1. Introduction
Malignant melanomas develop from the malignant transformation of melanocytes [
1,
2,
3]. Skin melanoma is characterized by high metastatic potential and significant morbidity and mortality [
2,
4]. The melanogenic activity and behavior of melanocytes are regulated by various factors, such as ultraviolet radiation (UVR) and a range of chemical and biological mediators, which consist of both hormonal and non-hormonal regulators, in addition to genetic and molecular ones [
5,
6,
7]. The most prevalent types of melanomas include cutaneous malignant melanomas which significantly impact large segments of the population with high incidence and mortality rates relative to those of other cancers [
8].
Skin melanoma is the 17th most prevalent cancer globally. Although Europe accounts for the highest absolute number of cases and death, the highest incidence and mortality rates per capita are reported in Australia and New Zealand [
8,
9]. Four major types of melanoma skin cancer exist. Superficial spreading melanoma, accounting for 60–70% of cases, typically grows outward on the skin and can occur anywhere on the body, while nodular melanoma (15–30%) tends to grow vertically into the skin; lentigo maligna melanoma, representing about 8% of cases, is often amelanotic and appears red or skin-colored, whereas acral lentiginous melanoma, the rarest subtype, presents in advanced stages as a palpable pigmented macule with variable coloration [
4,
8,
10,
11,
12].
Angiogenesis—the formation of new blood vessels from pre-existing vasculature—is a key process in tumor development and progression, supplying oxygen and other nutrients vital for tumor growth and survival [
13,
14,
15]. The prognostic significance of angiogenesis in melanoma has garnered increasing interest, with several studies linking enhanced angiogenic activity to tumor aggressiveness and poor clinical outcomes [
16,
17]. Recent advances in genomic and molecular biology have brought into the light the importance of angiogenesis-related genes (ARGs) in the growth and development of tumors, as well as potential therapeutic targeting [
15,
18]. Melanoma, recognized for its aggressive nature and rapid metastatic potential, remains a significant clinical challenge in terms of management and treatment [
4,
5]. Furthermore, ARGs play a role in regulating the tumor microenvironment (TME), modulating immune responses, and contributing to immune evasion mechanisms—factors that can profoundly influence the efficacy of immunotherapeutic approaches [
19,
20,
21,
22,
23]. Therefore, a comprehensive understanding of the molecular and functional characteristics of ARGs could provide valuable insights for developing novel targeted therapies and improving clinical outcomes for melanoma patients [
16,
24,
25,
26,
27,
28].
This study aims to comprehensively analyze the role of ARGs in skin melanoma, focusing on their prognostic significance, molecular characteristics, and interactions with tumor immunity.
2. Results
2.1. Gene Expression Prognositc Analysis of the Angiogenesis-Related Genes
