Evaluation of Colistin Susceptibility of Klebsiella pneumoniae Strains Exposed to Rotating Magnetic Field
Abstract
1. Introduction
2. Results
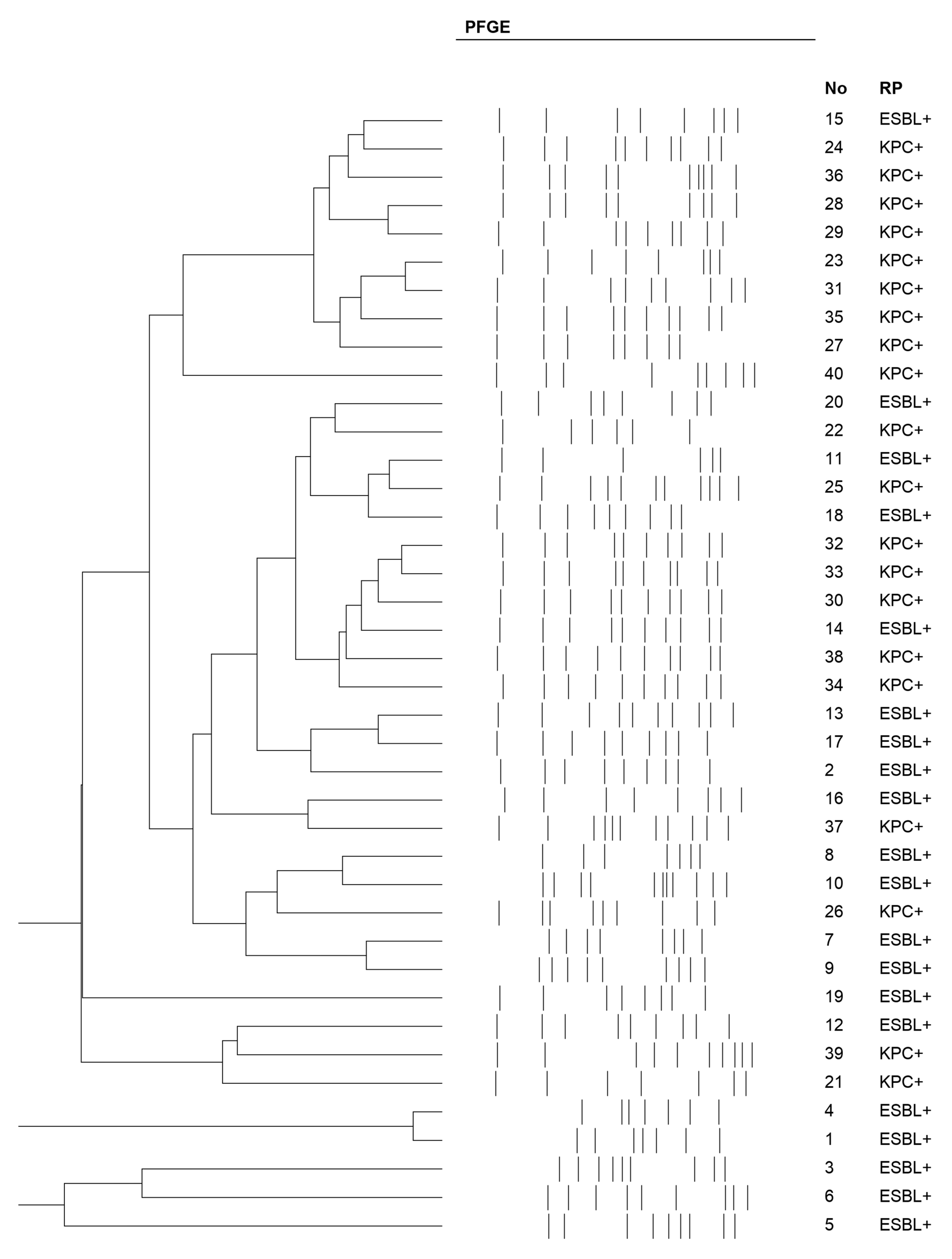
2.1. PFGE Results
2.2. Drug Susceptibility of (ESBLs)-Positive K. pneumoniae
2.3. Drug Susceptibility of (KPC)-Positive K. pneumoniae
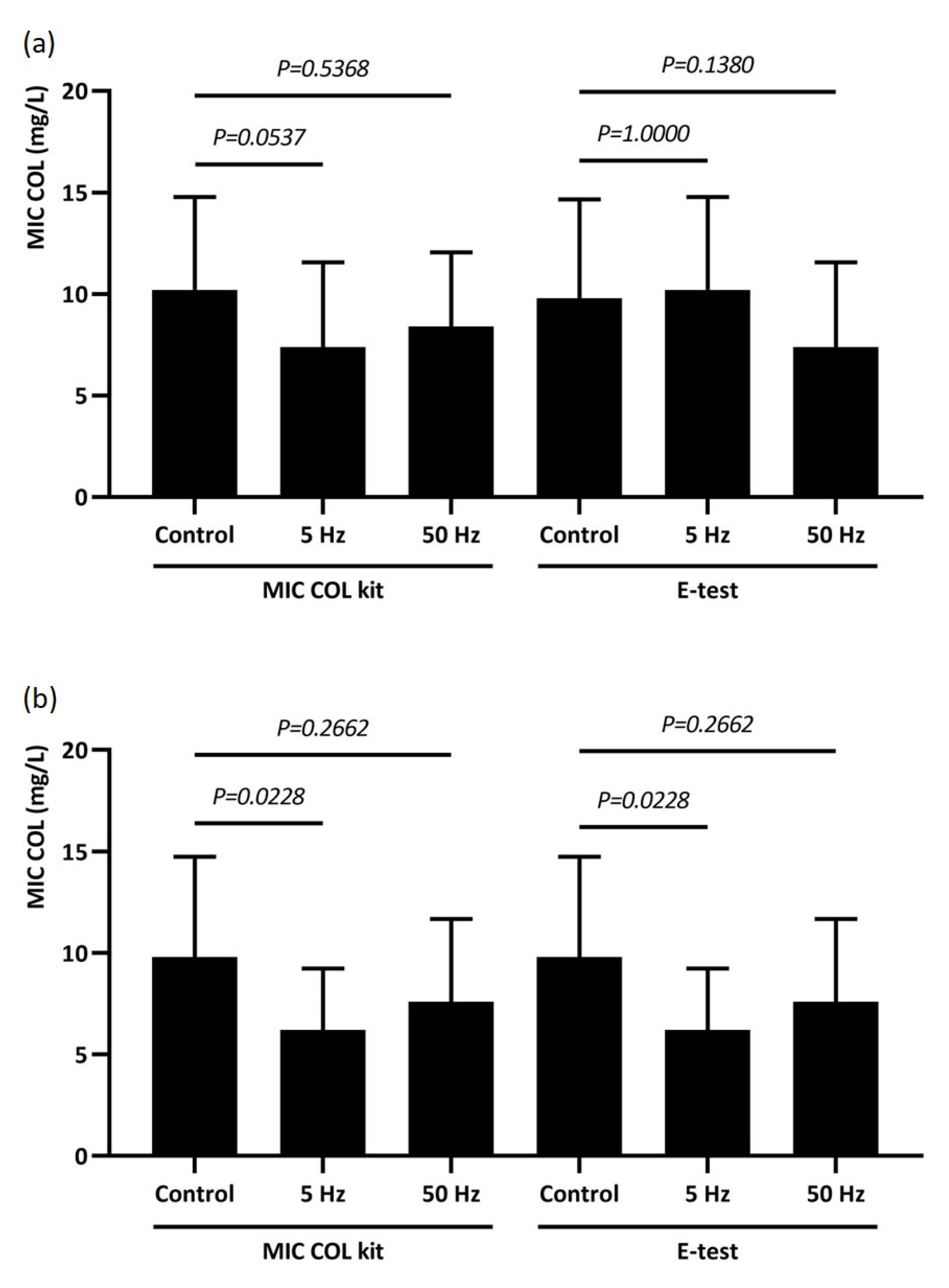
2.4. Quantitative Method for the MIC Determination of Colistin
2.5. Susceptibility to Colistin After RMF Exposure
3. Discussion
4. Materials and Methods
4.1. K. pneumoniae Strains
4.2. Rotating Magnetic Field Generator and Magnetic Exposure
4.3. Evaluation of the Effect of RMF on Susceptibility to Colistin
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ESBLs | Extended-spectrum beta-lactamases |
| KPC | Klebsiella pneumoniae carbapenemase |
| MDR | Multidrug-resistant |
| ECDC | European Centre for Disease Control |
| XDR | Extended multidrug-resistant |
| PDR | Pandrug-resistant |
| NDM | New Delhi Metallo-beta-lactamases |
| CHDL | Carbapenem-hydrolyzing class D beta-lactamases |
| MRSA | Methicillin-resistant Staphylococcus aureus |
| PFGE | Pulsed-field gel electrophoresis |
| AST | Antimicrobial susceptibility testing |
| EUCAST | European Committee on Antimicrobial Susceptibility Testing |
| DDST | Double disk diffusion method |
References
- Piperaki, E.T.; Syrogiannopoulos, G.A.; Tzouvelekis, L.S.; Daikos, G.L. Klebsiella pneumoniae: Virulence, biofilm and antimicrobial resistance. Pediatr. Infect. Dis. J. 2017, 36, 1002–1005. [Google Scholar] [CrossRef]
- Schroll, C.; Barken, K.B.; Krogfelt, K.A.; Struve, C. Role of type 1 and type 3 fimbriae in Klebsiella pneumoniae biofilm formation. BMC Microbiol. 2010, 10, 179. [Google Scholar] [CrossRef]
- Joseph, L.; Merciecca, T.; Forestier, C.; Balestrino, D.; Miquel, S. From Klebsiella pneumoniae colonization to dissemination: An overview of studies implementing murine models. Microorganisms 2021, 12, 1282. [Google Scholar] [CrossRef]
- Li, W.; Sun, G.; Yu, Y.; Li, N.; Chen, M.; Jin, R.; Jiao, Y.; Wu, H. Increasing occurrence of antimicrobial-resistant hypervirulent (hypermucoviscous) Klebsiella pneumoniae isolates in China. Clin. Infect. Dis. 2014, 58, 225–232. [Google Scholar] [CrossRef]
- Effah, C.Y.; Sun, T.; Liu, S.; Wu, Y. Klebsiella pneumoniae: An increasing threat to public health. Ann. Clin. Microbiol. Antimicrob. 2020, 19, 1. [Google Scholar] [CrossRef]
