Abstract
Although diagnostic criteria and research are constantly advancing, distinguishing between progressive supranuclear palsy (PSP) and corticobasal syndrome (CBS) remains a significant challenge. This difficulty stems from their similar clinical symptoms and the lack of reliable biomarkers. In this work, we present a detailed review of fluorodeoxyglucose (FDG)–positron emission tomography (PET), exploring its potential role in differentiating PSP and CBS, drawing on their established utility in other neurodegenerative diseases. We searched the PubMed database from its inception for original research articles assessing the utility of FDG-PET for the diagnosis or differential diagnosis of PSP and CBS from other neurodegenerative conditions. A total of 91 studies were eligible. These 91 studies were categorized as follows: (a) 20 studies included only patients with PSP, (b) 15 studies included only patients with CBS, (c) 39 studies involved patients with Parkinson’s disease and atypical Parkinsonian disorders, including subgroups of PSP and/or CBS, and (d) 17 studies compared patients with PSP and/or CBS to individuals with Alzheimer’s disease, frontotemporal dementia, or other dementias. Most FDG-PET studies involving PSP and CBS were not specifically designed for these disorders. An additional obstacle lies in the methodological variability across studies. Despite several studies achieving high diagnostic accuracy for PSP and/or CBS with specificity exceeding 90% using FDG-PET, sensitivity remains considerably lower. CBS appears to have a distinct hypometabolic pattern compared to PSP, marked by asymmetry and predominant cortical involvement. CBS more often affects posterior cortical regions (parietal and posterior parts of the frontal cortex, and sometimes temporal and occipital parts) and the thalamus, whereas PSP appears to affect the striatum, frontal cortex, anterior cingulate, and subtentorial structures, typically in a more symmetrical manner. Large, multicenter studies are needed, utilizing standardized imaging and protocols.
1. Introduction
Progressive supranuclear palsy (PSP) and corticobasal syndrome (CBS) are rare, rapidly progressive neurodegenerative disorders that belong to the spectrum of atypical parkinsonian disorders (APDs). Both conditions are characterized by distinctive clinical features, considerable overlap with other neurodegenerative diseases, and complex underlying neuropathology [1,2]. PSP is classically associated with early postural instability, supranuclear gaze palsy, axial rigidity, and cognitive impairment, while CBS is often defined by asymmetric motor symptoms, apraxia, dystonia, and cortical sensory deficits [1,2]. Despite these somewhat characteristic features, clinical differentiation between PSP, CBS, and other neurodegenerative diseases remains challenging, particularly in early disease stages [3,4]. Additionally, other neurodegenerative diseases, such as Alzheimer’s disease (AD), further contribute to diagnostic complexity (15%) [5]. Moreover, emerging perspectives categorize CBS and PSP within broader and more heterogeneous neurodegenerative spectrums, such as frontotemporal dementia (FTD) and posterior cortical atrophy (PCA), further complicating clinical diagnosis [6,7].
As a result, accurate biomarkers that can improve diagnostic certainty and provide insights into disease mechanisms are of great clinical and research importance [8,9]. Taking the lead from other neurological disorders, where imaging biomarkers continue to emerge [10], reliable biomarkers in PSP and CBS are essential, especially since an effective treatment is still lacking [11].
One of the most widely studied imaging biomarkers in PSP and CBS is fluorodeoxyglucose–positron emission tomography (FDG-PET) [12,13]. FDG-PET provides a measure of regional cerebral glucose metabolism, serving as a proxy for synaptic activity and neuronal integrity [13]. In neurodegenerative disorders, patterns of hypometabolism often precede overt structural changes and can reveal disease-specific signatures that aid in differential diagnosis [14]. Over the past two decades, FDG-PET has emerged as a powerful tool to complement clinical assessment, structural magnetic resonance imaging (MRI), and other functional imaging modalities, such as tau- and amyloid-positron emission tomography (PET) [15].
This narrative review aims to provide a comprehensive overview of FDG-PET findings in PSP and CBS. By synthesizing the current evidence, this review seeks to clarify the utility and limitations of FDG-PET in PSP and CBS, and to identify directions for future research.
2. Results
2.1. FDG-PET in Progressive Supranuclear Palsy
Several studies have investigated the diagnostic utility of FDG-PET in PSP, either alone or in combination with CBS, with or without healthy controls for comparison. Table 1 summarizes the main findings of these studies.

Table 1.
Studies analyzing FDG-PET 1 patterns in PSP 2.
Foster et al. used FDG-PET to study patients with PSP and compared them to healthy controls [16]. They observed reduced glucose metabolism in several subcortical structures—including the pons, thalamus, and caudate nucleus—but not in the cerebellum. In the cerebral cortex, multiple regions of interest (ROIs) showed hypometabolism, with the superior frontal cortex being the most severely affected. Notably, cortical hypometabolism was more pronounced than that observed in any subcortical region. Similarly, Goffinet et al. observed reduced glucose metabolism in the frontal lobe—particularly in the premotor and motor areas—of nine patients with PSP [17]. Karbe et al. also reported hypometabolism in the frontal lobe of PSP patients compared to normal controls [18]. However, they found more prominent hypometabolism in subcortical structures, including the upper midbrain, lentiform nucleus, and caudate nucleus—a pattern that differed slightly from that reported by Foster et al. The midbrain was the main ROI in the study of Mishina et al [21]. The researchers found hypometabolism in PSP patients compared to controls, independent of clinical deterioration.
Zhao et al. also reported decreased glucose metabolism in the midbrain, caudate nuclei, frontal lobes, and cingulate gyrus in patients with PSP [19]. Notably, midbrain hypometabolism was observed in 92.3% of PSP patients on FDG-PET, whereas only 57.9% showed the characteristic “hummingbird sign” in the same region on MRI. Yamauchi et al. combined FDG-PET and MRI to examine alterations in the corpus callosum of patients with PSP compared to healthy controls [20]. They observed corpus callosum atrophy on MRI in PSP patients, which correlated with frontal cortex hypometabolism, consistent with previous findings. Notably, these structural and metabolic changes were associated with poorer performance on neurocognitive tests of visuospatial ability and verbal fluency. Takahashi et al. compared MRI and FDG-PET in PSP vs. controls and found FDG-PET to be superior in detecting alterations in the middle frontal gyrus, medial/lateral frontal lobes, midbrain, and thalamus [22]. Notably, atrophy and hypometabolism of the frontal lobes were correlated with Mini-Mental State Examination (MMSE) scores.
Zwergal et al. also investigated hypometabolism in specific ROIs and its association with clinical feature severity in PSP [23]. Decreased FDG uptake in the thalami correlated with fall frequency, while increased metabolism in the precentral gyrus was also linked to increased fall risk. Subsequently, they showed that gait severity scales were correlated with hypometabolism in the prefrontal cortex, subthalamic nucleus, and pedunculopontine/cuneiform nucleus complex—findings that were confirmed both at rest and after walking [24]. Similarly, Amtage et al. examined gaze palsy patterns and their association with focal brain hypometabolism in PSP patients [25]. They found that downward gaze palsy correlated with bilateral hypometabolism in the anterior cingulate and the right lingual gyrus. On the other hand, Isella et al. focused on phonemic fluency in PSP and found that patients with reduced phonemic fluency exhibited decreased glucose metabolism in the dominant superior frontal cortex [26]. Doll-Lee et al. investigated cognitive impairment and cerebral glucose metabolism in patients with PSP [27]. Compared to healthy controls, PSP patients demonstrated reduced FDG uptake in the left inferior frontal gyrus and right angular gyrus. Notably, overall cognitive performance was correlated with metabolic alterations in the right frontal eye field. In addition, list learning was associated with metabolism in the left frontal eye field, while verbal fluency correlated with activity in the bilateral premotor and supplementary motor cortices.
Data on FDG-PET performance in specific PSP subtypes remains limited. Buchert et al. recruited patients with PSP–Richardson syndrome (PSP-RS) and other PSP subtypes, comparing them to healthy controls [28]. They evaluated visual analysis supported by voxel-based statistics alongside automatic covariance pattern analysis. While visual analysis demonstrated only modest sensitivity and specificity, performance improved significantly with automatic analysis. Notably, the rate of false-negative cases decreased overall and was reduced to zero specifically in the PSP-RS group. Subsequently, they employed MRI volumetric analysis, which demonstrated a comparable area under the curve (AUC) to that of automated FDG-PET analysis, but with higher specificity and lower sensitivity [35]. Black et al. compared metabolic patterns across the prefrontal, premotor, and sensorimotor cortices in 137 patients with various PSP subtypes, including PSP with predominant speech/language impairment (PSP-SL), corticobasal syndrome (PSP-CBS), frontal presentation (PSP-F), Parkinsonism (PSP-P), and progressive gait freezing (PSP-PGF) [30]. Notably, 43 patients underwent autopsy. They found frontal hypometabolism in 100% of patients with PSP-SL, PSP-CBS, and PSP-F, whereas lower rates were observed in other subtypes, such as 73% in PSP-PGF. Hypometabolism was most frequently asymmetric in the PSP-CBS, PSP-SL, PSP-P, and PSP-F groups. Additionally, Frontal Assessment Battery (FAB) scores were negatively correlated with glucose metabolism across all subtypes.
Garraux et al. were among the first to compare patients with PSP and CBD [29]. Compared to healthy controls, patients with CBD showed asymmetric hypometabolism affecting the putamen, thalamus, precentral gyrus, lateral premotor cortex, supplementary motor area, dorsolateral prefrontal cortex, and inferior parietal cortex. In contrast, when directly compared to PSP, CBD patients exhibited more posterior hypometabolism, including in the supplementary motor area. PSP patients, on the other hand, demonstrated more prominent hypometabolism in the midbrain, anterior cingulate cortex, and orbitofrontal cortex. Similarly, Hosaka et al. PSP showed hypometabolism in the medial and lateral frontal gyri, along with midbrain and basal ganglia alterations [31]. In contrast, CBS showed predominant parietal lobe hypometabolism, compared to both controls and PSP. Juh et al. conducted a study comparing 8 patients with CBD and 8 with PSP to 22 healthy controls [32]. When comparing the two patient groups directly, they found hypometabolism in the midbrain and thalamus in the PSP group, and in the parietal lobe in the CBD group. In addition, compared to controls, PSP patients exhibited hypometabolism in the orbitofrontal cortex, middle frontal cortex, and cingulate gyrus, while CBD patients showed asymmetric hypometabolism in the frontal and cingulate cortices, in addition to the parietal lobe involvement mentioned earlier.
Amtage et al. also compared PSP patients with either unilateral or bilateral motor impairment to patients with CBD and found similar hypometabolic patterns [33]. Interestingly, PSP patients with unilateral motor impairment showed hypometabolism in the contralateral thalamus, middle cingulate gyrus, and sensorimotor cortex compared to controls. Zalewski et al. evaluated FDG-PET findings in seven patients with autopsy-confirmed PSP, two cases of globular glial tauopathy (GGT), and one case of CBD [34]. They observed bilateral hypometabolism in the caudate, thalamus, and midbrain across all cases. No significant differences were found between the PSP and GGT cases, whereas the CBD case demonstrated more prominent and asymmetric hypometabolism, particularly in the parietal lobes.
Table 2 summarizes the main hypometabolic regions reported in each PSP study (when available). These regions are also illustrated in Figure 1.

Table 2.
FDG-PET 1 of the main hypometabolic regions in PSP 2.
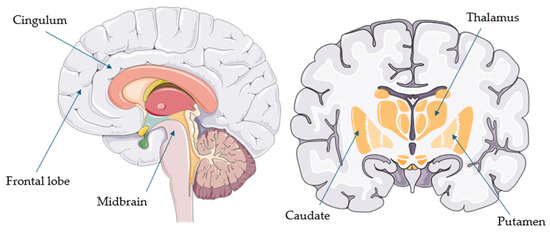
Figure 1.
Common hypometabolic regions in progressive supranuclear palsy (parts of the image provided by Servier Medical Art (https://smart.servier.com/ (accessed on 21 August 2025)), licensed under CC BY 4.0 (https://creativecommons.org/licenses/by/4.0/ (accessed on 21 August 2025)).
2.2. FDG-PET in Corticobasal Syndrome
Table 3 summarizes the main findings that utilize FDG-PET in CBS patients, either alone or in comparison with controls.

Table 3.
Studies analyzing FDG-PET 1 patterns in CBS 2.
