Molecular Mechanisms of Phthalates in Depression: An Analysis Based on Network Toxicology and Molecular Docking
Abstract
1. Introduction
2. Results
2.1. Target Retrieval
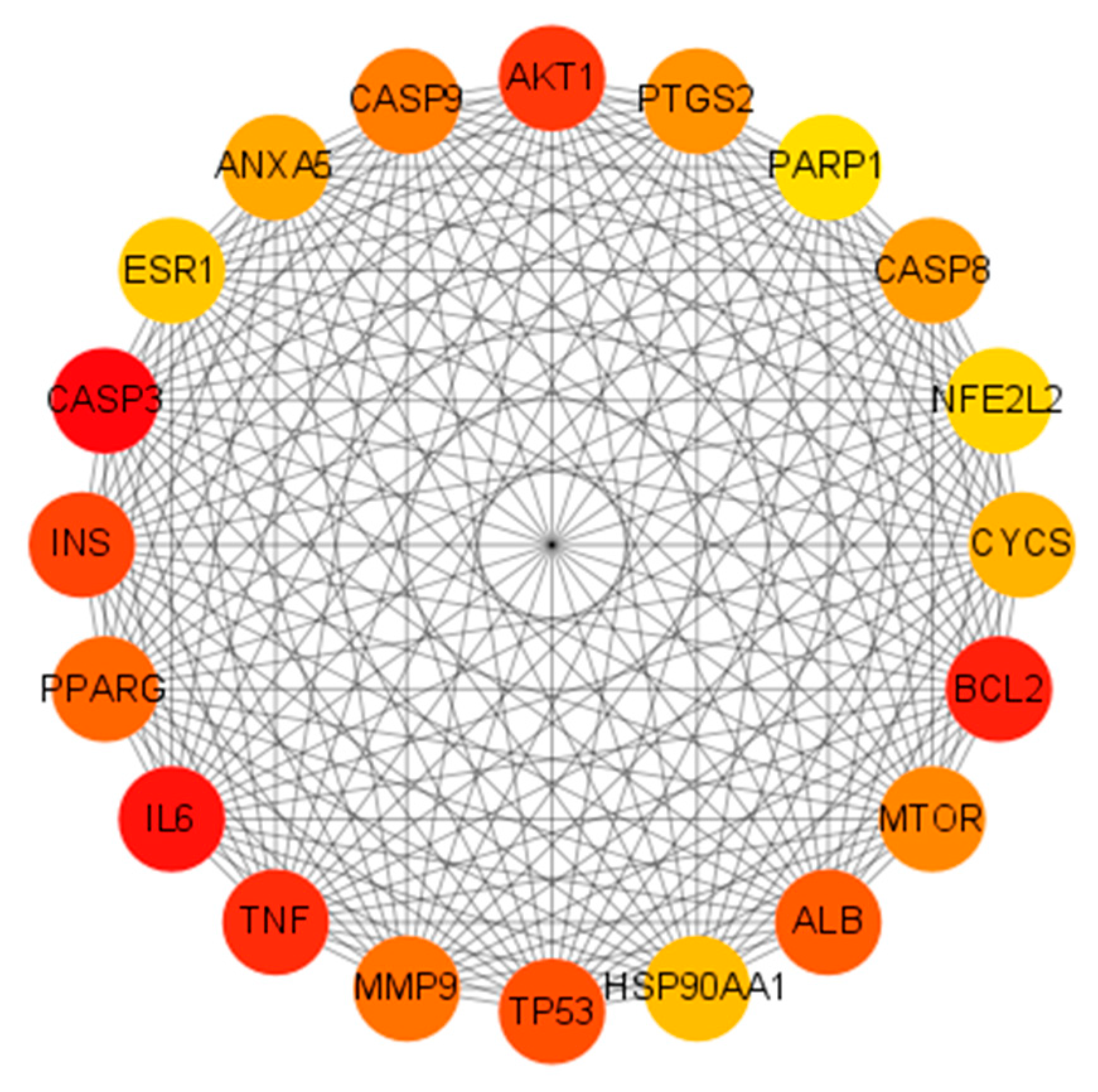
2.2. PPI Network of Shared Genes and Discovery of Core Targets
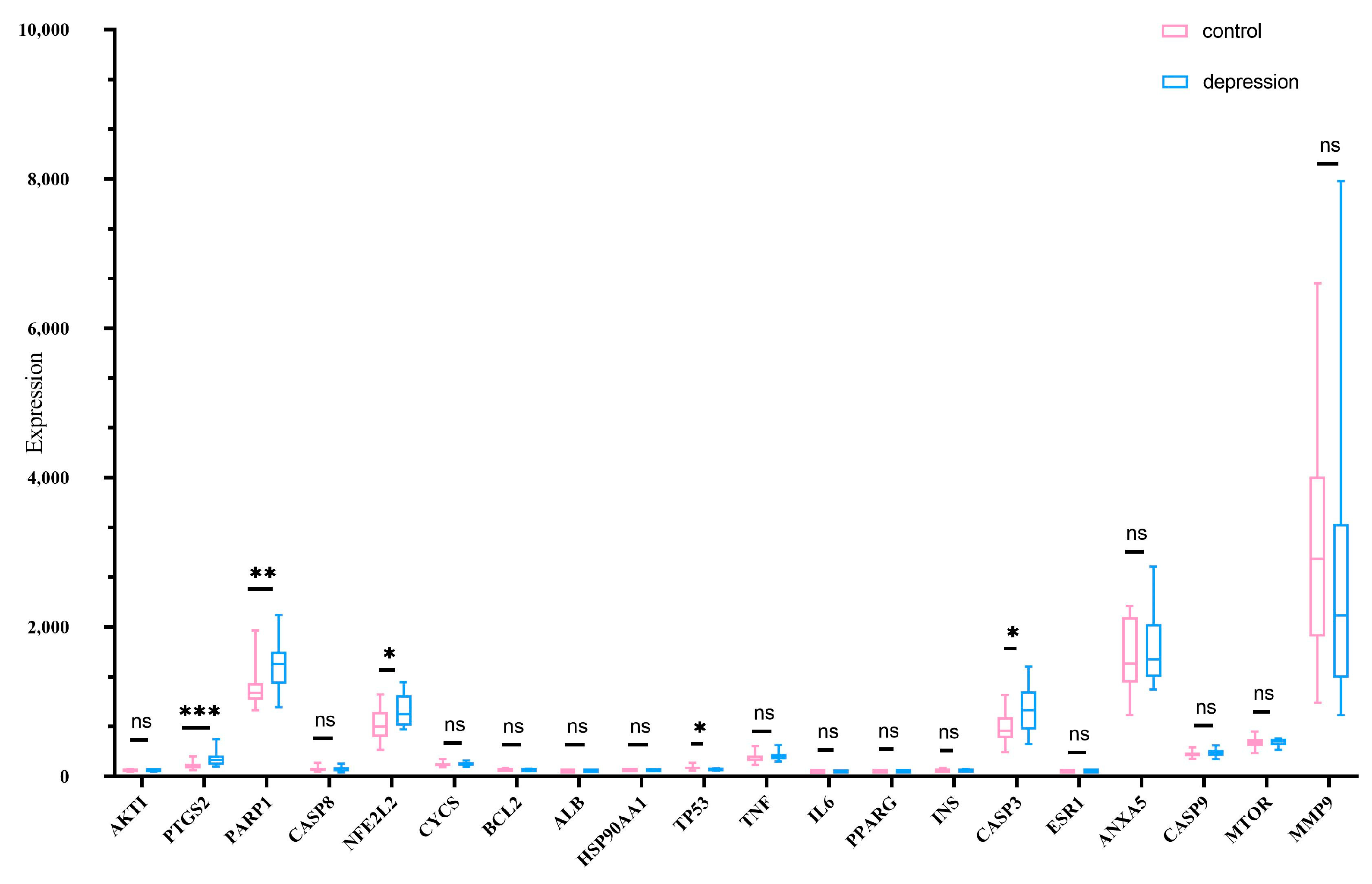
2.3. Randomized Dataset Validation of Core Genes
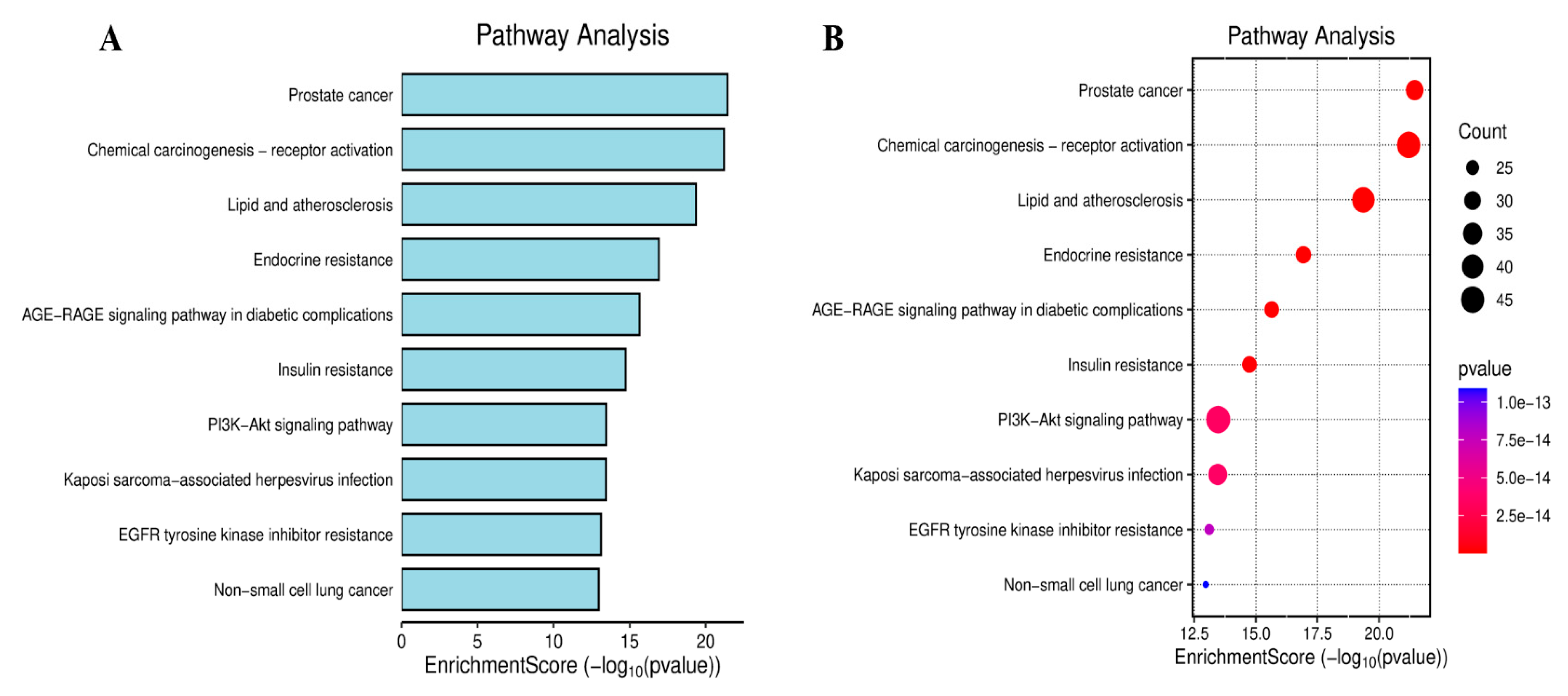
2.4. Enrichment Analyses for GO Terms and KEGG Pathways
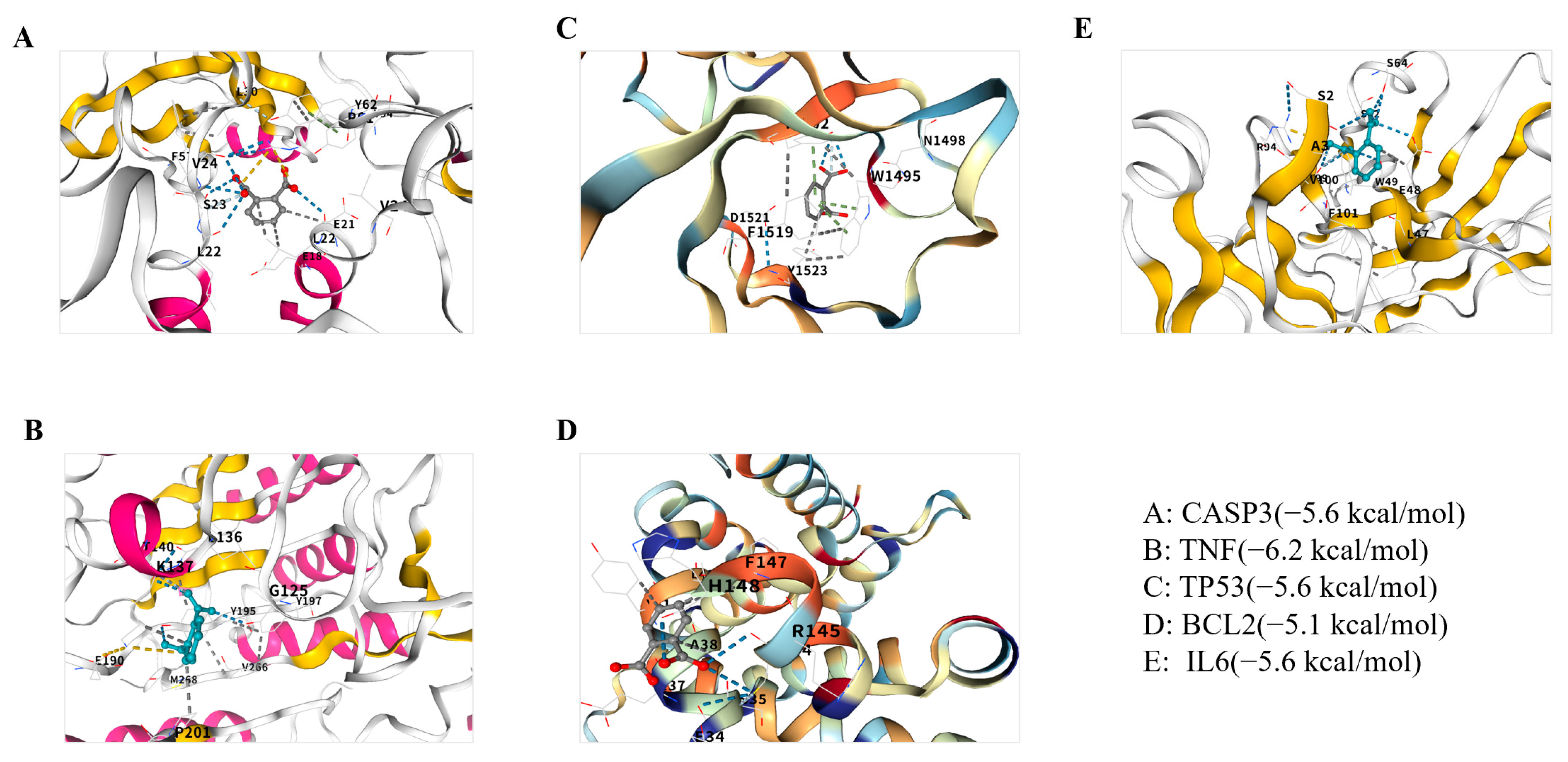
2.5. Molecular Docking of Phthalates with Depression Core Targets
3. Discussion
4. Methods and Materials
4.1. Collection of Phthalates Targets
4.2. Depression Target Retrieval
4.3. ”Phthalates-Depression” Network Plot
4.4. Construction of Protein-Protein Interaction Networks
4.5. External Gene Expression Dataset Validation
4.6. Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) Enrichment Analyses
4.7. Molecular Docking of Phthalates to Core Targets
4.8. Data Sources
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Petrick, L.M.; Wise, L.A.; Colicino, E.; Horton, M.K.; Rabinovici, J.; Strauss, T.; Sarna, B.; Lerner-Geva, L.; Elovitz, M.A.; Wright, R.J.; et al. The Chemical Exposome on Ovarian Aging in Adult Women: A Narrative Review. Curr. Pollut. Rep. 2025, 11, 13. [Google Scholar] [CrossRef]
- Barbieri, M.V.; Gonzalez, M.P.; Araya Pique, V.; Blasco, J.; Eljarrat, E. Contaminants in fish and seafood from the marine environment: A global overview of current status and future perspective. Mar. Pollut. Bull. 2025, 219, 118319. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Liu, B.; Jia, B.; Zhu, K.; Wu, Z.; Tan, H.; Morawska, L.; Wang, L.; Chen, J. Comprehensive profiling of phthalic acid esters (PAEs) in air-conditioning filters from diverse indoor environments across 12 major cities in China. Environ. Pollut. 2025, 382, 126714. [Google Scholar] [CrossRef] [PubMed]
