Effects of Nitric Oxide Expression on Hearing Loss
Abstract
1. Introduction
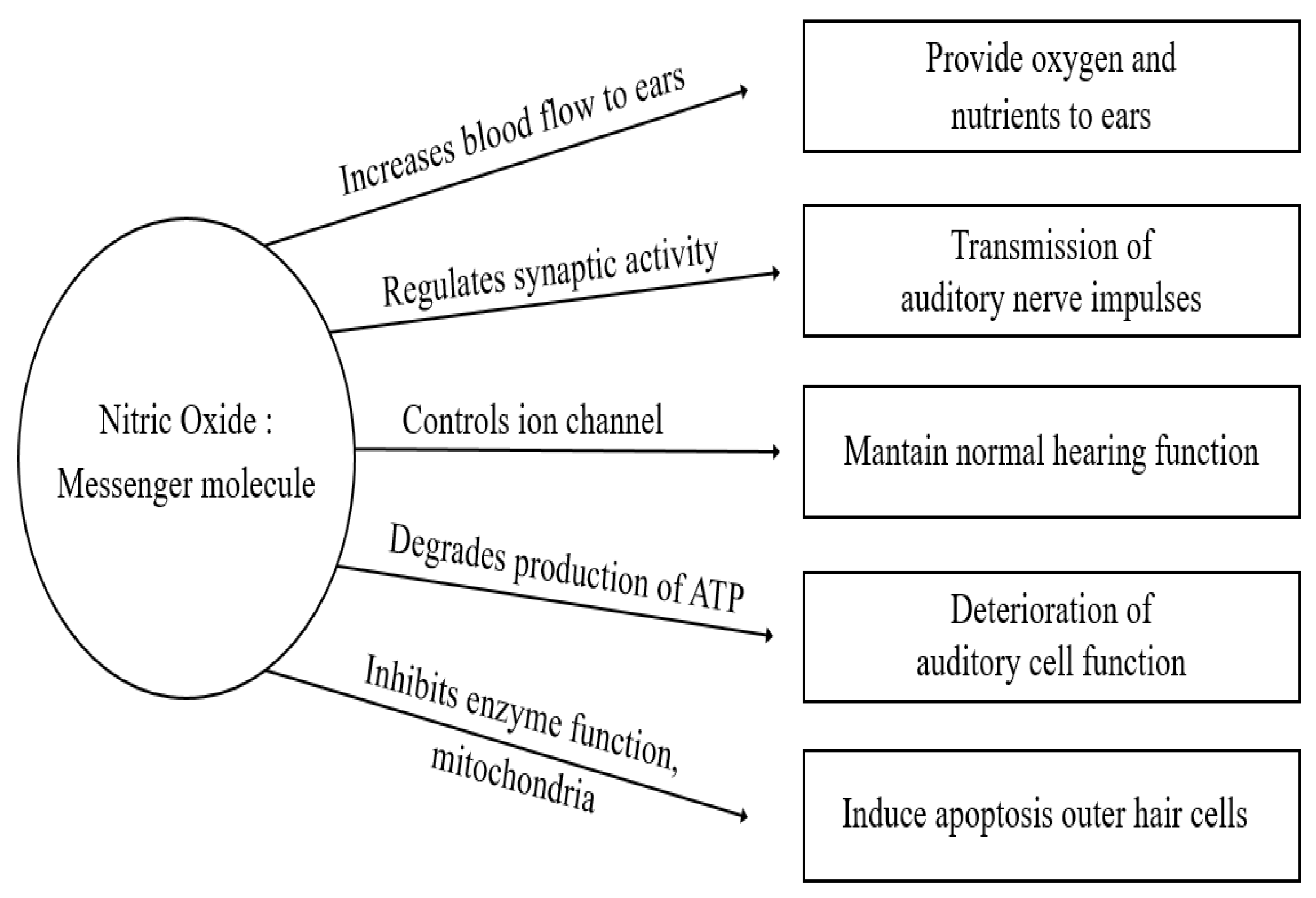
1.1. Nitric Oxide
1.2. Hearing Loss
2. Research Methods
3. Studies on the Role of NO in Hearing Loss
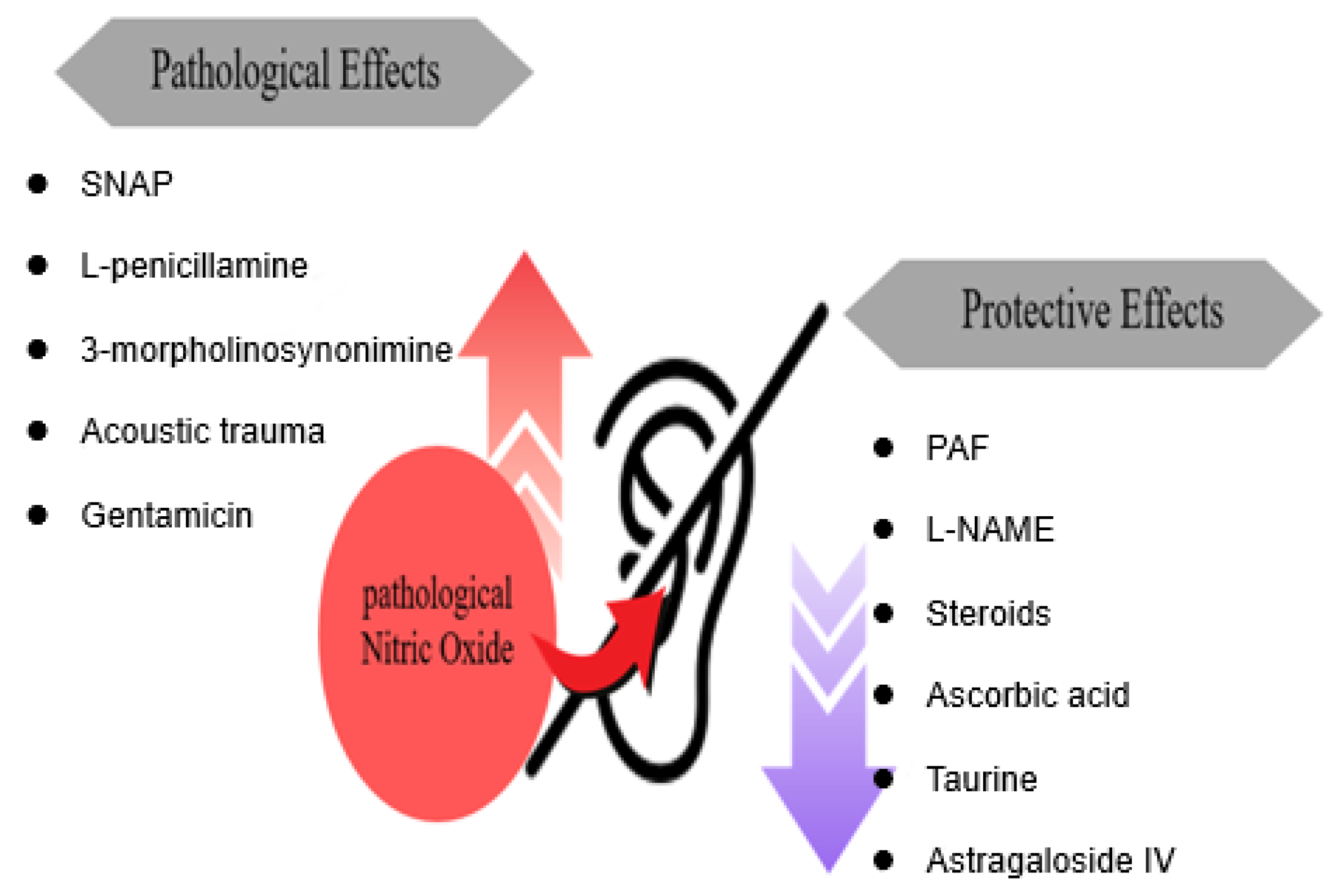
3.1. Studies Investigating the Role of Increased NO in the Pathogenesis of Hearing Loss
3.1.1. NO and NO Donor/NO-Producing Compounds
S-Nitroso-N-Acetylpenicillamine
SNAP-1/3-Morpholinosydnonimine
Acoustic Trauma or Noise
Gentamicin
3.1.2. NO Inhibitors
Platelet-Activating Factor and L-NAME
L-NAME
Steroids
Ascorbic Acid
Taurine
Astragaloside IV
3.1.3. eNOS Polymorphisms
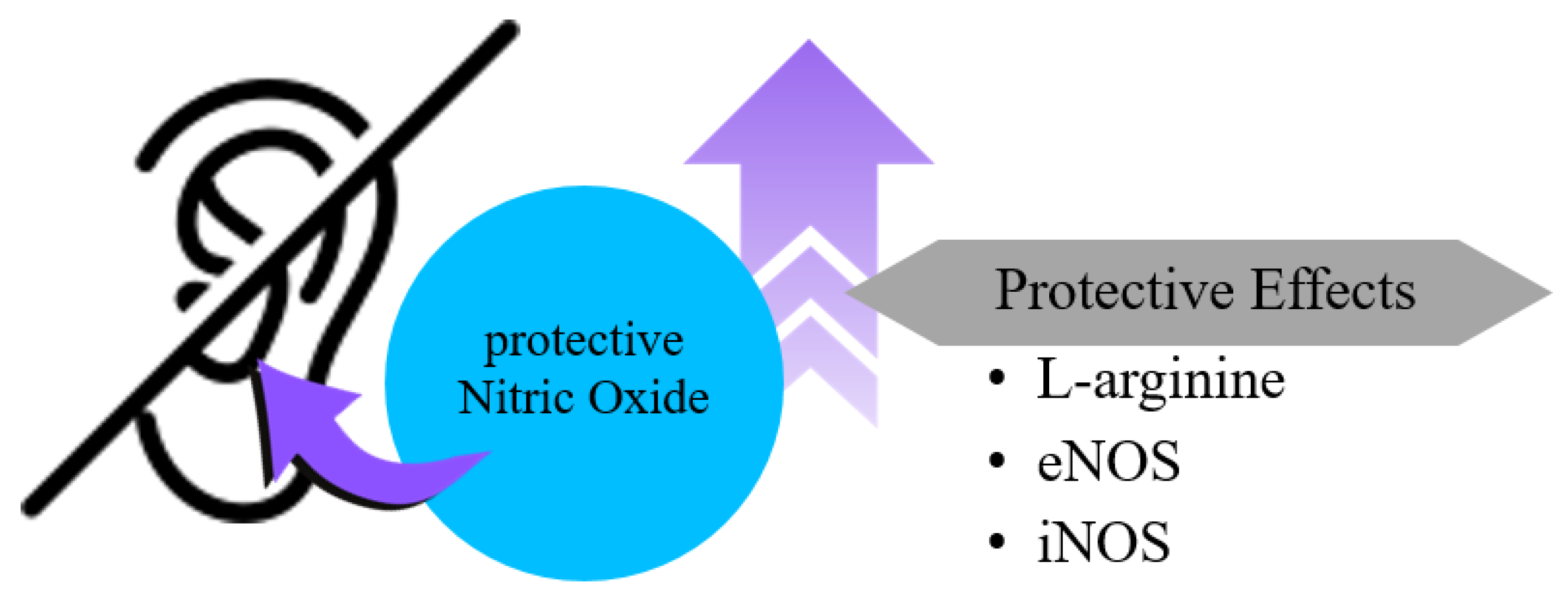
3.2. Studies Suggesting That Increased NO Prevents or Mitigates Hearing Loss
3.2.1. L-Arginine and NO Production
3.2.2. eNOS and Intrinsic Protection
3.2.3. nNOS Deficiency and Hearing Impairment
3.2.4. NO in Tinnitus and Auditory Modulation
3.2.5. iNOS and Age-Related Cochlear Protection
3.3. Studies Suggesting That Increased NO Is Associated with the Development of Hearing Loss, but with Uncertain or Inconclusive Evidence
3.3.1. NOS Isoforms and Cholesteatoma
3.3.2. NO Donors and Inhibitors
3.3.3. NO-GC Knockout Models
3.3.4. NOS Inhibitor
4. Limitations
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tenopoulou, M.; Doulias, P.-T. Endothelial Nitric Oxide Synthase-Derived Nitric Oxide in the Regulation of Metabolism. F1000Res 2020, 9, 1190. [Google Scholar] [CrossRef]
- Cinelli, M.A.; Do, H.T.; Miley, G.P.; Silverman, R.B. Inducible Nitric Oxide Synthase: Regulation, Structure, and Inhibition. Med. Res. Rev. 2020, 40, 158–189. [Google Scholar] [CrossRef]
- Cyr, A.R.; Huckaby, L.V.; Shiva, S.S.; Zuckerbraun, B.S. Nitric Oxide and Endothelial Dysfunction. Crit. Care Clin. 2020, 36, 307–321. [Google Scholar] [CrossRef]
- Srinivas, P.; Wink, D.; Mohanakumar, K.P.; Pillai, M.R. The Legacy of Nitric Oxide: Impact on Disease Biology. Nitric Oxide 2014, 43, 1–2. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, M.; Mukosera, G.T.; Borchardt, D.; Li, Q.; Tipple, T.E.; Ishtiaq Ahmed, A.S.; Power, G.G.; Blood, A.B. L-NAME Releases Nitric Oxide and Potentiates Subsequent Nitroglycerin-Mediated Vasodilation. Redox Biol. 2019, 26, 101238. [Google Scholar] [CrossRef] [PubMed]
- Kurabi, A.; Keithley, E.M.; Housley, G.D.; Ryan, A.F.; Wong, A.C.-Y. Cellular Mechanisms of Noise-Induced Hearing Loss. Hear. Res. 2017, 349, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Wagner, E.L.; Shin, J.-B. Mechanisms of Hair Cell Damage and Repair. Trends Neurosci. 2019, 42, 414–424. [Google Scholar] [CrossRef]
- Moser, T.; Starr, A. Auditory Neuropathy—Neural and Synaptic Mechanisms. Nat. Rev. Neurol. 2016, 12, 135–149. [Google Scholar] [CrossRef]
- Michels, T.C.; Duffy, M.T.; Rogers, D.J. Hearing Loss in Adults: Differential Diagnosis and Treatment. Am. Fam. Physician 2019, 100, 98–108. [Google Scholar]
- Vassallo, E.; Gatt, A.-S.; Grech, R.; Capasso, S.; Caranci, F.; Ugga, L. Imaging in Sensorineural and Conductive Hearing Loss—An Educational Review. Radiol. Med. 2024, 130, 46–62. [Google Scholar] [CrossRef] [PubMed]
- Jarman, F.C. Sensorineural Hearing Loss. J. Paediatr. Child. Health 1991, 27, 74–75. [Google Scholar] [CrossRef]
- Kopp-Scheinpflug, C.; Forsythe, I.D. Nitric Oxide Signaling in the Auditory Pathway. Front. Neural Circuits 2021, 15, 759342. [Google Scholar] [CrossRef] [PubMed]
