Integrative Evidence on Mulberry Extract for Modulating Metabolic Risk Factors Associated with Vascular Dementia
Abstract
1. Introduction
2. Results
2.1. Study Search and Characteristics of Included Patients
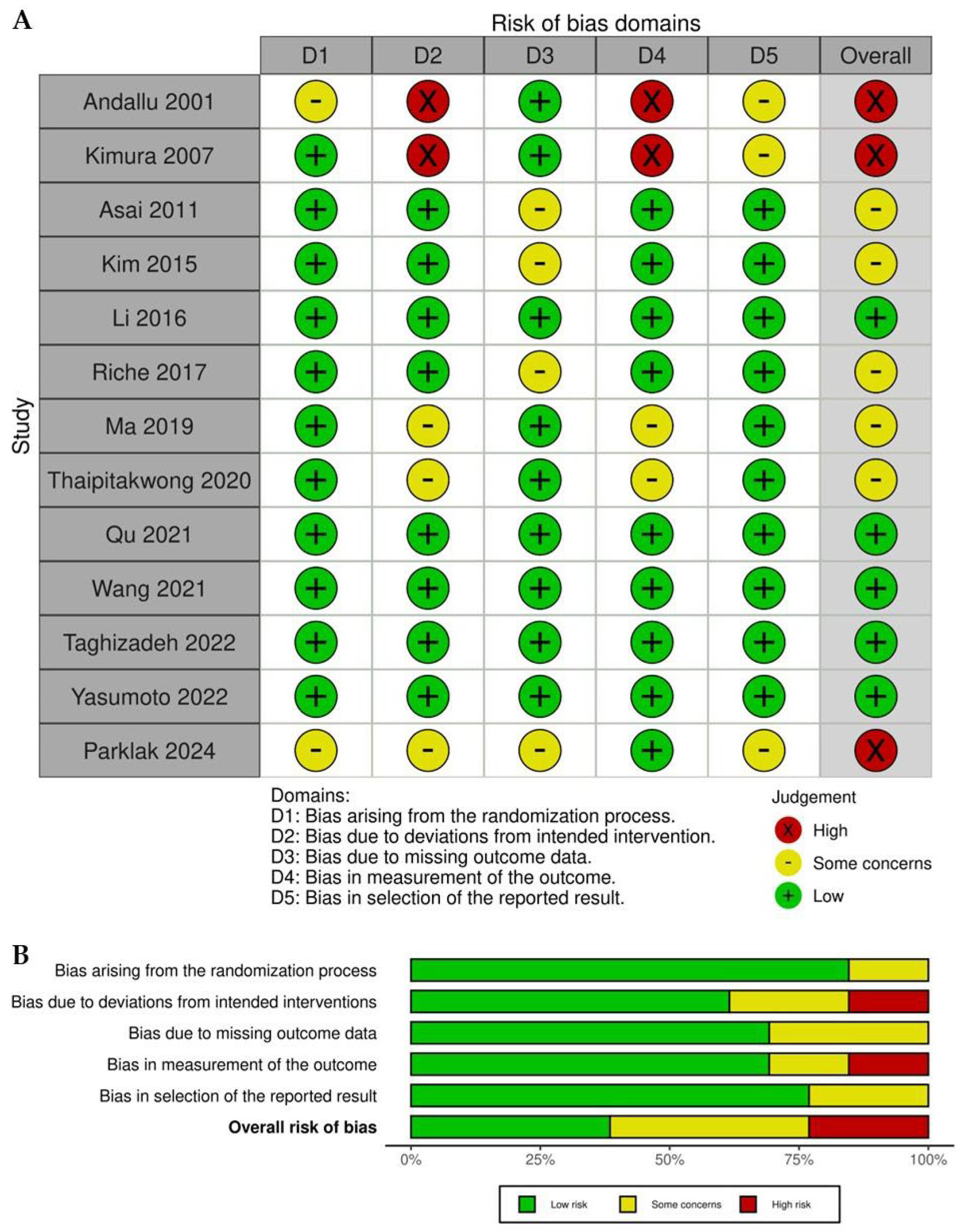
2.2. Quality Assessment
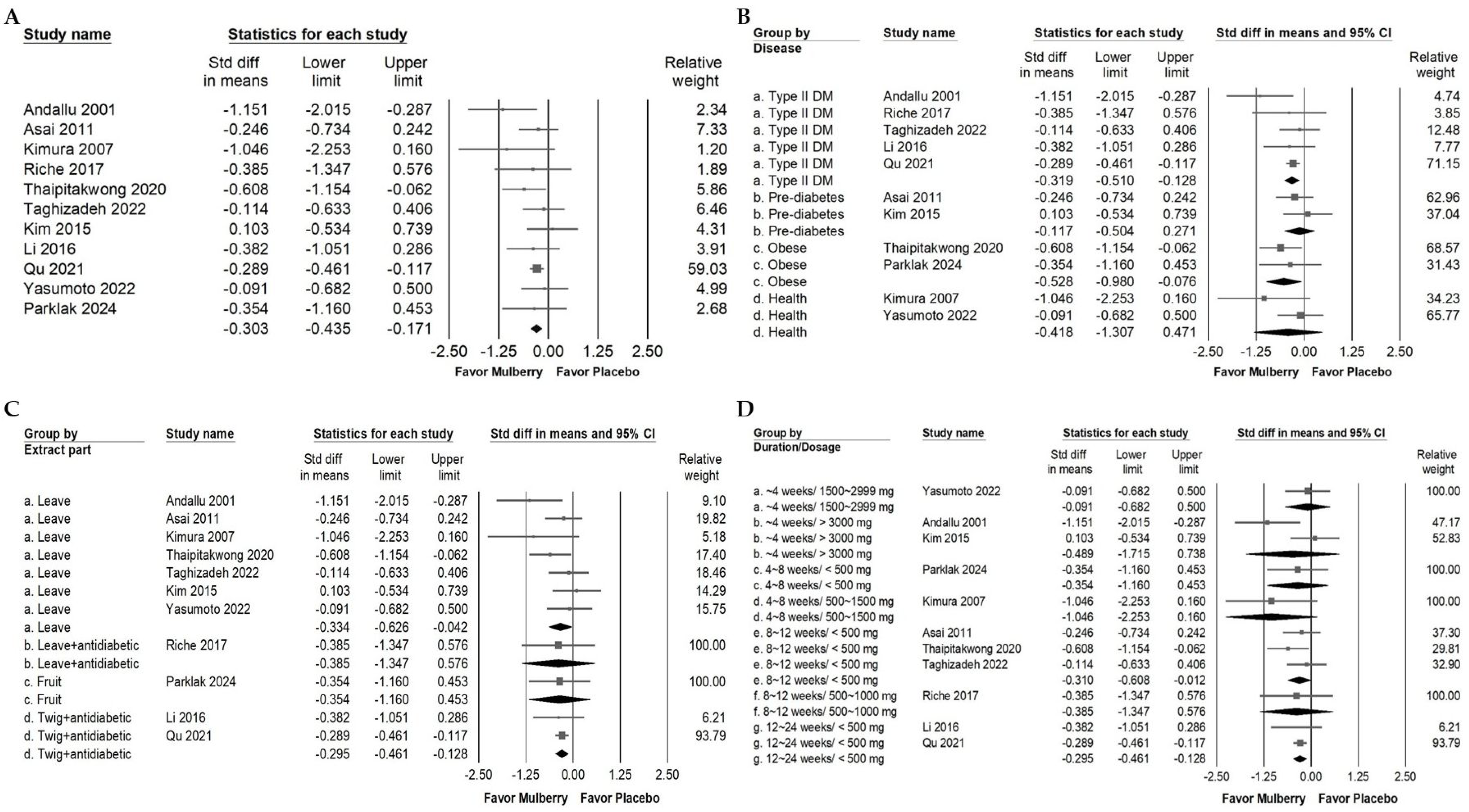
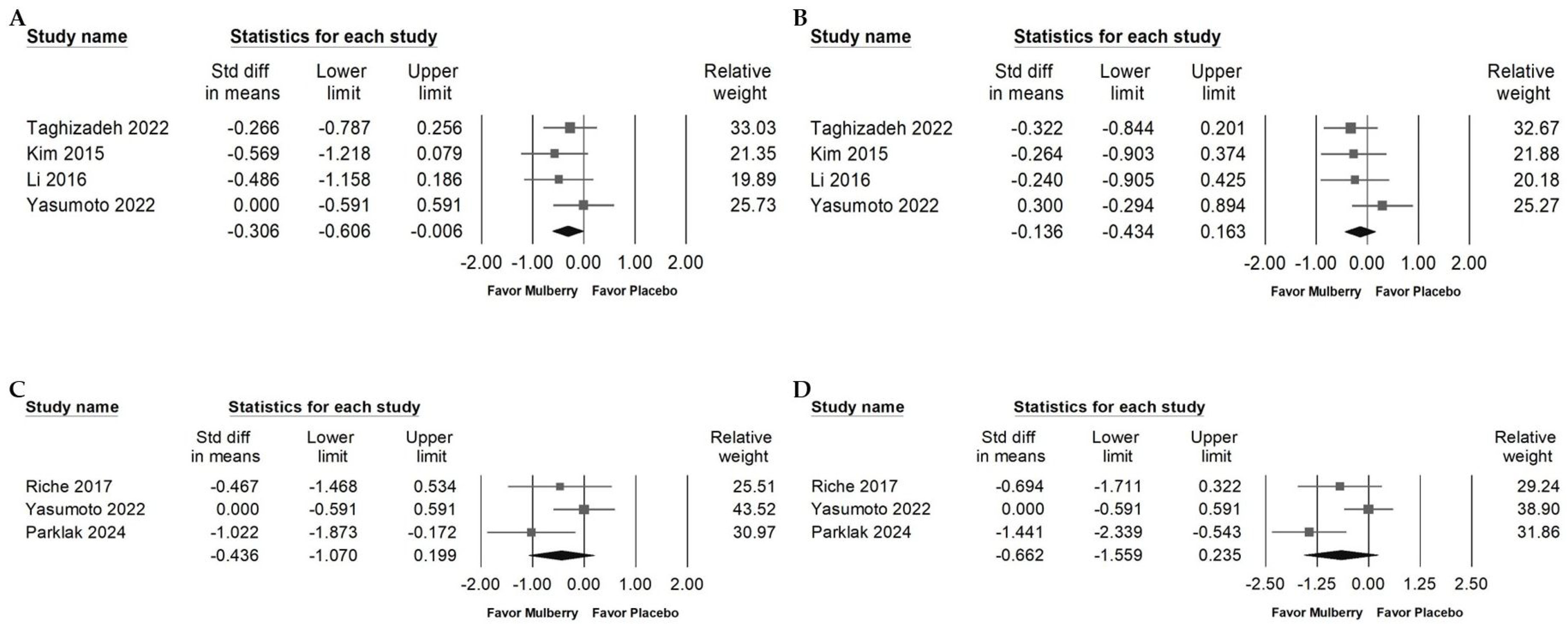
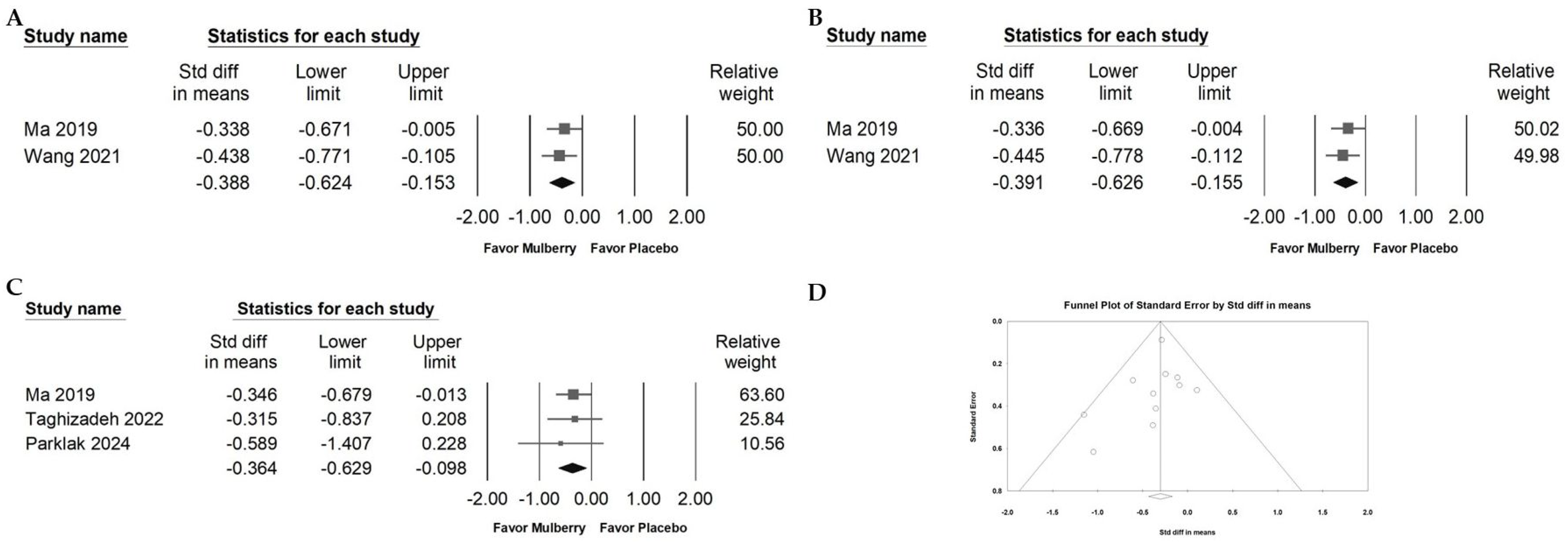
2.3. Impact of Mulberry Extract on Cholesterol
2.4. Influence of Mulberry Extract on TG, LDL, and HDL
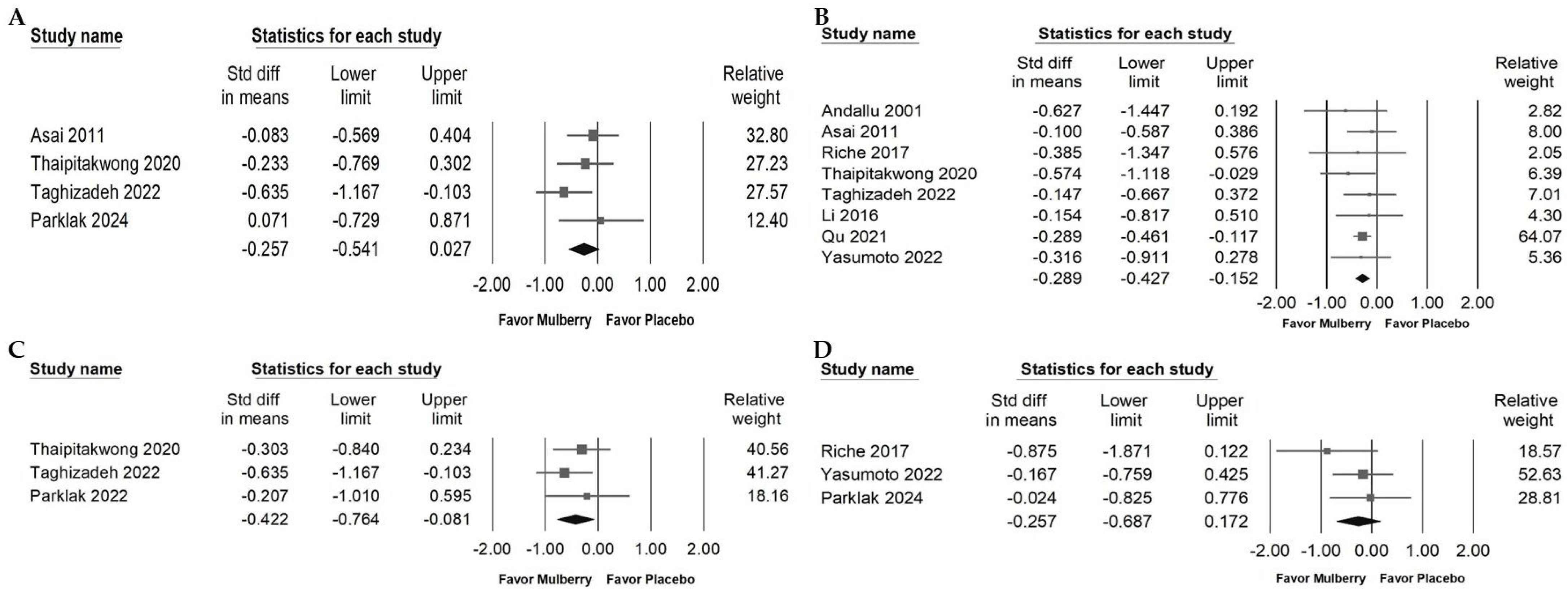
2.5. Impact of Mulberry Extract on Blood Fasting Glucose
2.6. Effects of Mulberry Extract on Serum Insulin, HbA1c, HOMA-IR, and BW
2.7. Impact of Mulberry Extract on Liver Injury Markers and Blood Pressure
2.8. Effect of Mulberry Extract on Inflammatory Cytokines
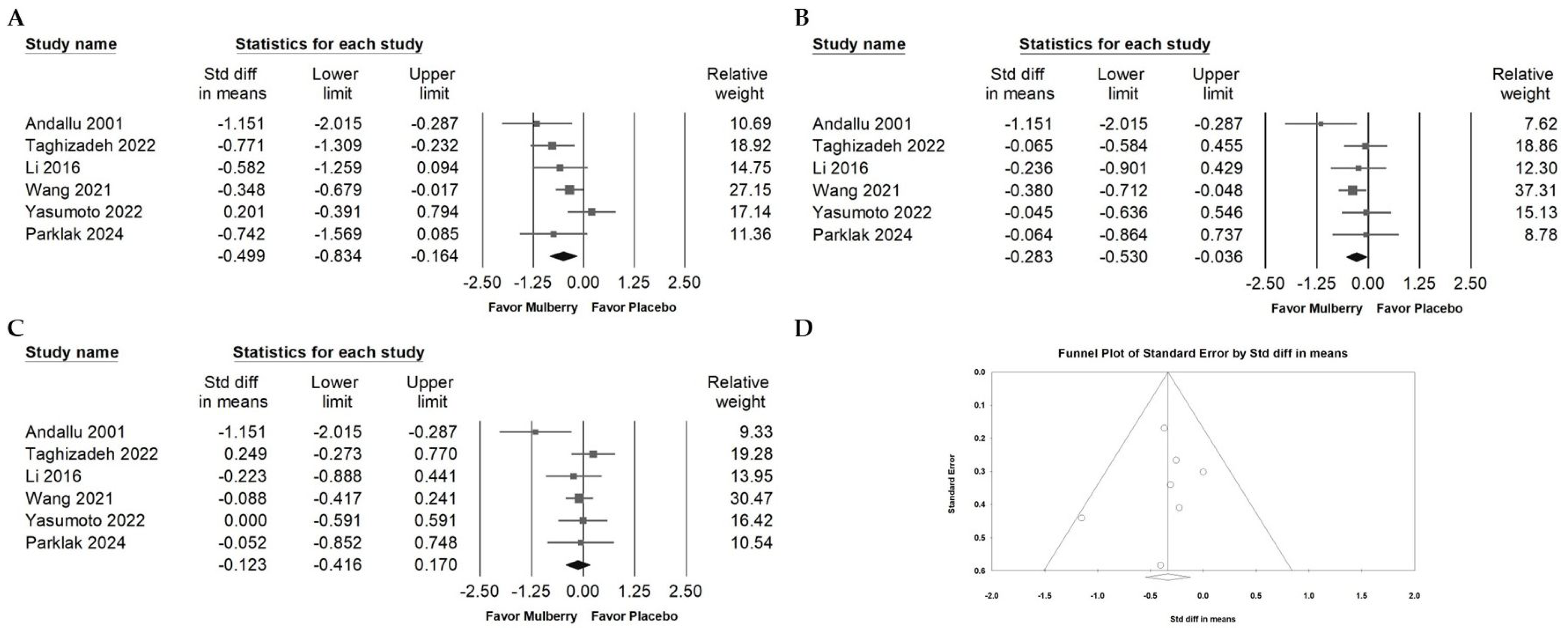
2.9. Publication Bias of Included RCTs Reporting Fasting Blood Glucose Data
2.10. Safety and Tolerability
3. Discussion
4. Materials and Methods
4.1. Data Sources and Selection Criteria
4.2. Selection of Studies
4.3. Data Extraction
4.4. Outcomes
4.5. Assessment of Methodological Quality
4.6. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Atti, A.R.; Valente, S.; Iodice, A.; Caramella, I.; Ferrari, B.; Albert, U.; Mandelli, L.; De Ronchi, D. Metabolic Syndrome, Mild Cognitive Impairment, and Dementia: A Meta-Analysis of Longitudinal Studies. Am. J. Geriatr. Psychiatry 2019, 27, 625–637. [Google Scholar] [CrossRef]
- Alberti, K.G.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.C.; James, W.P.; Loria, C.M.; Smith, S.C., Jr.; et al. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009, 120, 1640–1645. [Google Scholar] [CrossRef]
- Li, J.; Wang, Y.J.; Zhang, M.; Xu, Z.Q.; Gao, C.Y.; Fang, C.Q.; Yan, J.C.; Zhou, H.D.; Chongqing Ageing Study, G. Vascular risk factors promote conversion from mild cognitive impairment to Alzheimer disease. Neurology 2011, 76, 1485–1491. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, J.T.; Thomas, A. Vascular dementia. Lancet 2015, 386, 1698–1706. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.; Ali, M.U.; Kenny, M.; Mayhew, A.; Mokashi, V.; He, H.; Lin, S.; Yavari, E.; Paik, K.; Subramanian, D.; et al. Potentially Modifiable Risk Factors for Dementia and Mild Cognitive Impairment: An Umbrella Review and Meta-Analysis. Dement. Geriatr. Cogn. Disord. 2024, 53, 91–106. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, E.L.; Marcelino, G.; Silva, G.T.; Figueiredo, P.S.; Garcez, W.S.; Corsino, J.; Guimarães, R.D.C.A.; Freitas, K.D.C. Nutraceutical and Medicinal Potential of the Morus Species in Metabolic Dysfunctions. Int. J. Mol. Sci. 2019, 20, 301. [Google Scholar] [CrossRef]
- Chen, C.; Razali, U.H.M.; Saikim, F.H.; Mahyudin, A.; Noor, N.Q.I.M. Morus alba L. Plant: Bioactive Compounds and Potential as a Functional Food Ingredient. Foods 2021, 10, 689. [Google Scholar] [CrossRef]
- Yu, J.S.; Lim, S.H.; Lee, S.R.; Choi, C.I.; Kim, K.H. Antioxidant and Anti-Inflammatory Effects of White Mulberry (Morus alba L.) Fruits on Lipopolysaccharide-Stimulated RAW 264.7 Macrophages. Molecules 2021, 26, 920. [Google Scholar] [CrossRef]
- Andallu, B.; Varadacharyulu, N. Control of hyperglycemia and retardation of cataract by mulberry (Morus indica L.) leaves in streptozotocin diabetic rats. Indian. J. Exp. Biol. 2002, 40, 791–795. [Google Scholar]
- Parklak, W.; Chottidao, M.; Munkong, N.; Komindr, S.; Monkhai, S.; Wanikorn, B.; Makaje, N.; Kulprachakarn, K.; Chuljerm, H.; Somnuk, S. Nutraceutical Properties of Thai Mulberry (Morus alba L.) and Their Effects on Metabolic and Cardiovascular Risk Factors in Individuals with Obesity: A Randomized, Single-Blind Crossover Trial. Nutrients 2024, 16, 4336. [Google Scholar] [CrossRef]
- Thukham-Mee, W.; Wattanathorn, J.; Kirisattayakul, W.; Wannanon, P. Effect of Single Administration of Mulberry Milk on the Cognitive Function of 6-12-Year-Old Children: Results from a Randomized, Placebo-Controlled, Crossover Study. Oxid. Med. Cell Longev. 2020, 2020, 6123759. [Google Scholar] [CrossRef]
