Functionalized Indolizines as Potential Anticancer Agents: Synthetic, Biological and In Silico Investigations
Abstract
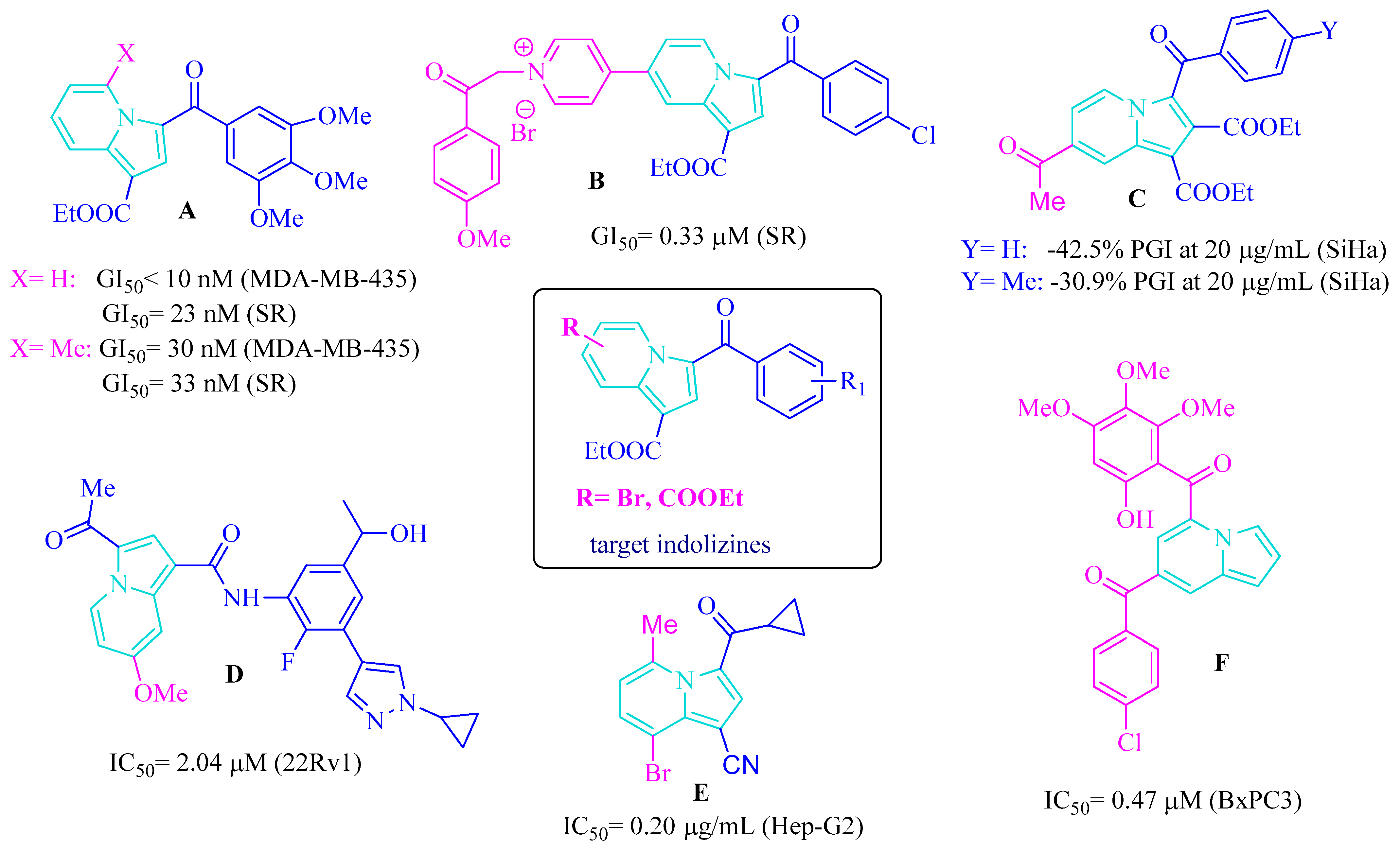
1. Introduction
2. Results
2.1. Chemistry
2.2. Biological Activity
2.2.1. Anticancer Activity
2.2.2. Molecular Docking
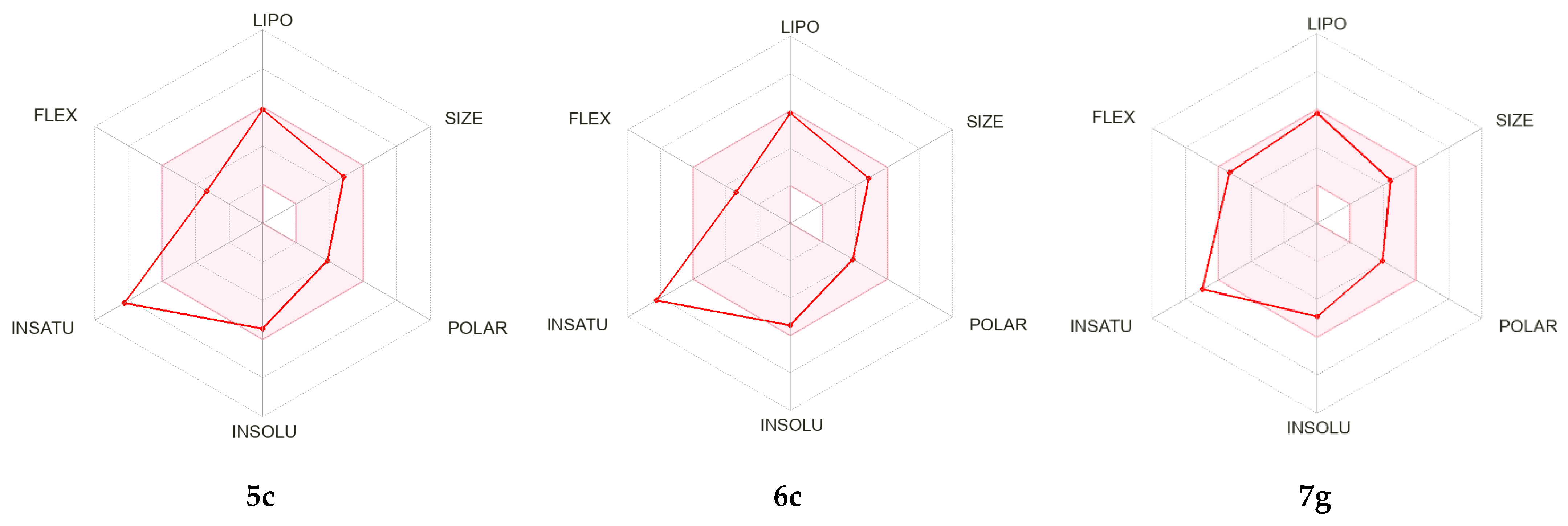
2.2.3. In Silico ADME [34] and Toxicity Predictions [35,36]
3. Materials and Methods
3.1. Chemistry
3.1.1. General Procedure for Quaternary Salts 1 and 2
3.1.2. General Procedure for Compounds 5, 6, 7
3.1.3. Spectral Data
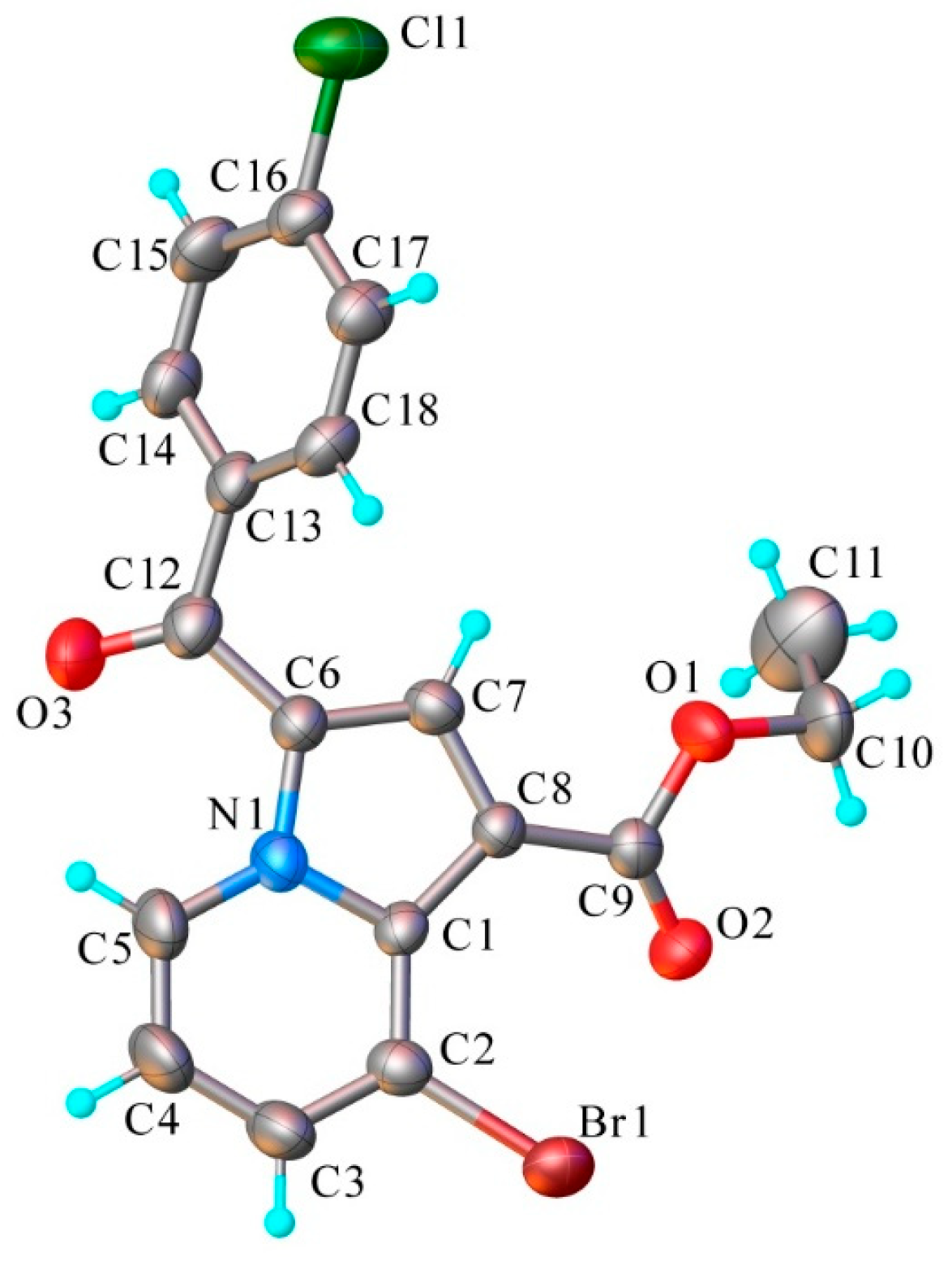
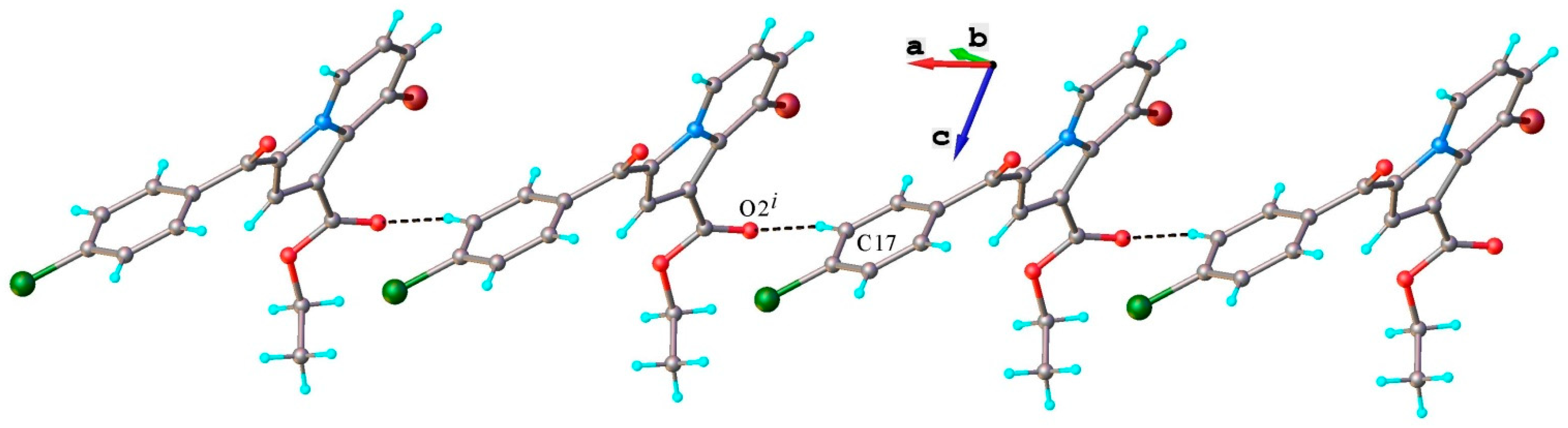
3.1.4. X-Ray Crystallography
3.2. Biological Activity
3.2.1. Cell Proliferation Assay
3.2.2. Molecular Docking
3.2.3. In Silico ADME and Toxicity Predictions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arora, P.; Arora, V.; Lamba, H.S.; Wadhwa, D. Importance of heterocyclic chemistry: A review. Int. J. Pharma Sci. Res. 2012, 3, 2947–2954. [Google Scholar] [CrossRef]
- Heravi, M.M.; Zadsirjan, V. Prescribed drugs containing nitrogen heterocycles: An overview. RSC Adv. 2020, 10, 44247–44311. [Google Scholar] [CrossRef]
- Marshall, C.M.; Federice, J.G.; Bell, C.N.; Cox, P.B.; Njardarson, J.T. An update on the nitrogen heterocycle compositions and properties of U.S. FDA-Approved Pharmaceuticals (2013–2023). J. Med. Chem. 2024, 67, 11622–11655. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Singh, A.A.K.; Singh, H.; Vijayan, V.; Kumar, D.; Naik, J.; Thareja, S.; Yadav, J.P.; Pathak, P.; Grishina, M.; et al. Nitrogen containing heterocycles as anticancer agents: A medicinal chemistry perspective. Pharmaceuticals 2023, 16, 299. [Google Scholar] [CrossRef] [PubMed]