Blin et al. initially studied five patients with a clinical diagnosis of CBD and compared them to healthy controls [36]. Although most cortical and subcortical regions showed reduced metabolism on FDG-PET in the CBD group, the greatest reductions in glucose uptake were observed in the temporal and sensorimotor cortices contralateral to the most affected side.
Multiple studies have compared FDG-PET with other imaging modalities in patients with CBS. Klaffke et al. reported contralateral hypometabolism in multiple cortical and subcortical regions in patients with CBD, while 123I-iodobenzamide (IBZM) SPECT imaging revealed reduced dopamine D2 receptor binding in only one patient, suggesting limited sensitivity of IBZM in detecting basal ganglia involvement in CBD [37]. Turaga et al. also observed asymmetrical hypometabolism involving the left basal ganglia and the inferior parietal, temporal, and frontal lobes [38]. These metabolic findings correlated with MRI-detected atrophy in the left temporal and parietal cortex as well as the basal ganglia—though notably, not in the parietal cortex—suggesting greater sensitivity of FDG-PET for detecting early or subtle changes. Importantly, FDG-PET abnormalities also correlated with deficits in neurocognitive assessments, particularly in frontal/executive function, speech, and visuospatial abilities. Franceschi et al. also employed FDG-PET and volumetric MRI to retrospectively assess patients with clinically established dementia [39]. Of the 12 patients later suspected to have CBD, 10 exhibited the previously described asymmetrical hypometabolic pattern involving both cortical and subcortical regions. This pattern corresponded to atrophy in the same areas on volumetric MRI. The remaining two patients demonstrated a hypometabolic pattern affecting the sensorimotor cortex bilaterally.
Sha et al. employed both FDG-PET and amyloid PET in a cohort of 25 patients with CBS, 8 of whom later had autopsy-confirmed diagnoses [40]. FDG-PET demonstrated higher sensitivity than amyloid PET in detecting CBS, but with lower specificity. On the other hand, Mille et al. combined FDG-PET and dopamine transporter imaging with single-photon emission computed tomography (DAT-SPECT) to assess CBS patients and found that, despite the hypometabolism of the frontal and parietal association cortices and putamen contralateral to most severely motor affected side, DAT-SPECT showed only slight asymmetry in DAT availability [51].
Isella et al. analyzed 35 patients with CBD and identified hypometabolism in the left inferior parietal, primary motor, primary sensory, and insular cortices [42]. Furthermore, they found an association between these hypometabolic ROIs and patients’ cognitive reserve, as measured by years of education. They also found that patients with CBS and asymmetric hypometabolism in the left hemisphere showed greater deficits in digit span, whereas those with right-hemisphere hypometabolism exhibited more pronounced visuospatial impairments [43].
Pardini et al. conducted one of the few studies involving only CBS patients with autopsy-confirmed diagnoses [44]. All patients showed hypometabolism in the perirolandic area, basal ganglia, and thalamus contralateral to the most affected side. Notably, patients with clinical CBS and CBD pathology demonstrated more pronounced and bilateral basal ganglia involvement, whereas those with underlying AD pathology exhibited more asymmetric hypometabolism in the lateral parietal and temporal lobes, as well as the posterior cingulate. In contrast, patients with PSP pathology had greater involvement of the anterior cingulate and medial frontal cortex. These findings suggest that distinct FDG-PET hypometabolic patterns in CBS may help differentiate the underlying pathology.
Data on the application of FDG-PET across specific subtypes of CBD or certain disease characteristics remain limited. Jo et al. investigated hypometabolic patterns in 52 patients with CBD by categorizing them into groups based on the presence of ideomotor apraxia, imitation apraxia, or both, and compared them to healthy controls [45]. Notably, patients with ideomotor apraxia were more likely to exhibit hypometabolism in the left angular gyrus, whereas those with imitation apraxia more commonly showed hypometabolism in the precuneus, postcentral gyrus, and posterior cingulate cortex. CBD was also associated with hypometabolism of the frontal gyrus and caudate, regardless of the apraxia. Parmera et al. employed FDG-PET, amyloid PET, and MRI with voxel-based morphometry to examine patients with probable CBS and specific neurocognitive deficits [46]. Interestingly, patients presenting with dysarthria showed hypometabolism in the left inferior frontal gyrus and premotor cortex, while MRI revealed pronounced atrophy in the frontal operculum and putamen. In contrast, phonemic fluency was positively correlated with glucose uptake in the frontal operculum as well as the inferior and middle temporal gyri, whereas semantic fluency correlated with glucose uptake in the lateral temporal lobe. Subsequently, they combined FDG-PET and amyloid PET imaging in patients with CBS, further categorizing them based on whether they fulfilled the Movement Disorder Society (MDS) criteria for 4R tauopathies. Interestingly, patients who met the criteria exhibited a distinct hypometabolic pattern involving the supplementary motor area, bilateral striatum, and anterior cingulate cortex—unlike those who did not meet the criteria [47]. Moreover, Nakano et al. combined FDG-PET, amyloid PET, and tau PET to investigate patients with CBS [49]. They found that patients with positive tau PET and negative amyloid PET expressed decreased glucose uptake in the precentral gyrus and thalamus compared to controls. Parmera et al. combined FDG-PET and amyloid PET to investigate patients with probable CBS [48]. They found that worse cognitive performance was most common in patients exhibiting an AD-like hypometabolic pattern on FDG-PET, whereas greater motor impairment was more often associated with a CBD-like pattern. Moreover, FDG-PET demonstrated very high specificity and positive predictive value, but only modest sensitivity in predicting amyloid positivity on amyloid PET based on its metabolic classification.
Similarly, Ghirelli et al. combined FDG-PET, tau PET, and amyloid PET to examine the association between tau and amyloid positivity and hypometabolism in patients with CBS [50]. They found that patients positive for both amyloid and tau exhibited more extensive hypometabolism in the lateral temporal, parietal, and occipital lobes compared to those who were tau-negative and either amyloid-positive or amyloid-negative. Additionally, this group showed more pronounced asymmetry, whereas metabolic patterns in the mesial temporal lobes and basal ganglia did not differ significantly across the three groups
Table 4 summarizes the main hypometabolic regions reported in each PSP study (when available). These regions are also illustrated in Figure 2.

Table 4.
FDG-PET 1 of the main hypometabolic regions in CBS 2.
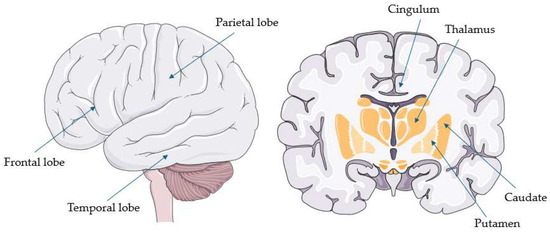
Figure 2.
Common hypometabolic regions in corticobasal syndrome (parts of the image provided by Servier Medical Art (https://smart.servier.com/ accessed on 21 August 2025), licensed under CC BY 4.0 (https://creativecommons.org/licenses/by/4.0/ accessed on 21 August 2025).
2.3. FDG-PET in Atypical Parkinsonian Disorders
FDG-PET has been extensively studied in Parkinson’s disease PD and APDs, with recommendations on its diagnostic utility and interpretation of findings [12]. Table 5 provides an overview of key studies comparing PSP and/or CBS with other Parkinsonian disorders.

Table 5.
Studies analyzing FDG-PET 1 patterns in PSP 2 and/or CBS 3 and Parkinsonian syndromes.
Eckert et al. used FDG-PET to examine hypometabolic patterns in patients with PSP, patients with multiple system atrophy (MSA), and healthy controls [52]. Their findings revealed a distinct hypometabolic pattern in PSP, primarily affecting the brainstem and bilateral medial frontal cortex. In contrast, MSA exhibited hypometabolism predominantly in the putamen and cerebellum. These characteristic patterns effectively distinguished both diseases from healthy controls (p < 0.001). Mudali et al. compared FDG-PET patterns among patients with PSP, PD, and MSA, as well as healthy controls [53]. Their computational analysis distinguished PSP from controls with moderate accuracy (AUC = 0.8) but showed relatively low accuracy in differentiating PSP from PD (AUC = 67.6) and MSA (AUC = 68.4).
Furthermore, Srulijes et al. compared FDG-PET patterns among patients with PSP-RS, PSP-P, and PD, as well as healthy controls [54]. They found thalamic hypometabolism in PSP-RS and putaminal hypometabolism in PSP-P compared to all other groups. Additionally, PSP-RS exhibited frontal hypometabolism. The putamen/thalamus uptake ratio effectively differentiated PSP-P from PSP-RS with good accuracy (AUC = 0.86) and from PD with an AUC of 0.80. Eckert et al. compared clinical and computerized assessments of patients with PSP, CBD, PD, and MSA, finding a high agreement rate of 92.4% [55]. Botha et al. investigated midbrain hypometabolism, known as the “pimple sign”, in PSP compared to CBS and MSA [90]. Their findings showed high specificity for PSP (100%), but very low sensitivity (29%)
Niethammer et al. investigated a pattern of bilateral, asymmetric hypometabolic dysfunction in the frontal and parietal cortices, as well as the caudate nuclei and thalami [56]. Their analysis revealed a significant overlap (24%) between CBD and PSP patients, but good discriminating ability between CBD and MSA. However, a logistic algorithm incorporating asymmetry scores and a distinct PSP pattern achieved high specificity for both CBD (92.7%) and PSP (94.1%). Similarly, in a follow-up period of 9 months, Hellwig et al. were able to discriminate PSP, CBD, PD, and MSA by utilizing distinctive hypometabolic patterns for each disease, achieving 90% diagnostic accuracy [57]. Comparing the same patient groups, Tripathi et al. achieved a high concordance between clinical and FDG-PET diagnoses (90.4% for PD, 80% for MSA, 93.3% for PSP, and 100% for CBS). Subsequently, Hellwig et al. have demonstrated moderate specificity and high sensitivity in diagnosing PSP (74% and 95%, respectively) and CBD (75% and 92%, respectively) in a comparative study with PD, dementia with Lewy bodies (DLB), and MSA patients [59]. In a reverse approach, Tang et al. enrolled patients with Parkinsonism and categorized them into PSP, PD, and MSA after calculating hypometabolic patterns based on logistic regression [60]. They then followed up with the patients under the care of a movement disorders specialist. PSP was predicted with 88% sensitivity and 94% specificity
Juh et al. compared patterns of hypometabolism in patients diagnosed with PSP, PD, and MSA, as well as in healthy controls [61]. All disease groups exhibited reduced metabolic activity in the neocortex. However, PSP was uniquely characterized by significant hypometabolism extending to subcortical regions, specifically the caudate nucleus, thalamus, midbrain, and anterior cingulum. These findings differentiated PSP not only from healthy controls but also from PD and MSA, suggesting distinct neuropathological processes.
Garraux et al. also utilized FDG-PET to compare patients with PSP, CBS, MSA, and PD [62]. However, classification accuracy was low for all disease study groups. Tripathi et al. continued their previous research by developing an algorithm with 94% specificity for differentiating PSP from PD and MSA [63]. Brajkovic et al. used FDG-PET hypometabolism to initially diagnose Parkinsonian syndromes [64]. After two years of clinical follow-up by a movement disorders specialist, the FDG-PET diagnosis was confirmed in 97% of PSP cases and 100% of CBS cases, compared to PD and MSA.
Furthermore, Marti-Andres et al. analyzed FDG-PET patterns in PSP subtypes, i.e., PSP-RS, PSP-P, PSP-PGF, PD, and controls [65]. PSP was distinguished from controls with 80% sensitivity and 96.9% specificity, and from PD with 80.4% sensitivity and 90.7% specificity. Notably, PSP-RS and PSP-P exhibited PSP-like hypometabolism more often than PD [65].
Shen et al. applied an MSA-defined FDG-PET hypometabolic pattern, involving decreased metabolism in the inferior frontal cortex, striatum, and cerebellum and increased metabolism in the occipital, parietal, and sensorimotor cortices, to differentiate patients with PSP, PD, and MSA from healthy controls [66]. The study demonstrated successful discrimination between all groups, highlighting the pattern’s diagnostic potential [66].