- Park, S.; Park, J.S.; Go, H.; Jang, B.H.; Shin, Y.; Ko, S.G. The efficacy and safety study of dietary supplement PURIAM110 on non-insulin taking Korean adults in the stage of pre-diabetes and diabetes mellitus: Protocol for a randomized, double-blind, placebo-controlled, and multicenter trial-pilot study. Trials 2011, 12, 38. [Google Scholar] [CrossRef] [PubMed]
- Kuwahara, M.; Kim, H.K.; Ozaki, M.; Nanba, T.; Chijiki, H.; Fukazawa, M.; Okubo, J.; Mineshita, Y.; Takahashi, M.; Shibata, S. Consumption of Biscuits with a Beverage of Mulberry or Barley Leaves in the Afternoon Prevents Dinner-Induced High, but Not Low, Increases in Blood Glucose among Young Adults. Nutrients 2020, 12, 1580. [Google Scholar] [CrossRef] [PubMed]
- Thukham-Mee, W.; Wattanathorn, J.; Paholpak, P.; Ransikachi, P.; Piyavhatkul, N. The Positive Modulation Effect of a 6-Week Consumption of an Anthocyanin-Rich Mulberry Milk on Working Memory, Cholinergic, and Monoaminergic Functions in Healthy Working-Age Adults. Oxid. Med. Cell Longev. 2021, 2021, 5520059. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Li, Y.; Mu, W.; Li, Z.; Sun, J.; Wang, B.; Zhong, Z.; Luo, X.; Xie, C.; Huang, Y. Mulberry leaf extract reduces the glycemic indexes of four common dietary carbohydrates. Medicine 2018, 97, e11996. [Google Scholar] [CrossRef] [PubMed]
- Demir Doğan, M.; Can, G.; Meral, R. Effectiveness of Black Mulberry Molasses in Prevention of Radiotherapy-Induced Oral Mucositis: A Randomized Controlled Study in Head and Neck Cancer Patients. J. Altern. Complement. Med. 2017, 23, 971–979. [Google Scholar] [CrossRef]
- Kalman, D.S.; Hewlings, S.J. The Effects of Morus alba and Acacia catechu on Quality of Life and Overall Function in Adults with Osteoarthritis of the Knee. J. Nutr. Metab. 2017, 2017, 4893104. [Google Scholar] [CrossRef]
- Costa, J.P.L.; Brito, H.O.; Galvão-Moreira, L.V.; Brito, L.G.O.; Costa-Paiva, L.; Brito, L.M.O. Randomized double-blind placebo-controlled trial of the effect of Morus nigra L. (black mulberry) leaf powder on symptoms and quality of life among climacteric women. Int. J. Gynaecol. Obstet. 2020, 148, 243–252. [Google Scholar] [CrossRef]
- Banu, S.; Jabir, N.R.; Manjunath, N.C.; Khan, M.S.; Ashraf, G.M.; Kamal, M.A.; Tabrez, S. Reduction of post-prandial hyperglycemia by mulberry tea in type-2 diabetes patients. Saudi J. Biol. Sci. 2015, 22, 32–36. [Google Scholar] [CrossRef]
- Derosa, G.; D’Angelo, A.; Maffioli, P. Ilex paraguariensis, white mulberry and chromium picolinate in patients with pre-diabetes. Phytother. Res. 2020, 34, 1377–1384. [Google Scholar] [CrossRef]
- Hu, M.; Zeng, W.; Tomlinson, B. Evaluation of a crataegus-based multiherb formula for dyslipidemia: A randomized, double-blind, placebo-controlled clinical trial. Evid.-Based Complement. Altern. Med. 2014, 2014, 365742. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Yoon, K.H.; Kang, M.J.; Yim, H.W.; Lee, K.S.; Vuksan, V.; Sung, M.K. A six-month supplementation of mulberry, korean red ginseng, and banaba decreases biomarkers of systemic low-grade inflammation in subjects with impaired glucose tolerance and type 2 diabetes. Evid.-Based Complement. Altern. Med. 2012, 2012, 735191. [Google Scholar] [CrossRef] [PubMed]
- Trimarco, V.; Izzo, R.; Stabile, E.; Rozza, F.; Santoro, M.; Manzi, M.V.; Serino, F.; Schiattarella, G.G.; Esposito, G.; Trimarco, B. Effects of a new combination of nutraceuticals with Morus alba on lipid profile, insulin sensitivity and endotelial function in dyslipidemic subjects. A cross-over, randomized, double-blind trial. High Blood Press. Cardiovasc. Prev. 2015, 22, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Aramwit, P.; Supasyndh, O.; Siritienthong, T.; Bang, N. Mulberry leaf reduces oxidation and C-reactive protein level in patients with mild dyslipidemia. BioMed Res. Int. 2013, 2013, 787981. [Google Scholar] [CrossRef]
- Aramwit, P.; Petcharat, K.; Supasyndh, O. Efficacy of mulberry leaf tablets in patients with mild dyslipidemia. Phytother. Res. 2011, 25, 365–369. [Google Scholar] [CrossRef]
- Sirikanchanarod, A.; Bumrungpert, A.; Kaewruang, W.; Senawong, T.; Pavadhgul, P. The effect of mulberry fruits consumption on lipid profiles in hypercholesterolemic subjects: A randomized controlled trial. J. Pharm. Nutr. Sci. 2016, 6, 7–14. [Google Scholar] [CrossRef]
