Yang, F.; Zhu, X.; Zhou, B.; Jin, K.; Dai, J.; Jiang, G. Effect of NLRP3 Inflammasome on Lung Cancer Immune Microenvironment Activation and Its Mechanism. Altern. Ther. Health Med. 2024, 30, 86–91. [Google Scholar] [PubMed]
- Ershaid, N.; Sharon, Y.; Doron, H.; Raz, Y.; Shani, O.; Cohen, N.; Monteran, L.; Leider-Trejo, L.; Ben-Shmuel, A.; Yassin, M.; et al. NLRP3 inflammasome in fibroblasts links tissue damage with inflammation in breast cancer progression and metastasis. Nat. Commun. 2019, 10, 4375. [Google Scholar] [CrossRef]
- Lv, Z.H.; Phuong, T.A.; Jin, S.J.; Li, X.X.; Xu, M. Protection by simvastatin on hyperglycemia-induced endothelial dysfunction through inhibiting NLRP3 inflammasomes. Oncotarget 2017, 8, 91291–91305. [Google Scholar] [CrossRef] [PubMed]
- Luo, B.; Li, B.; Wang, W.; Liu, X.; Liu, X.; Xia, Y.; Zhang, C.; Zhang, Y.; Zhang, M.; An, F. Rosuvastatin alleviates diabetic cardiomyopathy by inhibiting NLRP3 inflammasome and MAPK pathways in a type 2 diabetes rat model. Cardiovasc. Drugs Ther. 2014, 28, 33–43. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.T.; Koka, S.; Zhang, Y.; Hussain, T.; Li, X. Simvastatin improves lysosome function via enhancing lysosome biogenesis in endothelial cells. Front. Biosci. 2020, 25, 283–298. [Google Scholar] [CrossRef]
- Wondmkun, Y.T. Obesity, Insulin Resistance, and Type 2 Diabetes: Associations and Therapeutic Implications. Diabetes Metab. Syndr. Obes. 2020, 13, 3611–3616. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Gao, J.; Xu, M.; Ren, S.; Stefanovic-Racic, M.; O’Doherty, R.M.; Xie, W. PXR ablation alleviates diet-induced and genetic obesity and insulin resistance in mice. Diabetes 2013, 62, 1876–1887. [Google Scholar] [CrossRef]
- Thongnak, L.; Pengrattanachot, N.; Promsan, S.; Phengpol, N.; Sutthasupha, P.; Chatsudthipong, V.; Lungkaphin, A. The combination of dapagliflozin and statins ameliorates renal injury through attenuating the activation of inflammasome-mediated autophagy in insulin-resistant rats. J. Biochem. Mol. Toxicol. 2022, 36, e22978. [Google Scholar] [CrossRef]
- Henriksbo, B.D.; Lau, T.C.; Cavallari, J.F.; Denou, E.; Chi, W.; Lally, J.S.; Crane, J.D.; Duggan, B.M.; Foley, K.P.; Fullerton, M.D.; et al. Fluvastatin causes NLRP3 inflammasome-mediated adipose insulin resistance. Diabetes 2014, 63, 3742–3747. [Google Scholar] [CrossRef]
- Zhu, J.; Wu, S.; Hu, S.; Li, H.; Li, M.; Geng, X.; Wang, H. NLRP3 inflammasome expression in peripheral blood monocytes of coronary heart disease patients and its modulation by rosuvastatin. Mol. Med. Rep. 2019, 20, 1826–1836. [Google Scholar] [CrossRef]
- Altaf, A.; Qu, P.; Zhao, Y.; Wang, H.; Lou, D.; Niu, N. NLRP3 inflammasome in peripheral blood monocytes of acute coronary syndrome patients and its relationship with statins. Coron. Artery Dis. 2015, 26, 409–421. [Google Scholar] [CrossRef]
- Chen, J.; Yan, J.; Li, S.; Zhu, J.; Zhou, J.; Li, J.; Zhang, Y.; Huang, Z.; Yuan, L.; Xu, K.; et al. Atorvastatin inhibited TNF-alpha induced matrix degradation in rat nucleus pulposus cells by suppressing NLRP3 inflammasome activity and inducing autophagy through NF-kappaB signaling. Cell Cycle 2021, 20, 2160–2173. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.F.; Washko, G.R.; Nakahira, K.; Hatabu, H.; Patel, A.S.; Fernandez, I.E.; Nishino, M.; Okajima, Y.; Yamashiro, T.; Ross, J.C.; et al. Statins and pulmonary fibrosis: The potential role of NLRP3 inflammasome activation. Am. J. Respir. Crit. Care Med. 2012, 185, 547–556. [Google Scholar] [CrossRef]
- Saber, S.; Abd El-Fattah, E.E.; Yahya, G.; Gobba, N.A.; Maghmomeh, A.O.; Khodir, A.E.; Mourad, A.A.E.; Saad, A.S.; Mohammed, H.G.; Nouh, N.A.; et al. A Novel Combination Therapy Using Rosuvastatin and Lactobacillus Combats Dextran Sodium Sulfate-Induced Colitis in High-Fat Diet-Fed Rats by Targeting the TXNIP/NLRP3 Interaction and Influencing Gut Microbiome Composition. Pharmaceuticals 2021, 14, 341. [Google Scholar] [CrossRef]
- Khalifeh, M.; Penson, P.E.; Banach, M.; Sahebkar, A. Statins as anti-pyroptotic agents. Arch. Med. Sci. 2021, 17, 1414–1417. [Google Scholar] [CrossRef] [PubMed]
- Nieman, M.T. Protease-activated receptors in hemostasis. Blood 2016, 128, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Kagota, S.; Maruyama, K.; McGuire, J.J. Characterization and Functions of Protease-Activated Receptor 2 in Obesity, Diabetes, and Metabolic Syndrome: A Systematic Review. Biomed Res. Int. 2016, 2016, 3130496. [Google Scholar] [CrossRef]
- Johnson, J.J.; Miller, D.L.; Jiang, R.; Liu, Y.; Shi, Z.; Tarwater, L.; Williams, R.; Balsara, R.; Sauter, E.R.; Stack, M.S. Protease-activated Receptor-2 (PAR-2)-mediated Nf-kappaB Activation Suppresses Inflammation-associated Tumor Suppressor MicroRNAs in Oral Squamous Cell Carcinoma. J. Biol. Chem. 2016, 291, 6936–6945. [Google Scholar] [CrossRef]
- Oe, Y.; Hayashi, S.; Fushima, T.; Sato, E.; Kisu, K.; Sato, H.; Ito, S.; Takahashi, N. Coagulation Factor Xa and Protease-Activated Receptor 2 as Novel Therapeutic Targets for Diabetic Nephropathy. Arter. Thromb. Vasc. Biol. 2016, 36, 1525–1533. [Google Scholar] [CrossRef]
- Bagang, N.; Gupta, K.; Singh, G.; Kanuri, S.H.; Mehan, S. Protease-activated receptors in kidney diseases: A comprehensive review of pathological roles, therapeutic outcomes and challenges. Chem. Biol. Interact. 2023, 377, 110470. [Google Scholar] [CrossRef]
- Banfi, C.; Brioschi, M.; Lento, S.; Pirillo, A.; Galli, S.; Cosentino, S.; Tremoli, E.; Mussoni, L. Statins prevent tissue factor induction by protease-activated receptors 1 and 2 in human umbilical vein endothelial cells in vitro. J. Thromb. Haemost. 2011, 9, 1608–1619. [Google Scholar] [CrossRef]
- Lam, F.F. Role of protease-activated receptor 2 in joint inflammation. Arthritis Rheum. 2007, 56, 3514–3517. [Google Scholar] [CrossRef]
- Xue, M.; Chan, Y.K.; Shen, K.; Dervish, S.; March, L.; Sambrook, P.N.; Jackson, C.J. Protease-activated receptor 2, rather than protease-activated receptor 1, contributes to the aggressive properties of synovial fibroblasts in rheumatoid arthritis. Arthritis Rheum. 2012, 64, 88–98. [Google Scholar] [CrossRef]
- Patnaik, R.; Riaz, S.; Sivani, B.M.; Faisal, S.; Naidoo, N.; Rizzo, M.; Banerjee, Y. Evaluating the potential of Vitamin D and curcumin to alleviate inflammation and mitigate the progression of osteoarthritis through their effects on human chondrocytes: A proof-of-concept investigation. PLoS ONE 2023, 18, e0290739. [Google Scholar] [CrossRef] [PubMed]
- Jannati, S.; Patnaik, R.; Banerjee, Y. Beyond Anticoagulation: A Comprehensive Review of Non-Vitamin K Oral Anticoagulants (NOACs) in Inflammation and Protease-Activated Receptor Signaling. Int. J. Mol. Sci. 2024, 25, 8727. [Google Scholar] [CrossRef]
- Boileau, C.; Amiable, N.; Martel-Pelletier, J.; Fahmi, H.; Duval, N.; Pelletier, J.P. Activation of proteinase-activated receptor 2 in human osteoarthritic cartilage upregulates catabolic and proinflammatory pathways capable of inducing cartilage degradation: A basic science study. Arthritis Res. Ther. 2007, 9, R121. [Google Scholar] [CrossRef]
- Patnaik, R.; Varghese, R.; Jannati, S.; Naidoo, N.; Banerjee, Y. Targeting PAR2-mediated inflammation in osteoarthritis: A comprehensive in vitro evaluation of oleocanthal’s potential as a functional food intervention for chondrocyte protection and anti-inflammatory effects. BMC Musculoskelet. Disord. 2024, 25, 769. [Google Scholar] [CrossRef] [PubMed]
- Wojtukiewicz, M.Z.; Hempel, D.; Sierko, E.; Tucker, S.C.; Honn, K.V. Protease-activated receptors (PARs)–biology and role in cancer invasion and metastasis. Cancer Metastasis Rev. 2015, 34, 775–796. [Google Scholar] [CrossRef] [PubMed]
- Vetvicka, D.; Suhaj, P.; Olejar, T.; Sivak, L.; Benes, J.; Pouckova, P. Proteinase-activated Receptor 2: Springboard of Tumors. Anticancer Res. 2024, 44, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Shah, H.; Hill, T.A.; Lim, J.; Fairlie, D.P. Protease-activated receptor 2 attenuates doxorubicin-induced apoptosis in colon cancer cells. J. Cell Commun. Signal. 2023, 17, 1293–1307. [Google Scholar] [CrossRef]
- Morris, D.R.; Ding, Y.; Ricks, T.K.; Gullapalli, A.; Wolfe, B.L.; Trejo, J. Protease-activated receptor-2 is essential for factor VIIa and Xa-induced signaling, migration, and invasion of breast cancer cells. Cancer Res. 2006, 66, 307–314. [Google Scholar] [CrossRef]
- Wang, B.; Wu, M.D.; Lan, Y.J.; Jia, C.Y.; Zhao, H.; Yang, K.P.; Liu, H.N.; Sun, S.Z.; Tao, R.C.; Lu, X.D.; et al. PAR2 promotes malignancy in lung adenocarcinoma. Am. J. Transl. Res. 2024, 16, 7416–7426. [Google Scholar] [CrossRef]
- Patnaik, R.; Varghese, R.L.; Khan, S.; Huda, B.; Bhurka, F.; Amiri, L.; Banerjee, Y. Targeting PAR-2-driven inflammatory pathways in colorectal cancer: Mechanistic insights from atorvastatin and rosuvastatin treatment in cell line models. Transl. Cancer Res. 2025, 14, 1531–1566. [Google Scholar] [CrossRef] [PubMed]
- Ghesmati, Z.; Rashid, M.; Fayezi, S.; Gieseler, F.; Alizadeh, E.; Darabi, M. An update on the secretory functions of brown, white, and beige adipose tissue: Towards therapeutic applications. Rev. Endocr. Metab. Disord. 2024, 25, 279–308. [Google Scholar] [CrossRef]
- Joshi, M.; Patel, B.M. The burning furnace: Alteration in lipid metabolism in cancer-associated cachexia. Mol. Cell. Biochem. 2022, 477, 1709–1723. [Google Scholar] [CrossRef]
- Pare, M.; Darini, C.Y.; Yao, X.; Chignon-Sicard, B.; Rekima, S.; Lachambre, S.; Virolle, V.; Aguilar-Mahecha, A.; Basik, M.; Dani, C.; et al. Breast cancer mammospheres secrete Adrenomedullin to induce lipolysis and browning of adjacent adipocytes. BMC Cancer 2020, 20, 784. [Google Scholar] [CrossRef]
- Li, X.; Wang, G.; Liu, J.; Ding, G. Increased UCP1 expression in the perirenal adipose tissue of patients with renal cell carcinoma. Oncol. Rep. 2019, 42, 1972–1980. [Google Scholar] [CrossRef]
- Versteeg, H.H.; Schaffner, F.; Kerver, M.; Ellies, L.G.; Andrade-Gordon, P.; Mueller, B.M.; Ruf, W. Protease-activated receptor (PAR) 2, but not PAR1, signaling promotes the development of mammary adenocarcinoma in polyoma middle T mice. Cancer Res. 2008, 68, 7219–7227. [Google Scholar] [CrossRef] [PubMed]
- Balaz, M.; Becker, A.S.; Balazova, L.; Straub, L.; Muller, J.; Gashi, G.; Maushart, C.I.; Sun, W.; Dong, H.; Moser, C.; et al. Inhibition of Mevalonate Pathway Prevents Adipocyte Browning in Mice and Men by Affecting Protein Prenylation. Cell Metab. 2019, 29, 901–916.e8. [Google Scholar] [CrossRef]
- Ying, Z.; Tramper, N.; Zhou, E.; Boon, M.R.; Rensen, P.C.N.; Kooijman, S. Role of thermogenic adipose tissue in lipid metabolism and atherosclerotic cardiovascular disease: Lessons from studies in mice and humans. Cardiovasc. Res. 2023, 119, 905–918. [Google Scholar] [CrossRef]
- Atale, N.; Wells, A. Statins as Secondary Preventive Agent to Limit Breast Cancer Metastatic Outgrowth. Int. J. Mol. Sci. 2025, 26, 1300. [Google Scholar] [CrossRef]
- Myasoedova, V.A.; Parisi, V.; Moschetta, D.; Valerio, V.; Conte, M.; Massaiu, I.; Bozzi, M.; Celeste, F.; Leosco, D.; Iaccarino, G.; et al. Efficacy of cardiometabolic drugs in reduction of epicardial adipose tissue: A systematic review and meta-analysis. Cardiovasc. Diabetol. 2023, 22, 23. [Google Scholar] [CrossRef] [PubMed]
- Checa, J.; Aran, J.M. Reactive Oxygen Species: Drivers of Physiological and Pathological Processes. J. Inflamm. Res. 2020, 13, 1057–1073. [Google Scholar] [CrossRef] [PubMed]
- Caturano, A.; D’Angelo, M.; Mormone, A.; Russo, V.; Mollica, M.P.; Salvatore, T.; Galiero, R.; Rinaldi, L.; Vetrano, E.; Marfella, R.; et al. Oxidative Stress in Type 2 Diabetes: Impacts from Pathogenesis to Lifestyle Modifications. Curr. Issues Mol. Biol. 2023, 45, 6651–6666. [Google Scholar] [CrossRef]
- Jansen, F.; Yang, X.; Franklin, B.S.; Hoelscher, M.; Schmitz, T.; Bedorf, J.; Nickenig, G.; Werner, N. High glucose condition increases NADPH oxidase activity in endothelial microparticles that promote vascular inflammation. Cardiovasc. Res. 2013, 98, 94–106. [Google Scholar] [CrossRef] [PubMed]
- Kowluru, A.; Kowluru, R.A. Phagocyte-like NADPH oxidase [Nox2] in cellular dysfunction in models of glucolipotoxicity and diabetes. Biochem. Pharmacol. 2014, 88, 275–283. [Google Scholar] [CrossRef]
- Peng, J.J.; Xiong, S.Q.; Ding, L.X.; Peng, J.; Xia, X.B. Diabetic retinopathy: Focus on NADPH oxidase and its potential as therapeutic target. Eur. J. Pharmacol. 2019, 853, 381–387. [Google Scholar] [CrossRef]
- Farnaghi, S.; Prasadam, I.; Cai, G.; Friis, T.; Du, Z.; Crawford, R.; Mao, X.; Xiao, Y. Protective effects of mitochondria-targeted antioxidants and statins on cholesterol-induced osteoarthritis. FASEB J. 2017, 31, 356–367. [Google Scholar] [CrossRef]
- Davignon, J.; Jacob, R.F.; Mason, R.P. The antioxidant effects of statins. Coron. Artery Dis. 2004, 15, 251–258. [Google Scholar] [CrossRef]
- Vilas-Boas, E.A.; Almeida, D.C.; Roma, L.P.; Ortis, F.; Carpinelli, A.R. Lipotoxicity and beta-Cell Failure in Type 2 Diabetes: Oxidative Stress Linked to NADPH Oxidase and ER Stress. Cells 2021, 10, 3328. [Google Scholar] [CrossRef]
- Moon, D.O. NADPH Dynamics: Linking Insulin Resistance and beta-Cells Ferroptosis in Diabetes Mellitus. Int. J. Mol. Sci. 2023, 25, 342. [Google Scholar] [CrossRef]
- Iqbal, M.J.; Kabeer, A.; Abbas, Z.; Siddiqui, H.A.; Calina, D.; Sharifi-Rad, J.; Cho, W.C. Interplay of oxidative stress, cellular communication and signaling pathways in cancer. Cell Commun. Signal. 2024, 22, 7. [Google Scholar] [CrossRef] [PubMed]
- Moradi-Marjaneh, R.; Hassanian, S.M.; Mehramiz, M.; Rezayi, M.; Ferns, G.A.; Khazaei, M.; Avan, A. Reactive oxygen species in colorectal cancer: The therapeutic impact and its potential roles in tumor progression via perturbation of cellular and physiological dysregulated pathways. J. Cell. Physiol. 2019, 234, 10072–10079. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Li, Y.; Zamyatnin, A.A., Jr.; Werner, J.; Bazhin, A.V. Reactive oxygen species and colorectal cancer. J. Cell. Physiol. 2018, 233, 5119–5132. [Google Scholar] [CrossRef]
- Malla, R.; Surepalli, N.; Farran, B.; Malhotra, S.V.; Nagaraju, G.P. Reactive oxygen species (ROS): Critical roles in breast tumor microenvironment. Crit. Rev. Oncol. Hematol. 2021, 160, 103285. [Google Scholar] [CrossRef] [PubMed]
- Johar, R.; Sharma, R.; Kaur, A.; Mukherjee, T.K. Role of Reactive Oxygen Species in Estrogen Dependant Breast Cancer Complication. Anticancer. Agents. Med. Chem. 2015, 16, 190–199. [Google Scholar] [CrossRef]
- Franzoni, F.; Quinones-Galvan, A.; Regoli, F.; Ferrannini, E.; Galetta, F. A comparative study of the in vitro antioxidant activity of statins. Int. J. Cardiol. 2003, 90, 317–321. [Google Scholar] [CrossRef]
- Dos Anjos, P.M.F.; Volpe, C.M.O.; Miranda, T.C.; Nogueira-Machado, J.A. Atorvastatin Inhibited ROS Generation and Increased IL-1beta And IL-6 Release by Mononuclear Cells from Diabetic Patients. Endocr. Metab. Immune Disord. Drug Targets 2019, 19, 1207–1215. [Google Scholar] [CrossRef]
- Wagner, A.H.; Kohler, T.; Ruckschloss, U.; Just, I.; Hecker, M. Improvement of nitric oxide-dependent vasodilatation by HMG-CoA reductase inhibitors through attenuation of endothelial superoxide anion formation. Arter. Thromb. Vasc. Biol. 2000, 20, 61–69. [Google Scholar] [CrossRef]
- Xu, J.Z.; Chai, Y.L.; Zhang, Y.L. Effect of rosuvastatin on high glucose-induced endoplasmic reticulum stress in human umbilical vein endothelial cells. Genet. Mol. Res. 2016, 15. [Google Scholar] [CrossRef]
- Cheng, X.W.; Kuzuya, M.; Sasaki, T.; Inoue, A.; Hu, L.; Song, H.; Huang, Z.; Li, P.; Takeshita, K.; Hirashiki, A.; et al. Inhibition of mineralocorticoid receptor is a renoprotective effect of the 3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitor pitavastatin. J. Hypertens. 2011, 29, 542–552. [Google Scholar] [CrossRef] [PubMed]
- Piconi, L.; Corgnali, M.; Da Ros, R.; Assaloni, R.; Piliego, T.; Ceriello, A. The protective effect of rosuvastatin in human umbilical endothelial cells exposed to constant or intermittent high glucose. J. Diabetes Complicat. 2008, 22, 38–45. [Google Scholar] [CrossRef]
- Bruder-Nascimento, T.; Callera, G.E.; Montezano, A.C.; He, Y.; Antunes, T.T.; Nguyen Dinh Cat, A.; Tostes, R.C.; Touyz, R.M. Vascular injury in diabetic db/db mice is ameliorated by atorvastatin: Role of Rac1/2-sensitive Nox-dependent pathways. Clin. Sci. 2015, 128, 411–423. [Google Scholar] [CrossRef]
- Whaley-Connell, A.; DeMarco, V.G.; Lastra, G.; Manrique, C.; Nistala, R.; Cooper, S.A.; Westerly, B.; Hayden, M.R.; Wiedmeyer, C.; Wei, Y.; et al. Insulin resistance, oxidative stress, and podocyte injury: Role of rosuvastatin modulation of filtration barrier injury. Am. J. Nephrol. 2008, 28, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Chinen, I.; Shimabukuro, M.; Yamakawa, K.; Higa, N.; Matsuzaki, T.; Noguchi, K.; Ueda, S.; Sakanashi, M.; Takasu, N. Vascular lipotoxicity: Endothelial dysfunction via fatty-acid-induced reactive oxygen species overproduction in obese Zucker diabetic fatty rats. Endocrinology 2007, 148, 160–165. [Google Scholar] [CrossRef]
- Chen, Z.Y.; Liu, S.N.; Li, C.N.; Sun, S.J.; Liu, Q.; Lei, L.; Gao, L.H.; Shen, Z.F. Atorvastatin helps preserve pancreatic beta cell function in obese C57BL/6 J mice and the effect is related to increased pancreas proliferation and amelioration of endoplasmic-reticulum stress. Lipids Health Dis. 2014, 13, 98. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.H.; Chen, Y.Q.; Zhao, S.P. Simvastatin inhibits ox-LDL-induced inflammatory adipokines secretion via amelioration of ER stress in 3T3-L1 adipocyte. Biochem. Biophys. Res. Commun. 2013, 432, 365–369. [Google Scholar] [CrossRef] [PubMed]
- Barbalata, C.I.; Tefas, L.R.; Achim, M.; Tomuta, I.; Porfire, A.S. Statins in risk-reduction and treatment of cancer. World J. Clin. Oncol. 2020, 11, 573–588. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Peng, M.; Liu, Y.; Zhang, B.; Yi, L.; Long, Y. Simvastatin induces pyroptosis via ROS/caspase-1/GSDMD pathway in colon cancer. Cell Commun. Signal. 2023, 21, 329. [Google Scholar] [CrossRef]
- Bai, F.; Yu, Z.; Gao, X.; Gong, J.; Fan, L.; Liu, F. Simvastatin induces breast cancer cell death through oxidative stress up-regulating miR-140-5p. Aging 2019, 11, 3198–3219. [Google Scholar] [CrossRef]
- Thorand, B.; Lowel, H.; Schneider, A.; Kolb, H.; Meisinger, C.; Frohlich, M.; Koenig, W. C-reactive protein as a predictor for incident diabetes mellitus among middle-aged men: Results from the MONICA Augsburg cohort study, 1984–1998. Arch. Intern. Med. 2003, 163, 93–99. [Google Scholar] [CrossRef]
- Sproston, N.R.; Ashworth, J.J. Role of C-Reactive Protein at Sites of Inflammation and Infection. Front. Immunol. 2018, 9, 754. [Google Scholar] [CrossRef]
- Pickup, J.C. Inflammation and activated innate immunity in the pathogenesis of type 2 diabetes. Diabetes Care 2004, 27, 813–823. [Google Scholar] [CrossRef] [PubMed]
- Speelman, T.; Dale, L.; Louw, A.; Verhoog, N.J.D. The Association of Acute Phase Proteins in Stress and Inflammation-Induced T2D. Cells 2022, 11, 2163. [Google Scholar] [CrossRef] [PubMed]
- Stanimirovic, J.; Radovanovic, J.; Banjac, K.; Obradovic, M.; Essack, M.; Zafirovic, S.; Gluvic, Z.; Gojobori, T.; Isenovic, E.R. Role of C-Reactive Protein in Diabetic Inflammation. Mediat. Inflamm. 2022, 2022, 3706508. [Google Scholar] [CrossRef] [PubMed]
- Sipe, J.D. Acute-phase proteins in osteoarthritis. Semin. Arthritis Rheum. 1995, 25, 75–86. [Google Scholar] [CrossRef]
- Bu, F.; Cao, S.; Deng, X.; Zhang, Z.; Feng, X. Evaluation of C-reactive protein and fibrinogen in comparison to CEA and CA72-4 as diagnostic biomarkers for colorectal cancer. Heliyon 2023, 9, e16092. [Google Scholar] [CrossRef]
- Pierce, B.L.; Ballard-Barbash, R.; Bernstein, L.; Baumgartner, R.N.; Neuhouser, M.L.; Wener, M.H.; Baumgartner, K.B.; Gilliland, F.D.; Sorensen, B.E.; McTiernan, A.; et al. Elevated biomarkers of inflammation are associated with reduced survival among breast cancer patients. J. Clin. Oncol. 2009, 27, 3437–3444. [Google Scholar] [CrossRef]
- O’Hanlon, D.M.; Lynch, J.; Cormican, M.; Given, H.F. The acute phase response in breast carcinoma. Anticancer. Res. 2002, 22, 1289–1293. [Google Scholar]
- Yang, M.; Liu, F.; Higuchi, K.; Sawashita, J.; Fu, X.; Zhang, L.; Zhang, L.; Fu, L.; Tong, Z.; Higuchi, K. Serum amyloid A expression in the breast cancer tissue is associated with poor prognosis. Oncotarget 2016, 7, 35843–35852. [Google Scholar] [CrossRef]
- Sung, H.J.; Ahn, J.M.; Yoon, Y.H.; Rhim, T.Y.; Park, C.S.; Park, J.Y.; Lee, S.Y.; Kim, J.W.; Cho, J.Y. Identification and validation of SAA as a potential lung cancer biomarker and its involvement in metastatic pathogenesis of lung cancer. J. Proteome Res. 2011, 10, 1383–1395. [Google Scholar] [CrossRef] [PubMed]
- Albert, M.A.; Danielson, E.; Rifai, N.; Ridker, P.M.; PRINCE Investigators. Effect of statin therapy on C-reactive protein levels: The pravastatin inflammation/CRP evaluation (PRINCE): A randomized trial and cohort study. JAMA 2001, 286, 64–70. [Google Scholar] [CrossRef]
- Mashaba, G.R.; Phoswa, W.N.; Mokgalaboni, K. The Effect of Statins on Carotid Intima-Media Thickness and C-Reactive Protein in Type 2 Diabetes Mellitus: A Meta-Analysis. J. Cardiovasc. Dev. Dis. 2024, 11, 276. [Google Scholar] [CrossRef]
- Kandelouei, T.; Abbasifard, M.; Imani, D.; Aslani, S.; Razi, B.; Fasihi, M.; Shafiekhani, S.; Mohammadi, K.; Jamialahmadi, T.; Reiner, Z.; et al. Effect of Statins on Serum level of hs-CRP and CRP in Patients with Cardiovascular Diseases: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Mediat. Inflamm. 2022, 2022, 8732360. [Google Scholar] [CrossRef]
- Liu, C.; Chen, H.; Hu, B.; Shi, J.; Chen, Y.; Huang, K. New insights into the therapeutic potentials of statins in cancer. Front. Pharmacol. 2023, 14, 1188926. [Google Scholar] [CrossRef]
- Ahmadi, M.; Amiri, S.; Pecic, S.; Machaj, F.; Rosik, J.; Los, M.J.; Alizadeh, J.; Mahdian, R.; da Silva Rosa, S.C.; Schaafsma, D.; et al. Pleiotropic effects of statins: A focus on cancer. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165968. [Google Scholar] [CrossRef] [PubMed]
- Setiawan, B.; Budianto, W.; Sukarnowati, T.W.; Rizky, D.; Pangarsa, E.A.; Santosa, D.; Sudoyo, A.W.; Winarni, T.I.; Riwanto, I.; Setiabudy, R.D.; et al. The efficacy of atorvastatin on inflammation and coagulation markers in high-risk thrombotic cancer patients undergoing chemotherapy: A randomized controlled trial. Thromb. J. 2025, 23, 27. [Google Scholar] [CrossRef] [PubMed]
- Manthravadi, S.; Shrestha, A.; Madhusudhana, S. Impact of statin use on cancer recurrence and mortality in breast cancer: A systematic review and meta-analysis. Int. J. Cancer 2016, 139, 1281–1288. [Google Scholar] [CrossRef]
- Borgquist, S.; Bjarnadottir, O.; Kimbung, S.; Ahern, T.P. Statins: A role in breast cancer therapy? J. Intern. Med. 2018, 284, 346–357. [Google Scholar] [CrossRef]
