Determinants for Activation of the Ion Channel TRPV3 by Weak Acids
Abstract
1. Introduction
2. Results
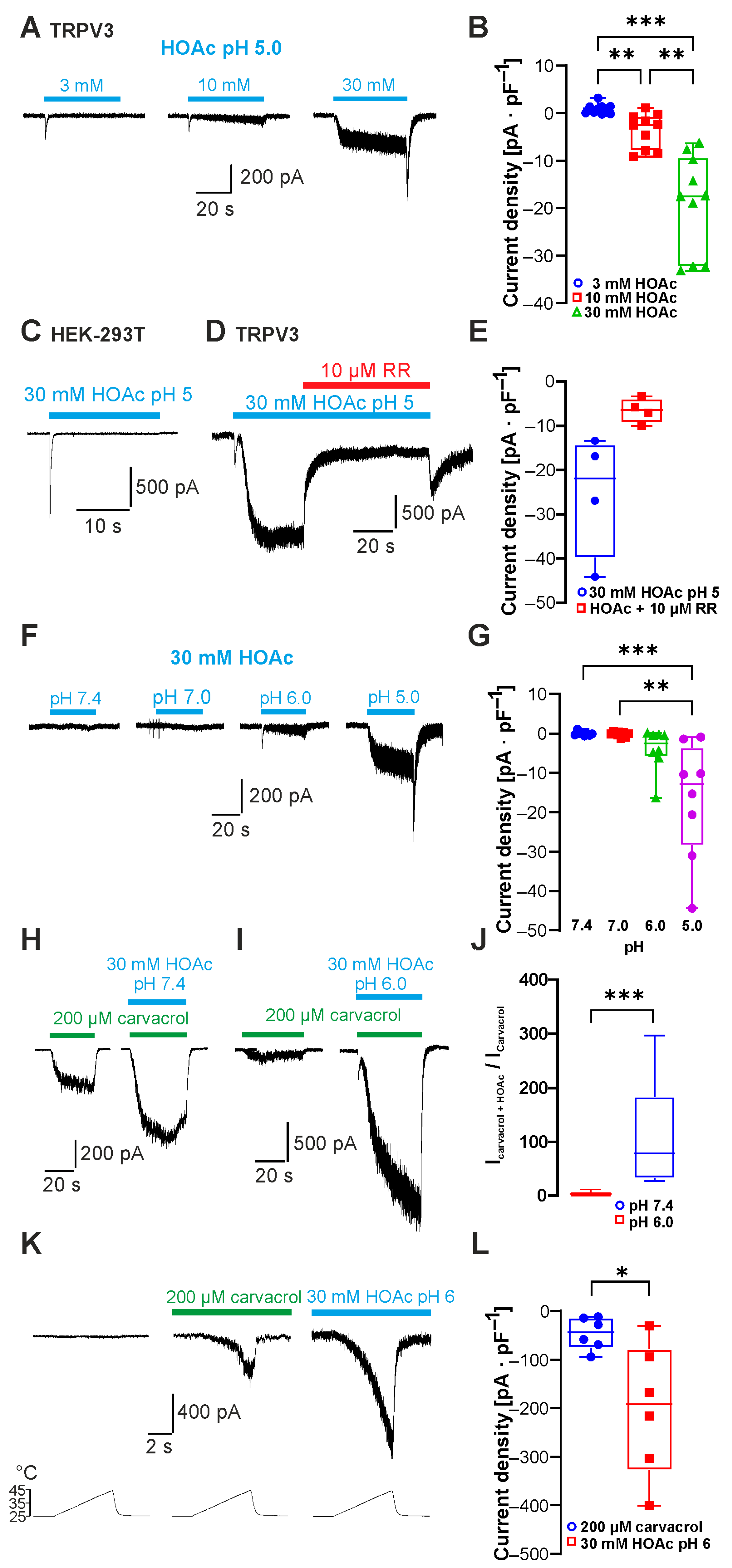
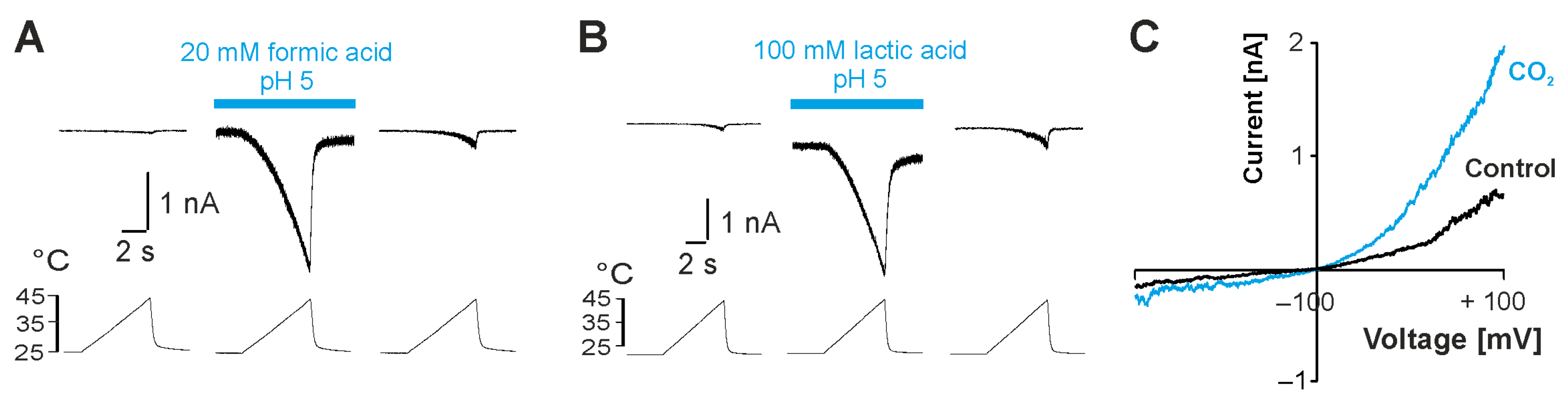
2.1. Weak Acids Sensitize and Activate TRPV3
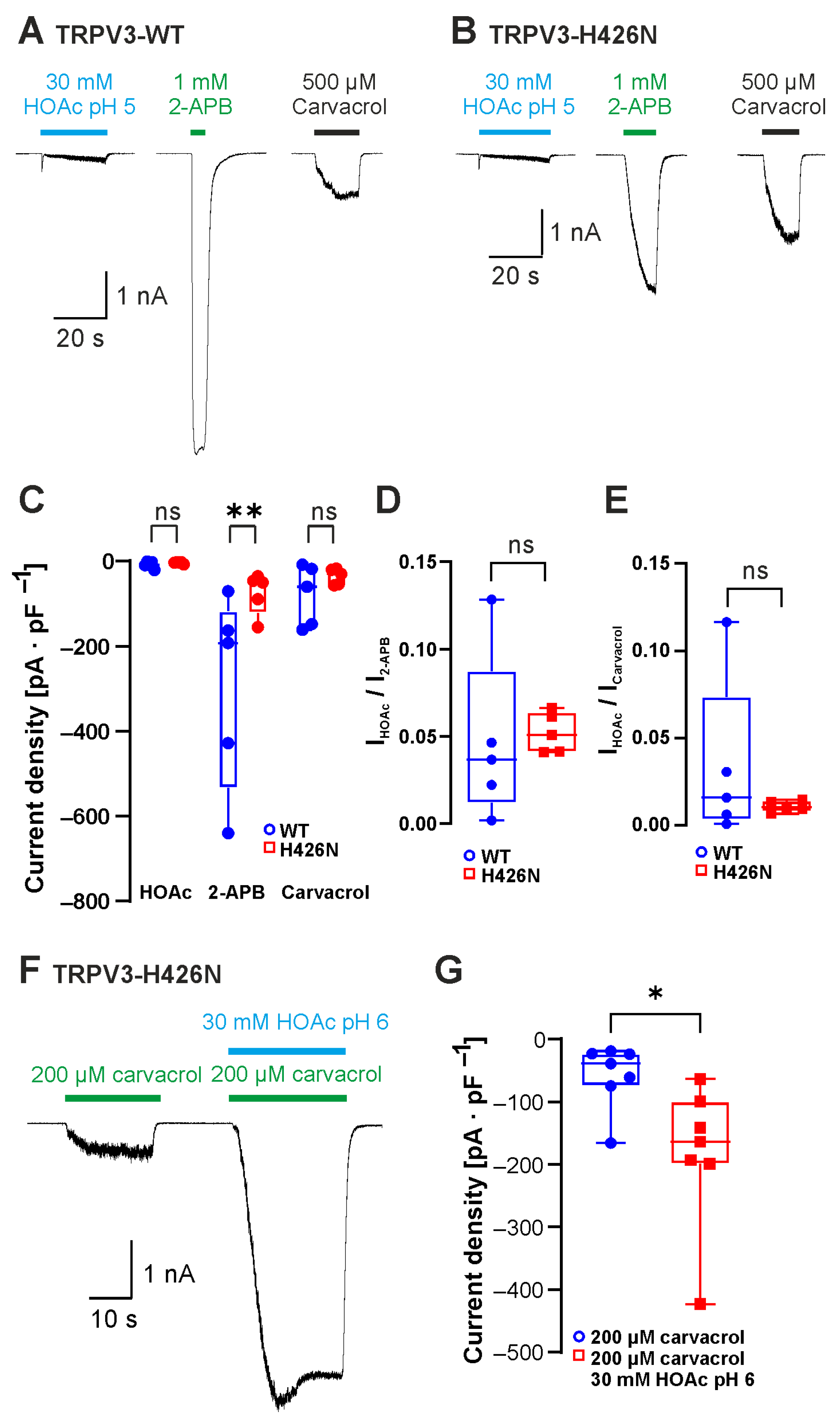
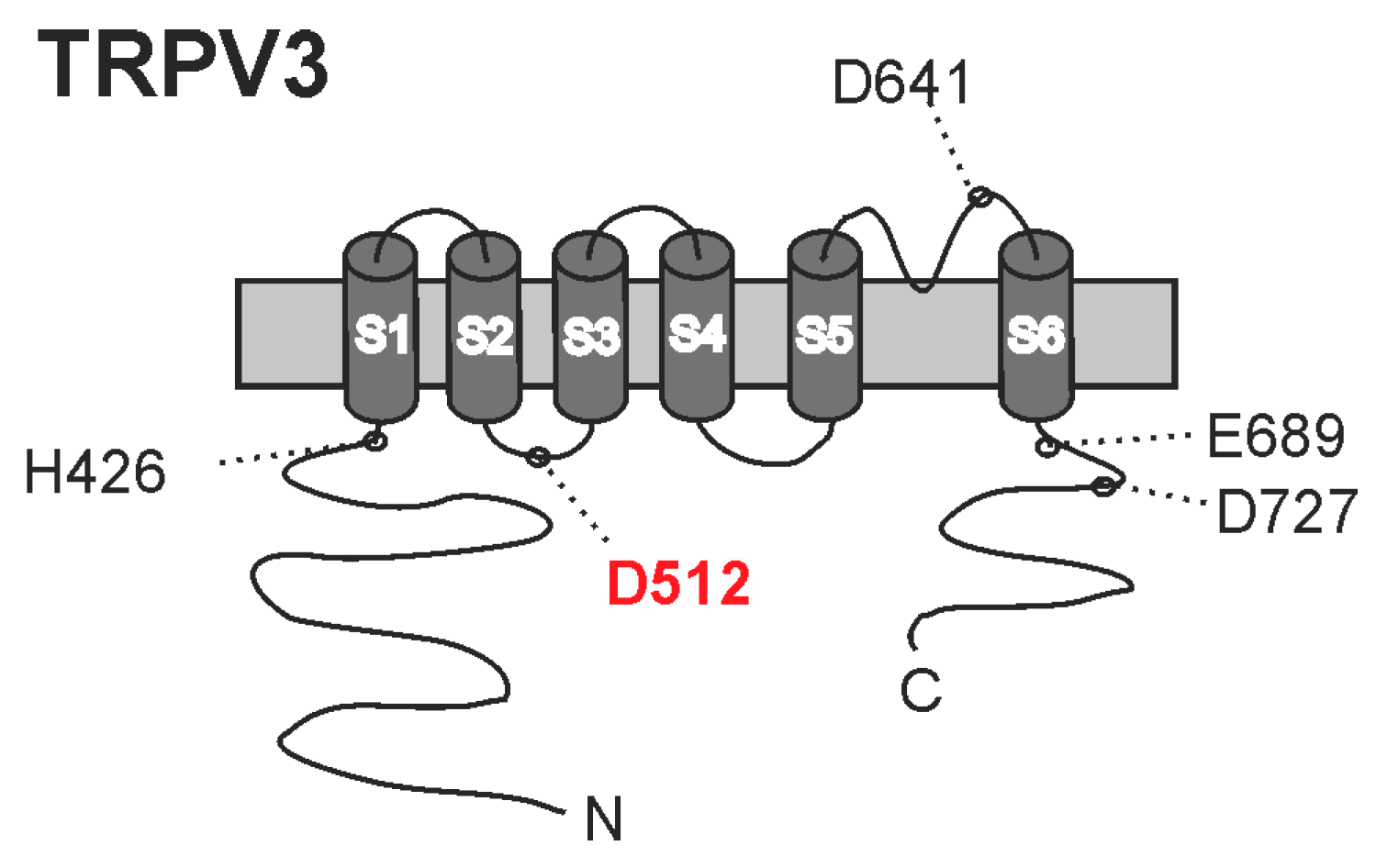
2.2. The 2-APB-Binding Site His426 Is Not Required for Sensitivity to Weak Acids
2.3. The Carvacrol-Binding Site Asp512 Is Required for Sensitivity to Weak Acids
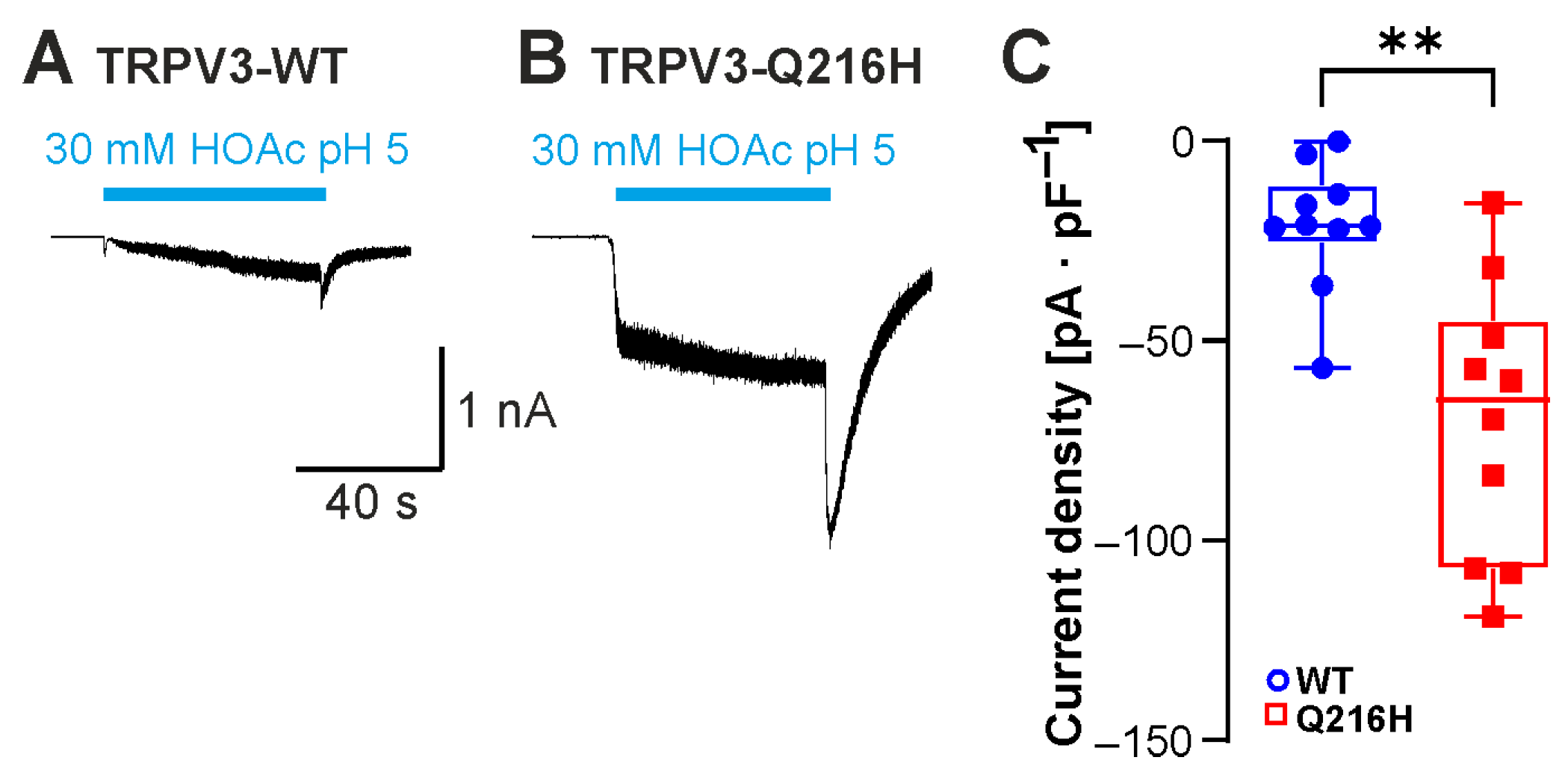
2.4. Neutralization of C-Terminal Protonatable Residues Increases Sensitivity to HOAc
2.5. Asp641 Is Relevant for Inhibition of Weak-Acid-Induced Currents by Protons and Calcium
2.6. Insertion of the Protonatable Probenecid-Binding Site from TRPV2 into TRPV3 Increases Weak-Acid Sensitivity
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Cell Culture
4.3. Mutagenesis
4.4. Patch Clamp
4.5. Carbon Dioxide Measurement
4.6. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TRPV3 | transient receptor potential vanilloid 3 |
| HEK293T | human embryonic kidney cells |
| 2-APB | 2-aminoethoxydiphenyl borate |
| HOAc | acetic acid |
| WT | wildtype |
| CO2 | carbon dioxide |
| FPP | farnesyl pyrophosphate |
| cryo-EM | cryo-electron microscopy |
References
