Papillary Thyroid Carcinoma and Body Mass Index: The Role of Immune System in Tumor Microenvironment
Abstract
1. Introduction
2. Results
2.1. Sample Features
2.2. Normalization and Gene Expression Data
2.3. Differential Analysis for Gene Functional Groups
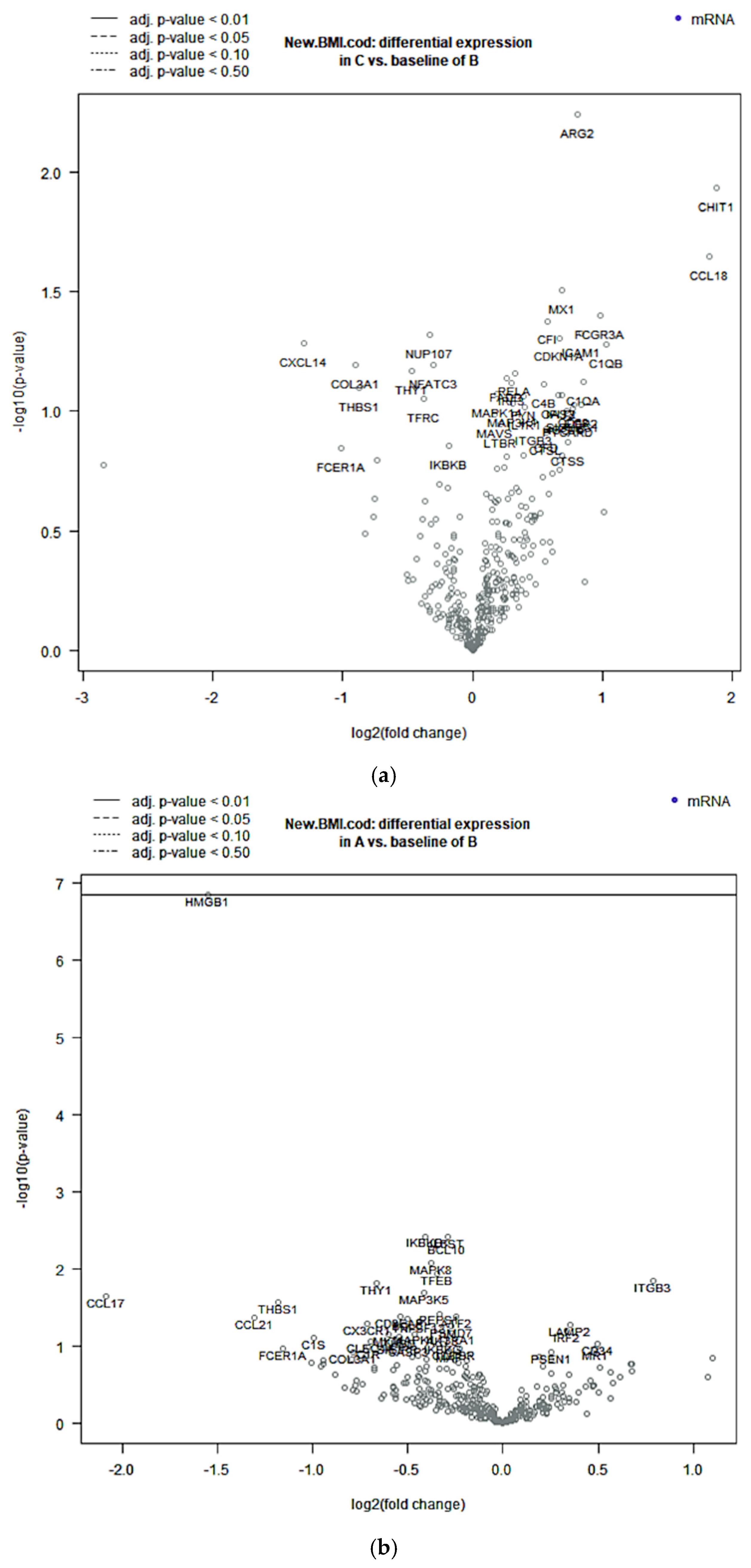
