Sex-Specific Differences in Adipose IRF5 Expression and Its Association with Inflammation and Insulin Resistance in Obesity
Abstract
1. Introduction
2. Results
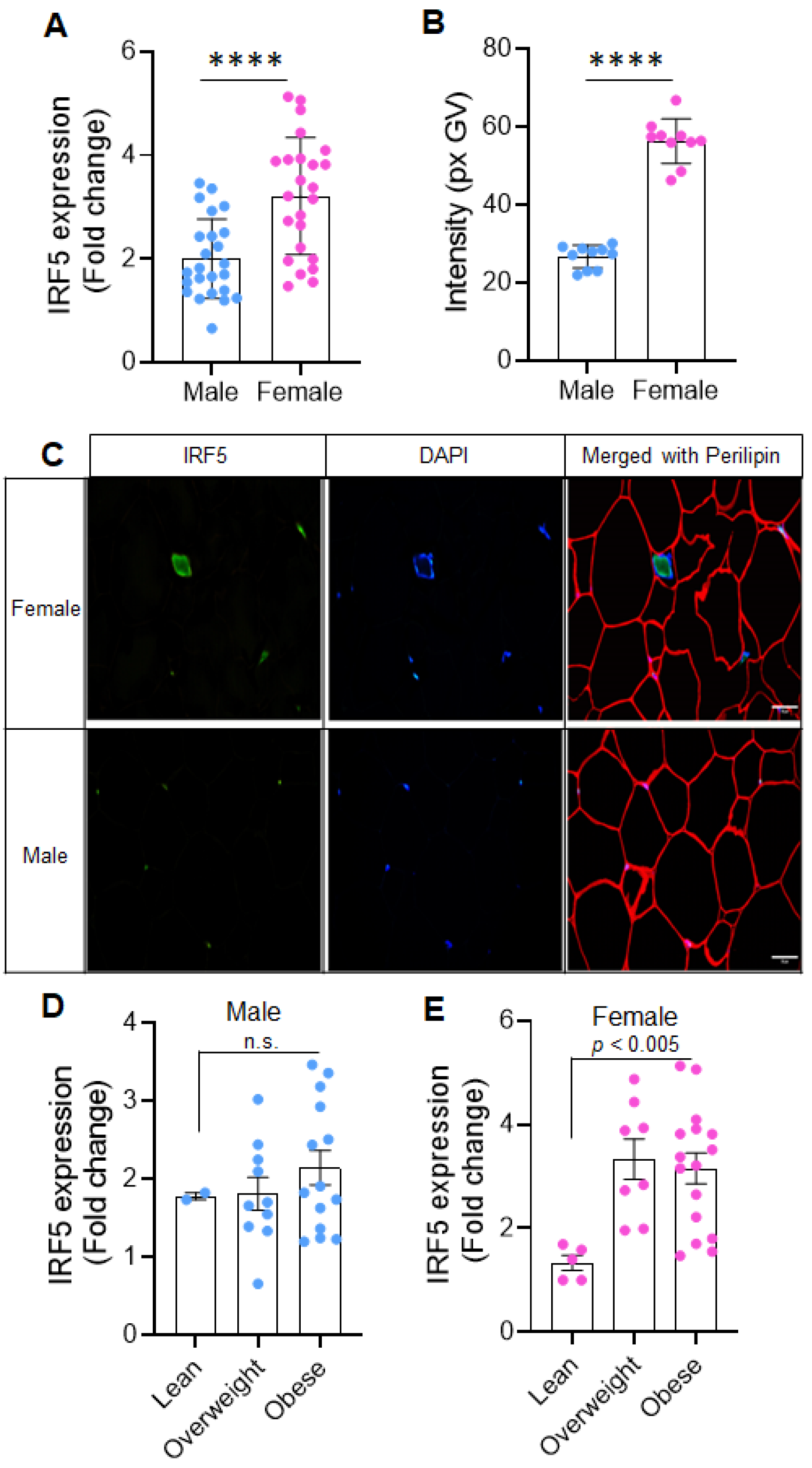
2.1. Sex-Related Differences in Adipose IRF5 Expression
2.2. Sex-Independent Correlation of IRF5 with Core Immune and Inflammatory Markers
2.3. Sex-Specific Associations of AT IRF5 with Inflammatory Markers
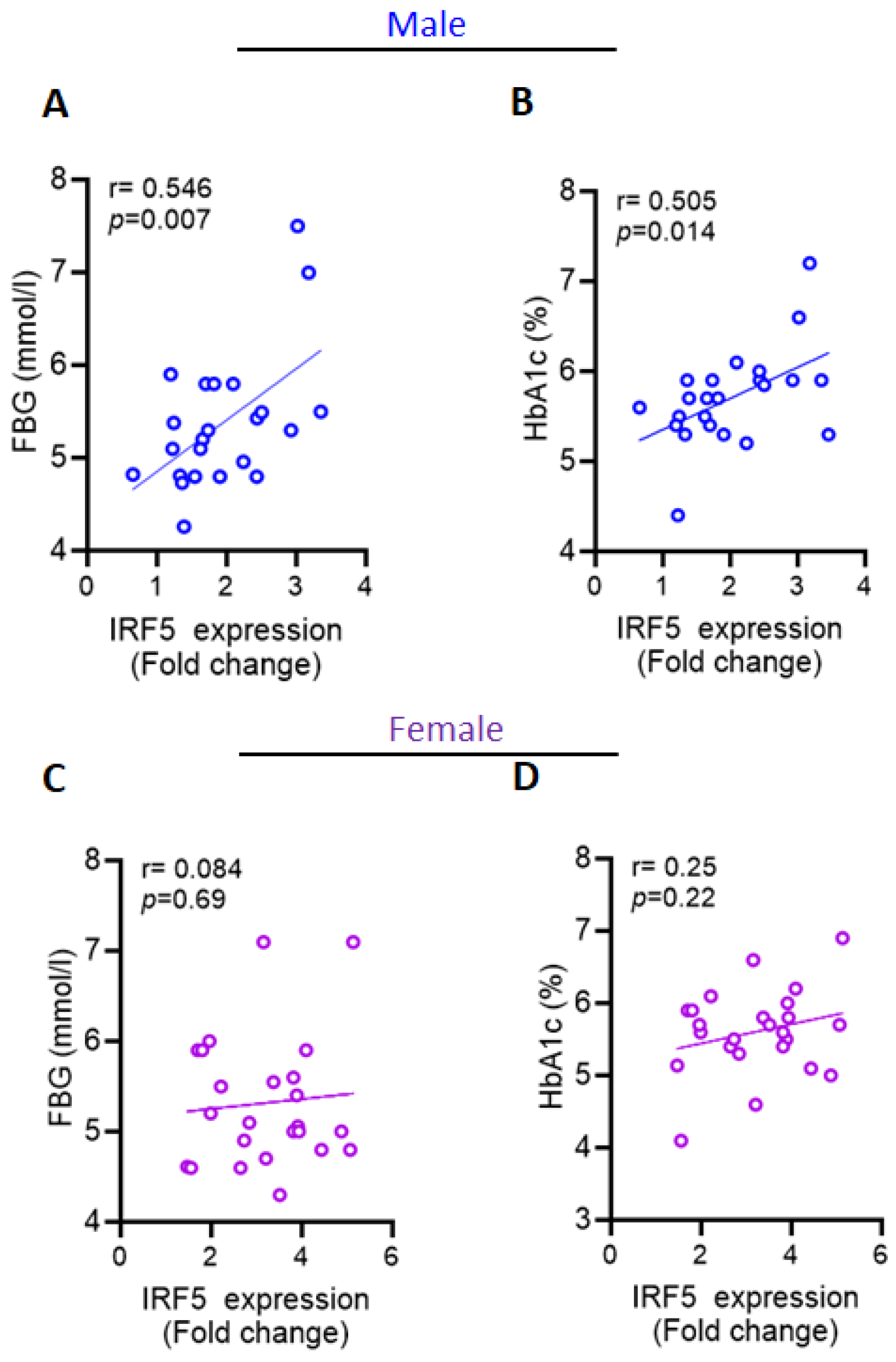
2.4. Increased AT IRF5 Gene Expression in Obesity Correlates with Diabetes Markers in Males
2.5. Independent Predictors of IRF5 Expression
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. Anthropometric and Physio-Clinical Measurements
4.3. Adipose Tissue Collection and Processing
4.4. Real-Time Reverse-Transcription Polymerase Chain Reaction (RT-PCR)
4.5. Immunofluorescence Microscopy
4.6. Statistical Analysis
5. Conclusions
6. Limitations and Future Directions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rohm, T.V.; Meier, D.T.; Olefsky, J.M.; Donath, M.Y. Inflammation in obesity, diabetes, and related disorders. Immunity 2022, 55, 31–55. [Google Scholar] [CrossRef] [PubMed]
- Alzaid, F.; Fagherazzi, G.; Riveline, J.P.; Bahman, F.; Al-Rashed, F.; Al-Mulla, F.; Ahmad, R. Immune cell-adipose tissue crosstalk in metabolic diseases with a focus on type 1 diabetes. Diabetologia 2025, 68, 1616–1631. [Google Scholar] [CrossRef] [PubMed]
- Weisberg, S.P.; McCann, D.; Desai, M.; Rosenbaum, M.; Leibel, R.L.; Ferrante, A.W., Jr. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Investig. 2003, 112, 1796–1808. [Google Scholar] [CrossRef]
- Xu, H.; Barnes, G.T.; Yang, Q.; Tan, G.; Yang, D.; Chou, C.J.; Sole, J.; Nichols, A.; Ross, J.S.; Tartaglia, L.A.; et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J. Clin. Investig. 2003, 112, 1821–1830. [Google Scholar] [CrossRef]
- Tramunt, B.; Smati, S.; Grandgeorge, N.; Lenfant, F.; Arnal, J.F.; Montagner, A.; Gourdy, P. Sex differences in metabolic regulation and diabetes susceptibility. Diabetologia 2020, 63, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Karastergiou, K.; Smith, S.R.; Greenberg, A.S.; Fried, S.K. Sex differences in human adipose tissues—The biology of pear shape. Biol. Sex Differ. 2012, 3, 13. [Google Scholar] [CrossRef]