- France, S.P.; Lindsey, E.A.; McInturff, E.L.; Berritt, S.; Carney, D.W.; DeForest, J.C.; Fink, S.J.; Flick, A.C.; Gibson, T.S.; Gray, K.; et al. Synthetic approaches to the new drugs approved during 2022. J. Med. Chem. 2024, 67, 4376–4418. [Google Scholar] [CrossRef]
- Al Shaer, D.; Al Musaimi, O.; Albericio, F.; de la Torre, B.G. 2019 FDA TIDES (Peptides and Oligonucleotides) Harvest. Pharmaceuticals 2020, 13, 40. [Google Scholar] [CrossRef]
- Akhtar, J.; Khan, A.A.; Ali, Z.; Haider, R.; Yar, M.S. Structure-activity relationship (SAR) study and design strategies of nitrogen-containing heterocyclic moieties for their anticancer activities. Eur. J. Med. Chem. 2017, 125, 143–189. [Google Scholar] [CrossRef]
- Ledade, P.V.; Lambat, T.L.; Gunjate, J.K.; Chopra, P.K.P.G.; Bhute, A.V.; Lanjewara, M.R.; Kadu, P.M.; Dongre, U.J.; Mahmood, S.H. Nitrogen-containing fused heterocycles: Organic synthesis and applications as potential anticancer agents. Curr. Org. Chem. 2023, 27, 206–222. [Google Scholar] [CrossRef]
- Biswas, T.; Mittal, R.K.; Kanupriya, V.S.; Mishra, I. Nitrogen-fused heterocycles: Empowering anticancer drug discovery. Med. Chem. 2024, 20, 369–384. [Google Scholar] [CrossRef]
- Ramalakshmi, N.; Amuthalakshmi, S.; Yamuna, R.; Anton, S.A.; Arunkumar, S. Indolizine- a privileged biological scaffold. Der. PharmaChemica 2021, 13, 60–69. [Google Scholar]
- Jadhav, M.; Mali, K.; Rajput, V.; Rudradip, D.; Shard, A. Exploring the decadal evolution of indolizine scaffold for anticancer innovations: A comprehensive analysis. Med. Chem. Res. 2024, 33, 1491–1510. [Google Scholar] [CrossRef]
- Amariucai-Mantu, D.; Antoci, V.; Sardaru, M.C.; Al Matarneh, C.M.; Mangalagiu, I.; Danac, R. Fused pyrrolo-pyridines and pyrrolo-(iso)quinoline as anticancer agents. Phys. Sci. Rev. 2023, 8, 2583–2645. [Google Scholar] [CrossRef]