- Kalinovskii, A.P.; Utkina, L.L.; Korolkova, Y.V.; Andreev, Y.A. Trpv3 ion channel: From gene to pharmacology. Int. J. Mol. Sci. 2023, 24, 8601. [Google Scholar] [CrossRef]
- Su, W.; Qiao, X.; Wang, W.; He, S.; Liang, K.; Hong, X. Trpv3: Structure, diseases and modulators. Molecules 2023, 28, 774. [Google Scholar] [CrossRef]
- Duchatelet, S.; Hovnanian, A. Olmsted syndrome: Clinical, molecular and therapeutic aspects. Orphanet J. Rare Dis. 2015, 10, 33. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Song, Y.; Liu, W.; Wang, T.; Ma, X.; Yu, Z. Novel insights into the role of keratinocytes-expressed trpv3 in the skin. Biomolecules 2023, 13, 513. [Google Scholar] [CrossRef] [PubMed]
- Lu, A.; Li, K.; Huang, C.; Yu, B.; Zhong, W. Pathogenesis and management of trpv3-related olmsted syndrome. Front. Genet. 2024, 15, 1459109. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, J.; Wang, K. Inhibition of intracellular proton-sensitive ca(2+)-permeable trpv3 channels protects against ischemic brain injury. Acta Pharm. Sin. B 2022, 12, 2330–2347. [Google Scholar] [CrossRef]
- Moussaieff, A.; Yu, J.; Zhu, H.; Gattoni-Celli, S.; Shohami, E.; Kindy, M.S. Protective effects of incensole acetate on cerebral ischemic injury. Brain Res. 2012, 1443, 89–97. [Google Scholar] [CrossRef]