- Nauli, A.M.; Matin, S. Why Do Men Accumulate Abdominal Visceral Fat? Front. Physiol. 2019, 10, 1486. [Google Scholar] [CrossRef]
- Blaak, E. Gender differences in fat metabolism. Curr. Opin. Clin. Nutr. Metab. Care 2001, 4, 499–502. [Google Scholar] [CrossRef]
- Kim, H.; Kim, S.-E.; Sung, M.-K. Sex and Gender Differences in Obesity: Biological, Sociocultural, and Clinical Perspectives. World J. Mens. Health 2025, 43, e49. [Google Scholar] [CrossRef]
- Zhang, Z.; He, Z.; Yang, H.; Li, D.; Duan, P.; Wei, X. The Accumulation of Visceral Fat in Postmenopausal Women: The Combined Impact of Prenatal Genetics, Epigenetics, and Fat Depot Heterogeneity—A Descriptive Review. Clin. Exp. Obstet. Gynecol. 2025, 52, 26194. [Google Scholar] [CrossRef]
- Hu, G.-B.; Lou, H.-M.; Dong, X.-Z.; Liu, Q.-M.; Zhang, S.-C. Characteristics of the interferon regulatory factor 5 (IRF5) and its expression in response to LCDV and poly I:C challenges in Japanese flounder, Paralichthys olivaceus. Dev. Comp. Immunol. 2012, 38, 377–382. [Google Scholar] [CrossRef]
- Yanai, H.; Chen, H.M.; Inuzuka, T.; Kondo, S.; Mak, T.W.; Takaoka, A.; Honda, K.; Taniguchi, T. Role of IFN regulatory factor 5 transcription factor in antiviral immunity and tumor suppression. Proc. Natl. Acad. Sci. USA 2007, 104, 3402–3407. [Google Scholar] [CrossRef] [PubMed]
- McNab, F.; Mayer-Barber, K.; Sher, A.; Wack, A.; O’Garra, A. Type I interferons in infectious disease. Nat. Rev. Immunol. 2015, 15, 87–103. [Google Scholar] [CrossRef] [PubMed]
- Krausgruber, T.; Blazek, K.; Smallie, T.; Alzabin, S.; Lockstone, H.; Sahgal, N.; Hussell, T.; Feldmann, M.; Udalova, I.A. IRF5 promotes inflammatory macrophage polarization and TH1-TH17 responses. Nat. Immunol. 2011, 12, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Eguchi, J.; Kong, X.; Tenta, M.; Wang, X.; Kang, S.; Rosen, E.D. Interferon regulatory factor 4 regulates obesity-induced inflammation through regulation of adipose tissue macrophage polarization. Diabetes 2013, 62, 3394–3403. [Google Scholar] [CrossRef]
