EphB2-Targeting Monoclonal Antibodies Exerted Antitumor Activities in Triple-Negative Breast Cancer and Lung Mesothelioma Xenograft Models
Abstract
1. Introduction
2. Results
2.1. Production of Mouse IgG2a-Type and Human IgG1-Type Anti-EphB2 mAbs from Eb2Mab-12
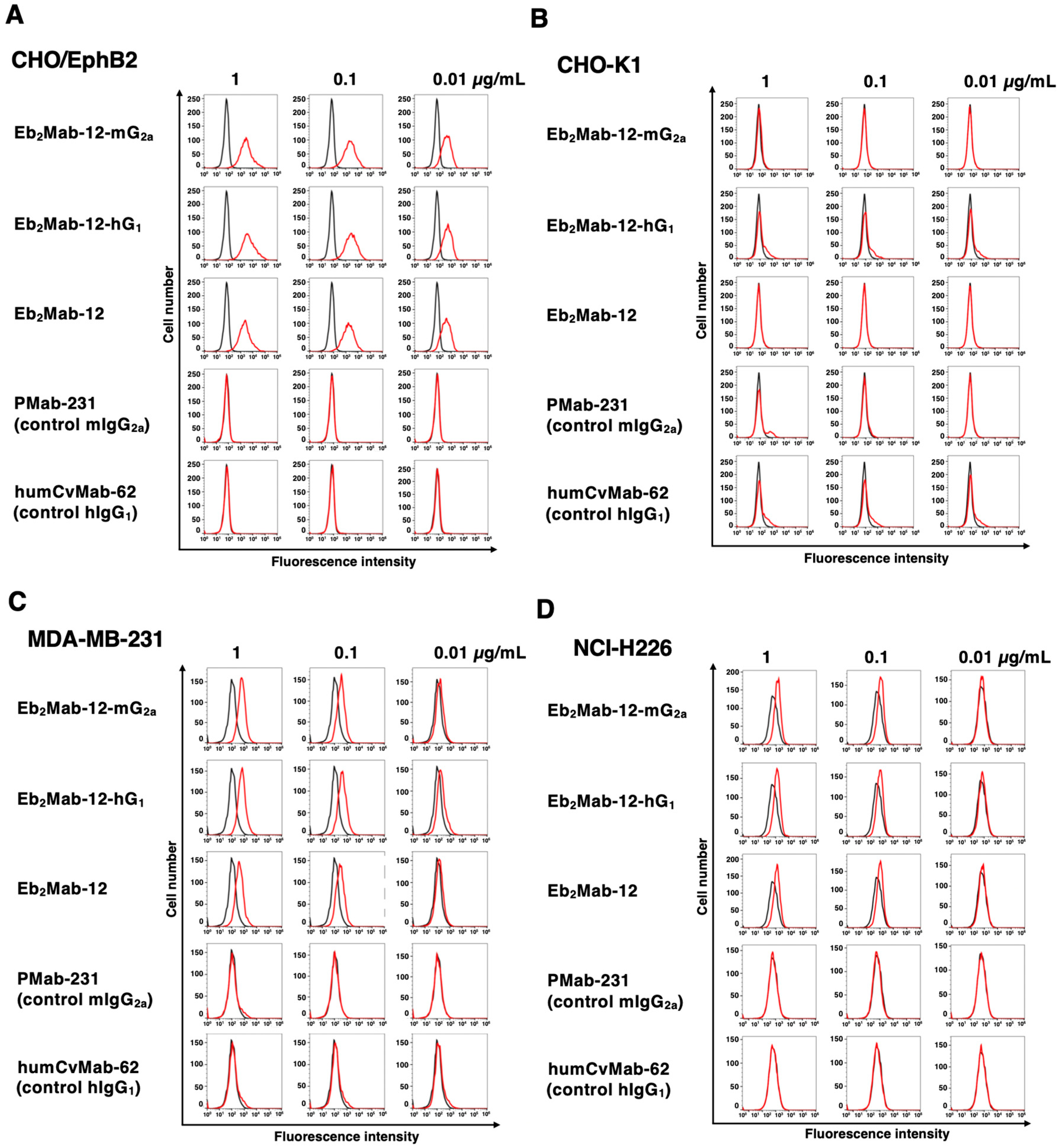
2.2. Flow Cytometry Using Eb2Mab-12-mG2a and Eb2Mab-12-hG1
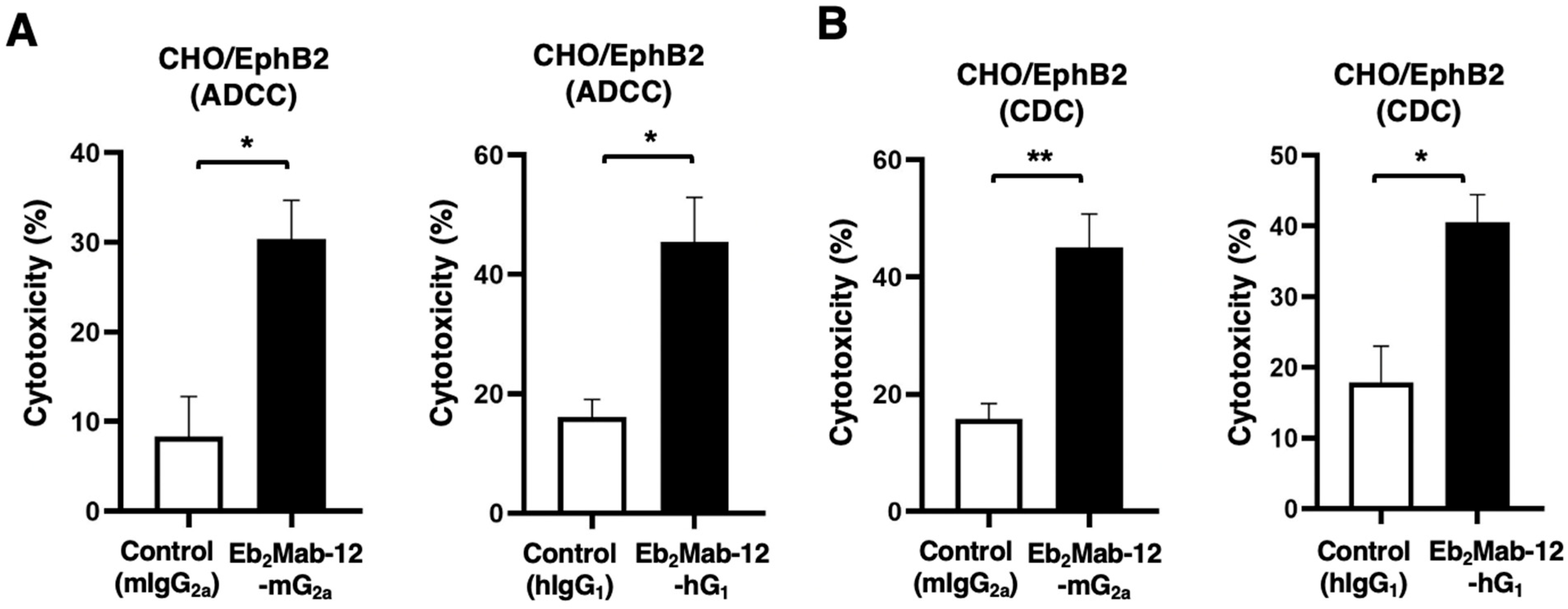
2.3. ADCC and CDC by Eb2Mab-12-mG2a and Eb2Mab-12-hG1 Against CHO/EphB2
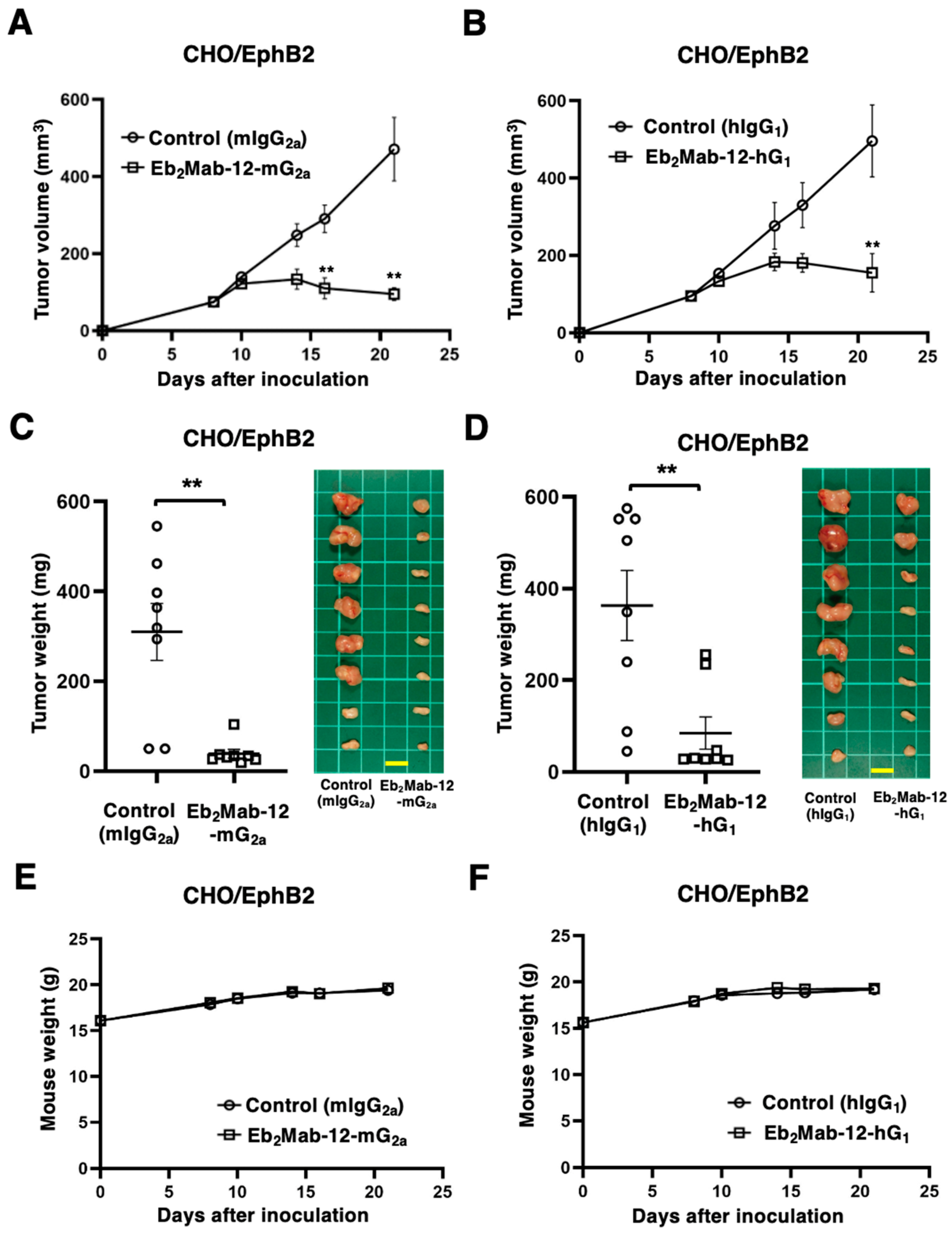
2.4. Antitumor Effect by Eb2Mab-12-mG2a and Eb2Mab-12-hG1 Against CHO/EphB2 Xenografts
2.5. ADCC and CDC by Eb2Mab-12-mG2a and Eb2Mab-12-hG1 Against Endogenous EphB2-Positive Cancer Cell Lines
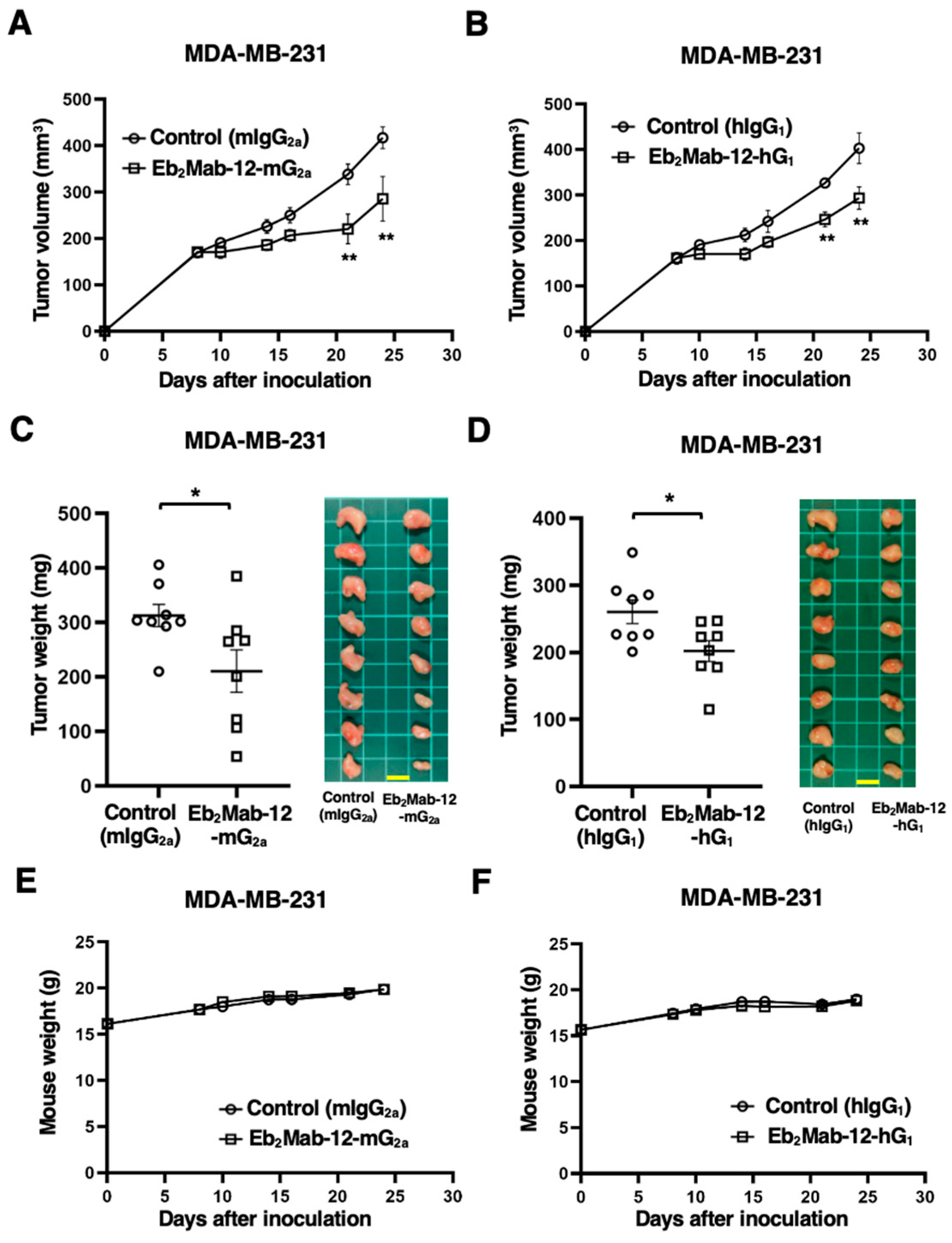
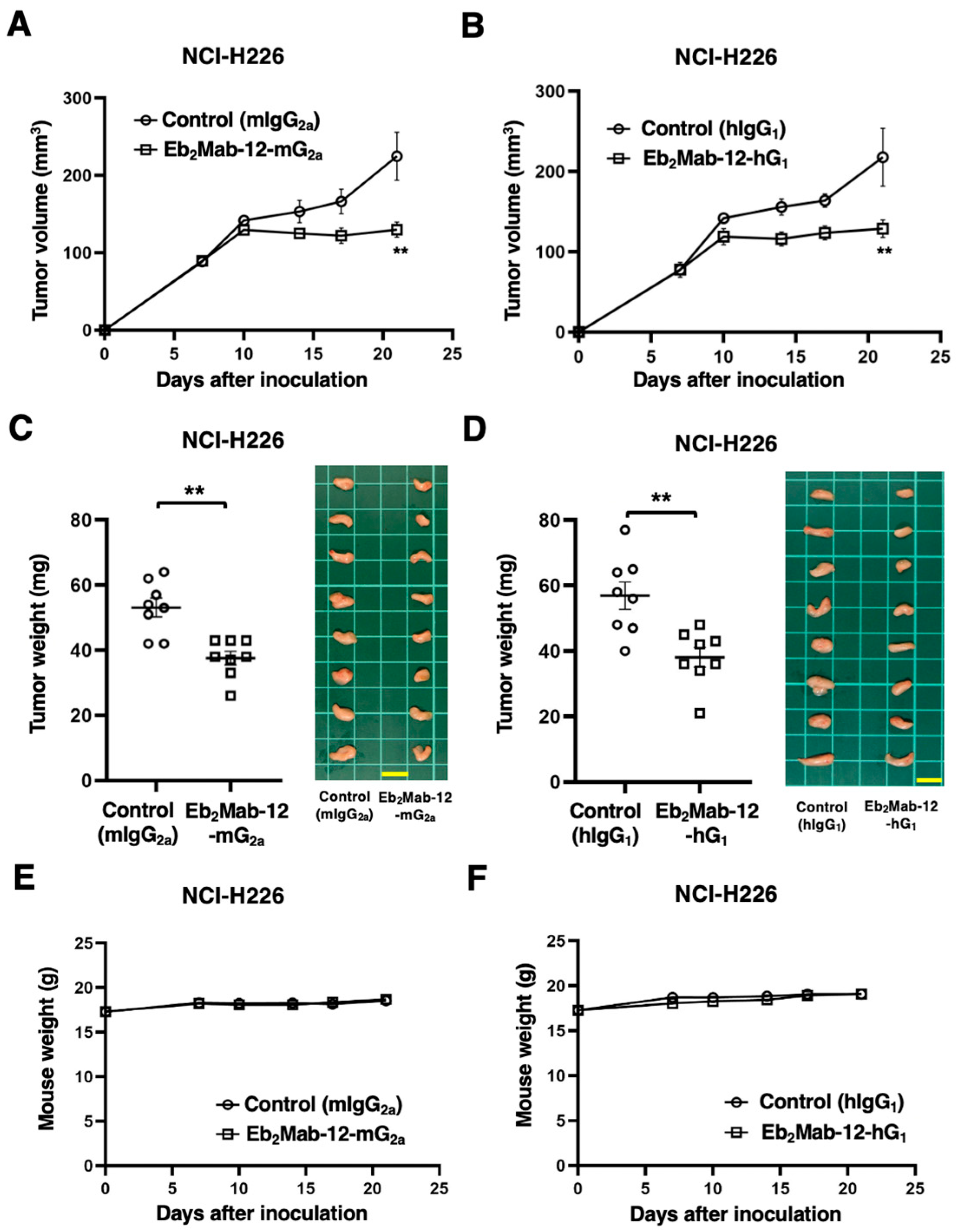
2.6. Antitumor Effect by Eb2Mab-12-mG2a and Eb2Mab-12-hG1 Against Endogenous EphB2-Positive Cancer Xenografts
3. Discussion
4. Materials and Methods
4.1. Cell Lines
4.2. Recombinant mAb Production
4.3. Flow Cytometry
4.4. ADCC by Eb2Mab-12-mG2a and Eb2Mab-12-hG1
4.5. CDC by Eb2Mab-12-mG2a and Eb2Mab-12-hG1
4.6. Antitumor Activity of Eb2Mab-12-mG2a and Eb2Mab-12-hG1
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Zhu, Y.; Su, S.A.; Shen, J.; Ma, H.; Le, J.; Xie, Y.; Xiang, M. Recent advances of the Ephrin and Eph family in cardiovascular development and pathologies. iScience 2024, 27, 110556. [Google Scholar] [CrossRef]
- Pasquale, E.B. Eph receptor signaling complexes in the plasma membrane. Trends Biochem. Sci. 2024, 49, 1079–1096. [Google Scholar] [CrossRef]
- Pasquale, E.B. Eph receptors and ephrins in cancer progression. Nat. Rev. Cancer 2024, 24, 5–27. [Google Scholar] [CrossRef]
- Anderton, M.; van der Meulen, E.; Blumenthal, M.J.; Schäfer, G. The Role of the Eph Receptor Family in Tumorigenesis. Cancers 2021, 13, 206. [Google Scholar] [CrossRef]
- Liang, L.Y.; Patel, O.; Janes, P.W.; Murphy, J.M.; Lucet, I.S. Eph receptor signalling: From catalytic to non-catalytic functions. Oncogene 2019, 38, 6567–6584. [Google Scholar] [CrossRef]