- Matsui, T.; Kadono-Maekubo, N.; Suzuki, Y.; Furuichi, Y.; Shiraga, K.; Sasaki, H.; Ishida, A.; Takahashi, S.; Okada, T.; Toyooka, K.; et al. A unique mode of keratinocyte death requires intracellular acidification. Proc. Natl. Acad. Sci. USA 2021, 118, e2020722118. [Google Scholar] [CrossRef]
- Peier, A.M.; Reeve, A.J.; Andersson, D.A.; Moqrich, A.; Earley, T.J.; Hergarden, A.C.; Story, G.M.; Colley, S.; Hogenesch, J.B.; McIntyre, P.; et al. A heat-sensitive trp channel expressed in keratinocytes. Science 2002, 296, 2046–2049. [Google Scholar] [CrossRef]
- Smith, G.D.; Gunthorpe, M.J.; Kelsell, R.E.; Hayes, P.D.; Reilly, P.; Facer, P.; Wright, J.E.; Jerman, J.C.; Walhin, J.P.; Ooi, L.; et al. Trpv3 is a temperature-sensitive vanilloid receptor-like protein. Nature 2002, 418, 186–190. [Google Scholar] [CrossRef]
- Xu, H.; Ramsey, I.S.; Kotecha, S.A.; Moran, M.M.; Chong, J.A.; Lawson, D.; Ge, P.; Lilly, J.; Silos-Santiago, I.; Xie, Y.; et al. Trpv3 is a calcium-permeable temperature-sensitive cation channel. Nature 2002, 418, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Yang, F.; Zheng, J.; Wang, K. Intracellular proton-mediated activation of trpv3 channels accounts for the exfoliation effect of alpha-hydroxyl acids on keratinocytes. J. Biol. Chem. 2012, 287, 25905–25916. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Grandl, J.; Bandell, M.; Petrus, M.; Patapoutian, A. Two amino acid residues determine 2-apb sensitivity of the ion channels trpv3 and trpv4. Proc. Natl. Acad. Sci. USA 2009, 106, 1626–1631. [Google Scholar] [CrossRef]