- Li, L.Y.; Li, Z.K.; Shu, J.; Fan, X.B.; Yu, X.F.; Wang, M.Q. A DIE responsive fluorescent probe for phthalate and its application in test paper and hydrogel detection platforms. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2025, 341, 126403. [Google Scholar] [CrossRef]
- Sakai, H.; Murakami, K.; Kobayashi, S.; Suga, H.; Sasaki, S.; Three-generation Study of Women on Diets and Health Study Group. Food-based diet quality score in relation to depressive symptoms in young and middle-aged Japanese women. Br. J. Nutr. 2017, 117, 1674–1681. [Google Scholar] [CrossRef]
- Chen, Y.; Weng, X.; Hu, Y.; Yin, J.; Liu, S.; Zhu, Q.; Hu, L.; Liao, C.; Jiang, G. Neonatal exposure to phthalates and their alternatives and associated thyroid disorders: Levels, potential health risks, and mechanisms. J. Environ. Sci. 2025, 156, 519–538. [Google Scholar] [CrossRef]
- Huo, C.Y.; Liu, L.Y.; Wang, L.; Sun, Y.; Du, S.; Song, L.; Li, Y.Y.; Wang, X.H.; Li, W.L. Distribution and partitioning behavior of phthalate esters (PAEs) and non-phthalate ester plasticizers (NPPs) in a home from northern China. Environ. Pollut. 2025, 381, 126532. [Google Scholar] [CrossRef]
- Fernandez-Arribas, J.; Moreno, T.; Eljarrat, E. Plastic additives in the diet: Occurrence and dietary exposure in different population groups. J. Hazard. Mater. 2025, 493, 138317. [Google Scholar] [CrossRef] [PubMed]
- Quasmi, M.N.; Singh, J.; Kumar, D.; Dhingra, D.; Jangra, A. Insights into the molecular mechanisms underlying phthalates-induced nephrotoxicity. Toxicology 2025, 516, 154187. [Google Scholar] [CrossRef]
- Mokdad, A.H.; Forouzanfar, M.H.; Daoud, F.; Mokdad, A.A.; El Bcheraoui, C.; Moradi-Lakeh, M.; Kyu, H.H.; Barber, R.M.; Wagner, J.; Cercy, K.; et al. Global burden of diseases, injuries, and risk factors for young people’s health during 1990–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2016, 387, 2383–2401. [Google Scholar] [CrossRef]
