Are an Aging Gut and a Decrease in Butyrate Production the Reasons for Atherosclerosis?
Abstract
1. Introduction
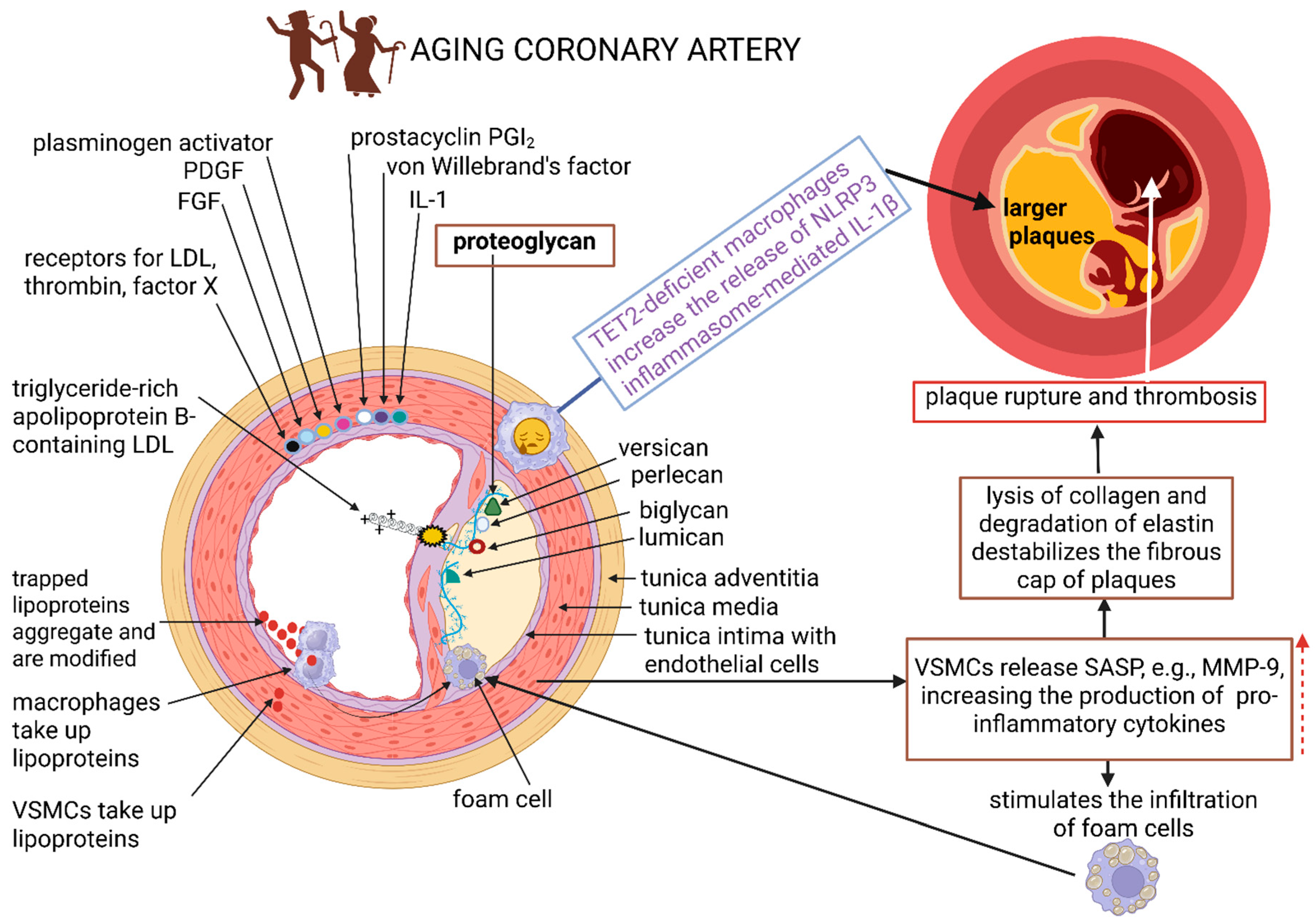
2. Changes in the Vessel Wall
3. Changes in the Gut Microbiome
4. Molecular Changes Driven by Gut Microbiota
5. Immunosenescence in Aging and Atherosclerosis
6. The Link Between Butyrate and Atherosclerosis (AS)
| Bacteria | Source | Methodology | References |
|---|---|---|---|
| Collinsella spp., Enterobacteriaceae, Streptococcaceae, Klebsiella spp. (Eubacterium spp., Roseburia spp., Ruminococcaceae) | Human | Metagenome sequencing | [130] |
| (Bacteroides xylanisolvens, Odoribacter splanchnicus, Eubacterium eligens, Roseburia inulinivorans, Roseburia intestinalis) | Human | Metagenome sequencing | [131] |
| Firmicutes phylum (Bacteroidetes) | Human | 16S rRNA sequencing | [132] |
| Streptococcus spp., Escherichia coli, Lactobacillus salivarius, Solobacterium moorei, Atopobium parvulum, Ruminococcus gnavus, Eggerthella lenta (Bacteroides spp., Prevotella spp., Roseburia intestinalis, Faecalibacterium prausnitzii, Bacteroides spp., Prevotella copri, Alistipes shahii) | Human | Metagenome sequencing | [133] |
| Escherichia coli, Shigella spp., Enterococcus spp. (Faecalibacterium spp., Subdoligranulum spp., Roseburia spp., Eubacterium rectale) | Human | 16S rRNA sequencing | [134] |
| Clostridium sp. HGF2, Streptococcus sp. M334, Streptococcus sp. M143 | Human | Metagenome sequencing | [135] |
| Faecalibacterium prausnitzii, Prevotella copri (Bacteroides vulgatus, Bacteroides dorei) | Human | 16S rRNA sequencing | [136] |
| (Bacteroides xylanisolvens, Odoribacter splanchnicus, Eubacterium eligens, Roseburia inulinivorans, Roseburia intestinalis) | Human | 16S rRNA sequencing | [137] |
| Enterorhabdus spp., Romboutsia spp., Proteus spp., Eubacterium nodatum, Escherichia spp., Shigella spp., Eubacterium coprostanoligenes, Parasutterella spp., Muribaculum spp., Enterococcus spp. (Bifidobacterium spp., Alistipes spp.) | Mice | 16S rRNA sequencing | [138] |
| Proteobacteria, Firmicutes, Romboutsia spp., Lactococcus spp. (Bacteroidetes, Actinobacteria, Faecalibaculum spp., Bifidobacterium spp., Bacteroides spp., Parabacteroides spp., Alloprevotella spp., Alistipes spp., Odoribacter spp., Allobaculum spp.) | Mice | 16S rRNA sequencing | [139] |
| Firmicutes, Faecalibaculum spp., Oscillibacter spp., Eubacterium coprostanoligenes-group, Blautia spp. (Muribaculaceae, Lactobacillus spp., Ileibacterium spp., Bifidobacterium spp.) | Mice | 16S rRNA sequencing | [140] |
| Firmicutes, Bacteroidota, Lactobacillaceae, Lactobacillus spp., Helicobacter spp. (Lachnospiraceae, Roseburia spp.) | Mice | 16S rRNA sequencing | [141] |
| Firmicutes, Bacteroidota, Verrucomicrobia, Ruminococcaceae, Bacteroidaceae, Bacteroides spp., Akkermansia spp. (Rikenellaceae) | Mice | 16S rRNA sequencing | [142] |
| Firmicutes, Lactobacillus spp. (Bacteroidetes; Bifidobacterium spp.) | Mice | 16S rRNA sequencing | [143] |
7. Limitations in Studies Linking Gut Microbiota to Atherosclerosis
8. Future Directions
9. Conclusions
Funding
Conflicts of Interest
References
- Wong, J.J.; Hong, R.; Teo, L.L.Y.; Tan, R.-S.; Koh, A.S. Atherosclerotic cardiovascular disease in aging and the role of advanced cardiovascular imaging. npj Cardiovasc. Health 2024, 1, 11. [Google Scholar] [CrossRef]
- Afzaal, M.; Saeed, F.; Shah, Y.A.; Hussain, M.; Rabail, R.; Socol, C.T.; Hassoun, A.; Pateiro, M.; Lorenzo, J.M.; Rusu, A.V.; et al. Human gut microbiota in health and disease: Unveiling the relationship. Front. Microbiol. 2022, 13, 999001. [Google Scholar] [CrossRef]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. Hallmarks of aging: An expanding universe. Cell 2023, 186, 243–278. [Google Scholar] [CrossRef]
- Lu, A.T.; Fei, Z.; Haghani, A.; Robeck, T.R.; Zoller, J.A.; Li, C.Z.; Lowe, R.; Yan, Q.; Zhang, J.; Vu, H.; et al. Universal DNA methylation age across mammalian tissues. Nat. Aging 2023, 3, 1144–1166, Correct in Nat. Aging 2023, 3, 1462. [Google Scholar] [CrossRef]
- Li, A.; Koch, Z.; Ideker, T. Epigenetic aging: Biological age prediction and informing a mechanistic theory of aging. J. Intern. Med. 2022, 292, 733–744. [Google Scholar] [CrossRef]
- Tenchov, R.; Sasso, J.M.; Wang, X.; Zhou, Q.A. Aging hallmarks and progression and age-related diseases: A landscape view of research advancement. ACS Chem. Neurosci. 2024, 15, 1–30. [Google Scholar] [CrossRef]
- Vellasamy, D.M.; Lee, S.J.; Goh, K.W.; Goh, B.H.; Tang, Y.Q.; Ming, L.C.; Yap, W.H. Targeting immune senescence in atherosclerosis. Int. J. Mol. Sci. 2022, 23, 13059. [Google Scholar] [CrossRef]
- Dicks, L.M.T. Butyrate produced by gut microbiota regulates atherosclerosis: A narrative review of the latest findings. Int. J. Mol. Sci. 2025, 26, 6744. [Google Scholar] [CrossRef]