- Mamun, A.A.; Liu, F. Role of IRF4-Mediated Inflammation: Implication in Neurodegenerative Diseases. Neurol. Neurother. Open Access J. 2017, 2, 107. [Google Scholar] [CrossRef]
- Dalmas, E.; Toubal, A.; Alzaid, F.; Blazek, K.; Eames, H.L.; Lebozec, K.; Pini, M.; Hainault, I.; Montastier, E.; Denis, R.G.; et al. Irf5 deficiency in macrophages promotes beneficial adipose tissue expansion and insulin sensitivity during obesity. Nat. Med. 2015, 21, 610–618. [Google Scholar] [CrossRef]
- Yunna, C.; Mengru, H.; Lei, W.; Weidong, C. Macrophage M1/M2 polarization. Eur. J. Pharmacol. 2020, 877, 173090. [Google Scholar] [CrossRef]
- Wang, L.; Zhu, Y.; Zhang, N.; Xian, Y.; Tang, Y.; Ye, J.; Reza, F.; He, G.; Wen, X.; Jiang, X. The multiple roles of interferon regulatory factor family in health and disease. Signal Transduct. Target. Ther. 2024, 9, 282. [Google Scholar] [CrossRef]
- Sindhu, S.; Kochumon, S.; Thomas, R.; Bennakhi, A.; Al-Mulla, F.; Ahmad, R. Enhanced Adipose Expression of Interferon Regulatory Factor (IRF)-5 Associates with the Signatures of Metabolic Inflammation in Diabetic Obese Patients. Cells 2020, 9, 730. [Google Scholar] [CrossRef]
- Sindhu, S.; Thomas, R.; Kochumon, S.; Wilson, A.; Abu-Farha, M.; Bennakhi, A.; Al-Mulla, F.; Ahmad, R. Increased Adipose Tissue Expression of Interferon Regulatory Factor (IRF)-5 in Obesity: Association with Metabolic Inflammation. Cells 2019, 8, 1418. [Google Scholar] [CrossRef] [PubMed]
- Griesbeck, M.; Ziegler, S.; Laffont, S.; Smith, N.; Chauveau, L.; Tomezsko, P.; Sharei, A.; Kourjian, G.; Porichis, F.; Hart, M.; et al. Sex Differences in Plasmacytoid Dendritic Cell Levels of IRF5 Drive Higher IFN-α Production in Women. J. Immunol. 2015, 195, 5327–5336. [Google Scholar] [CrossRef] [PubMed]
- Iyer, S.S.; Cheng, G. Role of interleukin 10 transcriptional regulation in inflammation and autoimmune disease. Crit. Rev. Immunol. 2012, 32, 23–63. [Google Scholar] [CrossRef]