- Sunder, S. Relevant topical skin care products for prevention and treatment of aging skin. Facial Plast. Surg. Clin. North. Am. 2019, 27, 413–418. [Google Scholar] [CrossRef]
- Tang, S.C.; Yang, J.H. Dual effects of alpha-hydroxy acids on the skin. Molecules 2018, 23, 863. [Google Scholar] [CrossRef]
- Gao, L.; Yang, P.; Qin, P.; Lu, Y.; Li, X.; Tian, Q.; Li, Y.; Xie, C.; Tian, J.B.; Zhang, C.; et al. Selective potentiation of 2-apb-induced activation of trpv1-3 channels by acid. Sci. Rep. 2016, 6, 20791. [Google Scholar] [CrossRef]
- Wang, H.; Yang, P.; Lu, Y.; Wang, J.; Jeon, J.; Wang, Q.; Tian, J.B.; Zang, B.; Yu, Y.; Zhu, M.X. Mechanisms of proton inhibition and sensitization of the cation channel trpv3. J. Gen. Physiol. 2021, 153, e202012663. [Google Scholar] [CrossRef]
- Niu, C.; Sun, X.; Hu, F.; Tang, X.; Wang, K. Molecular determinants for the chemical activation of the warmth-sensitive trpv3 channel by the natural monoterpenoid carvacrol. J. Biol. Chem. 2022, 298, 101706. [Google Scholar] [CrossRef]
- Xiao, R.; Tang, J.; Wang, C.; Colton, C.K.; Tian, J.; Zhu, M.X. Calcium plays a central role in the sensitization of trpv3 channel to repetitive stimulations. J. Biol. Chem. 2008, 283, 6162–6174. [Google Scholar] [CrossRef]
