Immune Checkpoint Dysregulation in Aneurysmal Subarachnoid Hemorrhage: A Prospective Study of sCTLA-4 and sPD-L1 as Biomarkers of Symptomatic Vasospasm
Abstract
1. Introduction
2. Results
2.1. Study Population
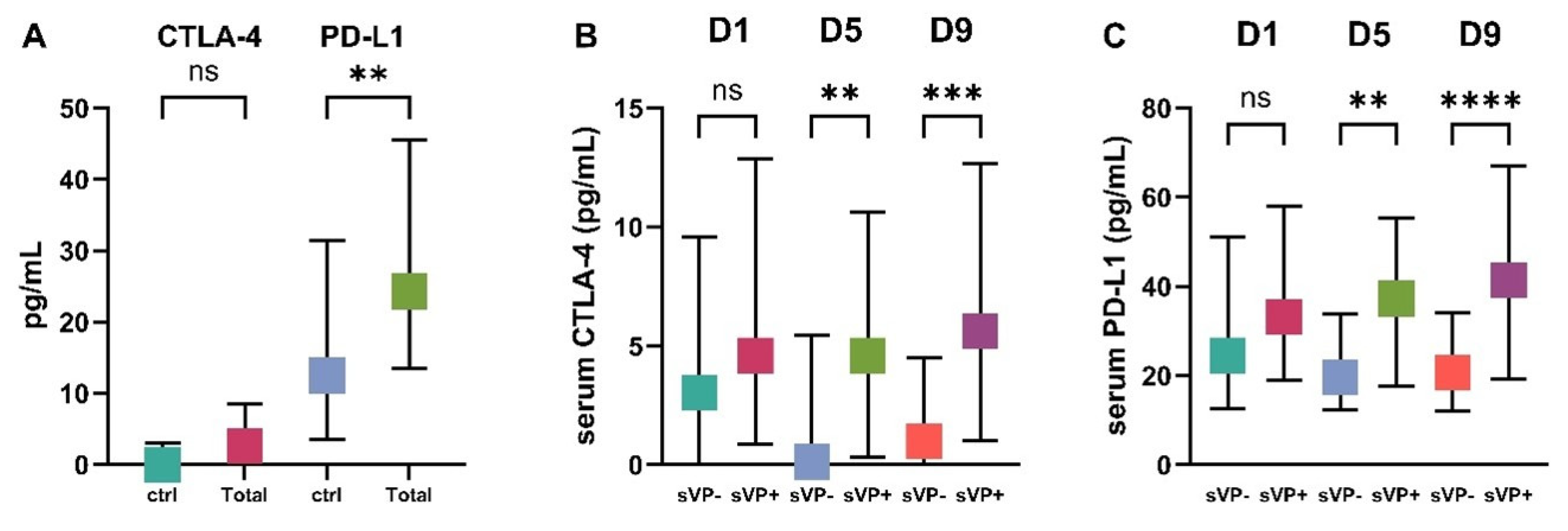
2.2. Serum CTLA-4 and PD-L1 Levels in sVP− and sVP+ Groups
2.3. Hierarchical Clustering of Serum CTLA-4 and PD-L1 Levels over Time
3. Discussion
4. Materials and Methods
4.1. Participants
4.2. Standard Protocol Approvals and Patient Consent
4.3. Serum CTLA-4 and PD-L1 Analysis
4.4. Statistical Analysis
4.5. Data Availability
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| aSAH | Aneurysmal Subarachnoid Hemorrhage |
| IA | Intracranial Aneurysm |
| DCI | Delayed Cerebral Ischemia |
| CTLA-4 | Cytotoxic T-lymphocyte Antigen-4 |
| PD-L1 | Programmed Death-Ligand 1 |
| sCTLA-4 | Soluble Cytotoxic T-lymphocyte Antigen-4 |
| sPD-L1 | Soluble Programmed Death-Ligand 1 |
| mPD-L1 | Membrane-bound Programmed Death-Ligand 1 |
| exoPD-L1 | Exosomal Programmed Death-Ligand 1 |
| MMP | Matrix Metalloproteinase |
| TLR | Toll-like Receptor |
| DAMP | Damage-Associated Molecular Pattern |
| SIRS | Systemic Inflammatory Response Syndrome |
| Treg | Regulatory T Cell |
| Th17 | T-helper 17 Cell |
| WFNS | World Federation of Neurological Societies |
| mRS | Modified Rankin Scale |
| CTA | Computed Tomography Angiography |
| DSA | Digital Subtraction Angiography |
| AVM | Arteriovenous Malformation |
| CRP | C-Reactive Protein |
| SD | Standard Deviation |
| IQR | Interquartile Range |
| NSCLC | Non-Small Cell Lung Cancer |
| CNS | Central Nervous System |
| ROS | Reactive Oxygen Species |
| HLA-DR | Human Leukocyte Antigen-DR isotype |
References
- Macdonald, R.L.; Schweizer, T.A. Spontaneous subarachnoid haemorrhage. Lancet 2017, 389, 655–666. [Google Scholar] [CrossRef] [PubMed]
- Etminan, N.; Macdonald, R.L. Management of aneurysmal subarachnoid hemorrhage. Nat. Rev. Neurol. 2014, 10, 432–442. [Google Scholar] [CrossRef]
- Roos, Y.B.W.E.M.; de Haan, R.J.; Groen, R.J.M.; Albrecht, K.W.; Vermeulen, M. Complications and outcome in patients with aneurysmal subarachnoid haemorrhage: A prospective hospital based cohort study in the Netherlands. J. Neurol. Neurosurg. Psychiatry 2000, 68, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Romoli, M.; Giammello, F.; Mosconi, M.G.; De Mase, A.; De Marco, G.; Digiovanni, A.; Ciacciarelli, A.; Ornello, R.; Storti, B. Immunological Profile of Vasospasm After Subarachnoid Hemorrhage. Int. J. Mol. Sci. 2023, 24, 8856. [Google Scholar] [CrossRef]
- Oaks, M.K.; Hallett, K.M.; Penwell, R.; Stauber, E.C.; Warren, S.J.; Tector, A.J. A native soluble form of CTLA-4. Cell. Immunol. 2000, 201, 144–153. [Google Scholar] [CrossRef]