- Alipour Talesh, G.; Trezeguet, V.; Merched, A. Hepatocellular Carcinoma and Statins. Biochemistry 2020, 59, 3393–3400. [Google Scholar] [CrossRef]
- Soulaidopoulos, S.; Nikiphorou, E.; Dimitroulas, T.; Kitas, G.D. The Role of Statins in Disease Modification and Cardiovascular Risk in Rheumatoid Arthritis. Front. Med. 2018, 5, 24. [Google Scholar] [CrossRef]
- De Vera, M.A.; Choi, H.; Abrahamowicz, M.; Kopec, J.; Lacaille, D. Impact of statin discontinuation on mortality in patients with rheumatoid arthritis: A population-based study. Arthritis Care Res. 2012, 64, 809–816. [Google Scholar] [CrossRef]
- Li, G.M.; Zhao, J.; Li, B.; Zhang, X.F.; Ma, J.X.; Ma, X.L.; Liu, J. The anti-inflammatory effects of statins on patients with rheumatoid arthritis: A systemic review and meta-analysis of 15 randomized controlled trials. Autoimmun. Rev. 2018, 17, 215–225. [Google Scholar] [CrossRef] [PubMed]
- McCarey, D.W.; McInnes, I.B.; Madhok, R.; Hampson, R.; Scherbakov, O.; Ford, I.; Capell, H.A.; Sattar, N. Trial of Atorvastatin in Rheumatoid Arthritis (TARA): Double-blind, randomised placebo-controlled trial. Lancet 2004, 363, 2015–2021. [Google Scholar] [CrossRef]
- Palmer, G.; Chobaz, V.; Talabot-Ayer, D.; Taylor, S.; So, A.; Gabay, C.; Busso, N. Assessment of the efficacy of different statins in murine collagen-induced arthritis. Arthritis Rheum. 2004, 50, 4051–4059. [Google Scholar] [CrossRef]
- Aranow, C.; Cush, J.; Bolster, M.B.; Striebich, C.C.; Dall’era, M.; Mackay, M.; Olech, E.; Frech, T.; Box, J.; Keating, R.; et al. A double-blind, placebo-controlled, phase II, randomized study of lovastatin therapy in the treatment of mildly active rheumatoid arthritis. Rheumatology 2020, 59, 1505–1513. [Google Scholar] [CrossRef] [PubMed]
- Takemoto, M.; Liao, J.K. Pleiotropic effects of 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibitors. Arter. Thromb. Vasc. Biol. 2001, 21, 1712–1719. [Google Scholar] [CrossRef]
- de Oliveira, P.S.S.; da Paixao, A.B.F.; da Rocha Junior, L.F.; Branco Pinto Duarte, A.L.; Pereira, M.C.; Barreto de Melo Rego, M.J.; da Rocha Pitta, I.; da Rocha Pitta, M.G. Atorvastatin inhibits IL-17A, TNF, IL-6, and IL-10 in PBMC cultures from patients with severe rheumatoid arthritis. Immunobiology 2020, 225, 151908. [Google Scholar] [CrossRef] [PubMed]
- Tang, T.T.; Song, Y.; Ding, Y.J.; Liao, Y.H.; Yu, X.; Du, R.; Xiao, H.; Yuan, J.; Zhou, Z.H.; Liao, M.Y.; et al. Atorvastatin upregulates regulatory T cells and reduces clinical disease activity in patients with rheumatoid arthritis. J. Lipid Res. 2011, 52, 1023–1032. [Google Scholar] [CrossRef] [PubMed]
- Maki-Petaja, K.M.; Booth, A.D.; Hall, F.C.; Wallace, S.M.; Brown, J.; McEniery, C.M.; Wilkinson, I.B. Ezetimibe and simvastatin reduce inflammation, disease activity, and aortic stiffness and improve endothelial function in rheumatoid arthritis. J. Am. Coll. Cardiol. 2007, 50, 852–858. [Google Scholar] [CrossRef]
- Xing, B.; Yin, Y.F.; Zhao, L.D.; Wang, L.; Zheng, W.J.; Chen, H.; Wu, Q.J.; Tang, F.L.; Zhang, F.C.; Shan, G.; et al. Effect of 3-hydroxy-3-methylglutaryl-coenzyme a reductase inhibitor on disease activity in patients with rheumatoid arthritis: A meta-analysis. Medicine 2015, 94, e572. [Google Scholar] [CrossRef]
- Lodi, S.; Evans, S.J.; Egger, P.; Carpenter, J. Is there an anti-inflammatory effect of statins in rheumatoid arthritis? Analysis of a large routinely collected claims database. Br. J. Clin. Pharmacol. 2010, 69, 85–94. [Google Scholar] [CrossRef][Green Version]
- Zhu, X.; Parks, J.S. New roles of HDL in inflammation and hematopoiesis. Annu. Rev. Nutr. 2012, 32, 161–182. [Google Scholar] [CrossRef]
- He, Y.; Wang, S.; Li, Y.; Deng, J.; Huang, L. Effects of atorvastatin in suppressing pulmonary vascular remodeling in rats with chronic obstructive pulmonary disease. Clinics 2023, 78, 100252. [Google Scholar] [CrossRef]
- Yildiz, T.; Tasdemir, M.S.; Tunik, S.; Ates, G.; Tekes, S.; Kaplanoglu, I.; Topcu, F.; Akkus, M. Effects of atorvastatin on smoking-induced alveolar injury in rat lungs. Curr. Ther. Res. Clin. Exp. 2009, 70, 366–376. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mroz, R.M.; Lisowski, P.; Tycinska, A.; Bierla, J.; Trzeciak, P.Z.; Minarowski, L.; Milewski, R.; Lisowska, A.; Boros, P.; Sobkowicz, B.; et al. Anti-inflammatory effects of atorvastatin treatment in chronic obstructive pulmonary disease. A controlled pilot study. J. Physiol. Pharmacol. 2015, 66, 111–128. [Google Scholar][Green Version]
- Wang, M.T.; Lo, Y.W.; Tsai, C.L.; Chang, L.C.; Malone, D.C.; Chu, C.L.; Liou, J.T. Statin use and risk of COPD exacerbation requiring hospitalization. Am. J. Med. 2013, 126, 598–606.e2. [Google Scholar] [CrossRef] [PubMed]
- Lahousse, L.; Loth, D.W.; Joos, G.F.; Hofman, A.; Leufkens, H.G.; Brusselle, G.G.; Stricker, B.H. Statins, systemic inflammation and risk of death in COPD: The Rotterdam study. Pulm. Pharmacol. Ther. 2013, 26, 212–217. [Google Scholar] [CrossRef]
- Cao, C.; Wu, Y.; Xu, Z.; Lv, D.; Zhang, C.; Lai, T.; Li, W.; Shen, H. The effect of statins on chronic obstructive pulmonary disease exacerbation and mortality: A systematic review and meta-analysis of observational research. Sci. Rep. 2015, 5, 16461. [Google Scholar] [CrossRef] [PubMed]
- Williams, E.J.; Baines, K.J.; Smart, J.M.; Gibson, P.G.; Wood, L.G. Rosuvastatin, lycopene and omega-3 fatty acids: A potential treatment for systemic inflammation in COPD; a pilot study. J. Nutr. Intermed. Metab. 2016, 5, 86–95. [Google Scholar] [CrossRef]
- Apostolopoulou, M.; Corsini, A.; Roden, M. The role of mitochondria in statin-induced myopathy. Eur. J. Clin. Investig. 2015, 45, 745–754. [Google Scholar] [CrossRef]
- Bouitbir, J.; Sanvee, G.M.; Panajatovic, M.V.; Singh, F.; Krahenbuhl, S. Mechanisms of statin-associated skeletal muscle-associated symptoms. Pharmacol. Res. 2020, 154, 104201. [Google Scholar] [CrossRef]
- Bell, G.; Thoma, A.; Hargreaves, I.P.; Lightfoot, A.P. The Role of Mitochondria in Statin-Induced Myopathy. Drug Saf. 2024, 47, 643–653. [Google Scholar] [CrossRef]
- Abd, T.T.; Jacobson, T.A. Statin-induced myopathy: A review and update. Expert Opin. Drug Saf. 2011, 10, 373–387. [Google Scholar] [CrossRef]
- Casula, M.; Gazzotti, M.; Bonaiti, F.; OImastroni, E.; Arca, M.; Averna, M.; Zambon, A.; Catapano, A.L.; PROSISA Study Group. Reported muscle symptoms during statin treatment amongst Italian dyslipidaemic patients in the real-life setting: The PROSISA Study. J. Intern. Med. 2021, 290, 116–128. [Google Scholar] [CrossRef] [PubMed]
- Stroes, E.S.; Thompson, P.D.; Corsini, A.; Vladutiu, G.D.; Raal, F.J.; Ray, K.K.; Roden, M.; Stein, E.; Tokgozoglu, L.; Nordestgaard, B.G.; et al. Statin-associated muscle symptoms: Impact on statin therapy-European Atherosclerosis Society Consensus Panel Statement on Assessment, Aetiology and Management. Eur. Heart J. 2015, 36, 1012–1022. [Google Scholar] [CrossRef]
- Sattar, N. Statins and diabetes: What are the connections? Best Pract. Res. Clin. Endocrinol. Metab. 2023, 37, 101749. [Google Scholar] [CrossRef] [PubMed]
- Criner, G.J.; Connett, J.E.; Aaron, S.D.; Albert, R.K.; Bailey, W.C.; Casaburi, R.; Cooper, J.A., Jr.; Curtis, J.L.; Dransfield, M.T.; Han, M.K.; et al. Simvastatin for the prevention of exacerbations in moderate-to-severe COPD. N. Engl. J. Med. 2014, 370, 2201–2210. [Google Scholar] [CrossRef]
- Thomson, N.C. Clinical Studies of Statins in Asthma and COPD. Curr. Mol. Pharmacol. 2017, 10, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Limburg, P.J.; Mahoney, M.R.; Ziegler, K.L.; Sontag, S.J.; Schoen, R.E.; Benya, R.; Lawson, M.J.; Weinberg, D.S.; Stoffel, E.; Chiorean, M.; et al. Randomized phase II trial of sulindac, atorvastatin, and prebiotic dietary fiber for colorectal cancer chemoprevention. Cancer Prev. Res. 2011, 4, 259–269. [Google Scholar] [CrossRef]
- Momeni, B.; Nazer, S.; Masoompour, S.M.; Geramizadeh, B.; Sajadi, S.V. The effect of atorvastatin on inflammatory markers in sulfur mustard gas induced bronchitis: A randomized double-blinded, placebo-control clinical trial. BMC Pulm. Med. 2021, 21, 112. [Google Scholar] [CrossRef]
- Boiati, R.F.; Manchini, M.T.; Jacobsen, O.; dos Santos Batista, J.G.; Silva Junior, J.A.; Nascimento, J.W. Evaluation of the Anti-inflammatory Activity of Atorvastatin and its Effect on Alveolar Diameter in a Model of Elastase-induced Emphysema in Rats. Drug Res. 2015, 65, 540–544. [Google Scholar] [CrossRef]
- Wang, J.; Dong, J.; Yang, J.; Wang, Y.; Liu, J. Association between statin use and incidence or progression of osteoarthritis: Meta-analysis of observational studies. Osteoarthr. Cartil. 2020, 28, 1170–1179. [Google Scholar] [CrossRef]
- Baek, W.Y.; Lee, S.M.; Lee, S.W.; Suh, C.H. Rosuvastatin treatment alone cannot alleviate lupus in murine model: A pilot study. J. Rheum. Dis. 2023, 30, 198–203. [Google Scholar] [CrossRef]
- Lai, S.W.; Lin, C.L.; Liao, K.F. Rosuvastatin and risk of acute pancreatitis in a population-based case-control study. Int. J. Cardiol. 2015, 187, 417–420. [Google Scholar] [CrossRef]
- Berk, M.; Mohebbi, M.; Dean, O.M.; Cotton, S.M.; Chanen, A.M.; Dodd, S.; Ratheesh, A.; Amminger, G.P.; Phelan, M.; Weller, A.; et al. Youth Depression Alleviation with Anti-inflammatory Agents (YoDA-A): A randomised clinical trial of rosuvastatin and aspirin. BMC Med. 2020, 18, 16. [Google Scholar] [CrossRef]
- Lee, E.B.; Daskalakis, N.; Xu, C.; Paccaly, A.; Miller, B.; Fleischmann, R.; Bodrug, I.; Kivitz, A. Disease-Drug Interaction of Sarilumab and Simvastatin in Patients with Rheumatoid Arthritis. Clin. Pharmacokinet. 2017, 56, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Al Salman, M.; Ghiasi, M.; Farid, A.S.; Taraz, M.; Azizpour, A.; Mahmoudi, H. Oral simvastatin combined with narrowband UVB for the treatment of psoriasis: A randomized controlled trial. Dermatol. Ther. 2021, 34, e15075. [Google Scholar] [CrossRef] [PubMed]
- Guan, F.; Liu, A.B.; Li, G.; Yang, Z.; Sun, Y.; Yang, C.S.; Ju, J. Deleterious effects of high concentrations of (-)-epigallocatechin-3-gallate and atorvastatin in mice with colon inflammation. Nutr. Cancer 2012, 64, 847–855. [Google Scholar] [CrossRef] [PubMed]
- Arian, A.; Mortazavi Moghadam, S.G.; Kazemi, T.; Zardast, M.; Zarban, A. Trial of Atorvastatin on Serum Interleukin-6, Total Antioxidant Capacity, C-Reactive Protein, and Alpha-1 Antitrypsin in Patients with Chronic Obstructive Pulmonary Disease. J. Res. Pharm. Pract. 2018, 7, 141–146. [Google Scholar] [PubMed]
- Cholesterol Treatment Trialists’ (CTT) Collaboration; Emberson, J.R.; Kearney, P.M.; Blackwell, L.; Newman, C.; Reith, C.; Bhala, N.; Holland, L.; Peto, R.; Keech, A.; et al. Lack of effect of lowering LDL cholesterol on cancer: Meta-analysis of individual data from 175,000 people in 27 randomised trials of statin therapy. PLoS ONE 2012, 7, e29849. [Google Scholar]
- Hoffmeister, M.; Jansen, L.; Rudolph, A.; Toth, C.; Kloor, M.; Roth, W.; Blaker, H.; Chang-Claude, J.; Brenner, H. Statin use and survival after colorectal cancer: The importance of comprehensive confounder adjustment. J. Natl. Cancer Inst. 2015, 107, djv045. [Google Scholar] [CrossRef]
- Lochhead, P.; Chan, A.T. Statins and colorectal cancer. Clin. Gastroenterol. Hepatol. 2013, 11, 109–118. [Google Scholar] [CrossRef]
- Walsh, A.; Perrem, L.; Khashan, A.S.; Henry, M.T.; Ni Chroinin, M. Statins versus placebo for people with chronic obstructive pulmonary disease. Cochrane Database Syst. Rev. 2019, 7, CD011959. [Google Scholar] [CrossRef]
- Ren, C.; Li, M. The efficacy of statins in the treatment of rheumatoid arthritis: A systematic review and meta-analysis. Medicine 2023, 102, e35088. [Google Scholar] [CrossRef]
- Naderi, S.H.; Bestwick, J.P.; Wald, D.S. Adherence to drugs that prevent cardiovascular disease: Meta-analysis on 376,162 patients. Am. J. Med. 2012, 125, 882–887.e1. [Google Scholar] [CrossRef]
- Toth, P.P.; Granowitz, C.; Hull, M.; Anderson, A.; Philip, S. Long-term statin persistence is poor among high-risk patients with dyslipidemia: A real-world administrative claims analysis. Lipids Health Dis. 2019, 18, 175. [Google Scholar] [CrossRef]
- Grundy, S.M.; Vega, G.L. Statin Intolerance and Noncompliance: An Empiric Approach. Am. J. Med. 2022, 135, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, Y.; Fard, J.K.; Ghafoor, D.; Eid, A.H.; Sahebkar, A. Paradoxical effects of statins on endothelial and cancer cells: The impact of concentrations. Cancer Cell Int. 2023, 23, 43. [Google Scholar] [CrossRef]
- Climent, E.; Benaiges, D.; Pedro-Botet, J. Hydrophilic or Lipophilic Statins? Front. Cardiovasc. Med. 2021, 8, 687585. [Google Scholar] [CrossRef]
- Mueller, A.M.; Liakoni, E.; Schneider, C.; Burkard, T.; Jick, S.S.; Krahenbuhl, S.; Meier, C.R.; Spoendlin, J. The Risk of Muscular Events Among New Users of Hydrophilic and Lipophilic Statins: An Observational Cohort Study. J. Gen. Intern. Med. 2021, 36, 2639–2647. [Google Scholar] [CrossRef]
- Irwin, J.C.; Khalesi, S.; Fenning, A.S.; Vella, R.K. The effect of lipophilicity and dose on the frequency of statin-associated muscle symptoms: A systematic review and meta-analysis. Pharmacol. Res. 2018, 128, 264–273. [Google Scholar] [CrossRef]
- Hou, Q.; Chen, Y.; Zhang, Y.; Pang, C. Comparative Muscle Tolerability of Different Types and Intensities of Statins: A Network Meta-Analysis of Double-Blind Randomized Controlled Trials. Cardiovasc. Drugs Ther. 2024, 38, 459–469. [Google Scholar] [CrossRef]
- Rea, F.; Biffi, A.; Ronco, R.; Franchi, M.; Cammarota, S.; Citarella, A.; Conti, V.; Filippelli, A.; Sellitto, C.; Corrao, G. Cardiovascular Outcomes and Mortality Associated With Discontinuing Statins in Older Patients Receiving Polypharmacy. JAMA Netw. Open 2021, 4, e2113186. [Google Scholar] [CrossRef] [PubMed]
- Khalaf, K.; Johnell, K.; Austin, P.C.; Tyden, P.; Midlov, P.; Perez-Vicente, R.; Merlo, J. Low adherence to statin treatment during the 1st year after an acute myocardial infarction is associated with increased 2nd-year mortality risk-an inverse probability of treatment weighted study on 54 872 patients. Eur. Heart J. Cardiovasc. Pharmacother. 2021, 7, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Nemes, K.; Aberg, F.; Gylling, H.; Isoniemi, H. Cholesterol metabolism in cholestatic liver disease and liver transplantation: From molecular mechanisms to clinical implications. World J. Hepatol. 2016, 8, 924–932. [Google Scholar] [CrossRef] [PubMed]
- Yorke, E. Co-Morbid Hypothyroidism and Liver Dysfunction: A Review. Clin. Med. Insights Endocrinol. Diabetes 2024, 17, 11795514241231533. [Google Scholar] [CrossRef]
- Duntas, L.H.; Brenta, G. A Renewed Focus on the Association Between Thyroid Hormones and Lipid Metabolism. Front. Endocrinol. 2018, 9, 511. [Google Scholar] [CrossRef]
- Scappaticcio, L.; Longo, M.; Maiorino, M.I.; Pernice, V.; Caruso, P.; Esposito, K.; Bellastella, G. Abnormal Liver Blood Tests in Patients with Hyperthyroidism: Systematic Review and Meta-Analysis. Thyroid 2021, 31, 884–894. [Google Scholar] [CrossRef] [PubMed]
- Bhuyan, A.K.; Sarma, D.; Kaimal Saikia, U.; Choudhury, B.K. Grave’s Disease with Severe Hepatic Dysfunction: A Diagnostic and Therapeutic Challenge. Case Rep. Med. 2014, 2014, 790458. [Google Scholar] [CrossRef]
- Yorke, E. Hyperthyroidism and Liver Dysfunction: A Review of a Common Comorbidity. Clin. Med. Insights Endocrinol. Diabetes 2022, 15, 11795514221074672. [Google Scholar] [CrossRef]
- Mansourian, A.R. Liver Functional Behavior During Thyrotoxicosis: A Review. J. Biol. Sci. 2013, 13, 665–678. [Google Scholar] [CrossRef]
- López-Castro, J.; Cid-Conde, L. Pharmacokinetic Aspects of Statins. In Cardiovascular Risk Factors in Pathology; IntechOpen: London, UK, 2020. [Google Scholar]
- Willrich, M.A.; Hirata, M.H.; Hirata, R.D. Statin regulation of CYP3A4 and CYP3A5 expression. Pharmacogenomics 2009, 10, 1017–1024. [Google Scholar] [CrossRef]
- Corticosteroids. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury; National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2012. [Google Scholar]
- Oluleye, O.W.; Kronmal, R.A.; Folsom, A.R.; Vaidya, D.M.; Ouyang, P.; Duprez, D.A.; Dobs, A.S.; Yarmohammadi, H.; Konety, S.H. Association Between Statin Use and Sex Hormone in the Multi-Ethnic Study of Atherosclerosis Cohort. J. Clin. Endocrinol. Metab. 2019, 104, 4600–4606. [Google Scholar] [CrossRef] [PubMed]
- Chihaoui, M.; Terzi, A.; Hammami, B.; Oueslati, I.; Khessairi, N.; Chaker, F.; Yazidi, M.; Feki, M. Effects of high-intensity statin therapy on steroid hormones and vitamin D in type 2 diabetic men: A prospective self-controlled study. Lipids 2024, 59, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Glina, F.P.A.; Lopes, L.; E Silva, R.S.; Barros, E.A.C.; Biselli, B.; Glina, S. Do statins decrease testosterone in men? Systematic review and meta-analysis. Int. Braz. J. Urol. 2024, 50, 119–135. [Google Scholar] [CrossRef]
- Trias, F.; Pinto, X.; Corbella, E.; Suarez-Tembra, M.; Ruiz-Garcia, A.; Diaz-Diaz, J.L.; Sanchez-Ruiz-Granado, E.; Sarasa, I.; Martinez-Porqueras, R.; Rodriguez-Sanchez, M.A.; et al. Differences in the diabetogenic effect of statins in patients with prediabetes. The PRELIPID study. Med. Clin. 2022, 158, 531–539. [Google Scholar] [CrossRef]
- Chacko, S.M.; Thekkekara, P.T. Combined Effect of Metformin and Statin. In Metformin—Pharmacology and Drug Interactions; IntechOpen: London, UK, 2021. [Google Scholar]
- Sun, J.; Yuan, Y.; Cai, R.; Sun, H.; Zhou, Y.; Wang, P.; Huang, R.; Xia, W.; Wang, S. An investigation into the therapeutic effects of statins with metformin on polycystic ovary syndrome: A meta-analysis of randomised controlled trials. BMJ Open 2015, 5, e007280. [Google Scholar] [CrossRef]
- Purwar, A.; Nagpure, S. Insulin Resistance in Polycystic Ovarian Syndrome. Cureus 2022, 14, e30351. [Google Scholar] [CrossRef]
- Mu, L.; Li, R.; Lai, Y.; Zhao, Y.; Qiao, J. Adipose insulin resistance is associated with cardiovascular risk factors in polycystic ovary syndrome. J. Endocrinol. Investig. 2019, 42, 541–548. [Google Scholar] [CrossRef]
- Ochoa-Rosales, C.; Portilla-Fernandez, E.; Nano, J.; Wilson, R.; Lehne, B.; Mishra, P.P.; Gao, X.; Ghanbari, M.; Rueda-Ochoa, O.L.; Juvinao-Quintero, D.; et al. Epigenetic Link Between Statin Therapy and Type 2 Diabetes. Diabetes Care 2020, 43, 875–884. [Google Scholar] [CrossRef]
- Cano-Corres, R.; Candas-Estebanez, B.; Padro-Miquel, A.; Fanlo-Maresma, M.; Pinto, X.; Alia-Ramos, P. Influence of 6 genetic variants on the efficacy of statins in patients with dyslipidemia. J. Clin. Lab. Anal. 2018, 32, e22566. [Google Scholar] [CrossRef]
- Niemi, M.; Pasanen, M.K.; Neuvonen, P.J. Organic anion transporting polypeptide 1B1: A genetically polymorphic transporter of major importance for hepatic drug uptake. Pharmacol. Rev. 2011, 63, 157–181. [Google Scholar] [CrossRef] [PubMed]
- Preissner, S.C.; Hoffmann, M.F.; Preissner, R.; Dunkel, M.; Gewiess, A.; Preissner, S. Polymorphic cytochrome P450 enzymes (CYPs) and their role in personalized therapy. PLoS ONE 2013, 8, e82562. [Google Scholar] [CrossRef] [PubMed]
- Mulder, A.B.; van Lijf, H.J.; Bon, M.A.; van den Bergh, F.A.; Touw, D.J.; Neef, C.; Vermes, I. Association of polymorphism in the cytochrome CYP2D6 and the efficacy and tolerability of simvastatin. Clin. Pharmacol. Ther. 2001, 70, 546–551. [Google Scholar] [CrossRef] [PubMed]
- Canestaro, W.J.; Austin, M.A.; Thummel, K.E. Genetic factors affecting statin concentrations and subsequent myopathy: A HuGENet systematic review. Genet. Med. 2014, 16, 810–819. [Google Scholar] [CrossRef]
- Zheng, E.; Madura, P.; Grandos, J.; Broncel, M.; Pawlos, A.; Wozniak, E.; Gorzelak-Pabis, P. When the same treatment has different response: The role of pharmacogenomics in statin therapy. Biomed. Pharmacother. 2024, 170, 115966. [Google Scholar] [CrossRef]
- Erickson, S.R.; Bravo, M.; Tootoo, J. Geosocial Factors Associated With Adherence to Statin Medications. Ann. Pharmacother. 2020, 54, 1194–1202. [Google Scholar] [CrossRef]
- Boruzs, K.; Juhasz, A.; Nagy, C.; Adany, R.; Biro, K. Relationship between Statin Utilization and Socioeconomic Deprivation in Hungary. Front. Pharmacol. 2016, 7, 66. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, R.W.; Johnsen, S.P.; Olesen, A.V.; Mortensen, J.T.; Boggild, H.; Olsen, J.; Sorensen, H.T. Socioeconomic gradient in use of statins among Danish patients: Population-based cross-sectional study. Br. J. Clin. Pharmacol. 2005, 60, 534–542. [Google Scholar] [CrossRef]
- Mohanty, D.; Padhee, S.; Sahoo, C.; Jena, S.; Sahoo, A.; Chandra Panda, P.; Nayak, S.; Ray, A. Integrating network pharmacology and experimental verification to decipher the multitarget pharmacological mechanism of Cinnamomum zeylanicum essential oil in treating inflammation. Heliyon 2024, 10, e24120. [Google Scholar] [CrossRef] [PubMed]
- De Giorgi, M.; Jarrett, K.E.; Burton, J.C.; Doerfler, A.M.; Hurley, A.; Li, A.; Hsu, R.H.; Furgurson, M.; Patel, K.R.; Han, J.; et al. Depletion of essential isoprenoids and ER stress induction following acute liver-specific deletion of HMG-CoA reductase. J. Lipid Res. 2020, 61, 1675–1686. [Google Scholar] [CrossRef] [PubMed]

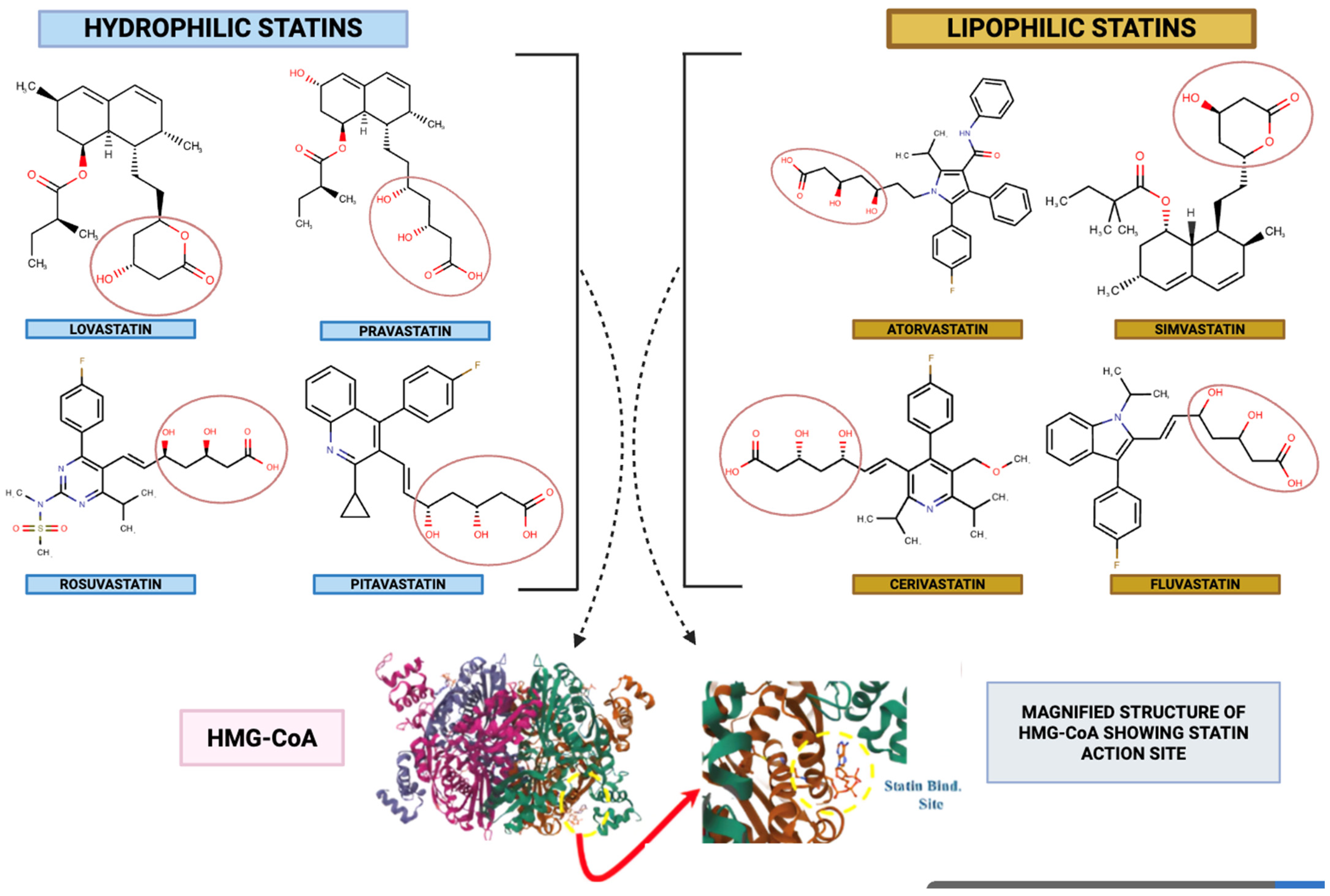
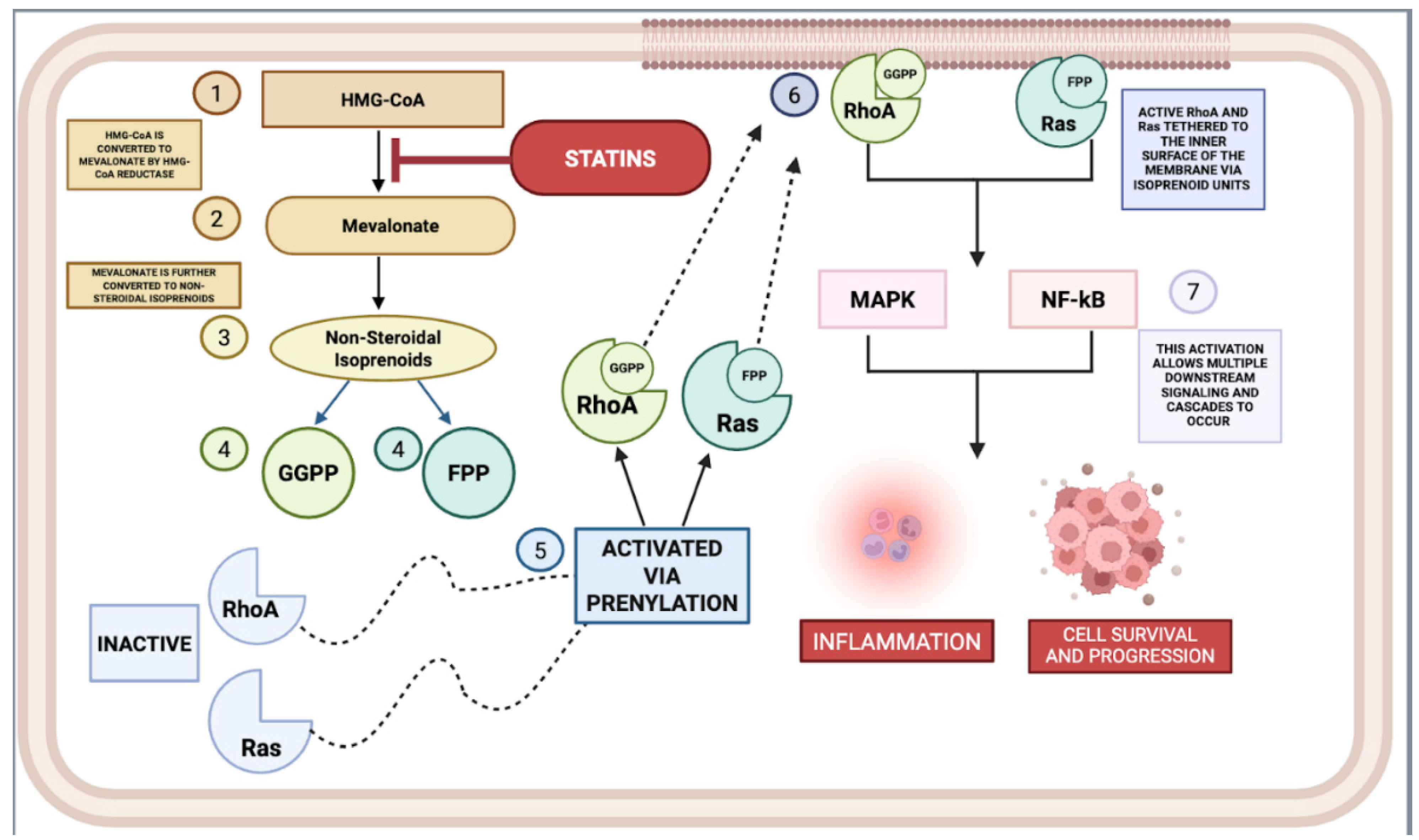
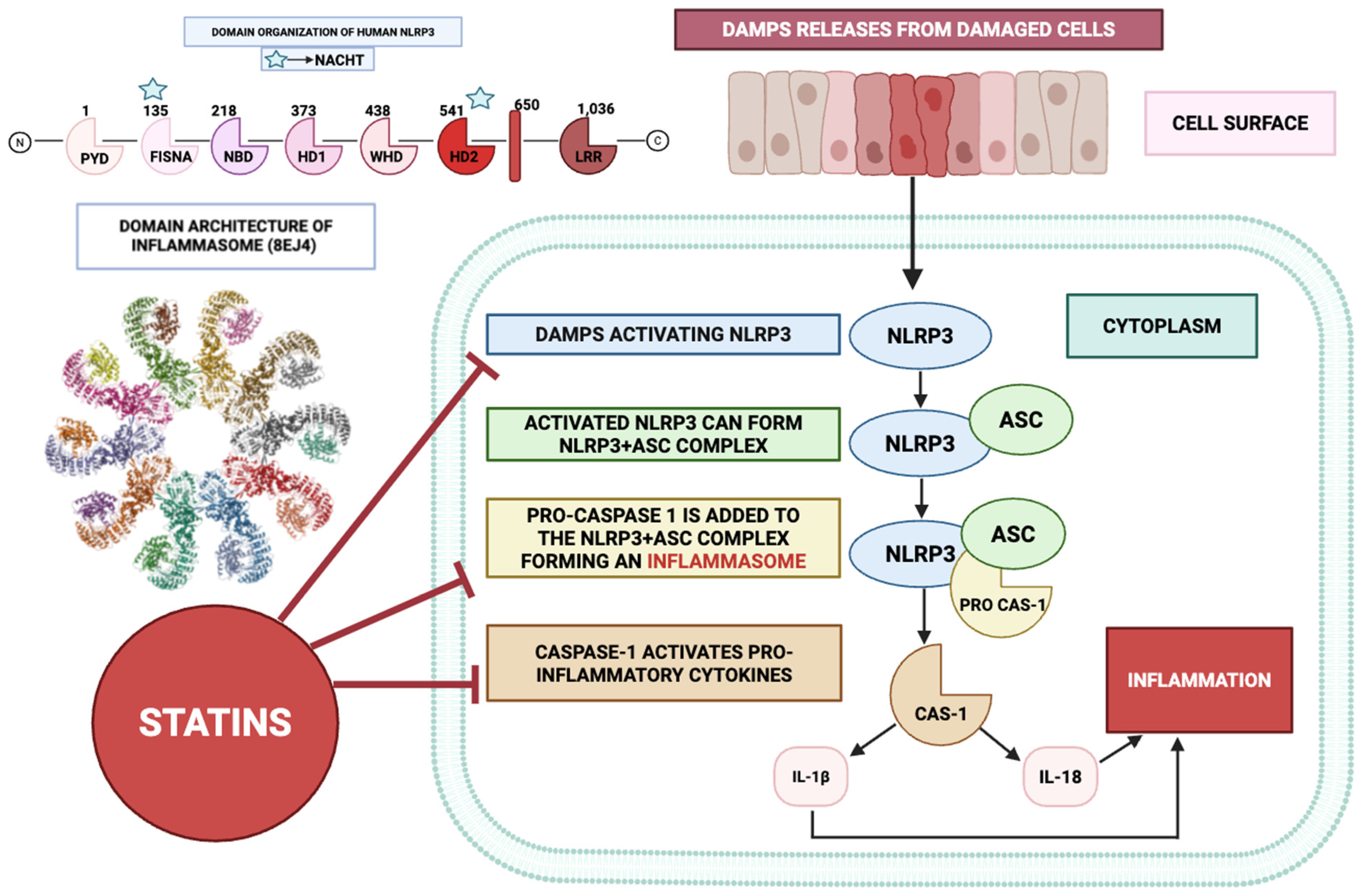


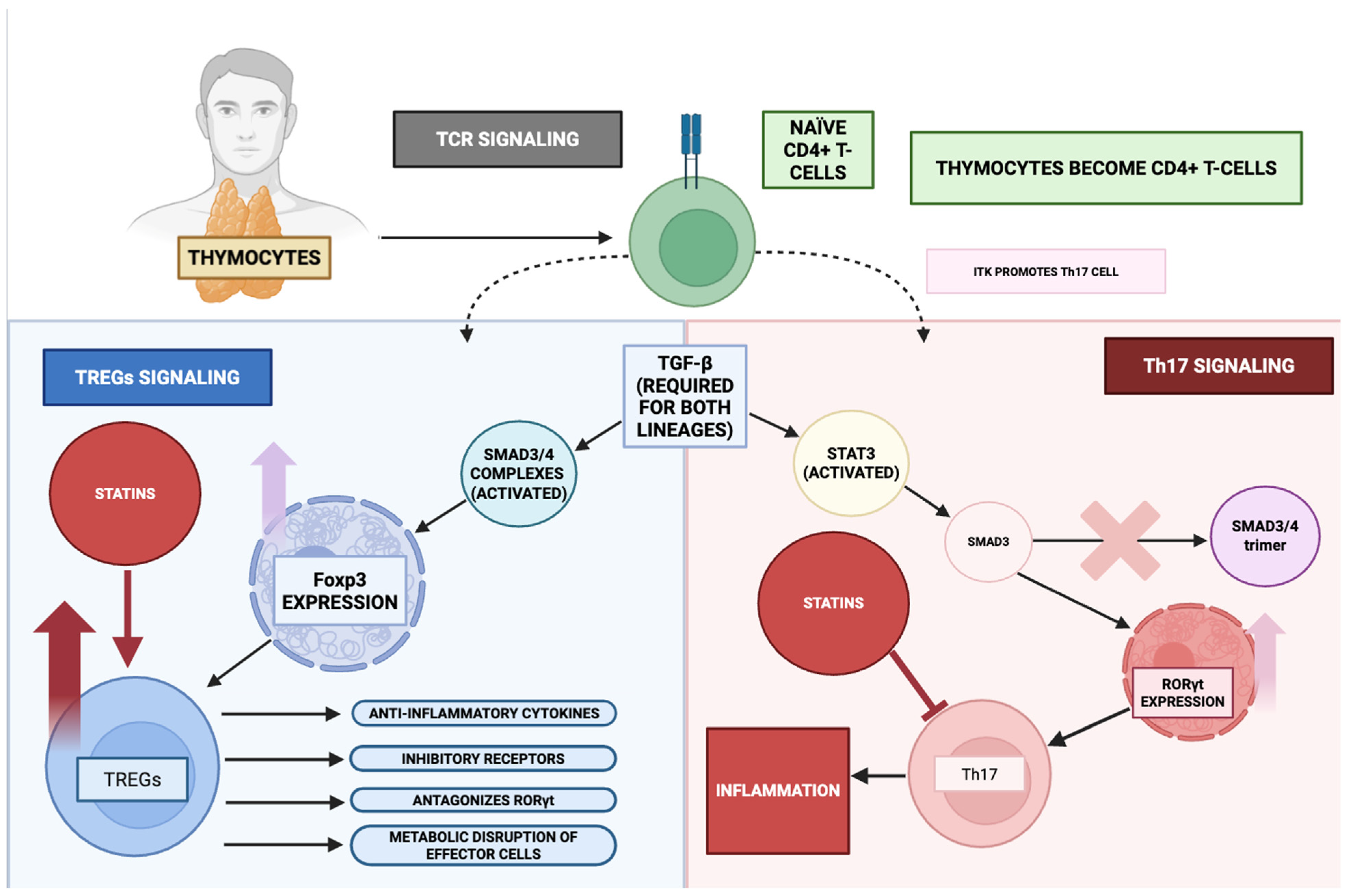
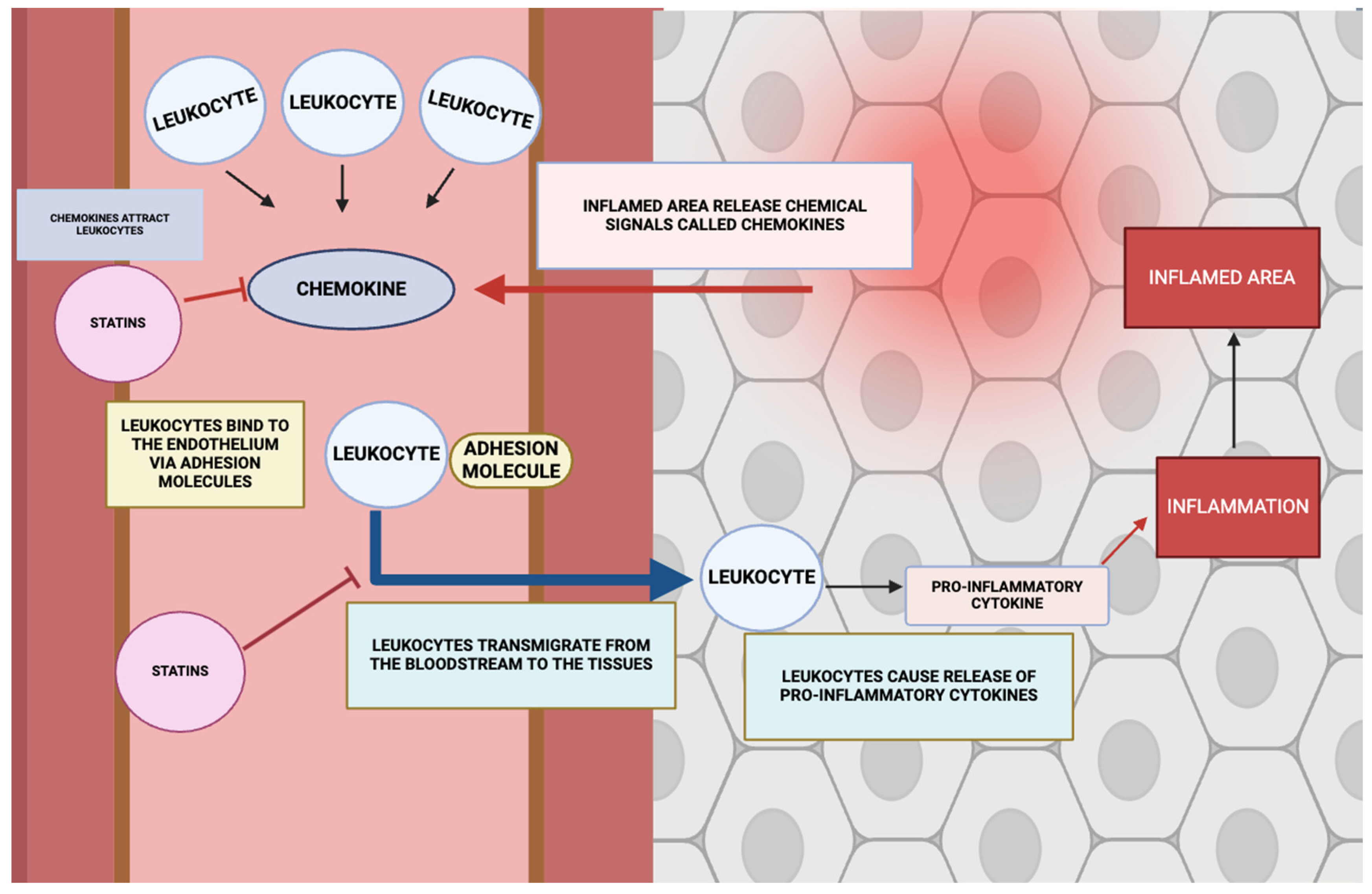
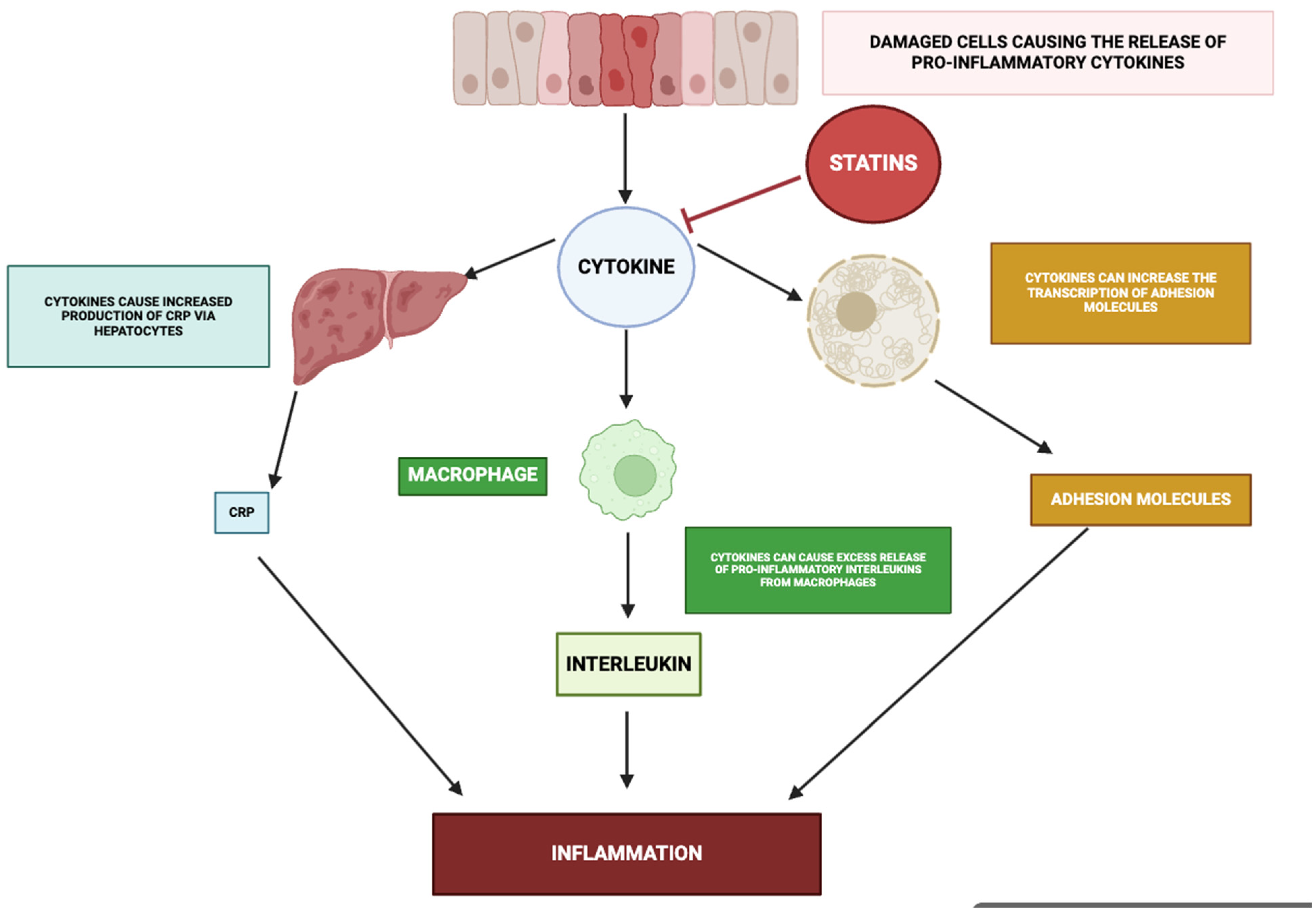
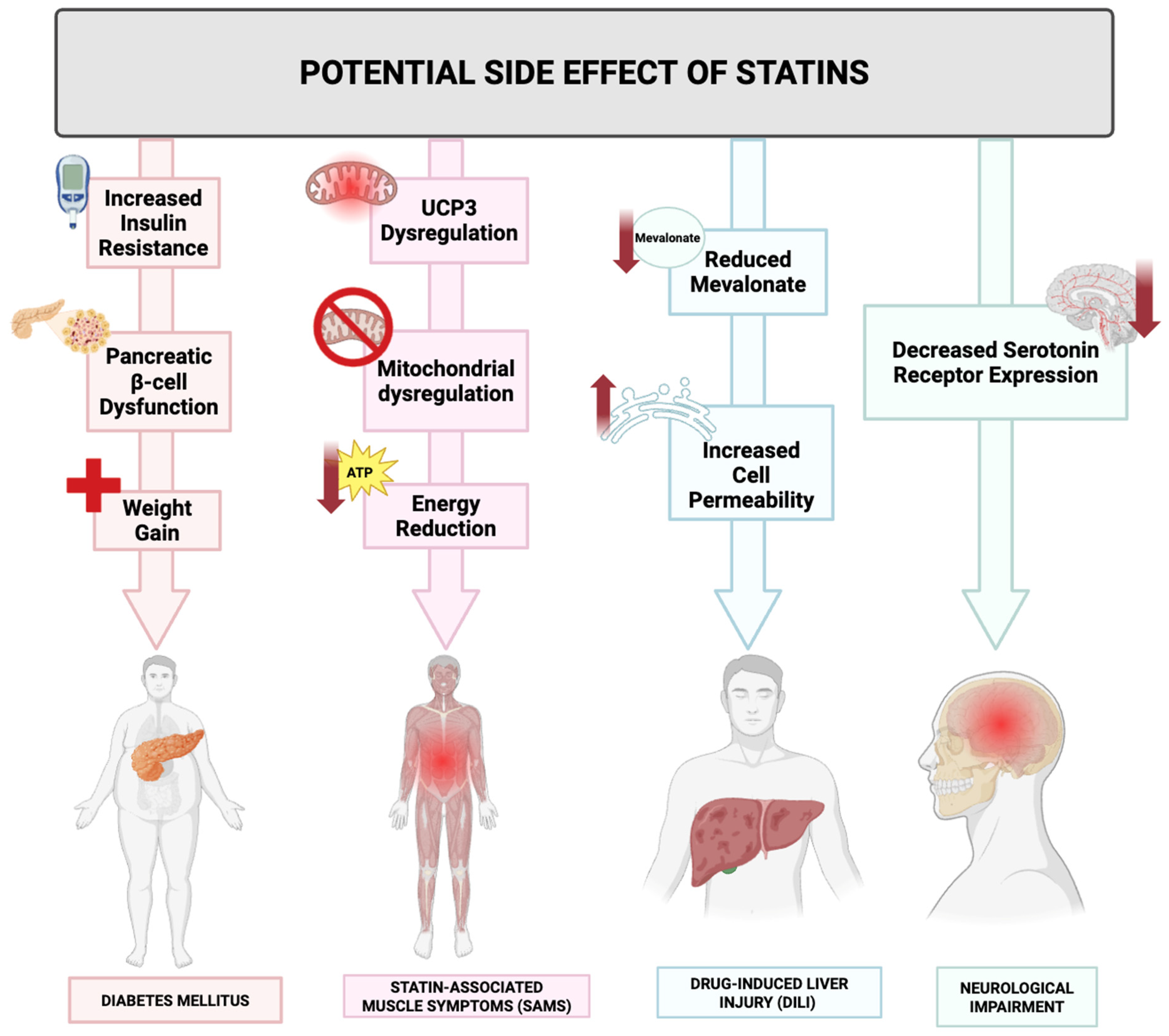
| Statin | Type | Hydrophilic/Lipophilic | Enzyme System for Metabolism | Conditions Used | Representative Trial | Remarks |
|---|---|---|---|---|---|---|
| Atorvastatin | Synthetic | Lipophilic | CYP3A4 | Hyperlipidemia, cardiovascular disease | ASCOT-LLA (Anglo-Scandinavian Cardiac Outcomes Trial—Lipid-Lowering Arm) | Highly potent, commonly used in primary and secondary prevention. |
| Simvastatin | Semi-synthetic | Lipophilic | CYP3A4 | Hyperlipidemia, cardiovascular disease | 4S (Scandinavian Simvastatin Survival Study) | Derived from fermentation, prodrug that requires activation. |
| Lovastatin | Natural | Lipophilic | CYP3A4 | Hyperlipidemia, cardiovascular disease | AFCAPS/TexCAPS (Air Force/Texas Coronary Atherosclerosis Prevention Study) | First statin discovered, naturally occurring in fungi. |
| Fluvastatin | Synthetic | Lipophilic | CYP2C9 | Hyperlipidemia, secondary prevention of CHD | LIPS (Lescol Intervention Prevention Study) | Less commonly used, shorter half-life. |
| Pravastatin | Semi-synthetic | Hydrophilic | Sulfation, CYP3A4 (minor) | Hyperlipidemia, cardiovascular disease | CARE (Cholesterol and Recurrent Events) | Lower muscle-related side effects, minimal metabolism by CYP450 |
| Rosuvastatin | Synthetic | Hydrophilic | CYP2C9 (minor), CYP2C19 (minor) | Hyperlipidemia, cardiovascular disease, inflammation | JUPITER (Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin) | Highly potent, longer half-life, minimal CYP450 interaction. |
| Pitavastatin | Synthetic | Lipophilic | CYP2C9, UGT1A3, UGT2B7 | Hyperlipidemia, primary prevention | LIVES (Livalo Effectiveness and Safety) | Newer statin, less effect on glucose metabolism. |