- Pahwa, R.; Goyal, A.; Jialal, I. Chronic Inflammation. In StatPearls; Updated 7 August 2023; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK493173/ (accessed on 4 July 2025).
- Ding, W.; Chen, J.; Zhao, L.; Wu, S.; Chen, X.; Chen, H. Mitochondrial DNA leakage triggers inflammation in age-related cardiovascular diseases. Front. Cell Dev. Biol. 2024, 12, 1287447. [Google Scholar] [CrossRef] [PubMed]
- Xia, T.; Yu, J.; Du, M.; Chen, X.; Wang, C.; Li, R. Vascular endothelial cell injury: Causes, molecular mechanisms, and treatments. MedComm 2025, 6, e70057. [Google Scholar] [CrossRef] [PubMed]
- Bleve, A.; Motta, F.; Durante, B.; Pandolfo, C.; Selmi, C.; Sica, A. Immunosenescence, inflammaging, and frailty: Role of myeloid cells in age-related diseases. Clin. Rev. Allergy Immunol. 2023, 64, 123–144. [Google Scholar] [CrossRef]
- Farooq, M.; Khan, A.W.; Kim, M.S.; Choi, S. The role of fibroblast growth factor (FGF) signaling in tissue repair and regeneration. Cells 2021, 10, 3242. [Google Scholar] [CrossRef]
- Eichhorn, T.; Weiss, R.; Huber, S.; Ebeyer-Masotta, M.; Mostageer, M.; Emprechtinger, R.; Knabl, L., Sr.; Knabl, L.; Würzner, R.; Weber, V. Expression of tissue factor and platelet/leukocyte markers on extracellular vesicles reflect platelet-leukocyte interaction in severe COVID-19. Int. J. Mol. Sci. 2023, 24, 16886. [Google Scholar] [CrossRef]
- Manetti, M.; Kahaleh, B. Mechanisms of vascular disease. In Scleroderma; Allanore, Y., Varga, J., Denton, C.P., Kuwana, M., Chung, L., Shah, A.A., Eds.; Springer: Cham, Switzerland, 2024. [Google Scholar] [CrossRef]
- Dunn, W.G.; McLoughlin, M.A.; Vassiliou, G.S. Clonal hematopoiesis and hematological malignancy. J. Clin. Investig. 2024, 134, e180065. [Google Scholar] [CrossRef] [PubMed]
- Barbu, E.; Popescu, M.R.; Popescu, A.C.; Balanescu, S.M. Inflammation as a precursor of atherothrombosis, diabetes and early vascular aging. Int. J. Mol. Sci. 2022, 23, 963. [Google Scholar] [CrossRef] [PubMed]
- Dicks, L.M.T. Cardiovascular disease may be triggered by gut microbiota, microbial metabolites, gut wall reactions, and inflammation. Int. J. Mol. Sci. 2024, 25, 10634. [Google Scholar] [CrossRef] [PubMed]
- Tacconi, E.; Palma, G.; De Biase, D.; Luciano, A.; Barbieri, M.; de Nigris, F.; Bruzzese, F. Microbiota effect on trimethylamine N-oxide production: From cancer to fitness—A practical preventing recommendation and therapies. Nutrients 2023, 15, 563. [Google Scholar] [CrossRef]
- Ramachandran, R.; Manan, A.; Kim, J.; Choi, S. NLRP3 inflammasome: A key player in the pathogenesis of life-style disorders. Exp. Mol. Med. 2024, 56, 1488–1500. [Google Scholar] [CrossRef]
- Puspitasari, Y.M.; Ministrini, S.; Schwarz, L.; Karch, C.; Liberale, L.; Camici, G.G. Modern concepts in cardiovascular disease: Inflamm-Aging. Front. Cell Dev. Biol. 2022, 10, 882211. [Google Scholar] [CrossRef]
- Pal, R.; Bhadada, S.L. AGEs accumulation with vascular complications, glycemic control and metabolic syndrome: A narrative review. Bone 2023, 176, 116884. [Google Scholar] [CrossRef]
- Booth, J.S.; Toapanta, F.R. B and T Cell Immunity in Tissues and Across the Ages. Vaccines 2021, 9, 24. [Google Scholar] [CrossRef] [PubMed]
- Taleb, S. Inflammation in atherosclerosis. Arch. Cardiovasc. Dis. 2016, 109, 708–715. [Google Scholar] [CrossRef]
- He, C.; Kim, H.I.; Park, J.; Guo, J.; Huang, W. The role of immune cells in different stages of atherosclerosis. Int. J. Med. Sci. 2024, 21, 1129–1143. [Google Scholar] [CrossRef]
- Zhou, B.; Lin, W.; Long, Y. Notch signaling pathway: Architecture, disease, and therapeutics. Signal Transduct. Target. Ther. 2022, 7, 95. [Google Scholar] [CrossRef]
- Qian, C.; You, X.; Gao, B.; Sun, Y.; Liu, C. The role of ROS in atherosclerosis and ROS-based nanotherapeutics for atherosclerosis: Atherosclerotic lesion targeting, ROS scavenging, and ROS-responsive activity. ACS Omega 2025, 10, 22366–22381. [Google Scholar] [CrossRef]
- Galimberti, F.; Casula, M.; Olmastrini, E. Apolipoprotein B compared with low-density lipoprotein cholesterol in the atherosclerotic cardiovascular diseases risk assessment. Pharmacol. Res. 2023, 95, 106873. [Google Scholar] [CrossRef]
- Picos, A.; Seoane, N.; Campos-Toimil, M.; Viña, D. Vascular senescence and aging: Mechanisms, clinical implications, and therapeutic prospects. Biogerontology 2025, 26, 118. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, X.; Liu, T.; Zhu, X.; Pan, X. The multifaceted role of the SASP in atherosclerosis: From mechanisms to therapeutic opportunities. Cell Biosci. 2022, 12, 74. [Google Scholar] [CrossRef] [PubMed]
- Cobo, I.; Tanaka, T.N.; Mangalhara, K.C.; Lana, A.; Yeang, C.; Han, C.; Schlachetzki, J.; Challcombe, J.; Fixsen, B.R.; Sakai, M.; et al. DNA methyltransferase 3 alpha and TET methylcytosine dioxygenase 2 restrain mitochondrial DNA-mediated interferon signaling in macrophages. Immunity 2022, 55, 1386–1401. [Google Scholar] [CrossRef]