- Jung, T.T.K.; Llaurado, R.J.; Nam, B.H.; Park, S.K.; Kim, P.D.; John, E.O. Effects of Nitric Oxide on Morphology of Isolated Cochlear Outer Hair Cells: Possible Involvement in Sensorineural Hearing Loss. Otol. Neurotol. 2003, 24, 682–685. [Google Scholar] [CrossRef]
- Heinrich, U.-R.; Helling, K.; Sifferath, M.; Brieger, J.; Li, H.; Schmidtmann, I.; Mann, W.J. Gentamicin Increases Nitric Oxide Production and Induces Hearing Loss in Guinea Pigs. Laryngoscope 2008, 118, 1438–1442. [Google Scholar] [CrossRef]
- Chen, Y.-S.; Tseng, F.-Y.; Liu, T.-C.; Lin-Shiau, S.Y.; Hsu, C.-J. Involvement of Nitric Oxide Generation in Noise-Induced Temporary Threshold Shift in Guinea Pigs. Hear. Res. 2005, 203, 94–100. [Google Scholar] [CrossRef]
- Tamura, A.; Matsunobu, T.; Tamura, R.; Kawauchi, S.; Sato, S.; Shiotani, A. Photobiomodulation Rescues the Cochlea from Noise-Induced Hearing Loss via Upregulating Nuclear Factor ΚB Expression in Rats. Brain Res. 2016, 1646, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Hanson, J.B.; Russell, P.T.; Chung, A.T.A.; Kaura, C.S.; Kaura, S.H.; John, E.O.; Jung, T.T.K. Effect of Round Window Membrane Application of Nitric Oxide on Hearing and Nitric Oxide Concentration in Perilymph. Int. J. Pediatr. Otorhinolaryngol. 2003, 67, 585–590. [Google Scholar] [CrossRef]
- Rhee, C.-K. Candidate’s Thesis: Platelet-Activating Factor-Induced Hearing Loss: Mediated by Nitric Oxide? Laryngoscope 2003, 113, 2059–2066. [Google Scholar] [CrossRef]
- Pudrith, C.; Martin, D.; Kim, Y.H.; Jahng, P.; Kim, B.; Wall, M.; Jung, T. Glucocorticoids Reduce Nitric Oxide Concentration in Middle Ear Effusion from Lipopolysaccharide Induced Otitis Media. Int. J. Pediatr. Otorhinolaryngol. 2010, 74, 384–386. [Google Scholar] [CrossRef]
- Heinrich, U.-R.; Fischer, I.; Brieger, J.; Rümelin, A.; Schmidtmann, I.; Li, H.; Mann, W.J.; Helling, K. Ascorbic Acid Reduces Noise-Induced Nitric Oxide Production in the Guinea Pig Ear. Laryngoscope 2008, 118, 837–842. [Google Scholar] [CrossRef] [PubMed]
- Diao, M.; Gao, W.; Sun, J. Nitric Oxide Synthase Inhibitor Reduces Noise-Induced Cochlear Damage in Guinea Pigs. Acta Otolaryngol. 2007, 127, 1162–1167. [Google Scholar] [CrossRef]
- Liu, H.-Y.; Chi, F.-L.; Gao, W.-Y. Taurine Attenuates Aminoglycoside Ototoxicity by Inhibiting Inducible Nitric Oxide Synthase Expression in the Cochlea. Neuroreport 2008, 19, 117–120. [Google Scholar] [CrossRef]
- Xiong, M.; Lai, H.; He, Q.; Wang, J. Astragaloside IV Attenuates Impulse Noise-Induced Trauma in Guinea Pig. Acta Otolaryngol. 2011, 131, 809–816. [Google Scholar] [CrossRef]
- Yazdani, N.; Kakavand Hamidi, A.; Soroush, N.; Jalili, N.; Vahidi, A.; Zarabi Ahrabi, N.; Tajdini, A.; Amoli, M. ENOS Gene Glu298Asp Variant Confer Risk in Sudden Sensorineural Hearing Loss. Acta Otolaryngol. 2018, 138, 904–908. [Google Scholar] [CrossRef] [PubMed]
- Teranishi, M.; Uchida, Y.; Nishio, N.; Kato, K.; Otake, H.; Yoshida, T.; Suzuki, H.; Sone, M.; Sugiura, S.; Ando, F.; et al. Polymorphisms in Genes Involved in the Free-Radical Process in Patients with Sudden Sensorineural Hearing Loss and Ménière’s Disease. Free Radic. Res. 2013, 47, 498–506. [Google Scholar] [CrossRef]
- Yang, H.; Zhu, Y.; Ye, Y.; Guan, J.; Min, X.; Xiong, H. Nitric Oxide Protects against Cochlear Hair Cell Damage and Noise-Induced Hearing Loss through Glucose Metabolic Reprogramming. Free Radic. Biol. Med. 2022, 179, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, U.R.; Schmidtmann, I.; Meuser, R.; Ernst, B.P.; Wünsch, D.; Siemer, S.; Gribko, A.; Stauber, R.H.; Strieth, S. Early Alterations of Endothelial Nitric Oxide Synthase Expression Patterns in the Guinea Pig Cochlea After Noise Exposure. J. Histochem. Cytochem. 2019, 67, 845–855. [Google Scholar] [CrossRef] [PubMed]