- Galli, M.; Baini, M.; Panti, C.; Concato, M.; Fossi, M.C. Microplastic ingestion and Phthalate ester levels as plastic tracers in the Mediterranean Velella velella: A candidate plastic indicator for the pelagic neustonic environment. Mar. Pollut. Bull. 2025, 217, 118133. [Google Scholar] [CrossRef]
- Meeker, J.D.; Ferguson, K.K. Relationship between urinary phthalate and bisphenol A concentrations and serum thyroid measures in U.S. adults and adolescents from the National Health and Nutrition Examination Survey (NHANES) 2007–2008. Environ. Health Perspect. 2011, 119, 1396–1402. [Google Scholar] [CrossRef]
- Modesto, T.; Tiemeier, H.; Peeters, R.P.; Jaddoe, V.W.; Hofman, A.; Verhulst, F.C.; Ghassabian, A. Maternal Mild Thyroid Hormone Insufficiency in Early Pregnancy and Attention-Deficit/Hyperactivity Disorder Symptoms in Children. JAMA Pediatr. 2015, 169, 838–845. [Google Scholar] [CrossRef]
- Sun, D.; Zhou, L.; Wang, S.; Liu, T.; Zhu, J.; Jia, Y.; Xu, J.; Chen, H.; Wang, Q.; Xu, F.; et al. Effect of Di-(2-ethylhexyl) phthalate on the hypothalamus-pituitary-thyroid axis in adolescent rat. Endocr. J. 2022, 69, 217–224. [Google Scholar] [CrossRef]
- Sjostrom, Y.; Holmes, B.; Ricklund, N.; Struwe, N.; Hagstrom, K.; Hagberg, J.; Larsson, M. Endocrine disruption potential of dust in children’s indoor environments: Associations with multiple chemicals from various compound classes across exposure matrices used for health risk assessment. Environ. Res. 2025, 278, 121614. [Google Scholar] [CrossRef] [PubMed]
- Miodovnik, A.; Engel, S.M.; Zhu, C.; Ye, X.; Soorya, L.V.; Silva, M.J.; Calafat, A.M.; Wolff, M.S. Endocrine disruptors and childhood social impairment. Neurotoxicology 2011, 32, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Wang, Y.X.; Chen, W.J.; Zhang, Y.; Li, H.H.; Xiong, L.; Zhu, H.P.; Chen, H.Y.; Peng, S.X.; Wan, Z.H.; et al. Associations of phthalates exposure with attention deficits hyperactivity disorder: A case-control study among Chinese children. Environ. Pollut. 2017, 229, 375–385. [Google Scholar] [CrossRef]
