Protective Antioxidant Potential of Argan Oil Versus Other Edible Oils in LPS-Challenged Mouse Heart and Kidney
Abstract
1. Introduction
2. Results
2.1. Composition of Oils
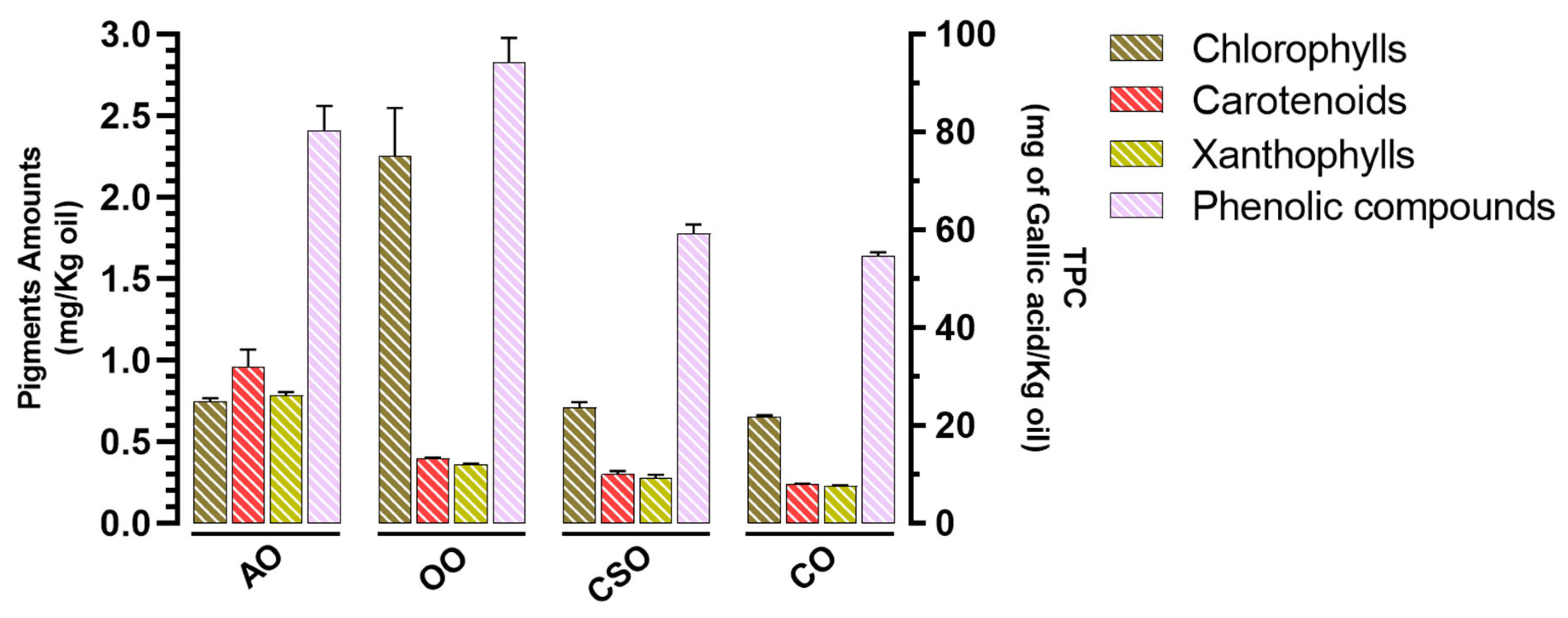
2.2. Pigment Amounts and Total Polyphenol Content
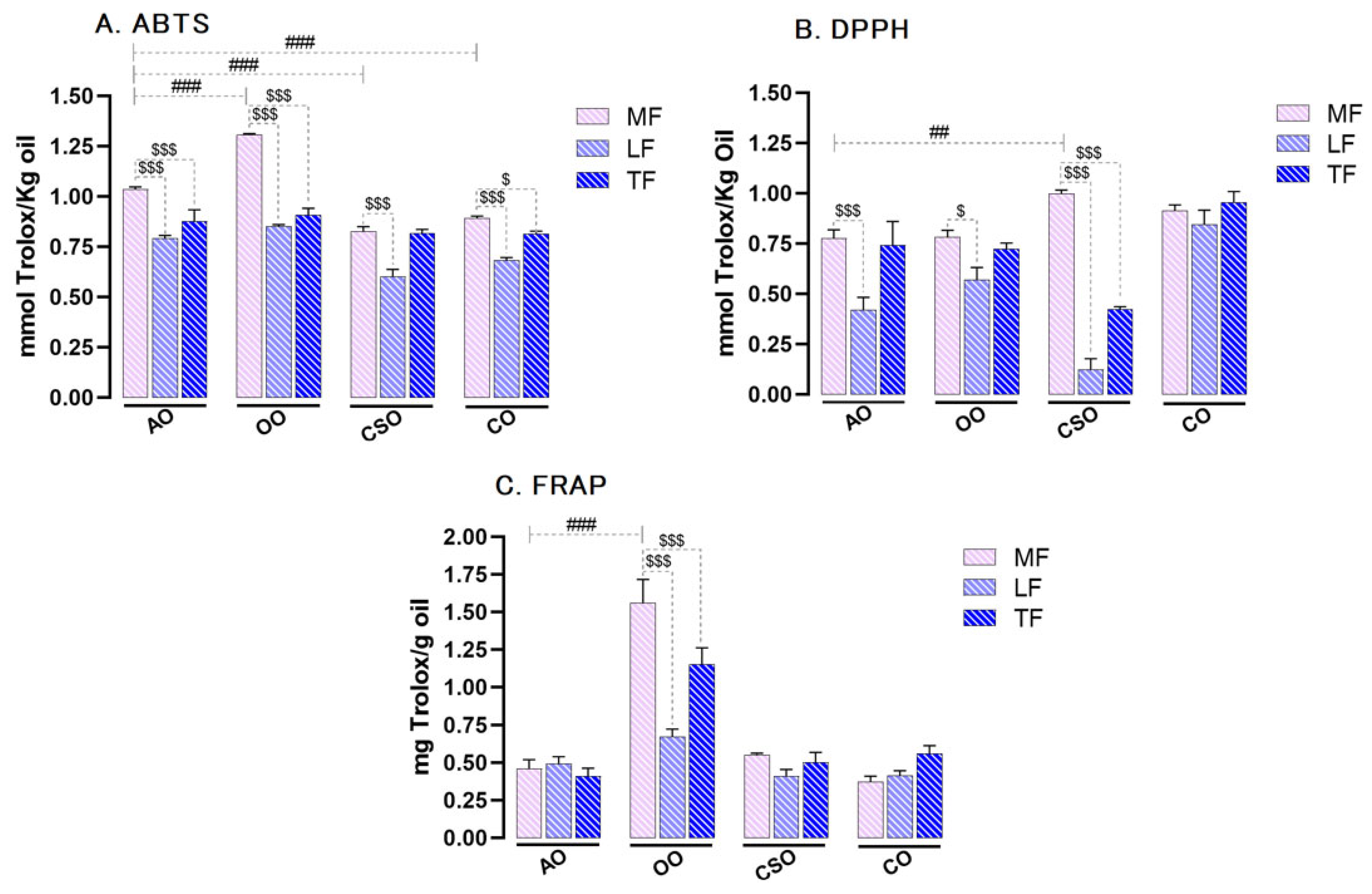
2.3. Antioxidant Activity of Methanolic, Lipophilic, and Total Fractions of Oils in Non-Cellular In Vitro Models
2.4. Effects of Argan Oil on LPS-Induced Heart and Kidney Oxidative Stress: In Vivo Approaches
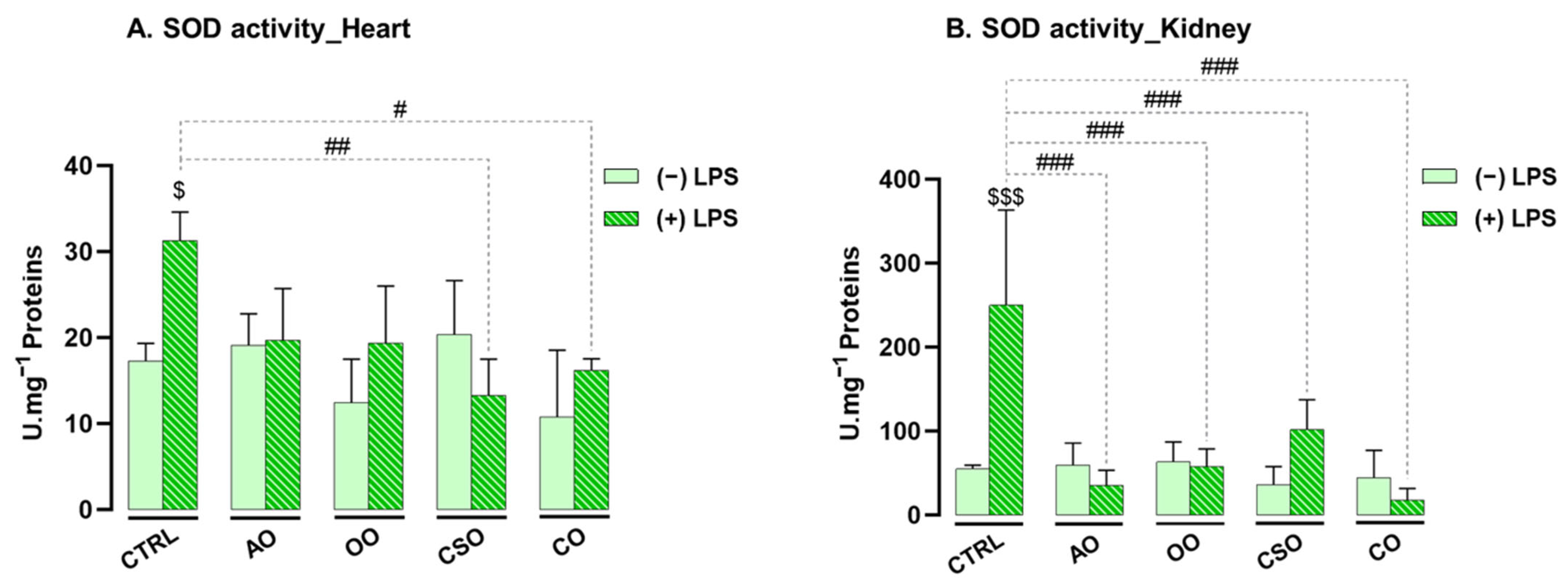
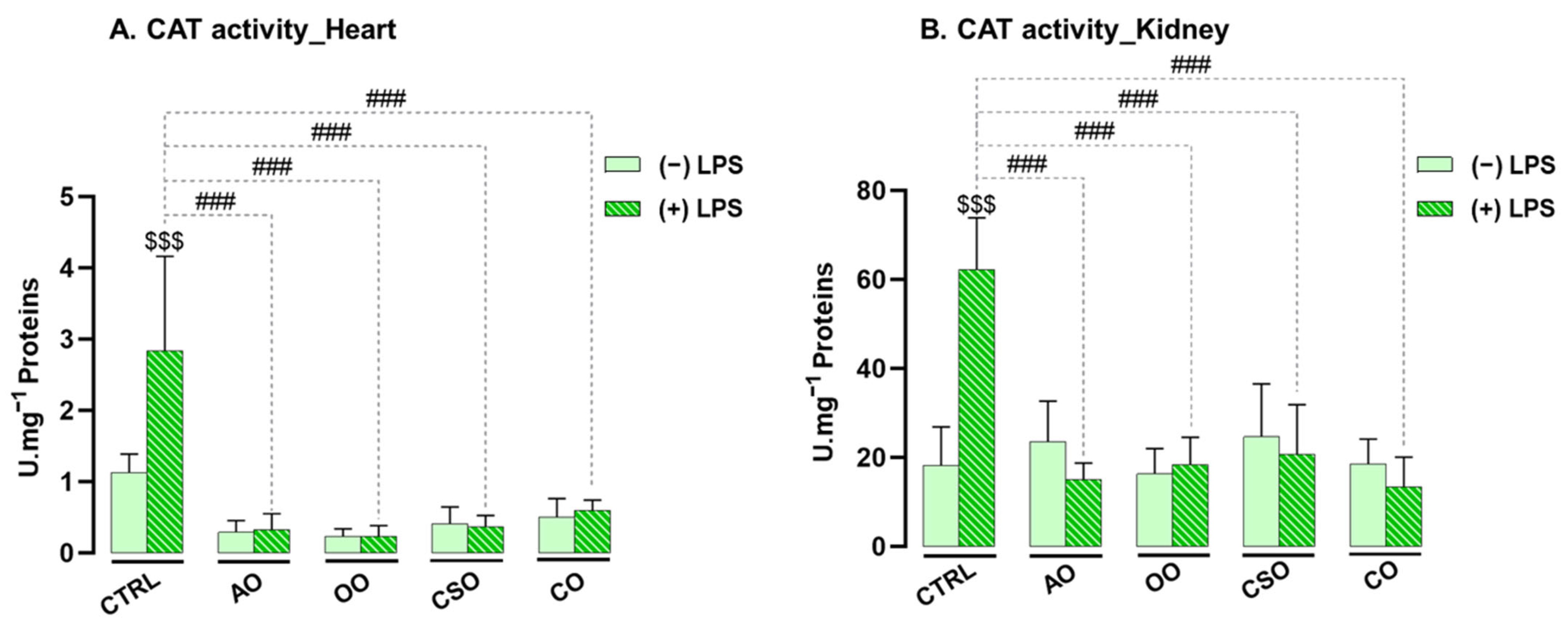
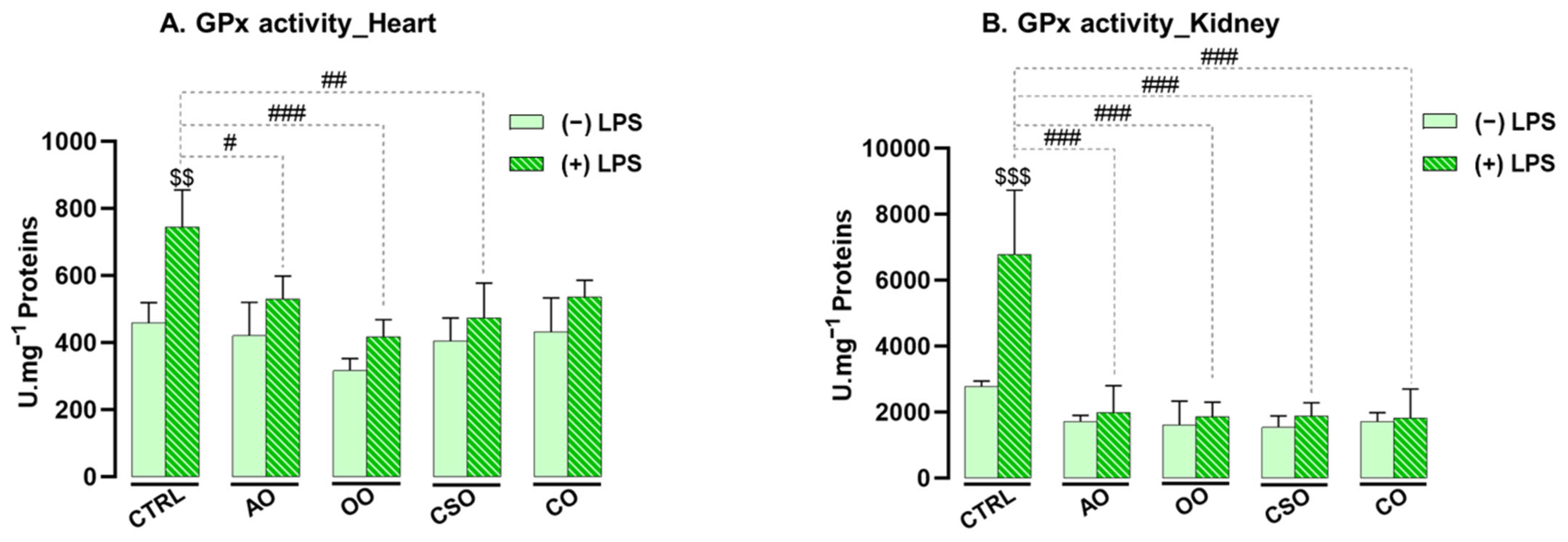
2.4.1. Effect of Argan Oil on the Regulation of Antioxidant Enzyme Activities in the Heart and Kidney
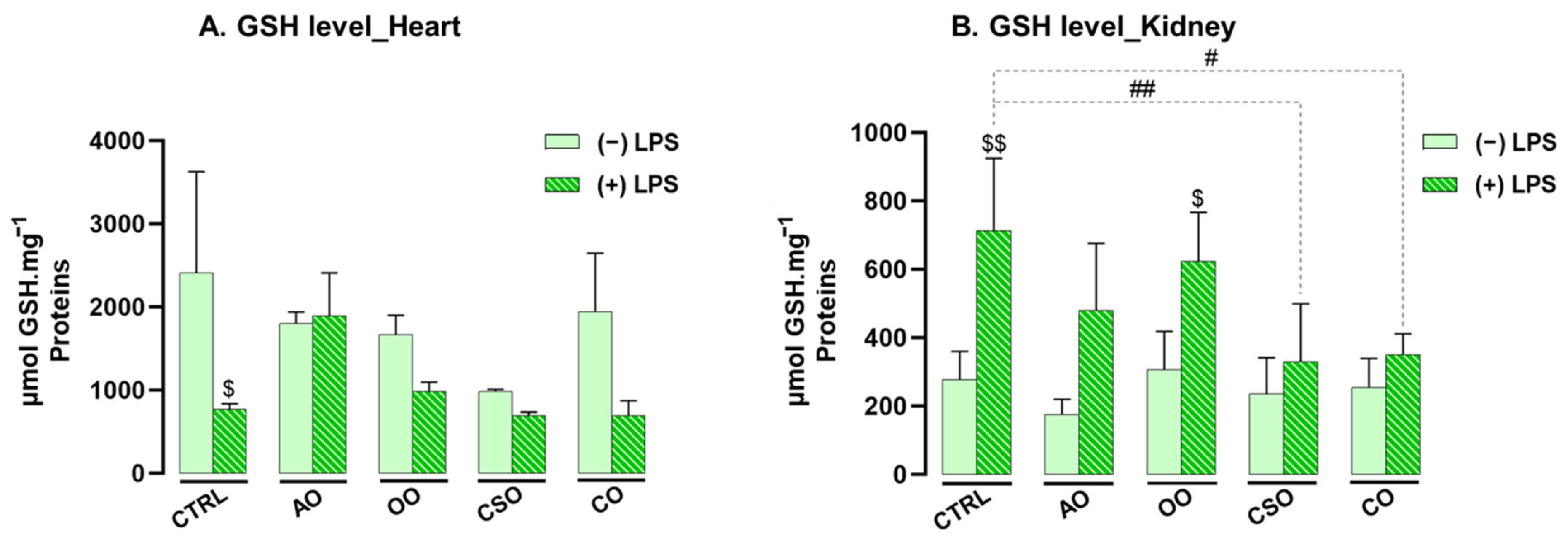
2.4.2. Effect of Argan Oil on the Regulation of Oxidative Stress Biomarkers in the Heart and Kidney
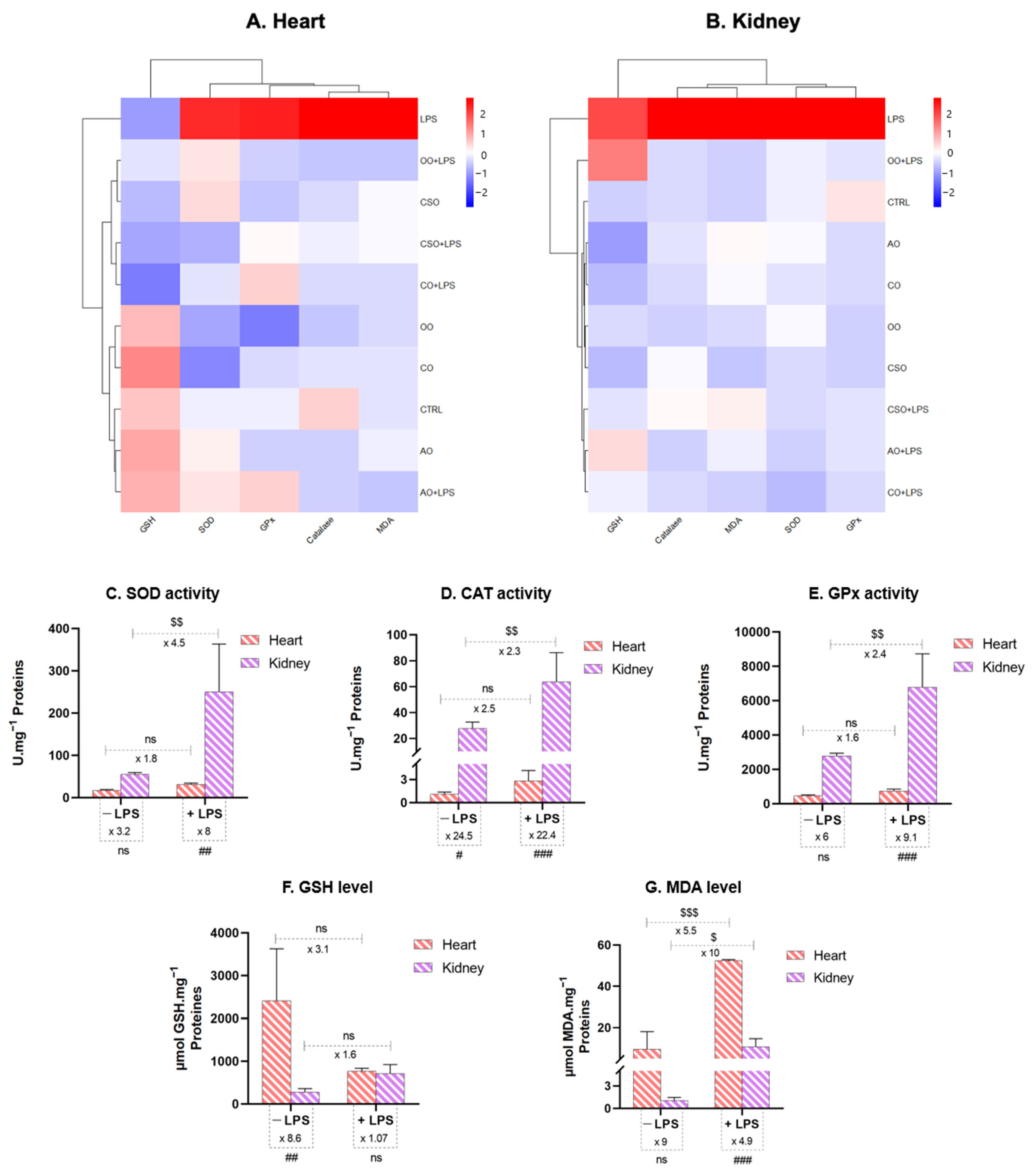
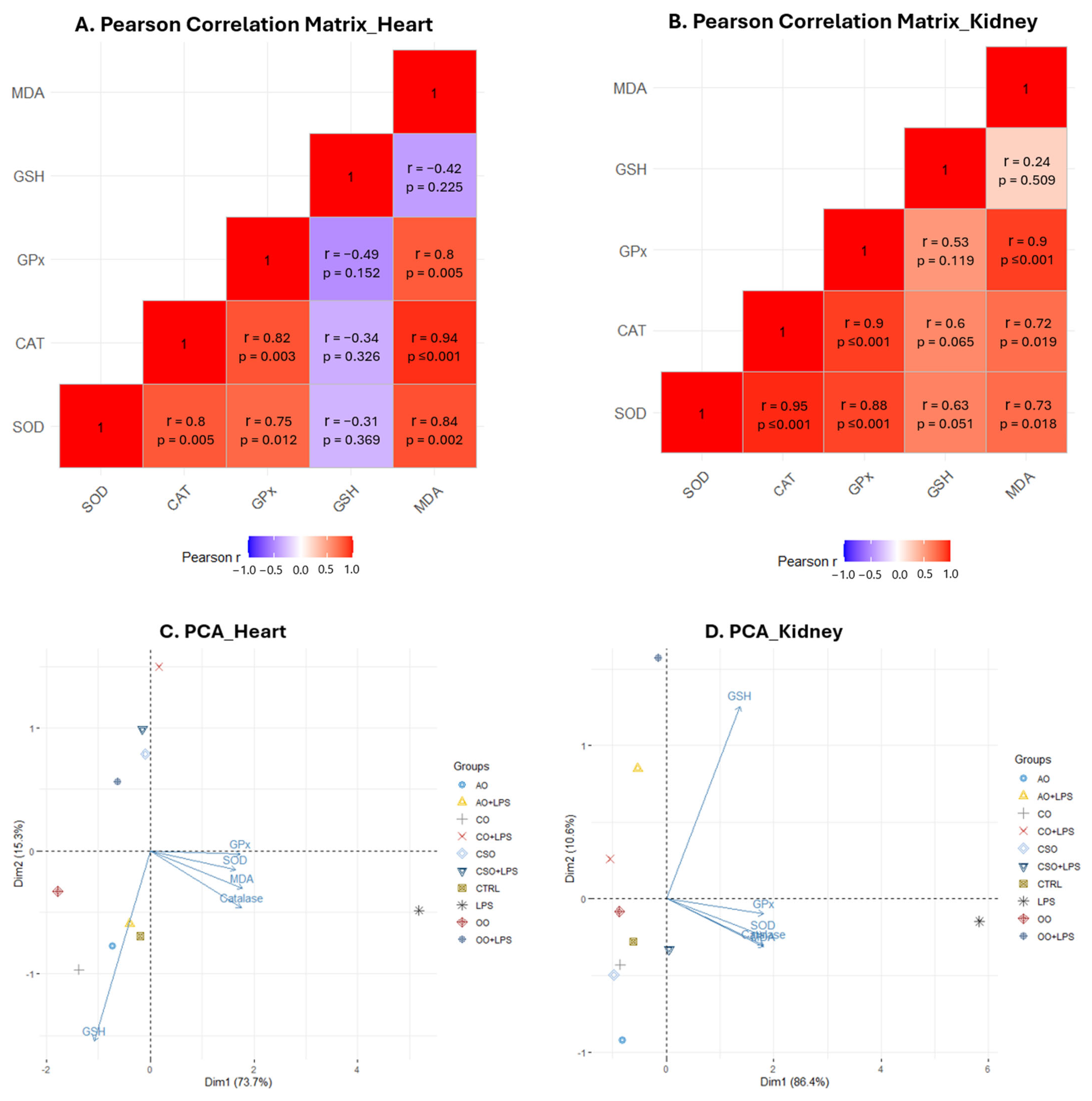
2.4.3. Tissue-Specific Oxidative Stress Profiles: Heatmap Analysis, Pearson’s Correlation, and PCA
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Origin and Extraction of Oils
4.3. Oils Characterization
4.3.1. Determination of Chlorophyll, Carotenoid, and Xanthophyll Contents
4.3.2. Extraction of Methanolic and Lipophilic Fractions of Oils
4.3.3. Determination of Total Phenolics Contents
4.3.4. Antioxidant Activities of Oils
4.4. In Vivo Study
4.4.1. Animal Model
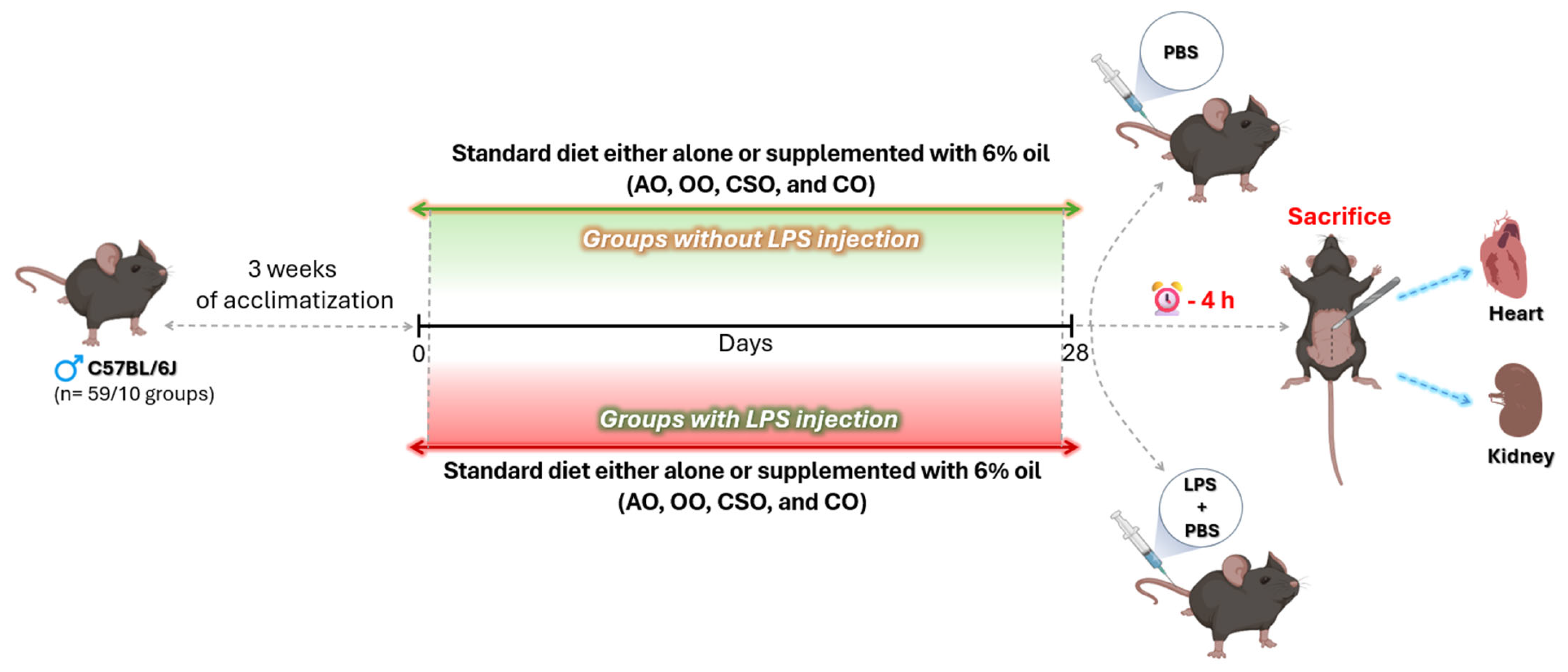
4.4.2. Experimental Design
4.4.3. Preparation of Tissue Homogenates
4.4.4. Quantification of Protein
4.4.5. Measurement of Enzymatic Antioxidants Defense
Superoxide Dismutase (SOD) Activity
Catalase (CAT) Activity
Glutathione Peroxidase (GPx) Activity
4.4.6. Measurement of Reduced Glutathione (GSH)
4.4.7. Measurement of Monodialdehyde (MDA)
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AO | Argan oil |
| OO | Olive oil |
| CSO | Cactus seed oil |
| CO | Colza oil |
| LPS | Lipopolysaccharide |
| MF | Methanolic fraction |
| LF | Lipidic fraction |
| TF | Total fraction |
| TPC | Total phenolic content |
| ROS | Reactive oxygen species |
| SOD | Superoxide dismutase |
| CAT | Catalase |
| GPx | Glutathione peroxidase |
| GSH | Reduced glutathione |
| MDA | Malondialdehyde |
| Nrf2 | Nuclear factor erythroid 2-related factor 2 |
| FOXO | Forkhead box O |
| HDL | High-density lipoprotein |
| LDL | Low-density lipoprotein |
| O2•− | Superoxide anion |
| H2O2 | Hydrogen peroxide |
| •OH | Hydroxyl radical |
| ABTS | 2,2′-azinobis(3 ethylbenzothiazoline-6-sulfonic acid) diammonium |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl radical |
| FRAP | Ferric reducing antioxidant power |
| TBA | Thiobarbituric acid |
| TCA | Trichloroacetic acid |
| DTNB | 5,5′-dithiobis(2-nitrobenzoic acid) |
| TNB− | 5-thio-2-nitrobenzoate |
| BCA | Bicinchoninic acid |
| ANOVA | Analysis of variance |
| SD | Standard deviation |
| PCA | Principal component analysis |
References
- Heurtaux, T.; Bouvier, D.S.; Benani, A.; Helgueta Romero, S.; Frauenknecht, K.B.M.; Mittelbronn, M.; Sinkkonen, L. Normal and Pathological NRF2 Signalling in the Central Nervous System. Antioxidants 2022, 11, 1426. [Google Scholar] [CrossRef]
- Juan, C.A.; Pérez de la Lastra, J.M.; Plou, F.J.; Pérez-Lebeña, E. The Chemistry of Reactive Oxygen Species (ROS) Revisited: Outlining Their Role in Biological Macromolecules (DNA, Lipids and Proteins) and Induced Pathologies. Int. J. Mol. Sci. 2021, 22, 4642. [Google Scholar] [CrossRef] [PubMed]
- Burton, G.J.; Jauniaux, E. Oxidative Stress. Best Pract. Res. Clin. Obstet. Gynaecol. 2011, 25, 287–299. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Fernández, S.; Gurpegui, M.; Garrote-Rojas, D.; Gutiérrez-Rojas, L.; Carretero, M.D.; Correll, C.U. Oxidative Stress Parameters and Antioxidants in Patients with Bipolar Disorder: Results from a Meta-Analysis Comparing Patients, Including Stratification by Polarity and Euthymic Status, with Healthy Controls. Bipolar Disord. 2021, 23, 117–129. [Google Scholar] [CrossRef]
- Liu, T.; Sun, L.; Zhang, Y.; Wang, Y.; Zheng, J. Imbalanced GSH/ROS and Sequential Cell Death. J. Biochem. Mol. Toxicol. 2022, 36, e22942. [Google Scholar] [CrossRef] [PubMed]
- Fransen, M.; Lismont, C. Peroxisomes and Cellular Oxidant/Antioxidant Balance: Protein Redox Modifications and Impact on Inter-Organelle Communication. Subcell. Biochem. 2018, 89, 435–461. [Google Scholar] [CrossRef]