- Ghinet, A.; Abuhaie, C.M.; Gautret, P.; Rigo, B.; Dubois, J.; Farce, A.; Belei, D.; Bicu, E. Studies on indolizines. Evaluation of their biological properties as microtubule-interacting agents and as melanoma targeting compounds. Eur. J. Med. Chem. 2019, 89, 115–127. [Google Scholar] [CrossRef]
- Shen, Y.M.; Lv, P.C.; Chen, W.; Liu, P.G.; Zhang, M.Z.; Zhu, H.L. Synthesis and antiproliferative activity of indolizine derivatives incorporating a cyclopropylcarbonyl group against Hep-G2 cancer cell line. Eur. J. Med. Chem. 2010, 45, 3184–3190. [Google Scholar] [CrossRef]
- Sardaru, M.C.; Craciun, A.M.; Al Matarneh, C.M.; Sandu, I.A.; Amarandi, R.M.; Popovici, L.; Ciobanu, C.I.; Peptanariu, D.; Pinteala, M.; Mangalagiu, I.I.; et al. Cytotoxic substituted indolizines as new colchicine site tubulin polymerisation inhibitors. J. Enzyme Inhib. Med. Chem. 2020, 35, 1581–1595. [Google Scholar] [CrossRef]
- Danac, R.; Al Matarneh, C.M.; Shova, S.; Daniloaia, T.; Balan, M.; Mangalagiu, I.I. New indolizines with phenanthroline skeleton: Synthesis, structure, antimycobacterial and anticancer evaluation. Bioorg. Med. Chem. 2015, 23, 2318–2327. [Google Scholar] [CrossRef]
- Sandeep, C.; Padmashali, B.; Venugopala, K.N.; Kulkarni, R.S.; Venugopala, R.; Odhav, B. Synthesis and characterization of ethyl 7-acetyl-2-substituted 3-(substituted benzoyl)indolizine-1-carboxylates for in vitro anticancer activity. Asian J. Chem. 2016, 28, 1043–10488. [Google Scholar] [CrossRef]
- Lee, Y.; Joshi, D.R.; Namkung, W.; Kim, I. Generation of a poly-functionalized indolizine scaffold and its anticancer activity in pancreatic cancer cells. Bioorg. Chem. 2022, 126, 105877. [Google Scholar] [CrossRef]