- Magistrelli, G.; Jeannin, P.; Herbault, N.; Benoit De Coignac, A.; Gauchat, J.F.; Bonnefoy, J.Y.; Delneste, Y. A soluble form of CTLA-4 generated by alternative splicing is expressed by nonstimulated human T cells. Eur. J. Immunol. 1999, 29, 3596–3602. [Google Scholar] [CrossRef]
- Khan, M.; Arooj, S.; Wang, H. Soluble B7-CD28 Family Inhibitory Immune Checkpoint Proteins and Anti-Cancer Immunotherapy. Front. Immunol. 2021, 12, 651634. [Google Scholar] [CrossRef]
- Esposito, L.; Hunter, K.M.D.; Clark, J.; Rainbow, D.B.; Stevens, H.; Denesha, J.; Duley, S.; Dawson, S.; Coleman, G.; Nutland, S.; et al. Investigation of soluble and transmembrane CTLA-4 isoforms in serum and microvesicles. J. Immunol. 2014, 193, 889–900. [Google Scholar] [CrossRef]
- Gerold, K.D.; Zheng, P.; Rainbow, D.B.; Zernecke, A.; Wicker, L.S.; Kissler, S. The soluble CTLA-4 splice variant protects from type 1 diabetes and potentiates regulatory T-cell function. Diabetes 2011, 60, 1955–1963. [Google Scholar] [CrossRef]
- Osaki, M.; Sakaguchi, S. Soluble CTLA-4 regulates immune homeostasis and promotes resolution of inflammation by suppressing type 1 but allowing type 2 immunity. Immunity 2025, 58, 889–908.e13. [Google Scholar] [CrossRef]
- Kim, J.E.; Patel, K.; Jackson, C.M. The potential for immune checkpoint modulators in cerebrovascular injury and inflammation. Expert Opin. Ther. Targets 2021, 25, 101–113. [Google Scholar] [CrossRef]
- Bailly, C.; Thuru, X.; Quesnel, B. Soluble Programmed Death Ligand-1 (sPD-L1): A Pool of Circulating Proteins Implicated in Health and Diseases. Cancers 2021, 13, 3034. [Google Scholar] [CrossRef] [PubMed]
- Dezutter-Dambuyant, C.; Durand, I.; Alberti, L.; Bendriss-Vermare, N.; Valladeau-Guilemond, J.; Duc, A.; Magron, A.; Morel, A.P.; Sisirak, V.; Rodriguez, C.; et al. A novel regulation of PD-1 ligands on mesenchymal stromal cells through MMP-mediated proteolytic cleavage. Oncoimmunology 2015, 5, e1091146. [Google Scholar] [CrossRef] [PubMed]
- Romero, Y.; Wise, R.; Zolkiewska, A. Proteolytic processing of PD-L1 by ADAM proteases in breast cancer cells. Cancer Immunol. Immunother. 2020, 69, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Ashrafizadeh, M.; Zarrabi, A.; Hushmandi, K.; Zarrin, V.; Moghadam, E.R.; Zabolian, A.; Tavakol, S.; Samarghandian, S.; Najafi, M. PD-1/PD-L1 axis regulation in cancer therapy: The role of long non-coding RNAs and microRNAs. Life Sci. 2020, 256, 117899. [Google Scholar] [CrossRef]
- Qu, S.; Jiao, Z.; Lu, G.; Yao, B.; Wang, T.; Rong, W.; Xu, J.; Fan, T.; Sun, X.; Yang, R.; et al. PD-L1 lncRNA splice isoform promotes lung adenocarcinoma progression via enhancing c-Myc activity. Genome Biol. 2021, 22, 104. [Google Scholar] [CrossRef]