- Vamecq, J.; Andreoletti, P.; El Kebbaj, R.; Saih, F.-E.; Latruffe, N.; El Kebbaj, M.H.S.; Lizard, G.; Nasser, B.; Cherkaoui-Malki, M. Peroxisomal Acyl-CoA Oxidase Type 1: Anti-Inflammatory and Anti-Aging Properties with a Special Emphasis on Studies with LPS and Argan Oil as a Model Transposable to Aging. Oxid. Med. Cell. Longev. 2018, 2018, 6986984. [Google Scholar] [CrossRef]
- Schrader, M.; Fahimi, H.D. Peroxisomes and Oxidative Stress. Biochim. Biophys. Acta 2006, 1763, 1755–1766. [Google Scholar] [CrossRef]
- El Kamouni, S.; El Kebbaj, R.; Andreoletti, P.; El Ktaibi, A.; Rharrassi, I.; Essamadi, A.; El Kebbaj, M.S.; Mandard, S.; Latruffe, N.; Vamecq, J.; et al. Protective Effect of Argan and Olive Oils against LPS-Induced Oxidative Stress and Inflammation in Mice Livers. Int. J. Mol. Sci. 2017, 18, 2181. [Google Scholar] [CrossRef]
- Tahri-Joutey, M.; Saih, F.-E.; El Kebbaj, R.; Gondcaille, C.; Vamecq, J.; Latruffe, N.; Lizard, G.; Savary, S.; Nasser, B.; Cherkaoui-Malki, M.; et al. Protective Effect of Nopal Cactus (Opuntia Ficus-Indica) Seed Oil against Short-Term Lipopolysaccharides-Induced Inflammation and Peroxisomal Functions Dysregulation in Mouse Brain and Liver. Int. J. Mol. Sci. 2022, 23, 11849. [Google Scholar] [CrossRef]
- Essadek, S.; Bouchab, H.; El Kebbaj, R.; Gondcaille, C.; El Kamouni, S.; Savary, S.; Vamecq, J.; Essamadi, A.; Cherkaoui-Malki, M.; Nasser, B.; et al. Effects of a Short-Term Lipopolysaccharides Challenge on Mouse Brain and Liver Peroxisomal Antioxidant and β-oxidative Functions: Protective Action of Argan Oil. Pharmaceuticals 2022, 15, 465. [Google Scholar] [CrossRef]
- Basauri, A.; González-Fernández, C.; Fallanza, M.; Bringas, E.; Fernandez-Lopez, R.; Giner, L.; Moncalián, G.; De La Cruz, F.; Ortiz, I. Biochemical Interactions between LPS and LPS-Binding Molecules. Crit. Rev. Biotechnol. 2020, 40, 292–305. [Google Scholar] [CrossRef]
- Ciesielska, A.; Matyjek, M.; Kwiatkowska, K. TLR4 and CD14 Trafficking and Its Influence on LPS-Induced pro-Inflammatory Signaling. Cell. Mol. Life Sci. 2021, 78, 1233–1261. [Google Scholar] [CrossRef]
- Atrooz, F.; Salim, S. Sleep Deprivation, Oxidative Stress and Inflammation. Adv. Protein Chem. Struct. Biol. 2020, 119, 309–336. [Google Scholar] [CrossRef] [PubMed]
- Paris-Robidas, S.; Bolduc, I.; Lapointe, V.; Galimi, J.; Lemieux, P.; Huppé, C.-A.; Couture, F. Impact of Time Intervals on Drug Efficacy and Phenotypic Outcomes in Acute Respiratory Distress Syndrome in Mice. Sci. Rep. 2024, 14, 20768. [Google Scholar] [CrossRef]
- Steven, S.; Dib, M.; Roohani, S.; Kashani, F.; Münzel, T.; Daiber, A. Time Response of Oxidative/Nitrosative Stress and Inflammation in LPS-Induced Endotoxaemia—A Comparative Study of Mice and Rats. Int. J. Mol. Sci. 2017, 18, 2176. [Google Scholar] [CrossRef] [PubMed]
- Seese, M.H.; Steelman, A.J.; Erdman, J.W. The Impact of LPS on Inflammatory Responses in Alpha-Tocopherol Deficient Mice. Curr. Dev. Nutr. 2024, 8, 104416. [Google Scholar] [CrossRef] [PubMed]
- Suliman, H.B.; Welty-Wolf, K.E.; Carraway, M.; Tatro, L.; Piantadosi, C.A. Lipopolysaccharide Induces Oxidative Cardiac Mitochondrial Damage and Biogenesis. Cardiovasc. Res. 2004, 64, 279–288. [Google Scholar] [CrossRef]
- Asgharzadeh, F.; Bargi, R.; Hosseini, M.; Farzadnia, M.; Khazaei, M. Cardiac and Renal Fibrosis and Oxidative Stress Balance in Lipopolysaccharide-Induced Inflammation in Male Rats. ARYA Atheroscler. 2018, 14, 71–77. [Google Scholar] [CrossRef]
- Ben-Shaul, V.; Lomnitski, L.; Nyska, A.; Zurovsky, Y.; Bergman, M.; Grossman, S. The Effect of Natural Antioxidants, NAO and Apocynin, on Oxidative Stress in the Rat Heart Following LPS Challenge. Toxicol. Lett. 2001, 123, 1–10. [Google Scholar] [CrossRef]
- Khodir, A.; Ghoneim, H.; Rahim, M.; Suddek, G. Montelukast Attenuates Lipopolysaccharide-Induced Cardiac Injury in Rats. Hum. Exp. Toxicol. 2016, 35, 388–397. [Google Scholar] [CrossRef] [PubMed]
- Feingold, K.R.; Wang, Y.; Moser, A.; Shigenaga, J.K.; Grunfeld, C. LPS Decreases Fatty Acid Oxidation and Nuclear Hormone Receptors in the Kidney. J. Lipid Res. 2008, 49, 2179–2187. [Google Scholar] [CrossRef] [PubMed]
- Sriskandan, S.; Altmann, D.M. The Immunology of Sepsis. J. Pathol. 2008, 214, 211–223. [Google Scholar] [CrossRef]
- Rabbaa, S.; Bouchab, H.; Laaziouez, Y.; Limami, Y.; Nasser, B.; Andreoletti, P.; Cherkaoui-Malki, M.; El Kebbaj, R. Argan Oil: A Natural Bioactive Lipid Modulating Oxidative Stress and Inflammation. Antioxidants 2025, 14, 515. [Google Scholar] [CrossRef]