- Rocereta, J.A.; Sturhahn, T.; Pumroy, R.A.; Fricke, T.C.; Herzog, C.; Leffler, A.; Moiseenkova-Bell, V. Structural insights into trpv2 modulation by probenecid. Nat. Struct. Mol. Biol. 2025, 32, 1019–1029. [Google Scholar] [CrossRef]
- Li, Y.; Lu, X.; Cheng, X.; Xia, K.; Hu, J.; Wang, P.; Pang, P.; Gao, B.; Sun, D.; Zhang, Z.; et al. Plant essential oil targets trpv3 for skin renewal and structural mechanism of action. Nat. Commun. 2025, 16, 2728. [Google Scholar] [CrossRef]
- Nadezhdin, K.D.; Neuberger, A.; Khosrof, L.S.; Talyzina, I.A.; Khau, J.; Yelshanskaya, M.V.; Sobolevsky, A.I. Trpv3 activation by different agonists accompanied by lipid dissociation from the vanilloid site. Sci. Adv. 2024, 10, eadn2453. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Hu, L.; Yue, Z.; Liao, D.; Guo, F.; Ke, H.; Jiang, D.; Yang, Y.; Lei, X. Structural basis of trpv3 inhibition by an antagonist. Nat. Chem. Biol. 2023, 19, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Neuberger, A.; Nadezhdin, K.D.; Sobolevsky, A.I. Structural mechanism of trpv3 channel inhibition by the anesthetic dyclonine. Nat. Commun. 2022, 13, 2795. [Google Scholar] [CrossRef]
- Neuberger, A.; Nadezhdin, K.D.; Zakharian, E.; Sobolevsky, A.I. Structural mechanism of trpv3 channel inhibition by the plant-derived coumarin osthole. EMBO Rep. 2021, 22, e53233. [Google Scholar] [CrossRef]