- Ghamar Talepoor, A.; Doroudchi, M. Immunosenescence in atherosclerosis: A role for chronic viral infections. Front. Immunol. 2022, 13, 945016. [Google Scholar] [CrossRef] [PubMed]
- Dicks, L.M.T. How important are fatty acids in human health and can they be used in treating diseases? Gut Microbes 2024, 16, 2420765. [Google Scholar] [CrossRef] [PubMed]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What is the healthy gut microbiota composition? A changing ecosystem across age, environment, diet, and diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef]
- de Crom, S.C.M.; Rossen, J.W.A.; van Furth, A.M.; Obihara, C.C. Enterovirus and parechovirus infection in children: A brief overview. Eur. J. Pediatr. 2016, 175, 1023–1029. [Google Scholar] [CrossRef]
- Kapoor, A.; Li, L.; Victoria, J. Multiple novel astrovirus species in human stool. J. Gen. Virol. 2009, 90, 2965–2972. [Google Scholar] [CrossRef]
- Ahmed, S.M.; Hall, A.J.; Robinson, A.E. Global prevalence of Norovirus in cases of gastroenteritis: A systematic review and meta-analysis. Lancet Infect. Dis. 2014, 14, 725–730. [Google Scholar] [CrossRef] [PubMed]
- Lecuit, M.; Eloit, M. The viruses of the gut microbiota. In The Microbiota in Gastrointestinal Pathophysiology; Academic Press: Cambridge, MA, USA, 2017; pp. 179–183. [Google Scholar]
- Rosenberg, E. Diversity of bacteria within the human gut and its contribution to the functional unity of holobionts. npj Biofilms Microbiomes 2024, 10, 134. [Google Scholar] [CrossRef] [PubMed]
- Coscia, A.; Bardanzellu, F.; Caboni, E.; Fanos, V.; Peroni, D.G. When a neonate is born, so is a microbiota. Life 2021, 11, 148. [Google Scholar] [CrossRef]
- Dicks, L.M.T.; Hurn, D.; Hermanus, D. Gut bacteria and neuropsychiatric disorders. Microorganisms 2021, 9, 2583. [Google Scholar] [CrossRef]
- Wang, J.; Lin, L. Early-life gut microbiota development from maternal vertical transmission. Gynecol. Obstet. Clin. Med. 2021, 1, 79–82. [Google Scholar] [CrossRef]
- Crovesy, L.; Masterson, D.; Rosado, E.L. Profile of the gut microbiota of adults with obesity: A systematic review. Eur. J. Clin. Nutr. 2020, 74, 1251–1262. [Google Scholar] [CrossRef]
- Roswall, J.; Olsson, L.M.; Kovatcheva-Datchary, P.; Nilsson, S.; Tremaroli, V.; Simon, M.-C.; Kiilerich, P.; Krämer, A.M.; Uhlén, M.; Gummesson, A.; et al. Developmental trajectory of the healthy human gut microbiota during the first 5 years of life. Cell Host Microbe 2021, 29, 765–776.e3. [Google Scholar] [CrossRef]
- Severino, A.; Tohumcu, E.; Tamai, L.; Dargenio, P.; Porcari, S.; Rondinella, D.; Venturini, I.; Maida, M.; Gasbarrini, A.; Cammarota, G.; et al. The microbiome-driven impact of western diet in the development of noncommunicable chronic disorders. Best Pract. Res. Clin. Gastroenterol. 2024, 72, 101923. [Google Scholar] [CrossRef]
- Magne, F.; Gotteland, M.; Gauthier, L.; Zazueta, A.; Pesoa, S.; Navarrete, P.; Balamurugan, R. The Firmicutes/Bacteroidetes ratio: A relevant marker of gut dysbiosis in obese patients? Nutrients 2020, 12, 1474. [Google Scholar] [CrossRef]
- Desai, M.S.; Seekatz, A.M.; Koropatkin, N.M.; Kamada, N.; Hickey, C.A.; Wolter, M.; Pudlo, N.A.; Kitamoto, S.; Terrapon, N.; Muller, A.; et al. A dietary fiber-deprived gut microbiota degrades the colonic mucus barrier and enhances pathogen susceptibility. Cell 2016, 167, 1339–1353. [Google Scholar] [CrossRef]
- Chen, D.; Wu, L.; Liu, X. Helicobacter pylori CagA mediated mitophagy to attenuate the NLRP3 inflammasome activation and enhance the survival of infected cells. Sci. Rep. 2024, 14, 21648. [Google Scholar] [CrossRef]
- Baragetti, A.; Severgnini, M.; Olmastroni, E.; Dioguardi, C.C.; Mattavelli, E.; Angius, A.; Rotta, L.; Cibella, J.; Caredda, G.; Consolandi, C.; et al. Gut microbiota functional dysbiosis relates to individual diet in subclinical carotid atherosclerosis. Nutrients 2021, 13, 304. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.; Chen, S.; Gu, G.; Zhou, J.; Wang, W.; Ren, J.; Wu, J.; Yang, D.; Zheng, Y. Exploration of crucial mediators for carotid atherosclerosis pathogenesis through integration of microbiome, metabolome, and transcriptome. Front. Physiol. 2021, 12, 645212. [Google Scholar] [CrossRef] [PubMed]
- Meslier, V.; Laiola, M.; Roager, H.M.; De Filippis, F.; Roume, H.; Quinquis, B.; Giacco, R.; Mennella, I.; Ferracane, R.; Pons, N.; et al. Mediterranean diet intervention in overweight and obese subjects lowers plasma cholesterol and causes changes in the gut microbiome and metabolome independently of energy intake. Gut 2020, 69, 1258–1268. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Lee, G.; Son, H.; Koh, H.; Kim, E.S.; Unno, T.; Shin, J.H. Butyrate producers, “The Sentinel of Gut”: Their intestinal 2ignificance with and beyond butyrate, and prospective use as microbial therapeutics. Front. Microbiol. 2023, 13, 1103836. [Google Scholar] [CrossRef]