- Meng, Z.; Xu, C.; Liu, H.; Gao, X.; Li, X.; Lin, W.; Ma, X.; Yang, C.; Hao, M.; Zhao, K.; et al. Effects of mulberry twig alkaloids (Sangzhi alkaloids) and metformin on blood glucose fluctuations in combination with premixed insulin-treated patients with type 2 diabetes. Front Endocrinol. 2023, 14, 1272112. [Google Scholar] [CrossRef]
- Andallu, B.; Suryakantham, V.; Lakshmi Srikanthi, B.; Reddy, G.K. Effect of mulberry (Morus indica L.) therapy on plasma and erythrocyte membrane lipids in patients with type 2 diabetes. Clin. Chim. Acta 2001, 314, 47–53. [Google Scholar] [CrossRef]
- Kimura, T.; Nakagawa, K.; Kubota, H.; Kojima, Y.; Goto, Y.; Yamagishi, K.; Oita, S.; Oikawa, S.; Miyazawa, T. Food-grade mulberry powder enriched with 1-deoxynojirimycin suppresses the elevation of postprandial blood glucose in humans. J. Agric. Food Chem. 2007, 55, 5869–5874. [Google Scholar] [CrossRef]
- Asai, A.; Nakagawa, K.; Higuchi, O.; Kimura, T.; Kojima, Y.; Kariya, J.; Miyazawa, T.; Oikawa, S. Effect of mulberry leaf extract with enriched 1-deoxynojirimycin content on postprandial glycemic control in subjects with impaired glucose metabolism. J. Diabetes Investig. 2011, 2, 318–323. [Google Scholar] [CrossRef]
- Li, M.; Huang, X.; Ye, H.; Chen, Y.; Yu, J.; Yang, J.; Zhang, X. Randomized, double-blinded, double-dummy, active-controlled, and multiple-dose clinical study comparing the efficacy and safety of mulberry twig (Ramulus Mori, Sangzhi) alkaloid tablet and acarbose in individuals with type 2 diabetes mellitus. Evid.-Based Complement. Altern. Med. 2016, 2016, 7121356. [Google Scholar] [CrossRef]
- Ma, Y.; Lv, W.; Gu, Y.; Yu, S. 1-deoxynojirimycin in mulberry (Morus indica L.) leaves ameliorates stable angina pectoris in patients with coronary heart disease by improving antioxidant and anti-inflammatory capacities. Front. Pharmacol. 2019, 10, 569. [Google Scholar] [CrossRef]
- Qu, L.; Liang, X.; Tian, G.; Zhang, G.; Wu, Q.; Huang, X.; Cui, Y.; Liu, Y.; Shen, Z.; Xiao, C.; et al. Efficacy and Safety of Mulberry Twig Alkaloids Tablet for the Treatment of Type 2 Diabetes: A Multicenter, Randomized, Double-Blind, Double-Dummy, and Parallel Controlled Clinical Trial. Diabetes Care 2021, 44, 1324–1333. [Google Scholar] [CrossRef]
- Riche, D.M.; Riche, K.D.; East, H.E.; Barrett, E.K.; May, W.L. Impact of mulberry leaf extract on type 2 diabetes (Mul-DM): A randomized, placebo-controlled pilot study. Complement. Ther. Med. 2017, 32, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Thaipitakwong, T.; Supasyndh, O.; Rasmi, Y.; Aramwit, P. A randomized controlled study of dose-finding, efficacy, and safety of mulberry leaves on glycemic profiles in obese persons with borderline diabetes. Complement. Ther. Med. 2020, 49, 102292. [Google Scholar] [CrossRef] [PubMed]
- Taghizadeh, M.; Mohammad Zadeh, A.; Asemi, Z.; Farrokhnezhad, A.H.; Memarzadeh, M.R.; Banikazemi, Z.; Shariat, M.; Shafabakhsh, R. Morus alba leaf extract affects metabolic profiles, biomarkers inflammation and oxidative stress in patients with type 2 diabetes mellitus: A double-blind clinical trial. Clin. Nutr. ESPEN 2022, 49, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yu, Z.; Jiang, J.; Li, Y.; Yu, S. Mulberry leaf attenuates atherosclerotic lesions in patients with coronary heart disease possibly via 1-Deoxynojirimycin: A placebo-controlled, double-blind clinical trial. J. Food Biochem. 2021, 45, e13573. [Google Scholar] [CrossRef]
- Yasumoto, K.; Hoshiko, H.; Sekiguchi, N.; Obata, H.; Takara, T. Safety evaluation of a beverage containing mulberry leaf extract. Biosci. Biotechnol. Biochem. 2022, 86, 519–527. [Google Scholar] [CrossRef]
- Kim, J.Y.; Ok, H.M.; Kim, J.; Park, S.W.; Kwon, S.W.; Kwon, O. Mulberry leaf extract improves postprandial glucose response in prediabetic subjects: A randomized, double-blind placebo-controlled trial. J. Med. Food 2015, 18, 306–313. [Google Scholar] [CrossRef]