- Hassounah, N.B.; Malladi, V.S.; Huang, Y.; Freeman, S.S.; Beauchamp, E.M.; Koyama, S.; Souders, N.; Martin, S.; Dranoff, G.; Wong, K.K.; et al. Identification and characterization of an alternative cancer-derived PD-L1 splice variant. Cancer Immunol. Immunother. 2019, 68, 407–420. [Google Scholar] [CrossRef]
- Raftery, M.J.; Abdelaziz, M.O.; Hofmann, J.; Schönrich, G. Hantavirus-driven PD-L1/PD-L2 upregulation: An imperfect viral immune evasion mechanism. Front. Immunol. 2018, 9, 2560. [Google Scholar] [CrossRef]
- Lee, S.H.; Park, H.J.; Moon, J.Y.; Kim, Y.T.; Lee, K.R.; Kwak, M.J.; Kim, Y.J. Soluble Programmed Cell Death Ligand-1 (sPD-L1) Levels in Various Cancer Types and Normal Populations. Clin. Lab. 2023, 69. [Google Scholar] [CrossRef]
- Zhu, X.; Lang, J. Soluble PD-1 and PD-L1: Predictive and prognostic significance in cancer. Oncotarget 2017, 8, 97671–97682. [Google Scholar] [CrossRef]
- Liu, C.; Jiang, J.; Gao, L.; Wang, X.; Hu, X.; Wu, M.; Wu, J.; Xu, T.; Shi, Q.; Zhang, X. Soluble PD-1 aggravates progression of collagen-induced arthritis through Th1 and Th17 pathways. Arthritis Res. Ther. 2015, 17, 340. [Google Scholar] [CrossRef]
- Greisen, S.R.; Rasmussen, T.K.; Stengaard-Pedersen, K.; Hetland, M.L.; Hørslev-Petersen, K.; Hvid, M.; Deleuran, B. Increased soluble programmed death-1 (sPD-1) is associated with disease activity and radiographic progression in early rheumatoid arthritis. Scand. J. Rheumatol. 2014, 43, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Kawamoto, E.; Masui-Ito, A.; Eguchi, A.; Soe, Z.Y.; Prajuabjinda, O.; Darkwah, S.; Park, E.J.; Imai, H.; Shimaoka, M. Integrin and PD-1 ligand expression on circulating extracellular vesicles in systemic inflammatory response syndrome and sepsis. Shock 2019, 52, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Yoshimoto, Y.; Tanaka, Y.; Hoya, K. Acute systemic inflammatory response syndrome in subarachnoid hemorrhage. Stroke 2001, 32, 1989–1993. [Google Scholar] [CrossRef] [PubMed]
- Kubota, T.; Handa, Y.; Tsuchida, A.; Kaneko, M.; Kobayashi, H. The kinetics of lymphocyte subsets and macrophages in subarachnoid space after subarachnoid hemorrhage in rats. Stroke 1993, 24, 1993–2001. [Google Scholar] [CrossRef]
- Wu, Y.; He, Q.; Wei, Y.; Zhu, J.; He, Z.; Zhang, X.; Guo, Z.; Xu, R.; Cheng, C.; Huang, Z.; et al. The association of neutrophil-to-lymphocyte ratio and delayed cerebral ischemia in patients with aneurysmal subarachnoid hemorrhage: Possible involvement of cerebral blood perfusion. Neuropsychiatr. Dis. Treat. 2019, 15, 1001–1007. [Google Scholar] [CrossRef]
- Neil-Dwyer, G.; Cruickshank, J. The blood leucocyte count and its prognostic significance in subarachnoid haemorrhage. Brain 1974, 97, 79–86. [Google Scholar] [CrossRef]
- Spallone, A.; Acqui, M.; Pastore, F.S.; Guidetti, B. Relationship between leukocytosis and ischemic complications following aneurysmal subarachnoid hemorrhage. Surg. Neurol. 1987, 27, 253–258. [Google Scholar] [CrossRef]