- Odamaki, T.; Kato, K.; Sugahara, H.; Hashikura, N.; Takahashi, S.; Xiao, J.Z.; Abe, F.; Osawa, R. Age-related changes in gut microbiota composition from newborn to centenarian: A cross-sectional study. BMC Microbiol. 2016, 16, 90. [Google Scholar] [CrossRef]
- Furusawa, Y.; Obata, Y.; Fukuda, S.; Endo, T.A.; Nakato, G.; Takahashi, D.; Nakanishi, Y.; Uetake, C.; Kato, K.; Kato, T.; et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 2013, 504, 446–450, Erratum in Nature 2013, 504, 254. [Google Scholar] [CrossRef]
- Salazar, N.; Arboleya, S.; Fernandez-Navarro, T.; de Los, C.G.; Gonzalez, S.; Gueimonde, M. Age-associated changes in gut microbiota and dietary components related with the immune system in adulthood and old age: A cross-sectional study. Nutrients 2019, 11, 11. [Google Scholar] [CrossRef] [PubMed]
- Ragonnaud, E.; Biragyn, A. Gut microbiota as the key controllers of “healthy” aging of elderly people. Immun. Ageing 2021, 18, 2. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Imlay, J.A. When anaerobes encounter oxygen: Mechanisms of oxygen toxicity, tolerance and defence. Nat. Rev. Microbiol. 2021, 19, 774–785. [Google Scholar] [CrossRef]
- DeJong, E.N.; Surette, M.G.; Bowdish, D. The gut microbiota and unhealthy aging: Disentangling cause from consequence. Cell Host Microbe 2020, 28, 180–189. [Google Scholar] [CrossRef]
- Kanimozhi, N.V.; Sukumar, M. Aging through the lens of the gut microbiome: Challenges and therapeutic opportunities. Arch. Gerontol. Geriatr. Plus 2025, 2, 100142. [Google Scholar] [CrossRef]
- Zhao, M.; Chu, J.; Feng, S.; Guo, C.; Xue, B.; He, K.; Li, L. Immunological mechanisms of inflammatory diseases caused by gut microbiota dysbiosis: A review. Biomed. Pharmacother. 2023, 64, 114985. [Google Scholar] [CrossRef]
- Xiao, M.; Zhang, C.; Duan, H. Cross-feeding of bifidobacteria promotes intestinal homeostasis: A lifelong perspective on the host health. npj Biofilms Microbiomes 2024, 10, 47. [Google Scholar] [CrossRef]
- Martins, D.; Silva, C.; Ferreira, A.C.; Dourado, S.; Albuquerque, A.; Saraiva, F.; Batista, A.B.; Castro, P.; Leite-Moreira, A.; Barros, A.S.; et al. Unravelling the gut microbiome role in cardiovascular disease: A systematic review and a meta-analysis. Biomolecules 2024, 14, 731. [Google Scholar] [CrossRef]
- Zhang, S.M.; Huang, S.L. The commensal anaerobe Veillonella dispar reprograms its lactate metabolism and short-chain fatty acid production during the stationary phase. Microbiol. Spectr. 2023, 11, e0355822. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, M.; Liu, Y.; Wei, F.; Li, X.; Feng, Y.; Jin, X.; Liu, D.; Guo, Y.; Hu, Y. Inulin-enriched Megamonas funiformis ameliorates metabolic dysfunction-associated fatty liver disease by producing propionic acid. npj Biofilms Microbiomes 2024, 9, 84, Correct in npj Biofilms Microbiomes 2024, 10, 9. [Google Scholar] [CrossRef]
- Sayols-Baixeras, S.; Dekkers, K.F.; Baldanzi, G.; Jönsson, D.; Hammar, U.; Lin, Y.T.; Ahmad, S.; Nguyen, D.; Varotsis, G.; Pita, S.; et al. Streptococcus species abundance in the gut is linked to subclinical coronary atherosclerosis in 8973 participants from the SCAPIS Cohort. Circulation 2023, 148, 459–472. [Google Scholar] [CrossRef]
- Wang, L.; Wang, S.; Zhang, Q.; He, C.; Fu, C.; Wei, Q. The role of the gut microbiota in health and cardiovascular diseases. Mol. Biomed. 2022, 3, 30. [Google Scholar] [CrossRef]
- Guamán, L.P.; Carrera-Pacheco, S.E.; Zúñiga-Miranda, J.; Teran, E.; Erazo, C.; Barba-Ostria, C. The impact of bioactive molecules from probiotics on child health: A comprehensive review. Nutrients 2024, 16, 3706. [Google Scholar] [CrossRef]
- Nie, K.; Ma, K.; Luo, W.; Shen, Z.; Yang, Z.; Xiao, M.; Tong, T.; Yang, Y.; Wang, X. Roseburia intestinalis: A beneficial gut organism from the discoveries in genus and species. Front. Cell. Infect. Microbiol. 2021, 11, 757718. [Google Scholar] [CrossRef] [PubMed]
- Rath, S.; Rox, K.; Kleine, B.S.; Schminke, U.; Dorr, M.; Mayerle, J.; Frost, F.; Lerch, M.M.; Karch, A.; Brönstrup, M.; et al. Higher trimethylamine-N-oxide plasma levels with increasing age are mediated by diet and trimethylamine-forming bacteria. mSystems 2021, 6, e94521. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.; Piceno, Y.M.; DeSantis, T.Z.; Pahl, M.; Andersen, G.L.; Vaziri, N.D. Expansion of urease- and uricase-containing, indole- and p-cresol-forming and contraction of short-chain fatty acid-producing intestinal microbiota in ESRD. Am. J. Nephrol. 2014, 39, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yang, S.; Li, S.; Zhao, L.; Hao, Y.; Qin, J.; Zhang, L.; Zhang, C.; Bian, W.; Zuo, L.; et al. Aberrant gut microbiota alters host metabolome and impacts renal failure in humans and rodents. Gut 2020, 69, 2131–2142. [Google Scholar] [CrossRef]