- Nadezhdin, K.D.; Neuberger, A.; Trofimov, Y.A.; Krylov, N.A.; Sinica, V.; Kupko, N.; Vlachova, V.; Zakharian, E.; Efremov, R.G.; Sobolevsky, A.I. Structural mechanism of heat-induced opening of a temperature-sensitive trp channel. Nat. Struct. Mol. Biol. 2021, 28, 564–572. [Google Scholar] [CrossRef]
- Shimada, H.; Kusakizako, T.; Dung Nguyen, T.H.; Nishizawa, T.; Hino, T.; Tominaga, M.; Nureki, O. The structure of lipid nanodisc-reconstituted trpv3 reveals the gating mechanism. Nat. Struct. Mol. Biol. 2020, 27, 645–652. [Google Scholar] [CrossRef]
- Jordt, S.E.; Tominaga, M.; Julius, D. Acid potentiation of the capsaicin receptor determined by a key extracellular site. Proc. Natl. Acad. Sci. USA 2000, 97, 8134–8139. [Google Scholar] [CrossRef]
- Caterina, M.J.; Schumacher, M.A.; Tominaga, M.; Rosen, T.A.; Levine, J.D.; Julius, D. The capsaicin receptor: A heat-activated ion channel in the pain pathway. Nature 1997, 389, 816–824. [Google Scholar] [CrossRef]
- Zhang, K.; Julius, D.; Cheng, Y. Structural snapshots of trpv1 reveal mechanism of polymodal functionality. Cell 2021, 184, 5138–5150 e5112. [Google Scholar] [CrossRef]
- Haug, F.M.; Pumroy, R.A.; Sridhar, A.; Pantke, S.; Dimek, F.; Fricke, T.C.; Hage, A.; Herzog, C.; Echtermeyer, F.G.; de la Roche, J.; et al. Functional and structural insights into activation of trpv2 by weak acids. EMBO J. 2024, 43, 2264–2290. [Google Scholar] [CrossRef] [PubMed]