| Cerivastatin | Synthetic | Lipophilic | CYP2C8, CYP3A4 | Hyperlipidemia | Withdrawn (due to risk of rhabdomyolysis) | Currently not in use |
| Statin Type | Population | Intervention | Major Outcomes | Study |
|---|---|---|---|---|
| Simvastatin | Control mice, Severe asthma mice (House dust mite (HDM)+ Ovalbumin (OVA)+ lipopolysaccharide (LPS)), Conventional asthma mice (Ovalbumin (OVA)). | Control mice treated with saline solution; Severe and conventional asthma mice received either: Cl-amidine 10 mg/kg, DNase I 1000 IU, or Simvastatin 40 mg/kg | Simvastatin-treated mice demonstrated anti-inflammatory activity by reducing the levels of pro-inflammatory cytokines like IL-1β in BALF, increase anti-inflammatory TREG cells and decrease pro-inflammatory Th2 and Th17 in severe asthma mice group. | Chen et al. [51] |
| Simvastatin | IEC-6 cell line exposed to TNF-α. Non-treated control mice group. Indomethacin only treated mice group. Indomethacin + PPI mice group. Indomethacin + Simvastatin mice group. | IEC-6 cells with inflammation treated with 0.5 μM simvastatin. In the in vivo part of the study, Group 1 was control mice group. Group 2 treated with 12 mg/kg of Indomethacin only. Group 3 with 12 mg/kg Indomethacin + 5 mg/kg of PPI. Group 4 with 12 mg/kg Indomethacin+ simvastatin 15 mg/kg. | In the in vitro experiment, the Simvastatin treated IEC-6 cells showed lower pro-inflammatory markers like IL-6, along with decreased inflammatory proteins like NF-κB. In the in vitro model, the mice treated with Simvastatin showed fewer intestinal bleeding and ROS production. | Kim et al. [52] |
| Atorvastatin | Rat cartilage explant in osteoarthritic model exposed to IL-1β | Control model. Cartilage model exposed to only IL-1β. Cartilage model exposed to IL-1β + 1, 3 or 10 μM of Atorvastatin. An additional set of each of these cartilage models were also incubated with 1400 W (selective iNOS inhibitor) | Atorvastatin group reduced levels of TNF-α and GAG release, suggesting less cartilage degradation. It also decreased MMP-13 and O2− levels. Furthermore, the source of NO in control and Atorvastatin group was eNOS, a beneficial form of NO. | Pathak et al. [53] |
| Rosuvastatin | Control Mice group. LPS only mice group. LPS+ Rosuvastatin mice group. Rosuvastatin only mice group. RAW264.7 macrophage cell line. | 5 mg/kg/day of Rosuvastatin was used. In the In vitro part of the study, RAW264.7 macrophages were cultured with LPS only. Another set with LPS+ 10 and 20 μM of Rosuvastatin | Rosuvastatin prevented sepsis-related death of mice and reduced pro-inflammatory markers like TNF-α. In the RAW264.7 macrophages, rosuvastatin prevented inflammation via downregulating NF-κB. | Tang et al. [54] |
| Rosuvastatin | Untreated mice group (Dextran sulphate sodium (DSS) only). Treated mice group (Dextran sulfate sodium (DSS)+Rosuvastatin). IEC-6 cell line exposed to TNF-α | Untreated mice group received only DSS. Treated mice group received DSS + 0.3 mg/kg/day of Rosuvastatin for 21 days. The IEC-6 cells were exposed to TNF-α and only some cell cultures were treated with 2 μmol/L of Rosuvastatin. | In the DSS-treated colitis mice, Rosuvastatin significantly reduced disease activity index compared to untreated group, and levels of pro-inflammatory cytokines like IL-6 were also attenuated. In vitro part of study showed that Rosuvastatin treated cell culture showed lower levels of inflammatory mediators and ROS. | Shin et al. [55] |
| Fluvastatin, Atorvastatin, Pravastatin | Murine RAW264.7 macrophages cell line | RAW264.7 macrophages treated with the following for 24 h: Fluvastatin at 1.25, 2.5, 5 and 10 μM, Atorvastatin at 10, 25, 50 and 100 μM. and Pravastatin at 25, 50 and 100 μM. | Fluvastatin and Atorvastatin increased H2S levels by Akt signalling. Pravastatin did not show a significant effect. Fluvastatin and Atorvastatin increased expression of cystathionine γ-lyase (CSE). Fluvastatin reduced IL-1β and MCP-1 mRNA levels. | Xu et al. [56] |
| Pravastatin | Mouse Podocytes | Cells treated with 0.01, 0.03, 0.1, 0.3 and1 mM Pravastatin | Carboxymethyllysine (CML)-induced MCP-1 was downregulated by Pravastatin. Pravastatin prevented phosphorylated ERK in podocytes and NF-ĸB and Sp1 translocation. | Gu et al. [57] |
| Simvastatin | M1 macrophage model stimulated with IFN-γ and LPS | 1.0, 2.5, 5.0 µmol/L of Simvastatin was cultured in M1 macrophages for 9 h | Simvastatin exhibited anti-inflammatory effects by stimulating M1 macrophages to switch to M2 phenotype. | Li et al. [58] |
| Simvastatin | Elastase-induced pulmonary emphysema in C57BL/6 mice | Mice were treated with 20 μg of Simvastatin or 200 μL of PBS every day for 2 weeks | Simvastatin reduced inflammation via reducing neutrophils and hydroxyproline levels | Takahashi et al. [59] |
| Atorvastatin | Murine RAW264.7 macrophages cell line | The cells were pre-treated with 0, 10, 20 and 40 mmol/L of Atorvastatin for 1 h. After, some cells were incubated with 40 mg/mL of ox-LDL for 24 h. | Atorvastatin downregulated ox-LDL-induced inflammatory cytokines, COX-2 expression, MAPK activation and IκB-α degradation. | Shao et al. [60] |
| Pravastatin, Simvastatin | Adult male cynomolgus monkeys | The monkeys consumed atherogenic diet for 12 months while receiving either no treatment, 40 mg/kg per day of pravastatin or 20 mg/kg per day of Simvastatin. | The statins reduced inflammation by lowering IL-1β levels | Sukhova et al. [61] |
| Statin Type | Condition | Phase | Duration | Eligibility | Major Outcome | Inflammatory Markers | Clinical Trial ID | Comments |
|---|---|---|---|---|---|---|---|---|
| Simvastatin | Breast Cancer | 2 | 24–48 weeks | Women aged ≥ 18 with early or advanced breast cancer, one healthy breast, not pregnant, non-smokers, non-drinkers, and not on any other medications (including cancer, hormonal, lipid-lowering, or antibiotics) for at least 3 months, with no significant health issues. Excludes men, women with bilateral breast cancer, those under 18, healthy volunteers, or those not meeting the above criteria. | Simvastatin significantly reduced total cholesterol, LDL, triglycerides, hsCRP, and estrone sulphate, particularly in postmenopausal women, indicating anti-inflammatory and anti-cancer potential. | hsCRP | NCT00334542 [62] | The researchers recorded that about 20% of the people in the trial underwent muscular-related issues after statin treatment. |
| Lovastatin | Breast cancer | 2 | 6 months | Inclusion criteria: 18 to 65 year old. Must have a high risk of developing breast cancer with known mutation in BRCA2, or other high-risk mutation. Must have family history of breast cancer. ECOG performance status 0 and no known other health conditions. Alcohol consumption must not exceed 3 alcoholic drinks per week. Exclusion criteria: Men. Women aged below 18 and above 65. Women with no risk of developing breast cancer/mutation in BRCA2, or other high-risk mutation or family history. Have had invasive breast cancer in past 2 years. Other known health conditions, especially muscular or liver related. Taking other medications like tamoxifen or allergic to statins. Alcoholic or breastfeeding. | No significant biomarker modulation | C-reactive protein (CRP) | NCT00285857 [63] | 30 women participated, with 27 completing the trial, hence the observation of no significant biomarker modulation may be due to the small sample size. [63] |
| Rosuvastatin | Breast cancer | 2 | 4 weeks | Inclusion Criteria: 18 year or above women with Metastatic breast cancer at stage IV. ECOG performance at 0, 1, 2. Currently receiving endocrine therapy with or without HER2 for at least 6 weeks. No other significant health condition. Exclusion criteria: below 18 women with non-metastatic breast cancer or metastatic breast cancer not at stage IV. Women participating in other clinical trials. Women completed HER2 or other chemotherapy. Have used statins recently or are taking other specific medication that interacts with the treatment drug. Have other health issues, including cardio, liver or muscular related problems. Asian descent women. Women who are alcoholic, smoke or pregnant/breastfeeding. | NRP | C-reactive Protein (CRP) | NCT01299038 | The sample size is too small for (19 participants), leaving room for possible statistical errors. |
| Simvastatin | Breast cancer | 2 | 14 days | Inclusion criteria: 18 or older post-menopausal women. Have history of invasive breast cancer, currently taking anastrozole for breast cancer treatment. Overall good health and no severe allergy to the medication. Exclusion criteria: younger than 18, no history of invasive breast cancer. Have other medical conditions and allergic to medication treatment. Currently using other statins, hormone therapy or antibiotics. Alcohol consumption exceeds 3 drinks a day. Pregnant or breastfeeding. | NRP | Serum Estradiol levels | NCT00354640 | Simvastatin was given with anastrozole |
| Atorvastatin | Prostate cancer | 2 | 6 months | Inclusion criteria: 18 years to 120 years old men. Have prostate cancer that has recurred after surgery or treatment. Rising PSA levels indicating cancer reoccurrence. No other cancer. Overall good health and platelet count. Exclusion Criteria: Younger than 18 years old or older than 120 years old. Have a history of heart conditions. Currently using other medication, including anti-thrombotic medications and statins. Ongoing cancer infection. | NRP | NF-κB, IL-6, Prostaglandin E2 | NCT01220973 | Atorvastatin was given with celecoxib. Although the trial period was relatively long, the sample size was small, with 27 participants at baseline and 1 withdrawing before therapy. |
| Lovastatin | Melanoma | 2 | 6 months | Patients eligible for enrolment comprised individuals in overall satisfactory systemic health presenting with multiple atypical melanocytic naevi or with completely resected stage I–II melanoma without adjuvant therapy within the preceding three months, using effective contraception if of reproductive potential, while excluding those with untreated or advanced (stage III–IV) melanoma, recent exposure to lipid-lowering agents, hypersensitivity to lovastatin or congeners, clinically significant comorbidities, or current pregnancy or lactation. | No significant results observed between Lovastatin group and placebo group. | C-reactive protein and RelA (a subunit of NF-κB) | NCT00462280 [64] | Possibly the reason for not achieving significant results might be due to the short duration of the trials [64]. |
| Rosuvastatin | Chronic Obstructive Pulmonary Disease | 2 | 12 weeks | Inclusion criteria: Stable COPD patients aged 40–80 years, COPD stages I to IV based on GOLD criteria, no COPD exacerbations within the last three weeks. Exclusion criteria: Other diagnosed lung diseases except certain bronchitis or mild bronchiectasis, significant cardiovascular diseases, severe hypertension, extreme obesity, uncontrolled diabetes, severe hypercholesterolemia, specific neuromuscular or renal conditions, abnormal liver function, pregnancy, substance abuse, recent statin use or reaction, recent malignancy, uncontrolled hypothyroidism, recent participation in other clinical trials, and use of medications interacting with Crestor. | No significant change in endothelial function or FEV1. Contradictory inflammatory response, marked by decreased CRP and increasing IL-6 | CRP, IL-6 | NCT00929734 [65] | The researchers published the trial (RODEO Trial, see section “Statins and COPD”) [65]. The contradictory inflammatory response may be due to the short trial period. |
| Atorvastatin | Rheumatoid arthritis | 3 | 6 months | Inclusion criteria: Adults aged 18–65 years with rheumatoid arthritis. Exclusion criteria: Pregnant or lactating individuals, and those with hepatic or renal impairment. | Recruiting | DAS-28 | NCT03770702 | The study is still recruiting. |
| Simvastatin | Pre-diabetic + Hypercholesterolemia + CVS Risk | N/A | 24 weeks | Adults aged 18–75 years with a BMI of 25–40 kg/m2 and pre-diabetes, defined by impaired glucose tolerance or fasting hyperglycaemia, were eligible, excluding individuals with triglycerides > 350 mg/dL, LDL cholesterol > 200 mg/dL, unstable blood pressure, cardiovascular, hepatic, or renal pathology, or current use of anti-inflammatory agents or other pharmacological modulators of lipid or glucose homeostasis. | The researchers observed reduction in total LDL cholesterol and apolipoprotein B levels. However, triglycerides only showed a decrease after combined therapy. Simvastatin reduced CRP levels, but no significant effect t was observed for IL-6 or TNF-α. | CRP, IL-6, TNF-α | NCT01103648 [66] | The researchers gave monotherapies of statin and ezetimibe but later gave combination therapy to both groups. Perhaps the reason the researchers obtained contradictory results for IL-6 and TNF-α may be due to the small sample size [66]. |
| Simvastatin | Stable Angina | N/A | 6 weeks | Inclusion criteria: Adults aged 18–80 years with stable angina and LDL cholesterol levels between 70 and 160 mg/dL. Exclusion criteria: Renal failure, current treatment with more than 20 mg of simvastatin, hepatic disease, inflammatory diseases, and age younger than 18 or over 80. | LDL-cholesterol was reduced by simvastatin; however, there was no significant differences in levels of hsCRP, ox-LDL, or sCD40L between the 2 treatment groups. | CRP, ox-LDL, MCP-1, sICAM-1, sCD40L, IL-6 | NCT00474123 [67] | One group of participants were treated with Statin and Ezetimibe [68]. The short duration of the clinical trial may present a limitation when assessing the effects on the inflammatory markers, as it may take longer to show results with the treatment. |
| Simvastatin | Type 1 Diabetes | 2 | N/A | Inclusion criteria: Adults over 20 years old with Type I diabetes (onset before 20 years and on insulin therapy since diagnosis), with diabetes duration over 1 year and no clinical macrovascular complications. Exclusion criteria: HbA1c > 10% in the last year, use of Glucophage or thiazolidinediones. Rheumatoid arthritis, abnormal liver function, thyroid disorders or other significant health conditions. Using steroid therapy or anti-inflammatory drugs except low-dose aspirin. Abnormal blood count. Pregnant/smoking or lactating. | NRP | CRP, IL-6, IL-8, TNF-α, NF-κB, monocyte cells, CD40 | NCT00441844 | It was a randomized double-blind trial, hence the probability of bias is reduced, and the results hold more validity. |
| Atorvastatin | Atrial Fibrillation | 4 | 1 year | Inclusion criteria: Elderly patients aged 68–82 years with atrial fibrillation, adequate oral anticoagulation therapy, and cholesterol levels between 4.5 mmol/L and 7 mmol/L. Exclusion Criteria: Indication for cholesterol-lowering treatment according to Dutch CBO-cholesterol guidelines (2004). | Atorvastatin significantly reduced the inflammatory markers and improved memory and cognition. MRI revealed les brain tissue loss in treatment group, particularly in hippocampus and amygdala. | Hs-CRP, IL-1RA. IL-9, IL-13, IL-17, IFNγ, IL-12, MIP-1β | NCT00449410 [69] | Atorvastatin was given along with Ezetimibe [70]. The areas with less tissue loss were associated with emotion as well, suggesting further research opportunity to investigate atorvastatin as an anti-inflammatory in emotional disorders. |
| Rosuvastatin | Non-familial hypercholesterolemia, physical inactivity | 4 | 20 weeks | Eligible participants comprised adults aged 40–65 years with nonfamilial hypercholesterolaemia, total cholesterol exceeding 200 mg/dL, LDL concentrations above 130 mg/dL, sedentary lifestyle, and moderate-to-low ethanol consumption, whereas exclusion encompassed hepatic or renal pathology, acute inflammatory or infectious states, other clinically significant morbidities, administration of agents such as corticosteroids or alternative lipid-lowering pharmacotherapies, active tobacco use, involuntary or intentional body mass reduction exceeding 2 kg within the preceding six months, and surgical intervention within the prior three months. | Rosuvastatin reduced TLR-4 expression on monocyte cells and TLR-4 activity. As a result of reduced TLR-4 activity, it reduced downstream inflammatory cytokines like IL-6, IL-12 and TNF-α | TLR4, CD14, IL-6, TNF-α, hsCRP, B7-1 | NCT00295373 [71] | Researchers published these results but also 2 more papers related to it [72]. The researchers also introduced a behavioural intervention (exercise). The use of inhibitors in this study gives more strength and reliability to statins effect on TLR-4 and its mediated processes. |
| Rosuvastatin | Heart Failure | 2 | 3 months | Adults aged 18–75 years with chronic heart failure classified as NYHA stage III–IV, peak oxygen uptake < 20 mL/min/kg, left ventricular ejection fraction < 30%, left ventricular end-diastolic diameter > 55 mm, and stable pharmacotherapy for ≥4 weeks were eligible, whereas exclusion applied to individuals with hepatic transaminase elevation (GOT, GPT), increased serum creatinine, insulin-dependent diabetes mellitus, arterial hypertension, myopathic disorders or elevated CK, fibrate therapy, concomitant administration of CYP3A4-metabolized agents, or any additional pathology precluding study participation. | Rosuvastatin improved vascular health by increasing levels of VEGF (a growth factor for blood vessels) and decreased levels of oxidized LDL. | Ox-LDL | NCT00176332 [73] | Reduced oxidized LDL suggests possible anti-inflammatory action and needs further investigation [73]. |
| Simvastatin | Septic Shock | 4 | N/A | Adults aged > 18 years with septic shock of <48 h’ duration were eligible, whereas exclusion applied to pregnancy, inability to tolerate enteral administration, anticipated survival < 72 h, recent exposure to simvastatin or other HMG-CoA reductase inhibitors, hypersensitivity to the investigational agent or structural analogues, known or suspected porphyria, elevated rhabdomyolysis risk, and haemorrhagic shock. | NRP | NA | NCT00450840 | N/A |
| Atorvastatin | Crohn’s Disease | 2 | 13 weeks | Adults aged 18–65 years with clinically confirmed Crohn’s disease, C-reactive protein > 2 mg/L in the absence of infection, and either fecal calprotectin > 250 mg/kg or CDAI > 150 were eligible, whereas exclusion applied to CDAI > 450 or prednisolone therapy exceeding 15 mg/day. | Atorvastatin reduced CXCL10 levels suggesting a decrease in CRP as they are correlated, therefore demonstrating atorvastatin’s anti-inflammatory effects. | CCL2, CCL4, CCL11, CCL13, CCL17, CCL22, CCL26, CXCL8, CXCL10, sP-selectin, sE-selectin, sICAM-3, thrombomodulin, CRP. | NCT00454545 [74] | The researchers published their results [75]. The trial only had 10 patients investigate the efficacy of the drug on, combined with the short trial period of 13 weeks. Hence, the trial should be replicated with higher number of participants and duration to achieve more reliable results. |
| Atorvastatin | Endometriosis | N/A | 6 months | Premenopausal women aged 18–45 years providing written informed consent, exhibiting clinical manifestations of endometriosis for ≥3 months confirmed via laparoscopy or laparotomy within the preceding 4 months to 5 years (preferably with histological corroboration), with moderate pain symptoms such as dysmenorrhoea and no evidence of sexually transmitted infection, were eligible. Exclusion encompassed malignancy of the ovary, adrenal gland, endometrium, cervix, or breast; pregnancy or lactation; unexplained uterine or cervical hemorrhage; hormonal therapy within the prior 3 months (6 months for GnRH analogues); menstrual cycle irregularity > 35 days or secondary amenorrhoea > 3 months; chronic disorders involving the pelvic or abdominal cavity; uncontrolled type I or type II diabetes mellitus; venous thromboembolism or other contraindications to the investigational medicinal product; and continuous administration of CYP3A4 inhibitors. | NRP | Inflammatory status | NCT00675779 | Atorvastatin was given either alone or in combination with oral contraceptives. |
| Atorvastatin | Dyslipidemia | 4 | 12–24 weeks | Inclusion criteria: Adults aged 21–75 years with LDL-C > 130 mg/dL and HDL-C ≤ 45 mg/dL in men or ≤ 55 mg/dL in women. Exclusion criteria: History of hypersensitivity to any statin, niacin, or aspirin; diagnosis of diabetes or fasting glucose > 125 mg/dL; untreated hyper or hypothyroidism (unless treatment is stable); and other health, medication, and logistical criteria. | Atorvastatin reduced LDL-cholesterol and increases HDL-cholesterol. Both treatments reduced inflammation markers like hs-CRP and TNF-α, with a more pronounced effect seen with the combination therapy. No change in IL-6 | hsCRP | NCT00194402 [76] | Atorvastatin was given with Slo-Niacin [77]. No difference in IL-6 was noted. |
| Atorvastatin | Heart Failure | 4 | 6 months | Adults ≥ 18 years with mild-to-moderate heart failure (NYHA class II–III) of any etiology, documented left ventricular systolic dysfunction (LVEF < 45%) by echocardiography within 3 months prior to randomisation, clinically stable, receiving optimized HF pharmacotherapy unchanged for ≥4 weeks before enrolment, and exhibiting normal fasting total cholesterol were eligible. Exclusion criteria comprised concurrent lipid-lowering therapy, absolute or relative contraindications to statins, established chronic inflammatory disorders, or medical conditions necessitating anti-inflammatory or immunosuppressive treatment. | Atorvastatin reduced expression of PIIINP, Hs-CRP, and BNP. The most reduced expression observed was for PIIINP | hsCRP, TNF-α, IL-6 | NCT00795912 [78] | Further investigations should be conducted on why PIIINP out of all was most reduced. [78] |
| Atorvastatin | Abdominal surgery | 3 | 5 weeks | Male and female patients aged 25–55 years undergoing non-bowel abdominal surgery involving an abdominal incision under spinal anesthesia, weighing 50–120 kg, capable of comprehending and adhering to all study procedures, and providing written informed consent were eligible. Exclusion criteria encompassed bowel surgery, procedures under general anaesthesia, emergency interventions, high-risk infection laparoscopic surgery, pregnancy or lactation (or absence of reliable contraception), active malignancy, uncontrolled diabetes mellitus, untreated hypertension, psychotic disorders or other significant psychiatric impairment, any condition predisposing to non-compliance or adversely influencing treatment outcomes, and known hypersensitivity to the investigational product or its constituents. | NRP | TNF-α, CRP | NCT00902967 | N/A |
| Atorvastatin | Sepsis | 2 | 10 days | Patients aged ≥ 15 years with a clinical diagnosis of severe sepsis or septic shock established within the preceding 24 h were eligible, whereas exclusion applied to prior statin use within 30 days, definitive clinical indication for statin therapy, ongoing immunosuppressive treatment, elevated risk of rhabdomyolysis, AIDS diagnosis, inability to tolerate enteral administration, pregnancy, or anticipated survival < 48 h. | NRP | N/A | NCT00452608 | N/A |