- Zhu, W.; Gregory, J.C.; Org, E.; Buffa, J.A.; Gupta, N.; Wang, Z.; Li, L.; Fu, X.; Wu, Y.; Mehrabian, M.; et al. Gut microbial metabolite TMAO enhances platelet hyperreactivity and thrombosis risk. Cell 2016, 165, 111–124. [Google Scholar] [CrossRef]
- Ding, L.; Chang, M.; Guo, Y.; Zhang, L.; Xue, C.; Yanagita, T.; Zhang, T.; Wang, Y. Trimethylamine-N-oxide (TMAO)-induced atherosclerosis is associated with bile acid metabolism. Lipids Health Dis. 2018, 17, 286. [Google Scholar] [CrossRef]
- Chen, M.L.; Zhu, X.H.; Ran, L.; Lang, H.D.; Yi, L.; Mi, M.T. Trimethylamine-N-oxide induces vascular inflammation by activating the NLRP3 inflammasome through the sirt3-sod2-mtros signaling pathway. J. Am. Heart Assoc. 2017, 6, e003698. [Google Scholar] [CrossRef]
- Ma, G.; Pan, B.; Chen, Y.; Guo, C.; Zhao, M.; Zheng, L.; Chen, B. Trimethylamine N-oxide in atherogenesis: Impairing endothelial self-repair capacity and enhancing monocyte adhesion. Biosci. Rep. 2017, 37, BSR20160244. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, L.; Ren, P.; Yang, Y.; Li, S.; Qin, X.; Zhang, M.; Zhou, M.; Liu, W. Qing-Xue-Xiao-Zhi formula attenuates atherosclerosis by inhibiting macrophage lipid accumulation and inflammatory response via TLR4/MyD88/NF-κB pathway regulation. Phytomedicine 2021, 93, 153812. [Google Scholar] [CrossRef]
- Yu, L.; Meng, G.; Huang, B.; Zhou, X.; Stavrakis, S.; Wang, M.; Li, X.; Zhou, L.; Wang, Y.; Wang, M.; et al. A potential relationship between gut microbes and atrial fibrillation: Trimethylamine N-oxide, a gut microbe-derived metabolite, facilitates the progression of atrial fibrillation. Int. J. Cardiol. 2018, 255, 92–98. [Google Scholar] [CrossRef]
- Baidoo, N.; Sanger, G.J. Age-related decline in goblet cell numbers and mucin content of the human colon: Implications for lower bowel functions in the elderly. Exp. Mol. Pathol. 2024, 139, 104923. [Google Scholar] [CrossRef] [PubMed]
- Buisine, M.P.; Devisme, L.; Savidge, T.C.; Gespach, C.; Gosselin, B.; Porchet, N.; Aubert, J.P. Mucin gene expression in human embryonic and fetal intestine. Gut 1998, 43, 519–524. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Kim, G.; Shafer, S.; Chen, Z.; Kubo, S.; Ji, Y.; Luo, J.; Yang, W.; Perner, S.P.; Kanellopoulou, C.; et al. Mucus sialylation determines intestinal host-commensal homeostasis. Cell 2022, 185, 1172–1188. [Google Scholar] [CrossRef]
- Choi, E.L.; Taheri, N.; Tan, E.; Matsumoto, K.; Hayashi, Y. The crucial role of the interstitial cells of Cajal in neurointestinal diseases. Biomolecules 2023, 13, 1358. [Google Scholar] [CrossRef]
- Broad, J.; Kung, V.W.S.; Palmer, A.; Elahi, S.; Karami, A.; Darreh-Shori, T.; Ahmed, S.; Thaha, M.A.; Carroll, R.; Chin-Aleong, J.; et al. Changes in neuromuscular structure and functions of human colon during ageing are region-dependent. Gut 2019, 68, 1210–1223. [Google Scholar] [CrossRef] [PubMed]
- Baidoo, N.; Sanger, G.J.; Belai, A. Effect of old age on the subpopulations of enteric glial cells in human descending colon. Glia 2023, 71, 305–316. [Google Scholar] [CrossRef]
- Palmer, A.; Epton, S.; Crawley, E.; Straface, M.; Gammon, L.; Edgar, M.M.; Xu, Y.; Elahi, S.; Chin-Aleong, J.; Martin, J.E.; et al. Expression of p16 within myenteric neurons of the aged Colon: A potential marker of declining function. Front. Neurosci. 2021, 15, 747067. [Google Scholar] [CrossRef] [PubMed]
- Baidoo, N.; Crawley, E.; Knowles, C.H.; Sanger, G.J.; Belai, A. Total collagen content and distribution is increased in human colon during advancing age. PLoS ONE 2022, 17, e0269689. [Google Scholar] [CrossRef]
- Shao, T.; Hsu, R.; Rafizadeh, D.L.; Wang, L.; Bowlus, C.L.; Kumar, N.; Mishra, J.; Timilsina, S.; Ridgway, W.M.; Gershwin, E. The gut ecosystem and immune tolerance. J. Autoimmun. 2023, 141, 103114. [Google Scholar] [CrossRef]
- Li, D.; Wu, M. Pattern recognition receptors in health and diseases. Signal Transduct. Target. Ther. 2021, 6, 291. [Google Scholar] [CrossRef]
- Holly, M.K.; Smith, J.G. Paneth cells during viral infection and pathogenesis. Viruses 2018, 10, 225. [Google Scholar] [CrossRef]
- Fernández-García, V.; González-Ramos, S.; Martín-Sanz, P.; García-del Portillo, F.; Laparra, J.M.; Boscá, L. NOD1 in the interplay between microbiota and gastrointestinal immune adaptations. Pharmacolog. Res. 2021, 171, 105775. [Google Scholar] [CrossRef]
- Ubeda, C.; Pamer, E.G. Antibiotics, microbiota, and immune defense. Trends Immunol. 2012, 33, 459–466. [Google Scholar] [CrossRef]
- Kulkarni, D.H.; Newberry, R.D. Intestinal macromolecular transport supporting adaptive immunity. Cell. Mol. Gastroenterol. Hepatol. 2019, 7, 729–737. [Google Scholar] [CrossRef]