- Chen, X.; Sohouli, M.H.; Nateghi, M.; Melekoglu, E.; Fatahi, S. Impact of mulberry consumption on cardiometabolic risk factors: A systematic review and meta-analysis of randomized-controlled trials. J. Clin. Pharm. Ther. 2022, 47, 1982–1993. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, H.; Tang, G.; Wang, L.; Tian, X.; Li, R. Risk factors for mild cognitive impairment in type 2 diabetes: A systematic review and meta-analysis. Front. Endocrinol. 2025, 16, 1617248. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.; Xu, W.; Ou, Y.N.; Cao, X.P.; Tan, M.S.; Tan, L.; Yu, J.T. Diabetes mellitus and risks of cognitive impairment and dementia: A systematic review and meta-analysis of 144 prospective studies. Ageing Res. Rev. 2019, 55, 100944. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; Yuan, Y.; Huang, R.; Tian, S.; Wang, J.; Lin, H.; An, K.; Han, J.; Wang, S. Association between plasma adipsin level and mild cognitive impairment in Chinese patients with type 2 diabetes: A cross-sectional study. BMC Endocr. Disord. 2019, 19, 108. [Google Scholar] [CrossRef] [PubMed]
- Chew, S.T.H.; Tan, N.C.; Cheong, M.; Oliver, J.; Baggs, G.; Choe, Y.; How, C.H.; Chow, W.L.; Tan, C.Y.L.; Kwan, S.C.; et al. Impact of specialized oral nutritional supplement on clinical, nutritional, and functional outcomes: A randomized, placebo-controlled trial in community-dwelling older adults at risk of malnutrition. Clin. Nutr. 2021, 40, 1879–1892. [Google Scholar] [CrossRef]
- Li, C.P.; Huang, S.C.; Hsiao, Y.; Tsai, R.Y. Evaluating the Role of Vitamin D in Alleviating Chronic Pruritus: A Meta-Analysis. Int. J. Mol. Sci. 2024, 25, 9983. [Google Scholar] [CrossRef]
- Blumberg, J.B.; Frei, B.B.; Fulgoni, V.L.; Weaver, C.M.; Zeisel, S.H. Impact of Frequency of Multi-Vitamin/Multi-Mineral Supplement Intake on Nutritional Adequacy and Nutrient Deficiencies in U.S. Adults. Nutrients 2017, 9, 849. [Google Scholar] [CrossRef]
- Batista-Jorge, G.C.; Barcala-Jorge, A.S.; Silveira, M.F.; Lelis, D.F.; Andrade, J.M.O.; de Paula, A.M.B.; Guimaraes, A.L.S.; Santos, S.H.S. Oral resveratrol supplementation improves Metabolic Syndrome features in obese patients submitted to a lifestyle-changing program. Life Sci. 2020, 256, 117962. [Google Scholar] [CrossRef]
- Farzin, L.; Asghari, S.; Rafraf, M.; Asghari-Jafarabadi, M.; Shirmohammadi, M. No beneficial effects of resveratrol supplementation on atherogenic risk factors in patients with nonalcoholic fatty liver disease. Int. J. Vitam. Nutr. Res. 2020, 90, 279–289. [Google Scholar] [CrossRef]
- Shao, Y.; Shi, J. Anti-inflammatory mechanism of resveratrol’s Triglyceride-lowering effect in hyperlipidemia: A meta-analysis integrated network pharmacology and molecular dynamics simulation. Clin. Exp. Med. 2025, 25, 262. [Google Scholar] [CrossRef]
- Sherratt, S.C.R.; Libby, P.; Budoff, M.J.; Bhatt, D.L.; Mason, R.P. Role of Omega-3 Fatty Acids in Cardiovascular Disease: The Debate Continues. Curr. Atheroscler. Rep. 2023, 25, 1–17. [Google Scholar] [CrossRef]
- Chen, H.; He, X.; Fan, J.; Mi, Y.; Li, F. The relationship between dietary niacin intake and the incidence of all-cause and cardiovascular mortality among chronic kidney disease patients. Am. J. Med. Sci. 2025, 369, 460–466. [Google Scholar] [CrossRef]
- Ndrepepa, G. Aspartate aminotransferase and cardiovascular disease—A narrative review. J. Lab. Precis. Med. 2021, 6, 6. [Google Scholar] [CrossRef]
- Dufour, D.R.; Lott, J.A.; Nolte, F.S.; Gretch, D.R.; Koff, R.S.; Seeff, L.B. Diagnosis and monitoring of hepatic injury. II. Recommendations for use of laboratory tests in screening, diagnosis, and monitoring. Clin. Chem. 2000, 46, 2050–2068. [Google Scholar] [CrossRef] [PubMed]
- Bahreini Boroujeni, L.Z.; Haghighat_Lari, M.M.; Ghandi, A.; Hashemian, S.M.; Shafabakhsh, R.; Banikazemi, Z.; Taghizadeh, M. Effects of black mulberry leaf extract on liver health and metabolic parameters in rats with high-fat diet-induced liver changes. Obes. Med. 2024, 50, 100554. [Google Scholar] [CrossRef]
- Cui, W.; Luo, K.; Xiao, Q.; Sun, Z.; Wang, Y.; Cui, C.; Chen, F.; Xu, B.; Shen, W.; Wan, F.; et al. Effect of mulberry leaf or mulberry leaf extract on glycemic traits: A systematic review and meta-analysis. Food Funct. 2023, 14, 1277–1289. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Zhang, Q.; Zhu, S.; Liu, B.; Liu, F.; Xu, Y. Mulberry leaf (Morus alba L.): A review of its potential influences in mechanisms of action on metabolic diseases. Pharmacol. Res. 2022, 175, 106029. [Google Scholar] [CrossRef]