- Arulselvan, P.; Fard, M.T.; Tan, W.S.; Gothai, S.; Fakurazi, S.; Norhaizan, M.E.; Kumar, S.S. Role of Antioxidants and Natural Products in Inflammation. Oxid. Med. Cell. Longev. 2016, 2016, 5276130. [Google Scholar] [CrossRef]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free Radicals, Antioxidants and Functional Foods: Impact on Human Health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef]
- Nesci, S.; Spagnoletta, A.; Oppedisano, F. Inflammation, Mitochondria and Natural Compounds Together in the Circle of Trust. Int. J. Mol. Sci. 2023, 24, 6106. [Google Scholar] [CrossRef]
- Sheikh, N.A.; Desai, T.R.; Tirgar, P.R. Evaluation of Iron Chelating and Antioxidant Potential of Epilobium Hirsutum for the Management of Iron Overload Disease. Biomed. Pharmacother. 2017, 89, 1353–1361. [Google Scholar] [CrossRef]
- Tabolacci, C.; Forni, C.; Jadeja, R.N.; Facchiano, F. Natural Compounds against Cancer, Inflammation, and Oxidative Stress. BioMed Res. Int. 2019, 2019, 9495628. [Google Scholar] [CrossRef]
- Minasyan, A.; Pires, V.; Gondcaille, C.; Ginovyan, M.; Mróz, M.; Savary, S.; Cherkaoui-Malki, M.; Kusznierewicz, B.; Bartoszek, A.; Andreoletti, P.; et al. Ribes Nigrum Leaf Extract Downregulates Pro-Inflammatory Gene Expression and Regulates Redox Balance in Microglial Cells. BMC Complement. Med. Ther. 2025, 25, 49. [Google Scholar] [CrossRef]
- El Kebbaj, R.; Bouchab, H.; Tahri-Joutey, M.; Rabbaa, S.; Limami, Y.; Nasser, B.; Egbujor, M.C.; Tucci, P.; Andreoletti, P.; Saso, L.; et al. The Potential Role of Major Argan Oil Compounds as Nrf2 Regulators and Their Antioxidant Effects. Antioxidants 2024, 13, 344. [Google Scholar] [CrossRef] [PubMed]
- Gabriele, M.; Pucci, L. Diet Bioactive Compounds: Implications for Oxidative Stress and Inflammation in the Vascular System. Endocr. Metab. Immune Disord. Drug Targets 2017, 17, 264–275. [Google Scholar] [CrossRef] [PubMed]
- Mattioli, R.; Mosca, L.; Sánchez-Lamar, A.; Tempera, I.; Hausmann, R. Natural Bioactive Compounds Acting against Oxidative Stress in Chronic, Degenerative, and Infectious Diseases. Oxid. Med. Cell. Longev. 2018, 2018, 3894381. [Google Scholar] [CrossRef]
- Liang, B.; Zhu, Y.-C.; Lu, J.; Gu, N. Effects of Traditional Chinese Medication-Based Bioactive Compounds on Cellular and Molecular Mechanisms of Oxidative Stress. Oxid. Med. Cell. Longev. 2021, 2021, 3617498. [Google Scholar] [CrossRef]
- Benayad, S.; Es-Sai, B.; Laaziouez, Y.; Rabbaa, S.; Wahnou, H.; Bouchab, H.; El Attar, H.; Benabdelkhalek, B.; Amahdar, L.; Abboussi, O.; et al. Protective Effects of Sodium Copper Chlorophyllin and/or Ascorbic Acid Against Barium Chloride-Induced Oxidative Stress in Mouse Brain and Liver. Molecules 2025, 30, 3231. [Google Scholar] [CrossRef]
- El Ghachi, H.; Oukhrib, M.; Aziz, F.; Benrazzouk, K.; Gamrani, H.; Soulimani, R.; Boukhzar, L. Exploring the Phytochemical and Toxicological Profile of Moroccan Cannabis Sativa L. Leaves Extract: Behavioral, Histological, and Oxidative Stress Assessments. J. Ethnopharmacol. 2025, 350, 120058. [Google Scholar] [CrossRef]
- Khan, M.Z.; Khan, A.; Huang, B.; Wei, R.; Kou, X.; Wang, X.; Chen, W.; Li, L.; Zahoor, M.; Wang, C. Bioactive Compounds Protect Mammalian Reproductive Cells from Xenobiotics and Heat Stress-Induced Oxidative Distress via Nrf2 Signaling Activation: A Narrative Review. Antioxidants 2024, 13, 597. [Google Scholar] [CrossRef]
- El Kebbaj, R.; Kamouni, S.E.; Hajj, H.I.E.; Andreoletti, P.; Gresti, J.; Latruffe, N.; El Kebbaj, M.S.; Vamecq, J.; Lizard, G.; Nasser, B.; et al. Modulation of Peroxisomes Abundance by Argan Oil and Lipopolysaccharides in Acyl-CoA Oxidase 1-Deficient Fibroblasts. Health 2013, 5, 62–69. [Google Scholar] [CrossRef]
- Kadda, S.; Belabed, A.; Loukili, E.H.; Hammouti, B.; Fadlaoui, S. Temperature and Extraction Methods Effects on Yields, Fatty Acids, and Tocopherols of Prickly Pear (Opuntia Ficus-Indica L.) Seed Oil of Eastern Region of Morocco. Environ. Sci. Pollut. Res. Int. 2022, 29, 158–166. [Google Scholar] [CrossRef]
- Al-Naqeb, G.; Fiori, L.; Ciolli, M.; Aprea, E. Prickly Pear Seed Oil Extraction, Chemical Characterization and Potential Health Benefits. Molecules 2021, 26, 5018. [Google Scholar] [CrossRef]
- Mechqoq, H.; El Yaagoubi, M.; El Hamdaoui, A.; Momchilova, S.; da Guedes Silva Almeida, J.R.; Msanda, F.; El Aouad, N. Ethnobotany, Phytochemistry and Biological Properties of Argan Tree (Argania spinosa (L.) Skeels) (Sapotaceae)—A Review. J. Ethnopharmacol. 2021, 281, 114528. [Google Scholar] [CrossRef]
- Charrouf, Z.; Guillaume, D. Should the Amazigh Diet (Regular and Moderate Argan-Oil Consumption) Have a Beneficial Impact on Human Health? Crit. Rev. Food Sci. Nutr. 2010, 50, 473–477. [Google Scholar] [CrossRef] [PubMed]