- Saraiva, M.; O’Garra, A. The regulation of IL-10 production by immune cells. Nat. Rev. Immunol. 2010, 10, 170–181. [Google Scholar] [CrossRef] [PubMed]
- Klein, S.L.; Flanagan, K.L. Sex differences in immune responses. Nat. Rev. Immunol. 2016, 16, 626–638. [Google Scholar] [CrossRef]
- Ghosh, A.R.; Bhattacharya, R.; Bhattacharya, S.; Nargis, T.; Rahaman, O.; Duttagupta, P.; Raychaudhuri, D.; Liu, C.S.; Roy, S.; Ghosh, P.; et al. Adipose Recruitment and Activation of Plasmacytoid Dendritic Cells Fuel Metaflammation. Diabetes 2016, 65, 3440–3452. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. The role of pattern-recognition receptors in innate immunity: Update on Toll-like receptors. Nat. Immunol. 2010, 11, 373–384. [Google Scholar] [CrossRef]
- Ahmad, Z.; Kahloan, W.; Rosen, E.D. Transcriptional control of metabolism by interferon regulatory factors. Nat. Rev. Endocrinol. 2024, 20, 573–587. [Google Scholar] [CrossRef]
- Lumeng, C.N.; Saltiel, A.R. Inflammatory links between obesity and metabolic disease. J. Clin. Investig. 2011, 121, 2111–2117. [Google Scholar] [CrossRef]
- de Bruijn, M.; Dzierzak, E. Runx transcription factors in the development and function of the definitive hematopoietic system. Blood 2017, 129, 2061–2069. [Google Scholar] [CrossRef]
- Regitz-Zagrosek, V.; Oertelt-Prigione, S.; Seeland, U.; Hetzer, R. Sex and gender differences in myocardial hypertrophy and heart failure. Circ. J. Off. J. Jpn. Circ. Soc. 2010, 74, 1265–1273. [Google Scholar] [CrossRef] [PubMed]
- Lovejoy, J.C.; Sainsbury, A. Sex differences in obesity and the regulation of energy homeostasis. Obes. Rev. Off. J. Int. Assoc. Study Obes. 2009, 10, 154–167. [Google Scholar] [CrossRef]
- Gannon, M.; Kulkarni, R.N.; Tse, H.M.; Mauvais-Jarvis, F. Sex differences underlying pancreatic islet biology and its dysfunction. Mol. Metab. 2018, 15, 82–91. [Google Scholar] [CrossRef]
- Bastard, J.P.; Maachi, M.; Lagathu, C.; Kim, M.J.; Caron, M.; Vidal, H.; Capeau, J.; Feve, B. Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur. Cytokine Netw. 2006, 17, 4–12. [Google Scholar]