- Han, T.S.; Lean, M.E.J. Metabolic syndrome. Medicine 2015, 43, 80–87. [Google Scholar] [CrossRef]
- Qi, F.X.; Hu, Y.; Li, Y.W.; Gao, J. Levels of anti-oxidative molecules and inflammatory factors in patients with vascular dementia and their clinical significance. Pak. J. Med. Sci. 2021, 37, 1509–1513. [Google Scholar] [CrossRef]
- Wang, F.; Zou, Z.; Gong, Y.; Yuan, D.; Chen, X.; Sun, T. Regulation of Human Brain Microvascular Endothelial Cell Adhesion and Barrier Functions by Memantine. J. Mol. Neurosci. 2017, 62, 123–129. [Google Scholar] [CrossRef]
- Zhang, H.; Ma, Z.F.; Luo, X.; Li, X. Effects of Mulberry Fruit (Morus alba L.) Consumption on Health Outcomes: A Mini-Review. Antioxidants 2018, 7, 69. [Google Scholar] [CrossRef]
- Borda, M.G.; Ramírez-Vélez, R.; Botero-Rodriguez, F.; Patricio-Baldera, J.; de Lucia, C.; Pola, I.; Barreto, G.E.; Khalifa, K.; Bergland, A.K.; Kivipelto, M.; et al. Anthocyanin supplementation in adults at risk for dementia: A randomized controlled trial on its cardiometabolic and anti-inflammatory biomarker effects. GeroScience 2025, 1–14. [Google Scholar] [CrossRef]
- Fang, Y.; Peck, M.R.; Quinn, K.; Chapman, J.E.; Medina, D.; McFadden, S.A.; Bartke, A.; Hascup, E.R.; Hascup, K.N. Senolytic intervention improves cognition, metabolism, and adiposity in female APP(NL)(-F/NL-F) mice. Geroscience 2025, 47, 1123–1138. [Google Scholar] [CrossRef]
- Ruggiero, A.D.; Vemuri, R.; Blawas, M.; Long, M.; DeStephanis, D.; Williams, A.G.; Chen, H.; Justice, J.N.; Macauley, S.L.; Day, S.M.; et al. Long-term dasatinib plus quercetin effects on aging outcomes and inflammation in nonhuman primates: Implications for senolytic clinical trial design. Geroscience 2023, 45, 2785–2803. [Google Scholar] [CrossRef]
- Tang, Q.; Xing, X.; Huang, H.; Yang, J.; Li, M.; Xu, X.; Gao, X.; Liang, C.; Tian, W.; Liao, L. Eliminating senescent cells by white adipose tissue-targeted senotherapy alleviates age-related hepatic steatosis through decreasing lipolysis. Geroscience 2024, 46, 3149–3167. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, M.; Katsube, T.; Koyama, A.; Itamura, H. Varietal differences in the flavonol content of mulberry (Morus spp.) leaves and genetic analysis of quercetin 3-(6-malonylglucoside) for component breeding. J. Agric. Food Chem. 2013, 61, 9140–9147. [Google Scholar] [CrossRef]
- van Dinther, M.; Voorter, P.H.M.; Zhang, E.; van Kuijk, S.M.J.; Jansen, J.F.A.; van Oostenbrugge, R.J.; Backes, W.H.; Staals, J. The neurovascular unit and its correlation with cognitive performance in patients with cerebral small vessel disease: A canonical correlation analysis approach. Geroscience 2024, 46, 5061–5073. [Google Scholar] [CrossRef]
- Csiszar, A.; Ungvari, A.; Patai, R.; Gulej, R.; Yabluchanskiy, A.; Benyo, Z.; Kovacs, I.; Sotonyi, P.; Kirkpartrick, A.C.; Prodan, C.I.; et al. Atherosclerotic burden and cerebral small vessel disease: Exploring the link through microvascular aging and cerebral microhemorrhages. Geroscience 2024, 46, 5103–5132. [Google Scholar] [CrossRef] [PubMed]
- Ting, K.K.; Coleman, P.; Kim, H.J.; Zhao, Y.; Mulangala, J.; Cheng, N.C.; Li, W.; Gunatilake, D.; Johnstone, D.M.; Loo, L.; et al. Vascular senescence and leak are features of the early breakdown of the blood-brain barrier in Alzheimer’s disease models. Geroscience 2023, 45, 3307–3331. [Google Scholar] [CrossRef]
- Xu, P.; Chen, Q.; Chen, X.; Qi, H.; Yang, Y.; Li, W.; Yang, X.; Gunawan, A.; Chen, S.; Zhang, H.; et al. Morusin and mulberrin extend the lifespans of yeast and C. elegans via suppressing nutrient-sensing pathways. Geroscience 2023, 45, 949–964. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Cochrane Collaboration. Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2019; p. xxviii. 694p. [Google Scholar]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]


| Author (Year)/Country | Diagnosis | Inclusion Criteria | Exclusion Criteria | Sample Size (% of Male)/Age | Study Design | Placebo Using | Intervention/Duration | Main Results | Secondary Results |
|---|---|---|---|---|---|---|---|---|---|