- Oliva, A.; Gizzi, F.; Mascellino, M.T.; Cipolla, A.; D’Abramo, A.; D’Agostino, C.; Trinchieri, V.; Russo, G.; Tierno, F.; Iannetta, M.; et al. Bactericidal and synergistic activity of double-carbapenem regimen for infections caused by carbapenemase-producing Klebsiella pneumoniae. Clin. Microbiol. Infect. 2016, 22, 147–153. [Google Scholar] [CrossRef]
- Tumbarello, M.; Trecarichi, E.M.; De Rosa, F.G.; Giannella, M.; Giacobbe, D.R.; Bassetti, M.; Losito, A.R.; Bartoletti, M.; Del Bono, V.; Corcione, S.; et al. Infections caused by KPC-producing Klebsiella pneumoniae: Differences in therapy and mortality in a multicentre study. J. Antimicrob. Chemother. 2015, 70, 2133–2143. [Google Scholar] [CrossRef]
- Cuzon, G.; Ouanich, J.; Gondret, R.; Naas, T.; Nordmann, P. Outbreak of OXA-48-positive carbapenem-resistant Klebsiella pneumoniae isolates in France. Antimicrob. Agents Chemother. 2011, 55, 2420–2423. [Google Scholar] [CrossRef]
- Sękowska, A.; Gospodarek, E.; Kamińska, D. Antimicrobial susceptibility and genetic similarity of ESBL-positive Klebsiella pneumoniae strains. Arch. Med. Sci. 2012, 8, 993–997. [Google Scholar] [CrossRef]
- Nirwati, H.; Sinanjung, K.; Fahrunissa, F.; Wijaya, F.; Napitupulu, S.; Hati, V.P.; Hakim, M.S.; Meliala, A.; Aman, A.T.; Nuryastuti, T. Biofilm formation and antibiotic resistance of Klebsiella pneumoniae isolated from clinical samples in a tertiary care hospital, Klaten, Indonesia. BMC Proc. 2019, 13 (Suppl. S11), 20. [Google Scholar] [CrossRef]
- Ordooei Javan, A.; Shokouhi, S.; Sahraei, Z. A review on colistin nephrotoxicity. Eur. J. Clin. Pharmacol. 2015, 71, 801–810. [Google Scholar] [CrossRef]
- Kwa, A.L.; Loh, C.; Low, J.G.; Kurup, A.; Tam, V.H. Nebulized colistin in the treatment of pneumonia due to multidrug-resistant Acinetobacter baumannii and Pseudomonas aeruginosa. Clin. Infect. Dis. 2005, 41, 754–757. [Google Scholar] [CrossRef]
- Petrosillo, N.; Taglietti, F.; Granata, G. Treatment options for colistin resistant Klebsiella pneumoniae: Present and future. J. Clin. Med. 2019, 8, 934. [Google Scholar] [CrossRef]
- Hafiz, T.A.; Alanazi, S.; Alghamdi, S.S.; Mubaraki, M.A.; Aljabr, W.; Madkhali, N.; Alharbi, S.R.; Binkhamis, K.; Alotaibi, F. Klebsiella pneumoniae bacteraemia epidemiology: Resistance profiles and clinical outcome of King Fahad Medical City isolates, Riyadh, Saudi Arabia. BMC Infect. Dis. 2023, 23, 579. [Google Scholar] [CrossRef]