- Chachlaki, K.; Messina, A.; Delli, V.; Leysen, V.; Maurnyi, C.; Huber, C.; Ternier, G.; Skrapits, K.; Papadakis, G.; Shruti, S.; et al. NOS1 Mutations Cause Hypogonadotropic Hypogonadism with Sensory and Cognitive Deficits That Can Be Reversed in Infantile Mice. Sci. Transl. Med. 2022, 14, eabh2369. [Google Scholar] [CrossRef]
- Hockley, A.; Berger, J.I.; Palmer, A.R.; Wallace, M.N. Nitric Oxide Increases Gain in the Ventral Cochlear Nucleus of Guinea Pigs with Tinnitus. Eur. J. Neurosci. 2020, 52, 4057–4080. [Google Scholar] [CrossRef]
- Shi, X.; Han, W.; Yamamoto, H.; Omelchenko, I.; Nuttall, A. Nitric Oxide and Mitochondrial Status in Noise-Induced Hearing Loss. Free Radic. Res. 2007, 41, 1313–1325. [Google Scholar] [CrossRef]
- Labbé, D.; Bloch, W.; Schick, B.; Michel, O. Hearing Impairment, Cochlear Morphology, and Peroxynitrite (ONOO(-)) Formation in Adult and Aging NOS II Knockout Mice. Acta Otolaryngol. 2016, 136, 991–998. [Google Scholar] [CrossRef]
- Jung, J.Y.; Pashia, M.E.; Nishimoto, S.Y.; Faddis, B.T.; Chole, R.A. A Possible Role for Nitric Oxide in Osteoclastogenesis Associated with Cholesteatoma. Otol. Neurotol. 2004, 25, 661–668. [Google Scholar] [CrossRef] [PubMed]
- Hockley, A.; Berger, J.I.; Smith, P.A.; Palmer, A.R.; Wallace, M.N. Nitric Oxide Regulates the Firing Rate of Neuronal Subtypes in the Guinea Pig Ventral Cochlear Nucleus. Eur. J. Neurosci. 2020, 51, 963–983. [Google Scholar] [CrossRef] [PubMed]
- Möhrle, D.; Reimann, K.; Wolter, S.; Wolters, M.; Varakina, K.; Mergia, E.; Eichert, N.; Geisler, H.-S.; Sandner, P.; Ruth, P.; et al. NO-Sensitive Guanylate Cyclase Isoforms NO-GC1 and NO-GC2 Contribute to Noise-Induced Inner Hair Cell Synaptopathy. Mol. Pharmacol. 2017, 92, 375–388. [Google Scholar] [CrossRef] [PubMed]
- Murashita, H.; Tabuchi, K.; Hoshino, T.; Tsuji, S.; Hara, A. The Effects of Tempol, 3-Aminobenzamide and Nitric Oxide Synthase Inhibitors on Acoustic Injury of the Mouse Cochlea. Hear. Res. 2006, 214, 1–6. [Google Scholar] [CrossRef]

| Author [Reference] | Study Design | Species and/or Sample | Detection Method | Causative Agent | Target Gene(s) | Results/Conclusion |
|---|---|---|---|---|---|---|
| Jung T.T. et al., 2003 [13] | Animal study | 58 chinchillas | A NO derivative was used to generate NO externally, and the corresponding change in the form of OHC was detected using the IMAGE Pro-Plus program. | SNAP | iNOS | OHCs exposed to either a standard bathing solution or sodium nitrite (Control Groups 1 and 2) did not exhibit any significant changes in cell shape or length. However, cells exposed to NO donors, such as SNAP or SIN-1, displayed ballooning and significant shortening in mean cell length (p < 0.01). /Exposure to NO induces irreversible morphological changes in isolated OHCs, suggesting that NO radicals may play a role in the development of SNHL as a consequence of chronic otitis media. |