2.4. Differential Analysis for Single Genes
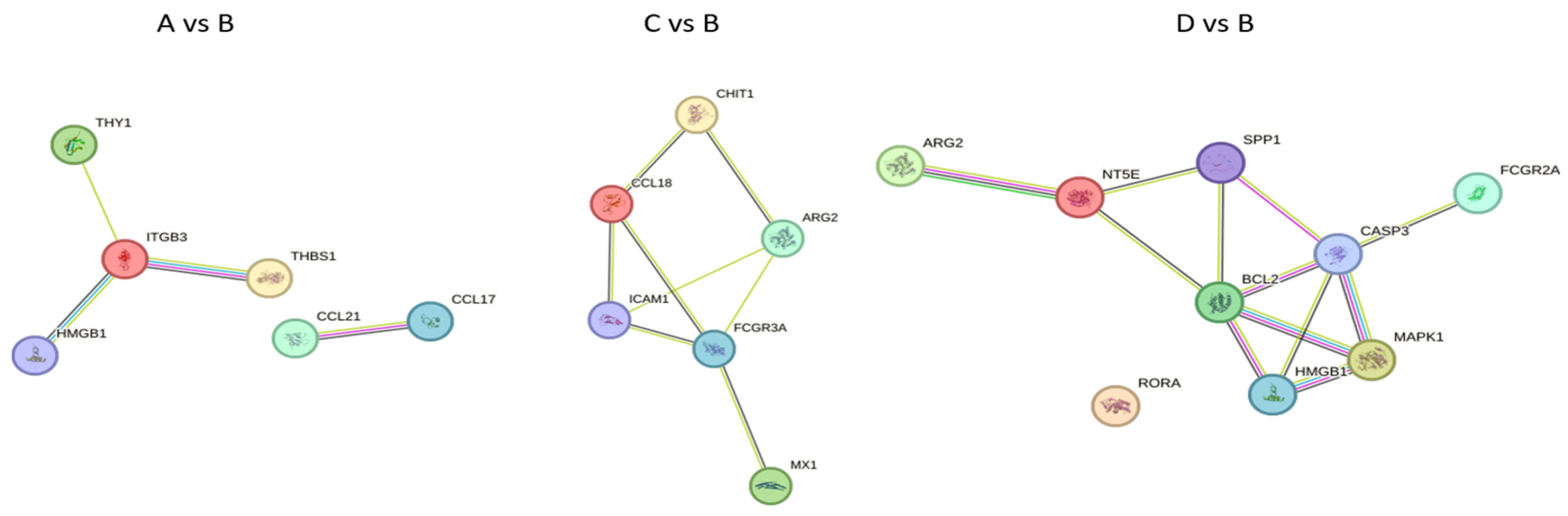
2.5. Functional Clustering Analysis
3. Discussion
4. Materials and Methods
4.1. Tissue Selection and Histologic Revision
- Underweight: BMI < 18.5 kg/m2;
- Normal weight: BMI 18.5–24.9 kg/m2;
- Overweight: BMI 25.0–29.9 kg/m2;
- Obesity: BMI ≥ 30.0 kg/m2.