- Zheng, D.; Liwinski, T.; Elinav, E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020, 30, 492–506. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, I.I. Review: Intestinal commensal microbes as immune modulators. Cell Host Microbe 2012, 12, 496–508. [Google Scholar] [CrossRef] [PubMed]
- Vanderpool, C.; Yan, F.; Polk, D.B. Mechanisms of probiotic action: Implications for therapeutic applications in inflammatory bowel diseases. Inflamm. Bowel Dis. 2008, 14, 1585–1596. [Google Scholar] [CrossRef]
- Fagundes, R.R.; Bravo-Ruiseco, G.; Hu, S.; Kierans, S.J.; Weersma, R.K.; Taylor, C.T.; Dijkstra, G.; Harmsen, H.J.M.; Faber, K.N. Faecalibacterium prausnitzii promotes intestinal epithelial IL-18 production through activation of the HIF1α pathway. Front. Microbiol. 2023, 14, 1298304. [Google Scholar] [CrossRef]
- Molla, M.D.; Akalu, Y.; Geto, Z.; Dagnew, B.; Ayelign, B.; Shibabaw, T. Role of caspase-1 in the pathogenesis of inflammatory-associated chronic noncommunicable diseases. J. Inflamm. Res. 2020, 13, 749–764. [Google Scholar] [CrossRef]
- Olivo-Martínez, Y.; Bosch, M.; Badia, J.; Baldomà, L. Modulation of the intestinal barrier integrity and repair by microbiota extracellular vesicles through the differential regulation of Trefoil Factor 3 in LS174T goblet cells. Nutrients 2023, 15, 2437. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Lin, Z.; Lin, Q.; Bei, W.; Guo, J. Pathological and therapeutic roles of bioactive peptide trefoil factor 3 in diverse diseases: Recent progress and perspective. Cell Death Dis. 2022, 13, 62. [Google Scholar] [CrossRef]
- Zia, S.; Tehreem, K.; Batool, S.; Ishfaq, M.; Mirza, S.B.; Khan, S.; Almashjary, M.N.; Hazzazi, M.S.; Qanash, H.; Shaikh, A.; et al. Epithelial cell adhesion molecule (EpCAM) expression can be modulated via NFκB. Biomedicines 2022, 10, 2985. [Google Scholar] [CrossRef] [PubMed]
- Mastrangelo, A.; Robles-Vera, I.; Mañanes, D.; Galán, M.; Femenía-Muiña, M.; Redondo-Urzainqui, A.; Barrero-Rodríguez, R.; Papaioannou, E.; Amores-Iniesta, J.; Devesa, A.; et al. Imidazole propionate is a driver and therapeutic target in atherosclerosis. Nature 2025. [Google Scholar] [CrossRef]
- Zhang, X.; Sergin, I.; Evans, T.D.; Jeong, S.J.; Rodriguez-Velez, A.; Kapoor, D.; Chen, S.; Song, E.; Holloway, K.B.; Crowley, J.R.; et al. High-protein diets increase cardiovascular risk by activating macrophage mTOR to suppress mitophagy. Nat. Metab. 2020, 2, 110–125, Correct in Nat. Metab. 2020, 2, 991. [Google Scholar] [CrossRef]
- Zhang, X.; Kapoor, D.; Jeong, S.J.; Fappi, A.; Stitham, J.; Shabrish, V.; Sergin, I.; Yousif, E.; Rodriguez-Velez, A.; Yeh, Y.S.; et al. Identification of a leucine-mediated threshold effect governing macrophage mTOR signalling and cardiovascular risk. Nat. Metab. 2024, 6, 359–377. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Evans, T.D.; Chen, S.; Sergin, I.; Stitham, J.; Jeong, S.-J.; Rodriguez-Velez, A.; Yeh, Y.-S.; Park, A.; Jung, I.-H.; et al. Loss of Macrophage mTORC2 Drives Atherosclerosis via FoxO1 and IL-1β Signaling. Circul. Res. 2023, 133, 200–219. [Google Scholar] [CrossRef]
- Aiello, R.J.; Bourassa, P.A.; Lindsey, S.; Weng, W.; Natoli, E.; Rollins, B.J.; Milos, P.M. Monocyte chemoattractant protein-1 accelerates atherosclerosis in apolipoprotein E-deficient mice. Arterioscler. Thromb. Vasc. Biol. 1999, 19, 1518–1525. [Google Scholar] [CrossRef]
- Tuomisto, S.; Huhtala, H.; Martiskainen, M.; Goebeler, S.; Lehtimäki, T.; Karhunen, P.J. Age-dependent association of gut bacteria with coronary atherosclerosis: Tampere Sudden Death Study. PLoS ONE 2019, 14, e0221345. [Google Scholar] [CrossRef] [PubMed]
- Koren, O.; Spor, A.; Felin, J.; Fåk, F.; Stombaugh, J.; Tremaroli, V.; Behre, C.J.; Knight, R.; Fagerberg, B.; Ley, R.E.; et al. Human oral, gut, and plaque microbiota in patients with atherosclerosis. Proc. Nat. Acad. Sci. USA 2011, 108 (Suppl. S1), 4592–4598. [Google Scholar] [CrossRef]
- Ott, S.J.; El Mokhtari, N.E.; Musfeldt, M.; Hellmig, S.; Freitag, S.; Rehman, A.; Kühbacher, T.; Nikolaus, S.; Namsolleck, P.; Blaut, M.; et al. Detection of diverse bacterial signatures in atherosclerotic lesions of patients with coronary heart disease. Circulation 2006, 113, 929–937. [Google Scholar] [CrossRef]
- Ho, Y.-H.; Del Toro, R.; Rivera-Torres, J.; Rak, J.; Korn, C.; Garcia-Garcia, A.; Macías, D.; González-Gómez, C.; del Monte, A.; Wittner, M.; et al. Remodeling of bone marrow hematopoietic stem cell niches promotes myeloid cell expansion during premature or physiological aging. Cell Stem Cell 2019, 25, 407–418.e6. [Google Scholar] [CrossRef]
- Linton, P.J.; Dorshkind, K. Age-related changes in lymphocyte development and function. Nat. Immunol. 2004, 5, 133–139. [Google Scholar] [CrossRef]
- Muggen, A.F.; de Jong, M.; Wolvers-Tettero, I.L.M.; Kallemeijn, M.J.; Teodosio, C.; Darzentas, N.; Stadhouders, R.; Ijspeert, H.; van der Burg, M.; van Ijcken, W.F.J.; et al. The presence of CLL-associated stereotypic B cell receptors in the normal BCR repertoire from healthy individuals increases with age. Immun. Ageing 2019, 16, 22. [Google Scholar] [CrossRef]