Moreover, Amod et al. employed FDG-PET to distinguish between patients with PSP, CBS, PD, MSA, and DLB, as well as healthy controls [67]. The study accurately identified 90% of PD patients and 93% of patients with PSP and CBS [67]. Lastly, Tomse et al. utilized PSP- and MSA-related pattern to compare patients with PSP, MSA—Parkinsonian type (MSA-P), and PD, as well as healthy controls. Both patterns were highly sensitive and specific (AUC 0.99 and 0.96, respectively) [68].
Eidelberg et al. were among the first to utilize FDG-PET to compare patients with CBD, patients with PD, and healthy controls [69]. Their findings revealed that the CBD group exhibited more pronounced and asymmetric hypometabolism in the thalamus, inferior parietal lobule, and hippocampus compared to the other groups [69]. Karbe et al. were among the first to compare patients with PSP, PD, and Parkinsonism (“PD plus dementia of Alzheimer type”) [70]. The latter group exhibited diffuse cortical hypometabolism, most prominently in the parietal lobes, whereas PD patients showed no significant cortical hypometabolism. In contrast, PSP patients demonstrated hypometabolism in the brainstem, putamen, caudate, and frontal cortex [70].
Klein et al. also compared PSP and PD patients. PSP patients showed hypometabolism in the dorsal midbrain and the caudal anterior cingulate, whereas PD patients exhibited hypometabolism in the lateral visual cortex and the right fusiform gyrus [71].
Herting et al. examined the relationship between brain hypometabolism and depression in patients with PSP, patients with MSA, and healthy controls. In PSP patients, reduced glucose metabolism was observed in the bilateral frontal cortex, right thalamus, and midbrain [72]. Additionally, in both PSP and MSA, depression severity was positively associated with hypometabolism in the dorsolateral prefrontal cortex. MSA patients also demonstrated bilateral hypometabolism in the cerebellar, frontal, and parietal cortices, as well as in the left putamen [72].
Park et al. compared patients with pure akinesia with gait freezing (PAGF) to those with PSP and PD, as well as healthy controls [73]. They found that PAGF patients exhibited hypometabolism in the midbrain, whereas PSP patients showed hypometabolism in both the midbrain and frontal cortex, consistent with prior findings. These results suggest one of the first in vivo pathophysiological links between PAGF and PSP, as demonstrated by FDG-PET imaging [73]. Likewise, in a notable prospective study, Josephs et al. followed 13 patients with primary progressive apraxia of speech (PPAOS) and found that 8 later progressed to PSP [78]. All patients demonstrated an expansion of their hypometabolic patterns over time [78].
Hellwig et al. sought to differentiate major neurodegenerative parkinsonian syndromes by combining FDG-PET and IBZM-SPECT imaging [74]. Their approach aimed to distinguish between PD, PD dementia (PDD), PSP, CBD, DLB, and MSA. The combined imaging yielded a sensitivity of 74% and specificity of 95% for PSP, and 75% sensitivity with 92% specificity for CBD. Notably, IBZM-SPECT was less effective than FDG-PET in distinguishing Lewy body disorders (PD, PDD, and DLB) from APDs [74]. Similarly, Teune et al. employed multivariate covariance analysis of FDG-PET data and demonstrated high discriminative accuracy for differentiating PSP, PD, and MSA based on their distinct metabolic patterns, even in early disease stages [75]. These findings were validated by long-term clinical follow-up confirming the diagnoses. However, Akdemir et al., using both visual and quantitative FDG-PET analysis, reported more heterogeneous results [76]. Most patients with PD, PSP, CBD, DLB, and MSA showed basal ganglia hypometabolism, with asymmetric patterns more frequently observed in PSP and PD. Additionally, some PSP and CBD patients exhibited thalamic hypometabolism, while cerebellar hypometabolism was predominant among MSA patients [76]. On the other hand, Baudrexel et al. combined FDG-PET with MRI diffusivity sequences and found that hypometabolism of the posterior putamen was associated with increased mean diffusivity in patients with MSA-P [77]. However, both imaging modalities demonstrated comparable discriminatory power in differentiating patients with PSP, patients with PD, and healthy controls [77].
Ko et al. combined FDG-PET and DAT-SPECT imaging to differentiate between PSP, CBS, PD, MSA, and DLB patients [79]. They found that metabolic and DAT binding patterns were correlated in CBS and PD, but not in PSP or MSA.
In addition, Ge et al. recruited patients with PSP, PD, and MSA from cohorts in both China and the United States [80]. They identified a characteristic hypometabolic pattern involving the cingulate, ventrolateral and middle prefrontal cortex, striatum, thalamus, and midbrain, along with relative hypermetabolism in the hippocampus and temporoparietal regions. This pattern effectively distinguished PSP from other groups with high reproducibility [80]. Similarly, Arnone et al. demonstrated that hypometabolic and hypermetabolic pattern maps improved diagnostic accuracy for non-experts when assessing patients with PSP, CBS, PD, and MSA [81]. In contrast, for expert clinicians, only the hypometabolic maps significantly enhanced diagnostic accuracy [81].
Lu et al. utilized two of the largest patient cohorts with Parkinsonism and FDG-PET and applied algorithms along with age- and gender-specific Z-scores to adjust for metabolic changes [82]. This approach achieved high diagnostic accuracy in distinguishing PSP, PD, and MSA [82].
On the other hand, in the study by Ouartassi et al., only half of the CBS patients exhibited a CBD-like hypometabolic pattern when compared to patients with PSP, patients with PD, and healthy controls [83]. In the study by Ali et al., FDG-PET was combined with volumetric MRI in patients with PSP, including both PSP-RS and other subtypes [84]. Clinical assessments included the Saccadic Impairment Scale (SIS) for vertical gaze palsy, the Montreal Cognitive Assessment (MoCA) for global cognition, and the MDS-Unified Parkinson’s Disease Rating Scale (MDS-UPDRS). The findings showed that both imaging modalities were more effective at predicting the presence of specific clinical features rather than their severity, suggesting their greater utility in assessing the state of the disease rather than its stage. FDG-PET was more sensitive to cortical-related clinical features, such as apraxia and executive dysfunction, while volumetric MRI was more effective in detecting subcortical-related features, such as Parkinsonism. The strongest predictive power for both modalities was observed for global cognitive performance as measured by MoCA. Furthermore, for specific symptoms, the imaging modality best predicted the corresponding anatomical substrates—for example, midbrain atrophy on MRI and frontal eye field hypometabolism on FDG-PET were both associated with vertical gaze palsy [84].
In a study by Du et al., the use of automated assessment tools—achieved very high diagnostic accuracy in differentiating patients with PD from those with PSP [85]. The study also included patients with MSA-P and vascular Parkinsonism [85]. Similarly, Ling et al. utilized a radiomics-guided deep learning model to differentiate patients from a large cohort with PD, PSP, MSA, and controls, achieving high sensitivity across all groups [86]. Subsequently, they applied a metabolic framework to the same cohort, which not only achieved high classification performance across all patient groups but also revealed disease-specific hypometabolic ROIs and networks—e.g., midbrain and midbrain-prefrontal disconnection in PSP [87]. Pillai et al. combined multiple MRI modalities with FDG-PET to study distinct PSP subtypes and patients with PD, demonstrating that FDG-PET could effectively differentiate PSP from PD [88]. Lastly, Stokelj et al. utilized FDG-PET data to develop an algorithm for differentiating patients with PD, PSP, and MSA [89]. However, while they achieved a high AUC for PSP, the correct identification rate of PSP patients was modest [89].
2.4. FDG-PET in Progressive Supranuclear Palsy and/or Corticobasal Syndrome Compared to Frontotemporal Dementia, Alzheimer’s Disease, and/or Neurodegenerative Diseases
FDG-PET has also been studied in FTD syndromes, including PSP and CBS. Table 6 provides an overview of key studies comparing PSP and/or CBS with other types of FTD, as well as AD and other neurodegenerative diseases.

Table 6.
Studies analyzing FDG-PET 1 patterns in PSP 2 and/or CBS 3 and frontotemporal syndromes.
Cerami et al. analyzed FDG-PET metabolic patterns in a large cohort of PPA patients [91]. This identified key ROIs associated with language impairment, including the left temporal (inferior, middle, and superior gyri, and pole), frontal (superior, middle, and inferior gyri), precentral gyrus, and parietal (inferior and superior lobules) regions. Specific hypometabolic patterns were observed for each PPA variant. Patients later diagnosed with CBD showed asymmetric parietal hypometabolism, while those meeting PSP criteria involved the midbrain and cerebellum. lvPPA patients presented with hypometabolism in the left temporal and parietal regions, with hippocampal involvement in 65% of cases. Clinical follow-up revealed progression to FTD in 6/11 svPPA patients, AD in 10/17 lvPPA patients, CBD in 11/19 nfvPPA patients, and PSP in 3/19 nfvPPA patients [91]. The same study group also stratified CBS patients by AD-related CSF biomarkers. Positive biomarkers correlated with typical AD hypometabolism (precuneus, posterior cingulate, and temporoparietal). Negative biomarkers correlated with bilateral frontoinsular and basal ganglia hypometabolism [92]. Similarly, Caminiti et al. studied the phenoconversion of MCI to AD and other dementias [14]. During clinical follow-up, 50 out of 80 MCI cases progressed to dementia, with 39 converting to AD dementia, 10 to FTD (including 2 to CBD and 1 to PSP), and 1 to DLB [14].
David Bergeron et al. have shown that, in patients with non-amnestic variants of AD and FTD, the dominant middle temporal gyrus showed the most hypometabolism in lvPPA, while the middle occipital gyrus showed the most hypometabolism in PCA, the middle temporal gyrus showed the most hypometabolism in frontal AD, and the angular gyrus showed the most hypometabolism in CBS associated with AD pathology [93].
Heikkinen et al. aimed to differentiate PSP, CBD, and bvFTD patients with extrapyramidal symptoms from those without extrapyramidal symptoms using FDG-PET and MRI biomarkers [94]. Patients with extrapyramidal symptoms demonstrated predominant hypometabolism in the left hemisphere, particularly in the temporal and medial frontal lobes. More specifically, in bvFTD, patients exhibited metabolic changes in the left temporal lobe, along with hypometabolism in the superior cerebellar peduncle, cerebellar lingula, and frontal lobes. In contrast, no cerebellar hypometabolism was observed in PSP or CBD [94]. Similarly, Isella et al. utilized FDG-PET to study hypometabolism in the precuneus, posterior cingulate, and temporoparietal cortex in cognitively impaired patients with a suspected underlying neurodegenerative cause [95]. Patients were divided into AD biomarker-positive and biomarker-negative groups, with metabolism estimated either visually or using a semi-quantitative tool. Interestingly, cases with an asymmetric temporoparietal profile and a CBS or PPA phenotype were more frequently classified as having an AD-like pattern, even in the absence of other AD biomarkers [95].
Teune et al. recruited early-stage patients with PSP, CBD, PD, DLB, MSA, AD, and FTD, and successfully discriminated between these conditions by analyzing disease-specific hypometabolic patterns—such as in the prefrontal cortex, caudate, thalamus, and midbrain for PSP, and the contralateral cortex for CBD [96]. However, when Wenzel et al. applied a b-spline method for stereotactic normalization of FDG-PET data from patients with CBD, AD, DLB, and FTD, as well as healthy controls, it had an insignificant impact on expert-based classification outcomes [97].
Garibotto et al. combined FDG-PET with DAT-SPECT to differentiate patients with CBD, AD, DLB, and PDD, and found that the combined approach offered greater diagnostic accuracy than either modality alone, both in discriminant analysis and cross-validation [98]. Similarly, Taswell et al. combined FDG-PET with amyloid PET to distinguish between patients with CBD, AD, lvPPA, nfvPPA, and svPPA, reporting that FDG-PET outperformed clinical assessment in the diagnostic accuracy of AD [99]. Moreover, Franceschi et al. combined FDG-PET with volumetric MRI to study patients with CBD, AD, DLB, and FTD [100]. They found a Pearson correlation coefficient of 0.58 (p < 0.05) between the metabolic z-score and lobar volume in the superior parietal lobule for CBD patients [100]. Subsequently, the same study group used the same imaging combination to investigate crossed cerebellar diaschisis in patients with neurodegenerative diseases. However, only 7.5% of patients exhibited crossed cerebellar diaschisis, three of whom showed imaging findings consistent with CBD [101]. They also applied the same modalities to patients within the FTLD and PPA spectrum. In these cases, patients with PSP showed hypometabolism in the posterior frontal cortex, thalamus, basal ganglia, and midbrain. In contrast, patients with CBD exhibited predominantly asymmetric hypometabolism in the sensorimotor cortex, basal ganglia, and thalamus [102].