- Xiang, Q.; Wang, C.; Wu, T.; Zhang, C.; Hu, Q.; Luo, G.; Hu, J.; Zhuang, X.; Zou, L.; Shen, H.; et al. Design, synthesis, and biological evaluation of 1-(Indolizin-3-yl)ethan-1-ones as CBP bromodomain inhibitors for the treatment of prostate cancer. J. Med. Chem. 2022, 65, 785–810. [Google Scholar] [CrossRef]
- Bedjeguelal, K.; Bienaymé, H.; Dumoulin, A.; Poigny, S.; Schmitt, P.; Tam, E. Discovery of protein–protein binding disruptors using multi-component condensation small molecules. Bioorg. Med. Chem. Lett. 2006, 16, 3998–4001. [Google Scholar] [CrossRef]
- Boruah, N.; Gogoi, P.P.; Sinha, U.B. Halogen Bonding: A new frontier in medicinal chemistry. In Integrationg Disciplines: A New Approach to Multidisciplinary Research; Upasana Bora Sinha’s Lab: Lumami, India, 2024; Volume 1, pp. 97–113. [Google Scholar]
- Cavallo, G.; Metrangolo, P.; Milani, R.; Pilati, T.; Priimagi, A.; Resnati, G.; Terraneo, G. The halogen bond. Chem. Rev. 2016, 116, 2478–2601. [Google Scholar] [CrossRef]
- Oniciuc, L.; Amariucai-Mantu, D.; Diaconu, D.; Mangalagiu, V.; Danac, R.; Antoci, V.; Mangalagiu, I.I. Benzoquinoline derivatives: An attractive approach to newly small molecules with anticancer activity. Int. J. Mol. Sci. 2023, 24, 8124. [Google Scholar] [CrossRef]
- Amarandi, R.M.; Al Matarneh, C.M.; Popovici, L.; Ciobanu, C.I.; Neamtu, A.; Mangalagiu, I.I.; Danac, R. Exploring pyrrolo-fused heterocycles as promising anticancer agents: An integrated synthetic, biological, and computational approach. Pharmaceuticals 2023, 16, 865. [Google Scholar] [CrossRef]
- Abramyants, M.G.; Lomov, D.A.; Lyashchuk, S.N.; Zaporozhets, O.O. Synthesis and properties of new indolizine derivatives. Russ. J. Org. Chem. 2018, 54, 593–600. [Google Scholar] [CrossRef]