We first examined the expression of ARGs in primary (SKCM-P) and metastatic (SKCM-M) skin melanoma samples, and found that several genes are strongly associated with survival outcomes. For instance, in SKCM-M,
S100A4,
ITGAV,
PF4,
TNFRSF21,
JAG2,
SERPINA5,
PDGFA and
KCNJ8 emerged as significant prognostic markers (
Figure 1). These genes—already linked to cell growth and angiogenesis—appear to influence disease progression and metastatic potential; therefore, their expression may help predict patient outcomes and guide personalized treatment strategies for advanced melanoma.
In contrast, COL3A1 and FSTL1 were significantly overexpressed in SKCM-P, pointing to a potential role in early-stage tumor development and offering targets for early detection and intervention.
COL3A1 and
FSTL1 showed prognostic significance for disease-specific survival, in the SKCM-P dataset. Known for regulating extracellular matrix dynamics and tissue remodeling, these genes are closely tied to early tumor development and may serve as markers for early detection and intervention. In SKCM-M,
PF4,
TNFRSF21,
JAG2,
SERPINA5,
CCND2, and
PDGFA were associated with survival outcomes (
Figure 2). These genes influence inflammation, cell proliferation, and angiogenesis—key processes in melanoma progression—and could inform targeted treatment strategies for metastatic disease. Notably,
TIMP1 emerged as a strong prognostic marker in both primary and metastatic melanoma. Its consistent expression across disease stages suggests it may be a valuable biomarker for tracking the tumor microenvironment (TME), monitoring disease progression, and evaluating treatment response throughout the course of the disease.
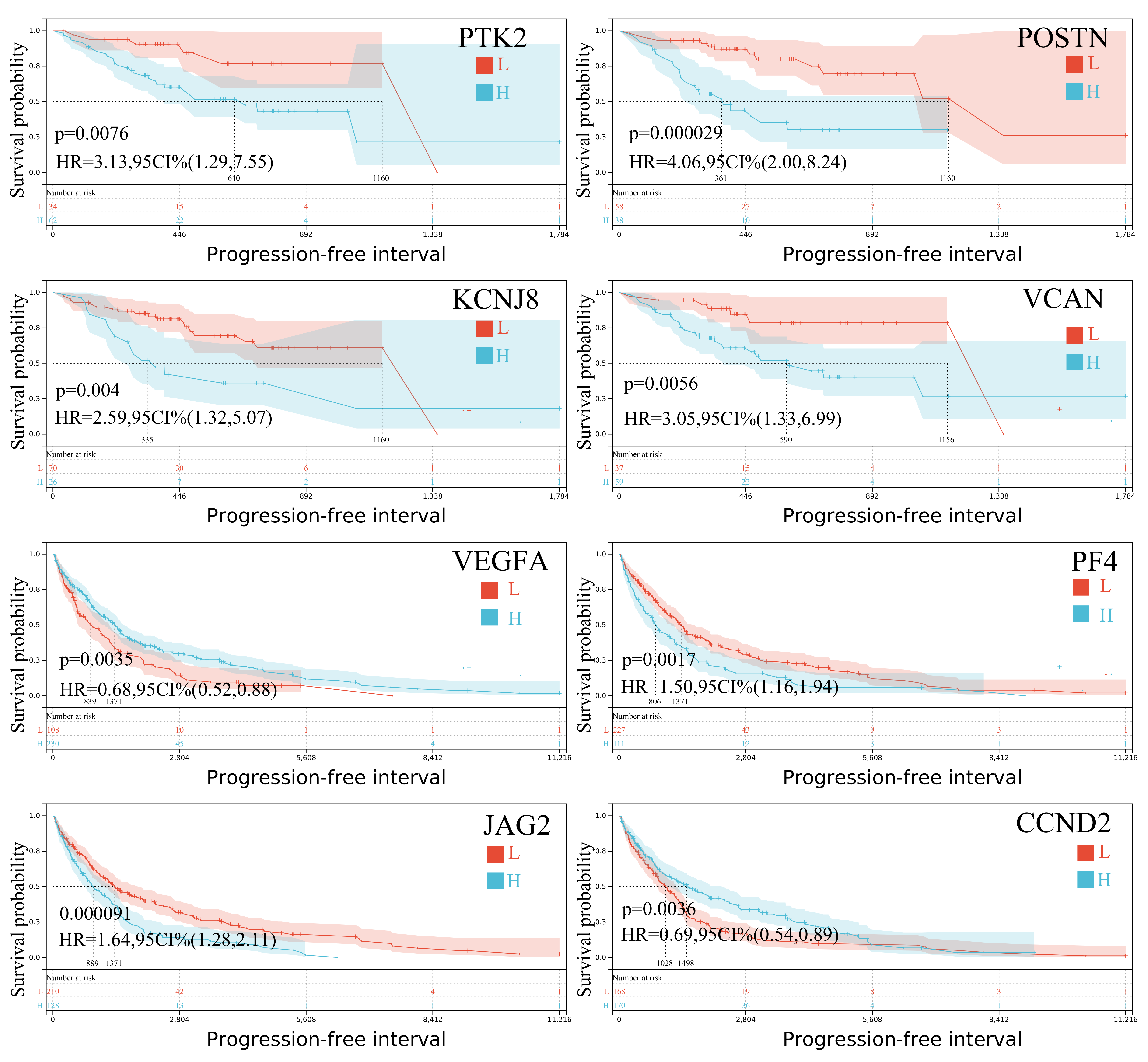
Progression-free interval analysis also revealed several prognostic genes. In SKCM-P,
PTK2,
VEGFA,
KCNJ8,
POSTN, and
VCAN were significantly associated with patient outcomes, while in SKCM-M,
VEGFA,
PF4,
JAG2,
CCND2, and
TIMP1 showed strong prognostic value (
Figure 3). Notably,
VEGFA and
SPP1 were significant prognosticators both in primary and metastatic tumors, underscoring their broader role in melanoma progression. These distinct expression patterns between primary and metastatic skin melanomas highlight the molecular complexity and heterogeneity of the disease, offering important clues for developing more precise, stage-specific therapeutic strategies.
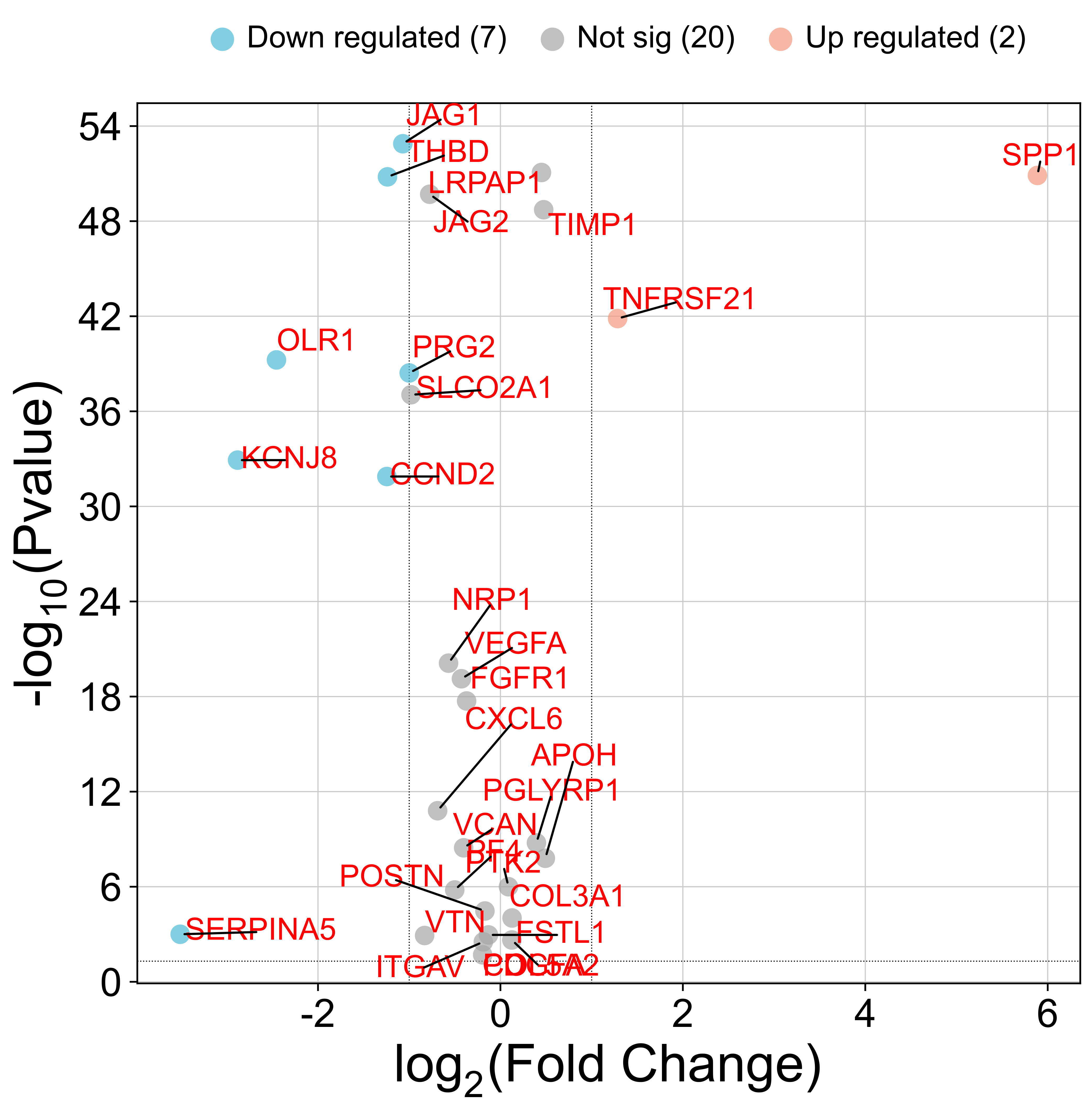
2.2. Differential Gene Expression Analysis of the Angiogenesis-Related Genes
We observed significant differences in ARG expression between melanoma and normal tissue. Eleven genes—including
TNFRSF21 and
SPP1—were markedly upregulated in tumors, suggesting active angiogenic signaling that may drive melanoma progression and metastasis (
Figure 4). Conversely, eighteen genes, including
SERPINA5,
CCND2,
KCNJ8,
PRG2,
OLR1,
THBD, and
JAG1, were downregulated (
Figure 4), pointing to the involvement of alternative, non-VEGFA-driven angiogenic pathways. These contrasting patterns underscore the complexity of angiogenesis regulation in melanoma and reveal potential therapeutic targets within these disrupted networks.