| Simvastatin | Cystic Fibrosis | 1 | 28 days | Patients aged ≥ 10 years with cystic fibrosis who were clinically stable and had an FEV1 > 50% of predicted were eligible, whereas exclusion applied to hepatic pathology, Burkholderia cepacia infection, corticosteroid therapy, and symptomatic allergic rhinitis. | NRP | IL-6, IL-8, and NOS2 mRNA | NCT00255242 | N/A |
| Atorvastatin | Dilated Cardiomyopathy | N/A | 6 months | Patients aged ≥ 18 years with dilated cardiomyopathy (per ESC 2007 criteria), EF ≤ 40% confirmed by echocardiography, absence of significant coronary artery stenosis > 30% on cardiac catheterization, and provision of written informed consent were eligible. Exclusion criteria encompassed abnormal blood pressure, concomitant cardiac disorders or heart failure, current statin therapy, hepatic or renal dysfunction, uncontrolled diabetes mellitus, alcohol or substance misuse, pregnancy or lactation, recent surgical procedures, thyroid disease, and immune system disorders. | Atorvastatin reduced IL-6 and TNF-α. Atorvastatin also improved heart function by decreasing NT-proBNP, which increased in control group but decreased in atorvastatin group. The atorvastatin group were able to walk more and had fewer hospitalization rates. | TNF-α, IL-6, IL-10 | NCT01015144 [79] | N/A |
| Simvastatin | Myocardial Infarction | 4 | 4 weeks | Eligible population comprised individuals aged 40–70 years presenting within a temporal window of ≤24 h from the inception of symptomatology consistent with acute myocardial infarction, exhibiting electrocardiographic perturbations characterized by ST-segment elevation ≥ 1 mm in the limb-lead vector orientation or ≥2 mm in the precordial-lead vector orientation across two anatomically contiguous leads, in conjunction with corroborative biochemical evidence of myocardial cytolysis, namely elevated creatine kinase–MB isoenzyme and troponin concentrations. Exclusionary parameters encompassed any antecedent exposure to statin-class pharmacotherapeutics within the six-month interval preceding the index myocardial infarction event. | NRP | CRP | NCT00905905 | One group was given statin with Ezetimibe. |
| Rosuvastatin | Abdominal Sepsis | 2 | 3 days | Eligibility parameters encompassed male and female patients aged 18–80 years presenting with abdominal sepsis, wherein a definitive diagnosis of diffuse peritonitis was surgically established within ≤48 h of clinical progression, including cases precipitated by penetrating trauma from metallic projectiles or bladed weaponry with concomitant contamination of the peritoneal cavity. Additional inclusionary requirements comprised an APACHE II severity index ≥ 8 and formal consent to enrolment. Exclusionary parameters included prior or current administration of pharmacological agents such as statins or fibrates; end-stage impairment of hepatic or renal function; ongoing pregnancy; recent traumatic insult; documented hypersensitivity to statin-class compounds; or concurrent management in a healthcare facility external to the study site. | NRP | IL-6, IL-1β, TNF-α, CRP | NCT00357123 | N/A |
| Pravastatin | Crohn’s Disease | N/A | 6 weeks | Men and non-pregnant women aged 18–65 years, adhering to an adequate contraceptive regimen, with active Crohn’s disease defined by HBI > 5 or serum CRP above the upper reference limit, receiving a stable pharmacological regimen for ≥4 weeks with continuation planned for 6 weeks, and maintained on an unchanged azathioprine/6-MP or methotrexate dosage for ≥8 weeks prior to enrolment were eligible. Exclusion applied to individuals < 18 or >65 years, concurrent use of alternative statins or other lipid-lowering agents, hypersensitivity to statin-class drugs, pregnancy or lactation, clinically significant hepatic or renal functional impairment, severe Crohn’s disease–related complications or other major comorbidities, and participation in other investigational therapeutic protocols. | NRP | CRP, ESR | NCT00599625 | N/A |
| Simvastatin | Myocardial Infarction | 4 | 5 days | Patients aged 45–70 years presenting within ≤24 h of the onset of clinical manifestations consistent with acute myocardial infarction, exhibiting ST-segment elevation across two anatomically contiguous electrocardiographic leads, and demonstrating biochemical confirmation of myocardial injury through elevated CK-MB and troponin concentrations were eligible. Exclusion encompassed any prior statin exposure within the preceding six months. | The researchers found that early and higher doses of simvastatin were more affective. Simvastatin reduced levels of CRP, IL-2 and TNF-α, along with improving blood vessel function and reducing oxidative stress | CRP | NCT00906451 [80] | The trials utilized a good sample size of 125 patients, allowing more reliable statistical analysis [80]. |
| Pitavastatin | Metabolic Syndrome | 4 | 12 weeks | Hypercholesterolemic individuals aged ≥ 20 years were eligible, whereas exclusion applied to those currently receiving lipid-lowering pharmacotherapy, diagnosed with familial hypercholesterolaemia, exhibiting renal pathology, disorders of the hepatic, gallbladder, or biliary systems, or pregnancy. | Pitavastatin reduced CRP levels and lower levels of adiponectin. | hsCRP, soluble ICAM-1 | NCT00444717 [81] | N/A |
| Atorvastatin | Surgery | N/A | 48 h | Patients aged > 45 years scheduled for elective high-risk surgery as per POISE criteria, and suitable for baseline brachial artery ultrasonography prior to the procedure, were eligible. Exclusion criteria comprised absence of informed consent, pregnancy, contraindications to the brachial artery ultrasound protocol (including intolerance to 0.4 mg sublingual nitroglycerin), concurrent participation in a conflicting clinical investigation, or prior enrolment in STAR-VaS or STAR-VaS2. | NRP | CRP | NCT00967252 | N/A |
| Atorvastatin + Simvastatin | N/A | 4 | 6 weeks | Inclusion Criteria: Adults aged 18 years or older with low HDL-c cholesterol levels (<40 mg/dL for men, <50 mg/dL for women) or elevated LDL-c or non-HDL-c levels (for TG 200–500 mg/dL) requiring therapy per NCEP guidelines, with an identifiable primary care provider and a working phone number for follow-up. Exclusion Criteria: Under 18, unstable heart condition, liver problem/other significant health condition. Alcoholic, pregnant or breastfeeding. Taking other medication that can interfere with the study or participating in other studies. | NRP | hsCRP | NCT00736463 | N/A |
| Rosuvastatin | Healthy | NA | NA | Healthy male, non-Asian individuals aged 19–39 years were eligible, whereas exclusion applied to those exhibiting elevated hepatic function indices, increased serum creatinine concentrations, or receiving anti-inflammatory or immunomodulatory pharmacotherapy. | NRP | NA | NCT00874757 | N/A |
| Simvastatin | Type 2 Diabetes | NA | 8 weeks | Inclusion Criteria: Adults aged 35–80 years with type 2 diabetes, HbA1c between 6.0% and 9.0%, and LDL-c > 100 mg/dL with no lipid-lowering treatment in the last six months. Exclusion Criteria: Refusal to provide informed consent. | NRP | NF-κB, IL-6, CRP | NCT01424891 | One group was given Simvastatin in combination with Ezetimibe |
| Atorvastatin | Polycystic Ovary Syndrome | NA | 6 months | Inclusion Criteria: Women aged 30–50 years diagnosed with PCOS (Rotterdam criteria) and using safe non-hormonal contraception. Exclusion Criteria: Use of cholesterol-lowering agents, antidepressants, oral cortisone medication, hormonal contraception, nursing, pregnancy, type 2 diabetes, liver disease, menopause, kidney or liver failure. | Atorvastatin improved lipid profile by decreasing LDL cholesterol and increasing HDL cholesterol. It reduced CRP levels significantly. Dehydroepiandrosterone sulphate (DHEA-S) decreased but Atorvastatin increased insulin resistance and had no effect on testosterone. | CRP | NCT01072097 [82] | More research needs to be conducted to find out the cause of the mixed effects on androgens |
| Rosuvastatin | Dietary selenium deficiency + dietary zinc deficiency | NA | 4 months | Eligibility parameters encompassed adult and geriatric cohorts with angiographically substantiated coronary atherosclerotic pathology and hemodynamically stable angina pectoris, characterized by ≥70% luminal calibre reduction in at least one segment of a principal epicardial coronary conduit, or ≥50% diminution in the intraluminal calibre of the left main coronary artery. Exclusionary determinants incorporated the presence of cardiac complications or any severe systemic comorbid condition, including but not limited to endocrine disorders of thyroidal origin, hematological dyscrasias, congenital anomalies, autoimmune-mediated hepatopathies, end-stage renal insufficiency, malignant neoplasia, concomitant infectious states, osteoporotic bone demineralisation, or post-surgical physiological stress states. Patients were also excluded if receiving pharmacological agents such as antacid formulations, antimicrobial agents, micronutrient supplementation, ethanol ingestion, or maintaining active tobacco use. | Rosuvastatin was effective in lowering LDL cholesterol and triglycerides. Rosuvastatin alone or in combination therapy has no effect on the level of antioxidant, zinc or selenium mineral. However, it did reduce hs-CRP levels | hsCRP, IL-6 | NCT01547377 [83] | One group was given rosuvastatin was co-treated with Zinc and Selenium [84]. Further studies need to be conducted with different dosages and population to reliably establish the possibility of using rosuvastatin in combination with zinc or selenium in conditions requiring antioxidant properties. |
| Atorvastatin | Inflammation | 4 | 16 weeks | Inclusion Criteria: Adults aged 18 years and older who are normocholesterolemic. Exclusion Criteria: Individuals with cardiovascular disease or risk equivalents, malignancy, active alcohol abuse, contraindications to statins, interacting drugs, or chronic use of anti-inflammatory drugs. | No significant change in ENA-78 cytokine at the end of the 16-week period | ENA-78 | NCT00361283 | Although the trial had a relatively large sample size of 108 individuals, 81 of them completed the trial and 27 were lost to follow-up. |
| Simvastatin | Depression + Coronary Artery Disease + Acute Coronary Syndrome | 1 | 20 weeks | Adults aged 18–60 years presenting with mild depressive symptomatology and elevated inflammatory indices (CRP > 2 mg/L) were eligible, whereas exclusion encompassed language or communication limitations, active substance misuse, clinically significant comorbidities such as hepatic or cardiovascular pathology, pregnancy, concurrent pharmacotherapy with potential to interfere with study parameters, or meeting established criteria for initiation of cholesterol-lowering therapy. | Terminated | CRP | NCT00208117 | The researchers were planning to compare outcomes in patients given either Simvastatin, Sertraline or placebo. However, the trial was terminated due to researchers being unable to enrol participants. |
| Atorvastatin | Rheumatoid Arthritis | 4 | 12 weeks | Adults aged ≥ 18 years meeting ACR classification criteria for rheumatoid arthritis, with disease duration ≥ 1 year, active disease status, and maintained on stable DMARD therapy for ≥3 months were eligible. Exclusion criteria comprised inability to provide informed consent, pregnancy or lactation, eligibility for or current use of lipid-lowering agents, documented hepatic pathology, elevated hepatic transaminases within the preceding two months, and recent exposure to hydroxychloroquine. | Atorvastatin reduced the inflammatory properties of HDL-cholesterol and hs-CRP. While there were improved, there was no significant improvement in the disease and ICAM-1. | hs-CRP, cytokine/ICAM-1 | NCT00356473 [85] | Further investigation needs to be conducted as to why there was no improvement in disease or ICAM-1 levels. |
| Simvastatin | Diabetes + Dyslipidemia | NA | 30 days | Inclusion Criteria: Adults aged 35–64 years with primary hyperlipidemia (total cholesterol > 200 mg/dL, triglycerides > 150 mg/dL), type 2 diabetes, and informed consent. For women: post-menopausal (>12 months), post-hysterectomy, or using mechanical contraception. Exclusion Criteria: Severe medical conditions like heart failure, kidney failure or cancer withing the last 5 years. secondary hyperlipidemia, morbid obesity (BMI > 40 kg/m2), alcohol or drug abuse, non-compliance, and specific laboratory abnormalities (elevated alanine transferase, creatine kinase, low hemoglobin, platelets, or white blood cells). | NRP | IL-1, TNF-α, IL-6, IL-10, hsCRP | NCT01101204 | The researcher gave combined treatment of 10 mg Simvastatin, 1000 mg Metformin, and 100 mg Fenofibrate. |
| Simvastatin | Community-acquired pneumonia | 3 | 30 days | Inclusion Criteria: Participants must be ≥60 years of age with a clinically and/or radiographically confirmed diagnosis of pneumonia. Exclusion Criteria: Exclusion criteria include the presence of severe sepsis or septic shock, hepatic cirrhosis (any etiology), acute coronary syndrome within the current admission or recent past, total serum cholesterol levels outside the reference safety range (marked hypercholesterolaemia or hypocholesterolaemia), ongoing therapeutic anticoagulation, current systemic corticosteroid therapy or other immunosuppressive pharmacotherapy, and declination of informed consent. | NRP | TNF-α, IFN-γ, CRP, PAI-1 | NCT01651728 | N/A |
| Simvastatin | Coronary Artery Disease | NA | 24 h | Inclusion Criteria: Adults aged ≥ 18 years scheduled to undergo percutaneous coronary intervention (PCI) or diagnostic coronary angiography, with no documented history of statin intolerance or adverse reactions, and who have provided written informed consent. Exclusion Criteria: Exclusion criteria comprise:
| Terminated | hsCRP | NCT00588471 | Study termination was necessitated by inadequate recruitment, resulting in failure to achieve the target enrolment required for statistical validity. |
| Atorvastatin | Atherosclerosis | 4 | 3 months | Inclusion Criteria: Subjects aged 18 to 80 years with accumulation of FDG-PET in the carotid artery or aorta. Exclusion Criteria: LDL cholesterol level outside 120–180 mg/dL, current use of statins or fibrates, symptomatic coronary or cerebrovascular diseases, myocardial infarction or stroke within 6 months, recent vascular interventions or operations, poorly controlled diabetes or hypertension, neoplasms, and systemic inflammatory diseases. | NRP | IL-6, soluble ICAM-1, hsCRP | NCT00920101 | The participants were given Atorvastatin along with lifestyle modifications (dietary). |
| NA | Coronary Artery Disease | 2/3 | 12 weeks | Inclusion Criteria: Patients aged 20–90 diagnosed with coronary artery disease (CAD) with at least 50% stenosis in one major coronary artery confirmed by cardiac catheterization, and currently receiving statin therapy. Exclusion Criteria: Individuals under 18 years of age, pregnant women, and those taking antioxidant vitamin supplements are excluded from the study. | The levels of the antioxidant enzymes activities were significantly higher in coenzyme Q10 group. The levels of inflammatory markers were significantly lower after coenzyme Q10 supplementation. | hsCRP, TNF-α, IL-6, adiponectin | NCT01424761 | The study published their results [86]. Although the researchers’ focus is on investigating Coenzyme Q10, all patients were undergoing statin therapy, suggesting possible anti-inflammatory benefits. |
| Atorvastatin | Acute Kidney Injury post Cardiac Surgery | NA | 72 h | Inclusion Criteria:
| NRP | IL-6, IL-10, TNF-α, hsCRP | NCT01547455 | The duration of the study was relatively short. With a sample size of only 96 participants, increasing the number of patients could help to enhance generalizability. |
| NA | Type 2 Diabetes + Atherosclerosis + Dyslipidemia | 4 | 5 days | Inclusion Criteria: Adults with an age range of 35–80 diagnosed with type 2 diabetes mellitus and eGFR > 30 mL/min/1.73 m2, on statin therapy for at least 6 months at specified minimal dosages. Exclusion Criteria: Type 1 diabetes mellitus, chronic renal failure (eGFR < 30 mL/min/1.73 m2), recent acute diseases, chronic inflammatory diseases, active cancer, LDL cholesterol > 160 mg/dL, significant atherosclerosis, use of ezetimibe, fibrates, or niacin, EP hormone therapy, pregnancy, lactation, or inability to provide informed consent. | NRP | M1/M2 polarization, hsCRP | NCT01600690 | This study investigates statin withdrawal. Duration of the study is relatively short with a small sample size of just 34 patients. |
| Atorvastatin | Bronchiectasis | 4 | 6 months | Inclusion Criteria:
| Between 23 June 2011, and 30 January 2011, 82 patients were screened for inclusion in the study and 22 were excluded before randomisation. 30 individuals were assigned atorvastatin and 30 were allocated placebo. The change from baseline to 6 months in LCQ score differed between groups, with a mean change of 1.5 units in patients allocated atorvastatin versus −0.7 units in those assigned placebo (mean difference 2.2, 95% CI 0.5–3.9; p = 0.01). 12 (40%) of 30 patients in the atorvastatin group improved by 1.3 units or more on the LCQ compared with five (17%) of 30 in the placebo group (difference 23%, 95% CI 1–45; p = 0.04). Ten (33%) patients assigned atorvastatin had an adverse event versus three (10%) allocated placebo (difference 23%, 95% CI 3–43; p = 0.02). No serious adverse events were recorded. | Assessment of sputum neutrophil numbers and apoptosis; neutrophil activation in the airway, measured by sputum myeloperoxidase, free elastase activity, and interleukin 8 (a key neutrophil chemoattractant in bronchiectasis); systemic inflammation, measured by white-blood-cell count, concentrations of C-reactive protein, and the erythrocyte sedimentation rate; other markers of systemic inflammation, including amounts of interleukins 1β, 6, 8, 10, and 12p70, and tumour necrosis factor α. | NCT01299181 | This study was referenced in the following research paper [87]. Had a small sample size of 60 participants |
| Atorvastatin | Type 2 Diabetes + Atherosclerosis | Withdrawn | 12 weeks | Inclusion Criteria:
| NRP | hs-CRP, adiponectin, MCP-1, PAI-1, TNF-α, IL-6 | NCT00932048 | Study was withdrawn |
| Atorvastatin | Vascular calcification + Atherosclerosis + Dyslipidemia+ Inflammation | 3 | 1 year | Inclusion Criteria:
| NRP | hsCRP | NCT00481364 | N/A |
| Simvastatin | Asthma | 3 | 8 weeks | Inclusion Criteria: Eligible participants will be male or female adults aged 18 to 70 years with forced expiratory volume in one second (FEV1) > 60% of the predicted value, as determined by standardized spirometric assessment, and requiring < 1000 µg/day of beclomethasone dipropionate or an equivalent inhaled corticosteroid dose for maintenance therapy. Exclusion Criteria: Subjects will be excluded if they have a documented history of chronic renal disease or a serum creatinine concentration > 2.0 mg/dL, or a history of chronic hepatic disease of any etiology. Additional exclusions encompass current pregnancy or lactation, prior exposure to statin pharmacotherapy, documented hypersensitivity or allergic reaction to statins, a history of statin-induced myositis, or any asthma exacerbation necessitating systemic corticosteroid therapy within the 3-month interval preceding study enrolment. | No Result Posted | Sputum eosinophil | NCT00792337 | Participants were given Simvastatin and Budenoside together. Duration of the study is relatively short. The study also utilized a small sample size of 53 enrollments. |
| Atorvastatin | Obstructive Sleep Apnea | 2 | 3 months | Inclusion Criteria: Eligible participants will comprise adult male and female subjects aged > 18 years with a confirmed diagnosis of obstructive sleep apnoea syndrome (OSAS), operationally defined by an apnoea–hypopnoea index (AHI) > 30 events/hour on polysomnography. Subjects must concurrently exhibit clinical arterial hypertension (ATH) of grade I or II, pharmacologically managed with monotherapy, with seated systolic arterial pressure (SAP) between 140 and 180 mmHg and diastolic arterial pressure (DAP) between 90 and 110 mmHg, measured under standardized conditions. Exclusion Criteria: Subjects will be excluded if they have a documented history of cerebrovascular accident or coronary ischaemic disease, chronic respiratory pathology (including, but not limited to, advanced chronic obstructive pulmonary disease or restrictive interstitial lung disorders), primary or secondary hypothyroidism, or are currently receiving statin therapy. Individuals requiring antihypertensive polypharmacy (i.e., >1 pharmacological agent for blood pressure control), pregnant or lactating women, and those with habitual ethanol consumption exceeding 3 units/day will be ineligible. Additional exclusions encompass concomitant treatment with pharmacological agents possessing significant cytochrome P450–mediated metabolic interactions or other clinically relevant drug–drug interactions, including azole antifungals (itraconazole, ketoconazole), antiprotease agents, fibrates, vitamin K antagonists, non-dihydropyridine calcium channel blockers (diltiazem, verapamil), macrolide antibiotics (erythromycin, clarithromycin), and cyclosporin. Hypersensitivity to investigational drug constituents, modification of existing therapeutic regimens within the preceding 3 months, severe excessive daytime somnolence with potential occupational safety hazards, or employment in safety-sensitive positions will also constitute exclusion criteria. | 51 severe OSA patients were randomized. Key demographics for the study population were age 54 ± 11 years, 21.6% female, and BMI 28.5 ± 4.5 kg/m2. In intention to treat analysis, mean PAT difference between atorvastatin and placebo groups was 0.008 (−0.29; 0.28), p = 0.979. Total and LDL cholesterol significantly improved with atorvastatin. Systolic BP significantly decreased with atorvastatin (mean difference: −6.34 mmHg (−12.68; −0.01), p = 0.050) whereas carotid atherosclerosis and PWV were unchanged compared to the placebo group. | hsCRP, LTE4, 11-DHTXB2 | NCT00669695 | The following paper references the clinical study showing a direct link to trial’s findings [88]. Study was terminated as the interim analysis was performed without efficient results. Statin treatment can be studied for a longer duration and with a larger sample size for more efficient data. |
| Atorvastatin | TB-IRS+ Immune Reconstitution Inflammatory Syndrome + Immune Reconstitution Syndrome | 2 + 3 | 7 days | Inclusion Criteria