- Pasquale, E.B. Eph receptors and ephrins in cancer: Bidirectional signalling and beyond. Nat. Rev. Cancer 2010, 10, 165–180. [Google Scholar] [CrossRef] [PubMed]
- Pasquale, E.B. Eph receptor signalling casts a wide net on cell behaviour. Nat. Rev. Mol. Cell Biol. 2005, 6, 462–475. [Google Scholar] [CrossRef] [PubMed]
- Arora, S.; Scott, A.M.; Janes, P.W. Eph Receptors in Cancer. Biomedicines 2023, 11, 315. [Google Scholar] [CrossRef] [PubMed]
- Lau, A.; Le, N.; Nguyen, C.; Kandpal, R.P. Signals transduced by Eph receptors and ephrin ligands converge on MAP kinase and AKT pathways in human cancers. Cell Signal 2023, 104, 110579. [Google Scholar] [CrossRef]
- Liu, W.; Yu, C.; Li, J.; Fang, J. The Roles of EphB2 in Cancer. Front. Cell Dev. Biol. 2022, 10, 788587. [Google Scholar] [CrossRef]
- Lam, S.; Wiercinska, E.; Teunisse, A.F.; Lodder, K.; ten Dijke, P.; Jochemsen, A.G. Wild-type p53 inhibits pro-invasive properties of TGF-β3 in breast cancer, in part through regulation of EPHB2, a new TGF-β target gene. Breast Cancer Res. Treat. 2014, 148, 7–18. [Google Scholar] [CrossRef]
- Zhou, F.; Wang, B.; Wang, H.; Hu, L.; Zhang, J.; Yu, T.; Xu, X.; Tian, W.; Zhao, C.; Zhu, H.; et al. circMELK promotes glioblastoma multiforme cell tumorigenesis through the miR-593/EphB2 axis. Mol. Ther. Nucleic Acids 2021, 25, 25–36. [Google Scholar] [CrossRef]
- Goparaju, C.; Donington, J.S.; Hsu, T.; Harrington, R.; Hirsch, N.; Pass, H.I. Overexpression of EPH receptor B2 in malignant mesothelioma correlates with oncogenic behavior. J. Thorac. Oncol. 2013, 8, 1203–1211. [Google Scholar] [CrossRef]
- Leung, H.W.; Leung, C.O.N.; Lau, E.Y.; Chung, K.P.S.; Mok, E.H.; Lei, M.M.L.; Leung, R.W.H.; Tong, M.; Keng, V.W.; Ma, C.; et al. EPHB2 Activates β-Catenin to Enhance Cancer Stem Cell Properties and Drive Sorafenib Resistance in Hepatocellular Carcinoma. Cancer Res. 2021, 81, 3229–3240. [Google Scholar] [CrossRef]
