Evaluation of the In Vitro Blood–Brain Barrier Transport of Ferula persica L. Bioactive Compounds
Abstract
1. Introduction
2. Results and Discussion
2.1. Evaluation of the Blood–Brain Barrier Permeability of F. persica Compounds Using the PAMPA-BBB Model
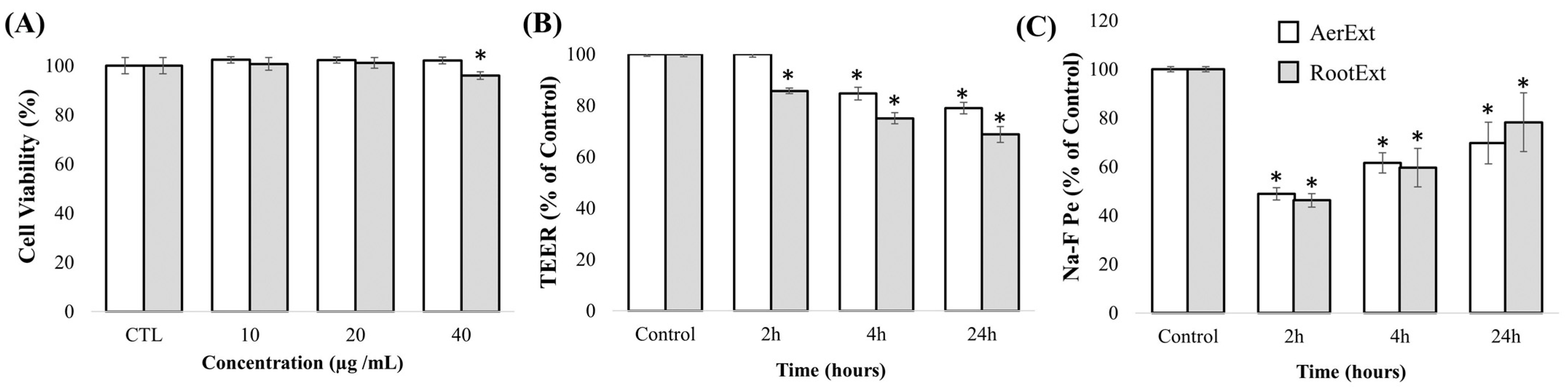
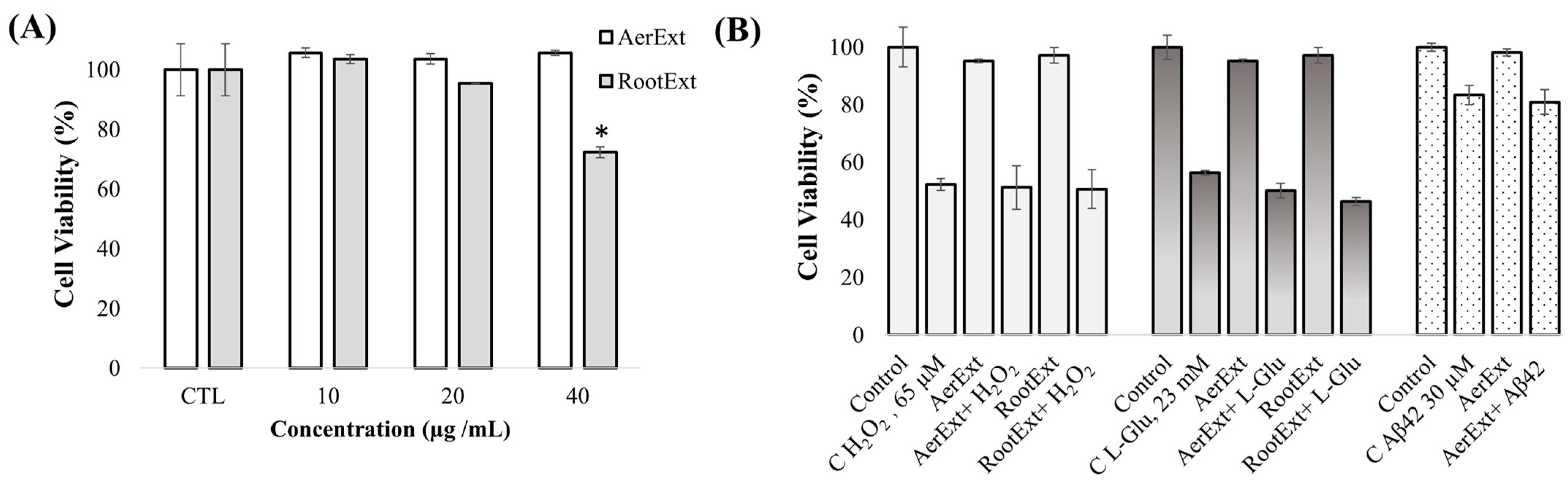
2.2. In Vitro Toxicity and Cell Barrier Integrity Assay of F. persica Extracts in HBMEC Cells
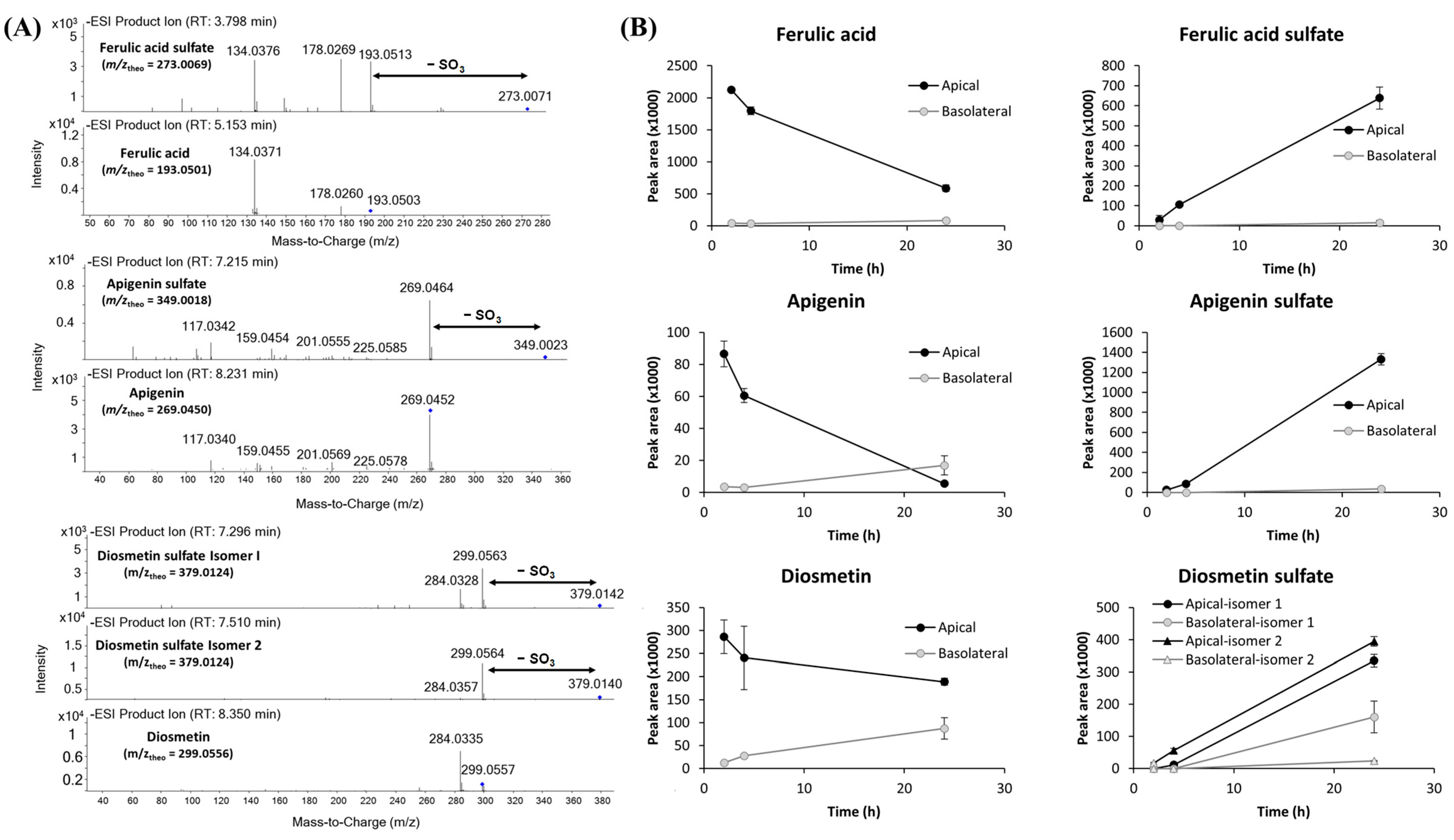
2.3. Evaluation of F. persica Compound Transport Across the BBB Endothelium
| Analytical Platform | Compound | RT (min) | Molecular Formula | Monoisotopic Mass | Subclass | MW | log p | TPSA | AerExt | RootExt | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Te (%) ± SD | Te (%) ± SD | |||||||||||||
| 2 h | 4 h | 24 h | 2 h | 4 h | 24 h | |||||||||
| LC-MS(+) | β-asarone | 9.032 | C12H16O3 | 208.10994 | Anisoles | 208.25 | 3.0 | 27.7 | 7.5 ± 2.0 | 15.0 ± 7.3 | 15.5 ± 1.0 | – | – | – |
| LC-MS(−) | 1,2,4-benzenetriol | 1.464 | C6H6O3 | 126.03169 | Benzenetriols and derivatives | 126.11 | 1.5 | 60.7 | – | – | – | n.q. | n.q. | n.q. |
| LC-MS(+) | Loliolide | 5.481 | C11H16O3 | 196.10994 | Benzofurans | 196.24 | 1.0 | 46.5 | 6.2 ± 0.7 | 12.1 ± 0.8 | 12.4 ± 0.8 | – | – | – |
| LC-MS(−) * | 2,3-dihydroxybenzoic acid | 3.572 | C7H6O4 | 154.02661 | BAs and derivatives | 154.12 | 1.5 | 57.5 | 17.5 ± 3.5 | 9.6 ± 0.6 | 14.0 ± 3.9 | 21.4 ± 5.3 | 23.9 ± 4.4 | 214.1 ± 45.4 |
| LC-MS(−) * | 4-hydroxybenzaldehyde | 3.771 | C7H6O2 | 122.03677 | Carbonyl compounds | 122.12 | 1.4 | 37.3 | 23.3 ± 3.1 | 18.5 ± 0.3 | 49.4 ± 5.9 | 62.1 ± 21.9 | 57.5 ± 1.7 | 184.6 ± 23.0 |
| LC-MS(−) | Protocatechuic aldehyde | 3.013 | C7H6O3 | 138.03169 | Carbonyl compounds | 138.12 | 1.3 | 57.5 | n.q. | n.q. | n.q. | n.q. | n.q. | – |
| LC-MS(−) * | Apigenin | 8.215 | C15H10O5 | 270.05282 | Flavones | 270.05 | 1.7 | 90.9 | 4.2 ± 0.9 | 5.1 ± 0.3 | 309.7 ± 93.0 | – | – | – |
| LC-MS(−) * | Luteolin | 7.553 | C15H10O6 | 286.04774 | Flavones | 286.24 | 1.4 | 111.1 | n.q. | n.q. | n.q. | – | – | – |
| LC-MS(−) * | Hesperidin | 6.254 | C28H34O15 | 610.18977 | Flavonoid glycosides | 610.6 | −1.1 | 234.3 | n.q. | n.q. | n.q. | n.q. | n.q. | n.q. |