- Chen, S.J.; Chen, G.S.; Deng, T.; Li, J.H.; He, Z.Q.; Liu, L.S.; Ren, H.; Liu, Y.L. 1,2-Dicarbofunctionalization of Trifluoromethyl Alkenes with Pyridinium Salts via a Cycloaddition/Visible-Light-Enabled Fragmentation Cascade. Org. Lett. 2022, 24, 702–707. [Google Scholar] [CrossRef]
- Zhang, D.; Dong, S.; He, Q.; Luo, Y.; Liu, Y.; Liu, X.; Feng, X. Enantioselective dicarbofunctionalization of (E)-alkenyloxindoles with pyridinium salts by chiral Lewis acid/photo relay catalysis. Chem. Commun. 2020, 56, 12757–12760. [Google Scholar] [CrossRef]
- Shoemaker, R.H. The NCI60 human tumour cell line anticancer drug screen. Nat. Rev. Cancer 2006, 6, 813–823. [Google Scholar] [CrossRef]
- Holbeck, S.L.; Camalier, R.; Crowell, J.A.; Govindharajulu, J.P.; Hollingshead, M.; Anderson, L.W.; Polley, E.; Rubinstein, L.; Srivastava, A.; Wilsker, D.; et al. The National Cancer Institute ALMANAC: A comprehensive screening resource for the detection of anticancer drug pairs with enhanced therapeutic activity. Cancer Res. 2017, 77, 3564–3576. [Google Scholar] [CrossRef]
- Mizuno, S.; Ikegami, M.; Koyama, T.; Sunami, K.; Ogata, D.; Kage, H.; Yanagaki, M.; Ikeuchi, H.; Ueno, T.; Tanikawa, M.; et al. High-throughput functional evaluation of MAP2K1 variants. Mol. Cancer Ther. 2023, 22, 227–239. [Google Scholar] [CrossRef]
- Prota, A.E.; Danel, F.; Bachmann, F.; Bargsten, K.; Buey, R.M.; Pohlmann, J.; Reinelt, S.; Lane, H.; Steinmetz, M.O. Tubulin–colchicine complex (PDB ID: 4O2B). Protein Data Bank 2014. [Google Scholar] [CrossRef]
- Kanakkanthara, A.; Miller, J.H. βIII-Tubulin Overexpression in cancer: Causes, consequences, and potential therapies. Biochim. Biophys. Acta - Rev. Cancer 2021, 1876, 188607. [Google Scholar] [CrossRef]