- Nakada, M.; Niska, J.A.; Miyamori, H.; McDonough, W.S.; Wu, J.; Sato, H.; Berens, M.E. The phosphorylation of EphB2 receptor regulates migration and invasion of human glioma cells. Cancer Res. 2004, 64, 3179–3185. [Google Scholar] [CrossRef] [PubMed]
- Nakada, M.; Niska, J.A.; Tran, N.L.; McDonough, W.S.; Berens, M.E. EphB2/R-Ras signaling regulates glioma cell adhesion, growth, and invasion. Am. J. Pathol. 2005, 167, 565–576. [Google Scholar] [CrossRef] [PubMed]
- Toracchio, L.; Carrabotta, M.; Mancarella, C.; Morrione, A.; Scotlandi, K. EphA2 in Cancer: Molecular Complexity and Therapeutic Opportunities. Int. J. Mol. Sci. 2024, 25, 12191. [Google Scholar] [CrossRef] [PubMed]
- Cha, J.H.; Chan, L.C.; Wang, Y.N.; Chu, Y.Y.; Wang, C.H.; Lee, H.H.; Xia, W.; Shyu, W.C.; Liu, S.P.; Yao, J.; et al. Ephrin receptor A10 monoclonal antibodies and the derived chimeric antigen receptor T cells exert an antitumor response in mouse models of triple-negative breast cancer. J. Biol. Chem. 2022, 298, 101817. [Google Scholar] [CrossRef]
- Xiao, T.; Xiao, Y.; Wang, W.; Tang, Y.Y.; Xiao, Z.; Su, M. Targeting EphA2 in cancer. J. Hematol. Oncol. 2020, 13, 114. [Google Scholar] [CrossRef]
- Tang, F.H.F.; Davis, D.; Arap, W.; Pasqualini, R.; Staquicini, F.I. Eph receptors as cancer targets for antibody-based therapy. Adv. Cancer Res. 2020, 147, 303–317. [Google Scholar]
- London, M.; Gallo, E. Critical role of EphA3 in cancer and current state of EphA3 drug therapeutics. Mol. Biol. Rep. 2020, 47, 5523–5533. [Google Scholar] [CrossRef] [PubMed]
- Buckens, O.J.; El Hassouni, B.; Giovannetti, E.; Peters, G.J. The role of Eph receptors in cancer and how to target them: Novel approaches in cancer treatment. Expert. Opin. Investig. Drugs 2020, 29, 567–582. [Google Scholar] [CrossRef]
- Saha, N.; Robev, D.; Mason, E.O.; Himanen, J.P.; Nikolov, D.B. Therapeutic potential of targeting the Eph/ephrin signaling complex. Int. J. Biochem. Cell Biol. 2018, 105, 123–133. [Google Scholar] [CrossRef]