- Won, E.K.; Kim, Y.; Ha, M.; Burm, E.; Kim, Y.S.; Lim, H.; Jung, D.E.; Lim, S.; Kim, S.Y.; Kim, Y.M.; et al. Association of current phthalate exposure with neurobehavioral development in a national sample. Int. J. Hyg. Environ. Health 2016, 219, 364–371. [Google Scholar] [CrossRef]
- Fan, Z.; Maisaidi, R.; Reheman, Y.; Li, Y.; Zhou, P.; Han, L. Shared molecular mechanisms of bisphenol A and phthalates in endometriosis: A bioinformatics and molecular docking study. Ecotoxicol. Environ. Saf. 2025, 299, 118388. [Google Scholar] [CrossRef]
- Shukla, R.; Kannan, A.; Laws, M.J.; Wagoner Johnson, A.; Flaws, J.A.; Bagchi, M.K.; Bagchi, I.C. Exposure to phthalates enhances estrogen and beta-catenin signaling pathways, leading to endometrial hyperplasia in mice. Toxicol. Sci. 2025, 206, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Gao, K.; Hua, K.; Wang, S.; Chen, X.; Zhu, T. Exploring the reproductive exposure risks of phthalates and organophosphates in atmospheric particulate matter based on quantitative structure-activity relationships and network toxicology models. J. Hazard. Mater. 2025, 488, 137395. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhou, W.; Wang, H.; Zhang, J.; Sun, X.; Qu, Z.; Yu, J.; Cai, Z.; Xu, X. Down-regulation of TET2 inhibits testosterone synthesis in offspring mice exposed to DBP during pregnancy through LH/cAMP/PKA/StAR signaling mediated by LHR. Ecotoxicol. Environ. Saf. 2025, 293, 118025. [Google Scholar] [CrossRef] [PubMed]
- Irniger, C.; Vetter, J.; Weidt, S.; Seifritz, E.; Holtforth, M.G.; Krahenmann, R. From past to present: The connection of traumatic childhood experiences, recent stressful life events, and treatment expectations in depression. Eur. J. Psychotraumatol. 2025, 16, 2518625. [Google Scholar] [CrossRef] [PubMed]