| LC-MS(−) * | Apigenin 7-glucoside | 6.281 | C21H20O10 | 432.10565 | Flavonoid glycosides | 432.38 | −0.1 | 170.1 | n.q. | n.q. | – | – | – | – |
| LC-MS(−) * | Ferulic acid | 5.167 | C10H10O4 | 194.05791 | HCAs and derivatives | 194.18 | 1.5 | 66.8 | 1.9 ± 0.2 | 1.9 ± 0.2 | 19.8 ± 10.3 | n.q. | n.q. | n.q. |
| LC-MS(−) | 2,5-dihydroxycinnamic acid | 4.068 | C9H8O4 | 180.04226 | HCAs and derivatives | 180.15 | 1.2 | 77.8 | n.d. | n.d. | n.d. | n.q. | n.q. | n.q. |
| LC-MS(−) * | 4-coumaric acid | 4.671 | C9H8O3 | 164.04734 | HCAs and derivatives | 164.04 | 1.5 | 57.5 | n.q. | n.q. | n.q. | n.q. | n.q. | n.q. |
| LC-MS(+) | 7-hydroxy-6-methoxycoumarin | 5.114 | C10H8O4 | 192.04226 | Hydroxycoumarins | 192.16 | 1.5 | 59.7 | 0.6 ± 0.2 | 1.9 ± 0.1 | 6.1 ± 1.5 | n.q. | n.q. | n.q. |
| LC-MS(+) | Isofraxidin | 5.410 | C11H10O5 | 222.05282 | Hydroxycoumarins | 222.19 | 1.5 | 68.9 | n.q. | n.q. | n.q. | n.q. | n.q. | 30.5 ± 6.6 |
| LC-MS(+) | Olivil glucoside isomer 1 | 4.298 | C26H34O12 | 538.20503 | Lignan glycosides | 538.50 | 0.1 | 187.8 | n.q. | n.q. | n.q. | – | – | – |
| LC-MS(+) | Olivil glucoside isomer 2 | 4.299 | C26H34O12 | 538.20503 | Lignan glycosides | 538.50 | 0.1 | 187.8 | n.q. | n.q. | n.q. | – | – | – |
| LC-MS(+) | Syringaresinol diglucoside | 5.117 | C34H46O18 | 742.26841 | Lignan glycosides | 742.74 | −1.4 | 254.1 | n.q. | n.q. | n.q. | – | – | – |
| LC-MS(−) | 2-hydroxy-5-methoxybenzaldehyde | 4.152 | C8H8O3 | 152.04734 | Methoxyphenols | 152.15 | 1.8 | 46.5 | 18.8 ± 2.3 | 9.1 ± 1.2 | 11.0 ± 1.3 | 31.8 ± 8.0 | 36.3 ± 10.9 | 164.8 ± 21.0 |
| LC-MS(−) | 4-methoxysalicylaldehyde | 4.843 | C8H8O3 | 168.04226 | Methoxyphenols | 152.15 | 1.5 | 46.5 | n.q. | n.q. | n.q. | n.q. | – | – |
| LC-MS(−) * | Diosmetin | 8.355 | C16H12O6 | 300.06339 | O-methylated flavonoids | 300.26 | 1.7 | 100.1 | 4.5 ± 0.2 | 12.1 ± 3.7 | 46.3 ± 14.1 | – | – | – |
| LC-MS(−) | Chrysoeriol | 8.683 | C16H12O6 | 300.06339 | O-methylated flavonoids | 300.26 | 1.7 | 100.1 | n.q. | n.q. | n.q. | – | – | – |
| LC-MS(+) * | Nobiletin | 8.976 | C21H22O8 | 402.13147 | O-methylated flavonoids | 402.4 | 3.0 | 85.6 | n.q. | n.q. | n.q. | n.q. | n.q. | n.q. |
| LC-MS(+) | Norharman | 4.120 | C11H8N2 | 168.06875 | Pyridoindoles | 168.2 | 3.2 | 41.9 | 2.3 ± 0.1 | 5.3 ± 1.4 | 4.1 ± 0.2 | – | – | – |