- Sindhu, S.; Thomas, R.; Shihab, P.; Sriraman, D.; Behbehani, K.; Ahmad, R. Obesity Is a Positive Modulator of IL-6R and IL-6 Expression in the Subcutaneous Adipose Tissue: Significance for Metabolic Inflammation. PLoS ONE 2015, 10, e0133494. [Google Scholar] [CrossRef]
- Kochumon, S.; Al-Rashed, F.; Abu-Farha, M.; Devarajan, S.; Tuomilehto, J.; Ahmad, R. Adipose tissue expression of CCL19 chemokine is positively associated with insulin resistance. Diabetes Metab. Res. Rev. 2019, 35, e3087. [Google Scholar] [CrossRef] [PubMed]
- Albeloushi, S.; Hasan, A.; Arefanian, H.; Sindhu, S.; Al-Rashed, F.; Kochumon, S.; Abukhalaf, N.; Jacob, T.; Shenouda, S.; Al Madhoun, A.; et al. Differential effects of fish-oil and cocoa-butter based high-fat/high-sucrose diets on endocrine pancreas morphology and function in mice. Front. Endocrinol. 2024, 15, 1265799. [Google Scholar] [CrossRef]
- Albeloushi, S.M. Effect of Branched-Chain Amino Acid Supplements on Pancreatic Development in Preterm Lambs. Ph.D. Thesis, The University of Auckland, Auckland, New Zealand, 2022. [Google Scholar]
- Cizkova, K.; Foltynkova, T.; Gachechiladze, M.; Tauber, Z. Comparative Analysis of Immunohistochemical Staining Intensity Determined by Light Microscopy, ImageJ and QuPath in Placental Hofbauer Cells. Acta Histochem. Cytochem. 2021, 54, 21–29. [Google Scholar] [CrossRef]
- Duplancic, R.; Kero, D. Novel approach for quantification of multiple immunofluorescent signals using histograms and 2D plot profiling of whole-section panoramic images. Sci. Rep. 2021, 11, 8619. [Google Scholar] [CrossRef] [PubMed]
| Inflammatory Markers | Male (Overweight + Obese) | Female (Overweight + Obese) | ||
|---|---|---|---|---|
| r-Value | p-Value | r-Value | p-Value | |
| Cytokines | ||||
| IL2 | 0.413 | 0.045 * | 0.277 | 0.212 |
| IL6 | 0.370 | 0.075 | 0.068 | 0.765 |
| IL8 | 0.458 | 0.032 * | 0.208 | 0.352 |
| IL10 | 0.107 | 0.635 | 0.542 | 0.008 ** |
| IL33 | 0.148 | 0.489 | 0.114 | 0.613 |
| TNFα | 0.474 | 0.019 * | 0.359 | 0.101 |
| TGFβ | 0.207 | 0.343 | 0.079 | 0.727 |
| CC and CXC chemokine ligands and receptors | ||||
