Real-World Laboratory Analysis of Molecular Biomarkers in Multiple Sclerosis Centers in Central-Eastern European Countries Covering 107 Million Inhabitants
Abstract
1. Introduction
2. Results
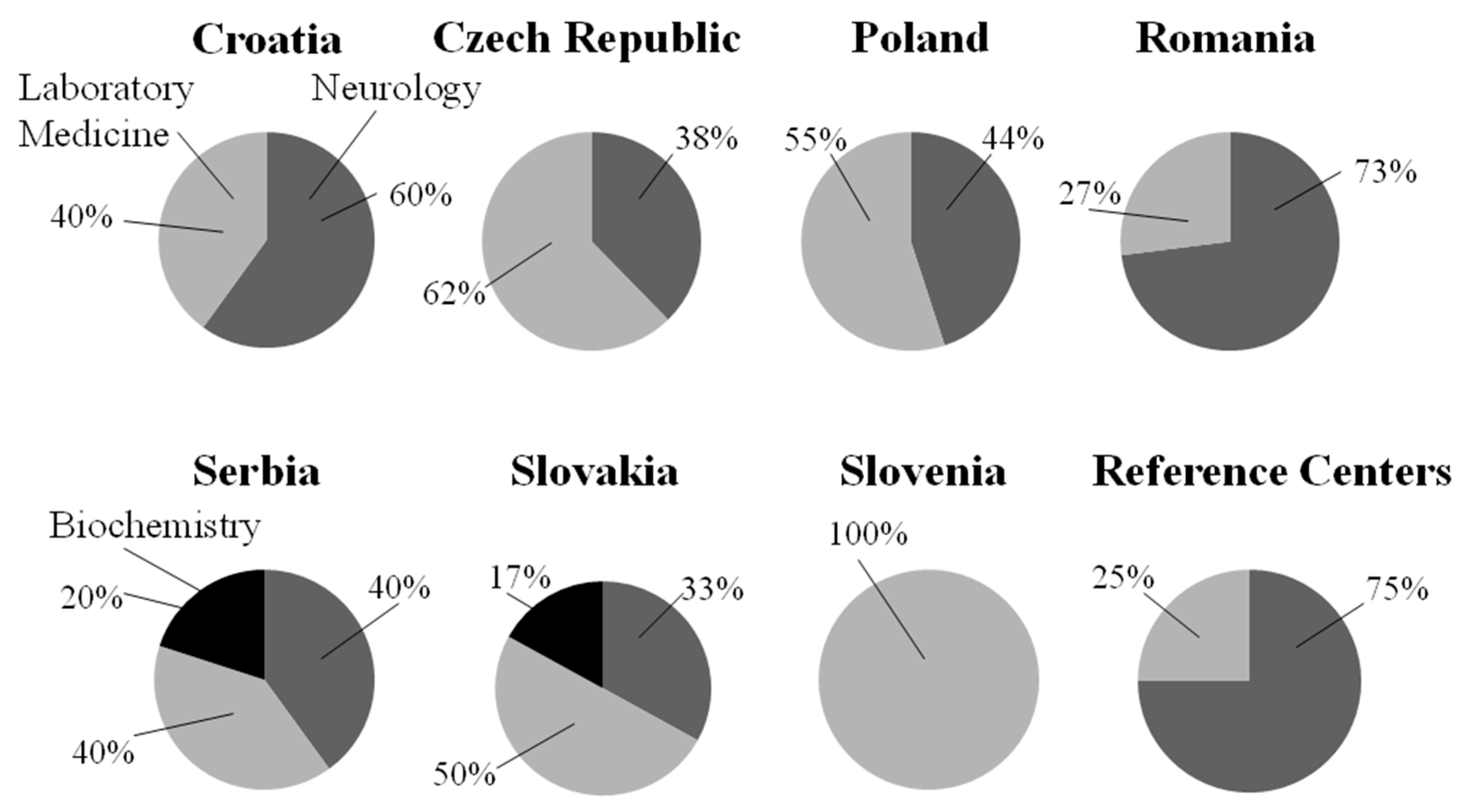
2.1. General Information About the Surveyed Centers
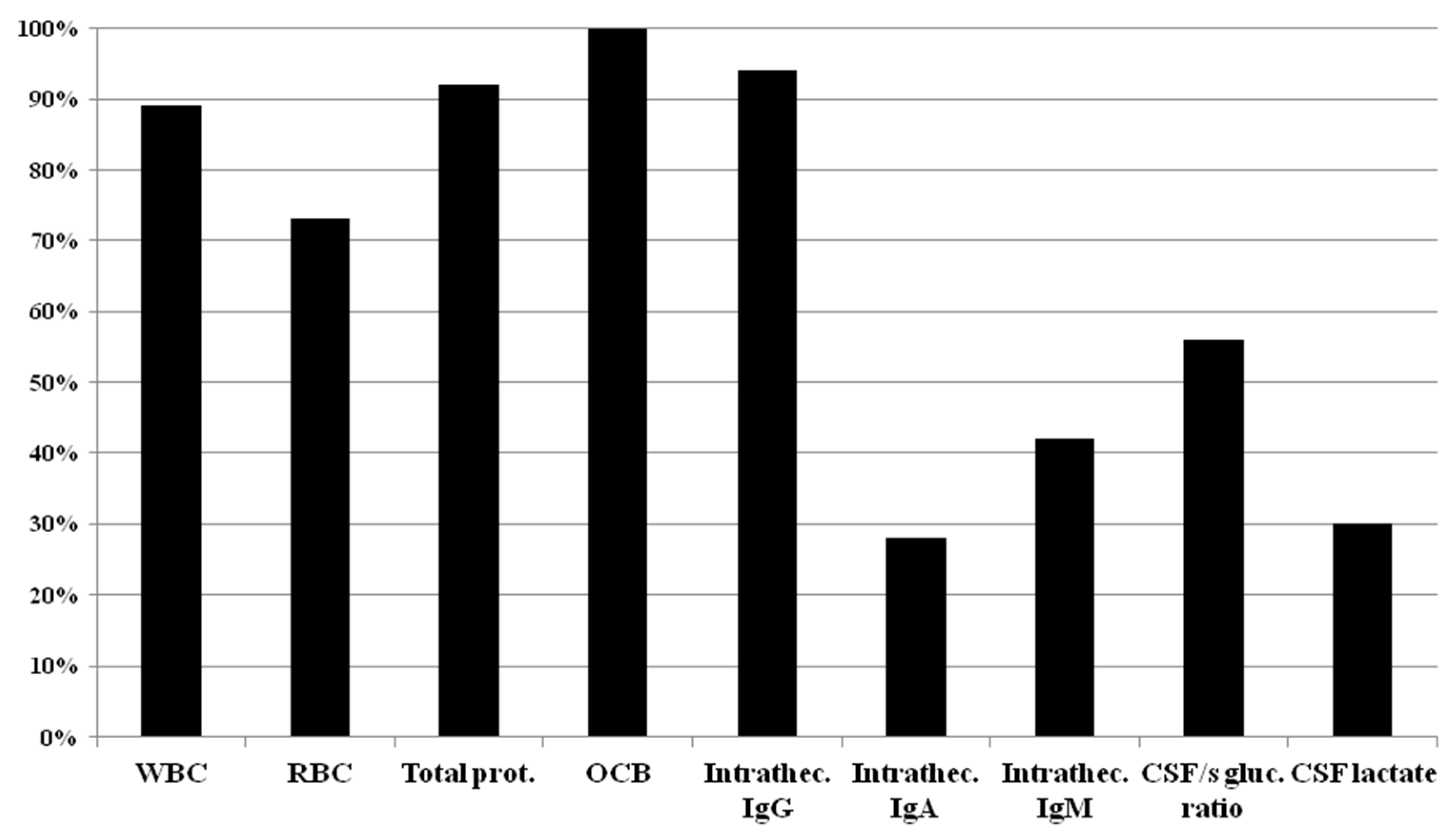
2.2. Cerebrospinal Fluid Analysis
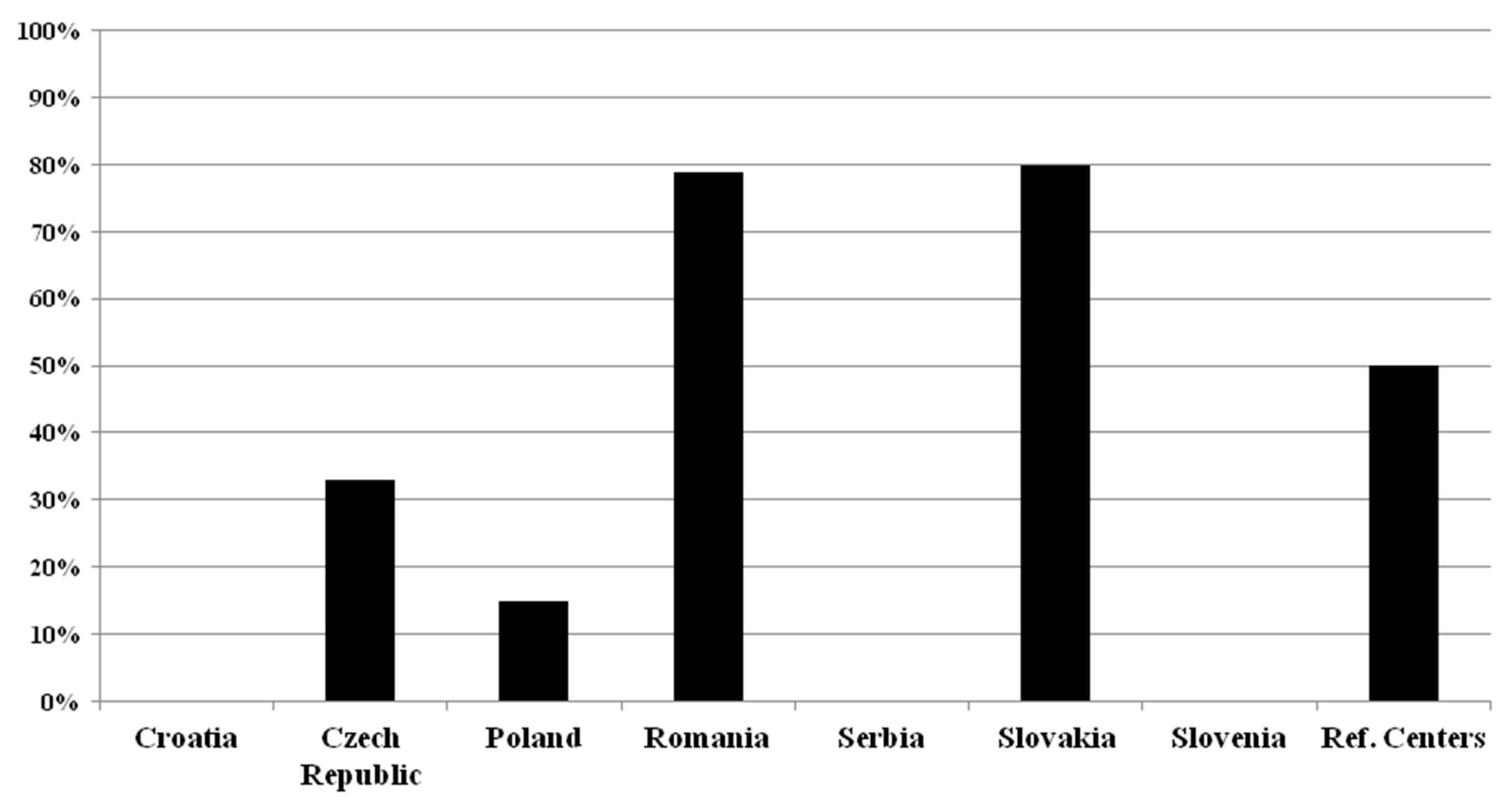
2.3. Determination of Kappa Free Light Chain (κFLC) in Patients with Suspected MS
2.4. Other CSF and/or Blood Biomarkers in Patients with MS
3. Discussion
4. Materials and Methods
4.1. Participating Centers and Data Collection
4.2. Data Statistical Analysis
4.3. Ethical Approval
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADA | Anti-drug antibodies |
| CIS | Clinically isolated syndrome |
| CNS | Central nervous system |
| CSF | Cerebrospinal fluid |
| DMT | Disease-modifying therapy |
| Ig | Immunglobulin |
| κFLC | Kappa free light chain |
| LP | Lumbar puncture |
| MRI | Magnetic resonance imaging |
| MS | Multiple Sclerosis |
| NfL | Neurofilament light chain |
| OCB | Oligoclonal band |
References
- Maroto-García, J.; Martínez-Escribano, A.; Delgado-Gil, V.; Mañez, M.; Mugueta, C.; Varo, N.; García de la Torre, Á.; Ruiz-Galdón, M. Biochemical biomarkers for multiple sclerosis. Clin. Chim. Acta 2023, 548, 117471. [Google Scholar] [CrossRef]
- Scalfari, A.; Traboulsee, A.; Oh, J.; Airas, L.; Bittner, S.; Calabrese, M.; Dominguez, J.M.G.; Granziera, C.; Greenberg, B.; Hellwig, K.; et al. Smouldering-Associated Worsening in Multiple Sclerosis: An International Consensus Statement on Definition, Biology, Clinical Implications, and Future Directions. Ann. Neurol. 2024, 96, 826–845. [Google Scholar] [CrossRef]
- Maglio, G.; D’Agostino, M.; Caronte, F.P.; Pezone, L.; Casamassimi, A.; Rienzo, M.; Di Zazzo, E.; Nappo, C.; Medici, N.; Molinari, A.M.; et al. Multiple Sclerosis: From the Application of Oligoclonal Bands to Novel Potential Biomarkers. Int. J. Mol. Sci. 2024, 25, 5412. [Google Scholar] [CrossRef]
- Thompson, A.J.; Banwell, B.L.; Barkhof, F.; Carroll, W.M.; Coetzee, T.; Comi, G.; Correale, J.; Fazekas, F.; Filippi, M.; Freedman, M.S.; et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018, 17, 162–173. [Google Scholar] [CrossRef]
- Huang, J.; Khademi, M.; Fugger, L.; Lindhe, Ö.; Novakova, L.; Axelsson, M.; Malmeström, C.; Constantinescu, C.; Lycke, J.; Piehl, F.; et al. Inflammation-related plasma and CSF biomarkers for multiple sclerosis. Proc. Natl. Acad. Sci. USA 2020, 117, 12952–12960. [Google Scholar] [CrossRef]
- Di Filippo, M.; Gaetani, L.; Centonze, D.; Hegen, H.; Kuhle, J.; Teunissen, C.E.; Tintoré, M.; Villar, L.M.; Willemse, E.A.J.; Zetterberg, H.; et al. Fluid biomarkers in multiple sclerosis: From current to future applications. Lancet Reg. Health Eur. 2024, 44, 101009. [Google Scholar] [CrossRef]
- Kennedy, P.G.E.; George, W.; Yu, X. The elusive nature of the oligoclonal bands in multiple sclerosis. J. Neurol. 2024, 271, 116–124. [Google Scholar] [CrossRef]
- Link, H.; Huang, Y.M. Oligoclonal bands in multiple sclerosis cerebrospinal fluid: An update on methodology and clinical usefulness. J. Neuroimmunol. 2006, 180, 17–28. [Google Scholar] [CrossRef]
- Saadeh, R.S.; Bryant, S.C.; McKeon, A.; Weinshenker, B.; Murray, D.L.; Pittock, S.J.; Willrich, M.A.V. CSF Kappa Free Light Chains: Cutoff Validation for Diagnosing Multiple Sclerosis. Mayo Clin. Proc. 2022, 97, 738–751. [Google Scholar] [CrossRef]
- Toscano, S.; Chisari, C.G.; Fermo, S.L.; Gulino, G.; Zappia, M.; Patti, F. A dynamic interpretation of κFLC index for the diagnosis of multiple sclerosis: A change of perspective. J. Neurol. 2023, 270, 6010–6020. [Google Scholar] [CrossRef]
- Izquierdo, G.; Angulo, S.; Garcia-Moreno, J.M.; Gamero, M.A.; Navarro, G.; Gata, J.M.; Ruiz-Peña, J.L.; Páramo, M.D. Intrathecal IgG synthesis: Marker of progression in multiple sclerosis patients. Acta Neurol. Scand. 2002, 105, 158–163. [Google Scholar] [CrossRef]