4.2. Nucleic Acid Extraction and Purification
4.3. Immune-Related Gene Expression Analysis
4.4. Differential Analysis for Gene Functional Groups and for Single Genes
4.5. Functional Clustering Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ARG2 | Arginase 2; extra-hepatic arginase; mitochondrial arginase |
| BY | Benjamini–Yekutieli |
| BMI | Body Mass Index |
| DTCs | Differentiated thyroid cells |
| ETE | Extrathyroid extension |
| FDR | False discovery rate |
| FFPE | Formalin-fixed paraffin-embedded |
| HGMB1 | High-mobility group box 1 |
| PTC | Papillary thyroid cancer |
| TILs | Tumor-infiltrating lymphocytes |
| TME | Tumor microenvironment |
| WHO | World Health Organization |
| NIS | Sodium/Iodide Symporter |
Appendix A
| Sample ID | Sex | BMI | BMI Category | Size Tumor | ETE | Embolism | Multifocality | T (8th ed) | N |
|---|---|---|---|---|---|---|---|---|---|
| PS7 | F | 10.8 | A | 1 | Yes | No | Unifocal | T1a(s) | Nx |
| PS8 | F | 18 | A | 1.2 | Yes | No | Multifocal | pT1b(m) | N1b |
| PS9 | F | 18 | A | 2.2 | No | No | Multifocal | pT2(m) | Nx |
| PS10 | M | 18.1 | A | 2.5 | No | No | Unifocal | pT2(s) | N1b |
| PS11 | F | 18.3 | A | 2.1 | No | No | Unifocal | pT2(s) | N1a |
| PS12 | F | 18.3 | A | 1.5 | Yes | No | Unifocal | pT1b(s) | N1a |
| PS13 | F | 18.8 | B | 1.3 | No | No | Multifocal | pT1b(m) | Nx |
| PS33 | F | 20 | B | 1.4 | No | No | Multifocal | pT1b(m) | Nx |
| PS34 | F | 20.2 | B | 2 | No | No | Unifocal | pT1b(s) | N1b |
| PS35 | F | 21.1 | B | 1.3 | Yes | No | Multifocal | pT1b(m) | Nx |
| PS17 | F | 21.2 | B | 1.2 | Yes | No | Multifocal | pT1b(m) | Nx |
| PS18 | F | 21.6 | B | 1.4 | Yes | No | Multifocal | pT1b(m) | N1a + N1b |
| PS1 | F | 21.7 | B | 2.5 | No | No | Unifocal | pT2(s) | Nx |
| PS19 | M | 22 | B | 3.5 | No | Yes | Unifocal | pT2(s) | N1b |
| PS36 | F | 22.3 | B | 2.8 | No | No | Multifocal | pT2(m) | Nx |
| PS30 | F | 22.4 | B | 2 | Yes | No | Multifocal | pT1b(m) | N1a + N1b |
| PS20 | F | 22.9 | B | 2.5 | Yes | No | Multifocal | pT2(m) | N1a |
| PS37 | F | 23.1 | B | 2.5 | No | No | Unifocal | pT2(s) | N1a |
| PS21 | F | 24 | B | 1.3 | Yes | No | Multifocal | pT1b(m) | N1a + N1b |
| PS22 | M | 24 | B | 2 | Yes | No | Multifocal | pT1b(m) | Nx |
| PS38 | F | 24.4 | B | 1.1 | No | No | Multifocal | pT1b(m) | Nx |
| PS31 | F | 24.6 | B | 1.7 | No | No | Unifocal | pT1b(s) | Nx |
| PS24 | M | 25.1 | C | 2.2 | No | No | Multifocal | pT2(m) | N1a |
| PS39 | F | 25.2 | C | 2 | No | No | Unifocal | pT1b(s) | Nx |
| PS40 | F | 26.2 | C | 2 | No | No | Unifocal | pT1b(s) | N0 |
| PS41 | F | 26.9 | C | 2.3 | Yes | No | Unifocal | pT2(s) | Nx |
| PS42 | F | 27 | C | 1.1 | No | No | Unifocal | pT1b(s) | Nx |
| PS26 | M | 27.9 | C | 1.8 | Yes | No | Unifocal | pT1b(s) | N1a + N1b |
| PS43 | F | 28 | C | 1.6 | No | No | Multifocal | pT1b(m) | Nx |
| PS27 | F | 28.2 | C | 1.6 | Yes | No | Multifocal | pT1b(m) | Nx |
| PS16 | F | 32.9 | D | 2.8 | Yes | No | Unifocal | pT2(s) | N1a |
| PS14 | M | 37.2 | D | 1.7 | No | No | Multifocal | pT1b(m) | Nx |
| PS6 | M | 37.4 | D | 1.5 | Yes | No | Multifocal | pT1b(m) | Nx |
| PS5 | M | 39.2 | D | 3 | No | No | Multifocal | pT2(m) | Nx |
| PS3 | F | 45 | D | 1.5 | Yes | No | Unifocal | pT1b(s) | Nx |
| PS2 | F | 62.8 | D | 2 | No | No | Multifocal | pT1b(m) | Nx |

References
- Siegel, R.; Naishadham, D.; Jemal, A. Cancer statistics, 2013. CA A Cancer J. Clin. 2013, 63, 11–30. [Google Scholar] [CrossRef]