| Heinrich U.R. et al., 2008 [14] | Animal study | 24 guinea pigs | Chemiluminescence | SNAP | eNOS | One day after injection, gentamicin caused a 1.34-fold increase in the production of NO2 (a stable oxidative product of NO) in the organ of Corti compared with control conditions (physiological saline). NO2 concentrations in the organ of Corti remained similar 2 and 7 days after gentamicin application. NO2 levels also significantly increased by a factor of 3.24 in the lateral wall 2 days post-injection, reaching a mean concentration of 874 nM. Gentamicin application led to a shift in the hearing threshold beginning on the second day after application. /Hearing impairment was correlated with an increase in NO2 content in the lateral wall. NO2 production increased slightly in the organ of Corti as early as day 1 after gentamicin injection. |
| Chen Y.S. et al., 2005 [15] | Animal study | 34 guinea pigs | NO Analyzer 280A | Acoustic trauma or noise | iNOS | An average 16.2 dB threshold shift was found immediately after noise exposure. The threshold returned to the pre-noise-exposed level on the second day post-exposure. Compared to unexposed control animals, the NO concentration increased nearly threefold immediately following noise exposure and decreased to twofold when the hearing threshold returned to the pre-noise-exposed level. /The increase in NO concentration after acoustic trauma is associated with hearing loss, indicating that a high concentration of NO is neurotoxic for cochlear function. |
| Atsushi Tamura et al., 2016 [16] | Animal study | 69 rats | Immunofluorescence | Acoustic trauma or noise | iNOS | Immunofluorescence image analysis of NF-κB, an upstream regulator of iNOS, revealed greater activation in the PBM group compared with naïve and non-treatment groups. Western blot analysis of NF-κB also showed stronger activation in cochlear tissues in the PBM group compared with naïve and non-treatment groups (p < 0.01 each). /PBM activates NF-κB, providing protection against iNOS-triggered oxidative stress and caspase-3-mediated apoptosis that accompanies NIHL. |
| Jonathan B. Hanson et al., 2003 [17] | Animal study | 18 chinchillas | Colorimetric assay | Gentamicin | iNOS | Comparing the mean ABR threshold measurements in the SNAP-operated (SNAP-OP) group with the SNAP non-operated (SNAP Non-OP) control group (contralateral) from 1 to 8 h following the application of a fresh SNAP solution on the round window membrane, there was a significant increase (p < 0.05) in the ABR threshold after 3 h in the experimental group compared to the control group. In the operated ears, levels of NO metabolites (NO2− + NO3−) in the perilymph were approximately three times higher (p = 0.02) than those in the non-operated ears. /While NO seems to play a crucial role in the development of middle ear effusions and can lead to cochlear damage, its impact on the inner ear as it moves from the middle ear space remains undocumented. |
| Chung-Ku Rhee et al., 2003 [18] | Animal study | 70 guinea pigs | Immunohistochemistry | PAF and NAME | iNOS | The ABR threshold and cochlear hair cell damage were notably higher in the PAF treatment group compared to the PBS control group. In the group treated with a PAF-antagonist, the threshold increased to 7 dB SPL at 3 h, fluctuating between 7 and 11 dB SPL throughout the 24 h period. /Application of PAF to the RWM resulted in hearing loss and damage to cochlear hair cells. However, the use of PAF-antagonists and L-NAME effectively prevented PAF-induced hearing loss and suppressed iNOS expression in the cochlea. |