| LC-MS(+) | α-cyperone | 9.871 | C15H22O | 218.16707 | Sesquiterpenoids | 218.33 | 3.8 | 17.1 | 12.2 ± 0.1 | 25.1 ± 1.4 | 56.5 ± 6.5 | 15.4 ± 2.6 | 20.1 ± 12.0 | 187.3 ± 12.5 |
| LC-MS(+) | (-)-caryophyllene oxide | 9.492 | C15H24O | 220.18272 | Sesquiterpenoids | 220.35 | 3.6 | 12.5 | 4.3 ± 0.1 | 8.6 ± 0.6 | 8.8 ± 0.7 | n.q. | n.q. | n.q. |
| LC-MS(+) | 8-hydroxyageraphorone | 8.088 | C15H24O2 | 236.17763 | Sesquiterpenoids | 236.35 | 2.9 | 37.3 | n.q. | n.q. | n.q. | n.q. | n.q. | 124.7 ± 16.9 |
| LC-MS(+) | Ophiopogonoside A | 6.235 | C21H38O8 | 418.25667 | Terpene glycosides | 418.50 | 0.4 | 139.8 | – | – | – | n.q. | n.q. | 66.0 ± 3.2 |
2.4. In Vitro Neuroprotective Potential of F. persica Extracts in SH-SY5Y Cells
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Sample Preparation and Extraction Conditions
3.3. Parallel Artificial Membrane Permeability Assay—Blood–Brain Barrier
3.4. Cell Culture Assays in HBMEC Cells
3.4.1. Toxicity Evaluation of F. persica Extracts
3.4.2. Blood–Brain Barrier Transport Study of F. persica Extracts
3.4.3. Cell Barrier Integrity
- Transendothelial electrical resistance (TEER)
- Sodium fluorescein (Na-F) paracellular permeability
3.5. Cell Culture Assays in SH-SY5Y Cells
3.5.1. Toxicity Evaluation of F. persica Extracts
3.5.2. Neuroprotection Evaluation of F. persica Extracts
3.6. Quantification of F. persica Compounds in Barrier Transport Assays
3.6.1. Compound Extraction
3.6.2. High Performance Liquid Chromatography–Tandem Mass Spectrometry (HPLC-Q-TOF-MS/MS) and Gas Chromatography–Mass Spectrometry (GC-Q-TOF-MS) Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brown, R.C.; Lockwood, A.H.; Sonawane, B.R. Neurodegenerative diseases: An overview of environmental risk factors. Environ. Health Perspect. 2005, 113, 1250–1256. [Google Scholar] [CrossRef]
- Omar, S.H.; Scott, C.J.; Hamlin, A.S.; Obied, H.K. The protective role of plant biophenols in mechanisms of Alzheimer’s disease. Nutr. Biochem. 2017, 47, 1–20. [Google Scholar] [CrossRef]
- World Health Organization. Fact Sheets of Dementia; World Health Organization: Geneva, Switzerland, 2021; Available online: https://www.who.int/news-room/fact-sheets/detail/dementia (accessed on 2 April 2025).