- Pellettieri, L.; Carlson, C.A.; Lindholm, L. Is the vasospasm following subarachnoidal hemorrhage an immunoreactive disease? Experientia 1981, 37, 1170–1171. [Google Scholar] [CrossRef]
- Ostergaard, J.R.; Kristensen, B.Ø.; Svehag, S.-E.; Teisner, B.; Miletic, T. Immune complexes and complement activation following rupture of intracranial saccular aneurysms. J. Neurosurg. 1987, 66, 891–897. [Google Scholar] [CrossRef]
- Hughes, J.T.; Schianchi, P.M. Cerebral artery spasm. A histological study at necropsy of the blood vessels in cases of subarachnoid hemorrhage. J. Neurosurg. 1978, 48, 515–525. [Google Scholar] [CrossRef]
- Hoshi, T.; Shimizu, T.; Kito, K.; Yamasaki, N.; Takahashi, K.; Takahashi, M.; Okada, T.; Kasuya, H.; Kitamura, K. Immunological study of late cerebral vasospasm in subarachnoid hemorrhage. Detection of immunoglobulins, C3, and fibrinogen in cerebral arterial walls by immunofluorescence method. Neurol. Med. Chir. 1984, 24, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, S.R.; Hafez, A.; Jahromi, B.R.; Kinfe, T.M.; Lamprecht, A.; Niemelä, M.; Muhammad, S. Role of damage associated molecular pattern molecules (DAMPs) in aneurysmal subarachnoid hemorrhage (aSAH). Int. J. Mol. Sci. 2018, 19, 2035. [Google Scholar] [CrossRef] [PubMed]
- Pluta, R.M.; Afshar, J.K.B.; Boock, R.J.; Oldfield, E.H. Temporal changes in perivascular concentrations of oxyhemoglobin, deoxyhemoglobin, and methemoglobin after subarachnoid hemorrhage. J. Neurosurg. 1998, 88, 557–561. [Google Scholar] [CrossRef]
- Jackson, C.M.; Choi, J.; Routkevitch, D.; Pant, A.; Saleh, L.; Ye, X.; Caplan, J.M.; Huang, J.; McDougall, C.G.; Pardoll, D.M.; et al. PD-1+ Monocytes Mediate Cerebral Vasospasm Following Subarachnoid Hemorrhage. Neurosurgery 2021, 88, 855–863. [Google Scholar] [CrossRef]
- Zhang, H.-F.; Zhao, M.-G.; Liang, G.-B.; Yu, C.-Y.; Li, Z.-Q.; Gao, X. Downregulation of T cell immunoglobulin and mucin protein 3 in the pathogenesis of intracranial aneurysm. Inflammation 2015, 38, 368–374. [Google Scholar] [CrossRef]
- Zhang, H.-F.; Liang, G.-B.; Zhao, M.-G.; Zhao, G.-F.; Luo, Y.-H. Patients with intracranial aneurysms presented defects in regulatory T cells, which were associated with impairment in Tim-3 upregulation. Int. Immunopharmacol. 2018, 64, 350–355. [Google Scholar] [CrossRef]
- Zhang, H.-F.; Liang, G.-B.; Zhao, M.-G.; Zhao, G.-F.; Luo, Y.-H. Regulatory T cells demonstrate significantly increased functions following stimulation with IL-2 in a Tim-3-dependent manner in intracranial aneurysms. Int. Immunopharmacol. 2018, 65, 342–347. [Google Scholar] [CrossRef]