- Kwiatkowski, P.; Pruss, A.; Grygorcewicz, B.; Wojciuk, B.; Dołęgowska, B.; Giedrys-Kalemba, S.; Kochan, E.; Sienkiewicz, M. Preliminary study on the antibacterial activity of essential oils alone and in combination with gentamicin against extended-spectrum β-lactamase-producing and New Delhi metallo-β-lactamase-1-producing Klebsiella pneumoniae isolates. Microb. Drug Resist. 2018, 24, 1368–1375. [Google Scholar] [CrossRef]
- Wei, Y.; Wang, X. Biological effects of rotating magnetic field: A review from 1969 to 2021. Prog. Biophys. Mol. Biol. 2023, 8, 103–115. [Google Scholar] [CrossRef]
- Fijałkowski, K.; Nawrotek, P.; Struk, M.; Kordas, M.; Rakoczy, R. Effects of rotating magnetic field exposure on the functional parameters of different species of bacteria. Electromagn. Biol. Med. 2015, 34, 48–55. [Google Scholar] [CrossRef]
- Woroszyło, M.; Ciecholewska-Juśko, D.; Junka, A.; Drozd, R.; Wardach, M.; Migdał, P.; Szymczyk-Ziółkowska, P.; Styburski, D.; Fijałkowski, K. Rotating magnetic field increases β-lactam antibiotic susceptibility of methicillin-resistant Staphylococcus aureus strains. Int. J. Mol. Sci. 2021, 22, 12397. [Google Scholar] [CrossRef]
- Ciecholewska-Juśko, D.; Żywicka, A.; Junka, A.; Woroszyło, M.; Wardach, M.; Chodaczek, G.; Szymczyk-Ziółkowska, P.; Migdał, P.; Fijałkowski, K. The effects of rotating magnetic field and antiseptic on in vitro pathogenic biofilm and its milieu. Sci. Rep. 2022, 12, 8836. [Google Scholar] [CrossRef]
- Moya, C.; Maicas, S. Antimicrobial resistance in Klebsiella pneumoniae strains: Mechanisms and outbreaks. Proceedings 2020, 66, 11. [Google Scholar] [CrossRef]
- Navon-Venezia, S.; Kondratyeva, K.; Carattoli, A. Klebsiella pneumoniae: A major worldwide source and shuttle for antibiotic resistance. FEMS Microbiol. Rev. 2017, 41, 252–275. [Google Scholar] [CrossRef]
- Kliebe, C.; Nies, B.A.; Meyer, J.F.; Tolxdorff-Neutzling, R.M.; Wiedemann, B. Evolution of plasmid-coded resistance to broad-spectrum cephalosporins. Antimicrob. Agents Chemother. 1985, 28, 302–307. [Google Scholar] [CrossRef]
- Livermore, D.M.; Woodford, N. The beta-lactamase threat in Enterobacteriaceae, Pseudomonas and Acinetobacter. Trends Microbiol. 2006, 14, 413–420. [Google Scholar] [CrossRef]