- Banerjee, S.; Dutta, S.; Ghosh, S.; Sil, P.C. Nutraceuticals in neurodegenerative diseases. Nutraceuticals Brain Health Beyond 2020, 14, 249–270. [Google Scholar] [CrossRef]
- Auti, S.T.; Kulkarni, Y.A. A systematic review on the role of natural products in modulating the pathways in Alzheimer’s disease. Vitam. Nutr. Res. 2017, 87, 99–116. [Google Scholar] [CrossRef]
- Soria Lopez, J.A.; González, H.M.; Léger, G.C. Alzheimer’s disease. In Handbook of Clinical Neurology; Dekosky, S.T., Asthana, S., Eds.; 2019; Volume 167, pp. 231–255. [Google Scholar] [CrossRef]
- Uddin, M.S.; Kabir, M.T. Oxidative Stress in Alzheimer’s Disease: Molecular Hallmarks of Underlying Vulnerability. In Biological, Diagnostic and Therapeutic Advances in Alzheimer’s Disease; Ashraf, G., Alexiou, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2019; pp. 91–115. [Google Scholar] [CrossRef]
- Cardoso, F.L.; Brites, D.; Brito, M.A. Looking at the blood–brain barrier: Molecular anatomy and possible investigation approaches. Brain Res. Rev. 2010, 64, 328–363. [Google Scholar] [CrossRef] [PubMed]
- Bicker, J.; Alves, G.; Fortuna, A.; Falcão, A. Blood-brain barrier models and their relevance for a successful development of CNS drug delivery systems: A review. Pharm. Biopharm. 2014, 87, 409–432. [Google Scholar] [CrossRef]
- Ureña-Vacas, I.; Aznar de la Riera, M.B.; Serrano, D.R.; González-Burgos, E. A new frontier in neuropharmacology: Recent progress in natural products research for blood–brain barrier crossing. Curr. Res. Biotechnol. 2024, 8, 100235. [Google Scholar] [CrossRef]
- Di, L.; Kerns, E.H.; Fan, K.; McConnell, O.J.; Carter, G.T. High throughput artificial membrane permeability assay for blood–brain barrier. Med. Chem. 2003, 38, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Chen, Q.; Chen, X.; Han, F.; Chen, Z.; Wang, Y. The blood-brain barrier: Structure, regulation, and drug delivery. Signal Transduct. Target. Ther. 2023, 8, 217. [Google Scholar] [CrossRef]
- Figueira, I.; Garcia, G.; Pimpão, R.C.; Terrasso, A.P.; Costa, I.; Almeida, A.F.; Tavares, L.; Pais, T.F.; Pinto, P.; Ventura, M.R.; et al. Polyphenols journey through blood-brain barrier towards neuronal protection. Sci. Rep. 2017, 7, 11456, Erratum in Sci. Rep. 2021, 11, 17112. [Google Scholar] [CrossRef] [PubMed]
- Palmela, I.; Correia, L.; Silva, R.F.M.; Sasaki, H.; Kim, K.S.; Brites, D.; Brito, M.A. Hydrophilic bile acids protect human blood-brain barrier endothelial cells from disruption by unconjugated bilirubin: An in vitro study. Front. Neurosci. 2015, 9, 80. [Google Scholar] [CrossRef] [PubMed]
- Eigenmann, D.E.; Xue, G.; Kim, K.S.; Moses, A.V.; Hamburger, M.; Oufir, M. Comparative study of four immortalized human brain capillary endothelial cell lines, hCMEC/D3, hBMEC, TY10, and BB19, and optimization of culture conditions, for an in vitro blood-brain barrier model for drug permeability studies. Fluids Barriers CNS 2013, 10, 33. [Google Scholar] [CrossRef]
- Kanjanasirirat, P.; Saengsawang, W.; Ketsawatsomkron, P.; Asavapanumas, N.; Borwornpinyo, S.; Soodvilai, S.; Hongeng, S.; Charoensutthivarakul, S. GDNF and cAMP significantly enhance in vitro blood-brain barrier integrity in a humanized tricellular transwell model. Heliyon 2024, 10, e39343. [Google Scholar] [CrossRef]
- Kovalevich, J.; Langford, D. Considerations for the use of SH-SY5Y neuroblastoma cells in neurobiology. Methods Mol. Biol. 2013, 1078, 9–21. [Google Scholar] [CrossRef]
- Dubey, S.K.; Ram, M.S.; Krishna, K.V.; Saha, R.N.; Singhvi, G.; Agrawal, M.; Ajazuddin; Saraf, S.; Saraf, S.; Alexander, A. Recent expansions on cellular models to uncover the scientific barriers towards drug development for Alzheimer’s disease. Cell. Mol. Neurobiol. 2019, 39, 181–209. [Google Scholar] [CrossRef]
- de Medeiros, L.M.; De Bastiani, M.A.; Rico, E.P.; Schonhofen, P.; Pfaffenseller, B.; Wollenhaupt-Aguiar, B.; Grun, L.; Barbé-Tuana, F.; Zimmer, E.R.; Castro, M.A.A.; et al. Cholinergic differentiation of human neuroblastoma SH-SY5Y cell line and its potential use as an in vitro model for Alzheimer’s disease studies. Mol. Neurobiol. 2019, 56, 7355–7367. [Google Scholar] [CrossRef]
- Huang, B.; Liu, J.; Fu, S.; Zhang, Y.; Li, Y.; He, D.; Ran, X.; Yan, X.; Du, J.; Meng, T.; et al. α-Cyperone attenuates H2O2-induced oxidative stress and apoptosis in SH-SY5Y cells via activation of Nrf2. Front. Pharmacol. 2020, 11, 281. [Google Scholar] [CrossRef] [PubMed]
- Gallego, R.; Valdés, A.; Sánchez-Martínez, J.D.; Suárez-Montenegro, Z.J.; Ibáñez, E.; Cifuentes, A.; Herrero, M. Study of the potential neuroprotective effect of Dunaliella salina extract in SH-SY5Y cell model. Anal. Bioanal. Chem. 2022, 414, 5357–5371. [Google Scholar] [CrossRef]
- de Matos, A.M.; Martins, A.; Man, T.; Evans, D.; Walter, M.; Oliveira, M.C.; López, Ó.; Fernandez-Bolaños, J.G.; Dätwyler, P.; Ernst, B.; et al. Design and synthesis of CNS-targeted flavones and analogues with neuroprotective potential against H2O2- and Aβ1-42-induced toxicity in SH-SY5Y human neuroblastoma cells. Pharmaceuticals 2019, 12, 98. [Google Scholar] [CrossRef] [PubMed]
- Sattar, Z.; Iranshahi, M. Phytochemistry and pharmacology of Ferula persica Boiss.: A review. Iran. J. Basic Med. Sci. 2017, 20, 1–8. [Google Scholar] [CrossRef]
- Karimi, G.; Iranshahi, M.; Hosseinalizadeh, F.; Riahi, B.; Sahebkar, A. Screening of acetylcholinesterase inhibitory activity of terpenoid and coumarin derivatives from the genus Ferula. Pharmacologyonline 2010, 1, 566–574. Available online: https://pharmacologyonline.silae.it/files/archives/2010/vol1/57.Karimi.pdf (accessed on 2 April 2025).