- Zheng, Y.; Cai, M.-T.; Yang, F.; Zhou, J.-P.; Fang, W.; Shen, C.-H.; Zhang, Y.-X.; Ding, M.-P. IgG Index Revisited: Diagnostic Utility and Prognostic Value in Multiple Sclerosis. Front. Immunol. 2020, 11, 1799. [Google Scholar] [CrossRef]
- Simonsen, C.S.; Flemmen, H.O.; Lauritzen, T.; Berg-Hansen, P.; Moen, S.M.; Gulowsen Celius, E.G. The diagnostic value of IgG index versus oligoclonal bands in cerebrospinal fluid of patients with multiple sclerosis. Mult. Scler. J. Exp. Transl. Clin. 2020, 6, 2055217319901291. [Google Scholar] [CrossRef]
- Shaw, F.; Chadwick, C. The diagnostic utility of IgG index and oligoclonal bands for multiple sclerosis in a neurology hospital patient population. Ann. Clin. Biochem. 2023, 60, 353–355. [Google Scholar] [CrossRef]
- Nakano, T.; Matsui, M.; Inoue, I.; Awata, T.; Katayama, S.; Murakoshi, T. Free immunoglobulin light chain: Its biology and implications in diseases. Clin. Chim. Acta 2011, 412, 843–849. [Google Scholar] [CrossRef]
- Esparvarinha, M.; Nickho, H.; Mohammadi, H.; Aghebati-Maleki, L.; Abdolalizadeh, J.; Majidi, J. The role of free kappa and lambda light chains in the pathogenesis and treatment of inflammatory diseases. Biomed. Pharmacother. 2017, 91, 632–644. [Google Scholar] [CrossRef]
- Hegen, H.; Berek, K.; Deisenhammer, F. Cerebrospinal fluid kappa free light chains as biomarker in multiple sclerosis-from diagnosis to prediction of disease activity. Wien Med. Wochenschr. 2022, 172, 337–345. [Google Scholar] [CrossRef]
- Hegen, H.; Walde, J.; Berek, K.; Arrambide, G.; Gnanapavan, S.; Kaplan, B.; Khalil, M.; Saadeh, R.; Teunissen, C.; Tumani, H.; et al. Cerebrospinal fluid kappa free light chains for the diagnosis of multiple sclerosis: A systematic review and meta-analysis. Mult. Scler. 2023, 29, 169–181. [Google Scholar] [CrossRef]
- Villar, L.M.; Espiño, M.; Costa-Frossard, L.; Muriel, A.; Jiménez, J.; Alvarez-Cermeño, J.C. High levels of cerebrospinal fluid free kappa chains predict conversion to multiple sclerosis. Clin. Chim. Acta 2012, 413, 1813–1816. [Google Scholar] [CrossRef]
- Makshakov, G.; Nazarov, V.; Kochetova, O.; Surkova, E.; Lapin, S.; Evdoshenko, E. Diagnostic and Prognostic Value of the Cerebrospinal Fluid Concentration of Immunoglobulin Free Light Chains in Clinically Isolated Syndrome with Conversion to Multiple Sclerosis. PLoS ONE 2015, 10, e0143375. [Google Scholar] [CrossRef] [PubMed]
- Gaetani, L.; Blennow, K.; Calabresi, P.; Di Filippo, M.; Parnetti, L.; Zetterberg, H. Neurofilament light chain as a biomarker in neurological disorders. J. Neurol. Neurosurg. Psychiatry 2019, 90, 870–881. [Google Scholar] [CrossRef]
- Khalil, M.; Teunissen, C.E.; Otto, M.; Piehl, F.; Sormani, M.P.; Gattringer, T.; Barro, C.; Kappos, L.; Comabella, M.; Fazekas, F.; et al. Neurofilaments as biomarkers in neurological disorders. Nat. Rev. Neurol. 2018, 14, 577–589. [Google Scholar] [CrossRef]
- Kouchaki, E.; Dashti, F.; Mirazimi, S.M.A.; Alirezaei, Z.; Jafari, S.H.; Hamblin, M.R.; Mirzaei, H. Neurofilament light chain as a biomarker for diagnosis of multiple sclerosis. EXCLI J. 2021, 20, 1308–1325. [Google Scholar] [CrossRef]
- Pijnenburg, Y.A.L.; Janssen, J.C.; Schoonenboom, N.S.M.; Petzold, A.; Mulder, C.; Stigbrand, T.; Norgren, N.; Heijst, H.; Hack, C.E.; Scheltens, P.; et al. CSF neurofilaments in frontotemporal dementia compared with early onset Alzheimer’s disease and controls. Dement. Geriatr. Cogn. Disord. 2007, 23, 225–230. [Google Scholar] [CrossRef]
- Lu, C.-H.; Macdonald-Wallis, C.; Gray, E.; Pearce, N.; Petzold, A.; Norgren, N.; Giovannoni, G.; Fratta, P.; Sidle, K.; Fish, M.; et al. Neurofilament light chain: A prognostic biomarker in amyotrophic lateral sclerosis. Neurology 2015, 85, 921, Erratum in: Neurology 2015, 84, 2247–2257. [Google Scholar] [CrossRef]
- Neselius, S.; Brisby, H.; Marcusson, J.; Zetterberg, H.; Blennow, K.; Karlsson, T. Neurological assessment and its relationship to CSF biomarkers in amateur boxers. PLoS ONE 2014, 9, e99870. [Google Scholar] [CrossRef]