- Navon-Venezia, S.; Hammer-Munz, O.; Schwartz, D.; Turner, D.; Kuzmenko, B.; Carmeli, Y. Occurrence and phenotypic characteristics of extended-spectrum beta-lactamases among members of the family Enterobacteriaceae at the Tel-Aviv Medical Center (Israel) and evaluation of diagnostic tests. J. Clin. Microbiol. 2003, 41, 155–158. [Google Scholar] [CrossRef]
- Chong, Y.; Ito, Y.; Kamimura, T. Genetic evolution and clinical impact in extended-spectrum β-lactamase-producing Escherichia coli and Klebsiella pneumoniae. Infect. Genet. Evol. 2011, 11, 1499–1504. [Google Scholar] [CrossRef]
- Cassini, A.; Högberg, L.D.; Plachouras, D.; Quattrocchi, A.; Hoxha, A.; Simonsen, G.S.; Colomb-Cotinat, M.; Kretzschmar, M.E.; Devleesschauwer, B.; Cecchini, M.; et al. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: A population-level modelling analysis. Lancet Infect. Dis. 2019, 19, 56–66. [Google Scholar] [CrossRef]
- Pakzad, I.; Zayyen Karin, M.; Taherikalani, M.; Boustanshenas, M.; Lari, A.R. Contribution of AcrAB efflux pump to ciprofloxacin resistance in Klebsiella pneumoniae isolated from burn patients. GMS Hyg. Infect. Control 2013, 8, Doc15. [Google Scholar] [CrossRef]
- Cantón, R.; Akóva, M.; Carmeli, Y.; Giske, C.G.; Glupczynski, Y.; Gniadkowski, M.; Livermore, D.M.; Miriagou, V.; Naas, T.; Rossolini, G.M.; et al. Rapid evolution and spread of carbapenemases among Enterobacteriaceae in Europe. Clin. Microbiol. Infect. 2012, 18, 413–431. [Google Scholar] [CrossRef]
- Sarowska, J.; Choroszy-Krol, I.; Jama-Kmiecik, A.; Mączyńska, B.; Cholewa, S.; Frej-Madrzak, M. Occurrence and characteristics of carbapenem-resistant Klebsiella pneumoniae strains isolated from hospitalized patients in Poland—A single centre study. Pathogens 2022, 11, 859. [Google Scholar] [CrossRef]
- Izdebski, R.; Sitkiewicz, M.; Urbanowicz, P.; Krawczyk, M.; Brisse, S.; Gniadkowski, M. Genomic background of the Klebsiella pneumoniae NDM-1 outbreak in Poland, 2012–2018. J. Antimicrob. Chemother. 2020, 75, 3156–3162. [Google Scholar] [CrossRef]
- Biedrzycka, M.; Izdebski, R.; Hryniewicz, W.; Gniadkowski, M.; Żabicka, D. Carbapenemase-producing Enterobacterales from patients arriving from Ukraine in Poland, March 2022–February 2023. Infect. Dis. Ther. 2025, 14, 401–419. [Google Scholar] [CrossRef]
- Antoniadou, A.; Kontopidou, F.; Poulakou, G.; Koratzanis, E.; Galani, I.; Papadomichelakis, E.; Kopterides, P.; Souli, M.; Armaganidis, A.; Giamarellou, H. Colistin-resistant isolates of Klebsiella pneumoniae emerging in intensive care unit patients: First report of a multiclonal cluster. J. Antimicrob. Chemother. 2007, 59, 786–790. [Google Scholar] [CrossRef]