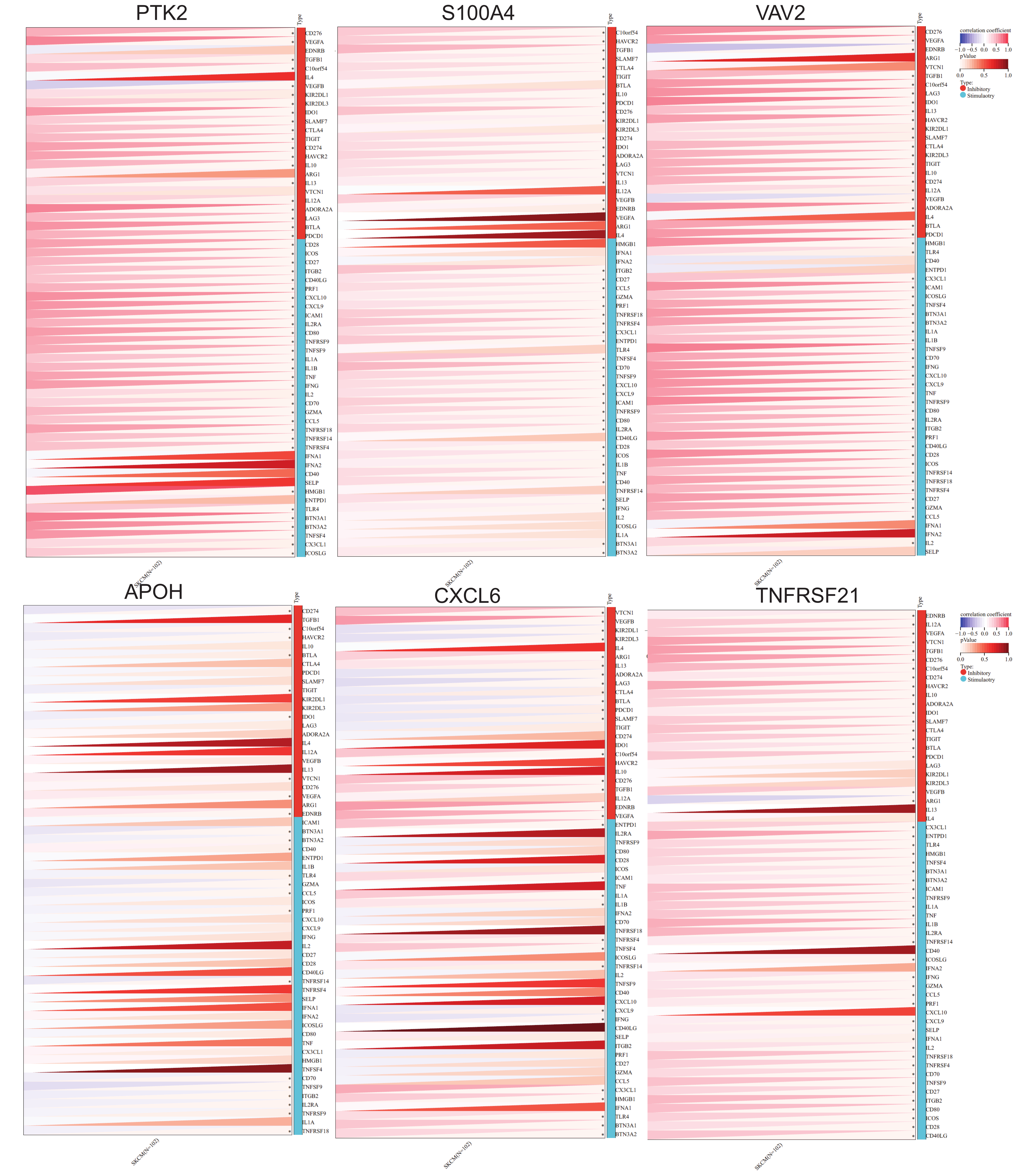
2.3. Immune Checkpoint Gene Analysis
Analysis of immune checkpoint gene expression revealed significant correlations between ARGs and immune regulation in SKCM. Several ARGs—including
PTK2,
S100A4,
CXCL6,
VAV2, APOH, and
TNFRSF21—showed strong positive correlations with inhibitory immune checkpoints such as
CD276, VEGFA, VEGFB, TGFB1, IDO1, TIGIT, CTLA4, CD274, SLAMF7, HAVCR2, IL10, IL13, IL12A, ADORA2A, LAG3, BTLA, and
PDCD1. This suggests these ARGs may contribute to enhanced immune checkpoint activity, potentially aiding tumor immune evasion. Interestingly,
CXCL6 and other ARGs also showed negative correlations with checkpoints like
VEGFB,
KIR2DL1,
LAG3,
CTLA4,
PDCD1, and
IFNG, indicating they may promote immune surveillance and anti-tumor responses (
Figure 5). These results highlight the intricate link between angiogenesis and immune modulation in melanoma and underscore the potential for ARGs to serve as dual biomarkers for angiogenic and immune-related therapeutic targeting.