- Stengel, C.; Newman, S.P.; Leese, M.P.; Potter, B.V.L.; Reed, M.J.; Purohit, A. Class III β-Tubulin expression and in vitro resistance to microtubule-targeting agents. Br. J. Cancer 2010, 102, 316–324. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Lagunin, A.A.; Dubovskaja, V.I.; Rudik, A.V.; Pogodin, P.V.; Druzhilovskiy, D.S.; Gloriozova, T.A.; Filimonov, D.A.; Sastry, N.G.; Poroikov, V.V. CLC-Pred: A freely available web-service for in silico prediction of human cell line cytotoxicity for drug-like compounds. PLoS ONE 2018, 13, e0191838. [Google Scholar] [CrossRef]
- Lagunin, A.A.; Rudik, A.V.; Pogodin, P.V.; Savosina, P.I.; Tarasova, O.A.; Dmitriev, A.V.; Ivanov, S.M.; Biziukova, N.Y.; Druzhilovskiy, D.S.; Filimonov, D.A.; et al. CLC-Pred 2.0: A freely available web application for in silico prediction of human cell line cytotoxicity and molecular mechanisms of action for druglike compounds. Int. J. Mol. Sci. 2023, 24, 1689. [Google Scholar] [CrossRef]
- Rigaku Oxford Diffraction. CrysAlis Pro Software System; Rigaku Corporation: Oxford, UK, 2015. [Google Scholar]
- Sheldrick, G.M. SHELXT - Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Crystallogr. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef]
- Morris, G.M.; Goodsell, D.S.; Halliday, R.S.; Huey, R.; Hart, W.E.; Belew, R.K.; Olson, A.J. Automated docking using a Lamarckian genetic algorithm and an empirical binding free energy function. J. Comput. Chem. 1998, 19, 1639–1662. [Google Scholar] [CrossRef]
- Dennington, R.; Keith, T.A.; Millam, J.M. GaussView, Version 5.0.9; Semichem Inc.: Shawnee Mission, KS, 2016. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision B.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- The PyMOL. Molecular Graphics System, Version 3.0; Schrödinger, LLC: New York, NY, USA, 2023. [Google Scholar]
- Schrödinger Release 2025-2. Maestro, Schrödinger, LLC: New York, NY, USA, 2025.