- Mohammadnezhad, P.; Valdés, A.; Cifuentes, A. Optimization and Chemical Characterization of Extracts Obtained from Ferula persica var. latisecta Aerial Parts and Roots and Their Neuroprotective Evaluation. Nutrients 2024, 16, 4210. [Google Scholar] [CrossRef]
- Chiu, Y.J.; Teng, Y.S.; Chen, C.M.; Sun, Y.C.; Hsieh-Li, H.M.; Chang, K.H.; Lee-Chen, G.J. A neuroprotective action of quercetin and apigenin through inhibiting aggregation of Aβ and activation of TRKB signaling in a cellular experiment. Biomol. Ther. 2023, 31, 285–297. [Google Scholar] [CrossRef] [PubMed]
- Lai, M.C.; Liu, W.Y.; Liou, S.-S.; Liu, I.-M. Diosmetin targeted at peroxisome proliferator-activated receptor gamma alleviates advanced glycation end products induced neuronal injury. Nutrients 2022, 14, 2248. [Google Scholar] [CrossRef]
- Chaves, J.S.; Leal, P.C.; Pianowisky, L.; Calixto, J.B. Pharmacokinetics and tissue distribution of the sesquiterpene alpha-humulene in mice. Planta Medica 2008, 74, 1678–1683. [Google Scholar] [CrossRef]
- Velásquez-Jiménez, D.; Corella-Salazar, D.A.; Zuñiga-Martínez, B.S.; Domínguez-Avila, J.A.; Montiel-Herrera, M.; Salazar-López, N.J.; Rodrigo-Garcia, J.; Villegas-Ochoa, M.A.; González-Aguilar, G.A. Phenolic compounds that cross the blood–brain barrier exert positive health effects as central nervous system antioxidants. Food Funct. 2021, 12, 10356–10369. [Google Scholar] [CrossRef]
- Jeong, K.-H.; Cho, S.-Y.; Hong, Y.-D.; Chung, J.-O.; Kim, K.-S.; Shim, S.-M. Transport of gallocatechin gallate and catechin gallate in high-temperature-processed green tea extract from gastrointestinal tract to brain by an in vitro bio-mimic model system coupled with sequential cell cultures. J. Funct. Foods 2018, 47, 83–90. [Google Scholar] [CrossRef]
- Correia-da-Silva, M.; Sousa, E.; Pinto, M.M.M. Emerging sulfated flavonoids and other polyphenols as drugs: Nature as an inspiration. Med. Res. Rev. 2014, 34, 223–279. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, H.A.; Ragab, E.A.; Dutta, A.; Othman, A.; Shaheen, U.; Jaremko, M.; Badshah, S.L.; Dhahri, M.; Al-Younis, I.; Al-Rimawi, F.; et al. Sulfated flavonoids: An updated and comprehensive review of their chemistry and bioactivities. Phytochem. Rev. 2025. [Google Scholar] [CrossRef]
- Zhu, J.; Yi, X.; Zhang, J.; Chen, S.; Wu, Y. Rapid screening of brain-penetrable antioxidants from natural products by blood-brain barrier specific permeability assay combined with DPPH recognition. J. Pharm. Biomed. Anal. 2018, 151, 42–48. [Google Scholar] [CrossRef]
- Cavalli, R.; Soster, M.; Argenziano, M. Nanobubbles: A promising efficient tool for therapeutic delivery. Ther. Deliv. 2016, 7, 117–138. [Google Scholar] [CrossRef]
- Hitchcock, S.A. Blood–brain barrier permeability considerations for CNS-targeted compound library design. Curr. Opin. Chem. Biol. 2008, 12, 318–323. [Google Scholar] [CrossRef]
- Agatonovic-Kustrin, S.; Chan, C.K.Y.; Gegechkori, V.; Morton, D.W. Models for skin and brain penetration of major components from essential oils used in aromatherapy for dementia patients. J. Biomol. Struct. Dyn. 2020, 38, 2402–2411. [Google Scholar] [CrossRef]
- Waterhouse, R.N. Determination of lipophilicity and its use as a predictor of blood–brain barrier penetration of molecular imaging agents. Mol. Imaging Biol. 2003, 5, 376–389. [Google Scholar] [CrossRef]
- Sánchez-Martínez, J.D.; Valdés, A.; Gallego, R.; Suárez-Montenegro, Z.J.; Alarcón, M.; Ibañez, E.; Alvarez-Rivera, G.; Cifuentes, A. Blood–brain barrier permeability study of potential neuroprotective compound recovered from plants and agri-food by-products. Front. Nutr. 2022, 9, 924596. [Google Scholar] [CrossRef]
- Yang, Y.; Bai, L.; Li, X.; Xiong, J.; Xu, P.; Guo, C.; Xue, M. Transport of active flavonoids, based on cytotoxicity and lipophilicity: An evaluation using the blood-brain barrier cell and Caco-2 cell models. Toxicol. Vitr. 2014, 28, 388–396. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J. Dietary flavonoid aglycones and their glycosides: Which show better biological significance? Crit. Rev. Food Sci. Nutr. 2017, 57, 1874–1905. [Google Scholar] [CrossRef] [PubMed]
- Shimazu, R.; Anada, M.; Miyaguchi, A.; Nomi, Y.; Matsumoto, H. Evaluation of blood-brain barrier permeability of polyphenols, anthocyanins, and their metabolites. J. Agric. Food Chem. 2021, 69, 11676–11686. [Google Scholar] [CrossRef] [PubMed]
- Mei, L.; Yang, Y.; Zhang, X.; Li, M.; Zhang, W.; Li, Y.; Zeng, X. Protective effects of polyphenol-rich black chokeberry (Aronia melanocarpa) extract against H2O2-induced oxidative damage and apoptosis in SH-SY5Y cells. Food Funct. 2022, 13, 1430–1444. [Google Scholar] [CrossRef]
- Zhang, T.; Su, J.; Guo, B.; Wang, K.; Li, X.; Liang, G. Apigenin protects blood-brain barrier and ameliorates early brain injury by inhibiting TLR4-mediated inflammatory pathway in subarachnoid hemorrhage rats. Int. Immunopharmacol. 2015, 28, 79–87. [Google Scholar] [CrossRef]