- Oh, W.J.; Lee, Y.S.; Cho, U.; Bae, J.S.; Lee, S.; Kim, M.H.; Lim, D.J.; Park, G.S.; Lee, Y.S.; Jung, C.K. Classic papillary thyroid carcinoma with tall cell features and tall cell variant have similar clinicopathologic features. Korean J. Pathol. 2014, 48, 201–208. [Google Scholar] [CrossRef]
- Ullmann, T.M.; Gray, K.D.; Moore, M.D.; Zarnegar, R.; Fahey, T.J., 3rd. Current controversies and future directions in the diagnosis and management of differentiated thyroid cancers. Gland. Surg. 2018, 7, 473–486. [Google Scholar] [CrossRef]
- Mele, C.; Samà, M.T.; Bisoffi, A.A.; Caputo, M.; Bullara, V.; Mai, S.; Walker, G.E.; Prodam, F.; Marzullo, P.; Aimaretti, G.; et al. Circulating adipokines and metabolic setting in differentiated thyroid cancer. Endocr. Connect. 2019, 8, 997–1006. [Google Scholar] [CrossRef]
- Franchini, F.; Palatucci, G.; Colao, A.; Ungaro, P.; Macchia, P.E.; Nettore, I.C. Obesity and Thyroid Cancer Risk: An Update. Int. J. Environ. Res. Public Health 2022, 19, 1116. [Google Scholar] [CrossRef]
- Basolo, A.; Poma, A.M.; Giannini, R.; Ceccarini, G.; Pelosini, C.; Fierabracci, P.; Castany, M.U.; Bechi Genzano, S.; Ambrosini, C.E.; Materazzi, G.; et al. Histological pattern and gene expression profiling of thyroid tissue in subjects with obesity. J. Endocrinol. Investig. 2022, 45, 413–423. [Google Scholar] [CrossRef]
- Piaggi, P.; Vinales, K.L.; Basolo, A.; Santini, F.; Krakoff, J. Energy expenditure in the etiology of human obesity: Spendthrift and thrifty metabolic phenotypes and energy-sensing mechanisms. J. Endocrinol. Investig. 2018, 41, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Masone, S.; Velotti, N.; Savastano, S.; Filice, E.; Serao, R.; Vitiello, A.; Berardi, G.; Schiavone, V.; Musella, M. Morbid Obesity and Thyroid Cancer Rate. A Review of Literature. J. Clin. Med. 2021, 10, 1894. [Google Scholar] [CrossRef] [PubMed]
- Khanna, D.; Khanna, S.; Khanna, P.; Kahar, P.; Patel, B.M. Obesity: A Chronic Low-Grade Inflammation and Its Markers. Cureus 2022, 14, e22711. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tumminia, A.; Vinciguerra, F.; Parisi, M.; Graziano, M.; Sciacca, L.; Baratta, R.; Frittitta, L. Adipose tissue, obesity and adiponectin: Role in endocrine cancer risk. Int. J. Mol. Sci. 2019, 20, 2863. [Google Scholar] [CrossRef]
- Hinshaw, D.C.; Shevde, L.A. The Tumor Microenvironment Innately Modulates Cancer Progression. Cancer Res. 2019, 79, 4557–4566. [Google Scholar] [CrossRef]
- Menicali, E.; Guzzetti, M.; Morelli, S.; Moretti, S.; Puxeddu, E. Immune Landscape of Thyroid Cancers: New Insights. Front. Endocrinol. 2021, 11, 637826. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shin, A.; Cho, S.; Jang, D.; Abe, S.K.; Saito, E.; Rahman, M.S.; Islam, M.R.; Sawada, N.; Shu, X.-O.; Koh, W.-P.; et al. Body Mass Index and Thyroid Cancer Risk: A Pooled Analysis of Half a Million Men and Women in the Asia Cohort Consortium. Thyroid 2022, 32, 306–314. [Google Scholar] [CrossRef]
- Yin, H.; Tang, Y.; Guo, Y.; Wen, S. Immune Microenvironment of Thyroid Cancer. J. Cancer 2020, 11, 4884–4896. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, S.; Han, H.; Yang, K.; Li, X.; Ma, L.; Yang, Z.; Zhao, Y.X. Emerging role of metabolic reprogramming in the immune microenvironment and immunotherapy of thyroid cancer. Int. Immunopharmacol. 2025, 144, 113702. [Google Scholar] [CrossRef] [PubMed]