| Charles Pudrith et al., 2010 [19] | Animal study | 53 chinchillas | Griess Reagent Assay | L-NAME | iNOS | At a 0.1% concentration, the three glucocorticoids—dexamethasone, fluticasone propionate, and rimexolone—demonstrated a numerical decrease in NO levels within middle ear effusions, with only fluticasone propionate achieving a statistically significant reduction. At a higher concentration of 1.0%, all three glucocorticoids markedly lowered NO concentrations, with an average reduction of 55.3%. /Glucocorticoid therapy effectively lowers NO levels in middle ear effusions within a lipopolysaccharide-induced otitis media model, implying that glucocorticoids might offer protection against SNHL by curbing NO-mediated ototoxicity. |
| Heinrich U.R. et al., 2008 [20] | Animal study | 54 guinea pigs | The amount of NO produced was indirectly evaluated by detecting nitrite, a stable oxidative metabolite of NO, by chemiluminescence. | Steroids | iNOS | Treatment with ascorbic acid led to a dose-dependent decrease in hearing thresholds following noise exposure. When administered in high doses, ascorbic acid notably reduced NO production in the lateral wall post-noise exposure and showed a tendency to lower NO production in the organ of Corti. /Oral supplementation with the natural radical scavenger ascorbic acid diminishes the rate of NO production in the inner ear under noisy conditions, supporting its protective role against inner ear damage. |
| Diao M. et al., 2007 [21] | Animal study | 70 guinea pigs | NO assay kit | Ascorbic acid | iNOS | Immediate pre-treatment with L-NAME (Group III) led to significantly greater outer hair cell (OHC) loss, threshold shifts, and NO levels when compared with 2-day pre-treatment (Group I) and noise-exposed animals without L-NAME (Group IV). The increase in NO levels in the cochlea due to noise was significantly reduced by L-NAME compared to Group III (p < 0.001). /L-NAME provides protection against cochlear damage from acoustic trauma by decreasing NO production. |
| Liu H.Y. et al., 2008 [22] | Animal study | 40 guinea pigs | Western blot, immunofluorescence | Taurine | iNOS | In an in vivo model of gentamycin/furosemide-induced ototoxicity, iNOS expression in the cochlea was significantly increased three days following treatment, correlating with severe hearing loss. Pretreatment with taurine or aminoguanidine, both of which are selective iNOS inhibitors, resulted in the downregulation of iNOS and prevented hearing damage. /Taurine offers protection against acute ototoxicity from gentamycin/furosemide, potentially by downregulating iNOS expression in the cochlea. |
| Min Xiong et al., 2011 [23] | Animal study | 36 guinea pigs | Immunohistochemistry | Astragaloside Ⅳ | iNOS | Astragaloside IV significantly mitigated ABR deficits, hair cell damage, iNOS expression, and RNS formation in a model of impulse noise-induced hearing loss. /The protective effect of Astragaloside IV against impulse NIHL may be due to its capacity to inhibit iNOS and prevent RNS formation. |
| Nasrin Yazdani et al., 2018 [24] | Case study | 77 patients | eNOS genetic analysis | NO polymorphisms | eNOS | A statistically significant correlation was identified between the genotype frequencies of the eNOS protein polymorphism, Glu298Asp, and the presence of SSNHL in the patient group compared to healthy controls. The TT genotype was notably more prevalent in the patient group than in the control group. /The eNOS protein polymorphism, Glu298Asp, shows a significant association with SSNHL in an Iranian population, suggesting that the TT genotype could be considered a risk factor for SSNHL. |