- Hasan, S.; Khatri, N.; Rahman, Z.N.; Menezes, A.A.; Martini, J.; Shehjar, F.; Mujeeb, N.; Shah, Z.A. Neuroprotective potential of flavonoids in brain disorders. Brain Sci. 2023, 13, 1258. [Google Scholar] [CrossRef] [PubMed]
- Kocsis, A.E.; Kucsápszky, N.; Santa-Maria, A.R.; Hunyadi, A.; Deli, M.A.; Walter, F.R. Much more than nutrients: The protective effects of nutraceuticals on the blood–brain barrier in diseases. Nutrients 2025, 17, 766. [Google Scholar] [CrossRef]
- El-Maraghy, S.A.; Reda, A.; Essam, R.M.; Kortam, M.A. The citrus flavonoid “Nobiletin” impedes STZ-induced Alzheimer’s disease in a mouse model through regulating autophagy mastered by SIRT1/FoxO3a mechanism. Inflammopharmacology 2023, 31, 2701–2717. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, A.; Yamakuni, T.; Haraguchi, M.; Omae, N.; Song, S.Y.; Kato, C.; Nakagawasai, O.; Tadano, T.; Yokosuka, A.; Mimaki, Y.; et al. Nobiletin, a citrus flavonoid that improves memory impairment, rescues bulbectomy-induced cholinergic neurodegeneration in mice. Pharmacol. Sci. 2007, 105, 122–126. [Google Scholar] [CrossRef]
- Durmaz, L.; Gulçin, İ.; Taslimi, P.; Tüzün, B. Isofraxidin: Antioxidant, anti-carbonic anhydrase, anti-cholinesterase, anti-diabetic, and in silico properties. Chem. Sel. 2023, 8, e202300170. [Google Scholar] [CrossRef]
- Lian, B.; Gu, J.; Zhang, C.; Zou, Z.; Yu, M.; Li, F.; Wu, X.; Zhao, A.Z. Protective effects of isofraxidin against scopolamine-induced cognitive and memory impairments in mice involve modulation of the BDNF-CREB-ERK signaling pathway. Metab. Brain Dis. 2023, 37, 2751–2762. [Google Scholar] [CrossRef]
- Carecho, R.; Carregosa, D.; Dos Santos, C.N. Low molecular weight (poly)phenol metabolites across the blood-brain barrier: The underexplored journey. Brain Plast. 2021, 6, 193–214. [Google Scholar] [CrossRef]
- Guo, C.; Valdés, A.; Sánchez-Martínez, J.D.; Ibáñez, E.; Bi, J.; Cifuentes, A. Neuroprotective potential of thinned peaches extracts obtained by pressurized liquid extraction after different drying processes. Foods 2022, 11, 2464. [Google Scholar] [CrossRef]
- Aboulaghras, S.; Piancatelli, D.; Oumhani, K.; Balahbib, A.; Bouyahya, A.; Taghzouti, K. Pathophysiology and immunogenetics of celiac disease. Clin. Chim. Acta 2022, 528, 74–83. [Google Scholar] [CrossRef]
- Yu, H.; Chen, Y.; Qu, T.; Hang, J.; Huang, X.; Hu, A.; Wang, T.; Gao, Y.; Shao, J.; Li, Y.; et al. α-Cyperone safeguards against cerebral ischaemia-reperfusion injury through the activation of the Nrf2 signalling pathway. Eur. J. Pharmacol. 2025, 1002, 177842. [Google Scholar] [CrossRef]
- Chen, X.; Murawski, A.; Patel, K.; Crespi, C.; Balimane, P.V.A. novel design of artificial membrane for improving the PAMPA model. Pharm. Res. 2008, 25, 1511–1520. [Google Scholar] [CrossRef]
- Sánchez-Martínez, J.D.; Garcia, A.R.; Alvarez-Rivera, G.; Valdés, A.; Brito, M.A.; Cifuentes, A. In vitro study of the blood–brain barrier transport of natural compounds recovered from agrifood by-products and microalgae. Int. J. Mol. Sci. 2023, 24, 533. [Google Scholar] [CrossRef] [PubMed]
- Pogačnik, L.; Pirc, K.; Palmela, I.; Skrt, M.; Kwang, K.S.; Brites, D.; Brito, M.A.; Ulrih, N.P.; Silva, R.F.M. Potential for Brain Accessibility and Analysis of Stability of Selected Flavonoids in Relation to Neuroprotection In Vitro. Brain Res. 2016, 1651, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Deli, M.A.; Ábrahám, C.S.; Kataoka, Y.; Niwa, M. Permeability Studies on in Vitro Blood-Brain Barrier Models: Physiology, Pathology, and Pharmacology. Cell. Mol. Neurobiol. 2005, 25, 59–127. [Google Scholar] [CrossRef] [PubMed]
- Cokdinleyen, M.; Valdés, A.; Kara, H.; Ibáñez, E.; Cifuentes, A. Neuroprotective Potential of Tetraselmis chuii Compounds: Insights into Blood–Brain Barrier Permeability and Intestinal Transport. Pharmaceuticals 2025, 18, 629. [Google Scholar] [CrossRef]
| Analytical Platform | Compound | RT (min) | Molecular Formula | Monoisotopic Mass | Subclass | MW | log p | TPSA | AerExt | RootExt | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| log Pe (cm−1) | RSD (%) | Cross BBB Potential | log Pe (cm−1) | RSD (%) | Cross BBB Potential | |||||||||
| LC-MS(−) * | Quinic acid | 0.694 | C7H12O6 | 192.06339 | Alcohols and polyols | 192.17 | −2.4 | 118.2 | −6.51 | 1.27 | + | n.d. | ||
| LC-MS(+) | β-asarone | 8.912 | C12H16O3 | 208.10994 | Anisoles | 208.25 | 3.0 | 27.7 | −4.86 | 1.40 | ++ | n.q. | – | |
| GC-MS | Elemicin | 11.731 | C12H16O3 | 208.10990 | Anisoles | 208.25 | 2.5 | 74.6 | −5.28 | 1.54 | ++ | −4.68 | 0.65 | ++ |
| LC-MS(−) * | 2,3-dihydroxybenzoic acid | 3.342 | C7H6O4 | 154.02661 | BAs and derivatives | 154.12 | 1.2 | 77.8 | n.q. | – | −5.42 | 2.53 | ++ | |
| LC-MS(−) | 3-formylsalicylic acid | 2.848 | C8H6O4 | 166.02661 | BAs and derivatives | 166.13 | 1.0 | 74.6 | n.d. | −5.50 | 1.70 | ++ | ||
| LC-MS(−) | 1,2,4-benzenetriol | 1.365 | C6H6O3 | 126.03169 | Benzenetriols and derivatives | 126.11 | 1.5 | 60.7 | n.q. | – | −5.19 | 1.60 | ++ | |