- Makbal, R.; Idrissi, F.E.J.; Ouchbani, T.; Tastift, M.A.; Kiai, H.; Hafidi, A.; Gadhi, C. Anti-Inflammatory, Antioxidant, Chemical Characterization, and Safety Assessment of Argania spinosa Fruit Shell Extract from South-Western Morocco. BioMed Res. Int. 2021, 2021, 5536030. [Google Scholar] [CrossRef] [PubMed]
- El Kharrassi, Y.; Maata, N.; Mazri, M.A.; El Kamouni, S.; Talbi, M.; El Kebbaj, R.; Moustaid, K.; Essamadi, A.K.; Andreoletti, P.; El Mzouri, E.H.; et al. Chemical and Phytochemical Characterizations of Argan Oil (Argania spinosa L. Skeels), Olive Oil (Olea europaea L. Cv. Moroccan picholine), Cactus Pear (Opuntia megacantha salm-dyck) Seed Oil and Cactus Cladode Essential Oil. J. Food Meas. Charact. 2018, 12, 747–754. [Google Scholar] [CrossRef]
- Mouas, N.T.; Kabouche, Z.; Bellel, N.; Chertout, L.K. Opuntia Ficus-Indica a Mediterranean Diet Product. In Proceedings of the 1st International Electronic Conference on Biological Diversity, Ecology and Evolution, Basel, Switzerland, 15–31 March 2021. [Google Scholar]
- El Kebbaj, R.; Andreoletti, P.; El Hajj, H.I.; El Kharrassi, Y.; Vamecq, J.; Mandard, S.; Saih, F.-E.; Latruffe, N.; El Kebbaj, M.S.; Lizard, G.; et al. Argan Oil Prevents Down-Regulation Induced by Endotoxin on Liver Fatty Acid Oxidation and Gluconeogenesis and on Peroxisome Proliferator-Activated Receptor Gamma Coactivator-1α, (PGC-1α), Peroxisome Proliferator-Activated Receptor α (PPARα) and Estrogen Related Receptor α (ERRα). Biochim. Open 2015, 1, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Derouiche, A.; Cherki, M.; Drissi, A.; Bamou, Y.; El Messal, M.; Idrissi-Oudghiri, A.; Lecerf, J.M.; Adlouni, A. Nutritional Intervention Study with Argan Oil in Man: Effects on Lipids and Apolipoproteins. Ann. Nutr. Metab. 2005, 49, 196–201. [Google Scholar] [CrossRef]
- Ursoniu, S.; Sahebkar, A.; Serban, M.-C.; Banach, M.; Lipid and Blood Pressure Meta-analysis Collaboration Group. The Impact of Argan Oil on Plasma Lipids in Humans: Systematic Review and Meta-Analysis of Randomized Controlled Trials: Lipid-Modifying Effects of Argan Oil. Phytother. Res. 2018, 32, 377–383. [Google Scholar] [CrossRef]
- Ould Mohamedou, M.M.; Zouirech, K.; El Messal, M.; El Kebbaj, M.S.; Chraibi, A.; Adlouni, A. Argan Oil Exerts an Antiatherogenic Effect by Improving Lipids and Susceptibility of LDL to Oxidation in Type 2 Diabetes Patients. Int. J. Endocrinol. 2011, 2011, 747835. [Google Scholar] [CrossRef]
- Podadera-Herreros, A.; Alcala-Diaz, J.F.; Gutierrez-Mariscal, F.M.; Jimenez-Torres, J.; de la Cruz-Ares, S.; Larriva, A.P.A.; Cardelo, M.P.; Torres-Peña, J.D.; Luque, R.M.; Ordovas, J.M.; et al. Long-Term Consumption of a Mediterranean Diet or a Low-Fat Diet on Kidney Function in Coronary Heart Disease Patients: The CORDIOPREV Randomized Controlled Trial. Clin. Nutr. 2022, 41, 552–559. [Google Scholar] [CrossRef]
- Doménech, M.; Roman, P.; Lapetra, J.; García de la Corte, F.J.; Sala-Vila, A.; de la Torre, R.; Corella, D.; Salas-Salvadó, J.; Ruiz-Gutiérrez, V.; Lamuela-Raventós, R.-M.; et al. Mediterranean Diet Reduces 24-Hour Ambulatory Blood Pressure, Blood Glucose, and Lipids: One-Year Randomized, Clinical Trial. Hypertens. Dallas Tex 1979 2014, 64, 69–76. [Google Scholar] [CrossRef]
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.-I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J.; et al. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with Extra-Virgin Olive Oil or Nuts. N. Engl. J. Med. 2018, 378, e34. [Google Scholar] [CrossRef]
- Covas, M.-I. Bioactive Effects of Olive Oil Phenolic Compounds in Humans: Reduction of Heart Disease Factors and Oxidative Damage. Inflammopharmacology 2008, 16, 216–218. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Morze, J.; Hoffmann, G. Mediterranean Diet and Health Status: Active Ingredients and Pharmacological Mechanisms. Br. J. Pharmacol. 2020, 177, 1241–1257. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Krause, M.; Schmucker, C.; Hoffmann, G.; Rücker, G.; Meerpohl, J.J. Impact of Different Types of Olive Oil on Cardiovascular Risk Factors: A Systematic Review and Network Meta-Analysis. Nutr. Metab. Cardiovasc. Dis. 2019, 29, 1030–1039. [Google Scholar] [CrossRef] [PubMed]
- Amiri, M.; Raeisi-Dehkordi, H.; Moghtaderi, F.; Zimorovat, A.; Mohyadini, M.; Salehi-Abargouei, A. The Effects of Sesame, Canola, and Sesame–Canola Oils on Cardiometabolic Markers in Patients with Type 2 Diabetes: A Tri-ple-Blind Three-Way Randomized Crossover Clinical Trial. Eur. J. Nutr. 2022, 61, 3499–3516. [Google Scholar] [CrossRef] [PubMed]