- Taki, S.; Kamada, H.; Inoue, M.; Nagano, K.; Mukai, Y.; Higashisaka, K.; Yoshioka, Y.; Tsutsumi, Y.; Tsunoda, S. A Novel Bispecific Antibody against Human CD3 and Ephrin Receptor A10 for Breast Cancer Therapy. PLoS ONE 2015, 10, e0144712. [Google Scholar] [CrossRef]
- Mao, W.; Luis, E.; Ross, S.; Silva, J.; Tan, C.; Crowley, C.; Chui, C.; Franz, G.; Senter, P.; Koeppen, H.; et al. EphB2 as a therapeutic antibody drug target for the treatment of colorectal cancer. Cancer Res. 2004, 64, 781–788. [Google Scholar] [CrossRef][Green Version]
- Janes, P.W.; Vail, M.E.; Gan, H.K.; Scott, A.M. Antibody Targeting of Eph Receptors in Cancer. Pharmaceuticals 2020, 13, 88. [Google Scholar] [CrossRef]
- Lehmann, B.D.; Bauer, J.A.; Chen, X.; Sanders, M.E.; Chakravarthy, A.B.; Shyr, Y.; Pietenpol, J.A. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J. Clin. Investig. 2011, 121, 2750–2767. [Google Scholar] [CrossRef]
- Yin, L.; Duan, J.J.; Bian, X.W.; Yu, S.C. Triple-negative breast cancer molecular subtyping and treatment progress. Breast Cancer Res. 2020, 22, 61. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, B.D.; Colaprico, A.; Silva, T.C.; Chen, J.; An, H.; Ban, Y.; Huang, H.; Wang, L.; James, J.L.; Balko, J.M.; et al. Multi-omics analysis identifies therapeutic vulnerabilities in triple-negative breast cancer subtypes. Nat. Commun. 2021, 12, 6276. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, B.D.; Jovanović, B.; Chen, X.; Estrada, M.V.; Johnson, K.N.; Shyr, Y.; Moses, H.L.; Sanders, M.E.; Pietenpol, J.A. Refinement of Triple-Negative Breast Cancer Molecular Subtypes: Implications for Neoadjuvant Chemotherapy Selection. PLoS ONE 2016, 11, e0157368. [Google Scholar] [CrossRef]
- Hubalek, M.; Czech, T.; Müller, H. Biological Subtypes of Triple-Negative Breast Cancer. Breast Care 2017, 12, 8–14. [Google Scholar] [CrossRef]