| Teranishi et a., 2013 [25] | Clinical study | 4160 participants | Polymerase Chain Reaction (PCR), Genotyping | NO polymorphisms | eNOS | Certain genetic variations, particularly in genes related to the body’s free-radical processes, are significantly associated with an increased risk of idiopathic sudden SSNHL and Ménière’s disease. Specifically, a variant in the NOS3 gene, which affects nitric oxide production, was notably linked to a higher risk of SSNHL. This was determined by comparing patients with these conditions to large control groups, highlighting the influence of these genetic factors. /Genetic differences influencing the free-radical process may play a role in the development of SSNHL and Ménière’s disease. These insights could pave the way for more personalized treatment approaches and help identify individuals at greater genetic risk for these conditions. The study also underscores the need for additional research to further explore these genetic influences and confirm the results in larger populations. |
| Author [Reference] | Study Design | Species and/or Sample | Detection Method | Causative Agent | Target Gene(s) | Results/Conclusion |
|---|---|---|---|---|---|---|
| Haidi Yang et al., 2021 [26] | Animal study | 30~40 mice | Colorimetric NO assay | L-argininge | eNOS | The cytosol of OHCs, IHCs, spiral ganglion cells, and stria vascularis cells exhibited strong immunostaining for eNOS. In response to noise exposure, NO levels in the cochlea increased as a compensatory mechanism. Supplementing with L-arginine further elevated NO levels and protected against NIHL in mice. Noise exposure led to a significant reduction in wave amplitude (from 60 to 80 dB SPL) compared to controls, a decrease that was mitigated by L-arginine supplementation. /NO helps to minimize noise-induced ROS buildup in the cochlea and reduces NIHL in mice. |
| Heinrich U.R. et al., 2019 [27] | Animal study | 24 guinea pigs | Immunohistochemistry, immunoelectron microscopy | eNOS | eNOS | Exposure to 90 dB for 1 or 2 h caused a rapid increase in eNOS immunostaining intensity in almost all cochlear regions compared to controls, aligning with previous findings that eNOS regulation occurs at transcriptional, translational, and post-translational levels. /Similarly to iNOS, eNOS can be upregulated, with its cochlear expression increasing in response to noise exposure. Additionally, the reticular lamina, which forms the endolymph-perilymph barrier at the apical side of the organ of Corti, participates in a swift, intrinsic cochlear otoprotective mechanism. |
| Konstantina Chachlaki et al., 2022 [28] | Animal study | Human and mice | Live-cell imaging using NO sensor (FlincG3), fluorometric nitrate assay | nNOS | iNOS | NOS1 was transiently expressed by GnRH neurons in the nasal region of both humans and mice. In mice, a deficiency in Nos1 led to abnormalities in sexual maturation, as well as impairments in olfaction, hearing, and cognitive functions. /The absence of timely NOS1 activity results in a GnRH deficiency, contributing to lifelong sensory and intellectual comorbidities in humans and mice. By addressing deficits in sexual maturation, olfaction, and cognition in Nos1-deficient mice within a critical period, NO treatment shows therapeutic promise for humans. |