- Nicol, K.; Mansoorian, B.; Latosinska, A.; Koutroulaki, A.; Mullen, B.; Combet, E. No Evidence of Differential Impact of Sunflower and Rapeseed Oil on Biomarkers of Coronary Artery Disease or Chronic Kidney Disease in Healthy Adults with Overweight and Obesity: Result from a Randomised Control Trial. Eur. J. Nutr. 2022, 61, 3119–3133. [Google Scholar] [CrossRef]
- Nielsen, N.S.; Pedersen, A.; Sandström, B.; Marckmann, P.; Høy, C.-E. Different Effects of Diets Rich in Olive Oil, Rapeseed Oil and Sunflower-Seed Oil on Postprandial Lipid and Lipoprotein Concentrations and on Lipoprotein Oxidation Susceptibility. Br. J. Nutr. 2002, 87, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Bakour, M.; Al-Waili, N.; El-Haskoury, R.; El-Menyiy, N.; Al-Waili, T.; AL-Waili, A.; Lyoussi, B. Comparison of Hypotensive, Diuretic and Renal Effects between Cladodes of Opuntia Ficus-Indica and Furosemide. Asian Pac. J. Trop. Med. 2017, 10, 900–906. [Google Scholar] [CrossRef]
- Marrone, G.; Murri, A.; Urciuoli, S.; Di Lauro, M.; Grazioli, E.; Vignolini, P.; Cornali, K.; Tranchita, E.; Masci, C.; Cerulli, C.; et al. Functional Foods and Adapted Physical Activity as New Adjuvant Therapy for Chronic Kidney Disease Patients. Nutrients 2024, 16, 2325. [Google Scholar] [CrossRef]
- Bouchab, H.; Ishaq, A.; Limami, Y.; Saretzki, G.; Nasser, B.; El Kebbaj, R. Antioxidant Effects of Cactus Seed Oil against Iron-Induced Oxidative Stress in Mouse Liver, Brain and Kidney. Molecules 2024, 29, 4463. [Google Scholar] [CrossRef]
- Miao, L.; St Clair, D.K. Regulation of Superoxide Dismutase Genes: Implications in Disease. Free Radic. Biol. Med. 2009, 47, 344–356. [Google Scholar] [CrossRef]
- Glorieux, C.; Calderon, P.B. Catalase, a Remarkable Enzyme: Targeting the Oldest Antioxidant Enzyme to Find a New Cancer Treatment Approach. Biol. Chem. 2017, 398, 1095–1108. [Google Scholar] [CrossRef]
- Lubos, E.; Loscalzo, J.; Handy, D.E. Glutathione Peroxidase-1 in Health and Disease: From Molecular Mechanisms to Therapeutic Opportunities. Antioxid. Redox Signal. 2011, 15, 1957–1997. [Google Scholar] [CrossRef]
- Forman, H.J.; Zhang, H.; Rinna, A. Glutathione: Overview of Its Protective Roles, Measurement, and Biosynthesis. Mol. Aspects Med. 2009, 30, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Khallouki, F.; Younos, C.; Soulimani, R.; Oster, T.; Charrouf, Z.; Spiegelhalder, B.; Bartsch, H.; Owen, R.W. Consumption of Argan Oil (Morocco) with Its Unique Profile of Fatty Acids, Tocopherols, Squalene, Sterols and Phenolic Compounds Should Confer Valuable Cancer Chemopreventive Effects. Eur. J. Cancer Prev. 2003, 12, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Khallouki, F.; Eddouks, M.; Mourad, A.; Breuer, A.; Owen, R.W. Ethnobotanic, Ethnopharmacologic Aspects and New Phytochemical Insights into Moroccan Argan Fruits. Int. J. Mol. Sci. 2017, 18, 2277. [Google Scholar] [CrossRef]
- Debbabi, M.; Zarrouk, A.; Bezine, M.; Meddeb, W.; Nury, T.; Badreddine, A.; Karym, E.M.; Sghaier, R.; Bretillon, L.; Guyot, S.; et al. Comparison of the Effects of Major Fatty Acids Present in the Mediterranean Diet (Oleic Acid, Docosahexaenoic Acid) and in Hydrogenated Oils (Elaidic Acid) on 7-Ketocholesterol-Induced Oxiapoptophagy in Microglial BV-2 Cells. Chem. Phys. Lipids 2017, 207 Pt B, 151–170. [Google Scholar] [CrossRef]
- Zeng, X.; Zhu, M.; Liu, X.; Chen, X.; Yuan, Y.; Li, L.; Liu, J.; Lu, Y.; Cheng, J.; Chen, Y. Oleic Acid Ameliorates Palmitic Acid Induced Hepatocellular Lipotoxicity by Inhibition of ER Stress and Pyroptosis. Nutr. Metab. 2020, 17, 11. [Google Scholar] [CrossRef]
- Wang, R.; Kern, J.T.; Goodfriend, T.L.; Ball, D.L.; Luesch, H. Activation of the Antioxidant Response Element by Specific Oxidized Metabolites of Linoleic Acid. Prostaglandins Leukot. Essent. Fat. Acids 2009, 81, 53–59. [Google Scholar] [CrossRef]
- Zhang, Q.; Jiang, Y.; Qin, Y.; Liu, J.; Xie, Y.; Zhang, L.; Li, K.; Wang, X.; Liu, G. Linoleic Acid Alleviates Lipopolysaccharide Induced Acute Liver Injury via Activation of Nrf2. Physiol. Res. 2024, 73, 381–391. [Google Scholar] [CrossRef]
- Duthie, G.G.; McPhail, D.B.; Morrice, P.C.; Arthur, J.R. Antioxidant Effectiveness of Tocopherol Isomers. In Lipid-Soluble Antioxidants: Biochemistry and Clinical Applications; Ong, A.S.H., Packer, L., Eds.; Birkhäuser: Basel, Switzerland, 1992; pp. 76–84. ISBN 9783034874328. [Google Scholar]
- Es-Sai, B.; Wahnou, H.; Benayad, S.; Rabbaa, S.; Laaziouez, Y.; El Kebbaj, R.; Limami, Y.; Duval, R.E. Gamma-Tocopherol: A Comprehensive Review of Its Antioxidant, Anti-Inflammatory, and Anticancer Properties. Molecules 2025, 30, 653. [Google Scholar] [CrossRef] [PubMed]