| LC-MS(−) | Catechol | 2.380 | C6H6O2 | 110.03678 | Benzenediols | 110.11 | 0.9 | 40.5 | −4.88 | 0.90 | ++ | −4.70 | 1.60 | ++ |
| GC-MS | Myristicin | 11.444 | C11H12O3 | 192.07860 | Benzodioxoles | 192.21 | 2.9 | 40.5 | n.d. | n.q. | – | |||
| LC-MS(+) | Loliolide | 5.162 | C11H16O3 | 196.10994 | Benzofurans | 196.24 | 1.0 | 46.5 | −4.98 | 2.17 | ++ | n.d. | ||
| LC-MS(−) * | 4-hydroxybenzaldehyde | 3.445 | C7H6O2 | 122.03677 | Carbonyl compounds | 122.12 | 1.4 | 37.3 | −4.52 | 0.46 | ++ | −4.33 | 1.76 | +++ |
| LC-MS(−) | Protocatechuic aldehyde | 2.745 | C7H6O3 | 138.03169 | Carbonyl compounds | 138.12 | 1.3 | 57.5 | −5.05 | 0.94 | ++ | −4.85 | 1.40 | ++ |
| LC-MS(−) | 3,4-dihydroxyacetophenone | 3.324 | C8H8O3 | 152.04734 | Carbonyl compounds | 152.15 | 1.5 | 57.5 | n.d. | −4.76 | 1.59 | ++ | ||
| LC-MS(+) | 9,19-cyclolanost-25-ene-3,24-diol | 9.900 | C30H50O2 | 442.38108 | Cycloartanols and derivatives | 442.70 | 8.6 | 40.5 | −5.60 | 1.25 | + | −5.79 | 1.93 | + |
| LC-MS(−) * | Apigenin | 8.074 | C15H10O5 | 270.05282 | Flavones | 270.24 | 1.7 | 90.9 | −4.01 | 1.07 | +++ | n.q. | – | |
| LC-MS(−) * | Luteolin | 7.268 | C15H10O6 | 286.04774 | Flavones | 286.24 | 1.4 | 111.1 | −4.58 | 0.83 | ++ | −4.68 | 0.72 | ++ |
| LC-MS(−) | Hispidulin 4′-glucoside | 6.302 | C22H22O11 | 462.11621 | Flavonoid glycosides | 462.40 | 0.8 | 179.3 | −6.14 | 2.04 | + | n.d. | ||
| LC-MS(−) * | Luteolin 7-glucoside | 5.489 | C21H20O11 | 448.10056 | Flavonoid glycosides | 448.40 | 0.5 | 190.3 | −6.92 | 1.30 | + | n.q. | – | |
| LC-MS(−) * | Apigenin 7-glucoside | 6.063 | C21H20O10 | 432.10565 | Flavonoid glycosides | 432.40 | −0.1 | 170.1 | −7.34 | 3.38 | + | n.q. | – | |
| LC-MS(−) | Isoquercetrin | 5.446 | C21H20O12 | 464.09548 | Flavonoid glycosides | 464.40 | −0.2 | 210.5 | n.q. | – | n.q. | – | ||
| LC-MS(−) * | Hesperidin | 6.050 | C28H34O15 | 610.18977 | Flavonoid glycosides | 610.60 | −1.1 | 234.3 | n.q. | – | n.q. | – | ||
| LC-MS(−) | Kaempferol 3-O-(3″,4″-di-O-acetyl-a-L-rhamnopyranoside) | 7.878 | C25H24O12 | 516.12678 | Flavonoid glycosides | 516.40 | −0.9 | 186.1 | n.q. | – | n.d. | |||
| LC-MS(+) | Harman | 4.273 | C12H10N2 | 182.08440 | Harmala alkaloids | 182.22 | 3.6 | 28.7 | −4.62 | 0.51 | ++ | n.d. | ||
| LC-MS(−) * | Ethyl caffeate | 7.188 | C11H12O4 | 208.07356 | HCAs and derivatives | 208.21 | 2.6 | 66.8 | −4.20 | 3.11 | +++ | n.d. | ||
| LC-MS(−) * | Ferulic acid | 4.905 | C10H10O4 | 194.05791 | HCAs and derivatives | 194.18 | 1.5 | 66.8 | −5.13 | 0.97 | ++ | n.q. | – | |
| LC-MS(−) * | Caffeic acid | 3.591 | C9H8O4 | 180.04226 | HCAs and derivatives | 180.16 | 1.2 | 77.8 | −5.16 | 1.89 | ++ | n.q. | – | |
| LC-MS(−) | 2,5-dihydroxycinnamic acid | 3.820 | C9H8O4 | 180.04226 | HCAs and derivatives | 180.16 | 1.2 | 77.8 | −5.68 | 3.34 | ++ | −5.22 | 1.61 | ++ |
| LC-MS(−) | 4-caffeoylquinic acid lactone | 4.477 | C16H16O8 | 336.08500 | HCAs and derivatives | 164.16 | 0.4 | 133.5 | −5.81 | 0.56 | + | −5.42 | 2.08 | ++ |
| LC-MS(−) * | 4-coumaric acid | 4.400 | C9H8O3 | 164.04734 | HCAs and derivatives | 336.29 | 1.5 | 57.5 | n.q. | – | n.q. | – | ||
| LC-MS(+) | 7-hydroxy-6-methoxycoumarin | 4.885 | C10H8O4 | 192.04226 | Hydroxycoumarins | 192.17 | 1.5 | 66.8 | −4.66 | 1.13 | ++ | −4.53 | 1.33 | ++ |
| LC-MS(+) | Fraxidin | 5.468 | C11H10O5 | 222.05282 | Hydroxycoumarins | 222.19 | 1.5 | 68.9 | −4.82 | 0.41 | ++ | −4.81 | 1.03 | ++ |
| LC-MS(+) | Isofraxidin | 5.142 | C11H10O5 | 222.05282 | Hydroxycoumarins | 222.19 | 1.5 | 68.9 | −5.20 | 0.38 | ++ | −5.03 | 2.17 | ++ |
| LC-MS(−) * | 6,7-dihydroxycoumarin | 3.528 | C9H6O4 | 178.02661 | Hydroxycoumarins | 178.14 | 1.2 | 70.7 | −5.60 | 0.72 | + | n.q. | – | |
| LC-MS(+) | 6″-O-acetylgenistin | 7.644 | C23H22O11 | 474.11621 | Isoflavonoid O-glycosides | 474.40 | 0.9 | 176.1 | n.q. | – | n.d. | |||
| LC-MS(+) | Olivil glucoside isomer 1 | 4.230 | C26H34O12 | 538.20503 | Lignan glycosides | 538.50 | 0.1 | 187.8 | n.q. | – | n.d. | |||
| LC-MS(+) | Olivil glucoside isomer 2 | 4.230 | C26H34O12 | 538.20503 | Lignan glycosides | 538.50 | 0.1 | 187.8 | n.q. | – | n.d. | |||
| LC-MS(+) | Syringaresinol glucoside | 5.997 | C28H36O13 | 580.21559 | Lignan glycosides | 580.60 | −1.4 | 254.1 | n.q. | – | n.q. | – | ||
| LC-MS(+) | Syringaresinol diglucoside | 5.008 | C34H46O18 | 742.26841 | Lignan glycosides | 742.70 | −1.4 | 254.1 | n.q. | – | n.q. | – | ||
| LC-MS(−) | 2-hydroxy-5-methoxybenzaldehyde | 3.857 | C8H8O3 | 152.04734 | Methoxyphenols | 152.15 | 1.8 | 46.5 | −4.71 | 0.65 | ++ | −4.40 | 1.27 | +++ |
| GC-MS | 4-vinylguaiacol | 8.757 | C9H10O2 | 150.0681 | Methoxyphenols | 150.17 | 2.4 | 46.5 | −6.04 | 2.03 | + | −5.36 | 4.47 | ++ |
| LC-MS(-) | 4-methoxysalicylaldehyde | 4.586 | C8H8O3 | 168.04226 | Methoxyphenols | 152.15 | 1.5 | 46.5 | n.q. | – | −3.95 | 3.50 | +++ | |
| GC-MS | trans-sinapyl alcohol | 16.498 | C11H14O4 | 210.08920 | Methoxyphenols | 210.23 | 1.3 | 66.8 | n.d. | −6.04 | 3.10 | + | ||