| Adam Hockley et al., 2020 [29] | Animal study | 26 guinea pigs | Iontophoresis | NO | nNOS | In the context of noise-induced tinnitus, a higher percentage of neural units responded to externally applied NO in both tinnitus (56%) and non-tinnitus groups (71%) compared to the control group (24%). Within the tinnitus group, endogenous NO increased the driven firing rate in 37% (7 out of 19) of neurons, seemingly restoring the mean driven firing rate to control levels via a mechanism involving NMDA receptors. Conversely, in the non-tinnitus group, endogenous NO enhanced the driven firing rate in only 5% (1 out of 22) of neurons and did not impact the driven firing rate in the control group. /NO may play a role in augmenting the gain on neurally driven activity, although other factors also contribute to the rise in spontaneous activity. |
| Shi et al., 2007 [30] | Animal study | 80 albino guinea pigs | Fluorescent dyes, Immunocytochemistry | NO | NO was predominantly localized in the mitochondria of OHCs in the cochlea. Exposure to loud noise and treatment with an NO donor both resulted in reduced mitochondrial membrane potential and increased NO levels. Additionally, there was an increase in nitrotyrosine, indicating the formation of peroxynitrite, a reactive nitrogen species, which suggests oxidative stress within the mitochondria. /NO plays a crucial role in regulating the energy status of OHCs and contributes to cellular damage under stress conditions like loud noise exposure. This interaction between NO and mitochondria could be a key factor in the pathology of NIHL, highlighting potential therapeutic targets to mitigate hearing damage by focusing on mitochondrial health and NO pathways. | |
| Daniel Labbé et al., 2016 [31] | Animal study | 30~50 mice | Immunohistochemistry | iNOS | iNOS | A study examining the role of iNOS (NOS2) in age-related cochlear regression revealed that NOS2 is upregulated in wild-type (WT) mice after 6 months of age. Nos2 knockout (NOS2-KO) mice displayed slightly impaired hearing in adulthood, with an accelerated decline in hearing as they aged, accompanied by increased nitrotyrosine formation and loss of OHCs. /The induction of NOS2 serves as a protective mechanism against age-related cochlear degeneration. |
| Author [Reference] | Study Design | Species and/or Sample | Detection Method | Causative Agent | Target Gene(s) | Results/Conclusion |
|---|---|---|---|---|---|---|
| Jung, Jae Y. et al., 2004 [32] | Animal study | 18 mouse model | Colorimetric assay, in vitro osteoclast culture method | NOS isoforms | iNOS | In a keratin implant-induced model of cholesteatoma-induced bone resorption, the expression levels of NOS1 and NOS3 remained low or very low throughout the study and did not change in response to the keratin implant. In contrast, NOS2 was upregulated due to the keratin implantation. While AG, a selective NOS2 inhibitor, reduced nitrite production in vitro, it did not affect the osteoclast response in vivo. /Although NOS2 was upregulated in vivo in this model, and AG suppressed nitrite production in vitro, the lack of effect of AG on the osteoclast response in vivo suggests that bone loss in this model is driven by a NOS-independent mechanism. |