| NRP | hsCRP | NCT01442428 | The trial was terminated prior to enrolment owing to unforeseen natural disaster–related disruptions. Specifically, the 2011 Thailand flooding resulted in the destruction of the Good Manufacturing Practice (GMP)–compliant pharmacy facility designated for investigational product handling and storage. This event precipitated substantial operational delays, compounded by associated project timeline overruns and regulatory compliance obstacles, ultimately rendering continuation of the study infeasible. |
| Simvastatin | Cystic Fibrosis + Systemic Inflammation | 1 + 2 | 12 weeks | Inclusion Criteria
| NRP | IL-6, TNF, IL-1β, LPS, LBP, sCD14, EndoCAB, SP-D, CCL-18 | NCT01092572 | Study was withdrawn due to lack of funding. |
| Simvastatin | COPD | 3 | Week 12 | Inclusion Criteria
| NRP | Systemic Inflammation | NCT02070133 | Short study duration yielded with a very small sample size of only 18 participants. |
| Simvastatin | COPD | 4 | 6 weeks | Inclusion Criteria: Male or female patients aged 45–80 years with confirmed COPD (FEV1 30–80% predicted, FEV1/FVC < 0.7, salbutamol reversibility < 12%), supportive smoking history, able to attend regular clinic appointments, and willing to comply with study requirements. Females of childbearing potential must have a negative pregnancy test at screening and use contraception. Exclusion Criteria: Known hypersensitivity to statins or related medications, clinically significant liver function abnormality, hypercholesterolemia (≥6.5 mmol/L), pregnancy or breastfeeding without contraception, conditions deemed detrimental to the study by the investigator, recent exacerbation, significant hypoxia, lactose intolerance, specific medical therapies or conditions as listed. | Total cholesterol dropped in the active group. There was no significant change in aortic PWV between the active group and the placebo group (−0.7 m/s, p = 0.24). In those with aortic stiffness > 10 m/s (n = 22), aortic PWV improved in the active group compared with the placebo group (−2.8 m/s, p = 0.03). Neither systemic nor airway inflammatory markers changed | MMP-9, hsCRP, NO | NCT01151306 [89] | Duration of the study was short with a sample size of 70. |
| Atorvastatin | Atrial Fibrillation + Arrhythmia + Inflammation+ Endothelial Dysfunction | 4 | 3 months | Inclusion Criteria
| No Results Posted | CRP | NCT00579098 | N/A |
| Pravastatin | Schizophrenia + Schizoaffective disorders | 4 | 12 weeks | Inclusion Criteria
| Increase in mean change in CRP was reported compared to placebo, but significance was not reported | CRP | NCT01082588 [90] | Duration of the study was short with a sample size of only 60 participants. |
| Lovastatin | Lung inflammation | 1 | 24 h | Inclusion Criteria: Healthy individuals aged 19–44 years, any race or ethnicity, with FEV1 and FVC > 80% of predicted and oxygen saturation > 97% on room air. Must be able to lie supine in a PET scanner for ~2.5 h and fast for 6 h. Exclusion Criteria: Pregnancy, lactation, actively menstruating at randomization, history of tobacco or illicit drug use in the past year, current prescription medication use, increased risk of radiation exposure, participation in another investigational drug study, known allergies to trimethoprim/sulfamethoxazole, amoxicillin, bronchoscopy drugs, lovastatin, or rhAPC, fasting glucose > 150 mg/dL. Additional exclusions for rhAPC and lovastatin use include recent internal bleeding, hemorrhagic stroke, severe trauma, anticoagulant or thrombolytic therapy, bleeding disorders, liver disease, certain medications, and specific lab abnormalities. | There was a statistically significant decrease in K(i) in the lovastatin-treated group that was not seen in the placebo-treated group, suggesting attenuation of inflammation by lovastatin treatment despite a small decrease in BAL total leukocyte and neutrophil counts that was not statistically significant. No significant decrease in K(i) was observed in the rhAPC-treated group, correlating with a lack of change in BAL parameters and indicating no significant anti-inflammatory effect with rhAPC. | Change in net influx constant (Kᵢ)—a quantitative index of [18F]fluorodeoxyglucose ([18F]FDG) uptake—in the right lung at 24 h post–lipopolysaccharide (LPS) instillation, as determined by Patlak graphical analysis of dynamic positron emission tomography (PET) imaging data. | NCT00741013 [91] | The study has a very short duration and has a very small sample size of 22 participants. |
| Atorvastatin | Atrial Fibrillation | 3 | 12 months | Inclusion Criteria: Patients aged ≥ 18 years with a clinical diagnosis of atrial fibrillation/flutter (ECG documented) and able to swallow pill form of drug. Exclusion Criteria: Patients < 18 years, enrolled in another trial, with paroxysmal atrial fibrillation, hemodynamic instability, recent atrial fibrillation ablation, contraindications for anticoagulation, severe valvular heart disease, single lead ICD, unstable angina, NYHA Class IV heart failure, hyperthyroidism, uncontrolled hypertension, limited life expectancy (<1 year), recent statin use, coronary artery disease requiring statin therapy, implanted arrhythmia management devices, no telephone access, illicit drug or alcohol abuse, atorvastatin hypersensitivity, pregnancy, sexually active females not on contraception, nursing mothers, chronic liver disease, severe renal disease, inflammatory muscle disease, CK > 3 times ULN, or concurrent use of cyclosporine, fibrates, or high-dose niacin. | Although no significant effect was seen on oxidative stress, 2 of 4 inflammatory markers, IL-6 (adjusted OR: 0.59, 95% CI: 0.35–0.97, p = 0.04) and hs-CRP (adjusted OR: 0.59, 95% CI: 0.37–0.95, p = 0.03) were significantly lowered with atorvastatin. Cholesterol levels significantly decreased with atorvastatin (p = 0.03). | Serum oxidative stress markers (ratios of oxidized to reduced glutathione and cysteine, derivatives of reactive oxygen species, isoprostanes) and inflammatory markers (high-sensitivity C- reactive protein [hs-CRP], interleukin-6 [IL-6], interleukin-1β[IL-1β], tumour necrosis factor α [TNFα]) | NCT00252967 [92] | Study was terminated due to insufficient power to show therapy difference at interim analysis [92]. |
| Atorvastatin | Arterial Occlusive Disease + Intermittent Claudication + Insulin Resistance | 2 + 3 | 1 h | Inclusion Criteria Eligible participants must meet all of the following:
Participants will be excluded if any of the following apply:
| Compared with healthy subjects, PAD subjects had higher levels of circulating TNF-α (p < 0.0001), CRP (p = 0.003), sICAM (p < 0.0001), and IL-6 (p < 0.0001). Expression of both IL-6 (p = 0.024) and CD36 (p = 0.018) was greater in PAD subjects than in healthy subjects. Among subjects with PAD, higher gene expression of TNF-α was associated inversely with MWT (p = 0.01). MWT was also associated inversely with greater levels of circulating TNF-α (p = 0.028), CRP (p = 0.024), IL-6 (p = 0.03), and sICAM (p = 0.018). | NA | NCT00153166 [93] | The study was conducted for a very short duration of time. |
| Simvastatin | Atrial Fibrillation | 2 | 6 months | Inclusion Criteria: Patients aged 18 years and older with paroxysmal atrial fibrillation (PAF) (≥3 episodes each > 15 min in length) over 6 months, on stable antiarrhythmic drug therapy, and with a life expectancy > 1 year. Exclusion Criteria: Patients with PAF due to a reversible cause, chronic inflammatory conditions, other medical conditions requiring statin therapy, on amiodarone or verapamil, elevated CK or ALT, life expectancy < 1 year, TAVN ablation, geographic isolation, or inability to give informed consent. | NRP | CRP, oxidative stress levels | NCT00321802 | Small sample size of only 40 participants |
| Fluvastatin | Ageing, Inflammation | 2 | 24 months | Inclusion Criteria: Patients aged 60–75 years, physically active less than 3 times a week, and able to provide written informed consent. Exclusion Criteria: Patients with heart disease requiring treatment, treatment with beta-receptor antagonists, statins, immunosuppressive drugs, or anti-inflammatory drugs, underlying hematological disease, alcohol or drug abuse, diabetes mellitus, recent study participation (within 30 days), known intolerance to the active agent, active liver disease, unexplained persistent elevation of serum transaminases or cholestasis, existing myopathy, pregnancy or nursing, absence of an ophthalmological examination within 12 months prior to inclusion, or known cataract. | NRP | Inflammatory parameters | NCT01045512 | The study was terminated due to insufficient recruitment of participants. |
| Rosuvastatin | HIV Infection + Cardiovascular disease | 2 + 3 | 3 months | Inclusion Criteria
| NRP | Primary and Secondary Biomarker Endpoints
| NCT00986999 | Study was terminated and no results were obtained due to poor enrollment of participants. |
| Atorvastatin | Renal Insufficiency (Graft Donor Kidney) | 3 | 12 months | Inclusion Criteria
| NRP | CRP, IL-6, TNF-α | NCT02355704 | Small sample of 48 participants was used. |
| Atorvastatin | Atherosclerotic Carotid Disease + Atheroma + Atherosclerosis | NA | 12 weeks | Inclusion Criteria
| Twenty patients completed 12 weeks of treatment in each group. A significant reduction from baseline in USPIO-defined inflammation was observed in the 80 mg group at both 6 weeks (DeltaSI 0.13; p = 0.0003) and at 12 weeks (DeltaSI 0.20; p < 0.0001). No difference was observed with the low-dose regimen. The 80-mg atorvastatin dose significantly reduced total cholesterol by 15% (p = 0.0003) and low-density lipoprotein cholesterol by 29% (p = 0.0001) at 12 weeks. | USPIO-enhanced MRI-defined inflammation | NCT00368589 | The following paper is linked to the clinical trial [94]. Short duration of study and a small sample size of 47 |
| Rosuvastatin | Carotid Artery Plaque + Ankylosing Spondylitis + Rheumatoid Arthritis | NA | 18 months | Inclusion Criteria: Men and women aged 35–80 with RA, AS, or other inflammatory arthritis forms, having cholesterol plaques in the carotid artery as shown by ultrasound, and who have given informed consent. Exclusion Criteria: Concomitant statin treatment, chronic irregular heart rhythm (e.g., atrial fibrillation), contraindications to statins (e.g., liver disease, statin-induced myopathy, hypersensitivity), raised creatinine, pregnancy or breastfeeding, lack of contraceptives in fertile women, cyclosporine treatment, interaction-prone medications, uncontrolled hypothyroidism, low creatinine clearance, secondary hyperlipidemia, uncontrolled diabetes, severe heart failure, gastrointestinal diseases affecting absorption, cancer, severe psychiatric illness, life-threatening arrhythmias, alcohol abuse, and participation in other studies. | No significant changes in inflammatory markers | ESR, CRP | NCT01389388 [95,96,97,98,99] | N/A |
| Atorvastatin | Type 2 Diabetes + Hypercholesterolemia | 4 | 30 days | Inclusion Criteria
| The atorvastatin-assigned group showed a significant and progressive reduction in urinary isoprostanes and serum Nox2, along with inhibition of platelet recruitment, platelet isoprostanes, Nox2, Rac1, p47(phox), and protein kinase C, starting 2 h after administration. Platelet phospholipase A(2) and thromboxane A(2) significantly decreased, and vasodilator-stimulated phosphoprotein and nitric oxide increased after 24 h. Low-density lipoprotein cholesterol decreased significantly after 72 h and further declined after 7 days. No changes were observed in the Mediterranean diet group. In vitro experiments demonstrated that atorvastatin dose-dependently inhibited platelet Nox2 and phospholipase A(2) activation, along with inhibition of platelet recruitment, platelet isoprostanes, and thromboxane A(2), and increased vasodilator-stimulated phosphoprotein and nitric oxide. | Oxidative stress, as assessed by serum Nox2 and urinary isoprostanes, and platelet activation, as assessed by platelet recruitment, platelet isoprostanes, and thromboxane A(2), platelet Nox2, Rac1, p47(phox), protein kinase C, vasodilator-stimulated phosphoprotein, nitric oxide, and phospholipase A(2) | NCT01322711 [100] | Brief study period, hence needs further research and follow up. Has a small sample size of only 60 participants. |
| Simvastatin | Atherosclerosis | NA | 3 months | Inclusion Criteria
| 18F-fluorodeoxyglucose positron emission tomography (FDG-PET) identified 117 FDG-avid atheromatous plaques in the simvastatin-treated cohort and 123 in the dietary-intervention cohort at baseline. Pharmacologic intervention with simvastatin, but not dietary modification alone, elicited a statistically significant attenuation of plaque FDG uptake, reflected by a reduction in standardized uptake values (SUVs) (p < 0.01). Simvastatin therapy achieved a 30% decrement in low-density lipoprotein cholesterol (LDL-C) (p < 0.01) and a 15% increment in high-density lipoprotein cholesterol (HDL-C) (p < 0.01), whereas no significant modulation of LDL-C or HDL-C was observed in the diet-only group. Within the simvastatin arm, the magnitude of SUV reduction exhibited a robust positive correlation with HDL-C elevation (p < 0.01), but no statistically significant association with the degree of LDL-C reduction. | hsCRP, plaque inflammation | NCT00114504 [101,102] | The following papers have referenced the trial. The study has a small sample size of only 43 participants. |
| Simvastatin | Rhinosinusitis | 2 | 60 days | Inclusion Criteria
| NRP | IL6, IL8, IL10, TNF | NCT01771198 | The study was terminated due to lack of efficacy with one month of treatment. |
| Atorvastatin, Simvastatin, Pravastatin, Fluvastatin, Lovastatin | Carotid Atherosclerosis | Withdrawn | 6 months | Inclusion Criteria: Adult patients (>21 years old) with diagnosed coronary artery disease or suspected cerebrovascular accident. Patients either having their statin dose or LDL lowering therapy increased to a high dose or maintained at a standard dose. Exclusion Criteria: Severe claustrophobia, implanted ferromagnetic materials unsuitable for MRI, anticipated carotid stenting or surgery within 6 months, inability to lie flat for 1 h, need for supplemental oxygen, pregnancy, and inability to follow up for 6 months. | NRP | Inflammatory Markers | NCT00388843 | No results published with the study being withdrawn. |
| Atorvastatin | Vascular Disease | 2 | 12 weeks | Inclusion Criteria: Adults aged 18–75 years with angiographically, ultrasonographically, or otherwise clinically documented atherosclerotic cardiovascular disease, presenting with fasting low-density lipoprotein cholesterol (LDL-C) concentrations between 70 and 130 mg/dL despite ongoing therapy with a stable regimen of low- to moderate-intensity statin for a protocol-defined pre-enrolment period. Eligible participants must exhibit a body mass index (BMI) within the range of 18–37 kg/m2, possess the ability to swallow oral solid dosage forms, and demonstrate adequate tolerance for all study-related interventions, including invasive or imaging-based procedures, administration of iodinated contrast agents, and trial pharmacotherapies. Exclusion Criteria: Documented statin intolerance or contraindication to continued statin therapy; clinically significant renal impairment, defined as a serum creatinine concentration > 1.5 mg/dL; known chronic viral hepatitis (e.g., hepatitis B or C) or biochemical/laboratory evidence of hepatic dysfunction; history of major infectious illness necessitating hospitalization or parenteral antimicrobial therapy within 2 months prior to initiation of study treatment. | Treatment with the p38 mitogen-activated protein kinase inhibitor BMS-582949 failed to elicit a statistically significant attenuation of arterial inflammation when compared with placebo, as evidenced by the change in target-to-background ratio (ΔTBR index: 0.10; 95% confidence interval [CI]: −0.11 to 0.30; p = 0.34) and in active slices (ΔTBR_(AS): −0.01; 95% CI: −0.31 to 0.28; p = 0.93). Similarly, there was no significant effect on high-sensitivity C-reactive protein (hs-CRP) concentrations (median percentage change [interquartile range, IQR]: 33.83% [153.91] for BMS-582949 vs. 16.71% [133.45] for placebo; p = 0.61). By contrast, statin intensification was associated with a statistically significant reduction in systemic inflammation, as reflected by hs-CRP (median % change [IQR]: −17.44% [54.68] vs. 16.71% [133.45] for placebo; p = 0.04), and a concomitant reduction in arterial inflammation within active slices (ΔTBR_(AS) = −0.24; 95% CI: −0.46 to −0.01; p = 0.04). | hsCRP, MMP9, IL-6, TNF-α | NCT00570752 [103] | Short study duration and small sample size of 72. |
| Pravastatin | HIV-1 Infection | Withdrawn | 24 weeks | Inclusion Criteria: Adults aged > 18 years with confirmed HIV-1 infection, demonstrating willingness to defer initiation of antiretroviral therapy (ART) and/or lipid-lowering therapy with statins for the study duration. Participants must present with a peripheral CD4+ T-lymphocyte count > 500 cells/mm3 and have no prior ART exposure exceeding 10 cumulative days, with the exception of short-course regimens administered for post-exposure prophylaxis, prevention of mother-to-child transmission, or other protocol-permitted circumstances. No lipid-lowering pharmacotherapy within 60 days and no ART within 30 days of enrolment are permitted. Eligibility requires capacity to provide written informed consent, absence of exclusionary medical or neuropsychiatric conditions, and fulfilment of protocol-defined laboratory thresholds for haematologic, hepatic, and renal function. Cardiovascular risk must meet one of the following criteria: (i) Framingham Risk Score (FRS) ≥ 10%, or (ii) FRS ≥ 6% in the context of high-sensitivity C-reactive protein (hsCRP) > 3.0 mg/L. Participants must have no exclusionary HIV-1 resistance mutations, must complete a pre-entry flow-mediated dilation (FMD) assessment, and—if female and of reproductive potential—must have a negative serum or urine pregnancy test and commit to effective contraception. All candidates must demonstrate capacity to complete the neuropsychological test battery. Exclusion Criteria: Pregnancy or lactation; documented hypersensitivity or intolerance to study drugs; established cardiovascular disease; uncontrolled type II diabetes mellitus; hepatic cirrhosis; chronic systemic inflammatory or autoimmune disorders; recent (within protocol-defined intervals) major infection, severe illness, or trauma necessitating hospitalization; febrile illness or acute intercurrent disease at screening; New York Heart Association (NYHA) class III/IV congestive heart failure; uncontrolled hypertension; untreated hypothyroidism; active malignancy; active CNS infection, neoplasm, or other neurological disorder impacting cognition; failure to maintain ≥ 8 h of fasting and abstinence from tobacco use or exercise prior to protocol assessments; anticipated initiation/cessation of smoking or major dietary/exercise modifications during the trial; active substance use disorder; psychiatric illness likely to impair adherence; exposure to hormonal anabolic agents or immunomodulators within 60 days; receipt of routine prophylactic or therapeutic vaccinations within 7 days of study procedures; high-dose supplementation with antioxidant vitamins E or C; systemic glucocorticoid therapy above physiologic replacement doses; concurrent hepatitis C pharmacotherapy; or investigational agents administered within 90 days prior to enrolment. | NRP | IL-6, hsCRP | NCT01515813 | The study was withdrawn as the trial is under revision. |
| Atorvastatin | Septic Shock | NA | 28 days | Inclusion Criteria: Clinical disease of septic shock, aged 18 years and above, admitted to ICU. Exclusion Criteria: Previous statin-induced myopathy or hypersensitivity reaction, liver transaminases greater than 2.5 times the normal limit, chronic liver disease, pregnant or lactating mothers. | Seventy-three septic shock patients with 36 and 37 included in the atorvastatin and placebo group, respectively. Both groups were equally matched. Twenty-eight-day mortality, event-free days, lipid profile, and adverse effects were also not significantly different between groups. Reduced levels of IL-1, IL-6, TNF-α, IFN, and CRP were observed in the atorvastatin group. Also observed were significant day-wise changes in inflammatory biomarkers. | IL-1, IL-6, TNF-α, IFN-γ, and CRP | NCT02681653 [104] | Short duration of study with only 40 enrollments. |
| Simvastatin | Asthma | 3 | 8 weeks | Inclusion Criteria: Patients aged 18–70 years old with mild-to-moderate persistent asthmatics, FEV1 ≥ 50% of predicted. Exclusion Criteria: Previous history of renal or liver disease, serum creatinine > 2 mg/dL, pregnancy or lactation, allergic to statins or have developed myositis, asthma exacerbation requiring oral corticosteroids in the past 3 months, treated with immunosuppressive agents, unwilling to cooperate with the study. | NRP | Sputum eosinophils, phosphorylated p38 MAPK | NCT01266434 | Short study duration with an enrollment of only 44 participants. |
| Simvastatin | Heart Failure | 1 | 3 years | Inclusion Criteria: Adults aged 18 to 85 years, symptomatic heart failure (NYHA class I to III), left ventricular ejection fraction < 0.40, and ability to give written informed consent. Exclusion Criteria: Pregnant or lactating women, heart failure due to specific conditions (e.g., active myocarditis, congenital heart disease), NYHA class IV symptoms, current or previous statin treatment, plasma LDL-C > 130 mg/dL with certain conditions (e.g., ischemic cardiomyopathy), progressive systemic diseases, uncorrected endocrine disorders, significant renal or hepatic disease, and inability or unwillingness to cooperate with the study. | NRP | Monocyte TF | NCT00769210 | The study had a small sample size of only 12 enrollments. |
| Rosuvastatin | Sepsis + Acute Lung Injury | 3 | 28 Days | Inclusion Criteria: Patients fulfilling the diagnostic parameters for systemic inflammatory response syndrome (SIRS)—operationalised by predefined thresholds for leukocyte count, core body temperature, and heart rate—concomitant with clinically suspected or microbiologically confirmed infection, and meeting the Berlin-derived definitional elements for acute lung injury (ALI), namely: (i) acute-onset hypoxaemia, (ii) radiographically confirmed bilateral pulmonary infiltrates, (iii) requirement for positive-pressure ventilatory support, and (iv) absence of haemodynamic evidence of left atrial hypertension. All ALI-defining parameters must manifest within a contiguous 24 h interval. Eligibility further requires enrolment within 48 h of ALI onset and ≤7 days from the initiation of invasive mechanical ventilation. Exclusion Criteria: Inability to secure informed consent; age < 18 years; duration of invasive mechanical ventilation exceeding 7 days; >48 h elapsed since fulfilment of ALI diagnostic criteria; designation for comfort measures only; inability to absorb enteral pharmacotherapy; prior statin exposure within the preceding washout interval; documented statin hypersensitivity or intolerance; attending physician–mandated statin therapy; elevated creatine kinase (CK), alanine aminotransferase (ALT), or aspartate aminotransferase (AST) beyond upper reference thresholds; untreated hypothyroidism; pregnancy or lactation; concurrent use of contraindicated pharmacologic agents; advanced chronic liver disease; moribund clinical status; chronic respiratory failure requiring long-term ventilatory support; home mechanical ventilation outside the context of obstructive sleep apnoea; diffuse alveolar hemorrhage; severe thermal injury; interstitial lung disease necessitating domiciliary oxygen therapy; unwillingness to adhere to the ARDS Network mechanical ventilation protocol; severe structural or ischaemic cardiac pathology; recent myocardial infarction; recent intracranial haemorrhage; or hyperpyrexia exceeding 40.3 °C. | No results pertaining to changes in inflammatory markers. | CRP | NCT00979121 [105,106,107,108] | The study was terminated and also had a short duration |