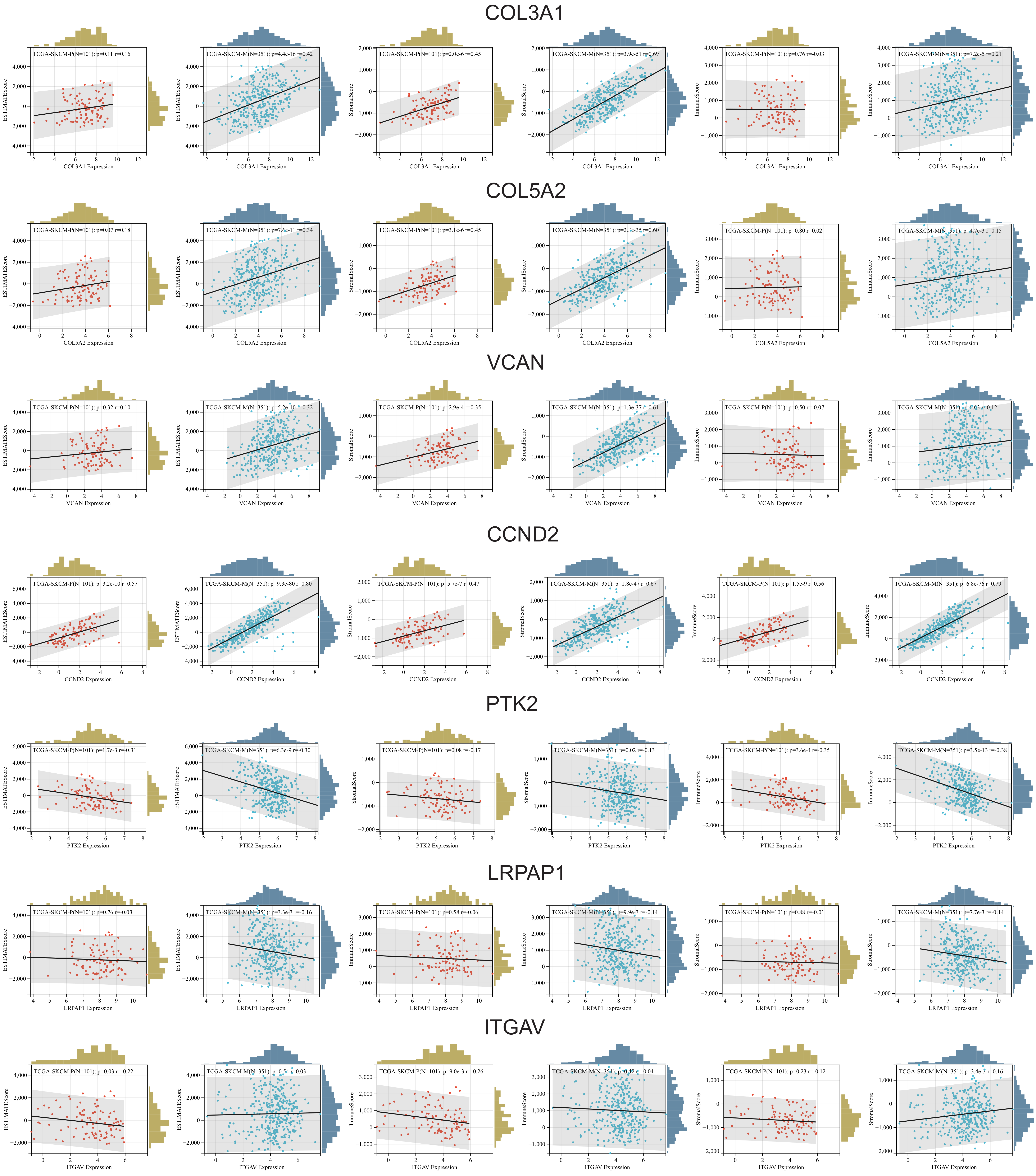
2.4. Immune Infiltration Analysis (ESTIMATE)
Immune infiltration analysis using ESTIMATE (Estimation of STromal and Immune cells in MAlignant Tumor tissues using Expression data), immune, and stromal scores revealed key associations between ARGs and the tumor microenvironment (TME) in skin melanoma.
COL3A1,
COL5A2,
VCAN,
CCND2,
JAG2, and
VTN showed consistent positive correlations with all three scores in both primary and metastatic tumors, suggesting their involvement in enhancing stromal support and recruiting immune cells. These genes were particularly linked to stromal components and the overall ESTIMATE score, indicating a strong association with the tumor stroma and its role in promoting tumor progression. Interestingly, some gene-TME interactions varied between primary and metastatic samples, especially regarding stromal and immune scores. In contrast,
PTK2 and
LRPAP1 showed negative correlations with immune-related scores, pointing to a role in dampening immune infiltration and enabling immune evasion.
ITGAV also exhibited negative correlations, particularly with the ESTIMATE and immune scores, hinting at a broader immunosuppressive effect that could hinder treatment response (
Figure 6).
Overall, these findings highlight the complex role of ARGs in shaping the immune landscape of SKCM. Genes positively correlated with stromal and immune scores may serve as biomarkers to identify patients more likely to benefit from immunotherapy. In contrast, genes negatively associated with immune scores could be promising targets for therapies aimed at boosting immune cell infiltration and enhancing anti-tumor immune responses.
2.5. Immune Cell Analysis (CIBERSORT)
We also used CIBERSORT to investigate the relationship between ARG expression and immune cell infiltration in the TME of both primary and metastatic skin melanomas.
PTK2 showed strong positive correlations with activated T cells and M2 macrophages, and negative correlations with naïve and memory B cells, activated dendritic cells, eosinophils, and neutrophils across both tumor types (
Figure S1).
PRG2 displayed distinct patterns—weakly positive in primary tumors and negative in metastatic ones—suggesting stage-specific immune interactions. In addition,
S100A4 was positively associated with M0 macrophages and negatively correlated with resting NK cells.
APOH showed notable associations with CD8+ T cells and Tregs, indicating a broader role in immune modulation.
VAV2 correlated positively with M0 macrophages, pointing to a role in macrophage-driven responses.
ITGAV and
SLCO2A1 were linked to multiple immune cell types, supporting their involvement in immune infiltration.
CXCL6 was positively correlated with macrophages and negatively correlated with T cells, while PGLYRP1 was associated with M1 macrophages, suggesting a role in pro-inflammatory responses. COL3A1 and VEGFA were both positively associated with M0 macrophages and negatively with CD8+ T cells, implying a contribution to an immunosuppressive microenvironment. Together, these findings reveal the diverse and context-dependent roles of ARGs in modulating immune cell infiltration in melanoma.
We also identified complex and gene-specific associations between ARGs and immune cell populations in both primary and metastatic tumors. FGFR1 showed a strong positive correlation with neutrophils, but negative correlations with dendritic cells and select macrophage subsets, suggesting it may influence immune suppression through targeted immune cell modulation. FSTL1 was positively associated with T follicular helper cells and negatively with γδ T cells, pointing to a potential role in regulating T cell-mediated immunity. In contrast, SERPINA5 correlated positively with activated dendritic cells and negatively with regulatory T cells, implying it may shape the immune microenvironment by enhancing antigen presentation while limiting immune suppression.