- Gattringer, T.; Pinter, D.; Enzinger, C.; Seifert-Held, T.; Kneihsl, M.; Fandler, S.; Pichler, A.; Barro, C.; Gröbke, S.; Voortman, M.; et al. Serum neurofilament light is sensitive to active cerebral small vessel disease. Neurology 2017, 89, 2108–2114. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, Y.; Tao, Q.; Lu, P.; Meng, F.; Zhuang, L.; Qiao, S.; Zhang, Y.; Luo, B.; Liu, Y.; et al. Diagnostic value of isolated plasma biomarkers and its combination in neurodegenerative dementias: A multicenter cohort study. Clin. Chim. Acta 2024, 558, 118784. [Google Scholar] [CrossRef]
- Ou, R.; Liu, K.; Lin, J.; Yang, T.; Xiao, Y.; Wei, Q.; Hou, Y.; Li, C.; Zhang, L.; Jiang, Z.; et al. Relationship between plasma NFL and disease progression in Parkinson’s disease: A prospective cohort study. J. Neurol. 2024, 271, 1837–1843. [Google Scholar] [CrossRef]
- Nakamura, T.; Kawarabayashi, T.; Shibata, M.; Kasahara, H.; Makioka, K.; Sugawara, T.; Oka, H.; Ishizawa, K.; Amari, M.; Ueda, T.; et al. High levels of plasma neurofilament light chain correlated with brainstem and peripheral nerve damage. J. Neurol. Sci. 2024, 463, 123137. [Google Scholar] [CrossRef]
- Rosén, C.; Mitre, B.; Nellgård, B.; Axelsson, M.; Constantinescu, R.; Munch Andersen, P.; Dalla, K.; Blennow, K.; Nilsson, G.; Zetterberg, H.; et al. High levels of neurofilament light and YKL-40 in cerebrospinal fluid are related to poor outcome in ALS. J. Neurol. Sci. 2024, 463, 123112. [Google Scholar] [CrossRef]
- Skillbäck, T.; Farahmand, B.; Bartlett, J.W.; Rosén, C.; Mattsson, N.; Nägga, K.; Kilander, L.; Religa, D.; Wimo, A.; Winblad, B.; et al. CSF neurofilament light differs in neurodegenerative diseases and predicts severity and survival. Neurology 2014, 83, 1945–1953. [Google Scholar] [CrossRef]
- Martínez, M.A.; Olsson, B.; Bau, L.; Matas, E.; Cobo Calvo, Á.; Andreasson, U.; Blennow, K.; Romero-Pinel, L.; Martínez-Yélamos, S.; Zetterberg, H. Glial and neuronal markers in cerebrospinal fluid predict progression in multiple sclerosis. Mult. Scler. 2015, 21, 550–561. [Google Scholar] [CrossRef]
- Kuhle, J.; Kropshofer, H.; Haering, D.A.; Kundu, U.; Meinert, R.; Barro, C.; Dahlke, F.; Tomic, D.; Leppert, D.; Kappos, L. Blood neurofilament light chain as a biomarker of MS disease activity and treatment response. Neurology 2019, 92, e1007–e1015. [Google Scholar] [CrossRef]
- Benkert, P.; Meier, S.; Schaedelin, S.; Manouchehrinia, A.; Yaldizli, Ö.; Maceski, A.; Oechtering, J.; Achtnichts, L.; Conen, D.; Derfuss, T.; et al. Serum neurofilament light chain for individual prognostication of disease activity in people with multiple sclerosis: A retrospective modelling and validation study. Lancet Neurol. 2022, 21, 246–257. [Google Scholar] [CrossRef]
- Højsgaard Chow, H.; Petersen, E.R.; Olsson, A.; Hejgaard Laursen, J.; Bredahl Hansen, M.; Bang Oturai, A.; Soelberg Sørensen, P.; Bach Søndergaard, H.; Sellebjerg, F. Age-corrected neurofilament light chain ratio decreases but does not predict relapse in highly active multiple sclerosis patients initiating natalizumab treatment. Mult. Scler. Relat. Disord. 2024, 88, 105701. [Google Scholar] [CrossRef]
- Pape, K.; Rolfes, L.; Steffen, F.; Muthuraman, M.; Korsen, M.; Meuth, S.G.; Zipp, F.; Bittner, S. Comparative effectiveness of natalizumab versus ocrelizumab in multiple sclerosis: A real-world propensity score-matched study. Ther. Adv. Neurol. Disord. 2022, 15, 17562864221142924. [Google Scholar] [CrossRef]
- Gunnarsson, M.; Malmeström, C.; Axelsson, M.; Sundström, P.; Dahle, C.; Vrethem, M.; Olsson, T.; Piehl, F.; Norgren, N.; Rosengren, L.; et al. Axonal damage in relapsing multiple sclerosis is markedly reduced by natalizumab. Ann. Neurol. 2011, 69, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Novakova, L.; Axelsson, M.; Khademi, M.; Zetterberg, H.; Blennow, K.; Malmeström, C.; Piehl, F.; Olsson, T.; Lycke, J. Cerebrospinal fluid biomarkers of inflammation and degeneration as measures of fingolimod efficacy in multiple sclerosis. Mult. Scler. 2017, 23, 62–71. [Google Scholar] [CrossRef]