| Cell Type | Compound → Cell Line ↓ | GI (%) (10–5 M) a | ||||||
|---|---|---|---|---|---|---|---|---|
| 1c | 5a | 5c | 6a | 6c | 7d | 7g | ||
| Leukemia | HL-60(TB) | 26 | 32 | 38 | 62 | 18 | 49 | 36 |
| K-562 | 61 | 22 | 25 | 24 | 40 | 0 | 0 | |
| MOLT-4 | 0 | 21 | 1 | 53 | 9 | 54 | 36 | |
| RPMI-8226 | 38 | 27 | 42 | 30 | 25 | 11 | 0 | |
| EKVX | 9 | 11 | 50 | 9 | 27 | 6 | 19 | |
| Non-small Cell Lung Cancer | HOP-62 | 54 | 55 | 100 b (34) | 78 | 97 | 21 | 100 b (15) |
| HOP-92 | 0 | 20 | 78 | 0 | 71 | 6 | 35 | |
| NCI-H226 | 31 | 16 | 100 b (14) | 19 | 54 | 8 | 47 | |
| NCI-H23 | 10 | 16 | 36 | 17 | 31 | 9 | 50 | |
| NCI-H522 | 6 | 21 | 60 | 19 | 52 | 54 | 44 | |
| Colon Cancer | HCT-116 | 11 | 21 | 29 | 23 | 21 | 4 | 41 |
| HCT-15 | 4 | 20 | 19 | 20 | 20 | 27 | 42 | |
| HT29 | 7 | 41 | 31 | 43 | 28 | 0 | 13 | |
| CNS Cancer | SF-268 | 13 | 19 | 59 | 9 | 45 | 0 | 25 |
| SNB-19 | 11 | 13 | 51 | 3 | 31 | 10 | 35 | |
| SNB-75 | 0 | 1 | 100 b (16) | 46 | 100 b (15) | 20 | 64 | |
| Melanoma | SK-MEL-2 | 18 | 0 | 100 b (7) | 11 | 80 | 6 | 15 |
| SK-MEL-28 | 17 | 9 | 45 | 4 | 26 | 0 | 32 | |
| SK-MEL-5 | 10 | 24 | 50 | 31 | 38 | 59 | 68 | |
| UACC-257 | 14 | 0 | 70 | 0 | 26 | 6 | 3 | |
| UACC-62 | 13 | 6 | 50 | 0 | 48 | 0 | 11 | |
| Ovarian Cancer | IGROV1 | 0 | 2 | 58 | 9 | 18 | 0 | 16 |
| OVCAR-3 | 15 | 0 | 47 | 6 | 36 | 33 | 54 | |
| OVCAR-4 | 9 | 6 | 67 | 8 | 60 | 1 | 20 | |
| OVCAR-8 | 8 | 21 | 65 | 7 | 54 | 6 | 16 | |
| SK-OV-3 | 0 | 0 | 52 | 15 | 30 | 10 | 0 | |
| Renal cancer | A498 | 7 | 2 | 41 | 0 | 33 | 2 | 16 |
| RXF 393 | 3 | 29 | 100 b (4) | 45 | 57 | 9 | 8 | |
| TK-10 | 2 | 0 | 52 | 14 | 47 | 10 | 10 | |
| Prostate cancer | PC-3 | 14 | 17 | 44 | 1 | 32 | 0 | 8 |
| Breast cancer | MCF7 | 14 | 5 | 46 | 18 | 35 | 13 | 62 |
| HS 578T | 24 | 10 | 68 | 13 | 68 | 10 | 11 | |
| Ligand | Lowest Binding Energy (kcal/mol) | Binding Conformation | 2D Interaction Diagram |
|---|---|---|---|
| Colchicine | −10.08 | 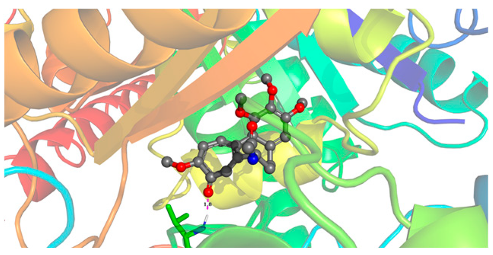 | 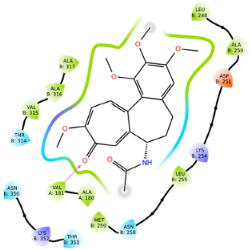 |
| Phenstatin | −8.04 | 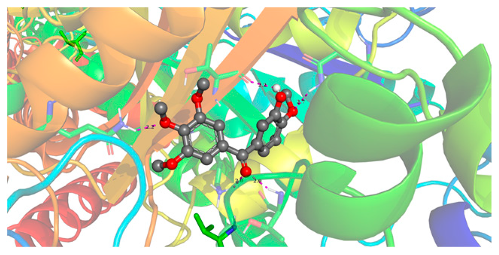 | 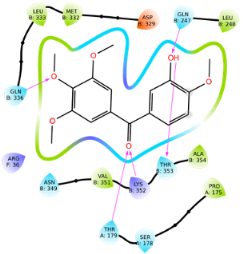 |
| 5c | −9.88 | 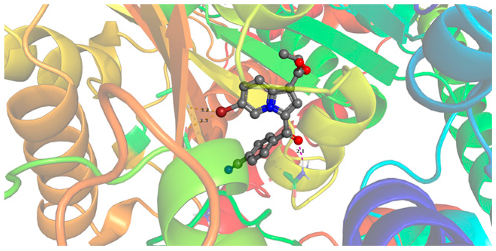 | 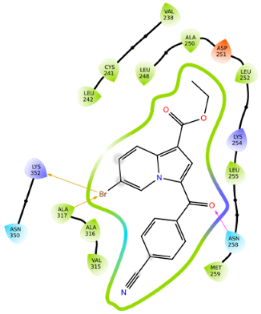 |
| 6c | −9.48 | 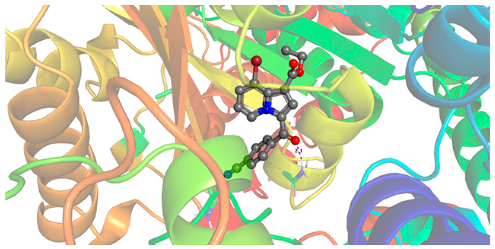 | 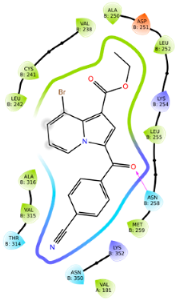 |
| 7g | −9.22 |  | 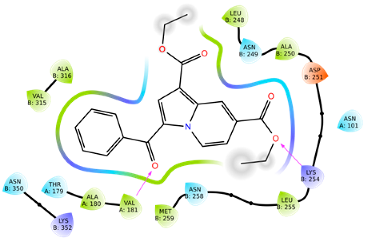 |
| Compound → ADME Parameter ↓ | 5c | 6c | 7g |
|---|---|---|---|
| Physicochemical Properties | |||
| Molecular weight | 397.22 g/mol | 397.22 g/mol | 365.38 g/mol |
| Log Po/w (MLOGP) | 2.26 | 2.26 | 2.37 |