- Kanchapogu, N.R.; Nandan Mohanty, S. Deep learning with ensemble-based hybrid AI model for bipolar and unipolar depression detection using demographic and behavioral based on time-series data. Dialogues Clin. Neurosci. 2025, 27, 16–35. [Google Scholar] [CrossRef]
- Chagraoui, A.; Thibaut, F.; De Deurwaerdere, P. 5-HT6 receptors: Contemporary views on their neurobiological and pharmacological relevance in neuropsychiatric disorders. Dialogues Clin. Neurosci. 2025, 27, 112–128. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, J.; Xin, Y.; Ma, J.; Chen, T.; Nie, J.; Niu, P. Associations between phenols, parabens, and phthalates and depressive symptoms: The role of inflammatory markers and bioinformatic insights. Ecotoxicol. Environ. Saf. 2024, 286, 117191. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Yan, B.; Yang, L.; Deng, L.; Xue, X.; Gao, M.; Wei, H.; Chen, S.; Wu, Y.; Yang, X.; et al. Prenatal co-exposure to diisodecyl phthalate and ozone contribute to depressive behavior in offspring mice through oxidative stress and TWIST1 participation. Sci. Total Environ. 2024, 928, 172411. [Google Scholar] [CrossRef]
- Hu, L.; Mei, H.; Feng, H.; Huang, Y.; Cai, X.; Xiang, F.; Chen, L.; Xiao, H. Exposure to bisphenols, parabens and phthalates during pregnancy and postpartum anxiety and depression symptoms: Evidence from women with twin pregnancies. Environ. Res. 2023, 221, 115248. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, Z.; Zhu, R.; Gou, D.; Jia, P.P.; Pei, D.S. An insight into sex-specific neurotoxicity and molecular mechanisms of DEHP: A critical review. Environ. Pollut. 2023, 316, 120673. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, J.; Fu, Q.; Zhao, H.; Wang, Z.; Gao, Y. Efficient evaluation of osteotoxicity and mechanisms of endocrine disrupting chemicals using network toxicology and molecular docking approaches: Triclosan as a model compound. Ecotoxicol. Environ. Saf. 2025, 293, 118030. [Google Scholar] [CrossRef]