On the other hand, Josephs et al. followed patients with PPAOS over a period of more than ten years, all of whom underwent autopsy. The majority were ultimately diagnosed with either PSP or CBD [103]. Interestingly, FDG-PET scans at the initial evaluation showed no significant metabolic differences between patients who later developed PSP or CBD. However, after four years, patients with CBD exhibited greater rates of metabolic decline and lower cortical metabolism. Performance on neurocognitive tests correlated with metabolism in the left Broca’s area in both groups, and additionally with the left superior temporal gyrus in the CBD group [103]. However, when Seckin et al. followed up patients with PPAOS, all of whom eventually developed either PSP or CBS, they observed only slight left-sided asymmetric hypometabolism in the CBS group, and absent or minimal midbrain hypometabolism in the PSP group [104].
Gan et al. applied FDG-PET in patients with various dementia syndromes, including PSP and CBS, and found that patients within the FTLD spectrum, including PSP and CBS, generally showed modest percentages of frontal and anterior temporal hypometabolism [105]. Lastly, Braun et al. combined FDG-PET with MRI to distinguish autopsy-confirmed PSP cases from a diverse cohort of neurodegenerative diseases—including CBD, AD, PCA, PPAOS, PD, DLB, MSA, and Pick’s disease—achieving high classification accuracy [106].
3. Discussion
Overall, FDG-PET appears to be a useful supportive biomarker for the diagnosis of PSP and CBS. This aligns with the latest European intersocietal recommendations, which suggest that FDG-PET should be the first assessment tool once a clinical syndrome of PSP or CBS has been recognized [13]. In PSP, the most common hypometabolic regions include the midbrain (particularly the tegmentum) and extensive areas of the frontal cortex (such as the sensorimotor, premotor, orbitofrontal, prefrontal, superior, middle, inferior, and medial regions), usually bilaterally [30,32]. These are followed by the cingulate cortex (especially the anterior cingulum), caudate, putamen, and thalamus [21]. Other regions—such as the globus pallidus, parietal and insular cortices, pons, cerebellar vermis, and superior cerebellar peduncles—are less commonly involved [16,21,24,27,29].
CBS appears to have a hypometabolic pattern that is not commonly encountered in PSP. Firstly, unlike PSP, hypometabolism in CBS is highly asymmetrical, predominantly affecting the hemisphere contralateral to the most clinically affected side [23,44,47]. Secondly, cortical and lobar structures appear to be more commonly affected than subcortical or subtentorial regions. Indeed, in contrast to PSP, hypometabolism of the midbrain or other subtentorial structures is only rarely reported [21,50], while among subcortical but supratentorial regions, the thalamus appears to be more frequently involved than the striatum [37,48,49]. Thirdly, whereas PSP more commonly affects the frontal cortex, CBS often extends to more posterior cortical regions, particularly the parietal lobe (especially the postcentral, inferior, and superior parietal gyri), and less frequently the lateral temporal and occipital cortices—areas rarely affected in PSP [26,42,44,45,46,48]. Moreover, when the frontal lobe is implicated in CBS, its more posterior regions (e.g., precentral gyrus, supplementary motor area, and inferior frontal gyrus) are typically involved, whereas, in PSP, frontal hypometabolism tends to be more generalized [26,42,44,45,48]. Similarly, while PSP more often affects the anterior cingulate, CBS also involves its posterior regions [23,24,45,47].
However, several methodological and interpretative issues are evident in many of the studies included in this review. The majority of FDG-PET studies involving PSP and CBS were not specifically designed for these disorders. Instead, their primary aim was to differentiate PD from APDs, which simply include PSP and CBS. As a result, PSP and CBS data were often grouped with other APDs, providing extensive comparative data between these disorders and PD but limiting direct comparisons with other Parkinsonian syndromes, such as MSA and DLB. This lack of direct comparison hinders efforts to achieve nuanced differential diagnoses.
A similar issue arises when comparing PSP and CBS to FTLD spectrum disorders, such as bvFTD and PPA. The substantial clinical overlap between these syndromes complicates biomarker studies. Current diagnostic criteria acknowledge that bvFTD and PPA can manifest as clinical presentations of underlying PSP or CBS pathology [1,2].
Therefore, studies including only autopsy-confirmed cases are essential to avoid grouping patients who share the same clinical syndrome but have different underlying pathologies. Nonetheless, even autopsy-confirmed cases may still be affected by selection bias [107].
Moreover, the methodology varies significantly, with studies using different FDG protocols or applying distinct FDG patterns associated with various neurodegenerative diseases [108]. Furthermore, these studies often begin with different baseline cognitive or neurodegenerative syndromes (e.g., MCI or PPA) and examine how they progress into more specific syndromes, such as PSP and CBS, over diverse follow-up periods. This variability is common in studies enrolling patients with FTD syndromes. Additionally, while some studies incorporate follow-up with repeated FDG-PET imaging, others rely solely on clinical re-evaluation. These methodological discrepancies greatly affect the generalizability of the results [109].
Furthermore, only studies with generally small sample sizes specifically focus on PSP and/or CBS subtypes [30,88,106], which exhibit distinct clinical courses and prognoses. Some subtypes may also benefit from different symptomatic treatment approaches, highlighting the need for more targeted research in this area. In addition, only a few studies validate biomarker- or clinically based diagnoses through autopsy, which remains the gold standard for confirming PSP and CBS. The lack of postmortem validation limits the ability to assess the true diagnostic accuracy of these approaches and underscores the need for more studies incorporating neuropathological confirmation.
Despite several studies achieving high diagnostic accuracy for PSP and/or CBS with specificity exceeding 90% using FDG-PET, sensitivity remains considerably lower [65,74,90]. Moreover, high specificity is more frequently attained with the aid of quantitative or semiquantitative computational analysis, whereas simple visual evaluation yields more modest results. Thus, computer-assisted analysis appears to provide high specificity, suggesting that FDG-PET could be a valuable tool in the differential diagnosis of PSP and CBS. Such a combination of fluid and imaging biomarkers may offer a more com-prehensive diagnostic approach while reducing the need for more invasive procedures, such as lumbar puncture for CSF biomarker analysis. A large-scale, multicenter cohort study specifically focused on PSP and CBS, incorporating both FDG-PET and serum biomarkers, could help to establish a more accurate diagnostic framework for these overlapping syndromes.
In addition, a combination of FDG-PET with other emerging techniques may boost its diagnostic utility. For example, combining FDG-PET with tau PET imaging may increase the diagnostic accuracy and understanding PSP and CBS. These imaging modalities provide complementary insights: tau PET identifies the distribution and burden of tau protein deposits, while FDG-PET assesses regional cerebral glucose metabolism, reflecting neuronal activity and integrity [110]. Thus, in PSP, tau PET imaging has demonstrated increased uptake in certain regions, such as the midbrain, thalamus, and basal ganglia, corresponding to areas affected by tau pathology [110,111]. FDG-PET in PSP typically shows hypometabolism in the frontal lobes, midbrain, and caudate nucleus, aligning with the regions of tau deposition [110,112]. The combination of these imaging techniques allows for a more comprehensive assessment of both the distribution of tau pathology and the functional consequences of neuronal degeneration [112].
Similarly, in CBS, tau PET imaging has revealed asymmetric tau deposition, particularly in the parietal and frontal cortices, which are associated with the clinical manifestations of the syndrome [113]. FDG-PET in CBS often shows hypometabolism in the affected cortical regions, correlating with the areas of tau accumulation [110,113,114]. The integration of tau PET and FDG-PET provides a dual perspective on the structural and functional abnormalities in CBS, aiding in differential diagnosis and monitoring disease progression [115].
Recent studies have highlighted the complementary value of combining tau PET with FDG-PET in these disorders. For instance, a study by Liang et al. emphasized the utility of this combined approach in distinguishing corticobasal degeneration from other neurodegenerative conditions, underscoring the importance of integrating structural and functional imaging to enhance diagnostic precision [114].
Furthermore, recent advances in MRI have explored the use of neural probes to investigate microstructural and biochemical changes in PSP and CBS. Techniques including diffusion tensor imaging and magnetic resonance spectroscopy enable the assessment of white matter integrity, iron deposition, and neurochemical alterations [116,117]. These MRI-based markers provide valuable insights into disease mechanisms, while also holding promise as diagnostic and progression-monitoring tools in PSP and CBS. Moreover, quantitative susceptibility mapping (QSM), an advanced MRI technique, has become instrumental in identifying abnormal iron deposition within the brain, offering insights into the pathophysiology of neurodegenerative disorders [10]. In PSP, QSM studies consistently reveal increased magnetic susceptibility in subcortical regions, notably the globus pallidus, substantia nigra, and red nucleus [118]. Additionally, Kawabata et al. observed distinctive deformation of the red nucleus in PSP patients, detectable via coronal QSM imaging, which may serve as a morphological marker for the disease. Moreover, combining QSM measurements with volumetric data from the subthalamic nucleus has shown promise in differentiating PSP from PD, even in the early stages [119]. CBS also exhibits unique QSM characteristics. Miyata et al. reported asymmetric increases in susceptibility within the cerebral gyri of CBS patients, a pattern not typically observed in other neurodegenerative disorders. This “three-layer” appearance, particularly evident in the left precentral gyrus, suggests a cortical involvement that may aid in distinguishing CBS from other conditions [120]. Furthermore, QSM findings in CBS patients have demonstrated the potential to complement other imaging modalities, including FDG-PET, enhancing diagnostic accuracy and providing a more comprehensive understanding of the disease’s progression [121].
Lastly, we would like to note that, as this is a narrative review, the reproducibility of the results presented is limited. A future systematic review on this topic would greatly enhance the scientific validity of the evidence.
4. Materials and Methods
4.1. Search Strategy
We searched the PubMed database from its inception until 5 July 2025, using the algorithm “fluorodeoxyglucose positron emission tomography AND (progressive supranuclear palsy OR corticobasal)”, according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines [122]. Studies were included if they met the following criteria: (a) original research articles; (b) full text available in English; (c) human studies; (d) reporting the number of patients with PSP and CBS that were included in the study; and (e) investigations assessing the utility of FDG-PET for diagnosis or differential diagnosis from other neurodegenerative conditions. Narrative reviews, systematic reviews, meta-analyses, case reports, brief communications, opinion papers, letters to the editors, etc., were excluded from the study.
4.2. Study Selection and Categorization
As depicted in Figure 3, our algorithm initially yielded 177 results. Five studies were excluded due to being either non-English or not involving human subjects. Forty-three articles were excluded as they were reviews, meta-analyses, case reports, opinion papers, or editorials. This left 129 studies for the full-text review. Following this, 38 studies were excluded for being irrelevant to the purpose of our review. Ultimately, 91 studies were eligible. These 91 studies were categorized as follows: (a) 20 studies included only patients with PSP, (b) 15 studies included only patients with CBS, (c) 39 studies involved patients with APDs, including subgroups of PSP and/or CBS, and (d) 17 studies compared patients with PSP and/or CBS to individuals with FTD, AD, or other dementias.
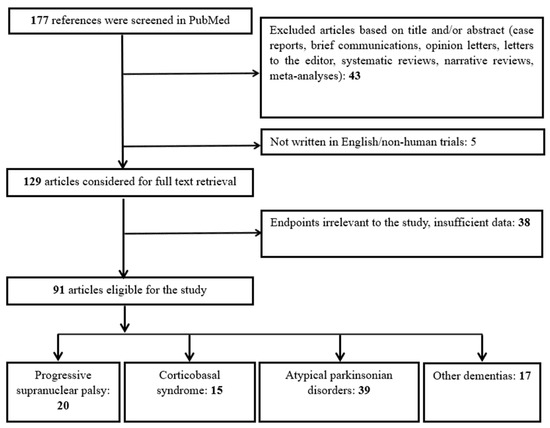
Figure 3.
Flowchart of study selection and categorization.
5. Conclusions
In conclusion, FDG-PET may hold potential for improving the differential diagnosis of PSP and CBS, as they achieve high specificity with the aid of computational analysis but generally exhibit low sensitivity. However, future studies should focus explicitly on PSP and CBS rather than using them as comparative references for other neurodegenerative disorders. Large-scale, multicenter cohort studies employing standardized methodologies are necessary to enhance validity and reproducibility. Given the frequent overlap in pathology between PSP and CBS, pathology-confirmed study cohorts should be prioritized. Implementing these strategies could improve diagnostic accuracy and deepen our understanding of these complex diseases, ultimately paving the way for effective treatments.