- Marchaim, D.; Chopra, T.; Pogue, J.M.; Perez, F.; Hujer, A.M.; Rudin, S.; Endimiani, A.; Navon-Venezia, S.; Hothi, J.; Slim, J.; et al. Outbreak of colistin-resistant, carbapenem-resistant Klebsiella pneumoniae in metropolitan Detroit, Michigan. Antimicrob. Agents Chemother. 2011, 55, 593–599. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Wang, Y.; Walsh, T.R.; Yi, L.X.; Zhang, R.; Spencer, J.; Doi, Y.; Tian, G.; Dong, B.; Huang, X.; et al. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: A microbiological and molecular biological study. Lancet Infect. Dis. 2016, 16, 161–168. [Google Scholar] [CrossRef]
- Azam, M.; Gaind, R.; Yadav, G.; Sharma, A.; Upmanyu, K.; Jain, M.; Singh, R. Colistin resistance among multiple sequence types of Klebsiella pneumoniae is associated with diverse resistance mechanisms: A report from India. Front. Microbiol. 2021, 12, 609840. [Google Scholar] [CrossRef]
- Zafer, M.M.; El-Mahallawy, H.A.; Abdulhak, A.; Amin, M.A.; Al-Agamy, M.H.; Radwan, H.H. Emergence of colistin resistance in multidrug-resistant Klebsiella pneumoniae and Escherichia coli strains isolated from cancer patients. Ann. Clin. Microbiol. Antimicrob. 2019, 18, 40. [Google Scholar] [CrossRef]
- Woroszyło, M.; Ciecholewska-Juśko, D.; Junka, A.; Pruss, A.; Kwiatkowski, P.; Wardach, M.; Fijałkowski, K. The impact of intraspecies variability on growth rate and cellular metabolic activity of bacteria exposed to rotating magnetic field. Pathogens 2021, 10, 1427. [Google Scholar] [CrossRef]
- Nawrotek, P.; Fijałkowski, K.; Struk, M.; Kordas, M.; Rakoczy, R. Effects of 50 Hz rotating magnetic field on the viability of Escherichia coli and Staphylococcus aureus. Electromagn. Biol. Med. 2014, 33, 29–34. [Google Scholar] [CrossRef]
- Hu, X.; Qiu, Z.; Wang, Y.; She, Z.; Qian, G.; Ren, Z. Effect of ultra-strong static magnetic field on bacteria: Application of Fourier-transform infrared spectroscopy combined with cluster analysis and deconvolution. Bioelectromagnetics 2009, 30, 500–507. [Google Scholar] [CrossRef]
- Woroszyło, M.; Ciecholewska-Juśko, D.; Junka, A.; Wardach, M.; Chodaczek, G.; Dudek, B.; Fijałkowski, K. The effect of rotating magnetic field on susceptibility profile of methicillin-resistant Staphylococcus aureus strains exposed to activity of different groups of antibiotics. Int. J. Mol. Sci. 2021, 22, 11551. [Google Scholar] [CrossRef]