- Kim, S.H.; Hong, S.M.; Ko, E.J.; Park, M.J.; Kim, J.Y.; Kim, S.Y. Neuroprotective Effects of a Combination of Dietary Trans-Resveratrol and Hesperidin Against Methylglyoxal-Induced Neurotoxicity in a Depressive Amnesia Mouse Model. Nutrients 2025, 17, 1548. [Google Scholar] [CrossRef]
- Zhou, J.; Wu, Z.; Zhao, P. Luteolin and its antidepressant properties: From mechanism of action to potential therapeutic application. J. Pharm. Anal. 2025, 15, 101097. [Google Scholar] [CrossRef]
- Aquino, G.A.; Sousa, C.N.S.; Santos Junior, M.A.D.; Cavalcante, L.R.L.; Forte, A.; Mota, M.R.L.; Martinez, F.E.O.; Almeida, T.S.; Leal, L.; Vasconcelos, S.M.M. Alpha-lipoic acid as a modulator of healing in an animal model of pressure injury: Inflammatory implications and its relationship with depressive disorders. Eur. J. Pharmacol. 2025, 1002, 177769. [Google Scholar] [CrossRef] [PubMed]
- Pienaar, L.; Ramsamy, A.; Millen, A.M.E.; Baijnath, S. Regional Molecular Changes in Chronic Lipopolysaccharide-Induced Neuroinflammation. Biol. Psychiatry Glob. Open Sci. 2025, 5, 100515. [Google Scholar] [CrossRef]
- Bai, Y.; Niu, L.; Song, L.; Dai, G.; Zhang, W.; He, B.; San, W.; Li, S. Uncovering the effect and mechanism of Jiawei Xiaoyao Wan in treating breast cancer complicated with depression based on network pharmacology and experimental analysis. Phytomedicine 2024, 128, 155427. [Google Scholar] [CrossRef]
- La Rosa, S.; Furlan, D.; Franzi, F.; Battaglia, P.; Frattini, M.; Zanellato, E.; Marando, A.; Sahnane, N.; Turri-Zanoni, M.; Castelnuovo, P.; et al. Mixed exocrine-neuroendocrine carcinoma of the nasal cavity: Clinico-pathologic and molecular study of a case and review of the literature. Head Neck Pathol. 2013, 7, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Li, H.; Du, H.; Sheng, L.; Huang, N.; Li, X.; Sun, R. Unravelling the mechanisms of Erchen decoction in the treatment of nonalcoholic fatty liver disease (NAFLD): An integrative study combining network pharmacology, molecular docking, molecular dynamics simulation, multi-omics analysis, and experimental validation. J. Ethnopharmacol. 