Author Contributions
Conceptualization, A.G.; methodology, S.K.; data curation, C.S.; writing—original draft preparation, A.G.; writing—original draft preparation, E.K.; writing—review and editing, L.P.; supervision and editing, A.P.K.; project administration, G.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data was created during the preparation of this manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| 4R | four-repeat |
| AD | Alzheimer’s disease |
| APD | atypical Parkinsonian disorder |
| AUC | area under the curve |
| bvFTD | behavioral variant frontotemporal dementia |
| CBD | corticobasal degeneration |
| CBS | corticobasal syndrome |
| CSF | cerebrospinal fluid |
| DaT-SPECT | dopamine transporter imaging with single-photon emission computed tomography |
| DLB | dementia with Lewy bodies |
| FAB | Frontal Assessment Battery |
| FDG-PET | fludeoxyglucose-18–positron emission tomography |
| FTD | frontotemporal dementia |
| GGT | globular glial tauopathy |
| IBZM | 123I-iodobenzamide |
| lvPPA | logopenic variant PPA |
| MCI | mild cognitive impairment |
| MDS | Movement Disorders Society |
| MDS-UPDRS | MDS-Unified Parkinson’s Disease Rating Scale |
| MMSE | Mini-Mental State Examination |
| MoCA | Montreal Cognitive Assessment |
| MRI | magnetic resonance imaging |
| MSA | multiple system atrophy |
| MSA-P | multiple system atrophy-parkinsonian type |
| nfvPPA | nonfluent variant PPA |
| PAGF | pure akinesia with gait freezing |
| PCA | posterior cortical atrophy |
| PD | Parkinson’s disease |
| PDD | Parkinson’s disease dementia |
| PET | Positron emission tomography |
| PPA | primary progressive aphasia |
| PPAOS | primary progressive apraxia of speech |
| PSP | progressive supranuclear palsy |
| PSP-CBS | PSP with predominant corticobasal syndrome |
| PSP-F | PSP with predominant frontal presentation |
| PSP-OM | PSP with predominant ocular motor dysfunction |
| PSP-P | PSP with predominant Parkinsonism |
| PSP-PGF | PSP with progressive gait freezing |
| PSP-PI | PSP with postural instability |
| PSP-PLS | PSP–primary lateral sclerosis |
| PSP-SL | PSP with predominant speech/language impairment |
| PSP-RS | PSP–Richardson syndrome |
| ROI | region of interest |
| SIS | Saccadic Impairment Scale |
| SPECT | single-photon emission computed tomography |
| svPPA | semantic variant PPA |
References
- Höglinger, G.U.; Respondek, G.; Stamelou, M.; Kurz, C.; Josephs, K.A.; Lang, A.E.; Mollenhauer, B.; Müller, U.; Nilsson, C.; Whitwell, J.L.; et al. Clinical Diagnosis of Progressive Supranuclear Palsy: The Movement Disorder Society Criteria. Mov. Disord. 2017, 32, 853–864. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, M.J.; Litvan, I.; Lang, A.E.; Bak, T.H.; Bhatia, K.P.; Borroni, B.; Boxer, A.L.; Dickson, D.W.; Grossman, M.; Hallett, M.; et al. Criteria for the Diagnosis of Corticobasal Degeneration. Neurology 2013, 80, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Giannakis, A.; Sioka, C.; Kloufetou, E.; Konitsiotis, S. Cognitive Impairment in Parkinson’s Disease and Other Parkinsonian Syndromes. J. Neural Transm. 2025, 132, 341–355. [Google Scholar] [CrossRef]
- Giannakis, A.; Konitsiotis, S. A New Paradigm for Neurodegenerative Diseases Classification: A Clinical Perspective. J. Clin. Neurosci. 2025, 134, 111099. [Google Scholar] [CrossRef]
- Shir, D.; Pham, N.T.T.; Botha, H.; Koga, S.; Kouri, N.; Ali, F.; Knopman, D.S.; Petersen, R.C.; Boeve, B.F.; Kremers, W.K.; et al. Clinicoradiologic and Neuropathologic Evaluation of Corticobasal Syndrome. Neurology 2023, 101, E289–E299. [Google Scholar] [CrossRef] [PubMed]
- Crutch, S.J.; Schott, J.M.; Rabinovici, G.D.; Murray, M.; Snowden, J.S.; van der Flier, W.M.; Dickerson, B.C.; Vandenberghe, R.; Ahmed, S.; Bak, T.H.; et al. Consensus Classification of Posterior Cortical Atrophy. Alzheimer’s Dement. 2017, 13, 870–884. [Google Scholar] [CrossRef]
- Heikkinen, S.; Katisko, K.; Haapasalo, A.; Portaankorva, A.; Hartikainen, P.; Solje, E. Overlap in the Diagnostic Criteria of Frontotemporal Dementia Syndromes with Parkinsonism. J. Alzheimer’s Dis. 2025, 104, 374–381. [Google Scholar] [CrossRef]
- Giannakis, A.; Konitsiotis, S.; Sioka, C. Differentiating Progressive Supranuclear Palsy and Corticobasal Syndrome: Insights from Cerebrospinal Fluid Biomarkers—A Narrative Review. Medicina. 2025, 61, 701. [Google Scholar] [CrossRef]
- Giannakis, A.; Konitsiotis, S.; Sioka, C. A Narrative Review of Serum Biomarkers in Progressive Supranuclear Palsy, Corticobasal Syndrome, and Related Disorders. J. Neural Transm. 2025. [Google Scholar] [CrossRef]
- Li, G.; Wu, R.; Tong, R.; Bo, B.; Zhao, Y.; Gillen, K.M.; Spincemaille, P.; Ku, Y.; Du, Y.; Wang, Y.; et al. Quantitative Measurement of Metal Accumulation in Brain of Patients With Wilson’s Disease. Mov. Disord. 2020, 35, 1787–1795. [Google Scholar] [CrossRef]
- Bellini, G.; Di Rauso, G.; Fontanelli, L.; Benevento, E.; Becattini, L.; Frosini, D.; Ceravolo, R.; Del Prete, E. Deep Brain Stimulation in Progressive Supranuclear Palsy: A Dead-End Story? A Narrative Review. J. Neural Transm. 2025. [Google Scholar] [CrossRef]
- Nobili, F.; Arbizu, J.; Bouwman, F.; Drzezga, A.; Agosta, F.; Nestor, P.; Walker, Z.; Boccardi, M. European Association of Nuclear Medicine and European Academy of Neurology Recommendations for the Use of Brain 18F-fluorodeoxyglucose Positron Emission Tomography in Neurodegenerative Cognitive Impairment and Dementia: Delphi Consensus. Eur. J. Neurol. 2018, 25, 1201–1217. [Google Scholar] [CrossRef] [PubMed]
- Frisoni, G.B.; Festari, C.; Massa, F.; Cotta Ramusino, M.; Orini, S.; Aarsland, D.; Agosta, F.; Babiloni, C.; Borroni, B.; Cappa, S.F.; et al. European Intersocietal Recommendations for the Biomarker-Based Diagnosis of Neurocognitive Disorders. Lancet Neurol. 2024, 23, 302–312. [Google Scholar] [CrossRef] [PubMed]
- Caminiti, S.P.; Ballarini, T.; Sala, A.; Cerami, C.; Presotto, L.; Santangelo, R.; Fallanca, F.; Vanoli, E.G.; Gianolli, L.; Iannaccone, S.; et al. FDG-PET and CSF Biomarker Accuracy in Prediction of Conversion to Different Dementias in a Large Multicentre MCI Cohort. Neuroimage Clin. 2018, 18, 167–177. [Google Scholar] [CrossRef]
- Saeed, U.; Lang, A.E.; Masellis, M. Neuroimaging Advances in Parkinson’s Disease and Atypical Parkinsonian Syndromes. Front. Neurol. 2020, 11, 572976. [Google Scholar] [CrossRef]
- Foster, N.L.; Gilman, S.; Berent, S.; Morin, E.M.; Brown, M.B.; Koeppe, R.A. Cerebral Hypometabolism in Progressive Supranuclear Palsy Studied with Positron Emission Tomography. Ann. Neurol. 1988, 24, 399–406. [Google Scholar] [CrossRef]
- Goffinet, A.M.; De Voider, A.G.; Gillain, C.; Rectem, D.; Bol, A.; Michel, C.; Cogneau, M.; Labar, D.; Laterre, C. Positron Tomography Demonstrates Frontal Lobe Hypometabolism in Progressive Supranuclear Palsy. Ann. Neurol. 1989, 25, 131–139. [Google Scholar] [CrossRef]
- Karbe, H.; Grond, M.; Huber, M.; Herholz, K.; Kessler, J.; Heiss, W.-D. Subcortical Damage and Cortical Dysfunction in Progressive Supranuclear Palsy Demonstrated by Positron Emission Tomography. J. Neurol. 1992, 239, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Zhang, B.; Gao, S.; Li, X. Clinical, MRI and 18F-FDG-PET/CT Analysis of Progressive Supranuclear Palsy. J. Clin. Neurosci. 2020, 80, 318–323. [Google Scholar] [CrossRef]
- Yamauchi, H.; Fukuyama, H.; Nagahama, Y.; Katsumi, Y.; Dong, Y.; Konishi, J.; Kimura, J. Atrophy of the Corpus Callosum, Cognitive Impairment, and Cortical Hypometabolism in Progressive Supranuclear Palsy. Ann. Neurol. 1997, 41, 606–614. [Google Scholar] [CrossRef]
- Mishina, M.; Ishii, K.; Mitani, K.; Ohyama, M.; Yamazaki, M.; Ishiwata, K.; Senda, M.; Kobayashi, S.; Kitamura, S.; Katayama, Y. Midbrain Hypometabolism as Early Diagnostic Sign for Progressive Supranuclear Palsy. Acta Neurol. Scand. 2004, 110, 128–135. [Google Scholar] [CrossRef]
- Takahashi, R.; Ishii, K.; Kakigi, T.; Yokoyama, K.; Mori, E.; Murakami, T. Brain Alterations and Mini-Mental State Examination in Patients with Progressive Supranuclear Palsy: Voxel-Based Investigations Using 18F-Fluorodeoxyglucose Positron Emission Tomography and Magnetic Resonance Imaging. Dement. Geriatr. Cogn. Dis. Extra 2011, 1, 381–392. [Google Scholar] [CrossRef]
- Zwergal, A.; La Fougère, C.; Lorenzl, S.; Rominger, A.; Xiong, G.; Deutschenbaur, L.; Linn, J.; Krafczyk, S.; Dieterich, M.; Brandt, T.; et al. Postural Imbalance and Falls in PSP Correlate with Functional Pathology of the Thalamus. Neurology 2011, 77, 101–109. [Google Scholar] [CrossRef]
- Zwergal, A.; Fougère, C.L.; Lorenzl, S.; Rominger, A.; Xiong, G.; Deutschenbaur, L.; Schöberl, F.; Linn, J.; Dieterich, M.; Brandt, T.; et al. Functional Disturbance of the Locomotor Network in Progressive Supranuclear Palsy. Neurology 2013, 80, 634–641. [Google Scholar] [CrossRef]
- Amtage, F.; Maurer, C.; Hellwig, S.; Tüscher, O.; Kreft, A.; Weiller, C.; Rijntjes, M.; Winkler, C.; Meyer, P.T. Functional Correlates of Vertical Gaze Palsy and Other Ocular Motor Deficits in PSP: An FDG-PET Study. Park. Relat. Disord. 2014, 20, 898–906. [Google Scholar] [CrossRef]