- Dittert, I.; Vlachova, V.; Knotkova, H.; Vitaskova, Z.; Vyklicky, L.; Kress, M.; Reeh, P.W. A technique for fast application of heated solutions of different composition to cultured neurones. J. Neurosci. Methods 1998, 82, 195–201. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rudolf, D.; Pombeiro Stein, I.C.A.; Sturhahn, T.; Wunder, J.; Hage, A.; Leffler, A. Determinants for Activation of the Ion Channel TRPV3 by Weak Acids. Int. J. Mol. Sci. 2025, 26, 8275. https://doi.org/10.3390/ijms26178275
Rudolf D, Pombeiro Stein ICA, Sturhahn T, Wunder J, Hage A, Leffler A. Determinants for Activation of the Ion Channel TRPV3 by Weak Acids. International Journal of Molecular Sciences. 2025; 26(17):8275. https://doi.org/10.3390/ijms26178275
Chicago/Turabian StyleRudolf, Daniel, Inês C. A. Pombeiro Stein, Toni Sturhahn, Julian Wunder, Axel Hage, and Andreas Leffler. 2025. "Determinants for Activation of the Ion Channel TRPV3 by Weak Acids" International Journal of Molecular Sciences 26, no. 17: 8275. https://doi.org/10.3390/ijms26178275
APA StyleRudolf, D., Pombeiro Stein, I. C. A., Sturhahn, T., Wunder, J., Hage, A., & Leffler, A. (2025). Determinants for Activation of the Ion Channel TRPV3 by Weak Acids. International Journal of Molecular Sciences, 26(17), 8275. https://doi.org/10.3390/ijms26178275