- de Flon, P.; Gunnarsson, M.; Laurell, K.; Söderström, L.; Birgander, R.; Lindqvist, T.; Krauss, W.; Dring, A.; Bergman, J.; Sundström, P.; et al. Reduced inflammation in relapsing-remitting multiple sclerosis after therapy switch to rituximab. Neurology 2016, 87, 141–147. [Google Scholar] [CrossRef]
- Axelsson, M.; Malmeström, C.; Gunnarsson, M.; Zetterberg, H.; Sundström, P.; Lycke, J.; Svenningsson, A. Immunosuppressive therapy reduces axonal damage in progressive multiple sclerosis. Mult. Scler. 2014, 20, 43–50. [Google Scholar] [CrossRef]
- Ashkar, A.A.; Ali Baig, M.M.; Arif, A.; Mazhar Ali, M.; Yousuf, F.; Ashkar, R. Prognostic significance of neurofilament light in Fingolimod therapy for Multiple Sclerosis: A systemic review and meta-analysis based on randomized control trials. Mult. Scler. Relat. Disord. 2023, 69, 104416. [Google Scholar] [CrossRef]
- Piehl, F.; Kockum, I.; Khademi, M.; Blennow, K.; Lycke, J.; Zetterberg, H.; Olsson, T. Plasma neurofilament light chain levels in patients with MS switching from injectable therapies to fingolimod. Mult. Scler. 2018, 24, 1046–1054. [Google Scholar] [CrossRef]
- Shahan, B.; Choi, E.Y.; Nieves, G. Cerebrospinal Fluid Analysis. Am. Fam. Physician 2021, 103, 422–428. [Google Scholar]
- Lo Sasso, B.; Agnello, L.; Bivona, G.; Bellia, C.; Ciaccio, M. Cerebrospinal Fluid Analysis in Multiple Sclerosis Diagnosis: An Update. Medicina 2019, 55, 245. [Google Scholar] [CrossRef]
- Deisenhammer, F.; Zetterberg, H.; Fitzner, B.; Zettl, U.K. The Cerebrospinal Fluid in Multiple Sclerosis. Front. Immunol. 2019, 10, 726. [Google Scholar] [CrossRef]
- Osenbrück, M.; Rao, M.L.; Quednau, H.D. Pattern of albumin, immunoglobulins, and glucose in cerebrospinal fluid and serum of patients with disorders of the central nervous system. Eur. Neurol. 1985, 24, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Haarmann, A.; Hähnel, L.; Schuhmann, M.K.; Buttmann, M. Age-adjusted CSF β2-microglobulin and lactate are increased and ACE is decreased in patients with multiple sclerosis, but only lactate correlates with clinical disease duration and severity. J. Neuroimmunol. 2018, 323, 19–27. [Google Scholar] [CrossRef]
- Tarhan, G.; Domaç, S.F.; Selek, S.; Gül, A.Z.; Demir, S. Utilizing metabolomic profiling as a supportive diagnostic tool for radiologically isolated syndrome. Mult. Scler. Relat. Disord. 2025, 94, 106250. [Google Scholar] [CrossRef]
- Abdelhak, A.; Hottenrott, T.; Mayer, C.; Hintereder, G.; Zettl, U.K.; Stich, O.; Tumani, H. CSF profile in primary progressive multiple sclerosis: Re-exploring the basics. PLoS ONE 2017, 12, e0182647. [Google Scholar] [CrossRef]
- Higgins, V.; Parker, M.L.; Beriault, D.L.; Mostafa, A.; Estey, M.P.; Agbor, T.; Ismail, O.Z. A survey of Canadian neurologists’ perspectives and preferences for laboratory reporting of CSF oligoclonal banding. Clin. Biochem. 2025, 135, 110855. [Google Scholar] [CrossRef]
- Link, H.; Tibbling, G. Principles of albumin and IgG analyses in neurological disorders. III. Evaluation of IgG synthesis within the central nervous system in multiple sclerosis. Scand. J. Clin. Lab. Investig. 1977, 37, 397–401. [Google Scholar] [CrossRef]
- Akaishi, T.; Takahashi, T.; Fujihara, K.; Misu, T.; Nishiyama, S.; Takai, Y.; Fujimori, J.; Abe, M.; Ishii, T.; Aoki, M.; et al. Impact of intrathecal IgG synthesis on neurological disability in patients with multiple sclerosis. Mult. Scler. Relat. Disord. 2020, 45, 102382. [Google Scholar] [CrossRef]
- Reiber, H.; Felgenhauer, K. Protein transfer at the blood cerebrospinal fluid barrier and the quantitation of the humoral immune response within the central nervous system. Clin. Chim. Acta 1987, 163, 319–328. [Google Scholar] [CrossRef]