In fact, several genes displayed dual or context-dependent roles in immune modulation. JAG1 was positively associated with macrophages and negatively with T cells, indicating a complex role in orchestrating immune infiltration. Similarly, LRPAP1 and KCNJ8 showed negative correlations with CD8+ T cells and positive correlations with macrophages and certain memory B cell subsets, suggesting that these genes may shift immune cell composition in ways that impact tumor immunity. SPP1 and TIMP1 followed a similar pattern—positively associated with macrophages and negatively with CD8+ T cells—supporting their potential role in promoting immune evasion via macrophage activation and T cell suppression.
Additional ARGs, including PDGFA, VTN, and POSTN, revealed intricate and subtype-specific correlations with both macrophages and T cells, reinforcing their involvement in shaping the tumor immune landscape. Notably, APP, VCAN, and STC1 exhibited strong positive correlations with macrophages and activated NK cells, but negative associations with memory B and resting T cells. These inverse patterns may signal immunosuppressive effects that hinder effective antitumor responses in melanoma.
Collectively, these findings underscore the diverse immunomodulatory roles of ARGs and their influence on immune cell dynamics in the TME.
2.6. Immune Cell Analysis (xCELL)
To further explore ARG–immune cell interactions, we used xCELL to analyze correlations between ARG expression and immune cell populations in both primary and metastatic melanoma. PTK2 expression showed a positive correlation with mast cells, but was negatively associated with CD8+ T cells, macrophages, and NK cells, suggesting a potential role in immune suppression across both tumor stages. PRG2 displayed a similar pattern—positively correlated with CD4+ central memory T cells and negatively with immature dendritic cells. S100A4 was positively linked to macrophages and regulatory T cells, while negatively associated with NK cells and eosinophils, highlighting its potential involvement in promoting immune evasion.
VAV2 and
ITGAV were associated with increased levels of activated T cells and macrophages, but showed negative correlations with B cells and CD8+ T cells, suggesting they may promote selective immune activation while suppressing cytotoxic responses.
SLCO2A1 was positively correlated with regulatory T cells and macrophages, and negatively with CD8+ T cells, reinforcing its possible immunosuppressive role (
Figure S2).
CXCL6 showed a strong positive correlation with immature dendritic cells and macrophages, but a negative correlation with CD8+ T cells, pointing to a dampening effect on cytotoxic immune activity.
PGLYRP1 revealed an intriguing profile—positively associated with innate immune cells like macrophages, but negatively with adaptive immune cells, indicating a potential role in balancing immune responses.
COL3A1 displayed diverse associations with various immune cell types, suggesting broad immunomodulatory effects.
VEGFA correlated positively with M2 macrophages—a subtype linked to immunosuppressive activity—and negatively with T cells, further supporting its role in blunting anti-tumor immunity (
Figure S3).
Together, these results underscore the intricate interplay between ARGs and immune effector cells in melanoma and they offer valuable insights into the immunological complexity of the tumor microenvironment.
In the metastatic tumors, VEGFA showed positive correlations with CD4+ T cells and M1 macrophages, but negative correlations with Tregs and B cells. In contrast, in the primary tumors, VEGFA was positively associated with endothelial cells and neutrophils, and negatively with NK cells, highlighting tumor stage-specific immune interactions. PF4 positively correlated with dendritic cells and negatively with CD4+ T cells and B cells, indicating a potential role in skewing immune activation. TNFRSF21 demonstrated complex associations: positive with Tregs and macrophages, but negative with NK cells and eosinophils, particularly in primary melanomas.
In the metastatic cohort,
JAG2 correlated positively with NKT cells and CD4+ memory T cells, while negatively with M2 macrophages, suggesting it may modulate both adaptive and immunosuppressive populations.
LRPAP1 showed weak positive correlations with macrophages and CD4+ T cells but was negatively associated with adipocytes.
MSX1 correlated with CD8+ T cells and M1 macrophages, whereas
NRP1 showed selective associations with M2 macrophages and other T cell subsets.
CCND2 consistently correlated positively with CD4+ central memory T cells across datasets, but showed inverse associations with other immune cell types (
Figure S3).
Further analyses revealed distinct gene-specific roles in immune modulation. NRP1 was positively correlated with macrophages and negatively with CD8+ T cells and NK cells, showing differing roles between metastatic and primary tumors. VTN showed positive associations with CD4+ T cells and dendritic cells in metastatic tumors, but negative correlations with NK cells and eosinophils. PDGFA correlated positively with macrophages and endothelial cells, but negatively with CD8+ T cells, suggesting a role in immune evasion. LUM expression was positively linked to smooth muscle cells and fibroblasts, and negatively to basophils in one dataset.
THBD showed positive correlations with M1 macrophages and negative with B cells. TIMP1 was strongly associated with M2 macrophages and negatively with multiple T cell populations, indicating an immunosuppressive function. SPP1 was positively linked to activated dendritic cells, and negatively to CD8+ T cells. OLR1 was positively correlated with macrophages, but negatively with both CD4+ and CD8+ T cells. APP showed positive associations with M2 macrophages, but inverse correlations with CD8+ T cells and T helper cells.
Arginine-regulated genes also showed significant relevance. LPL was positively correlated with macrophages, although its associations with other immune cells varied across datasets. VCAN consistently correlated positively with M2 macrophages and negatively with cytotoxic T cells, suggesting an immunosuppressive phenotype. STC1 generally exhibited positive immune cell correlations, except for a strong negative correlation with neutrophils in metastatic samples.
Collectively, these results emphasize the intricate and multifaceted relationships between ARG expression and immune cell infiltration in melanoma.
2.7. Immune Cell Analysis (TIMER)
Comprehensive correlation analyses between immune cell populations and gene expression across both datasets revealed intricate and subtype-specific interactions. In the metastatic dataset,
PTK2 consistently showed significant negative correlations with multiple immune cell types, including B cells, CD4+ T cells, CD8+ T cells, neutrophils, and dendritic cells. In contrast, in the primary dataset,
PTK2 generally exhibited positive correlations, particularly with macrophages, suggesting divergent roles in immune regulation between primary and metastatic melanoma (
Figure S4).
Similarly,
PRG2 demonstrated strong negative correlations with B cells in TCGA-SKCM-P, while showing positive associations with macrophages in both datasets. These patterns point to a potential role for
PRG2 in modulating innate immunity and suppressing adaptive immune responses (
Figure S4).