- Kamal, R.; Kharbach, M.; Vander Heyden, Y.; Doukkali, Z.; Ghchime, R.; Bouklouze, A.; Cherrah, Y.; Alaoui, K. In Vivo Anti-Inflammatory Response and Bioactive Compounds’ Profile of Polyphenolic Extracts from Edible Argan Oil (Argania spinosa L.), Obtained by Two Extraction Methods. J. Food Biochem. 2019, 43, e13066. [Google Scholar] [CrossRef] [PubMed]
- Ben Mansour, R.; Ben Slema, H.; Falleh, H.; Tounsi, M.; Kechebar, M.S.A.; Ksouri, R.; Megdiche-Ksouri, W. Phytochemical Characteristics, Antioxidant, and Health Properties of Roasted and Unroasted Algerian Argan (Argania spinosa) Oil. J. Food Biochem. 2018, 42, e12562, Erratum in J. Food Biochem. 2019, 43, 2. [Google Scholar] [CrossRef]
- Elbir, M.; Es-Safi, N.E.; Amhoud, A.; Mbarki, M. Characterization of Phenolic Compounds in Olive Stones of Three Moroccan Varieties. Maderas Cienc. Tecnol. 2015, 17, 479–492. [Google Scholar] [CrossRef]
- El haouhay, N.; Samaniego-Sánchez, C.; Asehraou, A.; Villalón-Mir, M.; López-García de la Serrana, H. Microbiological Characterization of Picholine Variety Olives and Analysis of Olive Oil Produced in Traditional Oil Mills in Morocco. CyTA-J. Food 2015, 13, 107–115. [Google Scholar] [CrossRef]
- Miliauskas, G.; Venskutonis, P.R.; van Beek, T.A. Screening of Radical Scavenging Activity of Some Medicinal and Aromatic Plant Extracts. Food Chem. 2004, 85, 231–237. [Google Scholar] [CrossRef]
- Nenadis, N.; Mastralexi, A.; Tsimidou, M.Z. Physicochemical Characteristics and Antioxidant Potential of the Greek PDO and PGI Virgin Olive Oils (VOOs). Eur. J. Lipid Sci. Technol. 2019, 121, 1800172. [Google Scholar] [CrossRef]
- Marfil, R.; Giménez, R.; Martínez, O.; Bouzas, P.R.; Rufián-Henares, J.A.; Mesías, M.; Cabrera-Vique, C. Determination of Polyphenols, Tocopherols, and Antioxidant Capacity in Virgin Argan Oil (Argania spinosa, Skeels). Eur. J. Lipid Sci. Technol. 2011, 113, 886–893. [Google Scholar] [CrossRef]
- Houshia, O.J.; Qutit, A.; Zaid, O.; Shqair, H.; Zaid, M. Determination of Total Polyphenolic Antioxidants Contents in West-Bank Olive Oil. J. Nat. Sci. Res. 2014, 4, 71. [Google Scholar]
- Zarrouk, A.; Martine, L.; Grégoire, S.; Nury, T.; Meddeb, W.; Camus, E.; Badreddine, A.; Durand, P.; Namsi, A.; Yammine, A.; et al. Profile of Fatty Acids, Tocopherols, Phytosterols and Polyphenols in Mediterranean Oils (Argan Oils, Olive Oils, Milk Thistle Seed Oils and Nigella Seed Oil) and Evaluation of their Antioxidant and Cytoprotective Activities. Curr. Pharm. Des. 2019, 25, 1791–1805. [Google Scholar] [CrossRef]
- Bouchab, H.; Essadek, S.; El Kamouni, S.; Moustaid, K.; Essamadi, A.; Andreoletti, P.; Cherkaoui-Malki, M.; El Kebbaj, R.; Nasser, B. Antioxidant Effects of Argan Oil and Olive Oil against Iron-Induced Oxidative Stress: In Vivo and In Vitro Approaches. Molecules 2023, 28, 5924. [Google Scholar] [CrossRef] [PubMed]
- Widomska, J.; Gruszecki, W.I.; Subczynski, W.K. Factors Differentiating the Antioxidant Activity of Macular Xanthophylls in the Human Eye Retina. Antioxidants 2021, 10, 601. [Google Scholar] [CrossRef] [PubMed]
- Stahl, W.; Sies, H. Antioxidant Activity of Carotenoids. Mol. Asp. Med. 2003, 24, 345–351. [Google Scholar] [CrossRef]
- Koubaa, M.; Mhemdi, H.; Barba, F.J.; Angelotti, A.; Bouaziz, F.; Chaabouni, S.E.; Vorobiev, E. Seed Oil Extraction from Red Prickly Pear Using Hexane and Supercritical CO2: Assessment of Phenolic Compound Composition, Antioxidant and Antibacterial Activities. J. Sci. Food Agric. 2017, 97, 613–620. [Google Scholar] [CrossRef]
- Chaalal, M.; Touati, N.; Louaileche, H. Extraction of Phenolic Compounds and in Vitro Antioxidant Capacity of Prickly Pear Seeds. Acta Bot. Gall. 2012, 159, 467–475. [Google Scholar] [CrossRef]
- Lumpuy-Castillo, J.; Amador-Martínez, I.; Díaz-Rojas, M.; Lorenzo, O.; Pedraza-Chaverri, J.; Sánchez-Lozada, L.G.; Aparicio-Trejo, O.E. Role of Mitochondria in Reno-Cardiac Diseases: A Study of Bioenergetics, Biogenesis, and GSH Signaling in Disease Transition. Redox Biol. 2024, 76, 103340. [Google Scholar] [CrossRef]
- Li, S.; Li, X.; Rozanski, G.J. Regulation of Glutathione in Cardiac Myocytes. J. Mol. Cell. Cardiol. 2003, 35, 1145–1152. [Google Scholar] [CrossRef] [PubMed]
- Lash, L.H. Role of Glutathione Transport Processes in Kidney Function. Toxicol. Appl. Pharmacol. 2005, 204, 329–342. [Google Scholar] [CrossRef]
- Kumar, P.; Osahon, O.W.; Sekhar, R.V. GlyNAC (Glycine and N-Acetylcysteine) Supplementation in Mice Increases Length of Life by Correcting Glutathione Deficiency, Oxidative Stress, Mitochondrial Dysfunction, Abnormalities in Mitophagy and Nutrient Sensing, and Genomic Damage. Nutrients 2022, 14, 1114. [Google Scholar] [CrossRef]