| Adam Hockley et al., 1997 [33] | Animal study | 27 male and female guinea pigs. | Iontophoresis, Immunohistochemistry, sound system response | L-NAME | nNOS | Inhibition of endogenous NO production using L-NAME led to a consistent increase in driven firing rates in 18% of neural units, while having minimal impact on spontaneous rates. This reduction in gain caused by endogenous NO was reflected in the effects of L-NAME on NMDA-evoked excitation, with 30% of units displaying enhanced NMDA-evoked excitation when NO levels were reduced by L-NAME application. Approximately 25% of neurons were nNOS-positive, and the NO they produced can modulate the firing rates of the main principal cells: medium stellates (choppers), large stellates (onset responses), and bushy cells (primary-like responses). /Endogenous NO seems to primarily function in suppressing driven firing rates linked to NMDA channel activity, but it also serves to increase neural gain when there are pathological increases in its production following hearing loss. |
| Dorit Möhrle et al., 2017 [34] | Animal study | 12 KO mice | Fluorescence resonance energy transfer, ABR, and DPOAE, RT-PCR. | NO-GC1, NO-GC2 | iNOS | A NO-induced increase in cGMP was observed in real-time within inner hair cells, but not in OHCs. Pharmacological long-term treatment with an NO-GC stimulator modified auditory nerve responses without affecting OHC function or hearing thresholds. Interestingly, NO-GC stimulation worsened the decline of auditory nerve response in older animals but mitigated it in younger animals. /NO-GC2 and, to a lesser extent, NO-GC1 may serve as targets for early pharmacological intervention to prevent auditory fiber loss (synaptopathy). Both isoforms offer selective advantages for hearing function by preserving the functional integrity of auditory nerve fibers during early life rather than in old age. |
| Murashita et al., 2006 [35] | Animal study | 67 female mice | Auditory brainstem response (ABR), cochlear surface preparation | Tempol, 3-aminobenzamide, and nitric oxide synthase inhibitors | NOS isoforms | Tempol, a superoxide anion scavenger, and 3-aminobenzamide (3AB), a PARS inhibitor, both significantly protected the cochlea from acoustic injury by reducing ABR threshold shifts and hair cell loss. The protective effects were dose-dependent. In contrast, NOS inhibitors did not show any protective effects. /The study suggests that ROS and PARS play crucial roles in acoustic injury, as evidenced by the protective effects of tempol and 3AB. The lack of effect from NOS inhibitors implies a lesser role for nitric oxide in such injuries. These findings point to ROS and PARS as potential therapeutic targets, but further research is needed to understand the underlying mechanisms. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cha, Y.J.; Yeo, J.H.; Kim, S.S.; Lee, J.M.; Oh, Y.J.; Yon, D.K.; Yeo, S.G. Effects of Nitric Oxide Expression on Hearing Loss. Int. J. Mol. Sci. 2025, 26, 8416. https://doi.org/10.3390/ijms26178416
Cha YJ, Yeo JH, Kim SS, Lee JM, Oh YJ, Yon DK, Yeo SG. Effects of Nitric Oxide Expression on Hearing Loss. International Journal of Molecular Sciences. 2025; 26(17):8416. https://doi.org/10.3390/ijms26178416
Chicago/Turabian StyleCha, Yoo Jin, Joon Hyung Yeo, Sung Soo Kim, Jae Min Lee, Yeon Ju Oh, Dong Keon Yon, and Seung Geun Yeo. 2025. "Effects of Nitric Oxide Expression on Hearing Loss" International Journal of Molecular Sciences 26, no. 17: 8416. https://doi.org/10.3390/ijms26178416
APA StyleCha, Y. J., Yeo, J. H., Kim, S. S., Lee, J. M., Oh, Y. J., Yon, D. K., & Yeo, S. G. (2025). Effects of Nitric Oxide Expression on Hearing Loss. International Journal of Molecular Sciences, 26(17), 8416. https://doi.org/10.3390/ijms26178416