Further analysis revealed gene-specific immune interactions that varied between primary and metastatic melanomas. In the TCGA-SKCM-M dataset, S100A4 was positively correlated with CD8+ T cells and neutrophils, whereas in the primary dataset it was primarily associated with macrophages. APOH showed strong negative correlations with B cells and CD4+ T cells in the primary tumors, while correlating positively with macrophages in the metastatic ones. In addition, VAV2 demonstrated significant positive associations with T cells and dendritic cells in the metastatic dataset but lacked notable positive correlations in the primary samples. ITGAV also displayed a mix of positive and negative associations, particularly involving T cells and macrophages in metastatic tumors. Lastly, SLCO2A1 showed consistent positive correlations with most immune cell types in both datasets—except dendritic cells, where no correlation was observed.
Notably,
CXCL6 exhibited strong negative correlations with B and T cells in primary melanomas, while
PGLYRP1 was positively associated with neutrophils and macrophages in metastatic ones.
COL3A1 showed moderate positive correlations with a variety of immune cell types, with patterns that varied between datasets (
Figure S5).
Deeper analysis of additional genes provided further insights.
COL3A1 displayed strong positive correlations with multiple immune cells in the TCGA-SKCM-M dataset, suggesting an immunoregulatory role.
COL5A2 showed variable associations, particularly with macrophages. Furthermore,
VEGFA was consistently correlated with neutrophils in both datasets, while
PF4 had negative associations with several T cell subsets. In addition,
TNFRSF21 showed specific relevance to T cells and neutrophils, whereas
JAG2 had predominantly negative correlations, especially with T cells in the metastatic cohort.
FGFR1 was positively correlated with macrophages, and
FSTL1 showed associations with both T cells and macrophages in the TCGA-SKCM-P dataset.
JAG1 had strong positive correlations with macrophages and dendritic cells, underscoring its immunomodulatory potential.
SERPINA5 demonstrated strong positive associations with CD4+ T cells, supporting its relevance in immune-targeted therapeutic strategies (
Figure S5).
Fixed correlation analysis in the TCGA-SKCM-M dataset revealed moderate but statistically significant positive correlations between
ARG expression and several immune cell types, including B cells, CD4+ and CD8+ T cells, neutrophils, macrophages, and dendritic cells. In contrast, the TCGA-SKCM-P dataset showed more selective trends, primarily involving CD4+ and CD8+ T cells, while neutrophil associations were less prominent compared to the metastatic dataset (
Figure S5).
2.8. Immune Cell Analysis (MCPcounter)
We used the MCPcounter algorithm as an additional way to determine the absolute abundance of various immune and stromal cell types in primary and metastatic melanomas, based on their ARG expression levels. We found that
PTK2 exhibited contrasting correlation patterns. In the TCGA-SKCM-M dataset, it was negatively associated with CD8+ T cells and other T cell subtypes, while in the TCGA-SKCM-P dataset, it showed positive correlations with dendritic cells, endothelial cells, and stronger associations with neutrophils. In addition,
PRG2 showed weak negative correlations with dendritic and endothelial cells in the primary tumors, but had no significant associations in the metastatic ones (
Figure S6).
Furthermore, S100A4 was negatively correlated with multiple immune cell types in the TCGA-SKCM-M dataset, but positively associated with macrophages in the TCGA-SKCM-P dataset. APOH also showed negative correlations with T cells in primary tumors and positive correlations with fibroblasts in metastatic ones. Genes such as VAV2, ITGAV and SLCO2A1 demonstrated consistent positive associations with endothelial cells, macrophages and fibroblasts, while CXCL6 and PGLYRP1 showed strong positive correlations with neutrophils across both cohorts. COL3A1 displayed robust positive correlations with a broad range of immune cells, reinforcing its role in the TME. Similar patterns were observed for COL5A2 and PF4, though PF4 showed contrasting correlations, with negative correlation with monocytes in primary tumors and positive ones with fibroblasts in metastatic ones. JAG2 and TNFRSF21 were correlated with dendritic cells and T cell subsets, while FGFR1 showed strong positive correlations with cytotoxic T cells. FSTL1, SERPINA5, and JAG1 also revealed significant correlations with immune cells including endothelial cells, fibroblasts and macrophages suggesting immunoregulatory potential.
Pearson correlation analysis confirmed these findings, with
JAG1 strongly correlating with endothelial and fibroblast cells, while
LRPAP1 showed positive correlations with endothelial cells and negative ones with CD8 T cells.
MSX1 and
CCND2 displayed opposing trends between datasets, underlining their context-specific roles.
NRP1 and
THBD both demonstrated broad positive associations with immune subsets, suggesting a role in immune regulation.
KCNJ8 showed consistent positive correlations with T cells, NK cells and cytotoxic lymphocytes across datasets.
LPL and
VCAN correlated with neutrophils, fibroblasts and dendritic cells, highlighting their influence on the immune landscape.
STC1 and
VCAN further showed strong correlations with NK cells, CD8 T cells and monocytic lineages, while T cells exhibited inverse correlations in both datasets (
Figure S6).
Gene-specific correlations suggest diverse roles in immune modulation and tumor progression, offering potential targets for immunotherapy.
2.9. Immune Cell Profiling (EPIC)
We further used EPIC, which includes RNA-seq-based gene expression reference profiles from immune cells and other nonmalignant cell types found in tumors. During the assessment of
PTK2 expression in the TCGA-SKCM-M dataset, significant positive correlations with CD8 T cells and negative correlations with B cells, CAFs, and endothelial cells were found; whereas no significant correlations were observed in the TCGA-SKCM-P dataset. In addition,
PRG2 showed significant negative and positive correlations with CAFs and macrophages, respectively in primary tumors; while no significant correlations were seen in metastatic ones (
Figure S7).
S100A4 correlated positively with B cells, CD8 T cells and macrophages, but negatively with NK cells. In the case of
APOH expression in CAFs, a significant negative correlation was seen only in the TCGA-SKCM-P dataset.
VAV2,
ITGAV, and
SLCO2A1 demonstrated positive correlations with multiple immune populations, including B cells, CAFs and endothelial cells.
CXCL6 was positively correlated with CAFs and CD4 T cells, while showing a negative correlation with CD8 T cells in the SKCM-M dataset.
PGLYRP1 correlated positively with B cells in the metastatic melanomas, but not in the primary ones (
Figure S8).
Across both datasets,
COL3A1 and
COL5A2 were strongly associated with CAFs; while
COL3A1 showed negative correlations with CD8 T cells.
VEGFA positively correlated with CD4 T cells in SKCM-P, but negatively with B cells in SKCM-M.
PF4,
TNFRSF21, and
JAG2 exhibited diverse correlations, notably with macrophages, T cells and endothelial cells.