- Zhang, W.; Deng, J.; Sunkara, M.; Morris, A.J.; Wang, C.; St Clair, D.; Vore, M. Loss of Multidrug Resistance-Associated Protein 1 Potentiates Chronic Doxorubicin-Induced Cardiac Dysfunction in Mice. J. Pharmacol. Exp. Ther. 2015, 355, 280–287. [Google Scholar] [CrossRef]
- Subramanyam, D.; Gurunathan, D.; Gaayathri, R.; Priya, V.V. Comparative Evaluation of Salivary Malondialdehyde Levels as a Marker of Lipid Peroxidation in Early Childhood Caries. Eur. J. Dent. 2018, 12, 067–070. [Google Scholar] [CrossRef]
- Soliman, M.M.; Alotaibi, S.S.; Sayed, S.; Hassan, M.M.; Althobaiti, F.; Aldhahrani, A.; Youssef, G.B.A.; El-Shehawi, A.M. The Protective Impact of Salsola imbricata Leaf Extract from Taif Against Acrylamide-Induced Hepatic Inflammation and Oxidative Damage: The Role of Antioxidants, Cytokines, and Apoptosis-Associated Genes. Front. Vet. Sci. 2022, 8, 817183. [Google Scholar] [CrossRef] [PubMed]
- Scapagnini, G.; Sonya, V.; Nader, A.G.; Calogero, C.; Zella, D.; Fabio, G. Modulation of Nrf2/ARE Pathway by Food Polyphenols: A Nutritional Neuroprotective Strategy for Cognitive and Neurodegenerative Disorders. Mol. Neurobiol. 2011, 44, 192–201, Erratum in Mol. Neurobiol. 2011, 44, 202. [Google Scholar] [CrossRef] [PubMed]
- Ashrafizadeh, M.; Ahmadi, Z.; Samarghandian, S.; Mohammadinejad, R.; Yaribeygi, H.; Sathyapalan, T.; Sahebkar, A. MicroRNA-Mediated Regulation of Nrf2 Signaling Pathway: Implications in Disease Therapy and Protection against Oxidative Stress. Life Sci. 2020, 244, 117329. [Google Scholar] [CrossRef] [PubMed]
- Bellezza, I.; Giambanco, I.; Minelli, A.; Donato, R. Nrf2-Keap1 Signaling in Oxidative and Reductive Stress. Biochim. Biophys. Acta BBA-Mol. Cell Res. 2018, 1865, 721–733. [Google Scholar] [CrossRef]
- Gorrini, C.; Harris, I.S.; Mak, T.W. Modulation of Oxidative Stress as an Anticancer Strategy. Nat. Rev. Drug Discov. 2013, 12, 931–947. [Google Scholar] [CrossRef]
- Cengiz, Z.T.; Yılmaz, H.; Beyhan, Y.E.; Ekici, A.; Çiçek, M.; Aydemir, S.; Cengiz, Z.T.; Yılmaz, H.; Beyhan, Y.E.; Ekici, A.; et al. The Importance of Antioxidant Enzymes and Oxidative Stress in Human Fascioliasis. Turk. Parazitol Derg. 2023, 47, 38–41. [Google Scholar] [CrossRef]
- Dubois-Deruy, E.; Peugnet, V.; Turkieh, A.; Pinet, F. Oxidative Stress in Cardiovascular Diseases. Antioxidants 2020, 9, 864. [Google Scholar] [CrossRef]
- Peoples, J.N.; Saraf, A.; Ghazal, N.; Pham, T.T.; Kwong, J.Q. Mitochondrial Dysfunction and Oxidative Stress in Heart Disease. Exp. Mol. Med. 2019, 51, 1–13. [Google Scholar] [CrossRef]
- Kim, J.M.; Kim, H.G.; Son, C.G. Tissue-Specific Profiling of Oxidative Stress-Associated Transcriptome in a Healthy Mouse Model. Int. J. Mol. Sci. 2018, 19, 3174. [Google Scholar] [CrossRef]
- Gagour, J.; Ahmed, M.N.; Bouzid, H.A.; Oubannin, S.; Bijla, L.; Ibourki, M.; Hajib, A.; Koubachi, J.; Harhar, H.; Gharby, S. Proximate Composition, Physicochemical, and Lipids Profiling and Elemental Profiling of Rapeseed (Brassica napus L.) and Sunflower (Helianthus annuus L.) Grown in Morocco. Evid.-Based Complement. Altern. Med. 2022, 2022, 3505943. [Google Scholar] [CrossRef]
- Minguez-Mosquera, M.I.; Gandul-Rojas, B.; Garrido-Fernandez, J.; Gallardo-Guerrero, L. Pigments Present in Virgin Olive Oil. J. Am. Oil Chem. Soc. 1990, 67, 192–196. [Google Scholar] [CrossRef]
- Espín, J.C.; Soler-Rivas, C.; Wichers, H.J. Characterization of the Total Free Radical Scavenger Capacity of Vegetable Oils and Oil Fractions Using 2,2-Diphenyl-1-Picrylhydrazyl Radical. J. Agric. Food Chem. 2000, 48, 648–656. [Google Scholar] [CrossRef]
- Dehpour, A.A.; Ebrahimzadeh, M.A.; Fazel, N.S.; Mohammad, N.S. Antioxidant Activity of the Methanol Extract of Ferula Assafoetida and Its Essential Oil Composition. Grasas Aceites 2009, 60, 405–412. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Ke, H.; He, J.; Ban, X.; Zeng, H.; Wang, Y. Extracts of Halenia Elliptica Exhibit Antioxidant Properties in Vitro and in Vivo. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2011, 49, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Oyaizu, M.; Oyaizu, M. Studies on Products of Browning Reaction Antioxidative Activities of Products of Browning Reaction Prepared from Glucosamine. Jpn. J. Nutr. 1986, 44, 307–316. [Google Scholar] [CrossRef]
- Masocha, W. Systemic lipopolysaccharide (LPS)-Induced Microglial Activation Results in Different Temporal Reduction of CD200 and CD200 Receptor Gene Expression in the Brain. J. Neuroimmunol. 2009, 214, 78–82. [Google Scholar] [CrossRef]