- Karamehic, J.; Delic-Sarac, M.; Subasic, D.; Jukic, T.; Coric, J.; Panjeta, M.; Drace, Z.; Zecevic, L.; Mutevelic, S.; Dzananovic, N.; et al. Reibergram and oligoclonal bands in diagnosis of multiple sclerosis. Med. Arch. 2012, 66, 222–225. [Google Scholar] [CrossRef]
- Auer, M.; Hegen, H.; Zeileis, A.; Deisenhammer, F. Quantitation of intrathecal immunoglobulin synthesis—A new empirical formula. Eur. J. Neurol. 2016, 23, 713–721. [Google Scholar] [CrossRef]
- Bernardi, G.; Biagioli, T.; Malpassi, P.; De Michele, T.; Vecchio, D.; Repice, A.M.; Lugaresi, A.; Mirabella, M.; Torri Clerici, V.; Ilaria Crespi, I. The contribute of cerebrospinal fluid free light-chain assay in the diagnosis of multiple sclerosis and other neurological diseases in an Italian multicenter study. Mult. Scler. 2022, 28, 1364–1372. [Google Scholar] [CrossRef]
- Castillo-Villalba, J.; Gil-Perotín, S.; Gasque-Rubio, R.; Cubas-Nuñez, L.; Carratalà-Boscà, S.; Alcalá, C.; Quintanilla-Bordás, C.; Pérez-Miralles, F.; Ferrer, C.; Martínez, A.C.; et al. High Levels of Cerebrospinal Fluid Kappa Free Light Chains Relate to IgM Intrathecal Synthesis and Might Have Prognostic Implications in Relapsing Multiple Sclerosis. Front. Immunol. 2022, 13, 827738. [Google Scholar] [CrossRef]
- Christiansen, M.; Gjelstrup, M.C.; Stilund, M.; Christensen, T.; Petersen, T.; Møller, H.J. Cerebrospinal fluid free kappa light chains and kappa index perform equal to oligoclonal bands in the diagnosis of multiple sclerosis. Clin. Chem. Lab. Med. 2018, 57, 210–220. [Google Scholar] [CrossRef]
- Domingues, R.B.; Dos Santos, M.V.; Salomão, D.; Senne, C. Concordance rate between oligoclonal bands and the Kappa index in patients with suspected multiple sclerosis (MS). Arq. Neuropsiquiatr. 2024, 82, s00441779690. [Google Scholar] [CrossRef]
- Ferraro, D.; Bedin, R.; Natali, P.; Franciotta, D.; Smolik, K.; Santangelo, M.; Immovili, P.; Camera Vitetta, F.; Gastaldi, M.; Trenti, T.; et al. Kappa Index Versus CSF Oligoclonal Bands in Predicting Multiple Sclerosis and Infectious/Inflammatory CNS Disorders. Diagnostics 2020, 10, 856. [Google Scholar] [CrossRef] [PubMed]
- Levraut, M.; Laurent-Chabalier, S.; Ayrignac, X.; Bigaut, K.; Rival, M.; Squalli, S.; Zéphir, H.; Alberto, T.; Pekar, J.D.; Ciron, J.; et al. Kappa Free Light Chain Biomarkers Are Efficient for the Diagnosis of Multiple Sclerosis: A Large Multicenter Cohort Study. Neurol. Neuroimmunol. Neuroinflamm. 2022, 10, e200049. [Google Scholar] [CrossRef]
- Marlas, M.; Bost, C.; Dorcet, G.; Delourme, A.; Biotti, D.; Ciron, J.; Renaudineau, Y.; Puissant-Lubrano, B. Kappa-index: Real-life evaluation of a new tool for multiple sclerosis diagnosis. Clin. Immunol. 2022, 241, 109066. [Google Scholar] [CrossRef]
- Desplat-Jégo, S.; Feuillet, L.; Pelletier, J.; Bernard, D.; Chérif, A.A.; Boucraut, J. Quantification of immunoglobulin free light chains in cerebrospinal fluid by nephelometry. J. Clin. Immunol. 2005, 25, 338–345. [Google Scholar] [CrossRef]
- Sarthou, A.; Chrétien, P.; Giorgi, L.; Chiron, A.; Leroy, C.; Horellou, P.; Krzysiek, R.; Deiva, K.; Hacein-Bey-Abina, S. The kappa free light chains index is an accurate diagnostic biomarker for paediatric multiple sclerosis. Mult. Scler. 2024, 30, 1436–1444. [Google Scholar] [CrossRef]
- Levraut, M.; Gavoille, A.; Landes-Chateau, C.; Cohen, M.; Bresch, S.; Seitz-Polski, B.; Mondot, L.; Lebrun-Frenay, C. Kappa Free Light Chain Index Predicts Disease Course in Clinically and Radiologically Isolated Syndromes. Neurol. Neuroimmunol. Neuroinflamm. 2023, 10, e200156. [Google Scholar] [CrossRef]
- Ayrignac, X.; Aouinti, S.; Vincent, T.; Carra-Dallière, C.; Charif, M.; Duflos, C.; Hirtz, C.; Dos Santos, A.; Menjot de Champfleur, N.; Labauge, P.; et al. Serum NfL and GFAP are weak predictors of long-term multiple sclerosis prognosis: A 6-year follow-up. Mult. Scler. Relat. Disord. 2024, 89, 105747. [Google Scholar] [CrossRef]