- Limsakul, P.; Choochuen, P.; Jungrungrueang, T.; Charupanit, K. Prognostic Markers in Tyrosine Kinases Specific to Basal-like 2 Subtype of Triple-Negative Breast Cancer. Int. J. Mol. Sci. 2024, 25, 1405. [Google Scholar] [CrossRef] [PubMed]
- Horbinski, C.; Berger, T.; Packer, R.J.; Wen, P.Y. Clinical implications of the 2021 edition of the WHO classification of central nervous system tumours. Nat. Rev. Neurol. 2022, 18, 515–529. [Google Scholar] [CrossRef]
- Johnson, R.A.; Wright, K.D.; Poppleton, H.; Mohankumar, K.M.; Finkelstein, D.; Pounds, S.B.; Rand, V.; Leary, S.E.; White, E.; Eden, C.; et al. Cross-species genomics matches driver mutations and cell compartments to model ependymoma. Nature 2010, 466, 632–636. [Google Scholar] [CrossRef] [PubMed]
- Pajtler, K.W.; Witt, H.; Sill, M.; Jones, D.T.; Hovestadt, V.; Kratochwil, F.; Wani, K.; Tatevossian, R.; Punchihewa, C.; Johann, P.; et al. Molecular Classification of Ependymal Tumors across All CNS Compartments, Histopathological Grades, and Age Groups. Cancer Cell 2015, 27, 728–743. [Google Scholar] [CrossRef]
- Arabzade, A.; Zhao, Y.; Varadharajan, S.; Chen, H.C.; Jessa, S.; Rivas, B.; Stuckert, A.J.; Solis, M.; Kardian, A.; Tlais, D.; et al. ZFTA-RELA Dictates Oncogenic Transcriptional Programs to Drive Aggressive Supratentorial Ependymoma. Cancer Discov. 2021, 11, 2200–2215. [Google Scholar] [CrossRef]
- Satofuka, H.; Suzuki, H.; Tanaka, T.; Li, G.; Kaneko, M.K.; Kato, Y. Development of an anti-human EphA2 monoclonal antibody Ea2Mab-7 for multiple applications. Biochem. Biophys. Rep. 2025, 42, 101998. [Google Scholar] [CrossRef]
- Satofuka, H.; Suzuki, H.; Hirose, M.; Shinoda, K.; Nakamura, T.; Tanaka, T.; Kaneko, M.K.; Kato, Y. Development of a specific anti-human EphA3 monoclonal antibody, Ea3Mab-20, for flow cytometry. Biochem. Biophys. Rep. 2025, 43, 102130. [Google Scholar] [CrossRef]
- Ubukata, R.; Suzuki, H.; Hirose, M.; Satofuka, H.; Tanaka, T.; Kaneko, M.K.; Kato, Y. Establishment of a highly sensitive and specific anti-EphB2 monoclonal antibody (Eb2Mab-12) for flow cytometry. Microbes Immun. 2025. [Google Scholar] [CrossRef]