- Hong, F.T. Magnetic field effects on biomolecules, cells, and living organisms. Biosystems 1995, 36, 187–229. [Google Scholar] [CrossRef]
- Sharpe, M.A.; Baskin, D.S.; Pichumani, K.; Ijare, O.B.; Helekar, S.A. Rotating magnetic fields inhibit mitochondrial respiration, promote oxidative stress and produce loss of mitochondrial integrity in cancer cells. Front. Oncol. 2021, 11, 768758. [Google Scholar] [CrossRef]
- Junka, A.F.; Rakoczy, R.; Szymczyk, P.; Bartoszewicz, M.; Sedghizadeh, P.P.; Fijałkowski, K. Application of rotating magnetic fields increase the activity of antimicrobials against wound biofilm pathogens. Sci. Rep. 2018, 8, 167. [Google Scholar] [CrossRef]
- EUCAST. Breakpoint Tables for Interpretation of MICs and Zone Diameters, Version 15.0; The European Committee on Antimicrobial Susceptibility Testing: Växjö, Sweden, 2025; Available online: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_15.0_Breakpoint_Tables.pdf (accessed on 1 January 2025).

| Antibiotic | Antibiotic Concentration (µg) | Resistant n (%) | Susceptibility n (%) |
|---|---|---|---|
| Piperacillin | 30 | 20 (100.0) | 0 (0.0) |
| Piperacillin/tazobactam | 30–6 | ||
| Amoxicillin with clavulanic acid | 20–10 | ||
| Ceftazidime | 10 | ||
| Cefotaxime | 30 | ||
| Amikacin | 30 | 8 (40.0) | 12 (60.0) |
| Gentamicin | 10 | 9 (45.0) | 11 (55.0) |
| Ciprofloxacin | 5 | 6 (30.0) | 14 (70.0) |
| Trimethoprim/sulfamethoxazole | 1.25–23.75 | 7 (35.0) | 13 (65.0) |
| Ertapenem | 10 | 0 (0.0) | 20 (100.0) |
| Meropenem | 10 | ||
| Imipenem | 10 |
| Antibiotic | Antibiotic Concentration (µg) | Resistant n (%) | Susceptibility n (%) |
|---|---|---|---|
| Piperacillin | 30 | 20 (100.0) | 0 (0.0) |
| Piperacillin/tazobactam | 30–6 | ||
| Amoxicillin with clavulanic acid | 20–10 | ||
| Ceftazidime | 10 | ||
| Cefotaxime | 30 | ||
| Meropenem | 10 | ||
| Imipenem | 10 | ||
| Ertapenem | 10 | ||
| Amikacin | 30 | 15 (75.0) | 5 (25.0) |
| Gentamicin | 10 | 16 (80.0) | 4 (20.0) |
| Ciprofloxacin | 5 | 12 (60.0) | 8 (40.0) |
| Trimethoprim/sulfamethoxazole | 1.25–23.75 | 17 (85.0) | 3 (15.0) |
| Mechanism | MIC COL Kit Control (mg/L) | MIC COL Kit + 5 Hz (mg/L) | Number of Strains n (%) | Effect After RMF Exposure | E-Test Control (mg/L) | E-Test + 5 Hz (mg/L) | Number of Strains n (%) | Effect After RMF Exposure |
|---|---|---|---|---|---|---|---|---|