- Candeloro, R.; Galloppa, M.; Lombardo, L.; Laudisi, M.; Ghisellini, S.; Negri, G.; Ferri, C.; Marcialis, C.; Bellini, T.; Pugliatti, M.; et al. Kappa Free Light Chains in Multiple Sclerosis as a Marker of Intrathecal Humoral Response: A Sex-Disaggregated Study. Diagnostics 2024, 14, 2798. [Google Scholar] [CrossRef] [PubMed]
- Vecchio, D.; Puricelli, C.; Virgilio, E.; Passarelli, F.; Guida, S.; Naldi, P.; Crespi, I.; Dianzani, U.; Comi, C. Kappa index for multiple sclerosis diagnosis: An accurate biomarker of intrathecal synthesis. J. Neurol. 2024, 272, 30. [Google Scholar] [CrossRef]
- Michetti, L.; Maffina, F.; Ravasio, R.; Barcella, V.; Radaelli, M.; Chiudinelli, L.; Sessa, M.; Alessio, M.G. Free light chains as a reliable biomarker of intrathecal synthesis in the diagnosis of CNS inflammatory diseases. J. Neuroimmunol. 2023, 379, 578091. [Google Scholar] [CrossRef]
- Agnello, L.; Lo Sasso, B.; Salemi, G.; Altavilla, P.; Pappalardo, E.M.; Caldarella, R.; Meli, F.; Scazzone, C.; Bivona, G.; Ciaccio, M. Clinical Use of κ Free Light Chains Index as a Screening Test for Multiple Sclerosis. Lab. Med. 2020, 51, 402–407. [Google Scholar] [CrossRef]
- Nicolella, V.; Fiorenza, M.; Monteiro, I.; Novarella, F.; Sirica, R.; D’Angelo, M.; Carbone, G.; La Civita, E.; Esposito, A.; Criscuolo, V.; et al. Clinical utility of the Lumipulse™ immunoassay for plasma neurofilament light chain in multiple sclerosis. J. Neurol. Sci. 2024, 463, 123115. [Google Scholar] [CrossRef]
- Ferriz, J.; Guallart, C.; Timoneda, P.; Fandos, M.; Lopez-Arqueros, J.; Sierra-Rivera, A.; Garcia-Hita, M.; Marcaida, G.; Carcelén-Gadea, M. Diagnostic approach for multiple sclerosis: Optimizing algorithms for intrathecal synthesis of immunoglobulins. Lab. Med. 2025, 56, 291–296. [Google Scholar] [CrossRef]
- Tortosa-Carreres, J.; Quiroga-Varela, A.; Castillo-Villalba, J.; Piqueras-Rodríguez, M.; Ramió-Torrenta, L.; Cubas-Núñez, L.; Gasqué-Rubio, R.; Quintanilla-Bordas, C.; Huertas-Pons, J.M.; Miguela, A.; et al. Improving the efficiency of free kappa light chains as diagnostic biomarker of Multiple Sclerosis by using a novel algorithm. Mult. Scler. Relat. Disord. 2023, 79, 104997. [Google Scholar] [CrossRef]
- Monreal, E.; Fernández-Velasco, J.I.; García-Soidán, A.; Sainz de la Maza, S.; Espiño, M.; Villarrubia, N.; Rodríguez-Jorge, F.; Chico-García, J.L.; Sainz-Amo, R.; Masjuan, J.; et al. Establishing the best combination of the kappa free light chain index and oligoclonal bands for an accurate diagnosis of multiple sclerosis. Front. Immunol. 2023, 14, 1288169. [Google Scholar] [CrossRef]
- Valencia-Vera, E.; Garcia-Ripoll, A.M.-E.; Enguix, A.; Abalos-Garcia, C.; Segovia-Cuevas, M.J. Application of κ free light chains in cerebrospinal fluid as a biomarker in multiple sclerosis diagnosis: Development of a diagnosis algorithm. Clin. Chem. Lab. Med. 2018, 56, 609–613. [Google Scholar] [CrossRef]
- Quintanilla-Bordás, C.; Cubas-Núñez, L.; Castillo-Villalba, J.; Carratalá-Boscá, S.; Gasque-Rubio, R.; Tortosa-Carreres, J.; Alcalá, C.; Forés-Toribio, L.; Lucas, C.; Gorriz, D.; et al. Clinical trajectories of patients with multiple sclerosis from onset and their relationship with serum neurofilament light chain levels. Front. Neurol. 2024, 15, 1477335. [Google Scholar] [CrossRef]
- Sainz-Amo, R.; Romero, A.R.; Monreal, E.; Chico García, J.L.; Fernández Velasco, J.I.; Villarrubia, N.; Veiga González, J.L.; Sainz de la Maza, S.; Jorge, F.L.; Masjuan, J.; et al. Effect of alemtuzumab over sNfL and sGFAP levels in multiple sclerosis. Front. Immunol. 2024, 15, 1454474. [Google Scholar] [CrossRef]
- Deisenhammer, F. Neutralizing antibodies to interferon-beta and other immunological treatments for multiple sclerosis: Prevalence and impact on outcomes. CNS Drugs 2009, 23, 379–396. [Google Scholar] [CrossRef]
- Hegen, H.; Auer, M.; Deisenhammer, F. Predictors of Response to Multiple Sclerosis Therapeutics in Individual Patients. Drugs 2016, 76, 1421–1445. [Google Scholar] [CrossRef]