- Isella, V.; Licciardo, D.; Ferri, F.; Crivellaro, C.; Morzenti, S.; Appollonio, I.; Ferrarese, C. Reduced Phonemic Fluency in Progressive Supranuclear Palsy Is Due to Dysfunction of Dominant BA6. Front. Aging Neurosci. 2022, 14, 969875. [Google Scholar] [CrossRef]
- Doll-Lee, J.; Klietz, M.; Greten, S.; Kopp, B.; Berding, G.; Brendel, M.; Wilkens, I.; Katzdobler, S.; Levin, J.; Danek, A.; et al. Associations between Neuropsychological Profile and Regional Brain FDG Uptake in Progressive Supranuclear Palsy. J. Park. Dis. 2025, 15, 904–912. [Google Scholar] [CrossRef]
- Buchert, R.; Wegner, F.; Huppertz, H.J.; Berding, G.; Brendel, M.; Apostolova, I.; Buhmann, C.; Dierks, A.; Katzdobler, S.; Klietz, M.; et al. Automatic Covariance Pattern Analysis Outperforms Visual Reading of 18F-Fluorodeoxyglucose-Positron Emission Tomography (FDG-PET) in Variant Progressive Supranuclear Palsy. Mov. Disord. 2023, 38, 1901–1913. [Google Scholar] [CrossRef] [PubMed]
- Garraux, G.; Salmon, E.; Peigneux, P.; Kreisler, A.; Degueldre, C.; Lemaire, C.; Destée, A.; Franck, G. Voxel-Based Distribution of Metabolic Impairment in Corticobasal Degeneration. Mov. Disord. 2000, 15, 894–904. [Google Scholar] [CrossRef] [PubMed]
- Black, J.A.; Pham, N.T.T.; Ali, F.; Machulda, M.M.; Lowe, V.J.; Josephs, K.A.; Whitwell, J.L. Frontal Hypometabolism in the Diagnosis of Progressive Supranuclear Palsy Clinical Variants. J. Neurol. 2024, 271, 4267–4280. [Google Scholar] [CrossRef] [PubMed]
- Hosaka, K.; Ishii, K.; Sakamoto, S.; Mori, T.; Sasaki, M.; Hirono, N.; Mori, E. Voxel-Based Comparison of Regional Cerebral Glucose Metabolism between PSP and Corticobasal Degeneration. J. Neurol. Sci. 2002, 199, 67–71. [Google Scholar] [CrossRef]
- Juh, R.; Pae, C.U.; Kim, T.S.; Lee, C.U.; Choe, B.; Suh, T. Cerebral Glucose Metabolism in Corticobasal Degeneration Comparison with Progressive Supranuclear Palsy Using Statistical Mapping Analysis. Neurosci. Lett. 2005, 383, 22–27. [Google Scholar] [CrossRef]
- Amtage, F.; Hellwig, S.; Kreft, A.; Spehl, T.; Glauche, V.; Winkler, C.; Rijntjes, M.; Hellwig, B.; Weiller, C.; Weber, W.A.; et al. Neuronal Correlates of Clinical Asymmetry in Progressive Supranuclear Palsy. Clin. Nucl. Med. 2014, 39, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Zalewski, N.; Botha, H.; Whitwell, J.L.; Lowe, V.; Dickson, D.W.; Josephs, K.A. FDG-PET in Pathologically Confirmed Spontaneous 4R-Tauopathy Variants. J. Neurol. 2014, 261, 710–716. [Google Scholar] [CrossRef]
- Buchert, R.; Huppertz, H.J.; Wegner, F.; Berding, G.; Brendel, M.; Apostolova, I.; Buhmann, C.; Poetter-Nerger, M.; Dierks, A.; Katzdobler, S.; et al. Added Value of FDG-PET for Detection of Progressive Supranuclear Palsy. J. Neurol. Neurosurg. Psychiatry 2024, 96, 287–295. [Google Scholar] [CrossRef]
- Blin, J.; Vidailhet, M.-J.; Pillon, B.; Dubois, B.; Feve, J.-R.; Agid, Y. Corticobasal Degeneration: Decreased and Asymmetrical Glucose Consumption as Studied with PET. Mov. Disord. 1992, 7, 348–354. [Google Scholar] [CrossRef]
- Klaffke, S.; Kuhn, A.A.; Plotkin, M.; Amthauer, H.; Harnack, D.; Felix, R.; Kupsch, A. Dopamine Transporters, D2 Receptors, and Glucose Metabolism in Corticobasal Degeneration. Mov. Disord. 2006, 21, 1724–1727. [Google Scholar] [CrossRef]
- Turaga, S.P.; Mridula, R.; Borgohain, R. Cerebral Glucose Metabolism, Clinical, Neuropsychological, and Radiological Profile in Patients with Corticobasal Syndrome. Neurol. India 2013, 61, 7–11. [Google Scholar] [CrossRef]
- Franceschi, A.; Clifton, M.; Naser-Tavakolian, K.; Ahmed, O.; Cruciata, G.; Bangiyev, L.; Clouston, S.; Franceschi, D. (18F)-Fluorodeoxyglucose Positron Emission Tomography/Magnetic Resonance Imaging Assessment of Hypometabolism Patterns in Clinical Phenotypes of Suspected Corticobasal Degeneration. World J. Nucl. Med. 2021, 20, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Sha, S.J.; Ghosh, P.M.; Lee, S.E.; Corbetta-Rastelli, C.; Jagust, W.J.; Kornak, J.; Rankin, K.P.; Grinberg, L.T.; Vinter, H.V.; Mendez, M.F.; et al. Predicting Amyloid Status in Corticobasal Syndrome Using Modified Clinical Criteria, Magnetic Resonance Imaging and Fluorodeoxyglucose Positron Emission Tomography. Alzheimers Res. Ther. 2015, 7, 8. [Google Scholar] [CrossRef] [PubMed]
- Boman, A.; Svensson, S.; Boxer, A.; Rojas, J.C.; Seeley, W.W.; Karydas, A.; Miller, B.; Kagedal, K.; Svenningsson, P. Distinct Lysosomal Network Protein Profiles in Parkinsonian Syndrome Cerebrospinal Fluid. J. Park. Dis. 2016, 6, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Isella, V.; Grisanti, S.G.; Ferri, F.; Morzenti, S.; Crivellaro, C.; Musarra, M.; Ferrarese, C. Cognitive Reserve Maps the Core Loci of Neurodegeneration in Corticobasal Degeneration. Eur. J. Neurol. 2018, 25, 1333–1340. [Google Scholar] [CrossRef] [PubMed]
- Isella, V.; Licciardo, D.; Ferri, F.; Crivellaro, C.; Morzenti, S.; Appollonio, I.M.; Ferrarese, C. Left and Right Corticobasal Syndrome: Comparison of Cognitive Profiles between Metabolic Imaging—Matched Groups. Neurol. Sci. 2024, 45, 1499–1506. [Google Scholar] [CrossRef]
- Pardini, M.; Huey, E.D.; Spina, S.; Kreisl, W.C.; Morbelli, S.; Wassermann, E.M.; Nobili, F.; Ghetti, B.; Grafman, J. FDG-PET Patterns Associated with Underlying Pathology in Corticobasal Syndrome. Neurology 2019, 92, E1121–E1135. [Google Scholar] [CrossRef]
- Jo, S.; Oh, J.S.; Cheong, E.N.; Kim, H.J.; Lee, S.; Oh, M.; Kim, J.S.; Chung, S.J.; Lee, C.S.; Kwon, M.; et al. FDG-PET Patterns Associated with Ideomotor Apraxia and Imitation Apraxia in Patients with Corticobasal Syndrome. Park. Relat. Disord. 2021, 88, 96–101. [Google Scholar] [CrossRef]
- Parmera, J.B.; de Almeida, I.J.; de Oliveira, M.C.B.; Silagi, M.L.; de Godoi Carneiro, C.; Studart-Neto, A.; Ono, C.R.; Reis Barbosa, E.; Nitrini, R.; Buchpiguel, C.A.; et al. Metabolic and Structural Signatures of Speech and Language Impairment in Corticobasal Syndrome: A Multimodal PET/MRI Study. Front. Neurol. 2021, 12, 702052. [Google Scholar] [CrossRef]
- Parmera, J.B.; de Godoi Carneiro, C.; de Almeida, I.J.; de Oliveira, M.C.B.; Barbosa, P.M.; Studart-Neto, A.; Ono, C.R.; Nitrini, R.; Buchpiguel, C.A.; Barbosa, E.R.; et al. Probable 4-Repeat Tauopathy Criteria Predict Brain Amyloid Negativity, Distinct Clinical Features, and FDG-PET/MRI Neurodegeneneration Patterns in Corticobasal Syndrome. Mov. Disord. Clin. Pract. 2024, 11, 238–247. [Google Scholar] [CrossRef]
- Parmera, J.B.; Coutinho, A.M.; Aranha, M.R.; Studart-Neto, A.; de Godoi Carneiro, C.; de Almeida, I.J.; Fontoura Solla, D.J.; Ono, C.R.; Barbosa, E.R.; Nitrini, R.; et al. FDG-PET Patterns Predict Amyloid Deposition and Clinical Profile in Corticobasal Syndrome. Mov. Disord. 2021, 36, 651–661. [Google Scholar] [CrossRef] [PubMed]
- Nakano, Y.; Shimada, H.; Shinotoh, H.; Hirano, S.; Tagai, K.; Sano, Y.; Yamamoto, Y.; Endo, H.; Matsuoka, K.; Takahata, K.; et al. PET-Based Classification of Corticobasal Syndrome. Park. Relat. Disord. 2022, 98, 92–98. [Google Scholar] [CrossRef]
- Ghirelli, A.; Goodrich, A.W.; Stephens, Y.C.; Graff-Radford, J.; Ali, F.; Machulda, M.M.; Schwarz, C.G.; Senjem, M.L.; Agosta, F.; Filippi, M.; et al. Relationships between Hypometabolism and Both β-Amyloid and Tau PET in Corticobasal Syndrome. Alzheimer’s Dement. 2025, 21, e70018. [Google Scholar] [CrossRef]
- Mille, E.; Levin, J.; Brendel, M.; Zach, C.; Barthel, H.; Sabri, O.; Bötzel, K.; Bartenstein, P.; Danek, A.; Rominger, A. Cerebral Glucose Metabolism and Dopaminergic Function in Patients with Corticobasal Syndrome. J. Neuroimaging 2017, 27, 255–261. [Google Scholar] [CrossRef]
- Eckert, T.; Tang, C.; Ma, Y.; Brown, N.; Lin, T.; Frucht, S.; Feigin, A.; Eidelberg, D. Abnormal Metabolic Networks in Atypical Parkinsonism. Mov. Disord. 2008, 23, 727–733. [Google Scholar] [CrossRef]
- Mudali, D.; Teune, L.K.; Renken, R.J.; Leenders, K.L.; Roerdink, J.B.T.M. Classification of Parkinsonian Syndromes from FDG-PET Brain Data Using Decision Trees with SSM/PCA Features. Comput. Math. Methods Med. 2015, 2015, 136921. [Google Scholar] [CrossRef]
- Srulijes, K.; Reimold, M.; Liscic, R.M.; Bauer, S.; Dietzel, E.; Liepelt-Scarfone, I.; Berg, D.; Maetzler, W. Fluorodeoxyglucose Positron Emission Tomography in Richardson’s Syndrome and Progressive Supranuclear Palsy-parkinsonism. Mov. Disord. 2012, 27, 151–155. [Google Scholar] [CrossRef]
- Eckert, T.; Barnes, A.; Dhawan, V.; Frucht, S.; Gordon, M.F.; Feigin, A.S.; Eidelberg, D. FDG PET in the Differential Diagnosis of Parkinsonian Disorders. Neuroimage 2005, 26, 912–921. [Google Scholar] [CrossRef]
- Niethammer, M.; Tang, C.C.; Feigin, A.; Allen, P.J.; Heinen, L.; Hellwig, S.; Amtage, F.; Hanspal, E.; Vonsattel, J.P.; Poston, K.L.; et al. A Disease-Specific Metabolic Brain Network Associated with Corticobasal Degeneration. Brain 2014, 137, 3036–3046. [Google Scholar] [CrossRef]
- Hellwig, S.; Reinhard, M.; Amtage, F.; Guschlbauer, B.; Buchert, R.; Tüscher, O.; Weiller, C.; Niesen, W.D.; Meyer, P.T. Transcranial Sonography and [18F]Fluorodeoxyglucose Positron Emission Tomography for the Differential Diagnosis of Parkinsonism: A Head-to-head Comparison. Eur. J. Neurol. 2014, 21, 860–866. [Google Scholar] [CrossRef]