- Tabibian-Keissar, H.; Hazanov, L.; Schiby, G.; Rosenthal, N.; Rakovsky, A.; Michaeli, M.; Shahaf, G.L.; Pickman, Y.; Rosenblatt, K.; Melamed, D.; et al. Aging affects B-cell antigen receptor repertoire diversity in primary and secondary lymphoid tissues. Eur. J. Immunol. 2016, 46, 480–492. [Google Scholar] [CrossRef] [PubMed]
- Gibson, K.L.; Wu, Y.C.; Barnett, Y.; Duggan, O.; Vaughan, R.; Kondeatis, E.; Nilsson, B.O.; Wikby, A.; Kipling, D.; Dunn-Walters, D.K. B-cell diversity decreases in old age and is correlated with poor health status. Aging Cell 2009, 8, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Stephan, R.P.; Reilly, C.R.; Witte, P.L. Impaired ability of bone marrow stromal cells to support B-lymphopoiesis with age. Blood 1998, 91, 75–88. [Google Scholar] [CrossRef]
- Snijckers, R.P.M.; Foks, A.C. Adaptive immunity and atherosclerosis: Aging at its crossroads. Front. Immunol. 2024, 15, 1350471. [Google Scholar] [CrossRef] [PubMed]
- Kumari, R.; Jat, P. Mechanisms of cellular senescence: Cell cycle arrest and senescence-associated secretory phenotype. Front. Cell Dev. Biol. 2021, 9, 645593. [Google Scholar] [CrossRef]
- Nordestgaard, B.G. Triglyceride-rich lipoproteins and atherosclerotic cardiovascular disease: New in-555 sights from epidemiology, genetics, and biology. Circ. Res. 2016, 118, 547–563. [Google Scholar] [CrossRef]
- Roy, P.; Orecchioni, M.; Ley, K. How the immune system shapes atherosclerosis: Roles of innate and adaptive immunity. Nat. Rev. Immunol. 2022, 22, 251–265. [Google Scholar] [CrossRef]
- Wolf, D.; Gerhardt, T.; Winkels, H.; Michel, N.A.; Pramod, A.B.; Ghosheh, Y.; Brunel, S.; Buscher, K.; Miller, J.; McArdle, S.; et al. Pathogenic autoimmunity in atherosclerosis evolves from initially protective apolipoprotein B100-reactive CD4+ T-regulatory cells. Circulation 2020, 142, 1279–1293. [Google Scholar] [CrossRef] [PubMed]
- Andreassi, M.G.; Botto, N.; Rizza, A.; Colombo, M.G.; Palmieri, C.; Berti, S.; Manfredi, S.; Masetti, S.; Clerico, A.; Biagini, A. Deoxyribonucleic acid damage in human lymphocytes after percutaneous transluminal coronary angioplasty. J. Am. Coll. Cardiol. 2002, 40, 862–868. [Google Scholar] [CrossRef]
- Liu, Z.; Liang, Q.; Ren, Y.; Guo, C.; Ge, X.; Wang, L.; Cheng, Q.; Luo, P.; Zhang, Y.; Han, X. Immunosenescence: Molecular mechanisms and diseases. Signal Transduct. Target. Ther. 2023, 8, 200. [Google Scholar] [CrossRef]
- Montenegro, J.; Armet, A.M.; Willing, B.P.; Deehan, E.C.; Fassini, P.G.; Mota, J.F.; Walter, J.; Prado, C.M. Exploring the influence of gut microbiome on energy metabolism in humans. Adv. Nutr. 2023, 14, 840–857. [Google Scholar] [CrossRef]
- Amiri, P.; Hosseini, S.A.; Ghaffari, S.; Tutunchi, H.; Ghaffari, S.; Mosharkesh, E.; Asghari, S.; Roshanravan, N. Role of butyrate, a gut microbiota derived metabolite, in cardiovascular diseases: A comprehensive narrative review. Front. Pharmacol. 2022, 12, 837509. [Google Scholar] [CrossRef]
- van den Munckhof, I.C.L.; Kurilshikov, A.; Ter Horst, R.; Riksen, N.P.; Joosten, L.A.B.; Rutten, J.H.W. Role of gut microbiota in chronic low-grade inflammation as potential driver for atherosclerotic cardiovascular disease: A systematic review of human studies. Obes. Rev. 2018, 19, 1719–1734. [Google Scholar] [CrossRef] [PubMed]
- Edfeldt, K.; Swedenborg, J.; Hansson, G.K.; Yan, Z.Q. Expression of toll-like receptors in human atherosclerotic lesions: A possible pathway for plaque activation. Circulation 2002, 105, 1158–1161. [Google Scholar] [CrossRef]
- Traore, K.; Zirkin, B.; Thimmulappa, R.K.; Biswal, S.; Trush, M.A. Upregulation of TLR1, TLR2, TLR4, and IRAK-2 expression during ML-1 cell differentiation to macrophages: Role in the potentiation of cellular responses to LPS and LTA. ISRN Oncol. 2012, 2012, 641246. [Google Scholar] [CrossRef]
- Dabravolski, S.A.; Sukhorukov, V.N.; Kalmykov, V.A.; Grechko, A.V.; Shakhpazyan, N.K.; Orekhov, A.N. The role of KLF2 in the regulation of atherosclerosis development and potential use of KLF2-558 targeted therapy. Biomedicines 2022, 10, 254. [Google Scholar] [CrossRef]
- Wang, L.; Huang, Z.; Huang, W.; Chen, X.; Shan, P.; Zhong, P.; Khan, Z.; Wang, J.; Fang, Q.; Liang, G.; et al. Inhibition of epidermal growth factor receptor attenuates atherosclerosis via decreasing inflammation and oxidative stress. Sci. Rep. 2017, 8, 45917. [Google Scholar] [CrossRef]
- Zou, Z.G.; Rios, F.J.; Neves, K.B.; Alves-Lopes, R.; Ling, J.; Baillie, G.S.; Gao, X.; Fuller, W.; Camargo, L.L.; Gudermann, T.; et al. Epidermal growth factor signaling through transient receptor potential melastatin cation channel regulates vascular smooth muscle cell function. Clin. Sci. 2020, 134, 2019–2035. [Google Scholar] [CrossRef]