| Rosuvastatin vs. Atorvastatin | Hyperlipidemia | 2 + 3 | 8 weeks | Inclusion Criteria: Adults aged 20–75 years, of either sex, with fasting low-density lipoprotein cholesterol (LDL-C) concentrations between 160 and 190 mg/dL and fasting triglyceride concentrations between 200 and 499 mg/dL at screening. Exclusion Criteria: Age < 20 years or >75 years; current use of lipid-lowering pharmacotherapy; concomitant intake of omega-3 fatty acid preparations or garlic-derived nutraceuticals; documented hypersensitivity to statin-class agents; concurrent use of systemic anti-inflammatory drugs (including corticosteroids or nonsteroidal anti-inflammatory drugs); intake of antioxidant vitamins A, C, or E; evidence of renal or hepatic impairment; pregnancy or lactation; active serious infection; or presence of a terminal illness. | NRP | Hs-CRP | NCT02979704 | Small sample size with only 90 enrollments. |
| Simvastatin | Sickle Cell Disease | 1 + 2 | 3 months | Inclusion Criteria: Patients aged ≥ 10 years with a confirmed diagnosis of sickle cell disease (HbSS genotype or S/β0-thalassemia), who have experienced ≥ 3 vaso-occlusive pain episodes within the 12 months preceding enrolment, and with a body mass ≥ 30 kg. Exclusion Criteria: Creatine kinase (CK) concentration exceeding the upper normal limit (UNL); total cholesterol < 90 mg/dL or triglycerides < 30 mg/dL; renal dysfunction defined as serum creatinine > 1.5 × UNL; hepatic dysfunction defined as alanine aminotransferase (ALT) > 2 × UNL; receipt of any pharmacologic agents with known metabolic interactions with statins within the preceding 30 days; hospitalization for vaso-occlusive pain within the preceding 30 days; receipt of red blood cell transfusion within the preceding 30 days; current pregnancy or lactation; musculoskeletal disorders associated with elevated CK; history of substance misuse; chronic pain of non–sickle cell etiology; or significant cognitive or neurological impairment precluding the use of smartphone technology or completion of electronic pain diaries. | Significant decrease in plasma high sensitivity C-reactive protein level after treatment with simvastatin | Hs-CRP | NCT01702246 [109] | Small sample size with only 24 enrollment. |
| Atorvastatin | Asthma + COPD + Smoking | 2 | 8 weeks | Inclusion Criteria: Adults aged 18–60 years with a confirmed clinical diagnosis of asthma of >1 year’s duration, currently symptomatic, and with a cumulative smoking exposure exceeding 5 pack-years. Eligible participants should be receiving only short-acting β2-adrenergic agonists as bronchodilator therapy; other asthma-related pharmacotherapies may be tapered or discontinued if the patient demonstrates clinical stability. Exclusion Criteria: Never-smokers or former smokers; current statin therapy; unstable or poorly controlled asthma; documented hypersensitivity or intolerance to statins; history of statin-associated myopathy or myositis; or concurrent use of pharmacological agents with known clinically significant interactions with statins. | At 4 weeks, there was no improvement in the atorvastatin group compared to the placebo group in morning peak expiratory flow [−10.67 L/min, 95% CI −38.70 to 17.37, p = 0.449], but there was an improvement with atorvastatin in asthma quality of life score [0.52, 95% CI 0.17 to 0.87 p = 0.005]. There was no significant improvement with atorvastatin and inhaled beclometasone compared to inhaled beclometasone alone in outcome measures at 8 weeks. | LB4, MPO, IL-6, IL-8, IL-10, hsCRP, sputum inflammatory cells | NCT00463827 [110] | Short duration of study with sample size of 71. |
| Atorvastatin | Bronchiectasis + Pseudomonas aeruginosa | 4 | 7.5 months | Inclusion Criteria: Patients aged 18–80 years with an established radiological diagnosis of bronchiectasis (CT of the chest) and colonized with Pseudomonas Aeruginosa. Patients must be able to give informed consent. Exclusion Criteria: Current smokers or ex-smokers of less than 1 year with a smoking history of more than 15 pack years, cystic fibrosis, active allergic bronchopulmonary aspergillosis, active tuberculosis, poorly controlled asthma, pregnancy or breastfeeding, known allergy to statins, active malignancy, chronic liver disease, established cardiovascular or cerebrovascular disease, or statin use in the last year. | Twenty-seven patients completed the study. Atorvastatin did not significantly improve the primary end point of cough as measured by the Leicester Cough Questionnaire (mean difference, 1.92; 95% CI for difference, −0.57–4.41; p = 0.12). However, atorvastatin treatment resulted in an improved St. Georges Respiratory Questionnaire (−5.62 points; p = 0.016) and reduced serum levels of CXCL8 (p = 0.04), tumour necrosis factor (p = 0.01), and intercellular adhesion molecule 1 (p = 0.04). There was a trend toward improvement in serum C-reactive protein and serum neutrophil counts (p = 0.07 and p = 0.06, respectively). We demonstrated in vitro that atorvastatin 10 μM reduced formyl-methionyl-leucyl phenylalanine-induced upregulation of CD11b expression and changes in calcium flux, reflecting an ability to decrease neutrophil activation. | Sputum neutrophil numbers and apoptosis; neutrophil activation in the airway measured by sputum myeloperoxidase, free elastase activity, and CXCL8; systemic inflammation measured by WBC count, CRP, and erythrocyte sedimentation rate; other markers of systemic inflammation, including concentrations of IL-1b, IL-6, CXCL8, IL-10, IL-12p70, TNF-α, and ICAM-1 | NCT01299194 [111] | Small sample size of only 32 participants. |
| Atorvastatin | Complications of Renal Dialysis | Withdrawn | 24 weeks | Inclusion Criteria: Adults aged 18–110 years, clinically stable on recombinant human erythropoietin (Epogen®) with maintained hemoglobin concentrations between 9.5 and 11.5 g/dL for ≥3 consecutive weeks prior to enrolment, no statin exposure within the preceding 3 months, and demonstrable adherence to prescribed haemodialysis regimens and concomitant medications. Exclusion Criteria: Documented history of myocardial infarction, cerebrovascular accident, or clinically significant peripheral or coronary vascular disease; hospitalization within 15 days prior to screening; major haemorrhagic events within the preceding 15 days (including traumatic, gastrointestinal, genitourinary, or menorrhagic bleeding); clinically evident hepatic dysfunction; active malignant neoplasm; or known haematologic pathology. | No Results Posted | Ferritin, CRP | NCT02764736 | Study was withdrawn as the enrollment did not progress as anticipated by the researchers. |
| Simvastatin | Perioperative Inflammatory Response | 4 | 72 h | Inclusion Criteria: Patients aged 18–80 years scheduled for elective major spine surgery (multilevel (2–6 level) open thoracic or lumbar spine surgery with instrumentation). Exclusion Criteria: Pregnancy; lactating females; oral or parenteral corticosteroid use in the past 30 days; elevation of AST or ALT > 3× normal; elevation of creatinine kinase > 2× normal; previous adverse drug reaction to any medication in the statin class; current use of fibrates, niacin, itraconazole, ketoconazole, macrolide antibiotics, HIV protease inhibitors, and/or nefazodone; active liver disease; current statin use; use of certain anti-inflammatory medications within the last 30 days and post-operative period. | Results were reported without significance level. | CRP, IL-6, TNF-α | NCT00656292 | Study duration is short with a sample size of only 61 participants. |
| Atorvastatin | Metabolic Syndrome | 1 + 2 | 6 weeks | Inclusion Criteria: Female participants aged 18–75 years meeting diagnostic criteria for metabolic syndrome, operationalised as central adiposity (abdominal circumference > 35 inches), hypertriglyceridemia (>150 mg/dL), reduced high-density lipoprotein cholesterol (HDL-C < 50 mg/dL), elevated blood pressure (>130/85 mmHg), and impaired fasting glycaemia (fasting plasma glucose > 100 mg/dL). Exclusion Criteria: Current pregnancy or intent to conceive within the ensuing 6–12 months; ongoing therapy with lipid-lowering agents; presence of obstructive hepatobiliary pathology or advanced hepatic insufficiency; established diabetes mellitus, clinically manifest cardiovascular disease, hypothyroidism, active infectious processes, or malignancy; major surgical intervention within the preceding months; eligibility for statin therapy based on low-density lipoprotein cholesterol thresholds, composite cardiovascular risk factors, or Framingham risk score; documented hypersensitivity or idiosyncratic reaction to statins; and unexplained creatine kinase elevation > 3 × the upper limit of normal. | At 6 weeks post-randomisation, the atorvastatin arm demonstrated pronounced and statistically significant decrements in total cholesterol, LDL-C, triglycerides, ApoB, and the ApoB/ApoA1 ratio compared with placebo, with these lipid-lowering effects persisting unabated through the 12-week follow-up. Glycaemic indices (fasting plasma glucose), HDL-C, hs-CRP, serum leptin, ApoA1, ICAM-1, and platelet activity metrics exhibited no significant between-group divergence. Intriguingly, VCAM-1 concentrations increased significantly within the atorvastatin cohort at both interim and terminal assessments, representing a paradoxical pro-adhesive signal in the context of otherwise favourable lipid modulation. Anthropometric indices (waist circumference) and haemodynamic parameters (systolic and diastolic blood pressure) remained stable across intervention arms, suggesting the observed biochemical shifts were independent of changes in adiposity or vascular tone. | Hs-CRP, sICAM, sVCAM, PAI-1, MPO | NCT01785615 [112] | Study was performed for a short duration. |
| Simvastatin | Septic Shock | 2 + 3 | 24 h | Inclusion Criteria: Adults aged > 18 years presenting with hypotension necessitating vasopressor support and clinical suspicion of infection. Exclusion Criteria: Pregnancy; hepatic failure defined by alanine aminotransferase (ALT) or aspartate aminotransferase (AST) concentrations exceeding 120 U/L; rhabdomyolysis with creatine phosphokinase (CPK) > 3× the upper limit of normal; designation for comfort measures only; chronic liver disease including cirrhosis; concurrent administration of cyclosporine, digoxin, or any statin therapy; and inability to receive enteral medications via oral or nasogastric tube routes. | In this cohort, 28 plasma specimens derived from 14 individuals meeting enrolment criteria underwent quantitative biochemical profiling. Circulating coenzyme Q10 (CoQ10) concentrations were profoundly diminished relative to physiologically normative values, with a median level of 0.49 μmol/L (interquartile range [IQR], 0.26–0.62), in stark contrast to healthy reference participants (0.95 ± 0.29 μmol/L; p < 0.0001). Temporal trajectory analysis demonstrated no statistically significant modulatory effect of statin administration on systemic CoQ10 abundance over the observation interval (p = 0.13). Bivariate correlation modelling revealed that CoQ10 concentrations exhibited statistically robust associations with a spectrum of endothelial activation and pro-/anti-inflammatory mediators, including vascular cell adhesion molecule-1 (VCAM-1; r2 = 0.20, p = 0.008), tumour necrosis factor-α (TNF-α; r2 = 0.28, p = 0.004), interleukin-8 (IL-8; r2 = 0.21, p = 0.015), interleukin-10 (IL-10; r2 = 0.18, p = 0.025), E-selectin (r2 = 0.17, p = 0.03), interleukin-1 receptor antagonist (IL-1ra; r2 = 0.21, p = 0.014), interleukin-6 (IL-6; r2 = 0.17, p = 0.029), and interleukin-2 (IL-2; r2 = 0.23, p = 0.009). Following multivariable adjustment for low-density lipoprotein cholesterol, inverse associations of biological and statistical significance persisted for VCAM-1 (r2 = 0.24, p = 0.01) and IL-10 (r2 = 0.24, p = 0.02), thereby implicating CoQ10 insufficiency in the modulation of both vascular adhesion molecule expression and counter-regulatory anti-inflammatory signalling in this patient population. | VCAM, TNF-α, IL-8, IL-10, E-selectin, IL-1ra, IL-6, IL-2 | NCT00676897 [113] | Small sample size of only 18 participants out of the expected 60. |
| Atorvastatin | Hip Fracture + Myocardial Ischemia + Inflammation | 2 | 2 days | Inclusion Criteria: Patients aged ≥ 65 years with a hip fracture or scheduled for elective hip or knee arthroplasty, and with an anticipated life expectancy > 3 months. Exclusion Criteria: Pathological hip fracture secondary to cancer; current or recent statin use; prior statin intolerance; acute myocardial infarction or unstable angina; history of myocardial infarction, acute coronary syndrome, angina, coronary or arterial revascularisation, peripheral arterial disease, stroke, or transient ischaemic attack; muscle disorders; significant hepatic disease or alanine aminotransferase (ALT) > 3× the upper limit of normal; severe renal impairment (creatinine clearance < 30 mL/min); treatment with HIV or Hepatitis C protease inhibitors, erythromycin, clarithromycin, niacin, or azole antifungals; and pregnancy, planned pregnancy, or breastfeeding. | From an initial screening cohort of 556 subjects, 22 individuals met the stringent eligibility criteria and were subsequently enrolled—comprising 4 cases of fragility hip fracture, 11 candidates for elective total hip arthroplasty, and 7 for total knee arthroplasty—of whom 2 participants later withdrew consent. Baseline preoperative assessment revealed that 80% of the study population exhibited quantifiable circulating concentrations of high-sensitivity cardiac troponin I (hs-cTnI) exceeding 1.1 pg/mL, indicative of subclinical myocardial injury. A perioperative increment in hs-cTnI of ≥10 pg/mL, fulfilling criteria for clinically relevant biomarker elevation, was documented in 20% of participants, with no evidence of pharmacological attenuation by perioperative atorvastatin exposure. Biomarker profiling demonstrated a postoperative surge in high-sensitivity C-reactive protein (hs-CRP) in 95% (19/20) of evaluable subjects, paralleled by a universal escalation in plasma interleukin-6 (IL-6) concentrations; neither inflammatory axis was modulated by statin therapy. On postoperative day two, IL-6 concentrations exhibited a statistically significant positive monotonic association with hs-cTnI levels (Spearman’s ρ = 0.59, p = 0.02), suggesting potential mechanistic interplay between systemic inflammatory activation and myocardial injury. Participant accrual was substantially constrained by the high baseline prevalence of antecedent statin therapy within the screened surgical population and by the disproportionately elevated rate of exclusion criteria fulfilment among patients presenting with hip fractures. | hs-CRP, IL-1β, IL-2, IL-2r, IL-4, IL-5, IL-6, IL-8, IL-10, IL-12, IL-13, IL-17, TNFα, IFNγ, sCD-40L | NCT02197065 [114] | Short duration of study and a small size of only 20 participants of the estimated 30. |
| Atorvastatin | Vascular Disease | NA | 10 Months | Inclusion Criteria: Adults aged ≥ 21 years, not currently receiving statins or other lipid-lowering agents, with no prior history of statin use, and presenting with at least two of the following cardiovascular risk factors:
Current or prior statin use; immediate clinical indication for statin therapy; use of investigational drugs; chronic or active hepatic or muscular disease; alcohol abuse; prior myocardial infarction; heart failure; uncontrolled arrhythmias; clinically significant hematological or biochemical abnormalities; history of cancer within the recent period; pregnancy or breastfeeding; or concomitant use of medications known to interact with statins (e.g., CYP3A4 inhibitors, nicotinic acid, gemfibrozil). | No Results Posted | Gene expression of inflammatory markers | NCT00293748 | N/A |
| Simvastatin | Heart Disease | NA | 12 months | Inclusion Criteria: Adults (>18 years) with documented atherosclerosis in at least one vascular territory, defined as:
Current statin therapy equivalent to or exceeding 80 mg simvastatin; presence of pacemaker, automated implantable cardioverter-defibrillator (AICD), or aneurysm clips; abnormal nasopharyngeal anatomy; active peptic ulcer disease; severe dysphagia; baseline liver transaminases or serum creatinine > 2× the upper limit of normal; decompensated congestive heart failure; or inability to provide informed consent. | Significant differences were observed between the low-dose and high-dose statin therapy in reduction in low-density lipoprotein cholesterol (LDL-c) (10 mg/dL, p = 0.001), total cholesterol (16.2 mg/dL, p < 0.001), vessel wall area (19.0 mm2, p < 0.001) and volume (343.4 mm3, p < 0.001), as well as increase in lumen area (54.4 mm2, p < 0.001) and volume (1038 mm3, p < 0.001). LDL-c lowering was significantly associated with aortic wall area and volume reduction in both groups. | CRP, IL-6, TNF-α | NCT00125060 [115] | Small sample size of 72 participants. |
| Simvastatin | Severe Asthma | 2 | 12 weeks | Inclusion Criteria: Statin-naïve adults aged > 18 years with severe asthma as defined by ATS; receiving inhaled corticosteroids (ICS) and long-acting β2-agonists (LABA); confirmed allergic asthma (serum IgE > 100 kU/L, positive RAST panel, and/or peripheral blood absolute eosinophil count ≥ 700/mm3); and clinically stable for at least 4 weeks. Exclusion Criteria: Baseline FEV1 < 30% predicted; current smokers or former smokers with >5 pack-years smoking history; pregnancy; lactation; women of childbearing potential actively attempting to conceive; nasal or sinus surgery or trauma within the past 3 months; ischemic heart disease; liver disease; and concurrent use of medications such as amiodarone, verapamil, diltiazem, gemfibrozil, cyclosporine, antifungal azoles, or danazol. | No Results Posted | Th2 gene expression, IL-13, eotaxin-1, eotaxin-2, eotaxin-3, STAT 6, NO | NCT02433535 | The study was performed for a short duration before it was withdrawn. |
| Simvastatin | Cystic Fibrosis | NA | 15 days | Inclusion Criteria: Adults aged 18–50 years, in good general health as determined by medical history; able to comprehend and sign the informed consent form; able to comply with study requirements; and willing to use an acceptable method of birth control. Exclusion Criteria: History of diabetes requiring insulin therapy; use of NSAIDs or corticosteroids (except for nasal steroid preparations); current use of statin medications; active gingival disease; presence of any chronic inflammatory condition compromising immune function; and pregnancy or intention to become pregnant. | Polymorphonuclear leukocytes (PMN) count decreased with simvastatin | Oral mucosal PMNs | NCT00531882 | Short study duration and has a small sample size of only 25 participants. |
| Atorvastatin | HIV + Atherosclerosis | NA | 1 year | Inclusion Criteria: Men and women aged 18–60 years with a prior diagnosis of HIV infection; evidence of subclinical coronary artery disease (plaque on coronary CTA in the absence of cardiac symptoms or events, and TBR > 1.6 on PET imaging); on stable antiretroviral therapy for >6 months without regimen changes; and LDL-cholesterol between 70 and 130 mg/dL. Exclusion Criteria: History of acute coronary syndrome; contraindications to statin therapy; current statin use; serum AST or ALT > 2× upper limit of normal; renal disease or serum creatinine > 1.5 mg/dL; infectious illness within the preceding 3 months; contraindications to beta-blocker or nitroglycerin use; body weight > 300 lbs; prior allergic reactions to iodine-containing contrast agents; significant radiation exposure within the past year; planned procedures involving substantial radiation exposure; pregnancy or breastfeeding; and coronary artery luminal narrowing > 70% on coronary CTA. | The study was conducted from 13 November 2009 to 13 January 2014. A total of 19 patients were assigned to atorvastatin and 21 to placebo. 37 (93%) of 40 participants completed the study, with equivalent discontinuation rates in both groups. Baseline characteristics were similar between groups. After 12 months, change in FDG-PET uptake of the most diseased segment of the aorta was not different between atorvastatin and placebo, but technically adequate results comparing longitudinal changes in identical regions could be assessed in only 21 patients (atorvastatin Δ −0.03, 95% CI −0.17 to 0.12, vs. placebo Δ −0.06, −0.25 to 0.13; p = 0.77). Change in plaque could be assessed in all 37 people completing the study. Atorvastatin reduced non-calcified coronary plaque volume relative to placebo: median change −19.4% (IQR −39.2 to 9.3) versus 20.4% (−7.1 to 94.4; p = 0.009, n = 37). The number of high-risk plaques was significantly reduced in the atorvastatin group compared with the placebo group: change in number of low attenuation plaques −0.2 (95% CI −0.6 to 0.2) versus 0.4 (0.0, 0.7; p = 0.03; n = 37); and change in number of positively remodelled plaques −0.2 (−0.4 to 0.1) versus 0.4 (−0.1 to 0.8; p = 0.04; n = 37). Direct LDL-cholesterol (−1.00 mmol/L, 95% CI −1.38 to 0.61 vs. 0.30 mmol/L, 0.04 to 0.55, p < 0.0001) and lipoprotein-associated phospholipase A2 (−52.2 ng/mL, 95% CI −70.4 to −34.0, vs. −13.3 ng/mL, −32.8 to 6.2; p = 0.005; n = 37) decreased significantly with atorvastatin relative to placebo. Statin therapy was well tolerated, with a low incidence of clinical adverse events. | Coronary and Aortic Plaque Inflammation, CRP, IL-6 | NCT00965185 [116,117] | Small sample size of 40 participants. |