| CCL2 | 0.251 | 0.236 | 0.197 | 0.355 |
| CCL5 | 0.394 | 0.077 | 0.265 | 0.273 |
| CCL7 | 0.255 | 0.241 | 0.530 | 0.009 ** |
| CCL19 | 0.368 | 0.084 | 0.195 | 0.396 |
| CXCL10 | −0.007 | 0.975 | 0.410 | 0.052 |
| CXCL11 | 0.137 | 0.534 | 0.431 | 0.040 * |
| Dectin-1 | 0.328 | 0.126 | 0.441 | 0.040 * |
| CCR1 | 0.031 | 0.895 | 0.369 | 0.083 |
| CCR2 | 0.403 | 0.070 | 0.375 | 0.085 |
| CCR5 | 0.434 | 0.034 * | 0.440 | 0.032 * |
| Macrophage markers | ||||
| CD11c | 0.677 | 0.000 ** | 0.639 | 0.001 ** |
| CD16 | 0.578 | 0.003 ** | 0.44 | 0.040 * |
| CD68 | 0.394 | 0.057 | 0.701 | 0.000 ** |
| CD86 | 0.195 | 0.385 | 0.637 | 0.001 ** |
| CD141 | 0.464 | 0.026 * | 0.032 | 0.883 |
| CD163 | 0.711 | 0.000 ** | 0.534 ** | 0.009 ** |
| Toll-like receptors (TLRs) signaling cascade | ||||
| TLR2 | 0.108 | 0.633 | 0.549 | 0.012 * |
| TLR4 | 0.385 | 0.094 | 0.257 | 0.262 |
| TLR8 | 0.169 | 0.431 | 0.751 | 0.000 ** |
| TLR9 | 0.355 | 0.089 | 0.432 | 0.035 * |
| TLR10 | 0.019 | 0.931 | 0.442 | 0.051 |
| MyD88 | 0.495 | 0.014 * | 0.584 ** | 0.003 |
| IRAK1 | 0.548 | 0.006 ** | 0.304 | 0.158 |
| TRAF6 | 0.087 | 0.688 | 0.087 | 0.687 |
| Transcription factors | ||||
| FOXP3 | 0.548 | 0.006 ** | 0.464 | 0.030 * |
| RUNX1 | 0.507 | 0.011 * | 0.681 | 0.000 ** |
| IRF4 | 0.110 | 0.626 | 0.414 | 0.044 * |
| IRF3 | 0.383 | 0.096 | 0.265 | 0.246 |
| Metabolic Markers | Male (Overweight + Obese) | Female (Overweight + Obese) | ||
|---|---|---|---|---|
| r-Value | p-Value | r-Value | p-Value | |
| Weight (kg) | 0.366 | 0.079 | −0.119 | 0.579 |
| Height (cm) | 0.347 | 0.097 | −0.158 | 0.461 |
| BMI (kg/m2) | 0.207 | 0.333 | −0.012 | 0.955 |
| PBF (number) | 0.179 | 0.463 | 0.002 | 0.992 |
| Waist (cm) | 0.412 | 0.071 | −0.075 | 0.741 |
| Cholestrol (mmol/L) | 0.121 | 0.573 | 0.136 | 0.525 |
| HDL (mmol/L) | −0.171 | 0.424 | −0.203 | 0.341 |
| LDL (mmol/L) | 0.014 | 0.947 | 0.230 | 0.279 |
| TGL (mmol/L) | 0.304 | 0.148 | −0.257 | 0.226 |
| FBG (mmol/L) | 0.546 | 0.007 ** | 0.084 | 0.696 |
| HbA1c (%) | 0.505 | 0.014 * | 0.255 | 0.229 |