- Nanamiya, R.; Suzuki, H.; Kaneko, M.K.; Kato, Y. Development of an Anti-EphB4 Monoclonal Antibody for Multiple Applications Against Breast Cancers. Monoclon. Antib. Immunodiagn. Immunother. 2023, 42, 166–177. [Google Scholar] [CrossRef]
- Tanaka, T.; Kaneko, Y.; Yamamoto, H.; Li, G.; Fujisawa, S.; Satofuka, H.; Shinoda, K.; Nakamura, T.; Kaneko, M.K.; Suzuki, H.; et al. Development of a novel anti-erythropoietin-producing hepatocellular receptor B6 monoclonal antibody Eb6Mab-3 for flow cytometry. Biochem. Biophys. Rep. 2025, 41, 101960. [Google Scholar] [CrossRef]
- Kaneko, M.K.; Suzuki, H.; Ohishi, T.; Nakamura, T.; Tanaka, T.; Kato, Y. A Cancer-Specific Monoclonal Antibody against HER2 Exerts Antitumor Activities in Human Breast Cancer Xenograft Models. Int. J. Mol. Sci. 2024, 25, 1941. [Google Scholar] [CrossRef]
- Kaneko, M.K.; Suzuki, H.; Ohishi, T.; Nakamura, T.; Yanaka, M.; Tanaka, T.; Kato, Y. Antitumor Activities of a Humanized Cancer-Specific Anti-HER2 Monoclonal Antibody, humH(2)Mab-250 in Human Breast Cancer Xenografts. Int. J. Mol. Sci. 2025, 26, 1079. [Google Scholar] [CrossRef]
- Overdijk, M.B.; Verploegen, S.; Ortiz Buijsse, A.; Vink, T.; Leusen, J.H.; Bleeker, W.K.; Parren, P.W. Crosstalk between human IgG isotypes and murine effector cells. J. Immunol. 2012, 189, 3430–3438. [Google Scholar] [CrossRef]
- Pegram, M.; Hsu, S.; Lewis, G.; Pietras, R.; Beryt, M.; Sliwkowski, M.; Coombs, D.; Baly, D.; Kabbinavar, F.; Slamon, D. Inhibitory effects of combinations of HER-2/neu antibody and chemotherapeutic agents used for treatment of human breast cancers. Oncogene 1999, 18, 2241–2251. [Google Scholar] [CrossRef]
- Pietras, R.J.; Pegram, M.D.; Finn, R.S.; Maneval, D.A.; Slamon, D.J. Remission of human breast cancer xenografts on therapy with humanized monoclonal antibody to HER-2 receptor and DNA-reactive drugs. Oncogene 1998, 17, 2235–2249. [Google Scholar] [CrossRef] [PubMed]