- Matrone, A.; Basolo, A.; Santini, F.; Elisei, R. Understanding the effect of obesity on papillary thyroid cancer: Is there a need for tailored diagnostic and therapeutic management? Expert. Rev. Endocrinol. Metab. 2022, 17, 475–484. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Li, X.; He, Y.; Wu, S.; Wang, S.; Sun, J.; He, Y.; Lun, Y.; Zhang, J. Immune Cell Confrontation in the Papillary Thyroid Carcinoma Microenvironment. Front. Endocrinol. 2020, 11, 570604. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kaymak, I.; Williams, K.S.; Cantor, J.R.; Jones, R.G. Immunometabolic Interplay in the Tumor Microenvironment. Cancer Cell 2021, 39, 28–37. [Google Scholar] [CrossRef]
- Carmona-Fontaine, C.; Deforet, M.; Akkari, L.; Thompson, C.B.; Joyce, J.A.; Xavier, J.B. Metabolic origins of spatial organization in the tumor microenvironment. Proc. Natl. Acad. Sci. USA 2017, 114, 2934–2939. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rodriguez, P.C.; Zea, A.H.; Culotta, K.S.; Zabaleta, J.; Ochoa, J.B.; Ochoa, A.C. Regulation of T cell receptor CD3zeta chain expression by L-arginine. J. Biol. Chem. 2002, 277, 21123–21129. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Hu, Y.; Gu, Y.; Lin, X.; Jiang, X.; Gong, C.; Fang, Z. Role of amino acid metabolism in tumor immune microenvironment of colorectal cancer. Am. J. Cancer Res. 2025, 15, 233–247. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Qi, D.; Wu, E. Cancer prognosis: Considering tumor and its microenvironment as a whole. EBioMedicine 2019, 43, 28–29. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Kumaravel, S.; Sharma, A.; Duran, C.L.; Bayless, K.J.; Chakraborty, S. Hypoxic tumor microenvironment: Implications for cancer therapy. Exp. Biol. Med. 2020, 245, 1073–1086. [Google Scholar] [CrossRef]
- Fussey, J.M.; Beaumont, R.N.; Wood, A.R.; Vaidya, B.; Smith, J.; Tyrrell, J. Does Obesity Cause Thyroid Cancer? A Mendelian Randomization Study. J. Clin. Endocrinol. Metab. 2020, 105, e2398–e2407. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bradley, D. Obesity, Thyroid Nodularity, and Thyroid Cancer: Epiphenomenon or Cause? J. Clin. Endocrinol. Metab. 2020, 105, e3010–e3012. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Strulov Shachar, S.; Williams, G.R. The Obesity Paradox in Cancer-Moving Beyond BMI. Cancer Epidemiol. Biomark. Prev. 2017, 26, 13–16. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mukherjee, S.; Seager, R.J.; Lee, Y.H.; Conroy, J.M.; Kalinski, P.; Pabla, S. Tumor Inflammation, Obesity, and Proliferative Status as Biomarkers in Gastroesophageal Adenocarcinoma. J. Pers. Med. 2021, 11, 1324. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zeigler-Johnson, C.; Morales, K.H.; Lal, P.; Feldman, M. The Relationship between Obesity, Prostate Tumor Infiltrating Lymphocytes and Macrophages, and Biochemical Failure. PLoS ONE 2016, 11, e0159109. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, S.; Zhang, Y. HMGB1 in inflammation and cancer. J. Hematol. Oncol. 2020, 13, 116. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chai, W.; Ye, F.; Zeng, L.; Li, Y.; Yang, L. HMGB1-mediated autophagy regulates sodium/iodide symporter protein degradation in thyroid cancer cells. J. Exp. Clin. Cancer Res. 2019, 38, 325. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zaytouni, T.; Tsai, P.Y.; Hitchcock, D.S.; DuBois, C.D.; Freinkman, E.; Lin, L.; Morales-Oyarvide, V.; Lenehan, P.J.; Wolpin, B.M.; Mino-Kenudson, M.; et al. Critical role for arginase 2 in obesity-associated pancreatic cancer. Nat. Commun. 2017, 8, 242. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- SClemente, G.; van Waarde, A.; FAntunes, I.; Dömling, A.; HElsinga, P. Arginase as a Potential Biomarker of Disease Progression: A Molecular Imaging Perspective. Int. J. Mol. Sci. 2020, 21, 5291. [Google Scholar] [CrossRef]