| ~8 weeks | |||||||||
| Andallu (2001)/India [28] | Male type 2 diabetes patients | 1. Age group: 40–60 years. 2. Fasting blood glucose > 150 mg/dL. 3. HbA1c > 12%. | Patients with severe complications or comorbidities. | P: 12 (100) I: 12 (100)/40 to 60 years. | RCT/not mentioned/placebo | Glibenclamide | Single Morus indica L. leaves/shade-dried, powdered, and provided in capsule form (3 g/day)/30 days | 1. 27% reduction in fasting blood glucose (p < 0.01). 2. Reduced total cholesterol (12%), LDL (23%), triglycerides (16%), and VLDL (17%) significantly (p < 0.01). | 1. Increased HDL by 18% (p < 0.01). 2. Significant reduction in plasma and erythrocyte membrane lipid peroxides. |
| Kimura (2007)/Japan [29] | Healthy volunteers | Aged around 25 years, with a BMI within the normal range. | Subjects with any underlying health conditions that could affect glucose metabolism or were currently taking any medication. | P: 6 I (DNJ 0.4 g): 6 I (DNJ 0.8 g): 6 I (DNJ 1.2 g): 6/average age of 25.3 years | RCT/not mentioned/placebo | 0 g of DNJ | DNJ-enriched Morus indica L. leaf powder/38 days | At doses of 0.8 g and 1.2 g, mulberry significantly reduced postprandial blood glucose, and DNJ intake suppressed plasma insulin secretion. Lipid profiles remained unchanged. | No adverse reactions in either group. |
| Kim (2015)/Korea [39] | Prediabetic subjects | Fasting blood glucose levels between 100 and 125 mg/dL and normal HbA1c levels (<6.5%). | Severe heart, liver, or kidney conditions, pregnant or breastfeeding women, and those taking medications or supplements that may influence glucose or lipid metabolism. | P: 19 (47.3) I: 19 (31.5)/P: 50.16 ± 7.83 I: 53.00 ± 7.20 | RCT/double-blind/placebo | Color-matched placebo (lactose tablets) | Single Morus indica L. leaf aqueous extract standardized to 3.6 mg/g of DNJ/4 weeks | Postprandial blood glucose significantly decreased at 30 and 60 min (p < 0.05), and insulin AUC was notably reduced after 4 weeks (p = 0.0207). | No significant changes in fasting blood glucose or HbA1c levels in either group. |
| Ma (2019)/China [32] | Stable angina pectoris in patients diagnosed with coronary heart disease and blood stasis syndrome | Aged 35–80 years with symptoms included chest pain, tightness, and shortness of breath. | Patients with stable angina pectoris caused by other heart diseases, a history of trauma or fever, severe heart failure (ejection fraction < 35%), malignant tumors, or other serious conditions were excluded. Pregnant or breastfeeding women were also not included. | P: 78 (53.8) I: 64 (68.7)/P: 68.16 ± 7.36 I: 65.42 ± 8.48 | RCT/double-blind/placebo | Conventional treatment | Daily oral administration of DNJ (10 mg) extracted from Morus indica L. leaves/4 weeks | Reduced inflammatory markers such as hs-CRP, IL-6, TNF-α, and malondialdehyde (MDA), and increased antioxidant SOD levels (p < 0.05). | 1. Increased left ventricular ejection fraction and reduced left ventricular mass index (p < 0.05). 2. Improved aortic elasticity and reduced atherosclerosis index (p < 0.05). |
| Yasumoto (2022)/Japan [38] | Healthy adults | Fasting glucose and 2 h post-OGTT in the normal or borderline range; able to attend visits; provided written informed consent. | On any medical treatment during the trial; systolic blood pressure < 90 mmHg; pregnancy or breastfeeding; recent blood donation beyond protocol limits; participation in other studies; cardiac, hepatic, or renal disorders, history of cardiac disease, diabetes mellitus, drug or food allergy, glaucoma, and hyponatremia. | P: 24 (50) I: 23 (52.2)/P: 46.4 ± 10.2 I: 43.3 ± 13.7 | RCT/double-blind/placebo | Matching 500 mL green tea beverage without mulberry leaf extract, identical in appearance and flavor | Green tea beverage containing mulberry leaf extract 550 mg per 500 mL, one bottle with each meal, three times daily, for 4 weeks | Intervention group decreases in ALP and urea nitrogen and an increase in uric acid and a decrease in LDL cholesterol; at 4 weeks, higher uric acid and HDL cholesterol and lower LDL cholesterol, non-HDL cholesterol, and HbA1c. | No adverse reactions in either group. |