FGFR1,
FSTL1, and
SERPINA5 displayed consistent positive correlations with CAFs, CD4 T cells and macrophages, respectively (
Figure S8).
JAG1,
LRPAP1, and
MSX1 correlated with CAFs and T cells particularly in metastatic cases.
CCND2 was broadly associated with immune populations, reinforcing its potential role in shaping the immune microenvironment.
NRP1,
VTN, and
PDGFA revealed significant correlations with fibroblasts, macrophages and CD4 T cells, respectively. Furthermore,
LUM,
THBD,
OLR1, and
KCNJ8 displayed distinctive immune signatures. Notably,
KCNJ8 showed strong positive correlations with CAFs and B cells, and weak negative ones with NK cells.
POSTN,
APP and
LPL were also positively correlated with CAFs and CD4 T cells, while
VCAN and
STC1 displayed consistent associations with CAFs, B cells and CD8 T cells, but were negatively correlated with NK cells (
Figure S8). These findings underscore the intricate immune dynamics driven by ARG expression, highlighting subtype-specific immune modulation across melanoma stages.
2.10. Immune Cell Profiling (QUANTISEQ)
We then used quanTIseq, a deconvolution pipeline that quantifies the fractions and densities of 10 different immune cell types relevant for cancer immunology, as well as the proportion of uncharacterized cells (e.g., malignant cells in bulk tumors). We found that
PTK2 displayed consistent positive correlations with B cells, NK cells and monocytes, and negative correlations with CAFs and endothelial cells in metastatic melanomas. In addition,
PRG2 was positively correlated with T cells and monocytes, and negatively with M2 macrophages (
Figure S9).
We also found that
S100A4 exhibited positive correlations with T cells and macrophages, but negative with monocytes.
APOH was negatively correlated with regulatory T cells in primary melanomas, whereas
VAV2 was positively correlated with CD8 T cells, but negatively with dendritic cells.
ITGAV was broadly associated with macrophages and inversely with T cells.
CXCL6,
SLCO2A1 and
PGLYRP1 showed varied correlations:
CXCL6 positively associated with neutrophils,
SLCO2A1 with B cells and monocytes, and
PGLYRP1 with multiple immune types, suggesting broad immune involvement.
COL3A1,
COL5A2 and
VEGFA showed dataset-specific correlations;
COL3A1 had strong associations with M1 macrophages, while
VEGFA was positively correlated with CD4 T cells in metastatic melanomas. Interestingly,
PF4 and
TNFRSF21 displayed contrasting roles, with
PF4 weakly correlating across immune types and
TNFRSF21 linking positively to B cells and M1 macrophages, but negatively to CD8 T cells.
FGFR1 had weak general correlations but a notable link with CD8 T cells. Furthermore,
FSTL1,
SERPINA5 and
JAG1 correlated positively with macrophages, B cells and dendritic cells, and negatively with monocytes and CD4 T cells. Likewise,
LRPAP1, MSX1, CCND2, and
NRP1 displayed strong associations with monocytes, Tregs and NK cells, especially in metastatic tumors.
VTN, PDGFA and
LUM also showed subtype-specific patterns with
PDGFA strongly linked to macrophages and LUM to B cells. Other ARGs such as
TIMP1, SPP1, OLR1, KCNJ8 and
POSTN demonstrated significant immune cell correlations. Notably
POSTN showed opposing roles, with positive correlations with M1/M2 macrophages and negative correlations with NK cells and Tregs.
VCAN, APP, LPL and
STC1 exhibited diverse associations, particularly in the TCGA-SKCM-M dataset, with
STC1 showing strong negative correlations with CD8 T cells and Tregs, but positive with NK cells and macrophages (
Figure S10).
These findings underline the gene-specific and dataset-dependent immune interactions within the melanoma TME.
2.11. Immune Cell Profiling (IPS)
We then re-estimated key components of the tumor immune microenvironment, including the MHC (antigen presentation capacity), EC (effector cells), SC (immune suppressor cells), CP (immune checkpoints), AZ (antigen zones or areas of immune activation) and the IPS (Immunophenoscore) infiltration scores per patient and tumor using gene expression data, summarizing the tumor’s overall immunogenic profile.
We found that
PTK2 showed differing associations: in metastatic tumors, it correlated positively with MHC, SC and CP, and negatively with EC and AZ, while in primary tumors, it was positively correlated with MHC and SC, but its correlations with AZ were mixed. On the other hand,
PRG2 consistently showed negative correlations with multiple immune components, suggesting a suppressive role (
Figure S11).
S100A4 and
ITGAV displayed dataset-specific patterns: negatively associated with SC and CP in metastatic melanomas, but positively with MHC and EC in primary ones.
APOH was weakly negatively correlated overall but had a stronger positive correlation with SC in primary tumors.
VAV2 was broadly negatively correlated in metastatic tumors, but displayed limited significance in primary ones.
SLCO2A1 was positively correlated with EC, but negatively with SC and IPS in metastatic tumors.
CXCL6 showed strong negative associations with immune cells in the SKCM-M dataset, with negligible correlations in SKCM-P.
PGLYRP1 exhibited only weak associations across datasets.
COL3A1 was negatively associated with EC and CP, indicating possible roles in immune modulation. Several ARGs, such as
COL5A2 and
VEGFA also demonstrated complex, context-specific correlations with immune cells. Notably,
VEGFA was negatively associated with several immune types in SKCM-M, indicating a possible immunosuppressive effect (
Figure S12).
JAG2 displayed strong negative correlations with MHC and EC in both datasets, suggesting a consistent inhibitory role.
FSTL1 had negative correlations in SKCM-M, but variable trends on SKCM-P.
SERPINA5 exhibited inconsistent profiles between datasets.
JAG1, LRPAP1, MSX1, CCND2, NRP1, VTN, PDGFA, LUM, THBD, and
TIMP1 each displayed unique patterns of association, often differing between metastatic and primary melanomas, underscoring the context-dependent nature of immune-gene interactions. Other ARGs, including
OLR1, KCNJ8, APP, POSTN, and
STC1 showed significant yet heterogenous relationships with immune cells subsets, reinforcing the complexity of immune regulation in melanoma (
Figure S12).
3. Discussion
Our comprehensive analysis of ARGs in skin cutaneous melanoma highlights their prognostic significance, molecular diversity, and immunological impact. By integrating gene expression profiles with clinical outcomes from both primary and metastatic tumor datasets, we identified distinct ARG expression patterns associated with overall survival, disease progression, and immune cell infiltration.