- Yu, Y.; Rajapakse, A.G.; Montani, J.P.; Yang, Z.; Ming, X.F. p38 mitogen-activated protein kinase is involved in arginase-II-mediated eNOS-uncoupling in obesity. Cardiovasc. Diabetol. 2014, 13, 113. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kim, H.J.; Kim, B.; Byun, H.J.; Yu, L.; Nguyen, T.M.; Nguyen, T.H.; Do, P.A.; Kim, E.J.; Cheong, K.A.; Kim, K.S.; et al. Resolvin D1 Suppresses H2O2-Induced Senescence in Fibroblasts by Inducing Autophagy through the miR-1299/ARG2/ARL1 Axis. Antioxidants 2021, 10, 1924. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kim, N.H.; Choi, S.H.; Yi, N.; Lee, T.R.; Lee, A.Y. Arginase-2, a miR-1299 target, enhances pigmentation in melasma by reducing melanosome degradation via senescence-induced autophagy inhibition. Pigment. Cell Melanoma Res. 2017, 30, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Rajapakse, A.G.; Riedo, E.; Fellay, B.; Bernhard, M.C.; Montani, J.P.; Yang, Z.; Ming, X.F. Targeting arginase-II protects mice from high-fat-diet-induced hepatic steatosis through suppression of macrophage inflammation. Sci. Rep. 2016, 6, 20405. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Giatromanolaki, A.; Harris, A.L.; Koukourakis, M.I. The prognostic and therapeutic implications of distinct patterns of argininosuccinate synthase 1 (ASS1) and arginase-2 (ARG2) expression by cancer cells and tumor stroma in non-small-cell lung cancer. Cancer Metab. 2021, 9, 28. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wei, Z.; Liu, X.; Cheng, C.; Yu, W.; Yi, P. Metabolism of Amino Acids in Cancer. Front. Cell Dev. Biol. 2021, 8, 603837. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. STRING v11: Protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019, 47, D607–D613. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sparavelli, R.; Giannini, R.; Signorini, F.; Materazzi, G.; Basolo, A.; Santini, F.; Ugolini, C. Papillary Thyroid Carcinoma and Body Mass Index: The Role of Immune System in Tumor Microenvironment. Int. J. Mol. Sci. 2025, 26, 8290. https://doi.org/10.3390/ijms26178290
Sparavelli R, Giannini R, Signorini F, Materazzi G, Basolo A, Santini F, Ugolini C. Papillary Thyroid Carcinoma and Body Mass Index: The Role of Immune System in Tumor Microenvironment. International Journal of Molecular Sciences. 2025; 26(17):8290. https://doi.org/10.3390/ijms26178290
Chicago/Turabian StyleSparavelli, Rebecca, Riccardo Giannini, Francesca Signorini, Gabriele Materazzi, Alessio Basolo, Ferruccio Santini, and Clara Ugolini. 2025. "Papillary Thyroid Carcinoma and Body Mass Index: The Role of Immune System in Tumor Microenvironment" International Journal of Molecular Sciences 26, no. 17: 8290. https://doi.org/10.3390/ijms26178290
APA StyleSparavelli, R., Giannini, R., Signorini, F., Materazzi, G., Basolo, A., Santini, F., & Ugolini, C. (2025). Papillary Thyroid Carcinoma and Body Mass Index: The Role of Immune System in Tumor Microenvironment. International Journal of Molecular Sciences, 26(17), 8290. https://doi.org/10.3390/ijms26178290