| Parklak (2024)/Thailand [10] | Adults with obesity and central obesity plus elevated blood pressure | BMI > 25 kg/m2; central obesity (waist > 90 cm men, >80 cm women); SBP > 130 mmHg and/or DBP > 85 mmHg; able to complete study procedures; provided consent. | Liver, kidney, heart, thyroid, or adrenal disease; cancer; antibiotics within 3 months; alcohol 2–3 times per week; established CVD, diabetes, or NAFLD; use of polyphenol-containing beverages or supplements. | n= 12 (58.3)/46.57 ± 8.49 | RCT/single-blind/crossover study | Placebo beverage of identical volume and calories, without mulberry extract | Concentrated mulberry drink (CMD); total 100 g/day; duration 6 weeks | In this crossover trial, CMD intake lowered systolic and diastolic blood pressure (p < 0.05), reduced triglycerides compared with placebo (p < 0.05), and kept fasting plasma glucose stable while it rose during placebo (p < 0.05). CRP protein was also lower with CMD than with placebo (p < 0.05), whereas LDL and HDL did not change significantly. | Body composition (weight, BMI, fat mass, WC/HC) and heart rate showed no meaningful changes across phases. |
| 12 weeks | |||||||||
| Asai (2011)/Japan [30] | Impaired glucose metabolism | Fasting plasma glucose between 100 and 140 mg/dL, indicating impaired glucose metabolism. | Severe medical illness, pregnancy, lactation, or those using any agent for blood glucose control were excluded. | P: 32 (68) I: 33 (63.6)/average age 53.5 ± 7.5 years | Crossover RCT/double-blind/placebo | Not mentioned | Study 1: single dose (DNJ); study 2: 12-week supplementation, 6 mg DNJ, three times daily | Significant improved postprandial glycemic control (p < 0.001). | No significant differences in fasting plasma glucose, HbA1c, or glycated albumin concentrations between groups. |
| Riche (2017)/United States [34] | Type 2 diabetes mellitus | Patients on monotherapy or oral combination therapy, with a stable hemoglobin A1C (7.0–8.0%) for at least 2 months. | Using insulin, alpha-glucosidase inhibitors, those with cardiovascular disease, hepatic or renal insufficiency, pregnant women, or those with non-compliance history. | P: 12 (42) I: 12 (42)/P: 56 ± 7.0 I: 57 ± 5.5 | RCT/double-blind/placebo | Matching placebo capsules | Single Morus indica L. leaf extract, 1000 mg standardized, taken three times daily with meals/3 months | Significant reduction in postprandial blood glucose (p < 0.05). | No significant changes in body weight, blood pressure, or fasting glucose were observed between groups. |
| Thaipitakwong (2020)/Thailand [35] | Borderline diabetes in obese individuals | Obese individuals aged 20–65 years with fasting plasma glucose of 100–140 mg/dL or 2 h postprandial plasma glucose of 140–199 mg/dL. | Taking antihyperglycemic agents, severe complications such as renal or hepatic impairments, or pregnant and lactating women were excluded. | P: 26 (23) I: 28 (32.1)/P: 52.0 ± 8.22 I: 53.14 ± 5.48 | RCT/controlled clinical trial | Maintained nutritional control only | Mulberry leaf powder containing 12 mg DNJ per dose, taken three times daily/12 weeks | 1. Significant reduction in fasting plasma glucose (p = 0.002). 2. Significant reduction in HbA1c levels (p = 0.011). | 1. Mild improvements in insulin resistance (HOMA-IR) with borderline significance (p = 0.057). 2. No changes in postprandial glucose or insulin levels. |
| Taghizadeh (2022)/Iran [36] | Type 2 diabetes mellitus | Aged 35–70 years with T2DM, diagnosed based on the American Diabetes Association criteria. | Taken mulberry extract within the last three months, those with changes in glucose-lowering medications, those using anticoagulants, pregnant or lactating women, or patients with malignancies and chronic liver diseases were excluded. | P: 28 (32.1) I: 29 (31.0)/P: 52.6 ± 6.95 I: 46.2 ± 20.1 | RCT/double-blind/placebo | Matching placebo twice daily | Morus alba extract (300 mg) taken twice daily/12 weeks | 1. Significant reduction in insulin levels (p = 0.026) and HOMA-IR (p = 0.02). 2. Significant increase in HDL (p = 0.001) and a reduction in malondialdehyde (MDA) levels (p < 0.0001). 3. No significant changes in fasting plasma glucose, triglycerides, or other lipid profiles between the groups. | No serious adverse effects were reported. |