- Chaudhry, S.R.; Kahlert, U.D.; Kinfe, T.M.; Endl, E.; Dolf, A.; Niemelä, M.; Hänggi, D.; Muhammad, S. Differential polarization and activation dynamics of systemic T helper cell subsets after aneurysmal subarachnoid hemorrhage (SAH) and during post-SAH complications. Sci. Rep. 2021, 11, 14226. [Google Scholar] [CrossRef]
- Feghali, J.; Jackson, C.M. Therapeutic implications for the PD-1 axis in cerebrovascular injury. Neurotherapeutics 2025, 22, e00459. [Google Scholar] [CrossRef]
- Barr, T.L.; VanGilder, R.; Rellick, S.; Brooks, S.D.; Doll, D.N.; Lucke-Wold, A.N.; Chen, D.; Denvir, J.; Warach, S.; Singleton, A.; et al. A genomic profile of the immune response to stroke with implications for stroke recovery. Biol. Res. Nurs. 2015, 17, 248–256. [Google Scholar] [CrossRef]
- Ma, J.; Shen, L.; Bao, L.; Yuan, H.; Wang, Y.; Liu, H.; Wang, Q. A novel prognosis prediction model, including cytotoxic T lymphocyte-associated antigen-4, ischemia-modified albumin, lipoprotein-associated phospholipase A2, glial fibrillary acidic protein, and homocysteine, for ischemic stroke in the Chinese hypertensive population. J. Clin. Lab. Anal. 2021, 35, e23756. [Google Scholar] [CrossRef]
- Kim, J.E.; Lee, R.P.; Yazigi, E.; Atta, L.; Feghali, J.; Pant, A.; Jain, A.; Levitan, I.; Kim, E.; Patel, K.; et al. Soluble PD-L1 reprograms blood monocytes to prevent cerebral edema and facilitate recovery after ischemic stroke. Brain Behav. Immun. 2024, 116, 160–174. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Akiyoshi, K.; Vandenbark, A.A.; Hurn, P.D.; Offner, H. Programmed death-1 pathway limits central nervous system inflammation and neurologic deficits in murine experimental stroke. Stroke 2011, 42, 2578–2583. [Google Scholar] [CrossRef] [PubMed]
- Yao, A.; Liu, F.; Chen, K.; Tang, L.; Liu, L.; Zhang, K.; Yu, C.; Bian, G.; Guo, H.; Zheng, J.; et al. Programmed death 1 deficiency induces the polarization of macrophages/microglia to the M1 phenotype after spinal cord injury in mice. Neurotherapeutics 2014, 11, 636–650. [Google Scholar] [CrossRef] [PubMed]
- Bodhankar, S.; Chen, Y.; Vandenbark, A.A.; Murphy, S.J.; Offner, H. PD-L1 enhances CNS inflammation and infarct volume following experimental stroke in mice in opposition to PD-1. J. Neuroinflamm. 2013, 10, 111. [Google Scholar] [CrossRef]
- Bodhankar, S.; Chen, Y.; Lapato, A.; Dotson, A.L.; Wang, J.; Vandenbark, A.A.; Saugstad, J.A.; Offner, H. PD-L1 monoclonal antibody treats ischemic stroke by controlling central nervous system inflammation. Stroke 2015, 46, 2926–2934. [Google Scholar] [CrossRef]
- Han, R.; Luo, J.; Shi, Y.; Yao, Y.; Hao, J. PD-L1 (programmed death ligand 1) protects against experimental intracerebral hemorrhage-induced brain injury. Stroke 2017, 48, 2255–2262. [Google Scholar] [CrossRef]