| LC-MS(−) * | Diosmetin | 8.214 | C16H12O6 | 300.06339 | O-methylated flavonoids | 300.26 | 1.7 | 100.1 | −3.83 | 3.27 | +++ | −4.17 | 1.41 | +++ |
| LC-MS(+) * | Nobiletin | 8.915 | C21H22O8 | 402.13147 | O-methylated flavonoids | 402.40 | 3.0 | 85.6 | −4.31 | 3.44 | +++ | −4.31 | 1.75 | +++ |
| LC-MS(−) | Chrysoeriol | 8.582 | C16H12O6 | 300.06339 | O-methylated flavonoids | 300.26 | 1.7 | 100.1 | n.q. | – | n.d. | |||
| LC-MS(+) | 3,7-epoxycaryophyllan-6-One | 7.431 | C15H24O2 | 236.17763 | Oxepanes | 236.35 | 2.8 | 26.3 | −4.98 | 0.52 | ++ | n.d. | ||
| LC-MS(+) | Norharman | 3.794 | C11H8N2 | 168.06875 | Pyridoindoles | 168.19 | 3.2 | 41.9 | −4.05 | 0.83 | +++ | −4.67 | 8.83 | ++ |
| LC-MS(+) | 8-hydroxyageraphorone | 7.814 | C15H24O2 | 236.17763 | Sesquiterpenoids | 236.35 | 2.9 | 37.3 | −4.65 | 0.91 | ++ | −4.23 | 1.65 | +++ |
| LC-MS(+) | (–)-caryophyllene oxide | 9.365 | C15H24O | 220.18272 | Sesquiterpenoids | 220.35 | 3.6 | 12.5 | −4.69 | 0.64 | ++ | −4.31 | 0.43 | +++ |
| LC-MS(−) | Pentalenic acid | 9.946 | C15H22O3 | 250.15689 | Sesquiterpenoids | 250.33 | 2.7 | 57.5 | −4.84 | 1.18 | ++ | −4.97 | 0.89 | ++ |
| GC-MS | Farnesyl acetate | 14.979 | C17H28O2 | 264.20890 | Sesquiterpenoids | 264.40 | 5.3 | 210.5 | −5.25 | 2.02 | ++ | n.d. | ||
| GC-MS | Valerianol | 12.973 | C15H26O | 222.19840 | Sesquiterpenoids | 222.37 | 3.5 | 77.8 | −5.35 | 1.45 | ++ | −5.59 | 5.60 | + |
| GC-MS | γ-eudesmol | 12.709 | C15H26O | 222.19840 | Sesquiterpenoids | 222.37 | 3.7 | 77.8 | −5.88 | 2.70 | + | −5.69 | 6.85 | + |
| GC-MS | epi-γ-eudesmol | 13.049 | C15H26O | 222.19840 | Sesquiterpenoids | 222.37 | 2.9 | 46.5 | −5.89 | 2.15 | + | −5.81 | 3.89 | + |
| GC-MS | α-bisabolol | 13.405 | C15H26O | 222.19840 | Sesquiterpenoids | 222.37 | 1.3 | 133.5 | −5.94 | 2.83 | + | −5.75 | 6.23 | + |
| LC-MS(+) | α-cyperone | 9.734 | C15H22O | 218.16707 | Sesquiterpenoids | 218.33 | 3.8 | 17.1 | −5.08 | 0.6 | ++ | −5.16 | 0.29 | ++ |
| GC-MS | γ-elemene | 10.220 | C15H24 | 204.18780 | Sesquiterpenoids | 204.35 | 5.4 | 29.5 | n.q. | – | n.q. | – | ||
| GC-MS | (–)-aristolene | 10.260 | C15H24 | 204.18780 | Sesquiterpenoids | 204.35 | 4.7 | 118.2 | n.q. | – | n.q. | – | ||
| GC-MS | α-humulene | 10.550 | C15H24 | 204.18780 | Sesquiterpenoids | 204.35 | 4.5 | 60.7 | n.q. | – | n.q. | – | ||
| GC-MS | Selina-3,7(11)-diene | 11.626 | C15H24 | 204.18780 | Sesquiterpenoids | 204.35 | 4.4 | 57.5 | n.q. | – | n.q. | – | ||
| GC-MS | (E)-nerolidol | 11.730 | C15H26O | 222.19840 | Sesquiterpenoids | 222.37 | 4.6 | 57.5 | n.q. | – | n.q. | – | ||
| GC-MS | Rosifoliol | 12.308 | C15H26O | 222.19840 | Sesquiterpenoids | 222.37 | 3.9 | 77.8 | n.q. | – | n.q. | – | ||
| GC-MS | Guaiol | 12.382 | C15H26O | 222.19840 | Sesquiterpenoids | 222.37 | 3.1 | 37.3 | n.q. | – | n.q. | – | ||
| GC-MS | α-epi-7-epi-5-eudesmol | 12.507 | C15H26O | 222.19840 | Sesquiterpenoids | 222.37 | 3.4 | 70.7 | n.q. | – | n.q. | – | ||
| GC-MS | Agarospirol | 12.848 | C15H26O | 222.19840 | Sesquiterpenoids | 222.37 | 3.7 | 77.8 | n.q. | – | n.q. | – | ||
| GC-MS | Bulnesol | 13.189 | C15H26O | 222.19840 | Sesquiterpenoids | 222.37 | 3.8 | 57.5 | n.q. | – | n.q. | – | ||
| GC-MS | Guaiol acetate | 13.914 | C17H28O2 | 264.20890 | Sesquiterpenoids | 264.40 | 3.6 | 46.5 | n.q. | – | −5.05 | 1.36 | ++ | |
| LC-MS(+) | Ophiopogonoside A | 6.047 | C21H38O8 | 418.25667 | Terpene glycosides | 418.50 | 0.4 | 139.8 | n.q. | – | n.q. | – | ||
| LC-MS(+) | 4-methyl-5-thiazoleethanol | 1.654 | C6H9NOS | 143.04048 | Thiazoles | 143.21 | 0.8 | 61.4 | n.d. | −5.04 | 1.25 | ++ | ||
| LC-MS(+) | Protopanaxadiol | 10.603 | C30H52O3 | 460.39165 | Triterpenoids | 460.70 | 7.2 | 60.7 | n.q. | – | n.q. | – | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohammadnezhad, P.; Valdés, A.; Cokdinleyen, M.; Mendiola, J.A.; Cifuentes, A. Evaluation of the In Vitro Blood–Brain Barrier Transport of Ferula persica L. Bioactive Compounds. Int. J. Mol. Sci. 2025, 26, 8017. https://doi.org/10.3390/ijms26168017
Mohammadnezhad P, Valdés A, Cokdinleyen M, Mendiola JA, Cifuentes A. Evaluation of the In Vitro Blood–Brain Barrier Transport of Ferula persica L. Bioactive Compounds. International Journal of Molecular Sciences. 2025; 26(16):8017. https://doi.org/10.3390/ijms26168017
Chicago/Turabian StyleMohammadnezhad, Pouya, Alberto Valdés, Melis Cokdinleyen, Jose A. Mendiola, and Alejandro Cifuentes. 2025. "Evaluation of the In Vitro Blood–Brain Barrier Transport of Ferula persica L. Bioactive Compounds" International Journal of Molecular Sciences 26, no. 16: 8017. https://doi.org/10.3390/ijms26168017
APA StyleMohammadnezhad, P., Valdés, A., Cokdinleyen, M., Mendiola, J. A., & Cifuentes, A. (2025). Evaluation of the In Vitro Blood–Brain Barrier Transport of Ferula persica L. Bioactive Compounds. International Journal of Molecular Sciences, 26(16), 8017. https://doi.org/10.3390/ijms26168017