- Qin, L.; Wu, X.; Block, M.L.; Liu, Y.; Breese, G.R.; Hong, J.-S.; Knapp, D.J.; Crews, F.T. Systemic LPS Causes Chronic Neuroinflammation and Progressive Neurodegeneration. Glia 2007, 55, 453–462. [Google Scholar] [CrossRef]
- Beyer, W.F.; Fridovich, I. Assaying for Superoxide Dismutase Activity: Some Large Consequences of Minor Changes in Conditions. Anal. Biochem. 1987, 161, 559–566. [Google Scholar] [CrossRef]
- Beers, R.F.; Sizer, I.W. A Spectrophotometric Method for Measuring the Breakdown of Hydrogen Peroxide by Catalase. J. Biol. Chem. 1952, 195, 133–140. [Google Scholar] [CrossRef]
- Mills, G.C. The Purification and Properties of Glutathione Peroxidase of Erythrocytes. J. Biol. Chem. 1959, 234, 502–506. [Google Scholar] [CrossRef]
- Ellman, G.L. Tissue Sulfhydryl Groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for Lipid Peroxides in Animal Tissues by Thiobarbituric Acid Reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]

| AO | OO | CSO [61] | CO [61] | |
|---|---|---|---|---|
| Fatty acids (%) | ||||
| Myristic acid C14:0 | 0.18 | nd | 0.14 | 0.12 |
| Pentadecanoic acid C15:0 | 0.06 | nd | nd | nd |
| Palmitic acid C16:0 | 12.99 | 6.02 | 13.85 | 6.48 |
| Stearic acid C18:0 | 6.71 | nd | 4.61 | 1.57 |
| Oleic acid C18:1, ∆9 | 43.52 | 62.31 | 9.95 | 77.18 |
| Linoleic acid C18:2, ∆6 | 35.39 | 0.69 | 67.32 | nd |
| Arachidic acid C20:0 | 0.55 | nd | 0.58 | 2.38 |
| Behenic acid C22:0 | 0.14 | nd | 0.28 | 1.32 |
| Lignoceric acid C24:0 | 0.05 | nd | 0.26 | 0.46 |
| Phytosterol (%) | ||||
| Stigmast-7-en-3-ol | 0.05 | nd | nd | nd |
| Tocopherol (%) | ||||
| γ-Tocopherol | 0.02 | nd | 0.08 | 0.15 |
| Triterpene (%) | ||||
| Squalene | 0.33 | 28.27 | nd | nd |
| TPC (mg GAE/Kg Oil) | ABTS (% Inhibition) | DPPH (% Inhibition) | FRAP (mg Trolox/g Oil) | ||
|---|---|---|---|---|---|
| MF | AO | 80 ± 5.6 | 74.4 ± 0.61 | 57.6 ± 2.73 | 0.46 ± 0.06 |
| OO | 94.3 ± 4.9 | 91 ± 0.41 | 69.1± 3.51 | 1.56 ± 0.15 | |
| CSO | 59.3 ± 1.8 | 60.8 ± 1.16 | 71.9 ± 1.2 | 0.44 ± 0.18 | |
| CO | 54.7 ± 0.8 | 65 ± 0.73 | 58 ± 2.15 | 0.37 ± 0.03 | |
| LF | AO | 57.7 ± 3.5 | 58.7 ± 0.8 | 34.8 ± 3.94 | 0.49 ± 0.04 |
| OO | 68.87 ± 0.6 | 62.4 ± 0.57 | 62.1 ± 4.46 | 0.67 ± 0.05 | |
| CSO | 55.8 ± 1.3 | 46.4 ± 2.26 | 15.7 ± 3.46 | 0.41 ± 0.04 | |
| CO | 45.3 ± 0.6 | 51.57 ± 0.96 | 44.28 ± 4 | 0.41 ± 0.03 | |
| TF | AO | 73.1 ± 1.8 | 64 ± 3.75 | 55.4 ± 7.6 | 0.41 ± 0.05 |
| OO | 82.1 ± 4.1 | 66.17 ± 2.13 | 66.4 ± 1.94 | 1.15 ± 0.1 | |
| CSO | 44 ± 0.15 | 60.2 ± 1.24 | 34.9 ± 0.9 | 0.50 ± 0.06 | |
| CO | 48.7 ± 0.8 | 60.1 ± 0.81 | 54.2 ± 1.91 | 0.55 ± 0.05 | |
| AO [44] | OO [44] | CSO [44] | CO [103] | |
|---|---|---|---|---|
| Acidity (%, as oleic acid) | 0.28 ± 0.00 | 1.05 ± 0.00 | 0.77 ± 0.00 | 1.41 ± 0.10 |
| Peroxide value (meq O2/kg oil) | 2.42 ± 0.04 | 2.26 ± 0.05 | 2.84 ± 0.05 | 3.7 ± 0.39 |
| K232 | 1.04 ± 0.09 | 1.21 ± 0.05 | 1.83 ± 0.06 | 2.555 ± 0.04 |
| K270 | 0.19 ± 0.00 | 0.14 ± 0.00 | 0.23 ± 0.00 | 0.544 ± 0.021 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rabbaa, S.; Bouchab, H.; Tahri-Joutey, M.; Laaziouez, Y.; Limami, Y.; Pires, V.; Nasser, B.; Andreoletti, P.; Cherkaoui-Malki, M.; El Kebbaj, R. Protective Antioxidant Potential of Argan Oil Versus Other Edible Oils in LPS-Challenged Mouse Heart and Kidney. Int. J. Mol. Sci. 2025, 26, 8300. https://doi.org/10.3390/ijms26178300
Rabbaa S, Bouchab H, Tahri-Joutey M, Laaziouez Y, Limami Y, Pires V, Nasser B, Andreoletti P, Cherkaoui-Malki M, El Kebbaj R. Protective Antioxidant Potential of Argan Oil Versus Other Edible Oils in LPS-Challenged Mouse Heart and Kidney. International Journal of Molecular Sciences. 2025; 26(17):8300. https://doi.org/10.3390/ijms26178300
Chicago/Turabian StyleRabbaa, Soufiane, Habiba Bouchab, Mounia Tahri-Joutey, Yassir Laaziouez, Youness Limami, Vivien Pires, Boubker Nasser, Pierre Andreoletti, Mustapha Cherkaoui-Malki, and Riad El Kebbaj. 2025. "Protective Antioxidant Potential of Argan Oil Versus Other Edible Oils in LPS-Challenged Mouse Heart and Kidney" International Journal of Molecular Sciences 26, no. 17: 8300. https://doi.org/10.3390/ijms26178300
APA StyleRabbaa, S., Bouchab, H., Tahri-Joutey, M., Laaziouez, Y., Limami, Y., Pires, V., Nasser, B., Andreoletti, P., Cherkaoui-Malki, M., & El Kebbaj, R. (2025). Protective Antioxidant Potential of Argan Oil Versus Other Edible Oils in LPS-Challenged Mouse Heart and Kidney. International Journal of Molecular Sciences, 26(17), 8300. https://doi.org/10.3390/ijms26178300