- Guo, S.B.; Hu, L.S.; Huang, W.J.; Zhou, Z.Z.; Luo, H.Y.; Tian, X.P. Comparative investigation of neoadjuvant immunotherapy versus adjuvant immunotherapy in perioperative patients with cancer: A global-scale, cross-sectional, and large-sample informatics study. Int. J. Surg. 2024, 110, 4660–4671. [Google Scholar] [CrossRef]
- Vergouwen, M.D.; Vermeulen, M.; van Gijn, J.; Rinkel, G.J.; Wijdicks, E.F.; Muizelaar, J.P.; Mendelow, A.D.; Juvela, S.; Yonas, H.; Terbrugge, K.G.; et al. Definition of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage as an outcome event in clinical trials and observational studies: Proposal of a multidisciplinary research group. Stroke 2010, 41, 2391–2395. [Google Scholar] [CrossRef]
- Gémes, N.; Balog, J.Á.; Neuperger, P.; Schlegl, E.; Barta, I.; Fillinger, J.; Antus, B.; Zvara, Á.; Hegedűs, Z.; Czimmerer, Z.; et al. Single-cell immunophenotyping revealed the association of CD4+ central and CD4+ effector memory T cells linking exacerbating chronic obstructive pulmonary disease and NSCLC. Front. Immunol. 2023, 14, 1297577. [Google Scholar] [CrossRef] [PubMed]
- Neuperger, P.; Szalontai, K.; Gémes, N.; Balog, J.Á.; Tiszlavicz, L.; Furák, J.; Lázár, G.; Puskás, L.G.; Szebeni, G.J. Single-cell mass cytometric analysis of peripheral immunity and multiplex plasma marker profiling of non-small cell lung cancer patients receiving PD-1 targeting immune checkpoint inhibitors in comparison with platinum-based chemotherapy. Front. Immunol. 2023, 14, 1243233. [Google Scholar] [CrossRef]

| Variable | Total (n = 179) | sVP− (n = 131) | sVP+ (n = 48) | p-Value |
|---|---|---|---|---|
| Age (mean ± SD) | 57.5 ± 12 | 58 ± 13 | 56 ± 12 | 0.374 |
| Female, N (%) | 130 (72) | 93 (71) | 37 (76) | 0.597 |
| Hypertension, N (%) | 95 (53) | 67 (51) | 28 (58) | 0.432 |
| Diabetes, N (%) | 18 (10) | 11 (8) | 6 (13) | 0.425 |
| Smoking, N (%) | 52 (29) | 39 (30) | 13 (27) | 0.863 |
| WFNS, median (IQR) | 2 (1–4) | 2 (1–4) | 2 (1–5) | 0.673 |
| mFisher score, median (IQR) | 3 (2–3) | 3 (2–3.5) | 3 (2–3) | 0.290 |
| Loss of consciousness during ictus, N (%) | 68 (38) | 50 (38) | 18 (38) | 1.000 |
| Seizure during ictus, N (%) | 16 (9) | 11 (9) | 5 (11) | 0.595 |
| Aneurysm location, N (%) | ||||
| internal carotid artery | 15 (9) | 10 (8) | 5 (11) | |
| middle cerebral artery | 51 (28) | 39 (30) | 12 (25) | |
| anterior communicating artery | 61 (34) | 42 (33) | 19 (39) | |
| posterior communicating artery | 20 (11) | 15 (12) | 5 (10) | |
| anterior cerebral artery | 7 (4) | 5 (4) | 2 (5) | |
| vertebrobasilar | 25 (14) | 20 (15) | 5 (10) | |
| C-reactive protein a, mg/L, median (IQR) | 15.3 (4–55) | 12 (4–60) | 22 (3–53) | 0.550 |
| Creatinine a, µmol/L, median (IQR) | 60 (49–72) | 61 (51–74) | 59 (43–70) | 0.100 |
| White blood cell count a, G/L, median (IQR) | 11 (9–13) | 11 (9–13) | 11 (9–15) | 0.317 |
| Neutrophile-lymphocyte ratio a, median (IQR) | 5.5 (4–10) | 5.4 (3–10) | 6.5 (4–10) | 0.271 |