| Insulin (mU/L) | 0.617 | 0.014 * | −0.363 | 0.167 |
| HOMA-IR | 0.657 | 0.008 ** | −0.283 | 0.289 |
| Adiponectin (ng/mL) | −0.543 | 0.068 | −0.371 | 0.130 |
| RANTES (ng/mL) | 0.515 | 0.086 | 0.073 | 0.774 |
| ANOVA (Sig) R2 = 082; p < 0.0001 | ||||
|---|---|---|---|---|
| Predictor Variable | Male | Female | ||
| Scandalized Confinement (β) | p-Value | Scandalized Confinement (β) | p-Value | |
| IRAK1 | 0.570 | 0.006 | _ | _ |
| HOMA-IR | 7.548 | 0.001 | _ | _ |
| MyD88 | 0.512 | 0.003 | 0.288 | 0.030 |
| RUNX1 | _ | _ | 0.399 | 0.005 |
| CD68 | _ | _ | 0.333 | 0.016 |
| CCL7 | _ | _ | 0.313 | 0.017 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kochumon, S.; Benobaid, N.; Al Madhoun, A.; Albeloushi, S.; Almansour, N.; Al-Rashed, F.; Sindhu, S.; Al-Mulla, F.; Ahmad, R. Sex-Specific Differences in Adipose IRF5 Expression and Its Association with Inflammation and Insulin Resistance in Obesity. Int. J. Mol. Sci. 2025, 26, 8229. https://doi.org/10.3390/ijms26178229
Kochumon S, Benobaid N, Al Madhoun A, Albeloushi S, Almansour N, Al-Rashed F, Sindhu S, Al-Mulla F, Ahmad R. Sex-Specific Differences in Adipose IRF5 Expression and Its Association with Inflammation and Insulin Resistance in Obesity. International Journal of Molecular Sciences. 2025; 26(17):8229. https://doi.org/10.3390/ijms26178229
Chicago/Turabian StyleKochumon, Shihab, Noelle Benobaid, Ashraf Al Madhoun, Shaima Albeloushi, Nourah Almansour, Fatema Al-Rashed, Sardar Sindhu, Fahd Al-Mulla, and Rasheed Ahmad. 2025. "Sex-Specific Differences in Adipose IRF5 Expression and Its Association with Inflammation and Insulin Resistance in Obesity" International Journal of Molecular Sciences 26, no. 17: 8229. https://doi.org/10.3390/ijms26178229
APA StyleKochumon, S., Benobaid, N., Al Madhoun, A., Albeloushi, S., Almansour, N., Al-Rashed, F., Sindhu, S., Al-Mulla, F., & Ahmad, R. (2025). Sex-Specific Differences in Adipose IRF5 Expression and Its Association with Inflammation and Insulin Resistance in Obesity. International Journal of Molecular Sciences, 26(17), 8229. https://doi.org/10.3390/ijms26178229