- Baselga, J.; Norton, L.; Albanell, J.; Kim, Y.M.; Mendelsohn, J. Recombinant humanized anti-HER2 antibody (Herceptin) enhances the antitumor activity of paclitaxel and doxorubicin against HER2/neu overexpressing human breast cancer xenografts. Cancer Res. 1998, 58, 2825–2831. [Google Scholar]
- Montero, J.C.; Seoane, S.; Ocaña, A.; Pandiella, A. Inhibition of SRC family kinases and receptor tyrosine kinases by dasatinib: Possible combinations in solid tumors. Clin. Cancer Res. 2011, 17, 5546–5552. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.; Fan, X.; Liu, H.; Liang, T. Advances in Trop-2 targeted antibody-drug conjugates for breast cancer: Mechanisms, clinical applications, and future directions. Front. Immunol. 2024, 15, 1495675. [Google Scholar] [CrossRef] [PubMed]
- Izci, H.; Punie, K.; Waumans, L.; Laenen, A.; Wildiers, H.; Verdoodt, F.; Desmedt, C.; Ardui, J.; Smeets, A.; Han, S.N.; et al. Correlation of TROP-2 expression with clinical-pathological characteristics and outcome in triple-negative breast cancer. Sci. Rep. 2022, 12, 22498. [Google Scholar] [CrossRef]
- Tanaka, T.; Suzuki, H.; Ohishi, T.; Kawada, M.; Kaneko, M.K.; Kato, Y. Antitumor Activities by a Humanized Cancer-Specific Anti-Podoplanin Monoclonal Antibody humPMab-117 Against Human Tumors. Cancer Sci. 2025, 116, 2232–2242. [Google Scholar] [CrossRef]
- Reusch, U.; Sundaram, M.; Davol, P.A.; Olson, S.D.; Davis, J.B.; Demel, K.; Nissim, J.; Rathore, R.; Liu, P.Y.; Lum, L.G. Anti-CD3 x anti-epidermal growth factor receptor (EGFR) bispecific antibody redirects T-cell cytolytic activity to EGFR-positive cancers in vitro and in an animal model. Clin. Cancer Res. 2006, 12, 183–190. [Google Scholar] [CrossRef]
- Hynes, N.E.; Lane, H.A. ERBB receptors and cancer: The complexity of targeted inhibitors. Nat. Rev. Cancer 2005, 5, 341–354. [Google Scholar] [CrossRef] [PubMed]