- Link, J.; Ramanujam, R.; Auer, M.; Ryner, M.; Hässler, S.; Bachelet, D.; Mbogning, C.; Warnke, C.; Buck, D.; Jensen, P.E.H.; et al. ABIRISK Consortium Clinical practice of analysis of anti-drug antibodies against interferon beta and natalizumab in multiple sclerosis patients in Europe: A descriptive study of test results. PLoS ONE 2017, 12, e0170395. [Google Scholar] [CrossRef]
- Deisenhammer, F.; Jank, M.; Lauren, A.; Sjödin, A.; Ryner, M.; Fogdell-Hahn, A.; Sievers, C.; Lindberg, R.; Jensen, P.E.; Sellebjerg, F.; et al. ABIRISK consortium Prediction of natalizumab anti-drug antibodies persistency. Mult. Scler. 2019, 25, 392–398. [Google Scholar] [CrossRef]
- Baker, D.; Asardag, A.N.; Quinn, O.A.; Efimov, A.; Kang, A.S. Anti-drug antibodies to antibody-based therapeutics in multiple sclerosis. Hum. Antibodies 2021, 29, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Kokas, Z.; Sandi, D.; Fricska-Nagy, Z.; Füvesi, J.; Biernacki, T.; Köves, Á.; Fazekas, F.; Jóri Birkás, A.; Katona, G.; Kovács, K.; et al. Do Hungarian multiple sclerosis care units fulfil international criteria? PLoS ONE 2022, 17, e0264328. [Google Scholar] [CrossRef]
- Kokas, Z.; Járdánházy, A.; Sandi, D.; Biernacki, T.; Fricska-Nagy, Z.; Füvesi, J.; Bartosik-Psujek, H.; Basic Kes, V.; Berger, T.; Berthele, A.; et al. Real-world operation of multiple sclerosis centres in Central-Eastern European countries covering 107 million inhabitants. Mult. Scler. Relat. Disord. 2023, 69, 104406. [Google Scholar] [CrossRef]

| Biomarkers used in clinical practice |
| IgG OCB [1,3,6] Kappa free light chain (κFLC) [1,3,6] Neurofilament (mainly neurofilament light chain(NfL)) (it is not yet in McDonald criteria (2017)) [1,3,5,6] |
| Potential biomarkers in MS |
| Tau protein [1,3] Glial fibrillary acidic protein (GFAP) [1,3,6] S100β [1,3] Myelin basic protein (MBP) [1,3] Chitinase-3-like-1 (CHI3L1) [1,3,6] Chitinase-1 (CHIT1) [6] Osteopontin (OPN) [1,3,5] Matrix metallopeptidase-9 (MMP-9) [5] Soluble form of myeloid cells 2 (sTREM2) [6] Chemokine ligand (CXCL9, CXCL12, CXCL13) [1,3,5,6] CD163 [1] CD5+ B cells [1] Tubulin β [1] Heat shock protein 70, 90 (HSP70, HSP90) [1,3] Oncostatin M (OSM), Hepatocyte growth factor (HGS) [5] Neuron-specific enolase (NSE) [3] Cytokines (IL-6, IL-15) [3] Other lyphocyte subpopulation [3] Autoantibodies [3] Several microRNAs [3,6] Differentially expressed genes or proteins [3] |
| Neurology in-Bed Patients | A Specialized MS Clinic | A Research Agenda for MS and Biomarker Development | |
|---|---|---|---|
| CROATIA | (5/5)100% | (3/5) 60% | (2/5) 40% |
| CZECH REPUBLIC | (8/9) 89% | (7/9) 78% | (3/9) 33% |
| POLAND | (18/20) 90% | (14/20) 70% | (5/20) 25% |
| ROMANIA | (14/14) 100% | (8/14) 57% | (4/14) 29% |
| SERBIA | (5/5) 100% | (5/5) 100% | (0/5) 0% |
| SLOVAKIA | (5/5) 100% | (4/5) 80% | (3/5) 60% |
| SLOVENIA | (2/2)100% | (2/2) 100% | (1/2) 50% |
| REFERENCE C. | (4/4) 100% | (4/4) 100% | (4/4) 100% |
| White Blood Cell Count | Red Blood Cell Count | Total Protein | OCB | Intrath IgG | Intrath IgA | Intrath IgM | CSF/se Glucose Ratio | CSF Lactate | |
|---|---|---|---|---|---|---|---|---|---|
| CROATIA | (4/5) 80% | (3/5) 60% | (5/5) 100% | (5/5) 100% | (5/5) 100% | (0/5) 0% | (1/5) 20% | (1/5) 20% | (1/5) 20% |
| CZECH REPUBLIC | (8/9) 89% | (7/9) 78% | (8/9) 89% | (9/9) 100% | (9/9) 100% | (6/9) 67% | (8/9) 89% | (5/9) 56% | (5/9) 56% |
| POLAND | (17/20) 85% | (13/20) 65% | (19/20) 95% | (20/20) 100% | (19/20) 95% | (4/20) 20% | (8/20) 40% | (11/20) 55% | (4/20) 20% |
| ROMANIA | (12/14) 86% | (10/14) 71% | (12/14) 86% | (14/14) 100% | (12/14) 86% | (1/14) 7% | (2/14) 14% | (9/14) 64% | (2/14) 14% |
| SERBIA | (5/5) 100% | (4/5) 80% | (5/5) 100% | (5/5) 100% | (4/5) 80% | (0/5) 0% | (0/5) 0% | (4/5) 80% | (1/5) 20% |
| SLOVAKIA | (5/5) 100% | (4/5) 80% | (5/5) 100% | (5/5) 100% | (5/5) 100% | (2/5) 40% | (3/5) 60% | (2/5) 40% | (3/5) 60% |
| SLOVENIA | (2/2) 100% | (2/2) 100% | (2/2) 100% | (2/2) 100% | (2/2) 100% | (2/2) 100% | (2/2) 100% | (2/2) 100% | (1/2) 50% |