- Tripathi, M.; Dhawan, V.; Peng, S.; Kushwaha, S.; Batla, A.; Jaimini, A.; D’Souza, M.M.; Sharma, R.; Saw, S.; Mondal, A. Differential Diagnosis of Parkinsonian Syndromes Using F-18 Fluorodeoxyglucose Positron Emission Tomography. Neuroradiology 2013, 55, 483–492. [Google Scholar] [CrossRef]
- Hellwig, S.; Frings, L.; Amtage, F.; Buchert, R.; Spehl, T.S.; Rijntjes, M.; Tüscher, O.; Weiller, C.; Weber, W.A.; Vach, W.; et al. 18F-FDG PET Is an Early Predictor of Overall Survival in Suspected Atypical Parkinsonism. J. Nucl. Med. 2015, 56, 1541–1546. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.C.; Poston, K.L.; Eckert, T.; Feigin, A.; Frucht, S.; Gudesblatt, M.; Dhawan, V.; Lesser, M.; Vonsattel, J.-P.; Fahn, S.; et al. Differential Diagnosis of Parkinsonism: A Metabolic Imaging Study Using Pattern Analysis. Lancet Neurol. 2010, 9, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Juh, R.; Kim, J.; Moon, D.; Choe, B.; Suh, T. Different Metabolic Patterns Analysis of Parkinsonism on the 18F-FDG PET. Eur. J. Radiol. 2004, 51, 223–233. [Google Scholar] [CrossRef]
- Garraux, G.; Phillips, C.; Schrouff, J.; Kreisler, A.; Lemaire, C.; Degueldre, C.; Delcour, C.; Hustinx, R.; Luxen, A.; Destée, A.; et al. Multiclass Classification of FDG PET Scans for the Distinction between Parkinson’s Disease and Atypical Parkinsonian Syndromes. Neuroimage Clin. 2013, 2, 883–893. [Google Scholar] [CrossRef]
- Tripathi, M.; Tang, C.C.; Feigin, A.; De Lucia, I.; Nazem, A.; Dhawan, V.; Eidelberg, D. Automated Differential Diagnosis of Early Parkinsonism Using Metabolic Brain Networks: A Validation Study. J. Nucl. Med. 2016, 57, 60–66. [Google Scholar] [CrossRef]
- Brajkovic, L.; Kostic, V.; Sobic-Saranovic, D.; Stefanova, E.; Jecmenica-Lukic, M.; Jesic, A.; Stojiljkovic, M.; Odalovic, S.; Gallivanone, F.; Castiglioni, I.; et al. The Utility of FDG-PET in the Differential Diagnosis of Parkinsonism. Neurol. Res. 2017, 39, 675–684. [Google Scholar] [CrossRef]
- Martí-Andrés, G.; van Bommel, L.; Meles, S.K.; Riverol, M.; Valentí, R.; Kogan, R.V.; Renken, R.J.; Gurvits, V.; van Laar, T.; Pagani, M.; et al. Multicenter Validation of Metabolic Abnormalities Related to PSP According to the MDS-PSP Criteria. Mov. Disord. 2020, 35, 2009–2018. [Google Scholar] [CrossRef]
- Shen, B.; Wei, S.; Ge, J.; Peng, S.; Liu, F.; Li, L.; Guo, S.; Wu, P.; Zuo, C.; Eidelberg, D.; et al. Reproducible Metabolic Topographies Associated with Multiple System Atrophy: Network and Regional Analyses in Chinese and American Patient Cohorts. Neuroimage Clin. 2020, 28, 102416. [Google Scholar] [CrossRef]
- Amod, F.H.; Bhigjee, A.I.; Nyakale, N. Utility of 18FFDG-PET in Parkinsonism in an African Population. eNeurologicalSci 2022, 27, 100399. [Google Scholar] [CrossRef]
- Tomše, P.; Rebec, E.; Studen, A.; Perovnik, M.; Rus, T.; Ležaić, L.; Tang, C.C.; Eidelberg, D.; Trošt, M. Abnormal Metabolic Covariance Patterns Associated with Multiple System Atrophy and Progressive Supranuclear Palsy. Phys. Medica 2022, 98, 131–138. [Google Scholar] [CrossRef]
- Eidelberg, D.; Dhawan, V.; Moeller, J.R.; Sidtis, J.J.; Ginos, J.Z.; Strother, S.C.; Cederbaum, J.; Greene, P.; Fahn, S.; Powers, J.M.; et al. The Metabolic Landscape of Cortico-Basal Ganglionic Degeneration: Regional Asymmetries Studied with Positron Emission Tomography. J. Neurol. Neurosurg. Psychiatry 1991, 54, 856–862. [Google Scholar] [CrossRef][Green Version]
- Karbe, H.; Holthoff, V.; Huber, M.; Herholz, K.; Wienhard, K.; Wagner, R.; Heiss, W.-D. Positron Emission Tomography in Degenerative Disorders of the Dopaminergic System. J. Neural Transm. Park. Dis. Dement. Sect. 1992, 4, 121–130. [Google Scholar] [CrossRef]
- Klein, R.C.; de Jong, B.M.; de Vries, J.J.; Leenders, K.L. Direct Comparison between Regional Cerebral Metabolism in Progressive Supranuclear Palsy and Parkinson’s Disease. Mov. Disord. 2005, 20, 1021–1030. [Google Scholar] [CrossRef] [PubMed]
- Herting, B.; Beuthien-Baumann, B.; Pöttrich, K.; Donix, M.; Triemer, A.; Lampe, J.B.; Von Kummer, R.; Herholz, K.; Reichmann, H.; Holthoff, V.A. Prefrontal Cortex Dysfunction and Depression in Atypical Parkinsonian Syndromes. Mov. Disord. 2007, 22, 490–497. [Google Scholar] [CrossRef] [PubMed]
- Park, H.K.; Kim, J.S.; Im, K.C.; Oh, S.J.; Kim, M.J.; Lee, J.H.; Chung, S.J.; Lee, M.C. Functional Brain Imaging in Pure Akinesia with Gait Freezing: [18F] FDG PET and [18F] FP-CIT PET Analyses. Mov. Disord. 2009, 24, 237–245. [Google Scholar] [CrossRef]
- Hellwig, S.; Amtage, F.; Kreft, A.; Buchert, R.; Winz, O.H.; Vach, W.; Spehl, T.S.; Rijntjes, M.; Hellwig, B.; Weiller, C.; et al. [18F]FDG-PET Is Superior to [123I]IBZM-SPECT for the Differential Diagnosis of Parkinsonism. Neurology 2012, 79, 1314–1322. [Google Scholar] [CrossRef]
- Teune, L.K.; Renken, R.J.; Mudali, D.; De Jong, B.M.; Dierckx, R.A.; Roerdink, J.B.T.M.; Leenders, K.L. Validation of Parkinsonian Disease-Related Metabolic Brain Patterns. Mov. Disord. 2013, 28, 547–551. [Google Scholar] [CrossRef]
- Akdemir, Ü.Ö.; Tokçaer, A.B.; Karakuş, A.; Kapucu, L.Ö. Brain 18F-FDG PET Imaging in the Differential Diagnosis of Parkinsonism. Clin. Nucl. Med. 2014, 39, e220–e226. [Google Scholar] [CrossRef]
- Baudrexel, S.; Seifried, C.; Penndorf, B.; Klein, J.C.; Middendorp, M.; Steinmetz, H.; Grünwald, F.; Hilker, R. The Value of Putaminal Diffusion Imaging versus 18-fluorodeoxyglucose Positron Emission Tomography for the Differential Diagnosis of the Parkinson Variant of Multiple System Atrophy. Mov. Disord. 2014, 29, 380–387. [Google Scholar] [CrossRef]
- Josephs, K.A.; Duffy, J.R.; Strand, E.A.; Machulda, M.M.; Senjem, M.L.; Gunter, J.L.; Schwarz, C.G.; Reid, R.I.; Spychalla, A.J.; Lowe, V.J.; et al. The Evolution of Primary Progressive Apraxia of Speech. Brain 2014, 137, 2783–2795. [Google Scholar] [CrossRef]
- Ko, J.H.; Lee, C.S.; Eidelberg, D. Metabolic Network Expression in Parkinsonism: Clinical and Dopaminergic Correlations. J. Cereb. Blood Flow. Metab. 2017, 37, 683–693. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Wu, J.; Peng, S.; Wu, P.; Wang, J.; Zhang, H.; Guan, Y.; Eidelberg, D.; Zuo, C.; Ma, Y. Reproducible Network and Regional Topographies of Abnormal Glucose Metabolism Associated with Progressive Supranuclear Palsy: Multivariate and Univariate Analyses in American and Chinese Patient Cohorts. Hum. Brain Mapp. 2018, 39, 2842–2858. [Google Scholar] [CrossRef]
- Arnone, A.; Allocca, M.; Di Dato, R.; Puccini, G.; Laghai, I.; Rubino, F.; Nerattini, M.; Ramat, S.; Lombardi, G.; Ferrari, C.; et al. FDG PET in the Differential Diagnosis of Degenerative Parkinsonian Disorders: Usefulness of Voxel-Based Analysis in Clinical Practice. Neurol. Sci. 2022, 43, 5333–5341. [Google Scholar] [CrossRef]
- Lu, J.; Wang, M.; Wu, P.; Yakushev, I.; Zhang, H.; Ziegler, S.; Jiang, J.; Förster, S.; Wang, J.; Schwaiger, M.; et al. Adjustment for the Age- and Gender-Related Metabolic Changes Improves the Differential Diagnosis of Parkinsonism. Phenomics 2023, 3, 50–63. [Google Scholar] [CrossRef]
- El Ouartassi, A.; Giordana, C.; Schiazza, A.; Chardin, D.; Darcourt, J. [18F]-FDopa Positron Emission Tomography Imaging in Corticobasal Syndrome. Brain Imaging Behav. 2023, 17, 619–627. [Google Scholar] [CrossRef]
- Ali, F.; Clark, H.; Machulda, M.; Senjem, M.L.; Lowe, V.J.; Jack, C.R.; Josephs, K.A.; Whitwell, J.; Botha, H. Patterns of Brain Volume and Metabolism Predict Clinical Features in the Progressive Supranuclear Palsy Spectrum. Brain Commun. 2024, 6, fcae233. [Google Scholar] [CrossRef]
- Du, X.; Zhao, H.; Li, Y.; Dai, Y.; Gao, L.; Li, Y.; Fan, K.; Sun, Z.; Zhang, Y. The Value of PET/CT in the Diagnosis and Differential Diagnosis of Parkinson’s Disease: A Dual-Tracer Study. NPJ Park. Dis. 2024, 10, 171. [Google Scholar] [CrossRef]
- Ling, R.; Wang, M.; Lu, J.; Wu, S.; Wu, P.; Ge, J.; Wang, L.; Liu, Y.; Jiang, J.; Shi, K.; et al. Radiomics-Guided Deep Learning Networks Classify Differential Diagnosis of Parkinsonism. Brain Sci. 2024, 14, 680. [Google Scholar] [CrossRef]
- Ling, R.; Cen, X.; Wu, S.; Wang, M.; Zhang, Y.; Jiang, J.; Lu, J.; Liu, Y.; Zuo, C.; Jiang, J.; et al. Explainable Graph Neural Network Based on Metabolic Brain Imaging for Differential Diagnosis of Parkinsonism. Front. Aging Neurosci. 2025, 17, 1580910. [Google Scholar] [CrossRef]
- Pillai, K.S.; Rajeswari, P.; Kamble, R.B.; Gopalan Nair Santhamma, S.; Chacko, M.; Jayakrishnan, V.; Ramachandran, R.; Avarachan, A.; Kishore, A. Multimodality Brain Imaging Markers in Progressive Supranuclear Palsy Subtypes and Parkinson’s Disease. Mov. Disord. Clin. Pract. 2025, 12, 664–669. [Google Scholar] [CrossRef]
- Štokelj, E.; Rus, T.; Jamšek, J.; Trošt, M.; Simončič, U. Multinomial Logistic Regression Algorithm for the Classification of Patients with Parkinsonisms. EJNMMI Res. 2025, 15, 24. [Google Scholar] [CrossRef]
- Botha, H.; Whitwell, J.L.; Madhaven, A.; Senjem, M.L.; Lowe, V.; Josephs, K.A. The Pimple Sign of Progressive Supranuclear Palsy Syndrome. Park. Relat. Disord. 2014, 20, 180–185. [Google Scholar] [CrossRef]