2025, 353, 120216. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, B.; Liu, L.; Huang, X.; Cai, Y.; Liao, L.; Min, X.; Gu, Y. The mechanistic study of quercetin in the treatment of alcoholic brain injury via the JNK/P38 MAPK signaling pathway. Apoptosis 2025, 30, 1875–1892. [Google Scholar] [CrossRef]
- Huo, K.; Xu, J.; Wei, M.; Ma, K.; Wang, J.; Han, J. Solasonine ameliorates cerebral ischemia-reperfusion injury via suppressing TLR4/MyD88/NF-kappaB pathway and activating AMPK/Nrf2/HO-1 pathway. Int. Immunopharmacol. 2023, 124, 110862. [Google Scholar] [CrossRef]
- Awad-Igbaria, Y.; Sakas, R.; Milhem, L.; Fishboom, T.; Ben-Menashe, A.; Edelman, D.; Shamir, A.; Soustiel, J.F.; Palzur, E. Mitochondrial translocator-protein ligand etifoxine reduces pain symptoms and protects against motor dysfunction development following peripheral nerve injury in rats. Neuropharmacology 2025, 273, 110456. [Google Scholar] [CrossRef]
- Vozella, V.; Borgonetti, V.; Cruz, B.; Onge, C.M.S.; Bullard, R.; Vlkolinsky, R.; Ceballos, D.G.; Ozburn, A.R.; Roberts, A.J.; Ciccocioppo, R.; et al. Apremilast reduces co-occurring alcohol drinking and mechanical allodynia and regulates central amygdala GABAergic transmission. JCI Insight 2025, 10, e189732. [Google Scholar] [CrossRef] [PubMed]
- De Souza, I.R.; Iulini, M.; Galbiati, V.; Rodrigues, A.C.; Gradia, D.F.; Andrade, A.J.M.; Firman, J.W.; Pestana, C.; Leme, D.M.; Corsini, E. The evaluation of skin sensitization potential of the UVCB substance diisopentyl phthalate by in silico and in vitro methods. Arch. Toxicol. 2024, 98, 2153–2171. [Google Scholar] [CrossRef]
- Lei, S.S.; Wang, Y.Y.; Huang, X.W.; Wang, X.P.; Gao, M.; Li, B. Epimedium brevicornum Maxim alleviates diabetes osteoporosis by regulating AGE-RAGE signaling pathway. Mol. Med. 2025, 31, 101. [Google Scholar] [CrossRef] [PubMed]