Several genes, including
S100A4,
ITGAV, and
COL3A1, previously linked to tumor progression and poor prognosis [
29,
30,
31,
32,
33], emerged in our study as robust prognostic indicators. These genes exhibited heterogeneous expression patterns and immune correlations across patient subgroups. Kaplan–Meier analyses confirmed significant associations between ARG expression and survival metrics, supporting their utility in patient stratification and prognostic modeling. Furthermore, differential expression analysis revealed upregulation of genes such as
PTK2,
PRG2, and
VEGFA in tumor samples compared to normal skin, alongside downregulation of other ARGs—reinforcing their involvement in melanoma pathogenesis.
VEGFA, a well-characterized pro-angiogenic driver, is known to promote tumor vascularization and immune evasion [
34,
35,
36,
37,
38,
39]. Our findings confirm its central role in melanoma biology and suggest it remains a viable therapeutic target. Importantly, our results also underscore the immunomodulatory functions of ARGs. For example,
JAG1 and
SERPINA5, which have been implicated in immune regulation and tumor-immune escape [
33,
40,
41], showed complex associations with immune cell subsets—positively correlating with macrophages and negatively with cytotoxic T cells—indicating their potential involvement in immune evasion mechanisms. The divergent infiltration patterns observed between primary and metastatic tumors further emphasize the importance of tailoring immunotherapeutic approaches to tumor stage and immune context [
42].
Leveraging advanced bioinformatics tools such as xCELL and TIMER enabled precise estimation of immune infiltration and detailed mapping of gene-immune interactions from bulk RNA-seq data [
43,
44]. These analyses revealed nuanced relationships between ARG expression and immune components. For instance,
CXCL6, known for its role in neutrophil and macrophage recruitment [
45,
46], showed consistent immunomodulatory activity in our datasets. Similarly,
TNFRSF21 correlated positively with B cells and M1 macrophages in metastatic samples, while
JAG2 showed strong negative correlations with CD8+ T cells and dendritic cells. Previous studies suggest that
JAG2, a Notch ligand, may contribute to immune evasion by impairing dendritic cell function and T cell priming [
47,
48,
49], which aligns with our findings.
Together, these insights reveal the multifaceted roles of ARGs in melanoma progression and immune modulation. They provide a valuable foundation for future studies and therapeutic strategies aimed at targeting angiogenesis and immune pathways in a personalized and stage-specific manner.
Several additional genes—including
FSTL1,
LRPAP1,
MSX1, and
APP—exhibited distinct immune interaction profiles, suggesting diverse roles in modulating tumor–immune dynamics. Notably,
FSTL1 showed positive correlations with M2 macrophages in primary melanoma, aligning with prior studies that link it to M2 polarization in other cancers [
50,
51].
SERPINA5 and
JAG1 were consistently negatively correlated with monocytes, indicating a potential role in immune suppression and myeloid cell regulation.
Other ARGs also demonstrated cell type-specific associations. For example,
POSTN was positively correlated with neutrophils, whereas
LPL and
VEGFA were linked to distinct immune subsets. Importantly,
CAF-1 showed a negative correlation with CD8+ T cells, a pattern observed in earlier studies and confirmed in our data [
52]. These findings underscore the multifaceted immunological effects of ARGs, reinforcing their dual role in regulating both angiogenesis and immune function.
Collectively, these immunogenomic insights highlight the potential of ARGs as actionable targets. Their expression patterns not only influence tumor vascularization, but also shape the immune microenvironment—affecting infiltration, immune suppression, and responsiveness to therapy. Modulating ARGs may enhance immune cell infiltration, boost responses to immune checkpoint inhibitors, and disrupt tumor-driven immune evasion mechanisms [
27,
53,
54,
55,
56].
Despite the significance of these findings, certain limitations must be acknowledged. Our analyses rely heavily on TCGA data, which includes a limited number of normal samples and may introduce sampling bias. Furthermore, the study is based on transcriptomic data, and experimental validation is essential to confirm the functional roles of the identified ARGs. In addition, although our study is correlative, the patterns observed suggest mechanistic links such as STAT3-mediated PD-L1 expression driven by ARGs. Experimental validation using knockdown and overexpression models is needed to confirm these causal pathways.
To minimize tool-specific bias, we used seven immune deconvolution algorithms. While discrepancies (e.g., TIMER vs. MCPcounter for PTK2) were observed in our findings, consistent trends across multiple tools strengthen the robustness of our key findings.
Future research should use spatial transcriptomics (e.g., Visium) or co-culture assays with PBMCs to confirm the immunosuppressive roles of TIMP1, S100A4 and other ARGs. We believe that our findings support a rationale for clinical trials testing anti-angiogenic agents (e.g., Bevacizumab) in combination with anti-PD1 therapy (e.g., Pembrolizumab) in VEGFA-high melanoma patients.
Importantly, while our study is based on correlative analyses, emerging evidence suggests that ARGs may influence immune responses via signaling pathways such as STAT3, TGF-β, and NF-κB. For example, COL3A1 and S100A4 may modulate PD-L1 expression through stromal remodeling and inflammatory cytokine activation. Gene-editing techniques and spatial transcriptomics will be able to delineate causal mechanisms in the future. Additionally, single-cell RNA-seq or multiplex immunohistochemistry (mIHC) would be more appropriate for mapping ARG expression in tumor niches and cell-specific interactions. Future studies may incorporate melanoma cell lines or mouse models (e.g., B16-F10) with ARG modulation and immune profiling to evaluate functional roles. In addition, although we did not include a direct analysis of immunotherapy response cohorts the identified ARGs (e.g., TIMP1 and VEGFA) have been independently implicated in resistance to immune checkpoint blockade. Further validation using independent datasets would enhance translational potential.
Our findings support a dual functional model where ARGs such as S100A4, TIMP1, and VEGFA modulate not only angiogenesis, but also immune cell infiltration and checkpoint gene expression. This duality reinforces their relevance as both prognostic and potentially therapeutic biomarkers in melanoma.
In addition, survival analyses were based on gene expression alone, without stratification for mutational profiles, Tumor Mutational Burden (TMB), or treatment history, due to limited metadata availability in TCGA.
In particular, evaluating ARGs within the context of combination immunotherapy and anti-angiogenic strategies could uncover new therapeutic avenues [
56,
57,
58,
59,
60]. Ultimately, integrating ARG expression profiles into clinical decision-making may support more precise, immune-informed treatment strategies for melanoma patients.