- Cerami, C.; Dodich, A.; Lettieri, G.; Iannaccone, S.; Magnani, G.; Marcone, A.; Gianolli, L.; Cappa, S.F.; Perani, D. Different FDG-PET Metabolic Patterns at Single-Subject Level in the Behavioral Variant of Fronto-Temporal Dementia. Cortex 2016, 83, 101–112. [Google Scholar] [CrossRef]
- Cerami, C.; Dodich, A.; Iannaccone, S.; Magnani, G.; Marcone, A.; Guglielmo, P.; Vanoli, G.; Cappa, S.F.; Perani, D. Individual Brain Metabolic Signatures in Corticobasal Syndrome. J. Alzheimer’s Dis. 2020, 76, 517–528. [Google Scholar] [CrossRef]
- Bergeron, D.; Beauregard, J.-M.; Jean-Guimond; Soucy, J.-P.; Verret, L.; Poulin, S.; Matias-Guiu, J.A.; Cabrera-Martín, M.N.; Bouchard, R.W.; Laforce, R. Posterior Cingulate Cortex Hypometabolism in Non-Amnestic Variants of Alzheimer’s Disease. J. Alzheimer’s Dis. 2020, 77, 1569–1577. [Google Scholar] [CrossRef] [PubMed]
- Heikkinen, S.; Cajanus, A.; Katisko, K.; Hartikainen, P.; Vanninen, R.; Haapasalo, A.; Krüger, J.; Remes, A.M.; Solje, E. Brainstem Atrophy Is Linked to Extrapyramidal Symptoms in Frontotemporal Dementia. J. Neurol. 2022, 269, 4488–4497. [Google Scholar] [CrossRef]
- Isella, V.; Crivellaro, C.; Formenti, A.; Musarra, M.; Pacella, S.; Morzenti, S.; Ferri, F.; Mapelli, C.; Gallivanone, F.; Guerra, L.; et al. Validity of Cingulate–Precuneus–Temporo-Parietal Hypometabolism for Single-Subject Diagnosis of Biomarker-Proven Atypical Variants of Alzheimer’s Disease. J. Neurol. 2022, 269, 4440–4451. [Google Scholar] [CrossRef]
- Teune, L.K.; Bartels, A.L.; de Jong, B.M.; Willemsen, A.T.M.; Eshuis, S.A.; de Vries, J.J.; van Oostrom, J.C.H.; Leenders, K.L. Typical Cerebral Metabolic Patterns in Neurodegenerative Brain Diseases. Mov. Disord. 2010, 25, 2395–2404. [Google Scholar] [CrossRef]
- Wenzel, F.; Young, S.; Wilke, F.; Apostolova, I.; Arlt, S.; Jahn, H.; Thiele, F.; Buchert, R. B-Spline-Based Stereotactical Normalization of Brain FDG PET Scans in Suspected Neurodegenerative Disease: Impact on Voxel-Based Statistical Single-Subject Analysis. Neuroimage 2010, 50, 994–1003. [Google Scholar] [CrossRef] [PubMed]
- Garibotto, V.; Montandon, M.L.; Viaud, C.T.; Allaoua, M.; Assal, F.; Burkhard, P.R.; Ratib, O.; Zaidi, H. Regions of Interest–Based Discriminant Analysis of DaTSCAN SPECT and FDG-PET for the Classification of Dementia. Clin. Nucl. Med. 2013, 38, e112–e117. [Google Scholar] [CrossRef]
- Taswell, C.; Villemagne, V.L.; Yates, P.; Shimada, H.; Leyton, C.E.; Ballard, K.J.; Piguet, O.; Burrell, J.R.; Hodges, J.R.; Rowe, C.C. 18F-FDG PET Improves Diagnosis in Patients with Focal-Onset Dementias. J. Nucl. Med. 2015, 56, 1547–1553. [Google Scholar] [CrossRef]
- Franceschi, A.; Naser-Tavakolian, K.; Clifton, M.; Ahmed, O.; Stoffers, K.; Bangiyev, L.; Cruciata, G.; Clouston, S.; Franceschi, D. Hybrid Imaging in Dementia: A Semi-Quantitative (18F)-Fluorodeoxyglucose Positron Emission Tomography/Magnetic Resonance Imaging Approach in Clinical Practice. World J. Nucl. Med. 2021, 20, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, A.M.; Clifton, M.A.; Naser-Tavakolian, K.; Ahmed, O.; Bangiyev, L.; Clouston, S.; Franceschi, D. FDG PET/MRI for Visual Detection of Crossed Cerebellar Diaschisis in Patients with Dementia. Am. J. Roentgenol. 2021, 216, 165–171. [Google Scholar] [CrossRef]
- Franceschi, A.M.; Naser-Tavakolian, K.; Clifton, M.; Bangiyev, L.; Cruciata, G.; Clouston, S.; Franceschi, D. Metabolic Positron-Emission Tomography/Magnetic Resonance Imaging in Primary Progressive Aphasia and Frontotemporal Lobar Degeneration Subtypes: Reassessment of Expected [18F]-Fluorodeoxyglucose Uptake Patterns. World J. Nucl. Med. 2021, 20, 294–304. [Google Scholar] [CrossRef]
- Josephs, K.A.; Duffy, J.R.; Clark, H.M.; Utianski, R.L.; Strand, E.A.; Machulda, M.M.; Botha, H.; Martin, P.R.; Pham, N.T.T.; Stierwalt, J.; et al. A Molecular Pathology, Neurobiology, Biochemical, Genetic and Neuroimaging Study of Progressive Apraxia of Speech. Nat. Commun. 2021, 12, 3452. [Google Scholar] [CrossRef] [PubMed]
- Seckin, Z.I.; Duffy, J.R.; Strand, E.A.; Clark, H.M.; Utianski, R.L.; Machulda, M.M.; Botha, H.; Ali, F.; Thu Pham, N.T.; Lowe, V.J.; et al. The Evolution of Parkinsonism in Primary Progressive Apraxia of Speech: A 6-Year Longitudinal Study. Park. Relat. Disord. 2020, 81, 34–40. [Google Scholar] [CrossRef]
- Gan, J.; Shi, Z.; Zuo, C.; Zhao, X.; Liu, S.; Chen, Y.; Zhang, N.; Cai, L.; Cui, R.; Ai, L.; et al. Analysis of Positron Emission Tomography Hypometabolic Patterns and Neuropsychiatric Symptoms in Patients with Dementia Syndromes. CNS Neurosci. Ther. 2023, 29, 2193–2205. [Google Scholar] [CrossRef]
- Braun, A.S.; Satoh, R.; Pham, N.T.T.; Singh-Reilly, N.; Ali, F.; Dickson, D.W.; Lowe, V.J.; Whitwell, J.L.; Josephs, K.A. Machine Learning Models of Voxel-Level [18F] Fluorodeoxyglucose Positron Emission Tomography Data Excel at Predicting Progressive Supranuclear Palsy Pathology. Ann. Neurol. 2025, 98, 410–420. [Google Scholar] [CrossRef]
- Armstrong, M.J. Lewy Body Dementias. Contin. Lifelong Learn. Neurol. 2019, 25, 128–146. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.-C.; Ye, Q.; Yuan, C.-X. Metabolic Pattern Analysis of 18F-FDG PET as a Marker for Parkinson’s Disease: A Systematic Review and Meta-Analysis. Rev. Neurosci. 2019, 30, 743–756. [Google Scholar] [CrossRef]
- Walker, Z.; Gandolfo, F.; Orini, S.; Garibotto, V.; Agosta, F.; Arbizu, J.; Bouwman, F.; Drzezga, A.; Nestor, P.; Boccardi, M.; et al. Clinical Utility of FDG PET in Parkinson’s Disease and Atypical Parkinsonism Associated with Dementia. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 1534–1545. [Google Scholar] [CrossRef] [PubMed]
- Alster, P.; Madetko, N.K.; Koziorowski, D.M.; Królicki, L.; Budrewicz, S.; Friedman, A. Accumulation of Tau Protein, Metabolism and Perfusion—Application and Efficacy of Positron Emission Tomography (PET) and Single Photon Emission Computed Tomography (SPECT) Imaging in the Examination of Progressive Supranuclear Palsy (PSP) and Corticobasal Syndrome (CBS). Front. Neurol. 2019, 10, 101. [Google Scholar] [CrossRef]
- Leuzy, A.; Chiotis, K.; Lemoine, L.; Gillberg, P.-G.; Almkvist, O.; Rodriguez-Vieitez, E.; Nordberg, A. Tau PET Imaging in Neurodegenerative Tauopathies—Still a Challenge. Mol. Psychiatry 2019, 24, 1112–1134. [Google Scholar] [CrossRef]
- Whitwell, J.L.; Höglinger, G.U.; Antonini, A.; Bordelon, Y.; Boxer, A.L.; Colosimo, C.; van Eimeren, T.; Golbe, L.I.; Kassubek, J.; Kurz, C.; et al. Radiological Biomarkers for Diagnosis in PSP: Where Are We and Where Do We Need to Be? Mov. Disord. 2017, 32, 955–971. [Google Scholar] [CrossRef]
- Vasilevskaya, A.; Taghdiri, F.; Multani, N.; Anor, C.; Misquitta, K.; Houle, S.; Burke, C.; Tang-Wai, D.; Lang, A.E.; Fox, S.; et al. PET Tau Imaging and Motor Impairments Differ Between Corticobasal Syndrome and Progressive Supranuclear Palsy With and Without Alzheimer’s Disease Biomarkers. Front. Neurol. 2020, 11, 574. [Google Scholar] [CrossRef]
- Liang, M.; Jia, C.; Yen, T.-C.; Liu, L.; Li, M.; Cui, R. Complementary Value of Metabolic and Tau PET Imaging in the Diagnosis of Corticobasal Degeneration. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 4286–4288. [Google Scholar] [CrossRef]
- Santibanez, R.A.; Mendez-Orellana, C.; Juri, C.; Escobar, S.; Bustamante, G.; Quiroga, C.; Contreras, N.; Haeger, A.; Amaral, H.; Kramer, V. Tau PET Imaging with 18F-PI-2620 in Patients with Corticobasal Syndrome: A Case Series. Alzheimer’s Dement. 2022, 18, e067105. [Google Scholar] [CrossRef]
- Giannakis, A.; Konitsiotis, S.; Xiromerisiou, G. Diffusion Tensor Imaging in Progressive Supranuclear Palsy Versus Other Neurodegenerative Diseases: A Review. J. Neuroimaging 2025, 35, e70063. [Google Scholar] [CrossRef]
- Klietz, M.; Mahmoudi, N.; Maudsley, A.A.; Sheriff, S.; Bronzlik, P.; Almohammad, M.; Nösel, P.; Wegner, F.; Höglinger, G.U.; Lanfermann, H.; et al. Whole-Brain Magnetic Resonance Spectroscopy Reveals Distinct Alterations in Neurometabolic Profile in Progressive Supranuclear Palsy. Mov. Disord. 2023, 38, 1503–1514. [Google Scholar] [CrossRef] [PubMed]
- Leng, F.; Gao, Y.; Li, F.; Wei, L.; Sun, Y.; Liu, F.; Zhu, Y.; Qiu, J.; Wang, Z.; Zhang, Y. Iron Deposition Is Associated with Motor and Non-Motor Network Breakdown in Parkinsonism. Front. Aging Neurosci. 2025, 16, 1518155. [Google Scholar] [CrossRef] [PubMed]
- Kawabata, K.; Banno, F.; Mizutani, Y.; Maeda, T.; Nagao, R.; Shima, S.; Murayama, K.; Ohno, Y.; Maeda, T.; Sasaki, M.; et al. Flattened Red Nucleus in Progressive Supranuclear Palsy Detected by Quantitative Susceptibility Mapping. Park. Relat. Disord. 2025, 131, 107251. [Google Scholar] [CrossRef]
- Miyata, M.; Kakeda, S.; Yoneda, T.; Ide, S.; Okada, K.; Adachi, H.; Korogi, Y. Signal Intensity of Cerebral Gyri in Corticobasal Syndrome on Phase Difference Enhanced Magnetic Resonance Images: Comparison of Progressive Supranuclear Palsy and Parkinson’s Disease. J. Neurol. Sci. 2020, 419, 117210. [Google Scholar] [CrossRef] [PubMed]
- Satoh, R.; Weigand, S.D.; Pham, N.T.T.; Ali, F.; Arani, A.; Senjem, M.L.; Jack, C.R.; Whitwell, J.L.; Josephs, K.A. Magnetic Susceptibility in Progressive Supranuclear Palsy Variants, Parkinson’s Disease, and Corticobasal Syndrome. Mov. Disord. 2023, 38, 2282–2290. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).