| REFERENCE CENTERS | (4/4) 100% | (4/4) 100% | (3/4) 75% | (4/4) 100% | (4/4) 100% | (3/4) 75% | (3/4) 75% | (2/4) 50% | (2/4) 50% |
| CSF-Restricted OCB | Intrathecal Kappa FLC | CSF Lymphocytosis | Other | None | |
|---|---|---|---|---|---|
| CROATIA | (5/5) 100% | (2/5) 40% | (0/5) 0% | (0/5) 0% | (0/5) 0% |
| CZECH REPUBLIC | (9/9) 100% | (9/9) 100% | (1/9) 11% | (0/9) 0% | (0/9) 0% |
| POLAND | (17/20) 85% | (3/20) 15% | (1/20) 5% | (1/20) 5% | (2/20) 10% |
| ROMANIA | (8/14) 57% | (4/14) 28.5% | (0/14) 0% | (0/14) 0% | (6/14) 43% |
| SERBIA | (4/5) 80% | (1/5) 20% | (0/5) 0% | (0/5) 0% | (1/5) 20% |
| SLOVAKIA | (3/5) 60% | (1/5) 20% | (1/5) 20% | (1/5) 20% | (2/5) 40% |
| SLOVENIA | (1/2) 50% | (1/2) 50% | (0/2) 0% | (0/2) 0% | (1/2) 50% |
| REFERENCE C. | (2/4) 50% | (1/4) 25% | (0/4) 0% | (0/4) 0% | (2/4) 50% |
| ≥1 CSF BANDS | ≥2 CSF BANDS | ≥3 CSF BANDS | ≥4 CSF BANDS | OTHER # | |
|---|---|---|---|---|---|
| CROATIA | (1/5) 20% | (2/5) 40% | (1/5) 20% | (0/5) 0% | (1/5) 20% |
| CZECH REPUBLIC | (0/9) 0% | (9/9) 100% | (0/9) 0% | (0/9) 0% | (0/9) 0% |
| POLAND * | (2/20) 10% | (12/20) 60% | (5/20) 25% | (1/20) 5% | (1/20) 5% |
| ROMANIA | (2/14) 14% | (4/14) 28% | (1/14) 7% | (1/14) 7% | (6/14) 43% |
| SERBIA | (0/5) 0% | (3/5) 60% | (2/5) 40% | (0/5) 0% | (0/5) 0% |
| SLOVAKIA | (1/5) 20% | (4/5) 80% | (0/5) 0% | (0/5) 0% | (0/5) 0% |
| SLOVENIA | (1/2) 50% | (0/2) 0% | (0/2) 0% | (1/2) 50% | (0/2) 0% |
| REFERENCE C. | (0/4) 0% | (1/4) 25% | (3/4) 75% | (0/4) 0% | (0/4) 0% |
| Czech Republic 3 = 100% | Poland 3 = 100% | Romania 11 = 100% | Slovakia 4 = 100% | Reference Centers 2 = 100% | |
|---|---|---|---|---|---|
| Diagnosis of MS | (0/3) 0% | (0/3) 0% | (1/11) 9% | (0/4) 0% | (0/2) 0% |
| Prognosis of disease course | (3/3) 100% | (3/3) 100% | (8/11) 73% | (0/4) 0% | (0/2) 0% |
| Monitoring disease course | (3/3) 100% | (2/3) 67% | (11/11) 100% | (3/4) 75% | (1/2) 50% |
| Evaluation of treatment response | (2/3) 67% | (2/3) 67% | (11/11) 100% | (3/4) 75% | (0/2) 0% |
| As additional information | (0/3) 0% | (1/3) 33% | (1/11) 9% | (1/4) 25% | (0/2) 0% |
| Other | (0/3) 0% | (0/3) 0% | (1/11) 9% | (1/4) 25% | (1/2) 50% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Járdánházy, A.; Berger, T.; Hegen, H.; Hemmer, B.; Bartosik-Psujek, H.; Kes, V.B.; Berthele, A.; Drulovic, J.; Habek, M.; Horakova, D.; et al. Real-World Laboratory Analysis of Molecular Biomarkers in Multiple Sclerosis Centers in Central-Eastern European Countries Covering 107 Million Inhabitants. Int. J. Mol. Sci. 2025, 26, 8274. https://doi.org/10.3390/ijms26178274
Járdánházy A, Berger T, Hegen H, Hemmer B, Bartosik-Psujek H, Kes VB, Berthele A, Drulovic J, Habek M, Horakova D, et al. Real-World Laboratory Analysis of Molecular Biomarkers in Multiple Sclerosis Centers in Central-Eastern European Countries Covering 107 Million Inhabitants. International Journal of Molecular Sciences. 2025; 26(17):8274. https://doi.org/10.3390/ijms26178274
Chicago/Turabian StyleJárdánházy, Anett, Thomas Berger, Harald Hegen, Bernhard Hemmer, Halina Bartosik-Psujek, Vanja Basic Kes, Achim Berthele, Jelena Drulovic, Mario Habek, Dana Horakova, and et al. 2025. "Real-World Laboratory Analysis of Molecular Biomarkers in Multiple Sclerosis Centers in Central-Eastern European Countries Covering 107 Million Inhabitants" International Journal of Molecular Sciences 26, no. 17: 8274. https://doi.org/10.3390/ijms26178274
APA StyleJárdánházy, A., Berger, T., Hegen, H., Hemmer, B., Bartosik-Psujek, H., Kes, V. B., Berthele, A., Drulovic, J., Habek, M., Horakova, D., Ledinek, A. H., Havrdova, E. K., Magyari, M., Rejdak, K., Tiu, C., Turcani, P., Bencsik, K., Kincses, Z. T., & Vécsei, L. (2025). Real-World Laboratory Analysis of Molecular Biomarkers in Multiple Sclerosis Centers in Central-Eastern European Countries Covering 107 Million Inhabitants. International Journal of Molecular Sciences, 26(17), 8274. https://doi.org/10.3390/ijms26178274