| Number of H-bond acceptors | 4 | 4 | 5 |
| Number of H-bond donors | 0 | 0 | 0 |
| Number of rotatable bonds | 5 | 5 | 8 |
| TPSA | 71.57 Å2 | 71.57 Å2 | 74.08 Å2 |
| Pharmacokinetics | |||
| Gastrointestinal (GI) absorption | high | high | high |
| Blood–brain barrier (BBB) permeant | yes | yes | yes |
| P-gp substrate | no | no | no |
| Drug likeness | |||
| Log S (ESOL) | −5.44 | −5.44 | −4.89 |
| Water solubility class | moderately soluble | moderately soluble | moderately soluble |
| Lipinski rule | no violation | no violation | no violation |
| Veber rule | no violation | no violation | no violation |
| Bioavailability | 0.55 | 0.55 | 0.55 |
| Medicinal Chemistry | |||
| PAINS alerts | 0 | 0 | 0 |
| Brenk alerts | 0 | 0 | 1: more_than_2_ esters |
| Synthetic accessibility | 2.67 | 2.74 | 2.82 |
| Compound | Pa | Pi | Cell-Line | Tissue/Organ | Type | IAP * |
|---|---|---|---|---|---|---|
| 5c | 0.578 | 0.002 | SNU-398 | Liver | Carcinoma | 0.970 |
| 0.530 | 0.016 | SNB-75 | CNS | Glioblastoma | 0.877 | |
| 6c | 0.545 | 0.003 | SNU-398 | Liver | Carcinoma | 0.970 |
| 0.510 | 0.013 | SNB-75 | CNS | Glioblastoma | 0.877 | |
| 7g | 0.578 | 0.002 | SNU-398 | Liver | Carcinoma | 0.970 |
| 0.574 | 0.012 | SNB-75 | CNS | Glioblastoma | 0.877 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciorteanu, R.; Ciobanu, C.I.; Cibotariu, N.; Shova, S.; Antoci, V.; Mangalagiu, I.I.; Danac, R. Functionalized Indolizines as Potential Anticancer Agents: Synthetic, Biological and In Silico Investigations. Int. J. Mol. Sci. 2025, 26, 8368. https://doi.org/10.3390/ijms26178368
Ciorteanu R, Ciobanu CI, Cibotariu N, Shova S, Antoci V, Mangalagiu II, Danac R. Functionalized Indolizines as Potential Anticancer Agents: Synthetic, Biological and In Silico Investigations. International Journal of Molecular Sciences. 2025; 26(17):8368. https://doi.org/10.3390/ijms26178368
Chicago/Turabian StyleCiorteanu, Roxana, Catalina Ionica Ciobanu, Narcis Cibotariu, Sergiu Shova, Vasilichia Antoci, Ionel I. Mangalagiu, and Ramona Danac. 2025. "Functionalized Indolizines as Potential Anticancer Agents: Synthetic, Biological and In Silico Investigations" International Journal of Molecular Sciences 26, no. 17: 8368. https://doi.org/10.3390/ijms26178368
APA StyleCiorteanu, R., Ciobanu, C. I., Cibotariu, N., Shova, S., Antoci, V., Mangalagiu, I. I., & Danac, R. (2025). Functionalized Indolizines as Potential Anticancer Agents: Synthetic, Biological and In Silico Investigations. International Journal of Molecular Sciences, 26(17), 8368. https://doi.org/10.3390/ijms26178368