- Stufchen, I.; Schweizer, J.; Volter, F.; Nowak, E.; Braun, L.; Kocabiyik, J.; Mederos, Y.S.M.; Williams, T.A.; Kunz, S.; Bidlingmaier, M.; et al. The impact of endocrine disrupting chemicals on adrenal corticosteroids—A systematic review of epidemiological studies. Environ. Res. 2025, 276, 121438. [Google Scholar] [CrossRef] [PubMed]


| Smile | Structure |
|---|---|
| C1=CC=C(C(=C1)C(=O)[O-])C(=O)[O-] | 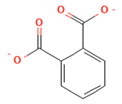 |
| Database Name | Access Address |
|---|---|
| TargetNet | Index-Home-TargetNet |
| PharmMapper | https://www.lilab-ecust.cn/pharmmapper/ |
| GeneCards | https://www.genecards.org/ |
| OMIM | https://www.omim.org/ |
| UniProt | https://www.uniprot.org/ |
| STRING | https://cn.string-db.org/ |
| GEO | https://www.ncbi.nlm.nih.gov/gds |
| CB-DOCK2 | CB-Dock2: An accurate protein-ligand blind docking tool |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, R.; Wen, H.; Lin, Z.; Li, B.; Zhou, X.; Wang, Q. Molecular Mechanisms of Phthalates in Depression: An Analysis Based on Network Toxicology and Molecular Docking. Int. J. Mol. Sci. 2025, 26, 8215. https://doi.org/10.3390/ijms26178215
Zhang R, Wen H, Lin Z, Li B, Zhou X, Wang Q. Molecular Mechanisms of Phthalates in Depression: An Analysis Based on Network Toxicology and Molecular Docking. International Journal of Molecular Sciences. 2025; 26(17):8215. https://doi.org/10.3390/ijms26178215
Chicago/Turabian StyleZhang, Ruiqiu, Hairuo Wen, Zhi Lin, Bo Li, Xiaobing Zhou, and Qingli Wang. 2025. "Molecular Mechanisms of Phthalates in Depression: An Analysis Based on Network Toxicology and Molecular Docking" International Journal of Molecular Sciences 26, no. 17: 8215. https://doi.org/10.3390/ijms26178215
APA StyleZhang, R., Wen, H., Lin, Z., Li, B., Zhou, X., & Wang, Q. (2025). Molecular Mechanisms of Phthalates in Depression: An Analysis Based on Network Toxicology and Molecular Docking. International Journal of Molecular Sciences, 26(17), 8215. https://doi.org/10.3390/ijms26178215