| Atorvastatin | Myocardial Infarction + Inflammation + Acute Coronary Syndrome + Reperfusion injury | 1 | 8 h | Inclusion Criteria: Males aged 18–40 years. Exclusion Criteria: Females, unwillingness to participate, inability to communicate or understand study instructions, participation in another ongoing trial, any chronic or acute disease within 30 days of inclusion, any regular or temporary medication within 15 days of inclusion, and current smokers. | NRP | IL-6, CRP, TNFR1, TNFR2, Fas, Fas ligand, MMP-2, TIMP-2, sP-selectin, PF-4, β-thromboglobulin | NCT02286544 | The study is mainly for testing for salmonella vaccine. Study performed for a short duration and sample size of only 36 participants. |
| Rosuvastatin | Coronary Artery Disease | 2 | 12 weeks | Inclusion Criteria: Adults > 18 years, fluent in English or Spanish, willing to participate, and clinically stable. Scheduled for cardiac catheterization and PCI with intent to stent; willing to receive high-dose cholesterol-lowering therapy for the study duration; able to provide signed informed consent. Women of childbearing potential must agree to use acceptable contraception. Presence of a proposed non-culprit YELLOW study lesion with maximum 4 mm LCBI ≥ 150. Exclusion Criteria: Acute myocardial infarction (ST-segment elevation, new Q waves, or non-ST-segment elevation with CK-MB > 5× upper normal limit within 72 h); cardiogenic shock; requirement for coronary artery bypass graft surgery; platelet count < 100,000 cells/mm3; comorbidities limiting life expectancy to ≤ 1 year; concurrent enrolment in another investigational study; liver disease; serum creatinine > 2.0 mg/dL; pregnancy or planned pregnancy during the trial; heart transplant recipients or candidates; active autoimmune disease; and breastfeeding women. | Baseline OCT minimal fibrous cap thickness (FCT) was 100.9 ± 41.7 μm, which increased to 108.6 ± 39.6 μm at follow-up, and baseline CEC was 0.81 ± 0.14, which increased at follow-up to 0.84 ± 0.14 (p = 0.003). Thin-cap fibroatheroma prevalence decreased from 20.0% to 7.1% (p = 0.003). Changes in FCT were independently associated with CEC increase by multivariate analysis (β: 0.30; p = 0.01). PBMC microarray analysis detected 117 genes that were differentially expressed at follow-up compared to baseline, including genes playing key roles in cholesterol synthesis (SQLE), regulation of fatty acids unsaturation (FADS1), cellular cholesterol uptake (LDLR), efflux (ABCA1 and ABCG1), and inflammation (DHCR24). Weighted coexpression network analysis revealed unique clusters of genes associated with favourable FCT and CEC changes. | hsCRP | NCT01837823 [118] | Study was performed for a short duration. |
| Simvastatin | Dyslipidemia | NA | 8 weeks | Inclusion Criteria: Patients aged 18 years or older, LDL cholesterol concentration between 160 and 190 mg/dL in patients with less than 2 cardiovascular risk factors, and LDL concentration between 130 and 160 mg/dL in patients with 2 or more cardiovascular risk factors (defined as age ≥ 45 years in men and ≥55 years in women, smoking habit, hypertension ≥ 140/90 mmHg, diabetes mellitus, HDL cholesterol ≤ 40 mg/dL, and family history of cardiovascular disease). Exclusion Criteria: Triglyceride concentration > 400 mg/dL, diabetes mellitus, kidney, liver, or thyroid disease. | Hs-CRP, ROS, ICAM-1 and E-selectin significantly decreased. IL-6 and TNF-α increased but p > 0.05. VCAM-1 decreased but p > 0.05 | hsCRP, IL-6, TNF-α, ROS, VCAM-1, ICAM-1, E-selectin | NCT02304926 | The study first assigned Simvastatin and Ezetimibe separately and after 4 weeks both groups were administered a combined therapy of both the drugs. Short study duration with sample size of 42 participants. |
| Atorvastatin | Myocardial Infarction (perioperative complications) + Myocardial Ischemia+ Inflammation | NA | 48 h | Inclusion Criteria: Adults aged > 45 years undergoing elective high-risk surgery as defined by the POISE criteria. Exclusion Criteria: Absence of informed consent; contraindications to statin therapy (including liver insufficiency, cirrhosis, active muscular disorders, myopathy, or prior adverse reaction to statins); pregnancy; concurrent enrolment in another conflicting study; prior enrolment in STAR VaS; current statin use. | Fifty-six participants completed the 30-day follow-up. The mean (standard deviation) changes in CRP levels from baseline at 48 h in Groups AA, PA, and PP were 141.0 (72.4), 153.5 (42.2), and 111.2 (84.6), respectively. The mean differences (95% confidence interval) at 48 h for AA vs. PA, AA vs. PP, and PA vs. PP were: −20.1 (−81.2 to 41.1), 22.7 (−31.7 to 77.2), and 42.8 (−20.0 to 105.7), respectively, adjusting for baseline CRP, type of procedure, presence of coronary artery disease, use of medications, and for multiple comparisons using Tukey’s method. | CRP | NCT00967434 [119] | Short study duration with sample size of 60 participants. |
| Atorvastatin | Chronic Kidney Disease | 3 | 12 weeks | Inclusion Criteria Patients with vitamin D–treated chronic kidney disease (CKD) undergoing haemodialysis for ≥3 months; presence of tunnelled permanent catheters for ≥6 months prior to enrolment; stable Kt/V > 45 litres; receiving atorvastatin therapy; no infectious or inflammatory episodes for ≥8 weeks; two consecutive measurements showing parathyroid hormone (PTH) < 400 pg/mL, serum calcium < 10.2 mg/dL, and serum phosphorus < 7.0 mg/dL. Exclusion Criteria Age < 18 years; pregnancy; hospitalization within the preceding 4 weeks; current use of immunosuppressive agents. | No Results Posted | IL-2, IL-4, IL-5, IL-6, IL-10, IL-13, TNF-β, CD3, CD4, CD8, CD19, CD25, CD56, CD69, CD95, COX-2, iNOS, PGE2, FGF23, | NCT01820767 | Atorvastatin is used as a comparator. Study has a small sample size of 31 participants. |
| Rosuvastatin | HIV + Cardiovascular Disease | 4 | 6 months | Inclusion Criteria Participants aged 40–90 years; documented HIV infection; on antiretroviral therapy (ART) for >1 year with undetectable viral load; CD4 count > 350 cells/μL; Framingham risk score between 10–20%. Exclusion Criteria Uncontrolled diabetes mellitus or hypertension; known coronary artery disease; chronic renal failure; total cholesterol > 5.8 mmol/L; LDL cholesterol > 4.0 mmol/L; current statin therapy for baseline dyslipidemia; pregnancy or lactation; untreated hepatitis B or C; inflammatory or autoimmune disorders; baseline regional perfusion abnormalities on stress myocardial contrast echocardiography (MCE). | NRP | Vascular inflammation (TBRmax) | NCT02234492 | Small sample size of 35 participants from the original estimated 82. |
| Simvastatin | Alzheimer’s Disease | 2 | 9 months | Inclusion Criteria: Ages 35–69 with a parent having Alzheimer’s disease. Exclusion Criteria: Current use of cholesterol-lowering medication, active liver disease, history of adverse reaction to statins, contraindication to lumbar puncture, elevated lab values, use of medications known to interact with statins, history of dementia, currently pregnant or planning to become pregnant, excessive grapefruit juice consumption, involvement in another investigational drug study | Reduction in hs-CRP but not significant | Hs-CRP | NCT00486044 | The non-significant results may be due to the short duration of trial. |
| Atorvastatin | HIV Dementia | 4 | 12 weeks | Inclusion Criteria: Living with HIV-1 infection, were on stable HIV treatment with controlled viral load, sufficient CD4 T-cell count, willing to comply with trial, elevated hs-CRP levels. Exclusion Criteria: Use of other lipid-lowering drugs, drugs that interact with the statin, pregnant, beast-feeding, active drug abuse, severe illness, allergic to statins, abnormal blood count. | No significant change in inflammatory markers | MCP-1, sCD14, sCD163, CD16 | NCT01263938 | The non-significant results may be due to small sample size (5 participants). |
| Atorvastatin | Type 1 Diabetes Mellitus + Hypercholesterolemia | 3 | 6 months | Inclusion Criteria
Severe dyslipidemia (LDL-C > 160 mg/dL, triglycerides > 400 mg/dL); smoking; pregnancy; current use of anti-inflammatory, immunomodulatory, lipid-lowering, or additional antidiabetic medications; hypertension and/or microalbuminuria (permitted only with balanced randomisation and standardized treatment). | No significant change in hsCRP | hsCRP | NCT01236365 | The non-significant results maybe due to the short duration of trial. |
| Rosuvastatin | Acute Coronary Syndrome | 4 | 6 months | Inclusion Criteria: Adults aged ≥ 19 years with acute coronary syndrome or carotid artery disease (20–50% stenosis) and at least one 18F-FDG–avid lesion (target-to-background ratio [TBR] ≥ 1.6) in the carotid artery confirmed by PET/CT imaging; provision of written informed consent. Exclusion Criteria: History of carotid endarterectomy or stenting; planned major surgery within the next 6 months; use of statin or ezetimibe therapy within the previous 4 weeks; chronic illness requiring steroid therapy; end-stage renal disease; chronic liver disease; malignancy within the past 3 years; pregnancy or breastfeeding; or life expectancy < 2 years. | NRP | hsCRP | NCT04056169 | High dose rosuvastatin was compared with a group that was given low rosuvastatin and ezetimibe. |
| Simvastatin/Atorvastatin/Rosuvastatin | Coronary Heart Disease + Dyslipidemia | NA | 36 Months | Inclusion Criteria: Male or female patients aged ≥ 18 years with high cardiovascular risk and otherwise good overall health. Exclusion Criteria: History of liver, kidney, pancreatic, or gallbladder disease; acute coronary syndrome within one month prior to enrolment; pregnancy; presence of inflammatory disease; or current treatment with medications known to affect triglyceride metabolism or concentration. | NRP | hsCRP | NCT02163044 [120] | N/A |
| Simvastatin | Metabolic Syndrome | 6 weeks | 4 | Participants enrolled in the study were men and women aged 21 years or older who had a confirmed diagnosis of metabolic syndrome. This diagnosis was established based on the presence of at least three out of five clinical features: abdominal obesity, triglyceride levels greater than 150 mg/dL, HDL cholesterol below 40 mg/dL for men or below 50 mg/dL for women, blood pressure exceeding 130/85 mm Hg, and fasting glucose levels above 100 mg/dL. Subjects were excluded if they had a history of bleeding disorders, drug or alcohol abuse, a prothrombin time more than 1.5 times the control value, a platelet count below 100,000/mm3, a hematocrit level under 25%, or a creatinine level above 4.0 mg/dL. Additional exclusion criteria included recent or planned surgery or angioplasty, any history of gastrointestinal or other bleeding events, drug-induced medical conditions, recent trauma, active malignancy, rheumatic or autoimmune diseases, coronary artery disease, prior stroke, participation in another drug trial within the preceding month, treatment with intravenous platelet glycoprotein IIb/IIIa inhibitors or thienopyridines within the past six months, and use of statins or aspirin within the previous four weeks. | Results posted but not analyzed | CRP, IL-6 | NCT00819403 | Short duration of study with a small sample size (15 participants). |
| Rosuvastatin | Preeclampsia | 2 | NA | Inclusion Criteria: Women aged 20–35 years with a singleton, non-anomalous pregnancy; normal lipid profile; normal liver transaminase levels; white blood cell count between 4–11 × 103/mm3; and C-reactive protein (CRP) < 3 mg/L. Exclusion Criteria: Refusal to participate; history of cardiac, respiratory, renal, neurological, or endocrine disorders; contraindications to statin therapy; concurrent treatment with fibrates, niacin, cyclosporine, clarithromycin, or erythromycin; inability to tolerate oral medications due to severe nausea or vomiting; multifetal gestation or intrauterine fetal demise; presence of fetal abnormalities; or requirement for emergency surgery. | NRP (Unknown Status) | CRP, IL-6 | NCT04303806 | N/A |
| Atorvastatin | Intracranial Aneurysm | 2 | 6 months | Inclusion Criteria: Adults aged 18 or over, male or non-pregnant female, with a saccular UIA ≥ 3 mm identified on imaging and wall enhancement of the aneurysm by MRI VWI, and able to understand the trial and sign informed consent. Exclusion Criteria: Patients with MRI contraindications, planned aneurysm treatment within 6 months, taking anti-inflammatory drugs, dyslipidemia, severely impaired liver or renal functions, recurrent aneurysm retreatment, pregnant or lactating, with malignant diseases or poor compliance. | NRP | CRP, TNF-α, IL-1β, IL-6 | NCT04149483 | N/A |
| Atorvastatin | Ageing-related Inflammation in HIV | 4 | 72 Weeks | Inclusion Criteria: Adults aged 45 years diagnosed with HIV-1 infection, receiving a specified antiretroviral therapy (ART) regimen for a minimum of 3 months, with documented undetectable plasma HIV-1 RNA levels for at least 12 consecutive months, and willing to provide voluntary written informed consent. Exclusion Criteria: History of virological failure to integrase inhibitor–based regimens; documented resistance mutations to integrase or nucleoside reverse transcriptase inhibitors (NRTIs); presence of systemic comorbid conditions (including hepatitis B or C co-infection, acute infections, or malignancy); current treatment with anti-inflammatory, anticoagulant, or antiplatelet agents; or use of statin therapy within the preceding 6 months. | Plasma inflammatory markers remained unchanged. Furthermore, no major change in T cells. However, there was a small decrease in number of CD38+ CD8 T cells and a small rise in the number of CD28-CD57+ T cells | IL-6, CRP | NCT02577042 [121,122] | One group had Raltegravir + atorvastatin. |
| Atorvastatin | HIV-1 Dementia | 4 | 12 Weeks | Inclusion Criteria: Adults aged 18 or older with chronic HIV-1 on stable HAART, HIV viral load < 200 copies/mL for over 6 months, nadir CD4 count < 350 and current CD4 count > 100 cells/ul, hs-CRP > 2 mg/L, Karnofsky score ≥ 80, willing to use contraception, comply with study evaluations, and if female, undergo monthly pregnancy testing. Exclusion Criteria: Use of lipid-lowering or anti-inflammatory drugs, pregnancy or breastfeeding, active drug or alcohol abuse, allergy to Atorvastatin, history of myositis or rhabdomyolysis, recent serious illness or infection, elevated liver enzymes or renal insufficiency, certain blood count abnormalities, HCV infection, severe heart failure, active IV drug use, coronary artery disease, or allergy to Lidocaine for LP sub-study. | No significant difference in CD16, MCP1, CD163, CCR2, sCD14, CD38 and sCD163. Significant difference in hsCRP | CD16, MCP1, CD163, CCR2, sCD14, hsCRP, CD38, sCD163 | NCT01600170 | Further research needs to be carried out regarding why only hs-CRP results were significant. |
| Atorvastatin | Healthy | NA | 7 days | Inclusion Criteria: Male or female, aged 18–50, fluent in English, BMI 18–30, willing and able to give informed consent, not on regular medications (except contraceptive pill). Exclusion Criteria: Regular medications (except contraceptive pill), significant psychiatric illness, alcohol or substance misuse disorder, significant hepatic or neurological conditions, hypersensitivity to atorvastatin, pregnant or breastfeeding, women not using contraception, recent participation in similar studies or studies involving medication. | NRP | hs-CRP | NCT03966859 | Although the study was assessing emotional processing, they also assessed inflammatory markers. |
| Atorvastatin + Rosuvastatin | Type 2 Diabetes | 4 | 6 months | Inclusion Criteria: Adults diagnosed with type II diabetes mellitus and documented hypercholesterolaemia. Exclusion Criteria: History or presence of hepatic impairment; renal insufficiency; coronary artery disease; other metabolic disorders; type I diabetes mellitus; autoimmune diseases; active or prior malignancy; current or recent infection; use of anti-inflammatory medications; major surgery within the preceding months; recent weight reduction or modification in antihypertensive therapy; prior or ongoing treatment with lipid-lowering agents; or any known contraindication to statin therapy. | NRP | Hs-CRP | NCT03784703 | N/A |
| Simvastatin | Sickle Cell Disease | 2 + 3 | 10 months | Inclusion Criteria: Children and adolescents aged 5 to 15 years presenting with a complete clinical manifestation of sickle cell disease, accompanied by an acute painful crisis. Exclusion Criteria: Presence of any other chronic medical condition; age less than 3 years or greater than 18 years; documented hepatic disease; renal impairment; or a diagnosis of diabetes mellitus. | NRP | hsCRP | NCT04301336 | Study is comparing Simvastatin with other treatments. |
| Pravastatin | Postmenopausal osteoporosis | 4 | 3 days | Inclusion Criteria: Chinese Han ethnic postmenopausal women with bone mineral density values less than 2.5 SD below the normal adult mean, willing to participate. Exclusion Criteria: Prior treatment with bisphosphonates, recent infections, evidence or history of cancer, contraindications to zoledronic acid or pravastatin, current use of certain medications (aminoglycosides, diuretics, thalidomide, fibrates, immunosuppressives, niacin), severe liver or renal insufficiency, and any condition precluding study participation. | NRP (Unknown Status) | CRP, IFN-γ, IL-6 | NCT04719481 | N/A |
| Atorvastatin, Rosuvastatin Simvastatin | Atherosclerosis | NA (Unknown Status) | 8 weeks | Inclusion Criteria: Patients over 40 years old with high- or very-high-risk CVD and LDL-Cholesterol ≤ 4.0 mmol/L (high risk) or ≤3.5 mmol/L (very high risk). Must have a stable CV disease history, stable statin therapy, and patients not taking Ezetimibe. Exclusion Criteria: Uncontrolled hypertension, significant hypertriglyceridemia, pregnancy, hypersensitivity to study drugs, significant liver function test abnormalities, recent acute CV events, chronic inflammatory conditions, severe renal impairment, recent malignancy, substance abuse, recent use of systemic corticosteroids, inability to provide informed consent, or medications causing significant drug interactions with study medications. | NRP | hsCRP, IL-2, IL-6, ox-LDL | NCT03355027 | Patients were given either Alirocumab and statin or Ezetimibe and statin |
| Atorvastatin | HIV-1 | 2 | 44 Weeks | Inclusion Criteria: HIV-1 infected individuals aged 18 or older on a stable ART regimen including a boosted PI for at least 6 months, with an undetectable HIV-1 RNA level (<40 copies/mL) and stable laboratory values within specified ranges. Participants must consent to contraception if of reproductive potential and agree to study requirements. Exclusion Criteria: Individuals with malignancy (excluding non-melanoma skin cancer), coronary artery disease, chronic hepatitis B or C, significant inflammatory conditions, pregnancy or breastfeeding, prior intolerance to statins, recent use of lipid-lowering or immunosuppressant therapies, heavy alcohol use, known coagulopathy, recent infections, or serious illness/trauma within 4 weeks prior to entry. | No significant change in IL-6. Other results were reported without significance level. | IL-6, MCP-1, CD40L, sCD14, sCD163, | NCT01351025 | NA |
| Atorvastatin, Rosuvastatin | Heart Failure | 4 | 6 months | Inclusion Criteria: Adults aged 18–65 with left ventricular ejection fraction < 40% and NYHA class II-III heart failure, optimized on standard CHF treatment for at least 1 month, and able to provide written informed consent. Exclusion Criteria: Hypersensitivity to statins, NYHA class IV, serum creatinine > 3 mg/dL, significant liver disease, current use of enzyme inducers/inhibitors, malignancy or chemotherapy, pregnancy or lactation, recent trial participation, uncontrolled diabetes or hypertension, or HIV/HBV/HCV infection. | NRP (Unknown Status) | hsCRP, IL-6 | NCT05072054 | NA |
| Rosuvastatin | Valvular Cardiac surgery | 3 | 3 months | Inclusion Criteria: Adults undergoing single or multiple valve repairs/replacements, Bentall procedure (without other aortic procedures), with or without MAZE procedure for atrial fibrillation. Exclusion Criteria: Age under 18, urgent/emergency surgery, inability to consent, current or recent statin use, chronic anti-inflammatory drug use, hypersensitivity to rosuvastatin, active liver disease, pregnancy or nursing, drug interactions, severe renal impairment (creatinine clearance < 30 mL/min/1.73 m2), known myopathy or inflammatory disorders, HIV. | NRP | Inflammatory markers | NCT01425398 | NA |
| Atorvastatin | Type 2 Diabetes, Hyperlipidemia, Hypertension | 4 | 5 weeks | Inclusion Criteria: Adults aged 19–74 with type 2 diabetes, hyperlipidemia, stage I hypertension, and adequately controlled hemoglobin levels, who have not used medications for hyperlipidemia and hypertension in the past 3 months and have provided written consent. Exclusion Criteria: Patients contraindicated for Rovelito, pregnant/nursing women, significant blood pressure discrepancies, recent use of specific medications (e.g., ARBs, ACE inhibitors, statins), previous antidiabetic use, intolerance to study medications, genetic angioedema, past adverse reactions to statins, CPK > 5× ULN, secondary hypertension, poorly controlled hypothyroidism, severe cardiac issues within 6 months, renal or hepatic disease, pancreatitis, major gastrointestinal surgeries, chronic inflammatory conditions, autoimmune diseases, recent substance abuse, history of malignant tumours within 5 years, recent investigational product use, and other factors deemed by the investigator. | NRP | ICAM-1, IL-6, CRP, | NCT02842359 | Study investigates combination with Ibersartan but compares with atorvastatin alone. Duration of the trial is short with only 11 participants actually enrolled from the original estimated 84 participants. |
| Simvastatin | Depression | 1 + 2 | 7 Days | Inclusion Criteria: Adults aged 18–50, fluent in English, BMI between 18 and 30, willing and able to give informed consent, and not taking any regular medications except contraceptive pills. Exclusion Criteria: Current use of regular medications (except contraceptive pills), significant psychiatric illness, alcohol or substance misuse disorder, significant hepatic or neurological conditions, history of hemorrhagic stroke or lacunar infarct, known hyperglycemia/pre-diabetes, hypersensitivity to simvastatin or sucrose, pregnant or breastfeeding women, women of childbearing potential not using appropriate contraception, recent participation in studies using similar tasks or medications. | Seven-day simvastatin 20 mg treatment, compared to placebo, did not lead to any statistically significant changes on the majority of the emotional processing, reward learning, and verbal memory outcomes measured, nor it did for most of the questionnaires administered and hs-CRP levels. | CRP | NCT04652089 [123] | Short study duration with a sample size of 53 participants. |