| Lumbal drain, N (%) | 90 (50) | 61 (47) | 29 (62) | 0.083 |
| Mechanical ventillation, N (%) | 81 (45) | 58 (44) | 23 (49) | 0.532 |
| Decompressive craniotomy, N (%) | 18 (10) | 14 (11) | 4 (9) | 0.802 |
| Extraventricular drainage, N (%) | 81 (46) | 56 (43) | 25 (51) | 0.346 |
| Infection, N (%) | 56 (31) | 34 (26) | 22 (46) | 0.011 |
| pg/mL | sVP− (n = 131) Median (IQR) | sVP+ (n = 48) Median (IQR) | Ctrl (n = 50) Median (IQR) | p-Value (sVP− vs. sVP+) | p-Value (sVP− vs. Ctrl) | p-Value (sVP+ vs. Ctrl) |
|---|---|---|---|---|---|---|
| CTLA-4-D1 | 3 (0–9.6) | 4.6 (1–12.5) | 0 (0–3.1) | NS | 0.073 | 0.002 |
| CTLA-4-D5 | 0.1 (0–5.1) | 4.6 (0.3–10.6) | 0 (0–3.1) | 0.001 | NS | 0.001 |
| CTLA-4-D9 | 1 (0–4.5) | 5.6 (1–12.7) | 0 (0–3.1) | <0.001 | NS | <0.001 |
| PD-L1-D1 | 24.5 (12.7–51.1) | 33.2 (19.4–54.2) | 12.5 (3.6–31.5) | NS | 0.006 | <0.001 |
| PD-L1-D5 | 19.7 (12.5–33.9) | 37.3 (17.6–55.4) | 12.5 (3.6–31.5) | 0.001 | NS | <0.001 |
| PD-L1-D9 | 20.7 (12.1–34.1) | 41.4 (19.3–67) | 12.5 (3.6–31.5) | <0.001 | NS | <0.001 |
| pg/mL | sVP−/Inf− | sVP−/Inf+ | sVP+/Inf− | sVP+/Inf+ | p-Value (sVP+/Inf− vs. sVP+/Inf+) |
|---|---|---|---|---|---|
| CTLA4D5 | 0.73 | 0.00 | 5.29 | 2.48 | NS |
| CTLA4D9 | 1.48 | 0.01 | 4.11 | 6.41 | NS |
| PDL1D5 | 19.86 | 16.59 | 38.29 | 37.30 | NS |
| PDL1D9 | 22.49 | 17.61 | 41.52 | 40.57 | NS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Varnai, R.; Szebeni, G.J.; Gémes, N.; Schwarcz, A.; Molnar, T.; Olah, C.; Csecsei, P. Immune Checkpoint Dysregulation in Aneurysmal Subarachnoid Hemorrhage: A Prospective Study of sCTLA-4 and sPD-L1 as Biomarkers of Symptomatic Vasospasm. Int. J. Mol. Sci. 2025, 26, 8228. https://doi.org/10.3390/ijms26178228
Varnai R, Szebeni GJ, Gémes N, Schwarcz A, Molnar T, Olah C, Csecsei P. Immune Checkpoint Dysregulation in Aneurysmal Subarachnoid Hemorrhage: A Prospective Study of sCTLA-4 and sPD-L1 as Biomarkers of Symptomatic Vasospasm. International Journal of Molecular Sciences. 2025; 26(17):8228. https://doi.org/10.3390/ijms26178228
Chicago/Turabian StyleVarnai, Reka, Gábor J. Szebeni, Nikolett Gémes, Attila Schwarcz, Tihamer Molnar, Csaba Olah, and Peter Csecsei. 2025. "Immune Checkpoint Dysregulation in Aneurysmal Subarachnoid Hemorrhage: A Prospective Study of sCTLA-4 and sPD-L1 as Biomarkers of Symptomatic Vasospasm" International Journal of Molecular Sciences 26, no. 17: 8228. https://doi.org/10.3390/ijms26178228
APA StyleVarnai, R., Szebeni, G. J., Gémes, N., Schwarcz, A., Molnar, T., Olah, C., & Csecsei, P. (2025). Immune Checkpoint Dysregulation in Aneurysmal Subarachnoid Hemorrhage: A Prospective Study of sCTLA-4 and sPD-L1 as Biomarkers of Symptomatic Vasospasm. International Journal of Molecular Sciences, 26(17), 8228. https://doi.org/10.3390/ijms26178228