| ESBLs | 4 | 4 | 11 (55.0) | ↔ | 4 | 4 | 11 (55.0) | ↔ |
| 8 | 8 | 8 | 8 | |||||
| 16 | 16 | 16 | 16 | |||||
| 8 | 4 | 9 (45.0) | ↓ | 6 | 4 | 9 (45.0) | ↓ | |
| 8 | 4 | 8 | 4 | |||||
| 16 | 8 | 16 | 8 | |||||
| 16 | 4 | 16 | 4 | |||||
| KPC | 4 | 4 | 10 (50.0) | ↔ | 4 | 4 | 10 (50.0) | ↔ |
| 8 | 8 | 8 | 8 | |||||
| 16 | 16 | 16 | 16 | |||||
| 8 | 4 | 10 (50.0) | ↓ | 8 | 4 | 10 (50.0) | ↓ | |
| 16 | 8 | 16 | 8 | |||||
| 16 | 4 | 16 | 4 | |||||
| 4 | 4 | 4 | 4 |
| Mechanism | MIC COL Kit Control (mg/L) | MIC COL Kit + 5 Hz (mg/L) | Number of Strains n (%) | Effect After RMF Exposure | E-Test Control (mg/L) | E-Test + 5 Hz (mg/L) | Number of Strains n (%) | Effect After RMF Exposure |
|---|---|---|---|---|---|---|---|---|
| ESBLs | 4 | 4 | 12 (60.0) | ↔ | 4 | 4 | 10 (50.0) | ↔ |
| 8 | 8 | 8 | 8 | |||||
| 16 | 16 | 16 | 16 | |||||
| 8 | 4 | 7 (35.0) | ↓ | 6 | 4 | 5 (25.0) | ↓ | |
| 16 | 8 | 16 | 8 | |||||
| 8 | 16 | 1 (5.0) | ↑ | 6 | 8 | 5 (25.0) | ↑ | |
| 8 | 16 | |||||||
| KPC | 4 | 4 | 11 (55.0) | ↔ | 4 | 4 | 11 (55.0) | ↔ |
| 8 | 8 | 8 | 8 | |||||
| 16 | 16 | 16 | 16 | |||||
| 8 | 4 | 8 (40.0) | ↓ | 8 | 4 | 8 (40.0) | ↓ | |
| 16 | 8 | 16 | 8 | |||||
| 4 | 8 | 1 (5.0) | ↑ | 4 | 8 | 1 (5.0) | ↑ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pruss, A.; Kobylińska, D.; Fijałkowski, K.; Masiuk, H.; Kwiatkowski, P. Evaluation of Colistin Susceptibility of Klebsiella pneumoniae Strains Exposed to Rotating Magnetic Field. Int. J. Mol. Sci. 2025, 26, 8281. https://doi.org/10.3390/ijms26178281
Pruss A, Kobylińska D, Fijałkowski K, Masiuk H, Kwiatkowski P. Evaluation of Colistin Susceptibility of Klebsiella pneumoniae Strains Exposed to Rotating Magnetic Field. International Journal of Molecular Sciences. 2025; 26(17):8281. https://doi.org/10.3390/ijms26178281
Chicago/Turabian StylePruss, Agata, Dagmara Kobylińska, Karol Fijałkowski, Helena Masiuk, and Paweł Kwiatkowski. 2025. "Evaluation of Colistin Susceptibility of Klebsiella pneumoniae Strains Exposed to Rotating Magnetic Field" International Journal of Molecular Sciences 26, no. 17: 8281. https://doi.org/10.3390/ijms26178281
APA StylePruss, A., Kobylińska, D., Fijałkowski, K., Masiuk, H., & Kwiatkowski, P. (2025). Evaluation of Colistin Susceptibility of Klebsiella pneumoniae Strains Exposed to Rotating Magnetic Field. International Journal of Molecular Sciences, 26(17), 8281. https://doi.org/10.3390/ijms26178281