- Anshory, M.; Effendi, R.M.R.A.; Kalim, H.; Dwiyana, R.F.; Suwarsa, O.; Nijsten, T.E.C.; Nouwen, J.L.; Thio, H.B. Butyrate properties in immune-related diseases: Friend or foe? Fermentation 2023, 9, 205. [Google Scholar] [CrossRef]
- Karlsson, F.H.; Fåk, F.; Nookaew, I.; Tremaroli, V.; Fagerberg, B.; Petranovic, D.; Bäckhed, F.; Nielsen, J. Symptomatic atherosclerosis is associated with an altered gut metagenome. Nat. Commun. 2012, 3, 1245. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhao, W.; Liu, X.; Cheng, L. Metagenomic analysis of the gut microbiome in atherosclerosis patients identify cross-cohort microbial signatures and potential therapeutic target. FASEB J. 2020, 34, 14166–14181. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Zhao, T.; Hu, H.; Zhang, W.; Hua, X. Association study of gut flora in coronary heart disease through high-throughput sequencing. BioMed Res. Int. 2017, 2017, 3796359. [Google Scholar] [CrossRef] [PubMed]
- Jie, Z.; Xia, H.; Zhong, S.-L.; Feng, Q.; Li, S.; Liang, S.; Zhong, H.; Liu, Z.; Gao, Y.; Zhao, H.; et al. The gut microbiome in atherosclerotic cardiovascular disease. Nat. Commun. 2017, 8, 845. [Google Scholar] [CrossRef]
- Zhu, Q.; Gao, R.; Zhang, Y.; Pan, D.; Zhu, Y.; Zhang, X.; Yang, R.; Jiang, R.; Xu, Y.; Qin, H. Dysbiosis signatures of gut microbiota in coronary artery disease. Physiol. Genom. 2018, 50, 893–903. [Google Scholar] [CrossRef]
- Feng, Q.; Liu, Z.; Zhong, S.; Li, R.; Xia, H.; Jie, Z.; Wen, B.; Chen, X.; Yan, W.; Fan, Y.; et al. Integrated metabolomics and metagenomics analysis of plasma and urine identified microbial metabolites associated with coronary heart disease. Sci. Rep. 2016, 6, 22525. [Google Scholar] [CrossRef]
- Yoshida, N.; Emoto, T.; Yamashita, T.; Watanabe, H.; Hayashi, T.; Tabata, T.; Hoshi, N.; Hatano, N.; Ozawa, G.; Sasaki, N.; et al. Bacteroides vulgatus and Bacteroides dorei reduce gut microbial lipopolysaccharide production and inhibit atherosclerosis. Circulation 2018, 138, 2486–2498. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Chen, X.; Hu, X.; Niu, H.; Tian, R.; Wang, H.; Pang, H.; Jiang, L.; Qiu, B.; Chen, X.; et al. Alterations in the gut microbiome and metabolism with coronary artery disease severity. Microbiome 2019, 7, 68. [Google Scholar] [CrossRef]
- Panyod, S.; Wu, W.K.; Peng, S.Y.; Tseng, Y.J.; Hsieh, Y.C.; Chen, R.A.; Huang, H.S.; Chen, Y.H.; Chuang, H.L.; Hsu, C.C.; et al. Ginger essential oil and citral ameliorates atherosclerosis in ApoE−/− mice by modulating trimethylamine-N-oxide and gut microbiota. npj Sci. Food 2023, 7, 19. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.-Y.; Wen, J.; Hou, J.; Zhang, S.-Q.; Sun, C.-B.; Zhou, L.-C.; Yin, W.; Pang, W.-L.; Wang, C.; Ying, Y.; et al. Gastrodia remodels intestinal microflora to suppress inflammation in mice with early atherosclerosis. Int. Immunopharmacol. 2021, 96, 107758. [Google Scholar] [CrossRef] [PubMed]
- Xie, B.; Zu, X.; Wang, Z.; Xu, X.; Liu, G.; Liu, R. Ginsenoside Rc ameliorated atherosclerosis via regulating gut microbiota and fecal metabolites. Front. Pharmacol. 2022, 13, 990476. [Google Scholar] [CrossRef]
- Yang, X.Y.; Yu, H.; Fu, J.; Guo, H.H.; Han, P.; Ma, S.R.; Pan, L.B.; Zhang, Z.W.; Xu, H.; Hu, J.C.; et al. Hydroxyurea ameliorates atherosclerosis in ApoE−/− mice by potentially modulating Niemann-Pick C1-like 1 protein through the gut microbiota. Theranostics 2022, 12, 7775–7787. [Google Scholar] [CrossRef]
- Liu, Q.; Li, Y.; Song, X.; Wang, J.; He, Z.; Zhu, J.; Chen, H.; Yuan, J.; Zhang, X.; Jiang, H.; et al. Both gut microbiota and cytokines act to atherosclerosis in ApoE−/− mice. Microb. Pathog. 2020, 138, 103827. [Google Scholar] [CrossRef]
- Zhu, B.; Zhai, Y.; Ji, M.; Wei, Y.; Wu, J.; Xue, W.; Tao, W.; Wu, H. Alisma orientalis beverage treats atherosclerosis by regulating gut microbiota in ApoE−/− mice. Front. Pharmacol. 2020, 11, 570555. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dicks, L.M.T. Are an Aging Gut and a Decrease in Butyrate Production the Reasons for Atherosclerosis? Int. J. Mol. Sci. 2025, 26, 8276. https://doi.org/10.3390/ijms26178276
Dicks LMT. Are an Aging Gut and a Decrease in Butyrate Production the Reasons for Atherosclerosis? International Journal of Molecular Sciences. 2025; 26(17):8276. https://doi.org/10.3390/ijms26178276
Chicago/Turabian StyleDicks, Leon M. T. 2025. "Are an Aging Gut and a Decrease in Butyrate Production the Reasons for Atherosclerosis?" International Journal of Molecular Sciences 26, no. 17: 8276. https://doi.org/10.3390/ijms26178276
APA StyleDicks, L. M. T. (2025). Are an Aging Gut and a Decrease in Butyrate Production the Reasons for Atherosclerosis? International Journal of Molecular Sciences, 26(17), 8276. https://doi.org/10.3390/ijms26178276