- Merchant, T.E.; Li, C.; Xiong, X.; Kun, L.E.; Boop, F.A.; Sanford, R.A. Conformal radiotherapy after surgery for paediatric ependymoma: A prospective study. Lancet Oncol. 2009, 10, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Amoozgar, Z.; Uccello, T.P.; Lei, P.J.; Zhao, Y.; Ho, W.W.; Huang, P.; Kardian, A.; Mack, S.C.; Duda, D.G.; et al. Targeting EPHB2/ABL1 restores antitumor immunity in preclinical models of ependymoma. Proc. Natl. Acad. Sci. USA 2025, 122, e2319474122. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Suzuki, H.; Ohishi, T.; Asano, T.; Tanaka, T.; Yanaka, M.; Nakamura, T.; Yoshikawa, T.; Kawada, M.; Kaneko, M.K.; et al. Antitumor activities of a defucosylated anti-EpCAM monoclonal antibody in colorectal carcinoma xenograft models. Int. J. Mol. Med. 2023, 51, 18. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ubukata, R.; Ohishi, T.; Kaneko, M.K.; Suzuki, H.; Kato, Y. EphB2-Targeting Monoclonal Antibodies Exerted Antitumor Activities in Triple-Negative Breast Cancer and Lung Mesothelioma Xenograft Models. Int. J. Mol. Sci. 2025, 26, 8302. https://doi.org/10.3390/ijms26178302
Ubukata R, Ohishi T, Kaneko MK, Suzuki H, Kato Y. EphB2-Targeting Monoclonal Antibodies Exerted Antitumor Activities in Triple-Negative Breast Cancer and Lung Mesothelioma Xenograft Models. International Journal of Molecular Sciences. 2025; 26(17):8302. https://doi.org/10.3390/ijms26178302
Chicago/Turabian StyleUbukata, Rena, Tomokazu Ohishi, Mika K. Kaneko, Hiroyuki Suzuki, and Yukinari Kato. 2025. "EphB2-Targeting Monoclonal Antibodies Exerted Antitumor Activities in Triple-Negative Breast Cancer and Lung Mesothelioma Xenograft Models" International Journal of Molecular Sciences 26, no. 17: 8302. https://doi.org/10.3390/ijms26178302
APA StyleUbukata, R., Ohishi, T., Kaneko, M. K., Suzuki, H., & Kato, Y. (2025). EphB2-Targeting Monoclonal Antibodies Exerted Antitumor Activities in Triple-Negative Breast Cancer and Lung Mesothelioma Xenograft Models. International Journal of Molecular Sciences, 26(17), 8302. https://doi.org/10.3390/ijms26178302