| Rosuvastatin | Ulcerative Colitis | 2 + 3 | 8 Weeks | Inclusion Criteria: Adults aged 18–70, Bact2 enterotyped one month prior to study, willingness to participate and sign informed consent (in Dutch), access to a −20 °C freezer. UC patients must be in remission or have mild to moderate active ulcerative colitis (Mayo score 4–10) with stable medication (8 weeks) and a Mayo endoscopic sub-score of 2–3 at week 0. Healthy Bact2 participants must have no physician-diagnosed diseases or disorders. Exclusion Criteria: Prior or ongoing use of statins, history of gastrointestinal surgery (except appendectomy), active liver disease, lactose intolerance, pre-diabetes, hereditary muscular disorders, alcohol abuse. UC patients: conditions causing profound immunosuppression, diagnosis of Crohn’s disease or indeterminate colitis, hypothyroidism, diabetes mellitus, severe renal impairment (creatinine clearance < 30 mL/min), myopathy, recent antibiotic use (past four months), steroid dependency requiring > 16 mg Medrol two weeks before week 0. Healthy Bact2 participants: family history of autoimmune chronic inflammatory diseases. | Recruiting | Hs-CRP | NCT04883840 | No results published as the trial is still recruiting participants. |
| Atorvastatin | Non-ST Segment Elevation Acute Coronary Syndrome | 2 | 30 Days | Inclusion Criteria: Adults (≥18 years) with non-ST elevation acute coronary syndrome who are statin-naive. Exclusion Criteria: Prior statin or colchicine therapy, known allergy to colchicine or statins, current treatment with potent CYP3A4 or P-glycoprotein inhibitors, previous or planned immunosuppressive therapy, active malignancy, severe kidney disease (creatinine > 3 mg/dL or dialysis), severe liver disease (ALT and/or AST > double normal values with total bilirubin > double normal values or altered coagulation), severe heart failure (NYHA class ≥ 3 or cardiogenic shock), severe acute or chronic gastrointestinal disease, pregnancy or lactation, current COVID-19 or other infectious disease, refusal of consent. | Recruiting | hsCRP | NCT05250596 | Study is investigating colchicine + atorvastatin. |
| Simvastatin | Chronic Obstructive Pulmonary Disease + Inflammation | 1 | 9 Weeks | Inclusion Criteria: Medically optimized COPD patients aged 40–79 years with serum CRP levels > 3 mg/L. Exclusion Criteria: Current smoker, COPD exacerbation in the last 2 months, active hepatic or severe renal dysfunction, connective tissue disease, chronic inflammatory disease, malignancy, acute illness, leukocytosis (>10,000 white blood cells), thrombocytosis (>450,000 platelets), recent history of myocardial infarction or angina in the last 6 months, pregnancy. | NRP | CRP | NCT00655993 | Small sample size of 30 participants from the original estimated 40. |
| Atorvastatin | Chronic Chagas Disease | 2 | 12 Months | Inclusion Criteria: Adults aged 18–50 years, weighing more than 40 kg, with confirmed T. cruzi infection and positive qPCR, normal lab values (except GGT > 2 × ULN), negative serum pregnancy test for women of reproductive age using effective contraception, and ability to comply with follow-up tests and visits. Exclusion Criteria: Signs of digestive form or chronic cardiac Chagas Disease stage II or higher, acute or chronic health conditions (including acute infections, HIV, diabetes, liver/kidney disease, hypothyroidism), family history of muscle disorders, pre-existing heart disease unrelated to Chagas, contraindications or hypersensitivity to study drugs, prior treatment for Chagas or with statins, concomitant use of antimicrobial agents or CYP3A4 modifiers, history of alcohol/drug abuse, inability to take oral medication, familial short QT syndrome, abnormal lab values (specific parameters), pregnancy or breastfeeding, refusal to use contraception during treatment. | Recruiting | TNF-α, IFN-γ, IL-10, IL-1β, IL-4, IL-17A, ICAM-1, VCAM-1, E-Selectin. | NCT04984616 | NA |
| Atorvastatin + Rosuvastatin | Acute Coronary Syndrome | 4 | 4 Weeks | Inclusion Criteria: Patients aged 18 and older, diagnosed with acute coronary syndrome, not previously taking statins, and who provide written informed consent. Exclusion Criteria: Patients on lipid-lowering drugs, with statin hypersensitivity or contraindications, undergoing surgical management, planned for coronary revascularization, pregnant or lactating women, and those with infections causing elevated inflammatory markers. | NRP | Hs-CRP | NCT06053983 | Short study duration. |
| Simvastatin | Atherosclerosis + Hypercholesterolemia + Inflammation | 4 | 6 weeks | Inclusion Criteria: Adults aged 18 to 75 years with obesity, defined as a body mass index (BMI) of ≥30 kg/m2, and low-density lipoprotein (LDL) cholesterol levels of ≥100 mg/dL. Eligible participants must not be taking vitamin or antioxidant supplements at the time of enrolment. Exclusion Criteria: Ongoing treatment with anti-hyperlipidaemic agents; serum triglyceride concentration > 500 mg/dL; history of myocardial infarction, percutaneous coronary intervention (angioplasty or stent placement), or coronary artery bypass graft surgery within the preceding six months; chronic administration of non-steroidal anti-inflammatory drugs (NSAIDs) or systemic corticosteroids; clinically significant hepatic disease or renal impairment; documented history of drug or alcohol misuse; concurrent participation in another clinical trial; receipt of an investigational medicinal product within 30 days prior to screening; active tobacco use; pregnancy; premenopausal women not employing reliable contraception; and anemia, defined as hemoglobin < 12 g/dL. | NRP | IL-1β, TNF-α, MMP-9 | NCT04638400 | The study was terminated due to lack of recruitment with the sample size being only 10. |
| Atorvastatin + Rosuvastatin | Clonal Cytopenia of Undetermined Significance, Myelodysplastic Syndromes | 2 | 3 Months | Inclusion Criteria: Adults aged 18 and older diagnosed with CCUS (presence of somatic mutations with VAF ≥ 2% and unexplained persistent cytopenia in at least one lineage for at least 6 months) or lower-risk MDS (IPSS-R score ≤ 3.5 with at least one mutation with VAF ≥ 2%). Patients must be transfusion-independent and able to provide informed consent. Exclusion Criteria: CCUS patients with cytogenetic change alone, recent use of disease-modifying therapy (within the last 3 months), prior statin use within 1 year, history of other malignancies (unless treatment was completed > 2 years ago), use of investigational agents within 5 half-lives of the agent, allergic reactions to statins, uncontrolled intercurrent illness, pregnant or breastfeeding women, and patients with HIV or HCV on active treatment. | Recruiting | Hs-CTRP | NCT05483010 | NA |
| Atorvastatin | Ulcerative Colitis | 2 | 6 Months | Inclusion Criteria: Adults aged 18 and older, both male and female, with a negative pregnancy test and effective contraception. Exclusion Criteria: Breastfeeding, significant liver and kidney function abnormalities, colorectal cancer, severe UC, use of rectal or systemic steroids, immunosuppressives, or biological therapies. | NRP (Recruiting) | IL-6, MPO | NCT05561062 | NA |
| Atorvastatin | Ulcerative Colitis | 2 | 6 Months | Inclusion Criteria: Adults aged 18 to 75 years, both male and female, with a negative pregnancy test and effective contraception. Exclusion Criteria: Breastfeeding, significant liver and kidney function abnormalities, colorectal cancer, severe UC, use of rectal or systemic steroids, immunosuppressives, or biological therapies. | NRP (Recruiting) | IL-18 | NCT05567068 | NA |
| Atorvastatin | Breast Cancer | 2 | 2 Years | Inclusion Criteria: Adults aged 18 years and older with a diagnosis of TNBC (including triple-negative inflammatory breast cancer), stage IIB or III disease, histological confirmation of breast carcinoma, residual disease post-neoadjuvant chemotherapy, ECOG performance status of 0–1, within 3 months from definitive surgery, and willing to take a statin for at least two years. Exclusion Criteria: Incomplete recovery from prior therapies, other active malignancies (except certain skin cancers or in situ cervical cancer), psychiatric or substance abuse disorders, recent statin therapy, use of other anti-lipidemic agents, hypersensitivity to statins, active liver disease, pregnancy or breastfeeding, distant metastasis, recent myocardial infarction, unstable cardiac conditions, and chronic steroid use. | NRP | ESR, CRP, IL-6 | NCT03872388 | Participants that were not eligible to receive atorvastatin will be enrolled into non-statin observation group with/without capecitabine treatment. Very small sample size of only 6 participants of the estimated 80. |
| Simvastatin | Obesity | NA | 6 Weeks | Inclusion Criteria: Adults between 18 and 65 years of age with a body mass index (BMI) exceeding 30 kg/m2 and low-density lipoprotein (LDL) cholesterol levels greater than 100 mg/dL. Participants must not be receiving vitamin or antioxidant supplementation and must be willing to provide written informed consent. Exclusion Criteria: Current or recent use of lipid-lowering agents; serum triglyceride levels exceeding 500 mg/dL; history of myocardial infarction, percutaneous coronary intervention (angioplasty with or without stent placement), or coronary artery bypass grafting within the recent past; chronic administration of non-steroidal anti-inflammatory drugs (NSAIDs) or corticosteroids; clinically significant hepatic disease or renal impairment; history of drug or alcohol misuse; concurrent enrolment in another clinical trial; administration of any investigational product within the preceding 30 days; active tobacco smoking; pregnancy; premenopausal women not using reliable contraception; and anemia defined by hemoglobin concentration below 12 g/dL. | Results were reported without significance level | CD16, IL-1β | NCT01420328 | N/A |
| Rosuvastatin | Coronary Artery Disease | 4 | 1 Year | Inclusion Criteria: Males and females aged 40–75 years, capable and willing to provide informed consent, with high CAD PRS and subclinical atherosclerosis (plaque visible on CCTA causing < 70% luminal stenosis). Exclusion Criteria: History of cardiovascular disease, liver disease, significant liver enzyme abnormalities, eGFR < 60 mL/min/1.73 m2, significant allergic reactions to iodinated contrast, colchicine, or statins, current use of LDL cholesterol-lowering or anti-inflammatory medications, need for potent CY2P inhibitors, pregnancy or breastfeeding, BMI ≥ 40 kg/m2, inability to provide informed consent, or inability to hold breath for 10 s. | NRP (Recruiting) | CRP, IL-1β, IL-6 | NCT05850091 | N/A |
| Pitavastatin | HIV + Cardiovascular Disease | 3 | 96 Months | Inclusion Criteria: HIV-1 infected individuals on combination ART for at least 180 days, with a CD4+ cell count > 100 cells/mm3, and acceptable screening labs, including specific LDL cholesterol and triglyceride levels based on ASCVD risk score. Exclusion Criteria: Clinical ASCVD, diabetes with LDL ≥ 70 mg/dL, ASCVD risk score > 15%, active cancer within 12 months (exceptions apply), known decompensated cirrhosis, recent myositis or myopathy, untreated symptomatic thyroid disease, severe allergy to statins, use of specific immunosuppressants, erythromycin, colchicine, or rifampin, use of statin drugs, gemfibrozil, or PCSK9 inhibitors within 90 days, recent serious illness or trauma, current pregnancy or breastfeeding, and conditions interfering with study completion. | NRP | CRP, Lp-PLA2, sCD163 | NCT02344290 | N/A |
| Statin Type and Dosage (If Specified) | Patient Population/Disease Context | Inflammatory Marker(s) Assessed | Observed Effect on Inflammatory Marker(s) | Cancer Outcome(s) Assessed | Observed Effect on Cancer Outcome(s) | Key Study/Evidence Type | Reference | Translational/Clinical Implication |
|---|---|---|---|---|---|---|---|---|
| Atorvastatin Simvastatin Rosuvastatin Fluvastatin Pravastatin Pitavastatin | Chronic Diseases | hsCRP, IL-6, TNF-α | Significant reduction (MD for CRP: −1.58 mg/L; IL-6: −0.24 ng/dL; TNF-α: −0.74 ng/dL) | N/A | N/A | Meta-analysis of RCTs | [224] | Statins exert broad anti-inflammatory effects across various chronic conditions. |
| Atorvastatin Simvastatin Pravastatin Rosuvastatin Fluvastatin Lovastatin Pitavastatin | Various (observational studies) | N/A | N/A | Cancer Incidence | Reduced incidence in 10/18 types, but evidence strength is low (p < 0.05 in some meta-analyses) | Umbrella Review (incl. observational) | [225] | Observational data suggests some incidence reduction, but this is not confirmed by higher-level evidence. |
| Atorvastatin Cerivastatin Fluvastatin Lovastatin Pravastatin Simvastatin | Various (RCTs) | N/A | N/A | Cancer Incidence, Cancer Deaths | No reduction (OR, 1.02 for incidence; OR, 1.01 for deaths) | Meta-analysis of RCTs | [226] | Randomized controlled trials do not support statins for primary cancer prevention. |
| Atorvastatin Cerivastatin Fluvastatin Lovastatin Pitavastatin Pravastatin Rosuvastatin Simvastatin | Advanced-stage Cancer Patients | N/A | N/A | Overall Survival, Cancer-Specific Survival, Progression-Free Survival | 26% decreased risk for OS/CSS (HR 0.74); 24% for PFS (HR 0.76) | Meta-analysis of RCTs and Observational Studies | [227] | Statins may improve survival in advanced cancers, suggesting a potential adjunctive role. |
| Rosuvastatin 20 mg/day | Healthy with elevated hsCRP (≥2 mg/L), low LDL-C | hsCRP | Significant 37% reduction | Major Cardiovascular Events, All-Cause Mortality | 44% reduction in CV events, 20% in all-cause mortality | JUPITER Trial (RCT) | [228] | Confirms hsCRP as a cardiovascular risk marker; Rosuvastatin demonstrates potent anti-inflammatory and cardiovascular protective effects. |
| Simvastatin 40 mg/day | Dyslipidemia or CHD | CRP | Most effective for lowering CRP (WMD = −4.07, 95% CI [−6.52, −1.77]) | N/A | N/A | Network Meta-analysis (RCTs) | [229] | Simvastatin 40 mg is a strong option for C-reactive protein reduction in cardiovascular contexts. |
| Atorvastatin 80 mg/day | Dyslipidemia or CHD | CRP | Best long-term effect on CRP | N/A | N/A | Network Meta-analysis (RCTs) | [229] | Atorvastatin 80 mg is suitable for sustained C-reactive protein reduction. |
| Atorvastatin | Chronic Diseases (e.g., RA, Crohn’s) | IL-6, TNF-α | Most significant reduction (MD for IL-6: −5.39 ng/dL; TNF-α: −3.32 ng/dL) | N/A | N/A | Meta-analysis of RCTs | [224] | Atorvastatin is particularly effective for reducing Interleukin-6 and Tumour Necrosis Factor-alpha. |
| Fluvastatin 80 mg | Acute Coronary Syndrome (ACS) | CRP, IL-6 | No significant difference on Day 2 and Day 30 | Major Adverse Cardiovascular Events | Reduced 1-year occurrence (OR 0.40, 95% CI 0.17–0.95) | FACS Trial (RCT) | [230] | Anti-inflammatory effects may be less pronounced or slower to manifest in acute settings; cardiovascular benefits are still observed. |
| Fluvastatin | Chronic Diseases | CRP | Most pronounced impact (MD = −7.36 mg/L) | N/A | N/A | Meta-analysis of RCTs | [224] | Fluvastatin is potent for C-reactive protein reduction in chronic inflammatory conditions. |
| Simvastatin 40 mg + Chemotherapy (XP) | Advanced Gastric Cancer | N/A | N/A | Progression-Free Survival | No significant increase (median PFS 5.2 vs. 4.63 months; HR 0.930, p = 0.642) | Phase III RCT | [231] | Simvastatin 40 mg did not improve progression-free survival in this specific context. |
| Simvastatin 40 mg + Gefitinib | Advanced NSCLC (wild-type EGFR nonadenocarcinomas) | N/A | N/A | Response Rate, Progression-Free Survival | Higher RR (40% vs. 0%), longer PFS (3.6 M vs. 1.7 M) in subgroup | Phase II RCT | [232] | Potential benefit in specific non-small cell lung cancer subgroups, suggesting a personalized approach. |
| Simvastatin 40 mg + Afatinib | Advanced Nonadenocarcinomatous NSCLC | N/A | N/A | Response Rate, Progression-Free Survival, Overall Survival | No significant differences (RR 5.7% vs. 9.4%; PFS 1.0 vs. 3.6 months; OS 10.0 vs. 7.0 months) | Phase II RCT | [233] | Simvastatin dose may have been insufficient for anti-cancer effect in this setting. |
| Simvastatin 40 mg + Chemotherapy (Carboplatin/Vinorelbine) | Metastatic Breast Cancer | Baseline hsCRP | Elevated baseline hsCRP (>10 mg/L) associated with shorter OS | Objective Response Rate, Overall Survival | No beneficial increase in tumour sensitivity or OS (HR 1.16, p = 0.57) | RCT (double-blind, placebo-controlled) | [234] | Safe profile but no direct anti-tumour benefit; baseline hsCRP is a strong prognostic marker in this population. |
| Pitavastatin | Chronic Inflammation (skin, pancreas) | IL-33 | Suppresses IL-33 expression by blocking TBK1-IRF3 pathway | Chronic Pancreatitis, Pancreatic Cancer | Linked to significantly reduced risk | Preclinical and Epidemiological Data | [235] | A novel mechanism for cancer prevention in inflammation-driven cancers, warrants further investigation. |
| Statin | Type of Study | Disease | Statin’s Mode of Action | Reference Number |
|---|---|---|---|---|
| Simvastatin | Clinical Trial | COPD | Regular administration of 40 mg Simvastatin had no significant effects on the exacerbation rates in high risk-exacerbation COPD patients. | [430] |
| Simvastatin | Review | COPD | A large RCT reviewed showed no reduction in exacerbations for patients with COPD with no cardiovascular indication for statin treatment. | [431] |
| Atorvastatin | Clinical trial | Colorectal Cancer (CRC) | The therapeutic intervention did not yield significant reductions in (rectal aberrant crypt foci ACF) number when compared with control in a sample of patients with an increased risk for sporadic CRC | [432] |
| Atorvastatin | Clinical trial | Chronic Bronchitis (exposed to sulphur mustard gas) | Patients with chronic bronchitis exposed to sulphur mustard gas showed no significant reduction in levels of pro-inflammatory markers when treated with atorvastatin including interleukin-6 (IL-6) and tumour-necrosis factor α (TNF-α) | [433] |
| Atorvastatin | In vivo study (animal trial) | COPD | In mice treated with atorvastatin, no significant reduction in alveolar diameter or leukocyte count was observed, indication that the anti-inflammatory effects of atorvastatin in mice with elastase- induced emphysema may not be negligible. | [434] |
| Rosuvastatin | Clinical trial | COPD + Chronic Heart failure (HF) | The study demonstrated that 10 mg rosuvastatin was not effective in all cause, cardiovascular, non-cardiovascular death rates and hospitalization rates. | [84] |
| Rosuvastatin | Meta-analysis | Osteoarthritis (OA) | Sub-group analysis showed that rosuvastatin was associated with a higher risk of hip, knee or hand osteoarthritis. | [435] |
| Rosuvastatin | In vivo study | Systemic lupus erythematosus (SLE) | The study observed that while serum LDL-cholesterol was reduced in SLE mice, the serum inflammatory cytokines, proteinuria and clinical manifestation remained unchanged, indicating rosuvastatin is not efficient in treating SLE. | [436] |
| Rosuvastatin | Case–control study | Pancreatitis | The study found an increased risk of acute pancreatitis with rosuvastatin use. | [437] |
| Rosuvastatin | Clinical trial | Major Depressive disorder (MDD) | There was no improvement in Major depressive disorder (MDD) with rosuvastatin. | [438] |
| Statin | Type of Study | Disease | Statin’s Mode of Action | Reference Number |
|---|---|---|---|---|
| Simvastatin + Sarilumab | Clinical Trial | Rheumatoid arthritis (RA) | Sarilumab antagonizing IL-6 allows for overactivity of CYP450 (specifically CYP3A4), suppression of simvastatin levels and potential reduced efficiency of the drug. | [439] |
| Simvastatin + Narrowband UBD phototherapy | Clinical Trial | Psoriasis | No significant difference was observed in improvement of psoriasis in treatment group and placebo group. | [440] |
| Atorvastatin + Epigallocatechin-3-gallate (EGCG) | In vivo study (animal trial) | Colorectal Cancer (CRC) | No carcinogenic-inhibitory activity was observed in the mice treated with the combination of 0.1% EGCG and 60 ppm ATST. Some of the treated group even demonstrated enhanced tumour proliferation. | [441] |
| Atorvastatin + Inhaled corticosteroid + long-acting β-agonist | Clinical trial | Chronic obstructive pulmonary disease (COPD) | In the case of patients treated with 40 mg of atorvastatin no reduction in serum C-reactive protein and total antioxidant capacity was observed. | [442] |
| Simvastatin + Rosuvastatin + Atorvastatin | Meta-analysis | Cancer | Statin therapy for a duration of 5 years showed no significant evidence on incident or mortality on any type of cancer. | [443] |
| Simvastatin + Atorvastatin | Observational study (prospective cohort study) | Colorectal Cancer (CRC) | In this cohort of colorectal cancer patients, statin use was not associated with improved overall cancer-specific or recurrence-free survival. | [444] |
| Simvastatin + Rosuvastatin + Atorvastatin | RCT | Colorectal Cancer (CRC) | When reviewing meta-analysis on observational study, a mild risk reduction was observed. However, when reviewing the Randomized control trials focusing on statin use and colorectal cancer, no association was found. Overall, inconsistent results from different studies were observed, hence further research needs to be conducted. | [445] |
| Simvastatin + Rosuvastatin + Atorvastatin | Review | Chronic obstructive pulmonary disease (COPD) | Found that statins reduced level of inflammation in people with COPD, but this did not result in any clear improvements in exacerbations, mortality, functional capacity, quality of life, or lung function | [446] |
| Simvastatin + Rosuvastatin + Atorvastatin | Meta-analysis | Rheumatoid arthritis (RA) | Interleukin- 6 (IL6) and swelling of the joint (SJ) levels were not significantly reduced in rheumatoid arteritis patients with statin therapy. | [447] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, S.; Huda, B.; Bhurka, F.; Patnaik, R.; Banerjee, Y. Molecular and Immunomodulatory Mechanisms of Statins in Inflammation and Cancer Therapeutics with Emphasis on the NF-κB, NLRP3 Inflammasome, and Cytokine Regulatory Axes. Int. J. Mol. Sci. 2025, 26, 8429. https://doi.org/10.3390/ijms26178429
Khan S, Huda B, Bhurka F, Patnaik R, Banerjee Y. Molecular and Immunomodulatory Mechanisms of Statins in Inflammation and Cancer Therapeutics with Emphasis on the NF-κB, NLRP3 Inflammasome, and Cytokine Regulatory Axes. International Journal of Molecular Sciences. 2025; 26(17):8429. https://doi.org/10.3390/ijms26178429
Chicago/Turabian StyleKhan, Sara, Bintul Huda, Farida Bhurka, Rajashree Patnaik, and Yajnavalka Banerjee. 2025. "Molecular and Immunomodulatory Mechanisms of Statins in Inflammation and Cancer Therapeutics with Emphasis on the NF-κB, NLRP3 Inflammasome, and Cytokine Regulatory Axes" International Journal of Molecular Sciences 26, no. 17: 8429. https://doi.org/10.3390/ijms26178429
APA StyleKhan, S., Huda, B., Bhurka, F., Patnaik, R., & Banerjee, Y. (2025). Molecular and Immunomodulatory Mechanisms of Statins in Inflammation and Cancer Therapeutics with Emphasis on the NF-κB, NLRP3 Inflammasome, and Cytokine Regulatory Axes. International Journal of Molecular Sciences, 26(17), 8429. https://doi.org/10.3390/ijms26178429