| 24 weeks | |||||||||
| Li (2016)/China [31] | Type 2 diabetes mellitus | Aged 18–70 years with T2DM (HbA1c between 7.0% and 10.0%) who were not using antidiabetic medications for at least 3 months before screening or had used antidiabetic medication for no more than 3 months in total. | Severe diabetes complications, gastrointestinal conditions, poor blood pressure control, and those with liver or kidney disease were excluded. Pregnant and lactating women were also excluded. | P: 15 (33.33) I: 23 (34.78)/P: 57 ± 6.70 I: 56 ± 9.71 | RCT/double-blind/double-dummy/active-controlled, and multiple-dose clinical trial | Matching mulberry twig alkaloid and acarbose tablets | Mulberry twig alkaloid tablet, 50 mg three times daily, increased to 100 mg three times daily after 4 weeks/24 weeks | 1. Significant reduction in HbA1c (p < 0.001). 2. 1 h and 2 h postprandial plasma glucose levels significantly decreased in both groups (p < 0.05). | No significant changes in fasting plasma glucose or lipid profiles in either group. |
| Qu (2021)/China [33] | Type 2 diabetes mellitus | Aged 18–70 years with T2DM (HbA1c between 7.0% and 10.0%) and fasting blood glucose less than 13 mmol/L. The patients had not received any antidiabetic therapy or had used it for less than 3 months. | Severe diabetic complications, liver or kidney dysfunction, cardiovascular diseases, gastrointestinal dysfunction, or those taking any medication affecting glucose metabolism were excluded. Pregnant women were also excluded. | P: 222 (53.2) I: 321 (48.6)/P: 54.2 ± 9.01 I: 54.9 ± 9.41 | RCT/double-blind/double-dummy/active-controlled, and multiple-dose clinical trial | Matching mulberry twig alkaloid and acarbose tablets | Sangzhi alkaloids from mulberry twigs, 50 mg three times daily for the first 4 weeks, then 100 mg three times daily for 20 more weeks/24 weeks | HbA1c significantly reduced. | No significant differences in fasting blood glucose, 1 h or 2 h postprandial glucose levels between the two groups. |
| 12 months | |||||||||
| Wang (2021)/China [37] | Coronary heart disease with atherosclerosis | Aged 40–80 years with coronary heart disease confirmed by coronary angiography, LDL cholesterol levels > 140 mg/dL, and a history of myocardial infarction or coronary artery interventions. | Severe hepatic or renal dysfunction, ongoing treatment with DNJ for more than one month, or allergic reactions to DNJ or the drugs used in the study. Pregnant or lactating women were also excluded. | P: 70 (20) I: 72 (27.7)/P: 57.45 ± 11.28 I: 58.93 ± 10.76 | RCT/double-blind/placebo | Placebo made of starch was administered | Mulberry leaf extract (DNJ 150 mg/day in three doses)/1 year | 1. Decreased inflammatory markers (TNF-α, IL-1β, IL-6) and increased IL-10 in the treatment group (p < 0.05). 2. Significant reductions in LDL-C and total cholesterol. | Significant reduction in carotid intima media thickness in the treatment group compared to the control group (p < 0.05). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, J.-T.; Li, C.-P.; Hsiao, Y.; Cheng, K.-P.; Tsai, R.-Y. Integrative Evidence on Mulberry Extract for Modulating Metabolic Risk Factors Associated with Vascular Dementia. Int. J. Mol. Sci. 2025, 26, 8380. https://doi.org/10.3390/ijms26178380
Yu J-T, Li C-P, Hsiao Y, Cheng K-P, Tsai R-Y. Integrative Evidence on Mulberry Extract for Modulating Metabolic Risk Factors Associated with Vascular Dementia. International Journal of Molecular Sciences. 2025; 26(17):8380. https://doi.org/10.3390/ijms26178380
Chicago/Turabian StyleYu, Jui-Ting, Chen-Pi Li, Yao Hsiao, Kuan-Po Cheng, and Ru-Yin Tsai. 2025. "Integrative Evidence on Mulberry Extract for Modulating Metabolic Risk Factors Associated with Vascular Dementia" International Journal of Molecular Sciences 26, no. 17: 8380. https://doi.org/10.3390/ijms26178380
APA StyleYu, J.-T., Li, C.-P., Hsiao, Y., Cheng, K.-P., & Tsai, R.-Y. (2025). Integrative Evidence on Mulberry Extract for Modulating Metabolic Risk Factors Associated with Vascular Dementia. International Journal of Molecular Sciences, 26(17), 8380. https://doi.org/10.3390/ijms26178380







